Dear Editor,
Transplantation of autologous hematopoietic stem/progenitor cells (HSPCs) derived from the adult peripheral blood has been widely used in the treatment of various hematological diseases 1. However, the small number of circulating HSPC is the major limitation and necessitates additional interventions such as G-CSF mobilization and leukapheresis. There have been several attempts to overcome the limitation with ex vivo expansion of HSPC. These strategies are largely based on supplementation of one or more “stem cell niche components” such as supporting-cells, growth factors, extracellular matrix (ECM) or physicochemical microenvironment in the bone marrow 2. Spheroid culture methods of stem cells from different tissues have been successfully used for expansion of cardiac and neural stem cells. These spheres sensitize target stem cells to growth factors and provide sufficient cell-to-cell and cell-to-matrix contacts, mimicking the in vivo stem cell niche 3, 4. Here we asked whether spheroid culture of blood mononuclear cells (MNCs) would potentiate the expansion of circulating blood HSPC.
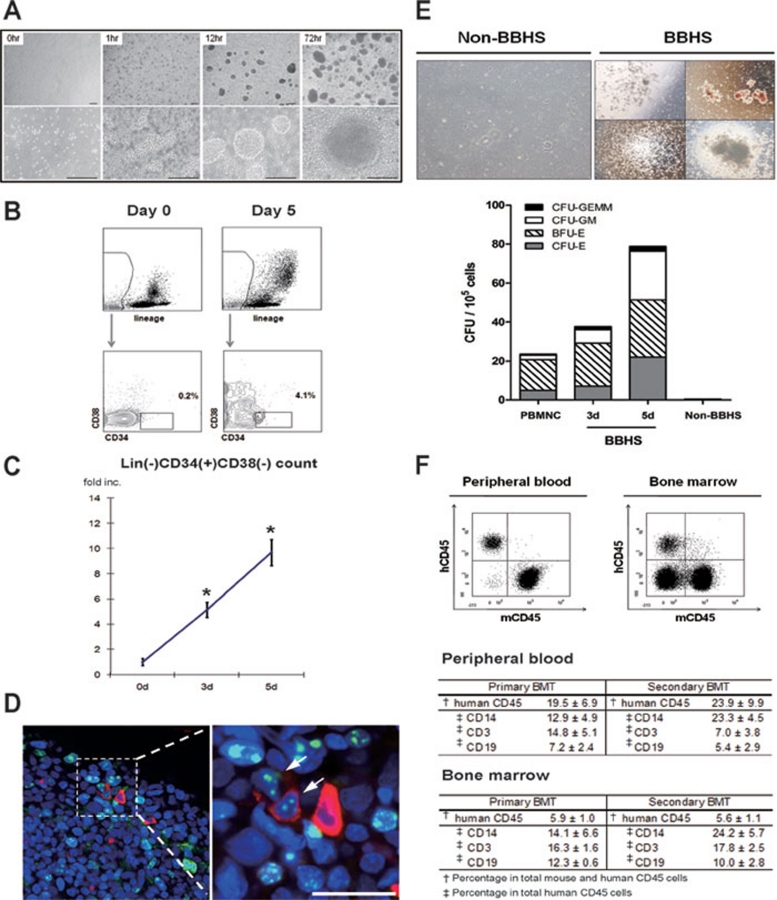
We isolated MNCs from peripheral blood of healthy donors without mobilization conditioning (Supplementary information, Table S1) 5. We found that MNCs organized into spheres spontaneously within 24 h when suspended on an ultra-low attach surface (NUNC) at a relatively high cell density (≥ 3 × 106 cells/ml; Figure 1A and Supplementary information, Figure S1A and S1B). These blood-born hematospheres (BBHSs) consisted of 5.0 ± 1.7 × 103 MNCs, and the diameters ranged from 50 to 250 μm with an average size of 219 ± 17 μm after 3 days when they reached a maximum count and formed spherical shape. Their sizes continued to increase gradually beyond this point and peaked on day 7 (Supplementary information, Figures S1C, S1D and S2). We used the basal EBM-2 media (Lonza) supplemented with 5% fetal bovine serum to remove the confounding effect of supplemented cytokines.
Figure 1.
