Background: We have studied the effect of caveolin-1 deficiency on the mechanisms that regulate free arachidonic acid availability.
Results: Macrophages from caveolin-1-deficient mice exhibit elevated fatty acid incorporation and remodeling and a constitutively increased CoA-independent transacylase activity.
Conclusion: Macrophages from caveolin-1 null mice show decreased arachidonate mobilization and eicosanoid production upon cell stimulation.
Significance: Caveolin-1 may play an important and previously unrecognized role in the eicosanoid biosynthetic response of macrophages.
Keywords: Arachidonic Acid, Eicosanoid, Inflammation, Phospholipid Metabolism, Phospholipid Turnover, Lipid Signaling, Lipidomics, Transacylase
Abstract
In this work we have studied the effect of caveolin-1 deficiency on the mechanisms that regulate free arachidonic acid (AA) availability. The results presented here demonstrate that macrophages from caveolin-1-deficient mice exhibit elevated fatty acid incorporation and remodeling and a constitutively increased CoA-independent transacylase activity. Mass spectrometry-based lipidomic analyses reveal stable alterations in the profile of AA distribution among phospholipids, manifested by reduced levels of AA in choline glycerophospholipids but elevated levels in ethanolamine glycerophospholipids and phosphatidylinositol. Furthermore, macrophages from caveolin-1 null mice show decreased AA mobilization and prostaglandin E2 and LTB4 production upon cell stimulation. Collectively, these results provide insight into the role of caveolin-1 in AA homeostasis and suggest an important role for this protein in the eicosanoid biosynthetic response.
Introduction
The potent biological activity of the eicosanoids, lipid mediators that play key roles in inflammation (1), compels the cells to tightly control the free levels of their common precursor, 5,8,11,14-eicosatetraenoic acid or arachidonic acid (AA,3 20:4n-6). AA is an intermediate of a deacylation/reacylation cycle of membrane phospholipids, the so-called Lands cycle, in which the fatty acid is cleaved from the sn-2 position of glycerophospholipids by the action of phospholipase A2 and is reacylated back to phospholipids by the combined action of acyl-CoA synthetases and CoA-dependent acyltransferases (2, 3). In resting unstimulated cells, the reacylation reactions dominate over the phospholipase A2-mediated deacylation step; thus, free AA is kept at very low levels. Stimulation of the cells by receptor agonists results in the activation of intracellular phospholipases A2 (4–8). Under these conditions, the rate of AA release clearly exceeds that of reincorporation into phospholipids; hence, net accumulation of free AA occurs that is followed by its conversion into various eicosanoids.
AA bound to phospholipid is also the subject of successive transacylation reactions aimed at ensuring the proper distribution of the fatty acid within the various cellular phospholipid pools (2). This appears to be important not only for membrane homeostasis but also for the execution of appropriate cell responses during physiological and pathophysiological activation (9–12). These transacylation reactions are catalyzed by CoA-independent transacylase (CoA-IT), an enzyme that transfers AA moieties preferentially from diacyl-PC species to PE plasmalogens (2).
Caveolin-1 is the main protein in the caveolae, membrane domains enriched in cholesterol and sphingolipids that play structural and signaling roles (13–15). Caveolin-1 is abundant in adipocytes, endothelial cells, and smooth muscle cells (14). Until recently, the presence of caveolae and caveolin-1 in immune cells was a controversial issue (16, 17), and probably as a consequence of this, little information exists on its involvement in macrophage immune responses. Recently, however, caveolae have been shown to be involved in the internalization of pathogens (18).
The generation of caveolin-1 null mice (19, 20) has permitted a better understanding of the specific functions of this protein as well as its physiological relevance. Caveolin-1-deficient mice are viable and fertile and exhibit resistance to diet-induced obesity and a tendency to be leaner. In addition, caveolin-1-deficient mice show alterations in the levels of plasma lipids and lipoproteins (21, 22).
Given that caveolin-1 displays fatty acid binding properties (23), in this work we have studied the possible participation of this protein on the mechanisms that control AA availability in immunoinflammatory cells. Our data suggest that the lack of caveolin-1 promotes marked changes in the distribution of AA among cellular phospholipids, with direct consequences on the eicosanoid biosynthetic response induced by innate immune stimuli.
MATERIALS AND METHODS
Reagents
RPMI 1640 medium was from Invitrogen. [5,6,8,9,11,12,14,15-3H]AA (specific activity 211 Ci/mmol) and radioactive substrates for enzyme assays were from GE Healthcare. Thin layer chromatography plates were from Scharlab (Barcelona, Spain). All other reagents were from Sigma.
Animals
Caveolin-1-deficient mice, strain Cav-1 tm1Mls/J, and their wild type controls, strain B6129SF2/J (20), were obtained from The Jackson Laboratory (Bar Harbor, ME). For some experiments, the caveolin-1-deficient mice produced by Drab et al. (19) were also used. Mice were maintained according to the animal care standards established by the European Union.
Cell Culture
Resident peritoneal macrophages were obtained by peritoneal lavage using 5 ml of cold PBS as described previously (24). The cells were plated at 2 × 106 per well (6-well plates) in 2 ml of RPMI 1640 medium with 10% heat-inactivated serum and allowed to adhere for 20 h in a humidified atmosphere of 5% CO2 at 37 °C. Wells were then extensively washed with PBS to remove nonadherent cells. Adherent macrophages were then used for experimentation.
Measurement of [3H]AA Incorporation into Cellular Lipids
Macrophages were either untreated or treated with exogenous [3H]AA (0.25 μCi/ml) at the indicated concentrations. At different times, the reactions were stopped by replacing the incubation medium with ice-cold 0.1% Triton X-100, and total lipids were then extracted according to the method of Bligh and Dyer (25) and separated by thin layer chromatography. Phospholipids and neutral lipids were separated with hexane/ether/acetic acid (70:30:1, v/v/v) as a mobile phase. The spots were cut out and analyzed for radioactivity by liquid scintillation counting (26). For analysis of the molecular species into which the exogenous fatty acid was incorporated, [2H]AA was used instead of [3H]AA, and the various [2H]AA-containing phospholipids were analyzed by LC/MS as described below.
Macrophage Stimulation
Cells were placed in serum-free medium for 30 min before addition of zymosan (1 mg/ml) for the time indicated. Subsequently, supernatants were used for PGE2 and LTB4 determination, and the cell monolayers were scraped twice with ice-cold water. Cell phospholipids were extracted and prepared for AA content analysis by GC/MS as described below. Zymosan was prepared as described elsewhere (27). Briefly, zymosan particles were suspended in PBS, boiled for 60 min, and washed three times. The final pellet was resuspended in PBS at 20 mg/ml and stored frozen. Zymosan aliquots were diluted in serum-free medium and sonicated before being added to the cells. No endogenous phospholipase A2 activity was detected in the zymosan batches used in this study, as assessed by in vitro activity assay utilizing substrates in the form of vesicles, mixed micelles, or natural membranes in the presence or absence of calcium (28–31).
Determination of PGE2 and LTB4 Production
Supernatants from zymosan-stimulated cells were collected and assayed for PGE2 or LTB4 using ELISA kits from Sapphire Bioscience (Waterloo, New South Wales, Australia), following the manufacturer's instructions.
