Abstract
Functional neuroimaging and related neuroimaging techniques are becoming important tools for rehabilitation research. Functional neuroimaging techniques can be used to determine the effects of brain injury or disease on brain systems related to cognition and behavior and to determine how rehabilitation changes brain systems. These techniques include: functional magnetic resonance imaging (fMRI), positron emission tomography (PET), electroencephalography (EEG), magnetoencephalography (MEG), near infrared spectroscopy (NIRS), and transcranial magnetic stimulation (TMS). Related diffusion weighted magnetic resonance imaging techniques (DWI), including diffusion tensor imaging (DTI) and high angular resolution diffusion imaging (HARDI), can quantify white matter integrity. With the proliferation of these imaging techniques in rehabilitation research, it is critical that rehabilitation researchers, as well as consumers of rehabilitation research, become familiar with neuroimaging techniques, what they can offer, and their strengths and weaknesses The purpose to this review is to provide such an introduction to these neuroimaging techniques.
Over the past 25 years, techniques to image brain structure and function have offered investigators in the cognitive neurosciences and related fields unprecedented opportunities to study how human brain systems work and are connected. Indeed, the number of peer-reviewed research articles using these techniques has grown at an exponential rate during this period. Inevitably, investigators have become interested in mapping neuroplastic changes that support learning and memory using functional neuroimaging, and concomitantly, rehabilitation researchers have become interested in mapping changes in brain systems responsible for treatment effects during the rehabilitation of patients with stroke, traumatic brain injury, and other brain injury or disease. This new rehabilitation research and development arena is important because a greater understanding of how and why brain systems remap in the service of rehabilitation will lead to the development of better treatments.
At the same time that functional neuroimaging methods have been developed, new structural neuroimaging techniques also have been added to the tool box of rehabilitation researchers. For example, diffusion tensor imaging (DTI) and related magnetic resonance (MR) techniques offer the ability to assess human white matter pathways in vivo. Not only can these techniques be used to estimate the integrity of a given volume of white matter, but they also can be used to trace fiber tracts within the brain. This latter development is exciting because most of what we know (or at least thought we knew) about the connections of the human cortex has actually come from research on nonhuman primates, leaving questions especially about the phylogenetically newer portions of the cortex. In the rehabilitation arena, a better understanding of how the brain’s connections are damaged could help us to predict what treatments are best for different research subjects and, eventually, might be useful in selecting the best treatment strategies for individual patients.
Because the newer functional and structural neuroimaging techniques have enormous implications for rehabilitation research and development, it is highly desirable that rehabilitation researchers be able to evaluate the usefulness of the techniques for rehabilitation research and that the consumers of rehabilitation research (i.e., clinicians and researchers) be able to evaluate research findings that have applied the techniques. The purpose of this article is to discuss functional and structural imaging techniques used in rehabilitation research. We will not cover routine clinical MR or x-ray computerized tomography (CT) images. Rather, we will concentrate on a variety of techniques used most frequently, though not necessarily exclusively, in research settings. The article will consist of two main sections: (1) Because of the extraordinary versatility or MR techniques, the large number of MR techniques will be discussed first. (2) Subsequently, other functional neuroimaging techniques will be discussed, including: Positron Emission Tomography (PET), Magnetoencephalography/Magnetic Source Imaging (MEG/MSI), Near Infrared Spectroscopy (NIRS), Transcranial Magnetic Stimulation (TMS), and Electroencephalography/Evoked Potentials (EEG/EPs). For each imaging modality, we will give a brief explanation of the modality, its uses/potential uses in rehabilitation research, its strengths and limitations, and an example of research in the area.
FUNCTIONAL IMAGING
Functional MRI (fMRI) is but one form of functional imaging. Before we discuss fMRI or the other forms of functional imaging covered after the discussion of MRI techniques, we must make several introductory comments about functional imaging. The working knowledge of functional imaging necessary to peruse the growing literature using functional imaging in rehabilitation requires familiarity (1) with the measures on which different kinds of functional imaging rely, (2) with the goals of functional imaging, and (3) with common methodological issues.
To begin, functional imaging attempts to measure neuronal activity. Some techniques measure neuronal activity relatively directly by measuring changes in electrical activity as clusters of neurons become active (electroencephalography: EEG) or by measuring the changes in magnetic fields related to electrical activity changes (magnetoencephalography: MEG). While these techniques measure activity of neuronal clusters in real time, they do have limitations. One such limitation is the difficulty measuring changes in electrical activity/magnetic fields in deep brain structures.
When neurons become active, their need for energy drives changes in metabolism. Hence, another form of functional imaging measures these metabolic changes as a proxy for neuronal activity. Regional metabolic changes have been measured with positron emission tomography (PET) by tagging fluorodeoxyglucose (FDG) with a positron emitting isotope (18F) or by using a positron emitting isotope of oxygen (15O). In practice, it takes a relatively long time (several minutes) to absorb enough labeled glucose to measure metabolic activity, and measuring oxygen metabolism has proven to be elusive.
Thus, many functional imaging techniques today do not rely on direct measures of metabolism. Rather, they rely on measuring hemodynamic responses. The increase in metabolic activity that supports regional increases in neuronal activity drives increases in blood flow to deliver the fuel for metabolic activity and carry away unneeded byproducts of metabolism. One PET method frequently used to measure changes in blood flow is to tag water with a positron emitting oxygen isotope (H215O) and inject it intravenously. It takes at least 40 seconds to accumulate enough positron emitting events and at least two scans under different conditions to measure changes in cerebral blood flow between the conditions. There are other ways to capture regional hemodynamic responses. For example, it has long been known that oxygenating blood changes its visible and infrared absorption spectrum. These changes can be measured near the surface of the brain with near infrared spectroscopy (NIRS). When a brain region becomes active, the increase in blood flow overshoots the metabolic need of the tissue for oxygen, and even though oxygen consumption increases, there is a higher level of blood oxygenation in blood leaving an active region than when it leaves that same region at rest. fMRI also relies on these changes in blood oxygenation to measure hemodynamic responses related to neuronal activity. For a relatively brief event of a second or two, the hemodynamic response takes about 12 to 15 seconds to resolve. While this is better temporal resolution than H215O, it is still relatively far from an instantaneous measure of neuronal activity.
Each of the functional imaging techniques mentioned above will be covered in greater detail below, with examples. The reader wishing to read further about the history of functional brain imaging is referred to a chapter by (Raichle, 2000a).
In general, functional imaging can be used in two ways. The first is to look at baseline levels of brain activity. In the early days of PET, 18F-FDG frequently was used to measure baseline regional cerebral metabolism. For example, Metter and his colleagues (Metter, Kempler, Jackson, Mazziotta, & Phelps, 1989) measured cerebral metabolism in stroke patients with aphasia, and they defined regions outside the area of infarcts that showed decreased metabolism, which helped explain behavioral deficits. More recently, MRI has been used to measure baseline cerebral perfusion. For example, Love and her colleagues (T. Love, D. Swinney, E. Wong, & R. Buxton, 2002) found they could explain the neural basis of reading deficits in a patient with left hemisphere lesion when in addition to structural MRI, they used arterial spin labeling (ASL), an MRI technique that allowed them to image hypoperfusion in the left inferior parietal lobule. This region did not show a lesion on standard structural images. While useful, such measures of baseline brain function do not define what brain areas are actually responsible for cognitive and behavioral activities. Over the past twenty years, functional imaging has been more commonly used to image regional changes in brain function that occur as a result of engaging in specific activities. Thus, the second way in which functional imaging has been used is to define regions that become active during specific behaviors and cognitive activities. At its simplest level, this form of imaging compares brain activity during contrasting states to define brain systems responsible for behavior. As examples, this form of functional imaging has been used to determine changes in motor cortex maps during rehabilitation (Dobkin, Firestine, West, Saremi, & Woods, 2004) or to map changes in brain systems responsible for language during rehabilitation (Breier et al., 2004; Meinzer et al., 2008). Most of our discussion on functional neuroimaging will cover this latter type of functional imaging, where brain regions responsible for specific behaviors are mapped.
As mentioned above, mapping brain regions responsible for cognitive or behavioral functions involves comparing images from two or more states. There is always a baseline or control image or state from which changes can be defined. For example, in order to map areas of cortex responsible for moving a limb, images acquired during movement are compared to images during which no movement is made. On the surface, the idea of comparing two or more images to derive a map of regions responsible for a specific kind of behavior seems relatively simple. In practice, it can be quite complex. One strategy for doing so involves taking a complex cognitive task with several components and comparing it to a baseline/control task with all of the same elements except the one of interest. Peck and his colleagues (Peck et al., 2004) showed one of the potential problems in using this strategy. In an fMRI experiment with neurologically normal subjects, they wished to isolate processes related to ordering the words in a sentence (i.e., syntax). Subjects were shown a picture depicting a subject, an action performed by the subject, and a recipient of the action. The process of generating a sentence involved not only ordering the words in the sentence correctly but also retrieving the proper words for the items pictured. Picture naming could be used as the control task for sentence generation since it included all of the elements of the sentence generation task except for ordering of words. However, when this was tried, Broca’s area, which has been shown to be involved in syntax in both lesion and functional imaging studies, did not show any activity. This is probably because Broca’s area is involved in word-finding processes as well as in syntax, although it could also be because these types of subtle difference comparisons result in changes in the BOLD fMRI signal below the detection or noise threshold. Hence, when two cognitive tasks use the same neural component for different kinds of processing, it is not a good idea to use one as a baseline or control task for the other. When these investigators used passive viewing of nonsense object pictures as a baseline task, activity in Broca’s area was visible. In another study with neurologically normal subjects, Newman and her colleagues (Newman, Twieg, & Carpenter, 2001) demonstrated greater extent of activity in language eloquent cortex when phoneme discrimination was compared to a resting baseline than when the baseline task was either tone discrimination or passive listening to the same stimuli as used in phoneme discrimination. Damage to the neural components of systems responsible for behavior, and especially cognitive functions like language, may produce unpredictable recruitment of undamaged structures to accomplish relevant tasks, even though such areas are not normally recruited. Hence, cognitively similar tasks are more likely to recruit overlapping areas in brain damaged subjects than in neurologically normal subjects. This reasoning suggests that caution should be used regarding the use of cognitively similar processes as baseline and experimental tasks in individuals with brain damage. Hence, selection of simpler baseline tasks may useful in functional imaging studies of rehabilitation. For readers wishing to explore the issue of baseline tasks more completely, Petersen and his colleagues (Petersen, van Mier, Fiez, & Raichle, 1998) have provided an excellent discussion of the matter.
Another important issue in studies of rehabilitation with brain damaged populations is mapping activity around lesions. An important issue in studies of aphasia is whether perilesional structures in the left hemisphere or structures in the previously nondominant right hemisphere are responsible for recovery of function or the effects of rehabilitation. This issue also has been addressed in studies of the motor system. In studies of subjects without well-demarcated areas of brain damage, the most frequently used analytic techniques involve deforming images of individual subjects into a common atlas space and averaging images across subjects to determine where significant activity differences between tasks or groups exist. A key assumption in this type of analysis is that tissue examined in any particular voxel for one subject corresponds to the tissue for that voxel in atlas space for any other subject in the sample. (Voxels are the basic 3-dimensional volume units containing the quantitative data from which functional images are made). The problem with this assumption for patients with strokes (ischemic or hemorrhagic) is that the lesions vary considerably in shape, size, and location. The same voxel that is occupied by perilesional activity in one subject may be occupied by lesion in other subjects, and combining images across subjects may average away important perilesional activity. Thus, when examining activity in the lesioned hemisphere is important, another data analysis approach must be used.
