Abstract
Nogo-66 receptor 1 (NgR1) is a glycosylphosphatidylinositol-anchored receptor for myelin-associated inhibitors that restricts plasticity and axonal regrowth in the CNS. NgR1 is cleaved from the cell surface of SH-SY5Y neuroblastoma cells in a metalloproteinase-dependent manner; however, the mechanism and physiological consequence of NgR1 shedding have not been explored. We now demonstrate that NgR1 is shed from multiple populations of primary neurons. Through a loss-of-function approach, we found that membrane-type matrix metalloproteinase-3 (MT3-MMP) regulates endogenous NgR1 shedding in primary neurons. Neuronal knockdown of MT3-MMP resulted in the accumulation of NgR1 at the cell surface and reduced the accumulation of the NgR1 cleavage fragment in medium conditioned by cortical neurons. Recombinant MT1-, MT2-, MT3-, and MT5-MMPs promoted NgR1 shedding from the surface of primary neurons, and this treatment rendered neurons resistant to myelin-associated inhibitors. Introduction of a cleavage-resistant form of NgR1 reconstitutes the neuronal response to these inhibitors, demonstrating that specific metalloproteinases attenuate neuronal responses to myelin in an NgR1-dependent manner.
Keywords: ADAM ADAMTS, Axon, Matrix Metalloproteinase (MMP), Neurodegeneration, Neurological Diseases, Neurons, Neuroscience, Shedding
Introduction
Nogo-66 receptor 1 (NgR1)2 was originally identified as a receptor for myelin-associated inhibitors (MAIs) (1). NgR1 is a glycosylphosphatidylinositol (GPI)-anchored protein that complexes with LINGO and p75NTR or TAJ/TROY to mediate neurite outgrowth inhibition of neurons (2). Neutralization of NgR1 attenuates the inhibitory effects of MAIs in vitro and promotes regeneration following CNS trauma in vivo (3). Further studies have suggested that NgR1 plays a protective role in Alzheimer disease (4), that NgR1 variants contribute to increased risk of schizophrenia (5), and that NgR1 limits plasticity in the hippocampus (6) and in the visual cortex (7). Understanding the mechanisms that regulate NgR1 will have important implications for CNS plasticity, disease, and regeneration.
NgR1 shed from the cell surface is a soluble fragment spanning amino acids 1–358 (8). This fragment is similar to NgREcto (amino acids 1–310), a dominant-negative protein that promotes axonal regeneration in vitro and in vivo by antagonizing MAI signaling (9, 10). The shed NgR1 product can be detected in human cerebral spinal fluid, indicative of a physiological role for this fragment.
NgR1 shedding is blocked by the metalloproteinase inhibitor PKF226–967. Metalloproteinases are a family of zinc-dependent proteases that include ADAM (a disintegrin and metalloproteinases) and secreted or membrane-type matrix metalloproteinases (MT-MMPs) (11). MT1-MMP (MMP-14), MT2-MMP (MMP-15), MT3-MMP (MMP-16), and MT5-MMP (MMP-24) anchor to the cell surface with a transmembrane domain, whereas MT4-MMP (MMP-17) and MT6-MMP (MMP-25) attach with a GPI anchor. MMPs have been widely studied for their capacity to degrade the extracellular matrix and to promote invasion and migration of tumor cells; however, it is also known that they can cleave a wide range of ligands and receptors. In fact, p75NTR is processed by the ADAM protein TNF-α-converting enzyme (TACE) (12). MT1-MMP cleaves the MAI NogoA (13), and chondroitin sulfate proteoglycans are processed by MT1-, MT2-, MT3-, MT5-, and MT6-MMPs (14). Thus, MMPs are interesting candidates to modulate inhibitory activity in the CNS in the context of disease, injury, and plasticity.
NgR1 shedding is blocked by endogenous tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3, but not by TIMP-1 (8). The distinct inhibitory profiles of the TIMPs suggest that the metalloproteinase responsible for NgR1 shedding is an MT-MMP or TACE (15). Recombinant TACE has been reported to enhance NgR1 cleavage (16), but the physiological regulator of NgR1 shedding has not been identified. Through a loss-of-function approach, we identified MT3-MMP as a regulator of basal NgR1 cleavage in cortical neurons and demonstrate that metalloproteinase cleavage of NgR1 attenuates growth cone collapse in response to myelin in an NgR1-dependent fashion. Our data demonstrate that specific MT-MMPs regulate NgR1-dependent processing, a finding that is likely to bear on recovery from a spectrum of CNS disorders and injuries.
EXPERIMENTAL PROCEDURES
Plasmids and Antibodies
Constructs for human MT-MMPs were provided by Dr. M. Seiki (University of Tokyo) (17). The human TACE construct was provided by Dr. P. Barker (McGill University). Constructs for soluble human MT1-MMP and MT2-MMP were described previously (18). Recombinant active human TACE was purchased from R&D Systems (Minneapolis, MN). Constructs for the production of soluble recombinant human MT3-MMP and MT5-MMP were generated by deleting the transmembrane domain (truncated at Asp-533 for MT3-MMP and Asn-570 for MT5-MMP) and subcloning into the psecTAGhygro A vector (Invitrogen). Antibodies were directed against mouse NgR1 (R&D Systems), rat NgR1 (Dr. R. J. Giger, University of Michigan), human MT1-MMP (Abcam, Cambridge, MA), human MT5-MMP (Dr. E. B. Ziff, New York University), TACE and TrkA (Dr. P. Barker), FLAG (Sigma), and the human EGF receptor (Santa Cruz Biotechnology). Anti-N-cadherin antibody was developed by M. Takeichi and H. Matsunami and was obtained from the Developmental Studies Hybridoma Bank.
