Abstract
A critical aspect in defining the utility of a vector for gene therapy applications is the cell tropism and biodistribution of the vector. Adeno-associated virus type 12 (AAV12) has several unique biological and immunological properties that could be exploited for gene therapy purposes, including a unique cell surface receptor, transduction of epithelial cells, and limited neutralization by pooled human antibodies. However, little is known about its cell tropism and biodistribution in vivo. In vivo biodistribution studies with AAV12 vectors encoding a cytomegalovirus promoted luciferase transgene indicated preferential transduction of the nasal epithelia which was not observed with AAV2-based vectors. Expression peaked 2 weeks postadministration, before decreasing to a persistent level. The level of neutralizing antibodies (Nab) induced was sevenfold lower for AAV12 than for AAV2, an advantage for use in repeat administration. Furthermore, vectors encoding influenza A nucleoprotein (NP), an antigen which has previously been shown to induce immune protection against challenge, resulted in generation of both anti-A/NP antibodies and lung anti-A/NP T cells. Our findings suggest further evaluation of AAV12 as a vector for gene therapy and as a potential nasal vaccine.
Introduction
Recombinant adeno-associated viruses (rAAVs) are promising vectors for gene therapy due to their unique life cycle, lack of pathogenicity, ability to infect both nondividing and dividing cells, and their persistent transgene expression.1,2 An important aspect of each vector is its cell tropism, which is determined by both cell surface and intracellular interactions with host proteins. Although in vitro assays using tissue culture cells have defined several important interactions, the use of in vivo bioluminescence imaging has greatly expanded our understanding of the behavior of vectors.3,4
Based on its in vitro interactions, AAV12 represents a unique class of AAV vectors. Originally isolated from a stock of simian adenovirus 18, it requires neither cell surface heparan sulfate proteoglycans nor sialic acid for transduction.5 The structural viral capsid protein, VP1, of AAV12 has 78% homology to AAV4; however, AAV12 shows little similarity in cell surface receptor binding properties and cell tropism. Unlike AAV4, it does not require sialic acid for transduction, shows a broader tropism for epithelial cells in vitro, and does require membrane-associated proteins for entry. Its mechanism of intracellular trafficking appears to be similar to that of AAV2, as it requires endosomal acidification for transduction. Another unique property of AAV12 is its low sensitivity to neutralization by pooled human antibodies compared with other AAVs, making it an attractive vector for in vivo gene therapy applications.5
In this study, we have examined the in vivo transduction and biodistribution of AAV12 following delivery by intraperitoneal (IP), intramuscular (IM), and intranasal (IN) injection. Although we saw no significant difference in tropism when vectors were administered by IP or IM routes, AAV12 did show strong, localized tropism for the nasal epithelia when administered IN compared with AAV2. Expression was shown to peak in this tissue before decreasing to a persistent level and resulted in a low level of neutralizing antibodies (Nab), allowing for repeat administration.
Furthermore, IN administration of AAV12 vector encoding influenza NP resulted in generation of anti-A/NP antibody and lung anti-A/NP T cell response. Previous studies with other recombinant viral vectors expressing NP have shown this induced T cell response to NP can be protective.6,7,8 Our findings suggest AAV12 may be a useful vector for vaccination.
Results
Comparison of rAAV12-Luc and rAAV2-Luc transduction and kinetics following IP and IM administration
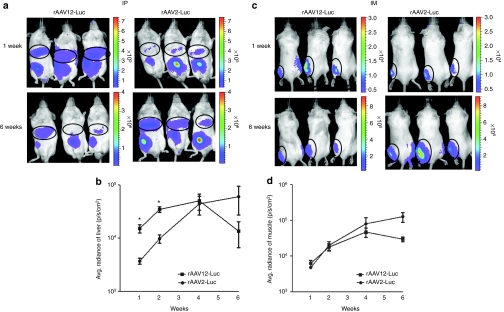
To examine the in vivo biodistribution of AAV12 by different routes of administration, we administered 1 × 1011 rAAV-Luciferase (rAAV-Luc) vector particles by IP injection and 1 × 1010 particles by IM injection, and measured expression by bioluminescence imaging of live animals at 1, 2, 4, and 6 weeks postvector administration. rAAV2-Luc was administered via the same routes as a control; the sequence of the AAV12 Rep gene places it in the same complementation group as AAV2 whose biodistribution has been widely studied, though its capsid bears only 60% similarity to that of AAV2.5 Overall no significant difference was seen in rAAV12 transduction tropism compared with rAAV2 after IP or IM administration (Figure 1). rAAV12 transduced the liver as efficiently as rAAV2, but showed no tropism for any other organ after IP administration (Figure 1a). Both vectors showed transduction at the site of injection. When luminescence in the liver was quantified and expressed over time as average radiance (photons/second/cm2), rAAV12 did show greater initial transduction of the liver 1 and 2 weeks postadministration (P < 0.05), but luciferase expression decreased by 6 weeks (Figure 1b). For IM administration of vector to the left anterior muscle, transduction by both vectors stayed localized to the site of injection in the muscle. Any expression seen on the right hand side of the animal was not transduction, but reflection of luciferase from the animal to its right (Figure 1c). rAAV12 transduction decreased by 6 weeks compared with rAAV2, which increased slightly over time (Figure 1d).
Figure 1.
Recombinant adeno-associated virus type 12 (rAAV12)-Luc transduction of the liver and muscle after intraperitoneal and intramuscular administration. Male BALB/cAnN mice were administered either 1 × 1011 rAAV12-Luc or rAAV2-Luc vector particles by intraperitoneal (IP) route, or 1 × 1010 rAAV12-Luc or rAAV2-Luc vector particles by intramuscular (IM) route. To visualize luciferase expression, mice underwent real time bioluminescence imaging using Xenogen IVIS-200 System. Images were captured and quantified using Living Image 2.60.1 software. Animals were exposed for 10 minutes before images were captured. (a) Images show luciferase expression in the liver, and at the site of injection, after IP administration 1 and 6 weeks after rAAV12-Luc vector administration, compared to rAAV2-Luc-treated mice. Bioluminescence scales indicate the intensity of luciferase expression in these animals. (b) To quantify luminescence in the liver, this region was selected on captured images and luminescence output was expressed as radiance (photons/second/cm2). Luciferase expression was quantified 1, 2, 4, and 6 weeks after rAAV-Luc vector administration and data is presented on a log scale to allow all time points to be included. Error bars represent the mean radiance + s.e.m, where n = 3. Statistical comparisons were performed by unpaired t-tests (*P < 0.05). (c) Images show luciferase expression in the left anterior muscle 1 and 6 weeks post rAAV-Luc vector administration by IM route. (d) Luciferase expression is quantified in the muscle as before, and presented on a log scale. Error bars represent the mean radiance + s.e.m., where n = 3. Statistical comparisons were performed by unpaired t-tests.