Assay for CoA-independent Transacylase Activity
CoA-independent transacylase activity was measured following the procedure originally described by Venable et al. (32) with slight modification. Briefly, the assay mixture was composed of 120 mm NaCl, 2 mm EGTA, 100 mm Tris-HCl (pH 7.5), cell homogenate (up to 100 μg of protein), and 5 μm 1-O-[3H]hexadecyl-2-lyso-sn-glycero-3-phosphocholine (lyso-platelet-activating factor, lyso-PAF) as a substrate, in a final volume of 0.2 ml. The assay mix also contained 10 μm thimerosal to inhibit endogenous CoA-dependent acyltransferase activity (33). Preliminary experiments demonstrated that at the indicated concentration, thimerosal quantitatively inhibited CoA-dependent acyltransferase activities in macrophage homogenates, as judged by in vitro assay (34). After incubation at 37 °C for 5 min, the reaction was stopped by the addition of 0.75 ml of chloroform/methanol (1:2). Chloroform (0.25 ml) and water (0.25 ml) were added, and the mixture was vortexed vigorously before centrifugation at 1000 × g for 5 min. The organic phase was evaporated and chromatographed on Silica Gel G plates with chloroform/methanol/ammonia 28% (65:25:5 by volume) as the developing solvent. PC and lyso-PAF were cut out of the plate and assayed for radioactivity by liquid scintillation counting.
Gas Chromatography/Mass Spectrometry Analysis (GC/MS) of Fatty Acid Methyl Esters
The phospholipid fraction, isolated by thin layer chromatography with hexane/ether/acetic acid (70:30:1, v/v/v) as a mobile phase, was transmethylated with 500 μl of 0.5 m KOH in MeOH for 30 min at 37 °C. One volume of 0.5 m HCl was added to neutralize, and fatty acid methyl esters were extracted twice with 2 volumes of n-hexane. Analysis of fatty acid methyl esters was carried out in an Agilent 6890N gas chromatograph coupled to an Agilent 5975 mass selective detector (MSD) operated in electron impact mode (EI, 70 eV), equipped with an Agilent DB23 column (60 m × 0.25 mm inner diameter × 0.15 μm film thickness), under the conditions described previously (35).
HPLC-MS Coupling
For HPLC separation of lipids, a Hitachi LaChrom Elite® L-2130 binary pump was used, together with a Hitachi Autosampler L-2200 (Merck). The HPLC system was coupled on-line to a Bruker esquire6000 ion-trap mass spectrometer (Bruker Daltonics, Bremen, Germany). The effluent was split, and 0.2 ml/min was introduced in the ESI interface of the mass spectrometer. The nebulizer was set to 30 p.s.i., the dry gas to 8 liters/min, and the dry temperature to 365 °C.
Analysis of PI, PE, and PC Species
Total lipid content corresponding to 2·106 cells was extracted according to Bligh and Dyer (25). After evaporation of the organic solvent under vacuum, the lipids were redissolved in methanol/water (9:1) and stored under nitrogen at −80 °C until analysis. The column was a Supelcosil LC-18 (5-μm particle size, 250 × 2.1 mm) (Sigma) protected with a Supelguard LC-18 20·2.1-mm guard cartridge (Sigma). Mobile phase was a gradient of solvent A (methanol, water, n-hexane, 30% ammonium hydroxide, 87.5:10.5:1.5:0.5, v/v/v) and solvent B (methanol, n-hexane, 30% ammonium hydroxide, 87.5:12:0.5, v/v/v). The gradient was started at 100% solvent A and was decreased linearly to 65% solvent A in 20 min, to 10% in 5 min, and to 0% in another 5 min. Flow rate was 0.5 ml/min, and 80 μl of the lipid extract was injected. PI and PE species were detected in negative ion mode with the capillary current set at +3500 V over the initial 22 min as [M − H]−. PC species were detected over the elution interval from 22 to 35 min in positive ion mode as [M + H]+ ion with the capillary current set at −4000 V.
AA-containing PI and PE species were identified by multiple reaction monitoring MS/MS experiments on chromatographic effluent by comparison with previously published databases (36–38). Cutoff parameter was set at m/z 150 and fragmentation amplitude at 1 arbitrary unit. Because of the lability of vinyl ether linkages in acid media, plasmanyl (1-alkyl) and plasmenyl (1-alk′1′-enyl) glycerophospholipids were distinguished by acidifying the samples before lipid extraction (39). For the identification of acyl chains of AA-containing PC species, ionization was carried out in negative mode with post-column addition of acetic acid at a flow rate of 100 μl/h as [M + CH3CO2]− adducts, and acyl chains were identified by MS3 experiments (40, 41).
Measurement of Lipid Phosphorus
Lipid phosphorus was measured as described previously (42), with a slight modification of the procedure. After digestion of lipids with 0.9 ml of 70% perchloric acid at 200 °C for 30 min, the samples were cooled in a water stream, and 4 ml of distilled water was added, followed by 0.5 ml of 2.5% ammonium molybdate and 0.5 ml of 10% ascorbic acid. This mixture was thoroughly mixed and incubated at 100 °C for 8 min, and absorbance was read at 820 nm.
Statistical Analysis
All experiments were carried out at least three times with incubations in duplicate or triplicate. Statistical analysis was carried out by the Student's t test, with p values < 0.05 taken as statistically significant.
RESULTS
Role of Caveolin-1 on Incorporation of AA and Other Fatty Acids in Mouse Peritoneal Macrophages
Because of its fatty acid-binding properties, caveolin-1 may be involved in cellular fatty acid uptake (23). To assess a possible role for caveolin-1 in AA incorporation into the various lipid classes of mouse peritoneal macrophages, cells from the caveolin-1 null mouse strain described by Lisanti and co-workers (20) and from their littermate wild type controls were exposed to various concentrations of [3H]AA, and the incorporation into phospholipid versus TAG was measured. The results are shown in Table 1 (top panel). In agreement with previous observations (43), at low levels of fatty acid there was negligible incorporation of AA into TAG, and most of the fatty acid was incorporated in phospholipids; however, increasing the concentration of the fatty acid resulted in a significant incorporation into TAG as well. This behavior was found in both control and caveolin-1-deficient cells and is fully consistent with previous results indicating the preferential incorporation of AA into phospholipids in macrophages via a high affinity pathway (the Lands cycle of deacylation/reacylation), followed by a second pathway of lower affinity (the de novo route) that primarily operates when the former has been saturated and leads to AA incorporation into TAG (2, 3). Strikingly, however, regardless of the concentration of AA utilized, the caveolin-1-deficient cells always incorporated significantly higher quantities of fatty acid than did the control cells in both phospholipid and TAG. The total amount of phospholipid and TAG did not significantly differ in control versus caveolin-1-deficient cells (phospholipid, 10.02 ± 0.56 nmol/106 cells in control cells and 9.96 ± 0.46 nmol/106 cells caveolin-1-deficient cells; TAG, 64.56 ± 0.99 nmol/106 cells in control cells and 67.46 ± 1.02 nmol/106 cells in caveolin-1-deficient cells; means ± S.E., n = 3). After addition of 10 μm AA, the amount of phospholipid or TAG did not significantly change in either control or caveolin-1-deficient cells.
