Abstract
Background
Nitric oxide sensitive guanylyl cyclase (NOsGC) is a heterodimeric enzyme formed by an α- and a β1-subunit. A splice variant (C-α1) of the α1-subunit, lacking at least the first 236 amino acids has been described by Sharina et al. 2008 and has been shown to be expressed in differentiating human embryonic cells. Wagner et al. 2005 have shown that the amino acids 61–128 of the α1-subunit are mandatory for quantitative heterodimerization implying that the C-α1-splice variant should lose its capacity to dimerize quantitatively.
Methodology/Principal Findings
In the current study we demonstrate preserved quantitative dimerization of the C-α1-splice by co-purification with the β1-subunit. In addition we used fluorescence resonance energy transfer (FRET) based on fluorescence lifetime imaging (FLIM) using fusion proteins of the β1-subunit and the α1-subunit or the C-α1 variant with ECFP or EYFP. Analysis of the respective combinations in HEK-293 cells showed that the fluorescence lifetime was significantly shorter (≈0.3 ns) for α1/β1 and C-α1/β1 than the negative control. In addition we show that lack of the amino-terminus in the α1 splice variant directs it to a more oxidized subcellular compartment.
Conclusions/Significance
We conclude that the amino-terminus of the α1-subunit is dispensable for dimerization in-vivo and ex-vivo, but influences the subcellular trafficking.
Introduction
Nitric oxide sensitive guanylyl cyclase is the physiological receptor for nitric oxide (NO) and nitric oxide releasing drugs. Its second messenger cyclic GMP is crucial for vasodilatation, penile erection, platelet disaggregation and neurotransmission. The heterodimeric enzyme is formed by either an α1- or an α2-subunit and a β1-subunit. Dimerization of the enzyme is a prerequisite for its catalytic activity, because both the α- as well as the β1-subunit provide essential residues for the conversion of GTP to cGMP [1]. There is conflicting evidence which parts of the subunits are mandatory for quantitative heterodimerization. Previous studies [2], [3] show inconsistent results with respect to the requirement of the α1 subunit amino-terminus which is missing in the naturally occurring C-α1 splice variant [4]. Since the occurrence of this splice variant has been linked to differentiation of human embryonic stem cells [5], the capacity of the C-α1 splice variant to heterodimerize and thus form a functionally active enzyme, is of biological importance.
Because of the controversial nature of the question, we investigated the dimerization capacity using two independent experimental approaches. First we used a purification method of the NOsGC β1-subunit and looked for co-purification of the α1 variants. With the second experimental approach we examined fluorescence resonance energy transfer (FRET) based on fluorescence lifetime imaging (FLIM) in intact cells using fusion proteins of the respective subunits with fluorescent proteins. While analyzing the fluorescence lifetimes of the respective NOsGC-subunits, we serendipitously discovered that the C-α1 splice isoform, shows a unique subcellular distribution. Using a fluorescent tagged marker for the endoplasmic reticulum (ER), we conclude, that the C-α1 isoform is located at the ER. As it is well known, that the redox state of the ER is relatively more oxidized than the cytosol [6], we performed a ratiometric analysis of the redox properties of the C-α1 protein by using a redox sensor (Grx1-roGFP2) and identified, that the protein is not only distributed in a different manner than the wild type, but also located in a more oxidized environment. Co-expression of the β1-subunit restored the cytosolic localization of the C-α1 splice isoform, but led to a nuclear localization that was not found for the canonical α1/β1 heterodimer. We could show that the C-α1 splice isoform retains its ability to heterodimerize quantitatively with the β1-subunit ex vivo and in intact cells, despite lack of amino-terminal amino acids that were thought to be important for heterodimerization [2]. In addition, we observed that the fluorescent fusion of the C-α1 splice subunit is directed to a more oxidized subcellular compartment, while the respective C-α1/β1 heterodimer shows a cytosolic and nuclear localization.
Results
α1ΔN259 will be expressed by the C-α1 splice form
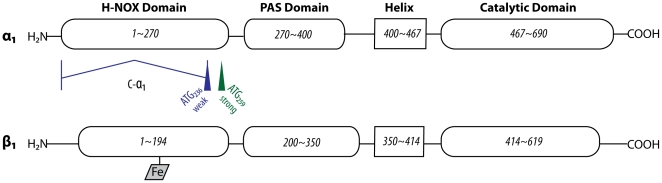
Analyzing the C-α1 splice-variant described by Sharina et al. [4] and designated α1ΔN240 shows that the first initiation codon after splicing would either form an α1ΔN236 or an α1ΔN259 variant because these represent the only methionines with an open reading frame in the human sequence (Fig. 1 and Data S2). In a recently published review by Sharina et al. 2011, the authors have adapted our numbering [7]. In a previous study by Koglin and Behrends 2003 [3], we investigated intensely the α1ΔN236 truncation in comparison to the α1ΔN259 truncation. Because neither enzyme activity, substrate-dependency (GTP), nor dose-effect-curve of nitric oxide differed significantly from α1ΔN259, it was suggested by the reviewers to remove the redundant data for the α1ΔN236 truncation. Since there was no difference in molecular weight between the α1ΔN236 and α1ΔN259 variant (Fig. 2), we now assume that the recognition of the translation initiation site of ATG259 is dominant over ATG236 in the human sequence. We thus suggest that the C-α1 splice variant leads to the formation of a subunit with an α1ΔN259 deletion.
Figure 1. Assumed formation of α1ΔN259 deletion through C-α1 splice variant of guanylyl cyclase.
The graphic is a modified version of the domain architecture model by Derbyshire and Marletta [34].
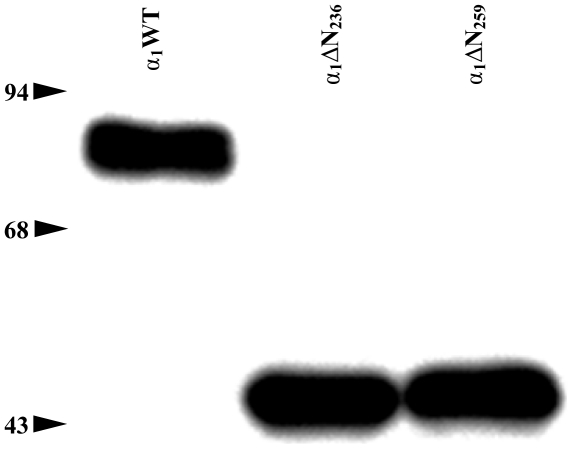
Figure 2. Characterization of expression of the α1-subunits by Western-blot analysis in cytosolic fractions of Sf-9 cells infected with the respective variants of the α1-subunits.
