Abstract
Heme-copper oxidases (HCuOs) are the last components of the respiratory chain in mitochondria and many bacteria. They catalyze O2 reduction and couple it to the maintenance of a proton-motive force across the membrane in which they are embedded. In the mitochondrial-like, A family of HCuOs, there are two well established proton transfer pathways leading from the cytosol to the active site, the D and the K pathways. In the C family (cbb3) HCuOs, recent work indicated the use of only one pathway, analogous to the K pathway. In this work, we have studied the functional importance of the suggested entry point of this pathway, the Glu-25 (Rhodobacter sphaeroides cbb3 numbering) in the accessory subunit CcoP (E25P). We show that catalytic turnover is severely slowed in variants lacking the protonatable Glu-25. Furthermore, proton uptake from solution during oxidation of the fully reduced cbb3 by O2 is specifically and severely impaired when Glu-25 was exchanged for Ala or Gln, with rate constants 100–500 times slower than in wild type. Thus, our results support the role of E25P as the entry point to the proton pathway in cbb3 and that this pathway is the main proton pathway. This is in contrast to the A-type HCuOs, where the D (and not the K) pathway is used during O2 reduction. The cbb3 is in addition to O2 reduction capable of NO reduction, an activity that was largely retained in the E25P variants, consistent with a scenario where NO reduction in cbb3 uses protons from the periplasmic side of the membrane.
Keywords: glutamate, nitric oxide, oxygen reduction
Heme-copper oxidases (HCuOs) are terminal oxidases catalyzing the reduction of oxygen to water (see 1), the last reaction of the respiratory chain. The HCuOs are located in the mitochondrial inner membrane in eukaryotes or the inner cell membrane in bacteria. HCuOs couple the exergonic O2 reduction to the maintenance of a proton electrochemical gradient across the membrane. This is achieved in two ways: First, electrons and protons used to reduce O2 to H2O (1) are derived from opposite sides of the membrane—electrons from donors (often a cyt. c) in the “outside” solution and protons from the “inside.” Second, protons are pumped through the protein across the membrane (2). In A-type HCuOs (see below), four protons are translocated across the membrane for every oxygen reduced to water (i.e., in 2, n = 4), but this number appears variable in the HCuO family (1) (see below).
| [1] |
| [2] |
The HCuO superfamily has previously been divided into subfamilies denoted A, B, and C type (2, 3). The A family comprises the enzyme found in mitochondria and other well-studied aa3-type (the names aa3 or cbb3 refer to the types of hemes found in the complexes) HCuOs from, e.g., Rhodobacter (R.) sphaeroides (4). The C family containing the cbb3 oxidases are found strictly in bacteria, and the cbb3 complex has been purified and characterized from, e.g., R. sphaeroides (5, 6) and Pseudomonas (P.) stutzeri (7). Also, bacterial NO-reductases (NOR), reducing NO to N2O (2NO + 2H+ + 2e- → N2O + H2O), belong to the HCuO family (8) and form a distinct subfamily. Among the O2-reducing HCuOs, the cbb3 type is the closest relative to NOR, and interestingly, cbb3-type oxidases, in contrast to A-type, have substantial NO-reduction activities (9, 10).
The A-type oxidases contain, in their catalytic subunit I, a low-spin heme a and a high-spin heme a3-CuB active site. Subunit II is a membrane-anchored protein, containing an additional redox cofactor, CuA, which is the initial acceptor of electrons from soluble cyt. c. Protons are transferred through two defined pathways from the negative (N, or in) side of the membrane up to the catalytic site, the D and the K pathways. The D pathway leads from an aspartate (D-132, R. sphaeroides aa3 numbering) at the cytoplasmic surface up to a glutamate (Glu-286) close to CuB, and the K pathway leads through a lysine (K-362) up to the active site. The D pathway transfers most of the protons, both for oxygen reduction and pumping, and the K pathway is used for only one or two protons that are taken up during reduction of the active site (11, 12).
Heme-copper oxidases of the C (or cbb3) type are generally expressed under low oxygen tension and have a lower Km for O2 than A-type HCuOs (13). In cbb3 the catalytic subunit CcoN contains the high-spin heme b3-CuB catalytic site and a low-spin heme b (for reviews on cbb3, see refs. 14 and 15). CcoO is a membrane-anchored protein containing one c-type heme. CcoP, anchored to the membrane via one transmembrane helix, contains two c-type hemes and is presumed to accept electrons from a soluble cyt. c. Very recently, the three-dimensional structure of the cbb3 oxidase from P. stutzeri was solved at 3.2-Å resolution (16). The residues constituting the D pathway are missing in cbb3s and cbb3s presumably have only one pathway (3, 16), spatially analogous to the K pathway in the A type, for delivering protons from the cytosol to the catalytic site. This feature is shared between the C- and B-type oxidases (17) and has been proposed to be linked to the lower pumping stoichiometry observed in these HCuOs (n = 2 in 2) (1, 18). In NOR, both the D and the K pathways are missing, and protons are supplied from the periplasmic side of the membrane in a reaction that does not conserve the energy available from NO reduction (19, 20).