Primary Cell Culture
Postnatal day (P) 8 cerebellar neurons were prepared as described previously (19). Rat cortical neurons were prepared from embryonic day 17–18 rat cortices. The cortex was dissected, dissociated with trypsin and mechanical trituration, and cultured for 7 days on dishes coated with 0.01% poly-l-lysine (Sigma). Dissociated P5 rat dorsal root ganglion (DRG) neurons were grown on chamber slides sequentially coated with 0.01% poly-l-lysine and 10 μg/ml laminin (BD Biosciences). P5 DRGs and embryonic day 17–18 cortical neurons were grown in Neurobasal medium, 2% B27 supplement, penicillin/streptomycin, and 1% glutamine. DRGs were supplemented with 50 ng/ml NGF, and cortical neurons with 1% N2 supplement (Invitrogen).
Reverse Transcription-PCR
For RT-PCR experiments, total RNA was isolated from human SH-SY5Y neuroblastoma cells, embryonic day 18–19 rat cortical neurons, P8 rat cerebellar neurons, P4 rat DRG neurons, and total P7 rat brain using an RNeasy kit (Qiagen, Mississauga, Ontario, Canada). cDNA was prepared with a ThermoScript reverse transcriptase kit (Invitrogen). Primers for PCR detection were as follows: rat MT1F, 5′-aagcttggctgcagcagtat; rat MT1R, 5′-taggcatagggcacttctcg; rat MT2F, 5′-ggctgcgactctatggctac; rat MT2R, 5′-tgtaccagcccagcttctct; rat MT3F, 5′-cagctctggaagaaggttgg; rat MT3R, 5′-gagctgcctcttgtttggtc; rat MT4F, 5′-agtttggctacctcccacct; rat MT4R, 5′-tccagactttgagggcgtag; rat MT5F, 5′-ggctatctgcttccctatga; rat MT5R, 5′-catgatgtctgcctccttcc; rat MT6F, 5′-cctgacctcctcgatcc; rat MT6R, 5′-cttgtgcatggtggctattg; rat TACEF, 5′-gaagcttgattctttgctctca; rat TACER, 5′-caccagcatcctttttatctttag; human MT1F, 5′-cactgcctacgagaggaagg; human MT1R, 5′-tcccttcccagactttgatg; human MT2F, 5′-ggccgacatcatggtactct; human MT2R, 5′-gtcaacgtccttccactggt; human MT3F, 5′-gctgacccaaggaaaaatga; human MT3R, 5′-gcattgggtatccatccatc; human MT4F, 5′-ccaccaagtggaacaagagg; human MT4R, 5′-gccttggagaagtcgatctg; human MT5F, 5′-tgaaggcattgacacagctc; human MT5R, 5′-cgctcagtttctggttgtca; human MT6F, 5′-gacctggacttttgggtcaa; human MT6R, 5′-tcctgagacaggcggtactt; human TACEF, 5′-tgctgcaacagcgactgc; and human TACER, 5′-agcactctgtttctttgctgtc.
Growth Cone Collapse
Rat P4–P6 DRGs were isolated from the lumbosacral spinal cord. After 13–16 h of incubation, the explants were pretreated with buffer, recombinant MT-MMP (0.75 μm MT1-MMP or 1.5 μm MT3-MMP), or 1 unit/ml phosphatidylinositol-specific phospholipase C (PI-PLC; Invitrogen) for 3 h prior to myelin treatment (100 μg/ml for 45 min). Bovine myelin extract was prepared as described previously (20). Explants were fixed with 4% paraformaldehyde, 0.1 m phosphate buffer, and 20% sucrose. Growth cones were visualized by rhodamine-phalloidin (Invitrogen).
Preparation of Recombinant Proteins
Constructs for soluble MT-MMPs were transfected into HEK293T cells. Serum-free medium was collected after 48 h, and recombinant MMPs were purified with M2 affinity gel (Sigma).
NgR1 Cleavage
In vitro MMP activity was assayed by incubating a 1:1 molar ratio of recombinant mouse NgR1 with recombinant MMP or TACE (R&D Systems) in 50 mm Tris, 200 mm NaCl, 5 mm CaCl2, and 0.005% Brij (pH 7.4) or in low salt buffer (25 mm Tris, 2.5 μm ZnCl2, and 0.005% Brij (pH 9.0)) for 6–8 h at 37 °C. NgR1 shedding from neurons was assayed by replacing the culture medium with serum-free medium for 4-6 h in the presence or absence of BB-94 (Tocris, Ellisville, MO) or GM6001 (Calbiochem) prior to collection. The medium was concentrated using Amicon Ultra Ultracel columns (Millipore, Billerica, MA). Shedding from P8 rat brain membrane was performed as described previously (21). Cell-surface labeling of FLAG-NgR1 was measured in COS-7 cells transfected with FLAG-WT-NgR1 or FLAG-CR4-NgR1 and treated with recombinant MMPs for 3 h. Immunodetection was done under non-permeabilizing conditions with antibody M2 at 4 °C prior to fixation with 4% paraformaldehyde. Cell-surface protein levels in COS-7 cells or in DRG or cortical neurons were assessed by cell-surface biotinylation. Sulfo-SHS-LC-biotin was added to neurons at 4 °C, and biotinylated proteins were isolated with streptavidin-agarose resin (Thermo Scientific).
