Abstract
TRIM28 is a transcriptional regulator that is essential for embryonic development and is implicated in a variety of human diseases. The roles of TRIM28 in distinct biological processes are thought to depend on its interaction with factors that determine its DNA target specificity. However, functional evidence linking TRIM28 to specific co-factors is scarce. chatwo, a hypomorphic allele of Trim28, causes embryonic lethality and defects in convergent extension and morphogenesis of extra-embryonic tissues. These phenotypes are remarkably similar to those of mutants in the Krüppel-associated box (KRAB) zinc finger protein ZFP568, providing strong genetic evidence that ZFP568 and TRIM28 control morphogenesis through a common molecular mechanism. We determined that chatwo mutations decrease TRIM28 protein stability and repressive activity, disrupting both ZFP568-dependent and ZFP568-independent roles of TRIM28. These results, together with the analysis of embryos bearing a conditional inactivation of Trim28 in embryonic-derived tissues, revealed that TRIM28 is differentially required by ZFP568 and other factors during the early stages of mouse embryogenesis. In addition to uncovering novel roles of TRIM28 in convergent extension and morphogenesis of extra-embryonic tissues, our characterization of chatwo mutants demonstrates that KRAB domain proteins are essential to determine some of the biological functions of TRIM28.
Keywords: KAP1, KRAB, Convergent extension, Extra-embryonic tissues, Gastrulation, Mouse
INTRODUCTION
Tripartite motif protein 28 (TRIM28), also known as KRAB-associated protein 1 (KAP1), KRAB interacting protein 1 (KRIP-1), or transcription intermediary factor 1 β (TIF1β), encodes a TRIM/RBCC motif (RING finger, B box, coiled coil), plant homeodomain (PHD) finger and bromodomain protein that functions as a strong transcriptional repressor when bound to DNA (Friedman et al., 1996; Kim et al., 1996; Moosmann et al., 1996). The ability of TRIM28 to repress transcription has been proposed to reside in its ability to recruit chromatin-modifying enzymes, including the SETDB1 histone 3 lysine 9 methyltransferase (Schultz et al., 2002) and CHD3, a component of the nucleosome remodeling and histone deacetylation (NuRD) complex (Schultz et al., 2001). TRIM28 also binds heterochromatin protein 1 (Nielsen et al., 1999; Ryan et al., 1999), an interaction that affects TRIM28 localization to heterochromatic regions (Cammas et al., 2002), as well as some TRIM28 biological functions (Cammas et al., 2004; Herzog et al., 2010). In addition to its well documented roles as a transcriptional repressor, TRIM28 has recently been proposed to activate transcription through its ability to bind transcription factors, such as OCT3/4 (POU5F1 – Mouse Genome Informatics) (Seki et al., 2010), NGFI-B (NR4A1 – Mouse Genome Informatics) (Rambaud et al., 2009) and C/EBPβ (CEBPB – Mouse Genome Informatics) (Chang et al., 1998), raising the possibility that the formation of multimeric complexes with other proteins can modulate TRIM28 transcriptional activity.
Trim28 knockout mouse mutants fail to gastrulate and they die at embryonic day (E) 5.5 (Cammas et al., 2000). Additionally, TRIM28 has essential roles in a broad range of biological processes including spermatogenesis (Weber et al., 2002), silencing of endogenous retroviral elements (Rowe et al., 2010; Wolf et al., 2008a; Wolf and Goff, 2007; Wolf et al., 2008b), maintenance of embryonic stem (ES) cell pluripotency (Hu et al., 2009; Seki et al., 2010), epigenetic phenotypic variation (Whitelaw et al., 2010), cancer metastasis (Ho et al., 2009; Yokoe et al., 2009) and anxiety disorders (Alter and Hen, 2008; Jakobsson et al., 2008). Even though the transcriptional targets and co-factors of TRIM28 involved in the control of these biological processes are largely unknown, the DNA-binding specificity of TRIM28 is believed to be provided through its interaction with proteins of the Krüppel-associated box (KRAB) zinc finger protein family (Urrutia, 2003), a large family of transcription factors found exclusively in tetrapod vertebrates (Agata et al., 1999; Gebelein and Urrutia, 2001; Iyengar et al., 2011; Moosmann et al., 1996; Urrutia, 2003). Proteins of this family contain a KRAB domain, which mediates interaction with the TRIM28 N-terminal RBCC domain (Germain-Desprez et al., 2003; Peng et al., 2000; Peng et al., 2002) and a variable number of zinc finger motifs, which are thought to provide DNA-binding specificity to different targets (Emerson and Thomas, 2009; Gebelein and Urrutia, 2001). On the basis of its ability to enhance KRAB-mediated transcriptional repression, TRIM28 has been proposed to function as the universal co-repressor of all KRAB domain-containing proteins (Abrink et al., 2001; Agata et al., 1999; Friedman et al., 1996; Moosmann et al., 1996). Although KRAB domain zinc fingers represent the largest family of transcription factors in mammals, comprising more than 300 genes (Emerson and Thomas, 2009; Huntley et al., 2006; Rowe et al., 2010), our current knowledge about the roles of individual KRAB domain proteins is limited to just a handful of these factors: ZFP568 is essential for embryo morphogenesis (Garcia-Garcia et al., 2008; Shibata and Garcia-Garcia, 2011), ZFP57 is required for the establishment and maintenance of genomic imprinting (Li et al., 2008), ZFP809 has been involved in silencing of retroviral elements in ES cells (Wolf and Goff, 2009), RSL1 and RSL2 regulate sex-specific gene expression in the liver (Krebs et al., 2003) and ZNF746 has been linked to parkin-dependent neurodegeneration (Shin et al., 2011). Even though several studies have demonstrated a functional link between some of these KRAB zinc finger proteins and TRIM28 (Gebelein and Urrutia, 2001; Li et al., 2008; Wolf and Goff, 2009), evidence supporting a role of TRIM28 as the universal co-repressor of all KRAB domain proteins is still limited. Furthermore, it is not yet clear whether TRIM28 is indispensable for the activity of individual KRAB domain proteins.
Our previous characterization of Zfp568chato mutants identified roles for the KRAB zinc finger protein ZFP568 in the control of convergent extension and morphogenesis of extra-embryonic tissues (Garcia-Garcia et al., 2008; Shibata and Garcia-Garcia, 2011). Zfp568 is widely and dynamically expressed during the early stages of mouse embryogenesis (Garcia-Garcia et al., 2008; Shibata and Garcia-Garcia, 2011). However, analysis of Zfp568 chimeric embryos revealed that Zfp568 is required in embryonic-derived cells to control morphogenesis of both embryonic and extra-embryonic tissues (Shibata and Garcia-Garcia, 2011). Here, we show that chatwo, an n-ethyl-n-nitrosourea (ENU)-induced mutation that causes a similar phenotype to Zfp568 mutants, is a hypomorphic allele of Trim28. Our comparative analysis of Trim28chatwo and Zfp568chato mutant phenotypes provides strong evidence that ZFP568 and TRIM28 control morphogenesis of embryonic tissues through a common molecular mechanism. Consistent with this, we found that TRIM28 binds to ZFP568 and is required to mediate ZFP568 transcriptional repression. We found that chatwo mutations affect TRIM28 protein stability and repressive activity, disrupting both ZFP568-dependent and ZFP568-independent TRIM28 functions. Together with the analysis of null Trim28KO mutants and embryos bearing a conditional inactivation of Trim28 in embryonic-derived tissues, our results demonstrate that TRIM28 is indispensable for ZFP568 activity during embryo morphogenesis, and that TRIM28 is differentially required by ZFP568 and other factors in a tissue-specific manner. Our results uncover novel roles of TRIM28 during early mouse embryogenesis and provide mechanistic insight into the functions of TRIM28 as a co-factor of KRAB domain proteins.
MATERIALS AND METHODS
Mouse strains
chatwo was characterized on FvB/NJ and C57BL/6 Mus musculus strain backgrounds. Phenotype expressivity was quantified in embryos from congenic FvB/NJ and N7 backcrossed C57BL/6 animals. D7Mit178 and D7Mit76 polymorphic markers were used for genotyping. Zfp568chato (Garcia-Garcia et al., 2008), Trim28 knockout and Trim28L2 conditional mice (Cammas et al., 2000) were previously described. Sox2Cre mice were obtained from Jackson Laboratory (Hayashi et al., 2002). Sox2Cre; Trim28L2/KO embryos were generated by crossing Trim28L2, Trim28L2/KO or Trim28L2/+ females to Sox2Cre; Trim28KO/+ males. Experiments with mice were carried out in accordance with institutional and national regulations.
Positional cloning of chatwo
The chatwo mutation was created on a C57BL6/J genetic background, and outcrossed to FVB as described (Liem et al., 2009). Using a whole genome single nucleotide polymorphism (SNP) panel (Moran et al., 2006), the chatwo mutation was mapped to a 20 Mb region on proximal chromosome 7. Further mapping of meiotic recombinants narrowed the interval to SNPs rs31712695 and rs31644455. Trim28 cDNA was sequenced as amplified (Superscript One-Step RT-PCR, Invitrogen) from wild-type and chatwo RNA extracted at E8.5 (RNA STAT-60, Tel-Test).
