Abstract
The GABA projection neurons of the substantia nigra pars reticulata (SNr) are output neurons for the basal ganglia and thus critical for movement control. Their most striking neurophysiological feature is sustained, spontaneous high frequency spike firing. A fundamental question is: what are the key ion channels supporting the remarkable firing capability in these neurons? Recent studies indicate that these neurons express tonically active TRPC3 channels that conduct a Na-dependent inward current even at hyperpolarized membrane potentials. When the membrane potential reaches −60 mV, a voltage-gated persistent sodium current (INaP) starts to activate, further depolarizing the membrane potential. At or slightly below −50 mV, the large transient voltage-activated sodium current (INaT) starts to activate and eventually triggers the rapid rising phase of action potentials. SNr GABA neurons have a higher density of (INaT), contributing to the faster rise and larger amplitude of action potentials, compared with the slow-spiking dopamine neurons. INaT also recovers from inactivation more quickly in SNr GABA neurons than in nigral dopamine neurons. In SNr GABA neurons, the rising phase of the action potential triggers the activation of high-threshold, inactivation-resistant Kv3-like channels that can rapidly repolarize the membrane. These intrinsic ion channels provide SNr GABA neurons with the ability to fire spontaneous and sustained high frequency spikes. Additionally, robust GABA inputs from direct pathway medium spiny neurons in the striatum and GABA neurons in the globus pallidus may inhibit and silence SNr GABA neurons, whereas glutamate synaptic input from the subthalamic nucleus may induce burst firing in SNr GABA neurons. Thus, afferent GABA and glutamate synaptic inputs sculpt the tonic high frequency firing of SNr GABA neurons and the consequent inhibition of their targets into an integrated motor control signal that is further fine-tuned by neuromodulators including dopamine, serotonin, endocannabinoids, and H2O2.
Keywords: action potential, basal ganglia, ion channel, Parkinson’s disease, substantia nigra, synapse
1. Introduction
The basal ganglia are a group of interconnected subcortical nuclei that include the caudate nucleus and putamen (the striatum), the globus pallidus external and internal segments (GPe and GPi), the subthalamic nucleus (STN), and the substantia nigra pars compacta and pars reticulata (SNc and SNr) (Fig. 1A; Parent et al., 2000; Gerfen and Bolam, 2010). The SNr and GPi (or entopeduncular nucleus in rodents) carry out an important role in basal ganglia physiology in that they are the output neurons for the basal ganglia network. Their projection neurons release GABA and subserve the important function of inhibiting and disinhibiting neurons in the main target nuclei which are the thalamus and superior colliculus. The spiking activity of SNr GABA neurons is shaped by a combination of intrinsic conductances that generate the tonic, high frequency, regular firing that is characteristic of these neurons under most conditions, and extrinsic influences that can increase or decrease their firing rate and in some cases change their firing pattern. Thus, an understanding of the intrinsic and extrinsic factors that influence the firing of nigral GABA neurons is central to elucidating how the basal ganglia control movement and other behaviors. Here we review the key anatomical and physiological characteristics of SNr GABA neurons that underlie the ability of these neurons to control target neurons and integrate input from other basal ganglia nuclei and elsewhere in the brain.
Fig. 1.
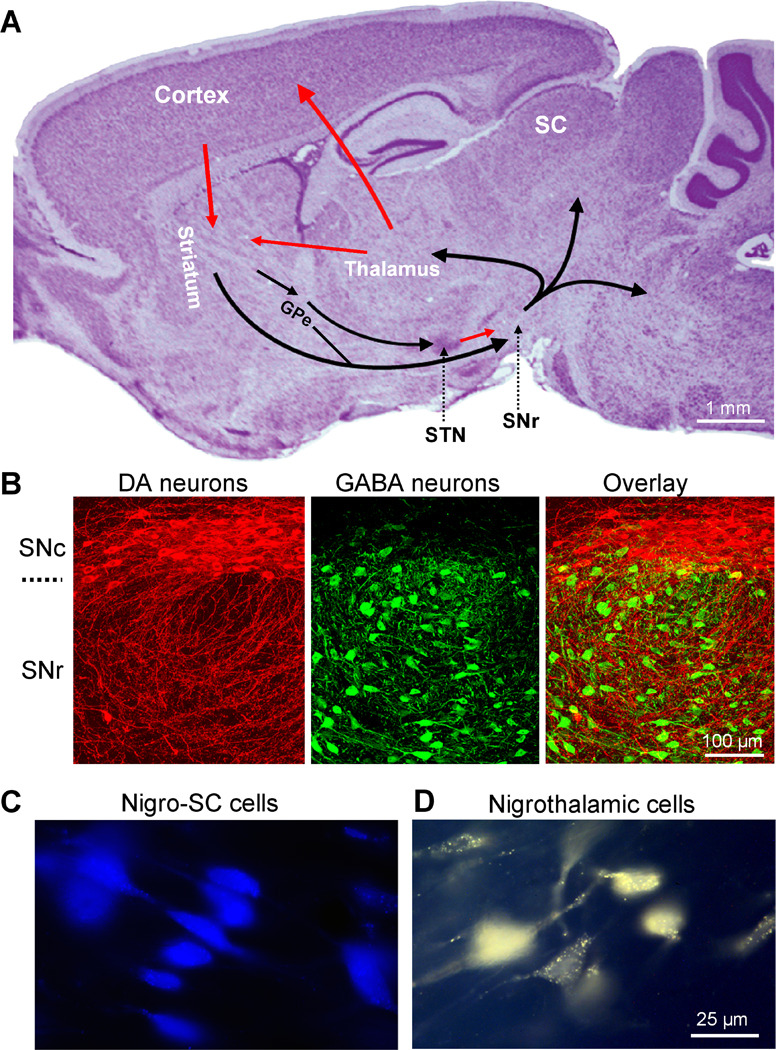
The substantia nigra pars reticulata (SNr) is a key output nucleus of the basal ganglia. (A) A Nissl-stained sagittal mouse brain section showing the location of SNr and other major components of the basal ganglia. Arrows indicate information flow directions. The SNr receives information from several components of the basal ganglia and sends output to the thalamus, superior colliculus (SC), and brainstem motor structures. Unpublished data of FMZ. (B) Confocal images of double immunohistochemical staining for tyrosine hydroxylase (TH, red, a key enzyme for dopamine synthesis) and parvalbumin (PV, green, expressed only in GABA neurons). Most TH-positive dopamine neurons are in SNc with their dendrites extending into SNr where most neurons are PV-positive GABA neurons. Modified from Zhou et al. 2009 with permission. (C) Examples of retrogradely labeled SNr neurons projecting to the superior colliculus (SC). Blue is a pseudocolor in this image for easier visualization. (D) Examples of retrogradely labeled SNr neurons projecting to the thalamus. C and D are modified from Lee and Tepper, 2007a with permission. Copyright 2006 Wiley-Liss, Inc.
2. Key anatomical features of SNr GABA projection neurons
The substantia nigra is located in the ventral midbrain and bordered dorsally by the medial lemniscus and ventrally by the cerebral peduncle. It is divided into the more dorsal SNc with primarily dopamine neurons and the more ventral SNr with primarily GABA neurons (Fig. 1B). There is, however, some intermixing of dopamine neurons within the SNr (González-Hernández and Rodríguez, 2000). SNr GABA neurons are medium-to large-sized and have mostly ovoid or fusiform somata that emit 2–6 primary dendrites which branch frequently (Grofová et al., 1982; Cebrián et al., 2007; Lee and Tepper, 2007a). A single main axon typically arises from the soma or a primary dendrite and courses through the substantia nigra issuing one or more local axon collaterals before leaving the nucleus (Deniau et al., 1982; Grofová et al., 1982; Kemel et al., 1988; Mailly et al., 2003).
One primary target of SNr GABA neurons is the thalamus (Fig. 1A, D) where they form most of their synapses in the ventromedial and intralaminar thalamic nuclei in rodents (Beckstead et al., 1979; Di Chiara et al., 1979; Herkenham, 1979; Nishimura et al., 1997) and the ventral anterior and ventral lateral nuclei in primates (Carpenter et al., 1976; Hazrati and Parent, 1991; François et al., 2002). Axons from SNr GABA neurons form synapses on the proximal dendrites and somata of relay neurons in the thalamus of both rodents and primates, thus forming a structural basis for nigral inhibition of thalamic neurons (Bodor et al., 2008). Indeed, electrical stimulation of nigrothalamic neurons produces a short latency, short duration inhibition of thalamic neurons (Deniau et al., 1978; MacLeod et al., 1980; Tanibuchi et al., 2009a,b). Conversely, inhibition of the SNr leads to increases in the spontaneous discharge of thalamic neurons demonstrating a tonic inhibition of those neurons by SNr GABA cells (MacLeod et al., 1980; Deniau and Chevalier, 1985). The other main target of SNr GABA neurons is the superior colliculus (Fig. 1C) (Beckstead et al., 1979; Di Chiara et al., 1979; Beckstead and Frankfurter, 1982; Williams and Faull, 1988; Hikosaka et al., 2000) where stimulation of SNr GABA neurons induces short latency inhibition of neurons in that structure (Chevalier et al., 1981; Hikosaka and Wurtz, 1983b; Hikosaka and Wurtz, 1985a,b). Although commonly projecting to one primary target nucleus, some SNr GABA neurons send axon collaterals to multiple targets (Anderson and Yoshida, 1980; Beckstead, 1983; Parent et al. 1983; Cebrián et al., 2005). There are also less well characterized projections from SNr GABA neurons to multiple brainstem and midbrain structures (e.g. Takakusaki et al., 2003, 2004). SNr GABA neurons also issue local axon collaterals that carry out an important role of inhibition within the substantia nigra itself. These local axon collaterals arborize throughout both segments of the substantia nigra and frequently have varicosities resembling both en passant and terminal boutons (Deniau et al., 1982; Grofová et al., 1982; Kemel et al., 1988; Mailly et al., 2003; Lee and Tepper, 2007a). Both SNr GABA neurons and SNc dopamine neurons are locally inhibited via axon collaterals from SNr GABA neurons (Deniau et al., 1982, Tepper et al., 1995; Saitoh et al., 2004). These efferent connections in combination with afferent inputs (discussed in sections on individual synaptic inputs) provide the anatomical bases for SNr GABA neurons to affect multiple aspects of movement control under physiological and pathological conditions.
3. SNr GABA projection neurons fire sustained autonomous high frequency spikes
A striking neurophysiological feature of SNr GABA neurons is their sustained high frequency spike firing (Fig. 2A). SNr GABA neurons fire spikes of short duration (~ 1 ms) spontaneously at very high frequencies (Fig. 2A–D) (around 25–30 Hz in awake rodents: Gulley et al., 1999; Gulley et al., 2004; Maurice et al., 2003; Windels and Kiyatkin, 2006a,b; Walters et al., 2007; around 65 Hz in awake primates: DeLong et al., 1983; Hikosaka and Wurtz, 1983a; Schultz, 1986; Wichmann et al., 1999; Nevet et al., 2004; Wichmann and Kliem, 2004). In contrast, nigral dopamine neurons fire low frequency (often at 1–4 Hz) and long duration spikes (about 2.5 ms) (Lacey et al., 1989; Yung et al., 1991; Richards et al., 1997; Hyland et al., 2002; Zhou et al., 2006; Guzman et al., 2009). After blockade of ionotropic receptor-mediated synaptic inputs in vitro, the spiking activity of SNr GABA neurons remains strikingly different from their neighboring dopamine neurons (Fig. 2B–D). In vitro, SNr GABA neurons fire around 10–15 Hz while dopamine neurons fire spontaneously around 2 Hz (Lacey et al., 1989; Richards et al., 1997; Yung et al., 1991; Blythe et al., 2007; Lee and Tepper, 2007b; Zhou et al., 2006; Seutin and Engel, 2010; Ding et al., 2011; Lee et al., 2011). The lower firing rate in vitro is largely due to low recording temperatures (commonly room temperature to 30 ˚C). Additionally, when compared with nigral dopamine neurons, the action potentials of SNr GABA neurons have a more negative threshold, a larger amplitude, and faster rise rate. Also, SNr GABA neurons show no or little adaptation in both firing frequency and spike amplitude, whereas nigral dopamine neurons are adaptive in both parameters. These striking differences in spike waveform and pattern indicate qualitative and/or quantitative differences in intrinsic ion channels in SNr GABA neurons and nigral dopamine neurons.
Fig. 2.
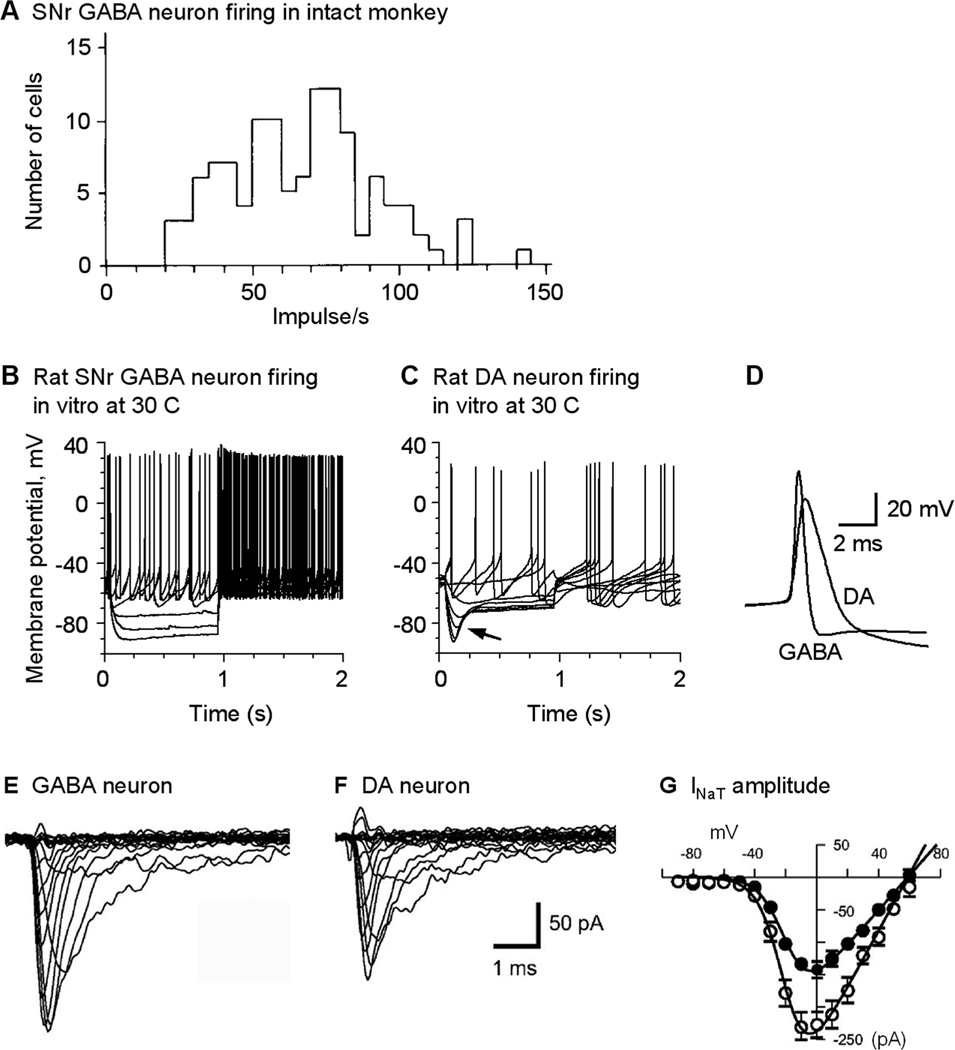
SNr GABA neurons fire sustained spontaneous high frequency spikes. (A) In intact primates, SNr GABA neurons fire tonic high frequency spikes. Modified from Schultz 1986 with permission. (B, C) In isolated preparations with fast synaptic inputs blocked and compared with nigral dopamine neurons, SNr GABA neurons still fire sustained high frequency spikes. In SNr GABA neurons, the Ih is weak whereas it is strong in nigral DA neurons as indicated by the arrow. Modified from Ding et al. 2011 with permission. (D) Spikes in SNr GABA neurons are larger in amplitude and shorter in duration than nigral DA neurons. (E, F, G) SNr GABA neurons have a higher density of the transient voltage-activated sodium current INaT than nigral DA neurons. E–G are modified from Seutin and Engel, 2010 with permission.
4. Key ion channels supporting the sustained autonomous high frequency firing in SNr GABA neurons
Several recent studies indicate that multiple ion channels are critical to the sustained high frequency firing in SNr GABA neurons, including a tonically active transient receptor potential (TRP) channel, voltage-gated sodium (NaV), potassium (Kv), and calcium (CaV) channels, and calcium-activated potassium (KCa) channels (Atherton and Bevan, 2005; Zhou et al., 2008; Ding and Zhou, 2010; Seutin and Engel, 2010; Ding et al., 2011). The sections below summarize some key recent findings and discuss the functional importance of these intrinsic ion channels in SNr GABA neurons.