TABLE 1.
Initial incorporation of [3H]AA in phospholipids and TAG in macrophages from caveolin-1-deficient and control cells
The cells were incubated with the indicated concentrations of [3H]AA (0.25 μCi/ml) for 30 min. After lipid extraction, the amount of radioactivity in phospholipids and TAG was measured. Panel A shows the results utilizing cells from the mice described by Lisanti and co-workers (20). Panel B shows the results utilizing cells from the mice described by Kurzchalia and co-workers (19). The results are given as pmol/106 cells and are means ± S.E. of three independent experiments with duplicate incubations.
| [3H]AA | Phospholipids |
TAG |
||
|---|---|---|---|---|
| Control | Cav-1-deficient | Control | Cav-1-deficient | |
| Panel A | ||||
| 0.001 μm | (11.4 ± 0.1)·10−3 | (21.9 ± 0.4)·10−3a | (0.8 ± 0.1)·10−3 | (0.9 ± 0.1)·10−3 |
| 0.10 μm | 1.1 ± 0.1 | 1.7 ± 0.1a | 0.1 ± 0.1 | 0.1 ± 0.1 |
| 1 μm | 9.1 ± 0.9 | 16.3 ± 1.5a | 1.1 ± 0.1 | 1.5 ± 0.2 |
| 10 μm | 57.8 ± 2.9 | 106.7 ± 2.6a | 10.1 ± 1.3 | 17.0 ± 0.3a |
| Panel B | ||||
| 0.001 μm | (16.2 ± 0.1)·10−3 | (18.4 ± 1.9)·10−3 | (1.3 ± 0.1)·10−3 | (1.6 ± 0.1)·10−3a |
| 0.10 μm | 1.3 ± 0.1 | 2.6 ± 0.1a | 0.1. ± 0.1 | 0.2 ± 0.1 |
| 1 μm | 11.3 ± 0.1 | 14.2 ± 0.9 | 1.7. ± 0.2 | 2.6 ± 0.2a |
| 10 μm | 69.2 ± 0.8 | 100.4 ± 4.9a | 23.5 ± 2.1 | 36.2 ± 1.9a |
a The data indicate significant differences in caveolin-1-deficient versus control cells (p < 0.05).
To confirm that the increase in the initial incorporation of AA in macrophage lipids is of biological significance and not the result of a peculiarity of the mouse strain utilized, we repeated the experiments described above with cells from another mouse strain lacking caveolin-1 by genetic ablation, i.e. that described by Kurzchalia and co-workers (19). The results, shown in Table 1 (bottom panel), were almost identical to those observed with the previous strain, i.e. a highly significant increase in the incorporation of AA into both phospholipid and TAG in caveolin-1-deficient cells versus control cells. In addition, the mass of fatty acid incorporated in both strains was very similar, albeit a bit lower in the cells from mice described by Lisanti and co-workers (20). Given these similarities, all successive experiments were carried out with the strain produced by Lisanti and co-workers (20), as this one was more accessible to us.
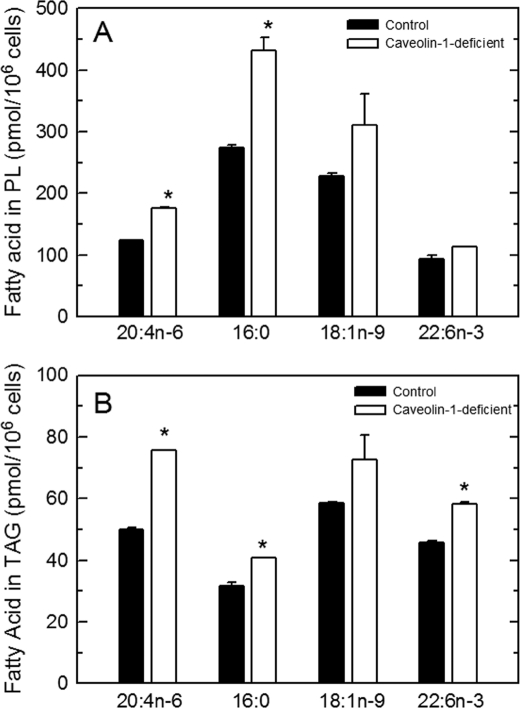
Fig. 1 shows that cells deficient in caveolin-1 not only displayed an increased incorporation of AA when exposed to this fatty acid but also when exposed to other fatty acids, whether saturated (palmitic acid, 16:0), monounsaturated- (oleic acid, 18:1n-9), or polyunsaturated (docosahexaenoic acid, 22:6n-3). Collectively, these results indicate that in peritoneal macrophages lacking caveolin-1, the initial incorporation of fatty acids into cellular lipids is more active than in cells expressing normal caveolin-1 levels.
FIGURE 1.
Incorporation of different fatty acids into cellular lipids of control and caveolin-deficient cells. The indicated fatty acids were given to the control (filled bars) or caveolin-1-deficient (open bars) cells at 10 μm for 30 min. Afterward, fatty acid incorporation into phospholipids (A) or TAG (B) was determined. Data are shown as means ± S.E. of three independent determinations (*, p < 0.05).
Metabolipidomic Analysis of AA Incorporation into Cell Phospholipids
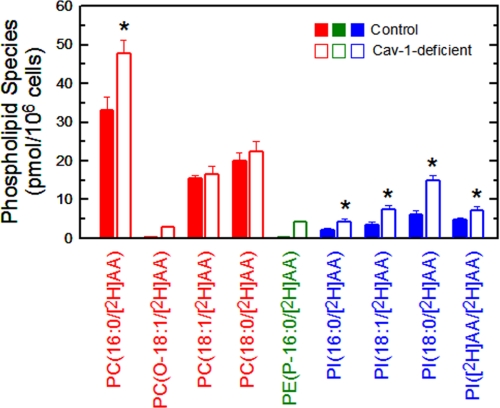
To study the phospholipids that initially incorporate AA immediately after addition of the fatty acid to the cells, a LC/MS/MS metabolipidomic approach was followed, where the cells were incubated with deuterated AA. Newly synthesized [2H]AA-containing phospholipids were unequivocally identified by their bell-shaped isotopic distribution with a maximum at +8 m/z apart from their native counterparts, due to the [2H]AA isotopomers (44). We have recently employed this technique to identify novel AA-containing phospholipids in human monocytes (40). Fig. 2 shows the phospholipid species that incorporated [2H]AA after a 30-min incubation of the cells with the fatty acid. Four [2H]AA-containing PC species were identified. The most abundant species, both in caveolin-1-deficient and control cells, was PC(16:0/[2H]AA), although it was significantly more abundant in the caveolin-1-deficient cells. Two other major PC species incorporating [2H]AA were PC(18:0/[2H]AA) and PC(18:1/[2H]AA), and these were found at similar levels in caveolin-1-deficient and control cells. Finally, the ether species PC(O-18:1/[2H]AA) was detected in caveolin-1-deficient cells but not in control cells (Fig. 2). As expected (45), no [2H]AA incorporation into PE species was detected in control cells expressing normal caveolin-1 levels (Fig. 2). Importantly, however, a very significant incorporation of the fatty acid was found in the plasmalogen species PE(P-16:0/[2H]AA) in caveolin-1-deficient cells (Fig. 2). Regarding PI species, four of these were detected in both caveolin-1-deficient and control cells, namely PI(18:0/[2H]AA), PI(18:1/[2H]AA), PI(16:0/[2H]AA), and PI([2H]AA/[2H]AA), and all of them were increased in the caveolin-1-deficient cells (Fig. 2).