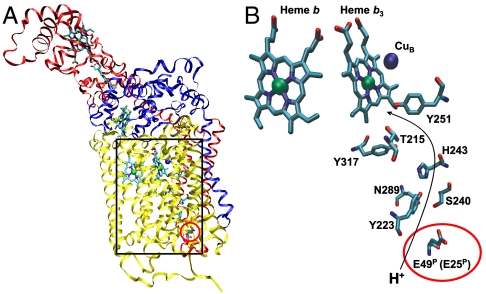
Fig. 1A shows the overall structure of the P. stutzeri cbb3 (16), and Fig. 1B shows a close-up of the suggested proton pathway (3, 16). The pathway is composed of residues entirely from the CcoN subunit, except for the suggested pathway entrance, which is at the Glu-49 in CcoP (corresponding to E-25 in the R. sphaeroides CcoP sequence). The pathway extends via a cluster of polar residues (Tyr-223, Ser-240, His-243, Tyr-317, and Thr-215, all conserved in R. sphaeroides cbb3; see Fig. S1) up to the Tyr-251, which is cross-linked to one of the histidine ligands to CuB (21) and is approximately 4 Å from the O2 binding site (16).
Fig. 1.
(A) Overview of the structure of cytochrome cbb3 from P. stutzeri (16) with the CcoN, O, and P subunits (A, B, and C chains in PDB ID code 3MK7) in yellow, blue, and red, respectively. (B) An enlargement of the region around the equivalent of the K path for proton transfer with the residue under investigation in this study, the Glu(E)-49 (numbering is according to the P. stutzeri sequences, only the R. sphaeroides equivalent of Glu-49 (E-25) is indicated in parenthesis) of the CcoP subunit marked by a red circle. The figure was produced using the VMD software (47). For the numbers of the corresponding residues in R. sphaeroides cbb3, see Fig. S1.
For the A-type HCuOs, the detailed mechanisms of oxygen reduction and the coupled proton transfer to the catalytic site and across the membrane have been extensively investigated using the so-called “flow-flash” technique. In this technique, the fully reduced HCuO with carbon monoxide (CO) bound to the active site heme is mixed in a stopped-flow apparatus with an oxygenated solution. The reaction is initiated by a short laser flash (∼10 ns) breaking the photolabile Fe–CO bond and allowing binding of dioxygen and its subsequent reduction. This reaction can be studied by time-resolved spectroscopy using various detection techniques, which has provided detailed information about the catalytic cycle of the A-type HCuOs (see, e.g., refs. 22–26).
In cbb3, the complex behavior of CO binding (15, 27) as well as the overlapping spectra of the three c-type hemes (see Figs. S2 and S3) have hampered detailed kinetic studies using the flow-flash technique. From our previous study (10), it is nevertheless clear that flow-flash studies are possible, and the fully reduced wild-type cbb3 reacts with O2, all redox-active groups oxidize and protons are taken up in a reaction with a time constant of approximately 1 ms. Furthermore, our results indicated that when reducing NO, the cbb3, like NOR, uses protons from the periplasmic side of the membrane (10).
In this work, we have studied the role of the Glu-25 of the CcoP subunit (called E25P) in the R. sphaeroides cbb3 by using site-directed mutagenesis, exchanging the glutamate for either alanine (A), asparagine (Q), or lysine (K). We investigated the reaction between the fully reduced cbb3 variants with O2 using the flow-flash technique. Our results strongly support a crucial role for the Glu-25P in proton uptake to the active site during O2 reduction. Also, in contrast, NO reduction is largely unaffected by the E25P substitutions, consistent with using a different proton transfer pathway during this reaction. Furthermore, in the E25PA and Q, when proton transfer is slow, an oxygen intermediate not seen with wild type is formed.
Results
Steady-State Activity and Respiratory Control Ratios (RCR).
Table 1 shows the O2- and NO-reduction activities of the investigated Glu(E)-25P variants in comparison to wild type. The E25PA and Q variants show low O2 activity, approximately 5% of wild type, whereas the E25PK variant retains approximately 40% of wild-type activity. In NO-reduction activity, all E25P variants retain 35–50% of the wild-type activity.
Table 1.
Comparison of activities and respiratory control ratio (RCR) of wild type and E25P variants before and after reconstitution
| In detergent | In vesicles | |||
| O2 (e-s-1) | NO (e-s-1) | O2 (%) | O2 RCR | |
| WT | 208 ± 4 (100%) | 2.3 ± 0.5 (100%) | 100 | 7 |
| E25K | 83 ± 0.5 (40%) | 0.8 ± 0.1 (35%) | 55 | 7 |
| E25A | 13 ± 0.4 (6%) | 1.1 ± 0.1 (48%) | 12 | 4 |
| E25Q | 10 ± 0.5 (5%) | 0.9 ± 0.1 (39%) | 12 | 5 |
The RCR, measured as the ratio of the O2-reduction activity of the liposome-reconstituted cbb3 in the presence and absence of uncouplers, was approximately 7 for wild-type cbb3. The RCR is expected to be lower in variants with a lower steady-state activity, because with a low activity, the rate of potential generation competes with the rate of proton back leak generating a smaller potential across the membrane. With this in mind, the RCRs were largely unaffected in the E25P variants; 7 for E25PK, 4 for E25PA, and 5 for E25PQ (see Table 1). We also note that there is a small increase in relative activity in liposomes for E25A and E25Q from approximately 5% of that for wild type in detergent to approximately 12% in (uncoupled) liposomes, possibly due to a small “rescuing” effect—i.e., the phospholipids acting as proton shuttles instead of the missing E25.