All lanes were loaded with 40 µg protein of the cytosolic fractions. The experiments were repeated at least three times and one representative result is shown. [molecular weights predicted from their amino acid sequences: α1WT, 77.6 kDa; α1ΔN236, 51.2 kDa; α1ΔN259, 48 kDa].
Establishment of a novel one-step-purification protocol
We performed a new one-step-purification of the soluble guanylyl cyclase using the Strep Tag II, which results in 1 mg purified NOsGC from 1,000 ml Sf-9 culture medium. The yield calculated by using the total activities of cytosol and purified protein is ≈25 %. The purification factor is ≈125-fold and results in a more than 95 % purity of the enzyme determined by Coomassie stained gels.
Analysis of the heterodimerization of C-α1 by co-purification with β1-S
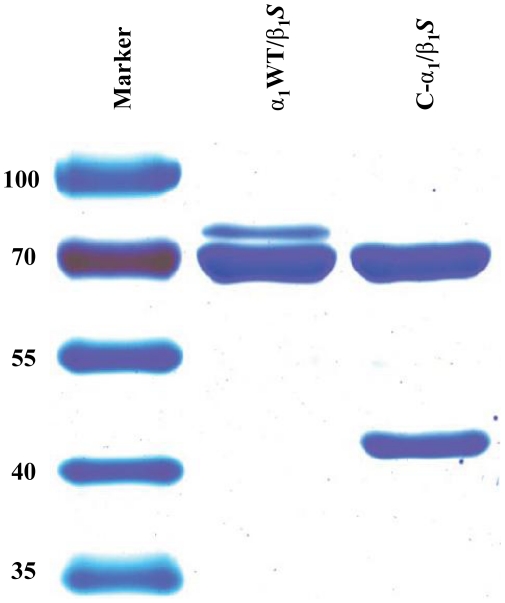
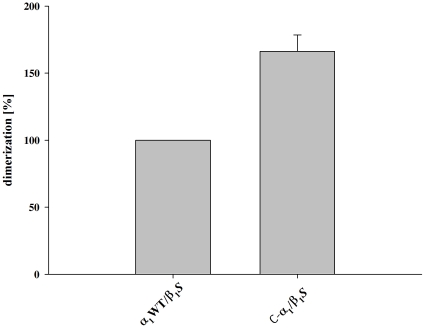
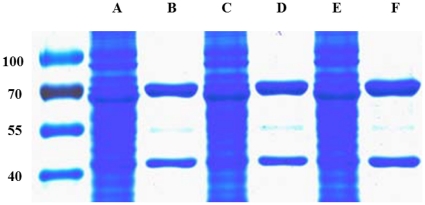
Using a carboxy-terminally Strep tagged β1-subunit of NOsGC, we could show that both α1-isoforms dimerize with the β1-subunit (Fig. 3). Densitometric analysis and graphical representation as described in [2] shows that C-α1 forms heterodimers not less but more effectively with a relative value of 166.2 % (±12.2 %) compared with α1WT (Fig. 4). Alternatively the ratio of the band intensities of the α1WT variants to the β1- bands were 0.75±0.11 for C-α1/β1 and 0.43±0.06 for α1/β1. The excess of the β1 subunit is due to the fact that purification was performed with a Strep Tag II attached to the β1-subunit as described in [2]. Because our findings contradict the central message of the paper by Wagner at al. 2005 although the experimental approach was very similar [2], we tested more subtle differences. We expressed Sf-9 cells without any supplement, only with hemin (4 mg/l) and with both hemin and lipid supplement as in [2], the latter being used to reduce the shear forces during Sf-9 cell culture. After expression under these conditions, we purified the enzyme complex and performed a SDS-PAGE and the gel was stained with Coomassie Blue according to the protocol by Kang et al. [8]. There was no negative effect of either of the supplements on the heterodimerization of C-α1/β1 S (Fig. 5) confirming our finding that the C-α1 splice-variant retains its ability to heterodimerize under different experimental conditions.
Figure 3. SDS-PAGE analysis of NOsGC variants.
5 µg of each purified enzyme was electrophoretically separated by SDS-Page and stained with Coomassie Blue. The experiments were repeated at least three times and one representative result is shown. [molecular weights predicted from their amino acid sequences: α1WT, 77.6 kDa; C-α1, 48 kDa; β1 S, 72.2 kDa].
Figure 4. Densitometric analysis of dimerization.
For quantification of the dimerization the optical densities of the Coomassie-stained bands were measured. A: The values obtained for α1 were normalized to β1 in the same lane. Dimerization was calculated in percentage of α1WT/β1 S. Data are expressed as means ± SEM.
Figure 5. Coomassie staining of cytosolic fractions and purified proteins.
50 µg Cytosol of cytosolic fraction or 5 µg of purified enzyme was electrophoretically separated by SDS-PAGE and stained with Coomassie Blue (a representative gel is shown). Lane A, cytosolic fraction of C-α1/β1 S expressed without heme-supplement and without lipid medium supplement. Lane B, purified C-α1/β1 S expressed without heme-supplement and without lipid medium supplement. Lane C, cytosolic fraction of C-α1/β1 S expressed only with heme-supplement and without lipid medium supplement. Lane D, purified C-α1/β1 S expressed with heme-supplement and without lipid medium supplement. Lane E, cytosolic fraction of C-α1/β1 S expressed only with heme-supplement and with lipid medium supplement. Lane F, purified C-α1/β1 S expressed with heme-supplement and with lipid medium supplement.
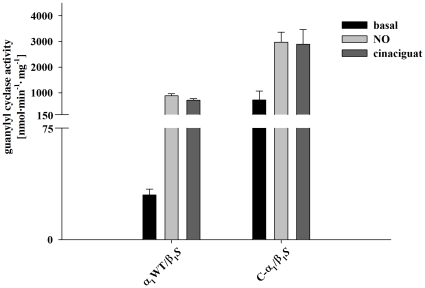
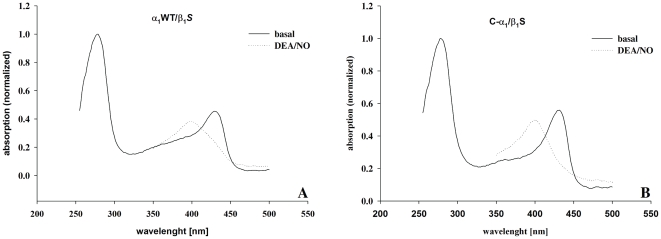
Guanylyl cyclase activity of the purified C-α1/β1 and α1/β1 heterodimers was measured under basal conditions, in the presence of NO (100 µM DEA/NO) or in the presence of 10 µM cinaciguat. Consistent with the formation of more functional heterodimers for C-α1/β1 versus α1/β1 in Sf-9 cell culture, enzyme activity was higher for the splice variant under all experimental conditions (Fig. 6). Spectroscopic analysis shows that both C-α1/β1 than α1/β1 contain a significant amount of heme (Fig. 7) which is consistent with their responsiveness to nitric oxide (see Fig. 6).
