Abstract
Background
In plants, nitrate (NO3-) nutrition gives rise to a natural N isotopic signature (δ15N), which correlates with the δ15N of the N source. However, little is known about the relationship between the δ15N of the N source and the 14N/15N fractionation in plants under ammonium (NH4+) nutrition. When NH4+ is the major N source, the two forms, NH4+ and NH3, are present in the nutrient solution. There is a 1.025 thermodynamic isotope effect between NH3 (g) and NH4+ (aq) which drives to a different δ15N. Nine plant species with different NH4+-sensitivities were cultured hydroponically with NO3- or NH4+ as the sole N sources, and plant growth and δ15N were determined. Short-term NH4+/NH3 uptake experiments at pH 6.0 and 9.0 (which favours NH3 form) were carried out in order to support and substantiate our hypothesis. N source fractionation throughout the whole plant was interpreted on the basis of the relative transport of NH4+ and NH3.
Results
Several NO3--fed plants were consistently enriched in 15N, whereas plants under NH4+ nutrition were depleted of 15N. It was shown that more sensitive plants to NH4+ toxicity were the most depleted in 15N. In parallel, N-deficient pea and spinach plants fed with 15NH4+ showed an increased level of NH3 uptake at alkaline pH that was related to the 15N depletion of the plant. Tolerant to NH4+ pea plants or sensitive spinach plants showed similar trend on 15N depletion while slight differences in the time kinetics were observed during the initial stages. The use of RbNO3 as control discarded that the differences observed arise from pH detrimental effects.
Conclusions
This article proposes that the negative values of δ15N in NH4+-fed plants are originated from NH3 uptake by plants. Moreover, this depletion of the heavier N isotope is proportional to the NH4+/NH3 toxicity in plants species. Therefore, we hypothesise that the low affinity transport system for NH4+ may have two components: one that transports N in the molecular form and is associated with fractionation and another that transports N in the ionic form and is not associated with fractionation.
Keywords: Low affinity ammonium transporters, Nitrogen isotopic signature, Ammonium/ammonia, Ammonium dissociation isotope factor, ammonia uptake
Background
Nitrogen (N) and carbon (C) are the main components of all living organisms and regulate the productivity of most ecosystems. In agriculture, N is by far the main nutrient in fertilisers, with nitrate (NO3-) and ammonium (NH4+) being the main N sources used by plants. However, relatively little is known about the isotopic fractionation during uptake of these ions. Assessment under natural conditions is difficult because, under most circumstances, NO3- and NH4+ are simultaneously present in the soil and their concentrations change both spatially and temporally over a wide range (e.g., 20 μM to 20 mM) [1,2]. Furthermore, this situation becomes even more complex if the rhizosphere and its symbiotic interactions (N2-fixing organisms or mycorrhiza) are taken into account.
The natural variation in stable N isotopes has been shown to be a powerful tool in several studies of plant and ecosystem N dynamics [3]. Generally, the global δ15N value of the plant biomass is determined by that of the primary N source (soil N, fertiliser, N2) [4]. Some studies assume that the δ15N of leaf tissue reflects that of the source in the soil (e.g., see [5]). This assumption implies that the isotope ratio of the N source is preserved during N absorption, assimilation and translocation. However, it is clear that physiological processes and biological mechanisms, such as N-uptake, assimilation through distinct pathways, internal N recycling in the plant and gaseous N exchange, can discriminate against 15N [4]. Furthermore, plant N fractionation is also dependent on the N availability. Thus, in the case of unlimited substrate (N) availability, an isotope effect will always be expressed, and therefore, the arising δ15N will be lower than in the N source if fractionation occurs [6]. In contrast, in a growth system where the quantity of substrate (N) is limited, and the organism exhausts the N source completely, the plant δ15N will be similar (or even identical) to the original N source [6,7]. Most studies concerning physiological and natural N fractionation have involved plants grown with NO3- as the only N source. A review of these studies [6] showed that N fractionation changes with plant age, the external NO3- concentration and the partitioning of N metabolism between the roots and shoots.
Similarly to NO3-, NH4+ influx through the membrane of plant cells exhibits a predominantly biphasic pattern. Thus, at concentrations up to 0.5-1 mM N, influx occurs via the high affinity transport system (HATS), which is saturable and energy dependent and has a Km in the submillimolar concentration range; the non-saturable low affinity transport system (LATS) operates with a Km in the millimolar concentration range, i.e., at N concentrations above 0.5-1 mM, for most plant roots [8,9].
While the proteins responsible for the high-affinity NH4+ transporters have been identified in many plant species, the low-affinity uptake system proteins have yet to be identified [9]. Recently, Loqué and von Wirén reviewed the different levels at which NH4+ transport is regulated in plant roots under HATS conditions [10]. A functional analysis of several ammonium transporters (AMTs) expressed in Xenopus oocytes showed evidence that NH4+, rather than NH3, uniport is the most likely transport mechanism for AMT1-type transporters from plants [11-13]. Nevertheless, individual plant AMT/Rh transporters may use different transport mechanisms [13] compared with the AMT2-type transporters, which recruit NH4+-mediated electroneutral NH4+ transport, probably in the form of NH3[14,15].
On the contrary, the molecular basis of transport under LATS conditions remains poorly understood. LATS for NH4+ operates when NH4+ is present at high concentrations in solution; under these conditions, several symptoms of toxicity have often been observed in a broad range of plant species [2]. Few studies have examined the natural isotopic signature of plants grown with NH4+ nutrition under LATS conditions and its relationship with sensitivity or tolerance to NH4+ nutrition. It has been speculated that NH3 could be the chemical species that enters the plant from the external medium via the plasma membrane [7,16]. Under conditions of high external pH and high NH4+, the transport of NH3 across membranes occurs, and it can become biologically significant [16,17]. In agro-ecosystems, in which the soils are currently fertilised with urea (50% of the total world fertiliser N consumption [18]) or (NH4)2SO4, emissions of N in the NH3 form take place (i.e., up to 10-20% of N in fertilisers applied as urea may be lost in the soil [19]). Thus, under these conditions, significant amounts of NH3 may be present in the soil and therefore enter the plant. When NH4+ is applied as the only N source or NH4+ is formed naturally in soils via mineralization of organic matter, the two forms, NH4+ and NH3, are present in the nutrient solution. The neutral and ionic forms do not have exactly the same natural isotopic signatures because there is a 1.025 thermodynamic isotope effect between NH3 (g) and NH4+ (aq), so NH3 (aq) is depleted for 15N by 20‰ relative to NH4+ (aq) [20]; in addition, the equilibrium fractionation factor for exchange of NH3 (aq) with NH3 (g) has been estimated as ~ 1.005 [21].
Thus, an understanding of the physiological processes that lead to variations in the stable isotopic composition is required. This work was intended to assess the natural δ15N dynamics for several plant species grown hydroponically under controlled conditions and with only one N source, namely NO3- or NH4+. Our working hypothesis for this study was that a part of NH4+ enters the plant root as neutral molecules (i.e. NH3) favouring the isotopic fractionation and this fractionation process during NH4+ uptake is related to the sensitivity of plants to NH4+ nutrition. Fractionation of the N source throughout the whole plant was interpreted on the basis of the relative transport of NH4+ and NH3. We also propose that LATS for NH4+ uptake may have two components, one that involves the ionic form (NH4+) and another that involves the molecular form (NH3).
Methods
Plant Culture
i) Isotopic signature experiment in several plant species
Nine species that show different NH4+ tolerances were grown hydroponically with NH4+ or NO3- as the sole N sources. Lettuce (Lactuca sativa L. cv. Marine), spinach (Spinacia oleracea L. cv. Spinner), tomato (Solanum lycopersicum L. cv. Trust), pea (Pisum sativum L. cv. Eclipse) and lupin (Lupinus albus L. cv. albus) plants were germinated, cultured and treated as described previously [22]. Carob (Ceratonia siliqua sp.) and Acacia aneura sp. plants were grown according to [23]. Perennial ryegrass (Lolium perenne L. cv. Herbus) and white clover (Trifolium repens L. cv. Huia) were cultured according to [24]. Pea plants (cv. Sugar-snap) were grown according to [25], and spinach (cv. Gigante de invierno) and pea plants (cv. Rondo) were cultured as described in [24]. Plants from each species were divided into two groups, each of which received different concentrations of N (0.5 to 6.0 mM) in the form of either NO3- or NH4+ (applied as Ca(NO3)2 or KNO3 and (NH4)2SO4, respectively). All seeds were surface-sterilised and plants were grown for several days (depending on the plant species) under hydroponic conditions. The pH of the nutrient solutions was buffered with CaCO3 (5 mM) to pH 6-7, depending on the plant species. The temperature of the solutions was between 18 and 20°C. Nutrient solutions were aerated vigorously (flow rate of 15 mL s-1) and replaced weekly to minimize the nitrification processes.
