Abstract
Our understanding of epithelial Na+ channel (ENaC) structure and function has been profoundly impacted by the resolved structure of the homologous acid-sensing ion channel 1 (ASIC1). The structure of the extracellular and pore regions provide insight into channel assembly, processing, and the ability of these channels to sense the external environment. The absence of intracellular structures precludes insight into important interactions with intracellular factors that regulate trafficking and function. The primary sequences of ASIC1 and ENaC subunits are well conserved within the regions that are within or in close proximity to the plasma membrane, but poorly conserved in peripheral domains that may functionally differentiate family members. This review examines functional data, including ion selectivity, gating, and amiloride block, in light of the resolved ASIC1 structure.
Keywords: amiloride, channel gating, homology models, blood pressure
na+ is the primary cation of extracellular fluids, and total body Na+ stores are inextricably related to extracellular fluid volume balance. The epithelial Na+ channel (ENaC) plays diverse roles in maintaining Na+ homeostasis through its functional expression in a variety of tissues. In the lingual epithelium, ENaC enables Na+-specific salt taste. In the apical membranes of cells in airway, alveoli, sweat glands, colon, and distal nephron of the kidney, ENaC facilitates transepithelial Na+ transport in conjunction with the Na+-K+-ATPase found in the basolateral membrane. These functions permit the control of extracellular fluid volume and blood pressure, as well as airway surface liquid volume and mucociliary clearance. ENaC activity is regulated by a number of factors that ultimately affect either the number of channels expressed at the cell surface (N) or channel open probability (Po). N is controlled by hormones and signaling proteins that affect either the transcription or trafficking of ENaC subunits. Po is regulated by many factors and is a focus of this review.
ENaCs are assembled from homologous α-, β-, and γ-subunits. Each member has two transmembrane helices (TM1 and TM2), relatively short amino and carboxyl termini, and a large extracellular region that encompasses several domains. ENaCs are members of the ENaC/degenerin family of ion channel proteins. Other members of this family include 1) genes identified in Caenorhabditis elegans that are involved in mechanosensation (mecs and degs) (45, 58, 102) or control of defecation rhythm (flrs) (137); 2) acid-sensing ion channels (ASICs) that are expressed in vertebrate central and peripheral nervous systems and have a role in nociception and mechanosensation (15, 17, 33, 34, 97, 104); 3) a family of proteins expressed in Drosophila that may have roles in airway fluid clearance (94), mechanosensation (3, 6), salt sensation (95), and detection of pheromones (91); and 4) peptide-gated channels expressed in marine snails (93). ENaC/degenerin channels have a variety of different cellular functions but have some common functional attributes. All members whose functional properties have been examined are Na+ selective and amiloride sensitive to various degrees, and each member is regulated by external ligands and/or is mechanosensitive. Compared with its family members, ENaC is highly Na+ selective (100 Na+ > 1 K+) and amiloride sensitive (EC50 of 150 nM). In addition, ENaC responds to both external mechanical and chemical stimuli.
Homology to Acid-Sensing Ion Channel 1
The Gouaux group has published two structures of the ENaC relative, acid-sensing ion channel 1 (ASIC1, see Fig. 1). The first structure (pdb code: 2QTS) exploited an ASIC1 construct where large portions of the intracellular amino and carboxyl termini were removed. The resulting protein was not active but formed crystals at pH 5.6 (63). The second structure (pdb code: 3HGC) employed an ASIC1 construct that included the amino terminus but was truncated shortly after TM2. This truncated ASIC1 had pH sensitivity and Na+ selectivity similar to wild-type ASIC1 but also had reduced activity. A structure of the minimally functional ASIC1 was determined at pH 6.5 (54). Given the low pH at which these proteins were crystallized, these structures are presumed to represent the desensitized state (i.e., nonconductive and nonresponsive to H+), for which there is no ENaC parallel. Both structures were homotrimers and included the extracellular domains and the TM helices, but not the intracellular termini. The structures of the extracellular domains in both models are essentially identical and exhibit threefold rotational symmetry about the long axis of the channel, normal to the membrane (Fig. 1A). However, the structures of the TM helices differ significantly.
Fig. 1.
Acid-sensing ion channel 1 (ASIC1) structure. A: ribbon diagram of ASIC1 trimer (pdb code: 3HGC)*. One subunit is shown as a solid ribbon, with domains in the extracellular region indicated. The other 2 subunits are shown as transparent ribbons. The approximate positions of the outer (Out) and inner (In) borders of the cell membrane are indicated. B: topology diagram of ASIC1. α-Helices and β-strands are represented by red cylinders and blue arrows, respectively. Labeling of secondary structure elements are as in Jasti et al. (63). This figure was adapted from a figure originally published in J Biol Chem 285: 35216–35223, 2010.
The ASIC1 structure revealed an extracellular region that resembled an outstretched hand containing a ball. It has clearly defined domains termed finger, thumb, palm, knuckle, and β-ball (Fig. 1) (63). The palm domain is a large central antiparallel β-sheet close to the threefold symmetry axis of the molecule (Fig. 1B). The β-ball domain is a smaller antiparallel β-sheet that is closely associated with the palm domain. The degree of sequence conservation between homologous ENaC subunits and ASIC1 conspicuously varies among these defined extracellular domains. Sequence identity between ENaC subunits and ASIC1 is highest (33–36%) in the palm and β-ball domains that form the inner core of the assembled channel complex. Sequence identity is much lower in the peripheral thumb, knuckle, and finger domains that are seemingly modular (Fig. 1B) and characterized by α-helices.
Despite the poor sequence conservation, there is ample evidence of structural homology between ENaC subunits and ASIC1 in most of these extracellular domains. For example, two antiparallel helices held together by five disulfide bonds characterize the thumb domain. These helices are connected to the palm domain by long loops that interact with the TM helices and are connected to each other at the opposite end by a long loop that interacts with the finger domain (Fig. 1B). ENaC subunits have a predicted secondary structure profile that matches the observed structures in the ASIC1 thumb domain (70). ENaC subunits also have 10 thumb domain Cys residues that align with their ASIC1 counterparts and are predicted to similarly form 5 disulfide bonds (52, 122). In addition, the loop connecting the two thumb helices in the ENaC α-subunit is likely to be in close proximity to the finger domain as both the thumb loop and finger domain interact with an inhibitory peptide (71). Two orthogonal short helices characterize the ASIC1 knuckle domain, which is situated near the threefold symmetry axis of the channel. The predicted secondary structures for the equivalent ENaC sequences align moderately well with the observed ASIC1 knuckle domain secondary structure (70). The finger domains of these proteins exhibit the greatest diversity of sequence and likely diverge in structure as well. The ASIC1 finger domain is composed of a three-helix bundle that is connected to the rest of the molecule via proximate attachment points in the palm and β-ball (Fig. 1B). The low sequence identity within the finger domain between ENaC subunits and ASIC1, as well as the large inserts (70–87 residues) in the ENaC finger domains, limit the utility of using the resolved ASIC1 finger domain structure to generate models of ENaC finger domains. However, there are some similarities in the ENaC and ASIC1 finger domains. For example, the finger domains of each ENaC subunit are also attached to the palm and β-ball, there is modest sequence identity between ASIC1 and ENaC subunits at the start of the finger domain, and secondary structure predictions suggest that there are several helices within the finger domains of ENaC subunits as observed in the ASIC1 structure (63, 70).
ENaC Subunit Stoichiometry
ENaC subunit stoichiometry has been the subject of a number of studies since the initial cloning of the α-, β-, and γ-ENaC subunits. Conclusions ranged from a heterotetramer with two α-subunits and single β- and γ-subunits, to larger assemblies with at least two copies of each subunit. At the heart of several studies were functional tests modeled after work by MacKinnon (99) that used wild-type and mutant subunits that exhibited different phenotypes. These studies estimated subunit stoichiometry by mixing wild-type and mutant subunits at various ratios and making assumptions about the freedom of these subunits to associate and the phenotypes of the possible subunit combinations (8, 19, 48, 51, 86, 128). These data were complemented by data from several other techniques, including sucrose gradient sedimentation (42, 128), surface expression (51), freeze-fracture electron microscopy (48), fluorescence resonance energy transfer (133), and fluorescence ratio analysis of total internal reflection fluorescence (132).