Optical Spectra and CO Recombination to the Fully Reduced cbb3.
The optical spectrum of the fully reduced wildtype and variant cbb3 oxidases were essentially the same (Fig. S2), showing that all heme groups are incorporated as in wild type.
CO recombination after flash photolysis of the fully reduced CO-bound cbb3 was studied as an indicator of the integrity of the active site. The results for wild-type and E25P variants, studied at 430 nm, are shown in Fig. S3. After flash photolysis, the solubilized wild-type cbb3 shows CO rebinding both to heme b3 and to one of the c-type hemes (15, 27), where the rapid phase represents the recombination to the c-type heme and the slow phase represents the b3-CO recombination. The E25P-A and -Q variants show essentially the same rates and relative amplitudes of these phases, indicating that CO binding is unchanged in these mutants. In the E25K, there is an increase in the relative degree of CO binding to the c-type heme. The reason for this is unknown, but because the change from glutamate to lysine represents a change in charge at the surface of the protein, it is perhaps related to the observation that changes in the lipid composition in liposome-reconstituted cbb3 can lead to changes in the relative c-type CO binding (15). It should be noted that these properties are unrelated to the turnover rates because the E25PK variant had the highest O2 reduction activity of the three E25P variants (see above).
Reaction of Fully Reduced cbb3 with O2.
We previously described the reaction between fully reduced wild-type R. sphaeroides cbb3 reconstituted into vesicles and O2 (10), where the reaction is characterized by a major oxidation phase (of all heme groups) with a time constant (τ) = 1 ms. In this work the solubilized cbb3 was studied, and the O2 concentration was 1 mM instead of 0.6 mM in the previous study. These changes made small changes in the reaction observed, and instead of one component, the reaction of wild-type cbb3 was fitted with two consecutive reaction steps with rate constants of k1 = 11,000 s-1 (τ = 90 μs) and k2 = 1,300 s-1 (τ = 0.8 ms). The faster process presumably represents oxygen binding to heme b3, because its kinetic difference spectrum (Fig. S4) is similar to that of O2 binding to cNOR (28), which also has a b3 heme in the active site. The k2 = 1,300 s-1 (τ = 0.8 ms) phase presumably corresponds to oxidation of all heme groups (see Fig. 2).
Fig. 2.
Reaction of the fully reduced wild type and E25P variant cbb3s with O2. A laser flash initiates the reaction at t = 0. Shown are the absorbance changes at (A) 420, (B) 430, (C) 550 (reporting oxidation of the c-type hemes) and (D) 560 nm (reporting on the low-spin heme b). Experimental conditions (after mixing): approximately 1 μM cbb3, 50 mM Hepes pH 7.5, 50 mM KCl, 0.03% DDM, [O2] = 1 mM, T = 298 K. The traces have been normalized using the initial absorbance change (from the CO dissociation) at 420 nm. E shows proton uptake from solution during the reaction of fully reduced wild type and E25P variants with O2. Experimental conditions: approximately 1 μM cbb3, 50 mM KCl, 50 μM EDTA, 0.03% DDM, 40 μM phenol red, [O2] = 1 mM, T = 298 K. Shown are the differences between the traces obtained in the absence of buffer and the ones obtained after adding 20 mM Hepes, pH 7.5. The traces for the different cbb3 variants have been normalised to the same absorbance change after 800 ms to emphasize the difference in rates.
The rapid k1 = 11,000 s-1 phase is essentially unchanged both in rate constant and kinetic difference spectrum in all E25P variants (Fig. S4); k varies between 12,000 s-1 and 19,000 s-1. There is also a reaction with a time constant in the ms range (k2 varying between 1,200 s-1 and 1,900 s-1) in all E25P variants (see Fig. 2), and its relative contribution at the wavelengths shown in Fig. 2 are essentially unchanged in the E25PK. However, in the E25PA and Q variants, in this phase only the heme b and presumably the binuclear site is oxidized (see Fig. 2 B and D) whereas it contains no contribution from oxidation of the low-spin hemes c (see Fig. 2 A and C), which occurs much later, with time constants of approximately 50 ms and 300 ms, in E25PA and Q, respectively. The extent of oxidation of heme b is similar in all variants (Fig. 2D), whereas in E25PA, the total amplitude of heme c oxidation is about 50% of that in wild type. In wild type, there is an additional phase with a τ ∼ 15 ms, which varies in amplitude between experiments, that we haven’t assigned to a specific reaction at present. We also note that in E25PK, on the slow time scale at 420 nm there is a signal with positive amplitude with τ ∼ 180 ms. The origin of this reaction has not been investigated but might be due to CO rebinding in a fraction of E25PK.
Proton Uptake from Solution During the Reaction of Fully Reduced cbb3 with O2.
In the wild-type R. sphaeroides cbb3, protons are taken up from solution with k = 1,800 s-1 (τ = 0.55 ms), approximately concomitantly with the τ = 0.8 ms phase of heme oxidation (see Fig. 2E and ref. 10). As also shown in Fig. 2E, the E25PK variant shows an essentially wild-type rate of proton uptake, whereas in the E25PA and Q variants, proton uptake is severely inhibited, occurring with k = 20 s-1 (τ = 50 ms) and 3 s-1 (τ = 300 ms), respectively, concomitant with the oxidation of the c-type hemes (Fig. 2 A and C).