FIGURE 2.
Analysis of the phospholipid species to which exogenous [2H]AA initially incorporates. Cells from control (filled bars) or caveolin-1-deficient (open bars) mice were exposed to [2H]AA for 30 min. The incorporation of [2H]AA into PC (red), PE (green), or PI (blue) species was determined by LC/MS/MS. Data represent the [2H]AA distribution after a 30-min period of exposure to the cells and are shown as means ± S.E. of five independent determinations (*, p < 0.05).
The total amount of PC, PE, and PI in control macrophages was 4.6 ± 0.2, 2.4 ± 0.1, and 0.8 ± 0.1 nmol/106 cells, respectively (means ± S.E., n = 3). These amounts did not significantly differ from those found in caveolin-1-deficient cells. Based on these amounts, we calculated that at 30 min, control cells incorporated ∼15 pmol of [2H]AA per nmol of PC and 22 pmol of [2H]AA per nmol of PI. In contrast, caveolin-1-deficient cells incorporated 20 pmol of [2H]AA per nmol of PC, 2 pmol of [2H]AA per nmol of PE, and 45 pmol of [2H]AA per nmol of PI. The ratio of AA incorporation in PC versus PE versus PI at 30 min was 79:0:21 in control cells and 71:4:25 in caveolin-1-deficient cells.
Lipidomic Profile of Endogenous Phospholipids in Caveolin-1-deficient Versus Control Cells
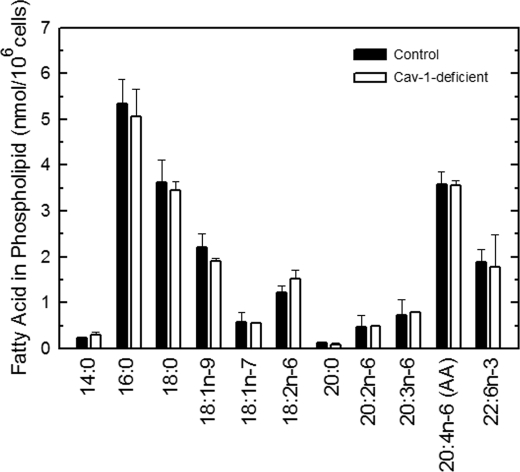
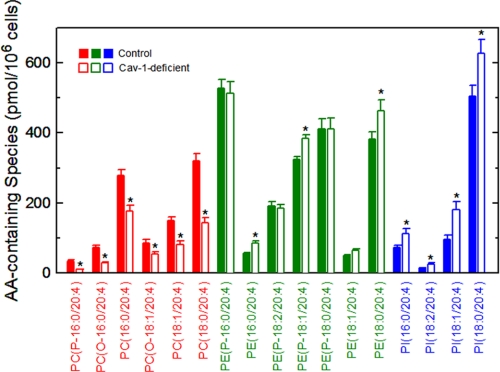
The results of Fig. 2 show that the profile of AA-containing phospholipids that are formed immediately after addition of the fatty acid to the cells is qualitatively and quantitatively different in caveolin-1-deficient versus control cells. It was therefore important to investigate whether these initial alterations persist over time. To this end, we conducted a full lipidomic analysis of endogenous phospholipid species in caveolin-1-deficient versus control cells under equilibrium, steady-state conditions. Fig. 3 compares the fatty acid content of phospholipids of control versus caveolin-1 null cells, as measured by gas chromatography/mass spectrometry. No significant changes were found in any of the fatty acids measured, including AA. The latter constituted 15–20% of total fatty acids present in the phospholipids of these cells, which is in accordance with previous estimates (46). Strikingly however, when endogenous AA-containing phospholipids were profiled by LC/MS/MS (Fig. 4), remarkable alterations were observed. Thus, although the lack of caveolin-1 did not modify the steady-state level of AA esterified in phospholipids, the distribution of this fatty acid between phospholipids was profoundly altered, resulting in significantly decreased levels of AA in PC which were counteracted by increases in PE and PI. The ratio of AA present in the different classes of phospholipids, PC versus PE versus PI, was changed from 27:54:19 in control cells to 14:59:27 in caveolin-1-deficient cells. Note that the relative amounts of AA in each phospholipid class in the equilibrium is substantially different from that observed in the metabolipidomic experiments at 30 min, both for control and caveolin-1-deficient cells (see previous paragraph).
FIGURE 3.
Fatty acid content of phospholipids from control and caveolin-1-deficient cells. The phospholipid fatty acid profile in control (filled bars) or caveolin-deficient (open bars) cells was determined by GC/MS after converting the fatty acid glyceryl esters into fatty acid methyl esters. Data are expressed as means ± S.E. of five independent determinations.
FIGURE 4.
AA-containing phospholipid species in control and caveolin-1-deficient cells. The profile of AA-containing PC (red), PE (green) and PI (blue) species in control (filled bars) or caveolin-1-deficient (open bars) cells was determined by LC/MS/MS. Data represent the equilibrium distribution of AA and are shown as means ± S.E. of five independent determinations (*, p < 0.05).
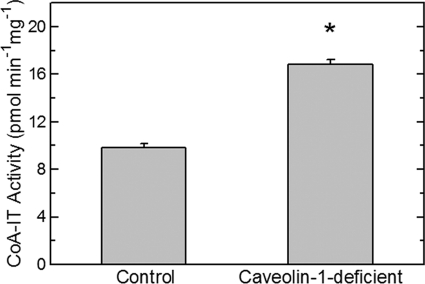
The distribution of AA among phospholipid classes ultimately depends on transacylation reactions that are catalyzed by CoA-IT. This enzyme continuously transfers AA moieties from PC species to PE species and is therefore responsible for maintaining the high level of AA in PE in phagocytes, despite that PC is the major acceptor for the initial incorporation of the fatty acid (Fig. 2) (2). The alterations in the distribution of AA among phospholipid classes in control versus caveolin-1-deficient cells, in particular the AA decrease in PC and increase in PE, are highly suggestive of alterations at the level of CoA-IT. Unfortunately, the nucleotide sequence of the gene coding for CoA-IT remains unknown, which prevents us from using genetic approaches to characterize the effect of caveolin-1 depletion on CoA-IT. Thus, in the current experiments, CoA-IT was studied by following its enzymatic activity by in vitro assay. The results are shown in Fig. 5 and indicate that the measurable CoA-IT activity of homogenates from caveolin-1 null cells was significantly higher than that of homogenates of control cells displaying normal caveolin-1 levels.
FIGURE 5.
CoA-IT activity is augmented in homogenates from caveolin-1-deficient cells. Homogenates were prepared from control cells and caveolin-1-deficient cells as indicated, and total CoA-IT activity was measured by in vitro assay. Data are given as means ± S.E. of five independent determinations (*, p < 0.05).