rAAV12-Luc transduction results in a strong transduction of the nasal cavity after IN administration
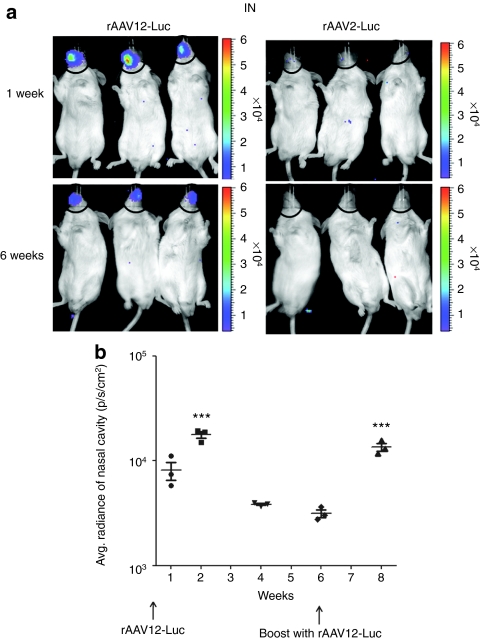
Although AAV2 is known to be a poor transducer of the respiratory epithelia due to the lack of heparan sulfate proteoglycans on the apical surface of human airway epithelia cells,9,10,11 little is known about the ability of AAV12 to transduce the upper or lower respiratory tract. In vivo biodistribution of rAAV12-Luc and rAAV2-Luc was compared following IN administration of 1 × 1011 vector particles. In this study, luciferin substrate was also administered IN, as previous studies have shown that luciferase detection is improved by this route of administration rather than IP administration.12 rAAV12 showed strong, localized tropism in the nasal cavity, indicated by significant luciferase expression in rAAV12-Luc-treated animals compared with the rAAV2-Luc group (Figure 2a). No luciferase expression was detected in the lungs of the animals. Luciferase expression was only observed in rAAV12-Luc animals when substrate was administered IN. When luminescence in the nasal cavity was quantified and expressed over time as average radiance (photons/second/cm2), expression peaked 2 weeks post-rAAV12-Luc administration before decreasing by week 6 (P < 0.001) (Figure 2b). The average radiance background in the nasal cavity region of rAAV2-Luc mice was 1 × 103 photons/second/cm2. The ability to readminister rAAV12-Luc was examined at this time point by treating animals with a second dose of 1 × 1011 rAAV12-Luc particle vectors. Average radiance was measured 2 weeks post-boost and was found to have increased significantly from the level seen in the same animals prior to the boost (P = 0.001), achieving a level similar to that observed 2 weeks after administration of the initial dose of vector. No significant difference was seen between peak expression after the first and second dose.
Figure 2.
Recombinant adeno-associated virus type 12 (rAAV12)-Luc transduction of the nasal cavity after intranasal administration. Male BALB/cAnN mice were administered two doses of 1 × 1011 rAAV12-Luc vector particles by the intranasal (IN) route. (a) Images show luciferase expression in the nasal cavity of rAAV12-Luc treated mice 1 and 6 weeks after initial vector administration compared to rAAV2-Luc-treated mice. To quantify luminescence, the nasal cavity region was selected on captured images and luminescence output was expressed as radiance (photons/second/cm2). (b) Luciferase expression was quantified 1, 2, 4, and 6 weeks after initial rAAV12-Luc vector administration, and 2 weeks post boost. Average background radiance in the nasal cavity region of rAAV2-Luc mice was 1 × 103 photons/second/cm2. Error bars represent the mean radiance + s.e.m., where n = 3. Statistical comparisons were performed by unpaired t tests. Peak expression 2 weeks postadministration was compared with initial expression after 1 week (P < 0.05), and diminished expression at 6 weeks (***P < 0.001). Luciferase expression 2 weeks post boost was compared with diminished expression 6 weeks after initial administration (***P = 0.001).
rAAV12-Luc transduces anterior nasal cavity tissue layers, but not the lungs
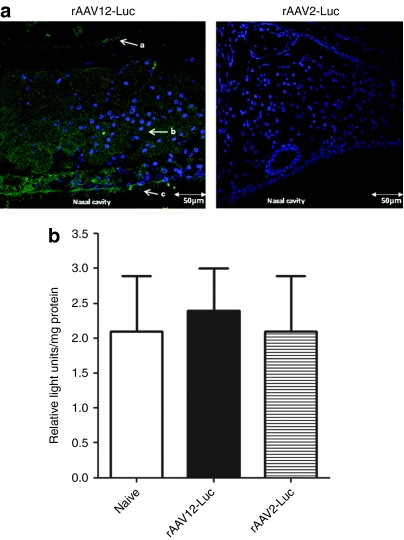
To determine exactly which cells of the nasal cavity were being transduced, we looked at luciferase expression in cross-sections of decalcified, paraffin-embedded nasal cavity tissue taken from IN-treated rAAV-Luc animals 5 days after vector administration by immunofluorescence, using a rabbit anti-luciferase antibody and 4′,6-diamidino-2-phenylindole counterstain. Luciferase expression was found to be extensive throughout the anterior nasal cavity epithelia, from the ciliated pseudostratified olfactory epithelium, through the lamina propria-containing Bowman's glands, to the respiratory epithelia covering the nasoturbinate bone (Figure 3a). Control rAAV2-Luc sections of the nasal cavity showed no luciferase staining. To confirm lack of expression in the lungs, ex vivo luciferase assay was performed on homogenized lung tissue removed from rAAV-Luc-treated animals (Figure 3b). No difference was seen between background levels in the lungs of naive mice compared to rAAV12-Luc- and rAAV2-Luc-treated mice when relative light units were expressed per mg of protein.
Figure 3.
Detection of luciferase expression by immunofluorescence in the anterior nasal cavity tissue of recombinant adeno-associated virus type 12 (rAAV12)-Luc treated mice. Mice were administered either 1 × 1011 rAAV12-Luc or rAAV2-Luc vector particles by the intranasal (IN) route. Five days after rAAV-Luc administration, two mice from each group were sacrificed, and nasal cavities were excised. (a) Cross-sections of nasal cavity tissue were immunostained for the presence of luciferase as described in the methods. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Sections were viewed by confocal microscopy and images captured at ×40 objective. Arrow a indicates respiratory epithelium surrounding nasoturbinate bone, arrow b indicates lamina propria with Bowman's glands, and arrow c indicates ciliated pseudostratified olfactory epithelium. Images are representative of luciferase staining in both mice sacrificed from each group. Bars = 50 µm. Cross-sections of nasal cavity tissue of naive mice were stained for luciferase expression as a negative control (data not shown). (b) To confirm lack of expression in the lungs of rAAV-Luc treated mice, ex vivo luciferase assay was performed on homogenized lung tissue and expression was measured as described in the Materials and Methods section. Tissue from a naive mouse was included as a negative control. Error bars represent the mean relative light units (RLU)/mg protein + s.e.m., where n = 3. Statistical comparisons were performed by unpaired t-tests.
