Abstract
Helicobacter pylori is the causative agent of a variety of gastric diseases, but the clinical relevance of bacterial virulence factors is still controversial. Virulent strains carrying the cag pathogenicity island (cagPAI) are thought to be key players in disease development. Here, we have compared cagPAI-dependent in vitro responses in H. pylori isolates obtained from 75 patients with gastritis, peptic ulcer, and gastric cancer (n = 25 in each group). AGS gastric epithelial cells were infected with each strain and assayed for (i) CagA expression, (ii) translocation and tyrosine phosphorylation of CagA, (iii) c-Src inactivation, (iv) cortactin dephosphorylation, (v) induction of actin cytoskeletal rearrangements associated with cell elongation, (vi) induction of cellular motility, and (vii) secretion of interleukin-8. Interestingly, we found high but similar prevalences of all of these cagPAI-dependent host cell responses (ranging from 56 to 80%) among the various groups of patients. This study revealed CagA proteins with unique features, CagA subspecies of various sizes, and new functional properties for the phenotypic outcomes. We further showed that induction of AGS cell motility and elongation are two independent processes. Our data corroborate epidemiological studies, which indicate a significant association of cagPAI presence and functionality with histopathological findings in gastritis, peptic ulcer, and gastric cancer patients, thus emphasizing the importance of the cagPAI for the pathogenicity of H. pylori. Nevertheless, we found no significant association of the specific H. pylori-induced responses with any particular patient group. This may indicate that the determination of disease development is highly complex and involves multiple bacterial and/or host factors.
Helicobacter pylori is a highly successful bacterial pathogen that persistently colonizes the human stomach. The bacterium has been recognized as the causative agent of chronic gastric inflammation, which can progress to a variety of other diseases, such as peptic ulcer, mucosa-associated lymphoid tissue lymphoma, or even gastric cancer (18, 19, 24, 28, 49, 57). Persistent colonization with H. pylori results in mucosal release of chemotactic factors that attract neutrophils and mononuclear cells, which enhance the inflammatory response (13, 42, 78). In particular, interleukin-8 (IL-8) secretion leads to recruitment and activation of neutrophils. In spite of accumulating knowledge about mechanisms involved in H. pylori-induced pathogenesis, it is not well understood why infected individuals develop one or the other disease. This enigma emphasizes the importance of multiple bacterial factors, differences among strains, differences in the host response to the bacteria, differences in host-microbe interaction, and/or the genetic susceptibility of the host in determining the course and outcome of H. pylori infections (23, 49, 57, 60).
The clinical isolates of H. pylori can be classified into two major types according to their degrees of pathogenicity. Virulent isolates are characterized by the presence of major disease-associated components, namely, the vacuolating cytotoxin (VacA) and the cag (cytotoxin-associated gene) pathogenicity island (cagPAI). H. pylori strains lacking the entire cagPAI resemble commensal bacteria rather than pathogens (18). cagPAI+ strains induce more notable phenotypic changes in vivo, such as higher levels of mucosal IL-8, than cagPAI− strains (20, 58), suggesting that IL-8 secretion is dependent upon particular cagPAI genes (11, 14, 27, 41, 64, 67, 73). In addition to IL-8 induction, attachment of cagPAI+ H. pylori to gastric epithelial cells in vitro induces several other responses, including (i) the activation of the Rho GTPases Rac1/Cdc42 and cellular motility (16, 17); (ii) the recruitment of the transcription factors NF-κB (42, 50, 68) and AP-1 (51); (iii) the activation (phosphorylation) of receptors, like the epidermal growth factor receptor (38) and c-Met (17); (iv) the activation of the proto-oncogenes c-Fos and c-Jun (44); (v) translocation and phosphorylation of the CagA protein (15); and (vi) the reorganization of the host cell actin cytoskeleton associated with the “hummingbird” phenotype (9, 10, 15, 32, 46, 63, 66).
H. pylori translocates the CagA protein into epithelial cells by a type IV secretion system (T4SS) encoded by the cagPAI (15, 27). Translocated CagA is tyrosine phosphorylated (CagAP-Tyr) on specific EPIYA sequence repeats by Src family tyrosine kinases and is capable of interfering with eukaryotic host cell signaling (65, 70). Transiently expressed CagA directly interacted with the tyrosine phosphatase SHP-2 and C-terminal Src kinase (Csk) via their Src homology 2 domains (32, 72). It was also shown that nonphosphorylated CagA binds to Grb2 (for growth factor receptor bound 2), a well-known adapter molecule in the c-Met/Ras signaling pathway (46). These binding factors were implicated in CagA-induced downstream signaling leading to the formation of very long spindle-like elongations, motility, and cell scattering (the hummingbird phenotype). Expression of CagA also increased serum response element- and serum response factor-driven gene transcription and enhanced phosphorylation of and DNA binding by Elk1 (33). CagA, in the presence of VacA, also plays an important role in the disruption of the epithelial barrier (3).
We have recently shown that CagAP-Tyr inhibits the catalytic activity of c-Src, an observation which can be explained by the activation of Csk (66). Nevertheless, we also detected Src inactivation in vitro in the absence of Csk. CagAP-Tyr induces both the dephosphorylation of the c-Src autophosphorylation site at tyrosine residue 418 (Y-418) and the phosphorylation of Y-527, a negative regulatory site, resulting in the inactivation of c-Src (66). c-Src inactivation in turn leads to a drastic reduction in the tyrosine phosphorylation of several host cell proteins, one of which is cortactin. This 80-kDa actin binding protein stimulates the actin nucleation activity of the Arp2/3 complex and is emerging as a central regulator of the actin cytoskeleton (74-76). Modification of both Src and cortactin requires the phosphorylation of Y-972 in CagA, as a point mutation at this site (CagA Y972F) blocked these alterations (66). Concomitantly, cortactin is specifically redistributed to the bases and tips of the actin-rich cellular elongations, an event which is required for rearrangements of the actin cytoskeleton.
Despite the significant research progress made with both in vitro and animal models (1, 54), the pathogenic role of the cagPAI in disease development remains to be fully understood (12, 18, 49). Epidemiological studies have established a strong association between cagPAI+ H. pylori and gastric disease based on either PCR data or the high seroprevalence for CagA (8, 25, 26, 30, 31, 40, 43, 45, 52, 55, 56, 58, 59, 69, 77). However, several factors may complicate the evaluation of the corresponding clinical studies. First, H. pylori can colonize as mixed populations (26, 39). Second, the pathogen is a highly diverse species with a high DNA recombination rate; thus, the emergence of recombinant strains and deletion of the cagPAI may account for the complex variety of phenotypic responses in infected patients (2, 7, 35, 39, 52, 61, 71). In vitro functional analyses of cagPAI-dependent host responses using multiple single clones from patients with different clinical outcomes are scarce (5, 69). Thus, there are still many open questions concerning the role of the H. pylori T4SS in disease development.
To further investigate the pathogenic role of the H. pylori T4SS, the main aim of the present study was to carry out a systematic functional analysis of H. pylori isolates from patients with specific clinical outcomes. Using in vitro T4SS-dependent host cell responses as readout systems, we evaluated the histopathological data obtained from multiple single clones isolated from biopsy samples from 75 German patients. The results show a high prevalence of the full set of cagPAI functions that were investigated in vitro in H. pylori isolates from gastritis, peptic ulcer, and gastric cancer patients. Furthermore, our data indicate that the cagPAI functions are important but not sufficient to explain the development of the different clinical outcomes.
MATERIALS AND METHODS
Patients.
In this study, >100 patients with gastric disorders underwent gastroduodenal endoscopy, and 75 H. pylori-positive biopsy specimens were investigated. The majority (32 specimens) of material was from the hospital in Groβhadern-Munich. Twenty-seven samples were from the Medical Center of the Virchow Clinics in Berlin (Charité); 15 specimens were from Medical Department I of the Hospital Carl Gustav Carus at the Technical University, Dresden; and two biopsies came from the gastroentrological doctor's practice Hoechter-Weingart in Munich. The clinical diagnosis (Table 1) was either gastritis (n = 25), peptic ulcer disease (n = 25; gastric ulcer or duodenal ulcer), or gastric cancer (n = 25; intestinal or diffuse type). All gastric cancers were distal adenocarcinomas. Most of the clinical data (age, sex, microscopy, histology, and antibody status) have been described in other studies (29, 31, 45). A list of the complete set of these data is available on request.
TABLE 1.