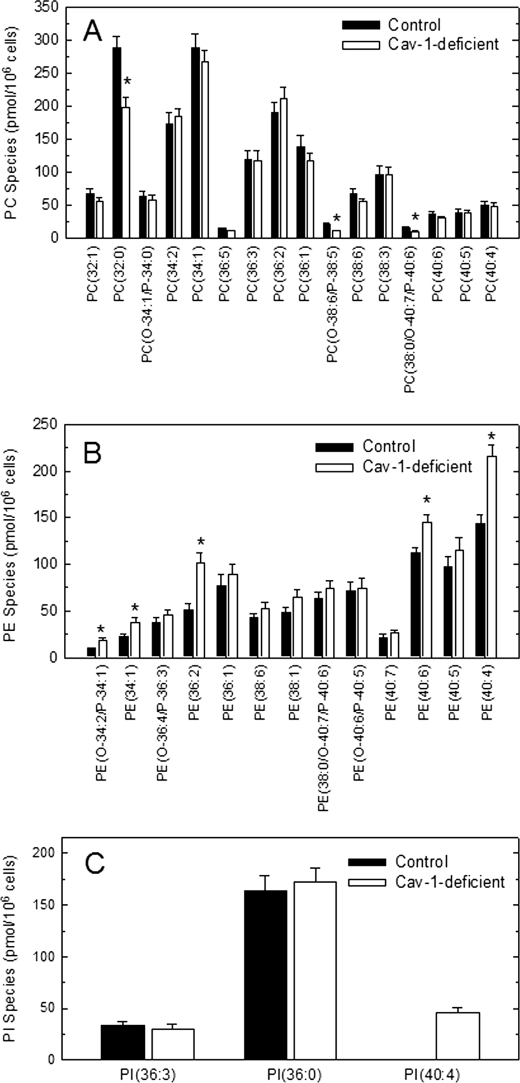
LC/MS/MS lipidomic analyses of major phospholipid species not containing AA were also conducted, and the results are shown in Fig. 6. Because in many cases fragmentation of the m/z peaks detected in MS analyses yielded fragments corresponding to various species, it was not always possible to unequivocally assign structures to these m/z peaks. Thus, the data are given in abbreviated form, indicating phospholipid class and number of carbon atoms and double bonds of the two lateral chains. Regarding PC species, the most striking difference was that of PC(32:0), which was strongly reduced in the caveolin-1-deficient cells. This species could be identified as PC(16:0/16:0) in fragmentation experiments. Regarding PE, various species were found to be significantly increased in caveolin-1-deficient cells. The most prominent were PE(34:1), PE(36:2), PE(40:6), and PE(40:4). The first two represent a mix of various species, but the latter two could be identified as PE(18:0/22:4) and PE(18:0/22:6) in fragmentation experiments. 22:4 is adrenic acid, the superior homologue of AA, and thus it is notable that the two closely related species PE(18:0/22:4) and PE(18:0/20:4) were found to be increased in caveolin-1-deficient cells (cf. Figs. 3 and 6). Regarding PI, only three species not containing AA were detected, one of which, PI(40:4), was found in caveolin-1-deficient but not in control cells. This species was unequivocally identified as PI(18:0/22:4) and, similar to the PE species described above, note that the species PI(18:0/20:4) was also increased in caveolin-1-deficient cells.
FIGURE 6.
Phospholipid species not containing AA in control and caveolin-1-deficient cells. The profile of PC (A), PE (B) and PI (C) species in control (filled bars) or caveolin-1-deficient (open bars) cells was determined by LC/MS/MS. Data are shown as means ± S.E. of five independent determinations (*, p < 0.05).
AA Mobilization and Eicosanoid Production in Caveolin-1-deficient Cells
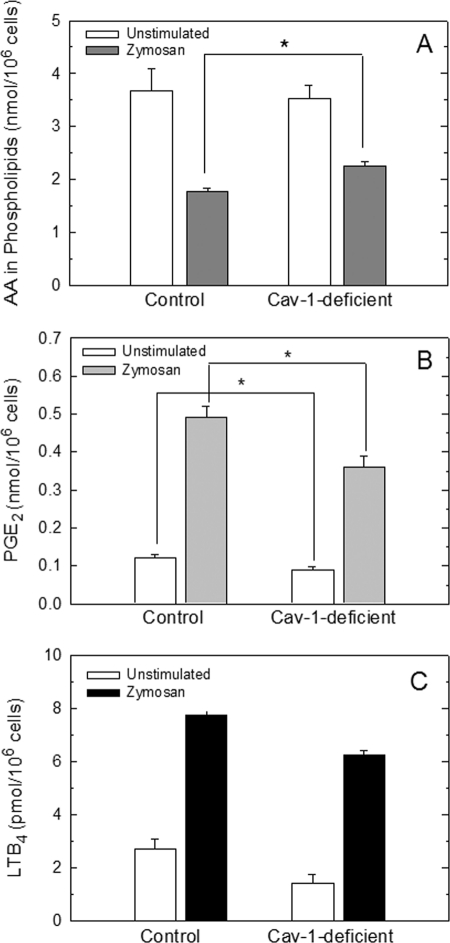
Free AA mobilization during cell activation represents a balance between what is released from phospholipids by phospholipase A2 values minus what is reincorporated back into phospholipids by acyltransferases and transacylases. Given the profound alterations detected in phospholipid fatty acid incorporation and remodeling in caveolin-1-deficient macrophages, it was of interest to explore the mobilization of free AA and attendant eicosanoid production when these cells were activated by an immunoinflammatory stimulus. The cells were challenged with yeast-derived zymosan, a potent inducer of the AA mobilization response in peritoneal macrophages (47–49), and total AA mobilized from cellular phospholipids after the challenge was analyzed by GC/MS. Caveolin-1-deficient cells retained more AA in phospholipids than did control cells after the challenge (Fig. 7A), indicating a considerably lower AA mobilization to the medium (17.9 ± 0.6% decrease; mean ± S.E., n = 6). This finding is consistent with the previous observations that caveolin-1-deficient cells incorporate AA into phospholipids at a higher rate than control cells (Table 1); hence, higher amounts of AA are retained by the cells. Analyses by GC/MS of the phospholipid classes that generated the AA, which was lost after stimulation of control and caveolin-1-deficient cells, indicated that both PC and PI behaved as sources of released AA. On the contrary, the levels of AA-containing PE remained fairly constant after the zymosan challenge. This situation is remarkably similar to that previously reported for zymosan-stimulated murine macrophages and RAW264.1 macrophage-like cells (50) and zymosan-stimulated human monocytes (41). It is remarkable that in terms of total mass, the amount of AA lost from PI in control and caveolin-1-deficient cells was very similar (0.67 ± 0.09 and 0.73 ± 0.11 nmol/106 cells for control and caveolin-1-deficient cells, respectively; means ± S.E., n = 3). The amount of AA lost from PC was 1.24 ± 0.18 and 0.54 ± 0.07 nmol/106 cells for control and caveolin-1-deficient cells, respectively; means ± S.E., n = 3).
FIGURE 7.
AA mobilization and eicosanoid production in control and caveolin-1-deficient mice. Peritoneal macrophages from control and caveolin-1-deficient mice were incubated in the absence (open bars) or presence of zymosan (filled bars) for 1 h. Afterward the supernatants were collected for PGE2 and LTB4 analyses by ELISA (B and C, respectively) and phospholipid AA content in the cell monolayers was determined by GC/MS (A). The data are shown as means ± S.E. of six independent determinations (*, p < 0.05).