Preparation of Recombinant Viral Preparation
Preparation of recombinant HSV or lentivirus was described previously (19, 22, 23). shRNA target sequences for MMPs were as follows: MT1-MMPa, AAACCATAGAACCTTTGCATG; MT1-MMPb, AACGCTGGCAGTAAAGCAGTC; MT2-MMPa, TAGAGTCGCAGCCAGTTCTCA; MT2-MMPb, ATCCCATAGAAACTCTGCATC; MT3-MMPa, TAGAGCTGCCTCTTGTTTGGT; MT3-MMPb, AAATGAGTATCGCCTCCAATT; MT5-MMPa, AACTGTTGCATAGTGGAGACT; MT5-MMPb, TTCATAGGCTGCGTCTATGCG; TACEa, CAAAGAATCAAGCTTCTCAAG; TACEb, TTAACAAACCTCCAAAGTGGC; and control, AATTCTCCGAACGTGTCACGT.
RESULTS
NgR1 Is Shed from Multiple Neuronal Cell Types
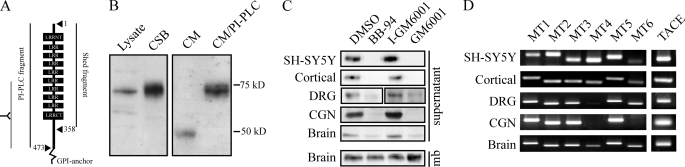
To assess shedding of endogenous NgR1, we used an antibody raised against the C-terminal half of rat NgR1 (24). Similar to previous findings in SH-SY5Y cells (8), an NgR1 fragment of ∼48 kDa could be detected in conditioned medium from cortical neurons (Fig. 1, A and B). The shed fragment represents a fraction of full-length NgR1 released from the cell surface into the conditioned medium by cleaving GPI-anchored proteins with PI-PLC. We found that NgR1 was constitutively shed from SH-SY5Y cells, cortical neurons, DRG neurons, cerebellar granule neurons, and brain lysates (Fig. 1C) and that shedding was blocked by the MMP inhibitors BB-94 and GM6001, but not by the inactive analog of GM6001.
FIGURE 1.
NgR1 is shed from multiple neuronal cell types. A, schematic representation of full-length NgR1, including the MMP cleavage site at Ala-358. B, Western blots with an anti-NgR1 antibody raised against the C-terminal portion of NgR1 reveal full-length NgR1 from cortical neuron lysates and from biotinylated cell-surface proteins (CSB). A 48-kDa shed NgR1 fragment is visible in the conditioned medium (CM) from cortical neurons. Full-length NgR1 was present in conditioned medium from PI-PLC-treated cortical neurons. C, Western blotting of the 48-kDa cleavage fragment in conditioned medium from SH-SY5Y cells and cortical, DRG, and cerebellar granule neurons (CGN). SH-SY5Y cells were transfected with mouse MycNgR1, and the shed product was detected with anti-mouse NgR1 antibody. Cells were grown in the presence or absence of dimethyl sulfoxide (DMSO), the metalloproteinase inhibitors BB-94 (5 μm) and GM6001 (10 μm), or the inactive analog of GM6001 (I-GM6001; 10 μm). NgR1 was also shed from crude membranes (mb) prepared from P5 rat brain. D, RT-PCR analysis for MT1-, MT2-, MT3-, MT4-, MT5-, and MT6-MMPs (MT1–MT6, respectively) and TACE from mRNA prepared from P5 rat brain, from SH-SY5Y cells, or from cortical, DRG, or cerebellar granule neurons. The human MT2-MMP product amplified from SH-SY5Y cells was larger than the rat MT2-MMP product amplified from cortical, DRG, or cerebellar granule neurons or from brain.
To identify candidate MMPs as mediators of NgR1 cleavage, we evaluated the expression profile of MT-MMPs and TACE in cellular populations that shed NgR1. mRNAs for MT-MMPs and TACE were present in SH-SY5Y cells, cortical neurons, DRG neurons, cerebellar granule neurons, and brain (Fig. 1D). MT4-MMP was relatively low in DRG neurons, and MT4-MMP and MT6-MMP were absent in cerebellar granule neurons. The profile of cleavage and expression identified MT1-, MT2-, MT3-, and MT5-MMPs and TACE as candidates to cleave NgR1.
MT1-, MT2-, MT3-, and MT5-MMPs Promote NgR1 Shedding
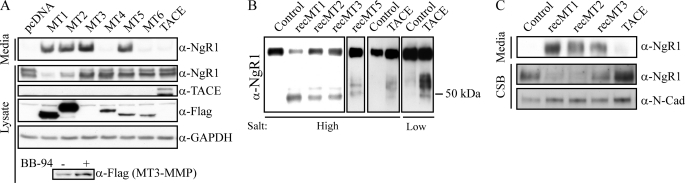
To determine which of these MMPs mediate NgR1 shedding, we cotransfected FLAG-tagged MMP constructs and NgR1 into COS-7 cells. Cells cotransfected with MT1-, MT2-, MT3-, and MT5-MMPs produced an NgR1 fragment in the conditioned medium, whereas MT4-MMP, MT6-MMP, and TACE had no effect (Fig. 2A). Differential expression of full-length MMPs in the cell lysate can be attributed in part to the autocatalytic nature of the MMPs. The most dramatic example is MT3-MMP, which could be detected with longer Western blot exposures, particularly in the presence of BB-94 (Fig. 2A, lower panel). In this cotransfection paradigm, we verified that the processed mature form of each MMP efficiently trafficked to the cell surface (supplemental Fig. S1A). We further validated the catalytic activity of TACE by demonstrating that it enhanced regulated intramembrane proteolysis of p75NTR as described previously (25) (Fig. S1B).
