Abstract
Although the systematic study of meditation is still in its infancy, research has provided evidence for meditation-induced improvements in psychological and physiological well-being. Moreover, meditation practice has been shown not only to benefit higher-order cognitive functions but also to alter brain activity. Nevertheless, little is known about possible links to brain structure. Using high-resolution MRI data of 44 subjects, we set out to examine the underlying anatomical correlates of long-term meditation with different regional specificity (i.e., global, regional, and local). For this purpose, we applied voxel-based morphometry in association with a recently validated automated parcellation approach. We detected significantly larger gray matter volumes in meditators in the right orbito-frontal cortex (as well as in the right thalamus and left inferior temporal gyrus when co-varying for age and/or lowering applied statistical thresholds). In addition, meditators showed significantly larger volumes of the right hippocampus. Both orbito-frontal and hippocampal regions have been implicated in emotional regulation and response control. Thus, larger volumes in these regions might account for meditators’ singular abilities and habits to cultivate positive emotions, retain emotional stability, and engage in mindful behavior. We further suggest that these regional alterations in brain structures constitute part of the underlying neurological correlate of long-term meditation independent of a specific style and practice. Future longitudinal analyses are necessary to establish the presence and direction of a causal link between meditation practice and brain anatomy.
Keywords: Thalamus, Orbital, Hippocampus, MRI, Plasticity, VBM
Introduction
Although the systematic study of meditation is still in its infancy, research has shown that an active meditation / mindfulness practice fosters attentional and emotional self-regulation, as well as behavioral flexibility, altogether promoting well-being (Brown and Ryan, 2003). Moreover, findings in clinical populations suggest that meditation is effective in reducing a number of psychological and physical symptoms, and even biological markers of disease progression (Baer, 2003; Grossman et al., 2004; Creswell et al., 2008). These outcomes are complemented by reports of alterations in physiological parameters and biochemical measures as a consequence of meditation (Solberg et al., 2004; Doraiswami and Xiong, 2007). Finally, meditation practice has been demonstrated to affect higher functions of the central nervous system, reflected in increased performances and altered brain activity (So and Orme-Johnson, 2001; Jha et al., 2007; Srinivasan and Baijal, 2007; Doraiswami and Xiong, 2007).
Less is known about the link between meditation and brain structure. Outcomes from cross-sectional studies in normative samples (i.e., non-meditators) have suggested changes in cerebral macro-structure, such as increased gray matter (GM), in response to intensive training in a number of cognitive, sensory, and motor domains (Maguire et al., 2000; Gaser and Schlaug, 2003; Mechelli et al., 2004). Evidences for experience-, stimulus-, and practice-induced alterations in brain anatomy have been further substantiated through longitudinal studies (Draganski et al., 2004; Draganski et al., 2006; May et al., 2007; Driemeyer et al., 2008; Boyke et al., 2008). Therefore, similar effects on cerebral macro-structure are likely as a consequence of meditation which “traditionally requires a long-term commitment to daily practice” (Pagnoni and Cekic, 2007). One recent morphometric study revealed altered age effects on GM volume in practitioners of Zen meditation, but did not provide direct evidence for structural GM differences between meditators and non-meditators (Pagnoni and Cekic, 2007). To our knowledge, there are only two studies1 that specifically compared aspects of brain anatomy between the two groups (Lazar et al., 2005; Holzel et al., 2008). More specifically, when examining differences in GM concentration (Holzel et al., 2008) and cortical thickness (Lazar et al., 2005) associated with Insight meditation (Vipassana), these analyses revealed significantly increased measurements in meditators in the left inferior temporal gyrus, right anterior insula, right hippocampus, and right middle / superior frontal cortex.
To further explore the associations between meditation and brain anatomy and expand the currently rather sparse literature on this field of research, we examined high-resolution MRI data in a well-matched sample of 22 meditators and 22 controls. We used voxel-based morphometry (VBM; Ashburner and Friston, 2000), in combination with a recently developed automated parcellation approach (Tu et al., 2008), to reveal possible links between meditation and brain structure on a global, regional, and very local level. That is, we complemented (i) overall volume measurements (total brain and GM) with (ii) volume measurements of pre-defined (sub)cortical regions of interest (ROIs), and with (iii) voxel-wise analyses of GM across the whole brain. Our study differs from previous analyses with respect to the mean duration of meditation practice, which is considerably longer in the current sample. Finally, we have included meditators who practice different styles of meditation to capture common elements among the immense variety of meditation practices (Doraiswami and Xiong, 2007), and thus reveal the underlying neural correlates of long-term meditation independent of a specific practice.