Figure 6. Guanylyl cyclase activity of purified variants of the enzyme.
Specific activity was measured under basal conditions (black column), in the presence of 100 µM NO (DEA/NO, white column) and in the presence of 10 µM cinaciguat (gray columns). Data are expressed as means ± SEM. The experiments were repeated at least three times and one representative result is shown.
Figure 7. Spectroscopic analysis of purified cGC enzyme complexes.
Spectroscopic analysis shows absorption values at basal (solid line) or NO-stimulated (100 µM DEA/NO, dotted line) conditions. A: α1WT/β1 S. B: C-α1/β1 S.
Analysis of the heterodimerization of C-α1 by FLIM-FRET
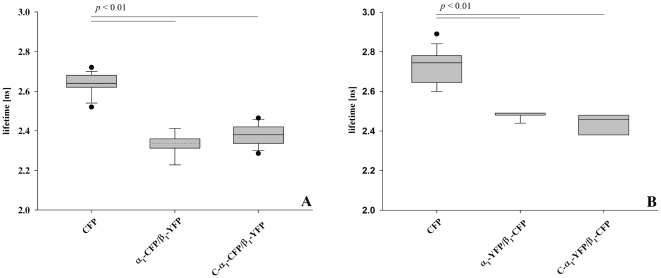
In order to test whether the unexpected formation of more functional heterodimers with the β1 subunit for C-α1 versus the canonical α1 subunit is also seen in intact cells we used fluorescence resonance energy transfer (FRET) based on fluorescence lifetime imaging. The α1-subunit and C-α1-subunit were fused with ECFP and the β1-subunit with EYFP in analogy to a previous FRET study by our group [9]. Analysis of the respective combinations in HEK-293 cells showed that the fluorescence lifetime was significantly shorter for α1/β1 and C-α1/β1 than the negative control (Fig. 8A). This indicates that heterodimerization of both α1 variants with the β1 subunit bring the respective fused fluorescent proteins in a physical distance below 80 Å [10]. As an additional control, FLIM-FRET experiments were performed with constructs where the fluorescent proteins were exchanged (Fig. 8B). Again there was evidence that the C-α1 splice variant and the canonical α1-subunit heterodimerize equally well in intact cells.
Figure 8. Fluorescence lifetimes of the FRET donor ECFP in HEK-293 cells, expressing donor only, and fluorescent tagged heterodimeric NOsGC variants.
Mean fluorescence lifetimes of ECFP were measured in HEK-293 cells at 37°C, 48 h post transfection with the respective recombinant constructs. A: α1-variants as fluorescence-donor. B: α1-variants as fluorescence-acceptor. Data are presented in box and whiskers plots showing the 25th percentiles, 75th percentile and median as box with the mean value as dotted line. Whiskers represent the 5th and 95th percentile, dots are outliers. Fluorescent tagged NOsGC variants show significantly reduced fluorescent lifetimes due to FRET.
The results are expressed as means ± SEM of at least three independent experiments. For minimum 20 cells were analyzed each. All results were controlled for their statistical significance by Student's t-test. A value of p<0.01 was considered to be statistically significant.
The magnitude of the change of the fluorescence-lifetime (≈0.3 ns) was expected to prove an interaction.
Subcellular distribution of C-α1
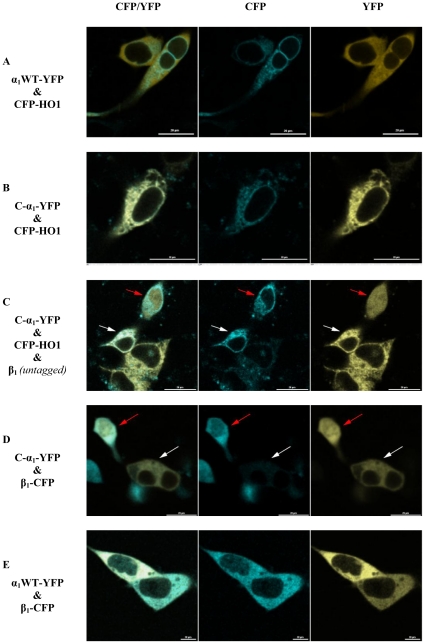
While performing expression controls for the FLIM-FRET experiments, we noticed a different subcellular localization of the fluorescent fusion proteins of the C-α1 splice variant versus the canonical α1 subunit each expressed in the absence of the β1-subunit. Because of the granular perinuclear appearance of the splice variant, we co-expressed human heme oxygenase 1 (HO-1) with an amino-terminal ECFP as a well-known marker for the endoplasmic reticulum [11] (Fig. 9A and B). While the C-α1 splice variant showed an exact co-localization with HO-1, the canonical α1 subunit showed a diverging homogenous distribution in the cytosol (see Fig. 9A and B). Co-expression of untagged β1-subunit led to a homogenous wild type like distribution of the C-α1 splice variant with an additional nuclear signal in some (Fig. 9C, red arrow) but not all cells (Fig. 9C, white arrow). Co-expression of CFP-tagged β1-subunit (and omission of CFP-HO1) demonstrates that expression of substantial amounts of the β1-subunit induces this translocation to the nucleus (Fig. 9D, red arrow). In contrast, no nuclear expression was detected for the canonical α1 subunit in the presence of β1 (Fig. 9E).
Figure 9. Expression of EYFP-marked α1 full-length (A) and C-α1 (B) in HEK-293 cells.
As a marker of the endoplasmic reticulum [11] ECFP tagged heme oxygenase 1 (human, HO-1) was co-transfected. A, α1 full-length shows cytosolic distribution. B, C-α1 shows a similar distribution like ECFP-HO-1. C, addition of an untagged β1-subunit led in some cells a similar subcellular distribution as the wild type. The red arrow denotes a cell likely co-transfected with β1, the white arrow denotes a cell likely not co-transfected with β1. D, only cells which express both subunits show homogenous subcellular distribution. The red arrow denotes a cell co-transfected with β1, the white arrow denotes a cell not co-transfected with β1. E, The subcellular localization of α1 full-length is not affected through coexpression of β1. Bar, 20 µm. CFP, ECFP channel; YFP, EYFP channel.