Upon publication of the trimeric ASIC1 structure, consideration of an ENaC heterotrimer was immediate (24) for good reason. In addition to belonging to the same superfamily, sequence identity is highest in the palm and β-ball domains that form the core of the ASIC1 extracellular structure and lie closest to the threefold symmetry axis. Sequence identity is also high in the TM2 helices that confer ion selectivity and amiloride sensitivity. Given the sequence conservation among the residues that form the core of the ASIC1 trimer, the ENaC structure likely features a similar core and assembles as a heterotrimer. It is unclear what accounts for the prior results regarding ENaC stoichiometry. Only one study using fluorescence ratio analysis of signals isolated from channels on the cell surface had a result consistent with an ENaC heterotrimer (132). As α-subunits expressed alone, or α-subunits expressed with either β- or γ-subunits evoke measurable currents (27), it is possible that contaminating noncanonical channel complexes influenced the results. Inaccurate assumptions may also account for some findings. While it is also possible that channel clustering may account for some results, individual functional channels are readily isolated in patch-clamp experiments, suggesting a lack of consistent clustering of functional channels. A moderate resolution structure of ENaC would formally resolve this issue.
Consideration of an ENaC heterotrimer leads to the question of circular permutation. From the extracellular perspective, is ENaC (clockwise) an α-β-γ trimer or an α-γ-β trimer? Or can both exist? This question is important for ENaC. Although the subunits are homologous and the trimer may exhibit pseudosymmetry, each possibility is a distinct species that is likely to have unique properties. Recent work examining Cl−-dependent inhibition of ENaC addressed this question (40). The structure of ASIC1 revealed three Cl− binding sites at symmetric positions at the subunit interfaces (63). Based on the ASIC1 structure and the initial observation that chloride inhibits ENaC, Collier and Snyder identified several residues that are likely involved in Cl− binding (38). An examination of the effects of mutations at these sites, both individually and in combination, led to the conclusion that ENaC is likely an α-γ-β trimer (clockwise, viewed from above), although an α-β-γ arrangement could not be excluded. A similar arrangement was recently suggested by Chen et al. (35) based on Cu2+ binding at a subunit interface.
ENaC Pore
The TM1 and TM2 helices that comprise the ENaC pore are homologous to their ASIC1 counterparts, with sequence identity for the TM2 segment being considerably higher than TM1 (Fig. 2A) (134). It follows that the architecture of the ENaC pore likely shares the basic features that were described in the ASIC1 pore. This prediction is supported by studies that examined the relative accessibility of TM2 residues (77, 88, 117, 121, 124, 130), which determined the relative orientation and secondary structure of TM2. It is also supported by studies that examined the roles of TM1 and TM2 in ion permeation (74, 77, 78, 88, 117, 120). The characteristic features of the ENaC pore are its ability to discriminate Li+ and Na+ from larger cations and all anions, specific block by amiloride, and the highly active phenotype of channels with mutations or modifications of introduced Cys residues at the degenerin site or at sites in proximity to the degenerin site.
Fig. 2.
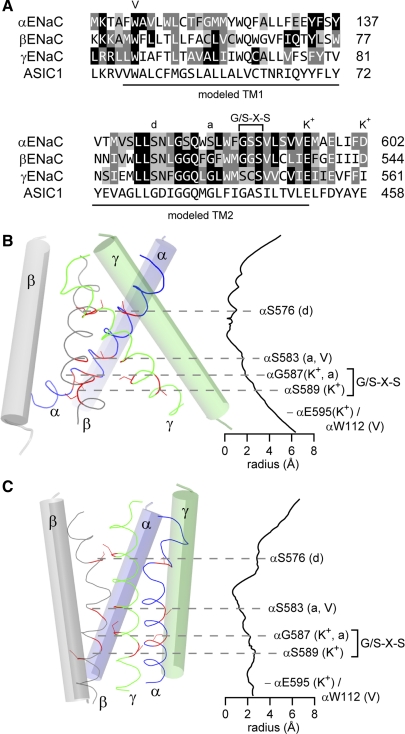
Epithelial Na+ channel (ENaC) pore comparative models. A: pore sequence alignment between chicken ASIC1 and mouse ENaC subunits. Identity (black background, white type), strong similarity (dark gray background, white type), and weak similarity (light gray background) are indicated. Comparative models of sequences indicated in A were generated using MODELLER (49), a clockwise α-γ-β-subunit arrangement, and either the 3HGC (B) or 2QTS (C) ASIC1 pdb coordinates. Seven different models are possible given this set of constraints: 1 for the symmetric 3HGC structure and 6 for the asymmetric 2QTS structure that presented 2 slightly different ASIC1 trimers (1.6 Å rmsd for pore Cα). For the sake of brevity, we made one model based on the 2QTS structure using the A, B, and C chains, and aligned the ENaC β-subunit to chain A based on previous work suggesting that the TM2 of the β-subunit is shifted toward the cytosol relative to the α- and γ-ENaC subunits (88). B and C: pore radius along the pore axis was calculated using HOLE (126). The Gly/Ser-X-Ser selectivity tract and mutations at select sites that affect ENaC open probability (Po), degenerin site (d), amiloride block (a), voltage sensitivity (V), and Na+/K+ selectivity (K+) are indicated. B and C: select sites are highlighted with dashed lines indicating the approximate level of homologous α, β, and γ residues in the pore.
The pore structures of the two resolved ASIC1 structures are divergent. In the structure of the minimally functional variant, the pore is symmetric, with TM2 helices from each subunit arranged around the threefold symmetry axis and tilted ∼50° relative to normal membrane. The three TM1 helices pack against the outside of the TM2 helices from the same subunit along their entire length and also interact with the TM helices of neighboring subunits. In the structure of the nonfunctional variant, the pore is similarly organized but increasingly skewed to one side toward the inner leaflet. Kinks at a glycine in the TM2 helices of two of the subunits promote this skew, which results in an asymmetric pore. To review studies that address features of the ENaC pore, in light of the ASIC1 pore structures, we constructed two homology models of the ENaC pore. These models were based on an α-γ-β arrangement of ENaC subunits and homology to ASIC1 and used the symmetric 3HGC model (Fig. 2B) or the asymmetric 2QTS model (Fig. 2C).
Ion selectivity.
ENaC conducts Li+ and Na+ at rates 100- to 1,000-fold higher than it conducts K+. While ENaC conducts K+ at a finite rate, ENaC is absolutely impermeable to larger ions (105). This level of Na+ over K+ selectivity is unique to ENaC and compares against a Na+:K+ selectivity ratio of 3–30 or less for ASICs and voltage-gated Na+ channels (50, 79). ENaCs conduct Li+ at 1.3–2 times the rate of Na+, with variability seemingly dependent on species and temperature (78, 105, 114, 120). Several groups studied ENaC Na+/K+ selectivity by mutating residues in the pore and assessing the resultant effects on relative Na+ and K+ currents. As a result, a Gly/Ser-X-Ser tract in TM2 of each ENaC subunit was identified, where mutations at either the first or third positions in the tract led to reduced Na+/K+ selectivity and occasionally resulted in finite currents of larger cations (e.g., Rb+, Cs+, NH4+) (74, 77, 78, 88, 120, 130). For example, modifying the α-subunit Gly-Ser-Ser tract to a K+ channel selectivity filter sequence of Gly-Tyr-Gly resulted in channels that were K+ selective, with a Na+/K+ selectivity of 1 to 4 (120).
