Abstract
Aims/hypothesis
In type 2 diabetes, aggregation of islet amyloid polypeptide (IAPP) into amyloid is associated with beta-cell loss. As IAPP is co-secreted with insulin, we hypothesised that IAPP secretion is necessary for amyloid formation, and treatments which increase insulin (and IAPP) secretion would thereby increase amyloid formation and toxicity. Further, we hypothesised that the unique properties of the glucagon-like peptide-1 (GLP-1) receptor agonist exendin-4, to maintain or increase beta-cell mass, would offset the amyloid-induced toxicity.
Methods
Islets from amyloid-forming human IAPP (hIAPP) transgenic and control non-transgenic mice were cultured for 48 hours in 16.7 mmol/l glucose alone (control) or with exendin-4, potassium chloride (KCl), diazoxide or somatostatin. hIAPP and insulin release, amyloid deposition, beta-cell area/islet area and apoptosis and AKT phosphorylation levels were determined.
Results
In control hIAPP transgenic islets, amyloid formation was associated with increased beta-cell apoptosis and beta-cell loss. Increasing hIAPP release with exendin-4 or KCl increased amyloid deposition. However, while KCl further increased beta-cell apoptosis and beta-cell loss, exendin-4 did not. Conversely, decreasing hIAPP release with diazoxide or somatostatin limited amyloid formation and its toxic effects. Treatment with exendin-4 was associated with an increase in AKT phosphorylation compared to control and KCl-treated islets.
Conclusions/interpretation
IAPP release is necessary for islet amyloid formation and its toxic effects. Thus, use of insulin secretagogues to treat type 2 diabetes may result in increased islet amyloidogenesis and beta-cell death. However, the AKT-associated anti-apoptotic effects of GLP-1 receptor agonists, such as exendin-4, may limit the toxic effects of increased islet amyloid.
Keywords: IAPP, insulin secretion, islet amyloid, GLP-1 receptor agonist, exendin-4, potassium, somatostatin, beta-cell apoptosis, beta-cell proliferation, beta-cell area
INTRODUCTION
The islet beta-cell lesion of type 2 diabetes is characterised by a failure to secrete sufficient insulin due to both decreased beta-cell mass and function [1]. Beta-cell failure is progressive and manifests clinically as failure of monotherapy over time and the need for additional therapies to maintain glycaemic control [2]. Several factors underlie the progressive loss of beta-cell mass and function in type 2 diabetes. One such factor is islet amyloid formation that has been shown to be toxic to beta cells and contributes to beta-cell loss [3–7].
The unique constituent of islet amyloid is islet amyloid polypeptide (IAPP), a normal product of the beta cell that is co-secreted with insulin [8]. Several studies have shown that increased production or secretion of IAPP per se is not sufficient for amyloid to form [9–11]. However, several pieces of evidence suggest that under pro-amyloidogenic conditions, the magnitude of IAPP secretion is an important determinant of islet amyloid formation. We have previously shown that glucose dose-dependently increases amyloid deposition in cultured islets with minimal amyloid forming at 5.5 and 11.1 mmol/l glucose and increased amyloid forming at 16.7 and 33.3 mmol/l glucose [7]. This suggests that one factor contributing to amyloid formation may be an increase in β-cell secretory demand. In line with this, in mice under conditions that favour amyloid formation, decreasing beta-cell secretory demand with insulin sensitisers results in decreased amyloid formation [12]. Additionally, increasing beta-cell secretion with glucose, leucine, tolbutamide, alpha-ketoisocaproic acid or glutamine has been shown to result in increased amyloid formation as measured by electron microscopy in cultured transgenic mouse islets [13]. As the formation of amyloid is toxic to the beta cell and is associated with beta-cell loss [3–7], it is of interest and perhaps even imperative to determine whether insulin secretagogues exacerbate beta-cell loss via their effect to increase amyloid formation.
A class of medications recently introduced for treating type 2 diabetes is the glucagon-like peptide 1 (GLP-1) receptor agonists [14]. These compounds have been shown to increase insulin secretion in a glucose-dependent manner, and in clinical trials have been found to lower HbA1c levels and induce weight loss [15–17]. It has also been suggested, based on in vitro studies with human islets, that GLP-1 receptor agonists are capable of preserving islet morphology [18], while both in vitro and in vivo studies examining rodent islets suggest that GLP-1 receptor agonists may preserve or even increase beta-cell mass [19–23]. Thus, it is possible that GLP-1 receptor agonists may have long-term beneficial effects on the beta cell. Evidence for GLP-1 receptor agonists protecting against amyloid-induced toxicity comes from a recent study where the GLP-1 receptor agonist exendin-4 was able to partially protect immortalized beta cells from apoptosis and growth inhibition induced by acute treatment with exogenously applied, supraphysiological concentrations of synthetic IAPP [24]. However, whether this protection occurs under conditions where amyloid forms from endogenously released IAPP is unknown.
While aggregation of IAPP is well established as being toxic to beta cells, the site of formation and toxicity of IAPP aggregates has been under much debate. There are studies suggesting that extracellular and intracellular deposits can exert beta-cell toxicity (reviewed in [25] and [26]). Thus, determining if secretion of IAPP is necessary for amyloid formation and beta-cell toxicity will also determine whether intracellular and/or extracellular IAPP aggregates are the species that cause beta-cell toxicity.