Discussion
Proton Transfer in cbb3.
In contrast to the D and K pathways for proton transfer in the A-type HCuOs, the C family was suggested to have only one functionally important proton transfer pathway with a spatial location similar to the K pathway in A-type oxidases (3, 16). Even within cbb3 subfamilies, no conserved residues that could form a second pathway are found (3). Although water molecules alone can form a proton pathway, the proton pathways found in membrane proteins all have at least a few polar/ionizable side chains, presumably in order to make the pathways specific for protons rather than other cations (29). In the A-type HCuOs the two different pathways (D and K) are used for proton transfer during different partial steps in the catalytic cycle; the D pathway is used for protons taken up during the oxidative phase (starting with the fully reduced enzyme), both the protons used in oxygen reduction and the pumped protons, a total of 6–7 H+ per O2 turnover. The K pathway is used for protons (1–2) taken up during reduction (see, e.g., refs. 23 and 30).
Fig. 1B shows a close-up of the suggested proton pathway in P. stutzeri cbb3 (3, 16). The pathway is composed of residues entirely from the CcoN subunit, except for the pathway entrance, which is at the Glu-49 (corresponding to Glu-25, called E25P, in the R. sphaeroides cbb3) in the CcoP subunit. Note that all residues suggested to form part of the proton transfer pathway in the P. stutzeri cbb3 are conserved to the R. sphaeroides cbb3 (see alignment in Fig. S1). This study investigated the role of the E25P by site-directed mutagenesis changing the Glu-25 into lysine, alanine, or glutamine, respectively. Our results show that in the E25PA and Q, catalytic O2-reduction activity is severely slowed, whereas it is largely retained in the E25PK variant. Furthermore, our time-resolved measurements show that rate constants for proton uptake during O2 reduction by the fully reduced cbb3 are severely slowed (see Fig. 2E) in the E25PA and Q variants, by factors of approximately 100 and 500, respectively, coupled to oxidation of the low-spin hemes c (Fig. 2 A and C). Because optical spectra, rates of CO and O2 binding (see Figs. S2–S4), as well as the rate constant for oxidation of the low-spin heme b (see Fig. 2D) are wild-type-like in E25PA and Q, this is a specific effect on proton transfer. In E25PK, the heme oxidation as well as proton uptake rate constants were unaffected. These results show that the amino acid at position 25 in CcoP needs to be protonatable for efficient proton transfer through the pathway but that a lysine can fulfill the role of the glutamate. It should be noted, however, that proton transfer per se could be slowed in the E25PK because the 1 ms phase in wild-type cbb3 is presumably not limited by the rate of proton transfer (15). Taken together, our results show that the suggested K pathway equivalent in cbb3 is the main pathway used during O2 reduction, thus fulfilling the role of the D pathway in A-type HCuOs. Also, our results show that in the E25PA and Q variants, even though the uptake of protons is severely slowed, it still occurs through the same path (from the inside), because the RCRs are still approximately 5 in the liposome-reconstituted E25PA and Q variants.
In the ba3 HCuO from T. thermophilus, which belongs to the B family, it was also suggested that there is only one proton pathway spatially equivalent to the K pathway (17), and this pathway was shown to be used during the oxidation of the fully reduced enzyme (31) and also during reduction of the enzyme (17), supporting this pathway being the only functional proton pathway.
Despite lacking the D pathway responsible for conducting the pumped protons in the A-type oxidases, the C and B oxidases have been shown to pump protons (1, 18, 32–35) but with a lower stoichiometry than in A-type HCuOs (1, 18, 35, but see refs. 32 and 34 where n = 4 was obtained on the whole cell level), suggesting that there is a link between using only one pathway and pumping fewer protons (1).
The lack of a D pathway in the B- and C-type oxidases might be linked to their higher O2 affinity because the hydrophilic D pathway overlaps with the suggested hydrophobic path for O2 (36–39). As the results in this study adds evidence that the C-type HCuOs, like the B type, uses only one proton transfer pathway, it appears that the A type is the “special case,” with two specialized proton transfer pathways where the D pathway can overlap with the path for oxygen delivery because the A-type oxidases work under conditions of higher O2 tensions.
In cNOR, even though NO reduction is similarly exergonic to O2 reduction, NORs do not pump protons, and substrate protons are taken from the periplasmic solution (19, 20, 40), as supported also by the recent crystal structure of cNOR from Pseudomonas aeruginosa, showing several possible proton pathways from the periplasm but none from the cytoplasm (41). The cbb3 oxidases from R. sphaeroides and P. stutzeri have been found to catalyze NO reduction (9, 10), and we recently investigated the coupling of NO reduction to proton translocation in the cbb3s by using the flow-flash technique in combination with both optical and electrometric detection in liposome-reconstituted cbb3 (10). When comparing the reaction of the fully reduced cbb3 with either O2 or NO, we found that oxidation of heme groups in cbb3 proceeded to the same extent with the two substrates, but that the buildup of electrical potential was much smaller with NO. This presumably means that no protons are pumped with NO, as is the case for NO reduction by NOR. Furthermore, our data was consistent with the chemical protons coming from the “outside.” The suggestion that NO reduction uses a different proton pathway than O2 reduction in cbb3 oxidases is consistent with the result in this study that the NO reduction is not significantly affected by the loss of the protonatable group at E25P, the E25PA and Q show 40–50% of wild-type NO-reduction activity, whereas O2 reduction is severely impaired with rates of approximately 5% of that in wild type. An alternative explanation of why O2 but not NO-reduction rates are affected in the E25P variants is that the much slower NO reduction is limited by a reaction much slower than the rate of proton transfer and hence unaffected in the E25P variants. Time-resolved studies aimed at clarifying this issue are under way in our laboratory.