Characteristics of H. pylori isolates and their respective source patients
| Statusa | Diagnosis from endoscopy and histology | No. of patients | Age (yr) (mean ± SD) | % Female |
|---|---|---|---|---|
| CagA+ | Gastritis | 20 | 60 ± 11 | 50 |
| Peptic ulcerb | ||||
| Gastric ulcer | 3 | 67 ± 18 | 33 | |
| Duodenal ulcer | 19 | 54 ± 17 | 47 | |
| Gastric cancer | 22c | 64 ± 10 | 55 | |
| Total | 64 | 60 ± 14 | 50 | |
| CagA− | Gastritis | 5 | 38 ± 23 | 50 |
| Peptic ulcer | ||||
| Gastric ulcer | 2 | 43 ± 18 | 0 | |
| Duodenal ulcer | 2 | 64 ± 13 | 100 | |
| Gastric cancer | 3c | 78 ± 14 | 0 | |
| Total | 12 | 54 ± 22 | 33 |
CagA status was verified by PCR and Western blot analyses. Both analyses gave the same results, with the exception of one H. pylori strain. In that strain (P318) from the gastric cancer group, the cagA gene is present but not expressed.
Histological examination revealed either gastric ulcer or duodenal ulcer. One patient had both gastric ulcer and duodenal ulcer (strain UH44).
From one gastric cancer patient, both a cagA-deficient and a cagA+ H. pylori strain were isolated (strains Ka148/1 and Ka148/2). Thus, we investigated 76 strains from 75 patients.
H. pylori isolates.
For culture, each biopsy specimen was carefully inoculated with a swab onto three different media: Wilkins-Chalgren agar containing 10% horse blood, Dent supplement (Oxoid, Wesel, Germany), and 0.4 g of KNO3 per liter; H. pylori agar (BioMérieux, Marcy L'Étoile, France); and nonselective Columbia chocolate agar (GCII; Becton Dickinson, Heidelberg, Germany) without antibiotics. All plates were incubated at 36°C for up to 10 days in anaerobic jars containing a gas mix of 11% O2, 9% CO2, and 80% N2 produced by gas insufflation. H. pylori was identified by morphology, Christensen urease (Bacto Urea Base; Difco, Sparks, Md.), oxidase (Oxidase Dry Slide; Difo), catalase (3% H2O2; Merck, Darmstadt, Germany), and microscopy. From each biopsy specimen, four isolated single colonies were selected, cultured, and then used for infection studies as described previously (9, 10). Isolates were stored at −70°C in brain heart infusion medium (Difco) plus 20% glycerol.
Synchronized infection assays.
AGS cells (ATCC CRL 1739, a human gastric adenocarcinoma epithelial cell line) were grown in 25-cm2 tissue culture flasks containing RPMI 1640 medium (Gibco BRL, Eggenstein, Germany) supplemented with 10% heat-inactivated fetal bovine serum (Biochrom, Berlin, Germany) for 2 days to reach monolayers with ∼70% cell confluence. H. pylori cells (2 × 108) were suspended in 0.5 ml of phosphate-buffered saline (PBS) and added to 2 × 106 AGS cells at a multiplicity of infection (MOI) of 100. Infection was synchronized by centrifugation for 5 min at 600 × g and incubation for various times.
Elongation phenotype and motility assays.
AGS cells were grown in six-well plates as monolayers with ∼70% cell confluence as described above. Synchronized infections were done for 4 h at an MOI of 100. For motility assays, AGS cells were washed with PBS 12 h before infection and supplemented with fresh RPMI medium without fetal bovine serum (17). One hundred randomly selected cells were counted and evaluated according to migration and elongation as follows. Particular sections of the plate containing ca. 100 to 200 AGS cells were marked and photographed before infection and 2 and 4 h after infection using an Olympus IX50 phase-contrast microscope. The photographs taken at the different time points were then projected onto each other using Adobe Photoshop version 6.0 software. Migration was considered to have occurred when the AGS cells in a certain cluster lost their cell-to-cell contact and moved a distance of at least 30 μm. The elongation phenotype is characterized by the production of thin needle-like cell protrusions 20 to 70 μm in length (9). Smaller protrusions (<10 μm) that were also occasionally seen in the uninfected control cells were not counted. All experiments were done in triplicate.
In vitro phosphorylation assays of CagA.
H. pylori cells (A550 = 0.9) were harvested in ice-cold kinase buffer (25 mM HEPES, pH 7.0, 150 mM NaCl, 10 mM MgCl2, 1% Nonidet P-40, 5 mM dithiothreitol, 1 mM Na3VO4, complete proteinase inhibitor mix) and lysed by 20 passages through a 20-gauge syringe. Five units of recombinant human c-Src (Upstate Technologies, Lake Placid, N.Y.) was mixed with 30 μl of the lysate. In a similar experiment, 107 AGS cells were lysed in 1 ml of ice-cold kinase buffer as described above. Twenty-five microliters of cell lysates was incubated with 25 μl of H. pylori lysate and 2 μl of 1 mM ATP for 30 min at 30°C. CagA proteins from TIGR strain 266695 (http://www.tigr.org/) and a phosphorylation-deficient mutant (with bp 2,366 to 3,024 deleted) were used as positive and negative controls, respectively. The reactions were stopped by the addition of the appropriate amount of 4× reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer (200 mM Tris-HCl, pH 6.8, 10% mercaptoethanol, 10% SDS, 0.4% bromphenol blue, 40% glycerol) and boiled for 5 min.
Determination of Src activity.
Src activity depends on autophosphorylation of the protein at Y-418 in the kinase domain (34). Src activity during infection of AGS cells was determined by Western blot experiments using a rabbit polyclonal anti-Src-PY-418 antibody (Biosource, Camarillo, Calif.). As a loading control, the total amount of Src was detected using a monoclonal anti-Src antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). Densitometric measurements of band intensities revealed the percentage of Src activity.
Phosphorylation status of cortactin.
Phosphorylated cortactin can be detected in uninfected AGS cells as a predominant band at 80 kDa by using a mouse monoclonal anti-phosphotyrosine antibody, PY99 (Santa Cruz), and a monoclonal anti-cortactin antibody (Transduction BD Biosciences, Mississauga, Canada). The phosphorylation status of cortactin was verified by immunoprecipitation experiments (66). Briefly, AGS cells (107) were lysed in Ripa buffer (25 mM HEPES, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 125 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, complete proteinase inhibitor mix) by 20 passages through a 20-gauge syringe. Insoluble material was removed by centrifugation at 12,000 × g for 10 min. The lysates were precleared with protein G-Sepharose (Amersham Pharmacia Biotech, Freiburg, Germany) for 30 min at 4°C. Two micrograms of anti-cortactin antibody was added to the supernatants, and the mixture was incubated overnight. Immune complexes were precipitated by incubation with protein G-Sepharose for 1 h and washed four times in Ripa buffer. The precipitates were boiled in 1× reducing SDS-PAGE buffer and analyzed by Western blotting as described above. Densitometric measurements of band intensities revealed the percentage of cortactin phosphorylation in comparison to uninfected AGS cells in the PBS control.
SDS-PAGE and immunoblot analysis.
Cell pellets with attached bacteria were mixed with equal amounts of 2× SDS-PAGE buffer and boiled for 5 min. Proteins were separated by SDS-PAGE on 6% polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, Mass.). Before addition of the antibodies, the membranes were blocked in TBS-T (140 mM NaCl, 25 mM Tris-HCl, pH 7.4, 0.1% Tween 20) with 3% bovine serum albumin for 1 h at room temperature. Phosphorylated and nonphosphorylated CagA proteins were detected by incubation of the membranes with a mouse monoclonal anti-phosphotyrosine antibody, PY99 (Santa Cruz Biotechnology), a rabbit polyclonal anti-CagA antibody (Ab-1), a mouse monoclonal anti-CagA antibody (Ab-2; Austral Biologicals, San Ramon, Calif.), and another rabbit polyclonal anti-CagA antibody (Ab-3). As a secondary antibody, horseradish peroxidase-conjugated anti-mouse or anti-rabbit polyvalent sheep immunoglobulin was used (Amersham Pharmacia Biotech). Antibody detection was performed with the Renaissance Western blot kit system for enhanced chemiluminescence immunostaining (ICN Biochemicals, Eschwege, Germany).
IL-8 ELISA.
The amount of IL-8 secreted into the cell culture medium after 24 h of infection was determined by sandwich enzyme-linked immunosorbent assay (ELISA) using the CytoSets system (BioSource International) according to the manufacturer's instructions. All samples were measured in triplicate in at least three independent experiments.
Statistical analysis.
Fischer's exact test and the chi-square test with Yates' continuity correction were applied using SPSS version 10.0 for Windows to compare differences in the prevalences of H. pylori phenotypic outcomes. P values of <0.05 were considered significant.