A major difference between human and rodent islets is the propensity of human islets to form islet amyloid [27]. However, as studying humans and examining pancreatic morphology is inherently limited, this necessitates the use of animal models. Since mouse IAPP is not amyloidogenic, we developed a transgenic mouse model that expresses human IAPP (hIAPP) in its beta cells and develops amyloid deposits morphologically indistinguishable from those in humans [28]. Amyloid formation in these mice is associated with beta-cell loss and secretory dysfunction in vivo [4, 29] and beta-cell apoptosis and oxidative stress in vitro [6]. Thus this mouse model recapitulates many features of the human islet lesion, making it particularly suited to study the effects of medications on islet amyloid formation and its consequences. Further, the non-amyloid forming non-transgenic islets provide the ideal control for the effects of the interventions independent of islet amyloid, something that cannot be done in studies using human islets.
We therefore used islets isolated from our hIAPP transgenic mice along with non-transgenic control islets to determine whether hIAPP secretion is a determinant of amyloid deposition and its toxic effects. Further, we determined whether anti-apoptotic and pro-proliferative effects of GLP-1 receptor agonists occur in the setting of amyloidogenesis and can offset the toxicity of islet amyloid formation and provide added benefit to the beta cell.
METHODS
Islet isolation and culture
Islets were isolated from 10-week-old hemizygous F1 C57BL/6 × DBA/2J hIAPP transgenic mice and their non-transgenic littermates, as previously described [7]. ‘Principles of laboratory animal care’ (NIH publication no. 85–23, revised 1985; http://grants1.nih.gov/grants/olaw/references/phspol.htm) were followed. Animal studies were approved by the Institutional Animal Care and Use Committee at the VA Puget Sound Health Care System.
Islets were cultured overnight in RPMI-1640 medium containing 10% foetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 11.1 mmol/l glucose, then cultured for 48 ours (or 144 hours in a subset of studies) in medium containing 16.7 mmol/l glucose with and without exendin-4 (American Peptide Company, Sunnyvale, CA, USA), or the secretion inhibitors diazoxide (Sigma-Aldrich, St. Louis, MO, USA) or somatostatin (Sigma-Aldrich). Concentration-response studies were performed (data not shown) and final concentrations chosen so that exendin-4 (10 nmol/l) maximally stimulated, while diazoxide (250 μmol/l) and somatostatin (100 nmol/l) maximally inhibited insulin release. We chose 16.7 mmol/l glucose for two reasons. First, we have previously shown that this is a pro-amyloidogenic condition that allows modulation of amyloid formation [7]. Second, exendin-4 acts to increase insulin release in a glucose-dependent manner; thus, this is an appropriate glucose concentration at which to determine the consequences of exendin-4 action.
To establish a secretagogue control that would match insulin output achieved by exendin-4 without beneficial effects on beta-cell apoptosis and/or replication, the sulphonylureas glyburide (Sigma-Aldrich) and tolbutamide (Sigma-Aldrich) as well as potassium chloride (KCl; Sigma-Aldrich) were tested (concentration-response data not shown). Glyburide (0.5 μmol/l) and tolbutamide (0.5 mmol/l) did not increase insulin release throughout the 48-hour culture period (31.1 glyburide vs. 32.0 nmol l−1 islet−1 control, p=0.99, n=4; 31.7 tolbutamide vs. 31.2 nmol l−1 islet−1 control, p=0.89, n=4), consistent with many studies showing a decreased effect of continued sulphonylurea treatment on insulin release [30, 31]. In contrast, KCl (10 mmol/l) was effective in increasing insulin release (37.8 KCl vs. 22.3 μmol l−1 islet−1 control, p=0.01, n=8). Thus, a second set of experiments was performed culturing islets with and without KCl.
To determine the effects of increasing insulin release under physiological glucose concentration, islets were also cultured in media containing 11.1 mmol/l glucose with and without exendin-4 (10 nmol/l) and KCl (10 mmol/l).
Determination of optimal study length
We have previously shown that amyloid is detectable as early as 24 hours after culture in 16.7 mmol/l glucose and its deposition increases progressively over 144 hours [7]. To determine the optimal culture time for the current study, islets were cultured for 144 hours with or without exendin-4 with samples taken at 48-hour intervals. In the first 48 hours, exendin-4 significantly increased insulin release (46.5±1.5 exendin-4 vs. 33.5±1.6 nmol l−1 islet−1 control, p=0.007, n=4). However, following this period exendin-4 no longer increased insulin release above control (48–96 hrs, 23.6±3.2 exendin-4 vs. 28.7±3.6 nmol l−1 islet−1 control, p=0.02; 96–144 hrs, 20.8±0.9 exendin-4 vs. 23.6±1.8 nmol l−1 islet−1 control, p=0.19), consistent with the documented effect of prolonged exendin-4 treatment to result in GLP-1 receptor desensitization in vitro [32]. Therefore, we determined that culture for 48 hours provided the best time period for assessing the effects of exendin-4.
Assessment of hIAPP and insulin release and content
For measurement of hormone release, five islets were cultured in 1 ml of media in triplicate for 48 hours as previously described [13] and the concentrations of hIAPP and insulin in the culture media measured. For content, 5 islets in triplicate were extracted with acid-ethanol [6]. hIAPP and insulin concentrations were determined by ELISA using the Human Amylin Immunoabsorbance Assay with F024 and F002 as the capture and detection antibodies respectively (a gift from Amylin Pharmaceuticals, San Diego, CA) and Insulin Ultrasensitive (Mouse) ELISA (Alpco, Salem, NH, USA).