O2 Reduction in cbb3.
In the aa3-type oxidases, when starting with the fully reduced enzyme, O2 reduction proceeds via a series of intermediates (see, e.g., ref. 42); first O2 binds to heme a3 with a time constant of approximately 10 μs (at 1 mM O2) forming the so-called A intermediate. The O–O bond is then reduced by four electrons in a single step forming the so-called peroxy (P) intermediate with a time constant of approximately 30 μs. Then, a proton is taken up (through the D pathway) with a time constant of 100 μs, forming the ferryl (F) intermediate, which is further reduced with a time constant of approximately 1 ms to form the oxidized (O) intermediate by transfer of the last electron (from CuA) together with a second proton (through the D pathway).
The fully reduced cbb3 contains six available electrons (on the two c hemes in CcoP, the heme c in CcoO, on the low-spin heme b as well as on the high-spin heme b3 and the CuB in CcoN), whereas the complete reduction of O2 to H2O requires four electrons, which means that presumably two electrons remain somewhere in the protein after a single turnover, or that the end product is a two-electron reduced O2 intermediate. In the wild-type cbb3, there is a single major phase of heme oxidation (for all hemes) and proton uptake from solution with τ = 1 ms (10). In addition, in the current study, we identified the A (oxygen adduct) intermediate in solubilized cbb3 (see Fig. S4) that forms with a rate constant of approximately 11,000 s-1, but no other intermediates are identified.
This is the first study to look at specific reaction steps during the reaction between fully reduced variant cbb3s and O2, and in the E25PA/Q variants there is still a τ = 1 ms heme oxidation phase, but only the b heme (and presumably the active site) oxidises and no protons are taken up. Proton uptake occurs much slower with τ = 50/300 ms (in E25PA and E25PQ, respectively) coupled to oxidation of the c-type hemes (see Fig. 2 C and E). As indicated in the schematic in Fig. 3, the intermediate formed with τ = 1 ms in the E25PA/Q is thus on the same reduction level as the so-called “P” intermediate in A-type HCuOs, that is with three electrons transferred to the active site and no bulk protons taken up. This intermediate in the E25P variants will be further studied to learn more about the catalytic cycle in cbb3s.
Fig. 3.
Schematic picture summarizing the observed events in wild type and E25PA/Q variants during O2 reduction by the fully reduced enzymes. The letters c and b refer to the hemes in the CcoP, O, and N subunits. A filled circle indicates a reduced redox-active site, and an empty circle an oxidized site. Half-full circles indicate partially reduced sites. The R-O2, I, P, and I’ indicates the binuclear site in its different redox states; R-O2 for the oxygen adduct of the reduced state formed similarly in wild type and E25P variants (see Fig. S4), I for the uncharacterized intermediate formed upon oxidation of the fully reduced wild type; P for the intermediate formed with τ = 1 ms in the E25PA and Q variants; and I’ for the intermediate formed after proton/electron transfer in E25PA and Q, which is possibly different from the final intermediate in wild type. When E25P is exchanged for a nonprotonatable group, proton uptake through the K-path equivalent is severely slowed, and presumably becomes rate-limiting for oxidation of the low-spin hemes c (see text for details).
Materials and Methods
Site-Directed Mutagenesis.
The QuikChange site-directed mutagenesis kit (Stratagene) was used to introduce the mutations using mutagenic primers from Eurofins. pU12803NHIS (43) was cut into pRK415 and ccoNOPQ by enzyme digestion (BamHI-EcoRI). The BamHI-EcoRI fragment containing ccoNOPQ was subcloned to pBluescript SK+ for easier site-directed mutagenesis. The mutant containing the ccoNOPQ fragment was then ligated back to the pRK415 expression vector and introduced into S-17-1 cells by electroporation. The plasmid was transferred into R. sphaeroides Δcbb3 strain by conjugation. Restriction enzymes were from Fermentas. Sequencing was performed by Eurofins.
Cell Culture and Protein Purification.
The R. sphaeroides Δcbb3 strain carrying pUI2803NHIS with mutations was grown under semianaerobic condition, where 3 L flasks were filled with 2 L of Sistrom’s minimum media supplemented with 1 μg/mL tetracycline. The cell cultures were shaken at 150 rpm at 30 °C. His-tagged wild type and variant enzymes were purified by histidine affinity chromatography essentially as described in refs. 10 and 43 but with the following modifications: (i) 1 mM EDTA, 8 mM MgSO4, DNAse, and protease inhibitor cocktail was added to the homogenizing buffer (20 mM Tris-HCl, 100 mM NaCl at pH 8.0); (ii) the dodecyl maltoside (DDM) concentration was increased to a final concentration of 1% for the membrane solubilization; (iii) the enzyme bound to the Ni2+ column was eluted with 100 mM imidazole in 25 mM Hepes, 100 mM KCl, 0.01% DDM at pH 8.0.