Materials and Methods
Subjects
A total of 25 active meditation practitioners were accrued through referrals and advertisements in various meditation venues. Three individuals showed macroscopic cerebral abnormalities without clinical significance and were excluded from the study. Our final sample included 22 active meditation practitioners and 22 controls from the International Consortium for Brain Mapping (ICBM) database of normal adults (http://www.loni.ucla.edu/ICBM/Databases/), matched for gender and age. There were 9 men and 13 women in each group. Age ranged between 30 and 71 years (meditators mean age: 53.00 years [SD: 11.54]; controls mean age: 53.09 years [SD: 11.38]). The maximum allowed age difference within a matched pair was one year. The level of education in meditators and controls was comparable (meditators / controls): 36% / 36% above college level; 45% / 54% college level; 14% / 9% below college level. All subjects were right-handed, except one control subject who was left-handed, where handedness was determined based on self-reports of hand preference for selected activities. All subjects were required to be free of any neurological disorders and gave informed consent according to institutional guidelines (Institutional Review Board of the University of Los Angeles, California [UCLA]).
Years of meditation practice ranged between five and 46 years (mean: 24.18 years [SD: 12.36]), where styles included Zazen, Samatha, Vipassana, and others. Although long-time practices can vary greatly (over time and with respect to the mental exercises performed), more than half of all meditators indicated deep concentration as being an essential part of their practice (63%). About a third of them engaged control of breath (36%), visualization (32%), as well as attention to external and internal stimuli / events (32%). Other elements, however less frequently indicated, included withdrawal of sensory perceptions (14%) and letting go of thoughts (18%). The length of formal meditation ranged from 10 to 90 minutes each session, with the majority of meditators (59%) having sessions daily.
Image Acquisition
Brain images were acquired on a 1.5-T MRI system (Siemens Sonata) using a 3D T1-weighted sequence (MPRAGE) with the following parameters: TR = 1900 ms; TE = 4.38 ms; flip angle = 15°; 160 contiguous 1 mm sagittal slices; FOV = 256 mm × 256 mm2; matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm.
Voxel-based GM Volume Analysis (Local Approach)
Data were processed and examined using the SPM5 software (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm), where we applied VBM standard routines and default parameters implemented in the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Images were bias field corrected, tissue classified, and registered using linear (12-parameter affine) and non-linear transformations (warping), within the same generative model (Ashburner and Friston, 2005). Subsequently, analyses were performed on GM segments, which were multiplied by the non-linear components derived from the normalization matrix in order to preserve actual GM values locally (modulated GM volumes). Importantly, GM segments were not multiplied by the linear components of the registration in order to account for individual differences in brain orientation, alignment, and size globally. Finally, the modulated GM volumes were smoothed with a Gaussian kernel of 14 mm full width at half maximum (FWHM). These smoothed modulated GM volumes are hereafter referred to as GM to simplify matters.
Voxel-wise GM differences between active meditators and controls were examined using independent-sample t-tests. In order to avoid possible edge effects between different tissue types, we excluded all voxels with GM values of less than 0.1 (absolute threshold masking). Statistical outcomes were corrected using small volume corrections, applying a 60 mm diameter of a sphere, and family-wise error (FWE) corrections for multiple comparisons. Significant outcomes were restricted to clusters exceeding 693 voxels (spatial extent threshold), in order to decrease the risk of detecting spurious effects due to noise. This spatial extent threshold corresponds to the expected number of voxels per cluster, calculated according to the theory of Gaussian random fields.
Supplemental Voxel-based GM Volume Analysis (Local Approach): Co-varying for Age
Although meditators and controls were carefully matched for age, we conducted an additional VBM analysis comparing meditators against controls (as described above), while co-varying for age. Again, we excluded all voxels with GM values of less than 0.1 (absolute threshold masking). Since these analyses were exploratory, we abstained from applying corrections for multiple comparisons (i.e., outcomes are presented as uncorrected findings at p<0.001) and applied an extent threshold of k=279 (corresponding to the expected number of voxels per cluster, re-calculated according to the new adjusted model).
Total Brain and GM Volume Analysis (Global Approach)
Using the tissue-classified partitions from the VBM analysis (i.e., GM, white matter [WM], and cerebrospinal fluid [CSF]), overall volumes were determined in cm3 as the sum of voxels representing GM+WM+CSF (total brain volume) or GM only (total GM volume). Both (a) total brain volumes and (b) total GM volumes were compared between meditators and control subjects using independent-sample t-tests.
Parcellated Volume Analysis (Regional Approach)
To optimize the automated structure parcellation, we applied a whole new series of preprocessing steps to all raw images. Specifically, image volumes were skull-stripped (Smith, 2002), bias-corrected (Shattuck et al., 2001), and registered to the ICBM-452 (Warp 5) atlas (http://www.loni.ucla.edu/ICBM/Downloads/Downloads_452T1.shtml) using affine parameter transformations (Woods et al., 1998).
Cortical and subcortical structures were then parcellated using a validated hybrid discriminative / generative model, as detailed elsewhere (Tu et al., 2008). Briefly, low-level information (i.e., signal intensity and local geometric properties) are used to determine the probability that a voxel belongs to a given structure. High-level information (i.e., global shape information), in connection with local smoothness constraints, are used to enforce the connectivity of each structure and its shape regularity. The applied hybrid model integrates both low- and high-level information into a unified system to reveal a gridface structure (voxel-wise labeling) which explicitly represents the three-dimensional topology of a particular region.