FIGURE 2.
NgR1 is cleaved at the cell surface by MT1-, MT2-, and MT3-MMPs. A, Western blot analysis of cell lysates and conditioned medium from COS-7 cells cotransfected with mouse NgR1 and FLAG-tagged MT1-, MT2-, MT3-, MT4-, MT5-, and MT6-MMPs (MT1–MT6, respectively) or TACE. Cotransfection with MT1-, MT2-, MT3-, and MT5-MMPs promoted NgR1 shedding. Anti-mouse NgR1 antibody was used to detect full-length and shed NgR1. MT3-MMP is apparent only in cell lysates with longer Western blot exposures (see lower panel). B, in vitro cleavage of recombinant NgR1 with recombinant (rec) MT1-, MT2-, MT3-, and MT5-MMPs or TACE. C, Western blot analysis of conditioned medium and cell-surface biotinylated proteins (CSB) from cortical neurons treated with 1 μm recombinant MT1-, MT2-, and MT3-MMPs or TACE.
To determine whether MT-MMPs cleave NgR1 directly, we purified recombinant MMPs (supplemental Fig. S1C) and mixed them with recombinant NgR1 in vitro. Consistent with the cotransfection experiment, MT1-, MT2-, MT3-, and MT5-MMPs cleaved recombinant NgR1 (Fig. 2B). Recombinant MT5-MMP had weak activity toward NgR1, but this may be because it undergoes considerable autocatalysis (supplemental Fig. S1C). TACE weakly cleaved NgR1 in vitro in 200 mm NaCl (Fig. 2C) and mediated a more robust cleavage in low salt buffer; however, the major cleavage fragments generated by TACE had a higher apparent molecular mass than the fragment generated in neurons (Fig. 1B versus Fig. 2B).
We then asked if recombinant MMPs can cleave endogenous NgR1 from the surface of neurons. Treatment of cortical neurons with recombinant MT1-, MT2-, and MT3-MMPs for 4 h resulted in accumulation of the NgR1 fragment in the medium and decreased full-length NgR1 at the cell surface (Fig. 2C). Recombinant TACE failed to enhance NgR1 cleavage from cortical neurons. Thus, MT1-, MT2-, and MT3-MMPs cleave NgR1 from the surface of neurons. MT5-MMP likely acts in a similar fashion, but we were unable to test this in primary neurons because recombinant MT5-MMP exhibits robust autocatalysis.
MT3-MMP Is a Physiological Regulator of NgR1 Shedding in Cortical Neurons
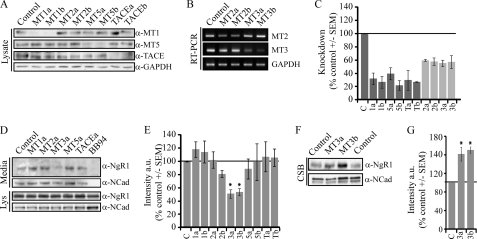
To assess which MT-MMPs regulate basal NgR1 shedding in vivo, we assessed shedding in cortical neurons transduced with lentiviruses expressing shRNA target sequences with a microRNA stem for knockdown of individual MMPs (Fig. 3). Knockdown of individual MMPs was validated by Western blotting for MT1-MMP, MT5-MMP, and TACE and by RT-PCR for MT2-MMP and MT3-MMP (Fig. 3, A–C). Knockdown of MT3-MMP significantly reduced NgR1 shedding into the medium (Fig. 3, D and E) and increased cell-surface NgR1 (Fig. 3, F and G), whereas loss of MT1-, MT2-, and MT5-MMPs and TACE had no effect on NgR1 processing. As a control for the cell-surface biotinylation, we assessed shedding of N-cadherin, a cell adhesion molecule known to be shed from the cell surface. N-cadherin shedding was blocked by BB-94, but not by knocking down expression of individual MT-MMPs. Our data identify MT3-MMP as a regulator of basal NgR1 shedding in cortical neurons.
FIGURE 3.
MT3-MMP regulates endogenous NgR1 cleavage in cortical neurons. A–G, P5 rat cortical neurons were transduced with lentiviruses for knockdown of individual MT-MMP isoforms, TACE, or a control sequence. A and B, cell lysates or mRNA extracts were analyzed by Western blotting or RT-PCR, respectively. a and b indicate separate target sequences for knockdown. C, quantification of MMP knockdown expressed as a percentage control ± S.E. for MMP protein (MT1-MMP (MT1), MT5-MMP (MT5), or TACE) or mRNA (MT2-MMP (MT2) or MT3-MMP (MT3)). Expression levels were normalized to the GAPDH signal for Western blotting and RT-PCR. D, Western blotting to detect full-length NgR1 or the NgR1 cleavage product was performed on cell lysates (Lys) and conditioned medium, respectively. N-cadherin (NCad) expression was analyzed as a control. E, quantification of NgR1 expression in conditioned medium as a percentage of control ± S.E. a.u., arbitrary units. F, Western blotting to detect full-length NgR1 from biotinylated cell-surface proteins (CSB). G, quantification of NgR1 from cell-surface biotinylated proteins as a percentage control ± S.E. All determinations are from three to six separate experiments. *, p < 0.05 by one-way analysis with Dunnett's post hoc test compared with the control (C).
