SUMMARY
Insulin-resistant, ‘type 2’ diabetes (T2D) results from a complex interplay between genes and environment. In particular, both caloric excess and obesity are strongly associated with T2D across many genetic backgrounds. To gain insights into how dietary excess affects insulin resistance, we studied the simple model organism Drosophila melanogaster. Larvae reared on a high-sugar diet were hyperglycemic, insulin resistant and accumulated fat – hallmarks of T2D – compared with those reared on control diets. Excess dietary sugars, but not fats or proteins, elicited insulin-resistant phenotypes. Expression of genes involved in lipogenesis, gluconeogenesis and β-oxidation was upregulated in high-sugar-fed larvae, as were FOXO targets, consistent with known mechanisms of insulin resistance in humans. These data establish a novel Drosophila model of diet-induced insulin resistance that bears strong similarity to the pathophysiology of T2D in humans.
INTRODUCTION
Diabetes mellitus is the fifth leading cause of death in the United States and the rate of diabetes continues to increase (Wild et al., 2004). Type 2 or insulin-resistant diabetes (T2D) is accompanied by a host of pathologies including obesity, cardiovascular disease, fatty liver, neuropathy, retinopathy and nephropathy. Insulin resistance develops as a result of several factors, including genetic susceptibility, obesity and diet (Muoio and Newgard, 2008). Large-scale genome-wide single nucleotide polymorphism (SNP) sequencing studies have identified susceptibility loci for T2D; together, these loci account for less than 10% of the risk of T2D (for a review, see Lusis et al., 2008). Obesity is the most obvious clinical risk factor for T2D in humans: 55% of T2D patients are obese (Mokdad et al., 2003), with caloric excess contributing to the recent epidemics of both obesity and T2D.
Most mouse, fly or worm models of T2D rely on single gene manipulations (e.g. of those encoding leptin, leptin receptor or insulin receptor) and provide insights into specific pathways of insulin resistance; however, monogenic forms of T2D are rare in humans. High-calorie diets are frequently used in rodents to elicit or enhance insulin resistance in mutants, but the ability of diet to induce T2D-like phenotypes in flies has not been described. Flies offer the ability to dissect the relative contributions of both genes and environment on metabolism. Drosophila has organs that are analogous to most of those involved in both human energy metabolism and the targets of diabetic complications: heart, brain, kidney (nephrocytes, Malpighian tubules), liver and adipose (fat body), gastrointestinal tract, and blood (hemolymph).
Metabolic regulation in Drosophila shows important similarities to mammals. Its genome contains conserved regulators of metabolism, including SirT, PGC-1α, FOXO, TOR, Akt and nuclear receptors (Baker and Thummel, 2007). Drosophila insulin-like peptides (DILPs) share sequence, structural and functional similarities with vertebrate insulin-like growth factor and insulin, regulating both growth and glucose homeostasis. DILPs and the glucagon analog adipokinetic hormone (AKH) function in the maintenance of hemolymph glucose levels in insects. Ablation of fly insulin-producing cells (IPCs), neuroendocrine cells that are analogous to pancreatic β-cells, leads to increased hemolymph sugar concentrations, modeling type 1 diabetes (Rulifson et al., 2002). By contrast, ablation of the corpora cardiaca, the organ that produces AKH, reduces the amount of hemolymph sugars (Kim and Rulifson, 2004). In this study, we demonstrate that a high-sugar diet induces phenotypes in Drosophila that model T2D: hyperglycemia, insulin resistance, and increased levels of triglycerides and free fatty acids.
RESULTS
We examined whether a high-calorie diet fed to developing larvae induced phenotypes that were consistent with insulin resistance. We used larvae for several reasons. First, larvae are constantly feeding, growing and storing energy, processes that are known to be controlled by the insulin signaling pathway; feeding rates are more reliable than in adults. Therefore, larvae served as a sensitive platform on which to identify relevant growth defects. Second, larvae are sexually immature, removing potentially confounding differences between adult male and female physiology. Third, one can study post-feeding physiology in wandering third instar larvae, and measure ‘fasting’ glucose levels and insulin sensitivity. In Drosophila, we define an insulin-resistant state as: hyperglycemia despite increased insulin production, blunted intracellular signaling response to insulin, and growth phenotypes consistent with the loss of insulin signaling, including developmental delay and small size.