Both Nab and total anti-AAV IgG antibodies are lower in rAAV12-Luc animals 4 weeks postvector administration compared with rAAV2-Luc
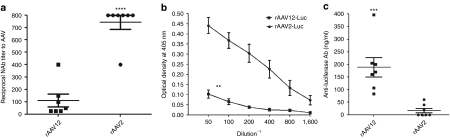
Previous studies have shown rAAV2 to be highly immunogenic when administered IN.11 rAAV12 has been shown to be relatively insensitive to neutralization by pooled human antibodies, making it potentially useful for in vivo gene therapy applications.5 To examine the in vivo immunological response to rAAV12-Luc after IN administration, we tested for the presence of Nab to AAV12 in serum taken from individual animals 4 weeks postadministration of 1 × 1011 vector particles. Some Nab to rAAV12 were present 4 weeks after initial IN administration of rAAV12-Luc, but overall levels were much lower than the Nab to AAV2 in rAAV2-Luc-treated mice (Figure 4a). Despite showing no detectable level of luciferase expression after IN administration, the majority of animals who received 1 × 1011 rAAV2-Luc vector particles showed reciprocal Nab titers of 800, significantly greater than those who received rAAV12-luc (P < 0.0001). Similar results were also observed with the total anti-AAV capsid immunoglobulin G (IgG) antibody response in rAAV12 and rAAV2 IN-treated mice. A significantly lower anti-AAV capsid-specific IgG response was induced in rAAV12-treated animals compared with rAAV2-treated animals after IN administration (P < 0.01) (Figure 4b).
Figure 4.
Detection of serum neutralizing antibodies, anti-AAV capsid immunoglobulin G (IgG), and anti-luciferase antibodies after intranasal (IN) administration of rAAV-Luc vectors. Mice were administered either 1 × 1011 recombinant adeno-associated virus type 12 (rAAV12)-Luc or rAAV2-Luc vector particles by IN route. (a) Serum neutralizing antibodies (Nab) to AAV12 and AAV2 were measured 4 weeks after initial vector administration as described in the Materials and Methods section. Neutralizing titers were expressed as the reciprocal of the highest serum dilution that still gave 50% transduction with rAAV-Luc vectors compared to naive control serum. No crossreactivity was seen between rAAV12 and rAAV2 antiserum. Error bars represent the mean reciprocal Nab titer to AAV + s.e.m., where n = 7. Statistical comparison was performed by unpaired t-test to compare rAAV12-Luc neutralizing titer with that of the rAAV2-Luc group (****P < 0.0001). (b) Anti-AAV capsid protein IgG titers were also measured 4 weeks post initial rAAV12-Luc and rAAV2-Luc vector administration by ELISA as described in methods. Optical density values of naive serum controls were subtracted from overall values. Error bars represent the mean optical density reading + s.e.m., where n = 7. Statistical comparison was performed by unpaired t-test to compare titer from rAAV12-Luc animals with those of the rAAV2-Luc group (**P < 0.01). (c) Anti-luciferase antibodies were measured in serum samples 2 weeks post boost by direct ELISA. Concentrations of anti-luciferase antibodies were estimated by comparison with a standard curve (anti-luciferase monoclonal antibody, 1 µg–0.001µg/ml range) and expressed in ng/ml. Serum from rAAV12-A/NP- and rAAV2-EGFP-treated animals were included as negative controls for rAAV12-Luc and rAAV2-Luc respectively, and showed no anti-luciferase antibody response. Error bars represent the mean anti-luciferase antibody titer (ng/ml) + s.e.m., where n = 7. Statistical comparison was performed by unpaired t-test to compare corresponding anti-luciferase antibody titers between the two groups (***P < 0.001).
Nab response in rAAV12-Luc- and rAAV2-Luc-treated animals after IP administration showed no significant difference between the two groups (Supplementary Figure S1). However, total anti-AAV capsid IgG levels were lower in the rAAV12-Luc-treated mice compared with the rAAV2-Luc group (P < 0.05) (Supplementary Figure S2). The immune response following IM injection was more similar to that seen with the IN route with an increase in both Nab (P < 0.05) and total anticapsid antibodies for the rAAV2-Luc-treated mice compared with the rAAV12-Luc group (Supplementary Figures S3 and S4).
rAAV12-Luc induces anti-luciferase antibodies
Given the ability to readminister AAV12 to animals, as seen in Figure 2b, and the lack of Nab to the vector capsid itself, we looked for the presence of anti-luciferase antibodies 2 weeks after repeat IN administration of rAAV-Luc vectors. Overall rAAV12-Luc-treated mice showed a significantly greater anti-luciferase antibody response compared with rAAV2-Luc-treated animals 2 weeks post-boost (P = 0.001) (Figure 4c). As a negative control, no anti-luciferase antibodies were seen in serum from rAAV12-A/NP or rAAV2-GFP treated mice (data not shown).
IN administration of rAAV12–A/NP vector induces an anti-influenza A NP antibody response
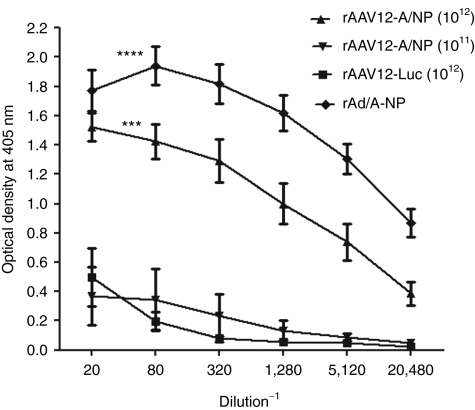
Viral vectors expressing conserved influenza A surface antigens, such the nucleoprotein (NP) and matrix (M) protein, have been used previously in immunization/challenge studies with varying degrees of success.6,7,8 Recent work has shown that mucosal routes of boosting are more effective in offering protection against H1N1 or H5N1 challenge in mice than parenteral routes such as IM. Based on its unique tropism for the nasal cavity, and the relatively low immunogenicity of its capsid, AAV12 might be useful as a localized upper respiratory tract vaccine. We constructed a rAAV12 vector expressing influenza A NP and examined its immunological properties in vivo. The NP coding region of influenza strain A/PR/8 was subcloned into an AAV expression plasmid containing ITRs for replication, from which rAAV12-A/NP vector particles were made. After confirming successful expression of the influenza A/NP gene from the AAV-A/NP plasmid following efficient transduction of Cos-7 cells with the vector (data not shown), we performed immunization studies to evaluate anti-A/NP antibody formation and T cell induction. Animals were treated with either 1 × 1011 or 1 × 1012 rAAV12-A/NP vector particles by IN administration at day 0, and again 5 weeks later. Anti-influenza A/NP responses were measured 2 weeks post-boost by enzyme-linked immunosorbent assay (ELISA) using serially diluted individual sera. Serum from animals who received 1 × 1012 rAAV12-Luc vector particles by IN administration at the same time points was included as a negative control. As a positive control, one group was treated with 1 × 1010 particles of recombinant adenoviral type 5 vector expressing influenza A/NP (rAd-A/NP), which induces a strong anti-A/NP response following IN delivery,13 and serum assayed 7 weeks after vector administration. A strong anti-NP response was seen 2 weeks post-boost in animals who received high dose rAAV12-A/NP, compared to the low-dose group (P = 0.01), and the rAAV12-Luc negative control group (P < 0.001). Similarly, rAd-A/NP treated mice also showed a significant anti-A/NP antibody response compared with low dose rAAV12-A/NP and the rAAV12-Luc control group (P < 0.0001) (Figure 5).
