Abstract
Eukaryotic cells use COPII-coated carriers for endoplasmic reticulum (ER)-to-Golgi protein transport. Selective cargo capture into ER-derived carriers is largely driven by the SEC24 component of the COPII coat. The Arabidopsis genome encodes three AtSEC24 genes with overlapping expression profiles but it is yet to be established whether the AtSEC24 proteins have overlapping roles in plant growth and development. Taking advantage of Arabidopsis thaliana as a model plant system for studying gene function in vivo, through reciprocal crosses, pollen characterization, and complementation tests, evidence is provided for a role for AtSEC24A in the male gametophyte. It is established that an AtSEC24A loss-of-function mutation is tolerated in the female gametophyte but that it causes defects in pollen leading to failure of male transmission of the AtSEC24A mutation. These data provide a characterization of plant SEC24 family in planta showing incompletely overlapping functions of the AtSEC24 isoforms. The results also attribute a novel role to SEC24 proteins in a multicellular model system, specifically in male fertility.
Keywords: AtSEC24, COPII, endoplasmic reticulum, pollen, protein traffic
Introduction
The evolutionarily conserved COPII (coat protomer complex II) generates intermediate carriers for protein export from the endoplasmic reticulum (ER) to the Golgi apparatus (Barlowe et al., 1994). The COPII coat is composed of two heterodimeric subcomplexes, SEC23/SEC24 and SEC13/SEC31, and the small GTPase SAR1. Activation of SAR1 by SEC12, an ER membrane-anchored GEF, leads to recruitment of the coat and selection of cargo into the COPII carriers (d'Enfert et al., 1991). Among eukaryotic genomes, there is a wide variation in the number of isoforms for each of the COPII proteins (Robinson et al., 2007). The Arabidopsis genome encodes a large number of COPII isoforms, often outnumbering many other eukaryotes; there are two SEC12, five SAR1, two SEC13, two SEC31, seven SEC23, and three SEC24 isoforms (Vernoud et al., 2003; Robinson et al., 2007). The significance of such diversification is not completely understood. Arabidopsis also has a number of putative COPII isoforms that might function in membrane transport in chloroplasts, highlighting the possibility of specific roles of COPII in plant-specific membrane traffic events (Andersson and Sandelius, 2004; Brandizzi, 2011).
Studies in non-plant systems clearly show that COPII isoforms have similar yet incompletely overlapping roles. For example, in the unicellular yeast, Saccharomyces cerevisiae, several SEC24 isoforms exist, one of which is essential. A knockout of the essential SEC24 gene can be partially rescued by over-expression of the non-essential SEC24 homologue ISS1 (Kurihara et al., 2000). However, Lst1p, another yeast SEC24 homologue, is specifically required for efficient traffic of Gas1p, a GPI-anchored protein, and Pma1p, a plasma membrane proton-ATPase (Shimoni et al., 2000; Peng et al., 2000). Similarly, using cell cultures, it has been demonstrated that mammalian SEC24 isoforms share a highly overlapping function in recognizing membrane cargo proteins, but that SEC24A is selectively required for the traffic of transmembrane proteins with a di-leucine (LL) signal in their cytosolic tail (Wendeler et al., 2007). Evidence is also emerging that plant COPII isoforms may have incompletely overlapping functions. In particular, it has been demonstrated that over-expression of fluorescent protein fusions of two nearly identical Arabidopsis SAR1 isoforms (AtSARA1A and AtSARA1B, 93% identical at the amino acid level) in tobacco protoplasts leads to different levels of inhibition of ER export of reporter-soluble cargo (Hanton et al., 2008). Furthermore, the two proteins exhibited a different localization in tobacco leaf epidermal cells, with AtSARA1B being associated with membranes to a larger extent than AtSARA1A (Hanton et al., 2008).
The availability of genetic tools and of sequenced genomes is enabling progress in probing the roles of different COPII coat proteins in intact metazoans and plants. For example, a forward genetics screen in mice has demonstrated that SEC24A, one of the three mammalian SEC24 isoforms, is critical for neural tube closure (Merte et al., 2010; Wansleeben et al., 2010). Similarly, a forward genetics screen in Arabidopsis has highlighted the possibility that AtSEC24 proteins may have only a partially functional overlap. In particular, an Arabidopsis mutant bearing a missense mutation in a conserved arginine residue to a lysine residue in position 693 of AtSEC24A has recently been isolated (Faso et al., 2009; Nakano et al., 2009). This site is highly conserved across SEC24 proteins from different organisms (Faso et al., 2009; Nakano et al., 2009) and is believed to be important for cargo selection in the process for ER export (Miller et al., 2003). The AtSEC24R693K caused alterations to the cortical ER network with the development of a large globular structure composed of convoluted ER tubules and entrapped organelles at the cell periphery, and with localized disruption of ER protein export at such structures (Faso et al., 2009; Nakano et al., 2009). These data were interpreted as a manifestation of ER export defects of an as yet unidentified AtSEC24A-specific cargo important for the maintenance of the functional and morphological integrity of the ER (Faso et al., 2009; Nakano et al., 2009). Lack of selective export of cargo suggests incomplete overlapping of function among the plant SEC24 isoforms, but, because the specific AtSEC24A cargo is still unidentified, the degree of functional overlap of the Arabidopsis SEC24 isoforms has yet to be experimentally defined. Nonetheless, lending support to the idea that AtSEC24 proteins may have unique biological roles, genetic analyses have not led to the identification of homozygous individuals bearing a T-DNA insertion in the second exon of AtSEC24A (Faso et al., 2009). Importantly, however, these data did not help to explain the causes of this phenotype, nor were they supported by complementation analyses that could exclude the possibility that the phenotype could be due to a tightly linked but unrelated mutation of AtSEC24A.