Embryo analysis
Embryos were dissected in 4% bovine serum albumin (BSA) in PBS. In situ hybridizations were conducted as previously described (Shibata and Garcia-Garcia, 2011). The Trim28 probe was synthesized from a PCR-amplified cDNA fragment. Embryos were imaged in methanol, and cryosectioned at a thickness of 16 μm. Immunohistochemistry was performed as described (Nagy, 2003) on 8 μm cryosections. All comparisons of wild-type and mutant embryos are at the same magnification unless otherwise noted. Whole-mount embryos and sagittal sections are shown with anterior to the left and extra-embryonic tissues up. For western blotting, embryos were dissected in PBS and protein levels were quantified using Photoshop and linear regression analysis.
Expression of Trim28 and intracisternal A-type particle (IAP) elements was tested by qRT-PCR on RNA samples extracted from independent pools of either wild-type, Zfp568chato, Trim28chatwo and/or Trim28KO embryos collected at E7.5 or E8.5 (RNA STAT-60, Tel-Test). SYBR Green real-time PCR was used to quantify cDNA samples synthesized using Superscript III First-Strand Synthesis (Invitrogen). Expression of IAP elements was tested on DNaseI-treated (Roche) RNA samples. The absence of contaminating genomic DNA was confirmed by performing the assay in absence of reverse transcriptase.
Yeast two-hybrid assays
Gal4DBD and AD fusion plasmids were sequentially transformed into AH109 yeast strain using Matchmaker GAL4 Two-Hybrid System 3 (Clontech). Colonies were re-plated onto Ade-His-Leu-Trp- or Leu-Trp-X-alpha-gal plates.
Cell culture
HEK293, HEK293T or NIH3T3 cells were transfected with FUGENE 6 (Roche) or Lipofectamine 2000 (Invitrogen). A Leica DMI6000B fluorescent microscope was used for imaging. For immunoprecipitation, cells were lysed in buffer containing 150 mM NaCl, 50 mM Tris pH 7.5, 1 mM EDTA, 1% Triton X-100, 0.05% SDS and protease inhibitors. Immunoprecipitations were performed using 2-3 μl of antibody and 25 μl protein A/G agarose beads (Santa Cruz Biotechnology). For luciferase assays, HEK293T cells were transfected with pGL35XUAS firefly luciferase reporter, a Gal4DBD effector and pRL Renilla luciferase plasmids. Total amount of DNA transfected was held constant by co-transfecting pCMV-MYC as needed. Cells were assayed with the Dual-Luciferase Reporter System (Promega) 24 hours after transfection. For small interfering RNA (siRNA) knockdown, 8 pmol of Trim28 siRNAs #1 (19779), #2 (19778) or non-silencing siRNA (Ambion) was transfected using Lipofectamine RNAiMax (Invitrogen). Cells were transfected with luciferase effectors and reporters 24 hours after siRNA transfection and luciferase was assayed after another 48 hours. For each luciferase assay, duplicate transfections and replicate lysates were measured for each condition (n=4). Firefly luciferase expression was normalized to Renilla to control for transfection efficiency. Percent luciferase expression was calculated compared with Gal4DBD. Lysates loaded for western blotting were normalized to Renilla expression. Statistical analysis was performed using paired, two-tailed t-test.
Reticulocyte translation assays
Translation was assayed using the TNT Coupled Reticulocyte Lysate and Transcend Non-Radioactive Translation Detection Systems (Promega) in the presence of 1 μg plasmid DNA and 1 μl of transcend tRNA (biotinylated lysine). Translated protein was visualized by western blotting using Streptavidin-HRP (1:10,000).
Antibodies
The following antibodies were used for western blotting, co-immunoprecipitation (co-IP) and/or immunofluorescence: anti-TRIM28 (4E6, Sigma-Aldrich; 1:500), anti-TRIM28 (H-300, Santa Cruz Biotechnology; 1:500), anti-TRIM28 (MA1-2023, Thermo Scientific; 1:25), anti-CHD3 (Abcam; 1:700), anti-SETDB1 (Millipore; 1:1000), anti-GAL4DBD (RK5C1, Santa Cruz Biotechnology; 1:500-1:800), anti-Myc (9e10, Hybridoma Bank; 1:250-1:1000), anti-Flag (M2, Sigma-Aldrich; 1:500-1:700), anti-HA (11, Covance 1:250), anti-HA (Y11, Santa Cruz Biotechnology; 1:500), anti-GAPDH (AB9482, Abcam; 1:8000), anti-mouse/rabbit HRP (Jackson ImmunoResearch; 1:10,000), anti-PECAM (eBioscience; 1:200), anti-rat Alexa 488 (Molecular Probes; 1:200), anti-mouse Alexa 568 (Molecular Probes; 1:200).
Constructs and primers
Plasmids pCDNA3.1-Gal4DBD-TRIM28, pCDNA3.1-Gal4DBD-TRIM286KR, pGL35XUAS firefly luciferase and pRL Renilla luciferase are described in Mascle et al. (Mascle et al., 2007). Other constructs were generated using primers as indicated in supplementary material Table S1.
RESULTS
chatwo causes embryonic and extra-embryonic defects similar to Zfp568 mutants
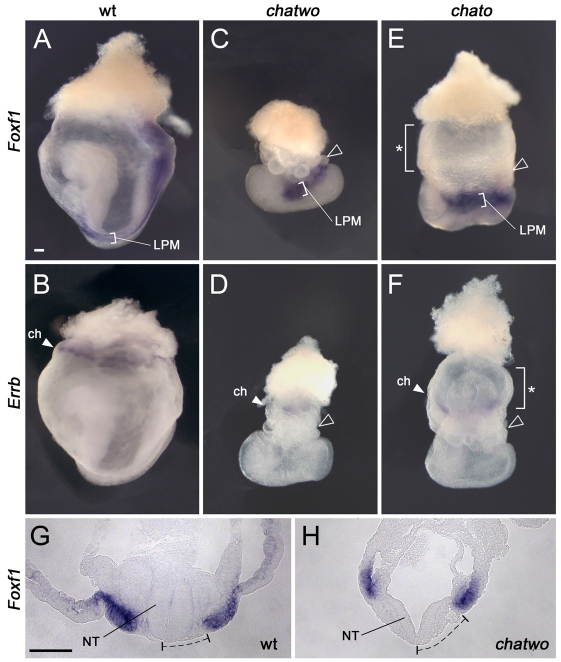
chatwo mutants were isolated in an ENU mutagenesis screen for recessive mutations affecting development of the mid-gestation mouse embryo. Embryos homozygous for chatwo arrested prior to E9 with severe convergent extension defects and disrupted morphogenesis of extra-embryonic tissues (Fig. 1). chatwo mutants had a short anterior-posterior axis and failed to undergo gut closure, giving chatwo embryos a characteristic U-shape (Fig. 1A-D). Additionally, extra-embryonic tissues in chatwo mutants appeared constricted and the yolk sac developed numerous bubble-like protrusions (Fig. 1C,D, arrowheads). These phenotypes were remarkably similar to those of chato mutants, which are homozygous for a null allele of Zfp568 (Fig. 1E,F) (Garcia-Garcia et al., 2008; Shibata and Garcia-Garcia, 2011), hence the name chatwo (cha-two; a second version of chato).
Fig. 1.
Embryonic and extra-embryonic defects in chatwo and Zfp568chato embryos. (A-F) Whole-mount in situ hybridizations with Foxf1 (A,C,E) and Errb (B,D,F) probes on wild-type (A,B), chatwo (C,D) and Zfp568chato (E,F) mouse embryos. Wild-type embryos are V-shaped, but chatwo and Zfp568chato embryos display a characteristic U-shape. Open arrowheads in C-F point to the blistered yolk sacs. Foxf1 expression (brackets in A,C,E) marks the lateral plate mesoderm (LPM). The bracket with an asterisk in E and F highlights the absence of blisters in distal yolk sac regions of Zfp568chato embryos. Filled arrowheads in D-F point to Errb expression in the chorion (ch). (G,H) Transverse sections of embryos assayed for Foxf1 expression. The dashed line highlights the distance between the embryonic midline and the LPM. NT, neural tube. Scale bars: 100 μm.
chatwo is a hypomorphic allele of Trim28
The chatwo mutation was mapped to a genetic interval on mouse chromosome 7 containing 9 genes (Fig. 2A). Trim28 stood out amongst these candidates, given its previous involvement as a co-repressor of KRAB domain proteins (Abrink et al., 2001; Agata et al., 1999; Friedman et al., 1996; Moosmann et al., 1996), a large family of transcriptional regulators that includes ZFP568 (Emerson and Thomas, 2009; Huntley et al., 2006). Sequencing Trim28 in chatwo mutants revealed two adjacent point mutations in the Trim28 open reading frame (ORF), which respectively caused Cys713Trp and His714Asn non-conservative amino acid changes (Fig. 2B). These amino acid residues are highly conserved in human and Xenopus TRIM28, as well as in other members of the TIF1 protein family (Fig. 2D). The chatwo mutations in Trim28 created a BslI restriction site that was used to confirm linkage of these sequence changes to the chatwo phenotype (Fig. 2C).
