Abstract
Mice are increasingly overtaking the rat model organism in important aspects of anxiety research, including drug development. However, translating the results obtained in mouse studies into information that can be applied in clinics remains challenging. One reason may be that most of the studies so far have used animals displaying ‘normal’ anxiety rather than ‘psychopathological’ animal models with abnormal (elevated) anxiety, which more closely reflect core features and sensitivities to therapeutic interventions of human anxiety disorders, and which would, thus, narrow the translational gap. Here, we discuss manipulations aimed at persistently enhancing anxiety-related behavior in the laboratory mouse using phenotypic selection, genetic techniques and/or environmental manipulations. It is hoped that such models with enhanced construct validity will provide improved ways of studying the neurobiology and treatment of pathological anxiety. Examples of findings from mouse models of enhanced anxiety-related behavior will be discussed, as well as their relation to findings in anxiety disorder patients regarding neuroanatomy, neurobiology, genetic involvement and epigenetic modifications. Finally, we highlight novel targets for potential anxiolytic pharmacotherapeutics that have been established with the help of research involving mice. Since the use of psychopathological mouse models is only just beginning to increase, it is still unclear as to the extent to which such approaches will enhance the success rate of drug development in translating identified therapeutic targets into clinical trials and, thus, helping to introduce the next anxiolytic class of drugs.
Keywords: anxiety disorders, anxiolytic, benzodiazepine, drug development, inborn anxiety, mutant mice, neurokinin 1 receptor, neuropeptide S, psychopathology, stress
From physiological to pathological anxiety
Anxiety and fear, along with happiness, sadness, anger and shame, are basic emotions accompanying human beings throughout their whole life [1]. A clear delineation between anxiety and fear has, in general, turned out to be difficult and often depends on the discipline [2]. In the neurosciences, anxiety is defined as the response to an undetermined, potentially dangerous situation, while fear is defined as the response to an explicit hazard [2]. Therefore, although anxiety and fear appear to be closely related, there is increasing evidence that, qualitatively, trait fear and anxiety are two largely distinct emotions in terms of behavioral responses [2-3]. From an evolutionary perspective, anxiety is a reasonable and useful effect, warning us about danger and initiating adequate somatic, cognitive, emotional and behavioral responses in order to avoid harm and, thus, elevate the chances of survival. Nowadays, most of the anxiety states we experience are acceptable providing that they quicken our responses so that we can get through the situation, adequately cope with stressful challenges and that they cease soon after. However, in some individuals, these anxiety reactions may become persistent, uncontrollable, excessive and inappropriate, even after the withdrawal of the stimulus, lacking any adaptive value, and negatively influencing the quality of their everyday life [601]. Such reactions, which persist for at least 6 months, characterize pathological anxiety [601]. Pathological anxiety can be described as either a quantitative or qualitative variation of a normal state; which it is, however, still a matter of debate [3-5]. What it is that causes the crossing from physiological to pathological anxiety is a subject sparking intense research interest.
There is evidence that the simultaneous occurrence of various intrinsic and extrinsic factors is important for the etiology of an anxiety disorder. Specifically, a 30–40% estimated heritability across anxiety disorders suggests a moderate risk factor in the manifestation of an anxiety dis order [6]. Moreover, environmental factors such as diverse (negative) experiences, including early life stress, have been demonstrated to exert a critical impact on the development of an anxiety disorder [7,8]. Interestingly, the individual’s susceptibility to environmental factors is modulated by a diverse range of genes [9-10].
Today, anxiety disorders are the most common neuropsychiatric disorders in the USA and Europe [11,12]. They represent some of the major health problems in the Western world in terms of costs of healthcare, sick-leave from work, disabilities and premature mortality [13]. Anxiety disorders encompass a spectrum of disorders exhibiting a wide range of symptoms and different degrees of severity, as well as variations in terms of age of onset, prevalence in males and females, and treatment responses. In addition, many patients with an anxiety disorder also suffer from other psychiatric disorders (e.g., 60% comorbidity with depression) and/or physical or organic diseases, further complicating the syndrome pattern [7,14,15]. The categorical classification systems of mental illnesses [16], both the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [17] and the International Statistical Classification of Diseases and Related Health Problems (ICD-10) [18], distinguish between several human anxiety syndromes, including generalized anxiety disorder, social phobia, specific phobias, panic disorder, post-traumatic stress disorder and obsessive-compulsive disorder. Interestingly, these classification systems do not distinguish categorically between anxiety and fear. These subdisorders have, in part, different sensitivities to anxiolytic treatments [19,20] and it is thought that they differ in their underlying neurobiology (e.g. [21,22]). However, there is also substantial comorbidity within these subcategories and what they do all have in common is an irrationally intense, uncontrollable feeling of anxiety, which suggests that these disorders have partly overlapping neurocircuitries (e.g., [21-23]).
Various classes of anxiolytic drugs are available for the treatment of anxiety disorders. The benzodiazepines and selective serotonin reuptake inhibitors (SSRIs) are the current first-line treatments in most anxiety disorders [19,20,24]. However, these drugs are far from being ideal as SSRIs are characterized by partial nonresponse, a delayed onset of therapeutic action, a considerable high rate of relapse and adverse side effects, while benzodiazepines induce sedation and have a risk of drug dependence and tolerance, limiting their use mainly to short-term treatment [19,20,24]. Therefore, within the last 20 years, both the pharmaceutical industry and academia have put great efforts into anxiety research and have invested heavily in it. To date, no novel anxiolytic class with an improved pharmacological profile has progressed from its discovery, via preclinical and clinical trials, onto the market. This fact suggests that the strategies followed so far are not optimal and, thus, need to be refined. In the course of drug development, the anxiolytic properties of substances are initially detected using mostly rodents in screening tests assessing anxiety-related behavior (see ‘The assessment of anxiety-related behavior in mice’). At the moment, it is still a matter of debate as to whether these anxiety tests merely elicit physiological responses to an anxiety-provoking situation rather than reflecting abnormal, pathological anxiety as observed in clinically manifested anxiety disorders [25]. Therefore, another idea is to create animal models that closely mimic clinically manifested forms of anxiety and that are quantitatively and/or qualitatively superior to anxiety tests according to a proposed theory of pathological anxiety [4]. In this respect, validated animal models of enhanced anxiety-related behavior with face, construct and predictive validity (see ‘Validity of mouse models of enhanced anxiety’) may be pivotal to an improved understanding of the neurobiological and genetic mechanisms underlying anxiety disorders, allowing causal hypotheses to be tested and, thus, leading to the discovery of new treatment targets. In this respect, the laboratory mouse has become an invaluable preclinical tool.
From the human anxiety disorder to the mouse model organism
The mouse in anxiety research
Rodents have always played a central role in neuropsychiatric research. In particular, the rat has been the species of choice for many years since the sampling of tissue and diverse fluids, such as blood, can be relatively easily accomplished using appropriate invasive techniques compared with the mouse. Furthermore, in behavioral pharmacology, rats usually perform well in many cognitive and operant tasks. Nevertheless, over the last two decades laboratory mice have become competitors of the rat in anxiety research and this appears to parallel the increasing use of sophisticated molecular techniques such as gene targeting [26]. These molecular techniques, which can be applied particularly to the mouse genome [27], allow scientists to introduce genetic modifications at will in order to study the neurobiological basis of an anxious phenotype [26,28] or to confirm identified genetic associations underlying enhanced anxiety-related behavior at a functional level [29]. Therefore, the laboratory mouse is recognized as the pre-eminent model for modern genetic research into psychiatric diseases. Apart from this genetic aspect, mice offer some practical and economic advantages over rats in terms of their small size (e.g., dosing) and their minimal requirement of space and food.
The evolutionary relationship between humans & mice in anxiety regulation
Although the mouse and the human genome diverged approximately 75 million years ago, they have similar gene functions and share a number of neuroanatomical, neurochemical and behavioral commonalities. The mouse genome is smaller than the human genome, but there are only a few cases, in which no mouse counterpart can be found for a particular human gene [30]. Furthermore, in both humans and mice, the brain is structured into cerebral hemispheres, with a forebrain, a diencephalon, a midbrain, a hindbrain and a cerebellum [31]. While subcortical structures processing anxiety, such as the amygdala, the hippocampus, the thalamus and the hypothalamus are well conserved between the two species, the murine cerebral cortex is clearly reduced in comparison to that of man. Nevertheless, it retains many features representing the fundamental principles of cortical organization, function and development [32]. In this respect, the brain regions of humans and mice implicated in the processing of anxiety, involving the prefrontal cortex, the amygdala and the hippocampus, among others, are phylogenetically related [33-35]. Within these structures the neurotransmitter/modulator systems involved in signaling anxiety-related information are highly preserved, with considerable functional homology. Finally, anxiety reactions are evolutionarily adaptive and, thus, generally highly conserved in mammals [36]. Indeed, when species-specific inborn characteristics are taken into consideration, analogous physiological and behavioral changes can be observed in mice and humans in response to an anxiety-provoking stimuli [33]. Typical anxiety features including the fight/flight responses, avoidance, freezing, urination/defecation, attention/vigilance, autonomic hyperarousal or muscular tension are present in both species.
The assessment of anxiety-related behavior in mice
In the laboratory mouse, an easily accessible behavioral readout, which is thought to reflect the emotional component of anxiety such as avoidance, escape or freezing behavior may be used as marker of a state of enhanced anxiety. Therefore, such behavior is often referred to as ‘anxiety-related’ or ‘anxiety-like’ behavior rather than as anxiety per se. Accordingly, numerous behavioral tests have been developed, originally in the rat, to assess the level of anxiety-related behavior. However, with the increasing use of the mouse it has become necessary to adapt these tests for the smaller species, and this has been done with varying degrees of success (e.g. [26,37]). In the field, these tests are often referred to as ‘model’, which can be misleading. As they elicit an acute emotional response, they are often considered as tests revealing ‘state’ anxiety [38] as opposed to ‘model’, which is thought to evoke pathology [39].
Tests of anxiety-related behavior can be roughly classified into unconditioned (ethologically-based procedures) and conditioned (learned procedures) (boxes 1 & 2) [37,40]. While unconditioned paradigms utilize the natural or spontaneous reactions of a mouse to an innate, aversive stimulus (e.g., avoidance of exposed, brightly lit spaces), conditioned responses involve training sessions in which usually a neutral or rewarding stimulus is paired with an aversive, mildly painful stimulus. Conditioning processes occur in the etiology of many anxiety disorders, including phobias and post-traumatic stress disorder [41,42]. The inhibition of the learned fear response is often disturbed in these disorders and this can be investigated – for example, in extinction paradigms in both humans [43] and animals demonstrating impaired extinction [44-46]. The key feature of all of these anxiety tests is their predictive validity (see following section) since in these paradigms the anxiety-related behavior can be reduced by the administration of clinically effective anxiolytics, especially benzodiazepines (e.g., [24,47-49]). Only a few studies have applied benzodiazepine-validated anxiety tests to investigate the anxiolytic effects of chronic antidepressant treatment (more than 2 weeks of treatment) [50]. The hyponeophagia test, the mouse defense test battery, stress induced hyperthermia and marble burying appear to be the most reliable paradigms in detecting anxiolytic properties of SSRIs in a manner consistent with the time-course of their effects in humans [48-52].
Box 1. Anxiety tests: acute anxious states (state anxiety).
Unconditioned tests
-
■Approach–avoidance conflict-based tests:
-
–Open-field test
-
–Light/dark test
-
–Elevated plus maze test
-
–Holeboard test
-
–
-
■Interaction-based conflict tests:
-
–Social interaction
-
–Resident intruder
-
–
-
■Others:
-
–Marble burying
-
–Hyponeophagia
-
–Stress-induced ultrasonic vocalizations
-
–Stress-induced hyperthermia
-
–
Conditioned (cognitive) tests
-
■Conflict-based:
-
–Geller–Seifter test
-
–Vogel conflict test
-
–Four-plate test
-
–Conditioned place aversion
-
–Conditioned taste aversion
-
–
-
■Others:
-
–Conditioned fear
-
–Conditioned emotional response
-
–
Box 2. Anxiety models: long-term enhanced anxious states (trait anxiety).
Inborn anxiety (e.g., strain, interindividual difference)
-
■
Strain differences
-
■
Interindividual differences
-
■
Genetic models
Environmental manipulations
-
■Adverse rearing:
-
–Maternal separation
-
–Early weaning
-
–Isolation housing
-
–
-
■Chronic stress exposure:
-
–Chronic mild stress
-
–Chronic social stress
-
–
Nutrient models
-
■
Magnesium deficiency
Pharmacological models
-
■
Chronic corticosterone treatment
-
■
Drug withdrawal
Short-term enhanced anxious states
-
■
Pharmacological models
Anxiety tests are frequently used in both industry and academia to screen for potential anxiolytic properties of compounds. Moreover, they fulfill face validity (see ‘Validity of mouse models of enhanced anxiety’), as the avoidance of the feared stimulus is a hallmark of many anxiety disorders. Indeed, these tasks are based on assumed analogies between human and mouse symptoms of anxiety; however, it is thought that anxiety-related measures, assessed in various tasks using mice as subjects, may reflect different features of murine anxiety [4,53]. The procedures, peculiarities, pitfalls and validity of these tests are discussed in detail in various excellent reviews and book chapters (e.g., [4,24,26,28,39,47-49,54]).
Anxiety tests can assess specific features of behavior, endocrinology or physiology that are symptomatic in anxiety disorders [25]. Therefore, in the present article both conditioned and unconditioned anxiety tests will be presented in terms of their behavioral characterization of the described mouse models of enhanced anxiety. Specifically, we focused on studies where the enhanced anxiety-related behavior of the mouse model was evaluated in tests of unconditioned anxiety that include the open-field test, the light/dark test, social interaction, the elevated plus or zero maze, ultrasonic vocalization and holeboard test, and in tests of conditioned anxiety that include the Vogel-conflict, fear-potentiated startle and conditioned-fear tasks. It should be emphasized that altered fear expression in the latter paradigm does not necessarily indicate altered fear but may be the result of changes in fear memory, and this must be controlled in any mouse model of enhanced anxiety-related behavior prior to further interpretation.
Mouse models of enhanced anxiety
A mouse model of enhanced anxiety-related behavior is aimed at reproducing the pathophysiology of the human anxiety disorder by experimentally manipulating the environment, the neurophysiology, the neurochemistry or the genetics (boxes 1 & 2) [25]. Such mouse models are thought to reflect ‘trait’ anxiety – that is, a persistent and enduring tendency of a genetically predisposed individual to demonstrate an increased anxiety response irrespective of whether it reflects quantitatively and/or qualitatively abnormal physiological anxiety [4]. Ideally, these murine models should be phenotyped using multiple tests for unconditioned and conditioned anxiety-related behaviors in order to account for the multifaceted nature of anxiety and, thus, for different forms of clinical anxiety. However, since unambiguous measures of anxiety disorder subcategory-specific endophenotypes correlated with neurobiological changes and/or biomarkers are not available, creating mouse models reflecting anxiety disorder subcategories is currently difficult.
Interestingly, many of the listed studies only chose a limited number of anxiety tests, with the majority focusing on anxiety-related behaviors related to exploration–avoidance conflict – most likely because they seem to be the easiest to perform. Other forms of anxiety, such as social conflict and learned fear (boxes 1 & 2), have been rarely considered. It should be noted that in all mouse models described in this section, potential discrepancies in observed anxiety-related traits may be explained by differences in the procedures prior to and/or during testing, including use of animals from different breeders, use of different sex and differences in the experimental setup as well as in the order in which the tests were carried out (e.g., [55-57]). These confounding factors may, thus, reduce the comparability of the results among different labs. In addition, the mouse models of enhanced anxiety-related behavior may also display signs of enhanced depression-related behavior, mirroring the high comorbidity between anxiety disorders and depression in humans [7,14]. Detailed discussion of aspects of this comorbidity, however, is beyond the scope of the present article.
Mice with naturally occurring high trait anxiety
Specific groups of mice display (genetically/epigenetically induced) enhanced levels of anxiety-related behavior. It may be that the anxious phenotype of a whole strain is elevated in comparison with that of another one, or that only individuals within one population are affected. This interindividual variability as a vulnerability factor for abnormal anxiety may provide the basis for a simple selection strategy, as well as for a breeding strategy. Since multiple genes (each with only little influence) are thought to contribute to the increased emotionality that represents the genetic risk factor of human anxiety disorders, pairs of mice exhibiting enhanced and normal anxiety-related behavior indeed form excellent models for investigating the genetic complexity of enhanced (pathological) anxiety.
Inbred mouse strains
With more than 450 strains accessible, inbred strains of mice provide a large natural source of a variety of genotypes as well as phenotypes for comparison [58]. A mouse strain is considered inbred when brother × sister mating has occurred for 20 or more generations and at least 98.6% homozygosity of the loci in each mouse is demonstrated [59]. The characteristic behavioral and physical phenotypes of inbred mice are very stable as they are inherited by multiple genes. Inbred strains with a distinct behavioral trait are widely used in neuroscience owing to various advantages, including allowing the possibility of overcoming the problem of high genetic heterogeneity that is found in human studies [60,61].
Numerous studies have evaluated the inborn level of anxiety-related behavior of different inbred mouse strains, mainly using unconditioned tests of anxiety including the elevated plus or zero maze, and open-field and light/dark tests. Table 1 summarizes strains of mice displaying enhanced anxiety-related behavior in comparison with C57Bl/6 mice. The C57Bl/6 strain was chosen as the comparator strain because it is the most popular common mouse strain used in anxiety research [62]. Furthermore, C57Bl/6 mice are categorized as a strain with moderate naturally occurring levels of anxiety despite some minor differences between the J and N subtypes [63]. Therefore, wherever it is possible, we use the correct name of C57Bl/6 mice. Any other mouse strain displaying modest anxiety-related behaviors, apart from the C57Bl/6 strain, may be used as the control group.
Table 1. Mouse models of enhanced anxiety-related behavior by strain adifferences.
| Anxious strain |
Comparator strain |
Anxiety-related behavior | |||||
|---|---|---|---|---|---|---|---|
| Unconditioned anxiety | Conditioned anxiety | ||||||
| 129/Sv | C57Bl/6 | ↑ | EPM | [334-335] | – | – | – |
| ~ | EPM, LD, OF | [334,336-337] | – | – | – | ||
| ↓ | EPM, SI | [338] | – | – | – | ||
| 129S1/ SvImJ |
C57Bl/6 | ↑ | OF, LD | [107,124,339] | ↑ | CF | [345] |
| ~ | EPM, EZM, LD, OF, SI | [124,339,341-342] | – | (imp-EXT) | [44,46,343] | ||
| 129/SvEv | C57Bl/6 | ↑ | EPM | [337] | – | – | – |
| 129S6/SvEv | C57Bl/6 | ↑ | EPM, LD, OF | [107,344-346] | ~ | CF | [346] |
| ↑ | CF | [345] | |||||
| A/J† | C57Bl/6† | ↑ | EPM, EZM, LD, HB, mHB, OF, SI, USV |
[60,68,107,339,341,342,344,345,347-351,369] | ↑ | CF, VC (imp-EXT) |
[347,349,352] [349] |
| ~ | EPM, HB, LD, SI | [340,348,349] | ~ | CF, FPS, VCT | [345,347,349] | ||
| – | ↓ | CF | [354] | ||||
| AKR/J | C57Bl/6 | ↑ | EPM, EZM, LD, OF | [342,344,347,348,355] | ↑ | CF, FPS | [347] |
| ~ | OF, EZM | [342,344,348] | – | – | – | ||
| BALB/c† | C57Bl/6† | ↑ | EPM, EZM, LD, OF, SI | [60,65,107,124,339,342,344,347,348,351, 356-365] |
↑ | CF, FPS (imp-EXT) |
[345,347,366] [354] |
| ~ | EPM, OF, SI | [65,124,336,337,341,344,345,367-370] | ~ | CF, FPS | [345,368] | ||
| ↓ | EPM, OF | [340,367,371,372] | ↓ | CF | [354] | ||
| C3H/He | C57Bl/6 | ↑ | EPM, LD, USV | [339,344,347,350,358,367,368] | ↑ | FPS | [368] |
| ~ | EPM, EZM, OF, SI | [65,335,336,339,342,344,345,350,367,369,372, 373] |
~ | CF | [347] | ||
| ↓ | LD, OF, EPM | [107,340,342,348] | ↓ | CF | [345] | ||
| DBA/2J† | C57BL/6† | ↑ | EPM, LD, OF, USV | [107,339,342,344,346- 348,349,350,361,362,365,368,370,373] |
↑ | CF, FPS | [345,347] |
| ~ | EPM, EZM, OF, SI | [336,340,342,345,347,350,370] | ~ | CF, FPS | [347,368,374] | ||
| ↓ | EPM, SI | [341,348] | ↓ | CF | [345,346,375] | ||
Inbred mouse lines with strongest evidence for increased anxiety-related behavior.
↑: Increased anxiety-related behavior; ↓: Decreased anxiety-related behavior; ~: Unaltered anxiety-related behavior; CF: Conditioned fear; EPM: Elevated plus maze; EZM: Elevated zero maze; FPS: Fear-potentiated startle; HB: Holeboard test; imp-EXT: Impaired extinction of conditioned fear; LD: Light/dark test; mHB: Modified holeboard test; OF: Open-field test; SI: Social interaction; USV: Stress-induced ultrasonic vocalization; VC: Vogel conflict test.
Signs of enhanced anxiety-related behavior have been reported in mice of the 129, A/J, AKR/J, BALB/c, C3H/He and DBA/2J strains, when compared with the C57Bl/6 strain, in some – though not all – tasks assessing unconditioned and conditioned anxiety (see Table 1). Of these, the BALB/c, the A/J and DBA/2J strains display the strongest evidence of increased emotionality in the elevated plus maze, open-field and light/dark tests compared with C57Bl/6 mice, while no differences and even lower levels of inborn anxiety have also been described in BALB/c mice or in the other two strains compared with C57Bl/6 mice that have undergone the same tests (Table 1). For further phenotypic characterizations of different mouse strains, the mouse phenome database may also be consulted, providing a useful tool with which to judge and compare anxiety-related traits of mouse strains [602]. Moreover, inbred strains of mice also display altered responsiveness to anxiolytics, although all strains seem to respond to the anxiolytic effects of benzodiazepines [4,64-67]. Despite some discrepancies between studies, BALB/c, A/J and DBA/2J strains are suggested to represent the best inbred mouse strain models for pathological anxiety [4,68].
In contrast to the mouse strains discussed so far, inbred 129P3/J mice initially show little anxiety-related behavior towards an aversive environment compared with the anxious BALB/c mice. However, repeated exposure to the environment does not cause a waning of the behavioral responses in mice, rather, anxiety-related behavior increases in 129P3/J mice compared with BALB/c mice [69]. Therefore, it is suggested that 129P3/J mice may be an example of a mouse model of nonadaptive anxiety. However, this proposed model has yet to be tested carefully before further conclusions can be drawn. Furthermore, C57Bl/6N mice have been suggested to represent a mouse model specific for post-traumatic stress disorder [70]. Subjects of this strain display persistent and increasingly sensitized fear after exposure to a single, brief electric footshock, which coincides with blunted emotionality in the modified holeboard test and the social interaction [70]. This mouse model enables vulnerable and resistant individuals to be distinguished for developing signs of post-traumatic stress disorder in response to an aversive encounter, mimicking important aspects of epidemiological observations in humans exposed to trauma [71].
Finally, crosses of phenotypically contrasting strains (e.g., of an anxious and a nonanxious inbred strain, such as A/J and C57Bl/6J) are the origin of recombinant inbred strains such as the AXB/BXA or BXD strains [72-73] and recombinant congenic mouse strains such as the AcB/BcA [72]. Intercrosses and backcrosses, together with inbred mouse and recombinant mouse lines, are widely used for the genetic dissection of anxiety traits in the mouse – as, for example, carried out within the scope of quantitative trait loci (QTL) mapping studies [74] (see ‘Genetic underpinnings of the anxiety trait’). In all of these approaches, one has to keep in mind that inbred strains, while differing in trait anxiety, additionally differ in an unknown number of other traits, which makes unambiguous interpretations of phenotype–genotype associations difficult, if not impossible.