We automatically generated labels for the following regions: (1) left inferior temporal gyrus; (2) right insula; (3) right hippocampus; (4) right superior frontal gyrus; and (5) right middle frontal gyrus. These regions were selected based on outcomes of the two existing morphometric studies comparing brain anatomy between meditators and non-meditators (Lazar et al., 2005; Holzel et al., 2008)1. Brain size-adjusted (i.e., scaled) volumes of these cortical and subcortical structures were determined in cm3 as the sum of voxels belonging to a particular label, followed by comparing regional volume measures between meditators and controls using independent-sample t-tests.
Relationships between local GM and Number of Meditation Years
Finally, in order to compare our findings with others in the literature indicating positive correlations between meditation experience and brain structure, we also set out to examine whether there is an effect of the duration of meditation practice (measured in years) on local GM. For this purpose, we conducted two different analyses: First, we examined voxel-wise correlations between local GM and the number of meditation years in a regression analysis. However, we also speculated that possible positive correlations between meditation experience and GM are likely to be modulated and/or canceled out by changes in brain structure that are shown to occur with age (i.e., older subjects have the longest meditation history but are more prone to brain atrophy (Sowell et al., 2003)). Unlike previous studies, the current study used a sample with a large age range (30–71 years), where 68 percent of all meditators were older than 50 years. Given that the trajectory of age effects varies considerably across different brain regions (Sowell et al., 2003), simply integrating age as a co-variate in the statistical model is likely to further bias outcomes.
Thus, we decided to take the alternative approach of splitting the meditation groups based on years of meditation experience, and compared the two groups of meditators against two age-matched control groups using a 2×2 ANOVA. More specifically, one meditation group (Sample A) included individuals with less than 20 years of meditation experience (n=13) and the second meditation group (Sample B) included individuals with more than 20 years of meditation experience (n=9). The separation of these two groups was based on plotting the number of meditation years, where the clustering of the data revealed the 20 years-marker as the main separator between data clouds. The two control groups (Sample A / Sample B) included n=13 / n=9 subjects, respectively. This analysis strategy allowed for the additional examination of interactions between group status (Meditators / Controls) and mediation experience (Sample A / Sample B).
For both approaches, in correspondence with the original VBM analysis (described above), we excluded all voxels with GM values of less than 0.1 and corrected statistical outcomes using small volume corrections as well as family-wise error (FWE) corrections for multiple comparisons.
Results
Local GM Volumes
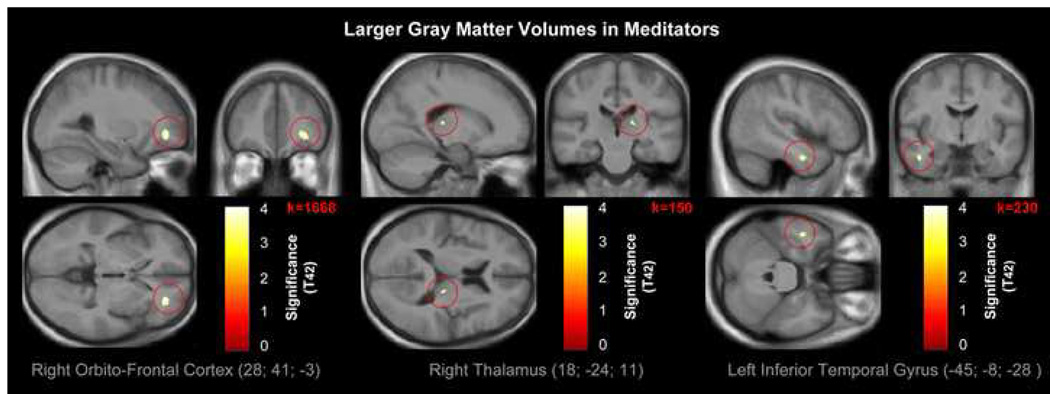
We detected one large cluster of significantly increased GM in meditators compared to controls in the right orbito-frontal cortex (Figure 1, left panel). More specifically, this cluster is located at the border between inferior and middle frontal gyrus (orbital sections) and in approximate distance to Brodmann areas (BA) 11, 12 and 47. Details with respect to cluster size (number of voxels), significance (p) and MNI coordinates (x; y; z) are provided in Table 1. A box plot illustrating the group difference (in percent) at the peak voxel with GM parameter estimates normalized to zero is provided in Supplemental Figure 1
Figure 1. Larger GM volumes in meditators.
Views of the right orbito-frontal cortex (left panel; p<0.04FEW-corr), right thalamus (middle panel; p<0.0005uncorr), and left inferior temporal gyrus (right panel; p<0.0005uncorr), where GM is larger in meditators compared to controls. The color intensity represents T-statistic values at the voxel level. The results are visualized on the mean image derived from the 44 T1-weighted scans of the subjects analyzed, and presented in neurological convention (right is right).