According to our models, the Gly/Ser-X-Ser tract resides on the cytosolic side of the TM2 helices (Fig. 2, B and C). In the vicinity of this tract, the pore widens and the TM2 helices are distorted in the symmetric (3HGC) pore model (Fig. 2B). In this configuration, the side chains of the first tract residues face the pore lumen while those of the third tract residues face TM1 of the same subunit. In contrast, the Gly/Ser-X-Ser tract inhabits a narrow region of the pore in the model based on the asymmetric 2QTS structure (Fig. 2C). The TM2 segments of each subunit exhibit good α-helical geometry in the vicinity of the selectivity tract. Upstream of the Gly/Ser-X-Ser tract, the TM2 helices of the α- and β-subunits have significant kinks in the portion that forms the outer vestibule of the pore. In this model (Fig. 2C), only the first residue in the β-subunit selectivity tract faces the pore lumen. The first residues of the α- and γ-selectivity tracts face the TM helices of neighboring subunits. The conserved Ser in the third tract position of each subunit largely faces lipid and the TM1 of the same subunit. It is important to bear in mind that neither structure likely represents an active conformation, based on the conditions under which both ASIC1 structures were determined. Nonetheless, we draw several conclusions based on published work regarding the Gly/Ser-X-Ser tract and the ENaC pore models that we generated. First, altering the pore diameter by mutation remains a satisfactory explanation for some of the functional data, reflecting the fact that altering residue side chains may modify interactions with neighboring TM helices, as had been proposed (74). Second, given the helical secondary structure of the TM segments, side chain functional groups of some pore-lining residues are likely to interact with permeant ions as previously suggested (124). This is in contrast to voltage-gated K+ channels, where backbone carbonyl groups have a major role in interacting with permeating ions (44). Third, our models are seemingly at odds with previously observed modifications of introduced Cys residues at key sites in the pore (i.e., Gly/Ser-X-Ser tract or residues in proximity to the amiloride binding and degenerin sites) by methanethiosulfonate derivatives or Cd2+ (88, 121, 124, 130). These inconsistencies reflect the expected need for side chains to face the lumen to react with permeating reagents, and that narrow regions preceding the Gly/Ser-X-Ser tract may hinder access of reagents to this tract (Fig. 2, B and C). However, the apparent conflict between our pore models and the chemical modification data is most easily resolved by the consideration that neither model likely represents an active conformation.
Conformational changes that occur in the pore in conjunction with channel activation have not been elucidated. Assuming the ENaC and ASIC1 pores have a similar conformation in closed and/or desensitized states, we propose that ENaC activation is accompanied by rotational movements within the pore helices, altering the orientation of residue side chains within the pore. A similar gating mechanism was recently proposed for ASIC1 (138). The observation by Snyder et al. (127) that a chemical modification of an ENaC pore residue occurred only while the channel was open supports this notion. In this regard, a concerted twist of pore helices was suggested by normal mode analysis of ASIC1 (143) and ENaC (70) models, with a local twist of as little as a quarter-turn. An unwinding of the TM2 helices that alters the orientation of pore residues provides an alternative conformational change that could be associated with channel gating. Such an unwound conformation may be stable, as suggested by the 3HGC ASIC1 structure (Fig. 2B) and would free backbone carbonyl groups to interact with permeant ions. Other mutagenesis studies have provided additional hints of the structure of ENaC's pore. For example, the inhibition of wild-type and mutant channels by amiloride (see below) suggests that the contour of the pore lumen of the open channel narrows from the extracellular vestibule to the first residue of the Gly/Ser-X-Ser tract, which is more similar to the asymmetric pore model presented in Fig. 2C than the symmetric model presented in Fig. 2B.
The introduction of mutations at a few specific sites distal to the Gly/Ser-X-Ser tract in the α-subunit also affected Na+/K+ selectivity (69, 123). For example, mutation of acidic residues that have homologous counterparts in ASIC1 at the C-terminal end of TM2 allowed for K+ permeation through the channel (Fig. 2) (123). These data raise the possibility that the ENaC pore might extend into the cytosolic space where additional sites could participate in ion discrimination. Indeed, Kuchner and colleagues (87) recently observed that mutations at a conserved His-Gly motif ∼15 residues before TM1 induced voltage-sensitive changes in Po, suggesting that the potential gradient typically associated with the pore extends to residues that precede TM1 (87).
Studies have identified numerous sites where introduced mutations alter Li+/Na+ selectivity. Unlike the narrow group of sites on TM2 where mutations affected Na+/K+ selectivity, the sites where mutations altered Li+/Na+ selectivity are widespread throughout the pore and extend beyond the pore. These sites include the aforementioned Gly/Ser-X-Ser tract (78, 120, 130) as well as other sites within TM2 (120, 130), sites just preceding and distal to TM2 (67, 123), and even sites throughout TM1 (72). Although size exclusion may be an important mechanism in discriminating K+ and larger cations from Na+ and Li+, it is clear that it is not the only mechanism at play in ENaC ion selectivity (74, 88, 120). The free energy landscape for the translocation of ions is determined by coordination geometries along the ion-permeation pathway. Between the energy minima that characterize the highest affinity binding sites lay local energy maxima that present kinetic barriers to permeant ions. Structural distortions through mutation likely influence this landscape and may account for the often subtle but readily measurable changes in Li+/Na+ selectivity observed. In such cases, rates of K+ permeation might also be affected, but resultant changes in K+ permeation may be undetectable due to the small baseline ENaC K+ current.
Amiloride binding.
The history of research regarding ENaC is intertwined with amiloride, as ENaC-mediated transepithelial or whole cell currents have been defined as the amiloride-sensitive component of the current by convention. Amiloride was developed as a K+-sparing natriuretic drug in the 1960s (14) that inhibited transepithelial Na+ transport (18). In the 1970s, Lindemann and Van Driessche (92) discovered that amiloride blocked Na+-selective channels in apical membranes, and Benos et al. (16) and Cuthbert (41) determined that only the monovalent cationic form of amiloride (pKa = 8.7) was active. In the 1980s, Palmer found that amiloride and other large cations blocked apical Na+ channels in a voltage-dependent manner, suggesting that amiloride block occurred within the pore of these channels (106). The subsequent cloning of ENaC subunits (25, 27) provided new tools to define where amiloride interacted within the channel. Schild et al. (117) found that mutations at equivalent positions in all three subunits (αSer583, βGly525, and γGly537 in mouse ENaC) that are four residues, or one helical turn, upstream of the Gly/Ser-X-Ser tract (Fig. 2, B and C) reduced the efficacy of amiloride block (117). While mutations of this site in the β- or γ-subunit resulted in a profound loss in amiloride efficacy, a mutation of this site in the α-subunit led to only modest changes. When Cys residues were introduced at these sites, they were susceptible to chemical modification by methanethiosulfonate compounds from the extracellular space (88, 121, 124, 130). We later found that mutation of αSer583 to His, Phe, or Tyr profoundly weakened amiloride block (73). In agreement with the earlier work, other mutations of the αSer583 had more modest effects. Interestingly, some of these mutations enhanced the voltage dependence of amiloride block. Furthermore, channels with a His residues at α583 displayed pH-dependent rectification. As the pH was reduced, presumably protonating the introduced His residue, channel Po fell with membrane hyperpolarization. In addition to these sites, we and others discovered that mutations within the Gly/Ser-X-Ser tract affected amiloride affinity (74, 78, 88, 124, 141). While published results are consistent with the notion that amiloride binds within the channel pore, it has also been noted that selected mutations of residues within the extracellular region influence the block of channels composed of α-subunits by amiloride (4, 80, 81).