Figure 5.
Detection of anti-influenza A nucleoprotein antibodies after recombinant adeno-associated virus type 12 (rAAV12)-A/NP administration. Mice were administered either 1 × 1011 or 1 × 1012 rAAV12-A/NP, or 1 × 1012 rAAV12-Luc control vector particles by intranasal (IN) route. Another group received one dose of 1 × 1010 rAd-A/NP by IN administration. Five weeks later rAAV12-treated mice were boosted IN with the same titer rAAV12 vector. Serum antibodies to NP protein were detected in all animals 2 weeks post boost by direct ELISA as described in the methods. Sera from the animals who received 1 × 1012 rAAV12-Luc at the same times by the IN route were included as negative controls. Average optical density values of wells which were treated with rNP, alkaline phosphatase labeled secondary immunoglobulin G (IgG) and substrate only, were subtracted from overall absorbance values as background. Error bars represent the mean optical density + s.e.m., where n = 9 for rAAV12-A/NP high dose group, n = 5 for rAAV12-A/NP low dose group, n = 5 for the rAd-A/NP group, and n = 4 for rAAV12-Luc group. Statistical comparison was performed by unpaired t-test to compare the high-dose rAAV12-A/NP group and the rAd-A/NP group, with the low-dose group, and the rAAV12-Luc negative control (***P < 0.001, ****P < 0.0001).
T cell responses to influenza A/NP dominant peptides are present in the lungs of animals treated with rAAV12-A/NP vector
Previous studies have shown the importance of a T-cell response to influenza NP in protection against influenza challenge.7,14,15,16,17 Studies using a DNA prime–recombinant adenoviral type 5 (rAd) boost immunization model indicated that antigen-specific CD8+ effector cells expressing T cell activation markers persisted in the lungs of immunized animals, 2 and 8 months after boosting with rAd-A/NP.15 rAd-A/NP alone, when given IN, can also achieve potent local immunity and protection.13
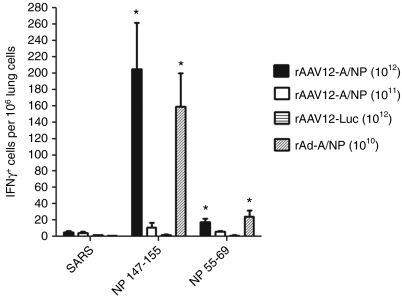
We looked at the response of lung T cells to stimulation with dominant influenza A/NP epitope peptides 3 weeks post-boost with rAAV12-A/NP. Interferon (IFN)-γ T cell enzyme-linked immunosorbent spot (ELISPOT) analysis was performed on individual lung cell suspensions. Mice treated with two doses of 1 × 1012 rAAV12-A/NP particles showed a strong T cell response to the dominant NP 147–155 peptide, compared to the rAAV12-Luc negative control group (P < 0.05) (Figure 6). Some response was also seen to the NP 55–69 peptide compared to the rAAV12-Luc control (P < 0.05), but this was not as high. A similar pattern of T cell response was also observed with recombinant Ad-A/NP-treated mice, with a stronger response to the NP 147–155 peptide compared with the NP 55–69. The group that received two doses of 1 × 1011 rAAV12-A/NP showed only minimal response to both peptides.
Figure 6.
Detection of T cell response to influenza A/NP peptides in the lungs of recombinant adeno-associated virus type 12 (rAAV12)-A/NP treated animals. Mice were administered either 1 × 1011 or 1 × 1012 rAAV12-A/NP, or 1 × 1012 rAAV12-Luc control vector particles by intranasal (IN) route. Another group received one dose of 1 × 1010 rAd-A/NP by IN administration. Five weeks later rAAV12 mice were boosted IN with the same titer rAAV12 vector. Three weeks post boost, lungs were harvested and interferon (IFN)-γ ELISPOT analysis was performed on individual lung suspensions, where T cell response to stimulation with NP peptides was measured as described in the Materials and Methods section. Negative control for the assay was an irrelevant SARS peptide. Error bars represent the mean IFN-γ producing cells per 106 lung cells + s.e.m., where n = 9 for rAAV12-A/NP high-dose group, n = 5 for rAAV12-A/NP low dose group, n = 5 for the rAd-A/NP group, and n = 4 for rAAV12-Luc group. Statistical comparison was performed by unpaired t-test to compare the response of the high-dose rAAV12-A/NP group and rAd-A/NP group to NP 147–155 and NP 55–69 peptides, with those of the low dose group and rAAV12-Luc control (*P < 0.05).
Discussion
Isolation of novel AAV serotypes as contaminants of adenoviral stocks has resulted in the development of new vectors with unique entry and binding properties that can be exploited for gene therapy and vaccination.18,19 Previous studies have indicated the potential of AAV vectors as gene therapy agents based on their tropism for distinct tissues, such as the liver, lung, and heart;20,21,22,23,24 however, an AAV vector with transduction activity in the nasal epithelia alone has not been reported, and as we demonstrate may likely be useful in both gene therapy and vaccination.
AAV12 is one of the only AAVs described so far that does not depend on either heparan sulfate proteoglycans or sialic acid for transduction, and that exhibits limited neutralization by human IgGs.5 It is likely its unique binding activities are responsible for its unique tropism for nasal tissue. Although not observed here, previous studies have shown that IN administration of AAV5 serotype, delivered in the same way as described here, is sufficient to result in transduction of the lungs.25 Other studies have shown expression in the lungs of animals when AAV serotypes, for example AAV6 and AAV9, have been directly delivered via intratracheal administration.11,26,27 AAV6 variants showed strong transduction of the nasal epithelia when delivered by the IN route, and the lungs when administered intratracheally.26 Further study will be required to define if AAV12 has a tropism for lung epithelia.