RESULTS
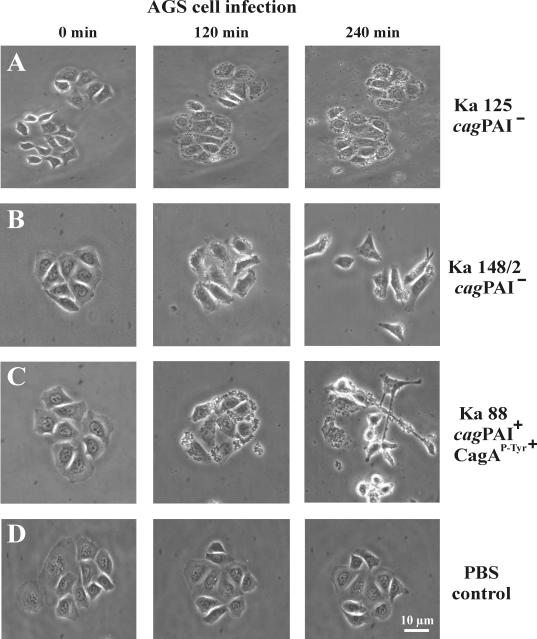
The H. pylori T4SS encoded by the cagPAI is thought to play an essential role in host-pathogen interaction. It has been shown to be essential for the development of severe gastritis, ulcer disease, and metaplasia in the Mongolian-gerbil model system (1, 54). In order to investigate the functional importance of this transporter system in the development of different gastric diseases in humans, we have chosen to examine H. pylori-positive patients with either gastritis, peptic ulcer disease, or gastric cancer. Antral biopsy specimens were taken from a total of 75 H. pylori-positive patients. The characteristics of the patients and the histopathology are summarized in Table 1. To examine the functionality of the T4SS in the corresponding H. pylori isolates, AGS gastric epithelial cells were infected with each strain, and the cellular responses were monitored in parallel experiments. The following different cellular responses served as readout systems: (i) CagA expression, (ii) translocation and tyrosine phosphorylation of CagA, (iii) c-Src inactivation, (iv) cortactin dephosphorylation, (v) the elongation phenotype, (vi) induction of cellular motility, and (vii) secretion of IL-8. Due to the large amount of data obtained, we show only representative findings in detail in the figures. The overall results are summarized in Fig. 1 and Tables 2 and 3 and are described below.
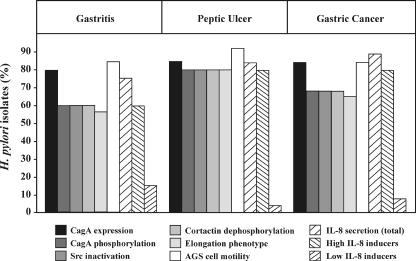
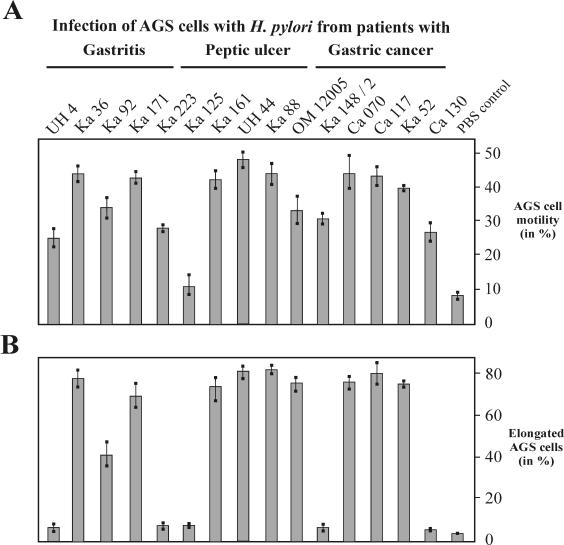
FIG. 1.
Comparison of cagPAI-dependent host cell responses in AGS cells after infection with H. pylori isolates obtained from 75 patients with gastritis, peptic ulcer, or gastric cancer disease (n = 25 in each group). Among the patients, we detected 64 cagPAI+ strains and 12 cagPAI− strains. The percentages of H. pylori isolates corresponding to the various parameters are shown. CagA expression, CagA phosphorylation, Src inactivation, and cortactin dephosphorylation were determined in Western blot analyses using specific antibodies. The elongation phenotype and cellular motility were quantified as described in Materials and Methods. IL-8 secretion was measured by standard ELISA. IL-8-inducing strains can be divided into high inducers (1.0 to 4.0 ng/ml) and low inducers (0.3 to 0.9 ng/ml). IL-8 induction of <0.3 ng/ml was in the range of PBS control levels. The numerical data and their respective percentages and P values are given in Table 2. Further details are provided in the text.
TABLE 2.
Summary of host cell responses during infection of AGS cells with H. pylori strains from 75 patients with different clinical outcomesa
| Characteristic | No. (%) of strains from patients with:
|
P value
|
||||
|---|---|---|---|---|---|---|
| Gastritis | Peptic ulcer | Gastric cancer | Gastritis vs. peptic ulcer | Gastritis vs. gastric cancer | Peptic ulcer vs. gastric cancer | |
| CagA expression | 20 (80) | 21 (84) | 21 (84) | 1.000 | 1.000 | 1.000 |
| CagA phosphorylation | 15 (60) | 20 (80) | 17 (68) | 0.217 | 0.520 | 0.769 |
| Src inactivation | 15 (60) | 20 (80) | 17 (68) | 0.217 | 0.520 | 0.769 |
| Cortactin dephosphorylation | 15 (60) | 20 (80) | 17 (68) | 0.217 | 0.520 | 0.769 |
| Elongation phenotype | 14 (56) | 20 (80) | 16 (64) | 0.128 | 0.345 | 0.773 |
| AGS motility | 21 (84) | 23 (92) | 21 (84) | 0.667 | 0.667 | 1.000 |
| IL-8 secretion (total) | 19 (76) | 21 (84) | 22 (88) | 0.725 | 1.000 | 0.463 |
| High IL-8 inducers | 15 (60) | 20 (80) | 20 (80) | 0.217 | 1.000 | 0.217 |
| Low IL-8 inducers | 4 (16) | 1 (4) | 2 (8) | 0.349 | 1.000 | 0.667 |
The numerical data presented in Fig. 1 are shown, together with their respective percentages and P values. All characteristics were determined as described in Materials and Methods.
TABLE 3.
Categorization of H. pylori strains according to their phenotypic outcomes
| Category | Phenotypea
|
No. (%) of strains from patients with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CagA expression | CagA phosphorylation | Src inactivation | Cortactin dephosphor. | Elongation phenotype | AGS cell motility | IL-8 secretion | Gastritis | Peptic ulcer | Gastric cancer | |
| I | + | + | + | + | + | + | + | 14 (56) | 20 (80) | 16 (64)b |
| II | + | + | + | + | − | + | + | 1 (4) | 0 (0) | 1 (4) |
| III | + | − | − | − | − | + | + | 0 (0) | 0 (0) | 4 (16) |
| IV | + | − | − | − | − | + | − | 2 (8) | 1 (4) | 1 (4) |
| V | + | − | − | − | − | − | + | 1 (4) | 0 (0) | 0 (0) |
| VI | + | − | − | − | − | − | − | 2 (8) | 0 (0) | 1 (4) |
| VII | − | − | − | − | − | + | + | 2 (8) | 1 (4) | 1 (4) |
| VIII | − | − | − | − | − | + | − | 2 (8) | 1 (4) | 0 (0) |
| IX | − | − | − | − | − | − | + | 1 (4) | 0 (0) | 1 (4) |
| X | − | − | − | − | − | − | − | 0 (0) | 2 (8) | 1 (4)b |
+, present; −, absent; dephosphor., dephosphorylation.
From one gastric cancer patient, both a cagPAI+ and a cagPAI− strain (Ka148/1 and Ka148/2) were isolated.
Presence of cagPAI, CagA expression, and tyrosine phosphorylation of CagA.