These are but a few examples of the complexities in functional neuroimaging research. In addition to these more general challenges, each functional imaging technique has its limitations and problems, some of which will be discussed in the following sections. However, it is difficult in this survey of functional neuroimaging to cover all of the difficulties that arise in research, or indeed in clinical applications. Instead, we will endeavor to cover the most commonly encountered problems with each technique.
MAGNETIC RESONANCE IMAGING AND SPECTROSCOPY
Basic Principles of Magnetic Resonance Imaging and Spectroscopy
Since a number of the techniques for brain imaging discussed in this review rely on magnetic resonance technology, it is worth some space to describe a few basic principles of magnetic resonance imaging and spectroscopy. Nuclei of atoms are comprised of protons and neutrons. Isotopes which have an odd number of either protons or neutrons have a non-zero spin and an associated nuclear magnetic moment (i.e., nuclear magnetism of specific strength and direction); those with an even number of both protons and neutrons have no magnetic moment and are not observable with nuclear magnetic resonance (NMR, often shortened to MR).
Nuclear magnets can align parallel or anti-parallel to the applied magnetic field, called B0. Since slightly more align with the field than against, a net macroscopic nuclear magnetization M exists. As a result of the property of spin, nuclear magnets have angular momentum causing them to precess around an applied magnet field (B0). Precession can be thought of as a wobbling of the magnetic moment around the magnetic field (B0), similar to the way a toy top both spins and precesses about its gravitational vector. The frequency of precession in a magnetic field of a specific strength is called the Larmor frequency. The direction of precession is dependent upon the parallel or anti-parallel orientation of the magnetic moment about the B0 axis, with precession occurring in opposite directions for the parallel vs anti-parallel orientations.
If a radiofrequency (rf) field with the Larmor frequency (rf pulse) is briefly applied to the sample, the spins can absorb energy and flip between the energy state levels. This moves the vector M out of alignment with B0, and it precesses around B0 and creates an oscillating magnetic field which can be detected with a coil in which a sinusoidal voltage is induced. The degree to which the vector M tips out of alignment with B0 (i.e., the flip angle) is dependent on the strength and duration of the rf pulse. The signal induced in the rf coil decays with a time constant referred to as T2. T1 is a time constant which describes the time it takes M to return, or relax, to its original state of alignment with B0. In biological samples, T2 is shorter than T1. By varying image acquisition parameters, one can produce T1-weighted images (where cerebrospinal fluid has low signal relative to grey and white matter) or T2-weighted images (where cerebrospinal fluid has high signal relative to grey and white matter). Other types of weighting are possible.
In general, many rf excitation pulses must be used in data acquisitions, and the time between excitation pulses for a given slice of tissue is referred to as the repetition time, or simply TR. In functional MRI, the TR determines how quickly a single brain image is acquired. To analyze how signal changes in concert with experimental manipulations, it is necessary to acquire many brain images during a functional MRI experiment. The time from application of the rf excitation pulse to the signal acquisition is referred to as the echo time or TE. In blood oxygenation level dependent (BOLD) functional MRI (see below for explanation), the most common form of functional MRI, a relatively long TE will yield greater changes in functional signal between activation and baseline states, but also will also cause greater artifacts near air-tissue interfaces and lessened overall signal intensity. Hence, the choice of a TE in BOLD functional MRI often will be a trade-off between the need to produce a relatively strong signal and the desire to limit artifacts.
Water, the most ubiquitous hydrogen-containing molecule in the body, offers a medium for magnetic resonance imaging and resonates at a single frequency in a uniform magnetic field. However, the Larmor frequency and phase of precession can be manipulated by creating slight gradients within the main magnetic field (B0), such that the strength of the field varies with a linear relationship to distance. Within an acquisition plane (image slice), the location of a signal in one direction is determined by slight changes in frequency and in the orthogonal direction by changes in phase of the induced signal. The third spatial dimension of the image is addressed by acquiring multiple relatively thin slices. Slice selection is also accomplished by creating a gradient in the magnetic field. All of these gradients in the magnetic field are created with gradient coils separate from the superconducting coil used to generate the B0 field.
Magnetic resonance spectroscopy can be used to detect some molecules in the brain other than water. Atomic nuclei in molecules have electrons associated with them in certain specific geometric spatial locations dictated by bonding forces to neighboring atoms. These orbitals constitute effective circuits through which the electrons circulate. In an applied magnetic field, these circulating currents induce a field in opposition to B0, which shields the nucleus from the applied B0 field. This means that, for example, hydrogen atoms in different parts of a molecule have different frequencies, or chemical shifts. Signals can be localized to voxels using selective excitation for single voxel spectroscopy or chemical shift imaging (CSI).
The physics of magnetic resonance imaging and spectroscopy is much more complex than can be described in the available space. We have endeavored to provide the reader with a brief description of some of the more common concepts. The reader wishing greater detail is referred to the excellent text by (Buxton, 2001).
Functional MRI
BOLD contrast fMRI
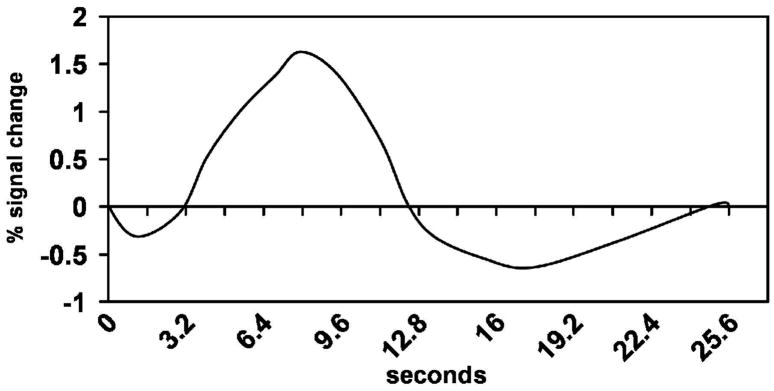
At the time of this review, BOLD contrast is the most common method for obtaining functional magnetic resonance images (fMRI). As noted above, BOLD is the acronym for blood oxygenation level dependent contrast. BOLD contrast is derived primarily from the paramagnetic properties of deoxygenated hemoglobin (deoxyhemoglobin). Paramagnetic materials tend to concentrate magnetic flux, decreasing the MR signal when a pulse sequence sensitive to magnetic susceptibility differences is used. Hence, regional cerebral increases in deoxyhemoglobin will cause decreases in the MR signal for the region. However, the underlying phenomena generating BOLD contrast are not as straightforward as it might seem at first glance. When an area of the brain becomes active, metabolism increases to support the activity. Aerobic metabolism increases oxygen extraction, and concentrations of deoxyhemoglobin transiently increase in active areas, thereby decreasing MR signal. However, rCBF then increases to deliver more oxygenated hemoglobin for the increased local metabolism. The increase in regional cerebral blood flow is in excess of what is needed to supply the active tissue with oxygen; therefore, the relative concentration of deoxyhemoglobin per unit volume in the active region decreases peaking roughly 6 seconds later due to this overcompensation in perfusion, and the MR signal increases as a result. Thus, the BOLD signal is the result of a complex interplay between changes in regional cerebral oxygen extraction, blood flow, and blood volume. A hemodynamic response triggered by an event taking less than a second to occur generates a hemodynamic response that typically shows an increase in signal lasting 10-12 sec to rise to peak and return to baseline. This positive change in signal may be followed by a negative phase of several seconds, but this phase is often considered of no interest. Figure 1 shows an example of a hemodynamic response derived from our research. The x-axis represents time, and the y-axis represents percent of change in MR signal. Other types of fMRI are covered below. The reader is referred to (Buxton, 2001) for a more detailed explanation of BOLD contrast.
Figure 1.
Hemodynamic response expressed as a percent change of the total signal. Note the positive phase of the response takes around 12 sec to resolve.
fMRI has specific advantages and disadvantages relative to other functional neuroimaging techniques. One advantage of BOLD-contrast fMRI over positron emission tomography, another form of functional imaging, is that BOLD contrast fMRI is noninvasive and does not expose the subject to radiation. The contrast in this form of fMRI is endogenous; i.e., BOLD-contrast takes advantage of existing substances in the body to produce images. PET images require the injection of a radioactive, positron-emitting contrast agent. Another advantage of fMRI over other imaging techniques is its spatial resolution. Spatial resolution refers to the degree of accuracy with which brain activity (i.e., hemodynamic responses) can be located in space. The spatial resolution of fMRI is limited by the voxel size in which images are acquired. The voxel size in fMRI images in human studies is most often driven by practical as opposed to physical considerations. Specifically, there is a trade off between spatial and temporal resolution. Acquiring data in smaller voxels takes more time than acquiring data in larger voxels because it takes more small than large voxels to cover the brain. Practically speaking, 2 mm cubed voxels are the lower limit of what it is practical to acquire in whole brain functional MR images on a conventional 1.5-3 Tesla scanner. However, it is possible to acquire smaller voxels than that, especially if whole-brain images are not needed or stronger magnets are available. Temporal resolution refers to the degree of accuracy on a temporal scale with which a functional imaging technique can describe when a neural event occurred. Temporal resolution is an advantage for fMRI over PET, but fMRI is at a disadvantage in temporal resolution compared to techniques using electroencephalography (EEG) or magnetoencephalography (MEG). PET’s ability to resolve neural events is on the order of tens of seconds, fMRI’s ability to do so is on the order of seconds, and the ability of EEG- and MEG-based techniques to do so is on the order of milliseconds. Since the cascade of events through neural systems happens on the millisecond scale, EEG- and MEG-based techniques are most likely to be useful when this kind of temporal information is needed. PET and fMRI can both image activity in deep, subcortical structures, whereas this is difficult if not impossible with EEG- and MEG-based techniques. Another advantage of fMRI is the ubiquity of MRI scanners, and most have the potential to be used for fMRI. By comparison, PET scanners are not as common, and to use PET as a functional imaging technique for cognitive processes, it is necessary to have a cyclotron and a radiochemist nearby. Another advantage for fMRI is that the same platform used to acquire functional images can be used to acquire high resolution anatomic images in the same space as functional images, so that functional images can be overlaid onto structural images to allow for precise anatomic localization. PET, MEG, EEG, TMS, and NIRS all have to rely on structural MR images and/or atlases to localize activity in the brain. Cost can be a disadvantage for fMRI if there is not a readily available instrument to acquire images. Relative to EEG-based techniques, fMRI is expensive. MRI scanners cost millions of dollars, and their maintenance can be expensive as well. However, PET and MEG-based techniques have similar costs for implementation. Other disadvantages for fMRI include the confined space in which participants must be placed, which can induce claustrophobia in susceptible participants, and the acoustic noise required to obtain scans, to which the brain inevitably reacts.
The use of fMRI in rehabilitation studies is just beginning. In locomotor rehabilitation, early evidence suggests that the amount of cortex involved in a related motor function (ankle dorsiflexion) expands as a 12-week rehabilitation program approaches its mid-point and then contracts again toward the end of treatment. It also appears that representations for the lower extremity can expand into cortex adjacent to their normal location (Dobkin et al., 2004). Findings in the aphasia rehabilitation literature are less clear, and there is a controversy regarding whether the right or the left hemisphere is the site of rehabilitation gain in premorbidly right-handed patients. A review of the literature suggests that both the right and the left hemisphere may make contributions under different circumstances, or indeed, within a single patient. The contribution of the left hemisphere may be greater in small lesions, while the contribution of the right hemisphere to treatment gains may be greater in large lesions (Crosson, McGregor et al., 2007). A number of technical obstacles must be overcome to use fMRI in aphasia treatment studies, particularly if language production is the target of treatment. The reader interested in more details is referred to the review by Crosson et al. (Crosson, McGregor et al., 2007).