A high-sugar diet induced hyperglycemia in Drosophila
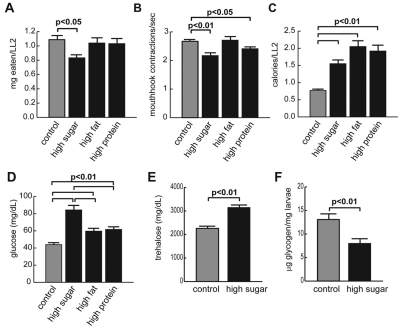
To examine diet-induced effects on metabolism, we fed wild-type Canton-S Drosophila a control diet (0.15 M sucrose) or high-caloric food containing high sugar (1 M sucrose), high fat or high protein, with all other ingredients kept constant (supplementary material Table S1). The high-sugar diet contained about 86.4% of calories from carbohydrates, similar in proportion to a banana (supplementary material Table S1, Fig. S1A) (Carsten et al., 2005). Larvae ate less calories on high-sugar compared with high-fat and -protein diets (Fig. 1A-C; supplementary material Table S1), but developed more severe hyperglycemia, or elevated hemolymph glucose, compared with other high-calorie diets (Fig. 1D). The concentration of trehalose, a glucose disaccharide synthesized from intracellular glucose in fat body cells and secreted into the hemolymph, was also increased in high-sugar-fed animals (Fig. 1E). The increase in trehalose was similar to that observed in both insulin-deficient and insulin-resistant Drosophila (Song et al., 2010; Rulifson et al., 2002). By contrast, the amount of glycogen decreased in high-sugar-fed larvae (Fig. 1F). The decrease in glycogen was similar to that observed in insulin pathway mutants (Bohni et al., 1999; Shingleton et al., 2005).
Fig. 1.
A high-sugar diet induces more severe hyperglycemia than do other high-calorie diets. Canton-S animals were fed a control diet or one of three high-calorie diets from embryo hatch until the post-feeding wandering L3 stage. The amount of food eaten by larvae on control, high-sugar, high-fat and high-protein foods was used to estimate the relative kilocalorie intake using the calorie data found in supplementary material Table S1. (A) Mass of each food consumed per larva from late L2 instar to the onset of metamorphosis on all foods. Developmental stage is used instead of time to account for the longer feeding time in high-sugar-fed larvae. (B) Number of mouth hook contractions per second in L3 larvae raised on each food. (C) Calories consumed on control, high-sugar, high-fat and high-protein diets. (D) Hemolymph glucose concentrations on all four diets in wandering L3 larvae; n>32. (E) Hemolymph trehalose levels; n≥3. (F) Whole body glycogen levels; n≥5. Error bars are ± s.e.m. An unpaired two-tailed t-test was used to derive all P-values.
High-sugar feeding resulted in insulin resistance
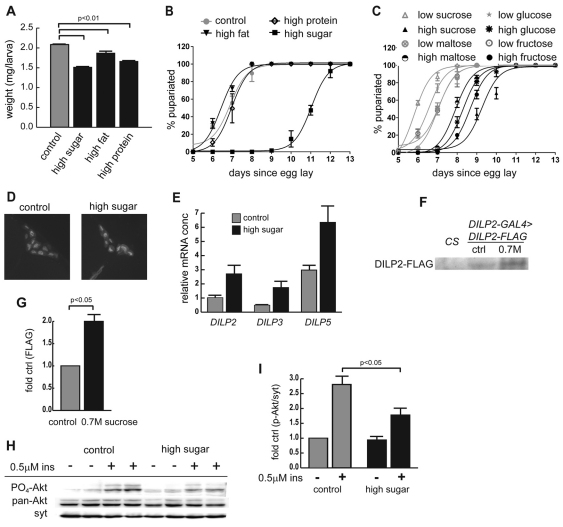
As a first step towards identifying the cause of hyperglycemia in high-sugar-fed larvae, we examined two phenotypes that are dependent on insulin signaling: developmental rate and larval size (Chen et al., 1996; Bohni et al., 1999; Shingleton et al., 2005). In the fly life cycle, larvae hatch from eggs and eat continuously, stopping only to molt twice after first instar (L1) and second instar (L2) stages. At 5–6 days after egg laying, third instar (L3) larvae leave the food and ‘wander’ as they prepare to undergo metamorphosis into the adult fly. Despite eating more calories, wild-type wandering L3 larvae raised on either high-sugar, high-fat or high-protein diets were reduced in size, with the greatest decrease in size observed for the high-sugar-fed animals (Fig. 2A). Adults reared on high-sugar food were also smaller than those reared on control food (data not shown). The decreases in both size and rate of larval development of the high-sugar-reared animals resembled those observed in mutations that reduced insulin receptor activity (Chen et al., 1996; Shingleton et al., 2005) or in IPC-ablated flies (Rulifson et al., 2002). Strikingly, high-sugar feeding resulted in a dramatic delay of 3–5 days in larval developmental rate when compared with those reared on control food (Fig. 2B). For the delay in the rate of development, the carbohydrate requirement was not strict, because glucose, fructose and maltose all produced similar results (Fig. 2C; supplementary material Fig. S1A-D). Of note, the disaccharides were present at half the osmolarity of the monosaccharides (1.0 vs 2.0 M), and thus the osmolarity of the food probably did not contribute to the delays observed on high sugar. To confirm that the phenotypes observed in larvae reared on elevated sucrose concentrations were not due to protein deficiency, we restored the protein composition of the diet to provide the same amount of protein per animal. This did not rescue any sugar-induced phenotypes (supplementary material Fig. S1E-F).
Fig. 2.