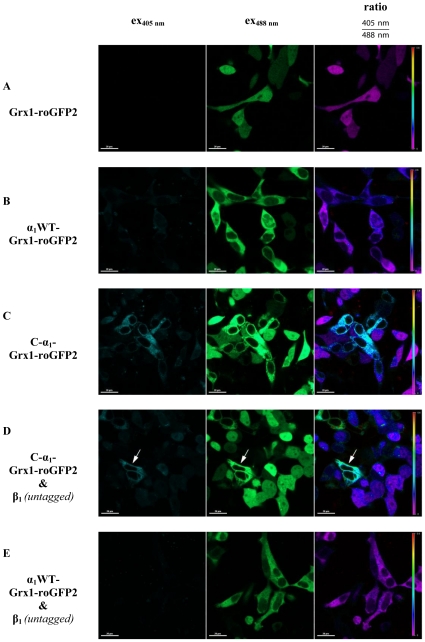
Because Sharin et al. [5] have shown that the C-α1 splice variant is “uniquely resistant to oxidative protein degradation” we analyzed the redox-state of both α1 variants by fusion with the redox-sensor Grx1-roGFP2. Ratiometric analysis showed no difference between Grx1-roGFP2 (Fig. 10A) and α1-Grx1-roGFP2 (Fig. 10B). In contrast, C-α1-Grx1-roGFP2 showed a higher ratiometric signal indicative of a more oxidized subcellular environment (Fig. 10C). This effect was attenuated by co-expression of the untagged β1-subunit (Fig. 10D). In contrast, co-expression of untagged β1-subunit with α1-Grx1-roGFP2 led to no change (Fig. 10E in comparison to Fig. 10B).
Figure 10. Redox-state analysis of the α1 full-length and C-α1 in HEK-293 cells, using the redox-sensor Grx1-roGFP2.
According to the settings a higher value means a more oxidized state of the protein.
Discussion
It has recently been shown that the α1-subunit of the nitric oxide receptor NOsGC undergoes splicing regulation in differentiating human embryonic cells [5]. The results indicated high levels of an amino-terminally deleted C-α1-splice form in differentiating cells that showed a different intracellular distribution in comparison to the canonical full-length α1-subunit. It has been published that the amino-terminus of the α1-subunit is important for quantitative dimerization with the β1-subunit [2]. This was in contrast to a study where we had shown co-purification of the β1-subunit with α1ΔN259 [3]. To explain the discrepancy, Wagner et al. [2] raised the argument that we had applied a purification method, where we pooled the fractions with catalytic activity [3]. Due to this approach, it was conceivable, that only a small fraction which has formed active heterodimers is considered, while non-heterodimerizing subunits are discarded. The present study circumvents this potential problem by using a purification procedure based on an affinity StrepTag attached to the β1-subunit in analogy to the study of Wagner et al. [2]. Our results obtained by using co-purification of the α1-subunit and the amino-terminally deleted C-α1-splice form with the StrepTagged β1-subunit indicate that the C-α1-splice form heterodimerizes quantitatively. Because of the controversial nature of the question, whether dimerization of nitric oxide sensitive guanylyl cyclase requires the α1 amino-terminus, we used an additional method based on fluorescence resonance energy transfer to demonstrate heterodimerization of the C-α1-splice form with the β1-subunit in intact cells. This corroborated our finding that lack of the amino-terminus of the α1-subunit does not influence quantitative dimerization with the β1-subunit. This is also consistent with a number of other studies [4], [12], [13], [14]. Our finding that α1-variants heterodimerize equally well in intact cells, but the C-α1 splice form seems to lead to more intact heterodimers upon purification is probably due to the greater stability of the C-α1 splice variant [5]. This may also be the reason for the higher enzyme activity of the C-α1/β1 heterodimer versus wild type. Alternatively, it is conceivable, that the amino-terminal region absent in C-α1 has a negative regulatory influence on basal activity. This would be in line with a proposed regulatory domain-scale mechanism of the amino-terminal domains of NOsGC [15] that are in proximity to the catalytic domain [9]. The splice variant would thus represent a disinhibited isoform under basal conditions.
Using fluorescent fusion proteins, we noticed a peculiar localization of the C-α1-splice form compared to the wild type. Comparison with fluorescent tagged heme oxygenase-1 which is known to be attached to the endoplasmic reticulum outer membrane [16], revealed a highly similar subcellular distribution. As the endoplasmic reticulum shows a relatively oxidizing thiol-disulfide milieu [17], we employed a novel method to measure the glutathione redox potential based on a fusion of the α1-subunit in its different splice forms with glutaredoxin-1 and roGFP2 [18]. This confirmed a more oxidizing thiol-disulfide milieu for the C-α1 splice variant compatible with localization at the endoplasmic reticulum. Co-expression of the non-tagged β1-subunit changed the subcellular localization as well as the thiol-disulfide milieu signal for the C-α1-splice form but had no effect on the α1-subunit. The finding that the α1/β1-heterodimer showed the expected diffuse cytosolic localization, while the C-α1/β1-heterodimer was also present in the nucleus, is interesting in the context of reports suggesting a role for the β1-subunit in the nucleus: Baltrons and colleagues have demonstrated that the β1-subunit translocates to the nucleus for proteosomal degradation in rat astrocytes after treatment with bacterial endotoxin [19]. In a subsequent report the β1-subunit was shown to be peripherally associated to chromosomes during mitosis and to play a role in chromatin condensation and cell cycle progression in rat C6 glioma cells [20]. The mechanism by which the β1-subunit enters the nucleus is unknown since the protein lacks a recognizable nuclear localization signal [19]. However, it is conceivable that regions of the β1-subunit that may be shielded in the classical α1/β1-heterodimeric enzyme are exposed in the C-α1/β1-heterodimer and the β1-homomer and are free to interact with proteins that regulate nuclear import [21].
Sharin et al. have analyzed the subcellular distribution of the native C-α1 splice form in differentiating human embryonic cells [5]: With biochemical methods the C-α1 splice form could be detected in small amounts in the fraction containing nuclei and to a greater extent in the cytosolic fraction [5]. This is consistent with our findings for the overexpressed fluorescent tagged C-α1/β1-heterodimer. Immunocytochemical analysis of differentiating human embryonic cells at day 12 with an antibody that recognizes both the α1-subunit and the C-α1 splice form showed a majority (88 %) of cells with a diffuse staining indicative of a predominant localization in the cytosol [5]. Again this is in line with our results for the fluorescent tagged C-α1/β1-heterodimer and the fluorescent tagged α1/β1-heterodimer. In a minority of cells (12 %), a filamentous staining was observed in human embryonic cells using an antibody that recognizes both the α1-subunit and the C-α1 splice form. These cells were not analyzed for the β1-subunit expression. So it remains a possibility that these cells do express no or less β1-subunit. This would be consistent with our finding that the fluorescently tagged C-α1 splice form shows a different subcellular localization and redox environment alone than in the presence of the β1-subunit.
