Abstract
The Kaposi’s sarcoma-associated herpesvirus (KSHV) is the causative agent of Kaposi’s sarcoma (KS), an important cause of morbidity and mortality in immunocompromised patients. KSHV interaction with the cell membrane triggers activation of specific intracellular signal transduction pathways to facilitate virus entry, nuclear trafficking, and ultimately viral oncogene expression. Extracellular heat shock protein 90 localizes to the cell surface (csHsp90) and facilitates signal transduction in cancer cell lines, but whether csHsp90 assists in the coordination of KSHV gene expression through these or other mechanisms is unknown. Using a recently characterized non-permeable inhibitor specifically targeting csHsp90, we show that csHsp90 inhibition suppresses KSHV gene expression during de novo infection, and that this effect is mediated largely through the inhibition of mitogen-activated protein kinase (MAPK) activation by KSHV. Moreover, we show that targeting csHsp90 reduces constitutive MAPK expression and the release of infectious viral particles by patient-derived, KSHV-infected primary effusion lymphoma cells. These data suggest that csHsp90 serves as an important co-factor for KSHV-initiated MAPK activation and provide proof-of-concept for the potential benefit of targeting csHsp90 for the treatment or prevention of KSHV-associated illnesses.
Keywords: Herpesvirus, heat shock protein, signal transduction
Introduction
In the modern era, cancer represents one of the leading causes of morbidity and mortality in patients infected with the human immunodeficiency virus (HIV) and in patients receiving solid organ transplants. (Bonnet et al., 2004; Engels et al., 2008; Lebbe et al., 2008). The Kaposi’s sarcoma-associated herpesvirus (KSHV) remains one of the most common etiologies of cancer in these populations (Bonnet et al., 2004; Engels et al., 2008; Lebbe et al., 2008), including primary effusion lymphoma (PEL) (Cesarman et al., 1995), multicentric Castleman’s disease (MCD) (Soulier et al., 1995), and Kaposi’s sarcoma (KS) (Chang et al., 1994). Use of cytotoxic chemotherapeutic agents or withdrawal of immune suppressive medications remain the preferred therapeutic approaches for patients with systemic HIV-associated and transplant-associated KS, respectively (Stallone et al., 2005; Vanni et al., 2006; Von Roenn., 2003). However, clinical responses to cytotoxic agents vary widely in published trials (Vanni et al., 2006; Von Roenn, 2003), and withdrawal of immune suppression in response to transplant-associated KS may lead to allograft rejection (Stallone et al., 2005).
Targeted approaches introduced thus far for the treatment of KSHV-associated cancers have included the inhibition of KSHV replication (Martin et al., 1999), or the inhibition of KSHV-induced signaling pathways (Chaisuparat et al., 2008; Stallone et al., 2005; Koon et al., 2005). Intracellular signaling events induced during KSHV infection and supporting viral gene expression include activation of the mammalian target of rapamycin (mTOR) (Sin et al., 2007; Sodhi et al., 2006) and mitogen-activated protein kinases (MAPK) (Sharma-Walia et al., 2005). However, the targeting of signal transduction pathways presents many challenges given the non-selectivity of kinase inhibitors, associated toxicities, and the development of resistance through multiple mechanisms, including the upregulation of alternative signaling pathways (Kerkela et al., 2006; Zhang et al., 2009; Chaisuparat et al., 2008). Existing data suggest that ongoing viral replication and infection of new target cells are important for KS progression (Boivin et al., 2000; Brown et al., 2006; Campbell et al., 2000; Dupin et al., 1999; Pak et al., 2005; Pyakurel et al., 2004; Quinlivan et al., 2002; Staskus et al., 1997; Aluigi et al., 1996; Grundhoff and Ganem, 2004; Lebbe et al., 1997; Salahuddin et al., 1988). Therefore, a more complete understanding of KSHV interactions with cell surface co-factors facilitating the initiation of signal transduction and viral gene expression during de novo infection may result in the development of novel targeted strategies for the treatment and/or prevention of KS.
Heat shock proteins (Hsp) modulate a wide variety of intracellular processes through the stabilization or regulation of protein folding (Tsutsumi and Neckers, 2007). In particular, the molecular chaperone Hsp90 plays an essential role in the protein maturation and subsequent activity of a multitude of signaling proteins pertinent to cancer pathogenesis (Tsutsumi and Neckers, 2007). Existing data also suggest that Hsp90 serves as a receptor for viruses (Lin et al., 2007; Reyes-Del Valle et al., 2005), as well as a critical co-factor for herpesvirus replication and nuclear localization of viral proteins (Basha et al., 2005; Burch and Weller, 2005; Li et al., 2004). Hsp90 inhibitors have proven beneficial for reducing solid tumor burden, and their validation for widespread use is ongoing in phase II clinical trials (Ramalingam et al., 2008). Moreover, recent identification of Hsp90 on the cell surface (csHsp90) (Eustace et al., 2004) has led to the observation that csHsp90 serves as a co-factor in the activation of specific intracellular signal transduction pathways in a more selective manner relative to the intracellular form of the protein (Tsutsumi et al., 2008).
In the present study, we used a cell-impermeable ansamycin derivative, DMAG-N-oxide (DNo), targeting the ATP-binding pocket of csHsp90 (Tsutsumi et al., 2008) as well as anti-Hsp90 antibodies to determine whether csHsp90 serves as a co-factor in KSHV activation of specific signal transduction pathways and KSHV gene expression during de novo infection.
Results
Hsp90 localizes to the cell surface on KSHV-susceptible cells
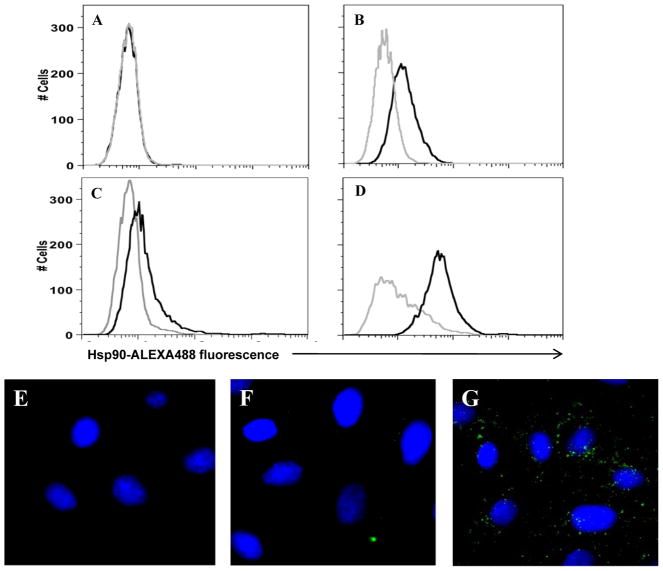
In order to determine whether KSHV-susceptible cells express csHsp90, we used a flow cytometry-based assay for quantification of csHsp90 for two KSHV-susceptible cell types—HeLa cells and DMVEC. Antibodies recognizing a C-terminal epitope for Hsp90 (SPS-830) failed to identify csHsp90 expression on a number of primary and transformed cell lines in our laboratory (data shown for HeLa cells in Fig. 1A). However, antibodies recognizing an N-terminal epitope (SPS-771) identified csHsp90-alpha expression by both HeLa cells (Fig. 1B) and pDMVEC (Fig. 1D). A second antibody recognizing a different epitope expressed by csHsp90-alpha (SPA-840) also identified csHsp90-alpha expression by HeLa cells (Fig. 1C). Immunofluorescence assays further validated the selectivity of the N-terminal antibody in identifying csHsp90 for HeLa cells (Fig. 1E–G).
Figure 1. KSHV-permissive cells express extracellular Hsp90.
(A–D) To identify cell surface localization of Hsp90 (csHsp90) by flow cytometry, HeLa (A–C) and primary dermal microvascular endothelial cells (pDMVEC) (D) were incubated with antibodies recognizing either the C-terminus (A), N-terminus (B and D), or an uncharacterized epitope (C) of the alpha isoform of Hsp90 followed by secondary antibodies conjugated to ALEXA-488 (black histograms). For controls, cells were incubated with secondary antibodies alone (gray histograms). (E–G) To validate these results, HeLa cells incubated with secondary antibodies conjugated to ALEXA-488 (E), anti-C-terminal Hsp90 Ab and secondary antibodies (F), or anti-N-terminal Hsp90 Ab and secondary antibodies (G) were examined by fluorescence microscopy. Original magnification × 60. Data shown represent one of three independent experiments.