In our models, the sites within the pore that alter the efficacy of amiloride block of ENaC currents are concentrated within a narrow group of residues consisting of the Gly/Ser-X-Ser selectivity tract and an upstream site one helical turn away (Fig. 2, B and C). In our symmetric model based on the 3HGC structure (Fig. 2B), the αSer583/βGly525/γGly537 sites face the pore lumen but lie on the cytosolic side of the most constricted part of the pore (Fig. 2B). The selectivity tract lies one turn further toward the cytosol. In contrast, our asymmetric model based on the 2QTS structure places these sites near the extracellular entry of the most constricted part of the pore (Fig. 2C). The requirement for amiloride addition to the extracellular side of the membrane for inhibition (18) is more consistent with the latter configuration. The asymmetric model positions the αSer583/βGly525/γGly537 trio away from the pore lumen and toward TM1 of neighboring subunits. Based on the requirement for Gly at β525 and γ537 and the tolerance of a wide variety of substitutions for Ser α583, it is unlikely that these residues present functional groups that interact directly with amiloride. Rather, these data suggest that mutations at these sites alter the shape of the amiloride-binding pocket. We proposed a model for amiloride binding where the pyrazine ring is positioned at the αSer583/βGly525/γGly537 site and the guanidinium group is positioned at the outer mouth of the Gly/Ser-X-Ser selectivity tract (124). As we lack an open-state structure, it is not surprising that the pore models do not allow for amiloride to interact with the channel at these sites, and that neither model is fully consistent with functional data. Both models in Fig. 2 are based on structures of ASIC1 in a nonconducting state, and amiloride blocks ENaC in the open state (36, 56, 89, 106).
Gating-pore.
ENaC subunits are structurally related to C. elegans polypeptides mec-4 and mec-10 (25). Mutations of a key residue in TM2 of these polypeptides result in degeneration of sensory neurons, which likely reflect constitutive activation of a mechanosensitive channel (45). Introduction of large groups at the equivalent site within ENaC subunits also results in channel activation, reflecting an increase in Po (76, 127). The “degenerin” site is seven residues, or two helical turns, upstream of the key amiloride binding site. The introduction of large groups by modifying introduced Cys residues at the degenerin site or other nearby sites activated ENaC by increasing channel Po (121, 127, 130). These experimental maneuvers destabilized the closed state relative to the open state of the channel and provide strong evidence for a conformational change in this portion of TM2 during gating transitions.
As movement of elements within the pore has a role in channel gating, there is the potential that charged groups in the pore could move relative to the electric field during gating and render ENaC sensitive to voltage. However, ENaC currents have been reported as voltage insensitive, suggesting that charged groups in the electric field maintain their positions during channel openings and closings. However, two groups have noted that specific mutations in the TMs induce voltage sensitivity. We found that the introduction of selected mutations at αSer583 (implicated in amiloride binding) changed the voltage sensitivity of amiloride binding (73). Whereas amiloride sensed 15–20% of the voltage when blocking wild-type channels, amiloride sensed 40–70% of the voltage when blocking selected mutants. We suggested that such mutations changed the potential landscape to shift the potential gradient toward the outer membrane leaflet. Such a shift could bring the movements of charged groups at the extracellular mouth of αTM1 and γTM2 under the influence of the electric field. Consistent with this notion, selected amiloride site (α583) mutants also exhibited inward rectification (i.e., more permeable at negative potentials). Pochynyuk et al. (113) later reported that substitution of a conserved tryptophan on TM1 at the intracellular mouth of the pore led to inwardly rectified ENaC currents. Interestingly, conserved Glu residues implicated in Na+/K+ selectivity are present at the cytosolic end of TM2 of each subunit and are adjacent to the TM1 tryptophan in our models (Fig. 2). Several acidic residues are also present in each subunit just preceding the start of TM1. Whether these acidic groups are required for the observed inward rectification remains to be determined. Intriguingly, Kuchner and colleagues (87) observed that mutations at the conserved His-Gly motif, which result in a loss of ENaC function and causes the renal Na+-wasting disorder pseudohypoaldosteronism type I, also induced inward rectification of ENaC currents. These data suggest that residues cytosolic to those in our ASIC1-based pore models may be closely associated with the pore.
ENaC Extracellular Region
Approximately 70% of the mass of each ENaC subunit is found in the extracellular regions. These regions are composed of core β-sheet domains (termed palm and β-ball) surrounded by peripheral α-helical domains (termed finger, thumb, and knuckle). A previously defined Cys-rich domain corresponds to the thumb domain, while the remaining Cys residues are dispersed among three of the other domains. The wrist connects the extracellular domains to the TM helices. The wrist is defined by the two short direct connections between the palm and the pore-forming TM segments. The β9-α4 loop links the thumb and palm domains and packs against these connectors (Fig. 1).
In addition to its fold, posttranslational processing of the extracellular regions further defines ENaC structure and influences the function of channels expressed on the cell surface. Posttranslational modifications include Asn-linked glycosylation and proteolytic processing. ENaCs are glycosylated at between 5 and 12 Asn residues per subunit (26, 129). This finding was used by several groups to determine the membrane topology of ENaC subunits. While removal of all of the α-subunit glycosylation sites through mutation of the Asn residues had no observed effect on ENaC currents (26, 129), preventing N-glycosylation altogether with tunicamycin dramatically reduced channel activity (81), suggesting that glycosylation affects ENaC function or trafficking.
ENaC responds to a number of extracellular factors. For example, proteolytic cleavage of ENaC at specific sites in the extracellular domains activates ENaC. Recent advances by our group and others have elucidated potential mechanisms by which proteases activate ENaC. Other relevant factors that interact with the extracellular regions and modulate channel activity include Na+, Cl−, protons, as well as laminar shear stress.
Proteolytic activation.
The observation that a protease inhibitor reduced amiloride-sensitive currents in epithelia provided the first hint that proteases have a role in regulating ENaC activity (103). Vallet and coworkers (139) subsequently provided compelling evidence that proteases activate ENaC. Several groups have since corroborated this observation, implicating numerous serine proteases (2, 22, 59, 61, 82, 139, 140). ENaC proteolysis occurs in two distinct steps. The first step likely takes place in the trans-Golgi network (TGN), where furin (and possibly other proprotein convertases) cleave the α-subunit twice (immediately following R205 and R231 in the mouse sequence) and the γ-subunit once (following R143 in mouse) (59, 60). Furin is a serine protease that localizes primarily to the TGN, but also cycles to the cell surface (64). We previously reported that furin and N-glycan terminal processing are all-or-none events within an individual channel oligomer (60), consistent with these processing events occurring within the biosynthetic pathway. Surprisingly, a population of channels lacking both cleavage and terminal N-glycan processing was found at the cell surface, suggesting that a pool of channels transits to the cell surface via a route that bypasses these processing steps (60). The route taken by these nonprocessed channels to reach the cell surface has not been defined. Subsequent proteolytic processing of channel subunits likely occurs within the biosynthetic pathway or at the cell surface. For example, glycosylphosphatidylinositol (GPI)-anchored or luminal proteases cleave the γ-subunit at sites distal to the furin cleavage site. Potentially relevant candidates for γ-subunit processing include the GPI-anchored channel activating protease 1 (CAP1, i.e., prostasin) (139), CAP2 (TMPRSS4), CAP3 (matriptase or epithin) (140), neutrophil and pancreatic elastase (5, 22), and plasmin (110, 135, 136) (for reviews, see Refs. 82, 83, 109, and 115).
Proteolytic cleavage increases channel Po, and does not affect single-channel conductance or ion selectivity (23, 37). However, there is evidence suggesting that the intracellular trafficking of proteolytically processed channels differs from that of nonprocessed channels. For example, Zhou and coworkers (144) suggested that the endosomal sorting complex component Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate) selectively targets the surface pool of cleaved channels for degradation. Soundararajan and coworkers (131) noted that it is the processed form of the α-subunit that selectively associates with glucocorticoid-induced leucine zipper protein 1 (GILZ1), a Raf-interacting protein that enhances ENaC surface expression by blunting the inhibitory effect of the Raf-Erk signaling pathway. In addition, it is interesting to note that a study from Knight et al. (84) suggests that channels with an increased residency time at the plasma membrane are more likely to be processed by proteases.
