Abstract
Multiple myeloma (MM) is a clonal plasma cell malignancy clinically characterized by osteolytic lesions, immunodeficiency, and renal disease. There are an estimated 750,000 people diagnosed with MM worldwide, with a median overall survival of 3 – 5 years. Besides chromosomal aberrations, translocations, and mutations in essential growth and tumor-suppressor genes, accumulating data strongly highlight the pathophysiologic role of the bone marrow (BM) microenvironment in MM pathogenesis. Based on this knowledge, several novel agents have been identified, and treatment options in MM have fundamentally changed during the last decade. Thalidomide, bortezomib, and lenalidomide have been incorporated into conventional cytotoxic and transplantation regimens, first in relapsed and refractory and now also in newly diagnosed MM. Despite these significant advances, there remains an urgent need for more efficacious and tolerable drugs. Indeed, a plethora of preclinical agents awaits translation from the bench to the bedside. This article reviews the scientific rationale of new therapy regimens and newly identified therapeutic agents – small molecules as well as therapeutic antibodies – that hold promise to further improve outcome in MM.
Keywords: bone marrow microenvironment, combination therapy, multiple myeloma
1. Background
Multiple myeloma (MM) is a clonal plasma cell malignancy with a highly heterogeneous genetic background, characterized by bone marrow (BM) plasmocytosis, production of monoclonal proteins, osteolytic bone lesions, renal disease, anemia, hypercalcemia, and immunodeficiency. Its development is a complex multistep process involving both early and late genetic changes in the tumor cell, as well as selective supportive conditions in the BM microenvironment. Specifically, MM cells disrupt homeostasis of stromal cell– stromal cell and stromal cell–extracellular matrix interactions and liquid factors (cytokines and growth factors). Tumor cells thereby induce direct as well as indirect signaling sequelae in the BM, which in turn supports MM cell proliferation, survival, migration, and drug resistance. MM bone disease, which occurs in 80% of MM patients, reflects an imbalance of osteoblast and osteoclast activity and is characterized by severe bone pain (25%), pathologic nonvertebral (12%) and vertebral (30%) fractures, and hypercalcemia (13%). These skeletal-related events (SRE) not only have a negative impact on patients’ quality of life, but also reduce their survival [1].
Although MM was first described in the mid 1850s, successful treatment was begun using a combination of melphalan and prednisone in the late 1960s and achieved a median survival of 3 – 4 years. Treatment regimens were further improved with the introduction of high-dose therapy with autologous stem cell transplantation (SCT). However, it was not until the late 1990s that a new era of MM treatment was initiated with the introduction of thalidomide (Thal), and later its analog lenalidomide (Len), as well as bortezomib. These compounds not only show activity in relapsed/refractory MM, but also demonstrate remarkable response rates when used in combination regimens to treat newly diagnosed transplant-eligible, as well as -ineligible, MM patients (Table 1) [2].
Table 1.
Chronology of advances in multiple myeloma treatment.
| Year | Advances in MM therapy |
|---|---|
| 1844 | Rhubarb and orange peel infusion Strengthening plaster, leeches and blisters |
| 1845 | Therapeutic bleeding, cupping, steel and quinine Camphor, warm baths, Dover’s powder |
| 1947 | Urethane |
| 1958, 1962, 1967 | Sarcolysin/melphalan (M)/L-phenylalanine mustard (L-PAM) |
| 1962, 1967 | Single-agent prednisone (P) |
| 1969 | Melphalan-prednisone (MP) |
| 1972 – 1977 | VBMCP, VMCP/VBAP, BCNU, ABCM, VCAP, VAD |
| 1982 – 1987 | Allogeneic SCT |
| 1983 – 1987 | Autologous SCT using different HDT protocols |
| 1995 | Biphosphonates |
| 1999 | Thalidomide (T) |
| 2006 | T/Dex – newly diagnosed MM |
| 2006 – 2007 | MPT |
| 2003 – 2004 | FDA: bortezomib (V) – relapsed and refractory MM (SUMMIT) and progressive MM after initial therapy (APEX) |
| 2006 | FDA: lenalidomide (Len) Dex – relapsed MM |
| 2007 | FDA: V/Doxil |
| 2008 | FDA: MPV – progressive MM (VISTA) |
Despite these dramatic advances disease relapse is inevitable, and MM remains incurable. Moreover, despite their emerging role in standard MM treatment regimens, Thal, Len, and bortezomib are associated with a variety of doselimiting adverse side effects. Moreover, although the novel drug combinations with conventional chemo therapy have resulted in better response rates, most of the studies show no benefit in terms of time to progression (TTP) or overall survival (OS). The identification of new therapeutic targets and derived more potent but less toxic agents is therefore urgently needed. Here we will describe the scientific rationale for some of the most important novel agents.
2. Medical need
The urgent need to improve patient survival and quality of life triggered the current evolution of MM therapies. Specifically, the identification of new therapeutic targets and the development of derived more potent and less toxic targeted agents is directed to decrease MM cell growth, survival, migration, and drug resistance and improve patient outcome. Indeed, the median survival of MM patients has been extended from 3 to 7 years, especially in patients aged < 50 years. Despite these significant advances, however, MM remains still incurable, and existing therapies can have dose-limiting adverse side effects (Table 2).
Table 2.
Medical needs.
| Existing therapy |
| Prevention/treatment of adverse side effects |
| Optimized therapeutic sequencing |
| Patient-specific tailoring of treatment regimens |
| Novel therapies |
| Identification of new therapeutic targets |
| Development of more potent, less toxic agents |
| Overall goal |
| Improvement of patient survival |
| Improvement of patient’s quality of life |
| Transition to chronic disease |
| Cure |
Myeloma was originally treated with therapeutic bleeding; application of leeches; steel and quinine; rhubarb pills and infusion of orange peel; strengthening plasters; alum; camphor; urethane; and stilbamidine. It was not until the discovery of sarcolysin (melphalan, L-phenylalanine mustard [L-PAM]) in 1958 that the first successful therapy in MM was reported by Blokhin and colleagues, and later by Bergsagel and co-workers (1962) and Hoogstraten and others (1967) [2]. The first classical treatment regimen in MM was defined with the introduction of melphalan plus prednisone (MP) [3]. Combination regimens based on the use of glucocorticoids and cytotoxic chemotherapeutics became the mainstay for nontransplantable MM patients. However, the prolonged use of alkylating agents such as melphalan is associated with an increased incidence of secondary malignancies, including myelodysplasia and acute leukaemia, and can also compromise subsequent collection of peripheral blood stem cells [4,5]. The introduction of high-dose therapy (HDT) with autologous SCT during the 1980s led again to a modest increase in OS of 3 – 5 years [6–10]; however, the proportion of patients proceeding to HDT and transplantation varies significantly (43 – 72%) dependent on age, co-morbidity, and failed stem cell mobilization [11]. Moreover, the majority of patients who undergo autologous SCT suffer from relapse.
Since the late 1990s, advances in our understanding of MM biology and the importance of the BM milieu have led to the identification of new therapeutic targets and agents. Thal, len, and bortezomib demosntrated significant anti-MM activity in preclinical models and have rapidly translated from bench to bedside, demonstrating efficacy first in relapsed/refractory MM and more recently in newly diagnosed disease. Ongoing studies are developing more potent and less toxic agents on the one hand and optimizing combination treatment regimens on the other. Parallel progress is ongoing to improve supportive therapies by delineating mechanisms causing MM bone disease and immune deficiency. Of note, these therapies may also have anti-MM activity.
3. Existing treatment
3.1 Treatment of relapsed and relapsed/refractory MM
Since the mid 1980s, pulsed high-dose Dex as well as combinations of various chemotherapeutic agents (e.g., VAD) have served as standard therapy for relapsed/refractory MM [12,13]. However, therapeutic options for relapsed/refractory MM have significantly changed with the introduction of Thal, Len, and bortezomib.
3.1.1 Thalidomide
Empirically tested as a single agent in relapsed/refractory MM patients, Thal achieved responses in approximately one-third of patients [14–16]. To enhance efficacy and reduce toxicity, Thal has been combined with a variety of agents including dexamethasone, cyclophosphamide, etoposide and liposomal doxorubicin (Dox) [17–23]. Despite high response rates, responses are transient and can be associated with significant toxicity.
3.1.2 Lenalidomide
Promising single-agent activity of Len was observed in Phase I trials even in MM refractory to Thal, without significant somnolence, constipation, or peripheral neuropathy [24,25]. These studies provided the framework for two Phase II trials, which confirmed its efficacy and lack of toxicity, as well as establishing the basis for adding Dex to enhance response. In 2006, the combination of Len plus high-dose Dex was approved by the FDA as therapy for relapsed and refractory MM based on two large, randomized, multicenter, double-blind, placebo-controlled Phase III trials which showed significantly increased response, progression-free survival (PFS) and OS of patients treated with Len/Dex versus Dex. However, in patients receiving Len/Dex, neutropenia and thromboembolic events (DVT and pulmonary emboli) occurred in 41 and 30% and 15 and 11%, respectively [26,27]. Therefore the use of antithrombotic prophylaxis is recommended. Other regimens that combine Len with other agents include: Len as well as DVd; Len plus adriamycin and Dex; and Len plus Dex and cyclophosphamide [28,29].
3.1.3 Bortezomib
Promising preclinical studies [30] and a Phase I trial [31] provided the framework for two multicenter clinical trials for relapsed/refractory MM patients (SUMMIT and the CREST trials), which demonstrated durable responses, including complete responses, associated with clinical benefit [32,33]. Based on these results, bortezomib was approved in 2003 by the FDA and EMEA (European Medicines Agency) for the treatment of relapsed/refractory MM. Subsequently, the international, randomized Phase III APEX trial compared bortezomib monotherapy versus highdose Dex in relapsed MM patients and revealed superior response rate and prolonged median OS [34,35]. Indeed, bortezomib is the only single agent to provide survival benefit and high overall response rate of 43% (partial and complete responses) in the setting of relapsed MM, leading to FDA approval of bortezomib in 2005. However, bortezomib has dose-limiting adverse side effects including peripheral neuropathy, gastrointestinal toxicity, and thrombocytopenia.
Again based on preclinical studies, a variety of combination therapies with bortezomib have been investigated. For exampple, bortezomib inhibits DNA damage repair and sensitizes or overcomes resistance to DNA-damaging agents [36]. The combination of bortezomib with pegylated liposomal doxorubicin (Doxil) is superior to bortezomib, and is now FDA-approved for the treatment of MM patients who have not previously received bortezomib and have had at least one prior line of anti-MM therapy [37]. Ongoing promising combinations to both enhance efficacy and reduce toxicity include bortezomib and heat shock protein inhibitors, AKT inhibitors or HDAC inhibitors.
3.2 Upfront MM therapy
The initial choice of current treatment options depends on whether or not the patient is eligible for SCT. Conventional MM therapies include melphalan and prednisone, Dex, as well as vincristine, adriamycin, Dex (VAD) and DVD regimens. Importantly, the incorporation of novel agents including Thal, Len, and bortezomib into initial MM therapy has great promise and has already markedly changed existing MM regimens. Indeed, high response rates of initial chemotherapeutic/novel agent combination regimens will allow for future studies to define the need of autologous SCT [38]. In addition to improved systemic therapies, supportive therapy with bisphosphonates has reduced bone complications, and several novel agents are under development.
3.2.1 Stem cell transplantation
Based on two large clinical trials (IMF90, MRC Myeloma VII) which demonstrated significant increases in response rates and durations of response, as well as OS, the standard of care for patients with newly diagnosed MM up to the age of 65 years is HDT followed by autologous SCT [9,10]. Fermand and colleagues confirmed the benefit of HDT with autologous SCT in terms of event-free survival (EFS) and treatment toxicity, but not OS [39]. Similarly, the US Intergroup trial demonstrated no benefit for HDT versus conventional therapy [40]. Moreover, HDT intensification significantly increased the complete response rate, but not PFS or OS, when given to MM patients who have responded to the initial chemotherapy [41].
Tandem autologous SCT [42,43], post-transplant maintenance strategies including immunotherapy, and most recently, integration of novel therapies, are under investigation to further improve response and OS rates. Attal and co-workers showed improvement in OS of patients receiving double versus single autologous SCT, especially in patients with less than very good partial response after the first transplantation (IFM94).
Myeloablative preparative regimens followed by allogeneic SCT in MM are generally limited to patients aged < 55 years [44–46]. Attempts to improve the efficacy of allografting (e.g., by exploiting alloimmune graft-versus-MM effects) and minimize high transplant-related mortality (e.g., graft-versus-host disease) include: T-cell depletion from allografts [47,48] and mini-allogeneic (nonmyeloablative/reduced-intensity) SCT [49]. Of note, autologous SCT followed by allografting with nonmyeloablative conditioning achieved dramatic reduction of transplant-related mortality with potent antitumor activity [50,51]. In contrast to the French IFM99 – 04 trial, which reported inferiority of autologous SCT followed by nonmyeloablative allogeneic SCT versus tandem autologous SCT [52], a study by Bruno and co-workers strongly indicated survival benefits of tandemautologous SCT: nonmyeloablative allogeneic transplant versus double autologous SCT [53]. Differences in these studies may be due to differences in conditioning and patient selection. Taken together, nonmyeloablative allografting regimens still remain investigational, but can be proposed to patients aged > 50 years with refractory MM who have HLA-matched donors.