Consistent with reduced mobilization of free AA, caveolin-1-deficient cells also produced reduced amounts of PGE2 and LTB4 after zymosan stimulation (Fig. 7, B and C).
DISCUSSION
Caveolin-1 is expressed in all cells and tissues, although it is especially abundant in adipocytes and endothelial cells (14). Caveolin-1 and caveolae are also present in all innate immune cells, although their expression level and distribution appear to strikingly depend on the activation and/or maturation state of the cell (16). Caveolin-1 has been unambiguously identified in murine macrophages and mast cells (18, 51–53) as well as human dendritic cells and neutrophils (54, 55).
It has been shown that both caveolin-1 isoforms, α and β, can bind free fatty acids to facilitate their uptake by adipocytes via the caveolae system with the participation of fatty acid transporters like FAT/CD36 (23, 56, 57). Given these previous data, we were interested in assessing the role of caveolin-1 in the uptake and esterification of AA into the phospholipids of macrophages. Although macrophages are not particularly enriched in caveolin-1 or caveolae (14), they do exhibit a high capacity to incorporate AA into membrane phospholipids (2, 3). Such a process is of great relevance from a pathophysiological point of view, because one of the limiting factors for the synthesis of bioactive eicosanoids during inflammation is the availability of free AA.
Studies on the initial incorporation of AA in macrophage lipids from caveolin null mice versus their wild type littermates indicate that most of the fatty acid is incorporated in phospholipids and only a minor fraction is incorporated in TAG, and this occurs at all concentrations tested. Strikingly, however, macrophages from the caveolin-1 null mice consistently incorporate more AA and other fatty acids, both saturated and unsaturated, than macrophages from wild type animals. Of note, identical results were obtained when the experiments were repeated utilizing a second and different caveolin-1 null mice strain, thus demonstrating that the effect is pathophysiologically relevant and not the result of a strain-specific peculiarity.
Caveolin-1 null mice have been found to exhibit severely elevated TAG and free fatty acid levels, especially in the post-prandial state (21, 58, 59). These metabolic defects observed in caveolin-1 null mice have underscored the role of this protein for proper lipid transport in the adipocyte (13, 58). Studies with mouse embrionary fibroblasts from caveolin-1 null mice have suggested that the lack of caveolae and caveolin-1 results in diminished incorporation of oleic acid into cellular lipids, a finding that was related to the loss of expression of the fatty acid transporter FAT/CD36 (57, 60). Therefore, the initial increase in the rate of incorporation of AA and other fatty acids in macrophages from caveolin-1 null mice is striking. We speculate that the relatively lower content of caveolin-1 and caveolae in macrophages compared with that of adipocytes and fibroblasts may likely explain these differences. Macrophages may exhibit regulatory features for the initial incorporation of potentially pro-inflammatory fatty acids such as AA, which are not present or are not operative in cells not directly implicated in innate immunity and inflammation (2, 3). In this regard, it is worth mentioning the work by Wu et al. (61) demonstrating that in mice lacking the fatty acid transporter FATP1, fatty acid incorporation into adipose tissue and muscle lipids is impaired, whereas in liver and heart the opposite occurs. Thus, clear mechanistic differences between tissues with regard to fatty acid transport appear to actually exist, and these may account for the differences observed regarding the effect of caveolin-1 on fatty acid incorporation in different cell types.
Metabolipidomic analyses of the initial phospholipid acceptors to which AA incorporates revealed at least three striking differences between macrophages from caveolin-1 null mice versus cells from control littermates that can be summarized as follows: (i) an increase in AA incorporation into some but not all PC species; (ii) the appearance of an AA-containing PE species in caveolin-1-deficient cells that is not present in control cells, namely PE(P-16:0/20:4); and (iii) a general increase in the incorporation of AA into PI species, particularly PI(18:0/20:4). It is well established that accumulation of AA into PE species is a delayed process that takes hours to develop and takes place mainly at the expense of the AA initially incorporated into PC species via CoA-independent transacylation reactions (2). Thus, the appearance of a PE species in the cells from caveolin-1 null mice as early as 30 min after addition of the fatty acid strongly indicates that an increased remodeling of AA-containing phospholipids via CoA-independent transacylase (CoA-IT) occurs in the caveolin-1-deficient cells.
With the recent cloning of mammalian Mg2+-dependent phosphatidate phosphatases (lipins) (62) and platelet-activating factor acetyltransferase (63), CoA-IT stands as the only lipid signaling and remodeling enzyme whose sequence remains unknown. Currently, the only manner to study the cell regulation of CoA-IT is by measuring its enzyme activity (35). By doing this, we demonstrate that CoA-IT activity levels are markedly elevated in the absence of caveolin-1. Thus, these results identify a novel molecular alteration in caveolin-1-deficient cells and suggest interesting implications as to the pathophysiological relevance of the finding. For example, it is well established that rapidly dividing cells such as tumor cells display higher CoA-IT-dependent phospholipid AA remodeling rates than nontumor cells, the inhibition of which results in apoptotic cell death (64–67). According to these data, it could be anticipated that cells from caveolin-1 null mice would be more susceptible to apoptosis than cells from wild type controls, and evidence in this regard has been put forth very recently (68).
Despite the increased rate of AA incorporation in caveolin-1-deficient versus wild type cells, the total mass of AA and other major fatty acids under equilibrium conditions (i.e. the endogenous fatty acid levels) did not significantly change. This is consistent with the notion that the initial rate of incorporation of a given fatty acid in cellular lipids does not determine the actual content of such fatty acid under steady-state conditions. Importantly, however, the distribution of AA among phospholipids in the equilibrium still exhibited very marked changes, some of which (increases in PE and PI), but not all (decreases in PC), had already been detected in the metabolipidomic experiments measuring initial rates of incorporation. This altered distribution of AA among phospholipids in caveolin-1 null cells under equilibrium conditions is again fully consistent with the increased CoA-IT activity that these cells exhibit. Moreover, accelerated remodeling due to increased CoA-IT activity may also help to explain the increased initial rate of incorporation of AA and other fatty acids into phospholipids, because the transfer of AA of PC to PE, and perhaps PI, will increase the availability of lyso-PC acceptors for the acylation of new molecules of fatty acid (2). That PI species, not only PE, also contribute to counteract the AA losses in PC seen in caveolin-1-deficient cells suggests that either lyso-PI could serve as an acceptor for the CoA-IT-mediated reaction, which would be remarkable, or that the channeling of AA from PC to PI is the consequence of a second independent molecular alteration of phospholipid AA remodeling. Studies are currently underway in our laboratory to explore these possibilities.
Another striking finding of this work is that the altered phospholipid composition of caveolin-1-deficient versus wild type cells is not limited to AA-containing phospholipids but to other species as well. In this regard, a major difference is the very reduced content of PC(16:0/16:0) in cells from caveolin-1-deficient cells. The significance of such a dramatic reduction in peritoneal macrophages is unclear at present, but PC(16:0/16:0) is a major constituent of the pulmonary surfactant, and it is known that caveolin-1 null mice suffer from respiratory problems (19). Thus it could be interesting to assess the levels of this phospholipid species and mechanisms of synthesis in cells from the lungs of caveolin-1 null mice to assess whether a relation exists.