Plants were harvested by separating the shoots and roots of each plant. The dry weight of each plant was obtained after drying in an oven at 75-80°C to a constant weight (48-72 h).
ii) Short-term control and 15N labelling experiments in spinach and pea plants
Spinach seeds (cv. Gigante de Invierno) were germinated and grown hydroponically as described by [26]. N-free Rigaud and Puppo solution [27], which had been diluted (1:2) and modified according to [25] was used during the growth period. The N-free solution was supplemented with 0.5 mM NH4NO3 as the only N source for the first 25 days of growth period. Then, spinach plants were fed with a Rigaud and Puppo solution containing 0.5 mM NH4Cl as the only N source for the last 5 days of the growth period. The pH of the solution was buffered with CaCO3 (0.25 mM) to pH 6-6.5.
Pea seeds (cv. Sugar-snap) were surface-sterilised according to [28] and then germinated as described in [25]. One-week-old pea seedlings were transferred into tanks (volume: 8 L) in groups of eight and grown in controlled-environment chambers at 275-300 μmol photons m-2 s-1, 22/18°C (day/night), 60/70% relative humidity and a 14 h light/10 h dark photoperiod for 1-2 weeks, until the second node stage was reached. The hydroponic vessels contained aerated (0.4 L air min−1 L−1) N-free Rigaud and Puppo solution [27], which had been diluted (1:2) and modified according to [25]. A solution of 0.5 mM NH4+ was supplied as NH4Cl during the growth period as the only N source. The pH of the solution was buffered with CaCO3 (2.5 mM) to 7-7.3.
Either spinach or pea plants were then transferred to a solution at pH 6 (KP buffer, 10 mM) or pH 9 (H3BO3/NaOH buffer, 50 mM) in a sealed 125-ml Erlenmeyer flask, such that the roots were fully immersed in 100 mL of solution. Fully 15N-labelled 15NH4Cl was injected and rapidly mixed to a final concentration of 10 mM NH4+. Plants from both pH levels were harvested by separating the shoots and roots of each plant at 0, 1, 7.5 (for spinach), 15, 30, 60 and 120 min after the 15NH4Cl injection. In order to evaluate how the pH increase affects ion uptake per se, we have used as control a nutrient solution containing RbNO3 (1 mM), instead of 15NH4Cl. This control was performed exclusively on spinach, which is considered a more sensitive species than pea. Internal Rb+ and NO3- contents were determined in shoots and roots at 7.5, 30 and 120 min after RbNO3 injection, as tracers of cation and anion uptake respectively in different pHs.
For the uptake experiments, the applied light intensity during the pH and RbNO3 or 15N-labelling short-term applications was 750-800 μmol photons m-2 s-1 to enhance the absorption process.
pH measurements were determined after the short-term experiments in order to verify that the pH of the solution was properly buffered and that there were no great changes in the pH due to the root ionic exchanges (ion influx/efflux) (Additional file 1).
Isotopic N Composition and N accumulation
Five to eight milligrams of powdered plant material from each sample (shoots and roots) was separately packed in tin capsules. The 15N/14N isotope ratios of these samples were determined by isotope ratio mass spectrometry (isoprime isotope ratio mass spectrometer - IRMS, Micromass-GV Instruments, UK). The N isotope composition results are expressed as δ15N, in parts per thousand (‰) relative to atmospheric N2: δ15N (‰) = [(Rsample/Rstandard)-1] * 1000, where Rsample is the 15N/14N ratio of the sample and Rstandard is the 15N/14N ratio of the atmospheric N2. Plant material that had previously been calibrated against a standard material of known isotope composition was used as a working standard for batch calibration during the isotope ratio analyses. The 15N contents (total, 15NH4+ and 15NH3) were obtained using δ15N and the total percentage of N for each plant tissue (leaves and roots), and 15N contents for the external NH4+ and NH3 were calculated using the Henderson-Hasselbalch equation, which takes into account the external pH. The percentages of NH3 molecules (relative to the total [NH4+ + NH3] molecules) at pH 6.08 and pH 9.0 were 0.0676% and 35.993%, respectively (see Additional file 2). Plant tolerance to NH4+ nutrition was calculated as the ratio between biomass accumulation of NH4+- and NO3--fed plants at the same N concentration [22]. The δ15N data corresponding to the N sources used ranged from +0.03 to +2.31 for NH4+ and -1.514 to +0.3 ‰ for NO3-.
Determination of inorganic soluble ion content
Plant extracts with soluble ionic contents from shoots and roots were obtained from dry tissues incubated in a bath in 1-2 mL of milli-Q water at 85°C for 10 min, followed by centrifugation (20,000×g, 30 min). The supernatants were stored at -20°C until analysis by ion chromatography. Soluble cation content (Rb+) was determined as described in [27] using an isocratic method with 20 mM metanosulphonic acid solution. Soluble anion content (NO3-) determination was carried out by the gradient method given by [27]. Rb+ content was below the detection limit in shoots.
Statistical analyses
All statistical analyses were performed with Statistical Product and Service Solutions (SPSS) for Windows, version 17.0.
i) Statistical analysis of the natural isotopic abundance experiment in several plant species
We examined results for nine species using analysis of variance to test for effects and interactions of the N treatments (source and concentration) and whether these changed according to the organ and species tested. Organ was included as a factor exclusively in the natural isotopic composition ANOVA test because it was meaningless to include it in the total biomass and total biomass ratio (NH4+/NO3-) ANOVA tests.
ii) Statistical analysis for short-term experiments in spinach and pea plants
One-way analysis of variance (ANOVA; factor: time) was performed. The homogeneity of variance was tested using the Levene test [29]. Least significant difference (LSD) statistics were applied for variables with homogeneity of variance, and the Dunnett T3 test [30] was used for cases of non-homoscedasticity. The pHs were compared using Student's t-test for each time point independently, and homoscedasticity was determined using the Levene test [29].
All statistical analyses were conducted at a significance level of 5% (P ≤ 0.05). The results of this study were obtained for plants cultured in several independent series. For the plant species lettuce (cv. Marine), spinach (cv. Spinner), tomato (cv. Trust), pea (cv. Eclipse) and lupin (cv. Albus), plant material from six plants was mixed and analysed in three independent series. For spinach (cv. Gigante de invierno), pea (cv. Sugar-snap and Rondo), carob, perennial ryegrass (cv. Herbus), white clover (cv. Huia) and Acacia sp., at least one sample was analysed for each of three independent series.