3.2.2 Treatment for newly diagnosed MM patients eligible for transplant
First utilized as a single agent to treat relapsed/refractory MM [14], Thal was then combined with Dex and achieved increased response compared with Dex alone in newly diagnosed transplant candidates. Based on these data, Thal–Dex was FDA-approved as first-line therapy in 2006 [54,55]. Most MM centers have since then replaced the classical VAD induction therapy regimen for autologous SCT of newly diagnosed MM patients with regimens of oral Thal–Dex or Thal–Dex with liposomal Dox, respectively, dependent on the aggressiveness of the disease [54–57]. The combination of Thal with Dex, cisplatin, Dox, cyclophosphamide, and etoposide (DTPACE) represents another promising induction therapy, especially for patients with high-risk features [23]. Of note, Thal increases the very good partial response rate (VGPRR) before and after HDT in previously untreated MM [58]. To overcome the risk of Thal-induced DVT, prophylaxis with aspirin is recommended in patients with one additional risk factor (age, central catheter, diabetes, cardiac disease, immobilization, hyperviscosity), or full-dose warfarin or LMWH in patients with > 1 additional risk factor [59].
Besides Thal, recent studies have also indicated a role of several other novel agents in conditioning treatment regimens for newly diagnosed transplant eligible patients including: Len plus Dex [60,61]; bortezomib plus Dex [62]; and the combination of Len–Bortezomib–Dex [63]. To overcome Len-induced decreases of CD34 + SC collection, early harvesting after induction therapy with Len using cyclophosphamide/G-CSF mobilization is recommended [64]. Of note, bortezomib–Dex combination achieves higher extent and frequency of response, both before and after high-dose melphalan, with 60% of patients achieving a VGPR or better [65] and therefore not candidates for second autologous SCT.
3.2.3 Treatment for newly diagnosed MM patients not eligible for transplant
Due to reduced morbidity and significant PFS in elderly patients ineligible for HDT, the oral regimen of MP–Thal replaced the standard combination of alkylating agents in 2006 [66]. While some investigators report that this regimen fails to demonstrate survival advantage [67], others report significant survival advantage, even in elderly patients aged > 75 years [68]. By contrast, Thal in combination with Dex did not show superiority to MP [69]. A promising alternative to MP–Thal for elderly MM patients is the combination of MP and Len (MP-R) [70,71]. Another alternative in elderly untreated MM patients is the combination bortezomib– melphalan and prednisone (MP-V). Importantly, bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in Phase II and three trials [72]. Most excitingly, San Miguel and colleagues (VISTA trial) have recently reported significantly increased overall and extent of response, as well as PFS and OS, when newly diagnosed patients ineligible for transplant are treated with MP-V versus MP, providing the basis for its FDA approval to treat newly diagnosed MM. Of note, partial response or better and complete response were noted in 71 and 30% of patients, respectively, treated with MP-V versus 35 and 4% of patients, respectively, in the MP-treated cohort. This magnitude of response is remarkable, previously achievable only in the context of high-dose therapy. Importantly, this response extent and frequency advantage translated into prolonged duration of response and PFS, as well as decreased death rate. The side-effect profile was as expected and not significantly different in the two arms. MP-V was superior to MP in patients with renal compromise and across all International Staging System groups. Importantly, high-risk cytogenetics, including t(4;14) or t(14;16), 17p deletion, or 13q deletion, did not affect response, TTP, or survival achieved with MP-V [73].
3.3 Existing treatment for MM-induced bone disease
Common approaches to treat MM bone disease include radiotherapy, surgery and medical management. Radiotherapy is mainly used to control bone fracture-related pain. Surgery, vertebroplasty and kyphoplasty, in particular, aim to restore vertebral integrity and height as well as offering pain relief. The medical management of MM bone disease is currently based on the clinical use of bisphosphonates (BP) including zoledronic acid and pamidronate, pyrophosphate derivatives that bind with high affinity to hydroxyapatite crystals. Based on the significant reduction in the incidence of skeletal related events (hypercalcemia, compression fractures, pain and the necessity of radiotherapy/surgical intervention), pamidronate and zoledronate received FDA approval for the treatment of MM-associated osteolytic lesions in 1996 and 2002, respectively [74]. Patients on bisphosphonates must be monitored for renal toxicity and osteonecrosis of the jaw (ONJ), characterized by exposed bone of the mandible and/or maxilla, severe pain and high risk of local infection. New guidelines to avoid ONJ include maintenance of optimal dental health and recommendations for duration of BP treatment [75]. Novel agents such as RANK-Fc are under development to reduce MM bone disease.
4. Market review
In 2008, Celgene projected Len sales growth by 140% to $770 million, thereby increasing the company’s overall revenue to $1.4 billion. Analysts have projected 2008 sales of more than $2 billion. Since its initial approval in 2003 for the treatment of relapsed/refractory MM, Velcade has demonstrated efficacy in both relapsed (2005) and newly diagnosed (2008) MM. Millennium reported a total revenue of $528 million for 2007, and Takeda Pharmaceutical Co. bought Millennium this year for $8.8 billion. Several other companies are now evaluating further proteasome inhibitors for their preclinical and clinical activity.
Although Thal, Len, and Velcade, especially when given in combination regimens, have significantly changed MM treatment for both relapsed/refractory and newly diagnosed patients, disease relapse is inevitable. Consequently, there is a clear opportunity for additional agents to enter the MM market. For example, two next-generation proteasome inhibitors, NPI0052 [76] and carfilzomib [77], overcome bortezomib resistance in preclinical in vitro and in vivo studies. Phase I/II clinical trials of both are ongoing. NPI 0052 will examine whether more broad proteasome inhibition is useful as it inhibits chymotryptic, tryptic, and caspase-like activities of the proteasome, whereas bortezomib targets primarily chymotrypic activity. In contrast, carlfizomib targets the chymotrpytic proteasome activity more potently than does bortezomib.
5. Research goals
Although the introduction of Thal, Len, and bortezomib into MM treatment regimens has significantly improved PFS and OS, MM still remains an incurable disease. Moreover, treatment with Thal, Len, and bortezomib can be associated with significant adverse side effects. Therefore ongoing research aims to further advance our understanding of MM pathogenesis in order to identify more potent and less toxic therapeutic compounds. Specifically, current research efforts focus on: i) agents that target signaling events in tumor cell development; ii) agents that target cytokines, growth factors and their receptors; iii) agents that target signaling sequelae in MM cells triggered by cytokines and growth factors, as well as MM cell–BMSC interactions; iv) agents that target molecules at the cell membrane; v) agents that specifically target the tumor-supportive MM microenvironment, including BM angiogenesis [78]; and vi) agents that target mechanisms of MM bone disease. Clinical trials using novel agents in each category are ongoing [79–81].
In addition, we aim to improve existing therapy regimens by identifying optimal treatment sequencing and designing patient-specific treatment plans based on proteomic and genomic data. For example, several preclinical reports strongly indicate benefits of long-term, low-dose, more frequent administration of conventional chemotherapeutics (metronomic chemotherapy) in combination with antiangiogenic agents, such as anti-VEGF [78,82] to enhance efficacy and prevent development of drug resistance. Within MM, patients with t(4;14) express cell surface FGFR3 and have been targeted with specific FGFR3 inhibitors.
6. Scientific rationale
The development of MM is a complex multistep process involving both early and late genetic changes in the tumor cell, as well as selective supportive conditions by the BM microenvironment. Indeed, it is now well established that MM cell-induced disruption of the BM homeostasis between the highly organized cellular and extracellular compartments supports MM cell proliferation, survival, migration, and drug resistance via activation of various signaling (e.g., PI3K–Akt, JAK–Stat, Raf–MEK–MAPK, NFκB, and Wnt) pathways. As a result of advances in oncogenomics on the one hand and increased understanding of the role of the BM in the pathogenesis of MM on the other, a new treatment paradigm targeting the tumor cell and its BM microenvironment to overcome drug resistance and improve patient outcome has now been developed in MM [83].
6.1 Targeting molecules that are dysregulated by genetic and epigenetic changes within the MM cell clone
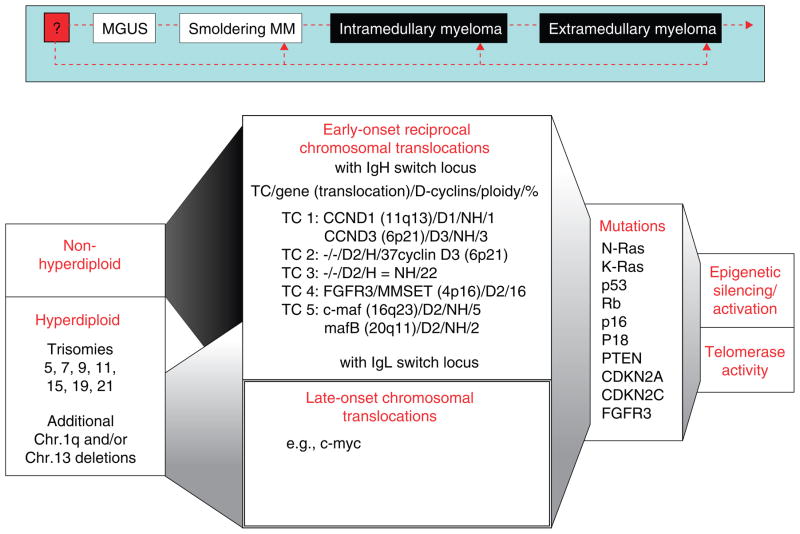
The MM cell clone is characterized by an increased frequency of complex heterogeneous genetic abnormalities and translocations that cause dysregulation of genes at breakpoints and include mutations in several proto-oncogenes and tumor suppressor genes. Dependent on chromosomal gains and losses, two cytogenetic patterns can be identified: a hyperdiploid pattern in the majority of cases; and more rarely, a non-hyperdiploid (pseudodiploid/hypodiploid/near-tetraploid) pattern with < 46 or > 74 chromosomes. Importantly, ploidy impacts prognosis, with longer OS in hyperdiploid patients versus non-hyperdiploid patients [83]. However, recent high-resolution genomic profiling of MM cells identified an additional subset of patients within the hyperdiploid group with additional gains on 1q and/or losses of chromosome 13, which has a worse prognosis than the non-hyperdiploid group. Indeed, a validated gene expression model of high-risk MM recently demonstrated that 30% of genes are located on chromosome 1 [84,85]. Early-onset reciprocal chromosomal translocations occur with significantly higher frequency in non-hyperdiploid versus hyperdiploid patients, and are linked to adverse prognosis; they most frequently involve the IgH switch locus 14q32.3, and less frequently, the IgL switch locus 2p12κ or 22q11λ. The five recurrent translocation partners commonly juxtaposed to the IgH enhancer locus elements include cyclin D1 t(11;14) (q13; q32) in 15 – 20%, cyclin D3 t(6;14)(p21;q32) in 5%, c-maf t(14;16) (q32;23) in 5 – 10%, FGFR3 and MMSET/WHSC1 t(4;14) (p16.3;q32) in 15%, and mafB t(14;20) (q32;q11) in 5%. Importantly, cyclin D is consistently dysregulated in both the hyperdiploid and the nonhyperdiploid groups, suggesting its key role in MM pathogenesis. Based on the five recurrent Ig translocations and cyclin D expression, a prognostic classification of five translocation and cyclin D (TC) groups was proposed, which also supported the cyclin D–Rb pathway as a potential therapeutic target in MM [86,87].
Signaling events triggered by these translocations remain elusive, with the exception of FGFR3 and c-Maf, and are under active investigation. Specifically, high expression of wild-type FGF3 receptor is observed in about two-thirds of patients with t(4;14)(p16;q32), while FGFR3-activating mutations are observed in a minority of cases. Dysregulation of FGFR3 confers poor prognosis [83]. It is likely that these patients, but not those with t(4;14)(p16;q32), who do not overexpress FGFR3 will benefit from FGFR3 blockade. Indeed, several studies have evaluated the preclinical efficacy of small-molecule FGFR3 inhibitors in MM cell lines carrying t(4;14)(p16;q32) including the specific inhibitors of FGF receptor tyrosine kinase SU5402 and SU10991, PD173074 (Pfizer, Ann Arbor, MI) and TKI258 (Novartis Oncology,) [88,89], as well as the inhibitory anti-FGFR3 antibody PRO-001 (ProChon Biotech Ltd, Rehovot, Israel) [90]. Target genes of c-maf include cyclin D2, β7-integrin, and CCR1, which mediate MM cell growth, adhesion to the BM stroma, and increased production of VEGF. Frequent overexpression of c-maf in MM makes it a potential new therapeutic target [91,92]. Translocations of c-Myc (8q24) are late secondary events and induce deregulation of c-Myc expression [93,94]. In addition to early- and late-onset translocations, many focal genetic lesions have been identified related to MM initiation and progression including: activating N- and K-Ras mutations; inactiva - ting mutations/deletions of tumor-suppressor genes p53, Rb/p18INK4c, p16INK4a and p18; as well as PTEN, cyclin-dependent kinase inhibitors CDKN2A and CDKN2C, and FGFR3-activating mutations [1,83].