Targeting csHsp90 reduces KSHV gene expression during de novo infection
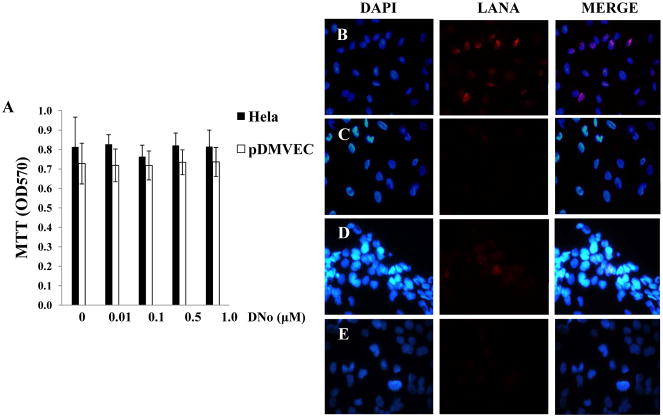
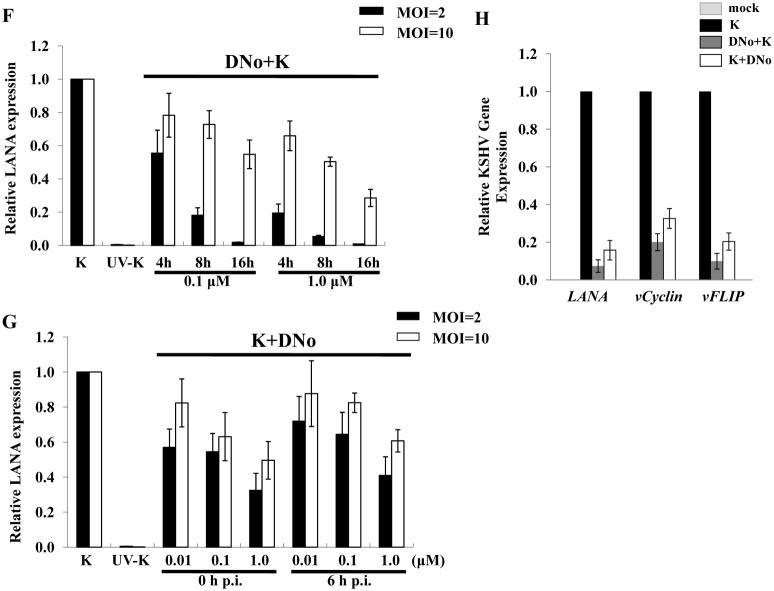
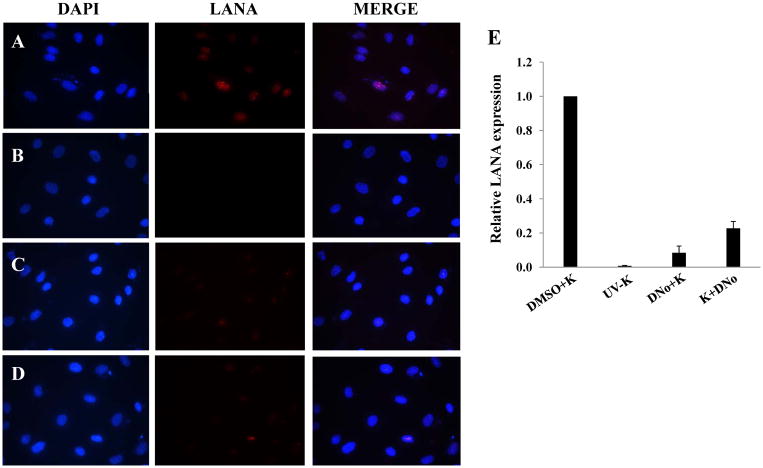
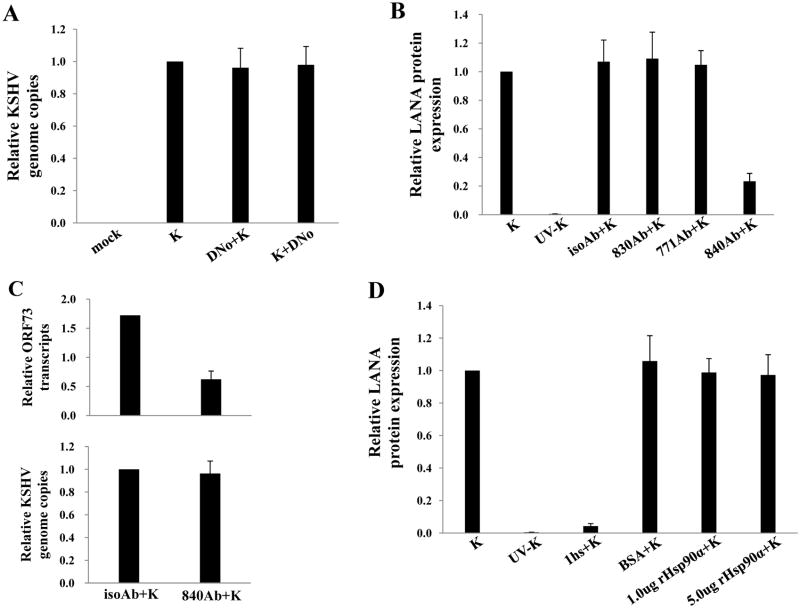
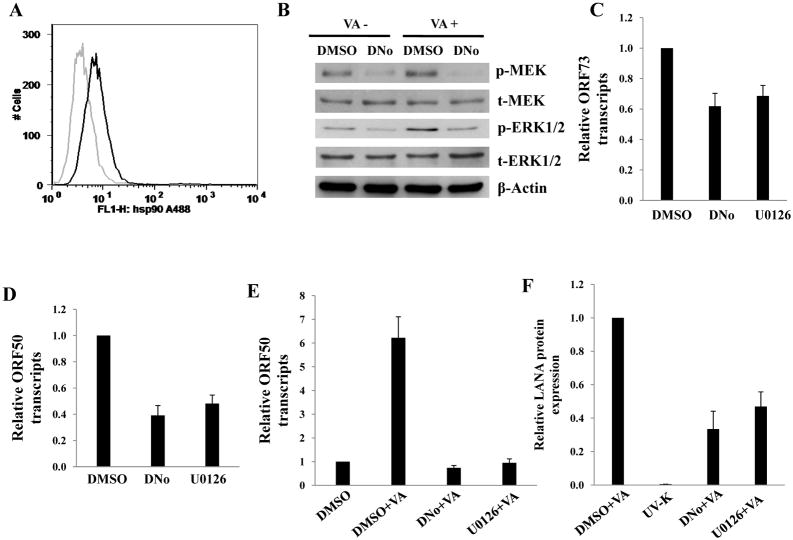
To determine initial DNo concentrations to be used for infection assays, we incubated HeLa cells and pDMVEC with DNo using a range of concentrations over which DNo inhibits intracellular signaling as shown previously (Tsutsumi and Neckers, 2007). DNo elicited no discernable toxicity over this range for either cell type (Fig. 2A). Next, to determine whether Hsp90 regulates KSHV gene expression during de novo infection, we quantified KSHV gene expression in KSHV-incubated, DNo-treated cells using an IFA for the KSHV-encoded latency-associated nuclear antigen (LANA) and qRT-PCR to amplify representative latent transcripts. We observed dose- and time-dependent reduction in the expression of LANA even if cells were incubated with DNo following viral incubation (Fig. 2B–G). In addition, latent transcripts representing 3 different open reading frames (ORF71-vFLIP, ORF72-vCyclin, and ORF73-LANA) were reduced in cells treated either before or after viral incubation with DNo (Fig. 2H). As further validation of these observations, we found that DNo treatment either before or after viral incubation reduced LANA expression in pDMVEC as well (Fig. 3A–E). Of note, DNo did not reduce intracellular KSHV genome copies in HeLa cells following viral incubation (Fig. 4A). In separate experiments to validate our findings with DNo, we incubated HeLa cells with anti-Hsp90 antibodies prior to viral incubation and found that a monoclonal antibody targeting Hsp90-alpha (SPA-840) also reduced LANA transcripts and protein expression in HeLa cells (Fig. 4B and C). Interestingly, a second antibody targeting Hsp90-alpha (SPS-771) had no effect (Fig. 4B and C). In addition, as with DNo, incubation of cells with the mAb SPA-840 failed to reduce intracellular KSHV genome copy number after viral incubation (Fig. 4C).
Figure 2. Extracellular Hsp90 acts as a co-factor for KSHV gene expression during de novo infection of HeLa cells.