Reconstitution of the cbb3 into Phospholipid Vesicles.
The R. sphaeroides cbb3 was reconstituted into vesicles as previously described (44). Briefly, purified soybean lipids (L-α-phosphatidylcholine, Sigma-Aldrich) were dissolved at the concentration of 80 mg/mL in 100 mM Hepes-KOH pH 7.4, and 2% cholic acid. Detergents were removed by adding biobeads over multiple steps as described. These treatments result in the incorporation of the enzyme into small unilamellar vesicles.
Steady-State Activity Measurement of O2 and NO Reduction.
The steady-state activities of cbb3 was determined by measuring the rate of O2 and NO reduction using a Clark-type electrode (Hansatech) with variable voltage setting as in ref. 45. The reaction chamber was filled (total of 1 mL) with 25 mM Hepes-KOH, pH 7.5, 100 mM KCl, 0.01% DDM, 5 mM ascorbate, 0.5 mM N,N,N’,N’-tetramethyl-p-phenylenediamine (TMPD), and 0.1 mM horse heart cytochrome c. For NO reduction, the buffer contained glucose (30 mM), glucose oxidase (1 unit/mL), catalase (20 units/mL) to remove any remaining O2. After removal of O2, the substrate NO was added by repeated injections of saturated NO buffer (2 mM) to a final concentration of 120 μM. Then the enzyme (50–200 nM) was added to initiate the reaction.
Flash-Photolysis Measurements.
The sample with a final cbb3 concentration of 1–5 μM was transferred to an anaerobic cuvette and the atmosphere was exchanged to N2 on a vacuum line. The sample was reduced with 2 mM ascorbate with 2 μM phenazine methosulphate (PMS) as mediator. The atmosphere was then exchanged to CO. The CO ligand was photolyzed by a 10 ns laser flash at 532 nm (Brilliant B, Quantel), followed by detection of absorbance changes by an apparatus from Applied Photophysics. See ref. 46 for a description of the equipment.
Flow-Flash Measurements; Optical Detection of Heme Oxidation and Proton Uptake.
Samples were prepared as for flash-photolysis measurements. Flow-flash measurements were performed as described in ref. 46. Briefly, fully reduced CO-bound cbb3 at a concentration of 5–10 μM in a buffer composed of 50 mM Hepes-KOH, 50 mM KCl, 0.03% DDM at pH 7.5, was mixed 1∶5, in a modified stopped-flow apparatus (Applied Photophysics), with an O2-saturated buffer of the same composition. About 200 ms after mixing the CO ligand was dissociated and the reaction with O2 was monitored optically as absorbance differences at single wavelengths.
The proton uptake measurements during oxidation of the cbb3 wild type and variants were performed essentially as described in ref. 10 with some modifications to the solutions. Briefly, buffer was removed in the solution by using a PD-10 column (Amersham Biosciences) equilibrated with 50 mM KCl, 0.03% DDM, 50 μM EDTA, pH approximately 7.6. Phenol red was added to 40 μM and the pH adjusted to approximately 7.6. Then the protein sample was reduced and CO was bound as described before. The O2 solution was prepared by bubbling O2 through 50 mM KCl, 50 μM EDTA, 0.03% DDM, and 40 μM phenol red, pH approximately 7.6. The pH change of phenol red in the solution was monitored at 570 nm. The buffered mixing solution containing 20 mM Hepes, 50 mM KCl, 50 μM EDTA, 0.03% DDM, pH 7.6, and 40 μM phenol red was used as control, measuring residual absorbance changes (unrelated to proton uptake) in cbb3 at 570 nm.
Supplementary Material
Acknowledgments.
H.J.L. is supported by a postdoctoral stipend from the Wenner-Gren foundation. P.Ä. is a Royal Swedish Academy of Sciences Research Fellow supported by a grant from the Knut and Alice Wallenberg Foundation. Our studies are supported by grants from the Swedish Research Council and the Faculty of Natural Sciences at Stockholm University.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107543108/-/DCSupplemental.