Epigenetic silencing/activation is another mechanism that influences the initial phase of MM pathogenesis. Hydroxamic acid derivatives such as suberoylanilide hydroxamic acid (SAHA) and pyroxamide are potent HDAC inhibitors at micromolar concentrations, as are the sulfonamide anilides; whereas the cyclic peptides, such as FK22816 and the hybrid cyclic hydroxamic acid peptide analogs, are active at nanomolar concentrations. Remarkable preclinical anti-MM activity was observed using the hydroxamic acid peptide analogs NVP-LAQ824 (Novartis, East Hanover, NJ), Vorinostat (rINN) or SAHA (Merck, Germany) and LBH589/panobinostat (Novartis, East Hanover, NJ), ITF2357 (Italfarmaco, Milano, Italy), belinostat/PXD101 (CuraGen Corp.,), and MS-275 [95–100]; as well as romidepsin (depsipeptide/FK228/FR901228) [101] when used alone or in combination with conventional or novel therapies.
Clinical studies to evaluate the efficacy of PXD101 in patients with advanced MM (Phase II) and MS-275 (Phase I) in hematologic cancers including MM have now been completed. A clinical Phase I study with vorinostat in MM showed modest activity. Clinical Phase II trials using LBH589 or romidepsin, and a clinical Phase I trial with a combination therapy of LBH589 or SAHA and bortezomib in patients with relapsed/refractory MM are ongoing. Indeed, significant anti-MM activity has already been observed using HDAC inhibitors in combination with proteasome inhibitors. Interestingly, HDAC6 inhibitors (e.g., tubacin) inhibit autophagic clearance and lysosomal degradation of polyubiquitinated proteins within the aggresome. Importantly, preclinical synergistic cytotoxicity of tubacin and bortezomib in MM cells provides further rationale for clinical evaluation of this combination [102]. Taken together, the identification of genetically and epigenetically dysregulated molecules within the MM cell provides the preclinical rationale for novel single-agent and combination clinical trials (Figure 1; Table 3).
Figure 1.
MM signaling pathways.
Table 3.
Small-molecule inhibitors.
| Agent | Company | Target | Phase | Remark |
|---|---|---|---|---|
| Targeting MM cell development | ||||
| SU5402 | Sugen Inc. | FGFR3 | ||
| SU10991 | Sugen Inc. | |||
| PD173074 | Pfizer | |||
| CHIR-258 | Novartis/Chiron | I/II | p.o. | |
| SAHA (Vorinostat) | Merck | HDAC | I | i.v. |
| NVP-LAQ824 | Novartis | p.o. | ||
| Panobinostat (LBH589) | Novartis | II | p.o. | |
| Romidepsin (depsipeptide, FK228) | Gloucester Pharm. | I/II | i.v. | |
| ITF2357 | Italfarmaco | p.o. | ||
| PXD101 | CuraGen | II | i.v. | |
| Belinostat | TopoTarget AIS | II | p.o. | |
| MS-275/SNDX-275 | Bayer Schering, Syndax | I | p.o. | |
| Tubacin | Broad Institute | HDAC6 | ||
| GRN163 | Geron Corp. | Telomerase | I | i.v. |
| TMPyP4 | ||||
| Telomestatin | ||||
| Epothilone B (KOS-862) | Novartis | microtubuli | i.v. | |
| Flavopiridol/alvocidib | Sanofi-Aventis | CDKs | II | i.v. |
| PD 0332991 | Pfizer | I/II | p.o. | |
| Targeting the cell membrane | ||||
| Statins | Merck | HMG-CoA | I/II | p.o. |
| Lovastatin | ||||
| Fluvastatin | ||||
| Simvastatin | ||||
| Targeting cytokines, growth factors and their receptors | ||||
| Sant7 (soluble IL-6 receptor) | Sigma-Tau | IL6-R | i.v. | |
| PTK787/ZK222584 (Vatalanib) | Novartis | VEGF | II | p.o. |
| Pazopanib (GW786034B) | GlaxoSmithKline | II | p.o. | |
| Sorafenib (BAY43-9006/Nexavar) | Bayer & Onyx | I | p.o. | |
| ZD6474 | AstraZeneca | II | p.o. | |
| SU5416 | Sugen, Inc. | II | i.v. | |
| Sunitinib (SU011248) | Pfizer | II | p.o. | |
| PI-88 | Progen | s.c. | ||
| XL999 | Exelixis | II | i.v. | |
| Neovastat (AE-941) | Aeterna Zentaris | II | p.o. | |
| BIBF100 | Boehringer Ingelheim | FGF, VEGF | p.o. | |
| AMD3100/perixafor/JM3100 | Genzyme | SDF-1 | II/III | i.v. |
| Chir12-12 | Chiron | i.v. | ||
| Atacicept | ZymoGenetics | TACI-Ig | i.v. | |
| NVP-ADW742 | Novartis | IGF-1 | p.o. | |
| JB-1 | Chiron | i.v. | ||
| Targeting downstream signaling pathways | ||||
| R115777 (Tipifarnib/Zarnestra) | J&J | FT | I/II | p.o. |
| SCH66336 (Lonafarnib) | Schering-Plough | p.o. | ||
| Sorafenib (BAY43-9006/Nexavar) | Bayer | Raf-1 | I/II | p.o. |
| AZD6244 (ARRY-14886) | AstraZeneca | MEK1/2 | p.o. | |
| Perifosine (KRX-0401) | Keryx | Akt | I/II | p.o. |
| Rapamycin, P70S6 | Genentech | mTOR | p.o. | |
| CCI-779 (temsirolimus) | Wyeth | II | i.v. | |
| RAD001 (Everolimus) | Novartis | II | p.o. | |
| AP23573 (Deforolimus) | Ariad | II | i.v. | |
| Atiprimod | Callisto Pharm. | JAK/Stat | I/II | p.o. |
| Aplidin (plitidepsin) | PharmaMar | SAPK/JNK | II | i.v. |
| SCIO-469 | SCIOS Inc. | p38 | II | p.o. |
| PS-1145 | Takeda | NF-κB, IκK | p.o. | |
| BAY11-7082 | Biomol | |||
| RTA 402 (CDDO-Me) | Reata | I | p.o. | |
| AS602868 | Merck | p.o. | ||
| MLN120B | Takeda | |||
| ACHP | Bayer | |||
| PKF115-584 | Wnt | |||
| Enzastaurin | Eli Lilly | PKC | I/II | p.o. |
| Midostaurin (PKC412) | Novartis | p.o. | ||
| Tanespimycin (KOS-953) | Kosan Biosciences | HSP90 | III | p.o. |
| Geldanamycin (17-AAG) | Kosan Biosciences | II | i.v. | |
| Inducing MM cell apoptosis | ||||
| LBW242 | Novartis | Smac agonist | ||
| 2ME2 | EntreMed | SOD | II | p.o. |
| B3139 (Genasense)/oblimersen | Genta Incorp. | Bcl2 | III | i.v. |
| Targeting MM cells and the MM BM microenvironment | ||||
| Bortezomibortezomibrtezomib | Takedaortezomibrtezomib | Proteasome | i.v. | |
| NPI-0052 | Nereus | I | i.v. | |
| PR-171 | Cephalon | I | i.v. | |
| CEP-18770 | Cephalon | i.v. | ||
| Thalidomide | Celgene | Thal | p.o. | |
| Lenalidomide | Celgene | IMiD | p.o. | |
| Pomalidomide/actimid | Celgene | II | p.o. | |
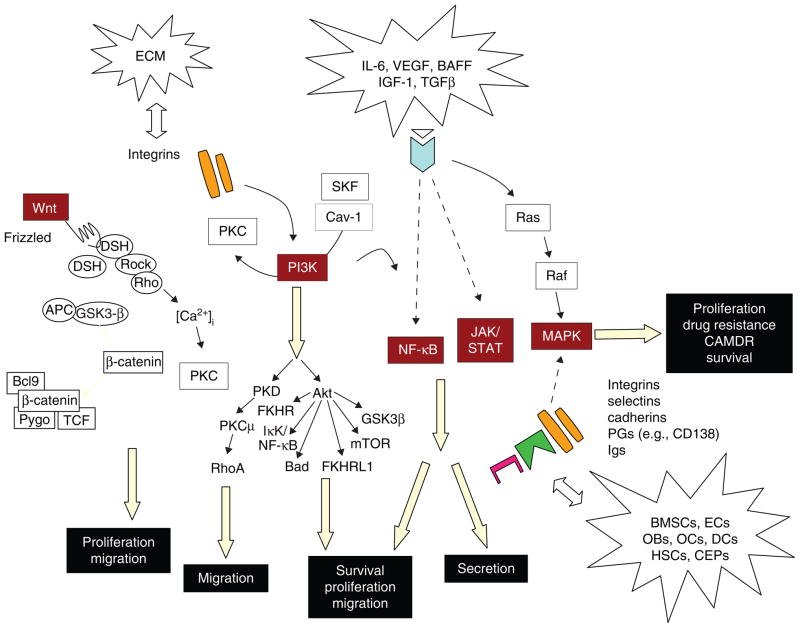
6.2 Targeting signaling pathways and signaling molecules within MM cells and the MM BM microenvironment
MM cell proliferation, survival, migration, and conventional drug resistance are regulated via different signaling cascades activated in the BM microenvironment including JAK–STAT, Ras–MEK–ERK, PI3K–Akt, NF-κB, Wnt–β-catenin, TGF-β–Smad, and Notch. Novel agents (Thal, immunomodulatory derivatives [IMiDs], proteasome inhibitors, and HSP90 inhibitors) are directed at molecular targets involved in these signaling cascades not only in MM cells, but also within the BM microenvironment (BM stromal cells, oeteoclasts, osteoblasts, and endothelial cells) (Figure 2; Tables 3 and 4).
Figure 2.
MM BM microenvironment.
Table 4.
Obstacles to effective antibody therapy.
| Immunogenicity of xenogeneic antibodies |
| Shedding of antigen into circulation |
| Heterogeneity of antigen on tumor surface |
| Limited numbers of effector cells at tumor |
| Immunosuppressive tumor microenvironment |
| Disordered vasculature in tumors |
| Expression of CD55, CD59, CD46 that interfere with CDC and allow tumor cells to escape complement attack |
6.2.1 Role of the BM microenvironment in signal transduction in MM cells
The BM microenvironment plays a crucial role in MM cell proliferation, survival, drug resistance, and migration mediated via many signaling pathways (phosphatidylinositide- 3 kinase [PI3K]–Akt; NF-κB; Ras–Raf–MAPK kinase [MEK]–extracellular signal-related kinase (ERK); Janus kinase [JAK]2–signal transducers and activators of transcription (STAT)3; Wnt–β-catenin; Notch; p38MAPK; and TGF-β– Smad). These signaling cascades are predominantly activated via soluble factors (cytokines, chemokines) including IL-6, IGF-1, VEGF, B-cell activating factor (BAFF), fibroblast growth factor (FGF), stromal cell-derived factor 1α (SDF-1α), TNF-α, and macrophage inflammatory protein-1α (MIP-1α). In addition, adherence of tumor cells to cellular components including BM stromal cells (SCs), osteoblasts, osteoclasts, and endothelial cells also activate these signaling pathways [83,103]. Among the cellular components, BMSCs are primarily implicated in cytokine- and cell adhesion-mediated signal transduction in MM cells. In addition to NF-κB, several signaling pathways are involved in this response: PI3K–Akt pathway; Ras–Raf–MEK–ERK pathway; JAK2–STAT3 pathway; Wnt–β-catenin pathway; and Notch pathway. These signaling pathways promote MM cell growth, survival, and migration, contributing to MM progression and drug resistance [30]. Moreover, many growth factors secreted by both MM and BMSCs trigger osteoclastogenesis (IL-6, IL-1, VEGF, SDF-1α, MIP-1α) and angiogenesis (VEGF). Importantly, genetic abnormalities in MM cells can modulate the ability of MM cells to interact with their BM milieu. For example, MM cells with t(14;16) translocation overexpress the transcription factor MAF [104,105], which not only transactivates the cyclin D2 promoter, but also upregulates β7-integrin expression and thereby enhances MM cell adhesion to BMSCs [92].