Eventually, it is possible that fMRI will be used to predict what treatment will work with which patient, to determine what structures can be recruited for rehabilitation, and/or to measure brain system changes that result from rehabilitation. However, a good deal of research must be accomplished before we reach that point.
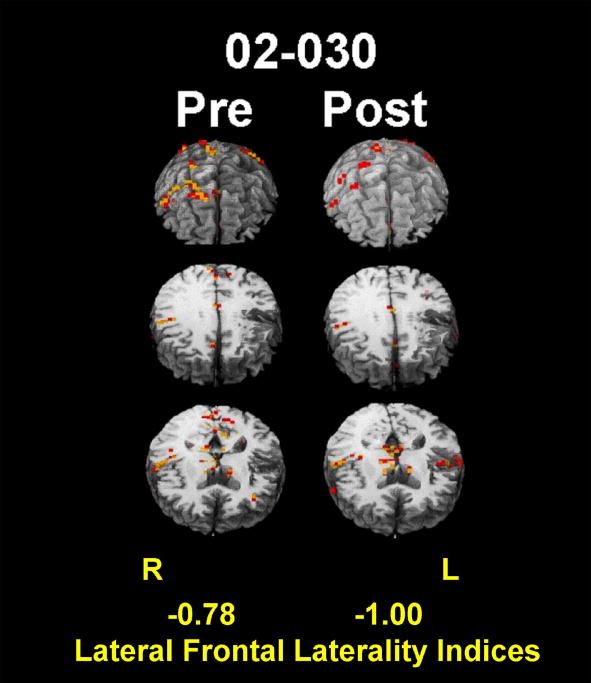
The following is an example of using fMRI in rehabilitation research from our own laboratory (Crosson et al., 2009). In 2007, we demonstrated that a treatment designed to relateralize language production mechanisms from the left to the right frontal lobe produced a faster rate of relearning of words than a similar control treatment (Crosson, Fabrizio et al., 2007). The treatment used an intention manipulation (complex movement of the left hand to initiate naming trials) because we thought that this manipulation would help to re-lateralize language production mechanisms. To assess whether or not the treatment actually re-lateralized frontal activity to the right hemisphere, we administered a category-member generation task to five patients during fMRI before and after the intention treatment. Four of the five patients improved during treatment. The four patients who improved in treatment showed a significant rightward shift in lateral frontal activity, which became more concentrated in the ventral portion of the right motor/premotor cortex or in pars opercularis just anterior to motor/premotor cortex. Although laterality of frontal activity for those four patients was not different from controls prior to treatment, it was significantly more lateralized to the right hemisphere than in controls after treatment. With the exception of the right hemisphere in one patient, activity in both frontal lobes decreased from pre- to post-treatment fMRI, suggesting greater efficiency of processing after treatment. The one patient who did not improve in treatment showed a leftward as opposed to a rightward shift in lateral frontal activity, and an increase in left frontal activity. Figure 2 shows the pre- and post-treatment activity most typical of those patients who improved in treatment. The images have been equated for sensitivity to BOLD responses between the two sessions. Note the total lack of right frontal activity in post- compared to pre-treatment images, indicating a rightward shift in lateral frontal laterality, and the reduction in activity in the right as well as the left frontal lobe, suggesting greater efficiency of processing post- compared to pre-treatment processing. This study is of interest because it indicates that it may be possible to target specific brain structures for rehabilitation and use functional imaging to confirm the success of the strategy. The ability to perform this kind of study eventually should lead to development of better treatments for aphasia.
Figure 2.
Pre- and post-treatment images for one subject who received the intention treatment. Red represents R2 ≥ .20; orange represents R2 ≥ .25. Note the lack of left frontal activity on post-compared to pre-treatment images, and the general reduction in frontal activity from pre to post treatment. Lateral frontal laterality indices are displayed at the bottom of the image, with 1.0 representing activity entirely lateralized to the left and -1.0 representing activity entirely lateralized to the right.
Other forms of fMRI
While BOLD contrast is by far the most common form of fMRI, it is important to note that there are other kinds of fMRI. The reasons that these forms of imaging have not gained the wide-spread usage and acceptance as that of BOLD contrast fMRI are variable. We briefly discuss these reasons and describe the following three techniques: arterial spin labeling, blood oxygenation sensitive steady-state, and dynamic susceptibility contrast. In addition, resting state or functional connectivity MRI (fcMRI) is described, which is fMRI without a specific task, and provides a passive means of interrogating functional brain networks and their connectivity.
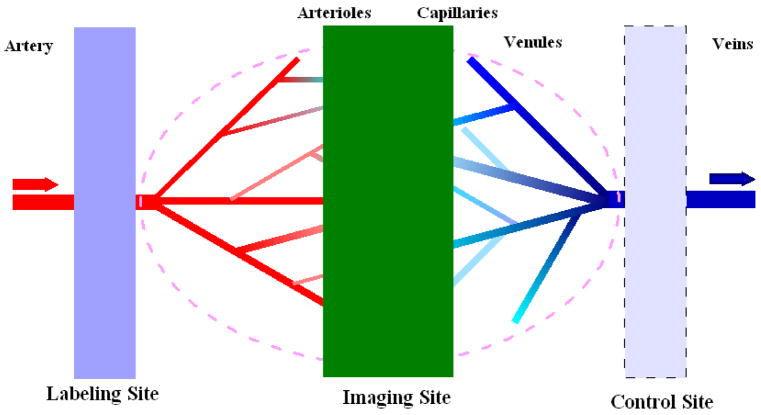
Arterial spin labeling (ASL) provides a measure of cerebral blood flow, using an endogenous tracer, blood water, to noninvasively measure tissue perfusion to evaluate the tissue viability or functionality (Golay, Hendrikse, & Lim, 2004; Liu & Brown, 2007). In ASL, two sets of images are alternately acquired, a set of labeling images and a set of control images. In the labeling experiment, arterial blood spins on the proximal side of the imaging slab are inverted. After a short delay of a second or so, to allow arterial blood that is tagged in this fashion to perfuse throughout the brain, images are acquired from the imaging slab. In the control imaging experiment, no arterial blood is inverted, and after the same short delay, images are acquired from the imaging slab. After pair-wise subtraction of control from labeling images, MRI signals from static brain tissue can be cancelled, leaving signal only from the labeled inflowing blood water perfusing brain tissue. The difference image from pair-wise subtraction of control images from labeling images is referred to as a perfusion-weighted image, or PWI (Figure 3). By using an appropriate perfusion model (Parkes, 2005), cerebral blood flow (CBF) maps can be reconstructed. There are several variants of ASL techniques (Liu & Brown, 2007).
Figure 3.
Schematic diagram for ASL imaging. The labeling of arterial blood is proximal to the tissue of interest, as shown by the blue plane at left. After a delay to let labeled blood to arrive at the tissue sites, imaging acquisitions will be performed. In some ASL techniques, control experiments will be done using the symmetric labeling RF pulse at the distal site to minimize the MT effects (light blue plane at right).
The application of inversion RF pulses can generate magnetization transfer (MT) effects (Wolff & Balaban, 1989) on tissue. In MT, irradiation of broad signals from macromolecules to which water is transiently binding changes the water signal intensity. If the MT effects are not the same between the labeling and control experiments, these MT effects can confound ASL perfusion signals. Using either an asymmetric control RF pulse in the same proximal site or a symmetric control RF pulse in the mirrored distal site can control MT effects by using the same RF pulses as in the labeling experiment, with adjusted radiation frequency.
ASL has become particularly useful in circumstances where baseline cerebral blood flow measures are useful. For example, ASL showed a hypoperfused area in the left parietal cortex of a stroke patient, consistent with the patient’s reading deficit, which appeared normal on conventional anatomic images (T. Love, D. Swinney, E. Wong, & R. B. Buxton, 2002). An example of this type of application of ASL from one of our own laboratories is shown in Figure 4. Measuring quantitative CBF changes induced by pharmaceutical agent challenge is also a promising for clinical application of ASL. For example, the specificity of cerebral perfusion changes under hypercapnia or hypocapnia states has been evaluated (Pollock et al., 2009). The effects on CBF of other drugs that can alter the physiological or neurophysiologic states of the brain have also been studied by ASL. For example, the global increase of brain perfusion with remifentanil challenge is mainly due to remifentanil’s depression of breathing and the consequent increase of blood PaCO2 (MacIntosh et al., 2008). In another ASL perfusion study, acetazolamide, a carbonic anhydrase inhibitor, was administrated to evaluate the augmentation of cerebral blood flow with patients with ischemic symptoms due to arterial stenosis (Detre et al., 1999).
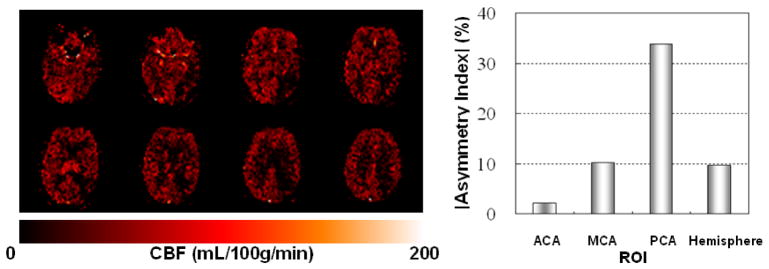
Figure 4.
CBF maps (left) and asymmetry analysis results (right) from ASL perfusion study of a 76 year-old female stroke patient. Asymmetry index (%) = (CBF(right) - CBF(left))/(CBF(right)+CBF(left)) × 100. Specific perfusion territories are designated by ACA (anterior cerebral arteries), MCA (middle cerebral arteries), and PCA (posterior cerebral arteries). In the bar graph, the negative index value for ACA is indicated with a different pattern. In the CBF maps, brighter colors indicate greater CBF. Note the decreased CBF in the posterior right side of the images, consistent with the greater asymmetry in CBF for the posterior cerebral artery (PCA) distribution compared to the anterior cerebral artery (ACA) and middle cerebral artery (MCA).
ASL also can be used for functional images, although fMRI has not become a wide-spread use for ASL. One problem with most ASL techniques is that it is hard to acquire whole-brain fMRI on a time scale that would allow for visualization of the hemodynamic response. Hence, the investigator has to be satisfied with a few image slices covering critical areas. However, in ASL fMRI, a much shorter TE is usually used compared to BOLD fMRI, which reduces the signal loss due to susceptibility effects and provides better spatial specificity than BOLD fMRI, where large draining veins can contribute artifactual signal. For example, (Kemeny, Ye, Birn, & Braun, 2005) demonstrated that ASL could be used to collect fMRI data during sentence production in such a way as to eliminate artifacts present on BOLD images when subjects speak.
Readers desiring more detail on ASL are referred to the article on techniques (Liu & Brown, 2007) and the companion article on applications (Brown, Clark, & Liu, 2007).