Selective breeding lines in mice
As an alternative approach to the uncontrolled inbreeding of mouse strains, selective breeding strategies for a specific endophenotype within one strain have proved to represent a powerful tool for investigating the genetic variability of complex, polygenetic traits such as anxiety [75]. The principle idea is to cluster a specific trait around the extremes of the whole spectrum typically observed in an outbred strain. The breeding protocol usually starts with a heterogeneous population of an outbred mouse strain that is tested for the particular trait of interest. Of those tested, individuals displaying the trait at the outermost ends of the response curve are mated. Their offspring, also displaying the extreme trait phenotypes, are further selectively bred. With every generation, the trait clusters more and more around the ‘high and low poles’ of the trait as demonstrated by the selective inbreeding of the high anxiety-related (HAB) and low anxiety-related (LAB) mouse lines. A specific advantage of selective breeding models is that they start from the same genetic background and any difference in the genome is then very likely to underlie their extreme behavioral trait, offering the possibility of investigating the heritability of this phenotype. While the confounding phenomenon of genetic drift can not entirely be eliminated, it is reduced – inter alia – by the parallel breeding of multiple families within a given line.
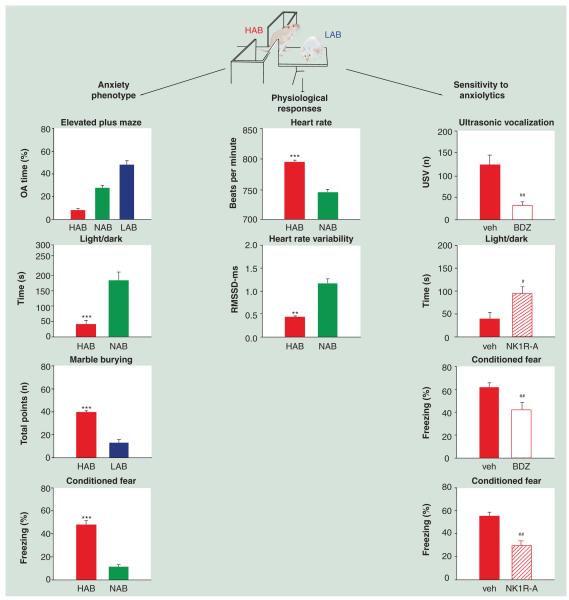
In the mouse, only a limited number of selective breeding models displaying signs of enhanced anxiety-related behavior are available (Table 2). At the Max-Planck-Institute of Psychiatry in Munich, the HAB, LAB and NAB mouse lines were initiated by deliberate selection and subsequent selective breeding of CD-1 mice for an extremely high, an extremely low and a normal level of anxiety-related behavior, displayed on the elevated plus maze (Figure 1 & Table 2). NAB mice thereby represent the population mean of unselected CD-1 mice. The high-anxiety trait of HAB animals has been confirmed in several other tests of anxiety including the light/dark, open-field and open-arm tests (Figure 1), as well as in different laboratories indicating a very robust phenotype [76-78] that appears to be largely independent of uterine environment, maternal care, sex and age [78]. Furthermore, HAB animals also display enhanced fear learning in classical cued and contextual fear conditioning paradigms suggesting that trait anxiety results in stronger fear memory and/or a weaker ability to inhibit fear responses in the HAB line (Figure 1) [79,80]. The positive association between learned (cued) fear acquisition and vulnerability to trait anxiety has very recently also been observed in healthy humans [81] and in patients with anxiety disorders [82]. The enhanced anxiety- and fear-related behavior of HAB mice is associated with reduced heart-rate variability in comparison with NAB mice (Figure 1), demonstrating that heart rate variability may be a sensitive, though not exclusive, biomarker for distinguishing between a normal and a high anxiety trait [80]. Enhanced anxiety- and fear-related behavior in HAB mice can both be reversed by anxiolytic drug treatment using either a benzodiazepine [76,79] or the selective neurokinin-1 receptor antagonist (NK1R-A) L-822,429 [79-80].
Table 2. Mouse models of enhanced anxiety-related behavior by selective breeding approaches.
| Mouse model | Comparator | Selection criterion | Background | Anxiety-related behavior | |||||
|---|---|---|---|---|---|---|---|---|---|
| Unconditioned anxiety | Conditioned anxiety | ||||||||
| Selection based on anxiety-related parameters | |||||||||
| HAB | NAB, LAB | Anxiety-related behavior on the EPM |
CD-1 | ↑ | LD, EPM, OA, USV | [76-77] | ↑ | CF (imp- EXT) |
[255, manuscript in prep., 375] [255, manuscript in prep.] |
| High contextua fear conditioning |
Low ocontextual fear conditioning |
Contextual fear conditioning |
C57Bl/6J × DBA/2J |
↑ | EZM, OF | [83] | ↑ | CF, FPS | [83] |
| ~ | LD | [7] | – | – | – | ||||
| AX | NAX | Anticipatory anxiety | MG15 × NMRI | ↑ | EPM, LD, OF | [84] | – | – | – |
| Selection based on other parameters | |||||||||
| NC900 | NC100 | Aggressive behavior towards a social partner |
ICR | ↑ | OF, LD, EZM | [87] | – | – | – |
| Increased wheel-running – line 8 |
Control line 2 | Voluntary wheel running | Hsd:ICR | ↑ | EPM | [376] | – | – | – |
| ~ | OF | [376] | – | – | – | ||||
| β-CCM- resistant line |
β-CCM- sensitive line |
Sensitivity to the to the convulsive effects of the inverse benzodiazepine receptor agonist β-CCM |
Outbreed stock of inbred mice |
↑ | EPM, HB, LD | [377] | – | – | – |
| HAP | LAP | Alcohol preference | HS/Ibg mice | – | – | – | ↑ | FPS | [378,379] |
| Light line | Control, heavy lines |
Bodyweight | Outbreed stock of inbred mice |
↑ | OF | [380] | – | – | – |
↑: Increased anxiety-related behavior; ↓: Decreased anxiety-related behavior; ~: Unaltered anxiety-related behavior; AX: Anxious line; β-CCM: β-carboline-3-carboxylate; CF: Conditioned fear; EPM: Elevated plus maze; EZM: Elevated zero maze; FPS: Fear-potentiated startle; HAB: High anxiety behavior line; HAP: High alcohol preference line; HB: Holeboard test; imp-EXT: Impaired extinction of conditioned fear; LAB: Low anxiety behavior line; LAP: Low alcohol preference line; LD: Light/dark test; NAB: Normal anxiety behavior line; NAX: Nonanxious line; NC: North Carolina line; OA: Open-arm exposure test; OF: Open-feld test; USV: Maternal separation-induced ultrasonic vocalization.
Figure 1. Validity of the high anxiety-related mouse model of high trait anxiety.
Outbred CD-1 mice were selectively bred according to their high, normal or low anxiety-related behavior displayed on the elevated plus maze test (percentage of OA time), resulting in the HAB, NAB and LAB lines [76]. The anxious phenotype of HAB mice is confirmed by reduced time spent in the aversive, lit chamber of the light/dark test [327], by increased marble burying as indicated by the number of points reflecting the number and extent of the buried marbles (Frank & Landgraf, Unpublished Data) and by increased freezing levels in response to a conditioned stimulus [79,80]. Physiological signs of abnormal anxiety-related behavior reflecting construct validity are evident in terms of altered autonomic hyperarousal (as indicated by an increased heart rate [in beats per min]) and reduced heart rate variability (in RMSSD, with R being the peak of the QRS complex of the ECG wave) in response to emotional trauma [80]. Predictive validity of the HAB mouse model is demonstrated by attenuation of the enhanced anxiety- and conditioned-fear states after treatment with either a BDZ or a selective NK1R-A in the ultrasonic vocalization test [76], the light/dark test [327] and the conditioned fear test [79,80].
*: p < 0.05; **: p < 0.01; ***: p < 0.001 HAB versus NAB or LAB mice; #: p < 0.05; ##: p < 0.01 drug versus vehicle-treated HAB mice. BDZ: Benzodiazepine; HAB: High anxiety-related; LAB: Low anxiety-related; NK1R-A: Neurokinin-1 receptor antagonist; OA: Open arm; RMSSD: Root mean square of successive R-R interval differences of the ECG signal.
As with the HAB mouse line, the divergent short-term selective breeding of mice for extremely high or low levels of fear conditioning results in two mouse lines that also differ in terms of both the fear-potentiated startle paradigm and unconditioned tests of anxiety (Table 2) [83], further supporting a relationship between high fear conditioning and greater anxiety-like behavior. Very recently, another anxious mouse line was established based on differences in the level of anticipatory anxiety displayed during a handling procedure [84]. Mice approaching the experimenter’s hand are the nonanxious NAX line while mice that do not volunteer themselves to be handled represent the anxious AX line. Relative to NAX mice, AX animals show enhanced anxiety-related behaviors in the elevated plus maze, light/dark and open-field tests (Table 2) [84].
As another example, the North Carolina (NC) lines are based upon differences in social behavior [85,86]. Specifically, animals of the NC900 line carry out unprovoked attacks on their social partners whereas NC100 mice rarely attack and become immobile in social situations. Relative to NC100 mice, the highly aggressive line NC900 displays increased anxiety-like behavior in the elevated zero maze and open-field test but not in the light/dark test (Table 2) [87]. Although defensive aggression is not considered to be a general symptom of anxiety disorders [17], it occurs in post-traumatic stress disorder [88-90] and borderline personality disorder [91,92]. NC900 mice are less sensitive to the anxiolytic effects of diazepam, which may be due to the reduced diazepam-sensitive binding in brain areas known to be part of the anxiety circuitry [87]. In addition, some forms of anxiety-related behavior seem to be increased in ‘mouse line 8’, selectively bred for high voluntary wheel running in comparison with ‘control line 2’ (Table 2). Finally, the selection of mice for high resistance to convulsive effects of the benzodiazepine receptor inverse agonist β-carboline-3-carboxylate (β-CCM) [93] coincides with increased anxiety-related behaviors in the light/dark and holeboard tests in comparison with the β-CCM sensitive line. Since benzodiazepine receptor binding sites are reduced in the β-CCM resistant line [93], this finding further supports a role played by benzodiazepine binding sites in the modulation of anxiety-related behavior. The NC900 line, the high voluntary wheel running line and the β-CCM-resistant line nicely demonstrate that the deliberate selection for one selected trait often results in changes in other behaviors, not necessarily directly associated with the feature of interest that was chosen.
On-site selection of mice with enhanced anxiety-related behavior
Selective breeding approaches are, of course, tedious and time-consuming. Therefore, some research groups opt for an acute selection (stratification) in terms of an enhanced emotional reactivity in response to an anxiety-provoking situation. Like the successful selection principle of the HAB and NAB mouse lines, the elevated plus maze was used to acutely preselect animals with high, intermediate and/or low anxiety as a means for studying variations in the anxiolytic effects of the benzodiazepine nitrazepam [94] and the relation between trait anxiety and ethanol [95]. Similarly, C57BL/6 male mice can be classified into high- or low-anxiety traits according to their latency to freely enter an unfamiliar arena from their home cage [96]. On the other hand, C57Bl/6J mice pre-selected for voluntary wheel-running also exhibit an anxiogenic phenotype in the open-field, light/dark and elevated O-maze tests compared with sedentary animals [97].
Mutants with enhanced anxiety-like behavior
In 1994 Stenzel-Poore described the first transgenic mouse with an anxiogenic phenotype caused by targeted overexpression of corticotropin-releasing hormone [98]. Since then, numerous genetically engineered mice in which a specific gene was either knocked-out, knocked-in, overexpressed or replaced have been generated, resulting in increased anxiety-related behaviors. Mutant mice with a relatively robust enhanced anxiety-related behavior are summarized in Table 3 – we have excluded those models with approximately equal numbers of reports describing an enhanced, unaltered or reduced anxious phenotype, such as with the GAT1 transgenic mice [99,100] and the Fmr1 knockout mice [101-104], as well as those where the evidence of an anxious phenotype was rather limited (e.g., [105,106]) – though we cannot exclude the possibility that some may have been missed. Genetic manipulation of many different systems, including monoamines, GABA, neuropepeptides or molecules of the immune system was shown to result in increased anxiety-related behaviors suggesting that these systems contribute to the anxious phenotype observed. Interestingly, the genetic background of the mutant seems to critically interfere with the genetic target and this, in many cases, determines whether or not enhanced emotionality could be monitored. This has been discussed in recent reviews [63,107].
Table 3. Mouse models of enhanced anxiety-related behavior by gene targeting.
| Mutant | Unconditioned anxiety-related behavior | Conditioned anxiety-related behavior | |||||
|---|---|---|---|---|---|---|---|
| Monoaminergic targets | |||||||
| 5-HT1A | KO | ↑ | EPM, OF, EZM | [381-383] | – | – | – |
| 5-HT2C | OE | ↑ | EPM, OF | [384] | – | – | – |
| α2A | KO | ↑ | EPM, LD | [385-386] | ↑ | CF | [387] |
| nAChRα4 | KO | ↑ | EPM, | [388] | – | – | – |
| COMT | KO | ↑ | LD, OF | [389], but see [390-391] | – | – | – |
| D1CT | TG | ↑ | LD, OF | [392-393] | – | – | – |
| D4 | KO | ↑ | EPM, LD, OF | [394-395], but see [396] | ~ | CF | [395] |
| SERT | KO | ↑ | EPM, EZM, LD, OF, SI | [397-399], but see [400] | – | – | – |
| Glutamatergic targets | |||||||
| NR2C-2B | RP | ↑ | EPM | [401] | – | – | – |
| GluR1 | KO | ↑ | EPM, LD, SI | [402-404] | ↓ | CF | [405-406] |
| mGluR5 | KO | ↑ | EPM, LD | [407] |
~
↓ |
CF CF (imp-EXT) |
[408] [409] [409] |
| mGluR8 | KO | ↑ | EPM, OF | [410-414], but see [415] | ↓ | CF | [415-416] |
| VGlutT1 | HET | ↑ | LD | [417] | – | – | – |
| GABAergic targets | |||||||
| GABA-Aγ2 | HET,KO | ↑ | EPM, LD | [418,419] | ↑ | TFC | [418] |
| GABA-Aγ2L | KO | ↑ | EPM | [420] | – | – | – |
| GABA-B1 | KO | ↑ | LD | [421] | – | – | – |
| GABA-B2 | KO | ↑ | LD | [421] | – | – | – |
| GAD65 | KO | ↑ | EZM, LD, OF | [422,423] |
~
↓ |
CF CF (imp-EXT) |
[424] [425] [424] |
| Neuropeptidergic targets | |||||||
| BDNFMet | KI | ↑ | OF, EPM | [426-427] | ↓ | CF | [427] |
| CB1 | KO | ↑ | EPM, LD, OF, SI | [428-433], but see [434] |
~
↓ |
CF CF (imp-EXT) |
[435-438] [439] [435,437,438] |
| CCK | KO | ↑ | EPM | [440] | – | – | – |
| CCK1R | KO | ↑ | EPM | [441,442] | – | – | – |
| CRH | OE | ↑ | EPM, LD, OF, SI | [98,353,443-446] | ~ | CF, VC | [347] |
| CRH-BP | KO | ↑ | EPM, LD, OF | [447-448] | – | – | – |
| CRH-R2 | KO | ↑ | EPM, LD, OF | [261,449-450] | – | – | – |
| αER | KO | ↑ | OF, LD | [451,452] | – | – | – |
| GR | OE | ↑ | EPM | [453,454], but see [455] | – | – | – |
| Mas | KO | ↑ | EPM | [456] | – | – | – |
| NPSR | KO | ↑ | OF, LD, EPM | [305], but see [306] | – | – | – |
| NPY | KO | ↑ | EPM, LD, OF | [457-459] | – | – | – |
| OFQ/N | KO | ↑ | EPM, LD, OF | [460-461] | ↑ | CF | [462] |
| PENK | KO | ↑ | EPM, EZM, LD, OF, SI, USV | [463-466] | ↑ | CF, FPS | [464,465] |
| Ucn | KO | ↑ | EPM, OF | [467], but see [468] | – | – | – |
| Immune system | |||||||
| IFN-γ | KO | ↑ | EPM, OF | [469-471] | – | – | – |
| TNF-α | OE | ↑ | HB, LD | [472] | – | – | – |
| Other targets | |||||||
| 3xTg-AD | TG | ↑ | LD, OF | [473] | ↑ | CF | [473] |
| APP,Sw,Ind | TG | ↑ | LD, OF | [473] | ↑ | CF | [473] |
| α-CamKII | OE | ↑ | EZM, LD, OF, SI | [474] | – | – | – |
| Dhh | KO | ~ | LD, OF | [475] | ↑ | VC | [475] |
| eNOS | KO | ↑ | EPM, OF | [476,477] | – | – | – |
| Fut9 | KO | ↑ | EPM, LD | [478] | – | – | – |
| Fyn | KO | ↑ | EPM, LD | [479,480] | ↑ | CF | [481], but see [482] |
| PAM | HET | ↑ | EZM | [483] | ↓ | FPS | [483] |
| PSA | KO | ↑ | EPM, OF | [484] | – | – | – |
| Rgs2 | KO | ↑ | LD | [244] | – | – | – |
| Schnurri-2 | KO | ↑ | OF | [485] | – | – | – |
| SF-1 | KO | ↑ | EPM, LD, OF | [486] | – | – | – |
| Sim2 | OE | ↑ | EZM, OF, SI | [487] | – | – | – |
| TAK1/TR4 | KO | ↑ | SI, LD, OF | [488] | – | – | – |
| TgNTRK3 | OE | ↑ | EPM | [490] | – | – | – |
| trkBCaMKII-CRE | KO | ↑ | OF | [491] | – | – | – |
↑: Increased anxiety-related behavior; ↓; Decreased anxiety-related behavior; ~: Unaltered anxiety-related behavior; 3xTg-AD: Alzheimer’s disease transgenic mice expressing taurine; 5-HT1A: Serotonin 1A receptor; 5-HT2C: Serotonin 2C receptor; α2A: α2A-adrenergic receptor; α-CamKII: α-calcium/calmodulin-dependent protein kinase II; αER: α-estrogen receptor; APPSw,Ind: Alzheimer’s disease transgenic mice expressing human mutant β-amyloid precursor protein; BDNF: Brain-derived neurotrophic factor; CB: Cannabinoid receptor; CCK: Cholecystokinin; CCK1R: Cholecystokinin 1 receptor; CF: Conditioned fear; COMT: Catechol-O-methyltransferase; CRH: Corticotropin-releasing hormone; CRH-BP: Corticotropin-releasing hormone binding protein; CRH-R2: Corticotropin-releasing hormone 2 receptor; D1CT: Expression of a neuro-potentiating cholera toxin transgene in dopamine D1 receptor-expressing neurons; D4: Dopamine D4 receptor; Dhh: Desert hedgehog; eNOS: Endothelial nitric oxide synthase; EPM: Elevated plus maze; EZM: Elevated zero maze; FPS: Fear-potentiated startle; Fut9: Fucosyltransferase IX; Fyn: Fyn tyrosine kinase; GABA: γ-amino butyric acid; GAD: Glutamic acid decarboxylase; GluR1: AMP-A GluR1 subunit; GR: Glucocorticoid receptor; HB: Holeboard test; imp-EXT: Impaired extinction of conditioned fear; KI: Knock-in; KO: Knockout; LD: Light/dark test; Mas: Mas proto-oncogene; mGluR: Metabotropic glutamate receptor; nAchRα4: α4-nicotinic acetylcholine receptor; NPSR: Neuropeptide S receptor; NPY: Neuropeptide Y; NR2C-2B: NMDA receptor 2C-2B; OE: Overexpression; OF: Open-field test; OFQ/N: Orphanin FQ, nociceptin; PAM: Peptidylglycine α-amidating monooxygenase; PENK: Prepro-enkephaline; PSA: Puromycin-sensitive aminopeptidase; Rgs2: Regulator of G-protein signaling; RP: Replacement; SERT: Serotonin transporter; SF: Steroidogenic factor; SI: Social interaction; Sim2: Single-minded 2 gene; TAK1/TR4: Nuclear orphan receptor; TFC: Trace fear conditioning; TG: Transgenic; TgNTRK3: Transgenic mice overexpressing the full-length neurotrophin-3 receptor TrkC; trkBCaMKII-CRE: Forebrain-specific knockout of the trkB receptor; Ucn: Urocortin; USV: Stress-induced ultrasonic vocalization; VC: Vogel conflict test; VGlutT1: Vesicular glutamate transporter 1.
Enhanced anxiety-related behavior observed in a mutant does not allow the final conclusion to be drawn that the altered expression of that gene underlies anxiety-related behavior. Anxiety disorders are complex mental diseases with a polygenetic etiology, while in mutants in most cases only one gene is intentionally affected. However, genetic manipulation performed in the laboratory usually induces large changes in gene function (e.g., the complete loss of function in knock-out animals) and congenic footprint phenomena [108], which may have potent effects on the anxiety phenotype. Therefore, the manipulation of genes and, subsequently, of their products in mutant mice can only provide incomplete information about their relation and contribution to anxiety and, potentially, to anxiety disorders. On the other hand, this polygenetic characteristic of anxiety disorders is indeed reflected by the large number of mutants displaying enhanced anxiety-related behaviors. Although the use of mouse mutants has proved to be a powerful tool in the identification and characterization of mechanisms controlling enhanced anxiety states, it must be considered that a targeted mutation affects the transcription of the gene of interest throughout the whole life of a mouse and, in particular, during its development. The mutation may thus trigger compensatory adaptations in other neuronal systems in an effort to maintain homeostasis, even if this proves to be unsuccessful. In both cases, it is not possible to ascertain whether the increased anxiety-related behavior is the resultant compensation or whether it is related to the function of the gene in terms of anxiety-related behavior.
As an alternative approach that overcomes many of these problems, conditional mutant mice have more recently been introduced using either gene mutation strategies or RNA interference (RNAi) techniques allowing temporal and regionally specific control of expression of the gene of interest. For example, using RNAi, the knockdown of phospholipase Cβ4 in the medial septum [109] and of calcineurin A in the amygdala [110] increased anxiety-like behavior in mice, while local knockdown of clock [111], corticotropin-releasing hormone receptor 1 [112], protein kinase Cε [113] or glyoxalase 1 [29] caused a reduction in anxiety-related measures. Therefore, these data point towards specific sites of action of target genes regulating emotionality, which may help to further advance novel treatment strategies in anxiety disorders.
Environmental manipulations
Negative environmental influences have been demonstrated to be intimately linked to vulnerability to neuropsychiatric disorders, including anxiety disorders [7,8].
Early life environment
Rearing conditions, including poor maternal care and adverse early-life events, are proposed to be critically involved in the development of mental disorders, including anxiety disorders (for review see [114,115]). While it is very difficult to control for differences in the human environment, rearing conditions can be carefully controlled and manipulated in the laboratory. Given that mother-pup interactions shape the emotional behavior of the pups when they mature into adult rodents [116,117], maternal separation for longer periods of time is used to model adverse early life events, negatively influencing emotional behavior in the adult rodent [118,119]. By contrast, maternal separation for short periods of time (~15 min) is used to elicit opposite effects on anxiety-related behavior. The maternal separation protocol has been successfully applied to the rat [116,118,119], while in the mouse the same attempts to do so have turned out to be challenging.
Following maternal separation, adult male mice have been shown to display increased [117,120-122], unaltered [121,123-127] and even reduced [127,128] anxiety-related behaviors using different established tests. Therefore, it seems that maternal separation does not produce robust and reliable effects on murine emotionality in the strains tested, including BALB/c, C57Bl/6J, CD-1, 129Sv and DBA/2J mice [124,127] and, thus, it may be necessary to modify current murine separation protocols. Indeed, in a first attempt to accomplish this, George and coworkers [129] recently demonstrated increased anxiety-related behavior as assessed in the open-field and elevated plus maze tasks in adult mice following maternal separation in combination with early weaning. Early weaning per se may be an example of a novel procedure that causes a persistent increase in anxiety-like and aggressive behavior [130,131].