4.1. Constitutively active classical type 3 transient receptor potential (TRPC3) channels depolarize SNr GABA neurons
SNr GABA neurons have a depolarized, non-oscillating membrane potential, unlike nigral DA neurons that have Ca2+-mediated membrane potential oscillations (Wilson and Callaway, 2000; Chan et al., 2007). Based on experiments using the NaV channel blocker tetrodotoxin (TTX) and Na replacement, Atherton and Bevan (2005) detected a Na-dependent, TTX-insensitive background cation conductance contributing to a tonic depolarization in SNr GABA neurons, but the molecular nature of this background conductance was not identified. Recent data indicate that TRPC3 channels are a potential candidate (Zhou et al., 2008). TRP channels are a class of depolarizing cation channels with mammals having at least 28 different TRP channel types (Hardie, 2007; Nilius et al., 2007; Birnbaumer, 2009; Clapham, 2009). Among these TRP channels, the TRPC3 channel is unique in that it has substantial constitutive activity (Dietrich et al., 2003; Albert et al., 2006; Eder et al., 2007). The constitutive activity is further stimulated by diacylglycerol (DAG) in response to phospholipase C (PLC) activation (Hofmann et al., 1999; Albert and Large, 2006). Therefore, neurons expressing TRPC3 channels may have a depolarized membrane potential. Also, neurotransmitter stimulation of the Gq/11-PLC signaling pathway may ultimately enhance TRPC3 channels, providing a molecular basis for TRPC3 channels to serve as an effector channel for G-protein coupled neurotransmitter receptors.
A combined molecular and electrophysiological analysis (Zhou et al., 2008) has provided evidence that the TRPC3 channel contributes to depolarization of SNr GABA neurons. Single cell reverse transcription polymerase chain reaction (scRT-PCR) consistently detected TRPC3 but not other TRP mRNA in SNr GABA neurons (Zhou et al., 2008), suggesting that TRPC3 channels are expressed at a relatively high level in these neurons. After blocking voltage-dependent Na currents with TTX, flufenamic acid (FFA), a broad spectrum TRP channel blocker, resulted in a non-desensitizing hyperpolarization (Fig. 3A). When voltage-clamped at − 70 mV, FFA induced an apparent outward current, or reduced a tonic inward current accompanied by decreased whole-cell conductance (Fig. 3B). This FFA-sensitive inward current was linear and reversed its polarity around −35 mV (Fig. 3C,D). These I–V characteristics and FFA-sensitivity together with the detection of TRPC3 mRNA indicate a potentially TRPC3 channel-mediated, tonic cation current in SNr GABA neurons. The tonic FFA-sensitive depolarization and inward current are also dependent on extracellular Na+. Furthermore, infusion of a TRPC3 antibody known to inhibit these channels (Albert et al., 2006; Amaral and Pozzo-Miller, 2007) into SNr GABA neurons substantially reduced the firing frequency and increased firing irregularity (Fig. 3E). Taken together, these recent studies suggest that SNr GABA neurons express a constitutively active, TRPC3 channel-mediated, Na+-dependent, voltage-independent inward current at membrane potentials negative to the action potential threshold. Because of their activity at hyperpolarized membrane potentials, these channels are particularly important in depolarizing SNr GABA neurons when voltage-dependent depolarizing ion channels are not active.
Fig. 3.
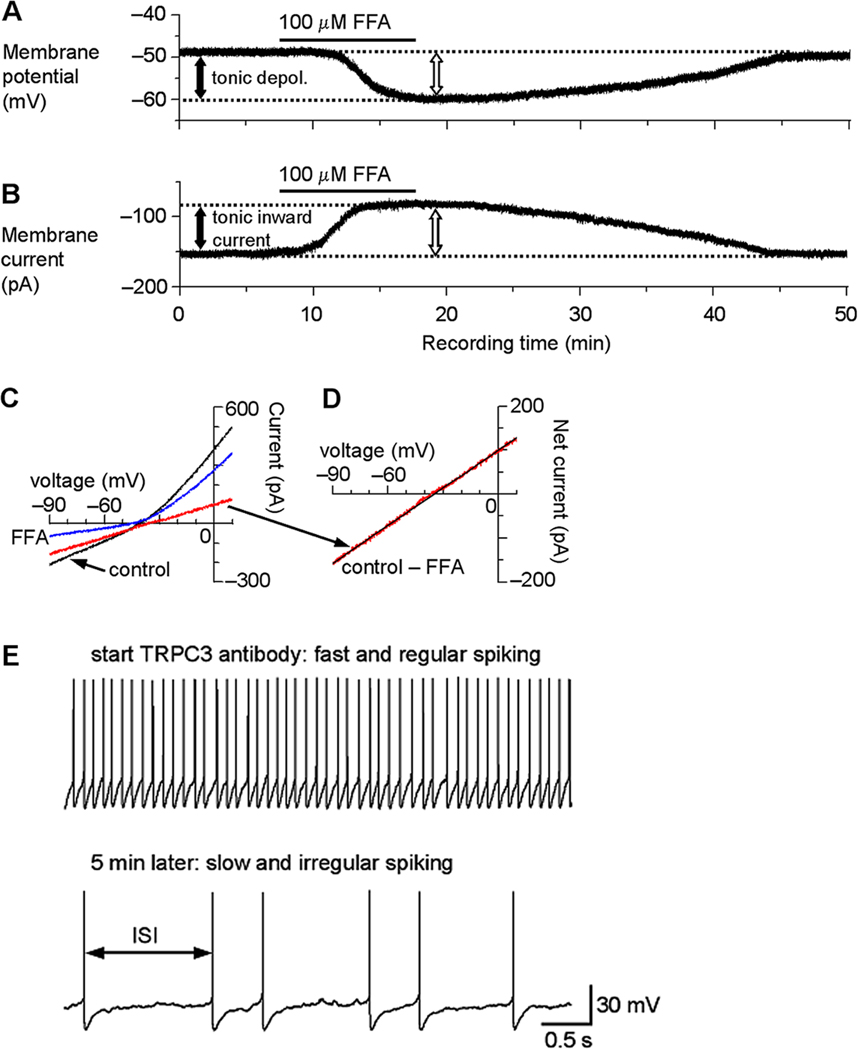
TRP channels mediate a tonic inward current and depolarization in SNr GABA neurons. (A) After blocking NaV channels with 1 µM TTX and under current clamp recording condition, bath application of 100 µM flufenamic acid (FFA) induced a hyperpolarization of about 10 mV (open arrow), indicating an FFA-sensitive tonic depolarization (depol., filled arrow). (B) When voltage clamped at −70 mV, 100 µM FFA induced an outward current (open arrow), or reduced a potential tonic inward current, as reflected by the apparent reduction of the holding current (filled arrow). (C) A linear voltage ramp from −90 mV to 10 mV was applied under control condition (black trace) and during 100 µM FFA application (blue trace). Digital subtraction (control – FFA) revealed the current inhibited by FFA (red trace) and its I–V relationship. The decreased current also indicates an increased input resistance or decreased whole cell conductance. (D) The FFA-inhibited current in C displayed at an enhanced scale. The I–V relationship is clearly linear with no signs of voltage-dependent activation or inactivation and reversed its polarity around −35 mV on average (intercept on X-axis in linear regression analysis as indicated by the black straight line). (E) Intracellular application of a TRPC3 antibody decreases the firing rate and increases the firing irregularity in SNr GABA neurons. Within the first minute of recording when the antibody was not likely to have diffused sufficiently into the cell, the firing was fast around 10 Hz and had a regular inter-spike interval (ISI). During the fifth minute of recording when a considerable amount of the antibody was likely to have diffused into the cell, the spontaneous firing became much slower and had an irregular ISI. Modified from Zhou et al., 2008 with permission.
4.2. Voltage-activated sodium (NaV) channels in SNr GABA neurons
NaV channels are the most important ion channel in the generation of action potentials (Hille, 2001; Bean, 2007). The remarkable capability of SNr GABA neurons to fire sustained high frequency action potentials indicates that their NaV channels must have suitable properties that meet this functional requirement. Recent studies indicate that SNr GABA neurons express a persistent sodium current (INaP) that induces subthreshold depolarization and a classical transient sodium current (INaT) that is larger and faster than INaT in the slow-spiking DA neurons (Atherton and Bevan, 2005; Ding and Zhou, 2010; Seutin and Engel, 2010).
4.2.1. A persistent sodium current (INaP) depolarizes SNr GABA neurons
The constitutively active TRPC3 channels can depolarize SNr GABA neurons at very negative membrane potentials. But since the TRPC3 current reversal potential is around −35 mV, the current becomes smaller as the neuron gets close to the action potential threshold, a situation that is not ideal for reliable spike generation. So other cation channels may join in depolarizing the membrane potential. The voltage-dependent INaP is an ideal candidate. INaP is generated when a small fraction of NaV channels remain open for long periods of time (Patlak and Ortiz, 1986; Alzheimer et al., 1993; Bant and Raman, 2010). Although the amplitude of INaP is only a fraction of INaT, its duration is much longer such that it can enhance neuronal excitability and contribute to the autonomous pacemaking activity (Crill, 1996; Do and Bean, 2003; Mercer et al., 2007; Khaliq and Bean, 2010).
Atherton and Bevan (2005) performed a detailed study on the INaP contribution to spontaneous spike firing in SNr GABA neurons. They found that spontaneous spiking continued almost unabated after blocking fast GABAergic and glutamatergic synaptic transmission, indicating that spontaneous high frequency firing is generated by intrinsic ion channels. They also found that blocking Ih at the native membrane potentials did not alter the firing, suggesting that the contribution of Ih is small under normal conditions. Using a voltage step protocol ranging from −80 mV to −50 mV, they detected a TTX-sensitive INaP that started to activate around −60 mV and peaked around −50 mV, indicating that INaP can depolarize the cell when it reaches −60 mV or more positive potentials and contributes to the generation of spontaneous firing in SNr GABA neurons.
4.2.3. The transient voltage-gated sodium current (INaT) is strong and fast in SNr GABA neurons
When a sufficient amount of the classical INaT is activated by subthreshold depolarization induced by TRPC3 channels and INaP, the regenerative fast rising phase of the action potential is triggered (Hille 2001; Bean 2007). SNr GABA neurons fire high frequency, short duration action potentials, whereas SNc DA neurons fire low frequency, long duration action potentials. The action potential threshold tends to be a few mV more negative in SNr GABA neurons than in nigral DA neurons, while action potential amplitude is about 10 mV larger in SNr GABA neurons than in nigral DA neurons (Richards et al., 1997; Ding et al., 2011). These observations indicate that INaT is different in these two cell types. Recent studies suggest that this is indeed the case (Ding and Zhou, 2010; Seutin and Engel, 2010). These studies showed that INaT has a higher density in SNr GABA neurons than in DA neurons (Fig. 2E–G), contributing to the faster rise and larger amplitude of action potentials in SNr GABA neurons. Additionally, the kinetics of INaT may also be different in these two neuron types (Ding and Zhou, 2010). Specifically, INaT has a steeper voltage-dependent activation and a faster deactivation in SNr GABA neurons than in nigral DA neurons, indicating that INaT in SNr GABA neurons can be activated more readily than those in nigral DA neurons, contributing to the fast rise and short duration of spikes in SNr GABA neurons. Equally important, INaT recovers more quickly from inactivation in SNr GABA neurons than in SN DA neurons, leading to less cumulative inactivation in SNr GABA neurons. Consequently, more functional NaV channels are available in SNr GABA neurons than in nigral DA neurons (Ding and Zhou, 2010). The different recovery from inactivation may contribute to the fact that spike firing either is not or is only slightly adaptive in amplitude and frequency in SNr GABA neurons even at very high frequency, whereas it is strongly adaptive in nigral DA neurons when the frequency is above 10 Hz. Similarly, Martina and Jonas (1997) found that rapid recovery of INaT from inactivation is important for the fast spiking properties in hippocampal GABA interneurons, indicating that there may be common ionic mechanisms among fast spiking neurons in different brain areas.
4.3. Voltage-activated potassium (Kv) currents in SNr GABA neurons
Kv currents are mainly responsible for repolarizing the membrane after the action potential has reached its peak and can activate even slightly before the peak (Hille, 2001; Bean, 2007). Therefore, a robust Kv current that activates quickly to rapidly repolarize the membrane and resists inactivation for continued availability is essential for sustained high frequency firing. A recent combined molecular and electrophysiological study indicates that SNr GABA neurons express a strong Kv3-like current with fast activation and slow inactivation kinetics that is required for the sustained high frequency firing capability in these neurons (Ding et al., 2011).
4.3.1. A robust Kv3-like fast delayed rectifier (IDR-fast) current in SNr GABA neurons
Tissue level studies have detected Kv3.1, Kv3.2, Kv3.3 and Kv3.4 mRNAs and also Kv3.1, Kv3.2, and Kv3.3 proteins in the SNr region, whereas these Kv channel signals are very low in SNc (Perney et al., 1992; Weiser et al., 1994, 1995; Ozaita et al., 2002; Chang et al., 2007). Kv4.3 mRNA is prominently expressed in the SNc region (Tsaur et al., 1997; Serodio et al., 1998). Since the main cell type in SNr and SNc are the GABA neurons and DA neurons, respectively, the Kv3 mRNAs are likely in SNr GABA neurons, whereas Kv4.3 mRNA is likely in SNc DA neurons. Recently, Ding et al. (2011) performed qRT-PCR analysis on the expression of Kv1.1–6, Kv2.1–2, Kv3.1–4, and Kv4.1–3 in immunohistochemically identified, laser capture-microdissected SNr GABA neurons and nigral DA neurons. They found that in comparison with slow-spiking nigral DA neurons, fast-spiking SNr GABA neurons express more Kv3.1 and Kv3.4 mRNAs that can form the fast delayed rectifier type K channels and conduct IDR-fast. In contrast, nigral DA neurons express more Kv4.2 and Kv4.3 mRNAs that may form IA type channels. Voltage clamp data showed that SNr GABA neurons express a prominent IDR-fast current that starts to activate near −30 mV, above the spike threshold (Fig. 4A). The activation was fast but the decay or inactivation was slow (Fig. 4A). These properties of this IDR-fast current are reminiscent of the currents mediated by cloned heteromeric Kv3.1 and Kv3.4 channels (Weiser et al., 1994; Rudy and McBain, 2001), indicating Kv3-like channels. The kinetic and pharmacological properties of the Kv3-like IDR-fast current in fast-spiking SNr GABA neurons are also similar to the Kv3-like IDR-fast currents in fast-spiking GABA neurons in other brain areas (Martina et al., 1998; Baranauskas et al., 2003; Lien and Jonas, 2003).
Fig. 4.
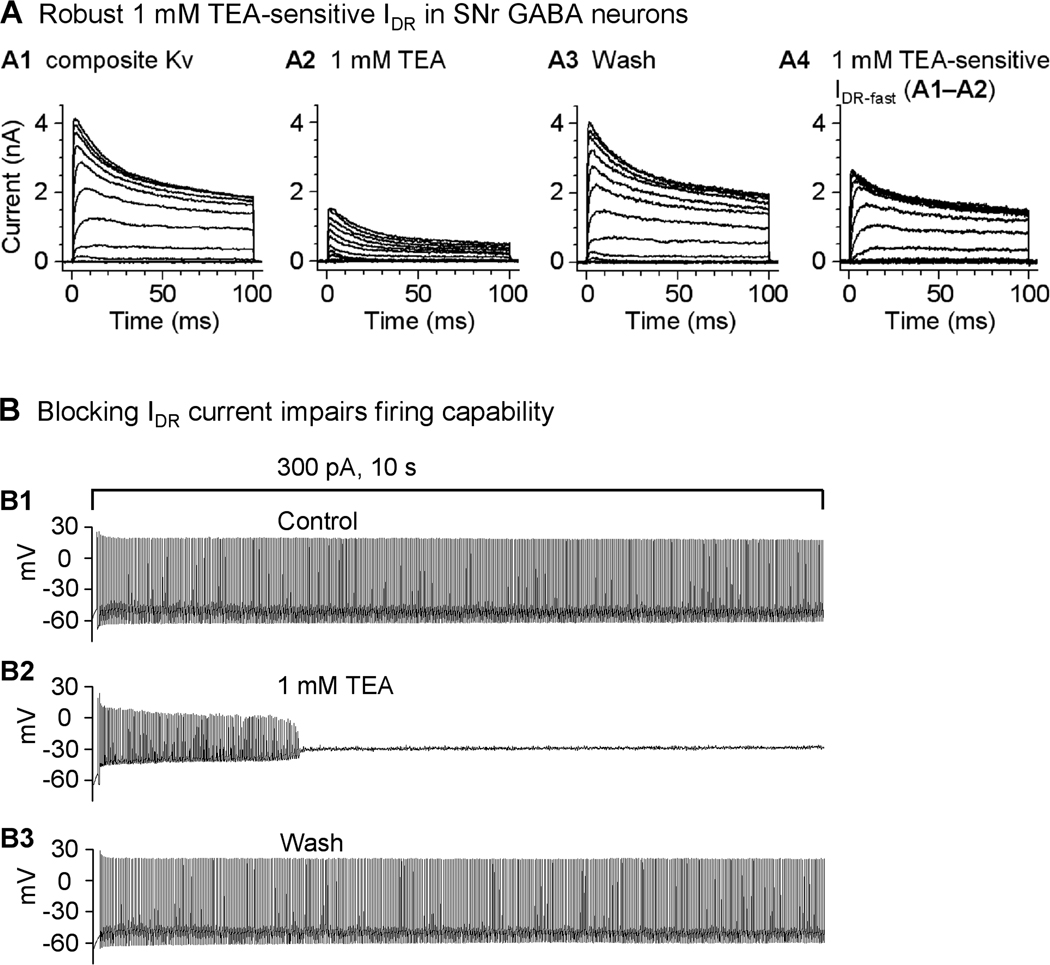
A robust, 1 mM TEA-sensitive IDR-fast current is critical to the sustained high frequency firing in SNr GABA neurons. (A) Representative traces of Kv currents in a nucleated membrane patch isolated from a GABA neuron under control condition (A1), in the presence of 1 mM TEA (A2) and after washing out 1 mM TEA (A3). The 1 mM TEA-sensitive IDR-fast was obtained by subtraction and displayed in A4. Holding potential was −100 mV, the first depolarizing step was to −80 mV and the last one was to +50 mV, and each step increment was 10 mV. (B) Inhibition of Kv3 channel-mediated IDR-fast by 1 mM TEA impairs the sustained high frequency firing in SNr GABA neurons. An example SNr GABA neuron was able to fire sustained high frequency (around 45 Hz) fast spikes upon injection of 300 pA current for 10 s (B1). In the presence of 1 mM TEA that blocks IDR-fast, the same 10 s 300 pA injection induced spike firing only for about 3 s or less (B2). Note the spike amplitude decreased progressively. The SNr neuron regained its sustained high frequency firing capability after washing out 1 mM TEA (B3). Modified from Ding et al. 2011 with permission.