Table 1.
Regions of increased gray matter in meditators (local approach)
| Region | Cluster Size (# of voxels) |
Significance (p) |
MNI coordinates (x; y; z) |
|---|---|---|---|
| Without co-varying for age | |||
| Right Orbito-frontal Cortex | 1668 | 0.04* | 28; 41; −3 |
| Left Inferior Temporal Lobe | 230 | 0.0005+ | −45; −8; −28 |
| Right Thalamus | 150 | 0.0005+ | 18; −24; 11 |
| When co-varying for age | |||
| Right Orbito-frontal Cortex | 745 | 0.0001+ | 28; 41; −3 |
| Left Inferior Temporal Lobe | 203 | 0.0003+ | −45; −8; −28 |
| Right Thalamus | 549 | 0.0003+ | 21; −22; 14 |
| Left Paracentral Lobe | 175 | 0.0004+ | −12; −9; 54 |
| Right Paracentral Lobe | 192 | 0.0005+ | 22; −23; 48 |
Corrected for multiple comparisons using family-wise error (FWE) and small volume corrections
Uncorrected for multiple comparisons
Of note, when we abstained from applying the specified spatial extent threshold and from applying corrections for multiple comparisons, we detected two additional clusters of increased GM in meditators. One was located in the right thalamus (Table 1 and Figure 1, middle panel). The other one was observed in the left inferior temporal gyrus (Table 1 and Figure 1, right panel). There were no regions where controls had significantly more GM than meditators.
Local GM Volumes: Co-varying for Age
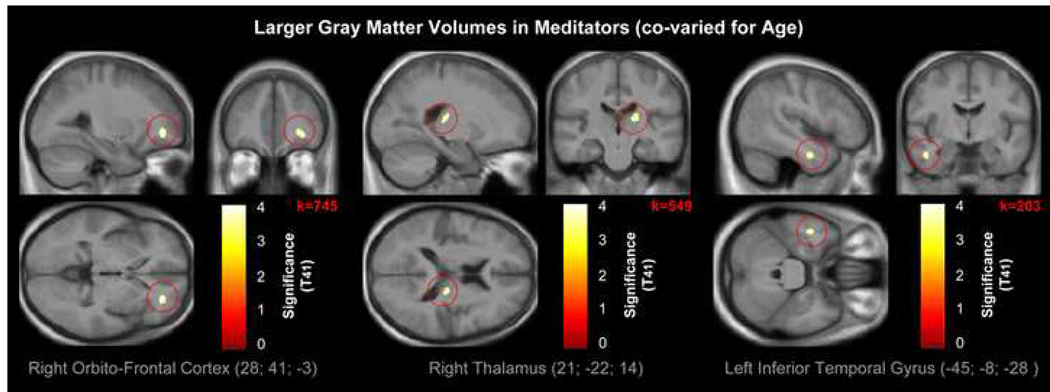
When controlling for age, we detected the same difference cluster in the right orbito-frontal cortex as described above, albeit cluster dimensions were diminished (Table 1 and Figure 2, left panel). Similarly, we observed a cluster of significantly increased GM in meditators compared to controls in the right thalamus (Table 1 and Figure 2, middle panel). However, cluster dimensions were increased compared to the outcomes without co-varying for age. When we lowered the applied spatial extent threshold down to 200 voxels, we also detected increased GM in meditators in the left inferior temporal gyrus (Table 1 and Figure 2, right panel), albeit cluster dimensions were diminished. There were no regions where controls had significantly more GM than meditators.
Figure 2. Larger GM volumes in meditators (co-varied for age).
Views of the right orbito-frontal cortex (left panel; p<0.0001uncorr), right thalamus (middle panel; p<0.0003uncorr), and left inferior temporal gyrus (right panel; p<0.0003uncorr), where GM is larger in meditators compared to controls. The color intensity represents T-statistic values at the voxel level. The results are visualized on the mean image derived from the 44 T1-weighted scans of the subjects analyzed, and presented in neurological convention (right is right).
When we abstained from applying the specified spatial extent thresholds altogether, there were two additional regions of increased GM in meditators. They were located in approximate distance of the paracentral lobes in the left and right hemisphere (Table 1 and Supplemental Figure 2).
Global and Regional Volumes
Meditators and controls did not differ with respect to total brain volume (p=0.98) and total GM volume (p=0.52), or any of the following ROI volumes: left inferior temporal gyrus (p=0.70); right insula (p=0.75); right superior frontal gyrus (p=0.10); and right middle frontal gyrus (p=0.14). However, meditators showed significantly larger volumes of the right hippocampus (p=0.01). There was no region where controls had significantly larger ROI volumes than meditators. Group-specific means and standard deviations of all morphological measures are shown in Table 2.
Table 2.