The endoplasmic reticulum is involved in heme trafficking, heme degradation and heme insertion into hemoproteins in eukaryotic cells [22]. Biochemical analysis in native human embryonic cells showed small amounts of the canonical α1-subunit in the fraction containing the endoplasmic reticulum. The function of the H-NOX domain of the α-subunits that is absent in the C-α1 splice variant is not clear. Very recently it has been suggested based on overexpression and purification of the H-NOX-α1-fragment in E. coli that it may bind heme via a non-covalent interaction [23]. It is possible that the lack of this domain in the C-α1 protein leads to trapping of the splice variant at the site of heme insertion, while the canonical α1-subunit or the α1/β1-heterodimer interacts only transiently with the endoplasmic reticulum e.g. during heme insertion or maturation.
Sharina et al. showed that the C-α1 splice variant is more stable in intact cells in the presence of ODQ, a well-known oxidant [24] while the canonical α1-subunit is more rapidly degraded under these conditions. We show in the current paper that fluorescent labeled overexpressed C-α1 protein is targeted to a more oxidized environment in comparison to the canonical α1-subunit. Thus the higher stability of the C-α1 protein in the presence of oxidants may represent an adaption to the redox environment at the endoplasmic reticulum because it is well known to be a more oxidized compartment than the cytosol [6]. NOsGC activators, like cinaciguat, which act at the H-NOX domain of the β1-subunit enhance the stability of the protein through inhibition of proteosomal degradation which can be induced by oxidants like ODQ in the cell [25], [26]. It is thus conceivable that heme or ligand free H-NOX domains not only of β1 but also of α1 subunits provide a signal that leads to more rapid degradation.
In summary, we present evidence that the amino-terminal H-NOX domain of the α1-subunit does not preclude quantitative dimerization with the β1-subunit to form a stable active, heme containing NOsGC-heterodimer but is important for subcellular localization.
Materials and Methods
Materials
Cinaciguat (BAY 58-2667) was a generous gift from Johannes-Peter Stasch (Bayer Pharma AG, Wuppertal, Germany). The Sf-9 cells were obtained from Invitrogen (Karlsruhe, Germany), the HEK-293 cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ) (Brunswick, Germany) and the Sf-9 Easy Titer cell line (Sf-9ET) was a generous gift from Dr. Dominic Esposito (National Institutes of Health, Rockville, USA). D-desthiobiotin, avidin and Strep-Tactin® Superflow® high capacity resin were purchased from IBA, Goettingen, Germany. 2-diethyl-1-nitroso-oxyhydrazine (DEA/NO), 2-(4-Hydroxyphenylazo)benzoic acid (HABA), creatine kinase, hemin, lipid medium supplement and all other chemicals, in the highest grade of purity, were obtained from Sigma-Aldrich, Munich, Germany. [α-32P]GTP (400 Ci/mmol) was purchased from Hartmann Analytic, Brunswick, Germany. All primers used for site directed mutagenesis, were obtained in HPLC purity grade from biomers.net, Ulm, Germany.
Cloning of α1 Deletion Mutants
For construction of the α1ΔN236 mutant, a PvuII/HindIII fragment of the α1 full-length (see ref. [3]) clone was ligated StuI/HindIII into pFastBac™1vector (Invitrogen, Karlsruhe, Germany). Cloning of the human amino-terminal deletion mutant α1ΔN259 has been described previously [3].
Cloning of carboxy-terminal Strep Tag II β1-subunit (Sf-9 system)
For construction of the carboxy-terminal Strep Tag II with the β1-subunit a PvuI/StuI fragment of the conjoined NOsGC construct β1α1-Strep [27] was cloned using PvuI/SmaI into the pFastBac™1 vector.
Cloning of α1WT and C-α1 fused with fluorescent proteins for determination of fluorescence lifetime
For construction of α1 full-length in pECFP-N1 (Clontech, Mountain View, CA, USA) the α1 cDNA in pcDNA3.1/V5/His-TOPO described by Haase et al. [27] was cloned HindIII/XhoI into HindIII/SalI pECFP-N1. Cloning of the C-α1 (rat α1ΔN258) was done by introduction of suitable restriction sites by site directed mutagenesis into this construct, restriction and religation. At the same time the Kozak consensus sequence [28] was optimized and a start-methionine had to be introduced. For construction of C-α1-ECFP the following primer pair was used: 5′-GAA CCA GCC CTA TTT GCT CGA GTC GGT CGC CAT G GA GAG CAC CAA GCC TTC TCT-3′ and 5′-AGA GAA GGC TTG GTG CTC TC C ATG GCG ACC GAC TCG AGC AAA TAG GGC TGG TTC-3′. The initiation codons or complementary sequence are bold type and the modified nucleotides are underlined. Using the respective restriction sites in pECFP before the insert XhoI digestion and religation led to C-α1-ECFP. Due to a slight difference in the amino acid sequence rat α1ΔN258 correspond to human α1ΔN259 (Data S1).
Cloning of β1 fused with EYFP for determination of fluorescence lifetime
The cloning has been described previously [27].
Exchange of ECFP and EYFP
The carboxy-terminal ECFP-fusions of α1 full-length and C-α1 were exchanged for EYFP and the carboxy-terminal EYFP-fusion of β1 was exchanged for ECFP using the pEYFP-N1 or pECFP-N1 vector (Clontech) and AgeI/BsrGI as restriction enzymes.
Cloning of β1 in pcDNA3.1/V5/His-TOPO for Expression in HEK-293-cells
The cloning has been described previously [27].
Cloning of ECFP-HO1 in pECFP-C1
The human heme oxygenase 1 (HO1) was amplified using the primer pair (5′-CCC AGC ACC GGC CGG ATG GAG-3′/5′-TTC AGT GCC CAC GGT AAG GAA GC-3′) und the FirstChoice™ PCR-Ready Human Placenta cDNA (Ambion, Austin, USA). The PCR product was subcloned into pCR®2.1-TOPO® vector. Through EcoRI/XbaI restriction the insertion into pFastBac™1 was performed. Using EcoRI/KpnI the cDNA was transferred into pECFP-C1 [29].