Chronic exposure to stress
It is not only early-life stress that has been associated with the manifestation of pathological anxiety in humans: repeated stressful experiences during later life have also been linked to this [7]. Therefore, mouse models of enhanced anxiety-related behavior may be generated by the repeated exposure of animals to stressful situations. Although most studies so far have been performed using rats, chronic stress usually results in some behavioral, neuroanatomical and neurochemical changes resembling those found in patients with anxiety disorders [132]. Various chronic stress procedures with different construct validity (see ‘Validity of mouse models of enhanced anxiety’) are applicable to mice. As social stressors are suggested to be very potent in enhancing the individual risk for developing pathological anxiety in both humans and animals [7], various paradigms have been developed taking into account this risk factor. Accordingly, increases in anxiety-related measures have been reported in mice in response to repeated exposure to an aggressive, dominant conspecific until attack and defeat occur [133-135], as a result of chronically subordinate housing [136-138], or by housing mice in a highly unstable social and hierarchical situation during their adolescence and young adult periods [139]. Indeed, the continuous disruption of social networks results, reproducibly, in enhanced anxiety-related behavior in adult male and female CD-1 mice, as assessed by the elevated plus maze and the novelty suppressed feeding paradigms [140-142]. Given that mice are social animals, long-term social isolation of mice is another chronic social stress model involving individual housing of mice in the post-weaning period. However, this procedure has revealed varying results in terms of anxiety-related behavior exhibited in the open-field, light/dark, social interaction and holeboard tests, and on the elevated plus maze where increases [122,134,143-145], no change [143-147] and even decreases [144,145,148] were observed. On the other hand, crowded social housing has also been shown to cause long-term effects in terms of increased anxiety-related behavior, depending on the strain involved [149].
In the unpredictable chronic mild stress paradigm animals are exposed to a choice of environmental stressors such as an empty cage or a tilted cage, cold or a reversed light–dark cycle and social stressors once per day for at least 2 weeks in a randomized, unpredictable order. Unpredictable chronic mild stress has been demonstrated to increase anxiety-related behavior in C57Bl/6N mice [150-152], BALB/c mice [153-154] and DBA/2 mice [152,153] as well as in some mutants [155], but not in C57Bl/6J mice [152,153]. However, anxiolytic-like effects of chronic mild stress have also been described in mice [152,156,157] and these findings are generally interpreted as anomalous [158].
To summarize, mouse models that involve chronic adverse environments, irrespective of whether they occur during early life or in adulthood, are of translational value in anxiety research. However, the anxiogenic effects of all the stress paradigms presented are variable. The inconsistencies may be due to methodological differences, such as duration and choice of stressors, the mouse strains used and the tests applied for revealing anxiety-related behavior. An alternative explanation may involve the complex interactions between genetic and non-genetic factors [159]. In contrast to these harmful surroundings, inanimate and social stimulation by environmental enrichment has been demonstrated to reduce anxiety in both mice with normal and enhanced levels of anxiety-related behavior (e.g., [112,160,161]). However, it has to be noted that, similar to adverse environments, the positive effects of environmental enrichment are not always reproducible [162].
Nutrient-induced enhanced anxiety-related behavior in mice
There is increasing evidence of a link between alterations in nutrition and psychiatric diseases [163,164]. Specifically, changes in values of diverse micronutrients, including electrolytes and vitamins, have been linked to symptoms in psychiatric patients [165-167]. Influenced by these studies, mouse models of enhanced anxiety-related behavior have been created utilizing this possibility.
Magnesium deficiency
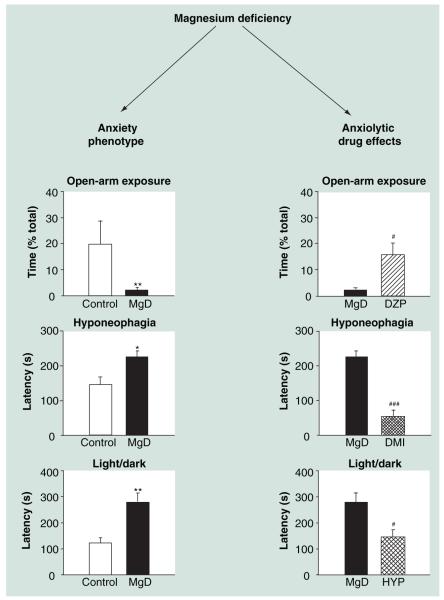
Magnesium (Mg) is an interesting candidate as it, among other actions, modulates NMDA receptor activity and NMDA-mediated mechanisms are suggested to contribute to (pathological) anxiety states [40]. An increasing number of clinical and preclinical studies proposes that changes in Mg homeostasis are involved in affective disorders [168], while relatively little is known about the influence of Mg on anxiety-related behavior. We have recently shown that feeding C57Bl/6J mice a low Mg-containing diet, providing approximately 10% of the daily requirement [169], changes the expression of proteins involved in NMDA signaling (amongst other processes) [170] and elicits enhanced anxiety-related behavior in several animal tests of anxiety, including the open-field and the light/dark tests (Figure 2) [171]. Confirming and corroborating these first findings, Mg-deficiency has also been found to enhance anxiety-related behavior in C57Bl/6N mice and the already highly emotional BALB/c mice (Figure 2) [172, manuscript in prep.]. The enhanced anxiety-related behavior induced by Mg-deficiency can be reversed by treatment with either the benzodiazepine diazepam, desipramine or hypericum perforatum (Figure 2) [171,172, manuscript in prep.].
Figure 2. Validity of the magnesium-deficiency model as a mouse model of enhanced anxiety-related behavior.
Compared with control mice, mice fed a low magnesium-containing diet (10% of the daily requirement) spend less time in the distal, aversive compartment of the open-arm exposure test and display enhanced latency to eat a pleasurable food in the hyponeophagia test [172, manuscript in prep.], as well as enhanced latency to enter the brightly lit compartment of the light/dark test [171], indicating enhanced anxiety-related behavior. The anxious phenotype of MgD mice is reduced by acute treatment with the classical benzodiazepine DZP [172, manuscript in prep.], as well as by chronic treatment with either the tricyclic antidepressant, DMI or HYP (St John’s wort) [171].
*: p < 0.05; **: p < 0.01 MgD versus control mice; #: p < 0.05; ###: p < 0.001 drug versus vehicle-treated MgD mice.
DMI: Desipramine; DZP: Diazepam; HYP: Hypericum perforatum;
MgD: Magnesium-deficiency model.
Others
PubMed searches aimed at finding additional nutrient-induced mouse models of enhanced anxiety-related behaviors proved fruitless. One potential candidate was zinc, which in a similar way to Mg, mildly antagonizes NMDA receptor signaling (amongst other processes) [173]. Mice fed a zinc-deficient diet were shown to display enhanced anxiety-related behavior in the novelty suppressed feeding paradigm but not in other anxiety tests, such as the elevated plus maze or light/dark tests [174]. Vitamin B is another suspect, since its deficiency is suggested to contribute to functional decline. Although folate deficiency does not seem to affect anxiety-related behavior in young adult BALB/c mice [175], the adult offspring of mothers under prenatal folate deficiency, which is repleted at birth, can manifest later with increased anxiety 9–12 weeks after birth [176]
Chemical & pharmacological manipulations
Acute challenge tests
In contrast to the mouse models of enhanced anxiety-related behavior discussed so far, challenge tests using anxiogenic substances elicit states of acutely enhanced anxiety-related behavior. These procedures are based, in part, on observations in human volunteers and/or patients in whom diverse chemical agents, including sodium lactate, cholecystokinin, caffeine, pentylenetetrazol, yohimbine and CO2 inhalation, provoke anxiety- or panic-like reactions [177-179]. Many of these agents have been shown to engage and activate relevant anxiety-related brain areas and circuitries centered on the amygdala [180-183]. Interestingly, in regard to CO2 stress, the amygdala has been identified as an important chemosensory site that detects hypercarbia and acidosis and initiates CO2-induced fear responses [184]. The same substances may be used to boost anxiety in the laboratory mouse. For example, pentylentetrazole increases the anxiety-related behavior of various mouse strains, including the CD-1, Swiss, DBA/2, and C57Bl/6 mice, as displayed on the elevated plus maze or in the light/dark test in a dose-dependent manner [185-191]. Similar effects have been described in the mouse after application of the 5-HT2C receptor agonist meta-chlorophphenylpiperazine (mCPP) [189,192-195], but see [196,197]). Other compounds, such as cholecystokinin, sodium lactate, caffeine, yohimbine, CO2 or the benzodiazepine inverse agonist FG-7142, have not been widely applied to mice for the induction of enhanced anxiety-related behavior.
The chronic corticosterone mouse model
On the basis of evidence of an association of some anxiety disorders with elevated glucocorticoid levels (Table 4) [8], long-term exposure to exogenous glucocorticoids in rodents is used to model chronic stress-induced changes in anxiety-related behaviors [198]. For that purpose, a low dose of corticosterone is administered via the drinking water to the mouse for several weeks. Then, increases in murine anxiety-related behaviors postregimen have been observed on the elevated plus maze [150,199] and in the open-field [150,200-202] and light/dark tests ([203], but see [198]).
Table 4. Comparison of neurobiological parameters between patients with anxiety disorder and mouse models of enhanced anxiety-related behavior.
| Patients† | Mouse models | |||
|---|---|---|---|---|
| Neuroanatomical (volume) alterations | ||||
| Anterior cingulate cortex | ↓ | [492–498] | – | – |
| Orbitofrontal cortex | ↓ | [497,499,500] | – | – |
| Hippocampus | ↓ | [497,501–503] | ↓ | [198,504] |
| Amygdala | ↓ | [494,498,505] | ↓ | [506] |
| Activity processing | ||||
| Amygdala | ↑(↓)‡ | [22] | ↑(↓)§ | [54,119,224,305,309,362,363,364] |
| Prefrontal cortex | ↑ dACC | [22] | – | – |
| ↑↓‡ rACC | [22] | ↑↓ Cg | [77,183,259,261,507] | |
| Neurochemistry | ||||
| GABA system | ↓ | [21] | ↓ | [87,508,509] |
| NA system | ↑ | [21] | ↑ | [136,510] |
| 5-HT system | Not clear | – | – | |
| DA system | Not clear | – | – | |
| Neuropeptides | ↑ CRH | [21] | ↑ CRH | [511–514] |
| ↑↓ NPY | [21] | ↓ NPY | [209] | |
| ↑ SP | [515,516] | ↑ SP | [328] | |
| Physiological parameters including autonomic arousal | ||||
| Autonomic arousal | ↑ | [517–518] | ↑ | [80,385,519] |
| Sleep disturbance/insomnia | Yes | [17,520,599] | Yes | [362,521,522] |
| Neuroendocrinology | ||||
| CORT | ↑ ↓ | [21] | ↑ ↓ | [136,140,511,512,514,523] |
Mainly post-traumatic stress disorder or panic disorder.
Based on a very small number of studies.
Increased activation mainly in the central amygdala (see. Table 5).
↑: Increased; ↓: Decreased; 5-HT: Serotonin; Cg: Cingulate cortex; CORT: Cortisol/corticosterone; CRH: Corticotropin-releasing hormone; DA: Dopamine; dACC: Dorsal anterior cingulate cortex; NA: Noradrenaline; NPY: Neuropeptide Y; rACC: Rostral anterior cingulate cortex; SP: Substance P.
High anxiety-related behavior induced by drug withdrawal
The initial mood-enhancing effects of recreational drugs are often followed by withdrawal symptoms with opposite effects on mood, including agitation, depression and anxiety and, as such, they have been utilized in order to induce an enhanced anxiety state in mice. Indeed, withdrawal of diverse psychostimulants after chronic administration has been shown to increase anxiety-related behavior in mice in a number of anxiety tests, inlcuding the elevated plus maze [204-206] and the light/dark test [205,207]. Specifically, (meth)amphetamine (e.g., [204]), ethanol (e.g., [205,208,209]) and nicotine (e.g., [206,207]) are the preferred substances used to induce a mouse model of prolonged enhanced anxiety after their removal.
N-ethyl-N-nitrosurea-induced random mutagenesis
The mutagen N-ethyl-N-nitrosurea (ENU) induces point mutations in murine genes at a high rate [210]. Following the injection of ENU into male mice, premeiotic stem cells are mutagenized, resulting in a large number of F1 animals carrying different mutations. ENU mutagenesis is a powerful phenotype-driven approach that involves identifying mutants with distinct behavioral patterns. In large-scale testing, pedigrees of ENU mutants have been identified displaying altered anxiety-related behaviors on the elevated plus maze [211], in the light/dark test [212], in fear conditioning [212-214], in the passive avoidance test [215] or in the open-field test [212,214-216]. So far, no studies of offspring of mice treated with ENU and displaying clear enhanced anxiety-related behavior have been published, but increased avoidance behavior towards an unprotected area has been reported in ENU pedigrees [217].
Validity of mouse models of enhanced anxiety
In 1984 Willner proposed three criteria, which valid animal models of any human psychiatric disease, including anxiety disorders, have to meet [218]. First, the behavioral and physiological responses observed in the model should reflect the hallmarks of the human condition, representing face validity. Second, construct including genetic validity requires similarity between humans and animals in terms of the neurobiological mechanisms, as well as in the analogy to the etiological causes underlying the behavioral changes. Third, the behavioral changes displayed by the model should be reversed, or at least reduced, by clinically effective pharmacotherapies, revealing predictive validity.
Of these criteria, true construct validity is probably the most difficult to fulfill for an animal model of enhanced anxiety-related behavior. Nevertheless, most of the presented mouse models meet construct validity quite well, reflecting that both genes and environment are suggested risk factors in the development of an anxiety disorder [6,219] and that neurobiological similarities between mouse models of enhanced anxiety and anxiety patients can also be found (Table 4), although it must be emphasized that our knowledge about the pathogenesis and pathophysiology of anxiety disorders is still incomplete in terms of neuroanatomy, neurochemistry and neuroendocrinology (see ‘Conclusion’ and ‘Future perspective’). Such models with enhanced construct validity could be well suited to be used as translational models and it is hoped that they will provide improved ways to study the neurobiology and treatment of pathological anxiety further.
Neurobiology of pathological anxiety: translational anxiety research
Genetic underpinnings of the anxiety trait
Both human and animal studies support the contribution of genes to the etiology of anxiety disorders. While it is clear that anxiety disorders are polygenic mental diseases, the success rate in identifying susceptibility genes of pathological anxiety is limited. Difficulties involved with gene mapping in human pathological anxiety include sample size, genetic heterogeneity, phenocopies as well as unknown genetic or haplotypic background. Many of these problems can be reduced using mouse models of enhanced anxiety-related behavior.
In mice, chromosomal loci, genes and polymorphisms for a variety of behavioral phenotypes relevant to humans have been identified, including anxiety and fear [220-224]. For example, in QTL studies, a significant association between an anxiety-related score and a genetic marker of known genomic location is drawn. Using recombinant inbred or congenic mice [72], F2 intercrosses from mice selected for either high or low anxiety-related behavior [225] and mice selected for high or low conditioned fear [83], small genetic effects underlying anxiety, fear and emotionality have been located. Specifically, on mouse chromosomes 1, 4, 5, 7, 8, 9, 10, 11, 13, 14, 18 and 19, loci contributing to multiple anxiety-related traits have been identified using a wide variety of anxiety tests ([83,225-230]; for review, see [231]). Considering the high homologies between mouse and human genotypes and phenotypes (see ‘The evolutionary relationship between humans & mice in anxiety regulation’), QTL analyses in mice with enhanced anxiety-related behavior may guide linkage analysis studies in patients with anxiety disorders. For example, Smoller and coworkers followed the results of murine QTL analysis studies in a large multiplex degree, segregating panic disorder and agoraphobia [232]. Indeed, they report a linkage of panic disorder and/or agoraphobia to a locus on chromosomes 12q13, while other loci on chromosome 10q25–26 and on chromosome 1 were associated with severe anxiety proneness [232]. Although these findings, unfortunately, did not reach genome-wide levels of statistical significance, they nicely demonstrate the potential power of such targeted genome screens. Nevertheless, translating mouse QTL data to humans turned out to be difficult. The difficulties of QTL studies in general are discussed elsewhere [61,233].
Despite all the progress achieved, the genetic loci identified so far account for only a small fraction of the total variation, and they rarely map to individual genes. The low contribution of chromosomal loci and individual genes, respectively, to trait anxiety, together with the fact that demonstrable genetic influences explain only a small fraction of estimated heritability in psychiatric conditions, indicates additional environmental/epigenetic effects (see ‘Environmental stimuli and epigenetic mechanisms contributing to anxiety-related behavior’). Obstacles in identifying genes that causally contribute to a given trait variation include:
-
■
Identification of potentially functional DNA polymorphisms between alternative alleles of the candidate gene;
-
■
Differences in mRNA expression profiles between genotypes;
-
■
Expression in brain areas thought to be relevant to the trait;
-
■
Association between polymorphisms and trait variation in a freely segregating panel;
-
■
Replication in independent studies.
Candidates that ‘survive’ multiple testing are worth pursuing as potential biomarkers and targets for psychotherapy.
The situation is further complicated by epistatic effects, occurring between closely linked QTLs and even polymorphisms at a single locus, which often mask locus effects, and pleiotropic effects with alleles simultaneously affecting multiple and often functionally unrelated traits. Pleiotropy is probably the rule rather than the exception for many traits [234,235]. QTLs typically contain multiple genes and disentangling coincidence of location from pleiotropic action is difficult [236].
While genetic differences in trait anxiety help to identify candidates that contribute to phenotypic variation, they approach causality at best. Therefore, similar to clinical studies, we run association studies to test for causality between genetic and phenotypic variation, which goes far beyond conventional two-group comparisons. For selectively- and bidirectionally-inbred mice, the original population (i.e., outbred CD-1) or a freely segregating F2 panel may be used to create and harness variations to the fullest possible extent. Although such approaches are often hampered by confounding factors that a priori limit success (e.g., reduced statistical power, owing to lower sample population compared to that commonly used in clinical samples, where often thousands of subjects are included), minor, though significant contributions of polymorphisms in the vasopressin and TMEM132D genes could recently be demonstrated to corroborate human studies [224,237]. Then, based on reliable association studies, causality can further be tested by appropriate approaches, including agonist/antagonist, RNAi, knockout and viral vector strategies. Nevertheless, it remains a challenge to handle large numbers of potential loci, candidate genes and polymorphisms and it is problematic to distinguish the genuine ones. It is of note in this context that the anxiety phenotype is at least as variable as the genotype with all its imponderabilities, including state versus trait, repeated testing, maternal influences and cage hierarchy among others, further complicating or even masking potentially significant associations.
Examples of bridging mouse & human neurogenetics
It is becoming widely accepted that the pathobiology of anxiety disorder – as that of comorbid depression – relies beyond dysfunction of monoamine systems [238] and that advances in the understanding of neurobiological underpinnings of anxiety can only be made by focusing on those candidates that do not necessarily belong to the ‘usual suspects’. For example, we have just begun to understand metabolic networks, for instance in mitochondria, as contributing to anxiety phenotypes.
One example, where the translational aspect from mouse to humans with pathological anxiety was quite successful, is the discovery of the regulator of G-protein signaling 2 (Rgs2) gene in anxiety-related behavior. Evidence for its possible involvement arose in QTL studies initially in mice and and then humans [226], suggesting Rgs2 as an anxiety-associated quantitative trait gene. Indeed, two polymorphisms in this gene, rs4606 and rs3767488, have been associated with patients suffering from panic disorder ([239], but see negative findings in a Japanese cohort [240]), post-traumatic stress disorders [241] and general anxiety disorder [242], as well as with trait anxiety in general [243]. In further support, Rgs2-knockout mice display enhanced anxiety-related behaviors [226,244]. These findings arising from the mouse model organism, thus, suggest that Rgs2 may play a role in the development of a nxiety in humans.
Despite the lack of evidence from QTL studies, altered expression of the zinc metallo-enzyme glyoxalase-1, which catalyzes the conversion of the highly reactive physiological metabolite, methylglyoxal, seems to impact anxiety-related behavior in inbred mouse strains [29] and in HAB mice selectively bred for high-trait anxiety [76,245], though in opposite ways. While Hovatta et al. [29] demonstrated elevated glyoxalase-1 gene expression to be linked to high anxiety levels, we described an inverse association, with HAB expressing less than LAB mice [76,245]. Support for the former finding came from Williams et al. [246], and for the latter from two recent papers. In the first, Fujimoto et al. described a reduced expression of glyoxalase-1 mRNA in mood disorder patients as compared with healthy subjects, a difference that disappeared in a remissive state [247]. In the second study, decreased levels of glyoxalase-1 were found in an inbred mouse strain selected for high anxiety-related behavior [84], further supporting its potential as molecular biomarker. Interestingly, an association between the Ala111Glu polymorphism of the glyoxalase-1 gene and panic disorder without agoraphobia has been previously found in an Italian population [248].
Evidence for another interesting candidate gene for anxiety phenotypes comes from a very recent paper summarizing mouse and human studies. Using genome-wide case-control association analysis in patients with panic disorder involving multiple psychiatric centers, two SNPs in the intron regions of TMEM132D gene were identified [237]. Furthermore, TMEM132D mRNA expression levels were found to be upregulated in the frontal cortex of post-mortem brains with risk genotypes for panic disorder. In an interspecies approach, the hypothesis that TMEM132D may be critically involved in the regulation of pathological anxiety and fear was tested by expression and association analyses in HAB mice selectively bred for high trait anxiety [76], confirming the human data. Indeed, TMEM132D mRNA expression was upregulated in the cingulate cortex of HAB mice and, remarkably, the SNP rs13478518, located in exon 9 of the TMEM132D gene, was significantly associated with the level of anxiety in a freely segregating F2 panel generated from cross-breeding HAB and LAB mice [237].
Another example for bridging mouse and human neurogenetics is the BDNF Met allele, likely to play an important role in the development of anxiety disorders [84]. Relative to the BDNF Val allele in humans and the wild-type gene in mice, the BDNF Met allele caused enhanced anxiety and impaired extinction of the conditioned fear response in both mice and humans (for review, see [249,250]). Using gene expression and MRI, respectively, the BDNF Met allele was further associated with decreased activity in the ventromedial prefrontal cortex, which is known to be critical in fear extinction, and increased activity in the amygdala, which is crucial for the acquisition and expression of fear conditioning. Although, according to Hariri [251] and Groves [252], the findings in human studies are, at best, inconclusive, this Met variant might determine how individuals respond to environmental stress exposure. Interestingly, deficits in extinction have recently been linked to reduced dendritic complexity of neurons in the prefrontal cortex [253,254], to abnormal processing in the prefrontal cortex [44-46] and to high trait anxiety [45,255, manuscript in prep.]. Moreover, in HAB mice, signs of hypoactivated prefrontal cortex and hyperactivated amygdala in response to mild anxiogenic stimulation were observed by Muigg et al. [77].
Together, data obtained independently in mice and humans suggest evolutionarily conserved neuroanatomical, genetic and neurochemical mechanisms underlying the regulation of anxiety-related behavior as well as their translational potential.
Alterations in functional neural circuits in pathological anxiety
Neuroimaging studies, in combination with symptom provocation, have revealed aberrant neuronal activation patterns in a number of anxiety disorders in brain areas implicated in the pathophysiology of anxiety disorders, including, for example, the amygdala, the prefrontal cortex, the hippocampus and the hypothalamus [22]. Interestingly, exaggerated amygdala reactivity is noted in social phobia, specific phobia and post-traumatic stress disorder [22]. Furthermore, an increased function of the dorsal anterior cingulate cortex is observed in specific phobias, in post-traumatic stress disorder and in generalized anxiety disorder, while diminished reactivity of the rostral anterior cingulate cortex may be specific to post-traumatic stress disorder [22]. Nevertheless, the information gained in such imaging studies is limited, owing to the presence of susceptibility artifacts and because of restricted spatial resolution even with the best (fMRI) techniques. Therefore, even up-to-date technologies of human imaging do not allow precise discrimination of small adjacent structures such as the hypothalamic and amygdaloid subnuclei or brain-stem regions.