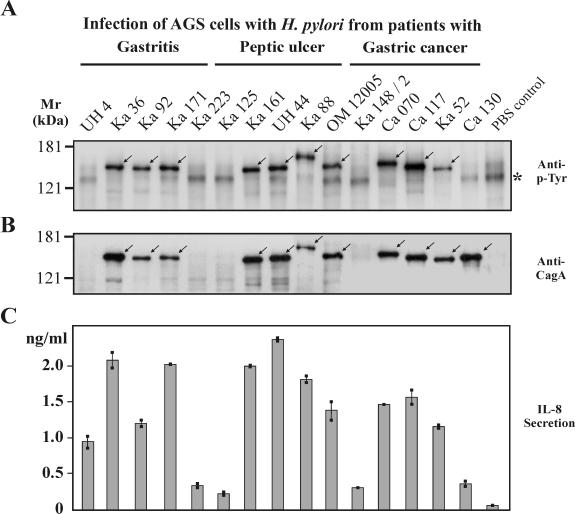
As shown in previous analyses, colonization with cagPAI+ H. pylori is associated with an increased risk for the development of gastritis, peptic ulcer disease, and gastric cancer (18, 19, 49, 57). Since expression of the CagA protein is a marker for the presence of the cagPAI, carriage of cagA gene-positive H. pylori strains was determined by (i) PCR of the cagA gene and (ii) detection of the CagA protein with three different anti-CagA antibodies using Western blotting experiments (Table 1). The presence of the cagA gene always correlated with the presence of the cagPAI sequences, since PCRs performed on the virB10 or virB11 gene were positive. The detailed findings from PCR typing will be published separately. To detect possible infections by multiple strains, four single colonies were selected from each cultured biopsy material sample and grown for further investigation. All single isolates from each biopsy sample contained either cagPAI+ or cagPAI− strains. With the exception of only one biopsy sample, which revealed both cagPAI+ and cagPAI− strains (Ka148) exhibiting considerably different host cell responses, similar responses were obtained for all four of the individual isolates from the other 75 biopsy samples. Hence, only one representative set of data is shown for each patient throughout this study (Tables 1 to 3 and Fig. 1). Altogether, we detected 64 cagPAI+ strains and 12 cagPAI− strains among the 75 patients. While the prevalence of CagA was very high throughout the groups of patients, the percentage of H. pylori isolates expressing CagA increased only slightly from patients with gastritis (80%) to patients with ulcer and cancer (both 84%) (Table 2 and Fig. 1). Within all but one strain carrying the cagA gene, the CagA protein was expressed, as determined by Western blotting with the anti-CagA antibodies. The CagA protein varied in mass from 120 to 160 kDa (Fig. 2B). In order to analyze the translocation and phosphorylation of CagA in individual H. pylori strains, AGS cells were infected for 4 h, and the tyrosine phosphorylation of CagA was analyzed by Western blotting using an antiphosphotyrosine antibody (Fig. 2A) and an anti-CagA antibody (Fig. 2B). Approximately 75 to 77% of the total CagA proteins from gastritis and gastric cancer patients were found to be tyrosine phosphorylated after the infection of AGS cells, indicating successful CagA translocation (Fig. 1). The highest percentage of phosphorylated CagA (95%) was found in H. pylori isolates from the peptic ulcer group of patients, with only one nonphosphorylated CagA protein (strain Ka61; data not shown). In the other groups of patients, we found nine strains in which CagA was not phosphorylated during infection. These included five strains from the gastritis group, namely, M15, M27, M33, M50, and M63 (data not shown), and four strains from the gastric cancer group, namely, MPI47, Ca115, Ca204 (data not shown), and Ca130 (Fig. 2A and B).
FIG. 2.
Phosphorylation of the CagA protein and induction of IL-8 during infection of AGS cells with 15 representative H. pylori isolates. (A) CagA tyrosine phosphorylation was analyzed in Western blots with a phosphotyrosine-specific antibody, PY-99 (arrows). The asterisk indicates the position of an unknown 125-kDa host cell protein that is phosphorylated in the PBS control. This protein changed its phosphorylation status during infection. (B) Stripping and reprobing of the blot with an anti-CagA antibody (Ab-1) indicated the positions of the different CagA protein species on the gel (arrows). UH4, Ka223, Ka125, and Ka148/2 are cagPAI− strains and did not express CagA. CagA of strain Ca130 was expressed but not phosphorylated. Infection was for 4 h at an MOI of 100. (C) In a parallel experiment, AGS cells were infected under the same conditions for 24 h and IL-8 release into the culture supernatant was measured by ELISA. The results shown are representative of three independent experiments. The error bars indicate standard deviations.
In vitro phosphorylation of CagA.
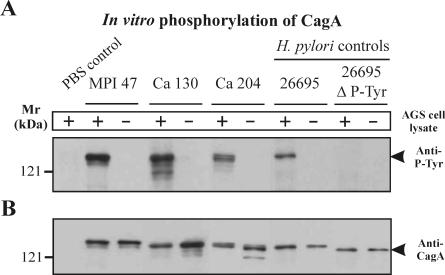
To investigate whether the phosphorylation deficiency of the nine CagA protein species was due to impaired translocation or the lack of EPIYA phosphorylation motifs in CagA (9, 70), we analyzed them in an in vitro phosphorylation assay. The results show that each of these CagA proteins can be phosphorylated in vitro (examples are shown in Fig. 3). As controls, wild-type CagA protein of the TIGR strain 26695 was tyrosine phosphorylated, whereas an EPIYA motif deletion mutant was not (Fig. 3). This result supports the view that CagA in these H. pylori strains may not be translocated, which can be explained by a T4SS defect or a missing translocation signal in the protein rather than a lack of phosphorylation sites. Indeed, while the cagPAI is largely inherited as a single block of 40 kb containing up to 31 genes (18), clinical H. pylori strains harboring only partial cagPAIs or exhibiting other T4SS defects have been identified in numerous studies (2, 7, 52, 61).
FIG. 3.
In vitro phosphorylation of CagA. (A) We observed nine nonphosphorylated CagA protein species during infection of AGS cells with the following H. pylori strains: M15, M27, M33, and M64 (gastritis group); Ka61 (peptic ulcer group); and MPI47, Ca115, Ca130, and Ca204 (gastric cancer group). These CagA proteins could be phosphorylated in an in vitro phosphorylation assay. The results are shown for three strains. Incubation of the H. pylori lysate with lysate of AGS cells or recombinant human c-Src (data not shown) resulted in specific CagA phosphorylation (arrowhead). The lysates of the TIGR H. pylori strain 26695 and a phosphorylation-deficient deletion mutant (ΔP-Tyr) were used as positive and negative controls, respectively. CagA phosphorylation was not detected either in the lysate of wild-type H. pylori incubated without c-Src or in the lysate of the CagAΔP-Tyr mutant incubated with c-Src. (B) An anti-CagA blot with antibody Ab-1 was performed as a control. +, present; −, absent.
IL-8 induction in AGS cells.
Gastric mucosal IL-8 levels correlate significantly with the extent of inflammatory cellular infiltration in gastric tissues of H. pylori-positive persons (4, 42, 58, 78), and the induction of IL-8 in vitro has been attributed to a functional cagPAI (11, 14, 27, 41, 48, 64, 67, 73). To assess strain- and group-specific IL-8 induction, each H. pylori isolate was incubated with AGS cells, and 24-h postinfection, the supernatants were assayed by ELISA for IL-8 release. Generally, H. pylori strains could be divided into high IL-8 inducers (range, 1.0 to 4.5 ng/ml) and low IL-8 inducers (0.3 to 0.9 ng/ml), as described by Ando and coworkers (5). All cagPAI+ strains in the patient groups induced significantly higher levels of IL-8 secretion than the cagPAI− isolates. However, the IL-8 induction did not vary significantly among clinical groups (Fig. 1 and Table 2). Among the eight low IL-8 inducer strains, we detected four cagPAI− H. pylori isolates. An example is H. pylori strain UH4 (Fig. 2C). Only two cagPAI+ strains were low IL-8 inducers (not shown), whereas six cagPAI+ strains did not induce IL-8 (e.g., strain Ca130 [Fig. 2C]). In these eight strains, there might be a T4SS defect, since in seven of the eight isolates CagA translocation and phosphorylation was impaired but the CagA proteins were tyrosine phosphorylated in vitro (Fig. 3).
Src inactivation and cortactin dephosphorylation by phosphorylated CagA.