The reduction in luciferase expression seen in the nasal cavity after IN administration was not due to an increase in Nab to AAV12 based on our findings. To ensure that this absence of Nab was not due to an inability to elicit antibodies as efficiently as AAV2, we examined whether AAV12 might be escaping the immune response more efficiently. AAV12 elicited a significantly lower AAV IgG response compared to AAV2, suggesting overall it is less immunogenic than AAV2 when delivered via this route. Further work will be required to determine the mechanism associated with this decrease in expression. While reduced, expression remained at a persistent level, and did not drop as low as background expression seen with AAV2. This level of expression may be biologically relevant and sufficient for some gene therapies. Transduction was seen throughout the anterior nasal cavity, suggesting transduction of multiple cell types; transduction may be primarily occurring in the horizontal basal cells, given the transient nature of expression. Luciferase expression from rAAV12 also decreased over time in transduced tissue when vector was administered IP and IM. Expression had decreased in the liver and the muscle by 6 weeks postadministration compared to AAV2 after IP and IM administration respectively. We do not believe that this decrease was due to increased adaptive immune response to AAV12 vector after administration, since we saw no increase in Nab response or AAV capsid IgG response in rAAV12 treated animals compared to those treated with rAAV2.
In general, administration of AAV vectors results in antivector capsid immune responses which preclude successful vector readministration,10,28 but AAV12, due to the low immunogenicity of its capsid after the first IN dose, can be successfully administered again. This suggests its potential in gene therapy applications that require repeat dosing. In comparison, AAV2 induced a significant Nab response after administration of one dose, indicating the immunogenic nature of its capsid. To date, many studies have speculated that AAV2 has the ability to induce a capsid-specific cytotoxic T-lymphocyte response in animal models, where CD4+ and CD8+ T cell epitopes for its capsid have been identified.29,30 Similarly, another study identified seven regions of the AAV capsid containing immunogenic epitopes by mapping of anti-AAV2 antibody epitopes.31 Previous studies have shown that even minor sequence changes in the capsid of AAV can affect antibody neutralization activity.32 Because AAV12 bears only 60% sequence homology to AAV2 capsid overall, these regions in the AAV12 capsid are likely either absent or significantly divergent to prevent induction of the same T and B cell response. Interestingly, although little capsid Nab was detected after AAV12 IN administration, a specific antibody response to the luciferase transgene product was seen. This antibody response may also be dose dependent, as administration of a higher dose rAAV12-Luc vector increased the antibody response to the transgene product (data not shown).
Previous studies show that the immune response to influenza NP protein can be protective in challenge models.7,14,15,16,17 In our study, a serum antibody response to A/NP was seen for the higher dose of vector used. Although intermediate doses were not studied, the significant increase in transduction we observed between the two doses may represent a threshold effect which has been previously observed with AAV2 in other tissues.33,34 In IFN-γ ELISPOT assays, we saw a strong T cell response to the NP 147–155 dominant peptide in the lung cells of mice receiving a high dose of rAAV12-A/NP vector given twice. This response was significantly greater than that seen in animals that received lower dose rAAV12-A/NP, and was comparable to the response seen 8 weeks after administration of one IN dose of rAd-A/NP vector. Previous studies have shown that responses elicited by administration of rAd-A/NP to mice are protective upon challenge with virulent H1N1.13 Many vaccination strategies utilize a prime boost approach. Boosting with rAd-A/NP and rAd-M2 provides protection in mice and ferrets from virulent H1N1 and H5N1 challenge.15 Further studies could identify an increase in immune response when rAAV12-A/NP vaccination is combined with DNA or rAd-A/NP vaccination. However, due to the ability to repeat dose with recombinant AAV12 vectors, boosting with recombinant AAV12 alone may be an attractive alternative.
In conclusion, the unique tropism of AAV12 for the nasal epithelia, the low immunogenicity of its capsid after IN administration, and its ability to elicit a stronger immune response to the transgene product than AAV2, provides another novel AAV serotype for gene therapy and vaccination testing. It is very important to establish what cell surface receptor(s) AAV12 binds, so it can be further utilized to transfer genes in a more cell-specific or tissue-specific manner.
Materials and Methods
Cell culture. Human embryonic kidney cells 293T and African green monkey Cos-7 cells were grown in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal bovine serum, 1% (vol/vol) penicillin and streptomycin, and 1% (vol/vol) -glutamine (Gibco, Carlsbad, CA).
Construction of AAV12-A/NP plasmid and in vitro expression of influenza A/NP protein. The full-length coding region of influenza strain A/PR/8 NP, 1.5 kb in size, was cloned into the PDT plasmid expressing AAV ITRs together with a region of the cytomegalovirus (CMV) promoter. VR1012-A/NP and PDT-AAV/ITR plasmids were each digested with KpnI and SnabI restriction enzymes, resulting in a 2.9 kbp size insert fragment and a 4.5-kbp size fragment, respectively, which were gel purified. Digested fragments were incubated together in an overnight ligation reaction at 15 °C in a ratio of 3:1 (insert to vector) using high concentration T4 DNA ligase (New England Biolabs, Ipswich, MA). Positive clones were identified by restriction digest with EcoRI and HindIII. Presence of AAV ITRs was confirmed by digestion with Sma1. Correct sequence was confirmed by sequencing with the following primers:
CMVF CATCTACGTATTAGTCATCGCTATTACCAT,
CMVR TGGAAATCCCCGTGAGTCA,
A/NP181F TTAACAATAGAGAGAATGGTGCTC,
A/NP1081F AGAGGAGTTCAAATTGCTTCCAAT,
A/NP120R GTAGAATCGTCCAATTCCACCAAT
PDT450R CGGCCTCAGTGAGCGAGCGAGCGC
In vitro expression of influenza A NP protein was confirmed by transfection of 2 × 105 Cos-7 cells with PDT-A/NP plasmid followed by immunofluorescence staining. Cos-7 cells were seeded in glass bottom microwell dishes and transfected according to effectene transfection reagent protocol (Qiagen, Valencia, CA) for 24 hours. Cells were fixed with 2% PFA for 15 minutes, blocked with 10% donkey serum in phosphate-buffered saline (PBS) for 30 minutes at room temperature, then incubated with mouse hybridoma supernatant containing anti-A/NP monoclonal antibody (HB65 H16-L10-4R5; ATCC, Manassas, VA) for 1 hour at room temperature followed by Alexa Fluor 488 donkey anti-mouse secondary antibody for 30 minutes. Negative controls included AAV2-luciferase plasmid transfected cells and an isotype control (ChromPure mouse IgG, unconjugated, 015-000-003; Jackson ImmunoResearch, West Grove, PA). All washes were carried out in PBS + 0.05% Tween-20. Hard set mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Labs, Burlingame, CA) was added to each dish and cells were visualized by confocal microscopy.