MT1-MMP and MT3-MMP Attenuate Myelin-dependent Growth Cone Collapse
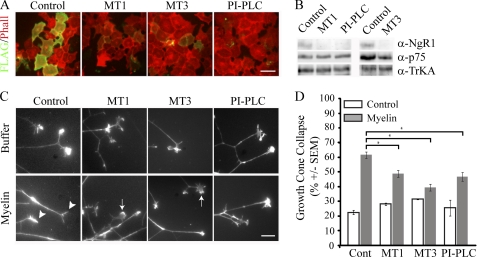
We next assessed if recombinant MT-MMPs that remove NgR1 from the cell surface attenuate NgR1-dependent neuronal responses to myelin. We chose to assess growth cone collapse in DRGs, widely accepted as an NgR1-dependent response to myelin (26, 27). We treated FLAG-NgR1-transfected COS-7 cells with recombinant MMPs and identified 0.75 μm recombinant MT1-MMP and 1.5 μm recombinant MT3-MMP as appropriate doses to remove NgR1 from the cell surface (Fig. 4A). These doses efficiently removed NgR1 from the surface of P5 rat DRG neurons without affecting the NgR1 coreceptor p75NTR or the neurotrophin receptor TrkA (Fig. 4B). When P5 rat DRGs were pretreated with recombinant MT1-MMP or MT3-MMP and subsequently treated with a myelin extract, growth cone collapse was significantly attenuated (Fig. 4, C and D). Importantly, the addition of these enzymes did not affect basal growth cone collapse. The metalloproteinase effect was similar to that observed with PI-PLC, which cleaved GPI-anchored proteins, including NgR1, from the cell surface (Fig. 4, B–D).
FIGURE 4.
MT1-MMP- and MT3-MMP-dependent NgR1 shedding attenuates myelin-dependent growth cone collapse. A, cell-surface FLAG and rhodamine-phalloidin (Phall) staining of COS-7 cells transfected with FLAG-NgR1 and treated with 0.75 μm MT1-MMP (MT1), 1.5 μm MT3-MMP (MT3), or 1 unit/ml PI-PLC for 3 h. Scale bar = 10 μm. B, Western blotting of biotinylated cell-surface proteins from P5 DRG neurons treated with recombinant MT-MMPs or PI-PLC. C and D, growth cones of P5 rat DRG explants treated with buffer or myelin extract. Explants were pretreated for 3 h with control buffer or MT1-MMP (0.75 μm), MT3-MMP (1.5 μm), or PI-PLC (1 unit/ml). Growth cones were stained with rhodamine-phalloidin (C; scale bar = 10 μm) and quantified for growth cone collapse (D). Arrows and arrowheads indicate examples of spread and collapsed growth cones, respectively. Growth cone collapse quantification is from three separate experiments performed in duplicate. All samples were blinded prior to quantification. *, p < 0.05 by one-way ANOVA with Tukey's post hoc test.
MT1-MMP and MT3-MMP Attenuate Myelin Responses by Cleaving NgR1
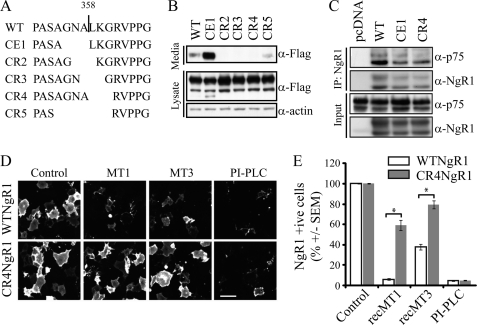
We then asked if the ability of MT1-MMP and MT3-MMP to attenuate myelin responses can be attributed to effects on NgR1 cleavage by introducing a cleavage-resistant form of NgR1 into DRG neurons and assessing the effects of MT-MMPs on myelin-dependent growth cone collapse. By deleting series of three residues surrounding the NgR1 cleavage site, we generated three cleavage-resistant forms of NgR1 (Fig. 5, A and B). Further analysis of CR4-NgR1 revealed that it retained its ability to co-immunoprecipitate with its p75NTR coreceptor (Fig. 5C) and efficiently trafficked to the cell surface (Fig. 5D). Recombinant MT1-MMP and MT3-MMP cleaved FLAG-WT-NgR1 from the surface of transfected COS-7 cells but failed to cleave FLAG-CR4-NgR1 to the same extent (Fig. 5, D and E). We then transduced P5 rat DRG neurons with WT-NgR1 and CR4-NgR1 to confer persistent NgR1 expression in the presence of MT-MMPs and assessed myelin-dependent growth cone collapse. MT1-MMP and MT3-MMP attenuated myelin-dependent growth cone collapse in P5 rat DRG neurons transduced with WT-NgR1, but not CR4-NgR1 (Fig. 6, A and B), demonstrating that the ability of these MT-MMPs to attenuate myelin responses can be directly attributed to their effects on NgR1 shedding. This is further supported by the failure of MT1-MMP and MT3-MMP to affect the cleavage of PirB (paired immunoglobulin-like receptor B), another receptor for the MAIs (supplemental Fig. S2). Although it is likely that myelin responses are transduced directly through CR4-NgR1-dependent signaling, it is also possible that this mutant could dimerize with endogenous NgR1, protecting it from proteolytic cleavage. Growth cone collapse responses in DRG neurons transduced with CE1-NgR1 were indistinguishable from those transduced with WT-NgR1 (supplemental Fig. S3), suggesting that insufficient levels of stable shed NgR1 fragment are released in the conditioned medium to function in a dominant-negative manner.
FIGURE 5.