References
- 1.Han H, et al. Adaptation of aerobic respiration to low O2 environments. Proc Natl Acad Sci USA. 2011;108:14109–14114. doi: 10.1073/pnas.1018958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta. 2001;1505:185–208. doi: 10.1016/s0005-2728(01)00169-4. [DOI] [PubMed] [Google Scholar]
- 3.Hemp J, et al. Comparative genomics and site-directed mutagenesis support the existence of only one input channel for protons in the C-family (cbb(3) oxidase) of heme-copper oxygen reductases. Biochemistry. 2007;46:9963–9972. doi: 10.1021/bi700659y. [DOI] [PubMed] [Google Scholar]
- 4.Svensson-Ek M, et al. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J Mol Biol. 2002;321:329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Horsman JA, Berry E, Shapleigh JP, Alben JO, Gennis RB. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 6.Oh JI, Ko IJ, Kaplan S. Reconstitution of the Rhodobacter sphaeroides cbb3-PrrBA signal transduction pathway in vitro. Biochemistry. 2004;43:7915–7923. doi: 10.1021/bi0496440. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher RS, Cheesman MR, Watmough NJ. Molecular and spectroscopic analysis of the cytochrome cbb3 oxidase from Pseudomonas stutzeri. J Biol Chem. 2002;277:31474–31483. doi: 10.1074/jbc.M204103200. [DOI] [PubMed] [Google Scholar]
- 8.van der Oost J, et al. The heme-copper oxidase family consists of three distinct types of terminal oxidases and is related to nitric oxide reductase. FEMS Microbiol Lett. 1994;121:1–9. doi: 10.1111/j.1574-6968.1994.tb07067.x. [DOI] [PubMed] [Google Scholar]
- 9.Forte E, et al. The cytochrome cbb3 from Pseudomonas stutzeri displays nitric oxide reductase activity. Eur J Biochem. 2001;268:6486–6491. doi: 10.1046/j.0014-2956.2001.02597.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Reimann J, Lepp H, Drici N, Ädelroth P. Vectorial proton transfer coupled to reduction of O2 and NO by a heme-copper oxidase. Proc Natl Acad Sci USA. 2008;105:20257–20262. doi: 10.1073/pnas.0805429106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ädelroth P, Gennis RB, Brzezinski P. Role of the pathway through K(I-362) in proton transfer in cytochrome c oxidase from R. sphaeroides. Biochemistry. 1998;37:2470–2476. doi: 10.1021/bi971813b. [DOI] [PubMed] [Google Scholar]
- 12.Konstantinov AA, Siletsky S, Mitchell D, Kaulen A, Gennis RB. The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer. Proc Natl Acad Sci USA. 1997;94:9085–9090. doi: 10.1073/pnas.94.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preisig O, Zufferey R, Thöny-Meyer L, Appleby CA, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitcher RS, Watmough NJ. The bacterial cytochrome cbb3 oxidases. Biochim Biophys Acta. 2004;1655:388–399. doi: 10.1016/j.bbabio.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Reimann J, Singh LM, Ädelroth P. Substrate binding and the catalytic reactions in cbb(3)-type oxidases: The lipid membrane modulates ligand binding. Biochim Biophys Acta. 2010;1797:724–731. doi: 10.1016/j.bbabio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Buschmann S, et al. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science. 2010;329:327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 17.Chang HY, Hemp J, Chen Y, Fee JA, Gennis RB. The cytochrome ba3 oxygen reductase from Thermus thermophilus uses a single input channel for proton delivery to the active site and for proton pumping. Proc Natl Acad Sci USA. 2009;106:16169–16173. doi: 10.1073/pnas.0905264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arslan E, Kannt A, Thöny-Meyer L, Hennecke H. The symbiotically essential cbb(3)-type oxidase of Bradyrhizobium japonicum is a proton pump. FEBS Lett. 2000;470:7–10. doi: 10.1016/s0014-5793(00)01277-1. [DOI] [PubMed] [Google Scholar]
- 19.Hendriks JH, Jasaitis A, Saraste M, Verkhovsky MI. Proton and electron pathways in the bacterial nitric oxide reductase. Biochemistry. 2002;41:2331–2340. doi: 10.1021/bi0121050. [DOI] [PubMed] [Google Scholar]
- 20.Reimann J, Flock U, Lepp H, Honigmann A, Ädelroth P. A pathway for protons in nitric oxide reductase from Paracoccus denitrificans. Biochim Biophys Acta. 2007;1767:362–373. doi: 10.1016/j.bbabio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Rauhamäki V, Baumann M, Soliymani R, Puustinen A, Wikström M. Identification of a histidine-tyrosine cross-link in the active site of the cbb3-type cytochrome c oxidase from Rhodobacter sphaeroides. Proc Natl Acad Sci USA. 2006;103:16135–16140. doi: 10.1073/pnas.0606254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proshlyakov DA, Pressler MA, Babcock GT. Dioxygen activation and bond cleavage by mixed-valence cytochrome c oxidase. Proc Natl Acad Sci USA. 1998;95:8020–8025. doi: 10.1073/pnas.95.14.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brzezinski P, Ädelroth P. Pathways of proton transfer in cytochrome c oxidase. J Bioenerg Biomembr. 1998;30:99–107. doi: 10.1023/a:1020567729941. [DOI] [PubMed] [Google Scholar]
- 24.Verkhovsky MI, Morgan JE, Verkhovskaya ML, Wikström M. Translocation of electrical charge during a single turnover of cytochrome-c oxidase. Biochim Biophys Acta. 1997;1318:6–10. [Google Scholar]
- 25.Faxén K, Gilderson G, Ädelroth P, Brzezinski P. A mechanistic principle for proton pumping by cytochrome c oxidase. Nature. 2005;437:286–289. doi: 10.1038/nature03921. [DOI] [PubMed] [Google Scholar]