Fig. 2.
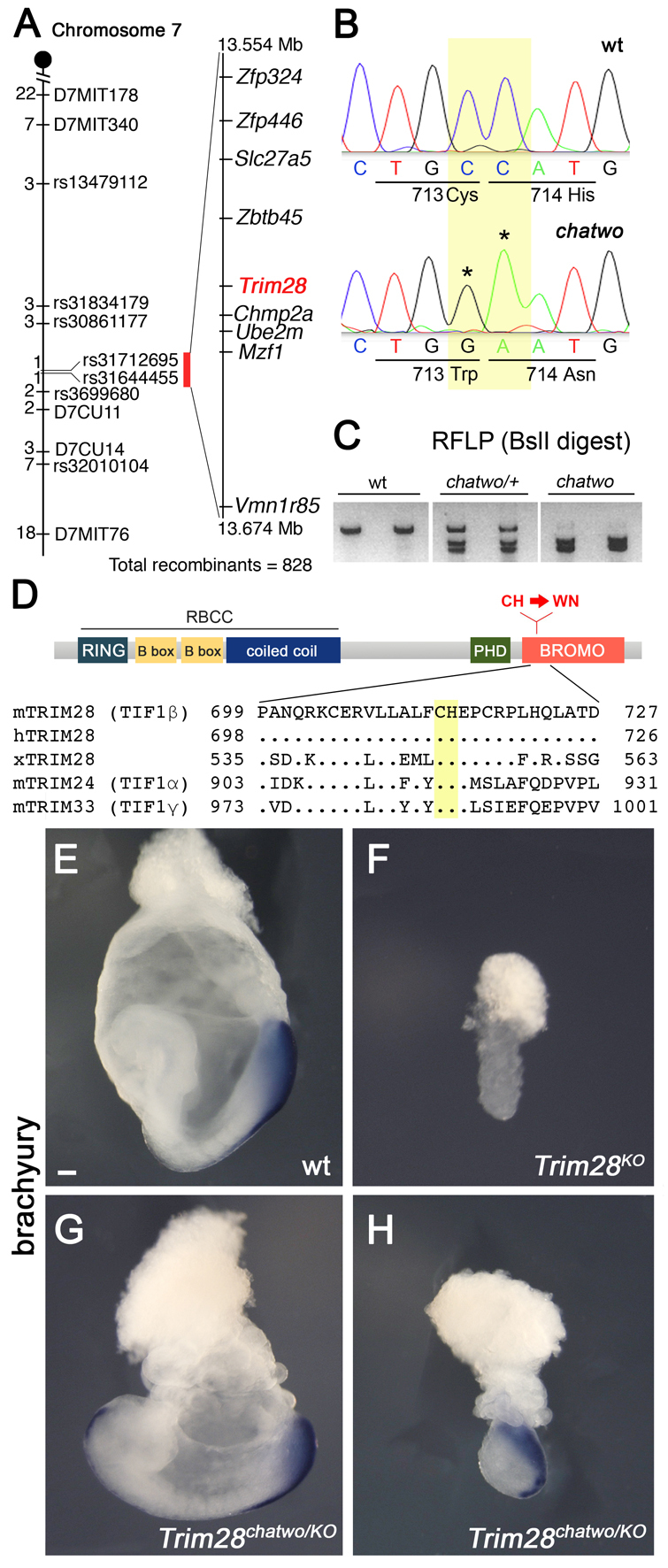
chatwo disrupts Trim28. (A) chatwo was mapped to a 120 kb interval containing nine genes. The number of independent recombinants separating the mutation from the corresponding polymorphic markers on mouse chromosome 7 is indicated to the left. (B) Chromatograms showing Trim28 cDNA sequence in wild type and chatwo mutants. Nucleotide positions 2142-2143 of Trim28 ORF (yellow) were mutated in chatwo embryos (asterisks), producing C713W and H714N amino acid substitutions. Although ENU generally introduces single point mutations at random genomic locations (Anderson, 2000), an incidence of two mutations in the same gene has been previously found in ENU-induced mutants (K. V. Anderson, personal communication). (C) chatwo mutations create a BslI restriction site. PCR-amplified wild-type DNA failed to digest with BslI, but digestion was observed in fragments from heterozygote carriers (chatwo/+) and chatwo mutant embryos. These results were consistent in a collection of more than six heterozygote chatwo carriers and over ten mutant embryos. (D) Domain structure of TRIM28 showing location of chatwo mutations (red). Sequence alignments show the conservation of the CH residues mutated in chatwo (highlighted in yellow) in mouse, human and Xenopus TRIM28, as well as in other mouse TIF1 family members. (E-H) Whole-mount in situ hybridizations on wild-type (E), Trim28KO (F) and Trim28chatwo/KO (G,H) embryos using a probe for brachyury. Scale bar: 100 μm.
Trim28-null embryos (Trim28KO) arrest at ∼E5.5, fail to gastrulate and lack expression of brachyury, a marker of the primitive streak (Fig. 2F) (Cammas et al., 2000; Wilkinson et al., 1990). We found that the chatwo allele had variable expressivity and sometimes caused developmental arrest at E7.5 (37.7%, n=182; supplementary material Fig. S1). However, the phenotype and stage of lethality of Trim28chatwo mutants were always different to those of Trim28KO embryos, as even chatwo embryos with an early developmental arrest expressed brachyury and were able to gastrulate (supplementary material Fig. S1C). These observations suggested that Trim28chatwo could be a hypomorphic allele. To obtain genetic confirmation that chatwo disrupts Trim28, we analyzed Trim28chatwo/KO embryos. Like Trim28chatwo mutants, some Trim28chatwo/KO embryos arrested at E7.5 (63.6%, n=7/11; Fig. 2H), whereas others survived until E8.5 (36.4%, n=4/11; Fig. 2G). Brachyury was expressed in all Trim28chatwo/KO embryos examined, regardless of their stage of lethality, indicating that phenotypes of Trim28chatwo/KO embryos are milder than those of Trim28KO/KO mutants (Fig. 2E-H). These results support the hypothesis that chatwo mutations create a hypomorphic allele of Trim28. Additionally, the early lethality of Trim28KO mice (Cammas et al., 2000) compared with that of Trim28chatwo mutants and the null Zfp568chato embryos suggests that TRIM28 is required during the early stages of mouse morphogenesis for processes other than those controlled by ZFP568.
chatwo affects ZFP568-dependent and ZFP568-independent roles of TRIM28
We showed previously that Zfp568chato embryos have strong convergent extension defects, including failure to undergo anterior-posterior axis elongation, mediolateral expansion of mesenchymal and epithelial tissues and an open neural tube (Garcia-Garcia et al., 2008). Phenotypic analysis confirmed that the phenotypes of Zfp568chato mutants and Trim28chatwo embryos with a late lethality were very similar. Analysis of Foxf1 expression, which marks the lateral plate mesoderm (Mahlapuu et al., 2001), showed that this tissue was shorter and wider in late-lethality Trim28chatwo mutants than in wild-type littermates (Fig. 1A,C, brackets), and was located further away from the midline (Fig. 1G,H, dashed line). Expression of Meox1 (Candia et al., 1992) and Twist (Quertermous et al., 1994), showed that the somitic mesoderm was also mediolaterally expanded in late-lethality Trim28chatwo embryos (not shown). Additionally, late-lethality Trim28chatwo mutants failed to close the neural tube (Fig. 1G,H). Expression of transthyretin (Ttr) (Cereghini et al., 1992), which labels visceral endoderm but not definitive endoderm, was used previously to evaluate convergent extension defects in Zfp568chato mutants (Garcia-Garcia et al., 2008). We found that in late-lethality Trim28chatwo embryos the definitive endoderm (Ttr-negative) failed to narrow (supplementary material Fig. S2E,G) to the same extent as Zfp568chato embryos (Garcia-Garcia et al., 2008). Altogether, our analysis shows that the embryonic defects in late-lethality Trim28chatwo embryos strongly resemble defects in Zfp568 mutants (Fig. 1E,F) (Garcia-Garcia et al., 2008).
Analysis of molecular markers in extra-embryonic tissues also highlighted similarities in the extra-embryonic phenotypes of Zfp568chato mutants and late-lethality Trim28chatwo embryos, although some phenotypic differences were notable. Like Zfp568chato embryos, the yolk sac of Trim28chatwo mutants had numerous bubble-like protrusions (Fig. 1C,D, arrowheads). However, yolk sac blisters in late-lethality Trim28chatwo embryos were found throughout the entire yolk sac, whereas they often clustered in a region proximal to the embryo in Zfp568chato mutants (Fig. 1C-F) (Shibata and Garcia-Garcia, 2011). In Zfp568chato embryos, defects in placental morphogenesis originate from the failure of the allantois to extend and contact the chorion, as well as from an expansion of the chorionic trophectoderm and failure of the ectoplacental cavity to collapse (Shibata and Garcia-Garcia, 2011). Similar to Zfp568chato mutants, we found that that the ectoplacental cavity failed to close in some late-lethality Trim28chatwo embryos (Fig. 1B,D; supplementary material Fig. S2B,D) and that the allantois was always underdeveloped (supplementary material Fig. S2B,D,F,H). However, late-lethality Trim28chatwo never showed an expansion of the chorionic trophectoderm similar to Zfp568chato mutants, as illustrated by the lack of the enlarged smooth yolk sac area characteristic of Zfp568chato embryos (Fig. 1E,F, bracket with asterisk) (Shibata and Garcia-Garcia, 2011). Instead, the yolk sac of Trim28chatwo mutants was covered with blisters and had a constricted appearance (Fig. 1C,D, arrowheads). Inspection of sagittal sections showed that all late-lethality Trim28chatwo mutants had a reduced exocoelomic cavity compared with wild-type littermates (supplementary material Fig. S2), a phenotype that is likely to have contributed to their distinct constricted and collapsed appearance compared with Zfp568chato embryos (Shibata and Garcia-Garcia, 2011).