We found that the activating effect of proteolysis required double cleavage in the finger domains of the α- and γ-subunits, and the removal of the intervening inhibitory tracts (26 residues in α, and 43 residues in γ when prostasin is the second protease) (21, 31, 119). Removal of either tract activates the channel, with removal of the γ tract having a greater effect on channel activity (28). Channels lacking both inhibitory tracts exhibit a remarkably high Po (21). Peptides corresponding to the α or γ excised sequences, or key subsets thereof, are inhibitory (21, 30, 31, 108). As homology in the finger domain among members of the ENaC/degenerin family is poor, the ASIC1 structure offers few clues as to how these inhibitory tracts and corresponding peptides inhibit ENaC. We recently examined how an α-inhibitory tract-derived peptide reduces channel currents. Scanning mutagenesis and double-mutant cycle analysis experiments pointed to a peptide binding site involving an interface between the finger and thumb domains (71). Using these data to complement ASIC1 homology, we built a comparative model of α-ENaC (Fig. 3) (70). This model posits that the inhibitory peptide binds to the finger and thumb interface and acts allosterically to close the channel by limiting relative movements of the thumb and finger domains, building upon an ASIC1-gating mechanism originally proposed by Jasti and colleagues (63). We proposed that proteolytic excision of the inhibitory tract facilitates relative movements between these two peripheral domains. Contrary to this model of proteolytic channel activation, catalytically inactive prostasin can also activate ENaC (10, 21). We have speculated that inactive prostasin binds to and sequesters the γ-subunit-inhibitory tract from its effector site (82).
Fig. 3.
ENaC α-subunit model highlighting proposed inhibitory peptide and Cl− binding sites. Shown is α trimer, with 1 subunit represented as a ribbon, and the other 2 subunits represented as a surface with relatively basic (blue) and acidic (red) areas indicated. Positions of bound Cl− (green spheres) were derived from the ASIC1 structure (pdb code: 3HGC). Ribbon is colored by domain: red, TM helices; yellow, palm, β-ball, and knuckle; green, finger; orange, thumb. Five disulfide bonds in the thumb are highlighted (orange spheres). The 8 inhibitory tract residues essential for peptide inhibition are highlighted (purple spheres), while the remaining residues of the furin-excised inhibitory tract are removed.
Recently, an alternative hypothesis proposed that cleavage induces channel activation through the dissociation of the amino-terminal fragment (i.e., residues 1–205 in α), including TM1, from the channel complex (57). A number of observations argue against this possibility. First, cleaved α-subunit fragments readily coimmunoprecipitate, suggesting that components of cleaved subunits remain in an assembled complex (61). Second, double cleavage is required for proteolytic activation of ENaC (31, 119), but under the alternative hypothesis a single cleavage should suffice. Third, cleavage is not required to induce channel activation, but inhibitory tract removal is sufficient (21, 31). This was demonstrated in mutants where the proximal furin site was defective and the inhibitory tract (including the distal furin site) was removed. Fourth, a disulfide bond between first and sixth Cys residues in the extracellular domain (52, 63, 70) links these two peptide fragments. This disulfide bond must be selectively reduced in order for cleaved fragments to dissociate. Finally, mutations both in αTM1 that alter channel ion selectivity or voltage sensitivity (72, 113) and in the conserved His-Gly motif of the α-subunit N terminus that reduce Po (55, 87) argue that the N terminus and TM1 of the α-subunit are components of functional channels.
Mechanosensitivity.
Renal tubular flow rates vary with time and expose channels on apical membranes to hydrodynamic forces. Early reports suggested that ENaC responded to changes in hydrostatic pressure (11, 62, 107), although some studies reported conflicting results (12, 68). Later reports implicated ENaC in the myogenic vasoconstriction response to increased pressure in cerebral and renal vessels (46, 65, 66). We and our colleagues found that increased flow rates increased ENaC-mediated Na+ transport in microperfused cortical collecting ducts (116) and that laminar shear stress increased ENaC-mediated Na+ currents in oocytes in a dose-dependent manner (32). The shear stress response was clearly a Po effect (7, 32, 100). The time constant for channel activation by shear stress was 7–8 s (32), remarkably similar to the time constants of inhibition observed with external Na+, Cl−, and the inhibitory peptides (31, 36, 38, 71, 118). Surprisingly, changing the membrane cholesterol composition had no effect on the shear stress response, despite measurable effects on membrane fluidity (29), suggesting that ENaC mechanosensitivity does not stem from deformations of the membrane. However, sites within TM2 have a role in determining the magnitude and time course of channel activation by shear stress (1). Recent work showed that mutations at sites in the β9-α4 loop (Fig. 1B) had effects on mechanosensitivity with parallel effects on allosteric Na+ inhibition (see below). This suggests that the mechanism for transducing mechanosensitivity converges with other allosteric transformations and hints at a role for structures in the extracellular domains in sensing mechanical forces (125).
Other channel activators.
Lowering extracellular pH activates ENaC (39). Interestingly, this is a species-specific response in that it occurs with human but not rodent ENaCs. Maximal channel activation was seen at a pH of 6.0. Activation reflects an increase in channel Po and a loss of Na+ self-inhibition. Lu and coworkers (96) identified a chemical activator of ENaC that also functioned in a species-specific manner, in that it also selectively activated human ENaC. The compound increased channel Po by interacting with the extracellular region of the β-subunit. Nie and coworkers (101) have shown that external 8-pCPT-cGMP also activates ENaC.
Channel inhibition by extracellular Na+.
Na+ inhibits ENaC currents through at least two distinct mechanisms that are differentiated by both the sites of action and the kinetics of inhibition. So-called Na+ feedback inhibition reflects increases in the cytosolic Na+ concentration and develops over the course of many minutes. The reduction in ENaC current has been attributed to decreases in both the cell surface channel density and the activity of individual channels (9, 75). The effect on channel density is mediated by the ubiquitin ligase Nedd4 (43). Knight et al. (85) showed that increases in intracellular Na+ reduced ENaC cleavage, providing a potential mechanism for an effect on single-channel activity. So called Na+ self-inhibition depends on increases in the extracellular Na+ concentration and takes full effect within seconds, rather than minutes. Na+ self-inhibition can be directly observed by rapidly increasing the bath Na+ concentration while measuring current in cells expressing ENaC (53). This maneuver results in an instantaneous increase in current resulting from the increased chemical driving force, which then declines to a steady-state value over the course of tens of seconds (118).
Na+ self-inhibition is a low-affinity (50–200 mM) (20, 36, 118), Na+-, or Li+-specific (20) inhibition of ENaC Po (98, 118, 119). The effect is dependent on the extent of proteolytic processing of ENaC subunits. Channels lacking both α- and γ-subunit-inhibitory tracts exhibit minimal Na+ self-inhibition (20), whereas channels that retain both inhibitory tracts have a markedly enhanced Na+ self-inhibition response (119). Channels lacking an α-subunit-inhibitory tract have an intermediate response to external Na+. It is likely that Na+ inhibition has a physiological role in modulating ENaC activity given the widely varied Na+ concentrations in the distal nephron. Na+ self-inhibition is neither voltage sensitive (20), nor does it depend on Na+ influx (36). Single-channel measurements using mutant channels that exhibit a markedly enhanced Na+ self-inhibition response demonstrated that Na+ self-inhibition reflects a reduction in channel Po (119), consistent with earlier work suggesting that external Na+ reduced ENaC currents by enhancing channel closure (53). These data imply that Na+ allosterically inhibits ENaC by binding to site(s) within the extracellular regions of ENaC. The time course of inhibition, with time constants of 2–8 s (13, 36, 118), is similar to the times reported for stochastic conductance changes in single-channel recordings (112). This is consistent with an allosteric effector model of Na+ self-inhibition where a conformational change is rate limiting.
Where are the Na+ effector site(s)? Efforts at functionally identifying sites where Na+ binding initiates conformational changes that lead to a reduction in channel Po have been complicated by experimental limitations. First, estimates of Na+ Ki have high uncertainty as experiments measuring Na+ self-inhibition rarely achieve saturation. Second, experiments employing mutagenesis cannot readily distinguish between direct effects at a putative Na+ binding site and indirect ones due to effects on the downstream allosteric machinery. This is true for mutations in the finger and thumb domains, where mutations at selected sites dramatically altered Na+ self-inhibition (13, 47, 71, 98, 118, 122, 142). Similar to ASIC1, ENaC subunits have an acidic cleft in the extracellular region (70). While the role of acidic residues within this cleft in Na+ binding remains to be determined, it is notable that mutations at select acidic residues in the extracellular region reduced Na+ self-inhibition (47).