The amount of AA mobilized from cells for eicosanoid synthesis during an immunoinflammatory challenge depends on the relative activity of the two competing reactions, i.e. deacylation (phospholipase A2) and reacylation (CoA-dependent acyltransferase and CoA-IT) (3). Hence, a likely consequence of the elevated phospholipid AA incorporation and remodeling that caveolin-1-deficient cells display would be a reduced AA mobilization and eicosanoid generation responses under stimulation conditions. We provide unambiguous evidence that this is exactly what happens when the cultured macrophages were exposed to the innate immunity stimulant zymosan. Analyses of the sources of the AA released upon zymosan challenge indicate that both in control and caveolin-1-deficient cells, PC and PI, but not PE, contribute to the response. Interestingly, stimulated caveolin-1-deficient cells appear to mobilize similar amounts of AA from PI but not from PC than control cells, which may reflect the increased rate of incorporation of AA into PC and the further phospholipid remodeling that these cells exhibit. Based on previous work by our and other groups (2, 9–11, 40, 50, 69–71), it seems possible that PE molecules also serve as immediate substrates for zymosan-induced AA mobilization but are rapidly reacylated using AA from other sources, i.e. PC, via CoA-IT. The stimulated entry of AA into PE via CoA-independent transacylation reactions has been demonstrated to occur in human neutrophils (9), human monocytes (41), and murine mast cells (70).
Acknowledgments
We thank Montse Duque and Yolanda Sáez for expert technical help. Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas is an initiative of Instituto de Salud Carlos III.
This work was supported by the Spanish Ministry of Science and Innovation Grants BFU2010-18826 and SAF2010-18831.
- AA
- arachidonic acid
- CoA-IT
- coenzyme A-independent transacylase
- PC
- choline glycerophospholipid
- PE
- ethanolamine glycerophospholipid
- PI
- phosphatidylinositol
- TAG
- triacylglycerol
- PG
- prostaglandin.
REFERENCES
- 1. Funk C. D. (2001) Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 2. Chilton F. H., Fonteh A. N., Surette M. E., Triggiani M., Winkler J. D. (1996) Biochim. Biophys. Acta 1299, 1–15 [DOI] [PubMed] [Google Scholar]
- 3. Pérez-Chacón G., Astudillo A. M., Balgoma D., Balboa M. A., Balsinde J. (2009) Biochim. Biophys. Acta 1791, 1103–1113 [DOI] [PubMed] [Google Scholar]
- 4. Balsinde J., Winstead M. V., Dennis E. A. (2002) FEBS Lett. 531, 2–6 [DOI] [PubMed] [Google Scholar]
- 5. Leslie C. C. (2004) Biochem. Cell Biol. 82, 1–17 [DOI] [PubMed] [Google Scholar]
- 6. Balsinde J., Balboa M. A. (2005) Cell. Signal. 17, 1052–1062 [DOI] [PubMed] [Google Scholar]
- 7. Balboa M. A., Balsinde J. (2006) Biochim. Biophys. Acta 1761, 385–391 [DOI] [PubMed] [Google Scholar]
- 8. Balsinde J., Pérez R., Balboa M. A. (2006) Biochim. Biophys. Acta 1761, 1344–1350 [DOI] [PubMed] [Google Scholar]
- 9. Nieto M. L., Venable M. E., Bauldry S. A., Greene D. G., Kennedy M., Bass D. A., Wykle R. L. (1991) J. Biol. Chem. 266, 18699–18706 [PubMed] [Google Scholar]
- 10. Balsinde J., Barbour S. E., Bianco I. D., Dennis E. A. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 11060–11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boilard E., Surette M. E. (2001) J. Biol. Chem. 276, 17568–17575 [DOI] [PubMed] [Google Scholar]
- 12. Balsinde J. (2002) Biochem. J. 364, 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. (2004) Physiol. Rev. 84, 1341–1379 [DOI] [PubMed] [Google Scholar]
- 14. Williams T. M., Lisanti M. P. (2004) Genome Biol. 5, 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parton R. G., Simons K. (2007) Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 16. Harris J., Werling D., Hope J. C., Taylor G., Howard C. J. (2002) Trends Immunol. 23, 158–164 [DOI] [PubMed] [Google Scholar]
- 17. Gargalovic P., Dory L. (2003) J. Lipid Res. 44, 11–21 [DOI] [PubMed] [Google Scholar]
- 18. Shin J. S., Gao Z., Abraham S. N. (2000) Science 289, 785–788 [DOI] [PubMed] [Google Scholar]
- 19. Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F. C., Schedl A., Haller H., Kurzchalia T. V. (2001) Science 293, 2449–2452 [DOI] [PubMed] [Google Scholar]
- 20. Razani B., Engelman J. A., Wang X. B., Schubert W., Zhang X. L., Marks C. B., Macaluso F., Russell R. G., Li M., Pestell R. G., Di Vizio D., Hou H., Jr., Kneitz B., Lagaud G., Christ G. J., Edelmann W., Lisanti M. P. (2001) J. Biol. Chem. 276, 38121–38138 [DOI] [PubMed] [Google Scholar]
- 21. Razani B., Combs T. P., Wang X. B., Frank P. G., Park D. S., Russell R. G., Li M., Tang B., Jelicks L. A., Scherer P. E., Lisanti M. P. (2002) J. Biol. Chem. 277, 8635–8647 [DOI] [PubMed] [Google Scholar]
- 22. Heimerl S., Liebisch G., Le Lay S., Böttcher A., Wiesner P., Lindtner S., Kurzchalia T. V., Simons K., Schmitz G. (2008) Biochem. Biophys. Res. Commun. 367, 826–833 [DOI] [PubMed] [Google Scholar]
- 23. Trigatti B. L., Anderson R. G., Gerber G. E. (1999) Biochem. Biophys. Res. Commun. 255, 34–39 [DOI] [PubMed] [Google Scholar]
- 24. Diez E., Balsinde J., Aracil M., Schüller A. (1987) Biochim. Biophys. Acta 921, 82–89 [DOI] [PubMed] [Google Scholar]
- 25. Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 26. Pérez-Chacón G., Astudillo A. M., Ruipérez V., Balboa M. A., Balsinde J. (2010) J. Immunol. 184, 1071–1078 [DOI] [PubMed] [Google Scholar]
- 27. Balsinde J., Balboa M. A., Dennis E. A. (2000) J. Biol. Chem. 275, 22544–22549 [DOI] [PubMed] [Google Scholar]
- 28. Balsinde J., Balboa M. A., Insel P. A., Dennis E. A. (1997) Biochem. J. 321, 805–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balboa M. A., Sáez Y., Balsinde J. (2003) J. Immunol. 170, 5276–5280 [DOI] [PubMed] [Google Scholar]