Blood oxygenation sensitive steady-state (BOSS) relies on a change in the MR signal frequency for deoxyhemoglobin relative to oxyhemoglobin. The BOSS acquisition capitalizes on this signal shift to collect images in which the signal from oxyhemoglobin will be positive and the signal from deoxyhemoglobin will be negative. BOLD and BOSS fMRI both measure signal changes resulting from the deoxyhemoglobin frequency shift. However, BOLD contrast fMRI measures that frequency shift indirectly as signal dephasing, and BOSS measures the shift directly. Because increased activity causes a drop in the proportion of negative, deoxyhemoglobin-related signal and an increase in the proportion of positive oxyhemoglobin-related signal, the net BOSS signal increases during activity (Miller et al., 2004). BOSS has advantages over BOLD. For example, BOSS has a higher signal-to-noise ratio than BOLD, with BOSS signal changes being roughly 2-3 times larger than BOLD. Further, BOSS is not subject to the signal drop out around air-tissue interfaces that plagues BOLD imaging. Among the reasons that BOSS fMRI has not become more popular are the facts that it has not found its way into the commercially available sequences on MR instruments and its implementation can be complex. Readers wishing more detailed treatment of BOSS are referred to the work of Miller and colleagues (Miller et al., 2004; Miller et al., 2003; Miller, Smith, Jezzard, & Pauly, 2006).
Dynamic susceptibility contrast (DSC) uses the paramagnetic property of gadolinium ions as a magnetic tracer. Gadolinium ions causes a transient disruption of the magnetic field around the vessels through which it flows, resulting in a transient signal loss proportional to the amount of gadolinium. The signal loss can be used to determine changes in relative cerebral blood volume at the voxel level. Advantages to this technique include its high contrast-to-noise ratio, which can be used to overcome artifacts during patient speech. The major drawback to DSC is its invasiveness, since intravenous injection of gadolinium is necessary. (Naeser et al., 2004) used DSC to study brain activity during narrative language in patients with chronic nonfluent aphasia. These authors also give a brief description of the technique.
Functional connectivity MRI (fcMRI) detects interconnected brain regions which activate together as a functional network. It is most often performed as resting state fMRI without a specific task, in which the brain pseudo-randomly activates under little or no guiding external influence. Since no task performance is required on the part of the subject, the resting state implementation has the advantage of being a passive method of interrogating functional brain networks and their functional connectivity. This is an obvious benefit for application to stroke and other pathologies that can make task performance and monitoring difficult.
The acquisitions for fcMRI should have about 300 volumes or serial time points, to adequately sample the low-frequency signal fluctuations (< 0.1 Hz) representative of vascular fcMRI responses to baseline neuronal activity “at rest”. Collecting cardiac and respiratory waveforms in conjunction with the fcMRI time course data is useful for regressing out signal fluctuations due to these non-neuronal sources of physiological variability, leaving signal fluctuations more nearly caused by vascular responses to neuronal activity (Glover et al. 2000). Calibration of the BOLD effect using breath hold (Thomason et al. 2007) or amplitude of resting state signal fluctuations (Biswal, Kannurpatti, & Rypma, 2007)reduces variability of individual vascular reactivity and improves group averages and comparisons.
Analyses of fcMRI data are typically done in one of two ways: unguided exploratory analyses by Independent Components Analysis, or ICA (De Luca et al. 2006), or seed-based analysis by which voxels whose signal time course correlates highly with those of the seed ROI are detected (DiMartino et al. 2008).
There are few examples in the literature of how fcMRI can be applied to stroke and rehabilitation. A left hemorrhagic posterior cerebral artery stroke produced increased functional connectivity between areas V4/V8 and V5 of the left hemisphere in comparison with the same areas in the intact hemisphere when the subject viewed changing colors, suggesting that visual perception after the V1 lesion is mediated by subcortical pathways that bypass V1 and project first to V5 and V4/V8 and subsequently to V2/V3 (Schoenfeld at al. 2002). Spatial neglect in stroke subjects was studied by fcMRI in both acute and chronic recovery stages in dorsal and ventral frontoparietal areas (He et al. 2007). Part of the lesioned ventral network was diffusely disrupted and showed no recovery, whereas in the structurally intact dorsal network, posterior parietal cortex connectivity was acutely disrupted but fully recovered. Disrupted connectivity in the ventral network correlated with impaired attentional processing, and disconnection of the frontal and parietal cortices was associated with more severe spatial neglect. A recent review (Damoiseaux and Greicius 2009) highlights studies which have combined structural (DTI, see following section) and functional (fcMRI) connectivity methods. Information about brain networks and functional connectivity affected by stroke and rehabilitation can also be obtained from EEG (Gerloff et al. 2006, Meister et al. 2006) and MEG (Schoenfeld at al. 2002).
One weakness in fcMRI is the certainty with which inferences can be made regarding functional networks for specific cognitive or behavioral functions when acquisitions are made in the resting state. Recently, techniques to address functional networks during performance of relevant tasks have been developed. These include adaptation of structural equation modeling (SEM) and dynamic causal modeling (DCM) to define relationships between components of functional networks. A potential drawback is that these techniques work best when investigators have a model to test. Abutalebi et al. (Abutalebi, Rosa, Tettamanti, Green, & Cappa, 2009) used DCM and fMRI to study recovery of functional connectivity in a bilingual patient who received therapy in his second language (L2) but not in his first language (L1). The functional connectivity of selected left-hemisphere language and cognitive control mechanisms increased across the course of therapy for L1, consistent with increasing activity and language skills in these areas for L1, while the functional connectivity of these structures decreased for L2, consistent with stable to decreasing performance in L1. Before therapy functional connections generally were stronger for L1 than L2, but the reverse was true after therapy. We expect that DCM and SEM will be increasingly applied to rehabilitation.
Diffusion Imaging and Diffusion Tensor Imaging
Although functional MRI has become a valuable tool for imaging changes in brain function as a result of rehabilitation, this versatile technology also has provided us with new methods of imaging brain structures, in particular the brain’s white matter. Diffusion-weighted magnetic resonance imaging (DW-MRI) is a non-invasive imaging technique that allows one to assess the structural integrity of tissues in-vivo. DW-MRI measures the diffusion of water molecules, random motion present due to thermal energy. In tissues, diffusion is restricted by cell membranes as well as tissue boundaries, resulting in diffusion anisotropy, or diffusion along one preferred direction. For example, in white matter fiber bundles, the myelin sheath surrounding axons prevents diffusion across the axon, restricting water diffusion only along the longitudinal axis of the axon. Therefore, the directionality of diffusion provides us with information about local fiber orientation.
Mathematically, diffusion in a given voxel can be characterized by an ellipsoid, or tensor, oriented along the direction of the fiber (Basser et al., 1994). The major axes of the ellipsoid, called eigenvectors, describe spatial orientation of the fibers. The lengths of the axes, known as the eigenvalues, describe the strength of diffusion along each of the three axes. The diffusion of water in white matter fiber bundles would be described by an ellipsoid elongated along the principle diffusion direction (PDD), or the longitudinal direction of the fiber. A scalar describing the proportion of the diffusion along a particular direction (relative to all other directions) is called fractional anisotropy, or FA. Values of FA range from 0 to 1, where 0 describes diffusion which is completely isotropic, or equal in all directions. For instance, isotropic diffusion is usually observed in the ventricles, as water molecules in CSF are able to disperse freely in all directions, described by FA values close to 0. White matter fiber bundles would exhibit FA values close to 1 as almost all diffusion is directed along the length of the axons. The average rate of diffusion, or mean diffusivity (MD) is another important scalar describing the average speed with which the molecules traveled during a given time interval. MD values are higher in the ventricles, as molecules are unrestricted and can travel larger distances than in gray or white matter. Together, FA and MD maps can be used in clinical settings to acquire information about the structural organization of tissues not present in conventional MRI. For example, the sensitivity of FA and MD measures have been shown to reveal damage in white matter in ischemic leukoariosis abnormalities not noticeable on T2-weighted MR scans, and to reveal Wallerian degeneration post-stroke months before it could be detected by T1-weighted MRI (O’Sullivan et al., 2001; Thomalla et al., 2004). Group studies investigating white matter abnormalities have used FA measures to localize differences in the white matter in patients with primary lateral sclerosis (PLS), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS), as well as to correlate these differences with the Expanded Disability Status Scale (EDSS) (Ciccarelli et al., 2009; Smith et al., 2007). In healthy populations, FA measures were shown to reflect effects of myelination correlated with hours of practice in professional piano players (Bengtsson et al., 2005).
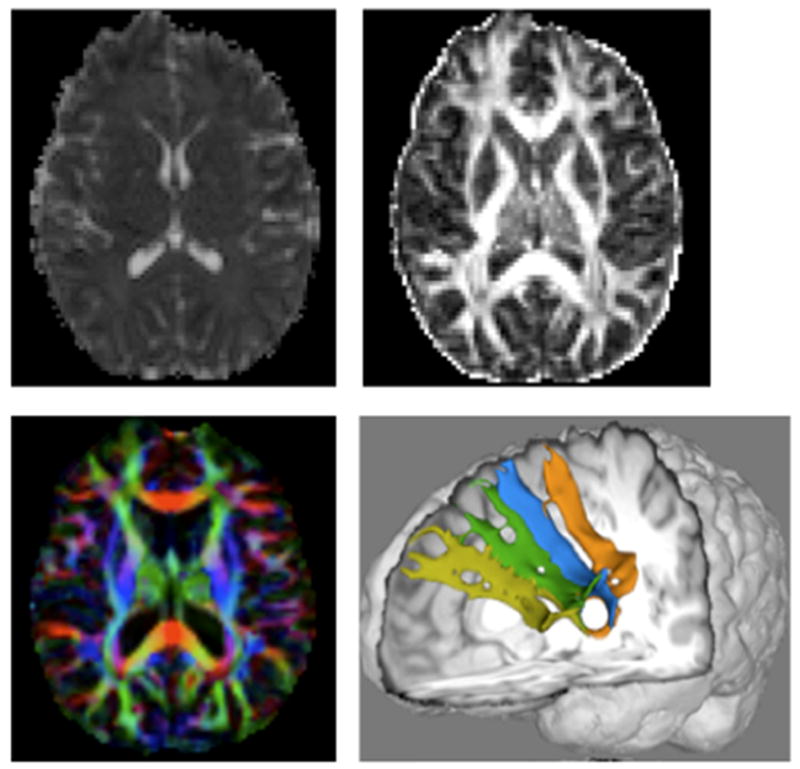
Generally, FA and MD values are negatively correlated with higher MD values and lower FA values indicating underlying tissue degeneration. However, an inverse relation between these measures is not always observed. Lower FA values indicate a reduction in preferred directionality of diffusion. However, decreased fiber integrity in secondary directions can artificially drive the FA value closer to 1 (Douaud et al., 2009) while MD is also increasing. In such cases, the FA value would not be a good indication of white matter integrity. Thus, to better describe structural changes in white matter in neurological disease, it is important to consider whether multiple DW-MRI measures provide converging evidence. Figure 5 shows images based on MD (top left), FA top Right, color coded PDD maps from FA (bottom left), and fiber tracts derived from DTI data (bottom right, see below for explanation).
Figure 5.
DW-MRI measures from left to right, top row: mean diffusivity (MD) map, fractional anisotropy (FA) map; bottom row: FA map overlaid with the principle diffusion direction (PDD) in color (red left to right, blue inferior to superior, green anterior to posterior). Bottom right: 3-dimensional rendering of previously undocumented fiber paths connecting Broca’s area (Brodmann’s areas 44/45) with Brodmann’s area 9 (light green).
