Abstract
Nectar drinkers must feed quickly and efficiently due to the threat of predation. While the sweetest nectar offers the greatest energetic rewards, the sharp increase of viscosity with sugar concentration makes it the most difficult to transport. We here demonstrate that the sugar concentration that optimizes energy transport depends exclusively on the drinking technique employed. We identify three nectar drinking techniques: active suction, capillary suction, and viscous dipping. For each, we deduce the dependence of the volume intake rate on the nectar viscosity and thus infer an optimal sugar concentration consistent with laboratory measurements. Our results provide the first rationale for why suction feeders typically pollinate flowers with lower sugar concentration nectar than their counterparts that use viscous dipping.
Keywords: biomechanics, biocapillarity, optimal concentration
Many insects and birds feed primarily or opportunistically on floral nectar. There are three principal techniques employed by nectar feeders: active suction, capillary suction, and viscous dipping. Lepidopterans (e.g., butterflies and moths) employ the former, sucking nectar through their probosci, along which a pressure gradient is generated by cibarial muscles (1, 2). Nectar-feeding birds (e.g., hummingbirds* and sunbirds) employ capillary suction, in which capillary pressure drives flow along the tongue once its tip touches the nectar (3). Most bees (except orchid bees) and some ants ingest nectar by dipping their tongue into, then extracting it from, the viscous nectar (4, 5). It is advantageous for creatures to ingest energy rapidly due to the threat of predation during feeding. Optimal conditions might thus be sought to maximize their energy intake rate. While the sweetest nectar offers the greatest energetic rewards, the exponential increase of viscosity with sugar concentration (2) also makes it the most difficult to transport. We here rationalize the different optimal concentrations reported for the different drinking strategies by developing a dynamic model for viscous dipping and comparing it to existing models of suction feeding. Our viscous dipping model indicates an optimal sugar concentration of 52%, which is higher than that for suction feeding, 33%. This result suggests a rationale for the fact that the nectar concentration of flowers pollinated by viscous dippers such as bees (35%) is typically higher than that of those pollinated by suction feeders such as hummingbirds or butterflies (20–25%) (6).
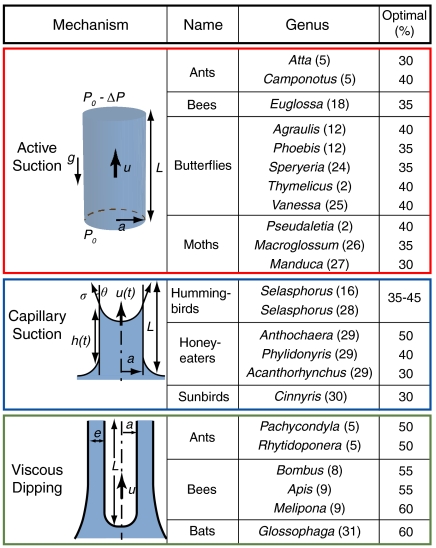
The sugar concentration that maximizes energy intake rate has been evaluated for a variety of nectar feeders in previous experimental studies (Fig. 1). Careful consideration of all of these results indicates that this so-called “optimal concentration” depends exclusively on feeding mechanism but not on body size, quantity of intake, or species. Roughly speaking, the optimal concentration for active or capillary suction feeders is 30–40% while that for creatures using viscous dipping is 50–60%. Optimal sugar concentrations for suction feeders have been previously rationalized by Kingsolver and Daniel, who established dynamic models for both active (1) and capillary (3) suction. Pivnick and McNeil (2) advanced the active suction model by introducing the assumption of constant power output for the suction pump, and so predicted an optimal concentration of approximately 35%, consistent with that observed. Daniel et al. further demonstrated how the optimal concentration of 35% emerges for active suction when muscular mechanics is considered (7). Kingsolver and Daniel (4) also suggested a dynamic model for bees that relies on capillary imbibition into the hairs of the tongue, a physical picture expected to be valid only in the limit of small nectar uptake rates. Owing to its reliance on capillary suction, their model implies an optimal concentration of 30–40%, identical to that for suction feeders. However, for larger uptake volumes (Fig. 1), this suction model is no longer expected to be valid and fails to rationalize the higher optimal concentrations of 50–60% reported, for example, for bees (8, 9). We proceed by briefly reviewing the active suction model developed by Pivnick and McNeil (2) and the capillary suction model of Kingsolver and Daniel (3), then developing a dynamic model for viscous dipping. The result is a global physical picture that describes all nectar feeders, and indicates that the optimal concentration depends exclusively on drinking style.