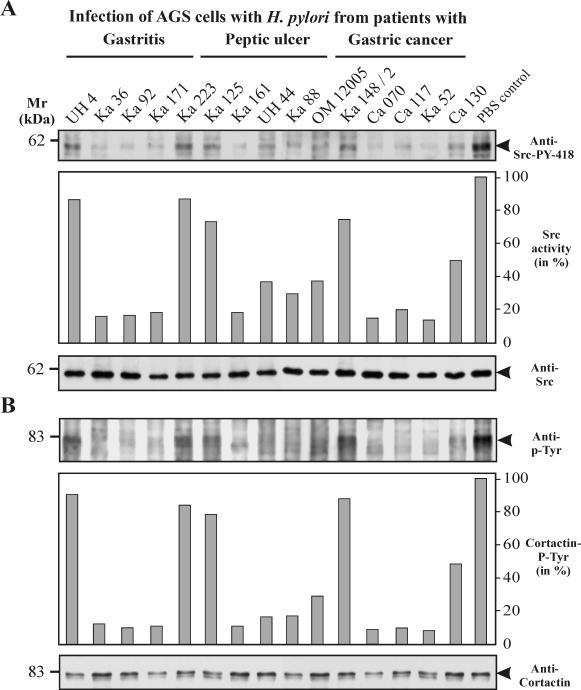
We have recently reported that CagAP-Tyr inactivates its cellular kinase, Src, via a negative feedback loop mechanism (66). The activity of Src can be assayed on the level of autophosphorylation at Y-418 in the kinase domain, which leads to the activation of the kinase (34). In order to investigate whether Src kinase inhibition is a common principle in H. pylori infections and whether there is strain-specific and patient group-specific capability in the inactivation of Src, we determined the phosphorylation of Y-418 with phosphospecific antibodies after 6 h of AGS cell infection. In agreement with a recent study showing that phosphorylation of CagA in the EPIYA motif (Y-972) is essential for inactivation of Src (66), we found that all H. pylori strains which produced CagAP-Tyr during infection resulted in a three- to sevenfold reduction in Src activity. With some strains that do not produce CagAP-Tyr during infection (for example, Ca130), Src activity was reduced only up to twofold. The extent of inhibition depended on the strain and the amount of CagAP-Tyr produced over time (Fig. 4A). Moreover, we have recently shown that CagAP-Tyr induces the dephosphorylation of the Src substrate, cortactin, by inhibiting the tyrosine kinase c-Src (66). In the present study, the observed drop in Src activity simultaneously resulted in a strong reduction of cortactin phosphorylation in all infections with H. pylori strains producing CagP-Tyr (Fig. 4B). This was observed in 80% of the strains from the peptic ulcer group versus 60 and 68% in the gastritis and gastric cancer patient groups, respectively (Table 2 and Fig. 1).
FIG. 4.
Induction of c-Src inactivation and cortactin dephosphorylation. (A) CagAP-Tyr-specific inactivation of c-Src. Western blotting with a phosphospecific anti-c-Src antibody revealed that some, but not all, H. pylori strains induce c-Src inactivation by dephosphorylation of residue Y-418 (top) in the catalytic kinase domain. Densitometric measurements of band intensities revealed the Src activity in comparison to uninfected AGS cells in the PBS control (middle). Stripping and reprobing of the blot with a monoclonal anti-Src antibody revealed that equal amounts of Src are present in all lanes (bottom). (B) Cortactin is a major phosphorylated protein in uninfected AGS cells and is specifically dephosphorylated during infection with some H. pylori strains (top). Cortactin was immunoprecipitated from infected AGS cells with a monoclonal anti-cortactin antibody, and the blots were probed with an anti-phosphotyrosine antibody (66). The phosphotyrosine pattern of cell lysates at 80 kDa shows dephosphorylation of cortactin in infections with those H. pylori isolates that induce CagA phosphorylation and Src inactivation (middle). Stripping and reprobing of the blot with a monoclonal anti-cortactin antibody revealed that similar amounts of cortactin were present in all lanes (bottom).
Induction of AGS cell motility and elongation phenotype.
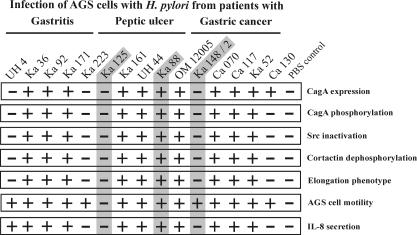
Next, we tested the motility of epithelial cells during infection with the various H. pylori strains. Comparison of the same AGS cell colony before and 4 h after infection with H. pylori revealed three different grades of cellular motility and phenotypic changes: very little or no motility (Fig. 5A), medium or strong motility without elongated AGS cells (Fig. 5B), and medium or strong motility with the presence of elongated AGS cells (Fig. 5C). Cells treated with PBS showed no effect (Fig. 5D). In addition, we quantified the cellular motility and found that the majority of H. pylori strains induced migratory behavior in ∼25 to 48% of the AGS cells (Fig. 6A). The prevalences of this stimulatory effect on AGS cells were very similar among the three groups of patients and ranged between 84 and 92% of the H. pylori strains (Fig. 1). Among the high-motility inducers were the complete set of cagPAI+ strains, as well as some of the cagPAI− H. pylori strains (Fig. 5 and 6B). In addition, we detected the elongation phenotype with only 56 to 80% of the H. pylori strains (Fig. 1 and 6B). In contrast to the motility phenotype, the elongation phenotype was dependent on CagA phosphorylation associated with Src inactivation and cortactin dephosphorylation, as summarized in Fig. 1 and 7. Table 3 shows a categorization of the strains (categories I to X) listed according to their phenotypic outcomes. Overall, we observed a trend toward a higher frequency of CagA phosphorylation, Src inactivation, cortactin dephosphorylation, elongation phenotype, and high IL-8 induction in strains obtained from patients with peptic ulcer and gastric cancer than in gastritis strains. However, our statistical analysis exhibited no significant association of the specific H. pylori-induced responses with any particular patient group (see the P values in Table 2).
FIG. 5.
Induction of two phenotypes associated with the stimulation of migratory behavior of H. pylori-infected host cells. Time lapse phase-contrast micrographs of AGS cells infected with wild-type strain Ka125 (A), Ka148/2 (B), and Ka88 (C) are shown as examples. Ka125 had no stimulatory effect on motility and elongation. In contrast, Ka148/2 induced a strong motility phenotype (B) and Ka88 induced motility and the elongation phenotype of AGS cells (C). (D) PBS as a control had no effect. In each experiment, H. pylori infection was for 4 h at an MOI of 100.
FIG. 6.
Quantification of motility (A) and elongation (B) phenotypes during infection with H. pylori. AGS cells were infected for 4 h at an MOI of 100. A single field of the coverslip was labeled and photographed before and after infection. One hundred cells from each photograph were counted and evaluated. The results are the means of three independent experiments. The error bars indicate standard deviations.
FIG. 7.
Summary of host cell responses during infection with 15 representative H. pylori isolates from patients with gastritis, peptic ulcer, or gastric cancer. The capacity of each wild-type strain to induce a certain response in infected AGS cells is represented as efficient induction (+) or no or drastically reduced induction (−). The chart demonstrates that translocation and phosphorylation of CagA correlates in each infection with Src inactivation, cortactin dephosphorylation, and induction of the elongation phenotype. AGS cell motility and IL-8 induction were observed with both the sets of H. pylori that induced the aforementioned responses and some cagPAI− strains. The phenotypic outcomes of the shaded strains are shown in Fig. 5. See the text for more details.
Detection of unique and multiple CagA protein subspecies.
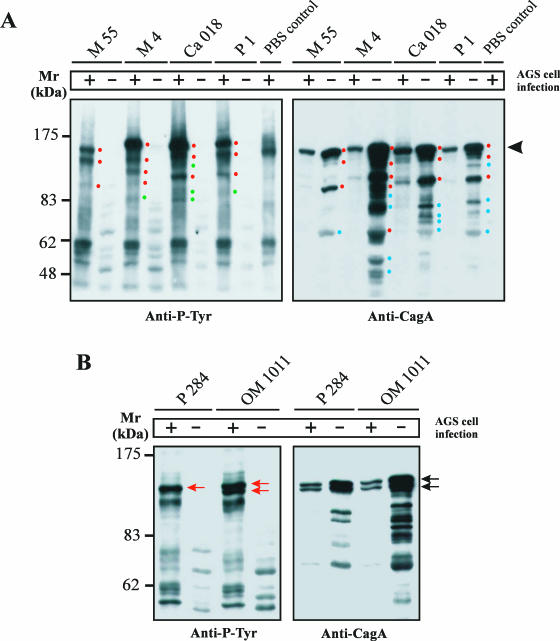
The majority of cagPAI+ strains produced full-length CagA as a major band on anti-CagA Western blots (Fig. 2B). However, there were some in which we detected a few new features of CagA. First, two CagA protein species (from strains P277 and P310) were tyrosine phosphorylated after the infection of AGS cells but did not induce the elongation phenotype (Table 3, category II). Second, in 10 of the H. pylori strains, we detected unique phosphotyrosine patterns with various phosphorylated proteins in the mass range between 70 and 140 kDa (examples are shown in Fig. 8A, left).
FIG. 8.