The functional role of the AtSEC24A isoform in planta has been experimentally addressed here. Using genetic analyses, pollen characterization, and complementation studies, it was found that AtSEC24A is indispensable for pollen. These data not only provide the genetic evidence that the AtSEC24A isoform is essential, they also attribute a novel role to SEC24 in multicellular organisms, specifically in male fertility.
Materials and methods
Plant materials and growth conditions
Plants used in this work were Arabidopsis thaliana (ecotype Columbia-0) either wild-type background or the atsec24a-1 mutant (GK-172F03; GABI-KAT, Max Planck Institut, Cologne, Germany). Seeds were surface-sterilized using 30% bleach plus 0.1% Triton-X 100 solution. Seeds were stratified onto 0.8% agar in MS medium supplemented with Gamborg's B5 vitamins and 1% (w/v) sucrose, then vernalized for at least 12 h followed by incubation at 21 °C under 16/8 h light/dark conditions. Two-week-old plants were then transferred to soil for crosses.
Molecular cloning
Genomic DNA was extracted using CTAB (hexadecyltrimethylammonium bromide) buffer protocol. The entire genomic AtSEC24A coding sequence was amplified from genomic DNA of wild-type Col-0 plants using Phusion® High-Fidelity DNA Polymerase (F530). The fragment was then subcloned in the binary vector pMDC107-Lat52Pro. This resulted in the vector bearing the Lat52::Atsec24A expression cassette for complementation analyses.
Bioinformatics analyses
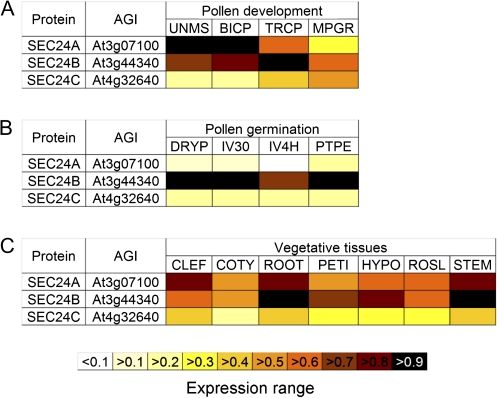
GCOS-normalized absolute expression levels for At3g07100 (AtSEC24A), At3g44340 (AtSEC24B), and At4g32640 (AtSEC24C) were extracted using the Arabidopsis eFP Browser (Winter et al., 2007). Experimental data sets for pollen development (Honys and Twell, 2004), pollen germination (Qin et al., 2009), and various vegetative tissues (Schmid et al., 2005) were each separately normalized to the AtSEC24 isoforms showing the highest expression in each experimental set.
PCR analysis of the atsec24a-1(+/m) progeny
Genomic DNA was extracted using an established protocol (Edwards et al., 1991). PCR experiments were performed in standard conditions and were carried out using 0.2 mM dNTPs, 0.2 μM primers, and 1 unit of Taq polymerase (Promega). Genotyping of the T-DNA insertion mutants was accomplished by genomic DNA extraction followed by DNA amplification with T-DNA (GABIo8409) and gene-specific primers (GABI_172F03RP and GABI_172F03LP), as described earlier (Faso et al., 2009). Two PCR products using GABI_172F03 RP paired with GABI_172F03 RP or GABI_172F03 RP paired with GABI_172F03 LP were sequenced to confirm the insertion site. Primer sequences used in this work are listed in Supplementary Table S1 at JXB online.
Arabidopsis stable transformation and complementation
Confirmed atsec24a-1(+/m) plants were transformed with pMDC107-Lat52-AtSEC24A by the floral dip method (Clough and Bent, 1998), and transformants were selected on Murashige and Skoog (MS) media supplemented with hygromycin (final concentration 50 μg ml−1) and 0.8% (w/v) agar.
Pollen germination assays
Pollen from open flowers was dipped on to semi-solid germination medium containing 1 mM MgSO4, 2.5 mM Ca(NO3)2, 2.5 mM CaCl2, 0.01% boric acid, 18% sucrose, and 0.7% fine agar (Li et al., 2008). The samples were then incubated at high humidity for 16 h, in the dark at 25 °C. The pollen grains were imaged using a Leica microscope.