Cleavage-resistant NgR1 is insensitive to MMPs. A, schematic of the WT-NgR1 cleavage site (indicated by a vertical line) and the mutations generated and tested for cleavage resistance. CE, cleavage enhanced. CR2–CR5, cleavage-resistant 2–5. B, Western blot analysis of conditioned medium and lysate from SH-SY5Y cells transfected with FLAG-tagged WT-NgR1 or mutant constructs. C, COS-7 cells were transfected with FLAG-NgR1 and p75 constructs. Cells were subjected to immunoprecipitation (IP) with an anti-FLAG antibody and analyzed for precipitating NgR1 and p75 by Western blotting. D, cell-surface FLAG staining of COS-7 cells transfected with FLAG-WT-NgR1 or FLAG-CR4-NgR1 and treated with 0.75 μm MT1-MMP (MT1), 1.5 μm MT3-MMP (MT3), or 1 unit/ml PI-PLC for 3 h. Scale bar = 10 μm. E, FLAG-positive cells were counted and are expressed as a percentage of total cells as identified by Hoechst counterstaining. Quantification is from three separate experiments. *, p < 0.001 by two-way ANOVA with a Bonferroni post hoc test. rec, recombinant.
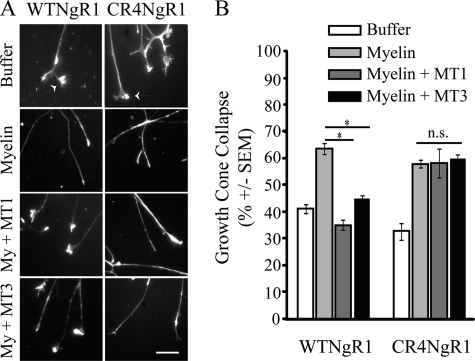
FIGURE 6.
MT1-MMP and MT3-MMP attenuate myelin responses by cleaving NgR1. A, growth cones of P5 rat DRG explants were transduced with HSV-WT-NgR1 or HSV-CR4-NgR1 and treated with buffer or myelin (My) extract. Explants were pretreated for 3 h with MT1-MMP (MT1; 0.75 μm) or MT3-MMP (MT3; 1.5 μm). Growth cones were stained with rhodamine-phalloidin (A; scale bar = 10 μm) and quantified for growth cone collapse (B). Growth cone collapse quantification is from three separate experiments performed in duplicate. *, p < 0.05 by one-way ANOVA with Tukey's post hoc test; n.s., not significant.
DISCUSSION
NgR1 undergoes cleavage at the cell surface, shedding its soluble ectodomain. Loss-of-function analysis indicates that MT3-MMP is responsible for basal NgR1 shedding in cortical neurons. By overexpression analysis, we have demonstrated that the NgR1 fragment can be generated by MT1-, MT2-, MT3-, and MT5-MMPs. Metalloproteinase-mediated removal of NgR1 from the cell surface renders neurons resistant to the growth cone-collapsing activity of myelin in an NgR1-dependent fashion.
Physiological Consequences of NgR1 Shedding
We have demonstrated that NgR1 can be shed from multiple neuronal cell types that respond to MAIs. The soluble fragment retains its ability to bind to Nogo-66 and is similar to NgREcto; thus, this product likely functions as a dominant-negative molecule by sequestering MAIs (9). It follows that NgR1 shedding is likely to attenuate MAI signaling through additional receptors, including PirB and NgR2 (24, 28). Thus, NgR1 shedding may have implications for a spectrum of CNS functions, including recovery from injury (29), CNS plasticity (6, 7), Alzheimer disease (4), and schizophrenia (5).
Our findings extend previous studies suggesting that MMPs may modulate the response to injury. MT-MMPs can target many inhibitory proteins, including MAIs and chondroitin sulfate proteoglycans, and we have shown that MMP activity could also impact regeneration or sprouting by affecting neuronal responses to the inhibitory environment. MT-MMPs are expressed in non-neuronal cells present at lesion sites, including resident and infiltrating immune cells (11). Furthermore, MT-MMP mRNA levels in total tissue and microglia are regulated in neuroinflammatory conditions such as experimental autoimmune encephalomyelitis (30). Several MMP transcripts are regulated in the spinal cord following a clip compression injury (31). Although MT-MMP transcripts are not regulated in this paradigm, it is possible that their proteolytic activity is affected by processing of pro-MT-MMPs to mature forms. A prediction from our data is that conditions that up-regulate the activity of MT-MMPs may promote nerve growth following spinal cord injury. It is also notable that immune cells express NgR1, providing an additional potential source of soluble NgR1 in the context of neuroinflammation (32). Thus, local expression of metalloproteinases at CNS injury sites is likely to impact both the inhibitory milieu and neuronal responses to this environment.
NgR1 has been recently shown to modulate synaptic plasticity, but the underlying mechanisms remain largely unknown (6, 7, 33). NgR1 influences hippocampal long-term potentiation, long-term depression, and dendritic spine morphology (6, 34). Local changes in NgR1 shedding could affect functional synaptic plasticity by affecting responses to synaptic NogoA and oligodendrocyte myelin glycoprotein. MMP inhibitors affect long-term potentiation, sprouting, and synaptogenesis (35). Furthermore, MT5-MMP localizes to the synapse of mature hippocampal neurons and is enriched in synaptosomes from mature brain (36). The expression pattern of MT5-MMP indicates that it may play a role in synapse remodeling, and our data raise the possibility that this may be through an NgR1-dependent mechanism. Detailed expression analysis of other MT-MMPs and their potential roles in synaptogenesis remain to be explored.
NgR1 can also interact with amyloid precursor protein to decrease the generation of the amyloid-β fragment (37). Several enzymes process full-length amyloid precursor protein, including MT1-, MT3-, and MT5-MMPs (38). An intriguing question is how parallel MT-MMP-dependent processing of NgR1 and amyloid precursor protein may ultimately affect amyloid precursor protein processing at the cell surface of neurons.