Detection of unique and multiple CagA protein species. (A) Anti-phosphotyrosine patterns of AGS infected with different H. pylori strains revealed unique phosphorylated protein species in each lane (left). The blot was stripped and reprobed with an anti-CagA antibody (right). This exposure revealed 5 to 13 CagA protein species with masses between 40 and 120 kDa, depending on the strain. The 25- to 35-kDa fragmentation products (10, 47, 53) were also observed but ran out of the gels shown. Full-length CagA is indicated by the arrowhead. The red dots indicate phosphorylated proteins corresponding in size to CagA protein species, the green dots indicate phosphorylated proteins not corresponding in size to CagA protein species, and the blue dots label CagA protein species with no detectable phosphorylated form during infection. H. pylori strain P1 served as a control because the fragmentation of 135-kDa wild-type CagA into an amino-terminal p100CagA fragment and a carboxy-terminal p35CagA fragment has been described (10). +, present; −, absent. (B) H. pylori strains P284 and OM1011 are unique and showed a double band of full-length CagA. This double band had nearly identical intensities in the anti-CagA blot (black arrows) but showed a different phosphorylation pattern during infection (red arrows).
Stripping and reprobing of the blot with an anti-CagA antibody revealed multiple and highly variable CagA protein species that are smaller than the full-length version of the protein (Fig. 8A, right). First, overlays of the exposures indicated that three, and possibly five, of the de novo tyrosine-phosphorylated proteins correspond in size to CagA protein species. Longer exposure of the Anti-CagA blot showed basically the same protein pattern of H. pylori cells with or without infection. Second, we detected a few more phosphorylated protein species with masses between 83 and 110 kDa in infected AGS cells with no corresponding CagA band, suggesting that they may correspond to phosphorylated host cell proteins appearing upon infection with H. pylori (Fig. 8A). Third, in the peptic ulcer group, but not in the other groups of patients, we detected four H. pylori strains, including OM1011 and P285, which produced a double band of full-length CagA protein with nearly identical intensities plus a few minor bands of lower molecular mass as described above (Fig. 8B, right). Interestingly, in strain OM1011, both of these full-length CagA proteins were phosphorylated, whereas for CagA from P284, only the upper band was phosphorylated (Fig. 8B, left). To exclude the possibility of mixed bacterial populations and protein degradation in our studies, H. pylori was repeatedly grown from single colonies and the cells were processed in the presence of complete proteinase inhibitor mix.
DISCUSSION
T4SSs from several pathogenic bacteria have been shown to deliver bacterial factors into the cytosol of eukaryotic cells (reviewed in references 15 and 18). Virulent H. pylori strains carry a T4SS encoded by the cagPAI, enabling the bacteria to inject CagA and possibly other unidentified virulence factors into infected host cells. Although cagA+ H. pylori strains are common worldwide, the factors accounting for the development of one or the other type of gastric disease are still unknown. Since previous functional studies of host cell responses in vitro have been carried out with only a small number of selected laboratory strains, our study sought to perform an extensive analysis of the diversity of host cell responses by using a large number of H. pylori strains isolated from patients with gastritis, peptic ulcer, or gastric cancer. The study was focused on cagPAI-induced host responses, and the results indicate that the cagPAI functions are very important for the induction of cellular signaling; however, we found no significant association of these specific H. pylori-induced responses with any particular patient group. This may indicate that the determination of disease development is highly complex and involves multiple factors apart from the cagPAI. Our data considerably extend the findings of other groups and shed new light on the role of the H. pylori T4SS during infection.
In our study, the presence of the cagA gene always correlated with the genetic presence of the cagPAI, and this prevalence was very high throughout all groups of patients and changed only slightly among patients with gastritis (80%), ulcer (84%), and cancer (84%). However, it is worth pointing out that while the cagA gene was commonly expressed, not all of the strains expressed the entire set of cagPAI-dependent responses (Fig. 1 and Tables 2 and 3). The percentages of H. pylori strains in category I with full cagPAI functionality were equally distributed and varied from 56 (gastritis group) to 80 (peptic ulcer group) and 64% (gastric cancer group). Similar results were obtained with the CagA-dependent responses. The percentage of expressed CagA that was phosphorylated during infection of cagPAI+ strains was highest in the peptic ulcer group (95%), followed by the gastric cancer group (81%) and the gastritis group (75%). The remaining CagA-expressing strains with no detectable CagAP-Tyr may have a T4SS defect or be missing a translocation signal, because in vitro phosphorylation of these CagA proteins was successful. Interestingly, such defects were detected mainly in the gastritis group (five strains) and the gastric cancer group (four strains) versus one strain in the peptic ulcer patient group.
In agreement with two recent studies showing that phosphorylation of CagA is essential for inactivation of Src (66, 72), we found that infection with all 52 H. pylori strains producing CagAP-Tyr resulted in a strong reduction of Src activity in the infected AGS cells. Also, as expected, the impaired Src kinase activity resulted in a strong reduction of cortactin phosphorylation in infections with the same strains. It is known from studies in other cell systems that cortactin binds F-actin and localizes to the sites of dynamic actin assembly, but the mechanism by which nonphosphorylated cortactin modulates the architecture of the actin cytoskeleton remains unknown (76). One hypothesis is that dephosphorylated cortactin could cross-link actin filaments into bundles, which are found within the characteristic extensions of elongated AGS cells. Alternatively, cortactin has been shown to activate actin polymerization by the Arp2/3 complex, which can also explain the production of elongated cells (74, 75). However, CagAP-Tyr-mediated inactivation of c-Src may also affect cytoskeletal rearrangements through a cortactin-independent mechanism(s).
The characteristic changes in the morphology of infected AGS cells as a consequence of CagA translocation and phosphorylation resemble the process of oncogenic transformation. Since cagA+ H. pylori strains have been associated with the onset of gastric cancer (8, 24, 43, 45, 49, 57, 77), it is tempting to speculate that CagAP-Tyr contributes to the oncogenic transformation of infected cells by interfering with c-Src signaling to cortactin. Interestingly, the cortactin-encoding gene is amplified in some other human cancers, and cortactin is suspected to play a role in tumor invasion (62). However, the present study revealed that cortactin is dephosphorylated during AGS cell infections with the majority of strains from all three groups: 68% of the strains from gastric cancer patients, 60% in the gastritis group, and up to 80% in the group of peptic ulcer patients. This suggests that H. pylori-induced targeting of eukaryotic tyrosine kinase and actin cytoskeletal signaling are common principles of infection and are not restricted to a specific group of patients. Nevertheless, in future experiments it will be interesting to study the general pathological effects of CagAP-Tyr in established animal models and in biopsy specimens from human patients.
The induction of motility (cell scattering) and cellular elongation were characterized as a single cagPAI- and CagA-dependent process in previous studies (9, 32, 46, 63, 70). Our present data indicate that induction of motility and the elongation phenotype are two independent host responses which occur in parallel in infections with many strains. The elongation phenotype is CagA dependent, whereas the induction of motility appears to be CagA independent. The presence of a functional cagPAI is not essential for the stimulation of the migratory response, since strains lacking the cagPAI also had a substantial stimulatory effect (Fig. 5B), which was observed in 84 to 92% of all H. pylori strains (Fig. 1). However, H. pylori strains harboring a functional cagPAI clearly contribute to and enhance AGS cell motility. In contrast, the elongation phenotype in infected AGS cells clearly depended on translocation and phosphorylation of CagA from 52 strains (Fig. 1 and Table 3), as has been shown by mutational analyses of CagA in single laboratory strains (9, 32, 66, 70). Interestingly, we detected two exceptions in this study in category II (Table 2). CagA proteins from strains P277 (gastritis group) and P310 (gastric cancer group) were tyrosine phosphorylated after infection but did not induce the elongation phenotype. This suggests that translocation and phosphorylation of CagA are essential but not sufficient for induction of this phenotype, as was previously shown (9). Alternatively, these CagA proteins might be mutated in a putative motif required for the binding of an adapter molecule that is involved in the signaling leading to the elongation phenotype. We are cloning and sequencing both cagA genes in order to further investigate this phenomenon.
Another interesting observation was that CagA proteins with multiple masses ranging from 30 to 120 kDa can exist in certain H. pylori strains. Previous reports have shown that CagA undergoes processing into p100CagA and p35CagA fragments, which have been observed during infection of both gastric epithelial cells (10) and phagocytic cells (47, 53). In this study, CagA proteins isolated from 10 H. pylori strains appeared as 6 to 11 discrete bands, corresponding to masses between 30 and 120 kDa, which produced reproducible patterns on Western blots. In three other strains from the peptic ulcer group of patients, full-length CagA was present as two dominant and equally expressed bands of 120 and 125 kDa, a phenomenon that has not yet been described. We do not know whether these multiple CagA masses originate from more than one cagA gene and/or from posttranslational modification of a single protein. Interestingly, we observed that some, but not all, of the CagA “fragments” undergo tyrosine phosphorylation, indicating that some fragments may be translocated by the T4SS. However, a translocation signal of CagA was not yet identified. Furthermore, this fragmentation of unknown nature did not seem to hamper induction of the elongation phenotype or the other responses observed.