Results
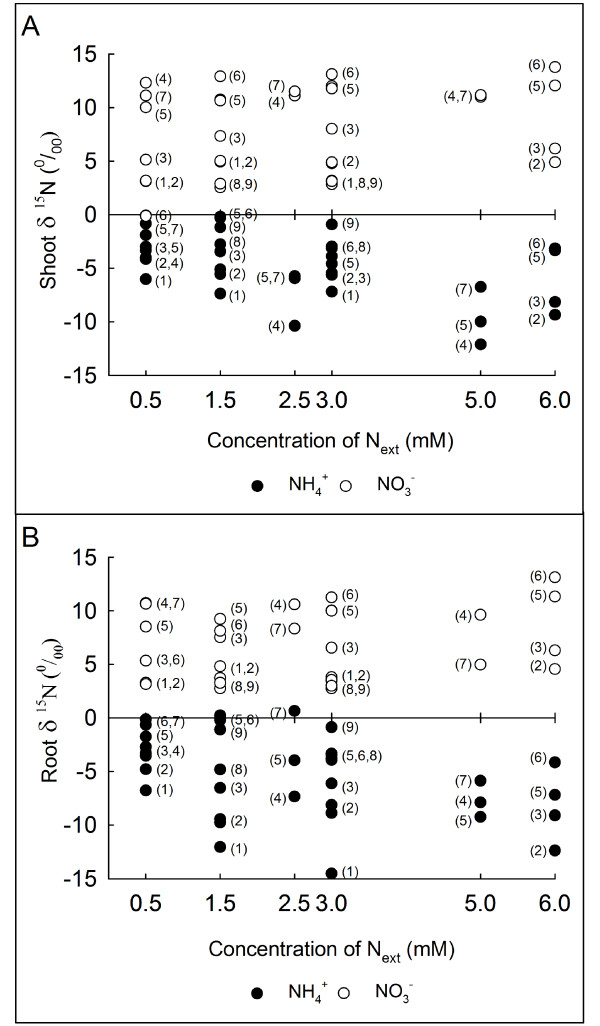
Although the δ15N values of the sources, NO3- and NH4+, similarly ranged from -1.514 to +2.31 ‰, the δ15N observed for several plant species was significantly different when N was provided either as NO3- or NH4+ (Table 1). In general, four trends emerged from the natural isotopic signature data (Figure 1): 1) NO3--fed plants tended to be enriched in the heavier N isotope, whereas NH4+-fed plants were depleted compared with their respective N sources; 2) for the same external N concentration, the degree of fractionation depended on the plant species; 3) the δ15N values of shoots and roots were not the same but followed similar patterns; and 4) in contrast to the NO3--fed plants, which had δ15N values that were insensitive to the N concentration, under NH4+ nutrition, fractionation tended to increase with the N concentration within plant species (Table 2). These four trends were supported by the results displayed in Tables 1 and 2 from the analyses of variance of N, species and organ effects. The source of N had a global effect on the isotopic composition (‰) and total biomass (g DW) (Table 1). Moreover, significant two-way interactions between the N source and N concentration (N source × N conc.) and the N source and species (N source × sp.) on the δ15N and the total biomass were observed (Table 1). Due to the strong effect of the N source on the δ15N, the main effects of N concentration, species and organ type was analysed in NO3-- and NH4+- fed plants separately (Table 2). In NH4+-fed plants, the N concentration, species and organ type had an effect on the natural isotopic abundance; however, in NO3-- fed plants, only the diversity (species) factor had an effect on the δ15N (Table 2).
Table 1.
Analysis of variance of the N sources, N concentrations and species.
| Global Effect | δ 15N
(‰) |
Total Biomass (g DW) |
||
|---|---|---|---|---|
| Factor | F | P > F | F | P > F |
| N Source | 1273.54 | < 0.0001 | 8.62 | 0.0043 |
| N Source × N Conc. | 19.95 | < 0.0001 | 16.01 | < 0.0001 |
| N Source × sp. | 10.01 | < 0.0001 | 39.71 | < 0.0001 |
| N Source × N Conc. × sp. | 1.23 | 0.2701 | 7.46 | < 0.0001 |
| Whole model R2 | 0.956 | 0.939 | ||
Global effects of N sources and interaction terms, including the N source effects, on isotopic composition (‰) and total biomass (g DW). N Conc.: N concentration; sp.: species. The main effects of the N concentration and species are not included because the results of the ANOVA test were masked by the strong N source effect. They are shown separately by the N source in Table 2. Significant effects (P ≤ 0.05) are shown in bold.
Figure 1.
Natural N isotopic composition of nine plant species with different sensitivity to NH4+ nutrition. Natural isotopic signatures (δ15N, ‰) of the shoots (A) and roots (B) of several plant species cultured under hydroponic conditions with different concentrations of NH4+ (●) or NO3- (○) as the sole N source. The following numbers indicate the species that correspond to each point: (1) Lactuca sativa L., (2) Spinacia oleracea L., (3) Solanum lycopersicum L., (4) Lolium perenne L., (5) Pisum sativum L., (6) Lupinus albus L., (7) Trifolium repens L., (8) Ceratonia siliqua sp., and (9) Acacia aneura sp. Each point is the average of several biological replicates (at least n = 3, depending on the species; see Methods). δ15N of the N sources: NO3- = +0.3 and -1.514 and NH4+ = +0.029, +0.5 and +2.31 ‰.
Table 2.
Analysis of variance of the N concentrations, species and organ effects.
| Factor | δ 15N (‰) |
Total Biomass (g DW) |
Total Biomass Ratio (NH4+/NO3- ) |
|||
|---|---|---|---|---|---|---|
| Effect on NO3--fed plants | F | P > F | F | P > F | F | P > F |
| N Conc. | 0.78 | 0.4743 | 38.53 | < 0.0001 | 10.92 | < 0.0001 |
| sp. | 13.20 | < 0.0001 | 80.73 | < 0.0001 | 64.81 | < 0.0001 |
| N Conc. × sp. | 1.18 | 0.3655 | 4.26 | < 0.0001 | 1.43 | 0.1912 |
| Organ | 1.80 | 0.1966 | - | - | - | - |
| Whole model R2 | 0.884 | 0.942 | 0.927 | |||
| Effect on NH4+-fed plants | F | P > F | F | P > F | F | P > F |
| N Conc. | 34.69 | < 0.0001 | 1.57 | 0.2183 | 8.93 | 0.0005 |
| sp. | 17.73 | < 0.0001 | 80.56 | < 0.0001 | 59.10 | < 0.0001 |
| N Conc. × sp. | 0.93 | 0.5418 | 6.84 | < 0.0001 | 1.40 | 0.1999 |
| Organ | 4.76 | 0.0392 | - | - | - | - |
| Whole model R2 | 0.916 | 0.936 | 0.908 | |||
The effects of N concentration and species (sp.) and the corresponding interactions are shown separately by the N source on the isotopic composition (‰), total biomass (g DW) and total biomass ratio (NH4+/NO3--fed plants). The organs did not influence the N concentration interaction (N Conc. × Organ; P > 0.8) or the species interaction (sp. × Organ; P > 0.05) or N Conc. × sp. interaction (N Conc. × Sp. × Organ; P > 0.8) with either N source. The interaction terms, including the organ effects, are therefore not shown above. Significant effects (P ≤ 0.05) are shown in bold text.
Biomass accumulation in NH4+- and NO3--fed plants at the same N concentration was dependent on the N concentration in the root medium and on the plant species concerned (Table 2). The degree of the effect of the N concentration on the total plant biomass (growth stimulation with NO3- nutrition or growth inhibition with NH4+ nutrition) depended on the species, as shown by the significant interaction of N conc. × sp. for both N sources (Table 2).
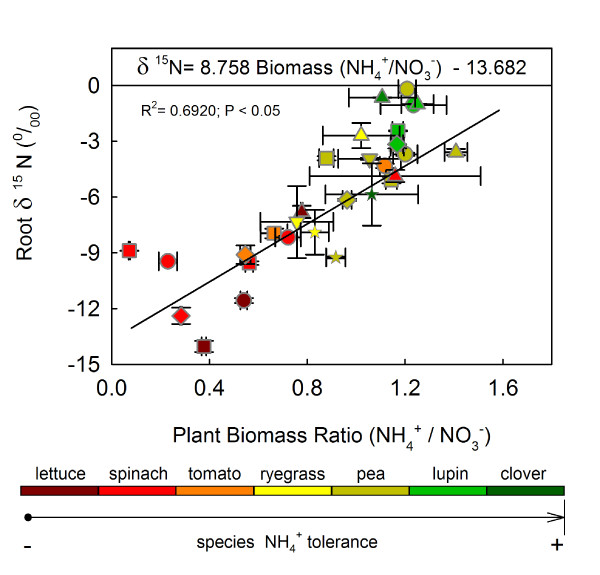
The ratio of biomass accumulations between the NH4+- and NO3--fed plants was therefore used as an indicator of each plant species' sensitivity (or tolerance) to NH4+ nutrition. The N concentration and diversity also influenced the total biomass ratio of NH4+- and NO3--fed plants (Table 2). A very strong correlation between the root δ15N of NH4+-fed plants and the ratio of biomass accumulation between the NH4+- and NO3--fed plants was observed (Figure 2). Thus, the lower biomass ratios (i.e., lower tolerance to NH4+) observed for seven species and cultivars, which presented different degrees of tolerance to NH4+ nutrition grown with several N concentrations, were associated with depletion of the heavier N isotope in the plant material studied (Figure 2). Hence, the most sensitive plants to NH4+ were the most depleted of 15N (Additional file 3 table S1). The Ceratonia species (carob) showed a unique behaviour relative to the other herbaceous species; its much higher biomass ratios for the negative δ15N values did not fit within the correlation (see Additional file 3, table S1). The ratio of the whole plant biomass accumulation (NH4+/NO3-) in Acacia species was not measured. Hence, they were excluded from the dataset in Figure 2.