Crosses, silique clearing, pollen staining, and microscopy analyses
Pollen from newly dehiscing flowers was deposited onto the stigmas of surgically emasculated flowers. Entire siliques were treated with a solution containing chloral hydrate:water:glycerol (8:2:1, by vol.) and cleared for 1–4 h at room temperature or overnight at 4 °C. Vitality stain was accomplished on pollen from newly dehiscent flowers as described by Alexander (1969). Pollen was observed with a Leica microscope. Electron microscopy analyses were conducted on dehiscent wild-type and mutant anthers fixed in 2.5% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 6.9, washed 3× in buffer and post-fixed in 2% aqueous osmium tetroxide for 90 min, washed with 0.1 M sodium cacodylate buffer and dehydrated in a graded acetone series. Samples were infiltrated and embedded in Poly/Bed 812 resin (Polysciences,). Sections were obtained with an RMC PowerTome XL (RMC, Boeckeler Instruments, Tucson, AZ) and stained with 2% uranyl acetate and lead citrate. Sections were observed using a JEOL 100CX (Japan Electron Optics Laboratory, Japan) transmission electron microscope. PaintShop Pro and Adobe Illustrator were used for further image handling.
Results
Although the AtSEC24 genes are ubiquitously expressed, a T-DNA insertion in AtSEC24A does not segregate with the expected Mendelian ratio
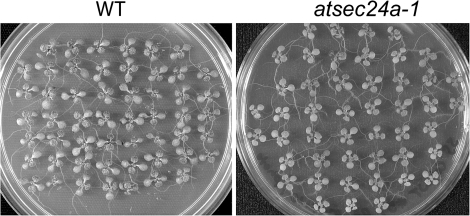
Microarray expression analyses of the pollen transcriptome have shown that the three AtSEC24 isoforms are all expressed in different stages of pollen development and germination (Honys and Twell, 2004; Qin et al., 2009) (Fig. 1A, B). Furthermore, the AtSEC24 proteins were also identified in proteomic analyses of mature pollen grains (Grobei et al., 2009). Analyses of microarray data from other experiments and experimental validation, as well as proteomic analyses indicate that the three isoforms are also expressed widely in vegetative tissues (Fig. 1C; Schmid et al., 2005; Winter et al., 2007; Baerenfaller et al., 2008; Faso et al., 2009). These data suggest that AtSEC24 isoforms may have overlapping functions in diverse developmental contexts. It has been shown, however, that a T-DNA insertion line in a Columbia (Col-0) background in the second exon of AtSEC24A (atsec24a-1) was not recovered in homozygosis (m/m) (Faso et al., 2009); thus, AtSEC24A may be essential for embryonic or reproductive development. Nonetheless, although these data suggest that AtSEC24A might be essential, they do not explain the causes of the phenotype. To exclude the possibility that atsec24a-1(m/m) could be lost during the process of collecting the seeds, due to various reasons including variations in seed size and/or fragility, the germination rate of seeds was examined from whole siliques from the progeny of the selfing of the atsec24a-1 heterozygous (+/m) line. It was found that all the seeds germinated comparably to wild-type (+/+) Col-0 (Fig. 2). Individual seedlings of the atsec24a-1(+/m) progeny populations were then genotyped by PCR, using primer sets that annealed to DNA within the insert (i.e. the T-DNA sequence) and on either side of the insertion site (see Supplementary Table S1 and Supplementary Fig. S1 at JXB online). Only heterozygous and wild-type individuals were found at a ∼50% ratio, rather than the expected 25(+/+):50(+/m):25(m/m) Mendelian ratio (Table 1A). While this experiment ensures that all the seedlings originating from seeds of whole siliques were analysed, it also indicates a non-Mendelian segregation of the mutant allele, evidence that homozygous atsec24a-1 mutants cannot be isolated (Faso et al., 2009).
Fig. 1.
Transcript and protein expression profiles of SEC24 homologues in various tissues. (A) Transcript expression values of SEC24 homologues in uninucleate microspores (UNMS), bicellular pollen (BICP), immature tricellular pollen (TRCP), and mature pollen grains (MPGR) were derived from the microarray experiments of Honys and Twell (2004). Data are expressed relative to the expression of SEC24A in UNMS (=1). (B) Transcript expression values of the SEC24 isoforms in dry pollen (DRYP), pollen germinated in vitro for 30 min (IV30), pollen germinated in vitro for 4 h (IV4H), and pollen tubes harvested after growth through pistil explants (PTPE) were derived from the microarray experiments of Qin et al. (2009). Data are expressed relative to the expression of SEC24B in PTPE (=1). (C) Transcript expression values of the AtSEC24 isoforms in various vegetative tissues including cauline leaves (CLEF), cotyledons (COTY), roots (ROOT), leaf 7 petioles (PETI), hypocotyls (HYPO), 2nd rosette leaves (ROSL), and second internodes of stems (STEM) were derived from the microarray experiments of Schmid et al. (2005). Data are expressed relative to the expression of SEC24B in ROOT (=1).
Fig. 2.
Germination of seeds from intact atsec24a-1(+/m) siliques. Plates with 2-week-old seedlings germinated from seeds of an individual silique of either a wild-type (A, WT) or an atsec24a-1(+/m) (B) plant. Seeds of an atsec24a-1(+/m) germinated similarly to the wild type (B).
Table 1.