Given that important basic neurocircuitries mediating anxiety are highly conserved among mammals [23,33-35], mouse models of enhanced anxiety-related behavior may be exploited in order to identify neuronal correlates of pathological anxiety at a subnuclear level. In the awake, freely-moving mouse neuronal activities can be measured, for example, by electrophysiological recordings or optical recordings of photons [256] and calcium [257]. However, these methods are very sophisticated and, to our knowledge, have not, or have only rarely, been exploited in mouse models of enhanced anxiety during emotional challenges. Alternatively, neuronal activation may be indirectly visualized by functional staining techniques of diverse neuronal activity markers whose expression has been proposed to correlate with the functional activation of neurons providing great spatial, even single-cell, resolution [23]. Such markers are, for example, immediate early genes, or cytochrome oxidase [258]. Of these, the immediate early gene c-Fos appears to be a widely used marker for neuronal activation in anxiety research [23].
Only a limited number of studies have addressed the question of where in the brain activation patterns differ between mice with enhanced anxiety-related behavior and those with normal anxiety-related behavior (Table 5). While in most of these studies, immediate early gene mappings were focused on one or just a few brain areas known to be part of the anxiety neurocircuitry, Muigg et al. provide a more complete picture through the rostro-caudal extent of the mouse brain [77]. In part, similar patterns of both hyper- and hypo-activation in specific brain areas have been revealed in different mouse models of enhanced anxiety-related behavior in response to diverse emotional challenges (Table 5) which have been previously shown to increase c-Fos expression in the normal rodent brain, though to different levels (for review see [23]).
Table 5. Functional mapping in mouse models of enhanced anxiety-related behavior.
| Mouse | model | Challenge | Marker | Cortex | Septum | BNST | Hippocampus | Hypothalamus | Thalamus | Amygdala | PAG | Hind- brain |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutants | |||||||||||||
| OXT KO | f | EPM | c-Fos | – | – | – | – | – | – | ↑ MeA | – | – | [524] |
| mGluR8 KO | m | Restraint | c-Fos | – | – | ↓ | – | – | – | ↓ MeA | – | – | [525] |
| f | Shaker | c-Fos | – | – | – | – | – | – | ↓ MeA | – | – | [526] | |
| m | EPM | c-Fos | – | – | – | – | – | ↑ CMT | – | – | – | [413] | |
| ATP1α2 HET | m | CF | c-Fos | ↑ Pir | – | – | – | – | – | ↑ | – | – | [527] |
| CRHR2 KO | m | EPM | pCREB | ↓ Cg | – | ↓ | – | ↓ VMH | – | ↓ BLA | – | – | [259] |
| Fyn KO | CF | c-Fos | – | – | – | – | ↑ PVN, LH | – | ↑ CeA, MeA, BMA, ACo ↓ LA, BLA |
↑ | – | [481] | |
| TgNTRK3 | m | Restraint | c-Fos | ↑ PrL | ↑ LS | – | – | – | – | – | – | – | [528] |
| Yohimbine | c-Fos | ↑ Cg | – | – | – | ↓ PVN | – | ↑ CeA | – | – | [183] | ||
| Caffeine | c-Fos | ↑ Cg | – | – | – | – | – | ↑ BLA | – | – | [183] | ||
| BAFF OE | m | EPM | c-Fos | ↑ Cg | – | – | ↑ DG | ↑ PVN | – | ↑ BLA | – | – | [507] |
| Environmental manipulation | |||||||||||||
| CSC | m | OAE | c-Fos | – | ↓ LS | – | ↓ CA3 | ↓ PVN | – | – | ↑ DMPAG | – | [138] |
| Mice with naturally occurring high trait anxiety | |||||||||||||
| HVWR | m | PO/OF | c-Fos | – | – | ↓ CA1, CA3, DG | – | – | – | – | – | [97] | |
| HAB | m | OAE | c-Fos | ↓ Cg | ↑ LS | – | ↓ DG | ↑ LH, DMH, VMH, MPA |
– | ↑ MeA, LA, ACo | ↑ DMPAG | ↑ LC | [77] |
| 129P3/J | f | mHB | c-Fos | ↓ PrL | ↓ LS | ↑ | ↓ DG | – | – | ↑ CeA ~ CeA, BLA |
– | – | [69,260] |
| BALB/c | m | Restraint | c-Fos | ↓ Cg | – | – | ↓ DG | – | ↑ PV | – | – | – | [257] |
↑: Hyperactivation; ↓: Hypoactivation; ~: Unaltered anxiety-related behavior; ACo: Cortical amygdala; ATP1a: Sodium pump α2 subunit; BAFF: B-cell activating factor; BLA: Basolateral amygdala; BMA: Basomedial amygdala; BNST: Bed nucleus of the stria terminalis; CA1: CA1 region of the hippocampus; CA3: CA3 region of the hippocampus; CeA: Central amygdala; CF: Conditioned fear expression; Cg: Cingulate cortex; CMT: Centromedial thalamus; CRHR2: Corticotropin-releasing hormone 2 receptor; CSC: Chronic subordinate colony; DG: Dentate gyrus; DMH: Dorsomedial hypothalamus; DMPAG: Dorsomedial PAG; EPM: Elevated plus maze; f: Female; Fyn: Fyn tyrosine kinase; HAB: High anxiety-related behavior; HET: Heterozygous; HVWR: High voluntary wheel running; KO: Knockout; LA: Lateral amygdala; LC: Locus coeruleus; LH: Lateral hypothalamus; LS: Lateral septum; m: Male; MeA: Medial amygdala; mGluR8: Metabotropic glutamate 8 receptor; mHB: Modified holeboard test; MPA: Medial preoptic area; OAE: Open-arm exposure; OE: Overexpressing; OXT: Oxytocin; PAG: Periaqueductal gray; Pir: Piriform cortex; PO/OF: Predator odor/open-field; PrL: Prelimbic cortex; PV: Paraventricular thalamus; PVN: Paraventricular hypothalamus; TgNTRK3: Transgenic mice overexpressing the full-length neurotrophin-3 receptor TrkC; VMH: Ventromedial hypothalamus.
In mice with high trait anxiety compared with their ‘normal’ anxiety controls, c-Fos induction in response to an anxiety-provoking stimulus is attenuated in the cingulate cortex [77,259-261], which seems to correlate to the rostral anterior cingulate cortex of humans [262], although determining exact homologies of cortical areas in humans and mice remains a difficult task [263]. Furthermore, in the dentate gyrus of the hippocampus, activation is attenuated in high anxiety animals. By contrast, neuronal activation is facilitated in the paraventricular hypothalamus, in the amygdala (in its central and medial parts in particular) and in the dorsomedial periaqueductal grey [77,97,138,259,260]. While these observations fit well with observations in humans with pathological anxiety (for review see [22]), there is some inconsistency within the different mouse models (Table 5). As with variations in the human data (for review, see [22]), discrepancies in neuronal activation patterns in mouse models with enhanced anxiety levels may be explained by the different models and challenge paradigms used, which, as it has been suggested, may all elicit specific facets of anxiety that engage distinct parts of the anxiety circuitry [23]. For example, it was shown in HAB rats that the open-arm challenge activates the central amygdala only slightly, obscuring activation-processing differences between high- and low-anxiety animals in this subarea [264]. If social defeat was used as a challenge, this difference became increasingly evident [265]. Furthermore, in mutant mice, direct local effects of the altered gene product must be considered.
Taken together, these findings support and corroborate the human data indicating that dysregulations in specific brain areas that are known to play critical roles in anxiety [266] and conditioned fear neurocircuitries [35,267], contribute to enhanced (pathological) anxiety. In addition, to enable a better understanding of the pathophysiology of anxiety, this approach can also be used for the screening of novel drugs by determining the ability of therapeutics to reverse (normalize) aberrant activation patterns and neurochemical changes associated with enhanced anxiety [268-271]. Attenuation of amygdala reactivity seems to be important in humans [272-275] and animals [268]. So far, unfortunately, this strategy has not been used in mouse models of enhanced anxiety.
Environmental stimuli & epigenetic mechanisms contributing to anxiety-related behavior
Gene–environment interactions in shaping the anxiety phenotype assume that multiple genes influence susceptibility to environmental risk factors, including stress, and that the latter finally cause psychopathology [276]. This complex interaction includes the fact that exposure to stressful events does not always generate the disorder, this response heterogeneity being under genetic control (for review, see [277]). While exposure to defined environmental stimuli can easily be manipulated under experimental control, short-comings of experimental gene–environment interactions must be acknowledged, as they do not necessarily reflect naturally occurring conditions [278].
Animal models of genetic susceptibility versus resilience to environmental factors offer a valuable window for studying the contribution of postnatal maternal factors [279] and the effects of risk exposure on psychopathological processes. Male HAB mice, for instance, seem to be largely resistant to classical antidepressant drug treatment and thus may mimic endophenotypes typical of drug nonresponding, genetically predisposed patients. These properties make them ideal for gene–environment interaction studies, as their genotype is relatively well characterized beyond single genetic polymorphisms (Filiou et al., Unpublished Data). The resultant changes in brain neurobiology that underlie and confer risks for anxious behavior have been studied at multiple levels to confirm face and construct validity (i.e., they are related to human anxiety and do reveal mechanisms derived from theory). Only with the focus on such complex interactions, the question can be approached as to why different individuals exposed to the same environmental challenge or, vice versa, genetically identical individuals, exposed to different environments, experience different levels of anxiety.
One candidate gene that has been associated with trait anxiety encodes the serotonin receptor 1A (5-HT1A) with a promoter polymorphism being related to anxiety-linked personality traits [280]. This receptor subtype provides an intriguing example of how genetic–maternal environmental risk may contribute to anxiety. In more detail, maternal deficiency in the 5-HT1A receptor resulted in an offspring phenotype reminiscent of psychopathology. More than the offspring’s own receptor deficiency, the genotype of the mother seemed to be the prevailing mechanism in producing an anxious phenotype as measured on the elevated plus-maze. However, in the absence of a maternal genotype effect, the offspring’s own receptor deficit was sufficient to elicit the phenotype, pointing to two different underlying mechanisms: one by inheritance of receptor deficiency and the other by nongenetic transmission associated with receptor deficiency in the mother, raising the possibility that maternal influences may increase the risk for anxiety via a nongenetic mechanism. This model of dual transmission of risk factors indicates that the overall effects of risk alleles can be higher than estimated by traditional genetic studies [280].
While epigenetic changes induced in the offspring remain to be elucidated, it becomes clear that complex behaviors such as anxiety are driven by multiple mechanisms. For studying the interaction between genetic and nongenetic factors in more detail, it might therefore be useful to generate models in which the genetic underpinnings of trait anxiety or, at least, the contribution of single polymorphisms or genes are well-described. Then, using a combination of expression, genotypic and epigenetic approaches, one could try to examine whether those genes are also prone to epigenetic modifications that either activate or silence them.
Epigenetics refers to stable changes in chromatin and DNA via acetylation and methylation that underlie long-lasting alterations in gene expression and that are not associated with changes in the primary DNA sequence itself [281]. While it is generally accepted that such phenomena may predispose an individual to anxiety disorders, in many cases methylation and acetylation profiles are not determined. Nevertheless, the implication of environmentally-induced behavioral alterations essentially suggests underlying mechanisms that are epigenetic in nature [282].
Childhood maltreatment, including early abuse and neglect, are predisposing factors for the development of psychopathology, leaving lasting imprints on mechanisms underlying cognition and emotionality. Key mediators of such neural plasticity are detectable particularly in the prefrontal cortex and hippocampus and include, among others, BDNF protein levels and indices of synaptic long-term potentiation. In an elegant series of experiments, Roth et al. succeeded in demonstrating that infant rat maltreatment results in reduced BDNF expression in the prefrontal cortex, owing to methylation of BDNF DNA through the lifespan to adulthood [283]. The epigenetic modification could be reduced with chronic treatment of a DNA methylation inhibitor. Interestingly, rats that have experienced adversity, mistreat their own offspring, with the latter also having significant DNA methylation, further highlighting the dynamic role of methylation in both gene regulation and transgenerational inheritance of phenotype. Along the same lines, Franklin et al. demonstrated that trait transmission in mice occurs through males, altering DNA methylation in the germline, and affects the offspring in a sex-dependent manner [284]. It is tempting to speculate that enriched environment might prove useful for reversing persisting effects of traumatic experiences in early life.
The experimental data suggest that, beginning early in development, an individual’s genes, including epigenetic events, induce distinct changes in expression profile that occur in susceptible individuals only, shaping neural circuitry and neurometabolism characteristic of trait anxiety. Epigenetic modification thus opens enormous combinatorial options upon control of a wide range.
Novel targets for potential clinical pharmacotherapeutics
Mice with targeted mutations in the GABA-A receptors provide the basis for the search for novel anxiolytics targeting specific GABA-A receptor subunits only, as this was suspected to result in a better side-effect profile [285]. However, although the further clincal development of some α2-subunit specific GABA-A receptor agonists such as MRK 409 or TPA023 was determined due to unexpected side effects, GABA-A receptor subtype selective modulators are still considered as interesting non-sedative anxiolytics [286]. The development of positive allosteric modulators of the mGluR8 receptor and of mGluR5, 5-HT1A and 5-HT2C receptor antagonists as potential novel anxiolytics was promoted by results in mouse models with enhanced anxiety-related behavior. Anxiolytic properties of these antagonists/modulators have been demonstrated in various rodent anxiety tests (e.g., [287-294], for review see [295,296]) but, so far, they have not been processed into clinical trials for the treatment of anxiety disorders. Here, we want to focus in more detail on two novel targets as examples of how mouse models of enhanced anxiety related behavior contributed to their identification and/or characterization.
Neuropeptide S: a potential novel anxiolytic
The neuropeptide S (NPS) gene and the gene encoding its receptor (NPSR) are two of the promising candidates that are just at the start of extensive testing – and first results are very promising (for review, see [297]). NPS consists of 20 amino acids [298] and it is known to activate a G protein-coupled receptor elevating intracellular Ca2+ and cAMP [297]. Whereas the distribution of NPS precursor mRNA is very restricted in the CNS [298,299], the NPSR is expressed in many of its parts, particularly in the cortex, the thalamus, the hypothalamus and the amygdala [299,300] – brain areas known to regulate stress responses and to be part of the anxiety circuitry (see previous section).
In normal mice, NPS has anxiolytic effects in various unconditioned tests of anxiety including the elevated plus maze test and the stress-induced hyperthermia test [298,301-303] while also increasing wakefulness and arousal [298]. Recently, we could demonstrate that in classical Pavlovian fear conditioning, NPS also reduced the exaggerated conditioned fear responses in HAB mice to levels displayed by NAB mice [304]. This effect may be mediated via the G-protein coupled NPSR, as knockout of this receptor enhances anxiety-related behaviors ([305], but see [306]). Hence, the pharmacological spectrum of NPS is quite unique in comparison with other transmitters or drugs that influence emotional behavior in terms of anxiolysis and wakefulness. In humans, a single A-to-T SNP in the NPSR gene resulting in a ten-fold higher receptor functionality in vitro has been associated with overinterpretation of fear reactions in humans [307], increased amygdala responsiveness to fear-related stimuli [308] and panic disorder [309-311], providing further evidence of the potential of the NPS system to impact anxiety. Although a gain in receptor functioning in anxiety disorders, together with anxiolytic effects of NPS in mice, seems paradoxical, it is speculated that NPS may have different qualities during developmental stages with beneficial effects in adulthood [308].
In addition to its strong influence on stress-induced anxiety-related behavior, the NPS and its NPSR have been shown to be involved in many other physiological and pathological processes including depression-like behavior [306], drug seeking [312], food intake [313], respiratory function [314], asthma/atopy [315-318] and inflammatory bowel disease [319]. Therefore, the role of the NPS/NPSR system in health and disease needs to be better characterized in order to appraise its usefulness as a potential anxiolytic drug target.
Neurokinin-1 receptor antagonists in clinical trials for anxiety disorders
Many different interesting therapeutic actions including anxiolytic, antidepressant, antiemetic, antimigraine, antiaddiction, analgesic, anti-inflammatory, anti-cancer effects have been attributed to NK1R-A [320]. Indeed, they have been investigated in clinical trials for many years and, until now, the only US FDA approved NK1R-A is aprepitant (Emend™, Merck Sharp & Dohme, USA) for the indication of postoperative and chemotherapy-induced nausea. In neuroscience, following disappointing results in depression trials, the focus moved to anxiety disorders, despite sparse evidence concerning involvement of the substance P (SP) and NK1Rs in pathological anxiety (for review see [321]). Indeed, in 2005 Furmark et al. [322] reported positive effects of NK1R-A in patients with social phobia during a stimulus-provocation task; this, however, was not confirmed in another study [323], while in patients with post-traumatic stress disorders, it was not found to be superior to a placebo after short-term treatment [324].
These clinical anxiety trials are based on evidence from preclinical research demonstrating that NK1R-A including the novel, potent compound vestipitant [325] exert anxiolytic (and anti-depressant) effects in various species including rats, gerbils and mice (for review see [326]). Since most of this evidence was obtained in ‘normal’ animals, we decided to use the HAB mouse model for antagonist testing. Indeed, treatment of HABs with the selective NK1R-A L-822,429 was found to attenuate exaggerated conditioned fear expression [79,80], as well as their unconditioned anxiety response of HABs in the light/dark test (Figure 1) [327]. In addition, the treatment also normalizes the reduced heart rate variability observed in HABs to levels displayed by normal anxiety NABs (Figure 1) [80]. To gain insight into the role of the SP system in pathological anxiety, we used molecular biological and microdialysis approaches. It was found that HABs display enhanced Tac1 gene expression, as well as exaggerated stress-induced SP release in the amygdala, a key brain area in fear and anxiety processing, suggesting dysregulated SP transmission as a result of genetic selection for an extremely anxious phenotype [328]. Thus, using a psychopathological mouse model that mimics important aspects of human anxiety disorders, we support and corroborate the so far limited human evidence of neurobiological and genetic mechanisms of SP (for review see [321]) underlying abnormal anxiety. This evidence derived from the use of HAB mice further strengthens the hope that NK1R antagonism may be a promising therapeutic approach for anxiety disorders, especially in individuals with a dysfunctional SP system, despite mixed clinical results so far [322-324].
Conclusion
Over the last two decades, building on data from human and rodent studies, our knowledge of the neurobiology of anxiety disorders has continuously increased, paralleling the increasing use of the laboratory mouse in anxiety research. In the present article, we have provided a summary of available mouse models of enhanced anxiety-related behavior and discussed examples of how and where such models are employed to advance the development of novel anxiolytic therapeutics (Figure 3). The discussed models mainly show improved validity, and in particular, face validity – that is, they closely reflect important symptomatology and they model aspects of human anxiety disorder endophenotypes. Of the models described so far, mice with inborn enhanced anxiety such as the HAB mouse line seem to meet translational value to a high degree, although it has to be noted that their validity is still incomplete. On the other hand, although astonishing homologies exist between mice and humans in terms of genes, brain mechanisms and anxiety-related responses, it will never be possible to model all aspects of clinically manifested anxiety disorders and even the most sophisticated mouse model of enhanced anxiety-related behavior will remain a reductionistic replication of the human disorder. This is also due to the fact that the integrity of the murine model depends very much on our status of knowledge about the disease itself.
Figure 3. Strategy for translating research from mice to humans with the ultimate goal of developing novel anxiolytic drugs.
Mouse models of enhanced anxiety-related behavior, induced by genetic or environmental manipulations and that closely resemble human symptomatology, increase the chances of identifying candidate genes for human anxiety disorders by reducing the problems of genetic heterogeneity and a variable environment. It is also important to study the involvement of these candidate genes in patients. Subsequently, elucidating the function of the identified gene using mouse models as well as the human population increases our knowledge of the neurobiology of anxiety disorders, which, at the same time, is necessary to improve the models. It is also necessary to test in mouse models the anxiolytic potential of possible novel anxiolytic drugs prior to clinical test phases. Dashed lines in the diagram indicate those aspects of anxiety research using mouse models of enhanced anxiety-related behavior that are just beginning to attract scientific interest, including gene-environment interactions and epigenetic modulations.
Bypassing this dilemma to a certain extent, the mouse provides a specific model organism to which partly unique (molecular) technologies can be applied in order to elucidate genetic mechanisms underlying anxiety disorders. Indeed, the involvement of TMEM132D and of the zinc metallo-enzyme, glyoxalase-1, in pathological anxiety represents two successful examples of bridging mouse and human neurogenetics [29,76,237,245]. Furthermore, mouse models of enhanced anxiety-related behavior are important for gaining insights into neurobiological mechanisms underlying pathological anxiety. Here, novel technologies may further help to limit brain regions to specific neuronal subpopulations modulating emotionality as demonstrated by Tye and coworkers, who identified amygdala projection neurons for reversible and bidirectional control of anxiety in mice by means of optogenetics [329]. The novel information can then be further tested in patients, using the technology that is available, such as imaging and post-mortem studies. For instance, an elevated expression of TMEM132D in the frontal cortex was observed in both HAB mice, selectively bred for high trait anxiety, and individuals with risk genotypes for panic disorder [237]. In addition, mouse models of enhanced anxiety-related behavior will be important for studying environmental stimuli and epigenetic modulations in the manifestation of an anxiety disorder. The information gained already and in the future will help to further improve subsequent mouse models of enhanced anxiety-related behavior (Figure 3). Using genetic tools, the mouse organism has proved ideal for elucidating the neurobiological function of identified susceptibility genes at specific loci in the brain. This has been, and will continue to be, particularly important, when classical pharmacological studies reach their limits, for example, when no selective ligands are available as demonstrated in the case of the GABA-A receptor subunits. Once the function of interesting systems has been elucidated, novel drug classes can be established (Figure 3).
Future perspective
It is clear that mouse models of enhanced anxiety-related behavior need to be refined continuously to fully reveal the therapeutic potential of a broad range of (novel) compounds. First, validation processes should be extended by means of objective, physiological readouts (e.g., [80]) and, wherever possible, pharmacological validation of the anxious phenotype, which may, however, interfere with the discovery of potentially novel targets. Thereby, intense collaborations between researchers from both the clinical and preclinical sides will be pivotal in order to improve the bidirectional characterization of patients and mouse models of enhanced anxiety. In the (near) future this may result in a better classification of anxiety disorders and may guide the development of mouse models of anxiety disorder subcategories (see initial attempts for post-traumatic stress disorder [70] or panic disorder [330,331]). Second, the mouse models described so far mostly reflect either genetic or environmental manipulations rather than mimicking the interaction of these two risk factors, though such interaction seems to be critical in the clinical manifestation of anxiety disorders [7,159]. Therefore, it is expected that the real clinical situation can be better modeled in mice by superimposing stressful environmental manipulations onto models with a well-defined genetic predisposition towards enhanced trait anxiety. Unfortunately, to date there have been only a limited number of such approaches whose findings have been published (e.g., [124,277,280,332,333]). What determines vulnerability to develop an anxiety disorder, together with treatment responses, may be causally addressed by genetic approaches in mouse models of enhanced anxiety-related behavior, providing fundamental insights into the construct of anxiety disorders. In the long run, clinical biological diagnostic tools will eventually be developed. Markers based on neurobiological and neurophysiological endophenotypes, as suggested for glyoxalase-1, could lead to a more precise classification and, thus, diagnosis of anxiety disorders, both of which at the moment are based on symptoms specified in DSM-IV and ICD-10 [17,18]. Such adjustments will enhance the models’ application for both the detection of novel targets with anxiolytic action and improve our understanding of the underlying pathophysiology of anxiety disorders, which is the basis for the development of novel anxiolytic drug classes with an improved pharmacologic profile (Figure 3).
The integration of mouse models of enhanced anxiety-related behavior into the drug discovery process will improve the screening for the drugs’ clear anxiolytic potential before it advances into clinical trials. In parallel, these models may be used for distinguishing responders to anxiolytic drugs from nonresponders in order to improve pharmacotherapy, as this is still an unresolved area. In the future, the pharmacotherapy will be combined with techniques recording changes in the brain, such as functional imaging or microdialysis. An important aspect of using mouse models of enhanced anxiety-related behavior is to prove whether normalization of specific brain activity alterations is necessary/sufficient for successful treatment to take place, as this information can be obtained in humans in a very crude manner only. In this respect, non-invasive imaging techniques with high temporal and spatial resolution, which can be applied without inducing stress to awake, freely moving animals, will need to be developed. Then, pinning down causality by targeting dysfunctions in brain-activity processing in animal models of enhanced anxiety will guide the development of novel anxiolytic pharmacotherapies.
