Abstract
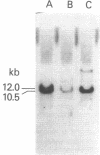
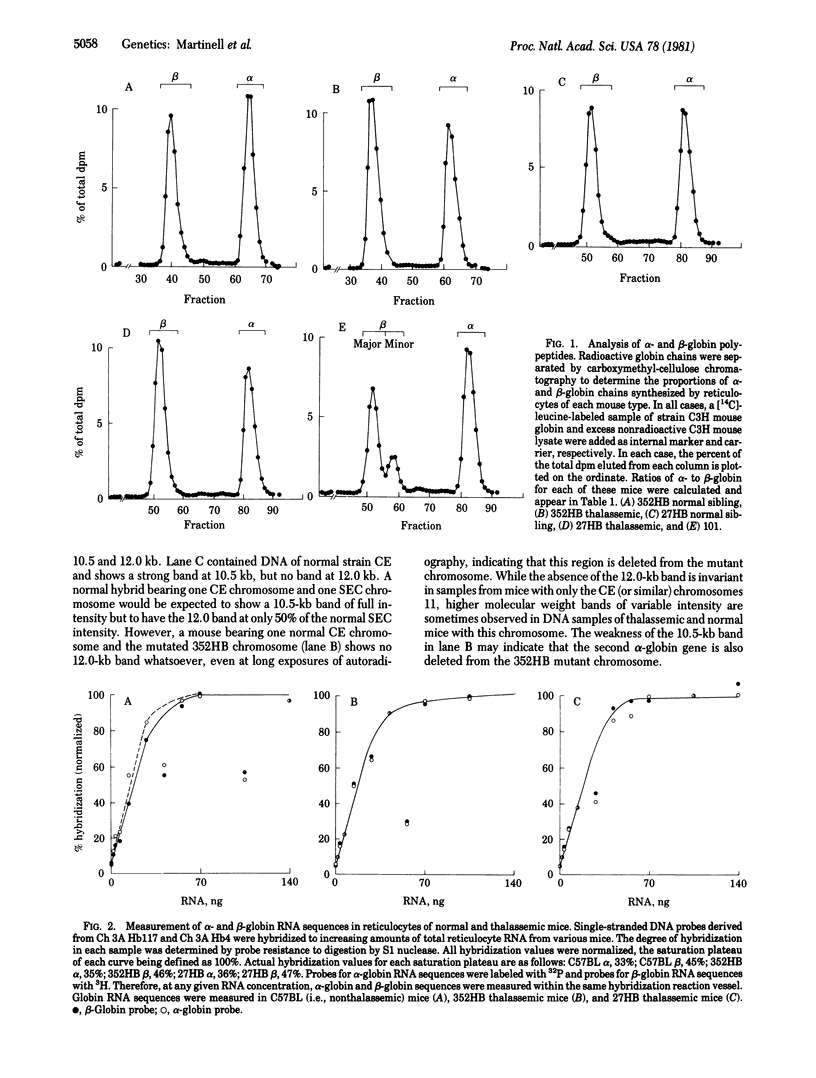
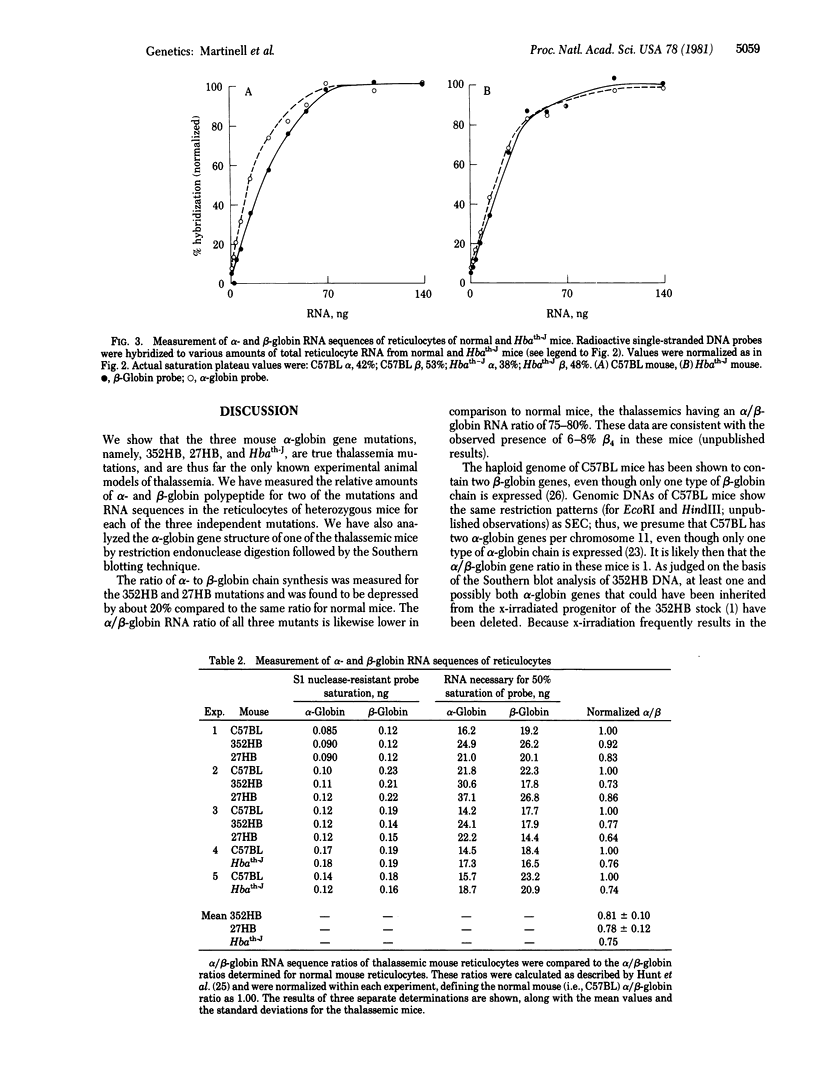
Three types of mice with globin gene mutations, called 352HB, 27HB, and Hbath-J, appear to be true animal models of human thalassemia. Expression of the alpha-globin genes in three stocks of mice, each one heterozygous for one of the alpha-globin mutations, was examined at the polypeptide, RNA, and DNA levels. alpha-Globin polypeptide chains, relative to beta-globin chains in heterozygous thalassemic mice, are present at approximately 80% of normal. The ratios of alpha-globin to beta-globin RNA sequences are also 75-80% of normal, exactly reflecting the alpha-globin to beta-globin chain ratios. In the case of mutant 352HB, at least one alpha-globin gene is deleted. Thalassemic mouse erythroid cells appear to compensate partially for the loss of half of their alpha-globin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Regulation of globin gene expression at the molecular level. Ann N Y Acad Sci. 1980;344:262–279. doi: 10.1111/j.1749-6632.1980.tb33667.x. [DOI] [PubMed] [Google Scholar]
- Benz E. J., Geist C. E., Steggles A. W., Barker J. E., Nienhuis A. W. Hemoglobin switching in sheep and goats. Preparation and characterization of complementary DNAs specific for the alpha-, beta-, and gamma-globin messenger RNAs of sheep. J Biol Chem. 1977 Mar 25;252(6):1908–1916. [PubMed] [Google Scholar]
- Benz E. J., Jr, Kretschmer P. J., Geist C. E., Kantor J. A., Turner P. H., Nienhuis A. W. Hemoglobin switching in sheep. Synthesis, cloning, and characterization of DNA sequences coding for the beta B, beta C, and gamma-globin mRNAs. J Biol Chem. 1979 Aug 10;254(15):6880–6888. [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. The isolation of messenger RNA from mammalian cells. Methods Enzymol. 1974;30:605–612. doi: 10.1016/0076-6879(74)30058-4. [DOI] [PubMed] [Google Scholar]