Fig. 1.
Optimal sugar concentrations for various nectar feeders (2, 5, 8, 9, 12, 16, 18, 22, 24–31). The optimal concentration is that for which the energy intake rate is highest based on drinking rates measured at various nectar concentrations in a laboratory setting.
Results
Suction feeding may be simply described mathematically. The pressure-driven flow of a fluid of density ρ and viscosity μ along a tube of radius a, with mean speed u, is described by Newton’s second law:
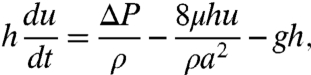 |
[1] |
where g is the gravitational acceleration and ΔP the pressure difference applied at the height h of the nectar. For active suction, ΔP is mainly generated by cibarial muscles (1, 2), while for capillary suction, ΔP ∼ σ/a results from curvature pressure, where σ is the surface tension (3). A cornerstone of biomechanics is that the force that a creature of characteristic size l can generate (10) F ∼ l2; thus, one expects the suction pressure generated by muscles, ΔP ∼ F/l2 ∼ l0, to be independent of scale and to be of comparable magnitude for all creatures [e.g., ΔP ∼ 10 kPa for both mosquitoes (4) and humans (11)]. One can thus assess the tube scale a ∼ σ/ΔP ∼ 10 μm below which curvature pressure dominates the applied suction pressure ΔP. For most suction feeders, the radius a of the proboscis is of order 100 μm (12, 13), so the curvature pressure is less than the pressure applied in active suction. Nevertheless, capillary suction is employed by certain creatures (Fig. 1) for which active suction is precluded by virtue of geometrical and physiological constraints such as the open, passive tongue of the hummingbird (13). We further note that most suction feeders have tubes of characteristic length L ∼ 1 cm (12, 13); consequently, ρgL/ΔP < 0.1, and the effect of gravity on the flows is negligible. Finally, the ratio of inertial to viscous terms scales as ρa2f/μ < 0.1, where f ∼ 10 Hz is the typical suction frequency (14, 15), indicating negligible inertial effects. Neglecting the gravitational and inertial terms in Eq. 1 yields 8μhu = a2ΔP.
In active suction, the nectar motion is described by Poiseuille flow, for which the volumetric flow rate is given by Q = πa2u = πa4ΔP/8 μL. By measuring the dependence of flow rate on sugar concentration, Pivnick (2) inferred that butterflies apply constant suction power in drinking, regardless of nectar concentration. The work per unit time required to overcome the viscous friction on the wall or power output 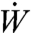 of the pump is given by
of the pump is given by 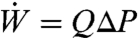 . Expressing ΔP in terms of Q then yields the dependence of volume flux on viscosity:
. Expressing ΔP in terms of Q then yields the dependence of volume flux on viscosity: 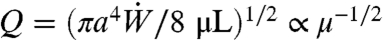 . In capillary suction, ΔP = 2σ cos θ/a, where θ denotes the contact angle, and the height of the nectar is time-dependent: h = h(t) and u = h′(t) (Fig. 1). The solution of the force balance, 4μhh′ = aσ cos θ, with initial condition h(0) = 0 is given by h(t) = (aσt cos θ/2μ)1/2. Capillary suction consists of repeated cycles of tongue insertion and retraction. The whole time for a cycle is thus the sum of the time to absorb the nectar, T, and the time to unload it, T0. The average volumetric flow rate per cycle,
. In capillary suction, ΔP = 2σ cos θ/a, where θ denotes the contact angle, and the height of the nectar is time-dependent: h = h(t) and u = h′(t) (Fig. 1). The solution of the force balance, 4μhh′ = aσ cos θ, with initial condition h(0) = 0 is given by h(t) = (aσt cos θ/2μ)1/2. Capillary suction consists of repeated cycles of tongue insertion and retraction. The whole time for a cycle is thus the sum of the time to absorb the nectar, T, and the time to unload it, T0. The average volumetric flow rate per cycle, 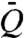 , is given by
, is given by 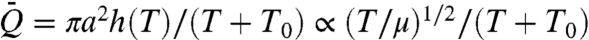 , where T1/2/(T + T0) depends weakly on viscosity (16), and so
, where T1/2/(T + T0) depends weakly on viscosity (16), and so 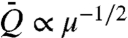 . Thus, for all suction mechanisms, we anticipate Q ∝ μ-1/2.
. Thus, for all suction mechanisms, we anticipate Q ∝ μ-1/2.