- 30. Pérez R., Balboa M. A., Balsinde J. (2006) J. Immunol. 176, 2555–2561 [DOI] [PubMed] [Google Scholar]
- 31. Balboa M. A., Pérez R., Balsinde J. (2008) FEBS J. 275, 1915–1924 [DOI] [PubMed] [Google Scholar]
- 32. Venable M. E., Nieto M. L., Schmitt J. D., Wykle R. L. (1991) J. Biol. Chem. 266, 18691–18698 [PubMed] [Google Scholar]
- 33. Kaever V., Goppelt-Strübe M., Resch K. (1988) Prostaglandins 35, 885–902 [DOI] [PubMed] [Google Scholar]
- 34. Balsinde J., Bianco I. D., Ackermann E. J., Conde-Frieboes K., Dennis E. A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8527–8531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Astudillo A. M., Pérez-Chacón G., Balgoma D., Gil-de-Gómez L., Ruipérez V., Guijas C., Balboa M. A., Balsinde J. (2011) Biochim. Biophys. Acta 1811, 97–103 [DOI] [PubMed] [Google Scholar]
- 36. Brügger B., Erben G., Sandhoff R., Wieland F. T., Lehmann W. D. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 2339–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy R. C. (2002) Mass Spectrometry of Phospholipids: Tables of Molecular and Product Ions, Illuminati Press, Denver, CO [Google Scholar]
- 38. Pulfer M., Murphy R. C. (2003) Mass Spectrom. Rev. 22, 332–364 [DOI] [PubMed] [Google Scholar]
- 39. Kayganich K. A., Murphy R. C. (1992) Anal. Chem. 64, 2965–2971 [DOI] [PubMed] [Google Scholar]
- 40. Balgoma D., Montero O., Balboa M. A., Balsinde J. (2008) FEBS J. 275, 6180–6191 [DOI] [PubMed] [Google Scholar]
- 41. Balgoma D., Astudillo A. M., Pérez-Chacón G., Montero O., Balboa M. A., Balsinde J. (2010) J. Immunol. 184, 3857–3865 [DOI] [PubMed] [Google Scholar]
- 42. Rouser G., Fleischer S., Yamamoto A. (1970) Lipids 5, 494–496 [DOI] [PubMed] [Google Scholar]
- 43. Balsinde J., Dennis E. A. (1996) Eur. J. Biochem. 235, 480–485 [DOI] [PubMed] [Google Scholar]
- 44. Balgoma D., Montero O., Balboa M. A., Balsinde J. (2010) Biochimie 92, 645–650 [DOI] [PubMed] [Google Scholar]
- 45. Balsinde J., Fernández B., Solís-Herruzo J. A. (1994) Eur. J. Biochem. 221, 1013–1018 [DOI] [PubMed] [Google Scholar]
- 46. Scott W. A., Pawlowski N. A., Murray H. W., Andreach M., Zrike J., Cohn Z. A. (1982) J. Exp. Med. 155, 1148–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balsinde J., Fernández B., Diez E. (1990) J. Immunol. 144, 4298–4304 [PubMed] [Google Scholar]
- 48. Balsinde J., Fernández B., Solís-Herruzo J. A., Diez E. (1992) Biochim. Biophys. Acta 1136, 75–82 [DOI] [PubMed] [Google Scholar]
- 49. Balsinde J., Fernández B., Solís-Herruzo J. A. (1994) Biochim. Biophys. Acta 1210, 195–201 [DOI] [PubMed] [Google Scholar]
- 50. Rouzer C. A., Ivanova P. T., Byrne M. O., Brown H. A., Marnett L. J. (2007) Biochemistry 46, 6026–6042 [DOI] [PubMed] [Google Scholar]
- 51. Kiss A. L., Kittel A. (1995) Cell Biol. Int. 19, 527–538 [DOI] [PubMed] [Google Scholar]
- 52. Kiss A. L., Geuze H. J. (1997) Eur. J. Cell Biol. 73, 19–27 [PubMed] [Google Scholar]
- 53. Balboa M. A., Shirai Y., Gaietta G., Ellisman M. H., Balsinde J., Dennis E. A. (2003) J. Biol. Chem. 278, 48059–48065 [DOI] [PubMed] [Google Scholar]
- 54. Yan S. R., Fumagalli L., Berton G. (1996) FEBS Lett. 380, 198–203 [DOI] [PubMed] [Google Scholar]
- 55. Werling D., Hope J. C., Chaplin P., Collins R. A., Taylor G., Howard C. J. (1999) J. Leukocyte Biol. 66, 50–58 [DOI] [PubMed] [Google Scholar]
- 56. Pohl J., Ring A., Ehehalt R., Schulze-Bergkamen H., Schad A., Verkade P., Stremmel W. (2004) Biochemistry 43, 4179–4187 [DOI] [PubMed] [Google Scholar]
- 57. Ehehalt R., Füllekrug J., Pohl J., Ring A., Herrmann T., Stremmel W. (2006) Mol. Cell. Biochem. 284, 135–140 [DOI] [PubMed] [Google Scholar]
- 58. Hnasko R., Lisanti M. P. (2003) Mol. Interv. 3, 445–464 [DOI] [PubMed] [Google Scholar]
- 59. Le Lay S., Kurzchalia T. V. (2005) Biochim. Biophys. Acta 1746, 322–333 [DOI] [PubMed] [Google Scholar]
- 60. Ring A., Le Lay S., Pohl J., Verkade P., Stremmel W. (2006) Biochim. Biophys. Acta 1761, 416–423 [DOI] [PubMed] [Google Scholar]
- 61. Wu Q., Ortegon A. M., Tsang B., Doege H., Feingold K. R., Stahl A. (2006) Mol. Cell. Biol. 26, 3455–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carman G. M., Han G. S. (2009) J. Biol. Chem. 284, 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morimoto R., Shindou H., Oda Y., Shimizu T. (2010) J. Biol. Chem. 285, 29857–29862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Surette M. E., Fonteh A. N., Bernatchez C., Chilton F. H. (1999) Carcinogenesis 20, 757–763 [DOI] [PubMed] [Google Scholar]
- 65. Trimboli A. J., Waite B. M., Atsumi G., Fonteh A. N., Namen A. M., Clay C. E., Kute T. E., High K. P., Willingham M. C., Chilton F. H. (1999) Cancer Res. 59, 6171–6177 [PubMed] [Google Scholar]
- 66. Pérez R., Matabosch X., Llebaria A., Balboa M. A., Balsinde J. (2006) J. Lipid Res. 47, 484–491 [DOI] [PubMed] [Google Scholar]
- 67. Pérez R., Melero R., Balboa M. A., Balsinde J. (2004) J. Biol. Chem. 279, 40385–40391 [DOI] [PubMed] [Google Scholar]
- 68. Bosch M., Marí M., Herms A., Fernández A., Fajardo A., Kassan A., Giralt A., Colell A., Balgoma D., Barbero E., González-Moreno E., Matias N., Tebar F., Balsinde J., Camps M., Enrich C., Gross S. P., García-Ruiz C., Pérez-Navarro E., Fernández-Checa J. C., Pol A. (2011) Curr. Biol. 21, 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Balsinde J., Dennis E. A. (1996) J. Biol. Chem. 271, 6758–6765 [DOI] [PubMed] [Google Scholar]
- 70. Fonteh A. N., Chilton F. H. (1992) J. Immunol. 148, 1784–1791 [PubMed] [Google Scholar]
- 71. Fonteh A. N., Chilton F. H. (1993) J. Immunol. 150, 563–570 [PubMed] [Google Scholar]