(A) HeLa cells and pDMVEC were incubated with the indicated concentrations of DNo and cell viability determined after 48 h by a standard MTT assay according to the manufacturer’s instructions. (B–E) HeLa cells were incubated with KSHV (MOI = 2) and DMSO vehicle (B) or UV-KSHV (C) or treated with 0.1 μM (D) or 1.0 μM (E) DNo for 16 h prior to KSHV incubation. Immunofluorescence assays (IFA) were performed 12 h after viral incubation to identify the typical punctate intranuclear expression of LANA (red dots) using an anti-LANA mAb and a secondary Ab conjugated to Texas Red, along with DAPI for nuclear co-localization (blue). (F, G) The number of LANA dots was determined for at least 100 cells for each DNo pre-treatment (DNo+K) and post-treatment (K+DNo) group and normalized to positive control cells. (H) qRT-PCR was used to determine relative transcript expression for ORF73 (LANA), ORF72 (vCyclin) and ORF71 (vFLIP) at 16 h after viral incubation from HeLa cells incubated with media alone (mock), KSHV alone (K), 1.0 μM DNo 16 h before KSHV (DNo+K) or for 16 h beginning immediately following KSHV incubation (K+DNo). Error bars represent the S.E.M. for three independent experiments.
Figure 3. Extracellular Hsp90 acts as a co-factor for KSHV gene expression during de novo infection of endothelial cells.
(A–D) pDMVEC were incubated with KSHV and DMSO (A), UV-KSHV (B), or 1.0 μM DNo for 16 h before (C) or beginning immediately after (D) their incubation with KSHV. LANA expression was determined by IFA 12 h after viral incubation as in Fig. 2. (E) Relative LANA expression in groups A–D was calculated as described in Figure 2. Error bars represent the S.E.M. for three independent experiments.
Figure 4. Direct KSHV interaction with extracellular Hsp90 is not required for KSHV entry.
(A) HeLa cells were incubated with media alone (mock), KSHV alone (K), 1.0 μM DNo 16 h before KSHV (DNo+K) or for 16 h beginning immediately following KSHV incubation (K+DNo), and qPCR was used to determine relative intracellular KSHV DNA content in the above groups. (B) Cells were incubated with KSHV alone, UV-KSHV alone, or 30 μg/mL monoclonal antibodies recognizing either the N-terminus (771Ab) C-terminus (830Ab), or an uncharactierized epitope (840Ab) of Hsp90 or isotype control Ab (isoAb) for 12 h prior to incubation with KSHV. LANA expression was quantified by IFA 12 h after viral incubation. (C) Cell-free KSHV pellets were incubated with 1.0 mg/mL of heparan sulfate (hs) for 1.5 h at 37°C, 1.0 μg BSA, 1.0 μg or 5.0 μg purified recombinant Hsp90-α for 1 h at 37°C, or media alone prior to infection. LANA expression was quantified by IFA 12 h later as above. Error bars represent the S.E.M. for three independent experiments.
Previous work has demonstrated that csHsp90 may serve as a receptor for viruses since viral binding and entry to host cells was reduced by either pre-incubation of cells with Hsp90-specific antibodies or pre-incubation of purified virus with recombinant Hsp90 (Lin et al., 2007; Reyes-Del Valle et al., 2005). Pre-incubation of purified KSHV with heparin sulfate as a positive control reduced viral genome copies (not shown) and LANA expression in our infection assays (Fig. 4D) as previously described (Birkmann et al., 2001; Akula et al., 2001), but pre-incubation of KSHV with concentrations of recombinant Hsp90 (rHsp90) previously shown to inhibit entry by other viruses (Lin et al., 2007; Reyes-Del Valle et al., 2005) did not reduce either viral genome copies (not shown) or LANA expression (Fig. 4D). Furthermore, we have not found a relationship between csHsp90 expression and cell permissiveness to KSHV (representative data are shown for HEK cells in Supplemental Fig. 1, as HEK are permissive for infection but express little to no csHsp90 upon passage in culture). Together with our data indicating that DNo reduces KSHV gene expression even if added following viral incubation, these data suggests that Hsp90 modulates KSHV gene expression largely through the regulation of post-entry events, and that this effect may be cell type-specific.
Targeting csHsp90 reduces KSHV-initiated MAPK activation
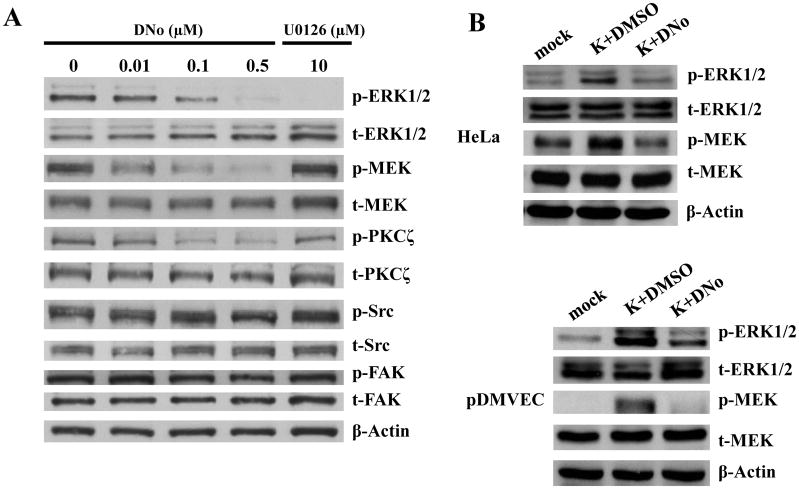
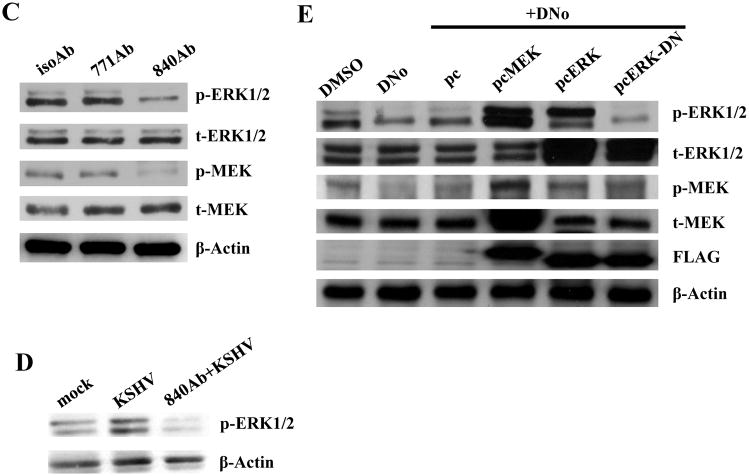
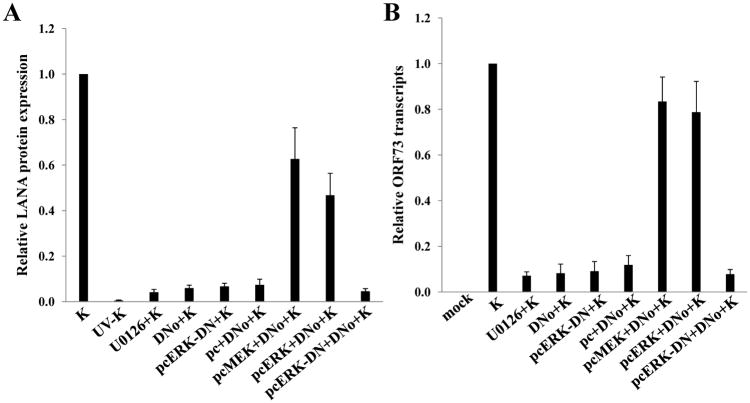
KSHV gene expression during de novo infection is regulated by a number of cell signaling intermediates that facilitate entry and intracellular trafficking of the virus following KSHV interactions with cell surface receptors, including α3β1 integrins (Krishnan et al., 2006; Sharma-Walia et al., 2004, 2005). To first determine whether csHsp90 regulates expression of α3β1 integrin-associated signaling intermediates, we quantified total and phosphorylated protein expression using immunoblot analyses for FAK, Src, PKC-ζ, MEK, and ERK during incubation of cells with DNo (Fig. 5). We observed dose-dependent DNo inhibition of basal MEK and ERK phosphorylation in HeLa cells to an extent comparable to that seen with a specific inhibitor of MAPK activation (U0126) shown previously to reduce ERK activation during de novo KSHV infection (Sharma-Walia et al., 2005). In addition, we observed inhibition of KSHV-initiated MEK and ERK phosphorylation in both HeLa cells and pDMVEC (Figs. 5B) with DNo, and validated these results with the observation of inhibition of both basal and KSHV-initiated MEK and ERK phosphorylation in HeLa cells with an Hsp90-specific antibody (Figs. 5C and D). Constitutive expression of MEK or ERK rescued MAPK activation during DNo treatment in HeLa cells (Fig. 5E), whereas transfection of cells with a construct encoding dominant-negative ERK reduced phosphorylated ERK levels below those of untreated or DNo-treated control cells (Fig. 5E). Therefore, we transfected KSHV-infected HeLa cells with the constructs described above to determine whether MEK or ERK overexpression rescued KSHV gene expression during DNo treatment. Compared to empty vectors that had no effect, MEK or ERK overexpression restored LANA protein (Fig. 6A) and transcript (Fig. 6B) expression in DNo-treated cells to approximately 50–80% of control levels, whereas control cells transfected with dominant-negative ERK constructs or incubated with U0126 revealed minimal LANA expression to levels similar to that seen with DNo. For verification, we obtained similar data for transcripts for two other latent genes expressed within KSHV-infected cells: vFLIP and vCyclin (Supplemental Fig. 2).