In addition to assessment of white matter integrity, DW-MRI also allows for white matter visualization using tractography. Tractography approaches that trace the fibers by following the PDD are called streamline. Although streamline methods produce robust results for large white matter tracts, such as the corticospinal tract (CST), they have difficulty accurately representing branching or crossing fibers, since in this case no single principal direction exists. In this case a second category, called probabilistic tractography, should be used to properly represent fiber architecture. Probabilistic tractography approaches trace the tracts in many possible directions, recording probabilities associated with each direction. Thus, at the intersection of two tracts, both fibers will have comparable probabilities associated with each corresponding direction, each of which will be traced by the algorithm. In stroke rehabilitation studies, ratios computed as the ipsi- over contra-lesional hemisphere tract volumes (numbers of voxels) were shown to correlate with recovery (Nelles et al., 2007). In patients with ALS disease, progression rates have been correlated with DW-MRI measures of structural integrity in the CST fibers, using connectivity measures generated by a probabilistic tractography approach (Ciccarelli et al., 2006).
In summary, DW-MRI provides clinicians with many useful tools sensitive to changes in the white matter. Moreover, these tools provide researchers with information, such as fiber tract visualization, that has previously been available only in primate studies and cadaver dissections. DW-MRI allows us to investigate not only changes in structural organization of known white matter projections, but also to ascertain the presence of new pathways, making it crucial to understanding mechanisms of neurological disease. For example, Figure 5 (bottom right) shows the visualization of fibers between medial frontal cortices and Broca’s area (Ford, unpublished data), both of which are involved in word production. One potential use of DW-MRI in rehabilitation involves the measurement of integrity of various tracts and correlating these measures with behavioral and cognitive measures or with measures of rehabilitation outcome.
MR Spectroscopy
Magnetic Resonance Spectroscopy (MRS) provides a non-invasive way of investigating brain chemistry. Although MR spectroscopy can be performed using a number of different nuclei, only the most clinically established method, 1H spectroscopy, will be described. With it, important brain metabolites such as N-acetylaspartate (NAA), creatine (Cr) and choline (Cho) can be measured, as well as molecules with weaker signals such as glutamate (Glu), glutamine (Gln), myo-inositol (mI), and lactate (Lac) (Govindaraju, Young, & Maudsley, 2000). Resonances (peaks) are identified by their position (chemical shift) in the spectra, expressed in parts per million (ppm) relative to a standard frequency. Peak areas are related to the metabolite concentrations. The dominant peaks are from the acetyl group of N-acetylaspartate (NAA) at 2.0 ppm, total creatine (Cr) at 3.0 ppm, and total choline (Cho) at 3.2 ppm (Figure 6).
Figure 6.
An example 1H spectrum, acquired at 3T, of the left basal ganglia of a normal control subject, post-processed in LCModel. Metabolites included in the modeled basis spectra are shown in the table on the right. Those detected with a reasonable confidence are in blue font. The %SD indicate Cramer-Rao lower error bounds.
NAA is an amino acid present mostly in neurons and is widely regarded as a marker of neuronal structural and functional health and integrity. Decrease of NAA is observed in various cerebral pathologies such as ischemia, brain tumors, gliosis, dementia, trauma, and multiple sclerosis. It is rarely increased, one exception being Canavan’s disease. Longitudinal MRS studies in stroke have found acute decreases in NAA, and a continuing fall during the first week after the onset (Gideon et al., 1992; Saunders, 2000). Recovery of the NAA levels has been observed in certain cases of reversible ischemia (Brulatout et al., 1996) and acute brain injury (De, Matthews, & Arnold, 1995), making NAA an important clinical outcome marker, as reviewed recently (Eliassen et al., 2008). Effects of rehabilitation and interventions have also been studied (Mountz, 2007), indicating relations between metabolite changes and brain reorganization and plasticity. N-acetylaspartate significance and changes under various conditions has been reviewed (Moffett, Ross, Arun, Madhavarao, & Namboodiri, 2007).
The total creatine (creatine and phosphocreatine) peak is a marker of the energetic status of cells, and is often used as a standard or reference for relative quantification of other metabolites, due to its relative consistency with location in the brain, age, and physiological conditions. However, caution needs to be exercised, since Cr levels do change in certain pathologies such as tumors and stroke.
The total choline peak (phosphocholine, free choline, and glycerophosphocholine) is a marker of cell membrane integrity and viability. It has been shown to be elevated in dementia, multiple sclerosis, aging, and tumors (Rudkin and Arnold 1999).
Lactate is below the MRS detection threshold in healthy brain, but is measurable in ischemia (Graham et al., 1992) and hypoxia since it is the end product of anaerobic processes.
Both data acquisition and analysis in MRS usually require significant technical expertise. In single voxel spectroscopy, a proper and reliable localization of the volume of interest is important, and usually requires an MR operator with a good knowledge of brain anatomy. The quality of the acquired spectra (the line-width and spectral resolution) is highly dependent on the homogeneity (shimming) of the of B0 magnetic field. Most of the MR vendors provide autoshim utilities, but in some brain areas a manual adjustment of the shim currents is often necessary. The water signal is orders of magnitude higher than the metabolite signals, so efficient water suppression is important as well. In CSI techniques extra effort needs to be taken for lipid signal suppression.
Processing the MRS data is in general complicated because of overlapping peaks and the limited signal to noise ratio of in vivo spectra; accurate quantification requires close attention to a number of details (Gillard, 2005). An automatic post-processing tool that has gained popularity in both clinical and research settings is LCModel (Provencher, 1993), which utilizes prior spectral knowledge by analyzing the in vivo spectrum as a linear combination of in vitro acquired metabolite model spectra (vendor provided or locally acquired). Simulated lipid and macromolecule signals are also included in the modeling. Accurate accounting for these signals is crucial for proper metabolite quantification especially at short echo times, which provide inherently higher signal-to-noise ratio.
Absolute metabolite quantification (Ernst, Kreis, & Ross, 1993; Kreis, Ernst, & Ross, 1993) requires internal (Barker et al., 1993) or external (Ernst, Kreis, & Ross, 1993; Kreis, Ernst, & Ross, 1993) reference standards. A number of instrument dependent factors as well as in vivo relaxation corrections complicate the quantification procedure. Since relaxation corrections (Traber, Block, Lamerichs, Gieseke, & Schild, 2004) are usually difficult to measure in vivo due to time constraints, it is often recommended to use sufficiently long repetition times (TR) and as short as possible echo times (TE) in the data acquisition.
Despite technical challenges, MRS is a valuable tool for measuring biochemical changes in the brain in various pathologies and in recovery and rehabilitation.
OTHER FUNCTIONAL NEUROIMAGING TECHNIQUES
Positron Emission Tomography
Although fMRI is today the dominant form of functional neuroimaging for cognition, the field owes much to scientists who used positron emission tomography (PET) as a tool for imaging cognitive systems before fMRI was a wide-spread technology. The technique relies on unique properties of short half-life, positron emitting radionuclides to map brain systems responsible for cognition. Specifically, a positron is a particle with the same mass as an electron, but differs from an electron in that its charge is positive, not negative. When emitted from the radionuclide, a positron will travel a very short distance before encountering an electron with which it undergoes annihilation. As a result of this process, two 511-KeV photons leave the site of annihilation traveling almost exactly 180 degrees from one another. PET images rely on rings of coincidence detectors and the concept that if two photons are detected coincidentally, it is highly likely that an annihilation event occurred on a straight line between the two points of detection. If enough of the events are detected, then a computer can be used to create images of the distribution of the radionuclide within the sample. 18F-labeled fluorodeoxyglucose (18FDG) was administered in many early PET studies to map baseline levels of regional glucose metabolism in the brain. 18F has a half-life of 110 minutes. Typically, 30 minutes or more was allowed for nonmetabolized 18FDG to clear the brain. With the remaining portion trapped in brain tissue, its distribution could be used as a good indicator regional glucose metabolism levels. However, the 30+ minutes needed to get a single image was not well suited to measure changes in brain activity related to changes in cognition. Fortunately, increases in metabolism that occur with neuronal activity also drive increases in blood flow. A technique of injecting 15O-labeled water (H215O) into the blood stream allowed PET investigators to image changes in regional cerebral blood flow due to cognitive activities performed during the roughly 40-second image acquisition. The relatively short half-life of 15O (123 seconds) allowed for multiple administrations of the radionuclide, and images of cognitive activity could be compiled by assessing differences in regional cerebral blood flow between images collected during two different cognitive states. For more information on PET technology and its history, the interested reader may wish to consult the chapter by Raichle (Raichle, 2000b).
While H215O-PET was better suited to measuring brain activity due to cognitive states than 18FDG-PET, there were still important limitations to the former technique. One drawback to the technique is the exposure of subjects to radiation and the limitation in the number of scans that this implies. From a practical standpoint, the most commonly used PET technique for exploring the brain’s cognitive systems, measurement of regional cerebral blood flow changes using H215O, is performed with a 123-second half-life contrast agent, which requires co-location with a cyclotron and a radiochemist. These requirements historically limited the availability of PET, whereas MRI scanners are much more common. Another drawback to PET, as noted above, is that subjects have to perform a task for roughly 40 seconds, rendering a temporal resolution in the tens of seconds. fMRI on the other hand has a resolution on the order of seconds for whole brain imaging. This temporal resolution for fMRI makes it possible to measure the effects of a series of single events with fMRI, whereas one can only measure long blocks of events with PET. Hence, many fMRI studies currently use event-related paradigms, which offer greater flexibility in experimental inquiry. PET is also at a disadvantage relative to fMRI in terms of spatial resolution. Nonetheless, one advantage that PET offers over fMRI is that it is not prone to the loss of signal near air-tissue interfaces as is the case with BOLD contrast fMRI. A second advantage is that the PET scanning environment suffers from less acoustic noise than the fMRI environment.
An interesting analogue study of aphasia rehabilitation with PET was done by (Musso et al., 1999). The authors performed 12 scans on each of four patients with Wernicke’s aphasia using a comprehension task. In between those scans, subjects were given intensive training in language comprehension that involved use of linguistic and non-linguistic cues in a variety of comprehension tasks. In addition to training in language comprehension, subjects received a language comprehension test (Token Test) between the PET scans. Hence, performance on the Token Test could be correlated with voxel-wise values for regional cerebral blood flow at 12 different time points to determine if changes in performance correlated with changes in regional cerebral blood flow. Regions best correlated with training-induced improvement in verbal comprehension included the posterior right superior temporal gyrus and the left precuneus (Musso et al., 1999). Findings were taken as an indication of participation of the right-hemsiphere homologue of Wernicke’s area in language comprehension gains.
Magnetoencephalography/Magnetic Source Imaging and Electroencaphalography/Evoked Potentails
Electroencephalography (EEG) and magnetoencephalography (MEG) represent two noninvasive functional brain imaging methods that work in a completely different manner than the above described functional imaging techniques (e.g., fMRI, PET). Both make use of the same neurophysiologic events, i.e., ionic currents caused by information exchange of neurons. Active neurons generate small, fluctuating electrical currents. During EEG, synchronized electrical activity of thousands of active neurons is detected by means of electrodes that are attached to the scalp. The electric currents inside the head also produce small magnetic field oscillations that are the sources of the MEG signal. The strength and orientation of these magnetic fields can be detected above the scalp by magnetometers inside the MEG system. As the magnetic fields measured during MEG are very weak (i.e., a fraction of the of the earth’s magnetic field) it needs to be assessed within a magnetically shielded room by using specific recording devices that are sensitive to extremely small magnetic fields (superconducting quantum interference devices, SQUID). As a result, MEG devices are extremely expensive (millions of dollars) while even advanced EEG systems are relatively inexpensive (several thousand dollars). EEG and MEG signals are mainly produced by postsynaptic ionic currents of synchronically active pyramidal cortical neurons. But due to the properties of the neural sources of the respective signals that are measured during EEG and MEG (electric current flow vs. magnetic fields that are oriented perpendicular to each other), EEG is most sensitive to activity generated on top of the cortical sulci, whereas MEG is more sensitive to activity in the sulci. Thus, both techniques can provide complimentary information about neural activity (Babiloni, Pizzella, Gratta, Ferretti, & Romani, 2009).