Results of the self- and outcrosses of plants heterozygous for AtSEC24A (+/–). Number of progeny indicates the number of seedlings that were genotyped from independent siliques developed upon successful crosses (No. of crosses). ++: Wild type; +/–: heterozygote; m/m homozygous for the T-DNA insertion. Results of the Chi-square distribution (χ2) analysis are indicated along with the probability value (P).
| Inheritance of AtSEC24A alleles | ||||||||
| (A) Self-cross of AtSEC24A(+/–) | atsec24a-1 | No. of crosses | No. of progeny | Genotypes of progeny |
χ2 |
P | ||
| +/+ | +/m | m/m | ||||||
| 25% | 50% | 25% | Expected | |||||
| m | 6 | 149 | 49.7% | 50.3% | 0% | 73.51 | <0.001 | |
| (B) Outcross of AtSEC24A(+/–) | atsec24a-1 | No. of crosses | No. of progeny | Genotypes of progeny | χ2 | P | ||
| +/+ | +/m | m/m | ||||||
| Pollen source: +/+; | 50% | 50% | – | Expected | ||||
| Pollen recipient: +/m | m | 6 | 96 | 50% | 50% | – | 0 | NSa |
| Pollen source: +/m; | 50% | 50% | – | Expected | ||||
| Pollen recipient: +/+ | m | 5 | 31 | 100% | – | – | 31 | <0.001 |
NS, Not statistically significant.
Developing embryos and seeds of the atsec24a-1(+/m) line are intact
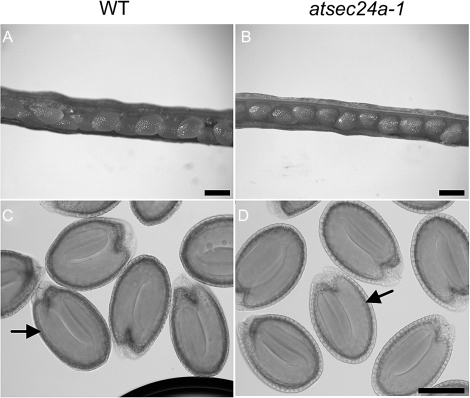
In flowering plants, the egg develops within a haploid embryo sac (female gametophyte) in the pistil. The haploid pollen grain (male gametophyte) germinates a pollen tube that carries two sperm cells to the embryo sac (Borg et al., 2009; Kagi and Gross-Hardt, 2009). Mutations that affect the function of the embryo sac or the pollen cannot be transmitted through the defective gametes; thus, such mutants can be carried only as heterozygotes. Because our genetic studies showed that the mutant allele was not transmitted to the progeny in the expected Mendelian ratio (Table 1A), and that the progeny of atsec24a-1(+/m) germinated similarly to wild-type Col-0 (Fig. 2), it was reasoned that the absence of atsec24a-1(m/m) individuals would be linked to defects in either the female or male gametophyte. To distinguish between these two possibilities, the first aim was to inspect the siliques, which form upon successful egg fertilization, from the selfing of atsec24a-1(+/m) plants. In particular, to check whether the siliques of the atsec24a-1(+/m) line carried abnormal seeds and/or empty spots that may form due to embryonic defects or lack of egg fertilization, seeds inside developing siliques were analysed. Similar to wild-type Col-0, non-dehiscent siliques (7 d after pollination; DAP) showed no obvious defects in the appearance of the maturing seeds and no empty spots (Fig. 3A, B). To ensure that the embryos in seeds of atsec24a-1(+/m) developed similarly to wild-type Col-0, cleared siliques at 10 DAP were analysed and no obvious defects were found in the embryos (Fig. 3C, D). Together with the evidence that the seed germination pattern of the progeny of atsec24a-1(+/m) was similar to wild-type Col-0 (Fig. 2), these data strongly suggest that the absence of atsec24a-1(m/m) individuals is not linked to defects in the female gametophyte or in embryonic development.
Fig. 3.
There are no visible defects in developing seeds and embryos of atsec24a-1(+/m) plants. Non-dehiscent siliques from wild-type (WT, A) and atsec24a-1(+/m) (B) plants showing no appreciable differences in the gross morphology of the maturing seeds. Scale bar in (A) and (B)=0.5 mm. Clarified 10-d-old seeds from siliques of wild-type (C) and atsec24a-1(+/m) (D) plants showing similar morphology of the embryos (arrows). Scale bar in (D) and (C)=0.2 mm.
The atsec24a-1 phenotype is linked to a male-specific transmission defect
To explore the possibility that lack of atsec24a-1(m/m) was associated with male transmission defects, reciprocal crosses of atsec24a-1(+/m) with wild-type Col-0(+/+) were performed. When pollen from atsec24a-1(+/m) plants was used for the crosses, no heterozygotes for the atsec24a-1 allele were identified, instead of the expected 50% of the progeny (Table 1B), suggesting that transmission of the mutation through the male gametophyte was impaired. To confirm that this was the case, the crosses with the atsec24a-1(+/m) female parent with wild-type Col-0 pollen(+/+) were analysed and it was found that 50% of the progeny were heterozygous for the mutation (Table 1B), as would be expected for a Mendelian segregation in crosses with functional gametes. This confirms a defective male transmission of the atsec24a-1 allele rather than a female defect.