Histological measurement of amyloid deposition, beta-cell area, beta-cell apoptosis and beta-cell proliferation
Islets were fixed in 4% (wt/vol) phosphate-buffered paraformaldehyde for 30 min and embedded in agar and then in paraffin [7]. 10 μm sections were labelled as described below and assessments made by an observer blinded to the status of the sample on an average of 20 islets per condition per experiment using Image Pro Plus (Media Cybernetics, Bethesda, MD, USA). For assessment of amyloid and beta-cell area/islet area sections were labelled with thioflavin S to visualise amyloid deposits, insulin antibody to visualise beta cells, and Hoechst to visualise nuclei and amyloid and insulin area expressed as a percentage of islet area. For quantification of apoptosis and proliferation, the number of apoptotic and proliferating beta cells were determined by manual counting of apoptotic nuclei (using propidium iodide labelling to identify condensed nuclei, Fig 3a) and Ki67-positive nuclei (Fig 4a) respectively in insulin-positive cells and expressed as a percentage of total cell number.
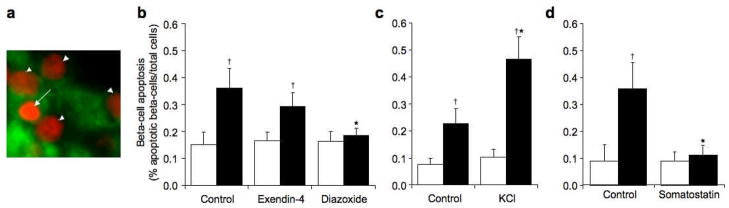
Figure 3.
Representative image of propidium iodide staining indicating an apoptotic nucleus (arrow) and non-apoptotic nuclei (arrow heads), propidium iodide = red, insulin = green (a). Beta-cell apoptosis (% apoptotic beta cells/total cells) in non-transgenic (open bars) and hIAPP transgenic (solid bars) islets cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4 or diazoxide (b), no drug (control) or KCl (c) or no drug (control) or somatostatin (d). *p<0.05 vs. control, †p<0.05 vs. non-transgenic, n=6–9.
Figure 4.
Representative image of Ki67 staining indicating a proliferating nucleus (arrow) and non-proliferating nuclei (arrow heads) Ki67 = red, insulin = green, nuclei = blue (a) Beta-cell proliferation (% Ki67 positive beta-cells/total cells) in non-transgenic (open bars) and hIAPP transgenic (solid bars) islets cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4 or diazoxide (b), no drug (control) or KCl (c). *p<0.05 vs. control, †p<0.05 vs. non-transgenic, n=6–9.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from 25 islets per condition (High Pure RNA isolation kit, Roche Applied Science, Indianapolis, IN, USA) and reverse transcribed (High Capacity cDNA Archive kit, Applied Biosystems, Foster City, CA, USA). mRNA expression of hIAPP and Insulin II was measured in triplicate using the TaqMan system (ABI Prism 7000; Applied Biosystems) with assays on demand (hIAPP, Hs00169095_m1; Insulin II, Mm00731595_gH, Applied Biosystems) and 18S as the endogenous control (Hs99999901_s1, Applied Biosystems). mRNA levels were calculated using the 2−ΔCt method and expressed relative to the experimental control (hIAPP transgenic no drug control for hIAPP and non-transgenic no drug control for insulin).
pAKT and AKT western blotting
Total protein was extracted from at least 200 islets per condition using the BioRad Cell Lysis kit (Bio-Rad Laboratories, Hercules, CA). 35 μg of islet protein per sample was separated by SDS-PAGE, protein transferred to PVDF membrane and then probed with a pAKT antibody (1:500; Cell Signaling, Danvers, MA) or total AKT antibody (1:500; Cell Signaling) followed by a goat anti-rabbit immunoglobulin/horseradish peroxidase antibody (1:50,000; Dako, Carpinteria, CA). Protein levels were normalised to the non-transgenic no drug control and pAKT expressed as a proportion of total AKT.
Statistical analyses
Data are expressed as mean ± SEM and were compared by analysis of variance with LSD post-hoc analysis or by Kruskal Wallis and Mann-Whitney U non-parametric tests if not normally distributed. A p value <0.05 was considered statistically significant.
RESULTS
hIAPP and insulin release and content
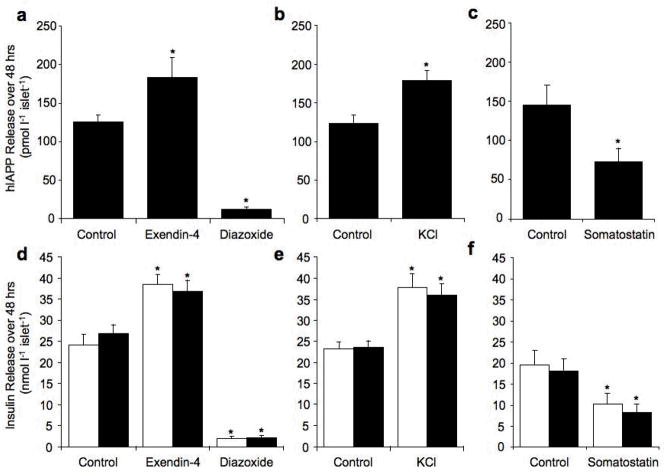
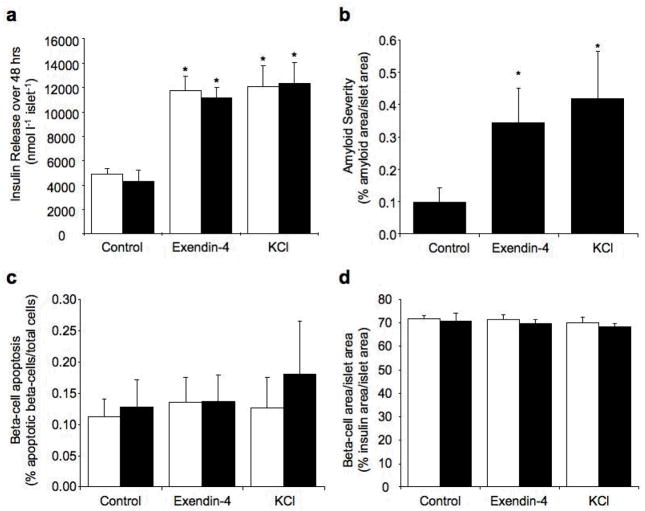
When hIAPP transgenic islets were cultured in 16.7 mmol/l glucose for 48 hours in the presence of exendin-4 or KCl, hIAPP release was similarly increased compared to hIAPP transgenic control islets (Fig. 1a and b). Conversely, culture with diazoxide or somatostatin decreased hIAPP release (Fig 1a and c). Insulin release was also increased by exendin-4 and KCl and decreased by diazoxide and somatostatin and did not differ between hIAPP transgenic and non-transgenic islets for any treatment (Fig 1d–f).