Taken together, results from our phenotypic characterization suggest that Trim28 is required to control the same morphogenetic processes as Zfp568 in embryonic tissues. However, the differences between the phenotype of late-lethality Trim28chatwo and Zfp568chato embryos in extra-embryonic tissues argue that chatwo disrupts morphogenetic processes in addition to those regulated by Zfp568.
Conditional inactivation of Trim28 in embryonic-derived tissues causes chatwo phenotypes
Our previous analysis of tetraploid chimeras showed that Zfp568 is required in embryonic-derived tissues to control morphogenesis of embryonic and extra-embryonic tissues (Shibata and Garcia-Garcia, 2011). If Trim28 is required to mediate Zfp568 function, we predicted that Trim28 should be required in the same tissues as Zfp568. We therefore used a floxed conditional allele of Trim28 (Trim28L2; Cammas et al., 2000; Weber et al., 2002) to conditionally inactivate Trim28 in embryonic-derived tissues using the Sox2Cre transgene, which mediates recombination in all embryonic cell types, as well as extra-embryonic mesoderm, from early developmental stages (Hayashi et al., 2002).
We found that Sox2Cre; Trim28L2/KO embryos escaped the early E5.5 lethality caused by complete loss of Trim28 function, and arrested at ∼E8.5. The embryonic phenotype of Sox2Cre; Trim28L2/KO mutants strongly resembled that of Zfp568chato and Trim28chatwo embryos, including a short anterior-posterior axis, a wavy neural tube and failure to undergo gut closure (Fig. 3A-D). Consistent with a role of Trim28 in convergent extension, analysis of Foxf1 expression showed that Sox2Cre; Trim28L2/KO embryos had a shorter and wider lateral plate mesoderm (Fig. 3A,B). Sox2Cre; Trim28L2/KO embryos did not show the yolk sac protrusions or trophoblast malformations characteristic of Trim28chatwo and Zfp568chato mutants (Fig. 3B,D, arrowheads). However, similar to Zfp568 mutant embryos (Shibata and Garcia-Garcia, 2011), yolk sac vasculogenesis was disrupted in Sox2Cre; Trim28L2/KO mutants, as visualized by retention of embryonic blood cells in blood islands (not shown) and PECAM staining (Fig. 3E-H).
Fig. 3.
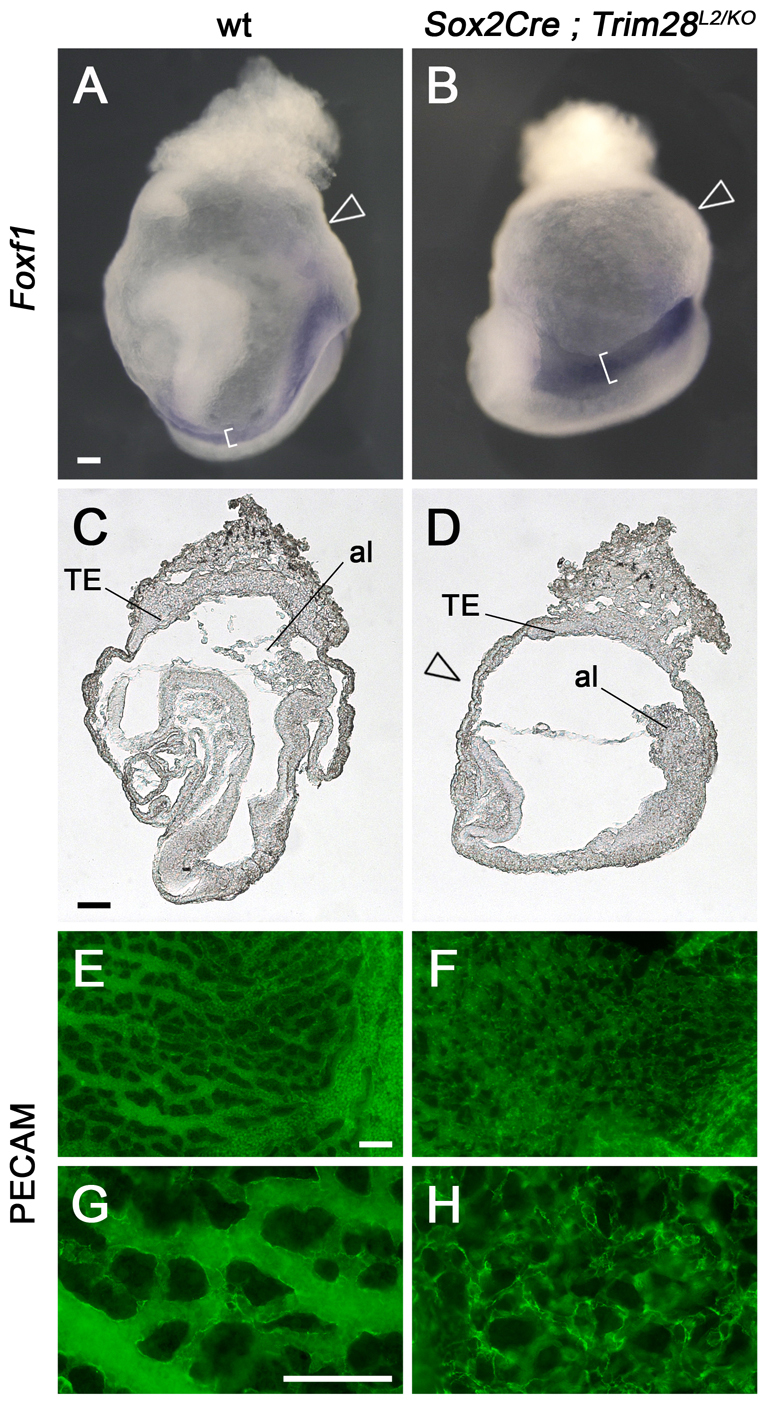
Trim28 is required in embryonic-derived cells. (A-H) Wild-type (A,C,E,G) and Sox2Cre; Trim28L2/KO (B,D,F,H) E8.5 mouse embryos were analyzed by whole-mount in situ hybridization with a Foxf1 probe (A,B) and in sagittal sections (C,D). Yolk sacs were stained with anti-PECAM antibodies to highlight the vascular plexus (E-H). Brackets in A and B highlight the lateral plate mesoderm. Arrowheads point to the yolk sac, which has a smooth appearance in Sox2Cre; Trim28L2/KO embryos. TE, trophectoderm; al, allantois. G and H are high magnifications of E and F, respectively. Scale bars: 100 μm.
During early embryogenesis, Trim28 is expressed in both embryonic and extra-embryonic tissues (supplementary material Fig. S3A) (Cammas et al., 2000). In situ hybridization experiments confirmed that Trim28 expression was effectively reduced in embryonic-derived tissues of Sox2Cre; Trim28L2/KO embryos (supplementary material Fig. S3A,B, solid arrowheads) and that, consistent with the lack of Sox2Cre expression in the trophectoderm (Hayashi et al., 2002), Trim28 expression was still expressed at high levels in this tissue (supplementary material Fig. S3A,B, open arrowheads). Western blotting indicated that although conditional inactivation of Trim28 with Sox2Cre substantially reduced the levels of TRIM28, a small amount of protein could still be detected in embryonic tissues (supplementary material Fig. S3C). Thus, it is possible that either Trim28 expression in the trophectoderm or the small amount of TRIM28 protein in embryonic tissues could be responsible for the milder yolk sac phenotype of Sox2Cre; Trim28L2/KO mutants compared with Trim28chatwo embryos. Regardless, the resemblance of Sox2Cre; Trim28L2/KO embryos to Zfp568 and Trim28chatwo mutants in embryonic tissues demonstrates further that TRIM28 is required for mammalian convergent extension.
TRIM28 forms transcriptional repressor complexes with ZFP568
The similarities between Trim28chatwo and Zfp568chato prompted us to examine whether TRIM28 interacts physically with ZFP568 and is required for ZFP568 transcriptional activity.