Recent studies have identified a small subpopulation (~ 5%) of high clonogenic postgerminal B cell-like CD138−/CD34−/CD19+ cells within CD138+/CD19− MM cell lines. These CD138− cells initiated MM following transplantation into non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice [106,107]. Expansion of these cells is mediated via the hedgehog (Hh) pathway. Conversely, inhibition of the Hh pathway using cyclopamine blocks clonal cell expansion and triggers terminal differentiation. In contrast, no effects of Hh inhibitors were observed on malignant MM cell growth [107]. Of clinical importance, the CD138− population is relatively chemoresistant, probably due to high drug efflux capacity and intracellular drug detoxification activity. Specifically, resistance has been observed to Len, bortezomib, Dex, and cyclophosphamide [108]. In summary, these data suggest that the existence of a proliferating self-renewing compartment (MM ‘stem cells’) indicates a potential therapeutic role for targeting molecules within the Hh pathway. Moreover, these studies recommend that novel agents should be evaluated for their potential to inhibit expansion of this cell subpopulation.
6.2.2 Ras–raf–MEK–ERK1/2 signaling
ERK1/2 (p42/44 MAPK) are a widely conserved family of serine/threonine protein kinases mediating cellular programs such as cell proliferation, differentiation, motility, and death. Upon stimulation, a sequential three-part protein kinase cascade is initiated consisting of MAP kinase kinase kinase (MAPKKK, Raf), MAP kinase kinase (MAPKK, MEK), and MAP kinase, ERK). The ERK1/2 signaling pathway is activated in response to a diverse range of extracellular stimuli including mitogens, cytokines, and chemokines. In MM cells, constitutive ERK1/2 activation can be further enhanced by many cytokines and/or chemokines in the BM microenvironment including IL-6, IGF-1, VEGF, BAFF, SDF1α, and Wnt [83,109].
Mutations in upstream kinases K-ras and N-ras contribute to constitutive activation of ERK, which is associated with progression of the disease [110–113]. Liu and colleagues reported that the mean tumor burden and median survival for patients with mutations of N-ras was indistinguishable from patients with no ras mutations; on the other hand, patients with K-ras mutations had a significantly higher mean BM tumor burden at diagnosis than patients with no ras mutations. In contrast, Martin and co-workers also reported the absence of mutations within either codon 12 of K-ras or codon 61 of N-ras in MGUS or MM, suggesting that Ras mutations do not play a significant role in the pathogenesis of MM [113].
Farnesyltransferase transfers the farnesyl group from farnesyl diphosphate to the CAAX motif of Ras, thereby facilitating its attachment to the inner plasma cell membrane and related signal transduction [114]. Inhibition of farnesylation is therefore a strategy to block Ras activity, and several farnesyltransferase inhibitors (FTI) inhibit tumor cell growth both in vitro and in vivo. In MM, two FTIs (FTI-277, R115777) have antitumor activities: FTI-277 inhibits growth and induces apoptosis even in drug-resistant MM tumor cells [115]. Although R115777 also induces apoptosis, its effects depend on the status of Ras mutation in cloned MM cells, but not on the status of N-Ras mutation in fresh MM cells [116]. Moreover, R115777 induces apoptosis in a Ras-independent fashion via multiple intrinsic pathways [117]. It shows clinical activity in patients with CML and MF; however, its clinical benefit in MM patients is still unclear [118]. Recently, we have shown that the MEK1/2 inhibitor AZD6244 shows remarkable anti-MM activities in vitro and in vivo in a xenograft mouse model of human MM. Specifically, AZD664 blocks phosphorylation of ERK triggered by IL-6, IGF-1, and CD40 with associated inhibition of MM cell growth, as well as inhibiting RANKLand M-CSF-induced differentiation of OCs from peripheral blood mononuclear cells. A derived clinical trial of AZD 2664 in MM will determine its efficacy in abrogating both tumor cell growth and bone disease [119].
6.2.3 JAK2/STAT3 signaling
JAK2 is a non-receptor tyrosine kinase (RTK) that is highly expressed and associated with gp130 (IL-6R β-subunit) in MM cells. After IL-6 binding to gp80 (IL-6R α-subunit), JAK2 is activated and induces phosphorylation of tyrosine residues of gp130 [120–123], followed by interaction and activation of STAT3, regulated by phosphorylation at tyrosine 705 and triggering dimerization and nuclear translocation of STAT3 [124]. The biologic sequelae of activation of the JAK2–STAT3 pathway in MM cells is to maintain survival by regulating expression of downstream antiapoptotic proteins, including Bcl-XL and Mcl-1. Many cytokines and chemokines trigger multiple signaling cascades; however, the JAK2–STAT3 pathway can be activated only by gp130 family member cytokines in MM cells. These cytokines include IL-6, leukemia inhibitory factor (LIF), and oncostatin M (OSM). Among these cytokines, IL-6 is the major trigger to activate JAK2–STAT3 in the BM milieu [84]. Multiple reports support an autocrine IL-6-mediated growth mechanism in MM, since some MM cells and derived cell lines both produce and respond to IL-6 in vitro [125]. Importantly, IL-6 in the BM milieu is predominantly secreted by BMSCs, and its tanscription and secretion in BMSCs is regulated by NF-κB [126]. IL-6 secretion is further augmented both by binding of MM cells to BMSCs, and by secretion of cytokines (ie, TGF-β, VEGF) within the BM microenvironment [126–131].
The JAK2–STAT3 pathway is therefore a promising therapeutic target in MM. Indeed, recent studies demonstrated significant anti-MM activities associated with STAT3 inhibition induced by azaspirane [132], pyridone-6 [133], ursidic acid [134], capsaicin [135], and the farnesoid X receptor antagonist guggulsterone [136].
6.2.4 Phosphoinositide 3-kinase (PI3K), Akt, and PKC signaling
Growth factors and hormones trigger PI3K activation and mediate cell growth, cell cycle entry, cell migration, and cell survival [137]. PI3K is composed of p85 (α, β) regulatory subunit and p110 (α, β, γ, δ) catalytic subunit; however, the biologic significance of each subunit in MM cells remains unclear. Phosphoinositide-dependent protein kinase 1 (PDK1) is a downstream kinase of PI3K that plays a crucial role in activating both Akt (PKB) and PKC isoenzymes p70 S6 kinase and RSK [138,139]. PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a major negative regulator of the PI3K–Akt signaling pathway [140]; however, mutation of PTEN is not frequent in MM cells.
In MM, PI3K–Akt signaling and its downstream target proteins can be activated by many cytokines including IL-6, IGF-1, VEGF, SDF1α, and BAFF [83,141]. This cascade regulates growth via downstream mammalian target of rapamycin (mTOR) and p70 S6 kinase pathway, and modulates cell cycle and proliferation both directly via activity on the CDK inhibitors p21WAF1/Cip1 and p27 Kip1, as well as indirectly by affecting the levels of p53 and cyclin D1. Akt (PKB) is also a major downstream mediator of cell survival directly by inhibiting proapoptotic protein Bad and forkhead family of transcription factors (FKHR), and indirectly by modulating two major regulators of cell death [137,142–147]. We have shown that IL-6 overcomes dexamethasone-induced apoptosis via activation of Akt in MM cells [148,149]; therefore PI3k/Akt signaling is also a promising therapeutic target in MM.
Recently, anti-MM activity of perifosine, a synthetic novel alkylphospholipid that potently inhibits Akt, has been reported. Perifosine inhibits cytokine- and BMSC-induced Akt activation of PDK1 without inhibiting PDK1 phosphorylation, and is associated with significant cytotoxicity in both MM cell lines and patient MM cells resistant to conventional therapeutic agents. Perifosine significantly downregulates expression of β-catenin and its downstream molecule, surviving, and induces MM cell cytotoxicity via caspase activation. Anti-MM activities of perifosine in a human MM cell xenograft mouse model are also remarkable [119,150–152], and Phase I/II clinical trials of perifosine with bortezomib are ongoing. As described above, mTOR is one of the major downstream molecules of Akt in MM, and previous studies have shown that inhibition of mTOR by rapamycin and other inhibitors (CCI-779), either alone or in combination with other agents (Dex, Len, Hsp90 inhibitor), results in significant anti-MM activities [153–156].
Members of the intracellular PKC family of serine/threonine kinases are also potential therapeutic targets in MM. Specifically, PKC isoform expression has been reported in several MM cell lines. Functionally, PKCs are: i) involved in MM cell apoptosis; ii) required for VEGF- and Wntinduced MM cell migration; and iii) associated with the control of IL-6 receptor-α shedding. Importantly, the unique gene signature of MM patients with the adverse prognostic t(4;14)(p16;q32) translocation shows marked upregulation of PKCβ [157]. Preclinical and clinical studies using the macrocyclic bisindolylmaleimide enzastaurin or the N -benzylstaurosporine midostaurin/PKC412 to target PKC pathways demonstrate promising activity in a variety of tumors including MM and Waldenstrom’s macro globulinemia (WM). Interestingly, the anti-MM activity of enzastaurin is mediated downstream of PKC via β-catenin upregulation by preventing phosphorylation required for its proteasomal degradation. In turn, upregulated β-catenin induces both early ER stress signaling via eIF2α, CHOP, and p21, leading to immediate growth inhibition; as well as later c-Jun/p73 induction, leading to MM cell death [158].
6.2.5 NF-κB signaling
NF-κB, a member of Rel family proteins including RelA (p65), RelB, c-Rel, NF-κB1 (p50) and NF-κB2 (p52), regulates protein expression mediating cell cycle/proliferation, antiapoptosis, and cytokine secretion in cancer. Recent studies have defined two different cascades mediating NF-κB activity: the canonical (classic) and non-canonical (alternative) pathways. Canonical NF-κB is typically a heterodimer composed of p50 and p65 subunits and is constitutively present in the cytosol and nucleus. In the cytosol, NF-κB is inactivated by its association with I κB family inhibitors [159]. I κBα therefore has a crucial role in regulating NF-κB activation. For example, various growth- and/or antiapoptosis-promoting cytokines trigger IκB protein phosphorylation by IκB kinases (IKKs), followed by its proteasomal degradation. These events allow translocation of NF-κB into the nucleus, where it binds to specific DNA sequences in the promoters of target genes, thereby stimulating transcription. The importance of the non-canonical pathway, predominantly mediated by p52/RelB, has been demonstrated in MM. Indeed, recent studies have defined genetic abnormalities associated with NF-κB activation in MM [160,161], confirming the biologic significance of non-canonical NF-κB signaling in MM pathogenesis. Specifically, the non-canonical NF-κB pathway is constitutively activated in MM cells with inactivation of TRAF3 [161], suggesting that the non-canonical pathway represents a novel therapeutic target.
Although the precise role of NF-κB activation in pathogenesis of MM has not been fully characterized, we have previously shown that MM cell adhesion to BMSCs induces NF-κB-dependent upregulation of IL-6 [126,162]. In addition, TNF-α secreted by BMSCs upregulates intracellular adhesion molecule-1 (CD54) and vascular cell adhesion molecule-1 (CD106) expression on both MM cells and BMSCs via NF-κB, thereby increasing MM cell to BMSC binding and related IL-6 secretion [131]. Since IL-6 is a major growth and survival factor in MM cells [163,164], blockade of NF-κB signaling represents a novel therapeutic strategy in MM.
We and others have shown that a variety of novel agents with both preclinical and early clinical anti-MM activity, including the proteasome inhibitor bortezomib [30], Thal and IMiDs [165], histone deacetylase inhibitors [93], TGF-β inhibitor [166], lysophosphatidic acid acyltransferase-β inhibitor [167], and 1′-acetoxychavicol acetate [168], inhibit both NF-κB activation and MM cell growth. Importantly, we have also shown that the small-molecule IKK-β inhibitors PS-1145 and MLN120B block MM cell growth in the context of BMSCs, associated with downregulation of IL-6 secretion from BMSCs [162,169]. MLN120B also inhibits MM cell growth in a clinically relevant SCID-hu mouse model, suggesting the potential utility of novel therapeutics targeting IKK-β in MM.
6.2.6 Wnt–β-catenin signaling
Wnts comprise a family of secreted proteins that interact with receptors consisting of a Frizzled (Fz) family member, alone or complexed with LDL receptor-related proteins (LRP5/6). Wnt signaling regulates various developmental processes and can lead to malignant tumor formation. Intracellularly, the Wnt signaling cascade blocks phosphorylation and degradation of β-catenin by proteasomes, thereby leading to accumulation of β-catenin in the cytoplasm [170]. In MM, the canonical Wnt signaling pathway is activated following treatment with Wnt-3a, associated with accumulation of β-catenin. Wnt-3a treatment also led to significant morphological changes in MM cells, accompanied by rearrangement of the actin cytoskeleton.
The biologic significance of Wnt/β-catenin signaling in MM has not been totally defined. Derksen and colleagues demonstrated that MM cells overexpress β-catenin, including its N-terminally unphosphorylated form, consistent with active β-catenin/T cell factor-mediated transcription. Accumulation and nuclear localization of β-catenin and/or increased cell proliferation resulted from stimulation of Wnt signaling via Wnt-3a, LiCl, or the constitutively active mutant of β-catenin [171]. Moreover, we have shown that F115 – 584, which disrupts the interaction of the transcriptionally active β-catenin–TCF protein complex, both blocks expression of Wnt target genes and induces cytotoxicity in patient MM cells and MM cell lines, without toxicity in normal plasma cells.