4.3.2. Inhibition of Kv3-like IDR-fast current impairs high frequency spiking in SNr GABA neurons
In other neuron types that are capable of high frequency firing, pharmacological inhibition (by 1 mM TEA) or genetic inactivation of members of Kv3 channel family has been shown to impair the ability of neurons to fire high frequency spikes (Wang et al., 1998; Erisir et al., 1999; Lau et al., 2000; Lien and Jonas, 2003; Macica et al., 2003). Similarly, Ding et al. (2011) found that blocking the Kv3-like IDR-fast also impaired the ability of SNr GABA neurons to fire high frequency spikes. As illustrated in Fig. 4B1, upon intracellular injection of depolarizing currents, SNr GABA neurons are able to fire sustained high frequency spikes, above the spontaneous firing frequency. The rise rate and the peak amplitude of these spikes are largely sustained, indicating that the Kv currents are able to quickly repolarize the cell and thus allow NaV channels to recover from inactivation. Bath application of 1 mM TEA progressively reduced the spike amplitude, eventually leading to a complete cessation of firing (Fig. 4B2), indicating that SNr GABA neurons are unable to fire sustained high frequency spikes when IDR-fast is blocked (Fig. 4A, B). As indicated by the delayed and shallow repolarization, blocking IDR-fast likely causes cumulative inactivation of NaV channels and eventual loss of spiking capability. Taken together, these results provide strong evidence that the Kv3-containing IDR-fast, due to its fast activation and slow inactivation kinetics, is critical for SNr GABA neurons to rapidly repolarize and fire sustained high frequency spikes.
4.3.3. The subthreshold IA is weak in SNr GABA neurons
In addition to the prominent IDR-fast, SNr GABA neurons also express small amounts of the classic subthreshold-activating, subthreshold-inactivating, and transient IA type current likely mediated by Kv4.2 and/or Kv4.3-containing channels (Ding et al., 2011). In contrast, nigral DA neurons express a robust IA. While a strong subthreshold IA can keep the firing of DA neurons at low rates, a weak IA allows SNr GABA neurons to fire at high frequencies (Liss et al., 2001; Koyama and Appel, 2006; Ding et al., 2011).
4.4. Voltage-gated calcium (CaV) channels and calcium-activated potassium (KCa) channels in SNr GABA neurons
4.4.1. CaV channels
CaV channels are among the intrinsic membrane channels influencing the excitability of the SNr neuronal membrane. CaV channels have been shown to influence both spontaneous activity in these neurons, as well as more complex behaviors such as burst firing (Atherton and Bevan, 2005; Ibáñez-Sandoval et al., 2007; Lee and Tepper, 2007b). Activation of CaV channels can lead to either increases or decreases in SNr neuron excitability. Calcium entry through N-type calcium channels (CaV2.2) causes activation of KCa channels that decrease the firing rate and increase the regularity of firing in SNr GABA neurons (Atherton and Bevan, 2005; Yanovsky et al., 2005). Blockade of CaV2.2 channels with ω-conotoxin GVIA exerts effects similar to blockade of KCa with apamin including loss of the medium afterhyperpolarization, an increase in firing rate, and an increase in firing irregularity (Atherton and Bevan, 2005; Yanovsky et al., 2005). The role of KCa channels in the regulation of nigral GABA neuron activity is discussed in more detail in the next section.
Other calcium channels contribute to depolarization of SNr GABA neurons, either directly or by activating another calcium-activated depolarizing conductance. Some SNr GABA neurons exhibit a depolarizing plateau potential following depolarization from a hyperpolarized membrane potential (Matsuda et al., 1987; Nakanishi et al., 1987; Lee and Tepper, 2007b). This plateau potential can be abolished in calcium-free conditions or by the L-type CaV channel (CaV1) blockers nimodipine or nifedipine (Fig. 5A,B) (Lee and Tepper, 2007b). Conversely, the L-type CaV channel activator, Bay K 8644 can evoke a plateau potential in response to depolarization if the neuron did not exhibit a plateau under control conditions (Fig. 5C) (Lee and Tepper, 2007b). The plateau potential can also be activated by calcium entry through ligand gated channels, such as those mediated by NMDA receptors, suggesting that the plateau potential is caused by a calcium-activated conductance rather than carried by L-type channels directly (Lee and Tepper, 2007b). The conductance underlying the plateau potential is carried primarily by sodium (Fig. 5D) and it can be blocked by FFA (Fig. 5E, F), suggesting that the conductance underlying the plateau is mediated by a TRP channel (Lee and Tepper, 2007b).
Fig. 5.
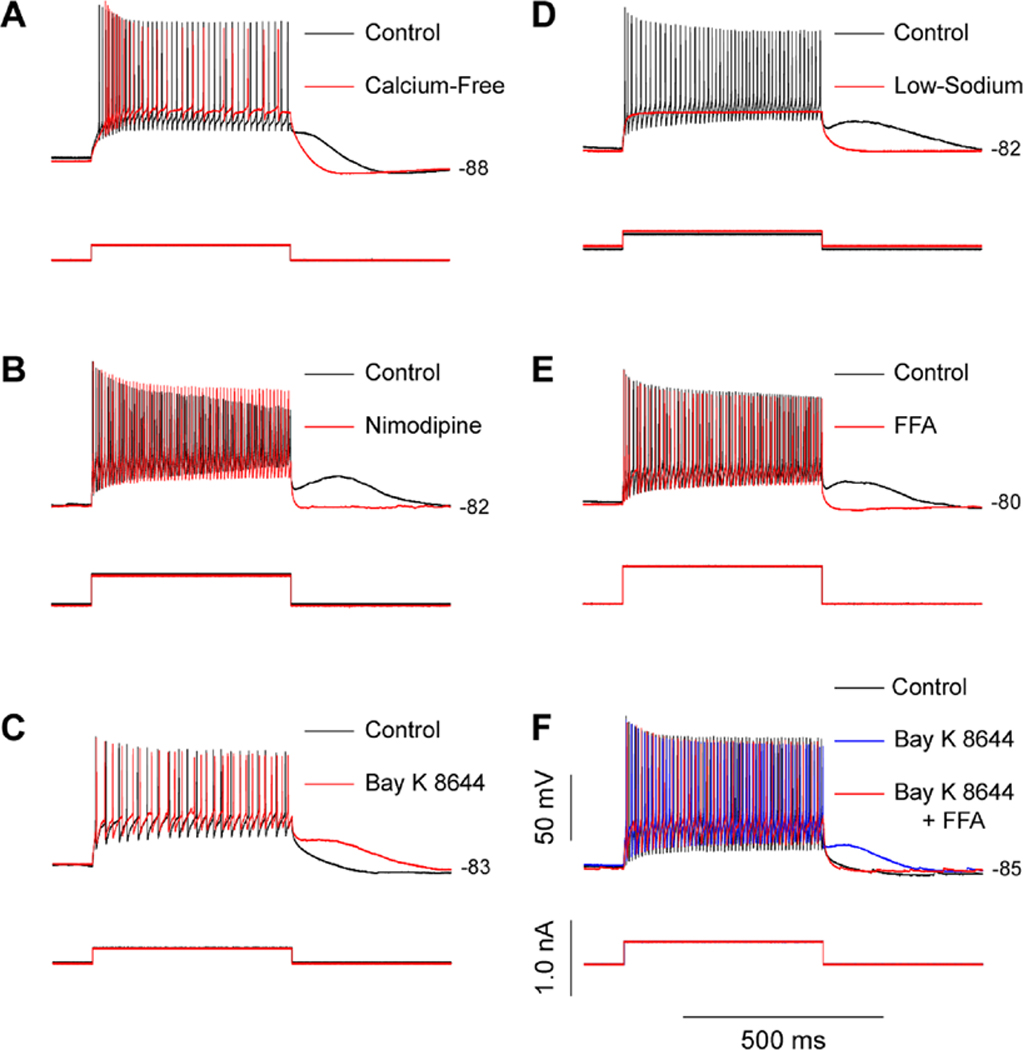
SNr GABA neurons have a calcium-activated plateau potential. (A) A representative SNr GABA neuron exhibiting a plateau potential that manifests as a prolonged membrane depolarization following a depolarizing current pulse delivered while the neuron is held hyperpolarized (black trace). The plateau potential is abolished in calcium-free conditions (red trace). (B) Another SNr GABA neuron exhibits a plateau potential under control conditions (black trace) that is abolished by nimodipine (10 µM; red trace) indicating the involvement of L-type calcium channels in plateau potential generation. (C) A SNr GABA neuron without a plateau potential under control conditions (black trace) is made to exhibit a plateau potential after activation of L-type calcium channels with Bay K 8644 (5 µM; red trace). (D) The plateau potential (black trace) is abolished in low-sodium conditions (red trace) suggesting that the conductance underlying the plateau potential is a calcium-activated nonselective cation conductance. (E) The plateau potential observed under control conditions (black trace) is abolished by the TRP channel blocker flufenamic acid (FFA; 200 µM; red trace). (F) A neuron not exhibiting a plateau potential under control conditions (black trace) exhibits a plateau potential in the presence of Bay K 8644 (blue trace). The evoked plateau potential is similarly abolished by FFA (red trace). Modified from Lee and Tepper, 2007b with permission. Copyright 2007 Society for Neuroscience.
Under certain conditions, basal ganglia output neurons can exhibit oscillatory burst firing that is observed in vivo most commonly in pathological conditions including Parkinson’s disease in humans (Wichmann and DeLong, 2006) and following dopamine denervation in animal models of the disease (Wichmann et al., 1999; Raz et al., 2000; Ruskin et al., 2002). Burst firing can also be observed during application of NMDA in vitro (Ibáñez-Sandoval et al., 2007). NMDA-induced burst firing in SNr GABA neurons appears to require activation of CaV channels, specifically T-type CaV channels (CaV3.2), as burst firing is abolished by their pharmacological blockade (Ibáñez-Sandoval et al., 2007). Blockade of presumed T-type CaV channels also results in a decrease in firing rate and firing regularity in SNr GABA neurons as well as an attenuation of the afterhyperpolarization suggesting that T-type CaV channels might both depolarize the neurons and also contribute to activation of KCa channels (Yanovsky et al., 2005).
4.4.2. Calcium-activated potassium (KCa) channels
SNr GABA neurons express KCa channels, likely consisting of SK2 subunits (Stocker and Pedarzani, 2000), that play a role in regulating the spontaneous firing rate and regularity of firing in these neurons. KCa channels are active during spontaneous activity in vitro, as blocking them with apamin reduces the amplitude of the spike afterhyperpolarization (AHP) and increases the firing rate of SNr GABA neurons (Atherton and Bevan, 2005; Yanovsky et al., 2005). KCa channel activity also contributes to firing regularity as the coefficient of variation, and thus firing irregularity is increased when these channels are blocked (Atheron and Bevan, 2005), whereas the firing rate decreases but regularity increases when KCa channels are pharmacologically activated (Yanovsky et al., 2006).
The source of the calcium entry required to activate KCa channels during spontaneous activity has been shown to be primarily N-type (CaV2.2) Ca channels (Atherton and Bevan, 2005; Yanovsky et al., 2005). There might also be a lesser contribution from T-type CaV channels as well (Yanovsky et al., 2005). KCa channels can also be activated by release of calcium from intracellular stores through ryanodine receptors, leading to a decrease firing rate following intracellular release of calcium induced by caffeine (Yanovsky et al., 2005). However, the release of intracellular calcium does not play a role in activation of KCa channels during spontaneous activity (Yanovsky et al., 2005). In addition to having an effect on firing rate and regularity, activation of KCa channels also prevents SNr GABA neurons from exhibiting burst firing, and it has been reported that burst firing can emerge following blockade of these channels with apamin (Yanovsky et al., 2005).
4.5. Summary of key intrinsic ion channels supporting sustained high frequency firing in SNr GABA neurons
Based on the above discussion, we can outline the key mechanisms underlying the autonomous high frequency spike firing in SNr GABA neurons. First, tonically active TRPC3 channels can depolarize these neurons even when they are at very negative membrane potentials. When the membrane potential reaches −60 mV, INaP starts to activate, further depolarizing the membrane potential. At or slightly below −50 mV, INaT starts to activate and eventually triggers the rapid rising phase of action potentials. SNr GABA neurons have a higher density of INaT, contributing to the faster rise and larger amplitude of action potentials, compared with the slow-spiking DA neurons. INaT also recovers from inactivation quickly in SNr GABA neurons, enabling the sustained high frequency firing in SNr GABA neurons. The rising phase of the action potential triggers the activation of high-threshold, inactivation-resistant Kv3-like channels in SNr GABA neurons that can rapidly repolarize the membrane, further supporting the sustained high frequency firing. Calcium contributes to the excitability of SNr GABA neurons by directly depolarizing the neuron or by activating other channels which either hyperpolarize and regularize firing of the neuron, as in the case of KCa channels, or depolarize the neuron by activating one or more TRP channels, inducing a plateau depolarization. Whether calcium entry causes activation of KCa channels or TRP channels appears to be dictated by the calcium channel with primarily N-type calcium channels activating KCa channels while calcium entry through L-type channels leads to activation of TRP channels. Together, these intrinsic ion channels provide SNr GABA neurons with the capabilities to fire autonomous, sustained high frequency spikes, the default mode of operation for these basal ganglia output neurons.
5. Synaptic inputs to SNr GABA projection neurons and their regulation
The autonomous high frequency firing in SNr GABA neurons, driven by intrinsic ion channels, provides a default tonic inhibitory signal to its targets. SNr GABA neurons also receive an array of synaptic inputs that sculpt SNr GABA neuron activity and hence its output. The discussion below seeks to highlight key recent advances in the field of synaptic regulation of SNr GABA neurons. For more comprehensive reviews, readers are referred to Misgeld (2004) and Deniau et al. (2007). For metabotropic glutamate receptors in SNr, see a comprehensive review by Conn et al. (2005).
5.1. GABA input and its regulation
5.1.1. GABAergic striatonigral input
Anatomical studies have established that dopamine D1 receptor (D1R)-expressing medium spiny neurons (D1-MSNs) but not D2 receptor-expressing D2-MSNs in the striatum project strongly and largely exclusively to the SNr, forming the direct striatonigral pathway (Alexander and Crutcher, 1990; Gerfen et al., 1990; DeLong and Wichmann, 2007; Gerfen and Bolam, 2010). These D1-MSN axons may also give off limited collaterals and innervate structures on its passage (Parent et al., 2000; Lévesque and Parent, 2005; Fujiyama et al., 2011). This D1-MSN-originated striatonigral projection is readily demonstrated with immunostaining for D1R protein (Fig. 6A) (Huang et al., 1992; Levey et al., 1993; Yung et al., 1995). Further confirming the direct striatonigral projection, in mice expressing D1R promoter-controlled green fluorescent protein (GFP) or red fluorescent protein (RFP), a prominent GFP or RFP-labeled axon projection to SNr can be easily seen (Gong et al., 2003; Shuen et al., 2008).
Fig. 6.