The aberrant male transmission of the atsec24a-1 allele is linked to defective pollen
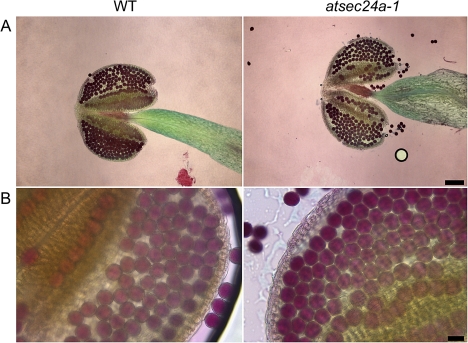
The next aim was to establish whether the atsec24a-1 allele could cause defects to the pollen. Newly dehiscent anthers of atsec24a-1(+/m) and wild-type Col-0 were stained with the Alexander stain that is commonly used to differentiate aborted from non-aborted pollen (Alexander, 1969). In the Alexander stain, two dyes are used: malachite green, which stains the pollen walls, and acid fuchsin, which stains cytoplasm and mitochondria (Alexander, 1969). It was found that atsec24a-1(+/m) pollen grains did not show obvious differences in staining compared with the wild type, that is, the majority of the grains appeared purple (Fig. 4). These data suggested that the atsec24a-1(+/m) pollen grains contained cytoplasm and organelles; staining of completely aborted pollen would appear light blue because only the walls would be coloured (Alexander, 1969) (see also Fig. 6). To characterize the pollen further and to establish whether the pollen had subcellular abnormalities, which cannot be distinguished with the Alexander stain, transmission electron microscopy analyses were conducted on newly dehiscent anthers from wild-type and atsec24a-1(+/m) plants. Pollen from wild-type plants showed dense cytoplasm with intact ER and Golgi (Fig. 5A, B). Pollen from atsec24a-1(+/m) was uniform in size with clearly defined walls (see Supplementary Fig. S2 at JXB online). However, while it was found that half of the atsec24a-1(+/m) grains appeared similar to the wild type (Fig. 5B, C; see Supplementary Fig. S2 at JXB online) with clearly visible organelles, such as the ER, Golgi, and mitochondria (Fig. 5D), the other half had a semi-translucent appearance, vacuolated structures, and abnormal mitochondria; organelles such as the ER and the Golgi were not clearly distinguishable (Fig. 5C, E, F). These data indicate that, in newly dehiscent anthers, half of the atsec24a-1(+/m) grains are abnormal although not completely devoid of cellular content.
Fig. 4.
Pollen from atsec24a-1(+/m) plants shows cellular activities as deduced by the Alexander stain. (A) Whole anthers from wild-type (WT) and atsec24a-1(+/m) dehiscent flowers showing similar pollen staining with the Alexander stain. Scale bar=100 μm. (B) Higher magnification images of the anthers of wild-type and mutant flowers, respectively. Scale bar=20 μm.
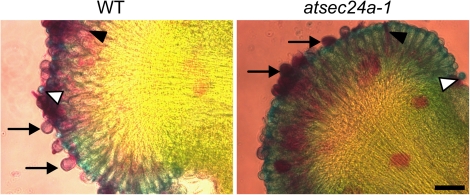
Fig. 6.
atsec24a-1 pollen can germinate in vivo. Alexander staining of wild-type and atsec24a-1 stigmas in contact for 4 h with the respective pollen. That the pollen can germinate is shown by the presence of pollen tubes (black arrowheads). Ungerminated and newly germinating grains (arrows) appear purple due to staining of the cytoplasm (Alexander, 1969). Fully germinated or aborted pollen grains appear light blue coloured due to the absence of cytoplasm (white arrowheads). Scale bar=40 μm.
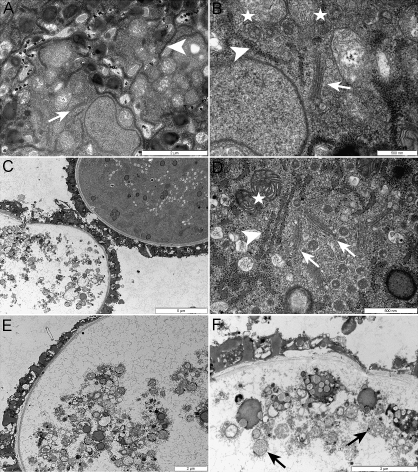
Fig. 5.
Pollen from atsec24a-1(+/m) plants has a heterogeneous appearance at an ultrastructural level. (A, B) Transmission electron micrograph of newly dehiscent wild-type anthers in which organelles are clearly visible (arrow, Golgi; arrowhead, ER, stars, mitochondria). Scale bars: 2 μm (A), 500 nm (B). (C–F) Analysis of pollen from newly dehiscent anthers of atsec24a-1 plants shows two types of grain. Although the pollen wall appears uniform among the grains, half of the pollen is similar to the wild type while the other half is more translucent (C). In the pollen grains that appear similar to the wild type, organelles such as the Golgi (arrows), ER (arrowhead), and mitochondria (star) are clearly visible (D). The content of the translucent pollen grains (E) is characterized by the presence of blebbing structures, including mitochondria (arrows) and other structures of unknown nature (F). Scale bars: 5 μm (C), 500 nm (D), 2 μm (E, F).