In xenograft models of human MM, PKF115 – 584 inhibits tumor growth and prolongs survival [172], suggesting that Wnt–β-catenin represents a therapeutic target in the treatment of MM. Interestingly, Qiang and co-workers demonstrated that Wnt-mediated migration is associated with the Wnt–RhoA pathway, but does not require signaling through β-catenin [173]. Moreover, Qiang and others reported that treatment of human MM in SCID-hu mice with recombinant Wnt3a attenuates MM cell growth, suggesting that Wnt3a signaling within the BM inhibits tumor growth [174]. Importantly, MM cells in BM-biopsy specimens contained detectable dickkopf 1 (DKK1), a negative regulator of Wnt signaling cascade and target of the β-catenin–TCF pathway. Moreover, elevated DKK1 levels in BM plasma and peripheral blood from patients with MM correlated with the DKK1 gene-expression patterns associated with focal bone lesions [175]. However, a recent study has shown that MM cells do not inhibit canonical Wnt signaling in the human BM microenvironment [176].
6.3 Focus on antibody-based therapies
In contrast to small-molecule inhibitors, therapeutic antibodies offer the potential not only to target tumor cells, but also to spare normal tissues and directly activate an immune response against tumor cells. However, they may also increase the risk of adverse immune reactions. The therapeutic success of the CD20-targeting antibody rituximab in non-Hodgkin’s lymphoma expanded the interest in unconjugated Abs for cancer therapy, including MM (Figure 3). However, to date, no mAb-based therapy has been approved for MM treatment. Indeed, studies in early 2000 showed only minimal activity of rituximab and anti-CD38 antibodies in MM. Despite these disappointing beginnings, > 10 potential mAb candidates targeting MM cells have entered clinical development in recent years (Table 4). Specifically, these mAbs directed against MM cell surface antigens (CD40, HM1.24, IGF-1R, CD56, CS1, CD138, CD74, IL-6R, CD38, TRAIL-R1) are being investigated as potential new therapies in MM. Therapeutic antibodies with great promise include a humanized anti-CD40 antibody [177], which both alone and with Len enhances antibody-dependent cellular cytotoxicity (ADCC) [178]; the humanized monoclonal antibody HuLuc63, which targets CS-1 and mediates selective ADCC in vitro [179]; as well as anti-FGFR3 antibody [87]. Moreover, mAb-based targeted therapies can also inhibit growth and survival advantages provided by cytokines and growth factors as well as the interaction of the MM cell with the BM microenvironment. For example, mAbs targeting IL-6, osteoprotegerin (OPG), DKK1, VEGF, and BAFF are among those under clinical evaluation (Table 5).
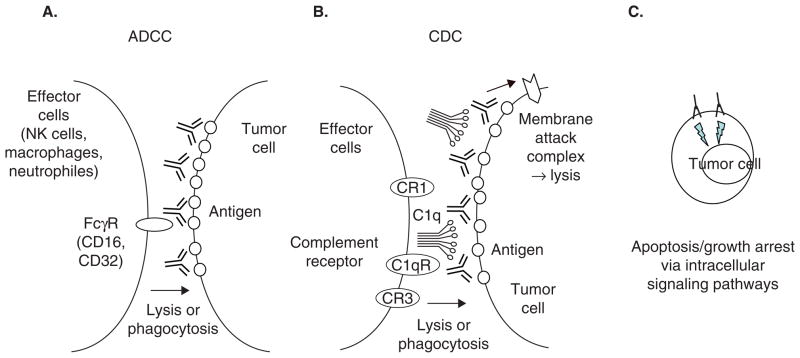
Figure 3. Mechanisms of action associated with unconjugated antibodies.
Chimeric/humanized or fully human IgG1 mAb induces antitumor activity mainly by: A. ADCC; B. CDC, through interaction between tumor cells and target tumor cells. Some Abs could directly induce apoptosis upon binding to cell surface antigen on tumor cells (C).
Table 5.
Antigens targeted by antibodies in MM in different stages of preclinical/clinical development.
| Target | Brand name | Company/Sponsor | Type of mAb (conjugate) | Indication | Literature | Phase | Remarks |
|---|---|---|---|---|---|---|---|
| CD138 | B-B4-DM1 | ImmunoGen | The maytansinoid immunoconjugate mouse IgG1 mAb B-B4 | Decreased growth and survival of MM cells in cell culture and in mice models | Tassone 2004, 2005 | Preclinical | |
| CD38 | MOR202 | Morphosys | Human | Kills CD38-expressing MM cells by ADCC and in a SCID-mouse xenograft model | Tesar 2007 | Preclinical | |
| HLA-DR | 1D09C3 | GPC Biotech, AG | Human | Fully human anti-HLA-DR antibody induces cell death; its anti-MM activity is enhanced by IFN-gamma | Carlo-Stella 2007 | Preclinical | |
| Kininogen | C11C1 | Temple University School of Medicine | Mouse | Monoclonal antibody to kininogen may improve the efficacy of conventional MM treatment with minimal side effects | Sainz 2006 | Preclinical | |
| HLA class I | 2D7-DB | Chugai Pharmaceutical Co. Ltd | Converted from mouse IgG2b | Single-chain Fv diabody | Sekimoto 2007 | Preclinical | |
| Beta2-microglobulin | Anti-B2M mAbs | MD Anderson Cancer Center | Mouse | Strong apoptotic effect on myeloma cells supports potential use as therapeutic agents | Yang 2006 | Preclinical | |
| CD38 | MOR202 | MorphoSys AG | Human | mAb induce ADCC and CDC against MM cells and block MM cell growth in vivo | Stevenson 2006 | Preclinical | |
| CD32B | MGA321 | MacroGenics | Humanized | mAb against low-affinity Fc receptor for use in the treatment of hematologic cancers by cell depleting, functional and adjuvant strategies | Rankin 2006 | Preclinical | |
| FGFR3 | PRO-001 | Prochon Biotech Ltd | Human | Anti-FGFR3 neutralizing antibody that is cytotoxic to t(4;14)-positive MM cells | Trudel 2006 | Preclinical | |
| mAb, ICAM-1 | UV3 | Abiogen | Mouse | UV3 significantly prolonged the survival of mice with either early or advanced stages of disease | Coleman 2006 | Preclinical | |
| BLyS | BLyS/rGel | Targa Therapeutics | Fusion protein of an antibody tethered to a toxin | Potential treatment of myeloma and autoimmune diseases | Nimmanapalli 2007 | Preclinical | |
| DKK | Anti-DKK1 | UAMS | Mouse | DKK1 neutralizing antibodies reduce osteolytic bone resorption, increase bone formation, and help control myeloma growth in lab mice | Yaccoby 2007 | Preclinical | |
| CD20 | Rituxan | NCI | Chimeric | High-dose cyclophosphamide in combination with rituximab in patients with primary refractory, high-risk, or relapsed myeloma; being studied for the treatment of peripheral neuropathy in patients with MGUS | NCT00258206 | II (ongoing) | The small phase II studies have evaluated rituximab in myeloma, each with conflicting results. i) Treon et al. conducted a Phase II study of 19 patients with previously treated myeloma, treated with rituximab. At 3 months, 32% had partial response or stable disease; of these, all had CD20+ bone marrow plasma cells. Median time to treatment failure was 5.5 months, ii) Zojer et al. performed a Phase II study of 10 patients (1 patient had CD20 expression on 10% of bone marrow plasma cells, another patient had CD20+ on 50% of marrow plasma cells). At 6 months, no patients had an objective response; 2 patients had stable disease, and 5 withdraw early for progressive disease. Rituximab treatment resulted in decrease in circulating B cells and IgM levels, but no effect on bone marrow plasma cells, suggesting an effect of rituximab on normal B cells but not on MM cells, iii) Moreau et al. conducted a Phase II study of 14 patients with myeloma (7 stage I never treated, 7 stage III previously treated), in which 33% of bone marrow plasma cells were CD20+, treatd with rituximab. One patient had response sustained to 18 months of rituximab treatment. Five patients had stable disease at follow-ups of 3 – 12 months. Three patients had stable disease at 3 months but progressed at 10 – 15 months. Five patients had no response despite partial clearance of CD20+ plasma cells in the bone marrow One patient had partial response sustained to 18 months of rituximab treatment. Five patients had stable disease at follow-ups of 3 – 12 months |
| CD20 | Zevalin (yttrium Y 90 ibritumomab tiuxetan) | NCI | Mouse IgG 1 antibody ibritumomab in conjunction with the chelator tiuxetan, to which a radioactive isotope yttrium-90 is added | Studying the side effects and best dose of yttrium Y 90 ibritumomab tiuxetan when given together with rituximab, melphalan, and autologous peripheral stem cell transplant in treating patients with previously treated MM | NCT00477815 | I (ongoing) | |
| CD20 | Bexxar (131I tositumomab) | GlaxoSmith Kline | Radioactive iodine 131 attaching to anti-CD20; mu IgG2a (131I) | Consolidation treatment with iodine I131 tositumomab | NCT00135200 | II (ongoing) | |
| CD40 | SGN-40 (a weak agonist) | Seatle Genetics, Genentech Inc. | Humanized | Multi-dose study of SGN-40, a weak agonist humanized IgG1 mAb, in patients with refractory or recurrent MM; SGN-40, lenalidomide (Revlimid®), and dexamethasone in MM patients is ongoing | Tai 2004, 2006; Hussein 2005 | Ib (ongoing). SGN-40 Phase lb open-label single-arm trial in combination with bortezomib was also recently initiated (2008) | |
| CD40 | HCD122 (an antagonist) | Novartis | Human | To determine the highest tolerated dose, safety and activity of HCD122 in relapsed MM patients | Tai 2005; Bensinger 2006 | I (ongoing) | |
| HM1.24 | AHM (humanized HM1.24) | Chugai Pharmaceutical Co. Ltd | Humanized | Induced ADCC that is enhanced by cytokine stimulation of effector cells | Ozaki 1999 | I | |
| MUC-1 (CD227) | BrevaRex®-AR20.5 | Altarex | Murine | BrevaRex®-AR20.5 mAb can elicit cancer-killing B and T cell immune responses to the target antigen, MUC1. This antibody is specific for a particular soluble and tumor-cell bound form of MUC1 that is preferentially expressed by myeloma cells | I/II (ongoing) | Treon et al. () reported that MUC-1 is expressed on myeloma cells. In a separate study, brevaRex mAb was shown to induce B- and T-cell antineoplastic responses to muc-1 in a Phase I study of 17 patients with advanced solid tumors (). A Phase II study of Brevarex, and a Phase I/II study of NM-3 (another agent that downregulates MUC-1 expression), are underway | |
| IGF-1R | CP-751,871 | Pfizer Inc. | Human IgG2 | CP-751,871 blocks ligand binding (IGF-1, IGF-2) and induces IGF-1R downregulation by promoting its internalization and degradation | Cohen 2005; Lacy 2008 | II (ongoing) | |
| CD56 (NCAM) | BB-10901 (huN901-DM1) | ImmunoGen, Vernalis | Humanized IgG1- (maytansine DM1) | Relapsed and relapsed refractory CD56-positive MM | Chanan-Khan 2006; NCT00346255 | I (ongoing) | |
| CS1 | Elotuzumab (HuLuc63) | PDL Biopharma, Inc. | Human | Elotuzumab triggers ADCC against myeloma cells and blocks myeloma growth in mice | Bensinger 2007; NCT00425347, NCT00726869, NCT00726869 | I/II (ongoing multicenter, open-label, dose-escalation study of HuLuc63, alone, with bortezomib/lenalidomide/dexamethasone, in MM) | |
| OPG (osteoprotegerin) | Denosumab (AMG162) | Amgen | Human | For the treatment of patients with relapsed or plateau-phase MM | Body 2006 | II (ongoing) | Body et al. () studied 29 patients with breast cancer and 25 patients with myeloma with bone lesions, who were treated with varying doses of denosumab versus pamidronate. Serum and urine markers of bone turnover decreased with both treatments. Denosumab conferred a prolonged, dose-dependent duration of 84 days at higher doses |
| VEGF | Avastin (becacizumab) | Genentech | Humanized | Given in combination with Velcade to patients with relapsed/refractory MM | Hoyer 2007 | II (ongoing) | Somlo et al. studied 12 patients with stages I-III refractory myeloma who were randomized to bevacizumab ± thalidomide. For bevacizumab monotherapy, median time to progression was 2 (range 1 – 4) months. For bevacizumab and thalidomide, median progression-free survival was 9 (range 6 – 30+) months. Study was closed because of slow accrual |
| CD52 | Campath-1H (alemtuzumab) | NCI and Fred Hutchinson Cancer Research Institute | Humanized | Used before allo transplant to prevent GVHD | NCT00040846 | II (ongoing) | A flow cytometry study by Kumar et al. reported that 53% of plasma cells isolated from patients with myeloma were positive for CD52, predominantly in the cd38_cd45+ plasma cell fraction A separate flow cytometry study by Westermann et al. reported only a small fraction of CD52+ cells among the CD38+CD45+ myeloma plasma cell population. Another study by Rawstron et al. found only low levels of CD52 expression on myeloma plasma cells. A study by Kroger et al. compared antithymocyte globulin (ATG) vs alemtuzumab in 73 patients with myeloma treated with reduced conditioning with melphalanlfludarabine, followed by allogeneic stem cell transplant; alemtuzumab, compared with ATG, had similar 2-year overall survival (54 vs 45%) and progression-free survival (30 vs 36%), lower incidence of GVHD, but higher probability of relapse (hazard ratio 2.37) |
| CD74 | Milatuzumab (hLL1 or IMMU-115) | Immunomedics, Inc. Morris Plains, NJ | Humanized; doxorubicin-conjugated | Humanized mAb conjugated with doxorubicin and targeted to CD74 to induce MM cell dealth. It caused growth inhibition and induction of apoptosis when cross-linked with an antihuman immunoglobulin G (IgG) second antibody. This mAb did not induce ADCC or CDC | Sapra 2005; NCT00421525 | I/II (ongoing, for patients with MM who have failed at least two prior standard systemic anti-myeloma treatment regimens) | |
| IL-6 | CNTO 328 | Centocor | Chimerized mouse (human-mouse) | Given in combination with Velcade to patients with relapsed/refractory MM | Trikha 2003; NCT00412321; NCT00401843 | I (ongoing trial NCT00412321 of CNTO328 in non-Hodgkin’s lymphoma, or Castleman’s desase, II (ongoing trial NCT00401843 of bortezomib ± CNTO 328 in relapsed or refractory myeloma) | |
| IL-6R | MRA (Tocilizumab) | Roche Pharmaceuticals | Humanized anti-IL-6R | Specifically blocks IL-6 actions and ameliorates the diseases with IL-6 overproduction, thus, blockade of IL-6R may prove effective in limiting MM cell growth | Nishimoto 2000 | I | |
| TRAIL-R1 | Mapatumumab (TRM-1) | Human Genome Sciences | Human | Binds to TRAIL, a TNF. Cleared by FDA for Phase I trial, Mapatumumab in Combination with Bortezomib (Velcade) and Bortezomib Alone | Menoret 2006; NCT00315757 | I/II (ongoing trial of bortezomib ± mapatumumab in relapsed or refractory myeloma) | |
| EGFR | Erbitux (EMMA-1, Cetuximab) | Imclone; Bristol-Myers Squibb; University of Cologne | Chimerized | Binding of erbitux to the EGFR blocks phosphorylation and activation of receptor-associated kinases, resulting in inhibition of cell growth, induction of apoptosis, and decreased matrix metalloproteinase and vascular endothelial growth factor production | NCT00368121 | II (ongoing trial of dexamethasone ± erbitux in refractory or relapsed MM) | |
| CD38 | HuMax-CD38 | Genmab A/S | Human | HuMax-CD38 triggers ADCC and CDC against myeloma cells and inhibits tumor growth in mice | http://www.genmab.com | I/II (ongoing, a safe and dose-finding study) | |
| DKK1 | BHQ880 | Novartis | Human | Potently and selectively neutralizes DKK1 suppression of Wnt signaling | Fulciniti 2007; Ettenberg 2008; NCT00741377 | Ib/II (ongoing, in combination with zoledronic acid in relapsed or refractory myeloma patients |
Every effort has been made to obtain reliable data from multiple sources (company and other web sites), but accuracy cannot be guaranteed.