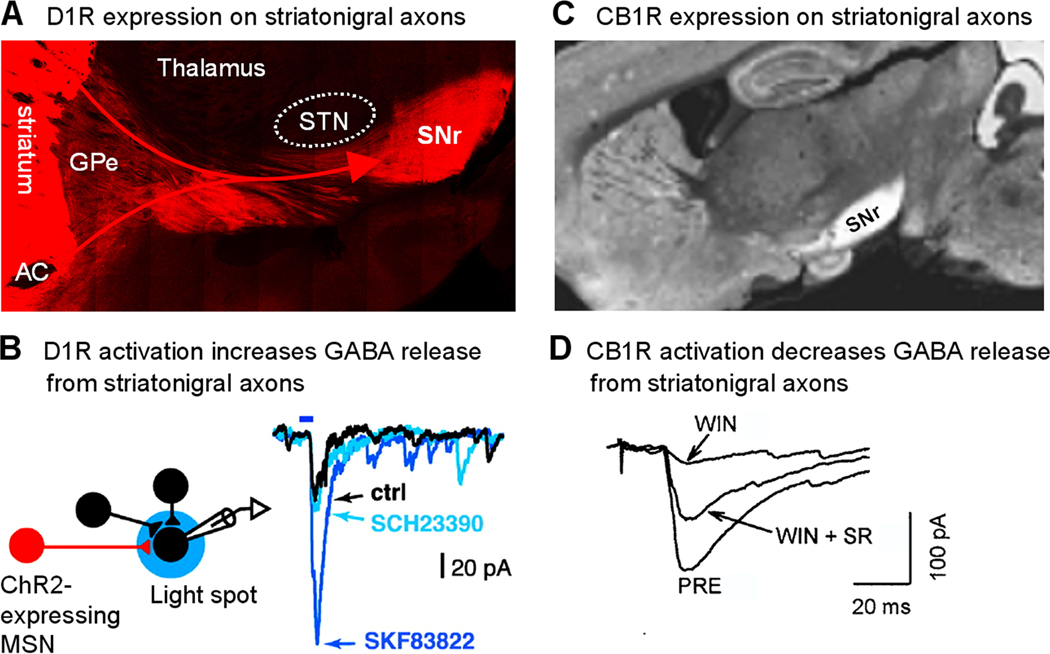
D1 receptor facilitation and CB1 receptor inhibition of the striatonigral axon terminals. (A) Immunostaining for D1 receptor protein reveals an intense expression of D1 receptors on striatonigral axons. Due to the curvature of the fibers in this saggital section, only part of the projection is captured in this image. AC: anterior commissure. Unpublished data of FMZ. (B) Selective photoactivation of ChR2-expressing MSN axons in SNr evoked IPSCs in SNr GABA neurons that was enhanced by a D1-like agonist SKF83822. This enhancing effect was blocked by the D1-like antagonist SCH23390. Adapted from Chuhma et al. 2011 with permission. (C) Immunostaining for CB1 receptor protein reveals an intense expression of CB1 receptors on striatonigral axons. Adapted from Fukudome et al. 2004 with permission. (D) The cannabinoid agonist WIN55212-2 (WIN) depressed striatum stimulation-evoked IPSCs in SNr GABA neurons and this effect was largely reversed by cannabinoid antagonist SR141716A (SR). PRE indicates pre-drug control. Adapted from Wallmichrath and Szabo, 2002b with permission.
Early electrophysiological recordings in intact animals have suggested that direct pathway MSNs inhibit SNr GABA neurons (see Hikosaka et al., 2000; Galvan and Wichmann, 2007 for review). Recordings in freely moving rats (Windels and Kiyatkin, 2004, 2006a) showed that SNr GABA neurons are sensitive to local application of the GABAA receptor blocker bicuculline, indicating that SNr GABA neurons are under the influence of tonic GABA inhibition. However, these electrophysiological recordings could not distinguish D1- or D2-MSNs or origins of the GABA inputs to SNr GABA neurons. Therefore, until recently, direct evidence for D1-MSN inhibition of SNr GABA neurons was lacking. Newly developed optogenetic techniques enable selective activation of specific groups of neurons by introducing light-sensitive ion channels (channelrhodopsin-2) into either D1-MSNs or D2-MSNs. Using this strategy, a recent study provides strong evidence that activation of D1-MSNs inhibits the spontaneous firing of SNr GABA projection neurons, whereas activation of D2-MSNs has the opposite effect (Kravitz et al., 2010). These new results confirm the classical basal ganglia model that the direct pathway D1-MSNs inhibit and the indirect pathway D2-MSNs excite GPi and SNr GABA neurons, respectively (Albin et al., 1989; DeLong, 1990).
5.1.2. Dopamine D1 receptor facilitation of GABAergic striatonigral axon terminals
Studies have established that striatonigral axon terminals strongly express D1Rs, providing the anatomical and molecular bases for dopamine regulation of the direct pathway MSN outputs (Fig. 6A) (Huang et al., 1992; Mansour et al., 1992; Levey et al., 1993; Yung et al., 1995; Smith and Kieval, 2000; Kliem et al., 2007; Kliem et al., 2010). These axons terminate mostly in SNr and generally do not innervate the neighboring areas. Due to its coupling to cAMP production that in turn increases neurotransmitter release (Missale et al., 1998; Yao and Sakaba, 2010), D1R activation should increase GABA release from striatonigral axon terminals, a prediction supported by microdialysis studies (Rosales et al., 1997; Trevitt et al., 2002; Kliem et al., 2007). However, despite these clear anatomical features and the neurochemical signaling pathway, functional physiological determination of presynaptic D1R-mediated facilitation of GABA release from striatonigral axon terminals had been controversial until recently. Cameron and Williams (1993) first reported that D1R activation enhanced GABAB receptor-mediated inhibitory postsynaptic potentials (IPSPs) in ventral tegmental dopamine neurons by presynaptically facilitating GABA release from forebrain afferents, although D1R expression is much weaker in VTA than in SNr. In 1998, two studies from 2 laboratories reached opposite conclusions. Radnikow and Misgeld (1998) reported that the D1-like agonist SKF38393 substantially increased the amplitude of evoked GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) in SNr GABA neurons. The frequency but not amplitude of the miniature IPSCs in the presence of TTX and Cd2+ (to block sodium spikes and Ca2+ channels, respectively) was also increased by the D1-like agonist SKF38393. These effects were blocked by the D1-like antagonist SCH23390, leading to the conclusion that D1R activation facilitates vesicular GABA release from striatonigral axon terminals. In contrast, Miyazaki and Lacey (1998) reported that D1-like receptor activation inhibited GABAA IPSCs in SNr GABA neurons by decreasing GABA release from striatonigral axon terminals. Which conclusion is correct? A recent study (Chuhma et al., 2011) provides an answer to this question. In this study, the authors used transgenic mice with conditional channelrhodopsin-2 (ChR2) expression restricted to striatal MSNs. Consequently, MSNs including striatonigral neurons and their axon terminals can be selectively photo-activated. Using this strategy, Chuhma et al. (2011) demonstrated unambiguously that D1-like ligand stimulation increased IPSC amplitude when these axon terminals were selectively photo-stimulated locally in the SNr (Fig. 6B). Another recent study has confirmed the presynaptic enhancement of striatonigral IPSCs by D1R activation, and has extended those findings by demonstrating a concentration-dependent shift from D1R-mediated IPSC enhancement to nonspecific inhibition with increasing concentrations of D1R agonist (de Jesús Aceves et al., 2011). These new neurophysiological data overwhelmingly support the original hypothesis, based on the intense D1R expression at striatonigral axon terminals, that D1R activation facilitates GABA release from striatonigral axon terminals. In hindsight, what led to the different conclusions of these two early studies were probably nonspecific effects of agonists and/or different stimulating methods used in these two studies. Radnikow and Misgeld (1998) used minimal stimulation with a patch pipette, whereas Miyazaki and Lacey (1998) used a much larger twisted wire bipolar stimulating electrode, likely leading to activation of unintended targets.
The intense D1R expression at striatonigral axon terminals underscores the physiological importance of D1R-mediated facilitation of this synapse. By directly enhancing GABA release, SNc DA neurons, via dendritically released DA, may decrease the activity of SNr GABA neurons and promote motor activity. Simultaneously in the striatum, dopamine released from SNc DA neurons increases D1-MSN activity and impulse output (West and Grace, 2002; Liang et al., 2008), thus increasing GABA output to SNr GABA neurons. These DA effects at D1-MSN somata and axon terminals may synergistically inhibit SNr GABA neurons.
5.1.3. Cannabinoid CB1 receptor inhibition of striatonigral axon terminals
The pioneering autoradiographic studies by Herkenham et al. (1990, 1991a,b) and subsequent immunohistochemical studies have established that cannabinoid CB1 receptors are intensely expressed on striatonigral axon terminals (Fig. 6C) (Tsou et al., 1998; Fukudome et al., 2004; Mátyás et al., 2006; see Freund et al., 2003 and Kano et al., 2009 for review). These CB1 receptors, together with the minor member CB2 of the cannabinoid receptor family, are coupled to Gi/o proteins. An established mode of operation is that CB1 receptor activation stimulates Gi/o that in turn inhibits Ca channels and Ca influx, leading to reduced neurotransmitter release. Evidence indicates that this mechanism operates at striatonigral axon terminals where CB agonists decrease the amplitude of GABAA IPSCs in SNr neurons (Fig. 6D) (Wallmichrath and Szabo, 2002a,b). At the same time, the paired pulse ratio is increased, indicating decreased vesicular GABA release. The presynaptic action of cannabinoids has been further confirmed by showing that CB receptor agonists neither affect the response of SNr neurons to exogenously applied GABA or a GABAA receptor agonist (Tersigni and Rosenberg, 1996; Chan et al., 1998; Wallmichrath and Szabo, 2002a,b) nor change the amplitude of Ca2+-independent miniature IPSCs (Wallmichrath and Szabo, 2002b).
More important, CB receptor antagonists have been reported to increase evoked IPSC amplitude, suggesting tonic presynaptic modulation of GABAergic input by endogenous cannabinoids (Wallmichrath and Szabo, 2002a; Yanovsky et al., 2003). Interestingly, IPSCs evoked in SNr neurons in mouse brain slices were insensitive to antagonist treatment in the absence of direct depolarization of postsynaptic SNr neurons (Wallmichrath and Szabo, 2002b). This finding indicates that postsynaptic SNr neurons are a source of endogenous cannabinoids and that depolarization augments endogenous cannabinoid signaling (Wallmichrath and Szabo, 2002b). Endogenous cannabinoids are also released from SNc dopamine neurons (Yanovsky et al., 2003). Since SNr GABA neurons are spontaneously active, their intrinsic activity might support endogenous cannabinoid production (Romo-Parra et al., 2009; Kano et al., 2009) resulting in an endogenous cannabinoid tone in the nucleus. The endogenous cannabinoid functioning at striatonigral synapses has been suggested to be 2-arachidonoylglycerol (Szabo et al., 2006). The functional importance of cannabinoid signaling in SNr is demonstrated by the fact that local or peripheral administration of CB agonists increases the spontaneous firing rate of nigral GABA neurons in vivo (Miller and Walker, 1995; Tersigni and Rosenberg, 1996). Conversely, local application of a cannabinoid receptor antagonist leads to a decrease in firing rate implicating endogenous cannabinoids as tonic modulators of basal ganglia output (Tersigni and Rosenberg, 1996).
5.1.2. GABA input from the globus pallidus external segment (GPe)
Another major GABAergic afferent to SNr GABA neurons is the GPe-SNr projection. Anatomical studies using axon tracing techniques have established that SNr GABA projection neurons receive GABA input from GPe neurons (Smith and Bolam, 1989, 1991; Bevan et al., 1994, 1996). GPe afferent terminals make large symmetrical synapses preferentially on the cell body and proximal dendrites, forming perisomatic baskets on SNr GABA neurons, whereas striatal afferents make smaller but apparently more numerous synapses preferentially on more distal dendrites. These anatomical data suggest that GPe input may powerfully influence SNr GABA neuron activity (Smith and Bolam, 1989, 1991), although physiologically demonstrating this idea was complicated by the difficulty in separately stimulating striatonigral axons and pallidonigral axons. To minimize this technical problem, a recent study used a sagittal brain slice preparation that allows stimulation of the striatum and GPe separately (Connelly et al., 2010). This study convincingly demonstrated that activation of even a single GPe neuron evoked large IPSCs in SNr GABA neurons, confirming the original prediction based on anatomical data. Equally important, Connelly et al. (2010) found that while the striatonigral synapse facilitates during repetitive activation (Fig. 7A), the pallidonigral synapse depresses (Fig. 7B). Thus, when the usually silent D1-MSNs activate and produce repetitive output upon receiving strong, converging glutamate inputs from cortical and thalamic areas (Kincaid et al., 1998; Zheng and Wilson 2002; Smith et al. 2004), D1-MSNs may induce robust IPSPs and effectively inhibit SNr GABA neurons. Weak glutamate inputs are less likely to activate D1-MSN somata and the facilitatory mechanism at the axon terminal may also not engage. Therefore, both the D1-MSN cell body and axon terminal allow only strong signals to pass, serving as a high-pass filter (Connelly et al., 2010). In contrast, due to the depressing nature of the pallidonigral synapse and the high frequency spontaneous firing of GPe neurons, the pallidonigral synapse is most effective in inhibiting SNr GABA neurons after a brief pause that allows the synapse to recover from the tonic depression (Connelly et al., 2010). Because of the powerful GPe-SNr projection, activation of D2-MSNs can increase SNr GABA activity by inhibiting GPe neurons (Chuhma et al., 2011; Kravitz et al., 2010).
Fig. 7.
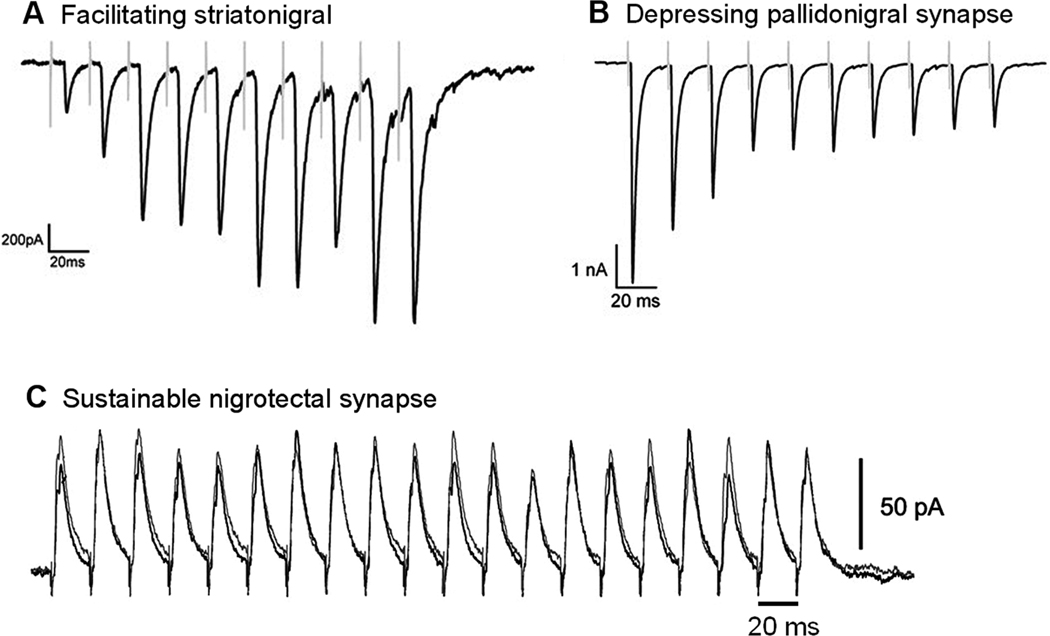
Striatonigral, pallidonigral, and nigrotectal synapses have different functional properties. The striatonigral synapse is facilitating (A), the pallidonigral synapse is depressing (B), and the nigrotectal synapse is sustainable or constant (C). A and B are adapted from Connelly et al. 2010 and C is adapted from Kaneda et al. 2008 with permission.
It is also necessary to point out that the axon terminal release properties of the fast-spiking GPe neurons are different from those of the fast-spiking SNr GABA neurons that can maintain almost constant release during high frequency repetitive stimulation (Kaneda et al., 2008) (Fig. 7C). Thus, the striatonigral, pallidonigral and nigrotectal synapses are endowed with distinct capabilities that serve their respective functions. The direct striatonigral input serves to inhibit the high frequency spontaneous firing of SNr GABA neurons and therefore release the thalamocortical motor circuit and brainstem motor nuclei from tonic inhibition, allowing movement to occur (Chevalier and Deniau, 1990; Hikosaka, 2000). Under basal conditions, striatonigral neurons are largely silent, and asynchronized, sporadic inhibitory output to SNr GABA neurons is probably not effective in inhibiting these neurons, especially given that striatonigral synapses are often at distal dendrites, allowing SNr GABA neurons to fire at high frequency. When behaviorally important, convergent glutamate input from the cortex and thalamus strongly activates striatonigral neurons, the sychronized inhibitory output to SNr GABA neurons is more effective in inhibiting these neurons. The facilitating nature of the striatonigral synapse further enhances this inhibition. The precise function of the GPe input and role of the indirect pathway is less clear. However, it seems that the powerful pallidonigral synapse is normally depressed to allow SNr GABA neurons to fire high frequency spikes. The pallidonigral synapse is further weakened by tonic activation of D2 class dopamine receptors that presynaptically decrease GABAergic pallidonigral input (de Jesús Aceves et al., 2011). When behaviorally necessary, however, the pallidonigral synapse is turned on to inhibit SNr GABA neurons. Indeed, Connelly et al. (2010) found that after a brief (~ 2 s) pause that is known to occur to GPe neurons in motor-related events, the pallidonigral synapse recovered to its full strength. Therefore, it appears that the pallidonigral synapse is normally in a depressed state that allows high frequency firing of SNr neurons, but can exert a powerful influence upon either increased activation of GPe (Celada et al., 1999) or immediately following phasic inhibition of GPe (Connelly et al., 2010). The recent finding demonstrating regulation of the pallidonigral synapse by dopamine (de Jesús Aceves, et al., 2011) sets the stage to investigate the role of other neuromodulators at this synapse as well.