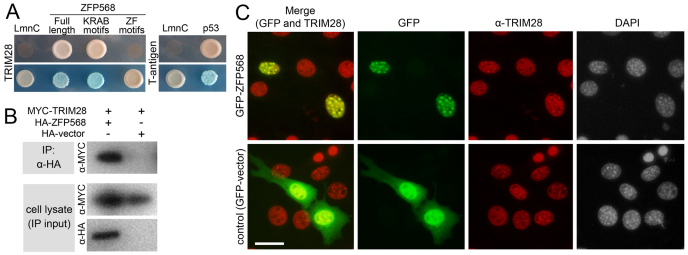
Yeast two-hybrid experiments showed that TRIM28 binds ZFP568 and that the interaction is mediated by the ZFP568 KRAB domain region (Fig. 4A). The interaction between TRIM28 and ZFP568 was also observed in mammalian cells, as revealed by co-immunoprecipitation experiments (Fig. 4B). TRIM28 has been shown to be enriched at heterochromatic puncta in the nuclei of NIH3T3 cells (Nielsen et al., 1999). We found that GFP-tagged ZFP568 colocalized with TRIM28 in the nucleus of NIH3T3 cells and was present in the same heterochromatic foci (Fig. 4C). Taken together, these results show that TRIM28 and ZFP568 form protein complexes in the nucleus, consistent with a role in regulation of gene expression.
Fig. 4.
ZFP568 interacts with TRIM28. (A) Yeast two-hybrid assays showed interaction of GAL4DBD-ZFP568 (full length and KRAB domains constructs) with GAL4AD-TRIM28, as indicated by growth in Ade-His-Leu-Trp-media (top panel) and blue colony color in Leu-Trp-X-alpha-gal plates (lower panel). GAL4DBD-ZFP568 zinc finger region did not interact with GAL4AD-TRIM28. Lamin C (LmnC) was used as a negative control. p53 interaction with SV40 large T-antigen was used as a positive control. (B) HA-ZFP568 and MYC-TRIM28 co-immunoprecipitate (IP) when transfected in HEK293 cells. (C) Subcellular localization of TRIM28 (red) and GFP-ZFP568 (top row, green) in NIH3T3 cells. An empty GFP vector was used as control (bottom panels, green). Samples were co-stained with DAPI. Scale bar: 50 μm.
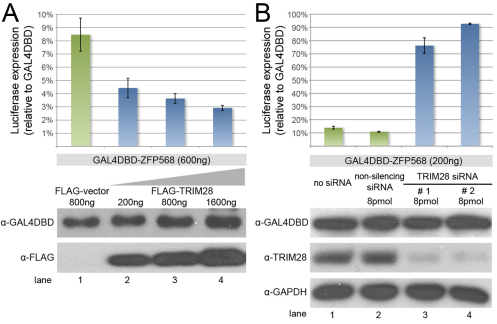
To determine whether TRIM28 mediates ZFP568 transcriptional activity, we used mammalian luciferase reporter assays. We found that a GAL4DBD-ZFP568 chimeric protein efficiently repressed expression of a 5xUAS-luciferase reporter in HEK293 cells (Fig. 5A, lane 1). This repression was enhanced in a dose-dependent fashion when increasing amounts of MYC-TRIM28 were transfected (Fig. 5A, lanes 2-4). Conversely, the ability of GAL4DBD-ZFP568 to repress transcription (Fig. 5B, lanes 1,2) was reduced when endogenous levels of TRIM28 were decreased using TRIM28 siRNAs (Fig. 5B, lanes 3,4). These experiments demonstrate that ZFP568 functions as a transcriptional repressor in vitro and that its ability to repress in these luciferase assays is dependent on the level of TRIM28.
Fig. 5.
TRIM28 mediates ZFP568 repressive activity. Quantification of luciferase expression from a 5xUAS-luciferase reporter in HEK293T cells, in the presence of GAL4DBD-ZFP568 and (A) absence (green)/presence (blue) of increasing amounts of FLAG-TRIM28 or (B) treatment with Trim28 siRNAs (blue). Luciferase expression is plotted as the percentage relative to Gal4DBD empty vector (100%). Error bars represent s.d. Western blots show levels of ZFP568-Gal4DBD and FLAG-TRIM28 protein normalized for transfection efficiency. GAPDH serves as loading control for the siRNAs, which were introduced through an independent transfection event (see Materials and methods). Results in B represent one of six experiments showing similar results.
chatwo mutations affect the protein stability and repressive activity of TRIM28
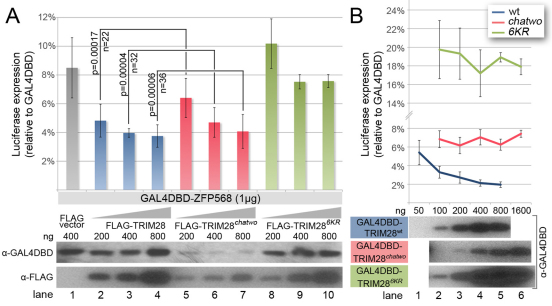
To determine the molecular basis for the effects of the hypomorphic chatwo mutation on TRIM28, we first tested the ability of TRIM28chatwo to provide ZFP568-mediated transcriptional repression in luciferase assays similar to those shown in Fig. 5. We found that GAL4DBD-ZFP568 repression increased in the presence of increasing amounts of FLAG-TRIM28chatwo (Fig. 6A; compare red and gray luciferase levels), indicating that TRIM28chatwo could bind to the KRAB domain of ZFP568 and mediate transcriptional repression. In agreement with this result, we found that TRIM28chatwo was still able to interact with ZFP568 in yeast two-hybrid assays (supplementary material Fig. S4A). GAL4DBD-ZFP568 transcriptional repression in the presence of ectopic FLAG-TRIM28chatwo was slightly reduced compared with when similar amounts of wild-type FLAG-TRIM28 were transfected (Fig. 6A, compare blue and red bars), but was not as severely affected compared with similar experiments using a sumoylation-deficient FLAG-TRIM286KR previously described to impair TRIM28 repressive activity (Mascle et al., 2007) (Fig. 6A, compare red and green bars). These results indicate that chatwo mutations decrease, but do not completely disrupt, TRIM28-mediated ZFP568 transcriptional repression activity.
Fig. 6.
chatwo mutations disrupt TRIM28 stability and repressive activity. Luciferase expression from a 5xUAS-luciferase reporter in HEK293T cells, (A) in the presence of GAL4DBD-ZFP568 and either FLAG empty vector (gray bar), increasing amounts of FLAG-TRIM28 (blue), Flag-TRIM28chatwo (red) or Flag-TRIM286KR (green); (B) in the presence of GAL4DBD-TRIM28wt (blue line), GAL4DBD-TRIM28chatwo (red line) or GAL4DBD-TRIM286KR (green line). Note that the dose-dependent effect of GAL4DBD-TRIM28wt (blue line) is lost in GAL4DBD-TRIM28chatwo and GAL4DBD-TRIM286KR mutants (red and green lines, respectively). Luciferase expression is plotted as the percentage relative to GAL4DBD empty vector (100%). Error bars represent s.d. Results in A represent one of nine experiments showing similar results. P-values were calculated using data from all nine experiments (n=total number of data points). Western blots show levels of chimeric proteins normalized for transfection efficiency. Levels of GAL4DBD-ZFP568 in the presence of FLAG-TRIM28chatwo were significantly lower compared with FLAG-vector conditions in six out of seven western blot experiments.
It is noteworthy that the amount of FLAG-TRIM28chatwo protein in cells was lower than in cells transfected with an equal amount of wild-type FLAG-TRIM28 (Fig. 6A, anti-FLAG western lanes 2-7). This result was not an artifact of the tagged forms of TRIM28 used in these experiments, as we obtained similar results with different FLAG- and MYC-tagged versions of TRIM28chatwo (Fig. 6; data not shown). Moreover, the reduced protein levels produced by TRIM28chatwo transgenes appeared to be specific to the chatwo mutations, as the sumoylation-deficient FLAG-TRIM286KR was expressed at similar levels to wild-type FLAG-TRIM28 (Fig. 6A, compare anti-FLAG western blot lanes 2-4 with lanes 8-10). Plasmids encoding FLAG-TRIM28 and FLAG-TRIM28chatwo produced similar protein levels in a reticulocyte translation system (supplementary material Fig. S5A), indicating that chatwo mutations do not affect the translation efficiency of these plasmids. Therefore, our results suggest that chatwo mutations affect the protein stability and/or rate of degradation of TRIM28.
We found that transfection of FLAG-TRIM28chatwo decreased the levels of GAL4DBD-ZFP568 protein in cells (Fig. 6A, anti-Gal4DBD western blot compare lanes 2-7 with lane 1), as well as protein levels from transgenes containing other KRAB domain proteins, including ZFP57 and ZFP809 (not shown). Because TRIM28chatwo was still able to interact with ZFP568 in yeast two-hybrid assays (supplementary material Fig. S4A), our results suggest that chatwo mutations affect the stability of TRIM28-KRAB domain proteins complexes, a hypothesis consistent with previous reports indicating that the stability of KRAB domain proteins depends on TRIM28 (Peng et al., 2000; Wolf and Goff, 2009).