A high-sugar diet induces insulin resistance. Canton-S larvae were reared on control and high-calorie diets. (A) Wandering L3 weights; n≥15. (B) Developmental time course to pupariation on all four diets; n=3. (C) Mono- and disaccharide sugars similarly elicit developmental delay. Sugar concentrations were 0.15 M (low sugar) and 1.0 M (high sugar) for disaccharides sucrose and maltose, and 2.0 M (high sugar) for monosaccharides fructose and glucose; n=3. (D) Young adult brains harboring DILP2-GAL4>UAS-GFP are highlighted in a 3D reconstruction of confocal images; n>10 brains per condition. (E) Quantitative RT-PCR of DILPs in 2- to 4-day-old adult male heads after rearing on both diets. DILP2, DILP3 and DILP5 transcripts were measured using actin5c as a control. P=0.06 for DILP2, P=0.04 for DILP3 and P=0.05 for DILP5 between control and high sugar; n≥7. A two-tailed t-test was used to derive P-values. (F) Western blot of hemolymph DILP2-FLAG from control- and high (0.7 M)-sugar-reared DILP2-GAL4>UAS-DILP2-FLAG wandering L3 larvae. Equal amounts of hemolymph were loaded in each lane. Canton-S (CS) served as a negative control. (G) Quantitation of high-sugar and control D2-FLAG signal on n=4 western blots. (H) Dissected organs from L3 male larvae raised on control or high-sugar diets were treated with insulin (0.5 μM) or vehicle and visualized using antibodies against Drosophila PO4-Akt, pan-Akt or syntaxin. (I) Bands from five western blot experiments were quantified, and PO4-Akt was normalized to syntaxin as a loading control; n=5. A one-tailed t-test was used to derive P-values. All error bars are ± s.e.m.
Of the high-calorie diets, high-sugar feeding had the most striking effects on hyperglycemia, larval size and developmental rate, and we focused on this diet for the remainder of our studies. Except where noted, wandering L3 larvae were used to examine persistent physiological changes resulting from the high-sugar diet. At this stage, larvae have reached maximal size and are no longer feeding.
Both insulin-deficient and insulin-resistant Drosophila exhibit growth defects, because the insulin signaling pathway serves both insulin- and IGF-like functions in the fly (Baker and Thummel, 2007). We examined whether high-sugar feeding led to either insulin deficiency or peripheral insulin resistance. To assess IPC integrity, we raised DILP2-GAL4, UAS-GFP Drosophila on control or high-sugar food. No significant change in the number or morphology of IPCs was observed (Fig. 2D). However, expression of multiple genes encoding DILPs increased after chronic high-sugar feeding (Fig. 2E), suggesting that larvae attempted to compensate for the increased glycemic load by increasing DILP levels. Because insulin secretion by IPCs has been shown to be controlled by diet (Geminard et al., 2009), we evaluated the levels of circulating DILP using DILP2-GAL4, UAS-DILP2-FLAG larvae. Increased levels of FLAG-tagged DILP2 were found in the hemolymph of high-sugar-reared larvae compared with larvae raised on control food (Fig. 2F,G). These data demonstrated that the hemolymph of high-sugar-reared larvae was not insulin deficient, and suggested that a central defect upon high-sugar feeding was peripheral insulin resistance.
To assess insulin resistance, we tested the ability of exogenous insulin to stimulate the phosphorylation of Akt at position Ser505. This residue is homologous to Ser473 in mammals, which acts as a marker of insulin pathway activity. Wandering L3 larvae raised on a control diet responded to 0.5 μM recombinant human insulin with an average 2.8-fold increase in phospho-Akt. By contrast, larvae raised on a high-sugar diet displayed a significantly diminished response to insulin compared with controls (1.8-fold; Fig. 2H,I). Taken together, high-sugar-fed larvae demonstrate criteria for a model of T2D: hyperglycemia, insulin resistance and insulin-defective growth phenotypes.
Insulin-resistant Drosophila accumulate stored fat
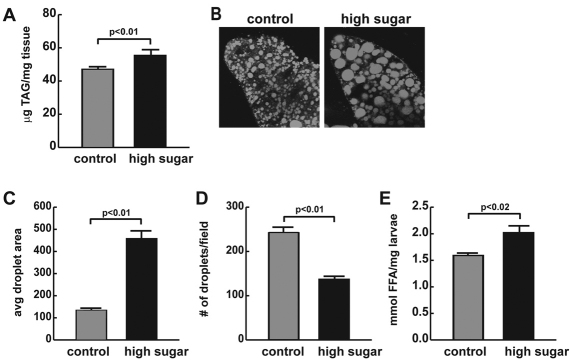
Insulin-resistant diabetes is often accompanied by obesity. To determine whether a high-sugar diet increased fat storage in Drosophila, we measured triglycerides (TAG), the main form of stored fat, in wandering L3 larvae. High-sugar-fed larvae experienced significant increases in percent body fat compared with normally fed animals (1.2 fold; Fig. 3A). The increase in TAG was similar to that observed in insulin pathway mutants (Bohni et al., 1999; Shingleton et al., 2005). The Drosophila fat body is the principal site of stored fat in the fly and functions as both adipose and liver. Although the amount of fat body tissue seemed to be unchanged, Nile Red staining revealed significantly larger lipid storage droplets in high-sugar-fed flies compared with control-fed animals, indicating a change in lipid storage in this organ upon high-sugar feeding (Fig. 3B,C). Droplets were larger but fewer in number in high-sugar-fed larvae (Fig. 3D), such that the total droplet area nearly doubled, and calculated volume was 2.7-fold increased compared with controls. In addition to the increased TAG levels, we also observed a significant increase in free fatty acids in high-sugar-fed animals (Fig. 3E).
