Abstract
The cytoplasmic C-ring of the flagellum consists of FliG, FliM and FliN and acts as an affinity cup to localize secretion substrates for protein translocation via the flagellar-specific type III secretion system. Random T-POP transposon mutagenesis was employed to screen for insertion mutants that allowed flagellar type III secretion in the absence of the C-ring using the flagellar type III secretion system-specific hook-β-lactamase reporter (Lee & Hughes, 2006). Any condition resulting in at least a 2-fold increase in flhDC expression was sufficient to overcome the requirement for the C-ring and the ATPase complex FliHIJ in flagellar type III secretion. Insertions in known and unknown flagellar regulatory loci were isolated as well as chromosomal duplications of the flhDC region. The 2-fold increased flhDC mRNA level coincided in a 2-fold increase in the number of hook-basal-bodies per cell as analyzed by fluorescent microscopy. These results indicate that the C-ring functions as a non-essential affinity cup-like structure during flagellar type III secretion to enhance the specificity and efficiency of the secretion process.
Keywords: flhDC regulation, flagellar type III secretion, C-ring affinity cup, transposon mutagenesis
Introduction
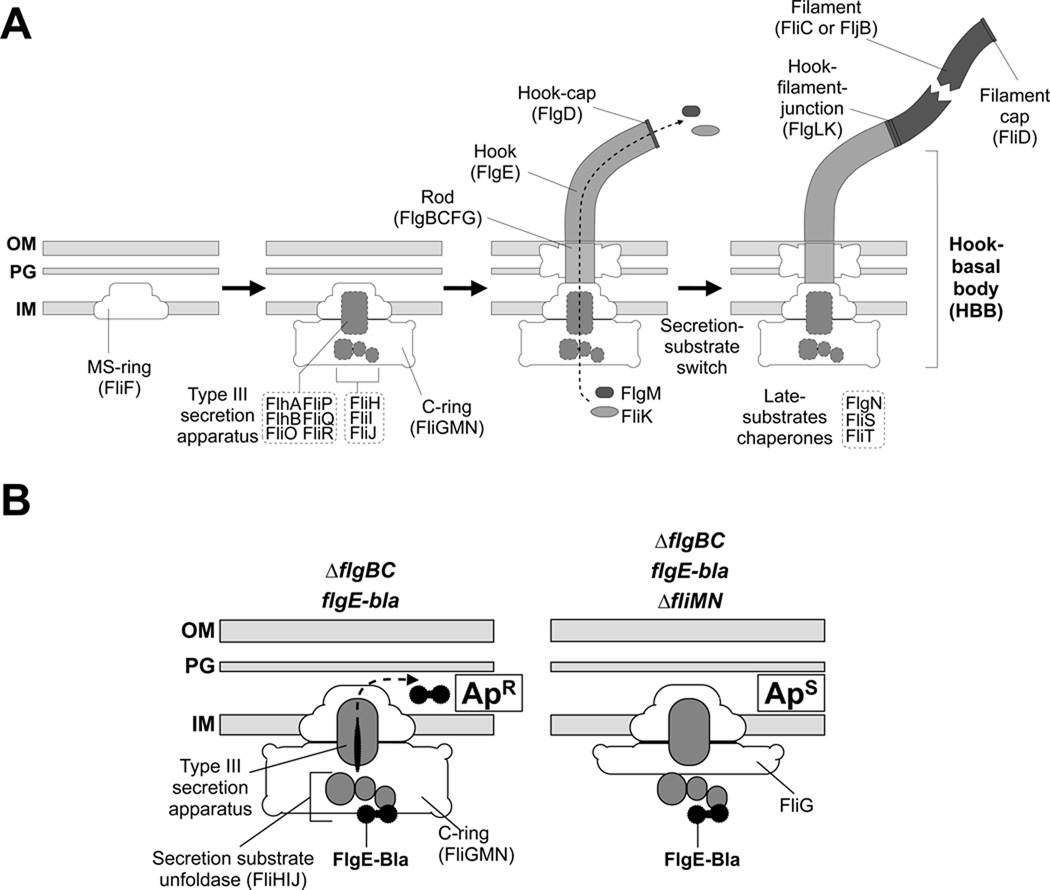
Many bacteria propel themselves in their environments by rotation of one or more propeller-like appendages called flagella (Berg & Anderson, 1973). The flagellum consists of mainly three structural parts: I) a basal body that spans the inner and outer membranes and is composed of a ion-powered (proton or sodium) rotary-motor, which incorporates a specific type III protein-secretion system, II) an external, flexible hook that acts as a universal joint between the rigid drive-shaft (rod) of the basal motor and III) the rigid, external filament (Berg, 2003, Chevance & Hughes, 2008, Macnab, 2003) (Figure 1A).
Figure 1.
(A) Steps in the assembly of the bacterial flagellum. The self-assembly process of the flagellum initiates with formation of the MS-ring (FliF) in the cytoplasmic membrane. Afterwards, a flagellar-specific type III secretion (T3S) apparatus assembles within a central pore of the MS-ring and the C-ring is attached to the cytoplasmic face of the MS-ring. At this point, flagellar secretion substrates are now selectively secreted via the T3S apparatus and coupled to the proton-motive force (Paul et al., 2008). The hook polymerizes to an app. length of 55 nm that is determined by the molecular ruler FliK and this triggers a secretion specificity switch from rod-hook-type substrates to late-secretion substrates. Upon completion of the hook-basal-body complex, the negative regulator of late substrates gene expression, the anti-σ28 factor FlgM, is secreted thereby freeing σ28 to initiate transcription of late substrate genes, like fliC or the genes of the chemosensory system.
(B) Hook-β-lactamase reporter system. Left panel: In a strain deleted for the proximal rod subunit genes, flgBC, hook-basal-body type substrates are secreted via the flagellar-specific T3S apparatus into the periplasm and subsequently degraded. β-lactamase (Bla) fused C-terminally to the hook protein FlgE is not degraded and confers resistance against lactam antibiotics, like ampicillin when secreted into the periplasm (ApR). Right panel: In a strain additionally deleted for two-thirds of the cytoplasmic C-ring (ΔfliMN), flagellar T3S is severely impaired and thus FlgE-Bla is not secreted into the periplasm and the strain is sensitive against ampicillin (ApS).
The flagellar-specific type III secretion (T3S) apparatus is believed to assemble at the base of the flagellar basal body within the MS-ring (consisting of FliF) in the inner membrane. The core T3S proteins include six integral membrane proteins (FlhA, FlhB, FliO, FliP, FliQ, FliR) and three cytoplasmic proteins (FliH, FliI, FliJ) (Minamino & Macnab, 1999). Recently it was discovered that translocation of substrates across the inner membrane was dependent on the proton motive force (PMF) (Paul et al., 2008, Minamino & Namba, 2008), and is presumably coupled to ATP-dependent substrate release and unfolding (Akeda & Galan, 2005). The ATPase complex FliH2IJ seems to function in cargo delivery to the C-ring and unfolding of the polypeptide prior to secretion. The FliH dimer has been shown to interact with the C-ring protein FliN (Gonzalez-Pedrajo et al., 2006), thereby presumably targeting substrates to the secretion system.
Beneath the MS-ring in the inner membrane, the cytoplasmic C-ring forms, which consists of FliG, FliM and FliN and is also referred to as the switch complex as this structure forms the rotor of the flagellar motor and controls the clockwise/counterclockwise rotation of the flagellum. The C-ring serves dual roles as the rotor of the flagellar motor and cup-like structure that possibly facilitates docking and secretion of flagellar substrates (Gonzalez-Pedrajo et al., 2006). Flagellar assembly is blocked at an early stage in strains deleted for fliG, fliM, or fliN (Kubori et al., 1992, Minamino & Macnab, 1999). Recently it has been shown that filament assembly in C-ring mutants is possible in a small fraction of the population upon overexpression of the T3S-specific ATPase FliI (Konishi et al., 2009).
The assembly of the flagellum is a highly regulated process. In Salmonella enterica and Escherichia coli, the flagellar regulon is controlled by a transcriptional hierarchy of three promoter classes for the expression of more than 30 structural and assembly-related proteins (Figure 1A) (Chevance & Hughes, 2008). At the top of the transcriptional hierarchy stands the class 1 promoter for transcription of the flagellar master operon, flhDC. Many different signals influence expression of the class 1 promoter to ultimately determine the level of flagellar gene expression. In S. enterica, six transcriptional start sites have been mapped within the flhDC promoter region (Yanagihara et al., 1999). The flhDC operon encodes the FlhD4C2 activator complex (Liu & Matsumura, 1994, Wang et al., 2006), which directs σ70-bound RNA polymerase to initiate transcription from class 2 promoters.
Class 2 gene products are required for the structure and assembly of the hook-basal body (HBB), which includes the T3S apparatus. Class 2 promoters also direct transcription of regulatory proteins such as the flagellum-specific σ-factor, σ28 (Ohnishi et al., 1990) and its cognate anti σ-factor, FlgM (Hughes et al., 1993). Upon completion of the HBB, substrate specificity of the flagellar-specific T3S system is switched from rod-hook substrate specificity to the secretion of late substrates like the filament subunits and the anti σ-factor FlgM. FlgM secretion after HBB completion releases σ28 to interact with RNA polymerase and activate transcription from class 3 promoters. Class 3 promoters control expression of late flagellar substrates like the filament subunits, the motor force generators (MotA and MotB) and the chemosensory system, but only in coordination with hook-basal body completion (Chevance & Hughes, 2008).
As mentioned above, many environmental signals are integrated on the level of the flhDC class 1 promoter to control the initiation or cessation of flagellar synthesis in S. enterica. Binding of the cyclic AMP-catabolite gene activator protein (CAP) complex to the flhDC promoter is required to activate transcription of flhDC (Komeda et al., 1976, Soutourina et al., 1999, Kutsukake, 1997). The iron-regulatory protein Fur, Fis and H-NS also activate flhDC transcription. Fis in S. enterica and Fur/H-NS in E. coli were shown to bind directly to the flhDC promoter (Kelly et al., 2004, Soutourina et al., 1999, Stojiljkovic et al., 1994). Another transcriptional regulator, SlyA that is required for virulence in Salmonella (Libby et al., 1994), has been shown to enhance flagellin expression (Spory et al., 2002).
Expression of flhDC is negatively regulated at both transcriptional and post-transcriptional levels by many other regulatory proteins. The RcsB regulator binds to the RcsAB box located within the flhDC promoter to inhibit motility (Wang et al., 2007). The RcsCDB system senses several external signals, like high osmolarity, desiccation and low temperature with high zinc concentrations (Majdalani & Gottesman, 2005). FimZ, a response regulator that activates type 1 fimbrial genes, inhibits motility by affecting class 1 gene expression (Clegg & Hughes, 2002). Similarly, the PefI/SrgD complex, which activates the fimbrial genes encoded on the S. enterica virulence plasmid, inhibits flhDC trancription in Salmonella (Wozniak et al., 2008). In E. coli, the LysR-type DNA-binding protein LrhA has been identified as another negative regulator of flhDC transcription that directly binds to the flhD promoter and thereby inhibits transcription (Lehnen et al., 2002). The binding of RtsB, another pathogenesis-related DNA-binding protein, also inhibits transcription of the class 1 flhDC promoter (Ellermeier & Slauch, 2003). EcnR, an uncharacterized regulatory protein was also identified as a novel negative regulator of class 1 transcription in S. enterica (Wozniak et al., 2008). Finally, deficiencies of guanosine tetraphosphate and guanosine pentaphosphate (ppGpp(p)) and the RNA-polymerase binding protein DksA respectively, have divergent effects on flagellar gene expression and motility (Aberg et al., 2009).
Following transcription, the RNA-binding protein CsrA, which is also involved in carbon storage regulation, stabilizes the flhDC transcript (Wei et al., 2001). The c-di-GMP-related protein YdiV negatively regulates FlhD4C2 activity on class 2 promoters at a post-transcriptional level (Wozniak et al., 2008). The FlhD4C2 complex is also degraded by the ClpXP protease (Tomoyasu et al., 2003) and the formation of active FlhD4C2 complex is promoted by the Hsp70 chaperone DnaK (Takaya et al., 2006).