Figure 1.
hIAPP release (pmol l−1 islet−1) from hIAPP transgenic islets (a–c) and insulin release (nmol l−1 islet−1) from non-transgenic (open bars) and hIAPP transgenic (solid bars) islets (d–f) cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4 (10 nmol/l) or diazoxide (250 μmol/l) (a and d), no drug (control) or KCl (10 mmol/l) (b and e) or no drug (control) or somatostatin (100 nmol/l) (c and f). *p<0.05 vs. control, n=6–9.
When hIAPP transgenic islets were cultured in the presence of exendin-4 or KCl, hIAPP content was significantly lower compared to hIAPP transgenic control islets (Table 1 and 2). Conversely, in the presence of diazoxide, hIAPP content was significantly increased (Table 1). Similarly insulin content was decreased by exendin-4 and KCl and increased by diazoxide and did not differ between hIAPP transgenic and non-transgenic islets for any given treatment (Table 1 and 2).
Table 1.
Content and mRNA levels of hIAPP and insulin in control, exendin-4 and diazoxide treated islets.
| Non-transgenic | hIAPP transgenic | |||||
|---|---|---|---|---|---|---|
| Control | Exendin-4 | Diazoxide | Control | Exendin-4 | Diazoxide | |
| hIAPP Content (pmol l−1 islet−1) | - | - | - | 64.7±8.1 | 34.3±4.3a | 131.6±19.0a |
| Insulin Content (nmol l−1 islet−1) | 12.4±3.7 | 5.1±1.3a | 27.5±6.7a | 10.2±1.7 | 5.8±1.5a | 22.7±5.1a |
| hIAPP mRNA (normalised to hIAPP Transgenic Control) | - | - | - | 1.00±0.28 | 0.93±0.37 | 0.91±0.34 |
| insulin mRNA (normalised to Non-transgenic Control) | 1.00±0.17 | 1.13±0.12 | 0.79±0.08 | 1.11±0.18 | 1.32±0.19 | 0.84±0.12 |
p<0.05 vs. Control.
Table 2.
Content and mRNA levels of hIAPP and insulin in control and KCl treated islets.
| Non-transgenic | hIAPP transgenic | |||
|---|---|---|---|---|
| Control | KCl | Control | KCl | |
| hIAPP Content (pmol l−1 islet−1) | - | - | 78.7±16.8 | 36.5±6.9a |
| Insulin Content (nmol l−1 islet−1) | 9.2±2.7 | 5.9±1.8a | 9.2±2.0 | 5.0±1.2a |
| hIAPP mRNA (normalised to hIAPP Transgenic Control) | - | - | 1.00±0.19 | 1.07±0.20 |
| insulin mRNA (normalised to Non-transgenic Control) | 1.00±0.21 | 0.92±0.20 | 0.99±0.13 | 1.01±0.12 |
p<0.05 vs. Control
hIAPP and insulin mRNA levels
To determine whether the selected treatments affected hIAPP or insulin mRNA expression, real-time PCR was performed. Islets cultured in 16.7 mmol/l glucose for 48 hours revealed no difference in expression of hIAPP or insulin under any treatment (Tables 1 and 2).
Islet amyloid deposition
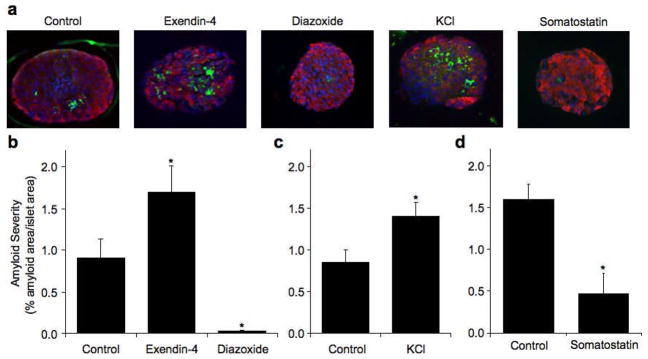
Culture of hIAPP transgenic islets for 48 hrs in 16.7 mmol/l glucose was associated with islet amyloid formation, while, as expected, no amyloid was present in non-transgenic islets (Fig 2a–d). Culture with either exendin-4 or KCl resulted in a similar increase in amyloid deposition relative to hIAPP transgenic control islets (1.9- fold exendin-4 vs. 1.7-fold KCl, p=0.25, Fig 2a–c). In contrast, diazoxide and somatostatin decreased amyloid deposition relative to control hIAPP transgenic islets (Fig 2a, b and d).
Figure 2.