Transmembrane MT-MMPs Can Target NgR1
Our data reveal that NgR1 can be cleaved by all four transmembrane MT-MMPs in a cis- or trans-fashion, making it a very susceptible target for MT-MMPs expressed by neurons and non-neuronal cells. The structurally distinct GPI-anchored MT4-MMP and MT6-MMP did not target NgR1. To our knowledge, only MT5-MMP protein has been found to be expressed and functional in neurons (36). The other MT-MMPs are present at the mRNA level in the brain, and we have confirmed that MT1-MMP protein is also expressed in cortical neurons. TACE is also expressed in the brain and has been shown to promote NgR1 shedding from DRG neurons (16). Here, we used the same recombinant active human TACE but observed only a relative inefficient cleavage of recombinant NgR1. We did not see any enhanced shedding of endogenous NgR1 from cortical neurons treated with active TACE. Our approach differed from that of Logan and co-workers (16), as we treated our neurons for 3–6 h instead of 2–3 days. This raises the possibility that NgR1 could be targeted by additional MMPs such as TACE if subjected to long-term exposure.
MT3-MMP, a Physiological Regulator of NgR1 Shedding
Several extracellular matrix proteins, including collagen, aggrecan, vitronectin, and laminin, can be cleaved by MT3-MMP (39). It can also target other cell-surface proteins such as syndecan-1 and CD44 (40, 41). Here, we have reported that exogenous and endogenous MT3-MMP can target neuronal NgR1. MT3-MMP levels are highly regulated by MMP activity and autocatalysis. NgR1 shedding is regulated by TIMP-2 and TIMP-3, which can also inhibit MT3-MMP activity (8, 11). MT3-MMP distribution in the mammalian brain and at the neuronal cell surface remains to be fully described, but our data suggest that MT3-MMP is expressed in cortical neurons at a level sufficient for NgR1 shedding. MT3-MMP mRNA is present in the postnatal CNS, and the protein has been detected in microglia in both white and gray matter (11, 42). Its expression also increases when P19 cells are differentiated into neurons (43). Intriguingly, MT3-MMP expression decreases following induction of experimental autoimmune encephalomyelitis or traumatic injury in the entorhinal cortex and spinal cord (30, 31), and this differs from the elevated expression of several other MMPs. The role of MT3-MMP in regulating NgR1-dependent effects on nerve repair, synaptic plasticity, and additional CNS functions remains to be explored.
Supplementary Material
Acknowledgments
We thank Wee Yong, Phil Barker, and Peter McPherson for insights regarding this project and manuscript.
This work was supported in part by a grant from the Canadian Institutes of Health Research (CIHR; to A. E. F.) and by a studentship from the Fonds de la Recherche en Santé du Québec (to G. B. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- NgR1
- Nogo-66 receptor 1
- MAI
- myelin-associated inhibitor
- GPI
- glycosylphosphatidylinositol
- MT-MMP
- membrane-type matrix metalloproteinase
- TACE
- TNF-α-converting enzyme
- TIMP
- tissue inhibitor of metalloproteinase
- P
- postnatal day
- DRG
- dorsal root ganglion
- PI-PLC
- phosphatidylinositol-specific phospholipase C
- ANOVA
- analysis of variance.
REFERENCES
- 1. Fournier A. E., GrandPre T., Strittmatter S. M. (2001) Nature 409, 341–346 [DOI] [PubMed] [Google Scholar]
- 2. McDonald C. L., Bandtlow C., Reindl M. (2011) Curr. Med. Chem. 18, 234–244 [DOI] [PubMed] [Google Scholar]
- 3. Nash M., Pribiag H., Fournier A. E., Jacobson C. (2009) Mol. Neurobiol. 40, 224–235 [DOI] [PubMed] [Google Scholar]
- 4. Park J. H., Strittmatter S. M. (2007) Curr. Alzheimer Res. 4, 568–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voineskos A. N. (2009) J. Neurosci. 29, 5045–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee H., Raiker S. J., Venkatesh K., Geary R., Robak L. A., Zhang Y., Yeh H. H., Shrager P., Giger R. J. (2008) J. Neurosci. 28, 2753–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGee A. W., Yang Y., Fischer Q. S., Daw N. W., Strittmatter S. M. (2005) Science 309, 2222–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walmsley A. R., McCombie G., Neumann U., Marcellin D., Hillenbrand R., Mir A. K., Frentzel S. (2004) J. Cell Sci. 117, 4591–4602 [DOI] [PubMed] [Google Scholar]
- 9. Fournier A. E., Gould G. C., Liu B. P., Strittmatter S. M. (2002) J. Neurosci. 22, 8876–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li S., Liu B. P., Budel S., Li M., Ji B., Walus L., Li W., Jirik A., Rabacchi S., Choi E., Worley D., Sah D. W., Pepinsky B., Lee D., Relton J., Strittmatter S. M. (2004) J. Neurosci. 24, 10511–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yong V. W., Power C., Forsyth P., Edwards D. R. (2001) Nat. Rev. Neurosci. 2, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weskamp G., Schlöndorff J., Lum L., Becherer J. D., Kim T. W., Saftig P., Hartmann D., Murphy G., Blobel C. P. (2004) J. Biol. Chem. 279, 4241–4249 [DOI] [PubMed] [Google Scholar]