- Embury S. H., Lebo R. V., Dozy A. M., Kan Y. W. Organization of the alpha-globin genes in the Chinese alpha-thalassemia syndromes. J Clin Invest. 1979 Jun;63(6):1307–1310. doi: 10.1172/JCI109426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Higgs D. R., Clegg J. B., Weatherball D. J., Marsh G. W. Determination of alpha thalassaemia phenotypes by messenger RNA analysis. Br J Haematol. 1980 May;45(1):53–64. doi: 10.1111/j.1365-2141.1980.tb03810.x. [DOI] [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAZAWA Y., THOMAS C. A., Jr NUCLEOTIDE COMPOSITION OF SHORT SEGMENTS OF DNA MOLECULES. J Mol Biol. 1965 Feb;11:223–237. doi: 10.1016/s0022-2836(65)80053-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Hemoglobin switching in sheep and goats: change in functional globin messenger RNA in reticulocytes and bone marrow cells. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2184–2188. doi: 10.1073/pnas.69.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Michelson A. Partial deletion of the alpha-globin structural gene in human alpha-thalassaemia. Nature. 1980 Jul 31;286(5772):538–540. doi: 10.1038/286538a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. The duplicated human alpha globin genes lie close together in cellular DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5950–5954. doi: 10.1073/pnas.75.12.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G. Sequence of amino acids in the major and minor chains of the diffuse hemoglobin from BALB-c mice. Biochim Biophys Acta. 1973 Mar 23;303(1):61–67. doi: 10.1016/0005-2795(73)90148-7. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Bradshaw B. S., Skow L. C. Effects of alpha thalassemia on mouse development. Differentiation. 1980;17(3):205–210. doi: 10.1111/j.1432-0436.1980.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Enlow M. K. Radiation-induced alpha-thalassemia in mice. Am J Vet Res. 1977 May;38(5):569–572. [PubMed] [Google Scholar]
- Popp R. A., Francis M. C., Bradshaw B. S. Erythrocyte life span in alpha thalassemic mice. Birth Defects Orig Artic Ser. 1978;14(1):181–185. [PubMed] [Google Scholar]