Cloning of Grx1-roGFP2-tagged α1 variants
Grx1-roGFP2 in pLPCX (Clontech) was a kind gift from Dr. Tobias Dick (DKFZ, Heidelberg, Germany) [18]. Through restriction with BglII/BsrGI the construct was ligated into pEGFP-N1. Using SmaI/XbaI ECFP in α1-ECFP (s.a.) was exchanged for Grx1-roGFP2 out of pLCPX (Eco47III/XbaI restricted). After ApaI restriction and religation the frame was restored.
Restriction of C-α1-EYFP and α1-Grx1-roGFP2 with Eco47III/BstEII led to an exchange of the full-length construct for the splice variant.
Control of sequences
All cloned constructs were verified by sequencing (GATC Biotech, Konstanz, Germany).
Baculovirus Generation
Recombinant baculoviruses of respective subunits were generated using the Bac-to-Bac® Baculovirus Expression System (Invitrogen).
Sf-9 Cell Culture, Expression of Recombinant Guanylyl Cyclase Subunits and Preparation of Cytosolic Fraction
Sf-9 cells were cultured in Sf-900™ II serum-free medium (Invitrogen) supplemented with 1 % penicillin/streptomycin (PAA Laboratories, Coelbe, Germany) and 10 % fetal bovine serum (Foetal Bovine Serum Gold, EU-approved, PAA Laboratories). Spinner cultures were grown at 27°C at 140 rpm shaking on a 50 mm orbit platform and diluted to 2 • 106 cells/ml for infection. 500 ml of cell solution were infected with the respective recombinant baculovirus stock with the multiplicities of infection (MOI) of 1. The MOI was determined using Sf-9ET cell line according to the recent published paper by Hopkins and Esposito [30]. After 72 h cells were harvested by centrifugation (4,000 g for 1 min at 4°C). All following steps were performed on ice. The cell pellet was resuspended in 30 ml of lysis buffer containing 50 mM TEA-HCl, 1 mM EDTA, 10 mM dithiothreitol (DTT), 250 nM Avidin, pH 7.4, and complete EDTA-free Protease Inhibitor Cocktail Tablets (Roche, Mannheim, Germany). The cells were lysed by sonication. Cytosolic fractions were obtained by centrifugation for 180 min at 15,000 g at 4 °C. The cytosolic fractions were filtered through a 0.2 µm syringe filter (Sartorius, Goettingen, Germany). 2 ml aliquots of cytosolic fractions were kept as reference for experiments to monitor the purification at all steps. For investigation of the influence of heme supplement and additionally of lipid medium supplement 4 mg/l hemin or/and 1 % lipid medium supplement were added.
One-Step-Purification of NOsGC
The purification was performed on ice or at 4°C. The chromatographic step was performed on an ÄKTApurifier 100 system (GE Healthcare, Munich, Germany). Cytosolic fractions were immediately applied to a Strep-Tactin® Superflow® high capacity resin (2 ml Volume in a Tricorn™ 10/20 column) at 1 ml/min [31]. Buffer W contained 100 mM Tris-HCl, 1 M NaCl, 1 mM EDTA, 1 mM benzamidine, 10 mM DTT, pH 8.0. Buffer E was prepared by adding 2.5 mM D-desthiobiotin to buffer W. The column was washed ad 1 ml/min with buffer W for 5 column volumes (CV). With 5 CV buffer E the elution of the NOsGC was performed. Monitoring the absorbance at 254 nm, 280 nm and 430 nm showed a single peak in the fraction which contained NOsGC. Regeneration of the column was performed by 15 CV buffer R (100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM HABA, pH 8.0) at 1 ml/min and 8 CV of buffer W at 1 ml/min The pooled fractions (approximately 15 ml) were concentrated to a volume of about 500 µl using an Amicon Ultra-15 centrifugal filter unit with 30 kDa cut-off (Millipore, Schwalbach, Germany).
Determination of Protein Concentration and Guanylyl Cyclase Activity Assay
Protein concentrations were determined by the Warburg-Christian method [32] using a NanoPhotometer™ (Implen, Munich, Germany). Guanylyl cyclase activity was measured as described previously [3]. Purified NOsGC was diluted with 50 mM TEA-HCl, 10 mM DTT, 1 mM EDTA, 0.5 µg/µl bovine serum albumin (Roth, Karlsruhe, Germany), pH 7.4, quick-frozen in liquid nitrogen with 10 % (v/v) glycerol and stored at -80°C. Enzyme activity of purified protein (50 ng of protein per assay tube) were determined by incubation for 10 min at 37°C in the presence of 1 mM cGMP, 0.5 mM [α-32P]GTP (about 0.2 µCi), 3 mM MgCl2, 50 mM TEA-HCl, pH 7.4, 0.25 g/liter creatine kinase, 5 mM creatine phosphate, and 1 mM 3-isobutyl-1-methylxanthine in a total volume of 100 µl as described by Schultz and Boehme [33]. Reactions were started by the addition of protein and incubation at 37°C. All experiments were stopped by ZnCO3 precipitation, and purification of the enzyme-formed cGMP was performed as described previously. Basal enzyme activity measurements were performed in the absence of NO or cinaciguat. Measurements of stimulated enzyme were performed in the presence of the NO donor DEA/NO or cinaciguat. DEA/NO was dissolved in 10 mM NaOH, which did not affect the enzyme activity (data not shown). Cinaciguat was dissolved in 100 % DMSO and then diluted in distilled water to a final concentration of 10 µM so that the final DMSO concentration in the enzyme assay did not exceed 2.5% (v/v). At this concentration no effects of DMSO on enzyme activity were observed (data not shown).