Fig. 3.
High-sugar-fed insulin-resistant Drosophila accumulate stored fat. Wandering L3 larvae raised on either control or high (1 M)-sucrose diets were analyzed for fat content. (A) Total TAG levels were assayed enzymatically in control and high-sugar-fed larvae, and normalized to weight; n≥11. (B) Nile Red staining of control and high-sugar larval fat bodies. (C) Quantification of lipid droplet area in two-dimensional confocal slices; n≥15. (D) Lipid droplet numbers in control and high-sugar-fed larval fat bodies; n≥15. (E) Free fatty acid (FFA) levels in control and high-sugar-fed larvae; n≥3. Error bars are ± s.e.m. An unpaired two-tailed t-test was used to derive P-values.
High-sugar feeding alters transcriptional control of fat and carbohydrate metabolism
Given that many targets are regulated by insulin signaling at the level of transcription in a variety of organisms, we examined whether there was evidence of conserved mechanisms for diet-induced insulin resistance by using transcriptional profiling. We were also interested in discovering pathways that are candidates for contributing to the observed insulin resistance.
We explored the mechanisms of insulin resistance in high-sugar-fed Drosophila using microarray analysis on male Canton-S larvae that were fed control or high-sugar food and harvested. We tested both lifespan-fed wandering L3 and 12-hour control and high-sugar-fed mid-L3 staged larvae to allow us to compare the effects of diet in both feeding and post-feeding larvae (supplementary material Fig. S2). Shorter-term feeding also allowed us to control for the age of larvae because lifespan feeding delayed larval development by 2–3 days (Fig. 2B). High-sugar feeding affected transcription in a greater number of genes over the 12-hour feeding when compared with chronic feeding (823 genes vs 309) but the average fold change was much higher in chronic high sugar (3.6- and −7.1-fold for up- and downregulated genes in chronic high sugar vs 2.3- and −2.6-fold for up- and downregulated genes in 12-hour feeding, respectively; Fig. 4A and supplementary material Table S2). A significant fraction of genes regulated by high-sugar feeding overlapped between these two data sets (Fig. 4A). Gene ontology analysis using GOstat (Beissbarth and Speed, 2004) revealed several metabolic pathways that were affected by high dietary sucrose in both paradigms, including glucose transport and metabolism, lipid synthesis and storage, trehalose synthesis and stability, and oxidative stress (Fig. 4A,B; supplementary material Table S2). These and other genes regulated by high-sugar feeding are likely to serve either as adaptive responses or as mediators of insulin resistance.
Fig. 4.
Expression analysis reveals potential mechanisms of insulin resistance at the transcriptional level in high-sugar-fed Drosophila. RNA was isolated from male control and high-sugar-fed Canton-S L3 larvae. Affymetrix GeneChips (Drosophila 2.0) were used to analyze gene expression. (A) Gene expression that changed more than 1.3-fold upon high-sugar feeding. The intersection of 12-hour and chronically high-sugar-fed datasets was modest, but significant for both up- and down-regulated genes; P<0.001 by a Pearson’s χ2 test; n=3 for each condition. (B) Gene expression changes that are consistent with known mechanisms of metabolic homeostasis or insulin resistance. Fold changes are only reported if statistically significant probe intensity differences exist; P<0.05 via unpaired two-tailed t-test. nc, no change.
DISCUSSION
We have developed a new model of T2D that facilitates an exploration of the interplay between diet and genetics using the powerful tools available for Drosophila. Our results in the fruit fly support the view that pathways leading to insulin resistance are evolutionarily conserved and that at least some of these pathways are dependent on dietary sugar.
A high-sugar diet leads directly to insulin resistance in Drosophila
We demonstrate a direct link between high-sugar feeding and insulin resistance in Drosophila. High-sugar-fed larvae were hyperglycemic, hyperinsulinemic and exhibited a defective response to insulin. We observed similar results in both male and female adult flies (data not shown). Interestingly, high-sugar feeding in adult flies also shortens lifespan and induces cardiac dysfunction and arrhythmias (Jianbo Na and Ross Cagan, personal communication). Our work extends previous studies in Drosophila that showed that excess dietary sugar increases TAG storage (Skorupa et al., 2008). A recent study examined the effects of modest dietary supplementation with sugar or fat in 146 inbred Drosophila strains and found that genotype contributed greater variance compared with diet when measuring metabolic parameters such as hemolymph sugar and total body triglyceride (Reed et al., 2010). The effects of high-calorie diets on hyperglycemia or on insulin sensitivity have not been previously described in flies.