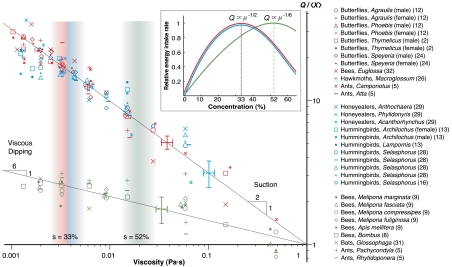
To test these proposed scalings against experimental data, we introduce a general relation between Q and μ: Q = Xμn, where X is a geometry-dependent prefactor that we expect to be different for each species. If we plot Q as a function of μ on a log scale, n and X represent the slope and the offset on the y axis, respectively. For each species, we calculate an average value 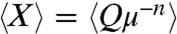 based on the measured dependence of flow rate on viscosity. In Fig. 2, red and blue points, respectively, indicate the dependence of
based on the measured dependence of flow rate on viscosity. In Fig. 2, red and blue points, respectively, indicate the dependence of 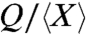 on μ for active and capillary suction. The convincing collapse of the data, plus the fact that, for each species, the slopes are close to -1/2, together support the proposed scalings.
on μ for active and capillary suction. The convincing collapse of the data, plus the fact that, for each species, the slopes are close to -1/2, together support the proposed scalings.
Fig. 2.
The dependence of scaled volumetric flow rate 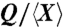 on nectar viscosity μ (2, 5, 8, 9, 12, 13, 16, 24, 26, 28, 29, 31, 32). The red points represent data for active suction, the blue points for capillary suction, and the green points for viscous dipping. The slopes of the expected lines for suction and viscous dipping are -1/2 and -1/6, respectively. Inset: Optimal concentrations for suction feeding (33%) and viscous dipping (52%), calculated from the dependence of relative energy intake rate on nectar viscosity, are denoted by vertical bands. Characteristic error bars are shown.
on nectar viscosity μ (2, 5, 8, 9, 12, 13, 16, 24, 26, 28, 29, 31, 32). The red points represent data for active suction, the blue points for capillary suction, and the green points for viscous dipping. The slopes of the expected lines for suction and viscous dipping are -1/2 and -1/6, respectively. Inset: Optimal concentrations for suction feeding (33%) and viscous dipping (52%), calculated from the dependence of relative energy intake rate on nectar viscosity, are denoted by vertical bands. Characteristic error bars are shown.
The energy intake rate 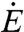 is given by the product of the energy content per unit mass of sugar c, the sucrose concentration s, and the volumetric flow rate
is given by the product of the energy content per unit mass of sugar c, the sucrose concentration s, and the volumetric flow rate 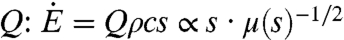 . For the sake of simplicity, density is treated as constant because its variation with sugar concentration is much less than that of viscosity. Considering the known dependence of nectar viscosity μ(s) on s (2), the dependence of
. For the sake of simplicity, density is treated as constant because its variation with sugar concentration is much less than that of viscosity. Considering the known dependence of nectar viscosity μ(s) on s (2), the dependence of 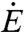 on s can be computed as shown in the inset of Fig. 2 and reveals an optimal concentration of 33% as inferred by Pivnick and McNeil for butterflies (2) and Kingsolver and Daniel for hummingbirds (3). These predicted optimal concentrations are consistent with the results from the experimental studies reported in Fig. 1.
on s can be computed as shown in the inset of Fig. 2 and reveals an optimal concentration of 33% as inferred by Pivnick and McNeil for butterflies (2) and Kingsolver and Daniel for hummingbirds (3). These predicted optimal concentrations are consistent with the results from the experimental studies reported in Fig. 1.
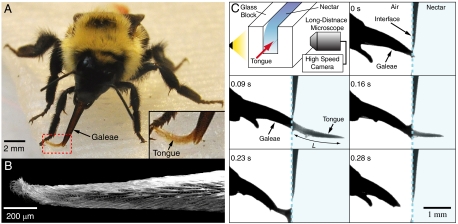
We proceed by presenting a model for feeding in which the nectar intake relies on viscous entrainment by the outer surface of the tongue (Fig. 3 and Movie S2). Viscous dipping is generally characterized by an extendible tongue being immersed into nectar, coated, then extracted as shown in Fig. 3C, where a honeybee (Apis) drinks nectar from a reservoir. One expects the volume entrained to be proportional to the area of the immersed tongue surface and the thickness e of the nectar layer. As in capillary suction, the feeding by viscous dipping consists of repeated cycles. If T and T0 represent, respectively, the time needed for tongue retraction and the interval between each cycle, then the volumetric flow rate is given by 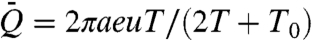 , where u represents the average tongue retraction speed.