Generation of recombinant vector. Recombinant AAV vectors were produced as previously described.5 For rAAV2-Luc production, 293T cells were co-transfected with pAAV2-CMV-Luc, pAAV2-Rep/Cap, and adenovirus helper plasmid 449B in a DNA ratio of 1:1:2 by calcium phosphate transfection. For rAAV12-Luc production cells were co-transfected with pAAV2-CMV-Luc, pAAV12Cap, pRepx26, and adenovirus helper plasmid 449B in a ratio of 1:1:1:2. For rAAV12-A/NP vector production cells were co-transfected with pPDT-CMV/A/NP, pAAV12Cap, pRepx26, and adenovirus helper plasmid 449B in a ratio of 1:1:1:2. All recombinant particles were purified by CsCl gradient centrifugation. Vector genome copy numbers for each vector stock were determined by quantitative-PCR using SYBR green detection with primers specific to CMV promoter. Titers were expressed as genomic copies/ml. Biological activity of rAAV-Luc vectors was determined by transduction of Cos-7 cells with serially diluted vector followed by measurement of luciferase expression using Bright Glo firefly luciferin assay reagent (Promega, Madison, WI) on a 96-well plate luminometer (Victor X2, Perkin Elmer 2030 multilabel reader). Luciferase expression was expressed as relative light units per well. Expression of rAAV12-A/NP vector was measured by transduction of Cos-7 cells followed by immunofluorescence staining for influenza A/NP protein as described above. rAd-A/NP vector was produced as previously described.7
In vivo administration of rAAV-Luciferase vectors. Male BALB/cAnN mice, aged 6–8 weeks, were obtained from the Division of Cancer Treatment, NCI, Bethesda, MD. All animal studies were approved by the NIDCR Animal Care and Use committee and the NIH Biosafety committee. Mice were mildly anaesthetized with 2% isofluorane and oxygen before vector administration. Groups of three mice received rAAV-Luc vectors as follows: single dose of 1011 vector particles by IP injection, single dose of 1010 vector particles by IM injection into the left anterior muscle, and two doses of 1011 vector particles by IN administration, on day 0 and again 6 weeks after initial administration. Vector was dialyzed in 0.9% sodium chloride and diluted to the appropriate concentration in saline prior to administration to animals. The total volume for IP injection was 100 µl, and 50 µl for IM or IN. For IN administration, vector was delivered directly to the nasal cavity, where mice inhaled 25 µl volume of dialyzed vector per nostril in droplet form, as it was applied to each nostril from a pipette tip. Animals who received vector IN were bled retro-orbitally 4 weeks after initial vector administration, and again 2 weeks post-boost.
In vivo bioluminescence imaging and quantification. Mice were anesthetized with 2% isofluorane and oxygen before administration of luciferin substrate (LUCK 5 g, reconstituted to 40 mg/ml in sterile PBS; Gold Bio Technology, St Louis, MO). Substrate was administered IP to mice who received rAAV-Luc vectors by IP and IM routes, at a dose of 4 mg/mouse (100 µl volume). For mice who received rAAV-Luc vector via IN administration, substrate was also administered IN, 2 mg/mouse (50 µl volume). Five minutes postsubstrate administration, each group of three mice underwent real time bioluminescence imaging using Xenogen IVIS-200 System (Hopkinton, MA). Images were captured and quantified using Living Image 2.60.1 software. For each group, animals were exposed for 10 minutes before images were captured. Animals were imaged 1, 2, 4, and 6 weeks postvector administration, and animals who received vector IN were imaged again 2 weeks post-boost. The experiment was repeated twice, and images shown are representative of the results, which consistently showed the same trend. To quantify luminescence, a region of interest was selected on the captured image and luminescence output was expressed as radiance (photons/second/cm2).
Detection of luciferase expression in nasal cavity. Mice were killed 5 days after IN administration of rAAV-Luc vectors. The nasal cavity was exposed and removed from the head by scalpel, submerged in 10% formalin-buffered saline overnight, and then later decalcified in 14% EDTA for 2 weeks before paraffin embedding and sectioning (Histoserv, Gaithersburg, MD). Cross-sections were de-waxed for 15 minutes at 65 °C, deparaffinized by submerging in xylene (2 × 5 min) followed by hydration in 100% ethanol (1 × 5 min), 95% ethanol (1 × 5 min), and 70% (1 × 5 min) before finally rinsing in distilled water. Sections were blocked with 10% donkey serum in 1% bovine serum albumin (BSA) solution overnight at 4 °C, incubated with rabbit anti-luciferase antibody (Sigma Aldrich, St Louis, MO), 10 µg/ml of 1% BSA, for 2 hours at room temperature, washed twice with PBS + 0.05% Tween 20 and then incubated with donkey anti-rabbit Alexa Fluor 488 secondary antibody (diluted 1/100 in 1% BSA) (Molecular Probes, Invitrogen, Carlsbad, CA) for 30 minutes. Sections were washed again before mounting with vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Labs), prior to adding cover slip. Sections were viewed by confocal microscopy and images captured at ×40 objective. Lungs were harvested from rAAV-Luc-treated mice 5 days after IN administration of rAAV-Luc vectors, and homogenized in cell culture lysis buffer (Cat E153A; Promega) using an Omni international homogenizer. Luciferase expression was measured in homogenized tissue using Bright Glo firefly luciferin assay reagent on a 96 well plate luminometer (Victor X2, Perkin Elmer 2030 multilabel reader). Luciferase expression was expressed as relative light units per mg of protein. Bio-rad protein assay was performed according to manufacturer's protocol to measure protein levels in homogenized tissue (BioRad, Hercules, CA). Lung tissue from a naive mouse was included as a background control.
Neutralization assay. Cos-7 cells were seeded at 5 × 103 cells per well of 96-well plates overnight. Sera from rAAV-Luc-treated animals was serially diluted (1/25–1/1600) in 1% FCS solution (50 µl volume) and preincubated with either 1 × 108 rAAV12-Luc or 1 × 107 rAAV2-Luc vector particles suspended in equal 50 µl volume for 1 hour at room temperature. Serum/vector mix was then added to cells, 100 µl total volume per well of an 96-well plate, in triplicate and incubated for 2 hours at 37 °C. Serially diluted serum from naive animals was preincubated with vector and the mixture added to cells as a control. After incubation, serum/vector mix was removed and replaced by Dulbecco's modified Eagle's medium complete medium. After 24 hours, transduction was determined by measurement of luciferase expression using Bright-Glo firefly luciferin assay reagent on a 96-well plate luminometer as before. Luciferase expression was measured as relative light units per well. The neutralizing titer is the highest serum dilution that still gives 50% transduction with rAAV-Luc vectors compared to naive control serum; therefore the lower the titer, the fewer antibodies is present. Neutralizing titers were expressed as the reciprocal of this dilution.