Executive summary.
Pathological anxiety
-
■
Anxiety disorders represent a quantitative and/or qualitative variation of ‘normal’ anxiety, which is still a matter of debate.
Pharmacotherapies used in anxiety disorders
-
■
Despite intense research, no true novel anxiolytic class with an improved pharmacotherapeutic profile has been introduced into therapy in the last four decades.
-
■
To date, after demonstration of its anxiolytic effects in preclinical trials, no novel, improved anxiolytic has so far made it via clinical trials onto the market. One explanation may be that their efficacy in animal testing was observed in physiological rather than pathophysiological anxiety states.
-
■
Animal models of enhanced anxiety-related behavior that mirror the pathophysiology of the human anxiety disorder may prove more effective in the development of novel anxiolytics.
Mouse models of enhanced anxiety-related behavior
-
■
Enhanced anxiety-related behavior is a persistent and enduring characteristic of animal models, reflecting trait anxiety.
-
■
Experimental manipulations reflecting the risk factors of anxiety disorders, including environmental and genetic manipulations, are used to induce enhanced anxiety-related behaviors in mice.
-
■
Mouse models of enhanced anxiety-related behavior demonstrate good face, and increasing construct validity and are hoped to improve the discovery of true novel anxiolytic drugs.
Translational anxiety research
-
■
The identification of candidate genes underlying the enhanced anxiety-related behavior of mouse models was successfully translated into information that can be applied to humans. By this means, an involvement of regulator of G-protein signaling 2, TMEM132D, glyoxalase-1 and BDNF in anxiety patients was demonstrated.
-
■
The demonstration in patients with anxiety of similar aberrant neuronal activation to that found in mouse models of enhanced anxiety-related behavior may be used for the screening of novel drugs by determining the ability of therapeutics to reverse (normalize) these changes associated with enhanced anxiety. So far, however, this strategy has not been used in mouse models of enhanced anxiety.
-
■
Mouse models of enhanced inborn anxiety-related behavior are ideal for studying genetic/epigenetic mechanisms contributing to pathological anxiety.
Novel targets for potential clinical pharmacotherapeutics
-
■
Mouse models of enhanced anxiety-related behavior have revealed interesting targets for novel anxiolytics, including specific GABA-A receptor subunits, the mGluR8, mGluR5, 5-HT1A, 5-HT2C and NK1 receptors as well as neuropeptide S. Their anxiolytic efficacy has been demonstrated in anxiety tests and has been partly confirmed in humans. However, none of these drugs has so far received approval for treating anxiety disorders.
Acknowledgments
Financial & competing interests disclosure
This work was supported by the Austrian Science Fund (FWF): SFB F4410-B19, NFN-S10202, P22931-B18 to N Singewald, and a Young Investigator Funding by the University of Innsbruck to SB Sartori. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Ortony A, Turner TJ. What’s basic about basic emotions? Psychol. Rev. 1990;97(3):315–331. doi: 10.1037/0033-295x.97.3.315. [DOI] [PubMed] [Google Scholar]
- 2.Sylvers P, Lilienfeld SO, Laprairie JL. Differences between trait fear and trait anxiety: implications for psychopathology. Clin. Psychol. Rev. 2011;31(1):122–137. doi: 10.1016/j.cpr.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Ohl F, Arndt SS, Van Der Staay FJ. Pathological anxiety in animals. Vet. J. 2008;175(1):18–26. doi: 10.1016/j.tvjl.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 2001;125(1–2):141–149. doi: 10.1016/s0166-4328(01)00291-1. [■ Review discussing the relevance of animal models of enhanced anxiety-like behavior. Includes selected mouse models of pathological anxiety.] [DOI] [PubMed] [Google Scholar]
- 5.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat. Rev. Genet. 2009;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 6.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am. J. Psychiatry. 2001;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 7.Merikangas KR, Pine D, American College of Neuropsychopharmacology . Genetic and Other Vulnerability Factors for Anxiety and Stress Disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; Nashville, TN, USA: 2002. p. 867. [Google Scholar]
- 8.Young EA, Abelson JL, Liberzon I. Stress Hormones and Anxiety Disorders. In: Blanchard RJ, Blanchard DC, Griebel G, Nutt D, editors. Handbook of Anxiety and Fear. Academic Press; Oxford: 2008. pp. 455–473. [Google Scholar]
- 9.Veenema AH, Meijer OC, De Kloet ER, Koolhaas JM. Genetic selection for coping style predicts stressor susceptibility. J. Neuroendocrinol. 2003;15(3):256–267. doi: 10.1046/j.1365-2826.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 10.Wermter AK, Laucht M, Schimmelmann BG, et al. From nature versus nurture, via nature and nurture, to gene x environment interaction in mental disorders. Eur. Child Adolesc. Psychiatry. 2010;19(3):199–210. doi: 10.1007/s00787-009-0082-z. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 12.Andlin-Sobocki P, Jonsson B, Wittchen Hu, Olesen J. Cost of disorders of the brain in Europe. Eur. J. Neurol. 2005;12(Suppl. 1):1–27. doi: 10.1111/j.1468-1331.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 13.WHO . Global Burden of Disease Report. WHO Press; Geneva, Switzerland: 2004. [Google Scholar]
- 14.Kessler RC, Ormel J, Petukhova M, et al. Development of lifetime comorbidity in the world health organization world mental health surveys. Arch. Gen. Psychiatry. 2011;68(1):90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott KM, Von Korff M, Alonso J, et al. Age patterns in the prevalence of DSM-IV depressive/anxiety disorders with and without physical co-morbidity. Psychol. Med. 2008;38(11):1659–1669. doi: 10.1017/S0033291708003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okasha A. Would the use of dimensions instead of categories remove problems related to subthreshold disorders? Eur. Arch. Psychiatry Clin. Neurosci. 2009;259(Suppl. 2):S129–S133. doi: 10.1007/s00406-009-0052-y. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- 18.WHO . The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD-10) WHO Press; Geneva, Switzerland: 1992. [Google Scholar]
- 19.Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin. Psychiatry. 2010;71(7):839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- 20.Plag J, Siegmund A, Strohle A. Pharmakotherapie bei Angsterkrankungen. Z. Psychiatr. Psychol. Psychother. 2009;57(3):185–194. [Google Scholar]
- 21.Charney DS, Drevets WC, American College of Neuropsychopharmacology . Neurobiological Basis of Anxiety Disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology – 5th Generation of Progress. Lippincott Williams & Wilkins; Nashville, TN, USA: 2008. [Google Scholar]
- 22.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singewald N. Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci. Biobehav. Rev. 2007;31(1):18–40. doi: 10.1016/j.neubiorev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01362.x. DOI: 10.1111/j.1476-5381.2011.01362.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steckler T, Stein MB, Holmes A. Developing Novel Anxiolytics: Improving Preclinical Detection and Clinical Assessment. In: Mcarthur RA, Borsini F, editors. Animal and Translational Models for CNS Drug Discovery. Academic Press; New York (NY, USA): 2008. [Google Scholar]
- 26.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4(9):775–790. doi: 10.1038/nrd1825. [■ Historical and practical review on mice in psychiatric research. A good place to start for a basc overview of mice in the field of anxiety and depression.] [DOI] [PubMed] [Google Scholar]
- 27.Joyner AL, Sedivy JM. Gene targeting: a practical approach. Oxford University Press; Oxford, NY, USA: 2000. [Google Scholar]
- 28.Crawley JN. What’s wrong with my mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss; 2000. [Google Scholar]
- 29.Hovatta I, Tennant RS, Helton R, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438(7068):662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 30.Carver EA, Stubbs L. Zooming in on the human-mouse comparative map: genome conservation re-examined on a high-resolution scale. Genome Res. 1997;7(12):1123–1137. doi: 10.1101/gr.7.12.1123. [DOI] [PubMed] [Google Scholar]
- 31.Tecott LH. The genes and brains of mice and men. Am. J. Psychiatry. 2003;160(4):646–656. doi: 10.1176/appi.ajp.160.4.646. [DOI] [PubMed] [Google Scholar]
- 32.Jones EG. The origins of cortical interneurons: mouse versus monkey and human. Cereb. Cortex. 2009;19(9):1953–1956. doi: 10.1093/cercor/bhp088. [DOI] [PubMed] [Google Scholar]
- 33.Belzung C, Philippot P. Anxiety from a phylogenetic perspective: is there a qualitative difference between human and animal anxiety? Neural Plast. 2007;2007:59676. doi: 10.1155/2007/59676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canteras NS, Resstel LB, Bertoglio LJ, Carobrez Ade P, Guimaraes FS. Neuroanatomy of anxiety. Curr. Top. Behav. Neurosci. 2010;2:77–96. doi: 10.1007/7854_2009_7. [DOI] [PubMed] [Google Scholar]
- 35.Pine DS. Integrating research on development and fear learning: a vision for clinical neuroscience? Depress. Anxiety. 2009;26(9):775–779. doi: 10.1002/da.20595. [DOI] [PubMed] [Google Scholar]
- 36.Stein DJ, Bouwer C. A neuro-evolutionary approach to the anxiety disorders. J. Anxiety Disord. 1997;11(4):409–429. doi: 10.1016/s0887-6185(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 37.Bourin M, Petit-Demouliere B, Dhonnchadha BN, Hascoet M. Animal models of anxiety in mice. Fundam Clin. Pharmacol. 2007;21(6):567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 38.Beuzen A, Belzung C. Link between emotional memory and anxiety states: a study by principal component analysis. Physiol. Behav. 1995;58(1):111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 39.Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007;179(1):1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Millan MJ. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70(2):83–244. doi: 10.1016/s0301-0082(03)00087-x. [■■ Exhaustive and scholarly piece on the mechanisms involved in the induction and inhibition of anxious states. Covers all classical neurotransmitters and many other neuromodulators.] [DOI] [PubMed] [Google Scholar]
- 41.Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press; London, UK: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson JB, Rayner R. Conditioned emotional reactions. J. Exp. Psychol. 1920;3(1):1–14. doi: 10.1037//0003-066x.55.3.313. [DOI] [PubMed] [Google Scholar]
- 43.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol. Psychol. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Hefner K, Whittle N, Juhasz J, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. 2008;28(32):8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muigg P, Hetzenauer A, Hauer G, et al. Impaired extinction of learned fear in rats selectively bred for high anxiety - evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur. J. Neurosci. 2008;28(11):2299–2309. doi: 10.1111/j.1460-9568.2008.06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J. Neurosci. 2010;30(41):13586–13596. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treit D, Engin E, McEown K. Animal Models of Anxiety and Anxiolytic Drug Action. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Springer; Heidelberg: 2010. pp. 121–160. [DOI] [PubMed] [Google Scholar]
- 48.Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur. J. Pharmacol. 2003;463(1–3):97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- 49.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005;29(4–5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl.) 2002;163(2):121–141. doi: 10.1007/s00213-002-1155-6. [■■ Details predictive validity of anxiety tests in response to chronic antidepressant treatment, particularly with selective serotonin reuptake inhibitors, which are clinically effective in the treatment of all anxiety disorders.] [DOI] [PubMed] [Google Scholar]
- 51.Dekeyne A. Behavioural models for the characterisation of established and innovative antidepressant agents. Therapie. 2005;60(5):477–484. doi: 10.2515/therapie:2005068. [DOI] [PubMed] [Google Scholar]
- 52.Vinkers CH, Van Bogaert MJ, Klanker M, et al. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. Eur. J. Pharmacol. 2008;585(2–3):407–425. doi: 10.1016/j.ejphar.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 53.Shekhar A, Mccann UD, Meaney MJ, et al. Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders. Psychopharmacology (Berl.) 2001;157(4):327–339. doi: 10.1007/s002130100859. [DOI] [PubMed] [Google Scholar]
- 54.Rodgers R. Animal Tests for Anxiety. In: Koo GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Academic Press; Oxford: 2010. pp. 90–100. [Google Scholar]
- 55.Clement Y, Joubert C, Kopp C, et al. Anxiety in mice: a principal component analysis study. Neural Plast. 2007;2007:35457. doi: 10.1155/2007/35457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones N, King SM. Influence of circadian phase and test illumination on pre-clinical models of anxiety. Physiol. Behav. 2001;72(1–2):99–106. doi: 10.1016/s0031-9384(00)00388-7. [DOI] [PubMed] [Google Scholar]
- 57.Post AM, Weyers P, Holzer P, et al. Gene–environment interaction influences anxiety-like behavior in ethologically based mouse models. Behav. Brain Res. 2011;218(1):99–105. doi: 10.1016/j.bbr.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 58.Beck JA, Lloyd S, Hafezparast M, et al. Genealogies of mouse inbred strains. Nat. Genet. 2000;24(1):23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 59.Davisson M. Rules for nomenclature of inbred strains. Oxford University Press; Oxford: 1996. [Google Scholar]
- 60.Crawley JN, Belknap JK, Collins A, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl.) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 61.Hovatta I, Barlow C. Molecular genetics of anxiety in mice and men. Ann. Med. 2008;40(2):92–109. doi: 10.1080/07853890701747096. [DOI] [PubMed] [Google Scholar]
- 62.Bouwknecht JA, Paylor R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav. Pharmacol. 2008;19(5–6):385–402. doi: 10.1097/FBP.0b013e32830c3658. [DOI] [PubMed] [Google Scholar]
- 63.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57(6):809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Crawley JN, Davis LG. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res. Bull. 1982;8(6):609–612. doi: 10.1016/0361-9230(82)90087-9. [DOI] [PubMed] [Google Scholar]
- 65.Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl.) 2000;148(2):164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 66.Rodgers RJ, Davies B, Shore R. Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus-maze: importance and implications of behavioural baseline. Genes Brain Behav. 2002;1(4):242–251. doi: 10.1034/j.1601-183x.2002.10406.x. [DOI] [PubMed] [Google Scholar]
- 67.Mathiasen LS, Mirza NR, Rodgers RJ. Strain- and model-dependent effects of chlordiazepoxide, L-838,417 and zolpidem on anxiety-like behaviours in laboratory mice. Pharmacol. Biochem. Behav. 2008;90(1):19–36. doi: 10.1016/j.pbb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Laarakker MC, Van Lith HA, Ohl F. Behavioral characterization of A/J and C57BL/6J mice using a multidimensional test: association between blood plasma and brain magnesium-ion concentration with anxiety. Physiol. Behav. 2011;102(2):205–219. doi: 10.1016/j.physbeh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 69.Salomons AR, Van Luijk JA, Reinders NR, Kirchhoff S, Arndt SS, Ohl F. Identifying emotional adaptation: behavioural habituation to novelty and immediate early gene expression in two inbred mouse strains. Genes Brain Behav. 2010;9(1):1–10. doi: 10.1111/j.1601-183X.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 70.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J. Psychiatr. Res. 2007;41(10):848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed AS. Post-traumatic stress disorder, resilience and vulnerability. Adv. Psyiatr. Treat. 2007;13:369–375. [Google Scholar]
- 72.Gill KJ, Boyle AE. Quantitative trait loci for novelty/stress-induced locomotor activation in recombinant inbred (RI) and recombinant congenic (RC) strains of mice. Behav. Brain Res. 2005;161(1):113–124. doi: 10.1016/j.bbr.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 73.Philip VM, Duvvuru S, Gomero B, et al. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010;9(2):129–159. doi: 10.1111/j.1601-183X.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flint J. Analysis of quantitative trait loci that influence animal behavior. J. Neurobiol. 2003;54(1):46–77. doi: 10.1002/neu.10161. [DOI] [PubMed] [Google Scholar]
- 75.Swallow JG, Garland T., Jr Selection Experiments as a tool in evolutionary and comparative physiology: insights into complex traits-an introduction to the symposium. Intr. Comp. Biol. 2005;45:387–390. doi: 10.1093/icb/45.3.387. [DOI] [PubMed] [Google Scholar]
- 76.Kromer SA, Kessler MS, Milfay D, et al. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J. Neurosci. 2005;25(17):4375–4384. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muigg P, Scheiber S, Salchner P, Bunck M, Landgraf R, Singewald N. Differential stress-induced neuronal activation patterns in mouse lines selectively bred for high, normal or low anxiety. PLoS One. 2009;4(4):E5346. doi: 10.1371/journal.pone.0005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Landgraf R, Kessler MS, Bunck M, et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 2007;31(1):89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Sartori SB, Hauschild M, Bunck M, Gaburro S, Landgraf R, Singewald N. Enhanced fear expression in a psychopathological mouse model of trait anxiety: pharmacological interventions. PLoS One. 2011;6:E16849. doi: 10.1371/journal.pone.0016849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaburro S, Stiedl O, Giusti P, Sartori SB, Landgraf R, Singewald N. A mouse model of high trait anxiety shows reduced heart rate variability that can be reversed by anxiolytic drug treatment. Int. J. Neuropsychopharmacol. 2011;15:1–15. doi: 10.1017/S1461145711000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69(3):563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lissek S, Powers AS, Mcclure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res. Ther. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Ponder CA, Kliethermes CL, Drew MR, et al. Selection for contextual fear conditioning affects anxiety-like behaviors and gene expression. Genes Brain Behav. 2007;6(8):736–749. doi: 10.1111/j.1601-183X.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 84.Szego EM, Janaky T, Szabo Z, et al. A mouse model of anxiety molecularly characterized by altered protein networks in the brain proteome. Eur. Neuropsychopharmacol. 2010;20(2):96–111. doi: 10.1016/j.euroneuro.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Gariepy JL, Hood KE, Cairns RB. A developmental-genetic analysis of aggressive behavior in mice (Mus musculus): III. Behavioral mediation by heightened reactivity or immobility? J. Comp. Psychol. 1988;102(4):392–399. doi: 10.1037/0735-7036.102.4.392. [DOI] [PubMed] [Google Scholar]
- 86.Cairns RB, Maccombie DJ, Hood KE. A developmental-genetic analysis of aggressive behavior in mice: I. Behavioral outcomes. J. Comp. Psychol. 1983;97(1):69–89. [PubMed] [Google Scholar]
- 87.Nehrenberg DL, Rodriguiz RM, Cyr M, et al. An anxiety-like phenotype in mice selectively bred for aggression. Behav. Brain Res. 2009;201(1):179–191. doi: 10.1016/j.bbr.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beckham JC, Feldman ME, Kirby AC, Hertzberg MA, Moore SD. Interpersonal violence and its correlates in Vietnam veterans with chronic posttraumatic stress disorder. J. Clin. Psychol. 1997;53(8):859–869. doi: 10.1002/(sici)1097-4679(199712)53:8<859::aid-jclp11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 89.Begic D, Jokic-Begic N. Aggressive behavior in combat veterans with post-traumatic stress disorder. Mil. Med. 2001;166(8):671–676. [PubMed] [Google Scholar]
- 90.Mcfall M, Fontana A, Raskind M, Rosenheck R. Analysis of violent behavior in Vietnam combat veteran psychiatric inpatients with posttraumatic stress disorder. J. Trauma Stress. 1999;12(3):501–517. doi: 10.1023/A:1024771121189. [DOI] [PubMed] [Google Scholar]
- 91.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364(9432):453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 92.Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I. psychopathology, comorbidity, and personality structure. Biol. Psychiatry. 2002;51(12):936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- 93.Chapouthier G, Launay JM, Venault P, Breton C, Roubertoux PL, Crusio WE. Genetic selection of mouse lines differing in sensitivity to a benzodiazepine receptor inverse agonist. Brain Res. 1998;787(1):85–90. doi: 10.1016/s0006-8993(97)01483-2. [DOI] [PubMed] [Google Scholar]
- 94.Reddy PV, Devi K. Intrastrain variations in anxiolytic effect of nitrazepam in mice. Indian J. Physiol. Pharmacol. 2006;50(3):309–312. [PubMed] [Google Scholar]
- 95.Correia D, Ribeiro AF, Brunialti Godard AL, Boerngen-Lacerda R. Trait anxiety and ethanol: anxiolysis in high-anxiety mice and no relation to intake behavior in an addiction model. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(5):880–888. doi: 10.1016/j.pnpbp.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 96.Jakovcevski M, Schachner M, Morellini F. Individual variability in the stress response of C57BL/6J male mice correlates with trait anxiety. Genes Brain Behav. 2008;7(2):235–243. doi: 10.1111/j.1601-183X.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 97.Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One. 2010;5(9):PII: E12769. doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J. Neurosci. 1994;14(5 Pt 1):2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu GX, Cai GQ, Cai YQ, et al. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32(7):1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- 100.Chiu CS, Brickley S, Jensen K, et al. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J. Neurosci. 2005;25(12):3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int. J. Neuropsychopharmacol. 2011;14(5):618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem. Pharmacol. 2010;79(4):632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 2009;46(2):94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 104.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4(7):420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 105.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport. 2004;15(8):1271–1274. doi: 10.1097/01.wnr.0000129370.04248.92. [DOI] [PubMed] [Google Scholar]
- 106.Holmes A, Kinney JW, Wrenn CC, et al. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003;28(6):1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- 107.Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav. Brain Res. 2002;136(2):489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 108.Schalkwyk LC, Fernandes C, Nash MW, Kurrikoff K, Vasar E, Koks S. Interpretation of knockout experiments: the congenic footprint. Genes Brain Behav. 2007;6(3):299–303. doi: 10.1111/j.1601-183X.2007.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shin J, Gireesh G, Kim SW, et al. Phospholipase C β 4 in the medial septum controls cholinergic theta oscillations and anxiety behaviors. J. Neurosci. 2009;29(49):15375–15385. doi: 10.1523/JNEUROSCI.3126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bahi A, Mineur YS, Picciotto MR. Blockade of protein phosphatase 2B activity in the amygdala increases anxiety- and depression-like behaviors in mice. Biol. Psychiatry. 2009;66(12):1139–1146. doi: 10.1016/j.biopsych.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Mukherjee S, Coque L, Cao JL, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol. Psychiatry. 2010;68(6):503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol. Psychiatry. 2010;15(9):905–917. doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- 113.Lesscher HM, Mcmahon T, Lasek AW, et al. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2008;7(3):323–333. doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 114.Nemeroff CB. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry. 2004;65(Suppl. 1):18–28. [PubMed] [Google Scholar]
- 115.Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci. Biobehav. Rev. 2009;33(4):573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holmes A, Le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci. Biobehav. Rev. 2005;29(8):1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 117.Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32(5):437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 118.De Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev. 2005;29(2):271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 120.Romeo RD, Mueller A, Sisti HM, Ogawa S, Mcewen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm. Behav. 2003;43(5):561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 121.Macri S, Laviola G. Single episode of maternal deprivation and adult depressive profile in mice: interaction with cannabinoid exposure during adolescence. Behav. Brain Res. 2004;154(1):231–238. doi: 10.1016/j.bbr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Niwa M, Matsumoto Y, Mouri A, Ozaki N, Nabeshima T. Vulnerability in early life to changes in the rearing environment plays a crucial role in the aetiopathology of psychiatric disorders. Int. J. Neuropsychopharmacol. 2011;14(4):459–477. doi: 10.1017/S1461145710001239. [DOI] [PubMed] [Google Scholar]
- 123.Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL. Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Res. 2004;1016(1):111–118. doi: 10.1016/j.brainres.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 124.Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes Brain Behav. 2006;5(4):346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 125.Venerosi A, Cirulli F, Capone F, Alleva E. Prolonged perinatal AZT administration and early maternal separation: effects on social and emotional behaviour of periadolescent mice. Pharmacol. Biochem. Behav. 2003;74(3):671–681. doi: 10.1016/s0091-3057(02)01068-7. [DOI] [PubMed] [Google Scholar]
- 126.Loizzo A, Loizzo S, Galietta G, et al. Overweight and metabolic and hormonal parameter disruption are induced in adult male mice by manipulations during lactation period. Pediatr. Res. 2006;59(1):111–115. doi: 10.1203/01.pdr.0000190575.12965.ce. [DOI] [PubMed] [Google Scholar]