Similar to other imaging techniques, spontaneous or evoked activity in response to specific behavior can be monitored (event-related electric potentials or magnetic fields; ERPs/ERFs). In combination with anatomical information (e.g., structural MRI) both techniques allow one to estimate the underlying neuronal generators of the recorded surface activity by using complex mathematical procedures. The main advantage of electrophysiological techniques is that they can detect changes in brain activity with millisecond temporal resolution. Thus, EEG and MEG are the most direct correlate of online brain processing that can be obtained non-invasively. On the other hand, the spatial resolution is in the range of centimeters and thus lower than that obtained during fMRI. MEG offers a slightly better spatial resolution than EEG, as the magnetic fields measured are not affected by the surrounding tissue (Johnsrude & Hauk, 2005)
Spontaneous neuroelectric or neuromagnetic activity in the healthy brain is characterized by rhythmic oscillatory activity in the frequency range above 8 Hz. Focal oscillations in lower frequency ranges (e.g., slow wave activity in the delta frequency range; 0.5-4 Hz) can be found in the vicinity of structural lesions (e.g., stroke, tumors) and have been interpreted as indicative of dysfunctional information processing capacity due to a loss of afferent input or due to a primary metabolic effect (Kamada et al., 1997). Mapping of slow-wave activity can be useful to identify dysfunctional network characteristics in neuropsychiatric disorders (Weisz, Moratti, Meinzer, Dohrmann, & Elbert, 2005)or to detect changes in brain activity over the course of recovery and in response to treatment. For example, (Lewine, Davis, Sloan, Kodituwakku, & Orrison, 1999) demonstrated that improved neuropsychological functioning after traumatic brain injury was associated with reduced MEG-slow wave activity. Hensel et al. (Hensel, Rockstroh, Berg, Elbert, & Schonle, 2004) reported reduced slow-wave activity to be associated with spontaneous recovery of language functions after stroke. Moreover, improvement of language functions after speech and language therapy has been shown to covary with changes in delta frequency in stroke sufferers (Meinzer et al., 2004) and (de Jongh et al., 2003) found reduced slow-wave activity after successful resection of brain tumors.
Due to the excellent temporal resolution EEG and MEG have widely been used to detect spatio-temporal distribution in the brain of higher cognitive processes, like language functions. For example, during picture naming, visual and conceptual processes take place within the first 175 msecs after stimulus presentation, followed by lexical retrieval (until ~250 msecs) and phonological encoding of the word form (250-450 msecs (Indefrey & Levelt, 2004). After neurological damage different aspects of word-retrieval can be impaired (e.g., post-stroke anomia). This has been shown by (Laganaro et al., 2009), who used EEG to compare temporal characteristics of evoked responses during picture naming in patients with two different types of anomia. Compared to a group of healthy subjects, the authors found early event-related potential abnormalities (100-250 msecs) in patients with lexical-semantic impairments. In contrast, a group of patients with predominantly phonological impairments evidenced abnormalities in later time windows associated with phonological processing (300-450 msecs). Electrophysiological techniques have also been used to track activity changes in response to treatment. For example, Cornelissen et al. (Cornelissen et al., 2003) assessed changes in brain activity after speech and language therapy that was designed to facilitate phonological encoding in patients with aphasia by means of MEG and magnetic source imaging (MSI). In line with studies in healthy subjects on phonological encoding (Indefrey & Levelt, 2004), treatment-induced activity changes were found in the time window between 300-600 msecs after picture presentation and were localized in the left inferior parietal cortex, an area associated with phonological encoding and storage.
More recently, researchers began to combine electrophysiological, blood-flow based techniques and structural imaging, as they provide converging lines of evidence to describe the neural substrates of normal cognitive functioning, changes in pathological conditions, and to assess treatment-induced recovery of functions. For example, Meinzer et al. (2008) used MEG-slow wave mapping prior to a two week treatment period in post-stroke aphasia to define areas of dysfunctional information processing. Subsequently, slow wave clusters were used as a region-of-interest to assess functional activity changes in these brain areas with fMRI. Moreover, EEG can be assessed simultaneously during fMRI. In the future, the combination of these two methods could be useful for a better description of pathological processes or the impact of rehabilitation efforts. As in fMRI, MEG can be used to assess functional connections between different brain areas (Schlee et al., 2009).
Near Infrared Spectroscopy
Near Infrared spectroscopy (NIRS), like the various MR techniques, is a noninvasive method of looking at brain tissue function. Although visible light is strongly absorbed by various components in body tissue, light in the near infrared frequency range (650-900 nm) is less strongly absorbed and thus can penetrate through the skin and skull and a centimeter or two into the typical adult brain. In addition to being absorbed by tissue, light is strongly scattered. This further reduces the light intensity and penetration and hinders the ability to precisely localize absorbing species. However, the scattered light can be used to measure cerebral blood flow.
Light absorption at a given wavelength follows the Beer-Lambert Law, Aλ = ελcl, where A is the absorption, λ is the wavelength, ε is absorption or molar extinction coefficient, c is the molar concentration of the absorbing species, and l is the optical path length. Molecules have different absorption spectra, which is the variability of the absorption coefficient with wavelength. The major species in tissue that absorb in the near infrared band and are present in significant quantities are cytochrome oxidase and hemoglobin, both of which exist in oxidized and reduced forms with unique absorption spectra.
It is possible to separately quantify the relative amounts of the reduced and oxidized forms of hemoglobin or cytochrome oxidase by using two measurement wavelengths (typically 760-780 nm for deoxyhemoglobin and 825-850 nm for oxyhemoglobin) at which the two forms have different absorption coefficients. A separate measurement at an isosbestic point (815 nm for hemoglobin), a wavelength at which the absorption coefficients of the two species are equal, provides the total amount of reduced and oxidized forms, which is proportional to blood volume. From absorptions measured at these three wavelengths, the concentrations of both oxidized and reduced species are calculated. Thus NIRS can measure amounts of hemoglobin and deoxyhemoglobin in tissue, which BOLD fMRI cannot, because BOLD fMRI signal changes arise from a complex mixture of CBF, CBV, and oxygenation changes.
In practice, NIRS is applied in vivo by placing a light source and a light detector adjacent to each other above the region to be measured. The light path through the tissue between and below them, a convex banana-shaped tissue region, is sampled.
A combined NIRS-fMRI study of MCA stroke patients in chronic recovery compared to control subjects demonstrated ipsilateral motor cortex compensation in the stroke patients (Kato et al. 2002). NIRS monitoring of mechanically- or therapist-assisted hemiplegic treadmill walking three months post-stroke showed stronger activation with therapist assistance and enhanced activation in the unaffected hemisphere (Miyai et al. 2002). A longitudinal study by the same group with NIRS monitoring at three and five months post-stroke showed decreased activation in the unaffected hemisphere and increased activation in the affected hemisphere in the second compared to the first session (Miyai et al. 2003); this shift towards normal lateralization has also been noted in fMRI studies of hand motor function. A similar trend towards increased, normal laterality was also noted in a longitudinal case study of NIRS monitoring of motor cortex activity during ten consecutive days of constraint-induced movement therapy (Park et al. 2004). A Japanese group used NIRS to monitor brain effects of 13 rehabilitation tasks in post-stroke hemiplegic patients (Saitou et al. 2000).
NIRS and transcranial Doppler ultrasound have been employed to monitor arterial blood flow and blood volume changes in response to hypercapnia in patients with lateralized stroke compared to normal controls (Vernieri et al. 1999). In the patients, NIRS showed an increase of blood volume with hypercapnia in the spared but not in the lesioned hemisphere. The two methods were also combined to monitor the effects of vinpocetine on cerebral blood flow and oxyhemoglobin, deoxyhemoglobin, and total hemoglobin in the stroke-affected hemisphere (Bonoczk, Panczel, & Nagy, 2002)); vinpocetine increased both CBF and oxygen extraction.
Reviews of NIRS and BOLD fMRI studies of stroke and brain tumors (Sakatani et el. 2007) and of NIRS studies of stroke rehabilitation (Strangman et al. 2006) provide an assessment of the opportunities and challenges. The Strangman (2006) paper also reports results of test-retest reliability for NIRS.
In summary, NIRS has spatial resolution of slightly less than a cm, temporal resolution of ms to tens of ms, light penetration of only about 1 cm into the superficial portions of the brain, and the ability to estimate amounts of oxyhemoglobin, deoxyhemoglobin, and total hemoglobin. Thus, for superficial brain regions, it is a useful complement to fMRI. Its portability, relatively low cost compared to MR, ability to be used in a normal and unconfined environment, and noninvasiveness make it potentially useful clinically.
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a painless, non-invasive neurophysiological technique during which a strong (typically 1 to 2 Tesla) focal magnetic pulse is directed at cortical areas from a stimulation coil placed directly above the scalp. The brief (measured in microseconds) change in magnetic field induces a corresponding electrical potential change in the affected cortex resulting in a rapid neuronal depolarization and the generation of action potentials. In the evaluation of the human motor system, typical output measures of TMS stimulation to the primary motor cortex (M1) are changes in the electromyographs (EMG) of target muscles including, if the stimulation is properly applied, the generation of EMG activity spikes known as magnetic evoked potentials (MEP). For the purposes of clinical research, TMS is typically delivered using one of three pulse (coil excitation) protocols: a single pulse, two pulses separated by a brief delay (paired pulse), or rapid repetitive pulses (rTMS). The utility of each within the domain of rehabilitative research will briefly be explored.
The oldest and most reported TMS procedure, single pulse TMS refers to the application of a single magnetic field change during stimulation trials. The technique has great clinical utility as a diagnostic tool for the motor system. A prevalent single pulse method in neurology is the assessment of an individual’s central motor conduction time (CMCT). In this procedure, a neurologist or technician uses single pulses to M1 representations of different muscles throughout the arm and hand. Delays or alterations in the onset and latency of MEP spikes (from coil stimulation events) can determine if an individual with suspected pathology deviates from age-matched normal values. Disorders such as multiple sclerosis, amyotrophic lateral sclerosis (ALS), stroke, leukoariaoisis, and traumatic brain injury can all affect neural conduction rates from pyramidal cell impetus to the measured muscle actuation (see Chen et al., 2008 for review). Another clinical use of single pulse TMS is for mapping of the motor cortex. This approach is particularly useful in the assessment of cortical plasticity in both healthy and pathological states. During the mapping procedure, the location of greatest sensitivity (requiring least stimulator current to produce MEP) to a target muscle is first identified using a thresholding procedure (see Kleim et al., 2007). After the “hotspot” is localized, stimulation proceeds at radially contiguous sites until the boundaries (areas that do not produce MEP) of the motor map are obtained. Figure 4 shows data from our lab combining both fMRI and TMS motor maps. Rehabilitation research has made use of single pulse cortical mapping to monitor change of maps over time in populations including stroke (Butler et al., 2005; Foltys et al., 2003), multiple sclerosis (Thickbroom et al., 2005), Parkinson’s disease (PD) (Fillippi et al., 2001), ALS (de Carvalho et al., 1999), and even those afflicted with polio (Oliveri et al., 1999).