Because the effects of chatwo mutations on the stability of TRIM28 and TRIM28-KRAB protein complexes could be responsible for the reduced transcriptional repression activity of FLAG-TRIM28chatwo in GAL4DBD-ZFP568 luciferase assays (Fig. 6A), we investigated further whether chatwo mutations disrupt the transcriptional repressor activity of TRIM28. For this, we tested the ability of wild-type, chatwo mutant and sumoylation-deficient versions of a GAL4DBD-TRIM28 chimeric protein to repress directly expression of the 5xUAS-luciferase reporter (Fig. 6B). As previously shown (Mascle et al., 2007), GAL4DBD-TRIM28 repressed luciferase reporter expression in a dose-dependent fashion (Fig. 6B, blue) and the sumoylation-deficient GAL4DBD-TRIM286KR was not able to repress as efficiently (Fig. 6B, green). Similar to the effect of chatwo mutations on TRIM28 stability observed previously (Fig. 6A), cells transfected with GAL4DBD-TRIM28chatwo contained lower levels of the chimeric protein than cells transfected with the same amount of wild-type GAL4DBD-TRIM28 (Fig. 6B, compare anti-GAL4DBD western blots). However, the ability of GAL4DBD-TRIM28chatwo to repress transcription was reduced compared with similar levels of wild-type GAL4DBD-TRIM28 protein (Fig. 6B, compare 800 ng GAL4DBD-TRIM28chatwo in lane 5 with 200 ng GAL4DBD-TRIM28 in lane 3), but was not as impaired as that of GAL4DBD-TRIM286KR. The ability of TRIM28chatwo to repress transcription in the context of our cell transfection experiments is consistent with results from co-immunoprecipitation experiments showing that MYC-tagged TRIM28chatwo could still recruit the chromatin-modifiers SETDB1 and CHD3 (supplementary material Fig. S4B). Altogether, these assays demonstrate that, independent of their effect on TRIM28 protein levels, chatwo mutations impair, but do not completely eradicate, TRIM28 repressive activity.
Repression of TRIM28 targets is disrupted in chatwo mutant embryos
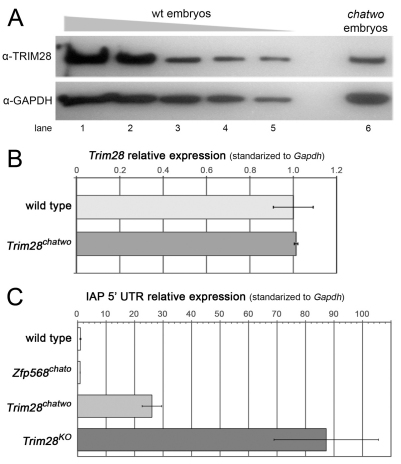
To determine whether results from the molecular characterization of TRIM28’s chatwo mutations held true in vivo, we sought to determine whether the protein levels and transcriptional repressive activity of TRIM28 were disrupted in chatwo mutant embryos. Western blotting determined that E7.5 chatwo mutants contained ∼40-55% the level of TRIM28 protein present in wild-type littermate embryos (Fig. 7A; supplementary material Fig. S6), but transcript levels were normal, as tested by qRT-PCR (Fig. 7B). Therefore, this result confirms our previous findings and provides evidence that chatwo mutations affect the stability and/or rate of degradation of TRIM28 during early mouse development.
Fig. 7.
chatwo mutations disrupt TRIM28 stability and repressive activity in vivo. (A) Western blot using an anti-TRIM28 antibody and lysates from a gradient of decreasing amounts of E7.5 wild-type extract (lanes 1-5) and a pool of six chatwo mouse embryos (lane 6). Lanes 1 to 5 represent lysates from approximately 4, 2, 1, 0.5 and 0.25 wild-type embryos, respectively. Anti-GAPDH antibody was used as a loading control (lower panel). The TRIM28 antibody was raised against part of the coiled-coil and HP1-binding domain and was still able to recognize the chatwo mutant protein. Similar results were obtained using an anti-TRIM28 polyclonal antibody (data not shown). Quantification of TRIM28 levels is shown in supplementary material Fig. S6. Note that mRNA and protein levels of Trim28 were unaffected in Zfp568chato mutants and that Zfp568 expression was normal in Trim28chatwo embryos (supplementary material Fig. S7). (B) qRT-PCR analysis of Trim28 expression in wild-type and Trim28chatwo embryos. (C) Expression of IAP elements quantified by qRT-PCR in wild-type, Zfp568chato, Trim28chatwo and Trim28KO embryos. qRT-PCR results show expression relative to the wild-type sample and normalized with respect to expression of Gapdh. Error bars represent s.d. between independent pools of embryos.
TRIM28 has been reported to repress expression of retrotransposons, including IAPs (Rowe et al., 2010). Therefore, we quantified the levels of IAP expression as a readout of TRIM28chatwo repressive activity. qRT-PCR showed that IAP elements were expressed at high levels in chatwo mutant embryos compared with wild-type littermate controls (26±3.45 fold; Fig. 7C, light gray). However, the levels of IAP expression in Trim28chatwo mutants were not as pronounced as in Trim28KO embryos (87.26±18.34 fold; Fig. 7C, dark gray). These experiments demonstrate that chatwo mutations disrupt, but do not completely eliminate the repressive activity of TRIM28 in vivo. Because IAP silencing was not disrupted in Zfp568chato mutants, these results further corroborate that chatwo mutations affect ZFP568-independent functions of TRIM28 (Fig. 7C).
DISCUSSION
ZFP568-TRIM28 complexes control convergent extension and morphogenesis of extra-embryonic tissues
On the basis of its ability to bind KRAB domains and mediate transcriptional repression, TRIM28 has been proposed to be the universal co-repressor of all KRAB domain proteins (Urrutia, 2003). However, the roles of TRIM28 as a universal KRAB co-repressor are poorly understood, partly owing to the lack of knowledge about the biological functions of individual KRAB domain proteins. The phenotypic similarities between Trim28chatwo and Zfp568chato mutants, together with the identification of chatwo as a hypomorphic allele of Trim28, provide strong genetic evidence that ZFP568 and TRIM28 control convergent extension and morphogenesis of extra-embryonic tissues through a common molecular mechanism. This conclusion is further supported by the phenotype of embryos with a conditional inactivation of Trim28 in embryonic-derived tissues (Sox2Cre; Trim28L2/KO embryos), which display defects similar to Trim28chatwo and Zfp568chato mutants in embryonic tissues (Fig. 3; supplementary material Table S2). We show that ZFP568 and TRIM28 interact physically and colocalize in heterochromatic foci, and demonstrate that TRIM28 is required to mediate ZFP568 transcriptional repression. These results demonstrate an essential role of TRIM28 as a co-factor of ZFP568, and are consistent with the notion that ZFP568-TRIM28 complexes control morphogenetic processes through transcriptional repression.
chatwo mutations disrupt TRIM28 protein stability and transcriptional repression activity
We have demonstrated genetically that chatwo is a hypomorphic allele of Trim28, and determined that chatwo mutations disrupt both TRIM28 protein stability and transcriptional activity in cell culture assays and in embryos.
Even though the effect of chatwo mutations on TRIM28 protein levels surely contributes to the phenotypes caused by this hypomorphic TRIM28 condition, mice with a 50% reduction in TRIM28 levels are viable (Whitelaw et al., 2010). Therefore, we find it unlikely that the 45-60% reduction in TRIM28 protein levels caused by chatwo mutations, alone, can explain the lethality and severity of morphological defects in Trim28chatwo embryos. Instead, we favor the hypothesis that the phenotype of Trim28chatwo mutants originates from the effects of chatwo mutations on both TRIM28 protein stability and transcriptional activity.
According to the available three dimensional structure of TRIM28’s PHD-bromodomain region (Zeng et al., 2008), chatwo mutations are located in an area of the bromodomain facing the adjacent PHD motif. We hypothesize that chatwo mutations might disrupt either the folding of the bromodomain or its interaction with the adjacent PHD motif, a configuration that might be important for the ability of TRIM28 to form functional transcriptional repressor complexes. The PHD domain of TRIM28 has been shown to promote intramolecular sumoylation of the neighboring bromodomain, a modification that impacts TRIM28 transcriptional activity (Ivanov et al., 2007; Mascle et al., 2007). We find it unlikely that the effect of chatwo mutations on TRIM28 repressive activity is due to lack of sumoylation, as chatwo mutations do not affect any of TRIM28’s sumoylated lysine residues, and the repressive activity of a sumoylation-deficient TRIM28 is far more reduced than that of TRIM28chatwo (Fig. 6B, compare TRIM28chatwo-red and TRIM286KR-green). Because TRIM28’s C-terminal bromodomain has been involved in recruitment of chromatin-modifying enzymes, including SETDB1 and CHD3 (Schultz et al., 2002; Sripathy et al., 2006), we favor the hypothesis that chatwo mutations affect TRIM28 repressive activity by interfering with the recruitment of these factors or other possible transcriptional co-factors that are as yet unknown.