In this work we sought mutants that allowed secretion of the flagellar hook protein fused to a β-lactamase reporter (deleted for its N-terminal Sec-dependent secretion signal) (FlgE-Bla) in strains defective in C-ring formation (ΔfliMN). We have shown previously that in strains deleted for flagellar rod genes (ΔflgBC), FlgE-Bla is efficiently secreted into the periplasm by the flagellar type III secretion system where it confers ampicillin resistance (ApR) (Lee & Hughes, 2006, Paul et al., 2008). Here we show that a ΔflgBC ΔfliMN double mutant will not secrete FlgE-Bla and the cells are ApS. Mutants able to secrete FlgE-Bla in the ΔflgBC ΔfliMN double mutant background (ApR) resulted in increased flhDC expression and HBB production supporting a role of the C-ring as an affinity cup that enhances the efficiency and specificity of flagellar T3S.
Results
Duplications of the flhDC operon overcome inhibition of FlgE-Bla secretion in a ΔfliMN C-ring mutant strain
For selective and quantitative measurement of flagellar T3S, we developed a reporter system consisting of the flagellar T3S-specific substrate FlgE (hook protein) fused to β-lactamase lacking its own Sec-dependent secretion signal (FlgE-Bla) (Lee & Hughes, 2006, Paul et al., 2008). In a mutant strain lacking the proximal rod subunits FlgB and FlgC, the hook-β-lactamase fusion protein is secreted into the periplasm conferring resistance to β-lactam antibiotics, like ampicillin (Figure 1B). Since the FlgE-Bla fusion protein is selectively secreted via the flagellar-specific T3S system, this powerful model system enables us to positively select for mutants with enhanced T3S by growth on otherwise inhibitory ampicillin concentrations. As mentioned earlier, the components of the cytoplasmic C-ring, FliG, FliM and FliN are needed for efficient T3S under wildtype conditions. In order to isolate mutants that allowed for secretion in the absence of the C-ring, overnight cultures of strain TH12470 (ΔflgBC ΔfliMN flgE-bla) were plated onto MacConkey ampicillin (15 ug/ml) selective medium. Ampicillin resistant (ApR) revertants arose at a frequency of ~10−5. This high frequency of reversion suggested that loss-of-function mutations in one or more genes would allow FlgE-Bla secretion in the ΔfliMN mutant strain.
We performed T-POP transposon mutagenesis in an attempt to isolate insertions that resulted in FlgE-Bla secretion in a rod-defective (ΔflgBC), C-ring-defective (ΔfliMN) strain by screening insertion mutants for those that resulted in an ApR phenotype. T-POP transposons are derivatives of the mini-Tn10 transposon Tn10dTc that encodes tetracycline resistance (TcR) (Lee et al., 2007, Rappleye & Roth, 1997). The T-POP transposon used, Tn10dTc[Δ25], is a Tn10dTc derivative that lacks Tn10 transposase and is deleted for the transcriptional terminator of the tetracycline resistance gene, tetA, within the transposon (the [Δ25] deletion). The divergently transcribed tetA and tetR gene encode an inner membrane efflux pump (TetA) and the TetR repressor of tetA and tetR transcription. When tetracycline (Tc) is present, it will bind to TetR and prevent DNA binding resulting in de-repression of the tetA and tetR genes. Some of the tetA and tetR transcripts will continue into adjacent chromosomal DNA flanking a Tn10dTc insertion. When Tc is added to strains that carry the Tn10dTc[Δ25] T-POP insertion, a substantial amount of transcription from the tetA promoter will continue into adjacent chromosomal DNA flanking the site of T-POP insertion (Rappleye & Roth, 1997).
The T-POP transposon was introduced into strain TH14953 (pNK2880Km/ΔflgBC ΔfliMN flgE-bla), which constitutively expresses a mutant Tn10 transposase from plasmid pNK2880Km. The mutant Tn10 transposase has lost target specificity and catalyzes random transposition into the chromosome by P22 transduction selecting for TcR and screening for ApR in the presence and absence of Tc. Of 24,000 TcR insertions, 91 were isolated that showed an ApR phenotype: 32 were ApR with or without added Tc, 23 were ApR only with added Tc and 36 were ApS when Tc was added to the medium. This is an unusually high mutation rate. The S. enterica chromosome has about 5000 genes. Assuming to the first approximation that the T-POP transposon inserts with an equal frequency in any given gene, 91/24,000 suggests a target of 18 genes that, when mutated, result in an ApR phenotype. During the handling of the ApR T-POP insertions, nine of the mutants segregated TcS ApS colonies at a high frequency (>10% from an overnight culture) when Tc and Ap were absent from the medium. Since duplication of that region of the chromosome was reported to occur at a frequency of ~10−4 (Anderson & Roth, 1978), these observations led us to conclude that ApR was occurring at a high frequency, independent of transposon mutagenesis by chromosomal duplications.
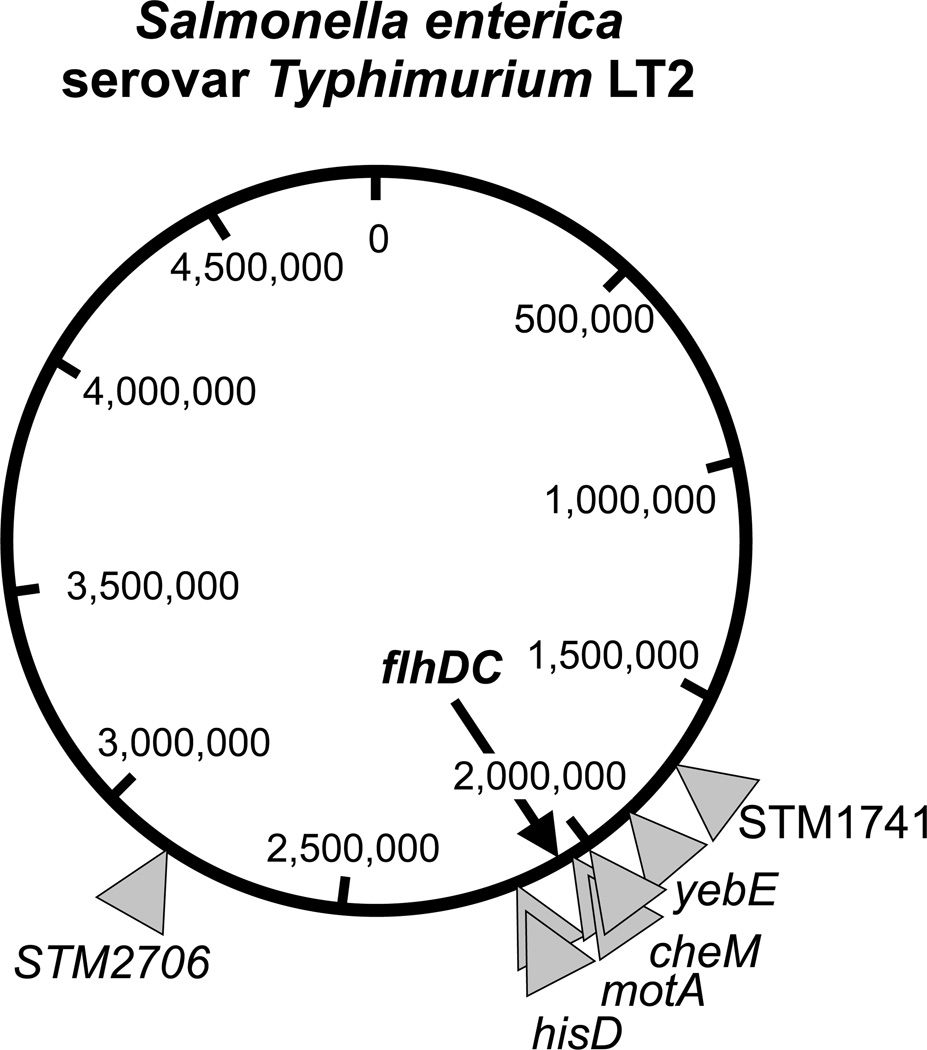
In order to test whether chromosomal duplications were giving rise to ApR in the ΔflgBC ΔfliMN flgE-bla background, MudJ transposon mutagenesis was performed on strain TH12470 (ΔflgBC ΔfliMN flgE-bla) to isolate insertions in duplicated regions that result in ApR. These would have the phenotype of losing the MudJ transposon at a high frequency when selection for maintaining the duplication is removed by growing these strains without added Ap to the medium. MudJ transposon mutagenesis was performed on strain TH12470 selecting for kanamycin resistance (KmR) in the presence of bromo-chloro-indolyl-galactopyranoside (XGal). The MudJ is a lac operon fusion vector. When MudJ inserts into a gene in the correct orientation the promoter of the inserted gene will transcribe promoterless lac operon within MudJ. We screened for ApR MudJ insertion mutants that showed an initial Lac+ phenotype in the presence of XGal (= blue colony), but upon restreaking on XGal medium lacking both Ap and Km segregated Lac− (white on XGal) colonies. This indicated that the MudJ had inserted within a duplicated region. A number of the unstable (duplication-held) ApR MudJ insertions were analyzed by DNA sequencing. As shown in Figure 2 and Table 1, these MudJ insertions were in a number of unrelated genes yet they localized to a region of the chromosome that includes the flhDC operon, suggesting that duplication of the region of the chromosome that includes flhDC resulted in the ApR phenotype. To verify this possibility, C-ring deletions strains (ΔfliMN, ΔfliG and ΔfliGMN), as well as deletions of the MS-ring (ΔfliF) and the ATPase complex (ΔfliHIJ) were constructed with an extra copy of the flhDC expressed from the arabinose promoter. Expression of flhDC from the arabinose promoter will provide about 60-fold more copies of flhDC mRNA than flhDC expressed from its native promoter (Figure 3B), however it has been shown previously that duplications tend to amplify to many more copies as well depending on selection (Kugelberg et al., 2006).
Figure 2. Locations of unstable Mud insertions in the Salmonella chromosome in strains duplicated for the flhDC region that conferred ampicillin resistance in the absence of the C-ring.
The positions of eight ApR Mud insertions in the Salmonella chromosome were determined by DNA sequence analysis. Individual Mud insertions are indicated by a grey triangle and additionally the chromosomal loci of the insertions. These unstable ApR Mud insertions resulted from transposition into duplications of the flhDC region of the chromosome thus conferring ampicillin resistance in the absence of the C-ring by increased flhDC expression. The precise insertion points are given in Table 1.
Table 1. Mud insertions and spontaneous mutations resulting in FlgE-Bla secretion in the absence of the C-ring.
Summary of isolated Mud insertions that transposed into duplicated flhDC regions of the chromosome and conferred ampicillin resistance in the C-ring deletion resulting from increased flhDC expression. Additionally shown are spontaneous mutations isolated in fliA and flhD promoter region that also allowed for FlgE-Bla secretion in the absence of the C-ring.
| Mud insertion | location of insertion |
|---|---|
| mud2 | 161 bp downstream of yebE stop, 39 bp upstream of ptrB start |
| mud4 | 204 bp downstream of hisD start |
| mud10 | 204 bp downstream of hisD start |
| mud13 | 439 bp downstream of STM2706 start |
| mud19 | 1308 bp downstream of cheM start |
| mud26 | 1323 bp downstream of cheM start |
| mud32 | 404 bp downstream of STM1741 start |
| mud36 | 703 bp downstream of motA start |
| allele | location of spontaneous mutation |
| fliA7463 | Q106:STOP (TH14683) |
| fliA7464 | Q106:STOP (TH14684) |
| PflhD7460 | −38G:A from AUG (TH14680) |
| PflhD7461 | −152C:T from AUG (TH14681) |
Figure 3.