5.2. Glutamate input and its regulation by dopamine
5.2.1. Excitatory glutamate input to SNr
The best characterized excitatory afferents to SNr GABA neurons are from glutamate-containing neurons in the neighboring subthalamic nucleus (STN) (Fig. 1A, Fig. 6A) (Smith and Parent, 1988; Deniau et al., 2007; Ammari et al., 2010). Anatomical studies show that both in rodents and primates, glutamatergic neurons in STN project heavily to SNr, forming excitatory, asymmetric synapses with SNr GABA neurons (Kanazawa et al., 1976; Kita and Kitai, 1987; Smith et al., 1990; Bevan et al., 1994; Sato et al., 2000). Axon tracing studies indicate that striatonigral axons making symmetric synapses and subthalamonigral axons making asymmetric synapses converge onto SNr GABA projection neurons (Bevan et al., 1994).
Electrophysiological recordings have demonstrated that STN neuron activation induces excitatory postsynaptic currents or potentials in SNr GABA neurons (Nakanishi et al. 1987; Bosch et al., 2011). Excitation of STN neuronal activity by local injection of the GABAA receptor blocker bicuculline increased metabolism in the SNr (Féger et al., 1991). Additionally, STN neurons apparently innervate neighboring STN glutamate neurons, causing recurrent excitation, and often producing polysynaptic EPSCs and burst spiking in SNr GABA neurons (Fig. 8A,B,C) (Shen and Johnson 2006, 2008; Ammari et al., 2010). Thus, STN neuron excitation may drive SNr GABA neurons into burst firing (Murer et al., 1997; Tseng et al., 2000, 2001; Shen and Johnson, 2006). Electrophysiological recordings in patients with Parkinson’s disease and in animal models indicate an excessive oscillatory burst firing in STN neurons (Bevan et al., 2006; Walters et al., 2000, 2007). STN lesion and functional inhibition reduce oscillatory firing and normalize the firing pattern in SNr GABA neuron in rat models of Parkinson’s disease, further demonstrating the influence of STN on SNr GABA neuron activity (Ryan and Sanders, 1993; Murer et al., 1997; Tseng et al., 2001).
Fig. 8.
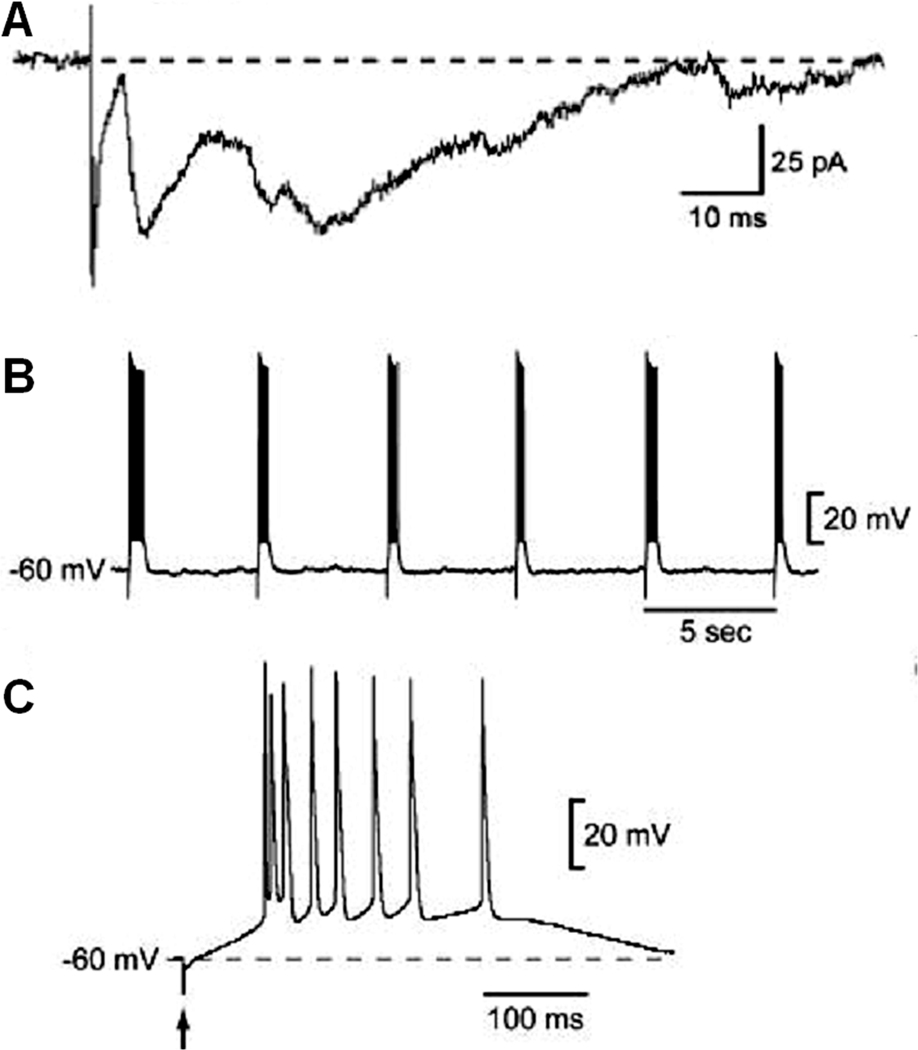
Excitatory synaptic input from STN neurons affects the firing of SNr GABA neurons. STN stimulation evokes complex polysynaptic EPSCs under voltage clamp at −70 mV (A) and burst spike firing in SNr GABA neurons (B, C). Adapted from Shen and Johnson 2006 with permission.
5.2.2. Presynaptic dopamine regulation of excitatory glutamate input
Dopamine may affect glutamate release by acting on subthalamonigral axon terminals. Electrophysiological studies have shown that D1-like agonist SKF38393 increased STN-evoked EPSC in SNr GABA neurons, whereas the D2-like receptor agonist quinpirole had the opposite effect (Ibanez-Sandoval et al., 2006). SKF38393 also decreased the paired pulse ratio whereas quinpirole increased it, indicating that D1 class receptor activation facilitates and D2-like receptor activation inhibits glutamate vesicle release at subthalamonigral axon terminals (Rosales et al., 1997; Ibanez-Sandoval et al., 2006). It is not clear why subthalamonigral axon terminals express both D1-like receptors and D2-like receptors that have opposing effects. One possibility is that these dopamine receptor subtypes have different affinity to dopamine resulting in differential activation by ambient dopamine levels. Thus, by acting on subthalamonigral axon terminals, dopamine can regulate the intensity and pattern of glutamate synaptic input to SNr GABA neurons.
5.3. Dopamine input
5.3.1. Dopamine dendrites in SNr
As shown in Fig. 1B, a prominent anatomical feature in SNr is the dopamine dendrites originating from SNc dopamine neurons (Bjorklund and Lindvall, 1975; Cheramy et al., 1981; Wassef et al., 1981). Evidence from fast cyclic voltammetry studies and other approaches indicates that nigral dopamine cell somata and dendrites may release DA largely via vesicular exocytosis (Groves et al., 1975; Geffen et al., 1976; Jaffe et al., 1998; Beckstead et al., 2004; Chen and Rice, 2001; John et al., 2006; Patel et al., 2009; Ford et al., 2010). Somatodendritic dopamine release can be triggered by action potential-evoked Ca2+ influx via voltage-activated Ca channels (Ford et al., 2010), but other mechanisms such as pacemaking activity-driven, L-type Ca2+ channel-mediated Ca2+ oscillation (Chan et al., 2007) and Ca2+ release from intracellular stores may also contribute to or facilitate dopamine release from dopamine neuron dendrites (Patel et al., 2009). Together, these release mechanisms may lead to an ambient extracellular dopamine level that is further regulated by reuptake (Cragg et al., 2001). Indeed, somatodendritically released dopamine induces a tonic autoinhibition of nigral dopamine neurons via the inhibitory D2 autoreceptors (Lacey et al., 1987; Pucak and Grace, 1994; Werkman et al., 2001). Endogenously released dopamine may also activate D1-like receptors on GABA and glutamate afferent terminals in the nigral areas (Misgeld et al., 2004; Ibanez-Sandoval et al., 2006).
5.3.2. Direct dopamine depolarization of SNr GABA neurons
Can dendritically released dopamine directly act on SNr GABA output neurons and influence their firing rate and pattern? Based on anatomical data, Cheramy et al. (1981) first suggested the possibility that dendritically released dopamine might directly act on SNr GABA neurons. Two iontophoretic studies also indicated that DA may exert an excitatory effect on SNr neurons in intact animals (Ruffieux and Schultz, 1980; Waszczak and Walters, 1983). However, these studies used extracellular recordings, did not remove synaptic inputs, and consequently could not determine if the effect was a direct excitation in SNr neurons or local circuit-mediated effect.
Recently, Zhou et al. (2009) have examined this question. scRT-PCR detected mRNAs for D1R and D5R in SNr GABA neurons, providing evidence that these basal ganglia output neurons may express D1R and D5R. These new results are consistent with two immuno-electron microscopy studies (Huang et al., 1992; Khan at al., 2000). Huang et al. (1992) detected D1Rs in the dendrites in SNr, even though the origin of the dendrites was not identified. However, other EM studies have reported no D1R signal in SNr neurons (Yung et al. 1995; Kliem et al. 2010). D5R has been detected in SNr GABA neurons (Khan et al., 2000; Kliem et al., 2010). Based on these different results, it is reasonable to conclude that the expression levels of D1R and D5R in SNr GABA neurons are probably low. However, even low levels of D1R and D5R may significantly influence basal ganglia output, because SNr GABA neurons are normally depolarized close to spike threshold such that their activity may be affected by even a small depolarization. Indeed, Zhou et al. (2009) found that the D1-like blocker SKF83566 induced a significant decrease in SNr GABA neuron firing frequency after blocking GABAA and ionotropic glutamate receptors and induced a clear hyperpolarization in SNr GABA neurons after blocking sodium spikes (Zhou et al., 2009). These results indicate that spontaneously released dopamine from dopamine dendrites induces tonic activation of D1–like receptors and exerts a tonic excitatory influence on SNr GABA neurons.
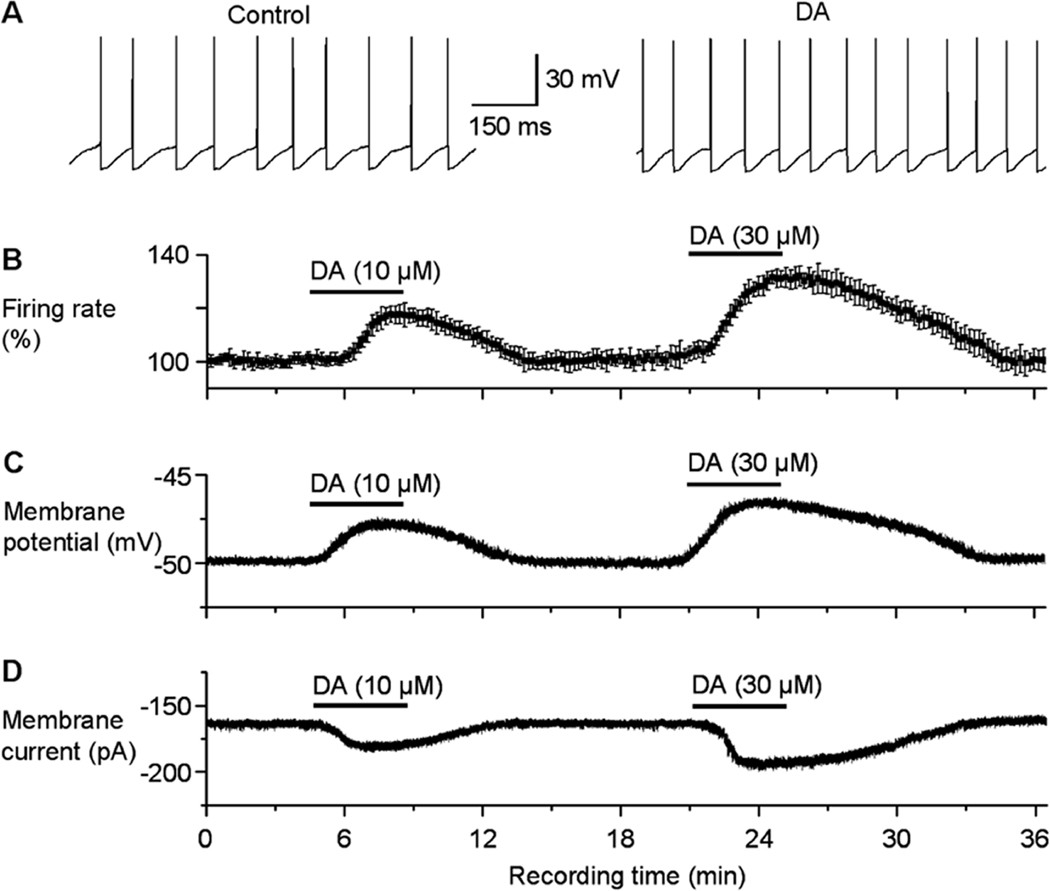
Direct excitation of SNr GABA neurons is readily observed with exogenous dopamine which increases the firing rate of SNr GABA neurons (Zhou et al., 2009) (Fig. 9A,B). After action potentials were blocked with TTX, bath application of exogenous dopamine induced a clear depolarization (Fig. 9C). When voltage clamped at −70 mV, dopamine also induced an inward current in a dose-dependent manner (Fig. 9D). Whole cell conductance was increased. Voltage ramp experiments show that the net dopamine-induced current I–V was linear with a reversal potential around −35 mV. These results suggest that dopamine was opening or enhancing a tonically active, mixed cation channel. Since D1R and D5R do not directly gate any ion channel, an important question is: what are the effector ion channels that mediate the depolarization? The fact that the dopamine current I–V curve was linear with a reversal potential around −35 mV suggested involvement of a constitutively active, mixed cation channel, similar to the I–V relationship of the TRPC3 channel-mediated current in SNr GABA neurons (Zhou et al. 2008, 2009). Indeed, blocking TRPC3 channels by intracellular infusion of an antibody known to inhibit TRPC3 channels also blocked the D1-like excitation (Zhou et al., 2009). The mechanisms coupling dopamine receptor activation to TRPC3 channel activation is unknown, but lipid signaling mechanisms may be involved. D1-like receptor agonists have been shown to stimulate PLC that in turn produces lipid signaling molecules including DAG, and this effect was eliminated in D5R null mice (Sahu et al., 2009). DAG is known to enhance TRPC3 channel activity (Hoffmann et al. 1999). It is reasonable to conclude that the apparent D1-D5 receptor activation in SNr GABA neurons may enhance the tonically active TRPC3 channels and that TRPC3 channels are the effector channel of direct dopamine excitation in these basal ganglia output neurons.
Fig. 9.
Direct dopamine excitation in SNr GABA neurons. All recordings were made in the presence of picrotoxin, D-AP5 and CNQX to block fast synaptic transmission. Sulpiride was present to prevent potential complications from D2 autoinhibition of DA neurons. (A) Examples of spontaneous spikes under control conditions (left) and during 10 µM DA application (right). (B) Group data of DA enhancement of spiking. (C) An example recording showing that, after blocking spikes with 1 µM TTX, DA induced a clear depolarization. (D) An example recording showing that, when voltage clamped at −70 mV, DA induced an inward current in the presence of 1 µM TTX. Note that SNr GABA neurons are normally depolarized and have a large holding current when clamped at −70 mV. Modified from Zhou et al., 2009 with permission.
The direct postsynaptic excitatory dopamine effect appears to counter the effects of presynaptic D1R facilitation of GABA release from striatonigral terminals. Why does dopamine need to have two opposing effects via pre- and post-synaptic mechanisms? One possibility is that the postsynaptic dopamine effect in SNr GABA neurons may prevent possible presynaptically mediated over-inhibition of SNr GABA neurons and the consequent over-disinhibition of the targets of SNr GABA neurons. A balance between the D1R-mediated presynaptic inhibition of SNr GABA neurons and the D1-like direct postsynaptic excitation of SNr GABA neurons, in concert with intrinsic ion channels and other synaptic inputs, may be important in maintaining the proper activity of SNr GABA neurons suitable for movement control.
5.4. Serotonin (5-HT) input
Histochemical studies have established that SNr receives dense 5-HT innervation originating from 5-HT neurons in raphe nuclei (Fig. 10A) (Steinbusch, 1981; Moukhles et al., 1997; Wallman et al., 2011), providing a rich anatomical basis for the 5-HT system to affect the motor control system (Hikosaka et al., 2006; Scholtissen et al., 2006; Nakamura et al., 2008; Fox et al., 2009). 5-HT neurons fire spontaneously at 1–2 Hz (Jacobs and Azmitia, 1992), leading to tonic 5-HT release and activation of 5-HT receptors (Rueter et al., 1997; Zhou et al., 2005). Over a dozen 5-HT receptors have been identified (Hannon and Hoyer, 2008). With the exception of 5-HT3 receptors coupling to a cation channel, all other 5-HT receptors are G-protein-coupled receptors (Barnes and Sharp, 1999; Zhou and Hablitz, 1999). SNr GABA neurons express multiple types of 5-HT receptors, particularly 5-HT2C receptors (5-HT2C-Rs) (Eberle-Wang et al., 1997; Clemett et al., 2000). 5-HT2C-Rs couple to Gq/11 protein and stimulate phospholipase C (PLC) that in turn generates diacylglycerol (DAG) and other lipid signaling molecules, providing a molecular basis for 5-HT2C-Rs to interact with other receptors and ion channels.
Fig. 10.