6.4 Focus on MM bone disease
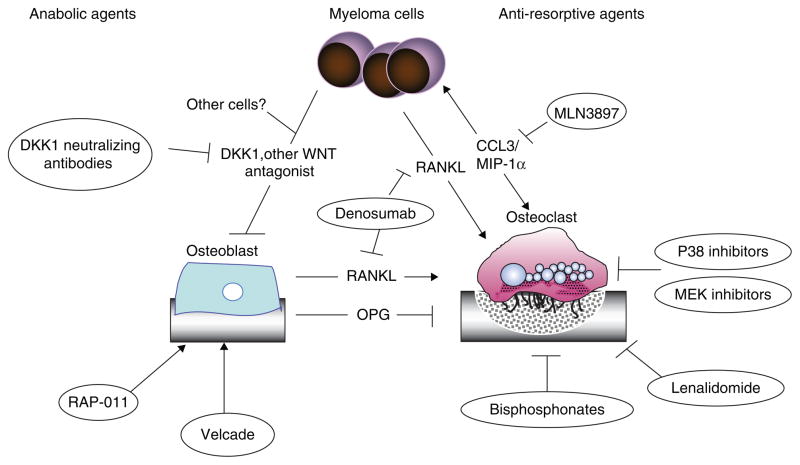
MM bone disease results from an unbalanced OC/OB axis, with enhanced bone resorption at the expense of bone deposition. MM cells directly interfere with physiologic bone remodeling by releasing OC-promoting cytokines such as receptor activator of NF-κB ligand (RANKL) [180], IL-1 [181], IL-6 [182], CCL3–MIP-1α [183], and CCL20 [184]. On the other hand, MM cells inhibit BM mesenchymal stem cell differentiation into OBs by releasing inhibitors of the WNT pathway, such as DKK1 and sFRP [175,176]. These MM-induced modifications in bone homeostasis lead to the development of osteolytic lesions, support tumor growth, and confer chemoresistance [185,186]. Therefore drugs such as the RANKL inhibitor denosumab (Amgen) and DKK1-neutralizing antibody, which aim to restore the balance of bone resorption and formation, may also target tumor growth, drug resistance, and tumor burden [187,188]. Of note, these novel compounds influence general bone homeostasis pathways and are often effective also in non-cancer settings such as osteoporosis. For example, neutralizing antibodies against DKK1 stimulate bone mass also independently of the presence of MM cells, suggesting that in addition to MM cells other sources of DKK1 production and secretion may be present in the BM microenvironment (Figure 4 and Table 6) [189].
Figure 4.
Targeting MM bone disease.
Table 6.
Targeting MM bone disease.
| Targeting MM bone disease | ||||
| RANKL: Fc | J&J | RANKL | I/II | i.v. |
| Denosumab | Amgen | RANKL | III | i.v. |
| OPG: Fc | Genway | OPG | ||
| AMGN-0007 | Amgen | OPG | ||
| PXD101 (belinostat) | Curagen | HDAC | II | p.o. |
| AZD6244/ARRY-142886 | AstraZeneca | MEK | p.o. | |
| Resveratrol | – | |||
| MLN3897 | Takeda | CCR1 | ||
| SDX-308 | Cephalon | COX | ||
| IMiDs: CC-4047, lenalidomide | Celgene | IMiDs | p.o. | |
| Bortezomib | Takeda | Proteasome DKK-1 |
i.v. | |
7. Competitive environment
Similar to Thal, Len, and bortezomib, additional novel therapies are directed at targets expressed by tumor cells or by other cells within the BM microenvironment in order to decrease MM–BM stromal cell interaction and inhibit tumor cell proliferation and drug resistance [80,103,141]. To date, the main class of anti-MM agents is represented by small-molecule inhibitors. Preclinical results also indicate a therapeutic role for targeted antibody-based therapy. Promising agents in clinical studies include HDAC inhibitors panobinostat (LBH-589), SAHA, and romidepsin; HSP90 inhibitor tanespimycin (KOS-953); immunomodulatory drug pomalidomide (IMiD CC4047; actimid); CDK inhibitor flavopiridol; mTOR inhibitor temsirolimus; the VEGF inhibitors aplidin, bevacicumab, and sorafenib; second-generation proteasome inhibitors carfilzomib and NPI-0052; FGFR3 inhibitors; CD40 antagonists; FTI and MAPK inhibitors; and PI3K inhibitors.
8. Potential development issues
Despite having a controversial history, Thal is one of the most widely prescribed anti-MM agents in the United States. Moreover, Pharmion Corp., which was bought by Celgene in 2008 for $2.9 billion, won recommended approval to reintroduce Thal to Europe as an anti-MM agent 50 years after its withdrawal from the market as a sedative and remedy for morning sickness (distributed by Grunenthal) in pregnant women. Celgene has developed IMiDs, including Len, in order to both increase potency and reduce toxicity. Revlimid in combination with Dex received marketing authorization for treatment of MM patients after at least one prior therapy in the United States, Australia, Canada, and Europe. Besides MM, Revlimid is also approved for use in the United States and Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndrome (MDS) associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities. However, the European Union, the EMEA’s Committee for Medicinal Products for Human Use (CHMP) issued a ‘Negative Opinion on the Company’s Marketing Authorisation Application’ for Len for this latter patient cohort due to insufficient data to support positive benefit–risk balance. Further trials are therefore needed.
Similar to Thal and Len, bortezomib also has dose-limiting adverse side effects. Further studies by Millenium/Takeda and other companies aim to develop more potent and less toxic proteasome inhibitors. It remains to be determined whether the therapeutic window of agents including tanespimycin, temsirolimus, sorafenib, enzastaurin or other smallmolecule inhibitors is favorable. Moreover, clinical trials are testing whether the preclinical promise of targeted antibody therapy translates into the clinical setting.
9. Expert opinion and conclusions
Significant advances in MM therapy during the last 8 years have been achieved with the introduction of Thal, Len, and bortezomib, which target the tumor cell in its microenvironment in both laboratory and animal models. Preclinical results have rapidly translated from the bench to the bedside: each of these agents was first used effectively to treat relapsed/refractory disease and then combined with Dex in transplant candidates and with MP in elderly patients as initial therapy to achieve increased frequency and extent of response. The most promising new bortezomib combinations include bortezomib with doxil, tanespimycin, Len, and LBH589 or SAHA, which are likely to further improve MM treatment regimens.
In the future, oncogenomics will be useful not only to identify novel therapeutic targets and to validate targeted therapies, but also to inform the design of clinical trials. Indeed, gene profiling and array comparative genomic hybridization are allowing for RNA- and DNA-based classifications of patients in order to identify those most likely to respond [190,191]. Remarkably, complete responses are achieved in up to 40% of newly diagnosed MM patients with combinations of chemotherapeutic/novel therapies, i.e., Len–bortezomib–Dex. These high response rates now allow for clinical trials to assess the added value of autologous SCT in initial therapy regimens [38].
There has been much progress in MM treatment options; many of the novel drug combinations with conventional chemotherapy have resulted in better response rates. However, most of the studies show no benefit in terms of TTP or OS, i.e., a recent update of the randomized Italian trial comparing MP with MPT [67]. Therefore there remains the urgent need for less toxic and more potent targeted therapies. The most promising novel agents target: i) signaling events in tumor cell development (e.g., FGFR3, PRO-001); ii) cytokines, growth factors and their receptors (e.g., IL-6, CD40, BAFF); iii) signaling sequelae triggered by cytokines and growth factors as well as MM cell–BMSC interactions (MEK: AZD644; PKC: Enzastaurin; NF-κB: MLN120B; Akt: perifosine; proteasomes: NPI0001, carfilzomib); iv) molecules at the cell membrane (e.g., CS-1); v) the tumor-supportive MM microenvironment, including BM angiogenesis (VEGF: avastin, pazopanib); and vi) mechanisms of MM bone disease (e.g., RANKL).
Although these compounds, especially when given in combination, show high activity in preclinical in vitro and in vivo settings, we eagerly await their clinical evaluation. Indeed, most of these agents are already under evaluation for their therapeutic potential in MM treatment either alone or in combination with other novel or conventional agents. Combination therapies have been curative in childhood acute lymphocyte leukemia and Hodgkin’s disease, and we are now poised to rationally combine novel and conventional therapies to similarly improve patient outcome in MM.
Acknowledgments
This work was supported by the Multiple Myeloma Research Foundation (MMRF) Senior Research Grant Award and Dunkin’ Donuts Rising Star Award (KP), MMRF Junior Research Grant Award (SV) as well as National Institutes of Health Grants IP50 CA100707, PO-1 78378, and RO-1 CA 50947; The Myeloma Research Fund; and the LeBow Family Fund to Cure Myeloma (KCA).
Footnotes
Declaration of interest: PR and KA have received support from Millennium-Takeda and Cellgene.