Morphological measurements (global and regional approach)
| Meditators (n=22) | Controls (n=22) | ||
|---|---|---|---|
| Global Volume Measures: Mean [SD] | |||
| (a) Total Brain Volume | 1774.17 cm3 [210.54] | 1732.97 cm3 [211.34] | |
| (b) Total GM Volume | 653.14 cm3 [90.75] | 652.53 cm3 [80.94] | |
| Regional Volume Measures: Mean [SD] | |||
| (1) Left Inferior Temporal Gyrus | 21.54 cm3 [1.22] | 21.38 cm3 [1.42] | |
| (2) Right Insula | 7.34 cm3 [0.60] | 7.28 cm3 [0.53] | |
| (3) Right Hippocampus* | 3.73 cm3 [0.21] | 3.53 cm3 [0.29] | |
| (4) Right Superior Frontal Gyrus | 57.31 cm3 [1.49] | 58.17 cm3 [1.85] | |
| (5) Right Middle Frontal Gyrus | 57.80 cm3 [1.58] | 58.50 cm3 [1.49] | |
SD: standard deviation
Significant: p<0.01
Relationships between local GM and Number of Meditation Years
Significant correlations between local GM and number of meditation years were absent. Similarly, there was no significant main effect of meditation experience (Sample A versus Sample B). Finally, there was no significant interaction between group status (Meditators / Controls) and meditation experience (Sample A / Sample B). Of note, significant main effects of group status (Meditators versus Controls) correspond to outcomes based on local GM volumes examined via independent sample t-tests (see above).
Discussion
We applied VBM in association with an automated parcellation approach to complement measurements of voxel-specific GM across the whole brain with measurements of structure-specific volumes in predefined regions. In agreement with previous studies, the present analyses revealed significantly larger cerebral measurements in meditators compared to controls. More specifically, we detected significantly increased GM in the right orbito-frontal cortex (as well as in the right thalamus and left inferior temporal lobe when co-varying for age and/or lowering applied statistical thresholds), and also significantly larger volumes of the right hippocampus. In addition, we observed increased GM in two clusters of the left and right paracentral lobe. However, given that these latter mentioned regions were not evident without co-varying for age even when statistical thresholds were lowered (k=0; p<0.001), we will abstain from commenting on these clusters, but provide them as preliminary findings as a potential reference for future studies.
Increased GM within the Orbito-Frontal Cortex
Structural associations with meditation in (pre)frontal regions have been reported previously, albeit data lack consistency with respect to the exact location. For example, Lazar and colleagues (2005) detected an increased cortical thickness in the middle and superior frontal gyrus in meditators compared to non-meditators. Holzel and colleagues (2008) observed meditation effects in the medial orbito-frontal cortex when establishing positive correlations between the cumulated hours of meditation and GM concentration. The current study revealed increased GM in meditators within an orbito-frontal region, located more inferiorly (compared to Lazar et al.) and more laterally (compared to Holzel et al.) than previous findings. Notwithstanding, the orbital effects detected by Holzel and colleagues are in approximate spatial correspondence with current outcomes along the y and z axes (peak x; y; z: 1; 45; −16 [Holzel et al., 2008] versus 28; 41; −3 [current study]). An even higher spatial correspondence with the location of the current cluster was revealed when examining functional correlates of mindfulness via functional MRI (fMRI) (Creswell et al., 2007). The authors reported that dispositional mindfulness was positively associated with activation in the right ventrolateral prefrontal cortex (peak x; y; z: 38; 44; 0 [Creswell et al., 2007]). Finally, Newberg and colleagues (2001) used single photon emission computed tomography (SPECT) and detected an increased regional cerebral blood flow during meditation in the inferior and orbital frontal cortices (no coordinates provided).
As summarized elsewhere (Davidson et al., 2000), a number of functional, behavioral, and lesion studies have provided evidence that the orbito-frontal cortex is closely linked with emotion. According to Quirk and Beer (2006), several analyses detected activation in orbito-frontal regions in association with suppressing or reappraising negative emotional stimuli and with suppressing the influence of negative emotional stimuli on subsequent behavior. Thus, the currently observed increased GM in the orbito-frontal cortex in active meditation practitioners might reflect meditators’ outstanding abilities linked to emotional self-regulation and behavioral flexibility (Brown and Ryan, 2003). Further support for the assumption that increased GM in the orbito-frontal cortex might be associated with less habitual or automatic functioning comes from studying the neurobiological basis of the framing effect (Tversky and Kahneman, 1981). It was demonstrated that individuals who were less susceptible to the framing effect (i.e., showed more consistency across decisions, regardless of the manner in which available choices are presented) had significantly enhanced activities in the right orbito-frontal cortex (DeMartino et al., 2006). Interestingly, the spatial correspondence of that cluster (peak x; y; z: 24; 30; −10) was noticeably similar to the observed cluster in the current study (peak x; y; z: 28; 41; −3). Thus, meditators might posses the underlying neuronal correlates that allow disengagement from automatic thoughts and habits, and therefore permit the consideration of options that would be more congruent with needs and values (Brown and Ryan, 2003). The specific nature of these underlying correlates (e.g., possibly enhanced neuropil, neuronal size, number, and density, size, and/or a particular wiring pattern of neuronal connections in meditators) remains to be established in future studies.