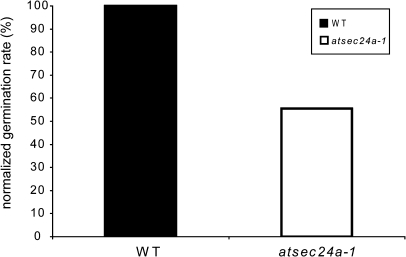
The evidence that half of the pollen appeared abnormal at an ultrastructural level suggested that half of the atsec24a-1(+/m) pollen could fail to germinate, thus explaining the aberrant male transmission verified in the crosses. To test this possibility, the ability of pollen to germinate was followed. It was found that atsec24a-1 pollen could germinate in vivo (Fig. 6), although it was not possible to accurately establish the germination rate on the stigmas because pollen grains were often released from the stigma surface during the Alexander stain procedure. Therefore, pollen germination was followed in vitro, which enables the careful estimation of germination percentages (Fig. 7; Li et al., 2008). The percentage of grains showing pollen tube emergence and of grains developing an elongated tube was recorded after 16 h of incubation on germination medium. An aberrant pattern of germination in pollen grains from atsec24a-1(+/m) anthers was found, with a germination rate close to 50% compared with that of wild-type grains (Fig. 7), indicating defects in the behaviour of half of the atsec24a-1(+/m) pollen. These data led us to suggest that the absence of viable atsec24a-1(m/m) mutants is most likely linked to aberrant transmission of the male gamete due to a pollen germination defect.
Fig. 7.
Pollen from atsec24a-1(+/m) plants has a reduced ability to form pollen tubes. Percentage of germination on solid medium of pollen grains from atsec24a-1(+/m) plants normalized to percentage of germination of wild-type pollen (WT) set to 100%. Data represent the average of two independent experiments for a total of counted pollen grains: 770 (wild type) and 2520 (atsec24a-1).
Absence of viable atsec24a-1(m/m) individuals is specifically linked to the AtSEC24A mutation
Having established that the transmission of the atsec24a-1 allele was linked to male defects, it could now be tested whether the lack of viable atsec24a-1(m/m) individuals was indeed due to the mutation of AtSEC24A rather than to a tightly linked but unrelated mutation. Therefore, a complementation experiment was performed, with a construct based on a fusion of the pollen promoter LAT52 (Twell et al., 1989) and the wild-type AtSEC24A coding sequence (LAT52::AtSEC24A) cloned in a binary vector for plant transformation. The construct was transformed into atsec24a-1(+/m) plants. Pollen from independent atsec24a-1-Lat52::AtSEC24A transformants, whose background was confirmed as atsec24a-1(+/m) through genotyping for the atsec24a-1 allele prior to the transformation (not shown), was outcrossed to wild-type Col-0(+/+). Genotyping of the progeny demonstrated complementation, with atsec24a-1 appearing in the progeny (Table 2). On the other hand, the controls, based on crosses of the atsec24a-1(+/m) on to Col-0, showed no transmission of the mutated atsec24a-1 allele, as expected (Table 2). These data allow us to conclude that the atsec24a-1 can be transmitted through the pollen of atsec24a-1(+/m)-Lat52::AtSEC24A plants, and therefore, that lack of atsec24a-1 transmission in atsec24a-1(+/m) individuals is specifically linked to the loss of AtSEC24A function.
Table 2.
Complementation test results. No heterozygous progeny were produced in an outcross to a wild type using pollen from atsec24a-1 heterozygotes (+/–). However, the atsec24a-1 allele was recovered in the progeny from individual outcrosses using pollen from an atsec24a-1(+/–) carrying a LAT52::AtSEC24A construct (+/mc), demonstrating that the male defective transmission of the atsec24a-1 mutant allele was complemented by the presence of LAT52::AtSEC24A.
| Inheritance of AtSEC24A alleles | |||||
| atsec24a-1(+/–) outcross | Allele | No. of crosses | No. of progeny | Genotypes of progeny (%) |
|
| Pollen source: +/m; | m | 5 | 31 | +/+=100 | +/m=0 |
| Pollen recipient: +/+ | |||||
| Pollen source: +/mc; | |||||
| Pollen recipient: +/+ | mc | 3 | 12 | +/+=83.3 | +/mc=16.7 |
m=atsec24a-1, mc=LAT52::AtSEC24A.
Discussion
Correct development of the male gametophyte, pollen germination, tube growth, and delivery of the sperm cells to the ovule are key factors for successful male fertility in plants. Evidence gathered in this study based on reciprocal crosses, pollen characterization, germination, and complementation tests demonstrates that lack of AtSEC24A is connected with defects in the male gametophyte that lead to reduced pollen germination. Our data provide a functional characterization in vivo of a plant SEC24, and provide experimental evidence for incompletely overlapping functions of the Arabidopsis SEC24 isoforms in plant growth and development. They also uncover a role of SEC24 proteins in fertility in a multicellular model system.