Channel inhibition by extracellular Cl−.
Collier and Snyder (38, 40) recently showed that Cl− inhibits ENaC currents with an apparent Ki of 30 mM, suggesting that urinary Cl− has a physiological role in modulating ENaC activity in the collecting duct. External Cl− inhibits ENaC by reducing Po with an apparent time course similar to the other allosteric inhibitors of ENaC. This inhibitory effect has a degree of anion selectivity, with Cl−, I−, and Br− inhibiting ENaC similarly, and F− and larger sulfates and phosphates inhibiting ENaC relatively poorly.
The resolved ASIC1 structure revealed a bound Cl− at each of the three subunit interfaces, with residues from the thumb and palm domains of different subunits defining each binding site (63). Collier and Snyder (38, 40) examined whether analogous sites within ENaC are involved in Cl− binding (Fig. 3). Mutations of key residues at these sites had differing effects on Cl− inhibition as well as Na+ self-inhibition (38, 40). Specifically, mutants at the αγ interface had little effect on Cl− inhibition, mutants at the βγ interface reduced Cl− inhibition, and mutants at the αβ interface reduced both Cl− inhibition and Na+ self-inhibition. The specificity of the effects of these mutants provided evidence for a preferential clockwise α-γ-β subunit arrangement, when viewed from the extracellular side. As no reported group of mutations completely abolished Cl− inhibition, it must be concluded that either these sites retained an impaired ability to bind Cl− or that additional Cl− effector site(s) exist.
Relating Structure to Function
How ligand binding events in the extracellular domains translate to conformational changes in the pore remains a key topic for future research. While mechanistic insights from studies of ASIC1 and other family members may extend to ENaC, there are certain limitations to such inferences. Specifically, sequence identity is poor in several regions, and ENaC and ASIC1 exhibit different gating characteristics. ENaC toggles between two functional states, open and closed, both of which are relatively stable. Stimuli simply shift the equilibrium between these states. ASIC1 cycles between three distinct states, i.e., resting, open, and desensitized, where the resting and desensitized states are nonconducting states. The resting state is the stable form at high pH, and the desensitized state is the stable form at low pH. The open state is only transiently populated upon a sudden drop in pH when starting from a resting state pH.
Various ligands and mechanical stress influence ENaC Po. Current evidence suggest that Cl− binds at the intersubunit thumb-palm αβ and βγ domain interfaces, that the α-subunit-derived inhibitory peptide binds at the finger-thumb interface of the α-subunit, and that Na+ binds at site(s) in the extracellular region. It is likely that conformational changes in the pore are associated with gating, based on the observations that certain mutations in the pore strongly increase Po and that amiloride blocks open channels. In the ASIC1 structure, the extracellular domains are directly connected to the pore-forming helices by the first and last β-strands of the palm domain (Fig. 1). The ASIC1 structure suggests that the β9-α4 loop between the palm and thumb is in close proximity to these connectors and the outer vestibule of the pore.
For binding events in the extracellular domains to affect the conduction status of the pore, ligand binding must lead to an applied force that eventually results in conformational changes in the pore. Such a force could conceivably be transmitted via the palm and the palm-TM linkers to the TM helices, or via the thumb and the β9-α4 loop to palm-TM linkers and the TM helices. For ASIC1, Jasti et al. (63) proposed that proton binding resulted in conformational changes that were transduced through the disulfide-stiffened thumb domain and eventually to the pore. Evidence from Li et al. (90) support this notion. Based on the finding that inhibitory peptide binding involves the thumb domain, we proposed a similar mechanism for peptide inhibition of ENaC (Fig. 3) (70). Cl− binding also involves the thumb domains and therefore could induce movement of the thumb and the β9-α4 loop. As Cl− binding also involves the palm domains, conformational changes may be directly transduced through the palm domains to the TM helices (Fig. 3). For ASIC1, movement of palm domain residues proximal to the TM helices has been associated with gating (111).
What are the conformational changes in the pore that result in channel opening or closure? Based on normal-mode analysis of ASIC1, Yang et al. (143) proposed that the pore undergoes a twist that dilates the pore from the nonconducting conformation observed in the crystal structures. This motion involves a small twist of each TM helix and an overall twist of the pore relative to the extracellular domains. Such a mechanism can account for the observation that an ASIC1 mutant with three intersubunit disulfide bonds across the extracellular mouth of the pore nonetheless gates in response to protons (138). When we performed normal-mode analysis on an ENaC model built on the basis of ASCI1 homology and peptide binding data, we observed that a similar motion in the pore was associated with motions near the peptide binding site (70). Such a mechanism for pore opening may account for ion selectivity and residue accessibility data that are seemingly inconsistent with the ASIC1 structures. Additional data characterizing the open state will be required to elucidate the conformational changes required to open these channels. In the absence of an open-pore model, the molecular details of ion discrimination and amiloride binding cannot be fully understood.
Summary
ENaC plays key roles in maintaining Na+ homeostasis, which is directly linked to extracellular fluid volume. In accord with its roles, ENaC is highly Na+ selective and channel activity is regulated at multiple levels, including biogenesis and assembly, intracellular trafficking, and gating. The resolved ASIC1 structure facilitates an examination of channel properties as they relate to the extracellular region and the pore, namely, Na+ selectivity, amiloride block, and allosteric regulation. The residues responsible for Na+ selectivity and amiloride block have been well characterized. ENaC models based on nonconducting ASIC1 structures (Fig. 2) reveal the relative positions of key residues. A detailed mechanistic understanding of selectivity and amiloride block is limited by the fact that these are properties of open channels. Reconciling functional data regarding open-pore properties and nonconducting pore models suggests conformational changes in the pore may entail a concerted twist that includes small rotations of the TM helices. Regardless of the nature of the pore conformational change, numerous allosteric effectors influence ENaC Po, seemingly at sites in the large extracellular regions of ENaC subunits. Binding sites for some of these have been described, and defining the effector sites for the other key ligands remains a focus of future research. How effector binding in the extracellular regions induces conformational changes in the pore is essential to an understanding of ENaC function. Here we note recent data suggesting that both an inhibitory peptide (70, 71) and Cl− (40) likely bind to sites partially defined by the thumb domain of ENaC subunits. These data are consistent with an important role for the thumb domain in transducing binding events to the pore as originally proposed by Jasti and colleagues (63) based on the ASIC1 structure.
GRANTS
This work is supported by National Institutes of Health Grants K01 DK078734, R01 DK065161, and R01 DK051391.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We dedicate this work in memory of Dale Benos, an exemplary physiologist who was a friend, colleague, and collaborator.