Representative images of hIAPP transgenic islets cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4, diazoxide, KCl or somatostatin, amyloid = green, insulin = red, nuclei = blue (a). Islet amyloid severity (% amyloid area/islet area) in hIAPP transgenic islets cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4 or diazoxide (b) no drug (control) or KCl (c) or no drug (control) or somatostatin (d). *p<0.05 vs. control, n=6–9. Non-transgenic islets did not form amyloid.
Beta-cell apoptosis
Amyloid formation in hIAPP transgenic control islets was associated with an increase in beta-cell apoptosis compared to non-transgenic control islets (Fig 3b–d). hIAPP transgenic islets treated with exendin-4 exhibited increased beta-cell apoptosis compared to exendin-4 treated non-transgenic islets (Fig 3b). However, despite increased amyloid formation, beta-cell apoptosis was not significantly different to hIAPP transgenic control islets (0.8-fold, p=0.35, Fig 3b). In contrast, the increase in amyloid observed in hIAPP transgenic islets with KCl was associated with a 2.1-fold increase in beta-cell apoptosis when compared to hIAPP transgenic control islets (Fig 3c). Despite similar increases in amyloid deposition, based on fold differences over controls, beta-cell apoptosis in exendin-4 treated hIAPP transgenic islets was reduced compared to KCl treated islets (p=0.007). The increased apoptosis with KCl was not due to a toxic effect of KCl treatment per se, as there was no difference in rates of beta-cell apoptosis in non-transgenic islets cultured with or without KCl (p=0.7). When amyloid formation was decreased with diazoxide or somatostatin, beta-cell apoptosis decreased compared to hIAPP transgenic control islets and was comparable to similarly treated non-transgenic islets (Fig 3b and d).
Beta-cell proliferation
No changes in beta-cell proliferation were detectable between non-transgenic and hIAPP transgenic islets or under any treatment condition (Fig 4b and c).
Beta-cell area/islet area
Islet area did not differ among any treatment group (p=0.53, average islet area 10757±356 μm2). Thus, any changes in beta-cell area did not result from alterations in islet size.
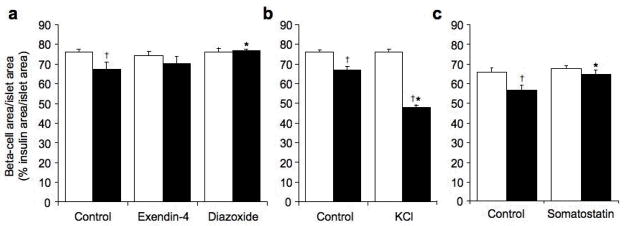
Amyloid formation together with increased beta-cell apoptosis in hIAPP transgenic control islets was associated with a significant reduction in beta-cell area/islet area when compared to non-transgenic control islets (Fig 5a–c). In contrast, despite increased amyloid formation in exendin-4 treated hIAPP transgenic islets, beta-cell area/islet area was not significantly different between hIAPP transgenic islets with or without exendin-4 treatment or between hIAPP transgenic and non-transgenic islets treated with exendin-4 (Fig 5a). On the other hand, hIAPP transgenic islets treated with KCl exhibited a marked reduction in beta-cell area/islet area compared to both control hIAPP transgenic islets and non-transgenic KCl treated islets consistent with the increased beta-cell apoptosis (Fig 5b). The decreased beta-cell area/islet area in the KCl-treated hIAPP transgenic islets was not due to an effect of KCl treatment per se, as non-transgenic islets with and without KCl treatment had similar beta-cell area/islet area (p=0.6). hIAPP transgenic islets treated with diazoxide or somatostatin, exhibited increased beta-cell area/islet area compared to hIAPP transgenic control islets, such that beta-cell area/islet area did not differ between similarly treated hIAPP transgenic and non-transgenic islets (Fig 5a and c).
Figure 5.
Beta-cell area/islet area (% insulin area/islet area) in non-transgenic (open bars) and hIAPP transgenic (solid bars) islets cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4 or diazoxide (a), no drug (control) or KCl (b) or no drug (control) or somatostatin (c). *p<0.05 vs. control, †p<0.05 vs. non-transgenic, n=6–8 (non-transgenic) and n=8–9 (hIAPP transgenic).
Effect of increased beta-cell secretion on amyloid formation at physiological glucose
In islets cultured in 11.1 mmol/l glucose for 48 hours, both exendin-4 and KCl treatment resulted in increased insulin release (Fig 6a).
Figure 6.
Insulin release (nmol l−1 islet−1) (a), islet amyloid severity (% amyloid area/islet area) (b), beta-cell apoptosis (% apoptotic beta cells/total cells) (c) and beta-cell area/islet area (% insulin area/islet area) (d) in non-transgenic (open bars) and hIAPP transgenic (solid bars) islets cultured for 48 hours in 11.1 mmol/l glucose with no drug (control), exendin-4 (10 nmol/l) or KCl (10 mmol/l). *p<0.05 vs. control, n=6. Non-transgenic islets did not form amyloid.
As expected, culture of hIAPP transgenic islets for 48 hrs in 11.1 mmol/l glucose was associated with minimal islet amyloid formation, while no amyloid was present in non-transgenic islets (Fig 6b). This minimal amyloid formation was not associated with any changes in beta-cell apoptosis or beta-cell area/islet area compared to non-transgenic islets (Fig 6c–d). Culture with exendin-4 and KCl resulted in a significant increase in amyloid deposition relative to hIAPP transgenic control islets (Fig 6b). Again however, this small increase in amyloid was not associated with a change in beta-cell apoptosis or beta cell area/islet area (Fig 6c–d).