Selective cargo capture into ER-derived carriers is driven by the SEC24 component of the COPII coat (Aridor et al., 1998; Kuehn et al., 1998). Structural analyses of the COPII components and selective mutagenesis studies in vitro in mammalian cell cultures and in the unicellular system Saccharomyces cerevisiae have enabled much progress in the understanding of the COPII assembly and cargo recruitment processes (Miller et al., 2003; Mossessova et al., 2003; Miller and Barlowe, 2010; Lee and Goldberg, 2010). With the availability of genetic tools and sequenced genomes it is now possible to gather important information on COPII in vivo. Analysing the functions of various COPII isoforms in intact animals and plants has the potential advantage to uncover novel roles of COPII components in relation to tissue and developmental specificity, which are hallmarks of multicellular organisms. For example, specific defects in multicellular organisms have been described for the COPII subunits, SAR1, SEC13, and SEC23, and suggested isoform-specific roles in the mechanisms for export of cargo during organ and tissue development (Jones et al., 2003; Boyadjiev et al., 2006; Townley et al., 2008). Defects linked to the lack of an isoform of SEC24 in plants are reported here and it is shown that AtSEC24A has a role in pollen. Mounting evidence gathered from experiments with the unicellular system in yeast and mammalian cell culture suggests that eukaryotic genomes express a diverse number of SEC24 isoforms, most probably to ensure efficient and selective transport of a variety of cargo proteins (Roberg et al., 1999; Peng et al., 2000; Miller et al., 2002, 2003, 2005; Mossessova et al., 2003; Wendeler et al., 2007). In multicellular organisms, the combinatorial assembly of diverse SEC24 isoforms may facilitate the export of cargo in defined developmental stages or tissues. This is supported by the findings in mice that loss of SEC24b, one of the four mammalian SEC24 isoforms, is critical for the incorporation in COPII carriers of Vangl2, a component of the planar cell polarity-signalling pathway (Merte et al., 2010; Wansleeben et al., 2010). Reduced export of Vangl2 causes defects in neuron tube closure (Merte et al., 2010; Wansleeben et al., 2010). It has been demonstrated here that AtSEC24A isoform is essential in the male gametophyte thus implicating a role of the COPII coat in fertility. This role is specific to AtSEC24A isoform since it is not compensated by the other two SEC24 isoforms.
Successful pollen tube germination requires the response of pollen grains to germination signals, followed by the generation and maintenance of polarized pollen tube growth for successful egg fertilization. Mutants with pollen germination defects can be identified in two broad categories: those that do not perceive signals for the receiving stigma to initiate germination, and those that have a defective response to these signals that result in the failure ofn pollen tube growth. Signals that are received from the stigma include hydration, which is mediated by proteins secreted by the stigma and the pollen coat, as well as downstream events mediated by proteins and secondary messengers (Hiscock and Allen, 2008). Our complementation tests with AtSEC24A expression driven by a pollen-promoter prove that defects of the transmission of the mutant allele are linked to the pollen. Defective germination in half of the pollen grain population is consistent with a Mendelian segregation of defects associated with one allele in 50% of haploid cells. The Alexander stain indicated that most of the pollen from atsec24a-1(+/m) plants has cellular activities; however, electron microscopy analyses showed subcellular defects in half the pollen from dehiscent anthers. Upon the second mitotic division, maturation of pollen depends on crucial changes that include rearrangement of the cytoplasmic content and development of storage vacuoles; pollen that fails to germinate undergoes a process of autolysis mediated by lysosomal structures with acid phosphatases (Yamamoto et al., 2003). In our electron microscopy analyses it was noted that the wild-type organization of the cellular content of half of the atsec24a-1(+/m) pollen is lost and that, in such pollen, the few distinguishable organelles such as mitochondria have pronounced blebbing profiles. The evidence that half of the atsec24a-1(+/m) pollen has a wild-type appearance but that the other half has an anomalous content indicates that the abnormal pollen is not linked to a physiological process of autolysis because this would occur in most of the pollen grains which are synchronous in the same anther. Rather, it is proposed that, in the absence of a functional atsec24A, pollen undergoes degeneration of its content, which may prevent both sensing and response to germination stimuli.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. List of primers used in the work.
Supplementary Fig. S1. Schematic of the AtSEC24A genomic fragment.
Supplementary Fig. S2. The appearance of the pollen of atsec24a-1(+/m) is not homogeneous at an ultrastructural level.
Acknowledgments
We are grateful to Ms Karen Bird for editing the manuscript, to Ms Linda Danhof, Ms Starla Zemelis, and Dr Inga Krassovskaya for technical help, and Dr Bruce McClure, University of Missouri for the generous gift of the LAT52 sequence. We acknowledge support by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, US Department of Energy (award number DE-FG02-91ER20021) and the National Science Foundation MCB 0948584 (FB).