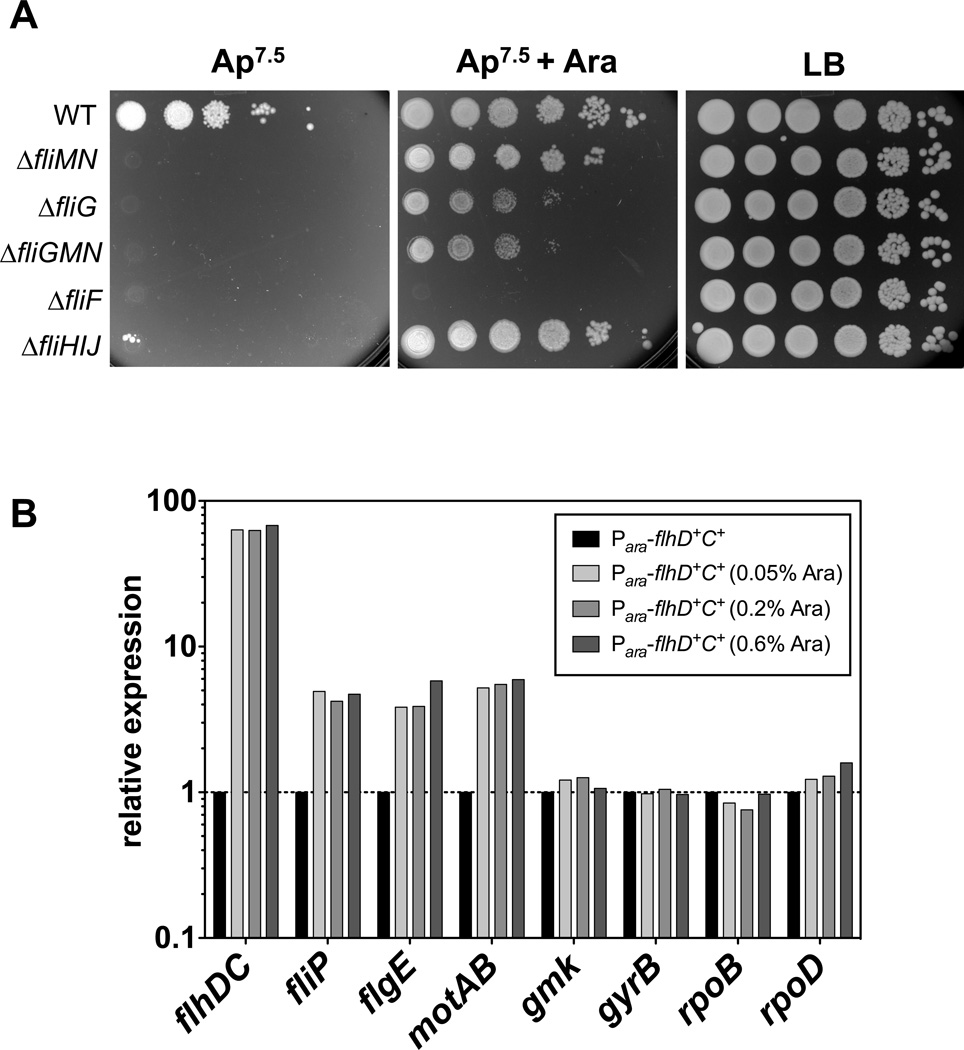
(A) Overexpression of flhDC from the arabinose promoter confers ampicillin resistance in deletion mutants of the C-ring and ATPase complex. Strains harboring an additional, functional copy of flhDC under the control of the arabinose promoter were grown overnight in the absence of arabinose. Equal volumes of ten-fold serial dilutions were spotted on LB plates and PPBS Ap7.5 plates in the presence or absence of 0.2 % arabinose. Mutant strains missing two-thirds (ΔfliMN) or the complete C-ring (ΔfliG and ΔfliGMN), as well as a strain deleted for the ATPase complex FliHIJ, but not a mutant strain missing the MS-ring (ΔfliF) are able grow in the presence of excess FlhDC. WT = TH14902; ΔfliMN = TH15498; ΔfliG = TH15497; ΔfliGMN = TH14906; ΔfliF = TH14903; ΔfliHIJ = TH14905.
(B) Effects of excess FlhDC on flagellar gene expression. Strain TH14156 (Para∷flhD+C+) was grown for 2.5 hours in LB media containing different concentrations of arabinose (0%, 0.05%, 0.2% and 0.6%) and total RNA was isolated of the pooled cultures of three biological replicates. Class 1 (flhDC), Class 2 (fliP and flgE), Class 3 (motAB) and rpoD transcript levels were analyzed by real-time qPCR as described in Experimental procedures. Relative gene expression was determined using the 2−ΔΔCT method (Livak & Schmittgen, 2001) and individual mRNA levels were normalized against multiple reference genes (rpoB, gyrB and gmk) and presented as fold change relative against the wildtype control (Vandesompele et al., 2002).
In the strain expressing flhDC from the arabinose promoter, the C-ring deletion strains were ApR in the presence of arabinose and ApS without added arabinose confirming that an at least two-fold excess flhDC was sufficient to allow FlgE-Bla secretion in the C-ring defective strain (Figure 3A). The strain deleted for the ATPase complex FliHIJ was also ApR in the presence of arabinose, confirming previous results that the ATPase function is not necessary for flagellar type III secretion (Paul et al., 2008, Minamino & Namba, 2008). While efficient secretion is possible under excess FlhDC in both the absence of the C-ring or ATPase complex, the deletion of the MS-ring prevented secretion. This indicates that the MS-ring has an essential function as a scaffold harboring the secretion apparatus, but the requirement of the C-ring and the ATPase complex can be overcome by excess substrate concentrations and increased number of potential secretion systems (see below).
It is important to note that the expression of flhDC from the arabinose promoter resulted in an about 60-fold upregulation of flhDC transcript levels if compared to wildtype flhDC expression (Figure 3B). However, different arabinose (0%, 0.05%, 0.2% and 0.6%) did neither change flhDC mRNA levels, nor upregulated Class 2 gene expression further than 4–5 fold. As shown below, consensus -10 box mutations of the flhD promoter result in only two-fold upregulation of flhDC, as well as Class 2 gene expression (Figure 6C). However, this change is sufficient to allow for efficient type III secretion in the absence of the C-ring. This indicates that activation of Class 2 gene expression by the FlhD4C2 complex is saturated early by only a small increase in flhDC levels an cannot be increased further. Importantly, this result explains why already a small increase in flhDC expression (e.g. a PflhD promoter up mutation or duplication of the flhDC operon) is sufficient to bypass the C-ring deletion.
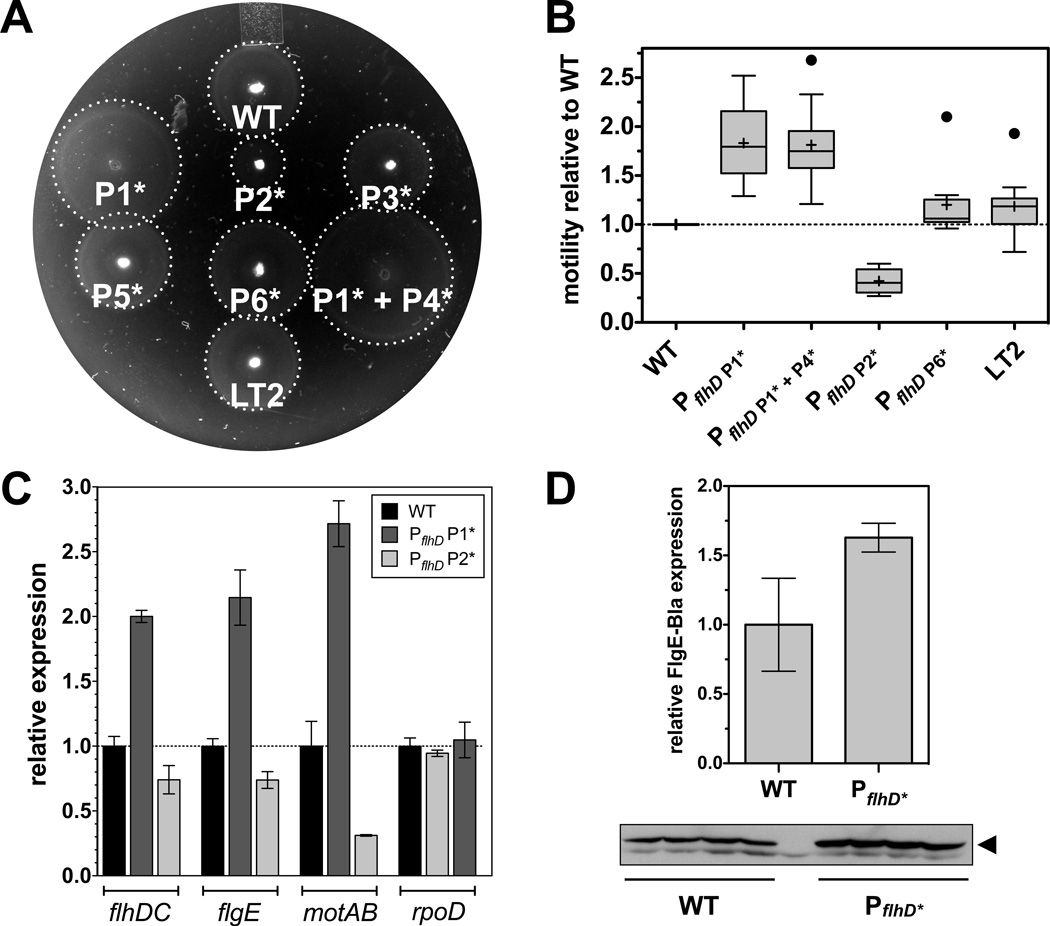
Figure 6. Motility, flagellar Class 1 and Class transcription and FlgE-Bla protein levels of PflhD promoter mutants.
(A) Motility of PflhD promoter mutants. A representative image shows the motility of different PflhD promoter mutants after 4 hours of incubation at 37°C. A dotted white line indicates the radius of the swarming circle. Consensus -10 box mutants of the P3, P5 and P6 flhD promoter display motility comparable to wildtype. The consensus -10 box mutant of the P1 flhD promoter and the combination of the consensus -10 box mutations of both the P1 and P4 flhD promoter showed increased swimming behavior, whereas the consensus -10 box mutation of the P2 flhD promoter displayed a decreased swimming speed. The PflhD promoter mutants were constructed in a strain harboring a functional fliM-gfp mutation (denoted here as WT). Motility was compared to S. enterica LT2 to show that the fliM-gfp mutation did not affect flagellar assembly or motility.
(B) Quantitative motility assay of PflhD promoter mutants. The motility of different mutant strains was measured by determining the motility diameter after 4 hours incubation at 37 °C. For each strain the diameter of 10 independent biological replicates was measured and normalized to the motility diameter of the wildtype control (fliM-gfp) on the same motility plate. Motility was compared to S. enterica LT2 to show that the fliM-gfp mutation did not affect flagellar assembly or motility. The data is presented as box diagrams with whiskers according to the Tukey method. A horizontal line indicates the median and a black cross the mean. Outliners are represented by a black dot.
(C) Relative Class 1, Class 2 and Class 3 mRNA levels as quantified by real-time qPCR. Class 1 (flhDC), Class 2 (flgE), Class 3 (motAB) and rpoD mRNA levels were analyzed by real-time qPCR as described in Experimental procedures. Relative gene expression was determined using the 2−ΔΔCT method (Livak & Schmittgen, 2001). Individual mRNA levels were normalized against rpoA, gyrB and gmk transcript levels and presented as fold change relative against the wildtype control (Vandesompele et al., 2002). The data shown represents the mean of three independent, biological samples ± SD.
(D) Relative FlgE-Bla protein levels analyzed by quantitative Western blotting. Whole cell lysates were prepared of four independent biological replicates of wildtype or P1 + P4 consensus -10 box flhD promoter mutant cells respectively. The experiment was performed in a ΔfliP background in order to analyze total protein levels. The reporter protein FlgE-Bla was expressed from its native promoter and FlgE-Bla was detected using FlgE-specific antibodies and quantified as described in Experimental procedures. Data shown are the mean of four replicates ± SD.
Characterization of T-POP insertions that allow hook-β-lactamase (FlgE-Bla) secretion in the absence of the C-ring
DNA-sequence analysis was performed on 42 independent ApR T-POP insertions in the ΔflgBC ΔfliMN flgE-bla background that did not appear to contain a duplication (they did not segregate ApS TcS segregants in the absence of selection) (Figure 4 and Table 2). Most T-POP transposon insertions resulted in an increase in FlhDC activity, by either downregulation of known transcriptional and post-transcriptional inhibitors of FlhDC (e.g. FliT, LrhA, ClpP) or upregulation of the flhDC operon itself (e.g. insertions in the flhDC promoter that increase flhDC expression).