TRIM28 influences the stability of KRAB domain proteins
Consistent with previous reports (Peng et al., 2000; Wolf and Goff, 2009), the molecular characterization of chatwo presented here indicates that the levels of TRIM28 in cells impinge on the stability of ZFP568 and other KRAB domain proteins (Fig. 6). We found that the effects of TRIM28 on KRAB domain proteins levels were dependent on the relative amount of each protein within the cell. Hence, increasing amounts of tagged-TRIM28 stabilized GAL4DBD-ZFP568 in a dose-dependent fashion only when the latter was transfected in excess (compare results in Fig. 6A with those of Fig. 5A). Notably, we did not observe destabilization of GAL4DBD-ZFP568 upon TRIM28 siRNA treatment (Fig. 5B), a surprising result given that stability of other KRAB domain proteins has been previously described to decrease upon TRIM28 knockdown (Wolf and Goff, 2009). We attribute this discrepancy to differences in the experimental design and/or in the affinity of distinct KRAB domain proteins for TRIM28 between our experiments and those previously published. Nevertheless, our cell culture experiments showed a consistent effect of chatwo mutations in destabilizing transfected GAL4DBD-ZFP568 (Fig. 6A, lanes 5-7).
Although an effect of Trim28chatwo mutations on ZFP568 protein stability could lead to a dominant loss-of-function effect in embryos, we have not observed any genetic evidence suggesting a possible antimorphic activity of the chatwo allele. Namely, we did not observe any dominant phenotype associated with chatwo heterozygote animals, nor did we observe a genetic interaction between Trim28chatwo and Zfp568chato in double heterozygote embryos (supplementary material Fig. S8). Therefore, further experiments using antibodies against ZFP568 and other specific KRAB domain proteins will be required to address whether the levels of TRIM28 within cells might be important to stabilize KRAB domain proteins in vivo.
chatwo mutations disrupt ZFP568-independent functions of TRIM28
Whereas the phenotypic similarities between Trim28chatwo and Zfp568chato mutants in embryonic tissues revealed a functional link between ZFP568 and TRIM28, the differences between the extra-embryonic phenotypes of Trim28chatwo and Zfp568chato embryos (Fig. 1; supplementary material Table S2) uncovered ZFP568-independent roles of TRIM28 in the development of the yolk sac and placenta. This is consistent with results from the double mutant analysis between Trim28chatwo and Zfp568chato mutants (supplementary material Fig. S8), which showed that embryos simultaneously mutant for Trim28chatwo and Zfp568chato have extra-embryonic defects more similar to those of Trim28chatwo embryos. Additionally, we have obtained evidence indicating that chatwo mutations affect ZFP568-independent functions of TRIM28 that relate to silencing of retroviral elements and gastrulation processes required for embryo survival past E7.5. We speculate that these ZFP568-independent roles of TRIM28 could be carried out through its ability to interact with other KRAB domain proteins or additional proteins that might determine its transcriptional activity and/or targets.
TRIM28 is differentially required during early mouse embryogenesis
Embryos homozygous for the Trim28chatwo hypomorph condition survive longer than Trim28KO mice, which fail to gastrulate and which die at E5.5 (Cammas et al., 2000). This observation implies that the reduced TRIM28 protein levels and repressive activity caused by chatwo mutations are sufficient to bypass TRIM28 requirements in pre-gastrula stage embryos, but not enough to fulfill the functions of TRIM28 past E7.5. Therefore, the identification and characterization of Trim28chatwo embryos revealed that TRIM28 has separate requirements during the early stages of mouse development. Analysis of Sox2Cre; Trim28L2/KO embryos also shed light on TRIM28 spatial and temporal requirements at early embryonic stages. The late lethality of Sox2Cre; Trim28L2/KO embryos indicates that TRIM28 might be required in embryonic tissues at early developmental stages prior to Sox2Cre activity (Hayashi et al., 2002). Alternatively, it is possible that Trim28 is required during pre-gastrula stages in the trophectoderm, a tissue that expresses high levels of Trim28 and is not affected in Sox2Cre; Trim28L2/KO embryos (supplementary material Fig. S3).
Because the DNA target specificity of TRIM28 is thought to depend on its interaction with other factors, mainly KRAB domain proteins (Urrutia, 2003), we hypothesize that the differences between phenotype and lethality of Trim28 hypomorphic (Trim28chatwo), conditional (Sox2Cre; Trim28L2/KO) and null (Trim28KO) conditions reflect that specific co-factors require different levels of TRIM28 activity.
Additionally, we obtained data indicating that TRIM28 requirements for a particular KRAB-domain protein might differ in a tissue-specific manner. Specifically, Sox2Cre; Trim28L2/KO embryos showed similar embryonic morphogenetic defects to Zfp568chato and late-arrest Trim28chatwo embryos, but lacked the extra-embryonic malformations characteristic of these mutants. Therefore, it is possible that ZFP568 requires higher levels of TRIM28 to control morphogenesis of embryonic tissues than to promote the development of the yolk sac and placenta. These differential requirements might be dictated by tissue-specific factors that could stabilize or modulate the activity of TRIM28 complexes with KRAB domain proteins or with other transcription factors.
In conclusion, the phenotypic and molecular characterization of the Trim28chatwo hypomorphic allele described here provides strong genetic evidence that TRIM28 is required for ZFP568 function, identifies a novel role of Trim28 in the control of mammalian convergent extension and reveals separate functions of Trim28 during early mouse embryogenesis. Recent studies to identify TRIM28 genomic targets have challenged the role of KRAB domain proteins as determining factors for the biological functions of TRIM28 (Iyengar and Farnham, 2011; Iyengar et al., 2011). However, our findings demonstrate that the interaction of TRIM28 with ZFP568 is essential for embryonic morphogenesis and suggest that particular KRAB domain proteins dictate TRIM28 involvement in specific biological processes in vivo. Future studies to identify additional binding partners of TRIM28 and elucidate the specific roles of KRAB domain proteins will be required to understand the complexity of TRIM28 functions.
Supplementary Material
Acknowledgments
We thank Kathryn Anderson, Tim Bestor, Mathieu Boulard, Ken Kemphues, John Schimenti, members of the laboratory and anonymous reviewers for helpful discussions and comments on the manuscript; Drs Ruth Arkell, Muriel Aubry, Florence Cammas, James Cross, Vincent Giguère, Leif Lundh, Xavier Mascle, Ling Qi, Shao-Cong Sun and Ken-ichi Yamamura for mice and reagents; and Cornell’s CARE staff for mice husbandry and care.
Footnotes
Funding
This work was supported by the National Institutes of Health [grant number R01HD060581 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development to M.J.G.G., R01NS044385 to K.F.L.]; and by the National Science Foundation [IOS-1020878 to M.J.G.G.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.072546/-/DC1
References
- Abrink M., Ortiz J. A., Mark C., Sanchez C., Looman C., Hellman L., Chambon P., Losson R. (2001). Conserved interaction between distinct Kruppel-associated box domains and the transcriptional intermediary factor 1 beta. Proc. Natl. Acad. Sci. USA 98, 1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata Y., Matsuda E., Shimizu A. (1999). Two novel Kruppel-associated box-containing zinc-finger proteins, KRAZ1 and KRAZ2, repress transcription through functional interaction with the corepressor KAP-1 (TIF1beta/KRIP-1). J. Biol. Chem. 274, 16412–16422 [DOI] [PubMed] [Google Scholar]
- Alter M. D., Hen R. (2008). Putting a KAP on transcription and stress. Neuron 60, 733–735 [DOI] [PubMed] [Google Scholar]
- Anderson K. V. (2000). Finding the genes that direct mammalian development: ENU mutagenesis in the mouse. Trends Genet. 16, 99–102 [DOI] [PubMed] [Google Scholar]
- Cammas F., Mark M., Dolle P., Dierich A., Chambon P., Losson R. (2000). Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development 127, 2955–2963 [DOI] [PubMed] [Google Scholar]
- Cammas F., Oulad-Abdelghani M., Vonesch J. L., Huss-Garcia Y., Chambon P., Losson R. (2002). Cell differentiation induces TIF1beta association with centromeric heterochromatin via an HP1 interaction. J. Cell Sci. 115, 3439–3448 [DOI] [PubMed] [Google Scholar]
- Cammas F., Herzog M., Lerouge T., Chambon P., Losson R. (2004). Association of the transcriptional corepressor TIF1beta with heterochromatin protein 1 (HP1): an essential role for progression through differentiation. Genes Dev. 18, 2147–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia A. F., Hu J., Crosby J., Lalley P. A., Noden D., Nadeau J. H., Wright C. V. (1992). Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development 116, 1123–1136 [DOI] [PubMed] [Google Scholar]
- Cereghini S., Ott M. O., Power S., Maury M. (1992). Expression patterns of vHNF1 and HNF1 homeoproteins in early postimplantation embryos suggest distinct and sequential developmental roles. Development 116, 783–797 [DOI] [PubMed] [Google Scholar]