Increased GM within the Thalamus and Inferior Temporal Gyrus
When controlling for age and/or lowering applied statistical thresholds, we detected not only group effects in the already discussed right orbito-frontal cortex, but also in the right thalamus, as well as in the left inferior temporal gyrus. Given the exploratory nature of this supplemental analysis2, the observed findings of increased thalamic and inferior temporal GM clearly require further investigation. Nevertheless, meditation-associated effects in the thalamus (Lou et al., 1999; Newberg et al., 2001; Newberg and Iversen, 2003; Lutz et al., 2008) and in the temporal lobe (Lou et al., 1999; Lazar et al., 2000; Holzel et al., 2007; Holzel et al., 2008) have been reported in prior studies.
In particular, Newberg and colleages (2001) detected an increased regional cerebral blood flow during meditation in the right thalamus. In a subsequent review on the neural basis of meditation, they comment on the potential role of the thalamus as a regulator of the flow of sensory information. They suggest, for example, that an increase in thalamic activation during meditation might result in a decrease of sensory input entering the posterior superior parietal lobule which, in turn, might lead to an enhanced sense of focus (Newberg and Iversen, 2003). The latter is often attained during the state of meditation, and is also known as a characteristic trait in active meditation practitioners.
With respect to the observed effect in the left inferior temporal gyrus, there is a striking spatial correspondence between the location of the current cluster (peak x; y; z: −45; −8; −28) and a cluster in a previous VBM analysis that showed a trend towards significance (peak x; y; z: −49; −9; −28) when comparing meditators against matched non-meditators (Holzel et al., 2008). Furthermore, in that study, the mean value of GM in the left inferior temporal gyrus was positively correlated with the amount of meditation training. Altogether, this emphasizes the relevance of the inferior temporal gyrus in the process of meditating, and/or the experience of a mindful state, and possibly also feelings of “deep pleasure and insights into the unity of all reality”, as further discussed by Holzel and colleagues (2008).
Larger Volumes of the Hippocampus
We observed significantly enlarged hippocampal volumes in the right hemisphere in meditators, which is in good agreement with the increased GM concentration in the right hippocampus in meditators, as reported by Holzel and colleagues (2008). Moreover, functional studies using positron emission tomography (PET) or fMRI revealed increased brain activation during meditation or mindfulness of breathing in hippocampal and parahippocampal regions (Lou et al., 1999; Lazar et al., 2000; Holzel et al., 2007).
Davidson and colleagues propose an active role of the hippocampus in emotional responding (2000). They hypothesize that individuals who habitually fail to regulate their affective responses in a context-sensitive fashion may have a functional impairment of the hippocampus. Thus, it is likely that the observed larger hippocampal volumes may account for meditators’ singular abilities and habits to cultivate positive emotions, retain emotional stability, and engage in mindful behavior. Aside from its involvement in emotional processes, the hippocampus has also been shown as relevant for attentional processes and “certain types of imagery”, as summarized by Newberg and Iversen (2003). Thus, the observed increased hippocampal volumes in meditators might be partly driven by subjects of the current sample who pay attention to external and internal stimuli / events and who engage visualization. Finally, the hippocampus has also been suggested to modulate cortical arousal and responsiveness via rich and extensive interconnections with the prefrontal cortex and in close interaction with the thalamus (Newberg and Iversen, 2003). Our observation of larger right hippocampal volumes together with increased voxel-wise GM in the right orbito-frontal cortex and in the right thalamus is in striking agreement with this postulate. Future analysis using fMRI, possibly in combination with diffusion tensor imaging (DTI), may further elucidate the functional interplay between these three regions and provide unique clues with respect to the fine architecture of their inter-regional connections in meditators.
Lack of Findings in Previously Reported Regions
We did not detect group differences in some regions previously reported to show associations between meditation practice and brain structure. That is, we did not identify any effects within the right anterior insula or in right middle and superior frontal regions corresponding to Brodmann areas (BA) 9 and 10, as observed by Lazar and colleagues (2005). Nevertheless, the investigated morphological substrates in both studies differed considerably, and although cortical thickness (Lazar et al., 2005) and local GM volume (current study) are likely to be somewhat related (Narr et al., 2005), they may reflect different aspects on a micro-anatomical level. In further support of this assumption, an independent VBM study (Holzel et al., 2008), measuring GM concentration, also failed to replicate effects in BA 9/10. However, in correspondence with Lazar et al. (2005), the VBM study by Holzel et al. (2008) revealed group differences in the anterior insula, though the detected cluster was very small (i.e., k=22). These undersized cluster dimensions might explain the lack of finding in the anterior insula in the current study. Moreover, it is possible that analyzing local GM concentration (Holzel et al., 2008) will reveal different outcomes than analyzing local GM volumes (current study). More specifically, while GM concentration is based on images where GM values are locally altered due to non-linear normalization effects, GM volumes are based on images without such local volume alterations (i.e., the actual amount of local GM is preserved due to multiplying GM segments by the non-linear components of the normalization matrix).