Figure 2.
Root isotopic signatures (δ15N, ‰) of NH4+-fed plants correlated with the plant NH4+ toxicity/tolerance indicator (plant biomass ratio NH4+/NO3- for each N concentration). The following N concentrations were represented in this analysis: 0.5 mM (upward triangle), 1.5 mM (circle), 2.5 mM (upside down triangle), 3 mM (square), 5 mM (star) and 6 mM (diamond). δ15N data of the (NH4)2SO4 used in NH4+-fed plants were +0.029, +0.5 and +2.31 ‰, and all three values fall within the area indicated (upper part of graph). The plant species that were cultured hydroponically and used for this statistical analysis were lettuce, spinach, tomato, ryegrass, pea, lupin and white clover. The dataset displayed represents the average values ± SE (at least n = 3, depending on species; see Methods). Linear regression was performed at P ≤ 0.05.
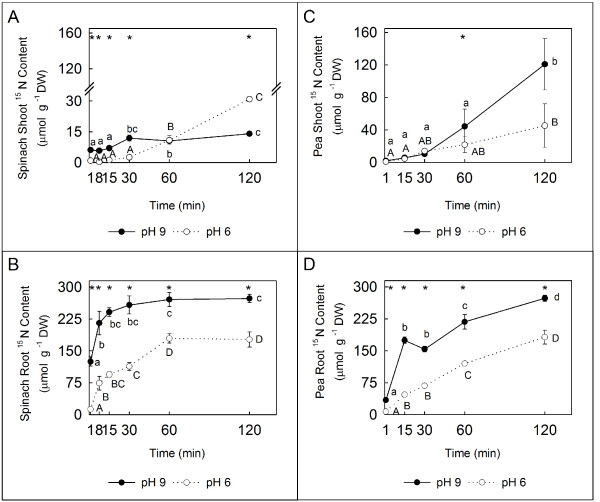
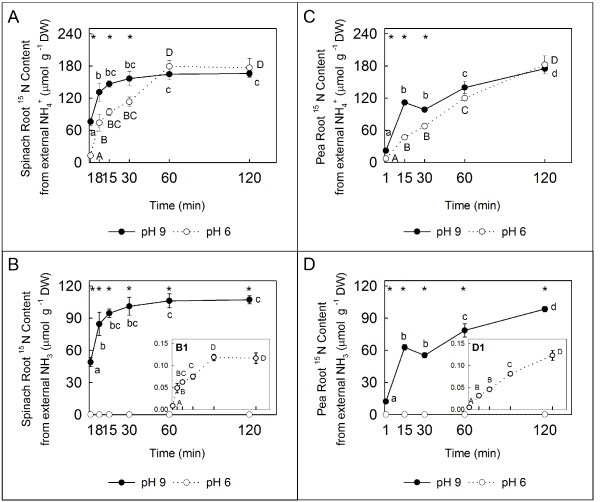
Natural soils rarely exhibit pH values close to the pKa of NH4+ (~ 9.25); therefore, NH3 is present in very small amounts under normal external pH conditions [2]. In the short-term experiments described herein, three- and four-week-old N-deficient pea and spinach plants, respectively, were transferred to a 100% 15N-labelled 10 mM NH4+ solution. δ15N was used as a tool to determine the amount of 15N that enters the plant roots under the experimental conditions, and a higher increase in the total 15N content was observed at pH 9 than at pH 6 in both plant species (Figure 3B and 3D). In plants with higher NH4+ sensitivity, i.e., spinach, the 15NH3/15NH4+ absorption reached the asymptotic trend moment in the curve in a shorter period of time than pea plants (Figure 3B and 3D). In shoots, the total 15N content per DW g was lower in spinach than in pea plants (Figure 3A and 3C). The content of 15N in spinach shoots was higher in pH 9 than in pH 6 (Figure 3A), whereas in pea plants no difference was observed between pHs during the initial 15 min (Figure 3C). This result indicates that in spinach plants the N is translocated immediately from the roots to the shoot, while in pea plants N translocation is delayed relative to N uptake. At 120 min, opposite effects between pHs were shown in both plant species. In spinach shoots, higher 15N content was displayed at pH 6, while pea shoots showed higher 15N content at pH 9 (Figure 3A and 3C). On the other hand, the internal root 15N content was related to the proportion of NH4+ and NH3 in the external solution at pH 6 and 9 (Figure 4), as calculated using the Henderson-Hasselbalch equation (see Additional file 2). In both plant species, some important differences were found between the plants at pH 6 and 9 in terms of the proportion of 15N uptake from the external NH4+ source during the initial 15 min after transfer to a different pH (Figure 4A and 4C), whereas the uptake rates of 15N from the external NH4+ were similar at both pH levels 60 min after the beginning of the experiment (Figure 4A and 4C). The most remarkable finding, however, was a drastic increase in 15N uptake from the external NH3 source at pH 9, which was maintained throughout the experiment (up to 120 min, Figure 4B and 4D).
Figure 3.
15N contents in tissues of spinach and pea plants. 15N content (μmol g-1 DW) calculated from the δ15N data, in shoots (A and C) and roots (B and D) of spinach (A and B) and pea (C and D) plants transferred from pH 7 to pH 6 (○) or pH 9 (●).
Figure 4.
Root 15NH4+ and 15NH3 contents calculated from the total 15N uptake. 15N content accumulated from 15NH4+ absorption (μmol g-1 DW) in spinach (A) and pea (C) plants. 15N content accumulated from 15NH3 absorption (μmol g-1 DW) in spinach (B) and pea (D) plants. (B1 and D1) Magnified portions of plots (B and D respectively) showing the 15N content that accumulated as a result of external 15NH3 absorption at pH 6 (μmol g-1 DW). The partitioning between NH3 and NH4+ has been calculated using the Henderson-Hasselbalch equation (see Additional file 2). Data represent the average values ± SE (n = 3). Letters represent significant differences (P ≤ 0.05) during exposure to pH 6 (A, B, C and D) and pH 9 (a, b, c and d). An asterisk (*) denotes significant differences between pH 6 and 9 (P ≤ 0.05).
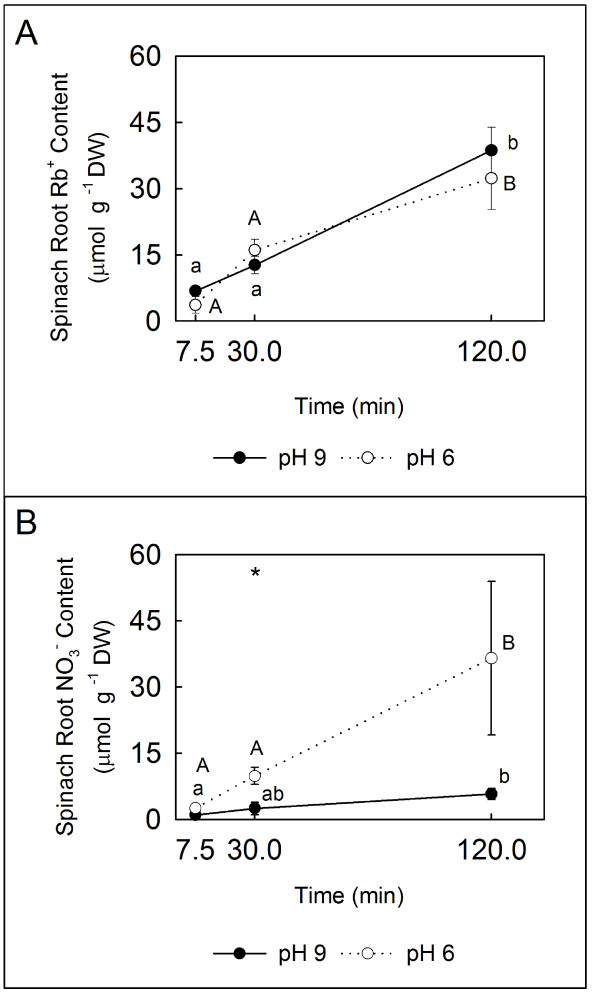
On the other hand, a broad range of K+ channels have been shown to allow significant levels of NH4+ to permeate [31], and at the same time Rb+ is commonly used as a K+ analogue in physiological studies [32], as its size and permeability characteristics are very similar to those of K+[33]. Thus we have used Rb+ as a tracer for evaluating the effect of pH increase in cation uptake. The uptake rates of Rb+ from the external RbNO3 source were similar at both pH levels throughout the experiment (Figure 5A). The anion (NO3-) absorption was lower under alkaline than acidic conditions (Figure 5B). In shoots, the internal NO3- contents were similar in both external pHs (not shown). Therefore, all the effects observed in this study under NH4+ nutrition and different pH conditions (Figures 3 and 4) can be just attributed to the ratio between NH3 and NH4+.