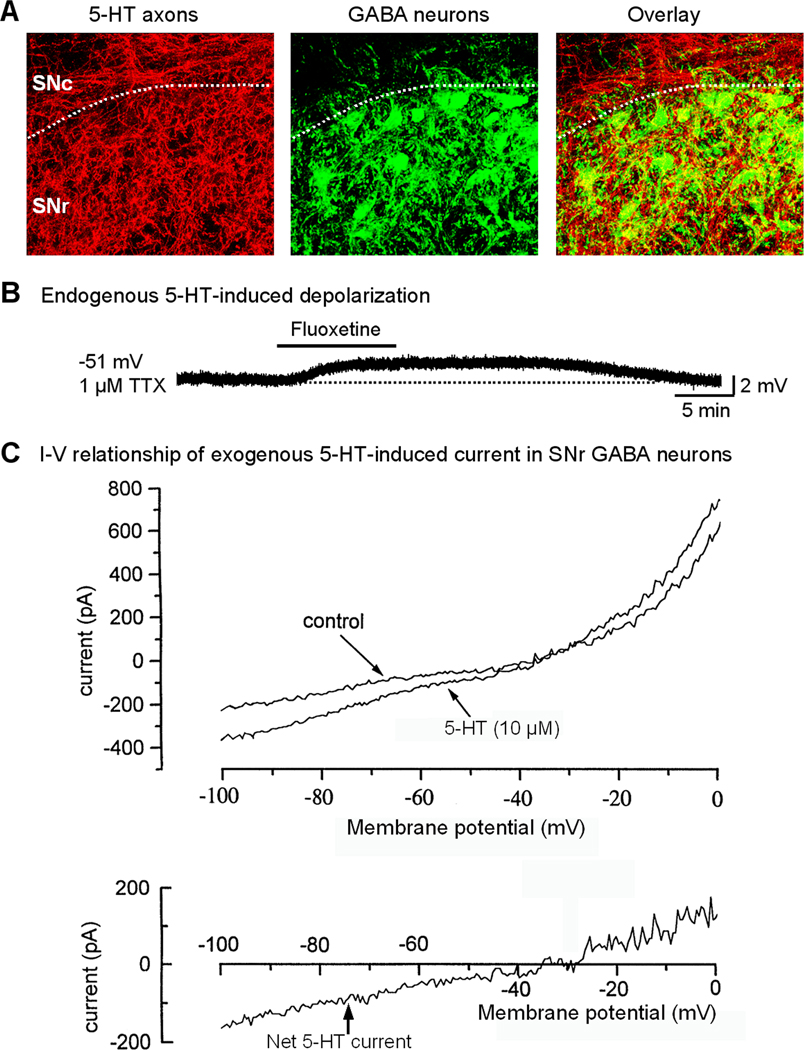
Direct 5-HT excitation in SNr GABA neurons. (A) Immunostaining for 5-HT transporter protein reveals a very dense 5-HT axonal network that intermingles with PV-positive GABA neurons in SNr. (B) Endogenous 5-HT induces a tonic depolarization in SNr GABA neurons that is enhanced by blocking 5-HT re-uptake with 2 µM fluoxetine. Action potentials were blocked by 1 µM TTX. Ionotropic glutamate receptors and GABAA receptors were blocked. (C) bath applied 5-HT induced a linear, voltage-independent inward current below action potential threshold. The whole cell conductance was increased during 5-HT application, indicating an opening of ion channels (e.g. TRPC3 channels), not a closing of background K channels. Note the remarkable similarity between this 5-HT current and the TRP current shown in Fig. 3C,D.A and B are unpublished data of FMZ. C is adapted from Stanford and Lacey, 1996a, with permission.
Studies indicate that exogenous 5-HT, via activating 5-HT2C-R, strongly excites SNr GABA neurons by inducing a linear inward current with a reversal potential around −30 mV (Fig. 10C) (Rick et al., 1995; Stanford and Lacey, 1996a; Zhou, unpublished data). This inward current is accompanied by an increased whole cell conductance, indicating an opening of cation channels that produces a next influx of cations and also excluding closing of K channels as the underlying ionic mechanism. Other excitatory 5-HT receptors (5-HT2A-R, 5-HT4-R) have also been detected in SNr, though the levels appear to be lower (Cornea-Hébert et al., 1999; Vilaró et al., 2005). The dense 5-HT axon terminals in the SNr release 5-HT spontaneously and induce tonic activation of 5-HT2C-Rs in SNr GABA neurons. Thus, blocking 5-HT reuptake induces excitation (Fig. 10B) and blocking 5-HT2C-R induces a hyperpolarization and decreases the firing rate in SNr GABA neurons (Zhou, unpublished data).
Since 5-HT2C-Rs do not directly gate any ion channels, a fundamental question is: what ion channels mediate the 5-HT2C-R activation-evoked inward current in SNr GABA neurons? As discussed above, constitutively active TRPC3 channels conduct a linear inward current with a reversal potential around −35 mV in SNr GABA neurons (Zhou et al., 2008). Unpublished data of Zhou indicate that the TRP channel blocker FFA inhibits 5-HT2C-R response. The I-V curves for 5-HT2C-R current and TRPC3 current are remarkably similar (Compare Fig. 3C,D with Fig. 10C). Generation of the 5-HT2C-R current is accompanied by increased conductance (Stanford and Lacey, 1996a; Zhou, unpublished data). Based on these data, it appears that TRPC3 channels may mediate the 5-HT2C-R activation-evoked inward current and excitation in SNr GABA neurons. This is entirely possible because 5-HT2C-R activation stimulates Gq/11 protein that in turn activates PLC, leading to the generation of DAG which enhances TRPC3 channel activity (Hofmann et al., 1999; Large et al., 2009). TRPC3 channels may thus serve as a common effector channel for 5-HT2C-Rs and potentially other G-protein coupled receptors that interact with lipid signaling mechanisms (Zhou, 2010).
5.5. Acetylcholine (ACh) input
Neurons in the SNr express nicotinic and muscarinic ACh receptors (nAChRs and mAChRs) (Nastuk and Graybiel, 1991; Klink et al., 2001; Wooltorton et al., 2003; Michel et al., 2004; Poisik et al., 2008). Locally applied ACh causes excitation of SNr GABA neurons in vivo (Collingridge and Davies, 1981; Pinnock and Dray, 1982). Application of ACh to SNr GABA neurons in vitro induces an inward current when mAChRs are blocked, indicating the presence of functional nAChRs on these neurons (Klink et al., 2001; Wooltorton et al., 2003; Poisik et al., 2008). Muscarinic stimulation similarly causes an increase in the firing rate of midbrain GABA neurons in vitro (Michel et al., 2004) via M3 receptor activation of protein kinase C followed by activation of a nonselective cationic conductance possibly mediated by TRPC channels (Michel et al., 2005).
Modulation of SNr GABA neuron activity by nAChRs plays a role in changes that result from nicotine exposure. nAChRs containing α4 subunits are up-regulated by chronic nicotine exposure in midbrain GABA neurons including SNr GABA neurons (Nashmi et al., 2007). Recorded in vitro, SNr GABA neurons exposed to chronic nicotine exhibit an increased tonic firing rate and a potentiated nicotine-induced increase in firing rate in response to further nicotine exposure when compared to control neurons (Nashmi et al., 2007). In contrast, midbrain dopamine neurons do not show a change in nAChR density in response to chronic nicotine exposure (Nashmi et al., 2007). Strikingly, SNc dopamine neurons exposed to chronic nicotine exhibit a decreased tonic firing rate and a muted response to further nicotine exposure in vitro possibly resulting from increased GABAergic input from neighboring SNr neurons (Nashmi et al., 2007).
In addition to being affected by exogenous ACh receptor agonists, SNr GABA neurons receive endogenous cholinergic input. The main cholinergic input to SN arises from the pedunculopontine nucleus (PPN) which also contains a significant number of glutamatergic and GABAergic neurons (Woolf and Butcher, 1986; Gould et al., 1989; Clements and Grant, 1990; Oakman et al., 1995; Charara et al., 1996; Wang and Morales, 2009). The PPN sends a dense projection to SNc suggesting preferential innervation of DA neurons (Saper and Loewy, 1982; Jackson and Crossman, 1983; Bolam et al., 1991), however there is also a projection to SNr (Saper and Loewy, 1982) and SNr GABA neurons receive synaptic contacts from PPN neurons (Lee and Tepper, 2009). Consistent with a direct innervation of SNr GABA neurons by the PPN, stimulation of the PPN in vivo most often produces short latency excitation of SNr GABA neurons (Scarnati et al., 1984; 1987). Further, PPN stimulation in organotypic cultures evokes EPSPs in SN GABA neurons that are attenuated by both Ach and glutamate receptor antagonists (Rohrbacher et al., 2000).
Surprisingly, lesioning the PPN increases SNr GABA neuron firing, but this effect is likely due to alterations in the basal ganglia network rather than a consequence of removing the monosynaptic projection from the PPN to the SNr (Breit et al., 2005). In addition, ACh might presynaptically alter afferent input to SNr through both mAChRs and nAChRs (Kayadjanian et al., 1994a,b; Michel et al., 2004) further complicating the interpretation of results obtained in vivo. In animals with dopaminergic lesion, however, additional lesion of the PPN leads to a decrease in SNr GABA neuron firing rate possibly suggesting an increased effect of the monosynaptic excitatory projection in Parkinsonian states, though the involvement of polysynaptic pathways is likely (Breit et al., 2006). The discovery of the PPN as an effective site of deep brain low frequency stimulation for the treatment of impaired gait and postural imbalance in Parkinson’s disease (Gubellini et al. 2009; Hamani et al., 2010) heightens the importance of studying the role and mechanisms of input from the PPN in the modulation of SNr GABA neuron activity, although the descending projection of PPN is also likely to be important.
5.6. Hydrogen Peroxide (H2O2)
H2O2 is emerging as an important neuromodulator in the substantia nigra where it affects both SNc dopamine (Rice, 2011) and SNr GABA neurons (Lee et al., 2011). Elevated H2O2 achieved by either exogenous application of H2O2 or by increasing endogenous levels by inhibiting the metabolizing enzyme glutathione peroxidase, increases the spontaneous firing rate of guinea-pig SNr GABA neurons recorded in vitro (Fig. 11A, B) and induces a decrease in input resistance (Lee et al., 2011). Conversely, depleting endogenous H2O2 with catalase decreases the spontaneous firing rate of these neurons and increases firing irregularity (Fig. 11C) (Lee et al., 2011). Thus, H2O2 plays a role in maintaining the spontaneous activity of SNr GABA neurons. The source of H2O2 involved in neuronal signaling is likely the electron transport chain in mitochondria, whose main role is the production of ATP during the process of oxidative phosphorylation (Boveris and Chance, 1973; Peuchen et al., 1997; Liu et al., 2002; Bao et al., 2009). Mitochondrial production of H2O2 increases when neuronal activity increases (Kann et al., 2003; Avshalumov et al., 2005, 2008), and the spontaneous activity of SNr GABA neurons is sufficient to drive modulatory H2O2 production, resulting in tonic H2O2 regulation of SNr neurons (Lee et al., 2011).
Fig. 11.
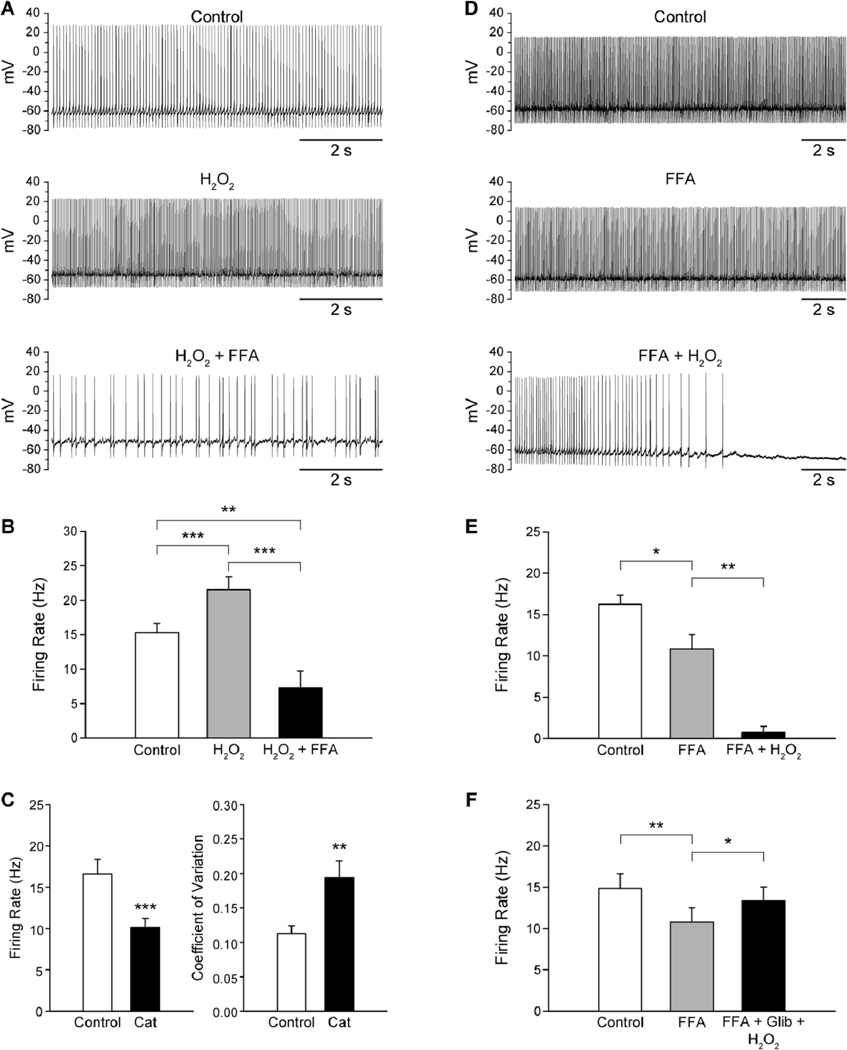
Modulation of guinea-pig SNr GABA neuron firing rate by H2O2. (A) Spontaneous activity of a SNr GABA neuron under control conditions, in the presence of H2O2 (1.5 mM), and with flufenamic acid (FFA; 20 µM) in the continued presence of H2O2. (B) H2O2 causes an increase in SNr GABA neuron firing rate that is reversed by the TRP channel blocker FFA, with the resulting firing rate falling below control levels. (C) Depletion of endogenous H2O2 with catalase (Cat; 500 U/mL) results in a decrease in firing rate and an increase in the coefficient of variation indicating that basal H2O2 levels play a role in maintaining the tonic firing of SNr GABA neurons. (D) Spontaneous activity of another SNr GABA neuron under control conditions, in the presence of FFA, and with H2O2 in the continued presence of FFA. (E, F) Blockade of TRP channels with FFA causes a decrease in SNr GABA neuron firing rate. Addition of H2O2 when TRP channels are blocked results in a suppression of firing, indicating activation of a hyperpolarizing conductance by H2O2 (E). The H2O2-induced suppression of firing is prevented by the KATP channel blocker glibenclamide (Glib; 3µM) (F). *p < 0.05; **p < 0.01; ***p < 0.001. Modified from Lee et al., 2011 with permission.
H2O2 exerts its excitatory effects on guinea-pig SNr GABA neurons by activating a FFA-sensitive channel which is likely to be a TRP channel (Fig. 11A, B) (Lee et al., 2011). The increase in firing rate induced by either exogenous H2O2 or elevated endogenous H2O2 is reversed by FFA (Lee et al., 2011). In fact, when FFA is applied in the continued presence of elevated H2O2, the firing rate falls below control levels (Fig. 11B), possibly reflecting a loss of tonic depolarization by presumed TRP channels or the additional activation of a hyperpolarizing channel by H2O2. These possibilities were examined by first blocking presumed TRP channels with FFA, and then elevating H2O2 levels in the continued presence of FFA. When this was done, SNr GABA neurons exhibited a decrease in firing rate in response to FFA, reflecting removal of a tonic depolarization mediated by TRP channels, followed by a suppression of firing when H2O2 levels were elevated demonstrating that H2O2 also activates a hyperpolarizing channel (Fig. 11 D , E) (Lee et al., 2011). H2O2 hyperpolarizes SNc dopamine neurons by activating ATP-sensitive potassium (KATP) channels (Avshalumov et al., 2005) that are also present on SNr GABA neurons (Schwanstecher and Panten, 1993; Stanford and Lacey, 1996b; Dunn-Meynell et al., 1998). When H2O2 was increased in the presence of both a TRP channel and KATP channel blocker, H2O2 did not suppress the firing of SNr GABA neurons (Fig. 11F), indicating that H2O2 can activate TRP channels as well as KATP channels in guinea-pig SNr GABA neurons (Lee et al., 2011). Other experiments showed that the H2O2-induced increase in firing rate was only modestly enhanced when KATP channels were blocked, suggesting that the main effect of H2O2 on guinea-pig SNr GABA neurons is to increase their firing rate.
It is likely that H2O2 plays a role in modulating the spontaneous firing rate of individual SNr GABA neurons and might also recruit increased activity in neighboring neurons given its ability to act as a diffusible messenger (Avshalumov et al., 2008). Further, as a part of abnormal reactive oxygen species production that may occur in Parkinson’s disease (Lin and Beal, 2006; Rice, 2011), increased H2O2 could contribute to pathological changes in basal ganglia output by increasing SNr GABA neuron activity.