- 13. Beliën A. T., Paganetti P. A., Schwab M. E. (1999) J. Cell Biol. 144, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hernandez-Barrantes S., Bernardo M., Toth M., Fridman R. (2002) Semin. Cancer Biol. 12, 131–138 [DOI] [PubMed] [Google Scholar]
- 15. Baker A. H., Edwards D. R., Murphy G. (2002) J. Cell Sci. 115, 3719–3727 [DOI] [PubMed] [Google Scholar]
- 16. Ahmed Z., Mazibrada G., Seabright R. J., Dent R. G., Berry M., Logan A. (2006) FASEB J. 20, 1939–1941 [DOI] [PubMed] [Google Scholar]
- 17. Egawa N., Koshikawa N., Tomari T., Nabeshima K., Isobe T., Seiki M. (2006) J. Biol. Chem. 281, 37576–37585 [DOI] [PubMed] [Google Scholar]
- 18. Morrison C. J., Overall C. M. (2006) J. Biol. Chem. 281, 26528–26539 [DOI] [PubMed] [Google Scholar]
- 19. Hsieh S. H., Ferraro G. B., Fournier A. E. (2006) J. Neurosci. 26, 1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Igarashi M., Strittmatter S. M., Vartanian T., Fishman M. C. (1993) Science 259, 77–79 [DOI] [PubMed] [Google Scholar]
- 21. Kalus I., Bormann U., Mzoughi M., Schachner M., Kleene R. (2006) J. Neurochem. 98, 78–88 [DOI] [PubMed] [Google Scholar]
- 22. Thomas S., Ritter B., Verbich D., Sanson C., Bourbonnière L., McKinney R. A., McPherson P. S. (2009) J. Biol. Chem. 284, 12410–12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allaire P. D., Marat A. L., Dall'Armi C., Di Paolo G., McPherson P. S., Ritter B. (2010) Mol. Cell 37, 370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venkatesh K., Chivatakarn O., Lee H., Joshi P. S., Kantor D. B., Newman B. A., Mage R., Rader C., Giger R. J. (2005) J. Neurosci. 25, 808–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Domeniconi M., Zampieri N., Spencer T., Hilaire M., Mellado W., Chao M. V., Filbin M. T. (2005) Neuron 46, 849–855 [DOI] [PubMed] [Google Scholar]
- 26. Chivatakarn O., Kaneko S., He Z., Tessier-Lavigne M., Giger R. J. (2007) J. Neurosci. 27, 7117–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim J. E., Liu B. P., Park J. H., Strittmatter S. M. (2004) Neuron 44, 439–451 [DOI] [PubMed] [Google Scholar]
- 28. Atwal J. K., Pinkston-Gosse J., Syken J., Stawicki S., Wu Y., Shatz C., Tessier-Lavigne M. (2008) Science 322, 967–970 [DOI] [PubMed] [Google Scholar]
- 29. Cafferty W. B., Strittmatter S. M. (2006) J. Neurosci. 26, 12242–12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toft-Hansen H., Babcock A. A., Millward J. M., Owens T. (2007) J. Neuroinflammation 4, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wells J. E., Rice T. K., Nuttall R. K., Edwards D. R., Zekki H., Rivest S., Yong V. W. (2003) J. Neurosci. 23, 10107–10115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pool M., Niino M., Rambaldi I., Robson K., Bar-Or A., Fournier A. E. (2009) Exp. Neurol. 217, 371–377 [DOI] [PubMed] [Google Scholar]
- 33. Karlén A., Karlsson T. E., Mattsson A., Lundströmer K., Codeluppi S., Pham T. M., Bäckman C. M., Ogren S. O., Aberg E., Hoffman A. F., Sherling M. A., Lupica C. R., Hoffer B. J., Spenger C., Josephson A., Brené S., Olson L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20476–20481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raiker S. J., Lee H., Baldwin K. T., Duan Y., Shrager P., Giger R. J. (2010) J. Neurosci. 30, 12432–12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reeves T. M., Prins M. L., Zhu J., Povlishock J. T., Phillips L. L. (2003) J. Neurosci. 23, 10182–10189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monea S., Jordan B. A., Srivastava S., DeSouza S., Ziff E. B. (2006) J. Neurosci. 26, 2300–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park J. H., Gimbel D. A., GrandPre T., Lee J. K., Kim J. E., Li W., Lee D. H., Strittmatter S. M. (2006) J. Neurosci. 26, 1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahmad M., Takino T., Miyamori H., Yoshizaki T., Furukawa M., Sato H. (2006) J. Biochem. 139, 517–526 [DOI] [PubMed] [Google Scholar]
- 39. Shimada T., Nakamura H., Ohuchi E., Fujii Y., Murakami Y., Sato H., Seiki M., Okada Y. (1999) Eur. J. Biochem. 262, 907–914 [DOI] [PubMed] [Google Scholar]
- 40. Endo K., Takino T., Miyamori H., Kinsen H., Yoshizaki T., Furukawa M., Sato H. (2003) J. Biol. Chem. 278, 40764–40770 [DOI] [PubMed] [Google Scholar]
- 41. Kajita M., Itoh Y., Chiba T., Mori H., Okada A., Kinoh H., Seiki M. (2001) J. Cell Biol. 153, 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshiyama Y., Sato H., Seiki M., Shinagawa A., Takahashi M., Yamada T. (1998) Acta Neuropathol. 96, 347–350 [DOI] [PubMed] [Google Scholar]
- 43. Hayashita-Kinoh H., Kinoh H., Okada A., Komori K., Itoh Y., Chiba T., Kajita M., Yana I., Seiki M. (2001) Cell Growth Differ. 12, 573–580 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.