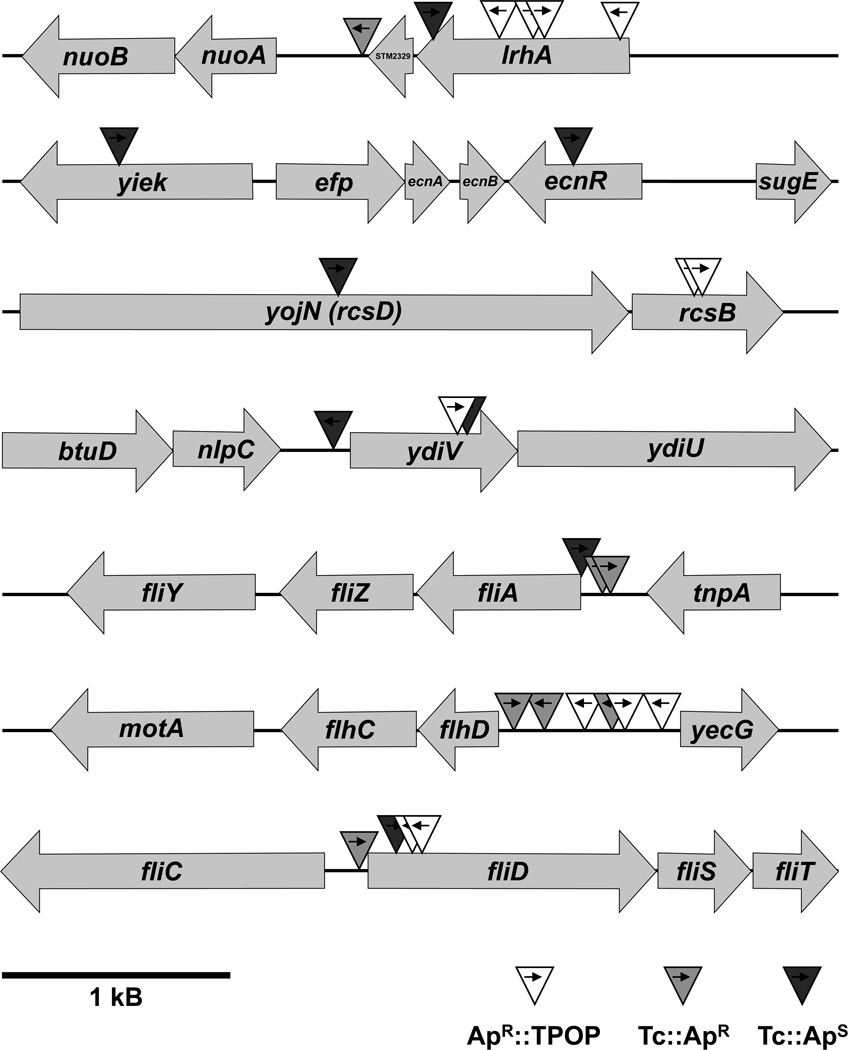
Figure 4. Schematic overview of T-POP insertions at seven different chromosomal loci that conferred ampicillin resistance in a C-ring deletion mutant.
Individual T-POP insertions are shown by a triangle with an arrow indicating the direction of the tetA transcript. A summary of the precise insertion point of every T-POP insertion and the effect of the tetA transcript reading in adjacent chromosomal genes is given in Table 2.
Table 2. T-POP transposon insertions that allow for FlgE-Bla secretion in the absence of the C-ring.
Summary of isolated T-POP transposon insertions in the C-ring deletion strain TH12470. The tetA gene of the T-POP transposon lacks a terminator sequence and therefore genes upstream of tetA can be induced by tetA expression. If the addition of tetracycline conferred ampicillin resistance, the Tc effect is indicated as Tc-ApR and if the addition of tetracycline resulted in an ampicillin sensitive phenotype, the Tc effect is indicated as Tc-ApS. In all other cases, the T-POP insertion conferred ampicillin resistance on its own and the addition of tetracycline showed no effect (ApR∷TPOP).
| allele | insertion | Tc effect |
|---|---|---|
| lrhA1 | lrhA coding region, 9 bp downstream of ATG | ApR∷TPOP |
| lrhA2 | lrhA coding region, 607 bp downstream of ATG | ApR∷TPOP |
| lrhA3 | lrhA coding region, 536 bp downstream of ATG | ApR∷TPOP |
| lrhA4 | lrhA coding region, 936 bp downstream of ATG | Tc-ApS |
| lrhA5 | STM2329 terminator, 9 bp downstream of stop | Tc-ApR |
| ecnR6 | ecnR coding region, 157 bp downstream of ATG | Tc-ApS |
| ecnR7 | yjek coding region, 859 bp downstream of ATG | Tc-ApS |
| slyA1 | slyA coding region, 170 bp downstream of ATG | ApR∷TPOP |
| rcsB131 | rcsB coding region, 332 bp downstream of ATG | ApR∷TPOP |
| rcsB132 | rcsB coding region, 332 bp downstream of ATG | ApR∷TPOP |
| yojN253 | yojN coding region, 1211 bp downstream of ATG | Tc-ApS |
| ydiV254 | ydiV coding region, 634 bp downstream of ATG | ApR∷TPOP |
| ydiV255 | ydiV coding region, 640 bp downstream of ATG | Tc-ApS |
| ydiV256 | ydiV promoter, 68 bp upstream of ATG | Tc-ApS |
| clpP71 | clpP coding region, 579 bp downstream of ATG | Tc-ApS |
| fliA7876 | fliA promoter, 30 bp upstream of ATG | Tc-ApR |
| fliA7877 | fliA promoter, 11 bp upstream of ATG | Tc-ApR |
| fliA7878 | fliA promoter, 1 bp upstream of ATG | Tc-ApS |
| flhDC7872 | flhD promoter, 426 bp upstream of ATG | ApR∷TPOP |
| flhDC7873 | flhD promoter, 625 bp upstream of ATG | ApR∷TPOP |
| flhDC7874 | flhD promoter, 799 bp upstream of ATG | ApR∷TPOP |
| flhDC7875 | flhD promoter, 615 bp upstream of ATG | Tc-ApR |
| flhDC5 | flhD promoter, 178 bp upstream of ATG | Tc-ApRR |
| flhDC6 | flhD promoter, 22 bp upstream of ATG | Tc-ApR |
| flgM1 | flgM coding region, 152 bp downstream of ATG | ApR∷TPOP |
| flgM2 | flgA promoter, 85 bp upstream of ATG | ApR∷TPOP |
| fliD7879 | fliD coding region, 93 bp downstream of ATG | ApR∷TPOP |
| fliD7880 | fliD coding region, 95 bp downstream of ATG | ApR∷TPOP |
| fliD7881 | fliD promoter, 30 bp upstream of ATG | Tc-ApR |
| fliD7882 | fliD coding region, 85 bp downstream of ATG | Tc-ApS |
| fliR1 | fliR coding region, 608 bp downstream of ATG | ApR∷TPOP |
| STM1856-1 | STM1854 coding region, 5 bp downstream of ATG | ApR∷TPOP |
| STM1856-2 | STM1856 promoter, 465 bp upstream of ATG | Tc-ApS |
| STM2011-1 | amn coding region, 5 bp downstream of ATG | Tc-ApR |
| STM2011-2 | STM2011 promoter, 272 bp upstream of ATG | Tc-ApR |
| rfbP1 | rfbP coding region, 1300 bp downstream of ATG | Tc-ApS |
| pgtE1 | pgtE coding region, 444 bp downstream of ATG | Tc-ApR |
| ddg/yfdZ1 | ddg/yfdZ terminator, 8 bp downstream of ddg stop | ApR∷TPOP |
| pykF1 | pykF terminator, 264 bp downstream of pykF stop | Tc-ApR |
| garL1 | garL coding region, 74 bp downstream of ATG | ApR∷TPOP |
| yieP1 | yieP coding region, 398 bp downstream of ATG | Tc-ApR |
| hpaX1 | hpaX coding region, 196 bp downstream of ATG | Tc-ApR |
Initially, the ApR T-POP mutants fell into three phenotypic classes (Table 2). (i) The first class of mutants was ApR solely by the T-POP insertion, indicating that the insertion knocked-out a gene whose product negatively influenced flagellar T3S in the absence of the C-ring (ApR∷T-POP). (ii) The ApR phenotype of the second class of mutants was dependent on addition of tetracycline, indicating that the transcriptional read-through from the tetA promoter induces expression of an adjacent gene that positively influences flagellar T3S in the absence of the C-ring (Tc-ApR). Alternatively, the transcriptional read-through from the tetA promoter could produce antisense RNA that would impair expression of adjacent genes. (iii) The last class of ApR mutants displayed an ApS phenotype in the presence of tetracycline (Tc-ApS). In this case the induction of tetA by addition of tetracycline presumably induces the expression of genes that negatively influence flagellar T3S in the absence of the C-ring.
In summary, we isolated three ApR∷T-POP insertions in the promoter region of flhDC that potentially disrupt binding sites of negative regulators of flhDC transcription. Three more T-POP insertions in the flhDC promoter displayed a Tc-dependent ApR phenotype (Tc-ApR), indicating an overexpression of flhDC upon Tc addition (Figure 4). Two ApR∷T-POP, one Tc-ApR and one Tc-ApS T-POP transposon insertion were in the coding region or promoter of fliD respectively (Figure 4). The transposon insertions in fliD presumably resulted in a polar effect on the expression of fliT, an anti-FlhD4C2 factor that prevents binding of the activator complex to class 2 promoters (Yamamoto & Kutsukake, 2006). This was verified by the introduction of a fliT deletion allele into strain TH12470 (ΔflgBC ΔfliMN flgE-bla), which resulted in an ApR phenotype. Additionally, we isolated T-POP insertions in known negative regulators of flhDC transcription or FlhDC levels, like lrhA, ecnR, rcsB, ydiV and clpP. Null alleles of these inhibitor loci presumably resulted in increased flhDC expression or increased stability of the FlhD4C2 activator complex. Accordingly, we isolated five T-POP insertions in the coding region or vicinity of lrhA, a LysR-type DNA-binding protein that was previously characterized as a regulator of flhDC transcription in E. coli (Lehnen et al., 2002) (Figure 4). One Tc-ApS T-POP insertion was found in the coding region of ecnR, a recently characterized regulator of flagellar gene expression (Wozniak et al., 2008). Another Tc-ApS T-POP insertion had been inserted in yjek, downstream of ecnR, thereby presumably negatively influencing the expression of ecnR (Figure 4). One ApR∷T-POP transposon had inserted in the coding region of slyA, a DNA-binding protein that has been previously shown to enhance expression of filament subunits (Spory et al., 2002). Two ApR∷T-POP transposons were isolated in the coding region of rcsB and one Tc-ApS transposon had inserted in rcsD (jojN), upstream of rcsB (Figure 4). RcsB is another negative regulator of flhDC expression and has been shown to directly bind the flhD promoter (Wang et al., 2007). We isolated one Tc-ApS T-POP insertion in the promoter region and two T-POP insertions (one ApR∷T-POP and one Tc-ApS T-POP) in the coding region of the c-di-GMP-related protein YdiV (Figure 4). YdiV negatively regulates FlhD4C2 activity at a post-transcriptional level (Wozniak et al., 2008). One Tc-ApS T-POP transposon had inserted in the coding region of clpP. It has been previously shown that the FlhD4C2 complex is degraded by the ClpXP protease (Tomoyasu et al., 2003).
T-POP insertions isolated in the promoter region of the fliAZ operon showed opposite effects: two were Tc-ApR and one was Tc-ApS (Figure 4). The fliZ gene encodes an activator of flhDC (Saini et al., 2008) and the SpiI virulence system. The fliA gene encodes σ28, which inhibits flhDC transcription (see below). Thus, these insertions could affect fliA, fliZ or both. We also isolated two ApR∷T-POP transposon insertions in the coding region of flgM and promoter region of flgA, upstream of flgM, respectively. Insertions in flgA are polar on flgM transcription. Kutsukake (Kutsukake, 1997) showed that the flhD operon is autogenously repressed in the presence of both σ28 and its cognate anti-sigma factor FlgM. However, flhDC expression is activated in the presence of σ28 and the absence of FlgM. As shown in Figure 5D, the overproduction of fliA from the arabinose promoter results in inhibition of flhC transcription in a strain harboring a functional copy of the flgM gene. This unknown mechanism of flhDC regulation would explain the effects on flhDC expression by our T-POP insertions in both flgM and the promoter region of the fliAZ operon.
Figure 5.