(A) Optimized spheroid culture of MNCs. Each panel depicts representative field of BBHS culture at designated time point. Scale bars, 100 μm. (B) Identification of Lin(−)CD34(+)CD38(−) population in BBHS. The number indicates the percentage of CD34(+)CD38(−) population in Lin(−) population. (C) Expansion of Lin(−)CD34(+)CD38(−) population in BBHS. Absolute counts of Lin(−)CD34(+)CD38(−) HSPC in BBHS culture at day 3 and day 5 were normalized to HSPC count at the start of culture to calculate the expansion fold. These experiments were done nine times with MNCs from different donors. Asterisks (*) denote statistically significant change from day 0 (P < 0.05). (D) HSPC localization and proliferation in BBHS. Whole mount stained BBHS with CD34-positive cells (red) in proliferation (Ki-67, green). Arrows indicate double-positive cells. Nuclei were counterstained with To-Pro-3 (blue). Scale bar, 20 μm. (E) Expansion of functional hematopoietic progenitors exclusively in BBHS-spheres. CFU counts are shown for day 0 MNC, BBHS at day 3 and day 5. Non-BBHS cells were quantified on day 5. Top panel: left panel shows representative field of non-BBHS group in CFU assay. Bottom panel: CFU count of each group. (F) BBHS-derived HSPC with long-term hematopoietic repopulating capability in vivo. NOG-SCID mice were injected with single-suspended BBHS cells after partial bone marrow ablation (primary BMT, n = 4). After 12 weeks, peripheral blood, bone marrow and other organs were analyzed for hematopoietic cell chimerism and lineage differentiation. Top panel shows representative FACS results analyzing human and mouse CD45 chimerism. To investigate the long-term repopulating capacity of BBHS-derived HSPC, human HSPC from primary BMT mice were sorted and injected into NOG-SCID mice (secondary BMT, n = 2). Hematopoietic cell chimerism and human hematopoietic lineage differentiation were assessed as above after 12 weeks. Tables summarize the results. Numbers indicate the mean ± SEM of each experiment.
To evaluate the change of Lin(−)CD34(+)CD38(−) HSPC population, we analyzed peripheral blood MNCs and whole BBHS culture with flow cytometry at day 3 and day 5. Peripheral blood MNCs contained scanty amount of Lin(−)CD34(+)CD38(−) cells. However, at day 5 of BBHS culture, 4-6% of Lin(−) cells were CD34(+)CD38(−). The expansion fold of Lin(−)CD34(+)CD38(−) cells at day 3 and day 5 were 5.2 and 9.4, respectively. Culture of 2.0 × 107 MNCs, which usually correspond to 10 ml of peripheral blood from healthy donor, yielded 1.5 ± 0.2 × 105 Lin(−)CD34(+)CD38(−) cells at day 5 which correspond to 1.6% of total cells and 7.2% of the lineage-negative fraction (Figure 1B, 1C and Supplementary information, Figure S3).
As the culture contains BBHS spheres and suspending single cells, we then asked if the expanding HSPC population resided in BBHS spheres. When we stained BBHS, most of CD34(+) cells were Ki-67 positive, indicating active cell proliferation (Figure 1D and Supplementary information, Figure S4). Furthermore, when we compared the number of colony-forming progenitors of BBHS at day 3 and day 5 to freshly isolated MNCs at day 0, we observed that BBHS at day 3 and day 5 gave rise to 1.6- and 3.4-fold increases in total CFU (Figure 1E). In vitro CFU assay also showed that only cells from the spheres could give rise to hematopoietic colonies. The results confirm that functional HSPC can be expanded through BBHS culture and that they are exclusively present inside the BBHS.