Figure 5. Extracellular Hsp90 serves as a co-factor for KSHV-induced MAPK activation.
(A) HeLa cells were pre-treated with the indicated concentrations of DNo for 16 h. Immunoblot analyses were performed 16 h later for total (t) or phosphorylated (p) signaling intermediates or β-Actin as an internal control. Control cells were treated with 10 μM of the MAPK-specific inhibitor U0126 for 1.5 h at 37°C. (B) Hela cells and pDMVEC were incubated with 1.0 μM DNo for 16 h beginning immediately following KSHV incubation, then immunoblot analyses were performed for signaling molecules as above. (C) HeLa cells were incubated with 30 μg/ml of either control isotype Ab (isoAb) or monoclonal anti-Hsp90 antibodies (771Ab and 840Ab) for 12 h at 37°C, then with purified KSHV for 2 h. Immunoblot analyses were performed 16 h later for signaling molecules as above. (D) HeLa cells were incubated with KSHV with or without pre-incubation with 30 μg/ml anti-Hsp90 antibodies (840Ab). Immunoblot analyses were performed 16 h later. (E) HeLa cells were incubated with DMSO or 0.5 μM DNo for 16 h, or first transiently transfected with 1 μg of pcDNA3.1 control (pc), pcDNA3.1-FLAG-MEK (pcMEK), pcDNA3.1-FLAG-ERK (pcERK) or pcDNA3.1-FLAG- dominant-negative-ERK (pcERK-DN) vectors prior to DNo treatment. 24 h post-transfection, cell lysates were collected and analyzed as above.
Figure 6. Constitutive MAPK expression restores KSHV gene expression during functional inhibition of extracellular Hsp90.
(A) HeLa cells were incubated with UV-KSHV, KSHV, 0.5 μM of DNo for 16 h followed by KSHV, or 0.5 μM of DNo following their initial transfection with a control vector (pc) or vectors encoding MEK (pcMEK), ERK (pcERK), or dominant-negative ERK (pcERK-DN). Control cells were incubated with 10 μM of U0126 for 1.5 h at 37°C or transfected with pcERK-DN alone. IFA were performed 16 h later to quantify LANA expression. Relative LANA expression was calculated as previously described. (B) qRT-PCR was used to determine relative transcripts expression for ORF73 (LANA) in the groups described above. Error bars represent the S.E.M. for two independent experiments.
Targeting csHsp90 reduces KSHV lytic transcripts and virion release by latently infected cells
Existing data suggest that reactivation of KSHV lytic replication from latency is dependent upon MAPK activation, and more specifically activation of ERK (Cohen et al., 2006; Ford et al., 2006; Xie et al., 2008; Yu et al., 2007). We sought to determine whether DNo treatment of BCBL-1 cells reduced lytic gene expression activation and virion release by these cells during induction of lytic replication. We first confirmed that BCBL-1 express of csHsp90 (Fig. 7A) and that 0.5 μM DNo induces no toxicity for BCBL-1 when incubated with these cells over 7 days based on MTT assays (data not shown). Next, we found that DNo reduces phosphorylation of MEK/ERK within BCBL-1 cells in the presence or absence of valproic acid (Fig. 7B). In addition, DNo reduces basal transcript expression of LANA and RTA (Figs. 7C and D), as well as valproic acid induction of RTA expression (Fig. 7E), so the same extent as observed for U0126. DNo also significantly reduced the number of infectious viral particles released by valproic acid-treated BCBL-1 cells in a manner similar to U0126 (Fig. 7F).
Figure 7. Targeting extracellular Hsp90 reduces KSHV lytic gene expression and release of infectious virions by PEL cells.
(A) BCBL-1 cells were prepared for flow cytometry experiments following their incubation with a monoclonal antibody recognizing Hsp90-alpha (771Ab) followed by secondary antibodies conjugated to ALEXA-488 (black histograms). For controls, cells were incubated with secondary antibodies alone (gray histograms). (B) BCBL-1 cells were incubated with 0.5 μM of DNo for 48 h with or without 0.6 mM valproic acid (VA), then immunoblot analyses were performed for signaling molecules as above and β-Actin used as an internal control. (C–D) BCBL-1 cells were incubated with 0.5 μM of DNo for 16 h, or with 10 μM U0126 for 1.5 h, then LANA (C) and RTA (D) transcripts quantified by qRT-PCR. (E) BCBL-1 cells were incubated with 0.6 mM valproic acid (VA) for 5 days along with either vehicle (DMSO), 0.5 μM of DNo or 1.0 μM U0126, and RTA transcripts quantified by qRT-PCR. (F) 200 μl of BCBL-1 culture supernatants were collected from groups of (E) then diluted 1:5 and incubated with HeLa cells in 8-well chamber slides for 16 h for LANA IFA as above. Controls included cells incubated with UV-KSHV (UV-K). Error bars represent the S.E.M. for two independent experiments.
Discussion
Existing data support a role for Hsp90 in mediating virus-host cell interactions for many different viruses, including herpesviruses. Events putatively mediated by Hsp90 include virus binding and entry, nuclear localization of viral proteins, and replication (Basha et al., 2005; Burch and Weller, 2005; Connor et al., 2007; Field et al., 2003; Li et al., 2004; Lin et al., 2007; Okamoto et al., 2006; Reyes-Del Valle et al., 2005). One study demonstrated that a cell-permeable Hsp90 inhibitor, geldanamycin, reduces HSV-1 replication (Li et al., 2004), and this may occur through the inhibition of nuclear localization of the viral DNA polymerase (Burch and Weller, 2005). Another study documented a role for Hsp90 in the expression of cytomegalovirus (CMV) genes during de novo infection, including genes representing all phases of the viral life cycle (Basha et al., 2005). Activation of the serine/threonine kinase Akt and nuclear factor-kappa B (NF-kappaB) following CMV infection was reduced in the presence of geldanamycin (Basha et al., 2005) suggesting that Hsp90 facilitates CMV-induced activation of these signaling pathways. Finally, geldanamycin was shown to inhibit IKK co-localization with Hsp90 in KSHV-infected BC-3 cells, as well as BC-3 cell growth (Field et al., 2003). These data provide rationale for performing additional studies to identify mechanisms for Hsp90 regulation of herpesvirus gene expression.
Recent identification of extracellular Hsp90 localized to the cell surface (Eustace et al., 2004) justifies further study of whether csHsp90 can be targeted to influence viral pathogenesis. DNo binds to the ATP-binding cleft within the N-terminal region of Hsp90 and inhibits ATPase activity to the same degree as for cell-permeable Hsp90 inhibitors (Tsutsumi et al., 2008). The non-permeable nature of DNo has been verified through its inability to compete for intracellular Hsp90 binding with tritium-labeled cell- permeable Hsp90 inhibitors, its inability to affect steady state levels of signaling molecules affected by cell-permeable Hsp90 inhibitors, and its inability to induce heat shock protein expression through the dissociation of intracellular Hsp90 with Hsp transcription factors (Tsutsumi et al., 2008). Unlike cell-permeable Hsp90 inhibitors, DNo does not affect steady-state levels of Akt and Raf-1 (Tsutsumi et al., 2008), supporting the notion that csHsp90 regulates a more limited array of intracellular signaling pathways and that targeting csHsp90 may induce less cellular toxicity. The advent of DNo compliments the use of functional anti-Hsp90 antibodies and has provided an important scientific advance for proof-of-concept experiments identifying cell functions regulated by csHsp90 and the potential utility of targeting csHsp90 for clinical applications. To our knowledge, the role of csHsp90 in pathogenesis related to oncogenic viruses has not been explored. DNo interrupts focal adhesion kinase (FAK) activation, focal adhesion formation, and cell migration by a fibrosarcoma cell line (Tsutsumi et al., 2008) which led us to hypothesize that csHsp90 facilitates KSHV-induced cell signaling and viral gene expression during de novo infection of target cells.