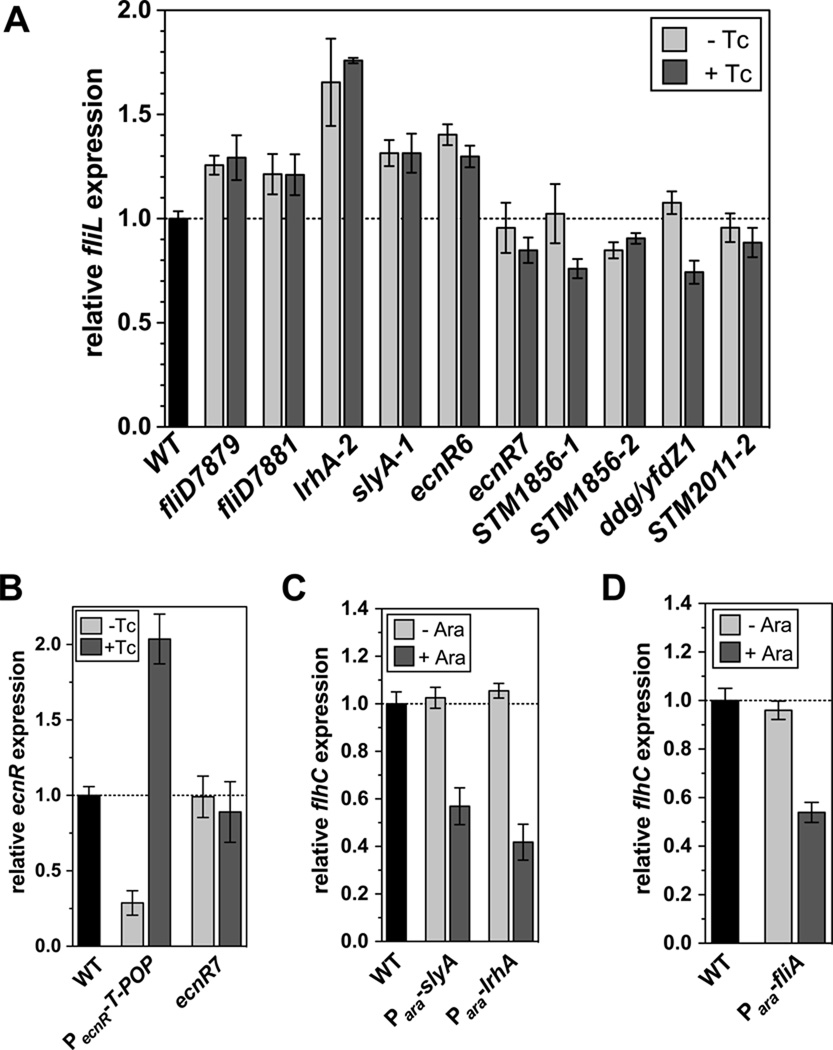
(A) Relative flagellar Class 2 transcription levels of selected, ampicillin-resistance conferring, T-POP insertions. Relative Class 2 transcription from the fliL∷MudJ transcriptional reporter was measured by β-galactosidase assays as described in Experimental procedures. Class 2 transcription was measured in the presence or absence of tetracycline and normalized against the wildtype control. Addition of tetracycline induces transcription from the tetA promoter, which proceeds into chromosomal DNA adjacent to the T-POP insertion. The data shown are the mean +/− SD of at least three independent, biological replicates.
(B) Relative ecnR transcription in a strain with a T-POP insertion in yiek near ecnR. Relative ecnR transcription from the ecnR∷MudJ transcriptional reporter was measured by β-galactosidase assays as described in Experimental procedures in strains harboring a T-POP insertion upstream of ecnR (PecnR∷T-POP) or in yiek near ecnR (ecnR7). ecnR transcription was measured in the presence or absence of tetracycline and normalized against the wildtype control. The data shown are the mean +/− SD of at least three independent, biological replicates.
(C) Relative flagellar Class 1 transcription in strains overexpressing the transcriptional regulators SlyA and LrhA. Relative Class 1 transcription from the flhC∷MudJ transcriptional reporter was measured by β-galactosidase assays as described in Experimental procedures in strains harboring slyA or lrhA under control of the arabinose promoter. Class 1 transcription was measured in the presence or absence of 0.2 % arabinose, respectively and normalized against the wildtype control. The data shown are the mean +/− SD of at least three independent, biological replicates.
(D) Relative flagellar Class 1 transcription in a strain overexpressing the flagellar-specific sigma factor σ28. Relative Class 1 transcription from the flhC∷MudJ transcriptional reporter was measured by β-galactosidase assays as described in Experimental procedures in a strain harboring fliA, the gene encoding for the flagellar-specific sigma factor σ28, under control of the arabinose promoter. Class 1 transcription was measured in the presence or absence of 0.2 % arabinose, respectively and normalized against the wildtype control. The data shown are the mean +/− SD of at least three independent, biological replicates.
Additionally, we isolated twelve T-POP transposon insertions in genes that are not obviously related to flhDC regulation. One ApR∷T-POP insertion was located after bp 608 of the 795 bp fliR gene. Two T-POP had inserted into the vicinity of sopE2, a type III-secreted effector protein. Two more Tc-ApR T-POP insertions were located to the coding region or vicinity of STM2011, a protein of unknown function. Seven more single T-POP insertions were found to be in rfbP (undecaprenol-phosphate galactosephosphotransferase/O-antigen transferase), pgtE (outer membrane protease), ddg/yfdZ (lipid A biosynthesis palmitoleoyl-acyltransferase and aminotransferase), pykF (pyruvate kinase), garL (alpha-dehydro-beta-deoxy-D-glucarate aldolase), yieP (putative regulatory protein) and hpaX (4-hydroxyphenylacetate permease) respectively. Each will require further characterization to determine how they result in ApR.
Effects of T-POP insertions on flagellar gene transcription
T-POP insertions in fliD presumably have a polar effect on fliT, a negative regulator of flhDC. Accordingly, we analyzed Class 2 transcription in various T-POP insertions that resulted in an ApR phenotype in the absence of the C-ring components FliM and FliN (Figure 5A). We found that the T-POP insertions in fliD, fliD7879 and fliD7881 respectively increased Class 2 transcription about 20 – 30%. T-POP insertions in the coding region of lrhA, slyA and ecnR had the most pronounced effect on Class 2 transcription. The loss-of-function T-POP insertion in lrhA2 increased Class 2 transcription about 1.7 fold, whereas the T-POP insertions in slyA and ecnR allowed for 1.3 – 1.4 fold enhanced Class 2 transcription.
Several other analyzed T-POP insertion had no apparent effect on Class 2 transcription, although the insertions resulted in an ApR phenotype in the C-ring deletion mutant. Surprisingly, the induction of down-and upstream genes of the respective T-POP insertions by induction with tetracycline, resulted in 10 – 30% decreased Class 2 transcription in the cases of ecnR7 (insertion in yjek, upstream of ecnR), STM1856, ddg/yfdZ1 and STM2011 (Figure 5A). We therefore asked the question whether the Tc-ApS phenotype of the ecnR7∷T-POP (insertion in yjek, upstream of ecnR) was due to Tc-dependent induction of ecnR. Accordingly, we analyzed ecnR transcription using a ecnR∷MudJ (lac transcriptional fusion) insertion described previously (Wozniak et al., 2008). As shown in Figure 5B, the T-POP insertion in yiek (ecnR7) displayed no apparent defect in ecnR transcription in the presence or absence of tetracycline.
Also, the effects of other T-POP insertions in less characterized genes or in intergenic regions remain elusive so far. Those T-POP insertions might confer ApR in the C-ring deletion mutant by the means of other mechanisms unrelated to regulation of flhDC. Possible reasons that would allow for elevated FlgE-Bla secretion include an increased proton-motive force gradient, less competition between secretion substrates or effects on localization of type III secretion substrates.
In conclusion, most of the analyzed T-POP insertions directly affect negative regulators of flhDC, thus resulting in increased Class 2 transcription and accordingly in enhanced secretion substrate levels. If the C-ring functions as an affinity cup that increases the efficiency of the secretion process, then elevated levels of substrates, like the hook-β-lactamase protein would still allow for sufficient secretion to confer ApR.
LrhA and SlyA negatively regulate flhDC transcription in Salmonella enterica
In our screen for mutants that allowed flagellar type III secretion in the absence of the C-ring, we isolated several T-POP insertions in two previously uncharacterized regulatory loci of flhDC expression in S. enterica, lrhA and slyA (Table 2). The LysR-type regulator LrhA has been previously shown to negatively regulate transcription of flhDC in E. coli (Lehnen et al., 2002). Conversely, the lack of the DNA-binding protein SlyA has been associated with increased flagellin expression (Spory et al., 2002). To analyze the effects of LrhA and SlyA in Salmonella, we cloned both putative regulators under the control of the arabinose promoter. As shown in Figure 5C, the expression of LrhA and SlyA respectively from the arabinose promoter decreases flhDC transcription 40 – 60%. Therefore, we conclude that both LrhA and SlyA act as negative regulators of flhDC transcription in S. enterica.
Null alleles in fliA and flhDC promoter mutants result in FlgE-Bla secretion in the absence of the C-ring
We attempted to isolate amino acid substitutions in flagellar genes that resulted in FlgE-Bla secretion in strains missing part (ΔfliMN) or all (ΔfliG) of the C-ring. Using the FlgE-Bla reporter construct in the rod-minus mutant background described above, we used transposons linked to the flh and fli flagellar regions to separate flh- and fli-linked ApR mutants from mixed pools of spontaneous ApR colonies. Spontaneous ApR mutants arose in strain TH12470 (ΔflgBC flgE-blaΔfliMN) at a frequency of 10−5 and in strain TH12466 (ΔflgBC flgE-blaΔfliG) at a frequency of 10−8. A mutS∷Km insertion was introduced into strain TH12466 and the frequency of spontaneous ApR mutants rose to 10−6. In the ΔfliG strain the complete C-ring is missing since FliG acts as a scaffold for FliMN assembly, whereas in the ΔfliMN strain two-thirds of the C-ring is not assembled. The lower frequency of mutation in the ΔfliG strain compared to the ΔfliMN strain can be explained by the following: 1) the physical C-ring structure may have importance in providing affinity sites for substrates or excluding non-substrates or 2) FliG in addition to FliMN provides additional interaction sites for secretion substrates. Accordingly, if the whole structure is missing (ΔfliG), one would expect a more pronounced effect than if two-thirds are missing (ΔfliMN). Additionally, we showed above that ΔfliMN displays increased FlgE-Bla secretion under excess FlhDC conditions compared to both ΔfliG and ΔfliGMN, where the complete C-ring is missing (Figure 3A). These results provide important evidence for our model of the C-ring acting as an affinity cup-like structure that locally increases secretion substrate concentration prior to secretion or excludes non-substrate interactions as discussed below.
Individual mutants that were linked to the flhDC operon were isolated in the ΔfliMN and ΔfliG backgrounds. DNA sequence analysis showed them to be single base pair changes in the flhD promoter region: −368G:A (relative to AUG start codon) in the ΔfliG background and −152C:T in the ΔfliMN background). The −368G:A allele changed the -10 sequence for the P4 promoter toward the consensus -10 σ70 binding site from TGGAAT to TAGAAT and the −152C:T allele change was outside any known promoter region, but presumably allowing for increased flhDC expression by affecting the binding site of a negative regulator of flhDC expression (Table 2). Two mutations that mapped to the fliAZY operon were both single base pair changes resulting in nonsense mutations of the fliA gene (Q106Stop, Table 2). This suggested that loss of σ28-dependent transcription increased FlhDC activity. We tested for the effect of increased fliA gene expression on an flhC∷MudJ reporter construct by over-expression of a second copy of fliA gene from the arabinose promoter. If the loss of σ28-dependent transcription would increase FlhDC activation of class 2 promoters, then increased fliA expression should have a negative effect on flhDC expression. As shown in Figure 5D, the addition of arabinose resulted in the inhibition of flhDC transcription. This result is an apparent contradiction to our earlier results that showed increased flhDC activity, as measured by FlgE-Bla secretion in the ΔflgBC ΔfliMN background in strains with reduced FlgM levels. One would expect that reduced FlgM levels result in increased σ28 activity, which in turn should negatively influence flhDC expression as described above. However, these results are consistent with previous results from K. Kutsukake (Kutsukake, 1997) who showed that the flhDC operon was autogenously repressed, except under the condition where flgM was inactivated in a fliA+ background. In the flgM-defective fliA+ background the flhDC operon was autogenously activated (Kutsukake, 1997). These apparent conflicting results of σ28-dependent inhibition of flhDC, and FlgM (anti-σ28)-dependent inhibition of flhDC warrant further study. It is possible that the FlgM/σ28 complex acts directly or indirectly to inhibit flhDC transcription.