We evaluated the hematopoietic function of the BBHS-derived HSPC in vivo with consecutive bone marrow repopulating experiment. NOD/Shi-scid IL2Rg(null) (NOG) mice were injected with single-cell suspension of spheres after partial bone marrow ablation (primary BMT) 6. At 12-week post-transplantation, peripheral blood, bone marrow-derived cells and splenocytes of xenotransplanted mice were analyzed for human and mouse hematopoietic cell chimerism. Notably, xenotransplanted human CD45(+) cells comprised 19.5 ± 6.9% of the hematopoietic pool of the recipient mice (Figure 1F and Supplementary information, Figure S5A). Further analysis of human CD45(+) cells showed that BBHS-derived HSPC gave rise to multiple hematopoietic lineages, such as T-, B-lymphoid and myeloid progeny (CD3: 14.8 ± 5.1%, CD19: 7.2 ± 2.4%, CD14: 12.9 ± 4.9%). To assess their long-term repopulating capacity, mouse and human Lin(−) HSCs, isolated from the primary bone marrow transplanted chimeric mouse, were transplanted into bone marrow-ablated NOG mice (secondary BMT). After 12 weeks, human and mouse hematopoietic chimerism was analyzed as above. The secondary transplanted NOG mice maintained mouse and human hematopoietic cell chimerism with similar hematopoietic lineage proportion (CD3: 7.0 ± 3.8%, CD19: 5.4 ± 2.9%, CD14: 23.3 ± 4.5%) and human cell engraftment was increased to 23.9 ± 9.9% in the secondary transplanted NOG mice (Figure 1F). The percentage of human CD45(+) cells in bone marrow is unusually lower than the percentage in peripheral blood in both transplantation experiments (primary BMT: 5.9%, secondary BMT: 5.6%). However, we could not find dominance of a specific lineage, which would suggest graft-versus-host-disease or malignant transformation. Interestingly, when we isolated MNCs from other potential homing sites, including liver, spleen, kidney and adipose tissue 7, we found higher percentage of human hematopoietic cell chimerism than in bone marrow or peripheral blood (Supplementary information, Figure S5). The above results confirm the existence of long-term SCID repopulating cells (SRCs) derived from unmobilized adult peripheral blood through BBHS spheres. However, quantitative analysis of SRC is required to confirm the expansion of functional HSPC in vivo. The unique organ preference of expanded HSPC as well as the source of expanded stem cells – unmobilized adult peripheral blood – distinguishes BBHS protocol from previous methods.
Here we described a new method that high-density suspension culture of peripheral blood MNCs results in spontaneous formation of hematopoietic cell spheroids (Materials and Methods are described in Supplementary information, Data S1). The culture system, which we termed BBHS, potentiates the expansion of rare circulating Lin(−)CD34(+)CD38(−) HSPC population in the sphere. The expanded HSPC remained functional in vitro and in vivo. BBHS culture expands phenotypic Lin(−)CD34(+)CD38(−) cells by average 10-fold and yields 6-fold expansion of colony-forming progenitors. Most of the attempts to expand human hematopoietic stem cells utilized cytokine cocktails, yielding up to 20-fold of SRC expansion 8, 9. However, comparing the expansion fold head-to-head would be inappropriate as the previous studies all used CD34(+)-enriched cord blood cells. Recently, Boitano et al. 10 described a novel molecule StemRegenin 1, which promoted 17-fold increase in SRC and 47-fold increase in mobilized peripheral blood CD34(+) cells in 5 weeks. The usage of unmobilized and unfractionated adult peripheral blood might have lowered the expanding potency of our protocol. Nevertheless, several aspects underscore the value of our method, such as, the simplicity of sampling procedure, short period of ex vivo culture and usage of autologous source.
To eliminate the differentiating effect of FBS on HSPC and to facilitate its potential clinical application, various kinds of serum-free media were tested for BBHS formation (Supplementary information, Figure S6). Among these media, we compared two commercially available HSPC support basal media to EBM/5% FBS. Of the two defined HSPC support media, StemSpan H3000 resulted in greater number of mature BBHS spheres than Stemline-II. Interestingly, only StemSpan medium was superior to EBM/5% FBS in HSPC expansion (17.0- vs 11.5-fold increase in Lin(−)CD34(+)CD38(−) HSPC and 7.73- vs 3.35-fold increase in CFU). Considering that the media did not include supplements, these finding suggests that the close cell-to-cell interaction and the tight spheroid structure itself are sufficient to maintain HSPC proliferation (Supplementary information, Figures S7 and S8). Most HSPC expansion methods allow ex vivo culture up to 5 weeks. To further utilize BBHS, improvement of culture protocol is needed to lengthen the period of culture, which is currently limited to 2 weeks (Supplementary information, Figures S8 and S9).