, where u represents the average tongue retraction speed.
Fig. 3.
Bees uptake nectar via viscous dipping. (A) A bumblebee (Bombus) drinking. While normally protruding straight from the galeae, the tongue here bends to lap up nectar from the substrate, a paper towel soaked in a sucrose solution. (B) Scanning Electron Microscope (SEM) image of the bumblebee’s tongue. (C) A schematic illustration of the experiment that allows us to visualize the viscous dipping of a honeybee (Apis) with a long-distance microscope and a high-speed camera operating at 250 frames per second. Here, the bee’s tongue is dipped into a 40% sucrose solution, then withdrawn (see Movie S2).
Encouraged by its success in the modeling of suction feeding, we introduce the assumption that the work rate applied in viscous dipping remains constant with respect to nectar concentration. The movement of the tongue in the fluid requires the power Pv ∼ μLu2 to overcome the viscous drag, where L is the tongue length (Fig. 3). The power required for tongue acceleration Pt ∼ mu′u ∼ ρa2u3, where m ∼ ρa2L is the tongue mass. The ratio Pt/Pv ∼ ρua2/μL ≪ 1, so the effect of Pt is negligible. Assuming constant applied power Pv thus suggests that u ∝ μ-1/2. One does not expect T/(2T + T0) to depend strongly on viscosity because if T is shorter in less viscous nectar due to a faster retraction, the unloading time T0 would also be shorter, so that T ∝ T0. Thus, the average volumetric flow rate may be expressed as 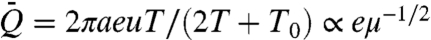 .
.
The thickness of the fluid layer entrained by a cylinder of radius a depends explicitly on three dimensionless groups: the Bond number Bo = ρga2/σ (the ratio of hydrostatic to capillary pressures), the Weber number We = ρu2a/σ (the ratio of inertial to curvature pressures), and the Capillary number Ca = μu/σ (the ratio of viscous stresses to curvature pressures). For bees, We ∼ 10-3 ≪ 1, Bo ∼ 10-3 ≪ 1, and Ca < 0.1 for s < 65%, so the thickness of the liquid layer on a tongue is prescribed by the Landau–Levich–Derjaguin theory (17) that predicts e ∼ Ca2/3a. We thus anticipate that 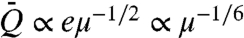 . In Fig. 2, this proposed scaling is validated by the data for all creatures that employ viscous dipping. The energy intake rate,
. In Fig. 2, this proposed scaling is validated by the data for all creatures that employ viscous dipping. The energy intake rate,  , thus scales as
, thus scales as 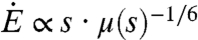 . In the inset of Fig. 2, the energy intake rate is plotted as a function of the sucrose concentration and peaks at a concentration of 52%, which is consistent with the data presented in Fig. 1. Our analysis thus provides rationale for the different optimal concentrations reported for creatures using suction and viscous dipping. For example, we can now rationalize the observation that orchid bees that employ active suction have optimal concentrations of 35%, while honeybees and bumblebees that use viscous dipping, 50–60% (18).
. In the inset of Fig. 2, the energy intake rate is plotted as a function of the sucrose concentration and peaks at a concentration of 52%, which is consistent with the data presented in Fig. 1. Our analysis thus provides rationale for the different optimal concentrations reported for creatures using suction and viscous dipping. For example, we can now rationalize the observation that orchid bees that employ active suction have optimal concentrations of 35%, while honeybees and bumblebees that use viscous dipping, 50–60% (18).
Discussion
A relatively complete physical picture of the fluid dynamics of nectar feeding has emerged. First and foremost, the optimal nectar concentration for a given creature depends only on its drinking style, being higher for viscous dippers than suction feeders. When considered in light of the coevolution between flowers and pollinators, this deduction provides rationale for the observation that the nectar concentration of flowers pollinated by bees (35%) is generally higher than that of those pollinated by butterflies and hummingbirds (20–25%) (6). Several caveats are in order, however. The optimal concentration in the laboratory might differ from that preferred in nature due to the limited availability of nectar in the wild. Specifically, in addition to energy intake rate, nectar feeders in the wild presumably consider competition between other individuals or colonies (9), and travel costs (19). Moreover, the nectar concentration proffered by flowers need not correspond to the optimal value owing to the pollination strategy of flowers (20). Indeed, it has been suggested that flowers try to keep their pollinators hungry and faithful (21) because too great an energy reward would decrease the interflower movement of pollinators, and too small a reward would bring about desertion of the pollinators. Hence, the optimal concentrations suggested by dynamic models still need to be carefully scrutinized in attempts to understand the cues of coevolution between flowers and nectar feeders.