Paired-pulse TMS is another technique that has been used in rehabilitation research. In a paired pulse TMS paradigm, two pulses are delivered to the motor cortex offset by a brief interstimulus interval. The initial pulse is called the conditioning pulse and is usually relative to the magnitude of current delivered to the coil during the second stimulation, which is called the test pulse. Researchers have discovered that variations in the temporal offset between the two pulses will alter the dynamics of the EMG output, as does variation of the relative magnetization strength between the conditioning and test stimulations. These changes can either produce a faciliatory or inhibitory effect deemed intracortical facilitation (ICF) or intracortical inhibition (ICI), respectively. Individuals afflicted with stroke (Manganotti et al., 2008; Swayne et al., 2008; Liepert, 2006) or Parkinson’s disease (Daeuper et al., 2002) appear to exhibit deviations in levels of both ICF and ICI as compared to healthy adults. Interestingly, however, treatments such as constraint-induced movement therapy (CIMT) in stroke (Liepert, 2006) or stimulation of the subthalamic nucleus after deep brain stimulation (Daeuper et al., 2002) have shown positive effect in normalizing ICI and ICF.
Repetitive TMS (rTMS) utilizes a train of TMS pulses delivered at either slow (up to 1Hz) or rapid (>1Hz) frequencies to alter the firing patterns of affected cortex. Slow frequency rTMS tends to depress the excitability of a target region, whereas high frequency has the opposite effect and tends to increase excitability in affected areas. As opposed to single or paired pulse TMS techniques, which are applied to the primary motor cortex and whose output measures are change in EMG signal, rTMS can be applied to any accessible cortical region including prefrontal, parietal, temporal or occipital areas. rTMS is being used with greater regularity as a treatment protocol for an increasing number of disorders. For example, in stroke recovery, Naeser et al. (2005) used low frequency rTMS with patients afflicted with Broca’s aphasia to improve naming ability without concomitant speech therapy. rTMS has also been used in the treatment depression (Paus & Barrett, 2004), Parkinson’s disease (Lefaucheur, 2006), chronic pain (Leo & Latif, 2007), dystonia (Lefaucheur et al., 2004), schizophrenia (Fitzgerald and Daskalakis, 2008) among many others.
Compared with other modalities of neurological research, TMS, as a technique, offers some significant advantages. First, single and paired-pulse TMS allows for direct inquiry into the activation of corticospinal tract, and its measure (EMG change) can increase or decrease in magnitude relative to the amount of current applied through the magnetic coil. Conversely, fMRI and PET are indirect measures of neural activity which rely on correlates of blood flow to index activity relative to a comparison state. Measured responses in these modalities are therefore dependent on and limited by the dynamics of the human cerebrovascular system. The motor-related TMS procedures also benefit from the TMS’ excellent temporal resolution in that MEP responses to TMS stimulation in target muscle typically occur from 18-30 milliseconds after stimulation. Responses in imaging modalities are limited to seconds (fMRI) or tens of seconds (PET) to derive their respective responses. Finally, TMS offers great practical advantages over other modalities in that the typical TMS apparatus is both inexpensive (tens of thousands as compared to millions for fMRI, MEG and PET) and quite portable (a single pulse system easily fits on a small cart).
However, TMS is beset by some limitations, as well. Primary amongst these is the fact that the magnetic field produced by TMS coils can only show effects at a depth of less than two centimeters from the stimulation site. TMS can therefore only be used with cortical areas, whereas fMRI and PET can also be used to probe subcortical processes. A second limitation of TMS is its spatial resolution, which due to size of the delivery coil and the diffusion of the generated magnetic field, is limited to about 1 cm2. Finally, great care has to be taken in the screening of participants and adherence to safety procedures due to the potential of headache and seizure from the stimulation, particularly for rTMS.
Given the strengths and limitations of TMS in comparisons with imaging modalities, many researchers have begun to combine the neurophysiological technique with fMRI, particularly when using single or paired pulse designs. Our lab has made use of the combination of the two techniques in the assessment of cortical maps of the FDI muscle (Figure 4). It is now possible to perform TMS stimulation while in the MRI scanner (Bohning et al., 1998), a procedure being employed with greater frequency as the technology becomes more widely available.
CONCLUSIONS
The potential impact of functional neuroimaging for rehabilitation research, and ultimately for rehabilitation care, can hardly be overestimated. If functional neuroimaging can help define how brain systems reorganize during rehabilitation, it may be possible to design treatments that enhance reorganization and thereby optimize rehabilitation. Further, it also is possible that functional imaging results will be found to predict rehabilitation outcome, including which treatments are optimal for patients with specific patterns of brain activity during tasks relevant to rehabilitation. Although the collection of a knowledge base adequate to impact clinical rehabilitation on a day-to-day basis may be years, or even decades away, important discoveries starting us down this path have already been made.
Advances in imaging over the past two or three decades have left rehabilitation investigators with a variety of tools to assess functional brain networks for behavior and cognition. These techniques include fMRI, PET, MEG, EEG, NIRS, and TMS, all of which were discussed briefly above. Each technique has strengths and weaknesses relating to cost, availability, temporal resolution, spatial resolution, and risk. For example, fMRI has become by far the most widely used technique for functional imaging because of its widespread availability, ability to perform structural images on the same acquisition platform as functional images, and excellent spatial resolution. However, fMRI does not have a temporal resolution capable of defining in real time the cascade of neuronal events in brain systems like EEG or MEG can, and the widely used BOLD contrast technique is vulnerable to signal loss near air-tissue interfaces. Although selection of an imaging technique for research is often a matter of instrument availability and convenience, the kind of information necessary to answer experimental questions also should be given some consideration in designing rehabilitation studies. In addition to functional neuroimaging techniques, new developments in structural neuroimaging, i.e., the use of diffusion imaging techniques to define degree of white matter loss/integrity, can also enhance our understanding of substrates for rehabilitation.
Rehabilitation clinicians and rehabilitation researchers who do not participate in imaging research are consumers of neuroimaging research in rehabilitation. To be discerning consumers, it is necessary to have some fundamental knowledge of functional neuroimaging techniques. We have tried not only to provide such fundamental information but also to refer the interested reader to additional sources of further information. The next decade or two is likely to bring important advances in our knowledge about brain systems, their plasticity, and their role in rehabilitation. Our ability to digest this information and use it in our clinical endeavors will enhance the lives of our patients.
Figure 7.
Cortical motor map of first dorsal interosseous (FDI) muscle using single pulse TMS overlaid onto 3-Dimensional rendering of fMRI activation during FDI exercise. Site 74 (blue circle) represents cortical site of maximal sensitivity to FDI activation (aka the “hotspot”). Green circles are areas that also generate MEP in the target muscle, while red circles are the map’s boundary. Orange areas represents active areas during fMRI at F > 5.0 (p<.0001, uncorrected).
Acknowledgments
Work on this manuscript was supported by Senior Research Career Scientist Award # B6364S (Crosson) and Center of Excellence Grant # B6793C from the VA Rehabilitation Research & Development Service, Grants # R01 DC007387 and # R01 DC009247 from the National Institute on Deafness and Other Communication Disorders, Grant # ME 3161/2-1 from German Science Foundation (DFG, Meinzer), and the Mobility Foundation Center, Dallas, TX. Also, Grant # DAMD17-01-1-0741 from the U.S. Army Medical Research and Materiel Command is acknowledged for the co-authors from the University of Texas Southwestern Medical Center. The content of this paper does not necessarily reflect the position or the policy of the U.S. government, and no official endorsement should be inferred.
References
- Abutalebi J, Rosa PA, Tettamanti M, Green DW, Cappa SF. Bilingual aphasia and language control: a follow-up fMRI and intrinsic connectivity study. Brain Lang. 2009;109:141–156. doi: 10.1016/j.bandl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Pizzella V, Gratta CD, Ferretti A, Romani GL. Fundamentals of electroencefalography, magnetoencefalography, and functional magnetic resonance imaging. Int Rev Neurobiol. 2009;86:67–80. doi: 10.1016/S0074-7742(09)86005-4. [DOI] [PubMed] [Google Scholar]
- Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, Bryan RN. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6:89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nature Neuroscience. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kannurpatti SS, Rypma B. Hemodynamic scaling of fMRI-BOLD signal: validation of low-frequency spectral amplitude as a scalability factor. Magn Reson Imaging. 2007;25:1358–1369. doi: 10.1016/j.mri.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, Haxthausen EU, Vincent DJ, George MS. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest Radiol. 1998;33:336–340. doi: 10.1097/00004424-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Bonoczk P, Panczel G, Nagy Z. Vinpocetine increases cerebral blood flow and oxygenation in stroke patients: a near infrared spectroscopy and transcranial Doppler study. Eur J Ultrasound. 2002;15:85–91. doi: 10.1016/s0929-8266(02)00006-x. [DOI] [PubMed] [Google Scholar]
- Breier JI, Castillo EM, Boake C, Billingsley R, Maher L, Francisco G, et al. Spatiotemporal patterns of language-specific brain activity in patients with chronic aphasia after stroke using magnetoencephalography. Neuroimage. 2004;23:1308–1316. doi: 10.1016/j.neuroimage.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Brown GG, Clark C, Liu TT. Measurement of cerebral perfusion with arterial spin labeling: Part2. Applications. Journal of the International Neuropsychological Society. 2007;13:526–538. doi: 10.1017/S1355617707070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulatout S, Meric P, Loubinoux I, Borredon J, Correze JL, Roucher P, Gillet B, Berenger G, Beloeil JC, Tiffon B, Mispelter J, Seylaz J. A one-dimensional (proton and phosphorus) and two-dimensional (proton) in vivo NMR spectroscopic study of reversible global cerebral ischemia. J Neurochem. 1996;66:2491–2499. doi: 10.1046/j.1471-4159.1996.66062491.x. [DOI] [PubMed] [Google Scholar]
- Butler AJ, Kahn S, Wolf SL, Weiss P. Finger extensor variability in TMS parameters among chronic stroke patients. J Neuroeng Rehabil. 2005;2:10. doi: 10.1186/1743-0003-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Introduction to functional magnetic resonance imaging: Principles and techniques. New York: Cambridge University Press; 2001. [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–32. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens TE, Altmann DR, Orrell RW, Howard RS, Johansen-Berg H, Miller DH, Matthews PM, Thompson AJ. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006;129:1859–1871. doi: 10.1093/brain/awl100. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, Nunes RG, Miller DH. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Human Brain Mapping. 2009;30:615–624. doi: 10.1002/hbm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen K, Laine M, Tarkiainen A, Jarvensivu T, Martin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15:444–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- Crosson B, Bacon Moore A, McGregor KM, Chang YL, Benjamin M, Gopinath K, et al. Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain and Language. 2009;111:73–85. doi: 10.1016/j.bandl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Fabrizio KS, Singletary F, Cato MA, Wierenga CE, Parkinson RB, et al. Treatment of naming in nonfluent aphasia through manipulation of intention and attention: a phase 1 comparison of two novel treatments. J Int Neuropsychol Soc. 2007;13:582–594. doi: 10.1017/S1355617707070737. [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang YL, et al. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17:157–177. doi: 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Däuper J, Peschel T, Schrader C, Kohlmetz C, Joppich G, Nager W, Dengler R, Rollnik JD. Effects of subthalamic nucleus (STN) stimulation on motor cortex excitability. Neurology. 2002;9:700–706. doi: 10.1212/wnl.59.5.700. [DOI] [PubMed] [Google Scholar]