Activation of AKT as a Molecular Mechanism for Exendin-4’s Protective Effect
Finally, we sought to elucidate the molecular mechanism that may explain the ability of exendin-4 to limit beta-cell apoptosis in the face of amyloid formation. Amyloid formation in hIAPP transgenic islets was not associated with decreased AKT phosphorylation when compared to non-transgenic islets (Fig 7). However, exendin-4 treatment was associated with a 1.5-fold increase in phosphorylation of AKT in hIAPP transgenic and non-transgenic islets (Fig 7).
Figure 7.
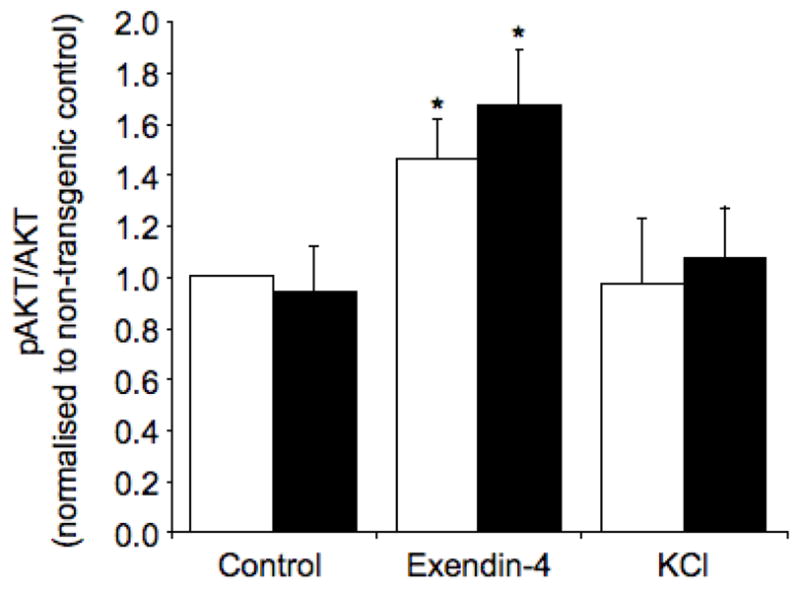
pAKT levels normalized to total AKT in non-transgenic (open bars) and hIAPP transgenic (solid bars) islets cultured for 48 hours in 16.7 mmol/l glucose with no drug (control), exendin-4 or KCl. *p<0.05 vs. control, n=5.
DISCUSSION
We have demonstrated that in hIAPP transgenic mouse islets cultured under amyloidogenic conditions induced by high glucose, decreasing hIAPP release with diazoxide or somatostatin resulted in decreased amyloid deposition and beta-cell apoptosis and preservation of beta-cell area/islet area to levels similar to non-transgenic islets. This indicates that hIAPP release from the beta cell is critical for amyloid formation. Further, as diazoxide treatment also resulted in increased cellular content of hIAPP, the intracellular accumulation of hIAPP is not sufficient to result in amyloid formation or induce beta-cell apoptosis. This supports the concept that extracellular aggregation of hIAPP is toxic and can result in beta-cell loss.
Under these same amyloidogenic conditions, treatment with the GLP-1 receptor agonist exendin-4 further increased amyloid deposition. However, beta-cell apoptosis was lower and beta-cell area/islet area was higher than would be expected for this increased level of amyloid formation. Therefore, while exendin-4 does result in increased amyloidogenesis, it also appears to offset the toxic effects of amyloid deposition by limiting beta-cell apoptosis, resulting in beta-cell preservation. Consistent with this hypothesis, increasing hIAPP release with KCl, which was not expected to exert anti-apoptotic effects, resulted in a similar increase in amyloid deposition as exendin-4 but simultaneously also increased beta-cell apoptosis, the net result being a decrease in beta-cell area/islet area. These results are also consistent with the effects of exendin-4 and KCl to increase amyloid deposition at lower (11.1 mmol/l) glucose. However, with this glucose concentration, only minimal amounts of amyloid formed, resulting in no detectible increase in beta-cell apoptosis or beta-cell loss.
GLP-1 receptor agonists have been shown to enhance beta-cell survival by decreasing apoptosis in many paradigms, including islet transplantation [33]. In vitro experiments have shown that GLP-1 receptor agonists protect the beta-cell from apoptosis resulting from insults including cytokines, ER stress, and glucolipotoxicity (reviewed in [34]). A major signalling molecule induced by GLP-1 receptor agonists is protein kinase A, which in turn activates the protein kinase AKT. Inhibition of AKT has been shown to abolish GLP-1 receptor agonist’s ability to protect against the deleterious effects of cytokines [35, 36], and gluco- and/or lipotoxicity [35, 37], indicating that activation of AKT is a major mechanism by which GLP-1 receptor agonists protect beta cells. On the other hand, GLP-1 receptor agonist protection from ER stress has been shown to occur via direct modulation of the ER stress pathway [38, 39]. As we have previously shown that ER stress is not induced in hIAPP transgenic or non-transgenic islets cultured in 16.7 mmol/l glucose [40], we focused on the AKT pathway as the mechanism by which exendin-4 could protect against amyloid associated apoptosis. We found no difference in AKT activation between hIAPP transgenic and non-transgenic islets indicating that decreased AKT activation is not a mechanism by which the amyloid formed from physiological amounts of endogenously produced hIAPP exerts its beta-cell toxicity. However, exendin-4 treatment significantly increased AKT activation in both hIAPP transgenic and non-transgenic islets. Therefore it is feasible that the effect of exendin-4 to limit beta-cell apoptosis occurred via increased AKT activation. Our observations are in keeping with a recent report that acute treatment of the INS-1 beta-cell line with exogenously applied, supraphysiological concentrations of synthetic hIAPP induced apoptosis and growth inhibition and exendin-4 partially protected from this toxicity by increasing AKT activation [24].
