Abstract
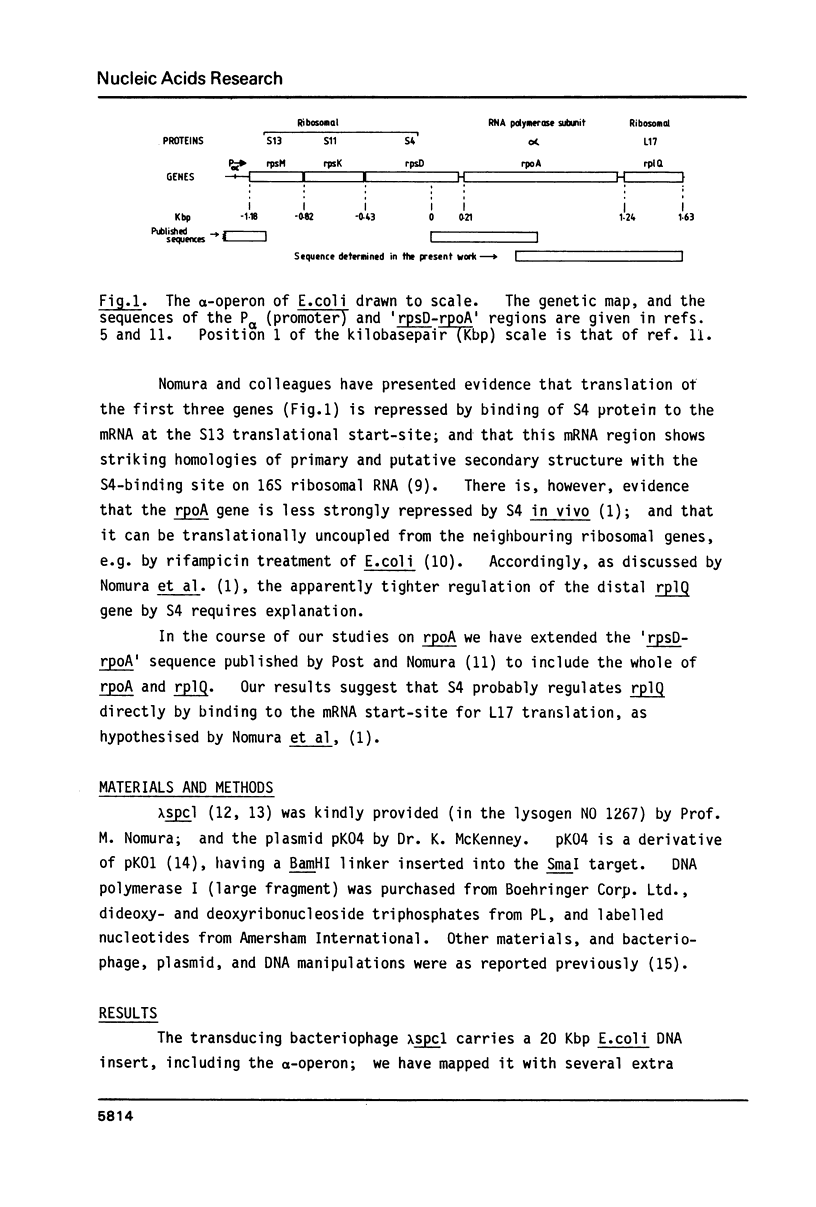
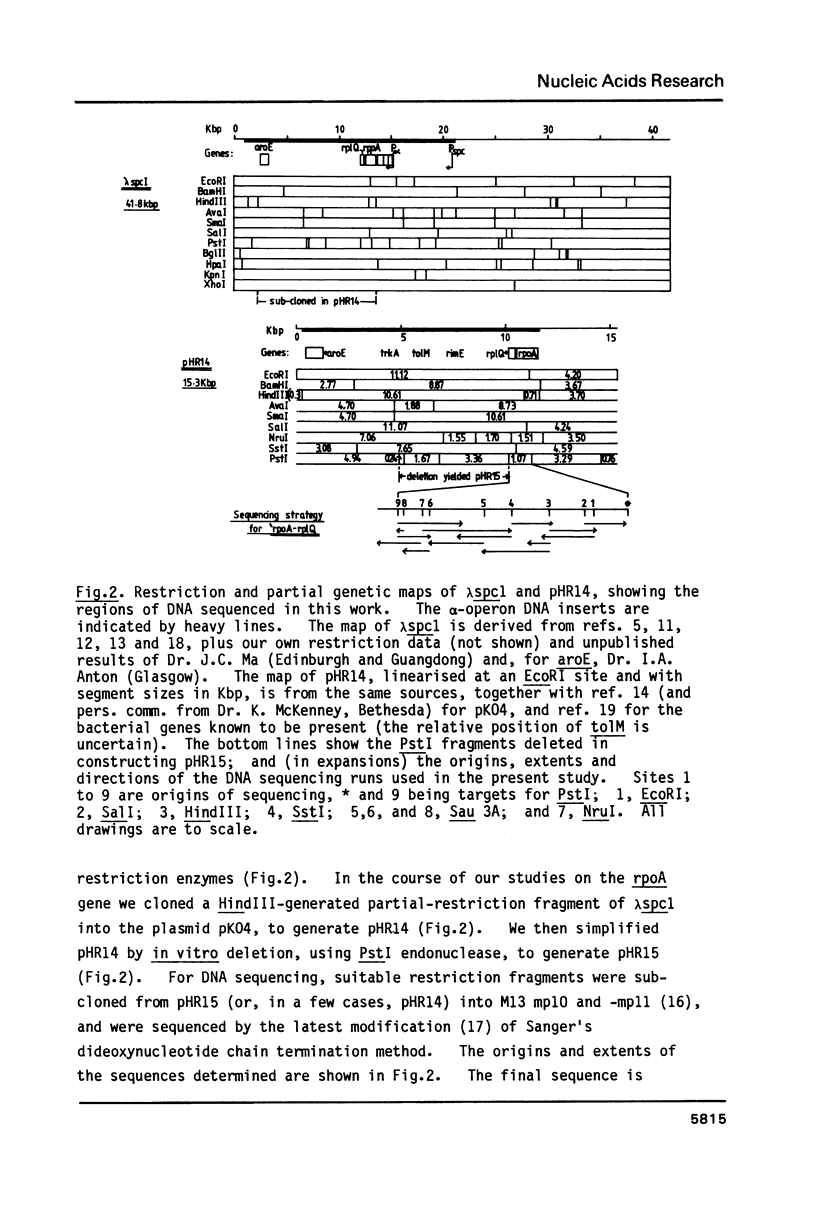
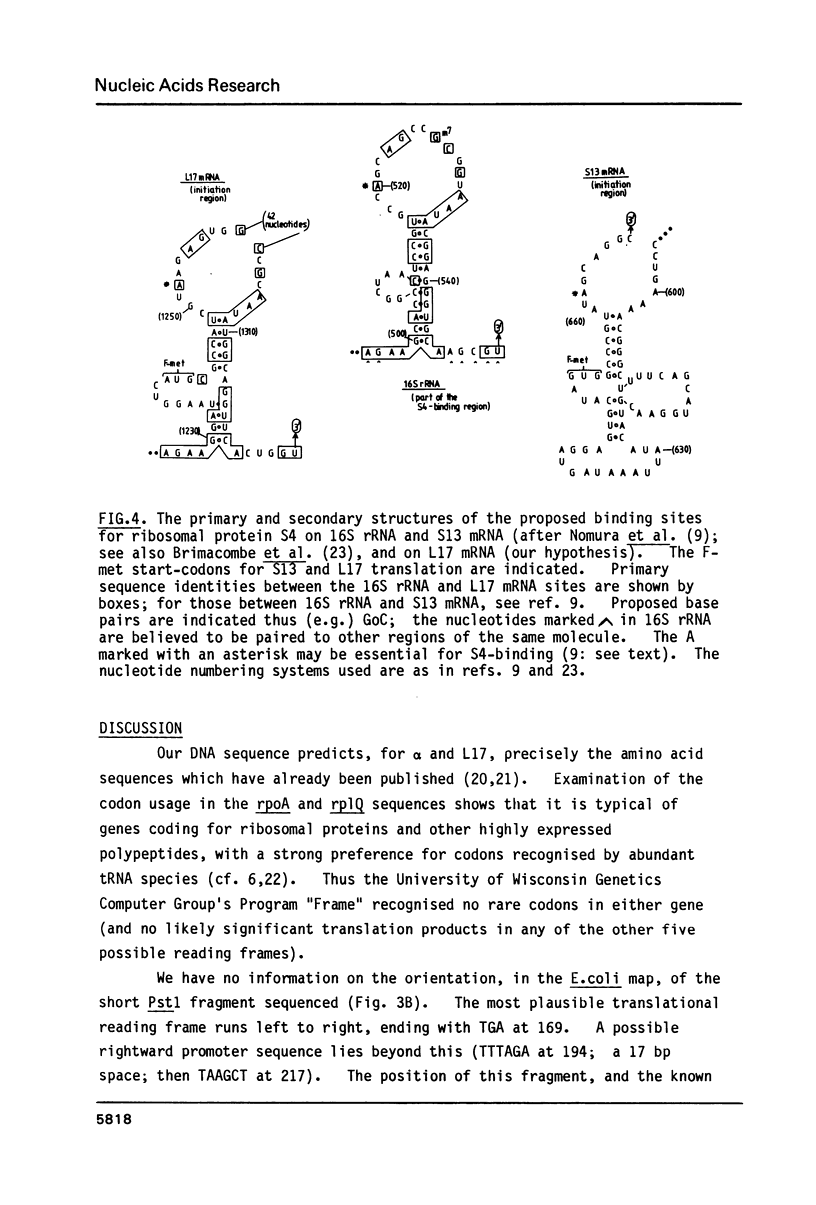
The "alpha-operon" of E.coli is a unit of regulation comprising the following known genes, mostly encoding ribosomal proteins (in order of transcription, and with their products named in brackets): rpsM (S13), rpsK (S11), rpsD (S4), rpoA (alpha-subunit of RNA polymerase), rplQ (L17). There is evidence that S4 tightly regulates all of these genes, except rpoA, by repressing translation of the polycistronic mRNA. Binding of S4 to the S13 start-site is thought to regulate the first three genes. We have extended the 'rpsD-rpoA' sequences previously determined by others, to include all of rpoA and rplQ. The rpoA-rplQ intercistronic region shows strong primary, and potential secondary structural homologies with the S4-binding sites on 16S rRNA and S13 mRNA. We suggest that S4 represses L17 translation directly.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Maly P., Zwieb C. The structure of ribosomal RNA and its organization relative to ribosomal protein. Prog Nucleic Acid Res Mol Biol. 1983;28:1–48. doi: 10.1016/s0079-6603(08)60081-1. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Blattner F. R., Jaskunas S. R., Lindahl L., Nomura M. Organization of ribosomal protein genes in Escherichia coli. I. Physical structure of DNA from transducing lambda phages carrying genes from the aroE-str region. J Mol Biol. 1976 Sep 25;106(3):817–835. doi: 10.1016/0022-2836(76)90267-9. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S., Nomura M. Ribosomal protein S4 acts in trans as a translational repressor to regulate expression of the alpha operon in Escherichia coli. J Bacteriol. 1982 Jul;151(1):193–202. doi: 10.1128/jb.151.1.193-202.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Archer R., Zengel J. M. Transcription of the S10 ribosomal protein operon is regulated by an attenuator in the leader. Cell. 1983 May;33(1):241–248. doi: 10.1016/0092-8674(83)90353-7. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Expression of ribosomal genes in bacteria. Adv Genet. 1982;21:53–121. doi: 10.1016/s0065-2660(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J., Nomura M. Organization of ribosomal protein genes in Escherichia coli. II. Mapping of ribosomal protein genes by in vitro synthesis of ribosomal proteins using DNA fragments of a transducing phage as templates. J Mol Biol. 1976 Sep 25;106(3):837–855. doi: 10.1016/0022-2836(76)90268-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Newman A. J., Ma J. C., Howe K. M., Garner I., Hayward R. S. Evidence that rifampicin can stimulate readthrough of transcriptional terminators in Escherichia coli, including the attenuator of the rpoBC operon. Nucleic Acids Res. 1982 Nov 25;10(22):7409–7424. doi: 10.1093/nar/10.22.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Lipkin V. M., Modyanov N. N., Chertov O. Y., Smirnov Y. V. Primary structure of alpha-subunit of DNA-dependent RNA polymerase from Escherichia coli. FEBS Lett. 1977 Apr 1;76(1):108–111. doi: 10.1016/0014-5793(77)80131-2. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. Functional mRNA half lives in E. coli. Mol Gen Genet. 1978 Nov 9;166(3):329–336. doi: 10.1007/BF00267626. [DOI] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Davis G. R., Nomura M. DNA sequence of the promoter region for the alpha ribosomal protein operon in Escherichia coli. J Biol Chem. 1980 May 25;255(10):4653–4659. [PubMed] [Google Scholar]
- Post L. E., Nomura M. Nucleotide sequence of the intercistronic region preceding the gene for RNA polymerase subunit alpha in Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):10604–10606. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Weissmann C. The making of a phage. FEBS Lett. 1974 Mar 23;40(0):suppl–suppl:S18. doi: 10.1016/0014-5793(74)80684-8. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Dean D., Strycharz W. A., Nomura M. E. coli ribosomal protein L10 inhibits translation of L10 and L7/L12 mRNAs by acting at a single site. Nature. 1981 Nov 12;294(5837):190–192. doi: 10.1038/294190a0. [DOI] [PubMed] [Google Scholar]