Figure 5.
Root ion contents of spinach plants. Root ion content (μmol g-1 DW) of plants transferred from pH 7 to pH 6 (○) or pH 9 (●). (A) Rb+ content. (B) NO3- content.
Discussion
Natural isotopic abundances of N in plants grown with NO3- or NH4+
An important degree of fractionation, determined as the difference between the δ15N of the N source and that of the plant, was observed when plants were grown hydroponically with a known concentration of a single N form in a controlled environment (Figure 1). Thus, NO3-- fed plants tended to be enriched in the heavier N isotope in relation to the source, whereas NH4+-fed plants tended to be depleted (Figure 1).
The degree of fractionation in the reaction rates of the two N isotopes (14N and 15N) reflects both their mass differences and the force constants of the bonds they form. A significant isotope effect due to ionisation would therefore not be expected [34].
The positive δ15N values for NO3--fed plants may be associated with N loss from the plant in the form of root efflux and exudates [6,7,35] or loss of NH3 through the stomata [36-39], which favours the lighter isotope [40]. The ratio between the root and shoot δ15N values may also depend on the partitioning of N metabolism between the roots and shoots. The isotopic effect for nitrate reductase enzyme is 1.015 (or higher, see [4] and references therein) and that associated with glutamine synthetase is 1.017 [41]; therefore, the resulting organic compounds (amino acids) would therefore be depleted of 15N in relation to the inorganic N pool. Thus, depending on the main site, shoots or roots, of N reduction and assimilation, the tissues would present distinct δ15N values. Since NO3- and NH4+ are not major constituents of the phloem, most of the N translocated into the plant in the organic form is likely to be depleted of 15N compared with N source. Because the main site of NO3- reduction for each species is dependent on the N status of the plant, the relationship between the δ15N of roots and shoots may vary for the same plant species according to the external N availability and for the same external conditions according to plant species (Figure 1) and phenological stage. Thus, under NO3- nutrition, there was no significant effect of the organ on the natural isotopic abundance of N (Table 2).
In contrast, the shoots of NH4+-fed plants were significantly enriched in 15N (Table 2) relative to the roots (see Additional file 3, tables S2 and S3). Among the various external factors, the source and concentration of N have an effect on stomatal NH3 emissions [36,37]. Thus, losses of NH3 from the stomata take place in NH4+-fed plants at high N concentrations [38,39]. This process will favour the lighter isotope emission and enrich the plant tissue (leaf specially) in 15N because the isotopic effect of NH3 (aq) exchange with NH3 (g) has been estimated to be 1.005. In other words, NH3 (g) is enriched in 14N by ~ 5 ‰ relative to NH3 (aq) [21]. In agreement with this reasoning, the nitrogen isotopic fractionation against 15N caused by volatilisation of NH3 has been shown in the aerial part of wheat plants [40]. Hence, in light of the N dynamics inside the plant, it is difficult to explain how the whole NH4+-fed plants can be depleted of the heavier N isotope.
N Isotopic fractionation and NH4+ toxicity mechanisms
Some studies have examined isotopic fractionation in plants grown with NH4+ nutrition under LATS controlled conditions, and contrasting results were obtained. For instance, isotopic fractionation in NH4+-fed (4.6 mM) Pinus sylvestris ranged from 0.9 to 5.8 [42]. For Oryza sativa L., the fractionation was dependent on the external NH4+ concentration, which ranged from -7.8 to -18 ‰ when the external NH4+ concentrations ranged from 0.4 to 7.2 mM [7]. In agreement with this latter trend in rice, our results showed that the fractionation tended to increase with the N concentration for most of the plant species studied under NH4+ nutrition (Figure 1, Table 2 and Additional file 3, tables S2 and S3). Hence, the organ δ15N values were closer to the source δ15N in low N availability conditions (at low N concentrations) for NH4+-fed plants [6] (Figure 1). Likewise, if the N concentration increases, the amount of substrate becomes unlimited and the isotope effect is observed [6] (Figure 1). However, the δ15N values from NO3--fed plants were almost insensitive to the N concentration (Figure 1 and Table 2), which agrees with experiments in rice [7]. Thus, even if organic N compounds were lost, this phenomenon would not be sufficient to explain the plant depletion of 15N as the assimilatory enzymes discriminate against the heavier N isotope [4].
If we consider the mechanisms of NH4+ toxicity, a recent study examined the causes of the primary root growth suppression by NH4+ nutrition [43]. It demonstrated that the NH4+-mediated inhibition of primary root growth is mostly due to a repression of cell elongation rather than cell division inhibition. Moreover, these authors linked this phenomenon to two mechanisms of NH4+ toxicity [44-46]. First, the futile plasma transmembrane cycle of NH4+ uptake and efflux through cell roots, with the subsequent high energetic cost, might explain the different tolerances exhibited by different plant species when NH4+ is supplied at high concentrations [44]. Hence, Li et al. [43] showed that NH4+ efflux is induced by high NH4+ concentrations in the Arabidopsis root elongation zone, which coincides with the inhibitory effect of NH4+ on cell length and primary root elongation. They also associated the NH4+-induced efflux in the root elongation zone with the enzyme GDP-mannose pyrophosphorylase (GMPase). The implication of GMPase in the NH4+ sensitivity of Arabidopsis roots represents the second (and last) mechanism of NH4+ toxicity [45,46]. Therefore, Li et al. pointed out that GMPase regulates the process of root NH4+ efflux, and showed that GMPase mutants had a higher net NH4+ efflux (1.8 fold) in the root elongation zone relative to wild-type Arabidopsis plants [43].
In our study, we did not determine the net NH4+ fluxes, but previous findings demonstrated that the root NH4+-induced efflux occurs in a broad range of plant species and are more or less significant depending on the NH4+ sensitivity of the plant species [44]. So, the mechanism of NH4+ ejection from the root cell, if it occurred, would significantly contribute towards the global 15N depletion of the NH4+-fed plants through a discriminatory mechanism against the lighter N isotope (i.e., favouring the 15N isotope). However, the fractionation mechanism against 14N is a thermodynamically unlikely event due to the differences in the physical and chemical properties of isotopic compounds. Thus, the heavier molecules have a lower diffusion velocity, and generally, the heavier molecules have higher binding energies [47].
Furthermore, the relative abundances of the stable isotopes in living organisms depend on the isotopic composition of their food sources and their internal fractionation processes [48]. Thus, taking into account the development of the relative abundance of the stable isotopes across the food web, internal fractionation generally leads to an enrichment of the heavier isotope in consumers relative to their diet [48]. The negative values for the natural isotopic fractionation observed in NH4+-fed plants must therefore be related to the chemical properties of the NH4+ ion in solution and the NH4+/NH3-uptake mechanisms. When NH4+ is applied as the only N source, the NH4+ and NH3 forms are present in the nutrient solution. However, these molecular and ionic forms do not have exactly the same natural isotopic signatures because there is a 1.020 thermodynamic isotope effect between NH3 (aq) and NH4+ (aq), such that NH3 (aq) is depleted of 15N by 20 ‰ relative to NH4+ (aq) [20]. To interpret the negative values of the whole plant δ15N, we hypothesise that a portion of the N enters the root as NH3, which leads to the depletion of the heavier isotope in the plant.