- 127.Savignac HM, Dinan TG, Cryan JF. Resistance to early-life stress in mice: effects of genetic background and stress duration. Front. Behav. Neurosci. 2011;5:13. doi: 10.3389/fnbeh.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fabricius K, Wortwein G, Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct. Funct. 2008;212(5):403–416. doi: 10.1007/s00429-007-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34(5):762–772. doi: 10.1016/j.psyneuen.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 131.Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 132.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1–2):172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 133.Dadomo H, Sanghez V, Di Cristo L, et al. Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.11.016. DOI:10.1016/j.pnpbp.2010.11.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 134.Adamcio B, Havemann-Reinecke U, Ehrenreich H. Chronic psychosocial stress in the absence of social support induces pathological pre-pulse inhibition in mice. Behav. Brain Res. 2009;204(1):246–249. doi: 10.1016/j.bbr.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 135.Erhardt A, Muller MB, Rodel A, et al. Consequences of chronic social stress on behaviour and vasopressin gene expression in the PVN of DBA/2OlaHsd mice – influence of treatment with the CRHR1-antagonist R121919/NBI 30775. J. Psychopharmacol. 2009;23(1):31–39. doi: 10.1177/0269881108089813. [DOI] [PubMed] [Google Scholar]
- 136.Reber SO, Birkeneder L, Veenema AH, et al. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148(2):670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- 137.Reber SO, Neumann ID. Defensive behavioral strategies and enhanced state anxiety during chronic subordinate colony housing are accompanied by reduced hypothalamic vasopressin, but not oxytocin, expression. Ann. NY Acad. Sci. 2008;1148:184–195. doi: 10.1196/annals.1410.003. [DOI] [PubMed] [Google Scholar]
- 138.Singewald GM, Nguyen NK, Neumann ID, Singewald N, Reber SO. Effect of chronic psychosocial stress-induced by subordinate colony (CSC) housing on brain neuronal activity patterns in mice. Stress. 2009;12(1):58–69. doi: 10.1080/10253890802042082. [DOI] [PubMed] [Google Scholar]
- 139.Schmidt MV, Sterlemann V, Ganea K, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32(5):417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 140.Schmidt MV, Scharf SH, Liebl C, et al. A novel chronic social stress paradigm in female mice. Horm. Behav. 2010;57(4–5):415–420. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 141.Schmidt MV, Scharf SH, Sterlemann V, et al. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology. 2010;35(5):635–643. doi: 10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 142.Sterlemann V, Ganea K, Liebl C, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm. Behav. 2008;53(2):386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 143.Koike H, Ibi D, Mizoguchi H, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav. Brain Res. 2009;202(1):114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 144.Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4(4):240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 145.Abramov U, Raud S, Koks S, et al. Targeted mutation of CCK(2) receptor gene antagonises behavioural changes induced by social isolation in female, but not in male mice. Behav. Brain Res. 2004;155(1):1–11. doi: 10.1016/j.bbr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 146.Ishihama T, Ago Y, Shintani N, et al. Environmental factors during early developmental period influence psychobehavioral abnormalities in adult PACAP-deficient mice. Behav. Brain Res. 2010;209(2):274–280. doi: 10.1016/j.bbr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 147.Gresack JE, Risbrough VB, Scott CN, et al. Isolation rearing-induced deficits in contextual fear learning do not require CRF(2) receptors. Behav. Brain Res. 2010;209(1):80–84. doi: 10.1016/j.bbr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bartolomucci A, Palanza P, Sacerdote P, et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 2003;28(4):540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 149.Reiss D, Wolter-Sutter A, Krezel W, Ouagazzal AM. Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav. Brain Res. 2007;184(2):167–173. doi: 10.1016/j.bbr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 150.Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral Z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J. Neurosci. Methods. 2011;197(1):21–31. doi: 10.1016/j.jneumeth.2011.01.019. [■■ Very recent article demonstrating a possibilty to overcome the problems of intrinsic variability of single anxiety tests by integrating measures along the same behavioral dimensions in different tests.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: Focus on individual differences. Pharmacol. Biochem. Behav. 2011;98(1):161–168. doi: 10.1016/j.pbb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 152.Schweizer MC, Henniger MS, Sillaber I. Chronic mild stress (CMS) in mice: of anhedonia, ‘anomalous anxiolysis’ and activity. PLoS One. 2009;4(1):E4326. doi: 10.1371/journal.pone.0004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175(1):43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 154.Surget A, Saxe M, Leman S, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry. 2008;64(4):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 155.Garcia-Garcia AL, Elizalde N, Matrov D, et al. Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol. Psychiatry. 2009;66(3):275–282. doi: 10.1016/j.biopsych.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 156.Rossler AS, Joubert C, Chapouthier G. Chronic mild stress alleviates anxious behaviour in female mice in two situations. Behav. Processes. 2000;49(3):163–165. doi: 10.1016/s0376-6357(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 157.Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80(15):1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 158.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 159.Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene–environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl.) 2011;214(1):175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin EJ, Choi E, Liu X, Martin A, During MJ. Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice. Behav. Brain Res. 2011;216(1):349–357. doi: 10.1016/j.bbr.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abramov U, Puussaar T, Raud S, Kurrikoff K, Vasar E. Behavioural differences between C57BL/6 and 129S6/SvEv strains are reinforced by environmental enrichment. Neurosci Lett. 2008;443(3):223–227. doi: 10.1016/j.neulet.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 162.Sztainberg Y, Chen A. An environmental enrichment model for mice. Nat. Protoc. 2010;5(9):1535–1539. doi: 10.1038/nprot.2010.114. [DOI] [PubMed] [Google Scholar]
- 163.Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J. Psychopharmacol. 2005;19(1):59–65. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- 164.Kroll JL. New directions in the conceptualization of psychotic disorders. Curr. Opin. Psychiatry. 2007;20(6):573–577. doi: 10.1097/YCO.0b013e3282f08759. [DOI] [PubMed] [Google Scholar]
- 165.Thys-Jacobs S. Micronutrients and the premenstrual syndrome: the case for calcium. J. Am. Coll. Nutr. 2000;19(2):220–227. doi: 10.1080/07315724.2000.10718920. [DOI] [PubMed] [Google Scholar]
- 166.Zender R, Olshansky E. Women’s mental health: depression and anxiety. Nurs. Clin. North Am. 2009;44(3):355–364. doi: 10.1016/j.cnur.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 167.Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin. Rheumatol. 2007;26(4):551–554. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 168.Murck H. Magnesium and affective disorders. Nutr. Neurosci. 2002;5(6):375–389. doi: 10.1080/1028415021000039194. [DOI] [PubMed] [Google Scholar]
- 169.Kantak KM. Magnesium deficiency alters aggressive behavior and catecholamine function. Behav. Neurosci. 1988;102(2):304–311. doi: 10.1037//0735-7044.102.2.304. [DOI] [PubMed] [Google Scholar]
- 170.Whittle N, Li L, Chen WQ, et al. Changes in brain protein expression are linked to magnesium restriction-induced depression-like behavior. Amino Acids. 2011 doi: 10.1007/s00726-010-0758-1. [DOI] [PubMed] [Google Scholar]
- 171.Singewald N, Sinner C, Hetzenauer A, Sartori SB, Murck H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice – influence of desipramine and Hypericum perforatum extract. Neuropharmacology. 2004;47(8):1189–1197. doi: 10.1016/j.neuropharm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 172.Sartori SB, Whittle N, Hetzenauer A, Singewald N. Mg deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.027. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr. Opin. Pharmacol. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 174.Whittle N, Lubec G, Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36(1):147–158. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- 175.Lalonde R, Barraud H, Ravey J, Gueant JL, Bronowicki JP, Strazielle C. Effects of a B-vitamin-deficient diet on exploratory activity, motor coordination, and spatial learning in young adult Balb/c mice. Brain Res. 2008;1188:122–131. doi: 10.1016/j.brainres.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 176.Ferguson SA, Berry KJ, Hansen DK, Wall KS, White G, Antony AC. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Res. A Clin. Mol. Teratol. 2005;73(4):249–252. doi: 10.1002/bdra.20111. [DOI] [PubMed] [Google Scholar]
- 177.Ballenger JC. Neurobiology of Panic Disorder. Wiley-Liss; NY, USA: 1990. [Google Scholar]
- 178.Balon R, Pohl R, Yeregani VK. The Provocation of Anxiety States in Humans and its Possible Significance for the Pathogenesis of These Disorders. In: Denboer JA, Sitsen JMA, editors. Handbook of Depression and Anxiety. Marcel Dekker; NY, USA: 1994. pp. 247–274. [Google Scholar]
- 179.Nutt DJ, Peters TJ. Alcohol: the drug. Br. Med. Bull. 1994;50(1):5–17. doi: 10.1093/oxfordjournals.bmb.a072883. [DOI] [PubMed] [Google Scholar]
- 180.Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53(4):275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 181.Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98(4):759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 182.Hackler EA, Turner GH, Gresch PJ, et al. 5-Hydroxytryptamine2C receptor contribution to m-chlorophenylpiperazine and N-methyl-β-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J. Pharmacol. Exp. Ther. 2007;320(3):1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- 183.Sahun I, Gallego X, Gratacos M, et al. Differential responses to anxiogenic drugs in a mouse model of panic disorder as revealed by Fos immunocytochemistry in specific areas of the fear circuitry. Amino Acids. 2007;33(4):677–688. doi: 10.1007/s00726-006-0464-1. [DOI] [PubMed] [Google Scholar]
- 184.Ziemann AE, Allen JE, Dahdaleh NS, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139(5):1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Angelis L. The anxiogenic-like effects of pentylenetetrazole in mice treated chronically with carbamazepine or valproate. Methods Find. Exp. Clin. Pharmacol. 1992;14(10):767–771. [PubMed] [Google Scholar]
- 186.De Angelis L, Furlan C. The anxiolytic-like properties of two selective MAOIs, moclobemide and selegiline, in a standard and an enhanced light/dark aversion test. Pharmacol. Biochem. Behav. 2000;65(4):649–653. doi: 10.1016/s0091-3057(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 187.Gavioli EC, Duarte FS, Guerrini R, Calo G, Rae GA, M De Lima TC. GABA(A) signalling is involved in N/OFQ anxiolytic-like effects but not in nocistatin anxiogenic-like action as evaluated in the mouse elevated plus maze. Peptides. 2008;29(8):1404–1412. doi: 10.1016/j.peptides.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 188.Kayir H, Uzbay IT. Nicotine antagonizes caffeine- but not pentylenetetrazole-induced anxiogenic effect in mice. Psychopharmacology (Berl.) 2006;184(3–4):464–469. doi: 10.1007/s00213-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 189.Rodgers RJ, Cole JC, Aboualfa K, Stephenson LH. Ethopharmacological analysis of the effects of putative ‘anxiogenic’ agents in the mouse elevated plus-maze. Pharmacol. Biochem. Behav. 1995;52(4):805–813. doi: 10.1016/0091-3057(95)00190-8. [DOI] [PubMed] [Google Scholar]
- 190.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994;61(1):59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 191.Lapin IP, Politi V. Antiethanol effects of indol-3-ylpyruvic acid in mice. Alcohol Alcohol. 1994;29(3):265–268. [PubMed] [Google Scholar]
- 192.Bert B, Felicio LF, Fink H, Nasello AG. The use of sudden darkness in mice: a behavioural and pharmacological approach. Psychopharmacology (Berl.) 2005;179(4):846–853. doi: 10.1007/s00213-004-2107-0. [DOI] [PubMed] [Google Scholar]
- 193.Cui XY, Zhao X, Chu QP, Chen BQ, Zhang YH. Influence of diltiazem on the behavior of zolpidem-treated mice in the elevated-plus maze test. J. Neural Transm. 2007;114(2):155–160. doi: 10.1007/s00702-006-0535-1. [DOI] [PubMed] [Google Scholar]
- 194.Griebel G, Misslin R, Pawlowski M, Vogel E. m-Chlorophenylpiperazine enhances neophobic and anxious behaviour in mice. Neuroreport. 1991;2(10):627–629. doi: 10.1097/00001756-199110000-00019. [DOI] [PubMed] [Google Scholar]
- 195.Navarro JF, Davila G, Pedraza C, Arias JL. Anxiogenic-like effects of γ-hydroxybutyric acid (GHB) in mice tested in the light–dark box. Psicothema. 2008;20(3):460–464. [PubMed] [Google Scholar]
- 196.Bourin M, Nic Dhonnchadha BA, Claude Colombel M, Dib M, Hascoet M. Cyamemazine as an anxiolytic drug on the elevated plus maze and light/dark paradigm in mice. Behav. Brain Res. 2001;124(1):87–95. doi: 10.1016/s0166-4328(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 197.Nic Dhonnchadha Ba, Bourin M, Hascoet M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav. Brain Res. 2003;140(1–2):203–214. doi: 10.1016/s0166-4328(02)00311-x. [DOI] [PubMed] [Google Scholar]
- 198.Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 2008;583(1):115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 199.Lee RS, Tamashiro KL, Yang X, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151(9):4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.David DJ, Samuels BA, Rainer Q, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Crupi R, Mazzon E, Marino A, et al. Hypericum perforatum treatment: effect on behaviour and neurogenesis in a chronic stress model in mice. BMC Complement. Altern. Med. 2011;11(1):7. doi: 10.1186/1472-6882-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Crupi R, Mazzon E, Marino A, et al. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. J. Pineal Res. 2010;49(2):123–129. doi: 10.1111/j.1600-079X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 203.Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav. Neurosci. 2006;120(2):249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- 204.Kitanaka N, Kitanaka J, Tatsuta T, et al. Withdrawal from fixed-dose injection of methamphetamine decreases cerebral levels of 3-methoxy-4-hydroxyphenylglycol and induces the expression of anxiety-related behavior in mice. Neurochem. Res. 2010;35(5):749–760. doi: 10.1007/s11064-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 205.Van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of δ opioid receptor-selective ligands for ethanol drinking and anxiety. J. Pharmacol. Exp. Ther. 2010;335(1):133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl.) 2010;210(2):285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mattioli L, Perfumi M. Evaluation of Rhodiola rosea L. extract on affective and physical signs of nicotine withdrawal in mice. J. Psychopharmacol. 2011;25(3):402–410. doi: 10.1177/0269881109348166. [DOI] [PubMed] [Google Scholar]
- 208.Verleye M, Heulard I, Gillardin JM. The anxiolytic etifoxine protects against convulsant and anxiogenic aspects of the alcohol withdrawal syndrome in mice. Alcohol. 2009;43(3):197–206. doi: 10.1016/j.alcohol.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 209.Eva C, Mele P, Collura D, et al. Modulation of neuropeptide Y and Y1 receptor expression in the amygdala by fluctuations in the brain content of neuroactive steroids during ethanol drinking discontinuation in Y1R/LacZ transgenic mice. J. Neurochem. 2008;104(4):1043–1054. doi: 10.1111/j.1471-4159.2007.05077.x. [DOI] [PubMed] [Google Scholar]
- 210.Balling R. ENU mutagenesis: analyzing gene function in mice. Annu. Rev. Genomics Hum. Genet. 2001;2:463–492. doi: 10.1146/annurev.genom.2.1.463. [DOI] [PubMed] [Google Scholar]
- 211.Keays DA, Nolan PM. N-ethyl-N-nitrosourea mouse mutants in the dissection of behavioural and psychiatric disorders. Eur. J. Pharmacol. 2003;480(1–3):205–217. doi: 10.1016/j.ejphar.2003.08.107. [DOI] [PubMed] [Google Scholar]
- 212.Cook MN, Dunning JP, Wiley RG, et al. Neurobehavioral mutants identified in an ENU-mutagenesis project. Mamm. Genome. 2007;18(8):559–572. doi: 10.1007/s00335-007-9035-3. [DOI] [PubMed] [Google Scholar]
- 213.Reijmers LG, Coats JK, Pletcher MT, Wiltshire T, Tarantino LM, Mayford M. A mutant mouse with a highly specific contextual fear-conditioning deficit found in an N-ethyl-N-nitrosourea (ENU) mutagenesis screen. Learn. Mem. 2006;13(2):143–149. doi: 10.1101/lm.98606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sayah DM, Khan AH, Gasperoni TL, Smith DJ. A genetic screen for novel behavioral mutations in mice. Mol. Psychiatry. 2000;5(4):369–377. doi: 10.1038/sj.mp.4000742. [DOI] [PubMed] [Google Scholar]
- 215.Wada Y, Furuse T, Yamada I, et al. ENU mutagenesis screening for dominant behavioral mutations based on normal control data obtained in home-cage activity, open-field, and passive avoidance tests. Exp. Anim. 2010;59(4):495–510. doi: 10.1538/expanim.59.495. [DOI] [PubMed] [Google Scholar]
- 216.Furuse T, Wada Y, Hattori K, et al. Phenotypic characterization of a new Grin1 mutant mouse generated by ENU mutagenesis. Eur. J. Neurosci. 2010;31(7):1281–1291. doi: 10.1111/j.1460-9568.2010.07164.x. [DOI] [PubMed] [Google Scholar]
- 217.Ohl F. Animal models of anxiety. Handb. Exp. Pharmacol. 2005;(169):35–69. doi: 10.1007/3-540-28082-0_2. [DOI] [PubMed] [Google Scholar]
- 218.Willner P. The validity of animal models of depression. Psychopharmacology (Berl.) 1984;83(1):1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 219.Clement Y, Chapouthier G. Biological bases of anxiety. Neurosci. Biobehav. Rev. 1998;22(5):623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 220.Flint J, Corley R, Defries JC, et al. A simple genetic basis for a complex psychological trait in laboratory mice. Science. 1995;269(5229):1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- 221.Gershenfeld HK, Neumann PE, Mathis C, Crawley JN, Li X, Paul SM. Mapping quantitative trait loci for open-field behavior in mice. Behav. Genet. 1997;27(3):201–210. doi: 10.1023/a:1025653812535. [DOI] [PubMed] [Google Scholar]
- 222.Talbot CJ, Nicod A, Cherny SS, Fulker DW, Collins AC, Flint J. High-resolution mapping of quantitative trait loci in outbred mice. Nat. Genet. 1999;21(3):305–308. doi: 10.1038/6825. [DOI] [PubMed] [Google Scholar]
- 223.Gale GD, Yazdi RD, Khan AH, Lusis AJ, Davis RC, Smith DJ. A genome-wide panel of congenic mice reveals widespread epistasis of behavior quantitative trait loci. Mol. Psychiatry. 2009;14(6):631–645. doi: 10.1038/mp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kessler MS, Murgatroyd C, Bunck M, et al. Diabetes insipidus and, partially, low anxiety-related behaviour are linked to a SNP-associated vasopressin deficit in LAB mice. Eur. J. Neurosci. 2007;26(10):2857–2864. doi: 10.1111/j.1460-9568.2007.05917.x. [DOI] [PubMed] [Google Scholar]
- 225.Henderson ND, Turri MG, Defries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav. Genet. 2004;34(3):267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- 226.Yalcin B, Willis-Owen Sa, Fullerton J, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat. Genet. 2004;36(11):1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- 227.Eisener-Dorman AF, Grabowski-Boase L, Steffy BM, Wiltshire T, Tarantino LM. Quantitative trait locus and haplotype mapping in closely related inbred strains identifies a locus for open field behavior. Mamm. Genome. 2010;21(5–6):231–246. doi: 10.1007/s00335-010-9260-z. [DOI] [PubMed] [Google Scholar]
- 228.Turri MG, Defries JC, Henderson ND, Flint J. Multivariate analysis of quantitative trait loci influencing variation in anxiety-related behavior in laboratory mice. Mamm. Genome. 2004;15(2):69–76. doi: 10.1007/s00335-003-3032-y. [DOI] [PubMed] [Google Scholar]
- 229.Turri MG, Henderson ND, Defries JC, Flint J. Quantitative trait locus mapping in laboratory mice derived from a replicated selection experiment for open-field activity. Genetics. 2001;158(3):1217–1226. doi: 10.1093/genetics/158.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Laarakker MC, Ohl F, Van Lith HA. Chromosomal assignment of quantitative trait loci influencing modified hole board behavior in laboratory mice using consomic strains, with special reference to anxiety-related behavior and mouse chromosome 19. Behav. Genet. 2008;38(2):159–184. doi: 10.1007/s10519-007-9188-6. [DOI] [PubMed] [Google Scholar]
- 231.Finn DA, Rutledge-Gorman MT, Crabbe JC. Genetic animal models of anxiety. Neurogenetics. 2003;4(3):109–135. doi: 10.1007/s10048-003-0143-2. [DOI] [PubMed] [Google Scholar]
- 232.Smoller JW, Acierno JS, Jr, Rosenbaum JF, et al. Targeted genome screen of panic disorder and anxiety disorder proneness using homology to murine QTL regions. Am. J. Med. Genet. 2001;105(2):195–206. doi: 10.1002/ajmg.1209. [DOI] [PubMed] [Google Scholar]
- 233.Fullerton J. New approaches to the genetic analysis of neuroticism and anxiety. Behav. Genet. 2006;36(1):147–161. doi: 10.1007/s10519-005-9000-4. [DOI] [PubMed] [Google Scholar]
- 234.Keller L. Adaptation and the genetics of social behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364(1533):3209–3216. doi: 10.1098/rstb.2009.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mackay TF, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 2009;10(8):565–577. doi: 10.1038/nrg2612. [DOI] [PubMed] [Google Scholar]
- 236.Flint J, Mackay TF. Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 2009;19(5):723–733. doi: 10.1101/gr.086660.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Erhardt A, Czibere L, Roeske D, et al. TMEM132D, a new candidate for anxiety phenotypes: evidence from human and mouse studies. Mol. Psychiatry. 2011;16(6):647–663. doi: 10.1038/mp.2010.41. [■ Recent paper combining research in mice and humans, which nicely demonstrates the potential of translational anxiety research.] [DOI] [PubMed] [Google Scholar]
- 238.Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 239.Leygraf A, Hohoff C, Freitag C, et al. Rgs 2 gene polymorphisms as modulators of anxiety in humans? J. Neural Transm. 2006;113(12):1921–1925. doi: 10.1007/s00702-006-0484-8. [DOI] [PubMed] [Google Scholar]
- 240.Mouri K, Hishimoto A, Fukutake M, Nishiguchi N, Shirakawa O, Maeda K. Association study of RGS2 gene polymorphisms with panic disorder in Japanese. Kobe J. Med. Sci. 2010;55(5):E116–E121. [PubMed] [Google Scholar]
- 241.Amstadter AB, Koenen KC, Ruggiero KJ, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J. Anxiety Disord. 2009;23(3):369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Koenen KC, Amstadter AB, Ruggiero KJ, et al. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress. Anxiety. 2009;26(4):309–315. doi: 10.1002/da.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Smoller JW, Paulus MP, Fagerness JA, et al. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Arch. Gen. Psychiatry. 2008;65(3):298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- 244.Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc. Natl Acad. Sci. USA. 2000;97(22):12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hambsch B, Chen BG, Brenndorfer J, et al. Methylglyoxal-mediated anxiolysis involves increased protein modification and elevated expression of glyoxalase 1 in the brain. J. Neurochem. 2010;113(5):1240–1251. doi: 10.1111/j.1471-4159.2010.06693.x. [DOI] [PubMed] [Google Scholar]
- 246.Williams RT, Lim JE, Harr B, et al. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One. 2009;4(3):E4649. doi: 10.1371/journal.pone.0004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fujimoto M, Uchida S, Watanuki T, et al. Reduced expression of glyoxalase-1 mRNA in mood disorder patients. Neurosci. Lett. 2008;438(2):196–199. doi: 10.1016/j.neulet.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 248.Politi P, Minoretti P, Falcone C, Martinelli V, Emanuele E. Association analysis of the functional Ala111Glu polymorphism of the glyoxalase I gene in panic disorder. Neurosci Lett. 2006;396(2):163–166. doi: 10.1016/j.neulet.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 249.Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann. NY Acad. Sci. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hashimoto K. BDNF variant linked to anxiety-related behaviors. Bioessays. 2007;29(2):116–119. doi: 10.1002/bies.20534. [DOI] [PubMed] [Google Scholar]
- 251.Hariri AR. Genetic polymorphisms: a cornerstone of translational biobehavioral research. Sci. Transl. Med. 2010;2(18):18PS16. doi: 10.1126/scitranslmed.3000811. [DOI] [PubMed] [Google Scholar]
- 252.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatry. 2007;12(12):1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 253.Yu H, Wang Y, Pattwell S, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J. Neurosci. 2009;29(13):4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yen YC, Mauch CP, Bunck M, et al. Acquisition and extinction of conditioned fear in a mouse model of extremes in trait anxiety. submitted. [Google Scholar]