REFERENCES
- 1. Abi-Antoun T, Shi S, Tolino LA, Kleyman TR, Carattino MD. Second transmembrane domain modulates epithelial sodium channel gating in response to shear stress. Am J Physiol Renal Physiol 300: F1089–F1095, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol 12: 1114–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol 140: 143–152, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams CM, Snyder PM, Welsh MJ. Paradoxical stimulation of a DEG/ENaC channel by amiloride. J Biol Chem 274: 15500–15504, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of γ ENaC mediates elastase activation of Na+ transport. J Gen Physiol 130: 611–629, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol 13: 1557–1563, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Anantharam A, Palmer LG. Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol 130: 55–70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol 574: 333–347, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol 17: 968–976, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Awayda MS, Ismailov II, Berdiev BK, Benos DJ. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am J Physiol Cell Physiol 268: C1450–C1459, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Awayda MS, Subramanyam M. Regulation of the epithelial Na+ channel by membrane tension. J Gen Physiol 112: 97–111, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Babini E, Geisler HS, Siba M, Grunder S. A new subunit of the epithelial Na+ channel identifies regions involved in Na+ self-inhibition. J Biol Chem 278: 28418–28426, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Baer JE, Jones CB, Spitzer SA, Russo HF. The potassium-sparing and natriuretic activity of N-amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride dihydrate (amiloride hydrochloride). J Pharmacol Exp Ther 157: 472–485, 1967 [PubMed] [Google Scholar]
- 15. Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem 272: 28819–28822, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Benos DJ, Simon SA, Mandel LJ, Cala PM. Effect of amiloride and some of its analogues of cation transport in isolated frog skin and thin lipid membranes. J Gen Physiol 68: 43–63, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bentley PJ. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol 195: 317–330, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berdiev BK, Karlson KH, Jovov B, Ripoll PJ, Morris R, Loffing-Cueni D, Halpin P, Stanton BA, Kleyman TR, Ismailov II. Subunit stoichiometry of a core conduction element in a cloned epithelial amiloride-sensitive Na+ channel. Biophys J 75: 2292–2301, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bize V, Horisberger JD. Sodium self-inhibition of human epithelial sodium channel: selectivity and affinity of the extracellular sodium sensing site. Am J Physiol Renal Physiol 293: F1137–F1146, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Canessa CM. Structural biology: unexpected opening. Nature 449: 293–294, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 361: 467–470, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel γ subunit has a dominant role in channel activation. J Biol Chem 283: 25290–25295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carattino MD, Liu W, Hill WG, Satlin LM, Kleyman TR. Lack of a role of membrane-protein interactions in flow-dependent activation of ENaC. Am J Physiol Renal Physiol 293: F316–F324, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the α-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 294: F47–F52, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its α subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J, Myerburg MM, Passero CJ, Winarski KL, Sheng S. External Cu2+ inhibits human epithelial Na+ channels by binding at a subunit interface of extracellular domains. J Biol Chem [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chraibi A, Horisberger JD. Na self-inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 120: 133–145, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol 111: 127–138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 284: 29320–29325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 284: 792–798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl− inhibitory residues suggests a trimeric αγβ channel architecture. J Biol Chem 286: 6027–6032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuthbert AW. Importance of guanidinium groups of blocking sodium channels in epithelia. Mol Pharmacol 12: 945–957, 1976 [PubMed] [Google Scholar]
- 42. Dijkink L, Hartog A, van Os CH, Bindels RJ. The epithelial sodium channel (ENaC) is intracellularly located as a tetramer. Pflügers Arch 444: 549–555, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+. Proc Natl Acad Sci USA 95: 7169–7173, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349: 588–593, 1991 [DOI] [PubMed] [Google Scholar]
- 46. Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Edelheit O, Hanukoglu I, Dascal N, Hanukoglu A. Identification of the roles of conserved charged residues in the extracellular domain of an epithelial sodium channel (ENaC) subunit by alanine mutagenesis. Am J Physiol Renal Physiol 300: F887–F897, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Eskandari S, Snyder PM, Kreman M, Zampighi GA, Welsh MJ, Wright EM. Number of subunits comprising the epithelial sodium channel. J Biol Chem 274: 27281–27286, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics: Chapter 5: Unit 5.6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Favre I, Moczydlowski E, Schild L. On the structural basis for ionic selectivity among Na+, K+, and Ca2+ in the voltage-gated sodium channel. Biophys J 71: 3110–3125, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC). EMBO J 17: 344–352, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Firsov D, Robert-Nicoud M, Gruender S, Schild L, Rossier BC. Mutational analysis of cysteine-rich domains of the epithelium sodium channel (ENaC). Identification of cysteines essential for channel expression at the cell surface. J Biol Chem 274: 2743–2749, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Fuchs W, Larsen EH, Lindemann B. Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J Physiol 267: 137–166, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grunder S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J 16: 899–907, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Helman SI, Baxendale LM. Blocker-related changes of channel density. Analysis of a three-state model for apical Na channels of frog skin. J Gen Physiol 95: 647–678, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hu JC, Bengrine A, Lis A, Awayda MS. Alternative mechanism of activation of the epithelial Na+ channel by cleavage. J Biol Chem 284: 36334–36345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367: 467–470, 1994 [DOI] [PubMed] [Google Scholar]
- 59. Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Ismailov II, Berdiev BK, Shlyonsky VG, Benos DJ. Mechanosensitivity of an epithelial Na+ channel in planar lipid bilayers: release from Ca2+ block. Biophys J 72: 1182–1192, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Jean F, Thomas L, Molloy SS, Liu G, Jarvis MA, Nelson JA, Thomas G. A protein-based therapeutic for human cytomegalovirus infection. Proc Natl Acad Sci USA 97: 2864–2869, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Ji HL, Bishop LR, Anderson SJ, Fuller CM, Benos DJ. The role of Pre-H2 domains of α- and δ-epithelial Na+ channels in ion permeation, conductance, and amiloride sensitivity. J Biol Chem 279: 8428–8440, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Ji HL, Fuller CM, Benos DJ. Osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes. Am J Physiol Cell Physiol 275: C1182–C1190, 1998 [DOI] [PubMed] [Google Scholar]
- 69. Ji HL, Parker S, Langloh AL, Fuller CM, Benos DJ. Point mutations in the post-M2 region of human α-ENaC regulate cation selectivity. Am J Physiol Cell Physiol 281: C64–C74, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286: 649–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kashlan OB, Boyd CR, Argyropoulos C, Okumura S, Hughey RP, Grabe M, Kleyman TR. Allosteric inhibition of the epithelial Na+ channel (ENaC) through peptide binding at peripheral finger and thumb domains. J Biol Chem 285: 35216–35223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kashlan OB, Maarouf AB, Kussius C, Denshaw RM, Blumenthal KM, Kleyman TR. Distinct structural elements in the first membrane-spanning segment of the epithelial sodium channel. J Biol Chem 281: 30455–30462, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Kashlan OB, Sheng S, Kleyman TR. On the interaction between amiloride and its putative α-subunit epithelial Na+ channel binding site. J Biol Chem 280: 26206–26215, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Kellenberger S, Auberson M, Gautschi I, Schneeberger E, Schild L. Permeability properties of ENaC selectivity filter mutants. J Gen Physiol 118: 679–692, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kellenberger S, Gautschi I, Rossier BC, Schild L. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest 101: 2741–2750, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kellenberger S, Gautschi I, Schild L. An external site controls closing of the epithelial Na+ channel ENaC. J Physiol 543: 413–424, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kellenberger S, Gautschi I, Schild L. A single point mutation in the pore region of the epithelial Na+ channel changes ion selectivity by modifying molecular sieving. Proc Natl Acad Sci USA 96: 4170–4175, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kellenberger S, Hoffmann-Pochon N, Gautschi I, Schneeberger E, Schild L. On the molecular basis of ion permeation in the epithelial Na+ channel. J Gen Physiol 114: 13–30, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Kelly O, Lin C, Ramkumar M, Saxena NC, Kleyman TR, Eaton DC. Characterization of an amiloride binding region in the α-subunit of ENaC. Am J Physiol Renal Physiol 285: F1279–F1290, 2003 [DOI] [PubMed] [Google Scholar]