- 26.Belevich I, Bloch DA, Belevich N, Wikström M, Verkhovsky MI. Exploring the proton pump mechanism of cytochrome c oxidase in real time. Proc Natl Acad Sci USA. 2007;104:2685–2690. doi: 10.1073/pnas.0608794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitcher RS, Brittain T, Watmough NJ. Complex interactions of carbon monoxide with reduced cytochrome cbb(3) oxidase from Pseudomonas stutzeri. Biochemistry. 2003;42:11263–11271. doi: 10.1021/bi0343469. [DOI] [PubMed] [Google Scholar]
- 28.Flock U, Watmough NJ, Ädelroth P. Electron/proton coupling in bacterial nitric oxide reductase during reduction of oxygen. Biochemistry. 2005;44:10711–10719. doi: 10.1021/bi050524h. [DOI] [PubMed] [Google Scholar]
- 29.Wraight CA. Chance and design—proton transfer in water, channels and bioenergetic proteins. Biochim Biophys Acta. 2006;1757:886–912. doi: 10.1016/j.bbabio.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Wikström M, Jasaitis A, Backgren C, Puustinen A, Verkhovsky MI. The role of the D- and K-pathways of proton transfer in the function of the haem-copper oxidases. Biochim Biophys Acta. 2000;1459:514–20. doi: 10.1016/s0005-2728(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 31.Smirnova I, et al. Functional role of Thr-312 and Thr-315 in the proton-transfer pathway in ba3 Cytochrome c oxidase from Thermus thermophilus. Biochemistry. 2010;49:7033–7039. doi: 10.1021/bi100749p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raitio M, Wikström M. An alternative cytochrome oxidase of Paracoccus denitrificans functions as a proton pump. Biochim Biophys Acta. 1994;1186:100–106. [Google Scholar]
- 33.de Gier JW, et al. Structural and functional analysis of aa3-type and cbb3-type cytochrome c oxidases of Paracoccus denitrificans reveals significant differences in proton-pump design. Mol Microbiol. 1996;20:1247–1260. doi: 10.1111/j.1365-2958.1996.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 34.Toledo-Cuevas M, Barquera B, Gennis RB, Wikström M, Garcia-Horsman JA. The cbb3-type cytochrome c oxidase from Rhodobacter sphaeroides, a proton-pumping heme-copper oxidase. Biochim Biophys Acta. 1998;1365:421–434. doi: 10.1016/s0005-2728(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 35.Kannt A, et al. Electrical current generation and proton pumping catalyzed by the ba3-type cytochrome c oxidase from Thermus thermophilus. FEBS Lett. 1998;434:17–22. doi: 10.1016/s0014-5793(98)00942-9. [DOI] [PubMed] [Google Scholar]
- 36.Riistama S, et al. Channelling of dioxygen into the respiratory enzyme. Biochim Biophys Acta. 1996;1275:1–4. doi: 10.1016/0005-2728(96)00040-0. [DOI] [PubMed] [Google Scholar]
- 37.Riistama S, Puustinen A, Verkhovsky MI, Morgan JE, Wikström M. Binding of O(2) and its reduction are both retarded by replacement of valine 279 by isoleucine in cytochrome c oxidase from Paracoccus denitrificans. Biochemistry. 2000;39:6365–6372. doi: 10.1021/bi000123w. [DOI] [PubMed] [Google Scholar]
- 38.Soulimane T, et al. Structure and mechanism of the aberrant ba(3)-cytochrome c oxidase from thermus thermophilus. EMBO J. 2000;19:1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salomonsson L, Lee A, Gennis RB, Brzezinski P. A single-amino-acid lid renders a gas-tight compartment within a membrane-bound transporter. Proc Natl Acad Sci USA. 2004;101:11617–11621. doi: 10.1073/pnas.0402242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell LC, Richardson DJ, Ferguson SJ. Identification of nitric oxide reductase activity in Rhodobacter capsulatus: The electron transport pathway can either use or bypass both cytochrome c2 and the cytochrome bc1 complex. J Gen Microbiol. 1992;138:437–443. doi: 10.1099/00221287-138-3-437. [DOI] [PubMed] [Google Scholar]
- 41.Hino T, et al. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science. 2010;330:1666–1670. doi: 10.1126/science.1195591. [DOI] [PubMed] [Google Scholar]
- 42.Ädelroth P, Ek M, Brzezinski P. Factors determining electron-transfer rates in cytochrome c oxidase: investigation of the oxygen reaction in the R. sphaeroides enzyme. Biochim Biophys Acta. 1998;1367:107–117. doi: 10.1016/s0005-2728(98)00142-x. [DOI] [PubMed] [Google Scholar]
- 43.Oh JI, Kaplan S. Oxygen adaptation. The role of the CcoQ subunit of the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1. J Biol Chem. 2002;277:16220–16228. doi: 10.1074/jbc.M200198200. [DOI] [PubMed] [Google Scholar]
- 44.Lee HJ, Öjemyr L, Vakkasoglu A, Brzezinski P, Gennis RB. Properties of Arg481 mutants of the aa3-type cytochrome c oxidase from Rhodobacter sphaeroides suggest that neither R481 nor the nearby D-propionate of heme a3 is likely to be the proton loading site of the proton pump. Biochemistry. 2009;48:7123–31. doi: 10.1021/bi901015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendriks J, et al. The active site of the bacterial nitric oxide reductase is a dinuclear iron center. Biochemistry. 1998;37:13102–13109. doi: 10.1021/bi980943x. [DOI] [PubMed] [Google Scholar]
- 46.Brändén M, et al. On the role of the K-proton transfer pathway in cytochrome c oxidase. Proc Natl Acad Sci USA. 2001;98:5013–5018. doi: 10.1073/pnas.081088398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.