Excess dietary carbohydrates have long been thought to contribute to the pathophysiology of diabetes, although definitive proof has been elusive (Sheard et al., 2004). Dietary excess and obesity often coexist in diabetics, making interpretations of the true cause of disease difficult. Elegant tracer studies in primary adipocytes from lean or obese individuals fed low- or high-carbohydrate diets demonstrated that diet had a greater effect on insulin sensitivity than did obesity (Salans et al., 1974). Two recent large epidemiological studies demonstrated that high glycemic load is a major risk factor for developing T2D, independent of obesity (Krishnan et al., 2007; Villegas et al., 2007). A meta-analysis of interventional studies concluded that insulin sensitivity was improved by lowering glycemic load (Livesey et al., 2008). High-sugar diets also lead to hyperglycemia and insulin resistance in rodents (Leiter et al., 1983; Fukuchi et al., 2004). In Drosophila, our studies demonstrate that a high-carbohydrate diet induces insulin resistance. Although evolution probably occurred outside the context of caloric excess for all animals, the response to excess dietary carbohydrate in many respects seems to be evolutionarily conserved.
The mechanisms of high-sugar-induced insulin resistance are evolutionarily conserved
We show a direct link between high-sugar feeding and insulin resistance in Drosophila. Importantly, insulin resistance in Drosophila seems to occur by evolutionarily conserved mechanisms, because the transcriptional effects of high-sugar feeding include pathways common to Caenorhabditis elegans, mouse and human insulin resistance. Several high-sugar-induced changes in mRNA levels are indicative of peripheral insulin resistance in our model. The T2D susceptibility gene hexokinase C was downregulated by high-sugar feeding, suggesting that glucose disposal through glycolysis might be impaired (Fig. 4B). According to the Randle hypothesis (Randle et al., 1963), increased β-oxidation of fatty acids leads to decreased glucose oxidation and to insulin resistance. Of note, high-sugar rearing led to transcriptional changes predicted to increase β-oxidation (Fig. 4B), suggesting that the Randle hypothesis might also be true in flies. Likewise, expression of the genes encoding the gluconeogenic enzymes PEPCK and fructose-1,6-bisphosphatase was upregulated by high-sugar feeding (Fig. 4B), consistent with observations in the gluconeogenic livers of insulin-resistant LIRKO mice (Michael et al., 2000) and T2D patients (O’Brien et al., 1990), although this paradigm has recently been challenged (Samuel et al., 2009). FOXO is an insulin-dependent transcription factor known to serve as a crucial regulator of insulin resistance. Several conserved FOXO targets, including panthothenate kinase and acyl CoA dehydrogenase, are altered in both insulin-resistant C. elegans and T2D Drosophila (Fig. 4B) (Lee et al., 2003). Genes encoding enzymes regulating glucose channeling into the sphingolipid, pentose phosphate, polyamine and polyol pathways were all affected by high-sugar feeding (supplementary material Table S2), suggesting that additional pathways function in both fly and mammalian models of glucotoxicity and insulin resistance.
Several of the transcriptional responses suggest potential metabolic toxicity mechanisms. Expression of some thioesterases, CoA ligases, and lipases were upregulated, whereas other lipases were downregulated, suggesting that a futile cycle of lipid hydrolysis and re-esterification might occur in the face of dietary excess (Guan et al., 2002). In addition, AKHR expression is increased (Fig. 4B) and could lead to a paradoxical mobilization of stored fat and carbohydrates (Lee and Park, 2004), which is surprisingly coincident with the upregulation of lipogenesis and trehalose synthesis (Fig. 1C, Fig. 3A). Bioactive lipids such as saturated fatty acids, ceramides and diacylglycerol (DAG) have been shown to play a role in rodent and human insulin resistance, broadly known as lipotoxicity (Yu et al., 2002; Holland et al., 2007; Brookheart et al., 2009; Junkin et al., 2009). Our data indicate that the expression of several enzymes involved in fatty acid synthesis and modification is upregulated by high-sugar feeding (Fig. 4B; supplementary material Table S2). In particular, the high-sugar diet might increase the production of DAG by upregulating TAG lipase expression, or produce more saturated fatty-acid-containing ceramides owing to the upregulation of sphingomyelinase expression (supplementary material Table S2). We hypothesize that such bioactive lipids are one source of cellular damage in high-sugar-fed Drosophila. High-carbohydrate-induced obesity could be protective against the accumulation of free long chain fatty acids or other lipotoxic intermediates that ensues when the capacity to safely store them is exceeded.