- 256.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 2010;13(11):1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lutcke H, Murayama M, Hahn T, et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Front. Neural Circuits. 2010;4:9. doi: 10.3389/fncir.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Harro J, Kanarik M, Matrov D, Panksepp J. Mapping patterns of depression-related brain regions with cytochrome oxidase histochemistry: relevance of animal affective systems to human disorders, with a focus on resilience to adverse events. Neurosci. Biobehav. Rev. 2011 doi: 10.1016/j.neubiorev.2011.02.016. DOI:10.1016/j.neubiorev.2011.02.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 259.O’Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF. Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav. Brain Res. 2010;213(2):148–154. doi: 10.1016/j.bbr.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 260.Salomons AR, Bronkers G, Kirchhoff S, Arndt SS, Ohl F. Behavioural habituation to novelty and brain area specific immediate early gene expression in female mice of two inbred strains. Behav. Brain Res. 2010;215(1):95–101. doi: 10.1016/j.bbr.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 261.Kishimoto T, Radulovic J, Radulovic M, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24(4):415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 262.Bissiere S, Mcallister KH, Olpe HR, Cryan JF. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav. Brain Res. 2006;175(1):195–199. doi: 10.1016/j.bbr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 263.Sotres-Bayon F, Cain CK, Ledoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry. 2006;60(4):329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 264.Salome N, Salchner P, Viltart O, et al. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol. Psychiatry. 2004;55(7):715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 265.Frank E, Salchner P, Aldag JM, et al. Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav. Neurosci. 2006;120(1):60–71. doi: 10.1037/0735-7044.120.1.60. [DOI] [PubMed] [Google Scholar]
- 266.Davis M. Neural circuitry of anxiety and stress disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology – 5th Generation of Progress. American College of Neuropsychopharmacology; Nashville, TN, USA: 2008. pp. 931–951. [Google Scholar]
- 267.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 1999;23(5):743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 268.Muigg P, Hoelzl U, Palfrader K, et al. Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol. Psychiatry. 2007;61(6):782–796. doi: 10.1016/j.biopsych.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 269.Keck ME, Sartori SB, Welt T, et al. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: effects of chronic paroxetine treatment. J. Neurochem. 2005;92(5):1170–1179. doi: 10.1111/j.1471-4159.2004.02953.x. [DOI] [PubMed] [Google Scholar]
- 270.Murphy SE. Using functional neuroimaging to investigate the mechanisms of action of selective serotonin reuptake inhibitors (SSRIs) Curr. Pharm. Des. 2010;16(18):1990–1997. doi: 10.2174/138161210791293051. [DOI] [PubMed] [Google Scholar]
- 271.Murphy SE, Mackay CE. Using MRI to measure drug action: caveats and new directions. J. Psychopharmacol. 2010 doi: 10.1177/0269881110372547. DOI: 10.1177/0269881110372547. Epub ahead of print. [■■ Recent review, which discusses the possibilites and caveats of pharmacological modulation of brain activity using MRI to bridge the gap between preclinical and clinical studies.] [DOI] [PubMed] [Google Scholar]
- 272.Paulus MP, Stein MB. An insular view of anxiety. Biol. Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 273.Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol. Sci. 2007;18(2):127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 274.Bryant RA, Felmingham K, Kemp A, et al. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol. Med. 2008;38(4):555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- 275.Goossens L, Sunaert S, Peeters R, Griez EJ, Schruers KR. Amygdala hyperfunction in phobic fear normalizes after exposure. Biol. Psychiatry. 2007;62(10):1119–1125. doi: 10.1016/j.biopsych.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 276.Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 277.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 279.Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 2003;6(5):445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- 280.Gleason G, Liu B, Bruening S, et al. The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc. Natl Acad. Sci. USA. 2010;107(16):7592–7597. doi: 10.1073/pnas.0914805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 282.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 283.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Franklin TB, Russig H, Weiss IC, et al. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry. 2010;68(5):408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 285.Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 286.Atack JR. GABAA receptor α2/α3 subtype-selective modulators as potential nonsedating anxiolytics. Curr. Top. Behav. Neurosci. 2010;2:331–360. doi: 10.1007/7854_2009_30. [DOI] [PubMed] [Google Scholar]
- 287.Griebel G, Rodgers RJ, Perrault G, Sanger DJ. The effects of compounds varying in selectivity as 5-HT(1A) receptor antagonists in three rat models of anxiety. Neuropharmacology. 2000;39(10):1848–1857. doi: 10.1016/s0028-3908(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 288.Duvoisin RM, Pfankuch T, Wilson JM, et al. Acute pharmacological modulation of mGluR8 reduces measures of anxiety. Behav. Brain Res. 2010;212(2):168–173. doi: 10.1016/j.bbr.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology. 2008;55(4):537–545. doi: 10.1016/j.neuropharm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rodriguez AL, Grier MD, Jones CK, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol. Pharmacol. 2010;78(6):1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mikulecka A, Mares P. Effects of mGluR5 and mGluR1 antagonists on anxiety-like behavior and learning in developing rats. Behav. Brain Res. 2009;204(1):133–139. doi: 10.1016/j.bbr.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 292.Griebel G, Rodgers RJ, Perrault G, Sanger DJ. Behavioural profiles in the mouse defence test battery suggest anxiolytic potential of 5-HT(1A) receptor antagonists. Psychopharmacology (Berl.) 1999;144(2):121–130. doi: 10.1007/s002130050984. [DOI] [PubMed] [Google Scholar]
- 293.Dekeyne A, Mannoury La Cour C, Gobert A, et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology (Berl.) 2008;199(4):549–568. doi: 10.1007/s00213-008-1177-9. [DOI] [PubMed] [Google Scholar]
- 294.Harada K, Aota M, Inoue T, et al. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur. J. Pharmacol. 2006;553(1–3):171–184. doi: 10.1016/j.ejphar.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 295.Spooren W, Gasparini F. mGlu5 receptor antagonists: a novel class of anxiolytics? Drug News Perspect. 2004;17(4):251–257. doi: 10.1358/dnp.2004.17.4.829052. [DOI] [PubMed] [Google Scholar]
- 296.Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann. NY Acad. Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- 297.Pape HC, Jungling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010;58(1):29–34. doi: 10.1016/j.neuropharm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Xu YL, Reinscheid RK, Huitron-Resendiz S, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43(4):487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 299.Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007;500(1):84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- 300.Leonard SK, Ring RH. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience. 2011;172:153–163. doi: 10.1016/j.neuroscience.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 301.Jungling K, Seidenbecher T, Sosulina L, et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59(2):298–310. doi: 10.1016/j.neuron.2008.07.002. [■■ Article that first described a functional role of neuropeptide S and underlying neurobiological mechanisms in fear-related processes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Leonard SK, Dwyer JM, Sukoff Rizzo SJ, et al. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl.) 2008;197(4):601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- 303.Vitale G, Filaferro M, Ruggieri V, et al. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides. 2008;29(12):2286–2291. doi: 10.1016/j.peptides.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 304.Hauschild M, Sartori SB, Gaburro S, et al. Altered fear response in a mouse model of trait anxiety; Soc. Neurosci; Chicago, USA. 17–21 October; 2009. Poster number: 191.7/EE70. [Google Scholar]
- 305.Duangdao DM, Clark SD, Okamura N, Reinscheid RK. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav. Brain Res. 2009;205(1):1–9. doi: 10.1016/j.bbr.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhu H, Mingler MK, Mcbride ML, et al. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology. 2010;35(8):1119–1132. doi: 10.1016/j.psyneuen.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Raczka K, Gartmann N, Mechias M, et al. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: a potential neurogenetic basis for catastrophizing. Mol. Psychiatry. 2010;15(11):1045, 1067–1074. doi: 10.1038/mp.2010.79. [DOI] [PubMed] [Google Scholar]
- 308.Dannlowski U, Kugel H, Franke F, et al. Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.73. DOI:10.1038/npp.2011.73. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Okamura N, Hashimoto K, Iyo M, et al. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(7):1444–1448. doi: 10.1016/j.pnpbp.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 310.Domschke K, Reif A, Weber H, et al. Neuropeptide S receptor gene – converging evidence for a role in panic disorder. Mol. Psychiatry. 2010 doi: 10.1038/mp.2010.81. DOI:10.1038/mp.2010.81. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 311.Donner J, Haapakoski R, Ezer S, et al. Assessment of the neuropeptide S system in anxiety disorders. Biol. Psychiatry. 2010;68(5):474–483. doi: 10.1016/j.biopsych.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 312.Cao J, De Lecea L, Ikemoto S. Intraventricular administration of neuropeptide S has reward-like effects. Eur. J. Pharmacol. 2011;658(1):16–21. doi: 10.1016/j.ejphar.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Smith KL, Patterson M, Dhillo WS, et al. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147(7):3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- 314.Zhu H, Perkins C, Mingler MK, Finkelman FD, Rothenberg ME. The role of neuropeptide S and neuropeptide S receptor 1 in regulation of respiratory function in mice. Peptides. 2011;32(4):818–825. doi: 10.1016/j.peptides.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hersh CP, Raby BA, Soto-Quiros ME, et al. Comprehensive testing of positionally cloned asthma genes in two populations. Am. J. Respir. Crit. Care Med. 2007;176(9):849–857. doi: 10.1164/rccm.200704-592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Laitinen T, Polvi A, Rydman P, et al. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304(5668):300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- 317.Melen E, Bruce S, Doekes G, et al. Haplotypes of G protein-coupled receptor 154 are associated with childhood allergy and asthma. Am. J. Respir. Crit. Care Med. 2005;171(10):1089–1095. doi: 10.1164/rccm.200410-1317OC. [DOI] [PubMed] [Google Scholar]
- 318.Kormann MS, Carr D, Klopp N, et al. G-Protein-coupled receptor polymorphisms are associated with asthma in a large German population. Am. J. Respir. Crit. Care Med. 2005;171(12):1358–1362. doi: 10.1164/rccm.200410-1312OC. [DOI] [PubMed] [Google Scholar]
- 319.D’amato M, Bruce S, Bresso F, et al. Neuropeptide s receptor 1 gene polymorphism is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2007;133(3):808–817. doi: 10.1053/j.gastro.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 320.Munoz M, Covenas R. NK-1 receptor antagonists: a new paradigm in pharmacological therapy. Curr. Med. Chem. 2011;18(12):1820–1831. doi: 10.2174/092986711795496746. [DOI] [PubMed] [Google Scholar]
- 321.Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Curr. Pharm. Des. 2009;15(14):1647–1674. doi: 10.2174/138161209788168074. [DOI] [PubMed] [Google Scholar]
- 322.Furmark T, Appel L, Michelgard A, et al. Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol. Psychiatry. 2005;58(2):132–142. doi: 10.1016/j.biopsych.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 323.Tauscher J, Kielbasa W, Iyengar S, et al. Development of the 2nd generation neurokinin-1 receptor antagonist LY686017 for social anxiety disorder. Eur. Neuropsychopharmacol. 2010;20(2):80–87. doi: 10.1016/j.euroneuro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 324.Mathew SJ, Vythilingam M, Murrough JW, et al. A selective neurokinin-1 receptor antagonist in chronic PTSD: a randomized, double-blind, placebo-controlled, proof-of-concept trial. Eur. Neuropsychopharmacol. 2011;21(3):221–229. doi: 10.1016/j.euroneuro.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Brocco M, Dekeyne A, Mannoury La Cour C, et al. Cellular and behavioural profile of the novel, selective neurokinin1 receptor antagonist, vestipitant: a comparison to other agents. Eur. Neuropsychopharmacol. 2008;18(10):729–750. doi: 10.1016/j.euroneuro.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 326.Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- 327.Sartori SB, Gaburro S, Landgraf R, Singewald N. Chronic treatment with a selective neurokinin-1 receptor antagonist in a mouse model of trait anxiety and depression: focus on behaviour and neuropeptidergic mechanisms. BMC Pharmacol. 2008;8(Suppl. 1):A19. [Google Scholar]
- 328.Singewald N, Gaburro S, Czibere L, Landgraf R, Ebner K, Sartori SB. The role of substance P in fear and anxiety. Summer Neuropeptide Conference; Salzburg, Austria. 20–23 July; 2009. [Google Scholar]
- 329.Tye KM, Prakash R, Kim SY, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gallego X, Murtra P, Zamalloa T, et al. Increased opioid dependence in a mouse model of panic disorder. Front. Behav. Neurosci. 2010;3:60. doi: 10.3389/neuro.08.060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sajdyk TJ, Keim SR, Thielen SR, Fitz SD, Shekhar A. Measurement of panic-like responses following intravenous infusion of sodium lactate in panic-prone rats. Curr. Protoc. Neurosci. 2003:1–19. doi: 10.1002/0471142301.ns0917s24. Chapter 9. [DOI] [PubMed] [Google Scholar]
- 332.Groenink L, Bijlsma EY, Van Bogaert MJ, Oosting RS, Olivier B. Serotonin(1A) receptor deletion does not interact with maternal separation-induced increases in startle reactivity and prepulse inhibition deficits. Psychopharmacology (Berl.) 2010;214(1):353–365. doi: 10.1007/s00213-010-1998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Neumann ID, Wegener G, Homberg JR, et al. Animal models of depression and anxiety: What do they tell us about human condition? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.11.028. DOI:10.1016/j.pnpbp.2010.11.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 334.Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol. Biochem. Behav. 1999;63(1):21–26. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- 335.Hagenbuch N, Feldon J, Yee BK. Use of the elevated plus-maze test with opaque or transparent walls in the detection of mouse strain differences and the anxiolytic effects of diazepam. Behav. Pharmacol. 2006;17(1):31–41. doi: 10.1097/01.fbp.0000189811.77049.3e. [DOI] [PubMed] [Google Scholar]
- 336.Ducottet C, Belzung C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav. Brain Res. 2005;156(1):153–162. doi: 10.1016/j.bbr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 337.Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol. Behav. 2002;77(2–3):301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- 338.Harms LR, Eyles DW, Mcgrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav. Brain Res. 2008;187(2):343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 339.Lad HV, Liu L, Paya-Cano JL, et al. Behavioural battery testing: evaluation and behavioural outcomes in 8 inbred mouse strains. Physiol. Behav. 2010;99(3):301–316. doi: 10.1016/j.physbeh.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 340.Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl.) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- 341.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav. Brain Res. 2007;176(1):21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7(4):496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 343.Camp M, Norcross M, Whittle N, et al. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes Brain Behav. 2009;8(8):744–752. doi: 10.1111/j.1601-183X.2009.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Liu X, Gershenfeld HK. An exploratory factor analysis of the tail suspension test in 12 inbred strains of mice and an F2 intercross. Brain Res. Bull. 2003;60(3):223–231. doi: 10.1016/s0361-9230(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 345.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp. Med. 2005;55(4):326–334. [PubMed] [Google Scholar]
- 346.Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1(1):55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 347.Solberg LC, Valdar W, Gauguier D, et al. A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm. Genome. 2006;17(2):129–146. doi: 10.1007/s00335-005-0112-1. [DOI] [PubMed] [Google Scholar]
- 348.Moy SS, Nadler JJ, Young NB, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Van Gaalen MM, Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav. Brain Res. 2000;115(1):95–106. doi: 10.1016/s0166-4328(00)00240-0. [DOI] [PubMed] [Google Scholar]
- 350.Rayburn WF, Christensen HD, Gold KM, Gonzalez CL. Neurobehavior effects in four strains of mice offspring exposed prenatally to alprazolam. Am. J. Obstet. Gynecol. 2002;187(4):968–972. doi: 10.1067/mob.2002.127133. [DOI] [PubMed] [Google Scholar]
- 351.Mozhui K, Karlsson RM, Kash TL, et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J. Neurosci. 2010;30(15):5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ponder CA, Munoz M, Gilliam TC, Palmer AA. Genetic architecture of fear conditioning in chromosome substitution strains: relationship to measures of innate (unlearned) anxiety-like behavior. Mamm. Genome. 2007;18(4):221–228. doi: 10.1007/s00335-007-9013-9. [DOI] [PubMed] [Google Scholar]
- 353.Van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur. J. Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- 354.Schimanski LA, Nguyen PV. Mouse models of impaired fear memory exhibit deficits in amygdalar LTP. Hippocampus. 2005;15(4):502–517. doi: 10.1002/hipo.20075. [DOI] [PubMed] [Google Scholar]
- 355.Gimsa U, Kanitz E, Otten W, Ibrahim SM. Behavior and stress reactivity in mouse strains with mitochondrial DNA variations. Ann. NY Acad. Sci. 2009;1153:131–138. doi: 10.1111/j.1749-6632.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 356.Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol. Biochem. Behav. 2000;67(4):739–748. doi: 10.1016/s0091-3057(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 357.Carola V, D’olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002;134(1–2):49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- 358.Kopp C, Vogel E, Misslin R. Comparative study of emotional behaviour in three inbredstrains of mice. Behav. Processes. 1999;47:161–174. doi: 10.1016/s0376-6357(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 359.Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J. Neurosci. Methods. 2008;171(1):48–52. doi: 10.1016/j.jneumeth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 360.Augustsson H, Meyerson BJ. Exploration and risk assessment: a comparative study of male house mice (Mus musculus musculus) and two laboratory strains. Physiol. Behav. 2004;81(4):685–698. doi: 10.1016/j.physbeh.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 361.Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav. Brain Res. 2002;136(2):555–569. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 362.Tang X, Xiao J, Liu X, Sanford LD. Strain differences in the influence of open field exposure on sleep in mice. Behav. Brain Res. 2004;154(1):137–147. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 363.Guillot PV, Chapouthier G. Intermale aggression and dark/light preference in ten inbred mouse strains. Behav. Brain Res. 1996;77(1–2):211–213. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 364.Ozawa M, Kikusui T, Takeuchi Y, Mori Y. Comparison of parental behavior and offspring’s anxiety behavior using a reciprocal F1 hybrid model. J. Vet. Med. Sci. 2010;72(12):1589–1596. doi: 10.1292/jvms.10-0173. [DOI] [PubMed] [Google Scholar]
- 365.Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci. Res. 2006;55(1):96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 366.Brinks V, Kloet ER, Oitzl MS. Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp. Neurol. 2009;216(2):375–382. doi: 10.1016/j.expneurol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 367.Rogers DC, Jones DN, Nelson PR, et al. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav. Brain Res. 1999;105(2):207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 368.Yilmazer-Hanke Dm, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav. Brain Res. 2003;145(1–2):145–159. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 369.Kliethermes CL, Finn DA, Crabbe JC. Validation of a modified mirrored chamber sensitive to anxiolytics and anxiogenics in mice. Psychopharmacology (Berl.) 2003;169(2):190–197. doi: 10.1007/s00213-003-1493-z. [DOI] [PubMed] [Google Scholar]
- 370.Popova NK, Naumenko VS, Tibeikina MA, Kulikov AV. Serotonin transporter, 5-HT1A receptor, and behavior in DBA/2J mice in comparison with four inbred mouse strains. J. Neurosci. Res. 2009;87(16):3649–3657. doi: 10.1002/jnr.22155. [DOI] [PubMed] [Google Scholar]
- 371.Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, Kudryavtseva NN. Features of the genetically defined anxiety in mice. Behav. Genet. 2000;30(2):101–109. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- 372.Livneh U, Dori A, Katzav A, Kofman O. Strain and regional dependence of alternate splicing of acetylcholinesterase in the murine brain following stress or treatment with diisopropylfluorophosphate. Behav. Brain Res. 2010;210(1):107–115. doi: 10.1016/j.bbr.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 373.Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm. Genome. 2000;11(7):555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- 374.Waddell J, Dunnett C, Falls WA. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behav. Brain Res. 2004;154(2):567–576. doi: 10.1016/j.bbr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 375.Stiedl O, Radulovic J, Lohmann R, et al. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav. Brain Res. 1999;104(1–2):1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 376.Jonas I, Schubert KA, Reijne AC, et al. Behavioral traits are affected by selective breeding for increased wheel-running behavior in mice. Behav. Genet. 2010;40(4):542–550. doi: 10.1007/s10519-010-9359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Suaudeau C, Rinaldi D, Lepicard E, et al. Divergent levels of anxiety in mice selected for differences in sensitivity to a convulsant agent. Physiol. Behav. 2000;71(5):517–523. doi: 10.1016/s0031-9384(00)00383-8. [DOI] [PubMed] [Google Scholar]
- 378.Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin. Exp. Res. 2007;31(7):1081–1088. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 379.Powers MS, Barrenha GD, Mlinac NS, Barker EL, Chester JA. Effects of the novel endocannabinoid uptake inhibitor, LY2183240, on fear-potentiated startle and alcohol-seeking behaviors in mice selectively bred for high alcohol preference. Psychopharmacology (Berl.) 2010;212(4):571–583. doi: 10.1007/s00213-010-1997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Wirth-Dzieciolowska E, Lipska A, Wesierska M. Selection for body weight induces differences in exploratory behavior and learning in mice. Acta Neurobiol. Exp. (Wars.) 2005;65(3):243–253. doi: 10.55782/ane-2005-1559. [DOI] [PubMed] [Google Scholar]
- 381.Heisler LK, Chu HM, Brennan TJ, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc. Natl Acad. Sci. USA. 1998;95(25):15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ramboz S, Oosting R, Amara DA, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc. Natl Acad. Sci. USA. 1998;95(24):14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc. Natl Acad. Sci. USA. 1998;95(18):10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kimura A, Stevenson PL, Carter RN, et al. Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur. J. Neurosci. 2009;30(2):299–306. doi: 10.1111/j.1460-9568.2009.06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Lahdesmaki J, Sallinen J, Macdonald E, Kobilka BK, Fagerholm V, Scheinin M. Behavioral and neurochemical characterization of α(2A)-adrenergic receptor knockout mice. Neuroscience. 2002;113(2):289–299. doi: 10.1016/s0306-4522(02)00185-9. [DOI] [PubMed] [Google Scholar]
- 386.Schramm NL, Mcdonald MP, Limbird LE. The α(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J. Neurosci. 2001;21(13):4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Davies MF, Tsui JY, Flannery JA, Li X, Delorey TM, Hoffman BB. Augmentation of the noradrenergic system in α-2 adrenergic receptor deficient mice: anatomical changes associated with enhanced fear memory. Brain Res. 2003;986(1–2):157–165. doi: 10.1016/s0006-8993(03)03248-7. [DOI] [PubMed] [Google Scholar]
- 388.Ross SA, Wong JY, Clifford JJ, et al. Phenotypic characterization of an α 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J. Neurosci. 2000;20(17):6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl Acad. Sci. USA. 1998;95(17):9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.O’tuathaigh CM, Hryniewiecka M, Behan A, et al. Chronic adolescent exposure to Δ-9-tetrahydrocannabinol in COMT mutant mice: impact on psychosis-related and other phenotypes. Neuropsychopharmacology. 2010;35(11):2262–2273. doi: 10.1038/npp.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Tammimaki A, Kaenmaki M, Kambur O, et al. Effect of S-COMT deficiency on behavior and extracellular brain dopamine concentrations in mice. Psychopharmacology (Berl.) 2010;211(4):389–401. doi: 10.1007/s00213-010-1944-2. [DOI] [PubMed] [Google Scholar]
- 392.Mcgrath MJ, Campbell KM, Veldman MB, Burton FH. Anxiety in a transgenic mouse model of cortical-limbic neuro-potentiated compulsive behavior. Behav. Pharmacol. 1999;10(5):435–443. doi: 10.1097/00008877-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 393.Mcgrath MJ, Campbell KM, Burton FH. The role of cognitive and affective processing in a transgenic mouse model of cortical-limbic neuropotentiated compulsive behavior. Behav. Neurosci. 1999;113(6):1249–1256. doi: 10.1037//0735-7044.113.6.1249. [DOI] [PubMed] [Google Scholar]
- 394.Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999;19(21):9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Falzone TL, Gelman DM, Young JI, Grandy DK, Low MJ, Rubinstein M. Absence of dopamine D4 receptors results in enhanced reactivity to unconditioned, but not conditioned, fear. Eur. J. Neurosci. 2002;15(1):158–164. doi: 10.1046/j.0953-816x.2001.01842.x. [DOI] [PubMed] [Google Scholar]