- Popp R. A. Hemoglobins of mice: sequence and possible ambiguity at one position of the alpha chain. J Mol Biol. 1967 Jul 14;27(1):9–16. doi: 10.1016/0022-2836(67)90347-6. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Skow L. C., Whitney J. B., 3rd Expression of embryonic hemoglobin genes in alpha-thalassemic and in beta-duplication mice. Ann N Y Acad Sci. 1980;344:280–283. doi: 10.1111/j.1749-6632.1980.tb33668.x. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Stratton L. P., Hawley D. K., Effron K. Hemoglobin of mice with radiation-induced mutations at the hemoglobin loci. J Mol Biol. 1979 Jan 15;127(2):141–148. doi: 10.1016/0022-2836(79)90235-3. [DOI] [PubMed] [Google Scholar]
- Pressley L., Higgs D. R., Aldridge B., Metaxatou-Mavromati A., Clegg J. B., Weatherall D. J. Characterisation of a new alpha thalassemia 1 defect due to a partial deletion of the alpha globin gene complex. Nucleic Acids Res. 1980 Nov 11;8(21):4889–4898. doi: 10.1093/nar/8.21.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILEY V. Adaptation of orbital bleeding technic to rapid serial blood studies. Proc Soc Exp Biol Med. 1960 Aug-Sep;104:751–754. doi: 10.3181/00379727-104-25975. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell L. B., Russell W. L., Kelly E. M. Analysis of the albino-locus region of the mouse. I. Origin and viability. Genetics. 1979 Jan;91(1):127–139. doi: 10.1093/genetics/91.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Russell W. L., Popp R. A., Vaughan C., Jacobson K. B. Radiation-induced mutations at mouse hemoglobin loci. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2843–2846. doi: 10.1073/pnas.73.8.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- Weaver S., Haigwood N. L., Hutchison C. A., 3rd, Edgell M. H. DNA fragments of the Mus musculus beta globin haplotypes Hbbs and Hbbd. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1385–1389. doi: 10.1073/pnas.76.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. B., 3rd, Russell E. S. Linkage of genes for adult alpha-globin and embryonic alpha-like globin chains. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1087–1090. doi: 10.1073/pnas.77.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Nienhuis A. W., Anderson W. F. Selective activation of human beta-but not gamma-globin gene in human fibroblast x mouse erythroleukaemia cell hybrids. Nature. 1979 Feb 15;277(5697):534–538. doi: 10.1038/277534a0. [DOI] [PubMed] [Google Scholar]