Conclusions
This study addresses a fundamental problem in human health: how does diet influence disease? This and other recent studies (Buch et al., 2008; Geminard et al., 2009) (for a review, see Baker and Thummel, 2007) establish Drosophila as a model system in which to study T2D. This model represents a simple and unique platform on which to identify gene products and drugs that can ameliorate the complications of T2D. Future studies will dissect the functional significance of different metabolic pathways in the production of toxic lipid mediators of sugar-induced pathophysiology.
METHODS
Fly stocks
The Canton-S and UAS-GFPnuc stocks were obtained from the Bloomington Drosophila Stock Center. DILP2-GAL4 flies were from Eric Rulifson (Rulifson et al., 2002), UAS-DILP2-FLAG flies were from Ernst Hafen (Honegger et al., 2008). Recipes for diets used are available on request.
Hemolymph glucose and trehalose measurements
Hemolymph was pooled from five to eight larvae to obtain 1 μl for assay. Glucose was measured by adding to 99 μl of Thermo Infinity Glucose Reagent (TR15321) frozen in a 96-well plate, then thawed to allow the detection reactions to occur simultaneously for all wells, and processed as per the manufacturer’s instructions. The level of trehalose was measured using the same reagent after digestion with trehalase, with a ten-fold dilution because trehalose levels are higher than those of glucose. 1 μl of hemolymph was incubated in 25 μl 0.25 M sodium carbonate at 95°C for 2 hours in a thermal cycler, cooled to room temperature, and 8 μl of 1 M acetic acid and 66 μl of 0.25 M sodium acetate (pH 5.2) were added to make a digestion buffer. 1 μl of porcine trehalase (Sigma T8778) was added to 40 μl of this mixture and incubated at 37°C overnight. The resulting glucose was analyzed using 10 μl reaction + 90 μl Infinity reagent as above. Glucose and trehalose standards were treated simultaneously and used to quantify the sugar levels in hemolymph.
Whole body carbohydrate and lipid measurements
Whole larvae were weighed and then homogenized in PBS for glycogen assays. Five larvae were homogenized in 50 μl PBS and then 5 μl of the homogenate was incubated with 5 μl of starch assay reagent containing amyloglucosidase (Sigma S9144) at 60°C for 15 minutes. 2 μl of this reaction was assayed for glucose using 98 μl of the Infinity reagent as above. Homogenates without amyloglucosidase were run in parallel to subtract free glucose and NADH.
For TAG, six to ten animals were homogenized in PBS + 0.1% Tween and heated for 5 minutes at 65°C to inactivate lipases. 2 μl of this homogenate was mixed with 198 μl of Thermo Infinity Triglyceride Reagent and analyzed as per the manufacturer’s instructions. Non-esterified fatty acids were extracted with chloroform and methanol (Marshall et al., 1999), and analyzed as per the manufacturers’ instructions [NEFA-HR(2), Wako Chemicals, Richmond, VA].
Quantitative RT-PCR
RNA was isolated from adult male heads using Tripure (Roche), DNase treated and then quantitative reverse transcriptase (RT)-PCR was performed using an ABI Prism 7000 as described (Broughton et al., 2005). A standard curve was used to enable quantitation of each messenger RNA.
Western blotting
For FLAG detection, wandering L3 larvae were bled and hemolymph diluted in 2× sample buffer + 25 mM EDTA. A volume containing 6 μl hemolymph was loaded in each lane. Sigma monoclonal M2 anti-FLAG antibody was used to detect the FLAG epitope. Note that these larvae were reared on 0.7 M sucrose because we had difficulty getting this genotype to thrive on 1 M sucrose, and many larvae were needed simultaneously to extract enough hemolymph for analysis.
For Akt detection, wandering L3 larvae were rinsed in PBS, bisected, and the anterior half was everted and placed in 100 μl oxygenated Buffer A on ice (Ceddia et al., 2003). Recombinant human insulin (Sigma I0259) or dilution buffer (10 mM HEPES) were added and incubated at room temperature for 15 minutes. Larvae were transferred to 100 μl sample buffer, homogenized and used to generate western blots. Cell Signaling antibodies against Drosophila PO4-Akt (#4054) or pan-Akt (#4691) were used to detect Akt, and anti-syntaxin (DSHB 8C-3) was used as a loading control. Secondary antibodies were from Santa Cruz.
Lipid droplet measurements
For Nile Red stains, larvae were dissected on a glass slide in 0.001% Nile Red (Sigma), 75% glycerol and incubated for 10 minutes before mounting. Slides were imaged at 60× magnification with an excitation wavelength of 543 nm and an emission of ∼626 nm within 1 hour of dissection, using a Nikon C1 confocal scanning system coupled to a Nikon Eclipse E-600 microscope. The NIH ImageJ software was used to quantify droplets and droplet size.
Microarrays
RNA was extracted from larvae using Tripure (Roche) and purified on RNeasy columns (Qiagen). Samples were prepared in triplicate to facilitate statistical analysis. Probe labeling, hybridization to Affymetrix GeneChip Drosophila Genome 2.0 Arrays and scanning were performed by the Washington University Biomedical Informatics Core Facility. Raw data was normalized using dChip (Li and Hung Wong, 2001) and analyzed using SAM 2.0 (Tusher et al., 2001), with a 5% false positive rate. Comparisons between microarray datasets were made using Microsoft Excel and Access. Microarray data from this study can be viewed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30020.