Other sources for discrepancies between findings may include sample characteristics in general, and aspects of meditation in particular; different meditation styles, for example, involve different mental exercises and thus may recruit different neural networks (Lazar et al., 2005; Holzel et al., 2008). Our study included not only practitioners of Vipassana (as in previous studies) but a conglomerate of different styles, including Vipassana but also Zazen, Samatha and others. This might have captured the underlying neuronal correlates of common elements (rather than specific elements inherent to certain styles) among the immense variety of meditation practices. In addition, the mean duration of meditation practice, was almost three-times as long compared to previous studies (24.2 years versus 8.6 years / 9.1 years), which may constitute a possible source for diverging findings per se, but also might have affected study outcomes via interacting with the higher mean age in the current study (~53 years versus ~34 years / ~37 years).
Summary and Outlook
We suggest that the observed regionally increased GM volumes in meditators constitute part of the underlying neurological correlate of long-term meditation independent of a specific style and practice. There were no differences between meditators and controls with respect to global cerebral measurements (i.e., total brain and GM volumes), suggesting that links between meditation and brain anatomy exist on a relatively small scale.
Obviously, the outcomes of the current study do not provide any indicators for a causal relationship between the long-term practice of meditation and brain structures. On the one hand, the observed increased regional volumes in meditators might constitute an innate extreme or specific pattern of normal anatomical variability, which may have drawn an individual to meditation and/or helped maintain an ongoing practice. On the other hand, it is possible that actively meditating over an extended period has altered specific brain regions routinely engaged in the activity of meditating. In support of the latter assumption, the human hippocampus was demonstrated to retain its ability to generate neurons throughout life (Eriksson et al., 1998), where regular practice may improve the rate of adult neurogenesis and foster the preservation of newly generated neurons (Gage, 2002). Thus, it is possible that the detected enlarged hippocampal volumes in meditators constitute practice-induced alterations, perhaps due to neurogenesis and/or other processes on the micro-anatomical level (e.g., dendritic branching; synaptogenesis; angiogenesis). These latter mentioned processes might also have provided the foundation for the larger GM volumes in meditators in the orbito-frontal cortex (and possibly in the thalamus and inferior temporal lobe). A wealth of evidence for structural plasticity in frontal and hippocampal regions due to environmental enrichment comes from research on animals (Davidson et al., 2000; Diamond, 2001; Kempermann et al., 2002). More specific evidence for use-dependent regional growth in hippocampal and frontal regions in humans has been provided in recent longitudinal designs using VBM (Draganski et al., 2004; Draganski et al., 2006; Boyke et al., 2008). The authors reported that only a few months of practicing how to juggle or learning for a medical exam appeared to have induced increased regional GM. Surprisingly, outcomes from follow-up analyses suggested that “the qualitative change (i.e. learning of a new task) is more critical for the brain to change its structure than continued training of an already-learned task” (Driemeyer et al., 2008).
Though the outcomes of these previous studies are intriguing, one can not reliably extrapolate findings and conclusions to individuals who have pursued an ongoing practice of meditation that lasted over decades. Importantly, Holzel and colleagues (2008) detected a positive correlation between regional GM concentration and the cumulated hours of meditation training in the medial orbito-frontal cortex, which might provide a hint that meditation can induce changes in brain structure. However, the findings of the current correlation analysis indicated a lack of significant relationships between local GM and the number of meditation years. Although these results might be explained by confounding age effects (i.e., older subjects have more years / hours of meditation practice but are more prone to brain atrophy), we also did not reveal a significant interaction between group status and meditation experience in the 2×2 ANOVA, which accounted for possible age-effects. However, we suggest being cautious in interpreting the lack of significant relationships, given that the mean duration of meditation practice in the current study was 24.18 years (compared to 8.60 years in Holzel et al., 2008). It is likely that only the first few years of meditation are crucial in inducing GM changes (Driemeyer et al., 2008), and that these changes then stabilize. Due to the distribution of meditation experience in our sample, there was no group with only little experience (i.e., even Sample A had up to 20 years of practice) and possibly relationships between GM and meditation experience might have been overseen. In strong support of this assumption, Vestergaard-Poulsen and colleagues (2008) also did not detect any significant changes in GM density as a function of total practice hours in a group of long-term meditators with 14–31 years of meditation experience. Clearly, longitudinal studies will be necessary to determine whether structural differences in meditators constitute adaptations to short-term or long-term meditation, or whether they are actually inherent prerequisites for the beginning and continuation of their practice.