In agreement with numerous studies revealing surface localization of Hsp90-alpha for a variety of cell types (Jin et al., 2003; Kotsiopriftis et al., 2005; Reyes-Del Valle et al., 2005; Triantafilou et al., 2001), we found that Hsp90-alpha is localized to the cell surface for both HeLa cells and DMVEC. Our subsequent infection assays revealed that csHsp90 inhibition with either DNo or an anti-Hsp90 monoclonal antibody reduces latent KSHV transcripts and LANA expression during de novo infection. Interestingly, DNo still inhibits KSHV gene expression if added to cells following the viral incubation period, and even if administered beginning 6 h after viral incubation. Limited published data support the possibility that Hsp90 serves as a receptor for viruses (Lin et al., 2007; Reyes-Del Valle et al., 2005), but neither DNo nor anti-Hsp90 antibodies reduced intracellular KSHV genome copies in our experiments. Furthermore, unlike pre-incubation of cells with heparin sulfate, pre-incubation of cells with antibodies recognizing Hsp90-alpha, or pre-incubation of purified KSHV with recombinant Hsp90-alpha, also failed to reduce intracellular viral genome copies during viral incubation. These methods have been shown to reduce infection mediated by Hsp90 interactions on the cell surface with other viruses (Lin et al., 2007; Reyes-Del Valle et al., 2005). These data further support the notion that Hsp90 localized to the cell surface facilitates KSHV gene expression primarily through the regulation of post-entry events, although they do not categorically exclude the possibility that csHsp90 inhibition reduces KSHV binding and entry mediated through KSHV receptor client proteins of Hsp90 localized to the cell surface, or possibly even Hsp90 itself. For cells passed in vitro, we have thus far found no relationship between cell permissiveness to KSHV infection and quantitative csHsp90 expression (data for HEK shown in Supplemental Fig. 1). In fact, the monoclonal anti-Hsp90 antibody eliciting functional repression of MAPK and KSHV gene expression in our experiments yielded the least robust flow cytometric identification of csHsp90-alpha by HeLa cells (Fig. 1). Furthermore, an antibody recognizing a different epitope for csHsp90-alpha and which yielded more robust identification of csHsp90-alpha by HeLa cells (Fig. 1) failed to repress MAPK or KSHV gene expression in these cells. Additional work is, therefore, needed to more thoroughly localize and characterize the functional epitopes of csHsp90 that mediate KSHV-initiated signal transduction and viral gene expression, and to determine whether paracrine factors associated with KSHV infection or the KS microenvironment regulate the surface localization of Hsp90 and cell susceptibility to infection.
The activation and function of a number of intracellular proteins important for KSHV gene expression have been linked to Hsp90, including FAK (Tsutsumi et al., 2008), PI3K (Basha et al., 2005), Akt (Basha et al., 2005), Src (Bijlmakers and Marsh, 2000), ERK (Georgakis et al., 2006; Koga et al., 2006), and NF-kB (Basha et al., 2005; Field et al., 2003). We found that targeting csHsp90 selectively reduces steady state MAPK activation (Fig. 5), and that restoration of MAPK activation partially restores latent KSHV transcripts and LANA protein expression in these cells. Moreover, this effect was comparable to that seen with a specific inhibitor of MAPK activation, U0126. To our knowledge, these are the first data suggesting that csHsp90 serves as a co-factor for MAPK activation or MAPK-associated viral gene expression. These data are in accordance with one previous study documenting a role for Hsp90 in the activation of ERK (Georgakis et al., 2006). Although conflicting data exist for the role for Hsp90 in the activation of MAPK pathways in cancer cells (Georgakis et al., 2006; Koga et al., 2006), conditional activation of ERK by viruses, and the differential regulation of signaling by csHsp90 relative to intracellular Hsp90 (Tsutsumi et al., 2008), may contribute to differences between virus-related Hsp90 chaperoning of ERK and constitutive ERK activation in tumor cells. Additional studies are ongoing to determine whether csHsp90 facilitates KSHV-induced ERK activation in other primary cell targets of the virus. Our data are also in agreement with previous data implicating ERK in the transcriptional activation of a number of different KSHV genes, including ORF73 and ORF50 (Sharma-Walia et al., 2005). The precise mechanism by which csHsp90 facilitates ERK-mediated KSHV gene expression remains to be determined, although ERK activation of the transcription factor AP-1 is one possibility (Sharma-Walia et al., 2005). We looked only at the expression of a few representative latent genes in these studies, but since the expression of all 3 latent genes we examined was reduced following csHsp90 inhibition, it is possible that inhibition of csHsp90 causes a —global reduction in viral gene expression during de novo infection. If this is true, it is unlikely that inhibition of ERK activation is the only mechanism through which this occurs, and this is supported by the fact that MEK or ERK overexpression only partially rescued latent gene expression in csHsp90-targeted cells. We also observed a reduction in the expression of PKCζ (see Fig. 5A), an —upstream molecule putatively involved in KSHV-induced ERK activation, and this observation requires additional characterization.
Our analyses were not exhaustive, as they did not include assessment of other potential mediators of ERK activation and KSHV gene expression, including PI3K or Rho-GTPase-activated microtubule assembly (Sharma-Walia et al., 2004). Hsp90 has been previously implicated in microtubule trafficking of intracellular proteins to the nucleus (Galigniana et al., 2004) and participates in the nuclear trafficking of the herpes simplex virus DNA polymerase (Burch and Weller, 2005). Therefore, it is possible that targeting csHsp90 restricts microtubule-associated KSHV protein trafficking to the nucleus, thereby reducing expression of an array of viral genes. csHsp90 regulates FAK recruitment and focal adhesion formation in some cell lines (Tsutsumi et al., 2008), and FAK activation has been linked previously to Rho-GTPase activation during KSHV infection (Sharma-Walia et al., 2004). Therefore, it is possible that although we observed no changes in total or phosphorylated FAK levels during csHsp90 targeting, this might reduce FAK recruitment following KSHV-receptor engagement, thereby reducing downstream signaling events pertaining to microtubule assembly. Hsp90 also regulates NF-κB activation (Basha et al., 2005; Field et al., 2003; Jin et al., 2003) which provides an important alternative pathway for transcriptional activation of KSHV gene expression independent of MAPK activation (Sadagopan et al., 2007). It is, therefore, conceivable that the coordinated regulation by csHsp90 of a number of different signaling intermediates facilitates the expression of multiple viral genes during KSHV infection. Studies are ongoing in our laboratory to determine the role of csHsp90 in facilitating KSHV gene expression during de novo infection through the regulation of NF-κB, and profiling a broader array of viral genes for these studies may shed additional light on these questions.
Previous studies have documented a role for ERK in the activation of lytic KSHV gene expression, including the activation of both ORF73 and ORF50 (Sharma-Walia et al., 2005). In our experiments using BCBL-1 cells, csHsp90 inhibition reduced MAPK activation, steady state expression of ORF50 and ORF73 transcripts, and valproic acid-induced activation of RTA to the same extent as observed using a specific inhibitor of MAPK activation. In addition, DNo suppressed the release of infectious viral particles by valproic acid-induced BCBL-1. Although other published data support a role for LANA in the regulation of RTA expression through interactions with its promoter (Li et al., Virology. 2008), it is plausible that reduction of ERK activation with csHsp90 inhibition reduces both LANA and RTA activation independent of one another. It also remains possible that inhibition of csHsp90 influences alternative signal transduction pathways in PEL cells that may independently influence expression of LANA and RTA, and further exploration of alternative signaling pathways and their relationship to csHsp90 function in these cells is warranted. One limitation of these experiments was that although DNo exhibited no toxicity for uninduced BCBL-1 cells over a range of concentrations, cell death induced by valproic acid (as occurs during induction of lytic replication in BCBL-1) obscured our ability to determine whether csHsp90 targeting accentuates cell death during lytic cycle induction.