Bibliography
- 1.Podar K, Richardson PG, Hideshima T, et al. The malignant clone and the bone-marrow environment. Best Pract Res. 2007;20:597–612. doi: 10.1016/j.beha.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208:1680–5. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- 4.Bergsagel DE, Bailey AJ, Langley GR, et al. The chemotherapy on plasma-cell myeloma and the incidence of acute leukemia. N Engl J Med. 1979;301:743–8. doi: 10.1056/NEJM197910043011402. [DOI] [PubMed] [Google Scholar]
- 5.Finnish Leukaemia Group. Acute leukaemia and other secondary neoplasms in patients treated with conventional chemotherapy for multiple myeloma: a Finnish Leukaemia Group study. Eur J Haematol. 2000;65:123–7. doi: 10.1034/j.1600-0609.2000.90218.x. [DOI] [PubMed] [Google Scholar]
- 6.McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–4. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 7.Barlogie B, Hall R, Zander A, et al. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67:1298–301. [PubMed] [Google Scholar]
- 8.Barlogie B, Alexanian R, Dicke KA, et al. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood. 1987;70:869–72. [PubMed] [Google Scholar]
- 9.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 10.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 11.Morris TC, Velangi M, Jackson G, et al. Less than half of patients aged 65 years or under with myeloma proceed to transplantation: results of a two region population-based survey. Br J Haematol. 2005;128 (4):510–2. doi: 10.1111/j.1365-2141.2004.05340.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexanian R, Barlogie B, Dixon D. High-dose glucocorticoid treatment of resistant myeloma. Ann Intern Med. 1986;105 (1):8–11. doi: 10.7326/0003-4819-105-1-8. [DOI] [PubMed] [Google Scholar]
- 13.Barlogie B, Smith L, Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984;310 (21):1353–6. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- 14.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341 (21):1565–71. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 15.Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98 (2):492–4. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Fonseca R, Dispenzieri A, et al. Thalidomide in the treatment of relapsed multiple myelome. Mayo Clin Proc. 2000;75 (9):897–901. doi: 10.4065/75.9.897. [DOI] [PubMed] [Google Scholar]
- 17.Alexanian R, Weber D, Anagnostopoulos A, et al. Thalidomide with or without dexamethasone for refractory or relapsing multiple myeloma. Semin Hematol. 2003;40 (4 Suppl 4):3–7. doi: 10.1053/j.seminhematol.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Zervas K, Kouvatseas G, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001;12 (7):991–5. doi: 10.1023/a:1011132808904. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo A, Falco P, Ambrosini MT, et al. Thalidomide plus dexamethasone is an effective salvage regimen for myeloma patients relapsing after autologous transplant. Eur J Haematol. 2005;75 (5):391–5. doi: 10.1111/j.1600-0609.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 20.Dimopoulos MA, Hamilos G, Zomas A, et al. Pulsed cyclophosphamide, thalidomide and dexamethasone: an oral regimen for previously treated patients with multiple myeloma. Hematol J. 2004;5 (2):112–7. doi: 10.1038/sj.thj.6200326. [DOI] [PubMed] [Google Scholar]
- 21.Moehler TM, Neben K, Benner A, et al. Salvage therapy for multiple myeloma with thalidomide and CED chemotherapy. Blood. 2001;98 (13):3846–8. doi: 10.1182/blood.v98.13.3846. [DOI] [PubMed] [Google Scholar]
- 22.Offidani M, Corvatta L, Marconi M, et al. Low-dose thalidomide with pegylated liposomal doxorubicin and high-dose dexamethasone for relapsed/refractory multiple myeloma: a prospective, multicenter, phase II study. Haematologica. 2006;91 (1):133–6. [PubMed] [Google Scholar]
- 23.Lee CK, Barlogie B, Munshi N, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21 (14):2732–9. doi: 10.1200/JCO.2003.01.055. [DOI] [PubMed] [Google Scholar]
- 24.Zangari M, Tricot G, Zeldis G, et al. Results of phase I study of CC-5013 for the treatment of multiple myeloma (MM) patients who relapse after high dose chemotherapy (HDCT) Blood. 2001;98:775a. [Google Scholar]
- 25.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100 (9):3063–7. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 26.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357 (21):2133–42. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357 (21):2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 28.Baz R, Walker E, Karam MA, et al. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: safety and efficacy. Ann Oncol. 2006;17 (12):1766–71. doi: 10.1093/annonc/mdl313. [DOI] [PubMed] [Google Scholar]
- 29.Morgan GJ, Schey SA, Wu P, et al. Lenalidomide (Revlimid), in combination with cyclophosphamide and dexamethasone (RCD), is an effective and tolerated regimen for myeloma patients. Br J Haematol. 2007;137 (3):268–9. doi: 10.1111/j.1365-2141.2007.06538.x. [DOI] [PubMed] [Google Scholar]
- 30.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61 (7):3071–6. [PubMed] [Google Scholar]
- 31.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20 (22):4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 32.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348 (26):2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 33.Jagannath J, Barlogie B, Berenson JR, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma [abstract #2717] Blood. 2007;110(11) doi: 10.1111/j.1365-2141.2008.07359.x. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352 (24):2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 35.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110 (10):3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101 (6):2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 37.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25 (25):3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 38.Lane SW, Gill D, Mollee PN, Rajkumar SV. Role of VAD in the initial treatment of multiple myeloma. Blood. 2005;106(10):3674M. doi: 10.1182/blood-2005-07-2610. author reply 3674–3675. [DOI] [PubMed] [Google Scholar]
- 39.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the group myelome-autogreffe. J Clin Oncol. 2005;23 (36):9227–33. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 40.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24 (6):929–36. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 41.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106 (12):3755–9. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 42.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89 (3):789–93. [PubMed] [Google Scholar]
- 43.Barlogie B, Jagannath S, Desikan KR, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93 (1):55–65. [PubMed] [Google Scholar]
- 44.Osserman EF, Dire LB, Dire J, et al. Identical twin marrow transplantation in multiple myeloma. Acta Haematol. 1982;68 (3):215–23. doi: 10.1159/000206984. [DOI] [PubMed] [Google Scholar]
- 45.Gahrton G, Tura S, Flesch M, et al. Bone marrow transplantation in multiple myeloma: report from the European Cooperative Group for Bone Marrow Transplantation. Blood. 1987;69 (4):1262–4. [PubMed] [Google Scholar]
- 46.Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88 (7):2787–93. [PubMed] [Google Scholar]
- 47.Alyea E, Weller E, Schlossman R, et al. T-cell-depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98 (4):934–9. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]
- 48.Lokhorst HM, Segeren CM, Verdonck LF, et al. Partially T-cell-depleted allogeneic stem-cell transplantation for first-line treatment of multiple myeloma: a prospective evaluation of patients treated in the phase III study HOVON 24 MM. J Clin Oncol. 2003;21 (9):1728–33. doi: 10.1200/JCO.2003.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Badros A, Barlogie B, Siegel E, et al. Improved outcome of allogeneic transplantation in high-risk multiple myeloma patients after nonmyeloablative conditioning. J Clin Oncol. 2002;20 (5):1295–303. doi: 10.1200/JCO.2002.20.5.1295. [DOI] [PubMed] [Google Scholar]
- 50.Kroger N, Schwerdtfeger R, Kiehl M, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100 (3):755–60. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 51.Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102 (9):3447–54. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 52.Moreau P, Hullin C, Garban F, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99–04 protocol. Blood. 2006;107 (1):397–403. doi: 10.1182/blood-2005-06-2573. [DOI] [PubMed] [Google Scholar]
- 53.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356 (11):1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 54.Weber D, Rankin K, Gavino M, et al. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21 (1):16–9. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 55.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24 (3):431–6. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 56.Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20 (21):4319–23. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 57.Zervas K, Mihou D, Katodritou E, et al. VAD-doxil versus VAD-doxil plus thalidomide as initial treatment for multiple myeloma: results of a multicenter randomized trial of the Greek Myeloma Study Group. Ann Oncol. 2007;18 (8):1369–75. doi: 10.1093/annonc/mdm178. [DOI] [PubMed] [Google Scholar]
- 58.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93 (1):124–7. doi: 10.3324/haematol.11644. [DOI] [PubMed] [Google Scholar]
- 59.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22 (2):414–23. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 60.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106 (13):4050–3. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82 (10):1179–84. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 62.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129 (6):776–83. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 63.Richardson PG, Lonial S, Jakubowiak A, et al. Safety and efficacy of lenalidomide (Len), bortezomib (Bz), and dexamethasone (Dex) in patients (pts) with newly diagnosed multiple myeloma (MM): A phase I/II study [abstract 8520] J Clin Oncol. 2008:26. [Google Scholar]
- 64.Paripati H, Stewart AK, Cabou S, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008;22 (6):1282–4. doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]
- 65.Harousseau JL, Mathiot C, Attal A, et al. VELCADE/Dexamethasone (Vel/D) versus VAD as induction treatment prior to autologous stem cell transplantion (ASCT) in newly diagnosed multiple myeloma (MM): updated results of the IFM 2005/01 trial [abstract #450] Blood. 2007;110(11) [Google Scholar]
- 66.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367 (9513):825–31. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 67.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized, controlled trial. Blood. 2008 doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 68.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370 (9594):1209–18. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 69.Ludwig H, Hajek R, Tothova E, et al. Thalidomide-dexamethasone compared to melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2008 doi: 10.1182/blood-2008-07-169565. [DOI] [PubMed] [Google Scholar]
- 70.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA – Italian Multiple Myeloma Network. J Clin Oncol. 2007;25 (28):4459–65. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 71.Bahlis NJ, Song K, Trieu Y, et al. Lenalidomide overcomes poor prognosis conferred by del13q and t(4; 14) but not del17p13 in multiple myeloma: results of the canadian MM016 trial [abstract #3597] Blood. 2007;110(11) [Google Scholar]
- 72.Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21 (1):151–7. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- 73.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359 (9):906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 74.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334 (8):488–93. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 75.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 Clinical Practice Guideline Update on the Role of Bisphosphonates in Multiple Myeloma. J Clin Oncol. 2007;25 (17):1–9. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 76.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8 (5):407–19. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110 (9):3281–90. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Podar K, Richardson PG, Chauhan D, Anderson KC. Targeting the vascular endothelial growth factor pathway in the treatment of multiple myeloma. Expert Rev Anticancer Ther. 2007;7 (4):551–66. doi: 10.1586/14737140.7.4.551. [DOI] [PubMed] [Google Scholar]
- 79.Available from: http://clinicaltrials.gov/
- 80.Available from: http://www.multiplemyeloma.org/
- 81.Availbale from: http://myeloma.org/
- 82.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4 (6):423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 83.Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7 (8):585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 84.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108 (6):2020–8. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109 (6):2276–84. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 86.Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23 (26):6333–8. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104 (3):607–18. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 88.Trudel S, Ely S, Farooqi Y, et al. Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4; 14) myeloma. Blood. 2004;103 (9):3521–8. doi: 10.1182/blood-2003-10-3650. [DOI] [PubMed] [Google Scholar]
- 89.Trudel S, Li ZH, Wei E, et al. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4; 14) multiple myeloma. Blood. 2005;105 (7):2941–8. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- 90.Trudel S, Stewart AK, Rom E, et al. The inhibitory anti-FGFR3 antibody, PRO-001, is cytotoxic to t(4;14) multiple myeloma cells. Blood. 2006;107 (10):4039–46. doi: 10.1182/blood-2005-10-4179. [DOI] [PubMed] [Google Scholar]
- 91.Chesi M, Nardini E, Lim RS, et al. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92 (9):3025–34. [PubMed] [Google Scholar]
- 92.Hurt EM, Wiestner A, Rosenwald A, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5 (2):191–9. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 93.Avet-Loiseau H, Gerson F, Magrangeas F, et al. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98 (10):3082–6. doi: 10.1182/blood.v98.10.3082. [DOI] [PubMed] [Google Scholar]
- 94.Shou Y, Martelli ML, Gabrea A, et al. Diverse karyotypic abnormalities of the c-myc locus associated with c-myc dysregulation and tumor progression in multiple myeloma. Proc Natl Acad Sci USA. 2000;97 (1):228–33. doi: 10.1073/pnas.97.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mitsiades N, Mitsiades CS, Richardson PG, et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood. 2003;101 (10):4055–62. doi: 10.1182/blood-2002-11-3514. [DOI] [PubMed] [Google Scholar]
- 96.Catley L, Weisberg E, Tai YT, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102 (7):2615–22. doi: 10.1182/blood-2003-01-0233. [DOI] [PubMed] [Google Scholar]
- 97.Catley L, Weisberg E, Kiziltepe T, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108 (10):3441–9. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maiso P, Carvajal-Vergara X, Ocio EM, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66 (11):5781–9. doi: 10.1158/0008-5472.CAN-05-4186. [DOI] [PubMed] [Google Scholar]