A proposal that relates N isotopic fractionation and NH4+ toxicity mechanism
When the whole plant is considered and NH4+ is the only available N source, the isotopic N signature of the plant would therefore be related to the amount of NH3 transported. Using the ratio between the biomass accumulations of NH4+- and NO3--fed plants as an indicator of NH4+ tolerance [22], we can relate NH4+ tolerance to the root δ15N of NH4+-fed plants. Plants that were less tolerant to NH4+ nutrition were the most depleted of the heavier isotope (Figure 2; Additional file 3, table S1), and presumably the uptake of NH3 was more important in those plants. According to our hypothesis, lettuce, spinach and tomato were the most sensitive to NH4+ nutrition of the plant species studied (Figure 2 and Additional file 3 table S1). Moreover, the "plant sensitivity to NH4+ nutrition" variable, expressed as the ratio of the biomasses of NH4+/NO3--fed plants, can explain 69% of the root δ15N variation observed in the dataset (Figure 2). Hence, although the fraction of NH3 in solution at pH 6-7 is very small (approx. 0.07-0.6%), the transient alkalinisation of the cytosol reported after NH3 uptake can be attributed to rapid diffusion of NH3 across the plasma membrane and its subsequent protonation within the cytosol [49,50]. The increased NH3 concentration will therefore consume the established Δ μH+, thereby contributing to a higher energetic cost to balance it. This may also be related to membrane depolarisation events observed after NH4+ application in NH4+-tolerant plants or to the higher energetic burden reportedly required to maintain membrane potentials in NH4+-sensitive species [44].
In order to test the viability of our hypothesis, short-term experiments were performed using two plant species that showed different tolerance to NH4+ nutrition at two pHs; a slightly acidic one pH (6.0), and an alkaline pH (9.0) which favoured the neutral form (NH3). Spinach (sensitive; Figure 2) and pea (tolerant; Figure 2) receiving 15NH4+ as the only N source showed that 2 h was sufficient to demonstrate that N uptake was faster in plants transferred from pH 6-7 to pH 9 than in those transferred from pH 6-7 to pH 6 (Figure 3B and 3C). The differences shown in shoot 15N contents between pHs and species (Figure 3A and 3C) suggest interesting dissimilarities in uptake and transport systems, linked to the degree of sensitivity/tolerance of these species to NH4+. This finding may be related to the different distribution of incorporated NH4+ reported in both species (shoot in spinach and root in pea plants) [51]. In this work it is proposed that differences in the site of NH4+ assimilation is linked to NH4+ tolerance. On the other hand, taking into consideration the N absorbed by the plants and the dissociation constant of the ionic form, most of the difference in N uptake at pH 6 and pH 9 is likely related to a higher proportion of NH3 under alkaline conditions (Figure 4B and 4D). These observations are consistent with the hypothesis that the NH3 form is involved in the uptake of reduced N by the cell in the LATS activity range.
Physiological studies have indicated that transport of NH3 across membranes occurs and may become significant at high NH4+ concentrations or at high pHs [16]. Indeed, NH3 transport has been described as a function of the HATS in Escherichia coli [52,53]. The first hints of protein involvement in plant NH3 transport came from nodules of legume rhizobia symbiosis and restoration of NH3 transport in yeast mutants complemented with three aquaporins from wheat roots. This complementation was found to be pH-dependent, with progressively better growth being observed at increasing pH, and was thus indicative of transport of neutral NH3 rather than charged NH4+[54]. Recently, the transport of NH3, rather than NH4+, by the AtAMT2 transporter was also shown [14,15]. Furthermore, the incubation of an illuminated suspension of mesophyll cell protoplasts from Digitaria sanguinalis, which had been preloaded with a pH-specific fluorescent probe, with 20 mM of NH4Cl showed rapid alkalinisation of the cytosolic pH [55], which may be explained on the basis of NH3 uptake. Further examples of transient alkalinisation of the cytosol have been reported in root hair cells of rice and maize after the addition of 2 mM NH4+ to a previously N-free bathing solution [50], which indicates that NH3 permeates cells [50,55]. This process will contribute to consumption of the established Δ μH+ and agrees with the hypothesis that the toxic effect of NH3 is associated with intracellular pH changes [44]. All of these studies together demonstrate that NH4+ may permeate cells in its neutral form (NH3) and therefore tends to increase cytosolic pH.
The level of GMPase activity has been proposed to be a key factor in the regulation of Arabidopsis sensitivity to NH4+[45]. Interestingly, these authors showed that GMPase activity is seemingly regulated by pH. Using in vitro experiments with recombinant wild-type and GMPase mutant proteins, GMPase activity was decreased by alkaline pH. In plants cultured on NO3-, a considerable decrease in GMPase activity was observed with increasing pHs from 5.7 to 6.7 of the plant growth medium. Moreover, plants grown in the presence of NH4+ showed lower GMPase activities relative to that shown by NO3--fed plants at the same external pH [45]. This could indicate that the transient cytosolic alkalinisation previously reported in NH4+ uptake (reviewed in [56]) may trigger the decrease of GMPase activity stimulated by NH4+ provision [45]. In fact, Qin et al. have hypothesised that this cytosolic alkalinisation may play a role in the inhibition of GMPase activity by NH4+[45].
Thus, in view of our results and these previous findings, we propose the existence of a mechanism that recruited the NH4+ in the molecular form (NH3) under LATS conditions, which would cause in parallel depletion in the heavier N isotope, as well as an alkalinisation of cytosol in root cells. It would trigger a decrease in GMPase activity and the subsequent downstream molecular events, i.e., deficiencies in protein N-glycosylation, the unfolded protein response and cell death in the roots [45], which are important for the inhibition of Arabidopsis growth by NH4+ application [45]. Moreover, reductions in cellulose biosynthesis, cell wall stability and cell viability shown in a null mutant of GMPase (cty1-2) are the result of an N-glycosylation deficiency [57]. The disturbance of cell wall biosynthesis caused by the decreased GMPase activity under NH4+ nutrition and the subsequent protein N-glycosylation deficiency [45] has been related to the NH4+ flux [43]. Our proposal, therefore, is compatible with the two related NH4+-toxicity mechanisms [43] proposed by Britto et al. [44] and Qin et al. [45].
On the other hand, several reports have suggested that K+ channels are an important component of the LATS for NH4+[58]. It has been shown that NH4+ produces similar, but weaker, currents compared to K+ in intact root cells or in protoplasts ([10] and references therein) and that a single amino acid substitution in a K+ channel can dramatically increase NH4+ permeability [59]. Indeed, a broad range of K+ channels have been shown to be permeable to NH4+[8,60], and most allow significant levels of NH4+ to permeate [31]. Alternatively, it might be expected that some channels and transporters poorly distinguish between K+ and NH4+. In fact, it has been shown that the futile NH4+ cycling, which was shown in NH4+-sensitive plants under NH4+ nutrition [44], is alleviated by elevated K+ levels and that low-affinity NH4+ transport is mediated by two components, one of which is K+ sensitive and the other is K+ independent [31]. As NH4+ transport through K+ channels would be in the ionic form, no 15N fractionation is expected to be associated with it.
Conclusions
Based on the results presented herein, we show that plants fed with NH4+ as the sole source of N are depleted of 15N in a concentration-dependent manner. We have observed a relationship between 14N/15N fractionation and the sensitivity of plants to NH4+ nutrition. We show that the most sensitive plants have the most negative δ15N values. Moreover, our data of 15N uptake at pH 6.0 and 9.0 together with other data found in the literature indicate that part of N uptake by the plant may occurs as NH3. Accordingly, current data has suggested that the LATS for NH4+ has at least two components. One component is involved in the transport of NH3 and would therefore indirectly discriminate against the heaviest N stable isotope due to the balance between ionic and molecular forms in the nutrient solution. This transport mechanism could correspond to the K+-independent component of NH4+ transport suggested previously [31]. The second component would be an NH4+-specific transport system, which interferes with K+ transport and does not discriminate against 15N. We propose that the negative values of δ15N observed in hydroponically grown plants are related to this NH3 uptake, which imprints a permanent N signature (δ15N) under steady-state external N conditions and contributes to the current understanding of the origin of NH4+ toxicity.
Authors' contributions
IA participated in experimental design and its coordination, carried out the short-term 15N labelling experiments and participated in isotopic signature experiments, analysed the data, performed the statistical analysis and wrote the paper. CC conceived of the study, carried out the isotopic signature experiments, analysed the data and wrote the manuscript. JFM conceived of the study and wrote the manuscript. MBG-M participated in the isotopic signature experiments and helped to draft the paper. CG-O performed the statistical analysis. CG-M carried out the isotopic signature experiments. MAM-L participated in isotopic signature experiments and helped to draft the paper. PMA-T conceived of the study, designed and coordinated the experiments, conducted the short-term 15N labelling and the isotopic signature experiments and helped to write the manuscript. All authors have read and approved the final manuscript.
Supplementary Material
Control measures of external pH in all short-term experiments. Initial and final pH values of the external solutions at pH 6 (panels A, C and E) and 9 (panels B, D and F).