TRANSLATIONAL IMPACT.
Clinical issue
It has long been recognized that obesity and type 2 diabetes (T2D) frequently co-occur in humans. The prevalence of both of these disorders is increasing rapidly in populations worldwide, but public programs to improve diet and nutrition have largely failed to reduce the problem. Although genome-wide association studies have identified susceptibility loci conferring a slight increase in risk that is present in <10% of T2D patients, the mechanisms by which dietary excess contributes to the disease are not well understood. There is therefore an urgent need to develop and study this disorder in model organisms. In particular, the role of high glycemic diets in the pathogenesis of insulin resistance has been debated and warrants investigation.
Results
To establish Drosophila as a simple model for the study of diet-induced T2D, the authors rear larvae on high-calorie diets. They find that excess dietary sugars, but not fats or proteins, elicit severe insulin-resistant phenotypes. Larvae reared on a high-sugar diet are developmentally delayed and small in size compared with those reared on control food, and are similar to mutant flies lacking the insulin receptor or insulin receptor substrate. Furthermore, larvae reared on a high-sugar diet develop hyperglycemia, obesity and insulin resistance, which are hallmarks of T2D in humans. Expression analysis of these larvae reveals that the genes and pathways that are differentially expressed in the context of diet-induced insulin resistance in flies are similar to those that have been implicated in the pathogenesis of T2D in humans.
Implications and future directions
The fact that this fly model of diet-induced T2D recapitulates many aspects of the human disease underscores the impact of a high-glycemic diet on the development of insulin resistance. In developing the first model of diet-induced T2D in a simple model organism, the authors provide a novel platform to screen for unidentified genes, pathways and drug candidates that influence the pathophysiology of the disorder. This system also enables discrete control of diet as well as the opportunity to carry out rapid dissection of the genetic mechanisms, neither of which is feasible in higher model organisms.
Supplementary Material
Acknowledgments
We thank Trey Coleman for expert technical assistance, and Aaron DiAntonio, Brian Finck, Susumu Hirabayashi, Irfan Lodhi, Jianbo Na, Clay Semenkovich and Carl Thummel for helpful discussions and reading our paper. We also thank the Bloomington Drosophila Stock Center and the Hafen and Rulifson labs for fly strains. This research was supported by NIH grants T32 GM007464 (L.P.M.) and R21 DK069940 (R.L.C. and T.J.B.), and Washington University DRTC P60 DK20579 (T.J.B.) and P20 RR020643 (T.J.B. and R.L.C.).
Footnotes
COMPETING INTERESTS
R.L.C. and T.J.B. are co-founders of Medros, Inc., which uses models of human disease for drug development. Some of this work was also supported by STTR 1R41DK76338 to Medros, Inc.
AUTHOR CONTRIBUTIONS
L.P.M., R.L.C. and T.J.B. conceived and designed the experiments. L.P.M., J.L.F., K.N., P.V.R. and S.S.H. performed the experiments. L.P.M., J.L.F., K.N., P.V.R. and S.S.H. analyzed the data. L.P.M. and T.J.B. wrote the paper.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.007948/-/DC1
REFERENCES
- Baker K. D., Thummel C. S. (2007). Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 6, 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissbarth T., Speed T. P. (2004). GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics 20, 1464–1465 [DOI] [PubMed] [Google Scholar]
- Bohni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., Andruss B. F., Beckingham K., Hafen E. (1999). Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875 [DOI] [PubMed] [Google Scholar]
- Brookheart R. T., Michel C. I., Schaffer J. E. (2009). As a matter of fat. Cell Metab. 10, 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S. J., Piper M. D., Ikeya T., Bass T. M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D. J., Leevers S. J., et al. (2005). Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA 102, 3105–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S., Melcher C., Bauer M., Katzenberger J., Pankratz M. J. (2008). Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 7, 321–332 [DOI] [PubMed] [Google Scholar]
- Carsten L. D., Watts T., Markow T. A. (2005). Gene expression patterns accompanying a dietary shift in Drosophila melanogaster. Mol. Ecol. 14, 3203–3208 [DOI] [PubMed] [Google Scholar]
- Ceddia R. B., Bikopoulos G. J., Hilliker A. J., Sweeney G. (2003). Insulin stimulates glucose metabolism via the pentose phosphate pathway in Drosophila Kc cells. FEBS Lett. 555, 307–310 [DOI] [PubMed] [Google Scholar]
- Chen C., Jack J., Garofalo R. S. (1996). The Drosophila insulin receptor is required for normal growth. Endocrinology 137, 846–856 [DOI] [PubMed] [Google Scholar]
- Fukuchi S., Hamaguchi K., Seike M., Himeno K., Sakata T., Yoshimatsu H. (2004). Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats. Exp. Biol. Med. (Maywood) 229, 486–493 [DOI] [PubMed] [Google Scholar]
- Geminard C., Rulifson E. J., Leopold P. (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 10, 199–207 [DOI] [PubMed] [Google Scholar]
- Guan H. P., Li Y., Jensen M. V., Newgard C. B., Steppan C. M., Lazar M. A. (2002). A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med. 8, 1122–1128 [DOI] [PubMed] [Google Scholar]
- Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., et al. (2007). Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- Honegger B., Galic M., Kohler K., Wittwer F., Brogiolo W., Hafen E., Stocker H. (2008). Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junkin K. A., Dyck D. J., Mullen K. L., Chabowski A., Thrush A. B. (2009). Resistin acutely impairs insulin-stimulated glucose transport in rodent muscle in the presence, but not absence, of palmitate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R944–R951 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Rulifson E. J. (2004). Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431, 316–320 [DOI] [PubMed] [Google Scholar]
- Krishnan S., Rosenberg L., Singer M., Hu F. B., Djousse L., Cupples L. A., Palmer J. R. (2007). Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch. Intern. Med. 167, 2304–2309 [DOI] [PubMed] [Google Scholar]
- Lee G., Park J. H. (2004). Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Kennedy S., Tolonen A. C., Ruvkun G. (2003). DAF-16 target genes that control C. elegans life-span and metabolism. Science 300, 644–647 [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Coleman D. L., Ingram D. K., Reynolds M. A. (1983). Influence of dietary carbohydrate on the induction of diabetes in C57BL/KsJ-db/db diabetes mice. J. Nutr. 113, 184–195 [DOI] [PubMed] [Google Scholar]
- Li C., Hung Wong W. (2001). Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2, RESEARCH0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey G., Taylor R., Hulshof T., Howlett J. (2008). Glycemic response and health-a systematic review and meta-analysis: the database, study characteristics, and macronutrient intakes. Am. J. Clin. Nutr. 87, 223S–236S [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Attie A. D., Reue K. (2008). Metabolic syndrome: from epidemiology to systems biology. Nat. Rev. Genet. 9, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. A., Tordjman K., Host H. H., Ensor N. J., Kwon G., Marshall C. A., Coleman T., McDaniel M. L., Semenkovich C. F. (1999). Relative hypoglycemia and hyperinsulinemia in mice with heterozygous lipoprotein lipase (LPL) deficiency. Islet LPL regulates insulin secretion. J. Biol. Chem. 274, 27426–27432 [DOI] [PubMed] [Google Scholar]
- Michael M. D., Kulkarni R. N., Postic C., Previs S. F., Shulman G. I., Magnuson M. A., Kahn C. R. (2000). Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 [PubMed] [Google Scholar]
- Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vinicor F., Bales V. S., Marks J. S. (2003). Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79 [DOI] [PubMed] [Google Scholar]
- Muoio D. M., Newgard C. B. (2008). Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 193–205 [DOI] [PubMed] [Google Scholar]
- O’Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. (1990). Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science 249, 533–537 [DOI] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. (1963). The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 [DOI] [PubMed] [Google Scholar]
- Reed L. K., Williams S., Springston M., Brown J., Freeman K., DesRoches C. E., Sokolowski M. B., Gibson G. (2010). Genotype-by-diet interactions drive metabolic phenotype variation in Drosophila melanogaster. Genetics 185, 1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson E. J., Kim S. K., Nusse R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 [DOI] [PubMed] [Google Scholar]
- Salans L. B., Bray G. A., Cushman S. W., Danforth E., Jr, Glennon J. A., Horton E. S., Sims E. A. (1974). Glucose metabolism and the response to insulin by human adipose tissue in spontaneous and experimental obesity. Effects of dietary composition and adipose cell size. J. Clin. Invest. 53, 848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V. T., Beddow S. A., Iwasaki T., Zhang X. M., Chu X., Still C. D., Gerhard G. S., Shulman G. I. (2009). Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc. Natl. Acad. Sci. USA 106, 12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard N. F., Clark N. G., Brand-Miller J. C., Franz M. J., Pi-Sunyer F. X., Mayer-Davis E., Kulkarni K., Geil P. (2004). Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American Diabetes Association. Diabetes Care 27, 2266–2271 [DOI] [PubMed] [Google Scholar]
- Shingleton A. W., Das J., Vinicius L., Stern D. L. (2005). The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 3, e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa D. A., Dervisefendic A., Zwiener J., Pletcher S. D. (2008). Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 7, 478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Ren D., Li W., Jiang L., Cho K. W., Huang P., Fan C., Song Y., Liu Y., Rui L. (2010). SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 11, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher V. G., Tibshirani R., Chu G. (2001). Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas R., Liu S., Gao Y. T., Yang G., Li H., Zheng W., Shu X. O. (2007). Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 167, 2310–2316 [DOI] [PubMed] [Google Scholar]
- Wild S., Roglic G., Green A., Sicree R., King H. (2004). Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053 [DOI] [PubMed] [Google Scholar]
- Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. (2002). Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.