- 99.Feng R, Oton A, Mapara MY, et al. The histone deacetylase inhibitor, PXD101, potentiates bortezomib-induced anti-multiple myeloma effect by induction of oxidative stress and DNA damage. Br J Haematol. 2007;139 (3):385–97. doi: 10.1111/j.1365-2141.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 100.Miller CP, Ban K, Dujka ME, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110 (1):267–77. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khan SB, Maududi T, Barton K, et al. Analysis of histone deacetylase inhibitor, depsipeptide (FR901228), effect on multiple myeloma. Br J Haematol. 2004;125 (2):156–61. doi: 10.1111/j.1365-2141.2004.04882.x. [DOI] [PubMed] [Google Scholar]
- 102.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005;102 (24):8567–72. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Podar K, Hideshima T, Chauhan D, Anderson KC. Targeting signalling pathways for the treatment of multiple myeloma. Expert Opin Ther Targets. 2005;9 (2):359–81. doi: 10.1517/14728222.9.2.359. [DOI] [PubMed] [Google Scholar]
- 104.Avet-Loiseau H, Li JY, Facon T, et al. High incidence of translocations t(11;14) (q13;q32) and t(4;14)(p16;q32) in patients with plasma cell malignancies. Cancer Res. 1998;58 (24):5640–5. [PubMed] [Google Scholar]
- 105.Chesi M, Nardini E, Brents LA, et al. Frequent translocation t(4;14) (p16.3;q32. 3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet. 1997;16 (3):260–4. doi: 10.1038/ng0797-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103 (6):2332–6. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA. 2007;104 (10):4048–53. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68 (1):190–7. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hideshima T, Podar K, Chauhan D, Anderson KC. Cytokines and signal transduction. Best pract Res. 2005;18 (4):509–24. doi: 10.1016/j.beha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 110.Neri A, Murphy JP, Cro L, et al. Ras oncogene mutation in multiple myeloma. J Exp Med. 1989;170 (5):1715–25. doi: 10.1084/jem.170.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu P, Leong T, Quam L, et al. Activating mutations of N- and K-ras in multiple myeloma show different clinical associations: analysis of the Eastern Cooperative Oncology Group Phase III Trial. Blood. 1996;88 (7):2699–706. [PubMed] [Google Scholar]
- 112.Tiedemann RE, Gonzalez-Paz N, Kyle RA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22 (5):1044–52. doi: 10.1038/leu.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin P, Santon A, Garcia-Cosio M, Bellas C. RAS mutations are uncommon in multiple myeloma and other monoclonal gammopathies. Int J Oncol. 2005;27 (4):1023–8. [PubMed] [Google Scholar]
- 114.Kato K, Cox AD, Hisaka MM, et al. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA. 1992;89 (14):6403–7. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bolick SC, Landowski TH, Boulware D, et al. The farnesyl transferase inhibitor, FTI-277, inhibits growth and induces apoptosis in drug-resistant myeloma tumor cells. Leukemia. 2003;17 (2):451–7. doi: 10.1038/sj.leu.2402832. [DOI] [PubMed] [Google Scholar]
- 116.Ochiai N, Uchida R, Fuchida S, et al. Effect of farnesyl transferase inhibitor R115777 on the growth of fresh and cloned myeloma cells in vitro. Blood. 2003;102 (9):3349–53. doi: 10.1182/blood-2003-03-0851. [DOI] [PubMed] [Google Scholar]
- 117.Beaupre DM, Cepero E, Obeng EA, et al. R115777 induces Ras-independent apoptosis of myeloma cells via multiple intrinsic pathways. Mol Cancer Ther. 2004;3 (2):179–86. [PubMed] [Google Scholar]
- 118.Cortes J, Albitar M, Thomas D, et al. Efficacy of the farnesyl transferase inhibitor R115777 in chronic myeloid leukemia and other hematologic malignancies. Blood. 2003;101 (5):1692–7. doi: 10.1182/blood-2002-07-1973. [DOI] [PubMed] [Google Scholar]
- 119.Tai YT, Fulciniti M, Hideshima T, et al. Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood. 2007;110 (5):1656–63. doi: 10.1182/blood-2007-03-081240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58 (3):573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 121.Nishimoto N, Ogata A, Shima Y, et al. Oncostatin M, leukemia inhibitory factor, and interleukin 6 induce the proliferation of human plasmacytoma cells via the common signal transducer, gp130. J Exp Med. 1994;179 (4):1343–7. doi: 10.1084/jem.179.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsuda T, Fukada T, Takahashi-Tezuka M, et al. Activation of Fes tyrosine kinase by gp130, an interleukin-6 family cytokine signal transducer, and their association. J Biol Chem. 1995;270 (19):11037–9. doi: 10.1074/jbc.270.19.11037. [DOI] [PubMed] [Google Scholar]
- 123.Kurth I, Horsten U, Pflanz S, et al. Activation of the signal transducer glycoprotein 130 by both IL-6 and IL-11 requires two distinct binding epitopes. J Immunol. 1999;162 (3):1480–7. [PubMed] [Google Scholar]
- 124.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264 (5155):95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 125.Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332 (6159):83–5. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 126.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87 (3):1104–12. [PubMed] [Google Scholar]
- 127.Uchiyama H, Barut BA, Mohrbacher AF, et al. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82 (12):3712–20. [PubMed] [Google Scholar]
- 128.Costes V, Portier M, Lu ZY, et al. Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production. Br J Haematol. 1998;103 (4):1152–60. doi: 10.1046/j.1365-2141.1998.01101.x. [DOI] [PubMed] [Google Scholar]
- 129.Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95 (8):2630–6. [PubMed] [Google Scholar]
- 130.Gupta D, Treon SP, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15 (12):1950–61. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 131.Hideshima T, Chauhan D, Schlossman R, et al. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20 (33):4519–27. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 132.Hamasaki M, Hideshima T, Tassone P, et al. Azaspirane (N-N-diethyl-8,8-dipropyl-2-azaspiro [4. 5]decane-2-propanamine) inhibits human multiple myeloma cell growth in the bone marrow milieu in vitro and in vivo. Blood. 2005;105 (11):4470–6. doi: 10.1182/blood-2004-09-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pedranzini L, Dechow T, Berishaj M, et al. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66 (19):9714–21. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- 134.Pathak AK, Bhutani M, Nair AS, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5 (9):943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 135.Bhutani M, Pathak AK, Nair AS, et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13 (10):3024–32. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 136.Ahn KS, Sethi G, Sung B, et al. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68 (11):4406–15. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 137.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296 (5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 138.Balendran A, Hare GR, Kieloch A, et al. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484 (3):217–23. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- 139.Williams MR, Arthur JS, Balendran A, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10 (8):439–48. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 140.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96 (8):4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2 (12):927–37. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 142.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96 (6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 143.Madrid LV, Wang CY, Guttridge DC, et al. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20 (5):1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madrid LV, Mayo MW, Reuther JY, et al. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276 (22):18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 145.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111 (3):293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 146.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2 (7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 147.Akiyama M, Hideshima T, Hayashi T, et al. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 2003;63 (1):18–21. [PubMed] [Google Scholar]
- 148.Puthier D, Bataille R, Amiot M. IL-6 up-regulates mcl-1 in human myeloma cells through JAK/STAT rather than ras/MAP kinase pathway. Eur J Immunol. 1999;29 (12):3945–50. doi: 10.1002/(SICI)1521-4141(199912)29:12<3945::AID-IMMU3945>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 149.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20 (42):5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 150.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkyl-phospholipid, inhibits Akt and induces in vitro and In vivo cytotoxicity in human multiple myeloma (MM) cells [abstract #250] Blood. 2005;106 (11):128. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hideshima T, Catley L, Raje N, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138 (6):783–91. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 152.Huston A, Leleu X, Jia X, et al. Targeting Akt and heat shock protein 90 produces synergistic multiple myeloma cell cytotoxicity in the bone marrow microenvironment. Clin Cancer Res. 2008;14 (3):865–74. doi: 10.1158/1078-0432.CCR-07-1299. [DOI] [PubMed] [Google Scholar]
- 153.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62 (17):5027–34. [PubMed] [Google Scholar]
- 154.Frost P, Moatomed F, Hoang B, et al. In vivo anti-tumor effects of the mTOR inhibitor, CCI-779, against human multiple myeloma cells in a xenograft model. Blood. 2004 doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 155.Raje N, Kumar S, Hideshima T, et al. Combination of the mTOR inhibitor Rapamycin and RevlimidTM(CC-5013) has synergistic activity in multiple myeloma. Blood. 2004 doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 156.Francis LK, Alsayed Y, Leleu X, et al. Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res. 2006;12 (22):6826–35. doi: 10.1158/1078-0432.CCR-06-1331. [DOI] [PubMed] [Google Scholar]
- 157.Podar K, Raab MS, Zhang J, et al. Targeting PKC in multiple myeloma: in vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin (LY317615. HCl) Blood. 2007;109 (4):1669–77. doi: 10.1182/blood-2006-08-042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Podar K, Raab MS, Chauhan D, Anderson KC. The therapeutic role of targeting protein kinase C in solid and hematologic malignancies. Expert Opin Investig Drugs. 2007;16 (10):1693–707. doi: 10.1517/13543784.16.10.1693. [DOI] [PubMed] [Google Scholar]
- 159.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nature reviews. 2005;5 (10):749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 160.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12 (2):115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12 (2):131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277 (19):16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 163.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85 (4):863–72. [PubMed] [Google Scholar]
- 164.Lichtenstein A, Tu Y, Fady C, et al. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162 (2):248–55. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 165.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99 (12):4525–30. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 166.Hayashi T, Hideshima T, Nguyen AN, et al. Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res. 2004;10 (22):7540–6. doi: 10.1158/1078-0432.CCR-04-0632. [DOI] [PubMed] [Google Scholar]
- 167.Hideshima T, Chauhan D, Hayashi T, et al. Antitumor activity of lysophosphatidic acid acyltransferase-beta inhibitors, a novel class of agents, in multiple myeloma. Cancer Res. 2003;63 (23):8428–36. [PubMed] [Google Scholar]
- 168.Ito K, Nakazato T, Xian MJ, et al. 1′-acetoxychavicol acetate is a novel nuclear factor kappaB inhibitor with significant activity against multiple myeloma in vitro and in vivo. Cancer Res. 2005;65 (10):4417–24. doi: 10.1158/0008-5472.CAN-05-0072. [DOI] [PubMed] [Google Scholar]
- 169.Hideshima T, Neri P, Tassone P, et al. MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res. 2006;12 (19):5887–94. doi: 10.1158/1078-0432.CCR-05-2501. [DOI] [PubMed] [Google Scholar]
- 170.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22 (10):1536–45. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 171.Derksen PW, Tjin E, Meijer HP, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101 (16):6122–7. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sukhdeo K, Mani M, Zhang Y, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA. 2007;104 (18):7516–21. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qiang YW, Walsh K, Yao L, et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106 (5):1786–93. doi: 10.1182/blood-2005-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Qiang YW, Shaughnessy JD, Jr, Yaccoby S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008;112 (2):374–82. doi: 10.1182/blood-2007-10-120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349 (26):2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 176.Giuliani N, Morandi F, Tagliaferri S, et al. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67 (16):7665–74. doi: 10.1158/0008-5472.CAN-06-4666. [DOI] [PubMed] [Google Scholar]
- 177.Tai YT, Catley LP, Mitsiades CS, et al. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64 (8):2846–52. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- 178.Tai YT, Catley L, Li XF, et al. Immunomodulatory drug Lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications [abstract 5150] Blood. 2005:106. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- 179.Tai YT, Dillon M, Song W, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2007 doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sezer O, Heider U, Zavrski I, et al. RANK ligand and osteoprotegerin in myeloma bone disease. Blood. 2003;101 (6):2094–8. doi: 10.1182/blood-2002-09-2684. [DOI] [PubMed] [Google Scholar]
- 181.Yao Z, Xing L, Qin C, et al. Osteoclast precursor interaction with bone matrix induces osteoclast formation directly by an interleukin-1-mediated autocrine mechanism. J Biol Chem. 2008;283 (15):9917–24. doi: 10.1074/jbc.M706415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146 (4):1991–8. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 183.Han JH, Choi SJ, Kurihara N, et al. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97 (11):3349–53. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- 184.Giuliani N, Lisignoli G, Colla S, et al. CC-Chemokine ligand 20/macrophage inflammatory protein-3alpha and CC-chemokine receptor 6 are overexpressed in myeloma microenvironment related to osteolytic bone lesions. Cancer Res. 2008;68 (16):6840–50. doi: 10.1158/0008-5472.CAN-08-0402. [DOI] [PubMed] [Google Scholar]
- 185.Abe M, Hiura K, Wilde J, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104 (8):2484–91. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 186.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91 (2):192–9. [PMC free article] [PubMed] [Google Scholar]
- 187.Choi SJ, Oba Y, Gazitt Y, et al. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108 (12):1833–41. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Edwards CM, Mueller G, Roelofs AJ, et al. Apomine, an inhibitor of HMG-CoA-reductase, promotes apoptosis of myeloma cells in vitro and is associated with a modulation of myeloma in vivo. Int J Cancer. 2007;120 (8):1657–63. doi: 10.1002/ijc.22478. [DOI] [PubMed] [Google Scholar]
- 189.Pinzone JJ, Hall BM, Thudi NK, et al. The role of Dickkopf-1 in bone development, homeostasis and disease. Blood. 2008 doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bergsagel PL, Kuehl WM, Zhan F, et al. Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106 (1):296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Carrasco DR, Tonon G, Huang Y, et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9 (4):313–25. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]