In summary, our data implicate csHsp90 as a co-factor in the establishment of KSHV gene expression during de novo infection through its regulation of MAPK activation. Future studies should clarify the role of csHsp90 in lytic reactivation of KSHV within latently infected cells and characterize additional signaling intermediates regulated by csHsp90 that impact KSHV gene expression. These studies provide support for the potential utility of targeting csHsp90 for reducing KSHV gene expression and KSHV-associated disease progression.
Materials and methods
Flow cytometry
HeLa and primary human dermal microvascular endothelial cells (pDMVEC) were first maintained at 50–70% confluence. Following trypsinization, cells were resuspended in staining buffer (0.1% sodium azide in 1X PBS) for 20 minutes, then incubated on ice for 30 min with 2.5 μg/mL of primary antibodies (Stressgen) recognizing either the C-terminus (SPS-830), N-terminus (SPS-771), or an uncharacterized epitope (SPA-840) of Hsp90-alpha. Following two subsequent wash steps, cells were incubated for an additional 30 min with secondary antibodies (Invitrogen) diluted 1:100 that recognize either the N-terminal primary antibody (goat anti-mouse IgG Alexa Fluor 488), C-terminal primary antibody (goat anti-rabbit IgG Alexa Fluor 488), or the mAb targeting an uncharacterized epitope (goat anti-rat IgG Alexa Fluor 488). Controls included cells incubated with secondary antibodies only. Cells were resuspended in 1X PBS and analyzed using a FACS Calibur 4-color flow cytometer and FlowJo software (TreeStar) to quantify cell surface localization of Hsp90.
Cell culture and infection assays
KSHV-infected body cavity-based lymphoma (BCBL-1) cells were maintained in RPMI 1640 media (Gibco) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES (pH 7.5), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 0.05 mM β-mercaptoethanol, and 0.02% (wt/vol) sodium bicarbonate. pDMVEC were maintained according to the manufacturer’s instructions (Lonza). HeLa and human embryonic kidney (HEK) 293A cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL streptomycin. To obtain KSHV for infection experiments, BCBL-1 cells were incubated with 0.6 mM valproic acid for 6 days, and purified virus was concentrated from culture supernatants as described previously (Qin et al., 2010; Parsons et al., 2006). For negative controls using ultraviolet light-inactivated KSHV (UV-KSHV), viral aliquots were exposed to 1200 J/cm2 UV light for 10 min using a CL-1000 Ultraviolet Crosslinker. Infectious titers (MOI) were determined using both pDMVEC and HeLa cells and methods previously described (Qin et al., 2010; Parsons et al., 2006) and approximated 4×106 infectious particles/ml routinely in these preparations. For competition assays, viral stocks were pretreated with medium only, recombinant Hsp90 proteins (1 h at 37°C), or 1 mg/mL of heparan sulfate (1.5 h at 37°C) prior to infection assays.
Immunofluorescence assays (IFA)
Briefly, 1×104 HeLa cells or pDMVEC per well were seeded in eight-well chamber slides (Nunc) and incubated with serial dilutions of viral stocks in the presence of 8 μg/mL Polybrene (Sigma-Aldrich) for 2 h at 37°C. After remaining in culture overnight, cells were incubated in 1:1 methanol-acetone at 20°C for fixation and permeabilization, then with a blocking reagent (10% normal goat serum, 3% bovine serum albumin, and 1% glycine) for an additional 30 minutes. Cells were then incubated for 1 h at 25°C with 1:1000 dilution of a rat anti-LANA monoclonal antibody (ABI) followed by a 1:100 dilution of a goat anti-rat secondary antibody conjugated to Texas Red (Invitrogen). For nuclear localization, cells were subsequently counterstained with 0.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma) in 180 mM Tris-HCl (pH 7.5). Relative LANA expression = #LANA dots per 100 cells in experimental group/#LANA dots per 100 cells in control group. For determination of Hsp90 surface localization, HeLa cells were incubated first at 37°C for 30 min with blocking reagent, and then for 1 h with 1:25 dilution of either SPS-771 or SPS-830 for negative controls. After two washes with 1X PBS, cells were incubated at 37°C for 45 min with 1:100 dilutions of respective secondary antibodies as for flow cytometry, then in cold 1:1 methanol/acetone for 10 min at −20°C. Some cells were incubated only with secondary antibodies for additional negative controls. For nuclear localization, cells were incubated at 4°C in the dark for 10 minutes with 0.5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Sigma) in 180 mM Tris-HCl (pH 7.5). Slides were washed once in 180 mM Tris-HCl for 15 min and prepared for visualization using a Leica TCPS SP2 AOBS confocal microscope.
Cell viability assays
Cell viability was assessed by MTT assay as previously described (Tsutsumi et al., 2008). A total of 5×103 Hela cells were incubated in each of individual wells in a 96-well plate for 24 h. Serial dilutions of DMAG-N-oxide (DNo) were then added and after 24–72 h, cells were incubated in 1 mg/ml of MTT solution (Sigma-Aldrich) at 37°C for 3 h, then 50% DMSO overnight and optical density at 570 nm determined by spectrophotometer (Thermo Labsystems). In parallel experiments to determine whether DNo influenced cell adherence (which may itself influence cell susceptibility to infection), Hela cells were incubated with serial dilutions of DNo and the number of adherent cells determined for different time points over 16 h following removal of media and non-adherent cells, trypsinization, and quantification of cells using a hemocytometer.
Hsp90/MAPK inhibition
DNo was dissolved in DMSO and aliquots frozen at −80°C. For DNo pre-treatment assays, 5×103 HeLa cells or pDMVEC were seeded per well in eight-well chamber slides (Nunc) and incubated with serial dilutions of DNo at 37°C. Viral aliquots were then added (MOI between 2–10) for 2 h at 37°C followed by an overnight incubation prior to IFA. For DNo post-treatment assays, the cells were incubated with viral aliquots for 2 h. Cells were washed three times, fresh media containing DNo was added immediately afterward (0 h) or 6 h later, and then cells were incubated for an additional 12 h prior to IFA. Control cells were treated with DMSO. As a control for MAPK activation experiments, cells were treated with the selective MAPK inhibitor U0126 (Sigma) for 1.5 h at 37°C.
Transfection assays
pcDNA3.1 control (pc), pcDNA3.1-FLAG-MEK (pcMEK), pcDNA3.1-FLAG-ERK (pcERK) and pcDNA3.1-FLAG-ERK dominant negative (pcERK-DN) vectors were kindly provided by Dr. Scott Eblen (Medical University of South Carolina, Charleston, SC). Cells were transfected with vectors in 12-well plates for 48 h using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Uniformity of transfection efficiency was confirmed through co-transfection of a lacZ reporter construct also kindly provided by Dr. Yusuf Hannun (Medical University of South Carolina), and β-galactosidase activity was determined using a commercially available β-galactosidase enzyme assay system according to the manufacturer’s instructions (Promega). 2–3 independent transfections were performed for each experiment, and all samples were analyzed in triplicate for each transfection.
Western blotting
Cells were lysed in buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% NP40, 1 mM EDTA, 5 mM NaF and 5 mM Na3VO4. Total cell lysates (30 μg) were resolved by 10% SDS–PAGE, transferred to nitrocellulose membranes, and immunoblotted with 100–200 μg/mL antibodies recognizing total and phosphorylated proteins as follows: phospho-p44/42 MAPK (Thr202/Tyr204), phospho-MEK1/2 (Ser217/221), phospho-PKCζ (Thr410/403), phospho-Src (Tyr416), p44/42 MAPK, MEK1/2, PKCζ and Src (Cell Signaling Technologies); phosphor-FAK (Tyr397) and FAK (Biosource). For loading controls, blots were also reacted with antibodies detecting the respective total protein or β-Actin (Sigma). To assess transfection efficiency, FLAG expression was detected with FLAG-specific antibodies. Immunoreactive bands were developed by enhanced chemiluminescence reaction (Perkin-Elmer) and visualized by autoradiography.