SDS-polyacrylamide electrophoresis gels and immunoblot analysis
Aliquots of 50 µg (cytosolic fractions) or 5 µg (purified enzyme) protein were heated for 3 min at 105°C with lidheat of 120°C (PCR-Cycler) in a modified Laemmli sample buffer (50 mM Tris-HCl, 1% SDS, 100 mM DTT, 30% Glycerol, pH 7.5). After the heat-incubation 1 µl of a blue sample puffer [10% (m/w) bromophenol blue solved in the modified Laemmli buffer] were added and the probes were resolved on 10% slab gels. Proteins were stained according to Kang et al. 2002 [8]. As protein markers predominantly PageRuler™ Prestained Protein Ladder and PageRuler™ Unstained Protein Ladder from Fermentas (St. Leon-Rot, Germany) were used. For immunoblotting, protein fractions were transferred electrophoretically to a nitrocellulose membrane [Amersham™ Hybond™ ECL (GE Healthcare)]. The membrane was reversibly stained with Ponceau S to evaluate the protein transfer. Unspecific binding sites were saturated by immersing the membrane for 1 h at room temperature in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% (v/v) Tween 20) containing 5% nonfat dry milk. The following antibodies against the two NOsGC subunits were used for detection: anti-α1 (1∶5,000) (Sigma, G4280) or α1-1200 (1∶1,000; as described in [3]) and anti-β1 (1∶4,000) (Sigma, G4530). Antibodies were incubated for 1 h in TBST-buffer at room temperature. The membranes were washed three times for 10 min with TBST and subsequently incubated for 1 h with horseradish-peroxidase-conjugated anti-rabbit IgG antibody (1∶2,000/Cell Signaling Technology, distributor: New England Biolabs, Frankfurt am Main, Germany or 1∶4,000/Sigma). After three washes with TBST-buffer the membranes were processed with the enhanced chemiluminescence western blotting detection system according to the recommendations of the manufacturer (Roche) and the signals were detected with a charge-coupled device camera (Intas, Goettingen, Germany) or the membranes were processed with ECL Western blotting detection system according to manufacturer's recommendations (Amersham Pharmacia Biotech, Piscataway, USA).
Quantification of heterodimerization
For quantification of subunit interaction, the Coomassie stained gels were either captured using a white trans-illuminator and a charge-coupled device camera (HighRes, Intas) or scanned using ScanMaker i900 (Microtek, Willich, Germany). The analysis was done using LabImage 1D (Kapelan, Leipzig, Germany). For the wild-type of the human α1-subunit and C-α1 the densitometric values of α1 full-length and the truncation were normalized to the value of β1 in the same lane and dimerization is given as a percentage of α1WT dimerization, which was set to 100%. Western blots were analyzed accordingly with similar results (data not shown).
Fluorescence Lifetime Imaging (FLIM) using a confocal laser scanning microscope
Determination of fluorescence lifetime was done as described previously [9]. For microscopy HEK-293 cells were seeded in 24-well imaging plates with special glass bottom (zell-kontakt, Noerten-Hardenberg, Germany; distributed by PAA Laboratories, Coelbe, Germany) and transfected with the cDNA coding for the respective constructs for the expression of fluorescent tagged NOsGC subunits using Lipofectamin™ LTX (Invitrogen). 48 h post transfection cells were imaged at 37°C on a Nikon Ti-E microscope equipped with an incubation chamber (Okolab, Naples, Italy) using a 60-fold immersion objective (NA 1.4, Nikon). For live cell imaging culture medium was removed, cells were washed twice and supplemented with Hank's balanced salt solution.
Fluorescence decays were measured in cells expressing the FRET donor alone (ECFP) using the vector pECFP-N1 (Clontech) as a negative control. The rat α1-subunits, wild-type and amino-terminal truncation are carboxy-terminally tagged with ECFP as FRET donor and the rat β1-subunit is carboxy-terminally tagged with FRET acceptor EYFP using the vector pEYFP-N1 (Clontech) or vice versa. Images were collected using a 405 nm pulsed laser. Emitted fluorescence signals were selected using a CFP bandpass filter (475/20 nm). FLIM images in the time domain from fluorescent cells were recorded with a 4 channel time gated detection system (LiMo module, Nikon). The images were 256×256 pixels in size and the acquisition time was 5 min.
Localization of α1 constructs by confocal laser scanning microscope
The cells were prepared as described above. Amino-terminally ECFP-tagged heme oxygenase 1 (human, HO-1) was used as a marker for the endoplasmic reticulum [11].
Analyzing the redox state of the C-α1 and the α1 wild type protein
Using fusion proteins of the respective α1 isoforms with the redox sensor Grx1-roGFP2 we performed a ratiometric measurement. The cells were simultaneously excited with a 405 nm and a 488 nm laser. The emission was collected by a GFP bandpass filter (525/50 nm). The ratio of the 405/488 emission corresponds to the redox state. A high value means a more oxidized state and vice versa.
Statistical analysis
The results are expressed as means ± SEM of at least three independent experiments. All results were controlled for their statistical significance by Student's t-test. A value of p<0.01 was considered to be statistically significant.
Supporting Information
Amino acid sequence alignment of human (Accession number: NP_000847.2) and rat (Accession number: NP_058786.2) α1-subunit to show sequence differences (performed with ClustalW2 at www.ebi.ac.uk ). Gaps are marked.
(PDF)
Nucleic acid sequence alignment (partly shown) of human α1 full-length (GB: Y15723) and both splice-variants C*-α1 (GB: BX649180)/C-α1 (GB: AK226125) (performed with ClustalW2 at www.ebi.ac.uk ). Initiation codons are marked.
(PDF)
Acknowledgments
We thank Gerlind Henze-Wittenberg, Carolin Rattunde, Anja Stieler and Ines Thomsen for their invaluable technical assistance. The generous gift of Grx1-roGFP2 by Dr. Tobias Dick (DKFZ, Heidelberg, Germany), of the Sf-9ET cell line by Dr. Dominic Esposito (National Institutes of Health, Rockville, USA) and of cinaciguat by Dr. Johannes-Peter Stasch (Bayer Pharma AG, Wuppertal, Germany) is gratefully acknowledged.
Footnotes
Competing Interests: MK is currently employed at HEPTARES therapeutics, but the data used in the manuscript were obtained during his doctoral thesis under SB's mentorship at the University Medical Center Hamburg-Eppendorf. Due to this fact, this does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials..