Methods
Five bumblebees were captured in Cambridge, MA, in April 2010. One of the bees was kept in a transparent 50 cm × 50 cm × 30 cm cage, with the bottom covered in paper towel soaked in a sucrose solution. Three hours after capture, we took still photographs (with a Nikon AF-S DX still camera) of the bee protruding its tongue to lap nectar from the substrate (Fig. 3A). The tongue of a deceased bee was cleaned in a methanol solution, naturally dried, and examined with an SEM (Zeiss ultra 55 FE-SEM) (Fig. 3B).
Live honeybees were purchased from a merchant in Paju, South Korea, in June 2010. One of the bees was confined to a cylindrical cage of inner diameter 15 mm. One end of the cylindrical cage had a window through which the bee could extrude its head. A feeder made with glass blocks was filled with a 40% (by mass) sucrose solution and placed sufficiently close to the window that the bee could drink from it. We filmed the drinking process with a high speed camera (Photron APX-RS) operating at 250 frames per second with a zoom lens (Navitar 12X Zoom) (Fig. 3C and Movie S2).
Supplementary Material
Acknowledgments.
The authors thank the STX Scholarship Foundation and the National Science Foundation for financial support, and H.-Y. Kim for use of high-speed video equipment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108642108/-/DCSupplemental.
*Rico-Guevara and Rubega (23) recently demonstrated that the hummingbird’s tongue folds around the nectar and suggested that this trapped fluid is then transported mouthward exclusively by tongue retraction. However, our recent observations indicate a meniscus rise speed of approximately 10 cm/s, greatly in excess of the tongue retraction speed, 2 cm/s, so clearly indicate capillary suction (Movie S1).
References
- 1.Kingsolver JG, Daniel TL. On the mechanics and energetics of nectar feeding in butterflies. J Theor Biol. 1979;76:167–179. doi: 10.1016/0022-5193(79)90368-0. [DOI] [PubMed] [Google Scholar]
- 2.Pivnick KA, McNeil JN. Effects of nectar concentration on butterfly feeding: Measured feeding rates for Thymelicus lineola (Lepidoptera: Hesperiidae) and a general feeding model for adult Lepidoptera. Oecologia. 1985;66:226–237. doi: 10.1007/BF00379859. [DOI] [PubMed] [Google Scholar]
- 3.Kingsolver JG, Daniel TL. Mechanical determinants of nectar feeding strategy in hummingbirds: Energetics, tongue morphology, and licking behavior. Oecologia. 1983;60:214–226. doi: 10.1007/BF00379523. [DOI] [PubMed] [Google Scholar]
- 4.Kingsolver JG, Daniel TL. In: Regulatory Mechanisms in Insect Feeding. Chapman RF, de Boer G, editors. New York: Chapman and Hall; 1995. pp. 32–53. [Google Scholar]
- 5.Paul J, Roces F. Fluid intake rates in ants correlate with their feeding habits. J Insect Physiol. 2003;49:347–357. doi: 10.1016/s0022-1910(03)00019-2. [DOI] [PubMed] [Google Scholar]
- 6.Pyke GH, Waser NM. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica. 1981;13:260–270. [Google Scholar]
- 7.Daniel TL, Kingsolver JG, Meyhöfer E. Mechanical determinants of nectar-feeding energetics in butterflies: Muscle mechanics, feeding geometry, and functional equivalence. Oecologia. 1989;79:66–75. doi: 10.1007/BF00378241. [DOI] [PubMed] [Google Scholar]
- 8.Harder LD. Effects of nectar concentration and flower depth on flower handling efficiency of bumble bees. Oecologia. 1986;69:309–315. doi: 10.1007/BF00377639. [DOI] [PubMed] [Google Scholar]
- 9.Roubik DW, Buchmann SL. Nectar selection by melipona and Apis mellifera (Hymenoptera: Apidae) and the ecology of nectar intake by bee colonies in a tropical forest. Oecologia. 1984;61:1–10. doi: 10.1007/BF00379082. [DOI] [PubMed] [Google Scholar]
- 10.McMahon TA, Bonner JT. On Size and Life. New York: Scientific Am Library; 1983. [Google Scholar]
- 11.Morrison NJ, Richardson J, Dunn L, Pardy RL. Respiratory muscle performance in normal elderly subjects and patients with copd. Chest. 1989;95:90–94. doi: 10.1378/chest.95.1.90. [DOI] [PubMed] [Google Scholar]