6. Summary
The most striking neurophysiological feature of SNr GABA neurons is their sustained, spontaneous high frequency spike firing. Tonically active TRPC3 channels and a subthreshold INaP together depolarize the membrane potential toward spike threshold. The large and fast INaT triggers the rapid rising phase of action potentials that in turn activates high-threshold, inactivation-resistant Kv3-like channels that can rapidly repolarize the membrane. The combination of these intrinsic ion channels provides these neurons with a remarkable spiking capability. Additionally, SNr GABA neurons receive and integrate excitatory, inhibitory and modulatory inputs that sculpt the default tonic high frequency firing into a meaningful motor control signal.
Highlights.
-
➢
SNr GABA projection neurons fire sustained autonomous high frequency spikes
-
➢
SNr GABA neurons have a higher density of Na currents than dopamine neurons
-
➢
Kv3-like current is essential for sustained fast spiking in SNr GABA neurons
-
➢
Dopamine D1 receptors facilitates the inhibitory striatonigral synapse
-
➢
Dendritically released dopamine directly depolarizes SNr GABA neurons
Acknowledgements
This work was supported by NIH grants R01NS058850 and R01DA021194 to FMZ and F32NS063656 to CRL.
Abbreviations
- ACh
Acetylcholine
- DA
dopamine
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- 5-HT2C-R
serotonin 5-HT2C receptor
- GABA
γ-aminobutyric acid
- GPe
globus pallidus external segment
- MSN
medium spiny neuron
- CaV channel
voltage-activated calcium channel
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- FFA
flufenamic acid
- GP
globus pallidus
- IA
transient A-type potassium current
- IDR-fast
fast, delayed rectifier type potassium current
- Ih
hyperpolarization-activation cation current
- INaP
persistent sodium current
- INaT
transient sodium current
- KATP channel
ATP-sensitive potassium channel
- KCa channel
calcium-activated potassium channel
- Kv channel
voltage-activated potassium channel
- NaV channel
voltage-activated sodium channel
- PPN
pedunculopontine nucleus
- PLC
phospholipase C
- scRT-PCR
single cell reverse transcription polymerase chain reaction
- SNr
substantia nigra pars reticulata
- SNc
substantia nigra pars compacta
- STN
subthalamic nucleus
- TRP channel
transient receptor potential channel
- TEA
tetraethylammonium
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert AP, Large WA. Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J Physiol. 2006;570:45–51. doi: 10.1113/jphysiol.2005.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Pucovský V, Prestwich SA, Large WA. TRPC3 properties of a native constitutively active Ca2+-permeable cation channel in rabbit ear artery myocytes. J Physiol. 2006;571:361–369. doi: 10.1113/jphysiol.2005.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Pozzo-Miller L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci. 2007;27:5179–5189. doi: 10.1523/JNEUROSCI.5499-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammari R, Lopez C, Bioulac B, Garcia L, Hammond C. Subthalamic nucleus evokes similar long lasting glutamatergic excitations in pallidal, entopeduncular and nigral neurons in the basal ganglia slice. Neuroscience. 2010;166:808–818. doi: 10.1016/j.neuroscience.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Yoshida M. Axonal branching patterns and location of nigrothalamic and nigrocollicular neurons in the cat. J Neurophysiol. 1980;43:883–895. doi: 10.1152/jn.1980.43.4.883. [DOI] [PubMed] [Google Scholar]
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci. 2005;25:8272–8281. doi: 10.1523/JNEUROSCI.1475-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Koós T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Patel JC, Rice ME. AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release. J Neurophysiol. 2008;100:1590–1601. doi: 10.1152/jn.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proc Natl Acad Sci U S A. 2010;107:12357–12362. doi: 10.1073/pnas.1005633107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci. 2009;29:9002–9010. doi: 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–266. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nature Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Beckstead RM. Long collateral branches of substantia nigra pars reticulata axons to thalamus, superior colliculus and reticular formation in monkey and cat. Multiple retrograde neuronal labeling with fluorescent dyes. Neuroscience. 1983;10:767–779. doi: 10.1016/0306-4522(83)90214-2. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience. 1982;7:2377–2388. doi: 10.1016/0306-4522(82)90202-0. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP, Crossman AR. Convergent synaptic input from the neostriatum and the subthalamus onto identified nigrothalamic neurons in the rat. Eur J Neurosci. 1994;6:320–334. doi: 10.1111/j.1460-9568.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Smith AD, Bolam JP. The substantia nigra as a site of synaptic integration of functionally diverse information arising from the ventral pallidum and the globus pallidus in the rat. Neuroscience. 1996;75:5–12. doi: 10.1016/0306-4522(96)00377-6. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Atherton JF, Baufreton J. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr Opin Neurobiol. 2006;16:621–628. doi: 10.1016/j.conb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- Bjöklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high frequency firing in substantia nigra dopamine neurons in vitro. J Neurophysiol. 2007;97:2837–2850. doi: 10.1152/jn.01157.2006. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Giber K, Rovó Z, Ulbert I, Acsády L. Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway. J Neurosci. 2008;28:3090–3102. doi: 10.1523/JNEUROSCI.5266-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Francis CM, Henderson Z. Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience. 1991;41:483–494. doi: 10.1016/0306-4522(91)90343-m. [DOI] [PubMed] [Google Scholar]
- Bosch C, Degos B, Deniau JM, Venance L. Subthalamic nucleus high frequency stimulation generates a concomitant synaptic excitation-inhibition in substantia nigra pars reticulata. J Physiol. 2011 doi: 10.1113/jphysiol.2011.211367. 2011 Jun 20. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Lessmann L, Benazzouz A, Schulz JB. Unilateral lesion of the pedunculopontine nucleus induces hyperactivity in the subthalamic nucleus and substantia nigra in the rat. Eur J Neurosci. 2005;22:2283–2294. doi: 10.1111/j.1460-9568.2005.04402.x. [DOI] [PubMed] [Google Scholar]
- Breit S, Lessmann L, Unterbrink D, Popa RC, Gasser T, Schulz JB. Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur J Neurosci. 2006;24:2275–2282. doi: 10.1111/j.1460-9568.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Nakano K, Kim R. Nigrothalamic projections in the monkey demonstrated by autoradiographic technics. J Comp Neurol. 1976;165:401–415. doi: 10.1002/cne.901650402. [DOI] [PubMed] [Google Scholar]
- Cebrián C, Parent A, Prensa L. Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J Comp Neurol. 2005;492:349–369. doi: 10.1002/cne.20741. [DOI] [PubMed] [Google Scholar]
- Cebrián C, Parent A, Prensa L. The somatodendritic domain of substantia nigra pars reticulata projection neurons in the rat. Neurosci Res. 2007;57:50–60. doi: 10.1016/j.neures.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chan PK, Chan SC, Yung WH. Presynaptic inhibition of GABAergic inputs to rat substantia nigra pars reticulata neurones by a cannabinoid agonist. Neuroreport. 1998;9:671–675. doi: 10.1097/00001756-199803090-00020. [DOI] [PubMed] [Google Scholar]
- Chang SY, Zagha E, Kwon ES, Ozaita A, Bobik M, Martone ME, Ellisman MH, Heintz N, Rudy B. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol. 2007;502:953–972. doi: 10.1002/cne.21353. [DOI] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM, Thierry AM, Feger J. The nigro-tectal pathway. An electrophysiological reinvestigation in the rat. Brain Res. 1981;213:253–263. doi: 10.1016/0006-8993(81)90232-8. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Transient Receptor Potential (TRP) Channels. In: Squire LR, editor. Encyclopedia of Neuroscience. volume 9. Oxford: Academic Press; 2009. pp. 1109–1133. [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett. 1990;120:70–73. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Davies J. The influence of striatal stimulation and putative neurotransmitters on identified neurones in the rat substantia nigra. Brain Res. 1981;212:345–359. doi: 10.1016/0006-8993(81)90467-4. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Schulz JM, Lees G, Reynolds JN. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J Neurosci. 2010;30:14854–14861. doi: 10.1523/JNEUROSCI.3895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME. Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J Neurophysiol. 2001;85:1761–1771. doi: 10.1152/jn.2001.85.4.1761. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- de Jesús Aceves J, Rueda-Orozco PE, Hernández R, Plata V, Ibañez-Sandoval O, Galarraga E, Bargas J. Dopaminergic presynaptic modulation of nigral afferents: its role in the generation of recurrent bursting in substantia nigra pars reticulata neurons. Front Syst Neurosci. 2011;5:6. doi: 10.3389/fnsys.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Relations between movement and single cell discharge in the substantia nigra of the behaving monkey. J Neurosci. 1983;3:1599–1606. doi: 10.1523/JNEUROSCI.03-08-01599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res. 1985;334:227–233. doi: 10.1016/0006-8993(85)90214-8. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Kitai ST, Donoghue JP, Grofova I. Neuronal interactions in the substantia nigra pars reticulata through axon collaterals of the projection neurons. An electrophysiological and morphological study. Exp Brain Res. 1982;47:105–113. doi: 10.1007/BF00235891. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Lackner D, Feger J. Effect of substantia nigra stimulation on identified neurons in the VL-VA thalamic complex: comparison between intact and chronically decorticated cats. Brain Res. 1978;145:27–35. doi: 10.1016/0006-8993(78)90793-x. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Prog Brain Res. 2007;160:151–172. doi: 10.1016/S0079-6123(06)60009-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 1979;176:273–284. doi: 10.1016/0006-8993(79)90983-1. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- Ding S, Zhou F-M. Molecular and functional differences in voltage-activated Na+ channels between GABA neurons and dopamine neurons in substantia nigra. Society for Neuroscience. 2010 doi: 10.1152/jn.00305.2011. Abstracts Abstract 339.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Matta SG, Zhou F-M. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol. 2011;105:554–570. doi: 10.1152/jn.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron. 2003;39:109–120. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol. 1997;384:233–247. [PubMed] [Google Scholar]
- Eder P, Poteser M, Groschner K. TRPC3: a multifunctional, pore-forming signalling molecule. Handb Exp Pharmacol. 2007;179:77–92. doi: 10.1007/978-3-540-34891-7_4. [DOI] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K+ channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol. 1999;82:2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- Féger J, Robledo P. The effects of activation or inhibition of the subthalamic nucleus on the metabolic and electrophysiological activities within the pallidal complex and substantia nigra in the rat. Eur J Neurosci. 1991;3:947–952. doi: 10.1111/j.1460-9568.1991.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Chuang R, Brotchie JM. Serotonin and Parkinson's disease: On movement, mood, and madness. Mov Disord. 2009;24:1255–1266. doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- Francois C, Tande D, Yelnik J, Hirsch EC. Distribution and morphology of nigral axons projecting to the thalamus in primates. J Comp Neurol. 2002;447:249–260. doi: 10.1002/cne.10227. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. GABAergic circuits in the basal ganglia and movement disorders. Prog Brain Res. 2007;160:287–312. doi: 10.1016/S0079-6123(06)60017-4. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Bolam JP. The neuroanatomical organization of the basal ganglia. In: Steiner H, Tseng KY, editors. Handbook of Basal Ganglia Structure and Function. Academic Press; 2010. pp. 3–28. [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- González-Hernández T, Rodríguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol. 2000;421:107–135. doi: 10.1002/(sici)1096-9861(20000522)421:1<107::aid-cne7>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolf NJ, Butcher LL. Cholinergic projections to the substantia nigra from the pedunculopontine and laterodorsal tegmental nuclei. Neuroscience. 1989;28:611–623. doi: 10.1016/0306-4522(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J Comp Neurol. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Young SJ, Rebec GV. Self-inhibition by dopaminergic neurons. Science. 1975;190:522–529. doi: 10.1126/science.242074. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Salin P, Kerkerian-Le Goff L, Baunez C. Deep brain stimulation in neurological diseases and experimental models: from molecule to complex behavior. Prog Neurobiol. 2009;89:79–123. doi: 10.1016/j.pneurobio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Kuwajima M, Mayhill E, Rebec GV. Behavior-related changes in the activity of substantia nigra pars reticulata neurons in freely moving rats. Brain Res. 1999;845:68–76. doi: 10.1016/s0006-8993(99)01932-0. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Reed JL, Kuwajima M, Rebec GV. Amphetamineinduced behavioral activation is associated with variable changes in basal ganglia output neurons recorded from awake, behaving rats. Brain Res. 2004;1012:108–118. doi: 10.1016/j.brainres.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sánchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Moro E, Lozano AM. The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm. 2010 doi: 10.1007/s00702-010-0547-8. 2010 Dec 31. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol. 1979;183:487–517. doi: 10.1002/cne.901830304. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991a;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991b;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983a;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983b;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. 1983. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in the monkey superior colliculus. J Neurophysiol. 1985a;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985b;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- Hille B. Sinauer. 3rd edition 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci USA. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Carrillo-Reid L, Galarraga E, Tapia D, Mendoza E, Gomora JC, Aceves J, Bargas J. Bursting in substantia nigra pars reticulata neurons in vitro: possible relevance for Parkinson disease. J Neurophysiol. 2007;98:2311–2323. doi: 10.1152/jn.00620.2007. [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Hernandez A, Floran B, Galarraga E, Tapia D, Valdiosera R, Erlij D, Aceves J, Bargas J. Control of the subthalamic innervation of substantia nigra pars reticulata by D1 and D2 dopamine receptors. J Neurophysiol. 2006;95:1800–1811. doi: 10.1152/jn.01074.2005. [DOI] [PubMed] [Google Scholar]
- Jackson A, Crossman AR. Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience. 1983;10:725–765. doi: 10.1016/0306-4522(83)90213-0. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the Somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Budygin EA, Mateo Y, Jones SR. Neurochemical characterization of the release and uptake of dopamine in ventral tegmental area and serotonin in substantia nigra of the mouse. J Neurochem. 2006;96:267–282. doi: 10.1111/j.1471-4159.2005.03557.x. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Marshall GR, Kelly JS. Afferents to the rat substantia nigra studied with horseradish peroxidase, with special reference to fibers from the subthalamic nucleus. Brain Res. 1976;115:485–491. doi: 10.1016/0006-8993(76)90364-4. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci. 2008;28:11071–11078. doi: 10.1523/JNEUROSCI.3263-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Schuchmann S, Buchheim K, Heinemann U. Coupling of neuronal activity and mitochondrial metabolism as revealed by NAD(P)H fluorescence signals in organotypic hippocampal slice cultures of the rat. Neuroscience. 2003;119:87–100. doi: 10.1016/s0306-4522(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kayadjanian N, Gioanni H, Ménetrey A, Besson MJ. Muscarinic receptor stimulation increases the spontaneous [3H]GABA release in the rat substantia nigra through muscarinic receptors localized on striatonigral terminals. Neuroscience. 1994a;63:989–1002. doi: 10.1016/0306-4522(94)90567-3. [DOI] [PubMed] [Google Scholar]
- Kayadjanian N, Rétaux S, Menétrey A, Besson MJ. Stimulation by nicotine of the spontaneous release of [3H]gamma-aminobutyric acid in the substantia nigra and in the globus pallidus of the rat. Brain Res. 1994b;649:129–135. doi: 10.1016/0006-8993(94)91056-1. [DOI] [PubMed] [Google Scholar]
- Kemel ML, Desban M, Gauchy C, Glowinski J, Besson MJ. Topographical organization of efferent projections from the cat substantia nigra pars reticulata. Brain Res. 1988;455:307–323. doi: 10.1016/0006-8993(88)90090-x. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, de la Calle A. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100:689–699. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-Like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;98:1489–1500. doi: 10.1152/jn.00171.2007. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Pare JF, Khan ZU, Wichmann T, Smith Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur J Neurosci. 2010;31:836–851. doi: 10.1111/j.1460-9568.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Appel SB. A-type K+ current of dopamine and GABA neurons in the ventral tegmental area. J Neurophysiol. 2006;96:544–554. doi: 10.1152/jn.01318.2005. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium. 2009;45:574–582. doi: 10.1016/j.ceca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–9085. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. Morphological and physiological properties of parvalbumin- and calretinin-containing gamma-aminobutyric acidergic neurons in the substantia nigra. J Comp Neurol. 2007a;500:958–972. doi: 10.1002/cne.21220. [DOI] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci. 2007b;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. Basal ganglia control of substantia nigra dopaminergic neurons. J Neural Transm Suppl. 2009;73:71–90. doi: 10.1007/978-3-211-92660-4_6. [DOI] [PubMed] [Google Scholar]