Funding: The study was supported by the Deutsche Forschungsgemeinschaft (INST 188/286-1; BE 1865/5-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu Y, Ruoho AE, Rao VD, Hurley JH. Catalytic mechanism of the adenylyl and guanylyl cyclases: modeling and mutational analysis. Proc Natl Acad Sci U S A. 1997;94:13414–13419. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner C, Russwurm M, Jager R, Friebe A, Koesling D. Dimerization of nitric oxide-sensitive guanylyl cyclase requires the alpha 1 N terminus. J Biol Chem. 2005;280:17687–17693. doi: 10.1074/jbc.M412099200. [DOI] [PubMed] [Google Scholar]
- 3.Koglin M, Behrends S. A functional domain of the alpha1 subunit of soluble guanylyl cyclase is necessary for activation of the enzyme by nitric oxide and YC-1 but is not involved in heme binding. J Biol Chem. 2003;278:12590–12597. doi: 10.1074/jbc.M212740200. [DOI] [PubMed] [Google Scholar]
- 4.Sharina IG, Jelen F, Bogatenkova EP, Thomas A, Martin E, et al. Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J Biol Chem. 2008;283:15104–15113. doi: 10.1074/jbc.M710269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharin VG, Mujoo K, Kots AY, Martin E, Murad F, et al. Nitric Oxide Receptor Soluble Guanylyl Cyclase Undergoes Splicing Regulation in Differentiating Human Embryonic Cells. Stem Cells and Development. 2011;20:1287–1293. doi: 10.1089/scd.2010.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer AJ, Dick TP. Fluorescent protein-based redox probes. Antioxid Redox Signal. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 7.Sharina IG, Cote GJ, Martin E, Doursout MF, Murad F. Nitric Oxide; 2011. RNA splicing in regulation of nitric oxide receptor soluble guanylyl cyclase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang D, Gho YS, Suh M, Kang C. Highly Sensitive and Fast Protein Detection with Coomassie Brilliant Blue in Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis. Bull Korean Chem Soc. 2002;23:1511–1512. [Google Scholar]
- 9.Haase T, Haase N, Kraehling JR, Behrends S. Fluorescent fusion proteins of soluble guanylyl cyclase indicate proximity of the heme nitric oxide domain and catalytic domain. PLoS One. 2010;5:e11617. doi: 10.1371/journal.pone.0011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong K, Ikura M. The use of FRET imaging microscopy to detect protein-protein interactions and protein conformational changes in vivo. Curr Opin Struct Biol. 2001;11:573–578. doi: 10.1016/s0959-440x(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 11.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 12.Wedel B, Harteneck C, Foerster J, Friebe A, Schultz G, et al. Functional domains of soluble guanylyl cyclase. J Biol Chem. 1995;270:24871–24875. doi: 10.1074/jbc.270.42.24871. [DOI] [PubMed] [Google Scholar]
- 13.Shiga T, Suzuki N. Amphipathic alpha-helix mediates the heterodimerization of soluble guanylyl cyclase. Zoolog Sci. 2005;22:735–742. doi: 10.2108/zsj.22.735. [DOI] [PubMed] [Google Scholar]
- 14.Rothkegel C, Schmidt PM, Atkins DJ, Hoffmann LS, Schmidt HH, et al. Dimerization region of soluble guanylate cyclase characterized by bimolecular fluorescence complementation in vivo. Mol Pharmacol. 2007;72:1181–1190. doi: 10.1124/mol.107.036368. [DOI] [PubMed] [Google Scholar]
- 15.Winger JA, Marletta MA. Expression and characterization of the catalytic domains of soluble guanylate cyclase: interaction with the heme domain. Biochemistry. 2005;44:4083–4090. doi: 10.1021/bi047601d. [DOI] [PubMed] [Google Scholar]
- 16.Lin Q, Weis S, Yang G, Weng YH, Helston R, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 17.Appenzeller-Herzog C. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J Cell Sci. 2011;124:847–855. doi: 10.1242/jcs.080895. [DOI] [PubMed] [Google Scholar]
- 18.Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, et al. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 19.Baltrons MA, Pifarre P, Berciano MT, Lafarga M, Garcia A. LPS-induced down-regulation of NO-sensitive guanylyl cyclase in astrocytes occurs by proteasomal degradation in clastosomes. Mol Cell Neurosci. 2008;37:494–506. doi: 10.1016/j.mcn.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Pifarre P, Baltrons MA, Foldi I, Garcia A. NO-sensitive guanylyl cyclase beta1 subunit is peripherally associated to chromosomes during mitosis. Novel role in chromatin condensation and cell cycle progression. Int J Biochem Cell Biol. 2009;41:1719–1730. doi: 10.1016/j.biocel.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2:a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz IJ, Chen C, Paw BH, Hamza I. Iron and porphyrin trafficking in heme biogenesis. J Biol Chem. 2010;285:26753–26759. doi: 10.1074/jbc.R110.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong F, Pan J, Liu X, Wang H, Ying T, et al. J Biol Inorg Chem; 2011. A novel insight into the heme and NO/CO binding mechanism of the alpha subunit of human soluble guanylate cyclase. [DOI] [PubMed] [Google Scholar]
- 24.Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- 25.Hoffmann LS, Schmidt PM, Keim Y, Schaefer S, Schmidt HH, et al. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br J Pharmacol. 2009;157:781–795. doi: 10.1111/j.1476-5381.2009.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, et al. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res. 2009;105:33–41. doi: 10.1161/CIRCRESAHA.109.198234. [DOI] [PubMed] [Google Scholar]
- 27.Haase N, Haase T, Kraehling JR, Behrends S. Direct fusion of subunits of heterodimeric nitric oxide sensitive guanylyl cyclase leads to functional enzymes with preserved biochemical properties: evidence for isoform specific activation by ciguates. Biochem Pharmacol. 2010;80:1676–1683. doi: 10.1016/j.bcp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeanner M, Kraehling JR, Busker M, Behrends S. Distinct NADPH-cytochrome P450 reductase dependence of heme oxygenase-1 and heme oxygenase-2. Basic & Clinical Pharmacology & Toxicology. 2010;107:162–692. [Google Scholar]
- 30.Hopkins R, Esposito D. A rapid method for titrating baculovirus stocks using the Sf-9 Easy Titer cell line. Biotechniques. 2009;47:785–788. doi: 10.2144/000113238. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt TG, Skerra A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat Protoc. 2007;2:1528–1535. doi: 10.1038/nprot.2007.209. [DOI] [PubMed] [Google Scholar]
- 32.Warburg O, Christian W. Isolierung und Kristallisation des Gärungsferments Enolase. Biochem Z. 1941;310:384–421. [Google Scholar]
- 33.Schultz G, Böhme E. Methods of Enzymatic Analysis. In: Bergmeyer HU, Bergmeyer J, Grassel M, editors. 3rd ed: Verlag Chemie. Weinheim, Germany: 1984. pp. 379–389. [Google Scholar]
- 34.Derbyshire ER, Marletta MA. Handb Exp Pharmacol; 2009. Biochemistry of soluble guanylate cyclase. pp. 17–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid sequence alignment of human (Accession number: NP_000847.2) and rat (Accession number: NP_058786.2) α1-subunit to show sequence differences (performed with ClustalW2 at www.ebi.ac.uk ). Gaps are marked.
(PDF)
Nucleic acid sequence alignment (partly shown) of human α1 full-length (GB: Y15723) and both splice-variants C*-α1 (GB: BX649180)/C-α1 (GB: AK226125) (performed with ClustalW2 at www.ebi.ac.uk ). Initiation codons are marked.
(PDF)