Effects of flhDC perfect -10 box promoter mutants on swimming motility and HBB number
Based on the discovery that single base pair changes in the flhD promoter are sufficient to allow for flagellar type III secretion in the absence of the C-ring, we further characterized the effects of flhD promoter mutants. The flhDC operon is transcribed from at least 6 different promoters and we constructed mutations in the known P1, P2, P3, P5 and P6 flhD promoter sequences to have a consensus -10 box (TATAAT) (Supplemental Figure 2). As shown in Figure 6A, the perfect -10 box mutations of the P3, P5 and P6 promoter had no apparent effect on swimming motility. However, the mutation of the P1 promoter resulted in an apparent 1.7 fold increased migration rate in the swim plate compared to the wildtype (Figure 6A and 6B). The combination of PflhDC P1 and P4 -10 box mutations did not further increase motility, whereas the perfect -10 box mutation of the P2 promoter decreased motility to about 50% of wildtype motility (Figure 6A and 5B). In order to further characterize the effects of the PflhDC promoter mutations on flagellar gene expression, we performed real-time quantitative PCR analysis of cDNA reversely transcribed from strains harboring the PflhDC P1 or the PflhDC P2 perfect -10 box mutation. As displayed in Figure 6C, the P1 mutation resulted in an about 2-fold upregulation of flhDC mRNA, whereas the P2 mutation resulted in a slight downregulation of flhDC mRNA levels. We additionally confirmed this finding using an flhC-lac fusion construct (data not shown). On the level of class 2 and class 3 flagellar genes expression, the P1 and P2 -10 box mutations also resulted in an up- or downregulation respectively (Figure 6C). In case of the P1 mutation, flgE expression is enhanced about 2-fold, whereas flgE expression in the P2 mutant is slightly decreased. Similarly, the mRNA levels of the motor force generator proteins MotAB are upregulated more than 2-fold in the P1 mutant and downregulated in the P2 mutant background (Figure 6C). Taken together, the increase in flhDC expression results in an about 2 – 4 fold increase in flagellar class 2 and class 3 gene expression that explains the differences in motility described above. To confirm the 2-fold upregulation of hook-basal-body (HBB) type secretion substrates on a protein level, we examined expression of the hook-β-lactamase fusion construct in the PflhDC P1 perfect -10 box mutation background by quantitative Western blot analysis. As shown in Figure 6D, FlgE-Bla is upregulated about 1.6 fold in the P1 promoter mutant.
Accordingly, every mutation that resulted in an increase in FlhD4C2 levels resulted in an about 2-fold upregulation of both structural components of the hook-basal-body complex, as well as HBB-type secretion substrates.
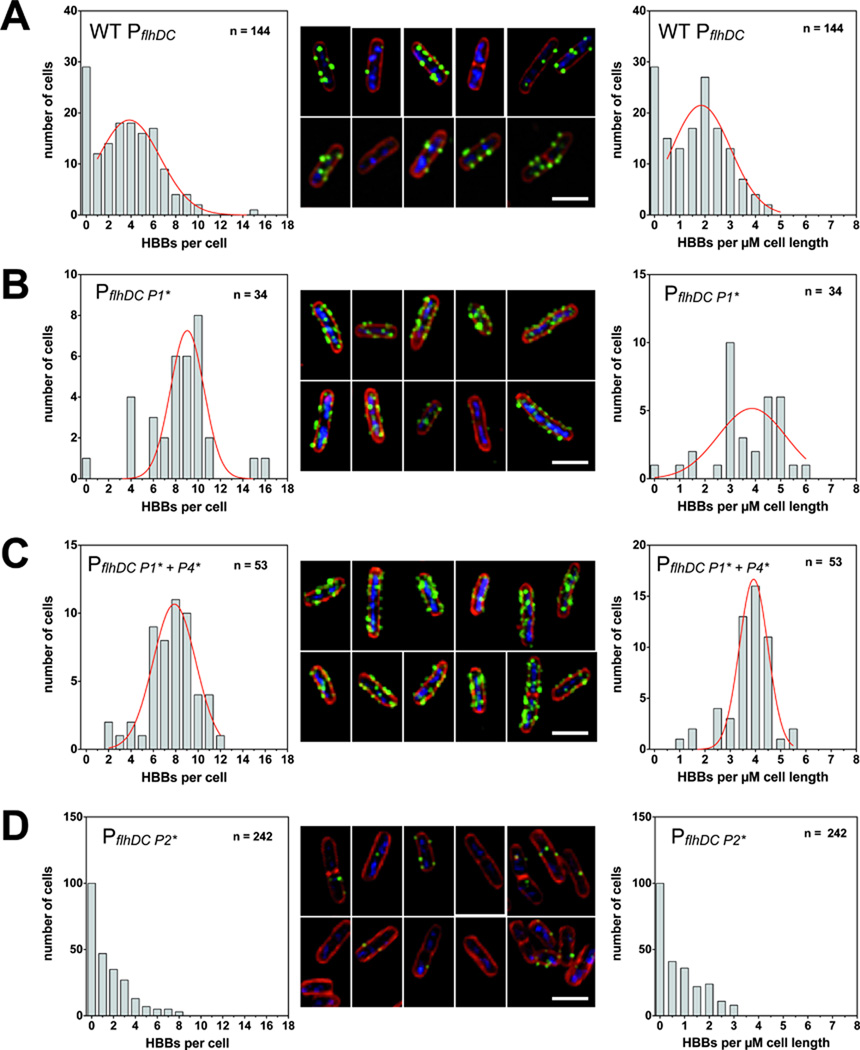
Based on the upregulation of HBB-type substrates we next investigated whether the number of hook-basal-bodies per cell is also increased. For this we utilized an FlgE variant harboring a hemagglutinin (HA) epitope tag for specific and sensitive immunodetection of completed hook-basal-body structures. We additionally used GFP fusions to components of the C-ring, FliM and FliG respectively, to analyze assembled C-rings by fluorescent microscopy. As shown in Figure 7A, the number of completed hook-basal-bodies per cell under wildtype flhDC levels peaks at about 2 HBB per µM cell length. Accordingly, wildtype S. enterica produces about 4 – 6 hook-basal-body structures per cell. We next investigated the effects of both the PflhDC P1 perfect -10 box mutation and the combination of the P1 + P4 perfect -10 box mutation on the number of hook-basal-body structures per cell. As displayed in Figure 7B and 7C, the number of HBBs doubles to about 4 HBBs per µM cell length in either the P1 or the P1 + P4 combination mutant. The PflhDC P1 perfect -10 box mutant therefore effectively doubles the number of available secretion systems (= HBBs) per cell, which is consistent with the gene expression data mentioned earlier. The number of completed HBBs in the PflhDC P2 perfect -10 box mutant however, are greatly reduced (Figure 7D). The majority of the cells do not complete a HBB, which might provide an explanation for the severe reduction in motility as shown in Figure 6A and 6B.
Figure 7. Number of assembled hook-basal-body (HBB) complexes as analyzed by hook immunostaining.
(A) Number of HBB complexes of the wildtype control. Left panel: distribution of HBBs per cell. Non-linear fitting of the Gaussian distribution was employed (red line) and the average HBB number per cell is 3.8 ± 2.8 (n = 144). Middle panel: hook immunostaining of exemplary cells expressing wildtype levels of flhDC. Green: HBB complexes (FlgE∷3×HA tag) labeled with anti-hemagglutinin antibodies coupled to Alexa Fluor 488. Red: cell membrane stained with FM-64. Blue: DNA stained with Hoechst. Right panel: distribution of HBBs per µM cell length. Cell length was measured based on the FM-64 membrane staining. Non-linear fitting of the Gaussian distribution was employed (red line) and the average HBB number per µM is 1.9 ± 1.2 (n = 144). Scale bar is 2 µM.
(B) Number of HBB complexes of the PflhD P1 consensus -10 box mutant. Left panel: distribution of HBBs per cell. Non-linear fitting of the Gaussian distribution was employed (red line) and the average HBB number per cell is 9.0 ± 1.5 (n = 34). Middle panel: hook immunostaining of exemplary PflhD P1 consensus -10 box mutant cells with increased flhDC expression levels. Green: HBB complexes (FlgE∷3×HA tag) labeled with anti-hemagglutinin antibodies coupled to Alexa Fluor 488. Red: cell membrane stained with FM-64. Blue: DNA stained with Hoechst. Right panel: distribution of HBBs per µM cell length. Cell length was measured based on the FM-64 membrane staining. Non-linear fitting of the Gaussian distribution was employed (red line) and the average HBB number per µM is 3.9 ± 1.4 (n = 34). Scale bar is 2 µM.
(C) Number of HBB complexes of the PflhD P1 + P4 consensus -10 box mutant. Left panel: distribution of HBBs per cell. Non-linear fitting of the Gaussian distribution was employed (red line) and the average HBB number per cell is 7.9 ± 1.9 (n = 53). Middle panel: hook immunostaining of exemplary PflhD P1 + P4 cinsensus -10 box mutant cells with increased flhDC expression levels. Green: HBB complexes (FlgE∷3×HA tag) labeled with anti-hemagglutinin antibodies coupled to Alexa Fluor 488. Red: cell membrane stained with FM-64. Blue: DNA stained with Hoechst. Right panel: distribution of HBBs per µM cell length. Cell length was measured based on the FM-64 membrane staining. Non-linear fitting of the Gaussian distribution was employed (red line) and the average HBB number per µM is 3.9 ± 0.6 (n = 53). Scale bar is 2 µM.
(D) Number of HBB complexes of the PflhD P2 consensus -10 box mutant. Left panel: distribution of HBBs per cell. Middle panel: hook immunostaining of exemplary PflhD P2 consensus -10 box mutant cells with decreased flhDC expression levels. Green: HBB complexes (FlgE∷3×HA tag) labeled with anti-hemagglutinin antibodies coupled to Alexa Fluor 488. Red: cell membrane stained with FM-64. Blue: DNA stained with Hoechst. Right panel: distribution of HBBs per µM cell length. Cell length was measured based on the FM-64 membrane staining. Scale bar is 2 µM.
We additionally confirmed the 2-fold upregulation of flagellar basal body structures using FliG-GFP and FliM-GFP protein fusions mentioned above (Supplemental Figure 1A and 1B). By overexpressing functional flhDC from the inducible arabinose promoter (ParaB-flhD+C+) we could furthermore confirm that elevated FlhD4C2 levels and thus increased expression of class 2 genes produces about 2-fold more HBB structures per cell compared to wildtype flhDC levels (Supplemental Figure 1C).
In summary, these data demonstrate that the upregulation of flhDC expression increases both the availability of secretion substrates and the number of export systems per se and taken together, these effects account for flagellar type III secretion even in the absence of the C-ring.
Discussion
In this work we employed a random screen for enhanced flagellar type III-specific protein secretion in a secretion deficient C-ring deletion mutant using the T-POP transposon. We utilized a hook-β-lactamase (FlgE-Bla) fusion construct in a strain deleted for the rod proteins FlgB and FlgC to positively select for flagellar type III secretion. The hook-β-lactamase reporter protein is selectively secreted via the flagellar T3S apparatus located at the base of the hook-basal-body and confers ampicillin resistance if secreted into the periplasm in a strain missing the proximal rod subunits FlgB and FlgC (Lee & Hughes, 2006). In a strain deleted for two thirds of the cytoplasmic C-ring (ΔfliMN), flagellar T3S is severely impaired and the FlgE-Bla reporter remains in the cytoplasm. Using a random T-POP transposon mutagenesis approach, we positively selected for T-POP transposon insertions that allowed for FlgE-Bla secretion even in the absence of the C-ring.