Detection of anti-AAV IgG by ELISA. Anti-AAV IgG was measured in serum samples by customized ELISA as previously described.35,36 Nunc ELISA plates were coated with 1 × 108 vector genomes of either rAAV12-Luc or rAAV2-Luc per well at 4 °C overnight. Plates were then washed four times with 0.05% Tween 20/PBS and blocked with 1% BSA/PBS for 2 hours at 37 °C. Serum samples were serially diluted (1/50–1/1,600) and added to wells in duplicate, 100 µl/well. Serum from rAAV12-Luc animals was added to rAAV12-coated plate. Serum from rAAV2-Luc animals was added to rAAV2-coated plate. Naive serum, in serial dilution, was added as a negative control to both plates. Plates were incubated at 4 °C overnight. Plates were washed four times before addition of alkaline phosphatase labeled goat anti-mouse IgG (4751-1806; KPL, Gaithersburg, MD) diluted 1:1,000, 100 µl/well. Plate was incubated at room temperature for 1 hour before washing and addition of phosphatase substrate according to manufacturer's protocol (KPL, 50-80-00). Optical density was read at a wavelength of 405 nm on a plate reader after 30 minutes.
Detection of serum antibodies to luciferase. Antibodies to expressed luciferase were measured in serum samples by direct ELISA. Immunosorbent NUNC ELISA plate was coated with recombinant luciferase protein (Promega) at a concentration of 1 µg/ml in coating buffer (100 mmol/l bicarbonate/carbonate buffer, pH 9.6). One hundred micro liter was added per well and plate was incubated at 4 °C overnight. Plate was washed with PBS + 0.05% Tween-20, blocked for 2 hours with 1% FCS in PBS (140 µl/well) and then washed again before incubation with diluted serum (1/20 dilution in 100 µl 1% FCS/PBS) for 2 hours at room temperature. Mouse anti-luciferase monoclonal antibody (L2164; Sigma Aldrich) was serially diluted (1 µg–0.001 µg/ml) and included as a standard curve. Plate was washed four times before addition of alkaline phosphatase labeled goat anti-mouse IgG (4751-1806, KPL) diluted 1:1,000, 100 µl/well. Plate was incubated at room temperature for 1 hour before washing and addition of phosphatase substrate according to manufacturer's protocol (KPL, 50-80-00). Plate was incubated for 10 minutes and reaction was stopped by addition of 100 µl stopping solution per well. Optical density was read at wavelength of 405 nm on plate reader and concentrations of anti-luciferase antibodies in serum samples were estimated from the standard curve and expressed as ng/ml. Serum from rAAV12-A/NP and rAAV2-EGFP-treated animals were used as background controls for rAAV12-Luc and rAAV2-Luc, respectively.
IN administration of rAAV12-A/NP vectors. Male BALB/cAnN mice, aged 6–8 weeks, were obtained from the Divison of Cancer Treatment, NCI, Bethesda, MD. Starting at 10 weeks of age, mice were immunized as follows: nine mice received two IN doses of 1 × 1012 rAAV12-A/NP, day 0 and 5 weeks later, five mice received two IN doses of 1 × 1011 rAAV12-A/NP at the same time points, and another five animals received two IN doses of 1 × 1012 rAAV12-Luc as a negative control. A remaining group of five animals received one IN dose of 1 × 1010 rAd-A/NP day 0 as a positive control. One mouse from the rAAV12-Luc negative control group died before evaluations. Two weeks postadministration of the second rAAV12 vector dose, all animals were bled retro-orbitally and sera isolated from whole blood for analysis of anti-NP antibody. Three weeks post repeat administration, animals were euthanized, and lungs were removed and harvested for use in IFN-γ ELISPOT.
Detection of serum antibodies to influenza A NP. Antibodies to expressed influenza NP were measured in serum samples by direct ELISA as previously described.37 Briefly ELISA plates were coated with recombinant NP protein (IMR-274, 1 mg/ml; IMgenex, San Diego, CA) at a concentration of 1 µg/ml in PBS, blocked for 2 hours with 1% FCS in PBS (140 µl/well), and then incubated with fourfold serially diluted serum (1/20–1/20,480) for 2 hours at room temperature. Alkaline phosphatase labeled goat anti-mouse (#1030-04; Southern Biotech, Birmingham, AL) diluted 1:1,000, was added to each plate, 100 µl/well. Plates were incubated at room temperature for 1 hour before washing and addition of phosphatase substrate according to manufacturer's protocol (KPL, 50-80-00). Optical density was read at wavelength of 405 nm on plate reader (Thermo Multiskan EX, Hudson New Hampshire) after 30 minutes
T-cell ELISPOT. IFN-γ T cell ELISPOT was performed as previously described on mouse lung cells.38 ELISPOT IP plates were coated with 50 µl of anti-IFN-γ (BD, 551309) at a concentration of 5 µg/ml in sterile Hank's balanced salt solution, and incubated overnight at 4 °C. Membranes were washed with sterile Hank's balanced salt solution and blocked with 10% fetal bovine serum/MEM media for at least 1 hour at room temperature. After blocking, 50 µl of either NP 147–155 or NP 55–69 peptide solution, final concentration 1 µg/ml in complete medium39—was added to each well in triplicate. Lung cell suspensions from individual animals were diluted in complete medium, and 200,000 cells were added to each well containing peptide solution in 50 µl volume by pipette. Plates were incubated at 37 °C for 36–48 hours and then washed with Hank's balanced salt solution. Fifty micro liter of filtered anti-IFN-γ biotin (BD, cat. 551506) was added to each well at 1 µg/ml in 10% fetal bovine serum/MEM. Plates were then incubated for 2 hours at room temperature. After washing with Hank's balanced salt solution, 50 µl of filtered alkaline phosphatase labeled Streptavidin (KPL, cat. 475-3000), diluted 1:1,000 in 10% fetal bovine serum/MEM, was added to each well and incubated for 2 hours at 37 °C. After washing, spots were developed by adding 100 µl of 5-bromo, 4-chloro, 3 indolylphosphate/nitroblue tetrazolium phosphatase substrate to each well (KPL, cat. 50-81-08) and counted with an ELISPOT reader (Zeiss Thornwood, NY). The T cell response was evaluated by measuring the number of IFN-γ producing cells per million in each pooled sample. Negative controls for the assay included an irrelevant SARS peptide.
Statistical analysis. Statistical comparisons were performed by unpaired Student's t-test using GraphPad Prism 5, where *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P < 0.0001.