Calculations appendix. The calculations used to achieve these results have been added to the manuscript to clarify the discussion and conclusions of this work. A) Calculations for obtaining the 15N content as μmol 15N·100 g-1 DW from the δ15N (‰) and total N content (% N). B) The 15N contents from the external NH4+ and NH3 were calculated using the Henderson-Hasselbalch equation to take into account the external pH conditions.
Natural isotopic signature data. Tables with plant biomass ratios of plants fed with NH4+/NO3- as the sole N source and δ15N values in shoots and roots of plants fed with NH4+ or NO3- as the sole N source.
Contributor Information
Idoia Ariz, Email: idoia.ariz@unavarra.es.
Cristina Cruz, Email: ccruz@fc.ul.pt.
Jose F Moran, Email: jose.moran@unavarra.es.
María B González-Moro, Email: mariabegona.gonzalez@ehu.es.
Carmen García-Olaverri, Email: mameng@unavarra.es.
Carmen González-Murua, Email: carmen.gmurua@ehu.es.
Maria A Martins-Loução, Email: maloucao@reitoria.ul.pt.
Pedro M Aparicio-Tejo, Email: pmapariciotejo@unavarra.es.
Acknowledgements
The authors wish to thank to Gustavo Garijo for technical assistance. This work was supported by the Spanish MICIIN (grant nos. AGL2006-12792-CO2-01 and 02 and AGL2009- 13339-CO2-01 and 02 [to P.A.-T. and C.G.M.] and AGL2007-64432/AGR [to J.F.M.]), by the Portuguese FCT (PTDC/BIA- BEC/099323/2008) and by the Basque Government IT526-10. IA was supported by a postdoctoral Fellowship from the Public University of Navarre. Technical support was provided by SGIker to the UPV/EHU researchers.
References
- Owen AG, Jones DL. Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol Biochem. 2001;33(4-5):651–657. doi: 10.1016/S0038-0717(00)00209-1. [DOI] [Google Scholar]
- Britto DT, Kronzucker HJ. NH4+ toxicity in higher plants: A critical review. J Plant Physiol. 2002;159(6):567–584. doi: 10.1078/0176-1617-0774. [DOI] [Google Scholar]
- Handley LL, Raven JA. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant, Cell Environ. 1992;15(9):965–985. doi: 10.1111/j.1365-3040.1992.tb01650.x. [DOI] [Google Scholar]
- Werner RA, Schmidt H. The in vivo nitrogen isotope discrimination among organic plant compounds. Phytochemistry. 2002;61(5):465–484. doi: 10.1016/S0031-9422(02)00204-2. [DOI] [PubMed] [Google Scholar]
- Denton TM, Schmidt S, Critchley C, Stewart GR. Natural abundance of stable carbon and nitrogen isotopes in Cannabis sativa reflects growth conditions. Funct Plant Biol. 2001;28(10):1005–1012. doi: 10.1071/PP01066. [DOI] [Google Scholar]
- Evans RD. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001;6(3):121–126. doi: 10.1016/S1360-1385(01)01889-1. [DOI] [PubMed] [Google Scholar]
- Yoneyama T, Matsumaru T, Usui K, Engelaar WMHG. Discrimination of nitrogen isotopes, during absorption of ammonium and nitrate at different nitrogen concentrations by rice (Oryza sativa L.) plants. Plant Cell Environ. 2001;24(1):133–139. doi: 10.1046/j.1365-3040.2001.00663.x. [DOI] [Google Scholar]
- Forde BG, Clarkson DT. Nitrate and ammonium nutrition of plants: Physiological and molecular perspectives. Adv Bot Res. 1999;30:1–90. [Google Scholar]
- Li B-, Merrick M, Li S-, Li H-, Zhu S-, Shi W-, Su Y-. Molecular basis and regulation of ammonium transporter in rice. Rice Sci. 2009;16(4):314–322. doi: 10.1016/S1672-6308(08)60096-7. [DOI] [Google Scholar]
- Loqué D, von Wirén N. Regulatory levels for the transport of ammonium in plant roots. J Exp Bot. 2004;55(401):1293–1305. doi: 10.1093/jxb/erh147. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Von Wiren N, Frommer WB. Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J Biol Chem. 2002;277(16):13548–13555. doi: 10.1074/jbc.M200739200. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Wilken S, Wu B, Jost W, Obrdlik P, El Bakkoury M, Marini A-, André B, Hamacher T, Boles E, Von Wirén N, Frommer WB. Homo- and Hetero-oligomerization of ammonium transporter-1 NH4+ uniporters. J Biol Chem. 2003;278(46):45603–45610. doi: 10.1074/jbc.M307424200. [DOI] [PubMed] [Google Scholar]
- Mayer M, Ludewig U. Role of AMT1;1 in NH4+ acquisition in Arabidopsis thaliana. Plant Biol. 2006;8(4):522–528. doi: 10.1055/s-2006-923877. [DOI] [PubMed] [Google Scholar]
- Neuhäuser B, Dynowski M, Ludewig U. Channel-like NH3 flux by ammonium transporter AtAMT2. FEBS Lett. 2009;583(17):2833–2838. doi: 10.1016/j.febslet.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Guether M, Neuhäuser B, Balestrini R, Dynowski M, Ludewig U, Bonfante P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009;150(1):73–83. doi: 10.1104/pp.109.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt SM, Udvardi MK. Structure, function and regulation of ammonium transporters in plants. Biochim Biophys Acta Biomembr. 2000;1465(1-2):152–170. doi: 10.1016/S0005-2736(00)00136-X. [DOI] [PubMed] [Google Scholar]
- Soupene E, He L, Yan D, Kustu S. Ammonia acquisition in enteric bacteria: Physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc Natl Acad Sci USA. 1998;95(12):7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Cobena A, Misselbrook TH, Arce A, Mingot JI, Diez JA, Vallejo A. An inhibitor of urease activity effectively reduces ammonia emissions from soil treated with urea under Mediterranean conditions. Agric Ecosyst Environ. 2008;126(3-4):243–249. doi: 10.1016/j.agee.2008.02.001. [DOI] [Google Scholar]
- Harrison R, Webb J. A review of the effect of N fertilizer type on gaseous emissions. Adv Agron. 2001;73:65–108. [Google Scholar]
- Hermes JD, Weiss PM, Cleland WW. Use of nitrogen-15 and deuterium isotope effects to determine the chemical mechanism of phenylalanine ammonia-lyase. Biochem. 1985;24(12):2959–2967. doi: 10.1021/bi00333a023. [DOI] [PubMed] [Google Scholar]
- Waser NAD, Harrison PJ, Nielsen B, Calvert SE, Turpin DH. Nitrogen isotope fractionation during the uptake and assimilation of nitrate, nitrite, ammonium, and urea by a marine diatom. Limnol Oceanogr. 1998;43(2):215–224. doi: 10.4319/lo.1998.43.2.0215. [DOI] [Google Scholar]
- Cruz C, Bio AFM, Domínguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Martins-Loução MA. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta. 2006;223(5):1068–1080. doi: 10.1007/s00425-005-0155-2. [DOI] [PubMed] [Google Scholar]
- Cruz C, Soares MIM, Martins-Loucção MA, Lips SH. Nitrate reduction in carob (Ceratonia siliqua L.) seedlings. New Phytol. 1991;119:413–419. doi: 10.1111/j.1469-8137.1991.tb00041.x. [DOI] [Google Scholar]
- Domínguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Cruz C, Martins-Loução MA, Moran JF. Nitrogen nutrition and antioxidant metabolism in ammonium-tolerant and - sensitive plants. Physiol Plant. 2008;132(3):359–369. doi: 10.1111/j.1399-3054.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Ariz I, Esteban R, García-Plazaola JI, Becerril JM, Aparicio-Tejo PM, Moran JF. High irradiance induces photoprotective mechanisms and a positive effect on NH4+ stress in Pisum sativum L. J Plant Physiol. 2010;167(13):1038–1045. doi: 10.1016/j.jplph.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Cruchaga S, Artola E, Lasa B, Ariz I, Irigoyen I, Moran JF, Aparicio-Tejo PM. Short term physiological implications of NBPT application on the N metabolism of Pisum sativum and Spinacea oleracea. J Plant Physiol. 2011;168(4):329–336. doi: 10.1016/j.jplph.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Ariz I, Artola E, Asensio AC, Cruchaga S, Aparicio-Tejo PM, Moran JF. High irradiance increases NH4+ tolerance in Pisum sativum: Higher carbon and energy availability improve ion balance but not N assimilation. J Plant Physiol. 2011;168(10):1009–1015. doi: 10.1016/j.jplph.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Labhilili M, Joudrier P, Gaultier M. Characterization of cDNA encoding Triticum durum dehydrins and their expression patterns in cultivars that differ in drought tolerance. Plant Sci. 1995;112:219–230. doi: 10.1016/0168-9452(95)04267-9. [DOI] [Google Scholar]