The mechanism by which BBHS supports HSPC expansion remains largely elusive, although we have recently investigated several clues. The sphere is entirely consisted of CD45(+) hematopoietic cells and most of them are CD14(+) monocytes (Supplementary information, Figures S10 and S11). Interestingly, when we investigated how these monocytes of hematopoietic origin could support HSPC expansion, some of the well-established components of bone marrow stem cell niche were found to be mimicked in BBHS. During BBHS culture, SDF-1 and CXCR4 mRNA expression was increased at day 3 and 5. Interestingly, staining of BBHS showed that the distribution of each molecule was distinct from one another (Supplementary information, Figure S12). Since ECM is known as a principal component of stem cell niche, we examined ECM components in the spheres. Various ECM components, such as collagen I, III, IV and laminin were present in BBHS. Fibronectin, one of the most important ECM to support HSPC, was profoundly secreted in the sphere and most BBHS cells expressed its receptor, Integrin αVβ1 (Supplementary information, Figures S13 and S14). The mRNA expression levels of ECM and integrins were also up-regulated during early BBHS culture (Supplementary information, Figure S15). As BBHS is composed of heterogeneous hematopoietic cell population, the comprehensive picture of the underlying interactions would be too complex to fully unveil. Thus, future investigation would be directed to dissecting the sphere with the help of marker-based sorting, finding the authentic niche-supporting population and delineating the major interaction between HSPC and the supporting hematopoietic niche.
Acknowledgments
We thank Suyeon Kim for her technical assistance. This study was supported by grants from the National Research Foundation funded by the Korea Government (MEST) (2010-0020257) and from the Innovative Research Institute for Cell Therapy, Republic of Korea. Dr Hyo-Soo Kim is also a professor of Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, sponsored by World Class University Program from the Ministry of Education, Science, and Technology of Korea.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Donor information on study PBMNC
Optimized spheroid culture of MNCs.
Morphology of typical BBHS with different sizes.
Absolute count of Lin(-)CD34(+)CD38(-) HSPC of individual donors during BBHS culture.
HSPC localization and expansion in BBHS.
BBHS-derived HSPC engraftment of solid organs.
Comparison of different media without serum supplement for BBHS culture.
Comparison of defined hematopoietic support media with EBM5%FBS. Sphere fromation in BBHS culture and CFU quantification.
Comparison of Lin(-)CD34(+)CD38(-) HSC expansion with defined hematopoietic support media.
Morphology of BBHS during 14-day culture
Whole-mount staining of BBHS with panleukocyte marker CD45.
Whole-mount staining of BBHS.
(A) CXCR4 expression and SDF-1 secretion of BBHS on day 5.
Whole-mount immunostaining of BBHSs.
Expression of fibronectin receptor Integrin αVβ1 in BBHS.
BBHS culture up-regulates gene expression of niche components.
Materials and methods
References
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Kirouac DC, Zandstra PW. Understanding cellular networks to improve hematopoietic stem cell expansion cultures. Curr Opin Biotechnol. 2006;17:538–547. doi: 10.1016/j.copbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, He X, Wilkie N, et al. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotech. 2001;19:475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Suemizu H, Yagihashi C, Mizushima T, et al. Establishing EGFP congenic mice in a NOD/Shi-scid IL2Rg(null) (NOG) genetic background using a marker-assisted selection protocol (MASP) Exp Anim. 2008;57:471–477. doi: 10.1538/expanim.57.471. [DOI] [PubMed] [Google Scholar]
- Han J, Koh YJ, Moon HR, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115:957–964. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- Robinson S, Niu T, de Lima M, et al. Ex vivo expansion of umbilical cord blood. Cytotherapy. 2005;7:243–250. doi: 10.1080/14653240510027172. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Donor information on study PBMNC
Optimized spheroid culture of MNCs.
Morphology of typical BBHS with different sizes.
Absolute count of Lin(-)CD34(+)CD38(-) HSPC of individual donors during BBHS culture.
HSPC localization and expansion in BBHS.
BBHS-derived HSPC engraftment of solid organs.
Comparison of different media without serum supplement for BBHS culture.
Comparison of defined hematopoietic support media with EBM5%FBS. Sphere fromation in BBHS culture and CFU quantification.
Comparison of Lin(-)CD34(+)CD38(-) HSC expansion with defined hematopoietic support media.
Morphology of BBHS during 14-day culture
Whole-mount staining of BBHS with panleukocyte marker CD45.
Whole-mount staining of BBHS.
(A) CXCR4 expression and SDF-1 secretion of BBHS on day 5.
Whole-mount immunostaining of BBHSs.
Expression of fibronectin receptor Integrin αVβ1 in BBHS.
BBHS culture up-regulates gene expression of niche components.
Materials and methods