- 396.Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol. Psychiatry. 2004;9(7):718–726. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- 397.Alexandre C, Popa D, Fabre V, et al. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J. Neurosci. 2006;26(20):5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6(4):389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 399.Kalueff AV, Jensen CL, Murphy DL. Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res. 2007;1169:87–97. doi: 10.1016/j.brainres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 400.Lira A, Zhou M, Castanon N, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry. 2003;54(10):960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 401.De Souza Silva MA, Marchetti L, Eisel UL, Huston JP, Dere E. NR2C by NR2B subunit exchange in juvenile mice affects emotionality and 5-HT in the frontal cortex. Genes Brain Behav. 2007;6(5):465–472. doi: 10.1111/j.1601-183X.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 402.Mead AN, Morris HV, Dixon CI, et al. AMPA receptor GluR2, but not GluR1, subunit deletion impairs emotional response conditioning in mice. Behav. Neurosci. 2006;120(2):241–248. doi: 10.1037/0735-7044.120.2.241. [DOI] [PubMed] [Google Scholar]
- 403.Wiedholz LM, Owens WA, Horton RE, et al. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol. Psychiatry. 2008;13(6):631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- 404.Bannerman DM, Deacon RM, Brady S, et al. A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behav. Neurosci. 2004;118(3):643–647. doi: 10.1037/0735-7044.118.3.643. [DOI] [PubMed] [Google Scholar]
- 405.Feyder M, Wiedholz L, Sprengel R, Holmes A. Impaired associative fear learning in mice with complete loss or haploinsufficiency of AMPA GluR1 receptors. Front. Behav. Neurosci. 2007;1:4. doi: 10.3389/neuro.08.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Humeau Y, Reisel D, Johnson AW, et al. A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J. Neurosci. 2007;27(41):10947–10956. doi: 10.1523/JNEUROSCI.2603-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wu LJ, Ko SW, Toyoda H, et al. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS One. 2007;2(1):E167. doi: 10.1371/journal.pone.0000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J. Neurosci. 2005;25(4):977–984. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 2009;29(12):3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Linden AM, Johnson BG, Peters SC, et al. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology. 2002;43(2):251–259. doi: 10.1016/s0028-3908(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 411.Duvoisin RM, Zhang C, Pfankuch TF, et al. Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur. J. Neurosci. 2005;22(2):425–436. doi: 10.1111/j.1460-9568.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- 412.Robbins MJ, Starr KR, Honey A, et al. Evaluation of the mGlu8 receptor as a putative therapeutic target in schizophrenia. Brain Res. 2007;1152:215–227. doi: 10.1016/j.brainres.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 413.Linden AM, Baez M, Bergeron M, Schoepp DD. Increased c-Fos expression in the centromedial nucleus of the thalamus in metabotropic glutamate 8 receptor knockout mice following the elevated plus maze test. Neuroscience. 2003;121(1):167–178. doi: 10.1016/s0306-4522(03)00393-2. [DOI] [PubMed] [Google Scholar]
- 414.Duvoisin RM, Villasana L, Pfankuch T, Raber J. Sex-dependent cognitive phenotype of mice lacking mGluR8. Behav. Brain Res. 2010;209(1):21–26. doi: 10.1016/j.bbr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Fendt M, Burki H, Imobersteg S, et al. The effect of mGlu8 deficiency in animal models of psychiatric diseases. Genes Brain Behav. 2010;9(1):33–44. doi: 10.1111/j.1601-183X.2009.00532.x. [DOI] [PubMed] [Google Scholar]
- 416.Gerlai R, Adams B, Fitch T, Chaney S, Baez M. Performance deficits of mGluR8 knockout mice in learning tasks: the effects of null mutation and the background genotype. Neuropharmacology. 2002;43(2):235–249. doi: 10.1016/s0028-3908(02)00078-3. [DOI] [PubMed] [Google Scholar]
- 417.Tordera RM, Totterdell S, Wojcik SM, et al. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur. J. Neurosci. 2007;25(1):281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- 418.Crestani F, Lorez M, Baer K, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999;2(9):833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 419.Chandra D, Korpi ER, Miralles CP, De Blas AL, Homanics GE. GABAA receptor γ 2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005;6:30. doi: 10.1186/1471-2202-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Homanics GE, Harrison NL, Quinlan JJ, et al. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the γ2 subunit of the γ-aminobutyrate type A receptor. Neuropharmacology. 1999;38(2):253–265. doi: 10.1016/s0028-3908(98)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Mombereau C, Kaupmann K, Gassmann M, Bettler B, Van Der Putten H, Cryan JF. Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport. 2005;16(3):307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- 422.Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA. 1999;96(4):1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Stork O, Ji FY, Kaneko K, et al. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865(1):45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- 424.Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC. Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J. Neurosci. 2009;29(50):15713–15720. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Stork O, Yamanaka H, Stork S, Kume N, Obata K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes Brain Behav. 2003;2(2):65–70. doi: 10.1034/j.1601-183x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 426.Li Wj, Yu H, Yang Jm, et al. Anxiolytic effect of music exposure on BDNFMet/Met transgenic mice. Brain Res. 2010;1347:71–79. doi: 10.1016/j.brainres.2010.05.080. [DOI] [PubMed] [Google Scholar]
- 427.Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002;16(7):1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 429.Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 2004;19(7):1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 430.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl.) 2002;159(4):379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 431.Uriguen L, Perez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46(7):966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 432.Hill MN, Hillard CJ, Mcewen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb. Cortex. 2011 doi: 10.1093/cercor/bhq280. DOI: 10.1093/cercor/bhq280. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav. Pharmacol. 2004;15(4):299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 434.Thiemann G, Watt CA, Ledent C, Molleman A, Hasenohrl RU. Modulation of anxiety by acute blockade and genetic deletion of the CB(1) cannabinoid receptor in mice together with biogenic amine changes in the forebrain. Behav. Brain Res. 2009;200(1):60–67. doi: 10.1016/j.bbr.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 435.Kamprath K, Plendl W, Marsicano G, et al. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 2009;8(2):203–211. doi: 10.1111/j.1601-183X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 436.Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J. Neurosci. 2010;30(14):4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 438.Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn. Mem. 2004;11(5):625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Mikics E, Dombi T, Barsvari B, et al. The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behav. Pharmacol. 2006;17(3):223–230. doi: 10.1097/00008877-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 440.Lo CM, Samuelson LC, Chambers JB, et al. Characterization of mice lacking the gene for cholecystokinin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(3):R803–810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- 441.Vasar E, Koks S, Beljajev S, Abramov U, Koovit I, Matsui T. CCKB receptor knockout mice: gender related behavioural differences. Eur. Neuropsychopharmacol. 2000;10(Suppl. 2):S69. [Google Scholar]
- 442.Miyasaka K, Kobayashi S, Ohta M, et al. Anxiety-related behaviors in cholecystokinin-A, B, and AB receptor gene knockout mice in the plus-maze. Neurosci Lett. 2002;335(2):115–118. doi: 10.1016/s0304-3940(02)01176-x. [DOI] [PubMed] [Google Scholar]
- 443.Heinrichs SC, Min H, Tamraz S, Carmouche M, Boehme SA, Vale WW. Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology. 1997;22(4):215–224. doi: 10.1016/s0306-4530(97)00030-9. [DOI] [PubMed] [Google Scholar]
- 444.Heinrichs SC, Stenzel-Poore MP, Gold LH, et al. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74(2):303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 445.Stenzel-Poore MP, Duncan JE, Rittenberg MB, Bakke AC, Heinrichs SC. CRH overproduction in transgenic mice: behavioral and immune system modulation. Ann. NY Acad. Sci. 1996;780:36–48. doi: 10.1111/j.1749-6632.1996.tb15110.x. [DOI] [PubMed] [Google Scholar]
- 446.Kasahara M, Groenink L, Breuer M, Olivier B, Sarnyai Z. Altered behavioural adaptation in mice with neural corticotrophin-releasing factor overexpression. Genes Brain Behav. 2007;6(7):598–607. doi: 10.1111/j.1601-183X.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 447.Karolyi IJ, Burrows HL, Ramesh TM, et al. Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc. Natl Acad. Sci. USA. 1999;96(20):11595–11600. doi: 10.1073/pnas.96.20.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Gammie SC, Seasholtz AF, Stevenson SA. Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience. 2008;157(3):502–512. doi: 10.1016/j.neuroscience.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24(4):410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 450.Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24(4):403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 451.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl Acad. Sci. USA. 1997;94(4):1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Tomihara K, Kaitsuka T, Soga T, et al. Abolition of sex-dependent effects of prenatal exposure to diethylstilbestrol on emotional behavior in estrogen receptor-α knockout mice. Neuroreport. 2006;17(11):1169–1173. doi: 10.1097/01.wnr.0000224771.82151.77. [DOI] [PubMed] [Google Scholar]
- 453.Wei Q, Lu Xy, Liu L, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc. Natl Acad. Sci. USA. 2004;101(32):11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Howell MP, Muglia LJ. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front. Neuroendocrinol. 2006;27(3):275–284. doi: 10.1016/j.yfrne.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 455.Ridder S, Chourbaji S, Hellweg R, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 2005;25(26):6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Walther T, Balschun D, Voigt JP, et al. Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J. Biol. Chem. 1998;273(19):11867–11873. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- 457.Bannon AW, Seda J, Carmouche M, et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868(1):79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- 458.Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog. Horm. Res. 1998;53:163–199. [PubMed] [Google Scholar]
- 459.Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur. J. Neurosci. 2008;28(1):173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- 460.Koster A, Montkowski A, Schulz S, et al. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc. Natl Acad. Sci. USA. 1999;96(18):10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Sakoori K, Murphy NP. Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology. 2009;56(5):896–904. doi: 10.1016/j.neuropharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 462.Higgins GA, Kew JN, Richards JG, et al. A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. Eur. J. Neurosci. 2002;15(5):911–922. doi: 10.1046/j.1460-9568.2002.01926.x. [DOI] [PubMed] [Google Scholar]
- 463.Konig M, Zimmer AM, Steiner H, et al. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383(6600):535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 464.Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC. Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J. Biomed. Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Bilkei-Gorzo A, Racz I, Michel K, Zimmer A, Klingmuller D. Behavioral phenotype of pre-proenkephalin-deficient mice on diverse congenic backgrounds. Psychopharmacology (Berl.) 2004;176(3–4):343–352. doi: 10.1007/s00213-004-1904-9. [DOI] [PubMed] [Google Scholar]
- 466.Ragnauth A, Schuller A, Morgan M, et al. Female preproenkephalin-knockout mice display altered emotional responses. Proc. Natl Acad. Sci. USA. 2001;98(4):1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Vetter De, Li C, Zhao L, et al. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat. Genet. 2002;31(4):363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- 468.Wang X, Su H, Copenhagen LD, et al. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol. Cell Biol. 2002;22(18):6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Kustova Y, Sei Y, Morse HC, Jr, Basile AS. The influence of a targeted deletion of the IFNγ gene on emotional behaviors. Brain Behav. Immun. 1998;12(4):308–324. doi: 10.1006/brbi.1998.0546. [DOI] [PubMed] [Google Scholar]
- 470.Litteljohn D, Cummings A, Brennan A, et al. Interferon-γ deficiency modifies the effects of a chronic stressor in mice: Implications for psychological pathology. Brain Behav. Immun. 2010;24(3):462–473. doi: 10.1016/j.bbi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 471.Litteljohn D, Mangano E, Shukla N, Hayley S. Interferon-γ deficiency modifies the motor and co-morbid behavioral pathology and neurochemical changes provoked by the pesticide paraquat. Neuroscience. 2009;164(4):1894–1906. doi: 10.1016/j.neuroscience.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 472.Fiore M, Alleva E, Probert L, Kollias G, Angelucci F, Aloe L. Exploratory and displacement behavior in transgenic mice expressing high levels of brain TNF-α. Physiol. Behav. 1998;63(4):571–576. doi: 10.1016/s0031-9384(97)00514-3. [DOI] [PubMed] [Google Scholar]
- 473.Espana J, Gimenez-Llort L, Valero J, et al. Intraneuronal β-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol. Psychiatry. 2010;67(6):513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 474.Hasegawa S, Furuichi T, Yoshida T, et al. Transgenic up-regulation of α-CaMKII in forebrain leads to increased anxiety-like behaviors and aggression. Mol Brain. 2009;2:6. doi: 10.1186/1756-6606-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Umehara F, Mishima K, Egashira N, Ogata A, Iwasaki K, Fujiwara M. Elevated anxiety-like and depressive behavior in Desert hedgehog knockout male mice. Behav. Brain Res. 2006;174(1):167–173. doi: 10.1016/j.bbr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 476.Frisch C, Dere E, Silva MA, Godecke A, Schrader J, Huston JP. Superior water maze performance and increase in fear-related behavior in the endothelial nitric oxide synthase-deficient mouse together with monoamine changes in cerebellum and ventral striatum. J. Neurosci. 2000;20(17):6694–6700. doi: 10.1523/JNEUROSCI.20-17-06694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Dere E, De Souza Silva Ma, Topic B, et al. Aged endothelial nitric oxide synthase knockout mice exhibit higher mortality concomitant with impaired open-field habituation and alterations in forebrain neurotransmitter levels. Genes Brain Behav. 2002;1(4):204–213. doi: 10.1034/j.1601-183x.2002.10402.x. [DOI] [PubMed] [Google Scholar]
- 478.Kudo T, Fujii T, Ikegami S, et al. Mice lacking α1,3-fucosyltransferase IX demonstrate disappearance of Lewis x structure in brain and increased anxiety-like behaviors. Glycobiology. 2007;17(1):1–9. doi: 10.1093/glycob/cwl047. [DOI] [PubMed] [Google Scholar]
- 479.Miyakawa T, Yagi T, Watanabe S, Niki H. Increased fearfulness of Fyn tyrosine kinase deficient mice. Brain Res. Mol. Brain Res. 1994;27(1):179–182. doi: 10.1016/0169-328x(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 480.Boehm SL, 2nd, Peden L, Chang R, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters behavioral sensitivity to ethanol. Alcohol Clin. Exp Res. 2003;27(7):1033–1040. doi: 10.1097/01.ALC.0000075822.80583.71. [DOI] [PubMed] [Google Scholar]
- 481.Kubota O, Hattori K, Hashimoto K, et al. Auditory-conditioned-fear-dependent c-Fos expression is altered in the emotion-related brain structures of Fyn-deficient mice. Brain Res. Mol. Brain Res. 2004;130(1–2):149–160. doi: 10.1016/j.molbrainres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 482.Isosaka T, Hattori K, Kida S, et al. Activation of Fyn tyrosine kinase in the mouse dorsal hippocampus is essential for contextual fear conditioning. Eur. J. Neurosci. 2008;28(5):973–981. doi: 10.1111/j.1460-9568.2008.06405.x. [DOI] [PubMed] [Google Scholar]
- 483.Gaier ED, Rodriguiz RM, Ma XM, et al. Haploinsufficiency in peptidylglycine α-amidating monooxygenase leads to altered synaptic transmission in the amygdala and impaired emotional responses. J. Neurosci. 2010;30(41):13656–13669. doi: 10.1523/JNEUROSCI.2200-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Osada T, Ikegami S, Takiguchi-Hayashi K, et al. Increased anxiety and impaired pain response in puromycin-sensitive aminopeptidase gene-deficient mice obtained by a mouse gene-trap method. J. Neurosci. 1999;19(14):6068–6078. doi: 10.1523/JNEUROSCI.19-14-06068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Takagi T, Jin W, Taya K, Watanabe G, Mori K, Ishii S. Schnurri-2 mutant mice are hypersensitive to stress and hyperactive. Brain Res. 2006;1108(1):88–97. doi: 10.1016/j.brainres.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 486.Zhao L, Kim KW, Ikeda Y, et al. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol. Endocrinol. 2008;22(6):1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Chrast R, Scott HS, Madani R, et al. Mice trisomic for a bacterial artificial chromosome with the single-minded 2 gene (Sim2) show phenotypes similar to some of those present in the partial trisomy 16 mouse models of Down syndrome. Hum. Mol. Genet. 2000;9(12):1853–1864. doi: 10.1093/hmg/9.12.1853. [DOI] [PubMed] [Google Scholar]
- 488.Kim YS, Harry GJ, Kang HS, et al. Altered cerebellar development in nuclear receptor TAK1/TR4 null mice is associated with deficits in GLAST+ glia, alterations in social behavior, motor learning, startle reactivity, and microglia. Cerebellum. 2010;9(3):310–323. doi: 10.1007/s12311-010-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Sekiyama K, Hashimoto O, Ushiro Y, et al. Abnormalities in aggression and anxiety in transgenic mice overexpressing activin E. Biochem. Biophys. Res. Commun. 2009;385(3):319–323. doi: 10.1016/j.bbrc.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 490.Dierssen M, Gratacos M, Sahun I, et al. Transgenic mice overexpressing the full-length neurotrophin receptor TrkC exhibit increased catecholaminergic neuron density in specific brain areas and increased anxiety-like behavior and panic reaction. Neurobiol. Dis. 2006;24(2):403–418. doi: 10.1016/j.nbd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 491.Zorner B, Wolfer DP, Brandis D, et al. Forebrain-specific trkB-receptor knockout mice: behaviorally more hyperactive than ‘depressive’. Biol. Psychiatry. 2003;54(10):972–982. doi: 10.1016/s0006-3223(03)00418-9. [DOI] [PubMed] [Google Scholar]
- 492.Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin. Neurosci. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Van Tol MJ, Van Der Wee NJ, Van Den Heuvel OA, et al. Regional brain volume in depression and anxiety disorders. Arch. Gen. Psychiatry. 2010;67(10):1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 494.Asami T, Yamasue H, Hayano F, et al. Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Res. 2009;173(2):128–134. doi: 10.1016/j.pscychresns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 495.Asami T, Hayano F, Nakamura M, et al. Anterior cingulate cortex volume reduction in patients with panic disorder. Psychiatry Clin. Neurosci. 2008;62(3):322–330. doi: 10.1111/j.1440-1819.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- 496.Uchida RR, Del-Ben CM, Busatto GF, et al. Regional gray matter abnormalities in panic disorder: a voxel-based morphometry study. Psychiatry Res. 2008;163(1):21–29. doi: 10.1016/j.pscychresns.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 497.Thomaes K, Dorrepaal E, Draijer N, et al. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J. Clin. Psychiatry. 2010;71(12):1636–1644. doi: 10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- 498.Rogers MA, Yamasue H, Abe O, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res. 2009;174(3):210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 499.Roppongi T, Nakamura M, Asami T, et al. Posterior orbitofrontal sulcogyral pattern associated with orbitofrontal cortex volume reduction and anxiety trait in panic disorder. Psychiatry Clin. Neurosci. 2010;64(3):318–326. doi: 10.1111/j.1440-1819.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- 500.Sobanski T, Wagner G, Peikert G, et al. Temporal and right frontal lobe alterations in panic disorder: a quantitative volumetric and voxel-based morphometric MRI study. Psychol. Med. 2010;40(11):1879–1886. doi: 10.1017/S0033291709991930. [DOI] [PubMed] [Google Scholar]
- 501.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 502.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog. Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2 Findings in neuropsychiatric disorders. Mol. Psychiatry. 2005;10(2):160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 504.Golub Y, Kaltwasser SF, Mauch CP, et al. Reduced hippocampus volume in the mouse model of posttraumatic stress disorder. J. Psychiatr. Res. 2011;45(5):650–659. doi: 10.1016/j.jpsychires.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 505.Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage. 2003;19(1):80–90. doi: 10.1016/s1053-8119(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 506.Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33(11):2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- 507.Crupi R, Cambiaghi M, Spatz L, et al. Reduced adult neurogenesis and altered emotional behaviors in autoimmune-prone B-cell activating factor transgenic mice. Biol. Psychiatry. 2010;67(6):558–566. doi: 10.1016/j.biopsych.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 508.Hode Y, Ratomponirina C, Gobaille S, Maitre M, Kopp C, Misslin R. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacol. Biochem. Behav. 2000;65(1):35–38. doi: 10.1016/s0091-3057(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 509.Robertson HA. Benzodiazepine receptors in ‘emotional’ and ‘non-emotional’ mice; comparison of four strains. Eur. J. Pharmacol. 1979;56(1–2):163–166. doi: 10.1016/0014-2999(79)90447-3. [DOI] [PubMed] [Google Scholar]
- 510.Tannenbaum B, Anisman H. Impact of chronic intermittent challenges in stressor-susceptible and resilient strains of mice. Biol. Psychiatry. 2003;53(4):292–303. doi: 10.1016/s0006-3223(02)01487-7. [DOI] [PubMed] [Google Scholar]
- 511.Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl.) 2008;201(1):15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149(6):2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- 513.Anisman H, Prakash P, Merali Z, Poulter MO. Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav. Brain Res. 2007;181(2):180–190. doi: 10.1016/j.bbr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 514.Chung S, Son GH, Park SH, et al. Differential adaptive responses to chronic stress of maternally stressed male mice offspring. Endocrinology. 2005;146(7):3202–3210. doi: 10.1210/en.2004-1458. [DOI] [PubMed] [Google Scholar]
- 515.Michelgard A, Appel L, Pissiota A, et al. Symptom provocation in specific phobia affects the substance P neurokinin-1 receptor system. Biol. Psychiatry. 2007;61(8):1002–1006. doi: 10.1016/j.biopsych.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 516.Geracioti TD, Jr, Carpenter LL, Owens MJ, et al. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am. J. Psychiatry. 2006;163(4):637–643. doi: 10.1176/ajp.2006.163.4.637. [DOI] [PubMed] [Google Scholar]
- 517.Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am. J. Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 519.Pattij T, Groenink L, Hijzen TH, et al. Autonomic changes associated with enhanced anxiety in 5-HT(1A) receptor knockout mice. Neuropsychopharmacology. 2002;27(3):380–390. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 520.Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567–590. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- 521.Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav. Brain Res. 2003;147(1–2):193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 522.Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/6J mice. Physiol. Behav. 2005;85(4):419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 523.Prakash P, Merali Z, Kolajova M, Tannenbaum BM, Anisman H. Maternal factors and monoamine changes in stress-resilient and susceptible mice: cross-fostering effects. Brain Res. 2006;1111(1):122–133. doi: 10.1016/j.brainres.2006.06.089. [DOI] [PubMed] [Google Scholar]
- 524.Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004;16(4):319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- 525.Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. J. Neuroendocrinol. 2003;15(11):1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- 526.Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(6):R1494–R1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- 527.Ikeda K, Onaka T, Yamakado M, et al. Degeneration of the amygdala/piriform cortex and enhanced fear/anxiety behaviors in sodium pump α2 subunit (Atp1a2)-deficient mice. J. Neurosci. 2003;23(11):4667–4676. doi: 10.1523/JNEUROSCI.23-11-04667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Amador-Arjona A, Delgado-Morales R, Belda X, et al. Susceptibility to stress in transgenic mice overexpressing TrkC, a model of panic disorder. J. Psychiatr. Res. 2010;44(3):157–167. doi: 10.1016/j.jpsychires.2009.07.008. [DOI] [PubMed] [Google Scholar]
Websites
- 601.National Institute of Mental Health This website provides information on symptoms, causes and treatments of the major anxiety disorders, with information on getting help and coping. www.nimh.nih.gov/health/publications/anxiety-disorders/introduction.shtml (Accessed March 2009)
- 602.Mouse Phenome Database A database by the Jackson Laboratory, which provides phenotype strain survey data. http://phenome.jax.org (Accessed March 2011)