PCR
Total cellular DNA was prepared using the QIAamp DNA Mini kit according to the manufacturer’s instructions (QIAGEN). Briefly, cells were trypsinized for 5 min at 37°C and collected with 1 ml of ice-cold DMEM. Cells were pelleted at 2,000 rpm for 5 min, washed, and resuspended in 200 μL of 1X PBS, and total DNA was prepared according to the manufacturer’s instructions. To ensure that viral DNA amplification in these experiments was not the result of —carry over viral DNA from culture supernatants rather than intracellular virus, cells were washed several times in fresh media prior to trypsinization, and samples from culture supernatants following these washes were assessed for viral DNA content. Total RNA was isolated from infected or uninfected cells using the RNeasy Mini kit according to the manufacturer’s instructions (QIAGEN). cDNA was synthesized from equal total RNA using SuperScript III First-Strand Synthesis SuperMix Kit (Invitrogen) according to the manufacturer’s procedures. Coding sequences for genes of interest and β-actin (loading control) were amplified from 200ng input cDNA or DNA and using iQ SYBR Green Supermix (Bio-Rad). Custom primer sequences used for amplification experiments (Operon) were as follows: LANA sense 5′ TCCCTCTACACTAAACCCAATA 3′; LANA antisense 5′ TTGCTAATCTCGTTGTCCC 3′; vFLIP sense 5′ GGGCACGGATGACAGGGAA 3′; vFLIP antisense 5′ TGTGATGGGCCGGAAAGG 3′; vCyclin sense 5′ CCCTCGGGACTTCTGGAT 3′; vCyclin antisense 5′ CGTCGCTAAGACTGCCTC 3′; RTA sense 5′ TAATGTCAGCGTCCACTCC 3′; RTA antisense 5′ TTCTGGCACGGTCAAAGC 3′; β-actin sense 5′ GGAAATCGTGCGTGACATT 3′; β-actin antisense 5′ GACTCGTCATACTCCTGCTTG 3′. Amplification experiments were carried out on an iCycler IQ Real-Time PCR Detection System, and cycle threshold (Ct) values were tabulated in triplicate (DNA) or duplicate (cDNA) for each gene of interest for each experiment. —No template (water) controls were also used to ensure minimal background contamination. Using mean Ct values tabulated for different experiments and using Ct values for β-actin as loading controls, fold changes for experimental groups relative to assigned controls were calculated using automated iQ5 2.0 software (Bio-rad).
Supplementary Material
(A,B) HEK cells were incubated with KSHV (A) or UV-KSHV (B), then IFA performed 12 h after viral incubation to identify the typical punctate intranuclear expression of LANA (red dots) using an anti-LANA mAb and a secondary Ab conjugated to Texas Red, along with DAPI for nuclear co-localization (blue). (C) HEK cells were incubated with anti-Hsp90 monoclonal antibodies (771Ab) and secondary antibodies (black histogram) or secondary antibodies alone (gray histogram) for flow cytometry. IFA images and flow cytometry data are representative of 3 independent experiments.
HeLa cells were incubated with UV-KSHV, KSHV, 0.5 μM DNo for 16 h followed by KSHV, or 0.5 μM of DNo following their initial transfection with a control vector (pc) or vectors encoding MEK (pcMEK), ERK (pcERK), or dominant-negative ERK (pcERK-DN). Control cells were treated with 10 μM U0126 for 1.5 h at 37°C or transfected with pcERK-DN alone. qRT-PCR was used to determine relative transcript expression for ORF72 (A) and ORF71 (B) in the groups described above. Error bars represent the S.E.M. for two independent experiments.
Acknowledgments
We would like to thank Dr. Charles Smith (Medical University of South Carolina) for providing DNo for these studies. We would also like to thank Dr. Scott Eblen (Medical University of South Carolina) for providing the MAPK expression vectors. This work was supported by grants from the National Institutes of Health (K08-1CA103858 to C.H.P. and a Medical Scientist Training Grant 5T32DE017551-03 for M.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akula SM, Wang FZ, Vieira J, Chandran B. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology. 2001;282(2):245–55. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- Aluigi MG, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147(5):267–75. doi: 10.1016/0923-2516(96)82285-0. [DOI] [PubMed] [Google Scholar]
- Basha W, Kitagawa R, Uhara M, Imazu H, Uechi K, Tanaka J. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibits gene expression and replication of human cytomegalovirus. Antivir Chem Chemother. 2005;16(2):135–46. doi: 10.1177/095632020501600206. [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck) Mol Biol Cell. 2000;11(5):1585–95. doi: 10.1091/mbc.11.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmann A, Mahr K, Ensser A, Yaguboglu S, Titgemeyer F, Fleckenstein B, Neipel F. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J Virol. 2001;75(23):11583–93. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G, Gaudreau A, Routy JP. Evaluation of the human herpesvirus 8 DNA load in blood and Kaposi’s sarcoma skin lesions from AIDS patients on highly active antiretroviral therapy. AIDS. 2000;14(13):1907–10. doi: 10.1097/00002030-200009080-00004. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, Costagliola D, Salmon D, Chene G, Morlat P. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317–24. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- Brown EE, Whitby D, Vitale F, Marshall V, Mbisa G, Gamache C, Lauria C, Alberg AJ, Serraino D, Cordiali-Fei P, Messina A, Goedert JJ. Virologic, hematologic, and immunologic risk factors for classic Kaposi sarcoma. Cancer. 2006;107(9):2282–90. doi: 10.1002/cncr.22236. [DOI] [PubMed] [Google Scholar]
- Burch AD, Weller SK. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J Virol. 2005;79(16):10740–9. doi: 10.1128/JVI.79.16.10740-10749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TB, Borok M, Gwanzura L, MaWhinney S, White IE, Ndemera B, Gudza I, Fitzpatrick L, Schooley RT. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi’s sarcoma clinical stage. AIDS. 2000;14(14):2109–16. doi: 10.1097/00002030-200009290-00006. [DOI] [PubMed] [Google Scholar]
- Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, Hamilton S, Huang ML, Wald A. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198(1):23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Chaisuparat R, Hu J, Jham BC, Knight ZA, Shokat KM, Montaner S. Dual inhibition of PI3Kalpha and mTOR as an alternative treatment for Kaposi’s sarcoma. Cancer Res. 2008;68 (20):8361–8. doi: 10.1158/0008-5472.CAN-08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Cohen A, Brodie C, Sarid R. An essential role of ERK signalling in TPA-induced reactivation of Kaposi’s sarcoma-associated herpesvirus. J Gen Virol. 2006;87(Pt 4):795–802. doi: 10.1099/vir.0.81619-0. [DOI] [PubMed] [Google Scholar]
- Connor JH, McKenzie MO, Parks GD, Lyles DS. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology. 2007;362(1):109–19. doi: 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96(8):4546–51. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6(6):507–14. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, Collins M. KSHV vFLIP binds to IKK-gamma to activate IKK. J Cell Sci. 2003;116(Pt 18):3721–8. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- Ford PW, Bryan BA, Dyson OF, Weidner DA, Chintalgattu V, Akula SM. Raf/MEK/ERK signalling triggers reactivation of Kaposi’s sarcoma-associated herpesvirus latency. J Gen Virol. 2006;87(Pt 5):1139–44. doi: 10.1099/vir.0.81628-0. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, O’Hagen HM, Ljungman M, Pratt WB. Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J Biol Chem. 2004;279(21):22483–9. doi: 10.1074/jbc.M402223200. [DOI] [PubMed] [Google Scholar]
- Georgakis GV, Li Y, Rassidakis GZ, Martinez-Valdez H, Medeiros LJ, Younes A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin’s lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin Cancer Res. 2006;12(2):584–90. doi: 10.1158/1078-0432.CCR-05-1194. [DOI] [PubMed] [Google Scholar]
- Grundhoff A, Ganem D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J Clin Invest. 2004;113(1):124–36. doi: 10.1172/JCI200417803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey RW, O’Brien TR, Newcomb FM, Nishihara H, Wyvill KM, Ramos GA, Saville MW, Goedert JJ, Straus SE, Yarchoan R. Kaposi’s sarcoma (KS)-associated herpesvirus-like DNA sequences in peripheral blood mononuclear cells: association with KS and persistence in patients receiving anti-herpesvirus drugs. Blood. 1996;88(1):297–301. [PubMed] [Google Scholar]
- Jin S, Song YC, Emili A, Sherman PM, Chan VL. JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90alpha and triggers signalling pathways leading to the activation of NF-kappaB and p38 MAP kinase in epithelial cells. Cell Microbiol. 2003;5 (3):165–74. doi: 10.1046/j.1462-5822.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc Natl Acad Sci U S A. 2006;103(30):11318–22. doi: 10.1073/pnas.0604705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon HB, Bubley GJ, Pantanowitz L, Masiello D, Smith B, Crosby K, Proper J, Weeden W, Miller TE, Chatis P, Egorin MJ, Tahan SR, Dezube BJ. Imatinib-induced regression of AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2005;23(5):982–9. doi: 10.1200/JCO.2005.06.079. [DOI] [PubMed] [Google Scholar]