- Lee CR, Witkovsky P, Rice ME. Regulation of Substantia Nigra Pars Reticulata GABAergic Neuron Activity by H2O2 via Flufenamic Acid-Sensitive Channels and KATP Channels. Front Syst Neurosci. 2011;5:14. doi: 10.3389/fnsys.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci USA. 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci. 2003;23:2058–2068. doi: 10.1523/JNEUROSCI.23-06-02058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and Kchip3.1 transcription. EMBO J. 2001;20:5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Macica CM, von Hehn CA, Wang LY, Ho CS, Yokoyama S, Joho RH, Kaczmarek LK. Modulation of the kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. J Neurosci. 2003;23:1133–1141. doi: 10.1523/JNEUROSCI.23-04-01133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Deniau JM. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J Neurosci. 2003;23:5247–5257. doi: 10.1523/JNEUROSCI.23-12-05247.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Fujimura K, Yoshida S. Two types of neurons in the substantia nigra pars compacta studied in a slice preparation. Neurosci Res. 1987;5:172–179. doi: 10.1016/0168-0102(87)90033-2. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol. 1997;505:593–603. doi: 10.1111/j.1469-7793.1997.593ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Thierry AM, Glowinski J, Deniau JM. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2003;23:9929–9936. doi: 10.1523/JNEUROSCI.23-30-09929.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci. 2007;27:13552–13566. doi: 10.1523/JNEUROSCI.3430-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod NK, James TA, Kilpatrick IC, Starr MS. Evidence for a GABAergic nigrothalamic pathway in the rat. II. Electrophysiological studies. Exp Brain Res. 1980;40:55–61. doi: 10.1007/BF00236662. [DOI] [PubMed] [Google Scholar]
- Michel FJ, Fortin GD, Martel P, Yeomans J, Trudeau LE. M3-like muscarinic receptors mediate Ca2+ influx in rat mesencephalic GABAergic neurones through a protein kinase C-dependent mechanism. Neuropharmacology. 2005;48:796–809. doi: 10.1016/j.neuropharm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Michel FJ, Robillard JM, Trudeau LE. Regulation of rat mesencephalic GABAergic neurones through muscarinic receptors. J Physiol. 2004;556:429–445. doi: 10.1113/jphysiol.2003.057737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AS, Walker JM. Effects of a cannabinoid on spontaneous and evoked neuronal activity in the substantia nigra pars reticulata. Eur J Pharmacol. 1995;279:179–185. doi: 10.1016/0014-2999(95)00151-a. [DOI] [PubMed] [Google Scholar]
- Misgeld U. Innervation of the substantia nigra. Cell Tissue Res. 2004;318:107–114. doi: 10.1007/s00441-004-0918-2. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Lacey MG. Presynaptic inhibition by dopamine of a discrete component of GABA release in rat substantia nigra pars reticulata. J Physiol. 1998;513:805–817. doi: 10.1111/j.1469-7793.1998.805ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukhles H, Bosler O, Bolam JP, Vallee A, Umbriaco D, Geffard M, Doucet G. Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience. 1997;76:1159–1171. doi: 10.1016/s0306-4522(96)00452-6. [DOI] [PubMed] [Google Scholar]
- Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 6-OHDA-lesioned rats: effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27:278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46:959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987;437:45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18:594–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk MA, Graybiel AM. Pharmacologically defined M1 and M2 muscarinic cholinergic binding sites in the cat's substantia nigra: development and maturity. Brain Res Dev Brain Res. 1991;61:1–10. doi: 10.1016/0165-3806(91)90108-u. [DOI] [PubMed] [Google Scholar]
- Nevet A, Morris G, Saban G, Fainstein N, Bergman H. Discharge rate of substantia nigra pars reticulata neurons is reduced in non-parkinsonian monkeys with apomorphine-induced orofacial dyskinesia. J Neurophysiol. 2004;92:1973–1981. doi: 10.1152/jn.01036.2003. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Takada M, Mizuno N. Topographic distribution and collateral projections of the two major populations of nigrothalamic neurons. A retrograde labeling study in the rat. Neurosci Res. 1997;28:1–9. doi: 10.1016/s0168-0102(97)01171-1. [DOI] [PubMed] [Google Scholar]
- Nitsch C, Riesenberg R. Immunocytochemical demonstration of GABAergic synaptic connections in rat substantia nigra after different lesions of the striatonigral projection. Brain Res. 1988;461:127–142. doi: 10.1016/0006-8993(88)90731-7. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Martone ME, Ellisman MH, Rudy B. Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channels subunit in brain. J Neurophysiol. 2002;88:394–408. doi: 10.1152/jn.2002.88.1.394. [DOI] [PubMed] [Google Scholar]
- Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 2000;23:S20–S27. doi: 10.1016/s1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak JB, Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986;87:305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J Neurophysiol. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Bolaños JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Pinnock RD, Dray A. Differential sensitivity of presumed dopaminergic and non-dopaminergic neurones in rat substantia nigra to electrophoretically applied substance P. Neurosci Lett. 1982;29:153–158. doi: 10.1016/0304-3940(82)90345-7. [DOI] [PubMed] [Google Scholar]
- Poisik OV, Shen JX, Jones S, Yakel JL. Functional alpha7-containing nicotinic acetylcholine receptors localize to cell bodies and proximal dendrites in the rat substantia nigra pars reticulata. J Physiol. 2008;586:1365–1378. doi: 10.1113/jphysiol.2008.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther. 1994;271:1181–1192. [PubMed] [Google Scholar]
- Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci. 1998;18:2009–2016. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME. H2O2: A dynamic neuromodulator. Neuroscientist. 2011 doi: 10.1177/1073858411404531. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immuno-cytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Rick CE, Stanford IM, Lacey MG. Excitation of rat substantia nigra pars reticulata neurons by 5-hydroxytryptamine in vitro: evidence for a direct action mediated by 5-hydroxytryptamine2C receptors. Neuroscience. 1995;69:903–913. doi: 10.1016/0306-4522(95)00283-o. [DOI] [PubMed] [Google Scholar]
- Rohrbacher J, Ichinohe N, Kitai ST. Electrophysiological characteristics of substantia nigra neurons in organotypic cultures: spontaneous and evoked activities. Neuroscience. 2000;97:703–714. doi: 10.1016/s0306-4522(00)00046-4. [DOI] [PubMed] [Google Scholar]
- Romo-Parra H, Misgeld U, Yanovsky Y. Regular firing of a single output neuron reduces its own inhibition through endocannabinoids in substantia nigra pars reticulata of juvenile mice. Neuroscience. 2009;160:596–605. doi: 10.1016/j.neuroscience.2009.02.062. [DOI] [PubMed] [Google Scholar]
- Rosales MG, Martinez-Fong D, Morales R, Nuñez A, Flores G, Góngora-Alfaro JL, Flóran B, Aceves J. Reciprocal interaction between glutamate and dopamine in the pars reticulata of the rat substantia nigra: a microdialysis study. Neuroscience. 1997;80:803–810. doi: 10.1016/s0306-4522(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- Ruffieux A, Schultz W. Dopaminergic activation of reticulata neurons in the substantia nigra. Nature. 1980;285:240–241. doi: 10.1038/285240a0. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Walters JR. Nigrostriatal lesion and dopamine agonists affect firing patterns of rodent entopeduncular nucleus neurons. J Neurophysiol. 2002;88:487–496. doi: 10.1152/jn.00844.2001. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sanders DJ. Subthalamic nucleus lesion regularizes firing patterns in globus pallidus and substantia nigra pars reticulata neurons in rats. Brain Res. 1993;626:327–331. doi: 10.1016/0006-8993(93)90596-f. [DOI] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res. 1982;252:367–372. doi: 10.1016/0006-8993(82)90404-8. [DOI] [PubMed] [Google Scholar]
- Saitoh K, Isa T, Takakusaki K. Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats. Eur J Neurosci. 2004;19:2399–2409. doi: 10.1111/j.0953-816X.2004.03337.x. [DOI] [PubMed] [Google Scholar]
- Sato F, Parent M, Levesque M, Parent A. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol. 2000;424:142–152. doi: 10.1002/1096-9861(20000814)424:1<142::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Campana E, Pacitti C. Pedunculopontine-evoked excitation of substantia nigra neurons in the rat. Brain Res. 1984;304:351–361. doi: 10.1016/0006-8993(84)90339-1. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Di Loreto S, Pacitti C. The reciprocal electrophysiological influence between the nucleus tegmenti pedunculopontinus and the substantia nigra in normal and decorticated rats. Brain Res. 1987;423:116–124. doi: 10.1016/0006-8993(87)90831-6. [DOI] [PubMed] [Google Scholar]
- Scholtissen B, Verhey FRJ, Steinbusch HWM, Leentjens AFG. Serotonergic mechanisms in Parkinson’s disease: Opposing results from preclinical and clinical data. J Neural Transm. 2006;113:59–73. doi: 10.1007/s00702-005-0368-3. [DOI] [PubMed] [Google Scholar]
- Schultz W. Activity of pars reticulata neurons of monkey substantia nigra in relation to motor, sensory, and complex events. J Neurophysiol. 1986;55:660–677. doi: 10.1152/jn.1986.55.4.660. [DOI] [PubMed] [Google Scholar]
- Schwanstecher C, Panten U. Tolbutamide- and diazoxide-sensitive K+ channel in neurons of substantia nigra pars reticulata. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:113–117. doi: 10.1007/BF00168546. [DOI] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Seutin V, Engel D. Differences in Na+ conductance density and Na+ channel functional properties between dopamine and GABA neurons of the rat substantia nigra. J Neurophysiol. 2010;103:3099–3114. doi: 10.1152/jn.00513.2009. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Subthalamic stimulation evokes complex EPSCs in the rat substantia nigra pars reticulata in vitro. J Physiol. 2006;573:697–709. doi: 10.1113/jphysiol.2006.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Complex EPSCs Evoked in Substantia Nigra Reticulata Neurons are Disrupted by Repetitive Stimulation of the Subthalamic Nucleus. Synapse. 2008;62:237–242. doi: 10.1002/syn.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Neurons of the substantia nigra reticulata receive a dense GABA-containing input from the globus pallidus in the rat. Brain Res. 1989;493:160–167. doi: 10.1016/0006-8993(89)91011-1. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience. 1991;44:45–73. doi: 10.1016/0306-4522(91)90250-r. [DOI] [PubMed] [Google Scholar]
- Smith Y, Hazrati LN, Parent A. Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. J Comp Neurol. 1990;294:306–323. doi: 10.1002/cne.902940213. [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23:S28–S33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res. 1988;453:353–356. doi: 10.1016/0006-8993(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996a;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata. Neuroscience. 1996b;74:499–509. doi: 10.1016/0306-4522(96)00151-0. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Okumura T, Sakamoto T. Evidence for a role of basal ganglia in the regulation of rapid eye movement sleep by electrical and chemical stimulation for the pedunculopontine tegmental nucleus and the substantia nigra pars reticulata in decerebrate cats. Neuroscience. 2004;124:207–220. doi: 10.1016/j.neuroscience.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Tanibuchi I, Kitano H, Jinnai K. Substantia nigra output to prefrontal cortex via thalamus in monkeys. I. Electrophysiological identification of thalamic relay neurons. J Neurophysiol. 2009a;102:2933–2945. doi: 10.1152/jn.91287.2008. [DOI] [PubMed] [Google Scholar]
- Tanibuchi I, Kitano H, Jinnai K. Substantia nigra output to prefrontal cortex via thalamus in monkeys. II. Activity of thalamic relay neurons in delayed conditional go/no-go discrimination task. J Neurophysiol. 2009b;102:2946–2954. doi: 10.1152/jn.91288.2008. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersigni TJ, Rosenberg HC. Local pressure application of cannabinoid agonists increases spontaneous activity of rat substantia nigra pars reticulata neurons without affecting response to iontophoretically-applied GABA. Brain Res. 1996;733:184–192. doi: 10.1016/0006-8993(96)00533-1. [DOI] [PubMed] [Google Scholar]
- Trevitt T, Carlson B, Correa M, Keene A, Morales M, Salamone JD. Interactions between dopamine D1 receptors and gamma-aminobutyric acid mechanisms in substantia nigra pars reticulata of the rat: neurochemical and behavioral studies. Psychopharmacology (Berl) 2002;159:229–237. doi: 10.1007/s002130100908. [DOI] [PubMed] [Google Scholar]
- Tsaur ML, Chou CC, Shih YH, Wang HL. Cloning, expression and CNS distribution of Kv4.3, an A-type K+ channel α subunit. FEBS lett. 1997;400:215–220. doi: 10.1016/s0014-5793(96)01388-9. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Riquelme LA, Belforte JE, Pazo JH, Murer MG. Substantia nigra pars reticulata units in 6-hydroxydopamine-lesioned rats: responses to striatal D2 dopamine receptor stimulation and subthalamic lesions. Eur J Neurosci. 2000;12:247–256. doi: 10.1046/j.1460-9568.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Pazo JH, Murer MG, Riquelme LA. Subthalamic nucleus lesions reduce low frequency oscillatory firing of substantia nigra pars reticulata neurons in a rat model of Parkinson's disease. Brain Res. 2001;904:93–103. doi: 10.1016/s0006-8993(01)02489-1. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Cortés R, Mengod G. Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: distribution and effects of neurotoxic lesions. J Comp Neurol. 2005;484:418–439. doi: 10.1002/cne.20447. [DOI] [PubMed] [Google Scholar]
- Wallman MJ, Gagnon D, Parent M. Serotonin innervation of human basal ganglia. Eur J Neurosci. 2011;33:1519–1532. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- Wallmichrath I, Szabo B. Analysis of the effect of cannabinoids on GABAergic neurotransmission in the substantia nigra pars reticulata. Naunyn Schmiedebergs Arch Pharmacol. 2002a;365:326–334. doi: 10.1007/s00210-001-0520-z. [DOI] [PubMed] [Google Scholar]
- Wallmichrath I, Szabo B. Cannabinoids inhibit striatonigral GABAergic neurotransmission in the mouse. Neuroscience. 2002b;113:671–682. doi: 10.1016/s0306-4522(02)00109-4. [DOI] [PubMed] [Google Scholar]
- Walters JR, Ruskin DN, Allers KA, Bergstrom DA. Pre- and postsynaptic aspects of dopamine-mediated transmission. Trends Neurosci. 2000;23:S41–S47. doi: 10.1016/s1471-1931(00)00024-0. [DOI] [PubMed] [Google Scholar]
- Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–776. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol. 1998;509:183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M, Berod A, Sotelo C. Dopaminergic dendrites in the pars reticulata of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience. 1981;6:2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Walters JR. Dopamine modulation of the effects of γ-aminobutyric acid on substantia nigra pars reticulata neurons. Science. 1983;220:218–221. doi: 10.1126/science.6828891. [DOI] [PubMed] [Google Scholar]
- Weiser M, Bueno E, Sekirnjak C, Martone ME, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J Neurosci. 1995;15:4298–4314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkman TR, Kruse CG, Nievelstein H, Long SK, Wadman WJ. In vitro modulation of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area by antipsychotic drugs. Neuropharmacology. 2001;40:927–936. doi: 10.1016/s0028-3908(01)00015-6. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Basal ganglia discharge abnormalities in Parkinson's disease. J Neural Transm Suppl. 2006;70:21–25. doi: 10.1007/978-3-211-45295-0_5. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA. Neuronal activity in the primate substantia nigra pars reticulata during the performance of simple and memory-guided elbow movements. J Neurophysiol. 2004;91:815–827. doi: 10.1152/jn.01180.2002. [DOI] [PubMed] [Google Scholar]
- Williams MN, Faull RL. The nigrotectal projection and tectospinal neurons in the rat. A light and electron microscopic study demonstrating a monosynaptic nigral input to identified tectospinal neurons. Neuroscience. 1988;25:533–562. doi: 10.1016/0306-4522(88)90257-6. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Callaway JC. Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol. 2000;83:3084–3100. doi: 10.1152/jn.2000.83.5.3084. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. GABA, not glutamate, controls the activity of substantia nigra reticulata neurons in awake, unrestrained rats. J Neurosci. 2004;24:6751–6754. doi: 10.1523/JNEUROSCI.1528-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. GABAergic mechanisms in regulating the activity state of substantia nigra pars reticulata neurons. Neuroscience. 2006a;140:1289–1299. doi: 10.1016/j.neuroscience.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. General anesthesia as a factor affecting impulse activity and neuronal responses to putative neurotransmitters. Brain Res. 2006b;1086:104–116. doi: 10.1016/j.brainres.2006.02.064. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sakaba T. cAMP modulates intracellular Ca2+ sensitivity of fast-releasing synaptic vesicles at the calyx of Held synapse. J Neurophysiol. 2010;104:3250–3260. doi: 10.1152/jn.00685.2010. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Mades S, Misgeld U. Retrograde signaling changes the venue of postsynaptic inhibition in rat substantia nigra. Neuroscience. 2003;122:317–328. doi: 10.1016/s0306-4522(03)00607-9. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Velte S, Misgeld U. Ca2+ release-dependent hyperpolarizations modulate the firing pattern of juvenile GABA neurons in mouse substantia nigra pars reticulata in vitro. J Physiol. 2006;577:879–890. doi: 10.1113/jphysiol.2006.117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky Y, Zhang W, Misgeld U. Two pathways for the activation of small-conductance potassium channels in neurons of substantia nigra pars reticulata. Neuroscience. 2005;136:1027–1036. doi: 10.1016/j.neuroscience.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Yung WH, Hausser MA, Jack JJ. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol. 1991;436:643–667. doi: 10.1113/jphysiol.1991.sp018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Wilson CJ. Corticostriatal combinatorics: the implications of corticostriatal axonal arborizations. J Neurophysiol. 2002;87:1007–1017. doi: 10.1152/jn.00519.2001. [DOI] [PubMed] [Google Scholar]
- Zhou F-M, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- Zhou F-M, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Co-release of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Zhou F-M. A transient receptor potential channel regulates basal ganglia output. Rev Neurosci. 2010;21:95–118. doi: 10.1515/revneuro.2010.21.2.95. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou F-M. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol. 2006;96:1581–1591. doi: 10.1152/jn.00148.2006. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Matta SG, Zhou F-M. Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci. 2008;28:473–482. doi: 10.1523/JNEUROSCI.3978-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Jin Y, Matta SG, Xu M, Zhou F-M. An ultra-short dopamine pathway regulates basal ganglia output neurons. J Neurosci. 2009;29:10424–10435. doi: 10.1523/JNEUROSCI.4402-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]