In vivo and in vitro studies have shown that the IL-8-inducing activities of some H. pylori strains with a series of cagPAI genes mutated was strongly reduced (11, 14, 27, 41, 47, 64, 67, 73). These data led to the hypotheses that H. pylori induces IL-8 secretion by an unidentified factor that is translocated through the T4SS or that the T4SS itself activates signaling via receptor activation (the receptor hypothesis). Indeed, in this study we found that the majority of strains, including cagPAI+ (55 strains) and cagPAI− (6 strains) H. pylori strains, strongly induced IL-8. The cagPAI+ strains include a subset of five isolates with a T4SS defect and a deficiency in CagA translocation. Interestingly, we found four cagPAI+ strains with no IL-8-inducing capacity, indicating the occurrence of strain-specific differences. In this context, it is noteworthy that there are also reports of studies in which the inactivation of the virD4, virB10, or virB11 gene of the cagPAI even enhanced IL-8 secretion (21, 22). Another group has reported that water-soluble extracts of H. pylori containing nonprotein low-molecular-weight compounds can induce IL-8 secretion (37). Other studies showed that an outer membrane protein encoded by oipA outside the cagPAI is involved in the induction of IL-8 release (77), whereas ΔoipA mutants from other strains induced IL-8 like wild-type bacteria (5). These phenomena appear to be very complex and are still poorly understood. Nevertheless, we have detected low IL-8 inducers in cagPAI− strains and in strains with a cagPAI defect, favoring the concept that at least two pathways of H. pylori-induced IL-8 induction may exist, a cagPAI-dependent pathway (high inducers) and a cagPAI-independent pathway (low inducers).
In conclusion, our study showed that the T4SS encoded by the cagPAI is a virulence component important for infection, but the determination of the direction of disease development (gastritis, ulcers, or cancer) is likely to involve a highly complex interplay of many bacterial and/or host factors. For example, colonization with cagPAI+ H. pylori affects the proliferation and apoptosis of human gastric epithelial cells. Alterations in these processes may be important in lowering the threshold for carcinogenesis (6, 36, 42). On the other hand, gastroduodenal illness associated with H. pylori may also be related to disturbed regulation of gastric immune responses (24, 78). It was also suggested that host inflammatory mediators, such as cytokines, prostaglandins, and hormones, might modulate H. pylori-induced alterations in cellular turnover (57). Clearly, host differences in the specific groups of patients may play a role in the different clinical outcomes for H. pylori-infected patients (60). Polymorphism of the human IL-1β gene promoter, a risk factor for both atrophic gastritis and gastric adenocarcinoma, is a good example (23). Another hypothesis to explain differences in the development of H. pylori-induced diseases is that colonizing strains may change their abilities to induce epithelial-cell responses, such as CagA delivery and IL-8 secretion, as part of their adaptation to the changing conditions within the host milieu. In order to unravel the hidden features of H. pylori-host interactions, future work should be directed toward the identification of both host cell factors and other translocated bacterial virulence factors.
Acknowledgments
We are grateful to Antonello Covacci and Stefano Censini (Siena, Italy) for providing us with anti-CagA antibody Ab-3 and to Matthias Selbach and Stefan Moese for discussion. We also thank Gaby Haas and Konstanze Vogt for providing information on the H. pylori strains collected in Berlin.
This work was supported through a grant from Priority Program SPP1150 of the Deutsche Forschungsgemeinschaft to S.B. and T.F.M. (Ba1671/3-1).
Editor: D. L. Burns
REFERENCES
- 1.Akanuma, M., S. Maeda, K. Ogura, Y. Mitsuno, Y. Hirata, T. Ikenoue, M. Otsuka, T. Watanabe, Y. Yamaji, H. Yoshida, T. Kawabe, Y. Shiratori, and M. Omata. 2002. The evaluation of putative virulence factors of Helicobacter pylori for gastroduodenal disease by use of a short-term Mongolian gerbil infection model. J. Infect. Dis. 185:341-347. [DOI] [PubMed] [Google Scholar]
- 2.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 3.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando, T., K. Kusugami, M. Ohsuga, M. Shinoda, M. Sakakibara, H. Saito, A. Fukatsu, S. Ichiyama, and M. Ohta. 1996. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am. J. Gastroenterol. 91:1150-1156. [PubMed] [Google Scholar]
- 5.Ando, T., R. M. Peek, Jr., Y. C. Lee, U. Krishna, K. Kusugami, and M. J. Blaser. 2002. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin. Diagn. Lab. Immunol. 9:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anti, M., A. Armuzzi, A. Gasbarrini, and G. Gasbarrini. 1998. Importance of changes in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut 43(Suppl. 1):S27-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audibert, C., C. Burucoa, B. Janvier, and J. L. Fauchere. 2001. Implication of the structure of the Helicobacter pylori cag pathogenicity island in induction of interleukin-8 secretion. Infect. Immun. 69:1625-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach, S., A. Makristathis, A. Pinto, M. Quina, M. Rotter, and A. M. Hirschl. 1999. Helicobacter pylori type I strains among Austrian and Portuguese patients with gastritis, peptic ulcer or gastric cancer. Eur. J. Clin. Microbiol. Infect. Dis. 18:807-810. [DOI] [PubMed] [Google Scholar]
- 9.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 10.Backert, S., E. C. Mueller, P. R. Jungblut, and T. F. Meyer. 2001. Tyrosine phosphorylation patterns and size modification of the Helicobacter pylori CagA protein after translocation into gastric epithelial cells. Proteomics 1:608-617. [DOI] [PubMed] [Google Scholar]
- 11.Beales, I. L., and J. Calam. 1997. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1β and TNF-α requires tyrosine kinase activity, but not protein kinase C. Cytokine 9:514-520. [DOI] [PubMed] [Google Scholar]
- 12.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodger, K., and J. E. Crabtree. 1998. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 54:139-150. [DOI] [PubMed] [Google Scholar]
- 14.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Censini, S., M. Stein, and A. Covacci. 2001. Cellular responses induced after contact with Helicobacter pylori. Curr. Opin. Microbiol. 4:41-46. [DOI] [PubMed] [Google Scholar]
- 16.Churin, Y., K. Kardalinou, T. F. Meyer, and M. Naumann. 2001. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol. Microbiol. 40:815-823. [DOI] [PubMed] [Google Scholar]
- 17.Churin, Y., L. Al-Ghoul, O. Kepp, T. F. Meyer, W. Birchmeier, and M. Naumann. 2003. The Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 19.Cover, T. L., and M. J. Blaser. 1999. Helicobacter pylori factors associated with disease. Gastroenterology 117:257-261. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree, J. E., D. Kersulyte, S. D. Li, I. J. Lindley, and D. E. Berg. 1999. Modulation of Helicobacter pylori induced interleukin-8 synthesis in gastric epithelial cells mediated by cag PAI encoded VirD4 homologue. J. Clin. Pathol. 52:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabtree, J. E., S. M. Farmery, I. J. Lindley, N. Figura, P. Peichl, and L. Tompkins. 1994. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J. Clin. Pathol. 47:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton, K. A., D. Kersulyte, M. Mefford, S. J. Danon, S. Krakowka, and D. E. Berg. 2001. Role of Helicobacter pylori cag region genes in colonization and gastritis in two animal models. Infect. Immun. 69:2902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2001. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 24.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 25.Evans, D. J., Jr., and D. G. Evans. 2001. Helicobacter pylori CagA: analysis of sequence diversity in relation to phosphorylation motifs and implications for the role of CagA as a virulence factor. Helicobacter 6:187-198. [DOI] [PubMed] [Google Scholar]