- 12.May PG. Nectar uptake rates and optimal nectar concentrations of two butterfly species. Oecologia. 1985;66:381–386. doi: 10.1007/BF00378303. [DOI] [PubMed] [Google Scholar]
- 13.Hainsworth FR. On the tongue of a hummingbird: its role in the rate and energetics of feeding. Comp Biochem Physiol A Comp Physiol. 1973;46:65–78. doi: 10.1016/0300-9629(73)90559-8. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Kim BH, Lee JY. Experimental study on the fluid mechanics of blood sucking in the proboscis of a female mosquito. J Biomech. 2009;42:857–864. doi: 10.1016/j.jbiomech.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Ewald PW, Williams WA. Function of the bill and tongue in nectar uptake by hummingbirds. Auk. 1982;99:573–576. [Google Scholar]
- 16.Roberts WM. Hummingbird licking behavior and the energetics of nectar feeding. Auk. 1995;112:456–463. [Google Scholar]
- 17.Quéré D. Fluid coating on a fiber. Annu Rev Fluid Mech. 1999;31:347–384. [Google Scholar]
- 18.Borrell BJ. Suction feeding in orchid bees (Apidae: Euglossini) Proc R Soc London Ser B. 2004;271:S164–S166. doi: 10.1098/rsbl.2003.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyneman AJ. Optimal sugar concentrations of floral nectars—dependence on sugar intake efficiency and foraging costs. Oecologia. 1983;60:198–213. doi: 10.1007/BF00379522. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich B, Raven PH. Energetics and pollination ecology. Science. 1972;176:597–602. doi: 10.1126/science.176.4035.597. [DOI] [PubMed] [Google Scholar]
- 21.Willmer PG, Stone GN. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv Study Behav. 2004;34:347–466. [Google Scholar]
- 22.Nicolson SW. In: Nectaries and nectar. Nicolson SW, Nep M, Pacinir E, editors. Dordrecht: Springer-Verlag; 2007. pp. 289–342. [Google Scholar]
- 23.Rico-Guevara A, Rubega MA. The hummingbird tongue is a fluid trap, not a capillary tube. Proc Natl Acad Sci USA. 2011;108:9356–9360. doi: 10.1073/pnas.1016944108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boggs CL. Rates of nectar feeding in butterflies: effects of sex, size, age and nectar concentration. Funct Ecol. 1988;2:289–295. [Google Scholar]
- 25.Hainsworth FR, Precup E, Hamill T. Feeding, energy processing rates and egg production in painted lady butterflies. J Exp Biol. 1991;156:249–265. [Google Scholar]
- 26.Josens RB, Farina WM. Nectar feeding by the hovering hawk moth Macroglossum stellatarum: Intake rate as a function of viscosity and concentration of sucrose solutions. J Comp Physiol. 2001;187:661–665. doi: 10.1007/s00359-001-0238-x. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson RD. Feeding rates of the tobacco hawkmoth Manduca sexta at artificial flowers. Am Zool. 1991;31:57A. [Google Scholar]
- 28.Tamm S, Gass CL. Energy intake rates and nectar concentration preferences by hummingbirds. Oecologia. 1986;70:20–23. doi: 10.1007/BF00377107. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell RJ, Paton DC. Effects of nectar volume and concentration on sugar intake rates of Australian honeyeaters (Meliphagidae) Oecologia. 1990;83:238–246. doi: 10.1007/BF00317758. [DOI] [PubMed] [Google Scholar]
- 30.Köhler A, Leseigneur CDC, Verburgt L, Nicolson SW. Dilute bird nectars: Visocity contrains food intake by licking in a sunbird. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1068–R1074. doi: 10.1152/ajpregu.00208.2010. [DOI] [PubMed] [Google Scholar]
- 31.Roces F, Winter Y, von Helversen O. In: Animal-plant Interactions in Tropical Environments. Barthlott W, editor. Bonn: Museum Koenig; 1993. pp. 159–165. [Google Scholar]
- 32.Borrell BJ. Mechanics of nectar feeding in the orchid bee Euglossa imperialis: Pressure, viscosity and flow. J Exp Biol. 2006;209:4901–4907. doi: 10.1242/jeb.02593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.