We have shown that while exendin-4 can decrease beta-cell apoptosis in hIAPP transgenic islets, there was no effect in non-transgenic islets. As exendin-4 is well documented to have anti-apoptotic effects in mouse islets [19, 41, 42], and we demonstrated that exendin-4 treatment resulted in increased AKT activation, this was somewhat surprising. However, the studies where a beneficial effect of exendin-4 was shown were performed under stress conditions where apoptosis was elevated. We have previously shown that non-transgenic islets cultured for 48 hours in 16.7 mmol/l glucose do not exhibit stress responses such as oxidative stress, ER stress or elevated beta-cell apoptosis compared to non-transgenic islets cultured in 5.5 mmol/l glucose [6, 40]. Thus, it is likely that exendin-4 treatment and/or elevated levels of phospho-AKT under these non-stress conditions does not decrease the low levels of basal beta-cell apoptosis.
Interestingly, in this study there was no effect of exendin-4 to increase beta-cell proliferation or insulin mRNA levels or content. Beta-cell proliferation has been shown to increase with exendin-4 treatment in many [19, 43] but not all rodent studies [43, 44]. Therefore the effects of exendin-4 on proliferation may depend on many factors including the diabetic milieu, the prevailing beta-cell replication rate and the animal strain used. Additionally, to our knowledge, no studies have shown an effect of exendin-4 to increase proliferation in vitro in cultured islets. In fact, one study demonstrated that GLP-1 receptor activation increased proliferation in vivo but did not affect proliferation when islets from the same mice were studied in vitro [43]. Thus it is possible that exendin-4 is unable to stimulate proliferation in vitro. Similarly, insulin mRNA and content have been shown to be increased with GLP-1 receptor activation in some studies [20, 45] but not others [46]. Thus, these effects of exendin-4 may also depend upon the diabetic milieu, animal strain or culture conditions used.
A limitation of using an in vitro culture system is that the effect of exendin-4 on pancreatic progenitor cell proliferation cannot be assessed. Studies have shown that GLP-1 signalling promotes neogenesis thereby increasing beta-cell mass [47–49]. Thus it is possible that in the setting of islet amyloidogenesis exendin-4 may still promote neogenesis and thus contribute to improved beta-cell mass and function in the long-term. Therefore, further work is required using an in vivo model in order to assess the combined effect of amyloid and exendin-4 on beta-cell neogenesis. An in vivo model would also allow further examination of the long-term consequences of exendin-4 treatment on amyloid deposition and its toxic effects.
We have shown that exendin-4 treatment is beneficial to maintain beta-cell area/islet area under amyloidogenic conditions as compared to KCl treatment. However, when compared to untreated hIAPP transgenic islets, exendin-4 seemed not to have a beneficial effect. This observation is comparable to findings from clinical studies which show a beneficial effect on glycaemic control during active treatment [15–17], but no persistent glucose-lowering benefit of treatment after withdrawal of the drug, consistent with a lack of clinically significant effect to improve beta-cell mass [50]. However, we have now shown that to correctly interpret the effects of exendin-4, a control that is appropriately matched for insulin release must be used. The possibility of a difference between GLP-receptor agonists and other insulin secretagogues such as sulphonylureas will need to be addressed in a long-term clinical study.
In summary, we have shown that hIAPP release is a critical determinant of amyloid formation in vitro and that increased hIAPP release with KCl results in increased amyloid formation, increased beta-cell apoptosis and decreased beta-cell area/islet area. However, exendin-4, likely via increased AKT activation, offsets the effect of the increased amyloid formation from increasing beta-cell apoptosis or decreasing beta-cell area. Thus, from the clinical perspective it is possible that treatment of type 2 diabetes with insulin secretagogues may result in increased amyloid formation and exacerbation of beta-cell death, but that use of GLP-1 receptor agonists, such as exendin-4, may offset some of these adverse effects and thus may be beneficial in terms of limiting loss of beta-cell mass and the progression of type 2 diabetes.
Acknowledgments
We thank C. Braddock, B. Barrow, M. Cone, J. Willard, M. Watts, M. Peters, C. Forsyth and R. Bhatti for excellent technical support. This work was supported by the Department of Veterans Affairs and National Institutes of Health grants DK-075998 (SEK), DK-074404 (RLH), DK-080945 (SZ), DK-007247 and DK-017047. K. Aston-Mourney was supported by an American Diabetes Association Mentor-Based Fellowship and the University of Washington McAbee Fellowship. J. Udayasankar was supported by a Juvenile Diabetes Research Foundation Postdoctoral Fellowship.
Abbreviations
- GLP-1
Glucagon like peptide-1
- hIAPP
human islet amyloid polypeptide
- IAPP
islet amyloid polypeptide
- KCl
potassium chloride
Footnotes
DUALITY OF INTEREST
The authors declare that there is no duality of interest associated with the manuscript.