- 26.Figura, N., C. Vindigni, A. Covacci, L. Presenti, D. Burroni, R. Vernillo, T. Banducci, F. Roviello, D. Marrelli, M. Biscontri, S. Kristodhullu, C. Gennari, and D. Vaira. 1998. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut 42:772-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 28.Forman, D., D. G. Newell, F. Fullerton, J. W. G. Yarnell, A. R. Stacey, N. Wald, and F. Sitas. 1991. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 302:1302-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerhard, M., N. Lehn, N. Neumayer, T. Boren, R. Rad, W. Schepp, S. Miehlke, M. Classen, and C. Prinz. 1999. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. USA 96:12778-12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunn, M. C., J. C. Stephens, J. A. Stewart, B. J. Rathbone, and K. P. West. 1998. The significance of cagA and vacA subtypes of Helicobacter pylori in the pathogenesis of inflammation and peptic ulceration. J. Clin. Pathol. 51:761-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. G. Goebel, K. Vogt, A. B. Roznowski, B. J. Wiedenmann, T. F. Meyer, T. Aebischer, and P. R. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 32.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 33.Hirata, Y., S. Maeda, Y. Mitsuno, K. Tateishi, A. Yanai, M. Akanuma, H. Yoshida, T. Kawabe, Y. Shiratori, and M. Omata. 2002. Helicobacter pylori CagA protein activates serum response element-driven transcription independently of tyrosine phosphorylation. Gastroenterology 123:1962-1971. [DOI] [PubMed] [Google Scholar]
- 34.Hunter, T. 1987. A tail of two src's: mutatis mutandis. Cell 49:1-4. [DOI] [PubMed] [Google Scholar]
- 35.Israel, D. A., N. Salama, C. N. Arnold, S. F. Moss, T. Ando, H. P. Wirth, K. T. Tham, M. Camorlinga, M. J. Blaser, S. Falkow, and R. M. Peek Jr. 2001. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J. Clin. Investig. 107:611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones, N. L., P. T. Shannon, E. Cutz, H. Yeger, and P. M. Sherman. 1997. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 151:1695-1703. [PMC free article] [PubMed] [Google Scholar]
- 37.Kassai, K., T. Yoshikawa, N. Yoshida, A. Hashiramoto, M. Kondo, and H. Murase. 1999. Helicobacter pylori water extract induces interleukin-8 production by gastric epithelial cells. Dig. Dis. Sci. 44:237-242. [DOI] [PubMed] [Google Scholar]
- 38.Keates, S., S. Sougioultzis, A. C. Keates, D. Zhao, R. M. Peek Jr., L. M. Shaw, and C. P. Kelly. 2001. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J. Biol. Chem. 276:48127-48134. [DOI] [PubMed] [Google Scholar]
- 39.Kersulyte, D., H. Chalkauskas, and D. E. Berg. 1999. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 31:31-43. [DOI] [PubMed] [Google Scholar]
- 40.Lai, Y. P., J. C. Yang, T. Z. Lin, J. T. Wang, and J. T. Lin. 2003. CagA tyrosine phosphorylation in gastric epithelial cells caused by Helicobacter pylori in patients with gastric adenocarcinoma. Helicobacter 8:235-243. [DOI] [PubMed] [Google Scholar]
- 41.Li, S. D., D. Kersulyte, I. J. Lindley, B. Neelam, D. E. Berg, and J. E. Crabtree. 1999. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect. Immun. 67:3893-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda, S., M. Akanuma, Y. Mitsuno, Y. Hirata, K. Ogura, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Distinct mechanism of Helicobacter pylori-mediated NF-κB activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 276:44856-44864. [DOI] [PubMed] [Google Scholar]
- 43.Matsukura, N., M. Onda, S. Kato, H. Hasegawa, K. Okawa, T. Shirakawa, A. Tokunaga, K. Yamashita, and A. Hayashi. 1997. Cytotoxin genes of Helicobacter pylori in chronic gastritis, gastroduodenal ulcer and gastric cancer: an age and gender matched case-control study. Jpn. J. Cancer Res. 88:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer-ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275:16064-16072. [DOI] [PubMed] [Google Scholar]
- 45.Miehlke, S., J. Yu, M. Schuppler, C. Frings, C. Kirsch, N. Negraszus, A. Morgner, M. Stolte, G. Ehninger, and E. Bayerdörffer. 2001. Helicobacter pylori vacA, iceA, and cagA status, and pattern of gastritis in patients with malignant and benign gastroduodenal disease. Am. J. Gastroenterol. 96:1008-1013. [DOI] [PubMed] [Google Scholar]
- 46.Mimuro, H., T. Suzuki, J. Tanaka, M. Asahi, R. Haas, and C. Sasakawa. 2002. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell 10:745-755. [DOI] [PubMed] [Google Scholar]
- 47.Moese, S., M. Selbach, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and S. Backert. 2001. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics 1:618-629. [DOI] [PubMed] [Google Scholar]
- 48.Moese, S., M. Selbach, T. F. Meyer, and S. Backert. 2002. cag+ Helicobacter pylori induces homotypic aggregation of macrophage-like cells by up-regulation and recruitment of intracellular adhesion molecule 1 to the cell surface. Infect. Immun. 70:4687-4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell. Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 50.Münzenmaier, A., C. Lange, E. Glocker, A. Covacci, A. Moran, S. Bereswill, P. A. Baeuerle, M. Kist, and H. L. Pahl. 1997. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor kappa B. J. Immunol. 159:6140-6147. [PubMed] [Google Scholar]
- 51.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, A. Covacci, R. Haas, and T. F. Meyer. 1999. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 274:31655-31662. [DOI] [PubMed] [Google Scholar]
- 52.Occhialini, A., A. Marais, M. Urdaci, R. Sierra, N. Munoz, A. Covacci, and F. Megraud. 2001. Composition and gene expression of the cag pathogenicity island in Helicobacter pylori strains isolated from gastric carcinoma and gastritis patients in Costa Rica. Infect. Immun. 69:1902-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell. Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 54.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbils. J. Exp. Med. 192:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owen, R. J., S. I. Sharp, S. A. Chisholm, and S. Rijpkema. 2003. Identification of cagA tyrosine phosphorylation DNA motifs in Helicobacter pylori isolates from peptic ulcer patients by novel PCR-restriction fragment length polymorphism and real-time fluorescence PCR assays. J. Clin. Microbiol. 41:3112-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan, Z. J., R. W. van der Hulst, M. Feller, S. D. Xiao, G. N. Tytgat, J. Dankert, and A. van der Ende. 1997. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis-associated dyspepsia. J. Clin. Microbiol. 35:1344-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 58.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73:760-770. [PubMed] [Google Scholar]
- 59.Peters, T. M., R. J. Owen, E. Slater, R. Varea, E. L. Teare, and S. Saverymuttu. 2001. Genetic diversity in the Helicobacter pylori cag pathogenicity island and effect on expression of anti-CagA serum antibody in UK patients with dyspepsia. J. Clin. Pathol. 54:219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rad, R., C. Prinz, B. Neu, M. Neuhofer, M. Zeitner, P. Voland, I. Becker, W. Schepp, and M. Gerhard. 2003. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J. Infect. Dis. 188:272-281. [DOI] [PubMed] [Google Scholar]
- 61.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. A. Falkow. 2000. Whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuuring, E., E. Verhoeven, W. J. Mooi, and R. J. Michalides. 1992. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7:355-361. [PubMed] [Google Scholar]
- 63.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveal VirD4/CagA-dependent and VirD4/CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277:6775-6778. [DOI] [PubMed] [Google Scholar]
- 66.Selbach, M., S. Moese, R. Hurwitz, C. R. Hauck, T. F. Meyer, and S. Backert. 2003. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 22:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma, S. A., M. K. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 69.Slater, E., R. J. Owen, M. Williams, and R. E. Pounder. 1999. Conservation of the cag pathogenicity island of Helicobacter pylori: associations with vacuolating cytotoxin allele and IS605 diversity. Gastroenterology 117:1308-1315. [DOI] [PubMed] [Google Scholar]
- 70.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43:971-980. [DOI] [PubMed] [Google Scholar]
- 71.Suerbaum, S., J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA × SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 278:3664-3670. [DOI] [PubMed] [Google Scholar]
- 73.Tummuru, M. K., S. A. Sharma, and M. J. Blaser. 1995. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 18:867-876. [DOI] [PubMed] [Google Scholar]
- 74.Uruno, T., J. Liu, P. Zhang, Y. X. Fan, C. Egile, R. Li, S. C. Mueller, and X. Zhan. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3:259-266. [DOI] [PubMed] [Google Scholar]
- 75.Weaver, A. M., A. V. Karginov, A. W. Kinley, S. A. Weed, Y. Li, J. T. Parsons, and J. A. Cooper. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11:370-374. [DOI] [PubMed] [Google Scholar]
- 76.Weed, S. A., A. V. Karginov, D. A. Schafer, A. M. Weaver, A. W. Kinley, J. A. Cooper, and J. T. Parsons. 2000. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamaoka, Y., D. H. Kwon, and D. Y. Graham. 2000. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 97:7533-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarrilli, R., V. Ricci, and M. Romano. 1999. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell. Microbiol. 1:93-99. [DOI] [PubMed] [Google Scholar]