- Chang C. J., Chen Y. L., Lee S. C. (1998). Coactivator TIF1beta interacts with transcription factor C/EBPbeta and glucocorticoid receptor to induce alpha1-acid glycoprotein gene expression. Mol. Cell. Biol. 18, 5880–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R. O., Thomas J. H. (2009). Adaptive evolution in zinc finger transcription factors. PLoS Genet. 5, e1000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. R., Fredericks W. J., Jensen D. E., Speicher D. W., Huang X. P., Neilson E. G., Rauscher F. J., 3rd (1996). KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 10, 2067–2078 [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M. J., Shibata M., Anderson K. V. (2008). Chato, a KRAB zinc-finger protein, regulates convergent extension in the mouse embryo. Development 135, 3053–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebelein B., Urrutia R. (2001). Sequence-specific transcriptional repression by KS1, a multiple-zinc-finger-Kruppel-associated box protein. Mol. Cell. Biol. 21, 928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain-Desprez D., Bazinet M., Bouvier M., Aubry M. (2003). Oligomerization of transcriptional intermediary factor 1 regulators and interaction with ZNF74 nuclear matrix protein revealed by bioluminescence resonance energy transfer in living cells. J. Biol. Chem. 278, 22367–22373 [DOI] [PubMed] [Google Scholar]
- Hayashi S., Lewis P., Pevny L., McMahon A. P. (2002). Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech. Dev. 119 Suppl. 1, S97–S101 [DOI] [PubMed] [Google Scholar]
- Herzog M., Wendling O., Guillou F., Chambon P., Mark M., Losson R., Cammas F. (2010). TIF1beta association with HP1 is essential for post-gastrulation development, but not for Sertoli cell functions during spermatogenesis. Dev. Biol. 350, 548–558 [DOI] [PubMed] [Google Scholar]
- Ho J., Kong J. W., Choong L. Y., Loh M. C., Toy W., Chong P. K., Wong C. H., Wong C. Y., Shah N., Lim Y. P. (2009). Novel breast cancer metastasis-associated proteins. J. Proteome Res. 8, 583–594 [DOI] [PubMed] [Google Scholar]
- Hu G., Kim J., Xu Q., Leng Y., Orkin S. H., Elledge S. J. (2009). A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 23, 837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley S., Baggott D. M., Hamilton A. T., Tran-Gyamfi M., Yang S., Kim J., Gordon L., Branscomb E., Stubbs L. (2006). A comprehensive catalog of human KRAB-associated zinc finger genes: insights into the evolutionary history of a large family of transcriptional repressors. Genome Res. 16, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. V., Peng H., Yurchenko V., Yap K. L., Negorev D. G., Schultz D. C., Psulkowski E., Fredericks W. J., White D. E., Maul G. G., et al. (2007). PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell 28, 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S., Farnham P. J. (2011). KAP1: an enigmatic master regulator of the genome. J. Biol. Chem. 286, 26267–26276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S., Ivanov A. V., Jin V. X., Rauscher F. J., 3rd, Farnham P. J. (2011). Functional analysis of KAP1 genomic recruitment. Mol. Cell. Biol. 31, 1833–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson J., Cordero M. I., Bisaz R., Groner A. C., Busskamp V., Bensadoun J. C., Cammas F., Losson R., Mansuy I. M., Sandi C., et al. (2008). KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron 60, 818–831 [DOI] [PubMed] [Google Scholar]
- Kim S. S., Chen Y. M., O’Leary E., Witzgall R., Vidal M., Bonventre J. V. (1996). A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc. Natl. Acad. Sci. USA 93, 15299–15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs C. J., Larkins L. K., Price R., Tullis K. M., Miller R. D., Robins D. M. (2003). Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev. 17, 2664–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Ito M., Zhou F., Youngson N., Zuo X., Leder P., Ferguson-Smith A. C. (2008). A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev. Cell 15, 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K. F., Jr, He M., Ocbina P. J., Anderson K. V. (2009). Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 106, 13377–13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M., Ormestad M., Enerback S., Carlsson P. (2001). The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128, 155–166 [DOI] [PubMed] [Google Scholar]
- Mascle X. H., Germain-Desprez D., Huynh P., Estephan P., Aubry M. (2007). Sumoylation of the transcriptional intermediary factor 1beta (TIF1beta), the co-repressor of the KRAB Multifinger proteins, is required for its transcriptional activity and is modulated by the KRAB domain. J. Biol. Chem. 282, 10190–10202 [DOI] [PubMed] [Google Scholar]
- Moosmann P., Georgiev O., Le Douarin B., Bourquin J. P., Schaffner W. (1996). Transcriptional repression by RING finger protein TIF1 beta that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 24, 4859–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J. L., Bolton A. D., Tran P. V., Brown A., Dwyer N. D., Manning D. K., Bjork B. C., Li C., Montgomery K., Siepka S. M., et al. (2006). Utilization of a whole genome SNP panel for efficient genetic mapping in the mouse. Genome Res. 16, 436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. (2003). Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Nielsen A. L., Ortiz J. A., You J., Oulad-Abdelghani M., Khechumian R., Gansmuller A., Chambon P., Losson R. (1999). Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18, 6385–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Begg G. E., Harper S. L., Friedman J. R., Speicher D. W., Rauscher F. J. (2000). Biochemical analysis of the Kruppel-associated box (KRAB) transcriptional repression domain. J. Biol. Chem. 275, 18000–18010 [DOI] [PubMed] [Google Scholar]
- Peng H., Feldman I., Rauscher F. J., 3rd (2002). Hetero-oligomerization among the TIF family of RBCC/TRIM domain-containing nuclear cofactors: a potential mechanism for regulating the switch between coactivation and corepression. J. Mol. Biol. 320, 629–644 [DOI] [PubMed] [Google Scholar]
- Quertermous E. E., Hidai H., Blanar M. A., Quertermous T. (1994). Cloning and characterization of a basic helix-loop-helix protein expressed in early mesoderm and the developing somites. Proc. Natl. Acad. Sci. USA 91, 7066–7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaud J., Desroches J., Balsalobre A., Drouin J. (2009). TIF1beta/KAP-1 is a coactivator of the orphan nuclear receptor NGFI-B/Nur77. J. Biol. Chem. 284, 14147–14156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H. M., Jakobsson J., Mesnard D., Rougemont J., Reynard S., Aktas T., Maillard P. V., Layard-Liesching H., Verp S., Marquis J., et al. (2010). KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463, 237–240 [DOI] [PubMed] [Google Scholar]
- Ryan R. F., Schultz D. C., Ayyanathan K., Singh P. B., Friedman J. R., Fredericks W. J., Rauscher F. J. (1999). KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell. Biol. 19, 4366–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. C., Friedman J. R., Rauscher F. J., 3rd (2001). Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev. 15, 428–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. C., Ayyanathan K., Negorev D., Maul G. G., Rauscher F. J., 3rd (2002). SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 16, 919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y., Kurisaki A., Watanabe-Susaki K., Nakajima Y., Nakanishi M., Arai Y., Shiota K., Sugino H., Asashima M. (2010). TIF1beta regulates the pluripotency of embryonic stem cells in a phosphorylation-dependent manner. Proc. Natl. Acad. Sci. USA 107, 10926–10931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Garcia-Garcia M. J. (2011). The mouse KRAB zinc-finger protein CHATO is required in embryonic-derived tissues to control yolk sac and placenta morphogenesis. Dev. Biol. 349, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. H., Ko H. S., Kang H., Lee Y., Lee Y. I., Pletinkova O., Troconso J. C., Dawson V. L., Dawson T. M. (2011). PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 144, 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripathy S. P., Stevens J., Schultz D. C. (2006). The KAP1 corepressor functions to coordinate the assembly of de novo HP1-demarcated microenvironments of heterochromatin required for KRAB zinc finger protein-mediated transcriptional repression. Mol. Cell. Biol. 26, 8623–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia R. (2003). KRAB-containing zinc-finger repressor proteins. Genome Biol. 4, 231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P., Cammas F., Gerard C., Metzger D., Chambon P., Losson R., Mark M. (2002). Germ cell expression of the transcriptional co-repressor TIF1beta is required for the maintenance of spermatogenesis in the mouse. Development 129, 2329–2337 [DOI] [PubMed] [Google Scholar]
- Whitelaw N. C., Chong S., Morgan D. K., Nestor C., Bruxner T. J., Ashe A., Lambley E., Meehan R., Whitelaw E. (2010). Reduced levels of two modifiers of epigenetic gene silencing, Dnmt3a and Trim28, cause increased phenotypic noise. Genome Biol. 11, R111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Herrmann B. G. (1990). Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 343, 657–659 [DOI] [PubMed] [Google Scholar]
- Wolf D., Goff S. P. (2007). TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 131, 46–57 [DOI] [PubMed] [Google Scholar]
- Wolf D., Goff S. P. (2009). Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 458, 1201–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Cammas F., Losson R., Goff S. P. (2008a). Primer binding site-dependent restriction of murine leukemia virus requires HP1 binding by TRIM28. J. Virol. 82, 4675–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Hug K., Goff S. P. (2008b). TRIM28 mediates primer binding site-targeted silencing of Lys1,2 tRNA-utilizing retroviruses in embryonic cells. Proc. Natl. Acad. Sci. USA 105, 12521–12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoe T., Toiyama Y., Okugawa Y., Tanaka K., Ohi M., Inoue Y., Mohri Y., Miki C., Kusunoki M. (2009). KAP1 is associated with peritoneal carcinomatosis in gastric cancer. Ann. Surg. Oncol. 17, 821–828 [DOI] [PubMed] [Google Scholar]
- Zeng L., Yap K. L., Ivanov A. V., Wang X., Mujtaba S., Plotnikova O., Rauscher F. J., 3rd, Zhou M. M. (2008). Structural insights into human KAP1 PHD finger-bromodomain and its role in gene silencing. Nat. Struct. Mol. Biol. 15, 626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.