- Levene H. In: Contributions to Probability and Statistic: essays in honor of Harold Hotelling. Ingram Olkin ea, editor. Stanford University Press; 1960. Robust tests for equality of variances; pp. 278–292. [Google Scholar]
- Dunnett CW. Pairwise multiple comparisons in the unequal variance case. J Am Statist Assoc. 1980;75(372):796–800. doi: 10.2307/2287161. [DOI] [Google Scholar]
- Szczerba MW, Britto DT, Balkos KD, Kronzucker HJ. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and - insensitive components of NH 4+ transport. J Exp Bot. 2008;59(2):303–313. doi: 10.1093/jxb/erm309. [DOI] [PubMed] [Google Scholar]
- Gierth M, Mäser P. Potassium transporters in plants - Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007;581(12):2348–2356. doi: 10.1016/j.febslet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Kohl DH, Shearer G. Isotopic fractionation associated with symbiotic N2 fixation and uptake of NO3- by plants. Plant Physiol. 1980;66:51–56. doi: 10.1104/pp.66.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb KJ, Evans RD. Influence of nitrogen source and concentration on nitrogen isotopic discrimination in two barley genotypes (Hordeum vulgare L.) Plant Cell Environ. 2003;26(9):1431–1440. doi: 10.1046/j.1365-3040.2003.01066.x. [DOI] [Google Scholar]
- Mattsson M, Schjoerring JK. Ammonia emission from young barley plants: Influence of N source, light/dark cycles and inhibition of glutamine synthetase. J Exp Bot. 1996;47(297):477–484. [Google Scholar]
- Mattsson M, Husted S, Schjoerring JK. Influence of nitrogen nutrition and metabolism on ammonia volatilization in plants. Nutr Cycl Agroecosyst. 1998;51(1):35–40. doi: 10.1023/A:1009796610912. [DOI] [Google Scholar]
- Schjoerring JK, Husted S, Mäck G, Nielsen KH, Finnemann J, Mattsson M. Physiological regulation of plant-atmosphere ammonia exchange. Plant Soil. 2000;221(1):95–102. doi: 10.1023/A:1004761931558. [DOI] [Google Scholar]
- Massad R-, Tuzet A, Loubet B, Perrier A, Cellier P. Model of stomatal ammonia compensation point (STAMP) in relation to the plant nitrogen and carbon metabolisms and environmental conditions. Ecol Model. 2010;221(3):479–494. doi: 10.1016/j.ecolmodel.2009.10.029. [DOI] [Google Scholar]
- O'Deen WA. Wheat volatilized ammonia and resulting nitrogen isotopic fractionation. Agron J. 1989;81:980–985. doi: 10.2134/agronj1989.00021962008100060027x. [DOI] [Google Scholar]
- Yoneyama T, Kamachi K, Yamaya T, Mae T. Fractionation of nitrogen isotopes by glutamine synthetase isolated from spinach leaves. Plant Cell Physiol. 1993;34:489–491. [Google Scholar]
- Högberg P, Högberg MN, Quist ME, Ekblad A, Näsholm T. Nitrogen isotope fractionation during nitrogen uptake by ectomycorrhizal and non-mycorrhizal Pinus sylvestris. New Phytol. 1999;142(3):569–576. doi: 10.1046/j.1469-8137.1999.00404.x. [DOI] [Google Scholar]
- Li Q, Li B-, Kronzucker HJ, Shi W-. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ. 2010;33(9):1529–1542. doi: 10.1111/j.1365-3040.2010.02162.x. [DOI] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA. 2001;98(7):4255–4258. doi: 10.1073/pnas.061034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Qian W, Wang W, Wu Y, Yu C, Jiang X, Wang D, Wu P. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105(47):18308–18313. doi: 10.1073/pnas.0806168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Gouzd ZA, Steele HP, Imperio RM. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot. 2010;61(2):379–394. doi: 10.1093/jxb/erp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook WG. In: Environmental isotopes in the hydrological cycle, principes and applications IHP-V. 39. Mook WG, editor. Vol. 1. Paris: UNESCO/IAEA; 2000. Introduction: Theory, Methods and Review; pp. 31–48. [Google Scholar]
- Rothe J, Gleixner G. In: Assembly Rules and Restoration Ecology - Bridging the Gap Between Theory and Practice. Temperton VM, Hobbs RJ, Nuttle T, Halle S, editor. Island Press; 2004. Application of stable nitrogen isotopes to investigate food-web development in regenerating ecosystems; pp. 245–264. [Google Scholar]
- Kosegarten H, Grolig F, Esch A, Glüsenkamp K-, Mengel K. Effects of NH4+, NO3- and HCO3-on apoplast pH in the outer cortex of root zones of maize, as measured by the fluorescence ratio of fluorescein boronic acid. Planta. 1999;209(4):444–452. doi: 10.1007/s004250050747. [DOI] [PubMed] [Google Scholar]
- Kosegarten H, Grolig F, Wieneke J, Wilson G, Hoffmann B. Differential ammonia-elicited changes of cytosolic pH in root hair cells of rice and maize as monitored by 2',7'-bis-(2-carboxyethyl)-5 (and -6)-carboxyfluorescein-fluorescence ratio. Plant Physiol. 1997;113(2):451–461. doi: 10.1104/pp.113.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa B, Frechilla S, Aparicio-Tejo PM, Lamsfus C. Role of glutamate dehydrogenase and phosphoenolpyruvate carboxylase activity in ammonium nutrition tolerance in roots. Plant Physiol Biochem. 2002;40(11):969–976. doi: 10.1016/S0981-9428(02)01451-1. [DOI] [Google Scholar]
- Khademi S, O'Connell J III, Remis J, Robles-Colmenares Y, Miercke LJW, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: Structure of AmtB at 135 Å. Science. 2004;305(5690):1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- Zheng L, Kostrewa D, Bernèche S, Winkler FK, Li X-. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci USA. 2004;101(49):17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn TP, Møller ALB, Zeuthen T, Holm LM, Klærke DA, Mohsin B, Kühlbrandt W, Schjoerring JK. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004;574(1-3):31–36. doi: 10.1016/j.febslet.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Giglioli-Guivarc'h N, Pierre J-, Brown S, Chollet R, Vidal J, Gadal P. The light- dependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis. Plant Cell. 1996;8(4):573–586. doi: 10.1105/tpc.8.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: New views on old paradigms. Plant Cell Environ. 2005;28(11):1396–1409. doi: 10.1111/j.1365-3040.2005.01372.x. [DOI] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA. 2001;98(5):2262–2267. doi: 10.1073/pnas.051625798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopen FT, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J Exp Bot. 2010;61(9):2303–2315. doi: 10.1093/jxb/erq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumí N, Gassmann W, Cao Y, Schroeder JI. Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutant selection in yeast. J Biol Chem. 1995;270(41):24276–24281. doi: 10.1074/jbc.270.41.24276. [DOI] [PubMed] [Google Scholar]
- White PJ. The permeation of ammonium through a voltage-independent K+ channel in the plasma membrane of rye roots. J Membr Biol. 1996;152(1):89–99. doi: 10.1007/s002329900088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control measures of external pH in all short-term experiments. Initial and final pH values of the external solutions at pH 6 (panels A, C and E) and 9 (panels B, D and F).
Calculations appendix. The calculations used to achieve these results have been added to the manuscript to clarify the discussion and conclusions of this work. A) Calculations for obtaining the 15N content as μmol 15N·100 g-1 DW from the δ15N (‰) and total N content (% N). B) The 15N contents from the external NH4+ and NH3 were calculated using the Henderson-Hasselbalch equation to take into account the external pH conditions.
Natural isotopic signature data. Tables with plant biomass ratios of plants fed with NH4+/NO3- as the sole N source and δ15N values in shoots and roots of plants fed with NH4+ or NO3- as the sole N source.