We isolated and analyzed by DNA-sequencing 42 independent T-POP insertions that we grouped into three different classes. The first class of T-POP insertions conferred ampicillin resistance without induction of the down- or upstream genes using tetracycline (ApR∷T-POP). The second class of mutants conferred ampicillin resistance after induction of down- or upstream genes using tetracycline (Tc-ApR). The mutants where induction with tetracycline resulted in an ampicillin sensitive phenotype were grouped into the third class of T-POP insertions (Tc-ApS). In summary, we obtained ApR∷T-POP insertions in the coding region of several negative regulators of flhDC, e.g. lrhA, slyA, ydiV, rcsB, ecnR and clpP. Those proteins have been previously shown to regulate flhDC expression or FlhD4C2 protein stability in E. coli or S. enterica. We additionally isolated several T-POP insertion in fliD, upstream of fliT, another negative regulator of flhDC. In a complimentary approach, we selected Mud insertions in the Salmonella chromosome that conferred ampicillin resistance in the absence of the C-ring by duplication of the flhDC operon. We confirmed this finding by expressing a second copy of flhDC from the arabinose promoter. Mutant strains (ΔflgBC flgE-bla Para-flhD+C+) missing two-thirds (ΔfliMN) or the complete C-ring (ΔfliG and ΔfliGMN) were found to be ApR in the presence of arabinose, but not without. Importantly, excess FlhDC also allows for efficient secretion in a ΔfliHIJ deletion mutant, confirming the previous finding that the ATPase complex FliHIJ is not needed for flagellar type III secretion per se (Paul et al., 2008, Minamino & Namba, 2008). Additionally, we obtained several spontaneous mutants of the flhD promoter region that conferred ampicillin resistance, as well as non-sense mutations in fliA. The sigma-factor σ28 has been shown to repress flhDC transcription in the presence of its cognate anti-sigma factor FlgM (Kutsukake, 1997). Additionally, σ28 activates the expression of negative regulators of flhDC, like FliT. Accordingly, the non-sense mutation of fliA allows for increased flhDC expression, which in turn confers ampicillin resistance using the same mechanism proposed below.
We analyzed several T-POP insertions for their effects on class 2 gene expression and found an increased class 2 expression by the T-POP insertions in lrhA, slyA and fliD. We additionally demonstrated that both LrhA and SlyA act as negative regulators of flhDC expression in S. enterica. LrhA (Lehnen et al., 2002) and SlyA (Spory et al., 2002) have been previously shown to act as regulators of flhDC and fliC respectively in E. coli.
In summary, we identified by our T-POP mutagenesis approach several known regulatory loci of flagellar Class 1 gene expression in S. enterica, like ecnR (Wozniak et al., 2008), ydiV and rcsB (Wang et al., 2007). We showed that both LrhA and SlyA act as negative regulators of flhDC expression also in S. enterica. We additionally identified several putative regulators of Class 2 transcription like STM1856 and STM2401/STM2402. A summary of the regulatory network influencing Class 1 gene expression is given in Figure 8A. Taken together we conclude that the upregulation of flhDC expression by impairing the function of the above mentioned negative regulators of flhDC expression is sufficient to answer the selective pressure of our FlgE-Bla secretion assay in the absence of the C-ring.
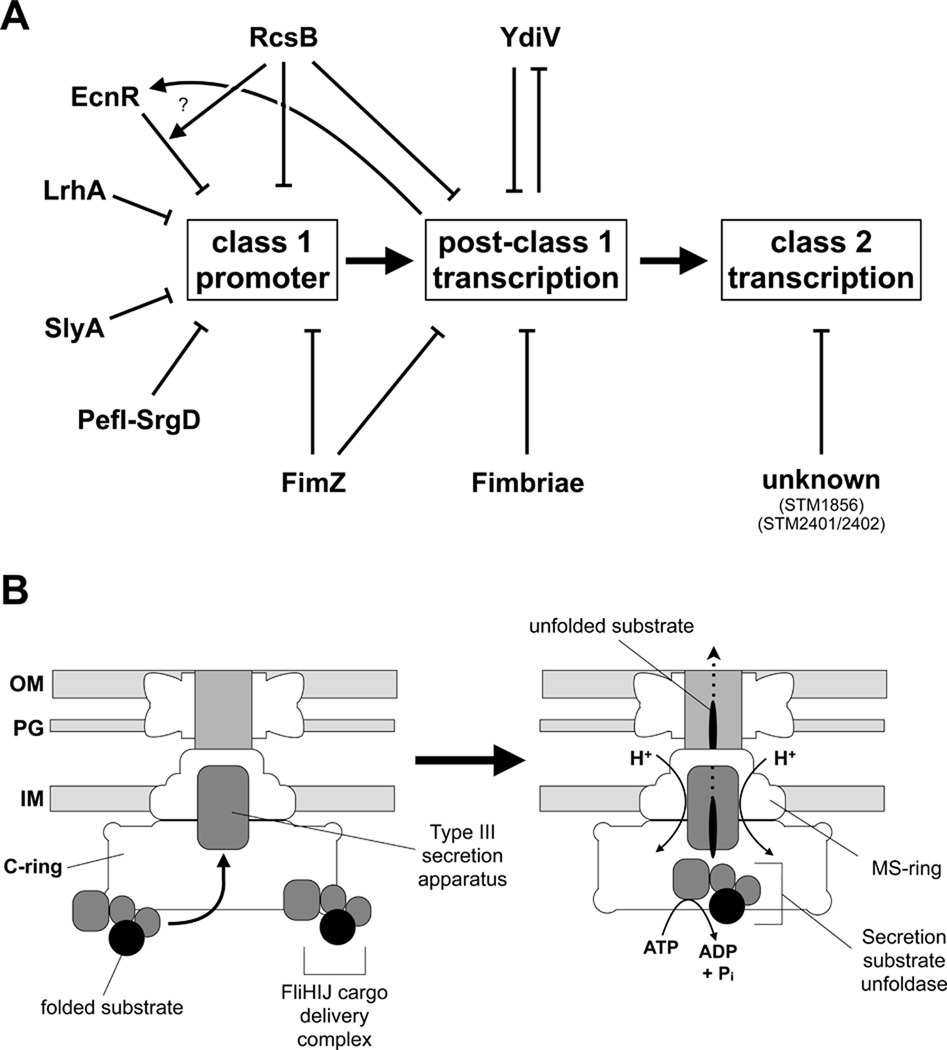
Figure 8. Regulatory network of flagellar Class 1 gene expression and model for flagellar type III secretion.
(A) Schematic representation of the regulatory network of flagellar Class 1 gene expression of Salmonella enterica. Arrows represent interactions between different regulators of flagellar Class 1 transcription or post-class 1 transcription. LrhA (Lehnen et al., 2002) and SlyA (Spory et al., 2002) have been previously identified as regulators of flhDC and FliC respectively in Escherichia coli. Our T-POP mutagenesis identified LrhA and SlyA as negative regulators of flhDC expression in Salmonella enterica, as well as several known regulators like EcnR (Wozniak et al., 2008) or RcsB (Wang et al., 2007). We also identified several putative regulators of Class 2 transcription like STM1856 and STM2401/STM2402.
(B) Model of the role of the C-ring affinity cup in flagellar type III secretion. Folded secretion substrates in complex with the cargo delivery complex FliHIJ dock to affinity sites of the C-ring awaiting secretion. Before PMF-dependent secretion, the secretion substrates are presumably unfolded using ATP-hydrolysis of the ATPase FliI thereby increasing the efficiency of the secretion process. The C-ring presumably acts as an affinity cup to enhance the specificity and efficiency of the flagellar type III secretion process under wildtype conditions by recruiting secretion substrates bound to the FliHIJ cargo delivery complex.
Flagellar type III secretion is possible in the absence of the C-ring and ATPase complex if FlhD4C2 levels are increased. Increased FlhD4C2 levels result in elevated level of Class 2 secretion substrates and additionally in more potential secretion systems by doubling the number of available basal bodies. Accordingly, increased substrate levels and more potential secretion systems overcome the requirement for both the C-ring and the ATPase complex in flagellar T3S.
To further analyze the effects of flhDC upregulation, we designed several flhD -10 box promoter mutants (Supplemental Figure 2). Using those mutants we demonstrate that single base pair changes in the P1 flhD promoter result in a significant upregulation of flhDC gene expression. The increased expression of flhDC results in 2 – 4 fold increase in class 2 and class 3 gene expression and additionally in an about 2-fold increased migration rate as analyzed using motility plates. The enhanced class 2 gene expression also translates in an almost 2-fold increase in protein levels of the reporter protein FlgE-Bla as analyzed by quantitative Western blotting. Interestingly, Salmonella cells harboring the perfect -10 box mutation of the P1 flhD promoter produce about 2 fold more HBB per cell if compared to cells expressing wildtype flhDC levels. A perfect -10 box mutation of the P2 flhD promoter however, resulted in a decrease of class 1, class 2 and class 3 gene expression, as well as in significantly reduced number of HBB per cell as analyzed by fluorescent microscopy. It was surprising to find that just a two-fold increase in the level of flhDC mRNA can circumvent the impaired secretion activity caused by the absence of the C ring. It suggests that the role of the C-ring as the rotor of the flagellar motor is its primary function and the C-ring plays only a minor role in the secretion process as an affinity site for substrate localization."
In summary, we propose the following mechanism for flagellar type III secretion in the absence of the C-ring by upregulation of flhDC: Every mutation that results in an increased level of functional FlhD4C2, like T-POP insertions in negative regulators of flhDC or mutations in the P1 promoter of flhD, will allow for FlgE-Bla secretion even in the absence of the C-ring by a combination of I) more available substrates (FlgE-Bla) and II) more potential secretion systems (more HBB complexes). Under conditions where the function of the C-ring is missing or impaired, flagellar type III secretion might still occur, albeit more inefficient, if the concentration of the secretion substrates are increased. This is the case in our isolated T-POP mutants that increase flhDC expression by impairing the function of negative regulators of flhDC, as well as in our flhDC promoter mutants that directly increase flhDC gene expression.
Accordingly, we propose that the C-ring acts under physiological conditions as an affinity cup that locally increases the secretion substrate concentration prior to type III secretion by either I) transiently binding secretion substrates or II) excluding non-secretion substrates interactions with the secretion apparatus (Figure 8B). Folded secretion substrates are bound by the cargo delivery complex FliHIJ and recruited to the C-ring affinity cup, thereby locally increasing the substrate concentration prior to secretion. Importantly, the function of the C-ring as an affinity cup in type III secretion seems to be highly conserved. Recently it has been shown that in case of the Chlamydia injectisome type III secretion system a multiple cargo secretion chaperone (Mcsc) bound to secretion substrates is recruited to the putative C-ring homolog CdsQ, thereby presumably allowing for a more efficient secretion process (Spaeth et al., 2009).
Experimental procedures
Bacterial strains, plasmids and media
All bacterial strains used in this study are listed in Supplemental Table 1. Cells were cultured in either Luria broth (LB) or peptone/protease-peptone/bile-salts (PPBS) (17 g/l peptone, 3 g/l protease peoptone, 1.5 g/l bile salts #3, 5 g/l sodium chloride, 10.8 g/l agar). A concentration of 5 µg/ml ampicillin and 15 µg/ml tetracycline was used in PPBS plates. Growth of strain TH12470 harboring the hook-β-lactamase reporter protein and missing two-thirds of the C-ring (ΔfliMN) was inhibited on PPBS plates containing 5 µg/ml ampicillin. Motility agar plates were prepared as described before (Gillen & Hughes, 1991, Wozniak et al., 2008). The generalized transducing phage of S. typhimurium P22 HT105/1 int-201 was used in all transductional crosses (Sanderson & Roth, 1983).
SDS-PAGE and Western Blotting
Whole-cell lysates of Salmonella were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting using anti-FlgE antibodies (rabbit) for detection of FlgE-β-lactamase. Specific protein detection of horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) was performed using ECL plus Western blotting detection reagents (Amersham Biosciences). Densitometric measurements of FlgE-β-lactamase bands were performed using ImageJ 1.42m for Mac OS X (Abramoff et al., 2004).
Isolation of random T-POP insertions spontaneous ApR mutants that allow Hook-β-lactamase secretion in the absence of the C-ring
The screen for random Tn10dTc[Δ25] transposon insertions allowing type III-specific secretion in the C-ring deletion mutant was essentially performed as described in (Wozniak et al., 2008, Lee et al., 2007) using strain TH12470 as the recipient on PPBS plates containing 5 µg/ml ampicillin.