Supplementary Material
Supplemental Figure 1: Larger GM volumes in meditators. Views of the right orbito-frontal region, where GM is larger in meditators compared to controls (top panel; p<0.04FEW-corr). The color intensity represents T-statistic values at the voxel level. The results are visualized on the mean image derived from the 44 T1-weighted scans of the subjects analyzed, and presented in neurological convention (right is right). The boxplots show the relative GM difference between groups in the peak voxel (global maximum) at x; y; z = 28; 41; −3 (bottom panel).
Supplemental Figure 2. Larger GM volumes in meditators (co-varied for age). Views of the left paracentral lobe (top panel; p<0.0004uncorr) and right parancentral lobe (bottom panel; p<0.0005uncorr), where GM is larger in meditators compared to controls. The color intensity represents T-statistic values at the voxel level. The results are visualized on the mean image derived from the 44 T1-weighted scans of the subjects analyzed, and presented in neurological convention (right is right).
Acknowledgments
We warmly thank all participants for their dedication and partaking in our study. The authors thank Zhuowen Tu for providing the automated structure parcellation approach. This work was supported by the National Institutes of Health through the NIH Roadmap for Medical Research, grant U54 RR021813 entitled Center for Computational Biology (CCB). Additional support was provided by the NIH grants P41 RR013642, M01 RR000865 and by BMBF grant 01EV0709.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Another study, that was only published after the current analyses were completed (Vestergaard-Poulsen et al., 2008), revealed increased GM density in meditators in the lower brain stem, the left superior and inferior frontal gyri, in the left fusiform gyrus, and the left and right anterior lobes of the cerebellum.
Because meditators and controls were closely matched for age, additionally co-varying for age when comparing groups might result in an over-correction for possible modulating influences of age. This issue is further complicated by the potential neuroprotective effects of meditation which were suggested to diminish the ‘normal’ age-related decline of GM in active meditators (Pagnoni and Cekic, 2007). Although group effects in the right thalamus and left inferior temporal gyrus also became evident without co-varying for age, these findings neither survived corrections for multiple comparisons nor the specified extent thresholds.
References
- Ashburner J, Friston KJ. Voxel-based morphometry - The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clinical Psychology: Science and Practice. 2003;10:125–143. [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers.Soc.Psychol. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: A small randomized controlled trial. Brain Behav.Immun. 2008 doi: 10.1016/j.bbi.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom.Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol.Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- DeMartino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC. Response of the brain to enrichment. An.Acad.Bras.Cienc. 2001;73:211–220. doi: 10.1590/s0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- Doraiswami PM, Xiong CL. Does Meditation Enhance Cognition and Brain Longevity? Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1393.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning--revisited. PLoS.ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat.Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. SCAN. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, Vaitl D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci.Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect.Behav.Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann.Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–1585. [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Hum.Brain Mapp. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS.ONE. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc.Natl.Acad.Sci.U.S.A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb.Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb.Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d'Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Med.Hypotheses. 2003;61:282–291. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol.Aging. 2007;28:1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr.Opin.Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum.Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So KT, Orme-Johnson DW. Three randomized experiments on the longitudinal effects of the Transcendental Meditation technique on cognition. Intelligence. 2001;29:419–440. [Google Scholar]
- Solberg EE, Ekeberg O, Holen A, Ingjer F, Sandvik L, Standal PA, Vikman A. Hemodynamic changes during long meditation. Appl.Psychophysiol.Biofeedback. 2004;29:213–221. doi: 10.1023/b:apbi.0000039059.20738.39. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Srinivasan N, Baijal S. Concentrative meditation enhances preattentive processing: a mismatch negativity study. Neuroreport. 2007;18:1709–1712. doi: 10.1097/WNR.0b013e3282f0d2d8. [DOI] [PubMed] [Google Scholar]
- Tu Z, Narr KL, Dollar P, Dinov I, Thompson PM, Toga AW. Brain anatomical structure segmentation by hybrid discriminative/generative models. IEEE Trans.Med.Imaging. 2008;27:495–508. doi: 10.1109/TMI.2007.908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P, van BM, Skewes J, Bjarkam CR, Stubberup M, Bertelsen J, Roepstorff A. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2008 doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J.Comput.Assist.Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Larger GM volumes in meditators. Views of the right orbito-frontal region, where GM is larger in meditators compared to controls (top panel; p<0.04FEW-corr). The color intensity represents T-statistic values at the voxel level. The results are visualized on the mean image derived from the 44 T1-weighted scans of the subjects analyzed, and presented in neurological convention (right is right). The boxplots show the relative GM difference between groups in the peak voxel (global maximum) at x; y; z = 28; 41; −3 (bottom panel).
Supplemental Figure 2. Larger GM volumes in meditators (co-varied for age). Views of the left paracentral lobe (top panel; p<0.0004uncorr) and right parancentral lobe (bottom panel; p<0.0005uncorr), where GM is larger in meditators compared to controls. The color intensity represents T-statistic values at the voxel level. The results are visualized on the mean image derived from the 44 T1-weighted scans of the subjects analyzed, and presented in neurological convention (right is right).