- Kotsiopriftis M, Tanner JE, Alfieri C. Heat shock protein 90 expression in Epstein-Barr virus-infected B cells promotes gammadelta T-cell proliferation in vitro. J Virol. 2005;79(11):7255–61. doi: 10.1128/JVI.79.11.7255-7261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HH, Sharma-Walia N, Streblow DN, Naranatt PP, Chandran B. Focal adhesion kinase is critical for entry of Kaposi’s sarcoma-associated herpesvirus into target cells. J Virol. 2006;80(3):1167–80. doi: 10.1128/JVI.80.3.1167-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krown SE. AIDS-associated Kaposi’s sarcoma: is there still a role for interferon alfa? Cytokine Growth Factor Rev. 2007;18(5–6):395–402. doi: 10.1016/j.cytogfr.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krown SE, Lee JY, Dittmer DP. More on HIV-associated Kaposi’s sarcoma. N Engl J Med. 2008;358(5):535–6. doi: 10.1056/NEJMc072994. author reply 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbe C, de Cremoux P, Millot G, Podgorniak MP, Verola O, Berger R, Morel P, Calvo F. Characterization of in vitro culture of HIV-negative Kaposi’s sarcoma-derived cells. In vitro responses to alfa interferon. Arch Dermatol Res. 1997;289(7):421–8. doi: 10.1007/s004030050215. [DOI] [PubMed] [Google Scholar]
- Lebbe C, Legendre C, Frances C. Kaposi sarcoma in transplantation. Transplant Rev (Orlando) 2008;22(4):252–61. doi: 10.1016/j.trre.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhou F, Ye F, Gao SJ. Genetic disruption of KSHV major latent nuclear antigen LANA enhances viral lytic transcriptional program. Virology. 2008;379(2):234–44. doi: 10.1016/j.virol.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Tao PZ, Liu YZ, Jiang JD. Geldanamycin, a ligand of heat shock protein 90, inhibits the replication of herpes simplex virus type 1 in vitro. Antimicrob Agents Chemother. 2004;48 (3):867–72. doi: 10.1128/AAC.48.3.867-872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TW, Lo CW, Lai SY, Fan RJ, Lo CJ, Chou YM, Thiruvengadam R, Wang AH, Wang MY. Chicken heat shock protein 90 is a component of the putative cellular receptor complex of infectious bursal disease virus. J Virol. 2007;81(16):8730–41. doi: 10.1128/JVI.00332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340(14):1063–70. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357(13):1352–3. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Nishimura Y, Ichimura T, Suzuki K, Miyamura T, Suzuki T, Moriishi K, Matsuura Y. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J. 2006;25(20):5015–25. doi: 10.1038/sj.emboj.7601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak F, Pyakural P, Kokhaei P, Kaaya E, Pourfathollah AA, Selivanova G, Biberfeld P. HHV-8/KSHV during the development of Kaposi’s sarcoma: evaluation by polymerase chain reaction and immunohistochemistry. J Cutan Pathol. 2005;32(1):21–7. doi: 10.1111/j.0303-6987.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Parsons CH, Adang LA, Overdevest J, O’Connor CM, Taylor JR, Jr, Camerini D, Kedes DH. KSHV targets multiple leukocyte lineages during long-term productive infection in NOD/SCID mice. J Clin Invest. 2006;116(7):1963–73. doi: 10.1172/JCI27249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyakurel P, Massambu C, Castanos-Velez E, Ericsson S, Kaaya E, Biberfeld P, Heiden T. Human herpesvirus 8/Kaposi sarcoma herpesvirus cell association during evolution of Kaposi sarcoma. J Acquir Immune Defic Syndr. 2004;36(2):678–83. doi: 10.1097/00126334-200406010-00004. [DOI] [PubMed] [Google Scholar]
- Qin Z, Kearney P, Plaisance K, Parsons CH. Pivotal advance: Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2010a;87(1):25–34. doi: 10.1189/jlb.0409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog. 2010b;6(1):e1000742. doi: 10.1371/journal.ppat.1000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan EB, Zhang C, Stewart PW, Komoltri C, Davis MG, Wehbie RS. Elevated virus loads of Kaposi’s sarcoma-associated human herpesvirus 8 predict Kaposi’s sarcoma disease progression, but elevated levels of human immunodeficiency virus type 1 do not. J Infect Dis. 2002;185(12):1736–44. doi: 10.1086/340652. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Egorin MJ, Ramanathan RK, Remick SC, Sikorski RP, Lagattuta TF, Chatta GS, Friedland DM, Stoller RG, Potter DM, Ivy SP, Belani CP. A phase I study of 17-allylamino-17-demethoxygeldanamycin combined with paclitaxel in patients with advanced solid malignancies. Clin Cancer Res. 2008;14(11):3456–61. doi: 10.1158/1078-0432.CCR-07-5088. [DOI] [PubMed] [Google Scholar]
- Reyes-Del Valle J, Chavez-Salinas S, Medina F, Del Angel RM. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol. 2005;79(8):4557–67. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces sustained NF-kappaB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol. 2007;81(8):3949–68. doi: 10.1128/JVI.02333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin SZ, Nakamura S, Biberfeld P, Kaplan MH, Markham PD, Larsson L, Gallo RC. Angiogenic properties of Kaposi’s sarcoma-derived cells after long-term culture in vitro. Science. 1988;242(4877):430–3. doi: 10.1126/science.2459779. [DOI] [PubMed] [Google Scholar]
- Schulz TF. The pleiotropic effects of Kaposi’s sarcoma herpesvirus. J Pathol. 2006;208(2):187–98. doi: 10.1002/path.1904. [DOI] [PubMed] [Google Scholar]
- Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79(16):10308–29. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78(8):4207–23. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin SH, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, Henry D, Harrington WJ, Jr, Damania BA, Dittmer DP. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007;109(5):2165–73. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Sawai ET, Molinolo A, Gutkind JS, Montaner S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10(2):133–43. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–80. [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Haase AT. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71(1):715–9. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2(4):338–45. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007;98(10):1536–9. doi: 10.1111/j.1349-7006.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S, Carreras C, Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27(17):2478–87. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni T, Sprinz E, Machado MW, Santana Rde C, Fonseca BA, Schwartsmann G. Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat Rev. 2006;32(6):445–55. doi: 10.1016/j.ctrv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Von Roenn JH. Clinical presentations and standard therapy of AIDS-associated Kaposi’s sarcoma. Hematol Oncol Clin North Am. 2003;17(3):747–62. doi: 10.1016/s0889-8588(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Cope A, Gill J, Bourboulia D, Hayes P, Imami N, Kubo T, Marcelin A, Calvez V, Weiss R, Gazzard B, Boshoff C, Gotch F. Identification of Kaposi’s sarcoma-associated herpesvirus (KSHV)-specific cytotoxic T-lymphocyte epitopes and evaluation of reconstitution of KSHV-specific responses in human immunodeficiency virus type 1-Infected patients receiving highly active antiretroviral therapy. J Virol. 2002;76(6):2634–40. doi: 10.1128/JVI.76.6.2634-2640.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. Reactivation of Kaposi’s sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 2008;371(1):139–54. doi: 10.1016/j.virol.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Harada JN, Brown HJ, Deng H, Song MJ, Wu TT, Kato-Stankiewicz J, Nelson CG, Vieira J, Tamanoi F, Chanda SK, Sun R. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog. 2007;3(3):e44. doi: 10.1371/journal.ppat.0030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A,B) HEK cells were incubated with KSHV (A) or UV-KSHV (B), then IFA performed 12 h after viral incubation to identify the typical punctate intranuclear expression of LANA (red dots) using an anti-LANA mAb and a secondary Ab conjugated to Texas Red, along with DAPI for nuclear co-localization (blue). (C) HEK cells were incubated with anti-Hsp90 monoclonal antibodies (771Ab) and secondary antibodies (black histogram) or secondary antibodies alone (gray histogram) for flow cytometry. IFA images and flow cytometry data are representative of 3 independent experiments.
HeLa cells were incubated with UV-KSHV, KSHV, 0.5 μM DNo for 16 h followed by KSHV, or 0.5 μM of DNo following their initial transfection with a control vector (pc) or vectors encoding MEK (pcMEK), ERK (pcERK), or dominant-negative ERK (pcERK-DN). Control cells were treated with 10 μM U0126 for 1.5 h at 37°C or transfected with pcERK-DN alone. qRT-PCR was used to determine relative transcript expression for ORF72 (A) and ORF71 (B) in the groups described above. Error bars represent the S.E.M. for two independent experiments.