Spontaneous mutations that conferred FlgE-Bla secretion in a C-ring deletion strain that were linked to either the flh or fli regions were isolated using transposons STM1911∷Tn10dTc and zec-3521∷Tn10dCm that are linked to the flh and fli regions, respectively. ApR mutants were pooled, a phage P22 transducing lysate was grown on the pooled cells and used to transduce either a ΔflgBC flgE-bla ΔfliG (TH12466) or ΔflgBC flgE-bla ΔfliMN (TH12470) recipient to either TcR or CmR. TcR transductants that also inherited ApR resulted from co-transduction of the ApR allele with flh-linked STM1911∷Tn10dTc. CmR transductants that also inherited ApR resulted from co-transduction of the ApR allele with fli-linked zec-3521∷Tn10dCm. Linked ApR mutants were characterized further using three-factor crosses and the mutations were determined using DNA sequencing analysis.
β-galactosidase assays
β-galactosidase assays were performed based on the protocol of Zhang and Bremer (Zhang & Bremer, 1995) with minor modifications. Briefly, logarithmic growing cells were permeabilized using 100 mM Na2HPO4, 20 mM KCl, 2 mM MgSO4, 0.08% CTAB (hexadecyl-trimethylammonium bromide), 0.04% sodium deoxycholate and 5.4 µl/ml β-mercaptoethanol. Afterwards, the reaction was started by addition of 60 mM Na2HPO4, 40 mM NaH2PO4, 1 mg/ml o-nitrophenyl-β-D-Galactoside (ONPG) and 2.7 µl/ml β-mercaptoethanol. The reaction was stopped using 1 M Sodium Carbonate (Na2CO3) and Miller units were calculated as described (Miller, 1972). For each strain, the assay was performed using three independent, biological replicates.
RNA isolation and quantitative real-time PCR
RNA was prepared from three independent, biological replicates essentially as described (Simm et al., 2009) using the SV Total RNA Isolation System (Promega) and on-column DNase I treatment. Alternatively, cultures of three independent, biological replicates were mixed equally and used for the subsequent purification of total RNA as described above. For complete removal of genomic DNA, purified RNA samples were treated a second time with DNase I for one hour at 37 °C (ZymoResearch). Afterwards, RNA samples were reverse transcribed using the RETROscript kit and random decamers (Ambion). qPCR reactions were performed using the EvaGreen qPCR master mix (BioRad) and primers 5’-GTAGGCAGCTTTGCGTGTAG + 5’-TCCAGCAGTTGTGGAATAATATCG (flhDC), 5’-GAACACGTTCGCGCAGTG + 5’-TAGGCAATTTTCCAGGAACCG (motAB), 5’-GATGGCGGCGAAATCG + 5’-AGGGTCCGTTGAGTTCAGGTT (flgE), 5’-GGCCAAAGCTGGTCATTATCC + 5’-TCGCCGGCAGAAACGT (fliP), 5’-CGCCCTGTTGACGATCTGG + 5’-TTTACCCAAGTTAGGCGTCTTAAG (rpoA), 5’-CAACCTGTTCGTACGTATCGAC + 5’-CAGCTCCATCTGCAGTTTGTTG (rpoB), 5’- CAACAGTATGCGCGTGATGAT + 5’-CGACGCAGAGCTTCATGATC (rpoD), 5’- CTGCTCAAAGAGCTGGTGTATCA + 5’- AGCGCGTTACAGTCTGCTCAT (gyrB) and 5’-TTGCAGAAATGAGCCATTACGCCG + 5’-GACGTTCAGCGCGAATGATGGTTT (gmk) on a CFX96 real-time PCR instrument (BioRad). Relative changes in mRNA levels were determined using the 2−ΔΔCT method described previously (Livak & Schmittgen, 2001) by simultaneous normalization against multiple reference genes rpoA, gyrB, and gmk transcript levels (Vandesompele et al., 2002). M values indicated in the figure legends were calculated according to Vandesompele et al. (Vandesompele et al., 2002).
Fluorescent microscopy
For fluorescent microscopy analysis, cells were grown to mid-log phase and fixed by addition of final 5% formaldehyde. Hooks were stained using monoclonal anti-hemagglutinin coupled to Alexa Fluor488 (Invitrogen). Fixed cells were immobilized using poly-L-lysine treated coverslips. DNA and membrane staining was performed using Hoechst (Invitrogen) and FM-64 (0.5 µg/ml, Invitrogen). Images were collected using an Applied Precision optical sectioning microscope with optical Z sections every 100 nm and deconvolved using softWoRx v.3.4.2 (Applied Precision). The pixel data of individual Z sections of the deconvolved images were projected on a single plane using the Quick Projection tool (settings: maximal intensity) of softWoRx Explorer v1.3 (Applied Precision) and used for quantitative scoring of the number of hook-basal-body complexes per cell.
Supplementary Material
Acknowledgements
This work was supported by PHS grant GM056141 from the National Institutes of Health. We thank Christopher E. Wozniak for helpful comments on how to quantify the number of hook-basal-body complexes and Fabienne F. V. Chevance and Christopher E. Wozniak for strain construction. We also thank Yichi Su and Anoush Emrazian for technical assistance and the Hughes lab for useful discussions of the manuscript. M.E. gratefully acknowledges scholarship support of the Boehringer Ingelheim Fonds.
References
- Aberg A, Fernandez-Vazquez J, Cabrer-Panes JD, Sanchez A, Balsalobre C. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J Bacteriol. 2009;191:3226–3236. doi: 10.1128/JB.01410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Akeda, Galan Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- Anderson RP, Roth JR. Tandem genetic duplications in Salmonella typhimurium: amplification of the histidine operon. J Mol Biol. 1978;126:53–71. doi: 10.1016/0022-2836(78)90279-6. [DOI] [PubMed] [Google Scholar]
- Berg The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- Berg HC, Anderson RA. Bacteria swim by rotating their flagellar filaments. Nature. 1973;245:380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Chevance FFV, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Micro. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S, Hughes KT. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:1209–1213. doi: 10.1128/jb.184.4.1209-1213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier CD, Slauch JM. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2003;185:5096–5108. doi: 10.1128/JB.185.17.5096-5108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen KL, Hughes KT. Molecular characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in Salmonella typhimurium. J Bacteriol. 1991;173:6453–6459. doi: 10.1128/jb.173.20.6453-6459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pedrajo B, Minamino T, Kihara M, Namba K. Interactions between C ring proteins and export apparatus components: a possible mechanism for facilitating type III protein export. Mol Microbiol. 2006;60:984–998. doi: 10.1111/j.1365-2958.2006.05149.x. [DOI] [PubMed] [Google Scholar]
- Hughes, Gillen, Semon, Karlinsey Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- Kelly A, Goldberg MD, Carroll RK, Danino V, Hinton JC, Dorman CJ. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology. 2004;150:2037–2053. doi: 10.1099/mic.0.27209-0. [DOI] [PubMed] [Google Scholar]
- Komeda Y, Suzuki H, Ishidsu JI, Iino T. The role of cAMP in flagellation of Salmonella typhimurium. Mol Gen Genet. 1976;142:289–298. doi: 10.1007/BF00271253. [DOI] [PubMed] [Google Scholar]
- Konishi M, Kanbe M, McMurry JL, Aizawa SI. Flagellar formation in C-ring defective mutants by overproduction of FliI, the ATPase specific for the flagellar type III secretion. J Bacteriol. 2009 doi: 10.1128/JB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S. Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol. 1992;226:433–446. doi: 10.1016/0022-2836(92)90958-m. [DOI] [PubMed] [Google Scholar]
- Kugelberg E, Kofoid E, Reams AB, Andersson DI, Roth JR. Multiple pathways of selected gene amplification during adaptive mutation. Proc Natl Acad Sci U S A. 2006;103:17319–17324. doi: 10.1073/pnas.0608309103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K. Autogenous and global control of the flagellar master operon, flhD, in Salmonella typhimurium. Mol Gen Genet. 1997;254:440–448. doi: 10.1007/s004380050437. [DOI] [PubMed] [Google Scholar]
- Lee, Wozniak, Karlinsey, Hughes Genomic screening for regulatory genes using the T-POP transposon. Meth Enzymol. 2007;421:159–167. doi: 10.1016/S0076-6879(06)21014-0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Hughes KT. Posttranscriptional control of the Salmonella enterica flagellar hook protein FlgE. J Bacteriol. 2006;188:3308–3316. doi: 10.1128/JB.188.9.3308-3316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnen D, Blumer C, Polen T, Wackwitz B, Wendisch VF, Unden G. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol. 2002;45:521–532. doi: 10.1046/j.1365-2958.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- Libby SJ, Goebel W, Ludwig A, Buchmeier N, Bowe F, Fang FC, Guiney DG, Songer JG, Heffron F. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci U S A. 1994;91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matsumura P. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J Bacteriol. 1994;176:7345–7351. doi: 10.1128/jb.176.23.7345-7351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Macnab R. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory, NY. 1972:352–355. [Google Scholar]
- Minamino, Macnab Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Namba K. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature. 2008;451:485. doi: 10.1038/nature06449. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Kutsukake K, Suzuki H, Iino T. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet. 1990;221:139–147. doi: 10.1007/BF00261713. [DOI] [PubMed] [Google Scholar]
- Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. Energy source of flagellar type III secretion. Nature. 2008;451:489–492. doi: 10.1038/nature06497. [DOI] [PubMed] [Google Scholar]
- Rappleye CA, Roth JR. A Tn10 derivative (T-POP) for isolation of insertions with conditional (tetracycline-dependent) phenotypes. J Bacteriol. 1997;179:5827–5834. doi: 10.1128/jb.179.18.5827-5834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S, Brown JD, Aldridge PD, Rao CV. FliZ Is a posttranslational activator of FlhD4C2-dependent flagellar gene expression. J Bacteriol. 2008;190:4979–4988. doi: 10.1128/JB.01996-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KE, Roth JR. Linkage map of Salmonella typhimurium, Edition VI. Microbiol. Rev. 1983;47:410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm R, Remminghorst U, Ahmad I, Zakikhany K, Romling U. A role for the EAL-like protein STM1344 in regulation of CsgD expression and motility in Salmonella enterica serovar Typhimurium. J Bacteriol. 2009;191:3928–3937. doi: 10.1128/JB.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutourina O, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth KE, Chen YS, Valdivia RH. The Chlamydia type III secretion system C-ring engages a chaperone-effector protein complex. PLoS Pathog. 2009;5:e1000579. doi: 10.1371/journal.ppat.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spory A, Bosserhoff A, von Rhein C, Goebel W, Ludwig A. Differential regulation of multiple proteins of Escherichia coli and Salmonella enterica serovar Typhimurium by the transcriptional regulator SlyA. J Bacteriol. 2002;184:3549–3559. doi: 10.1128/JB.184.13.3549-3559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic I, Baumler AJ, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- Takaya A, Matsui M, Tomoyasu T, Kaya M, Yamamoto T. The DnaK chaperone machinery converts the native FlhD2C2 hetero-tetramer into a functional transcriptional regulator of flagellar regulon expression in Salmonella. Mol Microbiol. 2006;59:1327–1340. doi: 10.1111/j.1365-2958.2005.05016.x. [DOI] [PubMed] [Google Scholar]
- Tomoyasu T, Takaya A, Isogai E, Yamamoto T. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol Microbiol. 2003;48:443–452. doi: 10.1046/j.1365-2958.2003.03437.x. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao Y, McClelland M, Harshey RM. The RcsCDB signaling system and swarming motility in Salmonella enterica serovar typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J Bacteriol. 2007;189:8447–8457. doi: 10.1128/JB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Fleming RT, Westbrook EM, Matsumura P, McKay DB. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Biol. 2006;355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Wei BL, Brun-Zinkernagel AM, Simecka JW, Pruss BM, Babitzke P, Romeo T. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol Microbiol. 2001;40:245–256. doi: 10.1046/j.1365-2958.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- Wozniak CE, Lee C, Hughes KT. T-POP array identifies EcnR and PefI-SrgD as novel regulators of flagellar gene expression. Journal of Bacteriology. 2008 doi: 10.1128/JB.01177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kutsukake K. FliT acts as an anti-FlhD2C2 factor in the transcriptional control of the flagellar regulon in Salmonella enterica serovar typhimurium. J Bacteriol. 2006;188:6703–6708. doi: 10.1128/JB.00799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Iyoda S, Ohnishi K, Iino T, Kutsukake K. Structure and transcriptional control of the flagellar master operon of Salmonella typhimurium. Genes Genet Syst. 1999;74:105–111. doi: 10.1266/ggs.74.105. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11189. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.