References
- 1.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52:1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner RC. The U.K. Prospective Diabetes Study. A review. Diabetes Care. 1998;21(S3):C35–38. doi: 10.2337/diacare.21.3.c35. [DOI] [PubMed] [Google Scholar]
- 3.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 4.Hull RL, Andrikopoulos S, Verchere CB, et al. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- 6.Zraika S, Hull RL, Udayasankar J, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zraika S, Hull RL, Udayasankar J, et al. Glucose- and time-dependence of islet amyloid formation in vitro. Biochem Biophys Res Commun. 2007;354:234–239. doi: 10.1016/j.bbrc.2006.12.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn SE, D’Alessio DA, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 9.Clark A, Saad MF, Nezzer T, et al. Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia. 1990;33:285–289. doi: 10.1007/BF00403322. [DOI] [PubMed] [Google Scholar]
- 10.Westermark G, Arora MB, Fox N, et al. Amyloid formation in response to beta cell stress occurs in vitro, but not in vivo, in islets of transgenic mice expressing human islet amyloid polypeptide. Mol Med. 1995;1:542–553. [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessio DA, Verchere CB, Kahn SE, et al. Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes. 1994;43:1457–1461. doi: 10.2337/diab.43.12.1457. [DOI] [PubMed] [Google Scholar]
- 12.Hull RL, Shen ZP, Watts MR, et al. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54:2235–2244. doi: 10.2337/diabetes.54.7.2235. [DOI] [PubMed] [Google Scholar]
- 13.MacArthur DL, de Koning EJ, Verbeek JS, Morris JF, Clark A. Amyloid fibril formation is progressive and correlates with beta-cell secretion in transgenic mouse isolated islets. Diabetologia. 1999;42:1219–1227. doi: 10.1007/s001250051295. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessio DA, Vahl TP. Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab. 2004;286:E882–890. doi: 10.1152/ajpendo.00014.2004. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 16.Nauck MA, Ratner RE, Kapitza C, Berria R, Boldrin M, Balena R. Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care. 2009;32:1237–1243. doi: 10.2337/dc08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 19.Farilla L, Hui H, Bertolotto C, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- 20.Kim JG, Baggio LL, Bridon DP, et al. Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003;52:751–759. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- 21.Rolin B, Larsen MO, Gotfredsen CF, et al. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 22.Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 23.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 24.Fan R, Li X, Gu X, Chan JC, Xu G. Exendin-4 protects pancreatic beta cells from human islet amyloid polypeptide-induced cell damage: potential involvement of AKT and mitochondria biogenesis. Diabetes Obes Metab. 2010;12:815–824. doi: 10.1111/j.1463-1326.2010.01238.x. [DOI] [PubMed] [Google Scholar]
- 25.Khemtemourian L, Killian JA, Hoppener JW, Engel MF. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Exp Diab Res. 2008;2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocrine Reviews. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verchere CB, D’Alessio DA, Palmiter RD, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udayasankar J, Kodama K, Hull RL, et al. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia. 2009;52:145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunbar JC, Foa PP. An inhibitory effect of tolbutamide and glibenclamide (glyburide) on the pancreatic islets of normal animals. Diabetologia. 1974;10:27–35. doi: 10.1007/BF00421411. [DOI] [PubMed] [Google Scholar]
- 31.Rabuazzo AM, Buscema M, Vinci C, et al. Glyburide and tolbutamide induce desensitization of insulin release in rat pancreatic islets by different mechanisms. Endocrinology. 1992;131:1815–1820. doi: 10.1210/endo.131.4.1396327. [DOI] [PubMed] [Google Scholar]
- 32.Baggio LL, Kim JG, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes. 2004;53(S3):S205–214. doi: 10.2337/diabetes.53.suppl_3.s205. [DOI] [PubMed] [Google Scholar]
- 33.Emamaullee JA, Merani S, Toso C, et al. Porcine marginal mass islet autografts resist metabolic failure over time and are enhanced by early treatment with liraglutide. Endocrinology. 2009;150:2145–2152. doi: 10.1210/en.2008-1116. [DOI] [PubMed] [Google Scholar]
- 34.Lavine JA, Attie AD. Gastrointestinal hormones and the regulation of beta-cell mass. Ann NY Acad Sci. 1212:41–58. doi: 10.1111/j.1749-6632.2010.05802.x. [DOI] [PubMed] [Google Scholar]
- 35.Bregenholt S, Moldrup A, Blume N, et al. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–584. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48:1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 37.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47:806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- 38.Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metabolism. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Cunha DA, Ladriere L, Ortis F, et al. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58:2851–2862. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL, Kahn SE. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia. 2009;52:1102–1111. doi: 10.1007/s00125-009-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferdaoussi M, Abdelli S, Yang JY, et al. Exendin-4 protects beta-cells from interleukin-1 beta-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes. 2008;57:1205–1215. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 43.Edvell A, Lindstrom P. Initiation of increased pancreatic islet growth in young normoglycemic mice (Umea +/?) Endocrinology. 1999;140:778–783. doi: 10.1210/endo.140.2.6514. [DOI] [PubMed] [Google Scholar]
- 44.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007;148:5136–5144. doi: 10.1210/en.2007-0358. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Perfetti R, Greig NH, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. 1997;99:2883–2889. doi: 10.1172/JCI119482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Haluzik M, Asghar Z, et al. Peroxisome proliferator-activated receptor-alpha agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes. 2003;52:1770–1778. doi: 10.2337/diabetes.52.7.1770. [DOI] [PubMed] [Google Scholar]
- 47.Hui H, Wright C, Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes. 2001;50:785–796. doi: 10.2337/diabetes.50.4.785. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Wang X, Pineyro MA, Egan JM. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48:2358–2366. doi: 10.2337/diabetes.48.12.2358. [DOI] [PubMed] [Google Scholar]
- 49.Hardikar AA, Wang XY, Williams LJ, et al. Functional maturation of fetal porcine beta-cells by glucagon-like peptide 1 and cholecystokinin. Endocrinology. 2002;143:3505–3514. doi: 10.1210/en.2001-211344. [DOI] [PubMed] [Google Scholar]
- 50.Bunck MC, Diamant M, Corner A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]