References
- Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technology. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Andersson MX, Sandelius AS. A chloroplast-localized vesicular transport system: a bio-informatics approach. BMC Genomics. 2004;5:40. doi: 10.1186/1471-2164-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE. Cargo selection by the COPII budding machinery during export from the ER. Journal of Cell Biology. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S. Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science. 2008;320:938–941. doi: 10.1126/science.1157956. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Borg M, Brownfield L, Twell D. Male gametophyte development: a molecular perspective. Journal of Experimental Botany. 2009;60:1465–1478. doi: 10.1093/jxb/ern355. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Fromme JC, Ben J, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nature Genetics. 2006;38:1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- Brandizzi F. Is there a COPII-mediated membrane traffic in chloroplasts? Traffic. 2011;12:9–11. doi: 10.1111/j.1600-0854.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- d'Enfert C, Wuestehube LJ, Lila T, Schekman R. Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. Journal of Cell Biology. 1991;114:663–670. doi: 10.1083/jcb.114.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Research. 1991;19:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faso C, Chen YN, Tamura K, et al. A missense mutation in the Arabidopsis COPII coat protein Sec24A induces the formation of clusters of the endoplasmic reticulum and Golgi apparatus. The Plant Cell. 2009;21:3655–3671. doi: 10.1105/tpc.109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U. Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Research. 2009;19:1786–1800. doi: 10.1101/gr.089060.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Matheson LA, Rossi M, Held MA, Brandizzi F. Plant Sar1 isoforms with near-identical protein sequences exhibit different localisations and effects on secretion. Plant Molecular Biology. 2008;67:283–294. doi: 10.1007/s11103-008-9317-5. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Allen AM. Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytologist. 2008;179:286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Jones EL, Bonney SA, et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nature Genetics. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- Kagi C, Gross-Hardt R. Analyzing female gametophyte development and function: there is more than one way to crack an egg. European Journal of Cell Biology. 2009;89:258–261. doi: 10.1016/j.ejcb.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Kuehn MJ, Herrmann JM, Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Hamamoto S, Gimeno RE, Kaiser CA, Schekman R, Yoshihisa T. Sec24p and Iss1p function interchangeably in transport vesicle formation from the endoplasmic reticulum in Saccharomyces cerevisiae. Molecular Biology of the Cell. 2000;11:983–998. doi: 10.1091/mbc.11.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Goldberg J. Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats. Cell. 2010;142:123–132. doi: 10.1016/j.cell.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gu Y, Yan A, Lord E, Yang ZB. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Molecular Plant. 2008;1:1021–1035. doi: 10.1093/mp/ssn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nature Cell Biology. 2010;12:41–46. doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO Journal. 2002;21:6105–6113. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Barlowe C. Regulation of coat assembly: sorting things out at the ER. Current Opinion in Cell Biology. 2010;22:447–453. doi: 10.1016/j.ceb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MC, Hamamoto S, Orci L, Schekman R. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- Miller EA, Liu Y, Barlowe C, Schekman R. ER-Golgi transport defects are associated with mutations in the Sed5p-binding domain of the COPII coat subunit, Sec24p. Molecular Biology of the Cell. 2005;16:3719–3726. doi: 10.1091/mbc.E05-03-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Nakano RT, Matsushima R, Ueda H, Tamura K, Shimada T, Li L, Hayashi Y, Kondo M, Nishimura M, Hara-Nishimura I. GNOM-LIKE1/ERMO1 and SEC24a/ERMO2 are required for maintenance of endoplasmic reticulum morphology in Arabidopsis thaliana. The Plant Cell. 2009;21:3672–3685. doi: 10.1105/tpc.109.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, De Antoni A, Gallwitz D. Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. Journal of Biological Chemistry. 2000;275:11521–11528. doi: 10.1074/jbc.275.15.11521. [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genetics. 2009;5:e1000621. doi: 10.1371/journal.pgen.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg KJ, Crotwell M, Espenshade P, Gimeno R, Kaiser CA. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. Journal of Cell Biology. 1999;145:659–672. doi: 10.1083/jcb.145.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C. Membrane dynamics in the early secretory pathway. Critical Reviews in Plant Science. 2007;26:199–225. [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nature Genetics. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Kurihara T, Ravazzola M, Amherdt M, Orci L, Schekman R. Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. Journal of Cell Biology. 2000;151:973–984. doi: 10.1083/jcb.151.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, Stephens DJ. Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. Journal of Cell Science. 2008;121:3025–3034. doi: 10.1242/jcs.031070. [DOI] [PubMed] [Google Scholar]
- Twell D, Wing R, Yamaguchi J, McCormick S. Isolation and expression of an anther-specific gene from tomato. Molecular and General Genetics. 1989;217:240–245. doi: 10.1007/BF02464887. [DOI] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiology. 2003;131:1191–1208. doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 2010;137:1067–1073. doi: 10.1242/dev.041434. [DOI] [PubMed] [Google Scholar]
- Wendeler MW, Paccaud JP, Hauri HP. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Reports. 2007;8:258–264. doi: 10.1038/sj.embor.7400893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An ‘electronic fluorescent pictograph’ browser for exploring and analyzing large-scale biological data sets. PLoS ONE. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T. Behavior of vacuoles during microspore and pollen development in. Arabidopsis thaliana. Plant and Cell Physiology. 2003;44:1192–1201. doi: 10.1093/pcp/pcg147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.