- 81. Kieber-Emmons T, Lin C, Foster MH, Kleyman TR. Antiidiotypic antibody recognizes an amiloride binding domain within the α subunit of the epithelial Na+ channel. J Biol Chem 274: 9648–9655, 1999 [DOI] [PubMed] [Google Scholar]
- 82. Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kleyman TR, Myerburg MM, Hughey RP. Regulation of ENaCs by proteases: an increasingly complex story. Kidney Int 70: 1391–1392, 2006 [DOI] [PubMed] [Google Scholar]
- 84. Knight KK, Olson DR, Zhou R, Snyder PM. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci USA 103: 2805–2808, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Knight KK, Wentzlaff DM, Snyder PM. Intracellular sodium regulates proteolytic activation of the epithelial sodium channel. J Biol Chem 283: 27477–27482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem 273: 13469–13474, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Kucher V, Boiko N, Pochynyuk O, Stockand JD. Voltage-dependent gating underlies loss of ENaC function in pseudohypoaldosteronism type 1. Biophys J 100: 1930–1939, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li J, Sheng S, Perry CJ, Kleyman TR. Asymmetric organization of the pore region of the epithelial sodium channel. J Biol Chem 278: 13867–13874, 2003 [DOI] [PubMed] [Google Scholar]
- 89. Li JH, Lindemann B. Competitive blocking of epithelial sodium channels by organic cations: the relationship between macroscopic and microscopic inhibition constants. J Membr Biol 76: 235–251, 1983 [DOI] [PubMed] [Google Scholar]
- 90. Li T, Yang Y, Canessa CM. Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J Biol Chem 284: 4689–4694, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci USA 102: 12831–12836, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lindemann B, Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science 195: 292–294, 1977 [DOI] [PubMed] [Google Scholar]
- 93. Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature 378: 730–733, 1995 [DOI] [PubMed] [Google Scholar]
- 94. Liu L, Johnson WA, Welsh MJ. Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc Natl Acad Sci USA 100: 2128–2133, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron 39: 133–146, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Lu M, Echeverri F, Kalabat D, Laita B, Dahan DS, Smith RD, Xu H, Staszewski L, Yamamoto J, Ling J, Hwang N, Kimmich R, Li P, Patron E, Keung W, Patron A, Moyer BD. Small molecule activator of the human epithelial sodium channel. J Biol Chem 283: 11981–11994, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64: 885–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Maarouf AB, Sheng N, Chen J, Winarski KL, Okumura S, Carattino MD, Boyd CR, Kleyman TR, Sheng S. Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J Biol Chem 284: 7756–7765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350: 232–235, 1991 [DOI] [PubMed] [Google Scholar]
- 100. Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Nie HG, Zhang W, Han DY, Li QN, Li J, Zhao RZ, Su XF, Peng JB, Ji HL. 8-pCPT-cGMP stimulates αβγ-ENaC activity in oocytes as an external ligand requiring specific nucleotide moieties. Am J Physiol Renal Physiol 298: F323–F334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8: 43–50, 2005 [DOI] [PubMed] [Google Scholar]
- 103. Orce GG, Castillo GA, Margolius HS. Inhibition of short-circuit current in toad urinary bladder by inhibitors of glandular kallikrein. Am J Physiol Renal Fluid Electrolyte Physiol 239: F459–F465, 1980 [DOI] [PubMed] [Google Scholar]
- 104. Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain 133: 150–160, 2007 [DOI] [PubMed] [Google Scholar]
- 105. Palmer LG. Ion selectivity of the apical membrane Na channel in the toad urinary bladder. J Membr Biol 67: 91–98, 1982 [DOI] [PubMed] [Google Scholar]
- 106. Palmer LG. Voltage-dependent block by amiloride and other monovalent cations of apical Na channels in the toad urinary bladder. J Membr Biol 80: 153–165, 1984 [DOI] [PubMed] [Google Scholar]
- 107. Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. J Gen Physiol 107: 35–45, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 299: F854–F861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens 19: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the γ subunit. J Biol Chem 283: 36586–36591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Passero CJ, Okumura S, Carattino MD. Conformational changes associated with proton-dependent gating of ASIC1a. J Biol Chem 284: 36473–36481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- 113. Pochynyuk O, Kucher V, Boiko N, Mironova E, Staruschenko A, Karpushev AV, Tong Q, Hendron E, Stockand J. Intrinsic voltage dependence of the epithelial Na+ channel is masked by a conserved transmembrane domain tryptophan. J Biol Chem 284: 25512–25521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Puoti A, May A, Canessa CM, Horisberger JD, Schild L, Rossier BC. The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am J Physiol Cell Physiol 269: C188–C197, 1995 [DOI] [PubMed] [Google Scholar]
- 115. Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71: 361–379, 2009 [DOI] [PubMed] [Google Scholar]
- 116. Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 117. Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the α, β, and γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 109: 15–26, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sheng S, Bruns JB, Kleyman TR. Extracellular histidine residues crucial for Na+ self-inhibition of epithelial Na+ channels. J Biol Chem 279: 9743–9749, 2004 [DOI] [PubMed] [Google Scholar]
- 119. Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Sheng S, Li J, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelial sodium channel. J Biol Chem 275: 8572–8581, 2000 [DOI] [PubMed] [Google Scholar]
- 121. Sheng S, Li J, McNulty KA, Kieber-Emmons T, Kleyman TR. Epithelial sodium channel pore region. Structure and role in gating. J Biol Chem 276: 1326–1334, 2001 [DOI] [PubMed] [Google Scholar]
- 122. Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of the epithelial Na+ channel in Na+ self-inhibition. J Biol Chem 282: 20180–20190, 2007 [DOI] [PubMed] [Google Scholar]
- 123. Sheng S, McNulty KA, Harvey JM, Kleyman TR. Second transmembrane domains of ENaC subunits contribute to ion permeation and selectivity. J Biol Chem 276: 44091–44098, 2001 [DOI] [PubMed] [Google Scholar]
- 124. Sheng S, Perry CJ, Kashlan OB, Kleyman TR. Side chain orientation of residues lining the selectivity filter of epithelial Na+ channels. J Biol Chem 280: 8513–8522, 2005 [DOI] [PubMed] [Google Scholar]
- 125. Shi S, Ghosh DD, Okumura S, Carattino MD, Kashlan OB, Sheng S, Kleyman TR. Base of the thumb domain modulates epithelial sodium channel gating. J Biol Chem 286: 14753–14761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Smart OS, Goodfellow JM, Wallace BA. The pore dimensions of gramicidin A. Biophys J 65: 2455–2460, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Snyder PM, Bucher DB, Olson DR. Gating induces a conformational change in the outer vestibule of ENaC. J Gen Physiol 116: 781–790, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Snyder PM, Cheng C, Prince LS, Rogers JC, Welsh MJ. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem 273: 681–684, 1998 [DOI] [PubMed] [Google Scholar]
- 129. Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem 269: 24379–24383, 1994 [PubMed] [Google Scholar]
- 130. Snyder PM, Olson DR, Bucher DB. A pore segment in DEG/ENaC Na+ channels. J Biol Chem 274: 28484–28490, 1999 [DOI] [PubMed] [Google Scholar]
- 131. Soundararajan R, Melters D, Shih IC, Wang J, Pearce D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proc Natl Acad Sci USA 106: 7804–7809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J 88: 3966–3975, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Staruschenko A, Medina JL, Patel P, Shapiro MS, Booth RE, Stockand JD. Fluorescence resonance energy transfer analysis of subunit stoichiometry of the epithelial Na+ channel. J Biol Chem 279: 27729–27734, 2004 [DOI] [PubMed] [Google Scholar]
- 134. Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life 60: 620–628, 2008 [DOI] [PubMed] [Google Scholar]
- 135. Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Svenningsen P, Uhrenholt TR, Palarasah Y, Skjodt K, Jensen BL, Skøtt O. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol 297: R1733–R1741, 2009 [DOI] [PubMed] [Google Scholar]
- 137. Take-Uchi M, Kawakami M, Ishihara T, Amano T, Kondo K, Katsura I. An ion channel of the degenerin/epithelial sodium channel superfamily controls the defecation rhythm in Caenorhabditis elegans. Proc Natl Acad Sci USA 95: 11775–11780, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1A. J Biol Chem 286: 16297–16307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 140. Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol 120: 191–201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Waldmann R, Champigny G, Lazdunski M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+ channel function. J Biol Chem 270: 11735–11737, 1995 [DOI] [PubMed] [Google Scholar]
- 142. Winarski KL, Sheng N, Chen J, Kleyman TR, Sheng S. Extracellular allosteric regulatory subdomain within the γ subunit of the epithelial Na+ channel. J Biol Chem 285: 26088–26096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yang H, Yu Y, Li WG, Yu F, Cao H, Xu TL, Jiang H. Inherent dynamics of the acid-sensing ion channel 1 correlates with the gating mechanism. PLoS Biol 7: e1000151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhou R, Kabra R, Olson DR, Piper RC, Snyder PM. Hrs controls sorting of the epithelial Na+ channel between endosomal degradation and recycling pathways. J Biol Chem 285: 30523–30530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]