SUPPLEMENTARY MATERIAL Figure S1. Detection of serum neutralizing antibodies after IP administration of rAAV-Luc vectors. Figure S2. Detection of anti-AAV capsid IgG antibodies after IP administration of rAAV-Luc vectors. Figure S3. Detection of serum neutralizing antibodies after IM administration of rAAV-Luc vectors. Figure S4. Detection of anti-AAV capsid IgG antibodies after IM administration of rAAV-Luc vectors.
Acknowledgments
We thank Sandra Wainer and Beverly Handelman (MPTB, NIDCR, NIH) for their expertise in AAV vector production, Giovanni DiPasquale (MPTB, NIDCR, NIH) for assistance with Xenogen Imaging, William Swaim (MPTB, NIDCR, NIH) for help with immunofluorescence and confocal microscopy, and Milton Papa (VRC, NIDCR, NIH) for assistance with retro-orbital bleeding. This work was funded by an NIH intramural grant, to J.A.C.
Supplementary Material
Detection of serum neutralizing antibodies after IP administration of rAAV-Luc vectors.
Detection of anti-AAV capsid IgG antibodies after IP administration of rAAV-Luc vectors.
Detection of serum neutralizing antibodies after IM administration of rAAV-Luc vectors.
Detection of anti-AAV capsid IgG antibodies after IM administration of rAAV-Luc vectors.
REFERENCES
- Berns KI., and, Linden RM. The cryptic life style of adeno-associated virus. Bioessays. 1995;17:237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- Grimm D., and, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G., and, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Koch WJ., and, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3:81–89. doi: 10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D., and, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot BE, Sample CJ., and, Ramsburg EA. Recombinant vesicular stomatitis virus expressing influenza nucleoprotein induces CD8 T-cell responses that enhance antibody-mediated protection after lethal challenge with influenza virus. Clin Vaccine Immunol. 2009;16:488–498. doi: 10.1128/CVI.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L.et al. (2005Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein Vaccine 235404–5410. [DOI] [PubMed] [Google Scholar]
- Breathnach CC, Rudersdorf R., and, Lunn DP. Use of recombinant modified vaccinia Ankara viral vectors for equine influenza vaccination. Vet Immunol Immunopathol. 2004;98:127–136. doi: 10.1016/j.vetimm.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Kelley WM, Burda JF., and, Wilson JM. A novel adenovirus-adeno-associated virus hybrid vector that displays efficient rescue and delivery of the AAV genome. Hum Gene Ther. 1996;7:2079–2087. doi: 10.1089/hum.1996.7.17-2079. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW., and, Miller AD. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Allen JM., and, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley SM, Howe SJ, Rahim AA, Buning H, McIntosh J, Wong SP.et al. (2008Luciferin detection after intranasal vector delivery is improved by intranasal rather than intraperitoneal luciferin administration Hum Gene Ther 191050–1056. [DOI] [PubMed] [Google Scholar]
- Price GE, Soboleski MR, Lo CY, Misplon JA, Quirion MR, Houser KV.et al. (2010Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses PLoS ONE 5e13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C.et al. (1998Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA J Virol 725648–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GE, Soboleski MR, Lo CY, Misplon JA, Pappas C, Houser KV.et al. (2009Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses Vaccine 276512–6521. [DOI] [PubMed] [Google Scholar]
- Schotsaert M, Ibañez LI, Fiers W., and, Saelens X. Controlling influenza by cytotoxic T-cells: calling for help from destroyers. J Biomed Biotechnol. 2010;2010:863985. doi: 10.1155/2010/863985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Verhoeven D, Page CA, Turner D., and, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Afione S, Schmidt M., and, Chiorini JA. Identification of adeno-associated virus contamination in cell and virus stocks by PCR. BioTechniques. 2004;36:676–680. doi: 10.2144/04364DD01. [DOI] [PubMed] [Google Scholar]
- Atchison RW, Casto BC., and, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang L, Johnson JS, Zhijian W, Grieger JC, Ping-Jie X.et al. (2009Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium Mol Ther 172067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR.et al. (2010IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia Gene Ther 17567–576. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M.et al. (2010High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines Mol Ther 182048–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JD, Thesier DM, Swain JB, Katz MG, Tomasulo C, Henderson A.et al. (2011Myocardial gene delivery using molecular cardiac surgery with recombinant adeno-associated virus vectors in vivo Gene Ther 18546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL.et al. (2000Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer J Virol 743852–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ., and, Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP., and, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Rutledge EA, Allen JM, Russell DW., and, Miller AD. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino DE, Mingozzi F, Hui DJ, Chen H, Colosi P, Ertl HC.et al. (2005Identification of mouse AAV capsid-specific CD8+ T cell epitopes Mol Ther 121023–1033. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu Q, Yang P, Hsu HC., and, Mountz JD. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG.et al. (2000Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure J Virol 741761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ.et al. (2003Adeno-associated viruses undergo substantial evolution in primates during natural infections Proc Natl Acad Sci USA 1006081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro P, Downey HD, Zhou JS, Wright JF, Hauck B, Mingozzi F.et al. (2009Host and vector-dependent effects on the risk of germline transmission of AAV vectors Mol Ther 171022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettrumpf J, Liu JH, Couto LB, Addya K, Leonard DG, Zhen Z.et al. (2006Inadvertent germline transmission of AAV2 vector: findings in a rabbit model correlate with those in a human clinical trial Mol Ther 131064–1073. [DOI] [PubMed] [Google Scholar]
- Ito T, Yamamoto S, Hayashi T, Kodera M, Mizukami H, Ozawa K.et al. (2009A convenient enzyme-linked immunosorbent assay for rapid screening of anti-adeno-associated virus neutralizing antibodies Ann Clin Biochem 46Pt 6508–510. [DOI] [PubMed] [Google Scholar]
- Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V.et al. (2008Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential Mol Vis 141760–1769. [PMC free article] [PubMed] [Google Scholar]
- Lo CY, Wu Z, Misplon JA, Price GE, Pappas C, Kong WP.et al. (2008Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies Vaccine 262062–2072. [DOI] [PubMed] [Google Scholar]
- Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z.et al. (2007Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1 Emerging Infect Dis 13426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Marshall D, Coleclough C., and, Woodland DL. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J Immunol. 2000;164:3274–3282. doi: 10.4049/jimmunol.164.6.3274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of serum neutralizing antibodies after IP administration of rAAV-Luc vectors.
Detection of anti-AAV capsid IgG antibodies after IP administration of rAAV-Luc vectors.
Detection of serum neutralizing antibodies after IM administration of rAAV-Luc vectors.
Detection of anti-AAV capsid IgG antibodies after IM administration of rAAV-Luc vectors.