- De SN, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, Miranda PC, Luís ML, Ducla-Soares E. Cortical muscle representation in amyotrophic lateral sclerosis patients: changes with disease evolution. Muscle Nerve. 1999;22:1684–1692. doi: 10.1002/(sici)1097-4598(199912)22:12<1684::aid-mus10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- de Jongh A, de Munck JC, Baayen JC, Puligheddu M, Jonkman EJ, Stam CJ. Localization of fast MEG waves in patients with brain tumors and epilepsy. Brain Topogr. 2003;15:173–179. doi: 10.1023/a:1022658217474. [DOI] [PubMed] [Google Scholar]
- Detre JA, Samuels OB, Alsop DC, Gonzalez-At JB, Kasner SE, Raps EC. Noninvasive magnetic resonance imaging evaluation of cerebral blood flow with acetazolamide challenge in patients with cerebrovascular stenosis. J Magn Reson Imaging. 1999;10(5):870–875. doi: 10.1002/(sici)1522-2586(199911)10:5<870::aid-jmri36>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- DiMartino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state fMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23(1):370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Behrens TE, Poupon C, Cointepas Y, Jbabdi S, Gaura V, Golestani N, Krystkowiak P, Verny C, Damier P, Bachoud-Levi A, Hantraye P, Remy P. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage. 2009;46:958–966. doi: 10.1016/j.neuroimage.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Tenforde A, et al. To modulate or not to modulate: differing results in uniquely shaped Williams syndrome brains. Neuroimage. 2006;32:1001–7. doi: 10.1016/j.neuroimage.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Eliassen JC, Boespflug EL, Lamy M, Allendorfer J, Chu WJ, Szaflarski JP. Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: a review. Top Stroke Rehabil. 2008;15:427–450. doi: 10.1310/tsr1505-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst TR, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson, Series B. 1993;102:1–8. [Google Scholar]
- Filippi MM, Oliveri M, Pasqualetti P, Cicinelli P, Traversa R, Vernieri F, Palmieri MG, Rossini PM. Effects of motor imagery on motor cortical output topography in Parkinson’s disease. Neurology. 2001;57:55–61. doi: 10.1212/wnl.57.1.55. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Daskalakis ZJ. A review of repetitive transcranial magnetic stimulation use in the treatment of schizophrenia. Can J Psychiatry. 2008;53:567–576. doi: 10.1177/070674370805300903. [DOI] [PubMed] [Google Scholar]
- Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, Töpper R. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114:2404–2415. doi: 10.1016/s1388-2457(03)00263-3. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Gideon P, Henriksen O, Sperling B, Christiansen P, Olsen TS, Jorgensen HS, rlien-Soborg P. Early time course of N-acetylaspartate, creatine and phosphocreatine, and compounds containing choline in the brain after acute stroke. A proton magnetic resonance spectroscopy study. Stroke. 1992;23:1566–1572. doi: 10.1161/01.str.23.11.1566. [DOI] [PubMed] [Google Scholar]
- Gillard JH, Waldman A, Barker P. In: Clinical MR Neuroimaging: Diffusion, Perfusion, and Spectroscopy. Gillard Jonathan H, Waldman Adam D, Barker Peter B., editors. Cambridge ; New York, NY: Cambridge University Press; 2005. [Google Scholar]
- Glover GH, Li T-Q, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI; RETOICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15(1):10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Graham GD, Blamire AM, Howseman AM, Rothman DL, Fayad PB, Brass LM, Petroff OA, Shulman RG, Prichard JW. Proton magnetic resonance spectroscopy of cerebral lactate and other metabolites in stroke patients. Stroke. 1992;23:333–340. doi: 10.1161/01.str.23.3.333. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hensel S, Rockstroh B, Berg P, Elbert T, Schonle PW. Left-hemispheric abnormal EEG activity in relation to impairment and recovery in aphasic patients. Psychophysiology. 2004;41(3):394–400. doi: 10.1111/j.1469-8986.2004.00164x. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Johnsrude I, Hauk O. Neuroimaging: Techniques for examininghuman brain function. In: Braisby N, editor. Cognitive Psychology: A Methods Companion. New York: Oxford University Press; 2005. [Google Scholar]
- Kamada K, Saguer M, Moller M, Wicklow K, Katenhauser M, Kober H, et al. Functional and metabolic analysis of cerebral ischemia using magnetoencephalography and proton magnetic resonance spectroscopy. Ann Neurol. 1997;42(4):554–563. doi: 10.1002/ana.410420405. [DOI] [PubMed] [Google Scholar]
- Kato H, Izumiyama M, Koizumi H, Takahashi A, Itoyama Y. Near-infrared spectroscopic topography as a tool to monitor motor reorganization after hemiparetic stroke: a comparison with functional MRI. Stroke. 2002;33:2032–2036. doi: 10.1161/01.str.0000021903.52901.97. [DOI] [PubMed] [Google Scholar]
- Kemeny S, Ye FQ, Birn R, Braun AR. Comparison of continuous overt speech fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2005;24(3):173–183. doi: 10.1002/hbm.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II Metabolite concentrations. J Magn Reson, Series B. 1993;102:9–19. [Google Scholar]
- Laganaro M, Morand S, Schwitter V, Zimmermann C, Camen C, Schnider A. Electrophysiological correlates of different anomic patterns in comparison with normal word production. Cortex. 2009;45(6):697–707. doi: 10.1016/j.cortex.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Leo RJ, Latif T. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. J Pain. 2007;8:453–459. doi: 10.1016/j.jpain.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Davis JT, Sloan JH, Kodituwakku PW, Orrison WW., Jr Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR Am J Neuroradiol. 1999;20(5):857–866. [PMC free article] [PubMed] [Google Scholar]
- Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19:41–7. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc. 2007;13(3):517–525. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D, Wong E, Buxton R. Perfusion imaging and stroke: A more sensitive measure of the brain bases of cognitive deficits. Aphasiology. 2002;16:873–883. doi: 10.1080/02687030244000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh BJ, Pattinson KT, Gallichan D, Ahmad I, Miller KL, Feinberg DA, et al. Measuring the effects of remifentanil on cerebral blood flow and arterial arrival time using 3D GRASE MRI with pulsed arterial spin labelling. J Cereb Blood Flow Metab. 2008;28(8):1514–1522. doi: 10.1038/jcbfm.2008.46. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Elbert T, Wienbruch C, Djundja D, Barthel G, Rockstroh B. Intensive language training enhances brain plasticity in chronic aphasia. BMC Biol. 2004;2:20. doi: 10.1186/1741-7007-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39(4):2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Meister IG, Sparing R, Foltys H, Gebert D, Huber W, Topper R, Boroojerdi B. Functional connectivity between cortical hand motor and language areas during recovery from aphasia. Journal of the Neurological Sciences. 2006;247:165–168. doi: 10.1016/j.jns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Kempler D, Jackson C, Hanson WR, Mazziotta JC, Phelps ME. Cerebral glucose metabolism in Wernicke’s, Broca’s, and conduction aphasia. Archives of Neurology. 1989;46:27–34. doi: 10.1001/archneur.1989.00520370029014. [DOI] [PubMed] [Google Scholar]
- Miller KL, Hargreaves BA, J L, Ress D, deCharms RC, Pauly JM. Functional brain imaging with BOSS FMRI. Proceedings of the 26th Annual International Conference of the IEEE EMBS. 2004 September 1-5;:5234–5237. doi: 10.1109/IEMBS.2004.1404463. [DOI] [PubMed] [Google Scholar]
- Miller KL, Hargreaves BA, Lee J, Ress D, deCharms RC, Pauly JM. Functional brain imaging using a blood oxygenation sensitive steady state. Magnetic Resonance in Medicine. 2003;50:675–683. doi: 10.1002/mrm.10602. [DOI] [PubMed] [Google Scholar]
- Miller KL, Smith SM, Jezzard P, Pauly JM. High-resolution FMRI at 1.5 T using balanced SSFP. Magnetic Resonance in Medicine. 2006;55:161–170. doi: 10.1002/mrm.20753. [DOI] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, Kubota K. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52:188–94. doi: 10.1002/ana.10274. [DOI] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34:2866–2870. doi: 10.1161/01.STR.0000100166.81077.8A. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz JM. Nuclear medicine in the rehabilitative treatment evaluation in stroke recovery. Role of diaschisis resolution and cerebral reorganization. Eura Medicophys. 2003;43:221–239. [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122(Pt 9):1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22(1):29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Nelles M, Gleseke J, Flacke S, Lachenmayer L, Schild HH, Urbach H. Diffusion tensor pyramidal tractography in patients wit anterior choroidal artery infarcts. American Journal of Neuroradiology. 2008;29:488–493. doi: 10.3174/ajnr.A0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Twieg DB, Carpenter PA. Baseline conditions and subtractive logic in neuroimaging. Hum Brain Mapp. 2001;14:228–235. doi: 10.1002/hbm.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Brighina F, La Bua V, Buffa D, Aloisio A, Fierro B. Reorganization of cortical motor area in prior polio patients. Clin Neurophysiol. 1999;110:806–12. doi: 10.1016/s1388-2457(98)00055-8. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SCR, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: A diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- Park SW, Butler AJ, Cavalheiro V, Alberts JL, Wolf SL. Changes in serial optical topography and TMS during task performance after constraint-induced movement therapy in stroke: a case study. Neurorehabil Neural Repair. 2004;18:95–105. doi: 10.1177/0888439004265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes LM. Quantification of cerebral perfusion using arterial spin labeling: two-compartment models. J Magn Reson Imaging. 2005;22(6):732–736. doi: 10.1002/jmri.20456. [DOI] [PubMed] [Google Scholar]
- Paus T, Barrett J. Transcranial magnetic stimulation (TMS) of the human frontal cortex: implications for repetitive TMS treatment of depression. J Psychiatry Neurosci. 2004;29:268–279. [PMC free article] [PubMed] [Google Scholar]
- Peck KK, Wierenga CE, Moore AB, Maher LM, Gopinath K, Gaiefsky M, et al. Comparison of baseline conditions to investigate syntactic production using functional magnetic resonance imaging. Neuroimage. 2004;23(1):104–110. doi: 10.1016/j.neuroimage.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95(3):853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JM, Deibler AR, Whitlow CT, Tan H, Kraft RA, Burdette JH, et al. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. AJNR Am J Neuroradiol. 2009;30(2):378–385. doi: 10.3174/ajnr.A1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Raichle ME. A brief history of human functional brain mapping. In: Toga AW, Mazziota JC, editors. Brain Mapping: The Systems. New York: Academic Press; 2000a. pp. 33–75. [Google Scholar]
- Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999;56:919–926. doi: 10.1001/archneur.56.8.919. [DOI] [PubMed] [Google Scholar]
- Saitou H, Yanagi H, Hara S, Tsuchiya S, Tomura S. Cerebral blood volume and oxygenation among poststroke hemiplegic patients: effects of 13 rehabilitation tasks measured by near-infrared spectroscopy. Arch Phys Med Rehabil. 2000;81:1348–1356. doi: 10.1053/apmr.2000.9400. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Murata Y, Fujiwara N, Hoshino T, Nakamura S, Kano T, Katayama Y. Comparison of blood-oxygen-level–dependent functional magnetic resonance imaging and near-infrared spectroscopy recording during functional brain activation in patients with stroke and brain tumors. J Biomed Optics. 2007;12:062110-1–062110-8. doi: 10.1117/1.2823036. [DOI] [PubMed] [Google Scholar]
- Saunders DE. MR spectroscopy in stroke. Br Med Bull. 2000;56:334–345. doi: 10.1258/0007142001903256. [DOI] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld MA, Noesselt T, Poggel D, Tempelmann C, Hopf J-M, Woldorff MG, Heinze H-J, Hillyard SA. Analysis of pathways mediating preserved vision after striate cortex lesions. Ann Neurol. 2002;52:814–824. doi: 10.1002/ana.10394. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TEJ. Acquisition and voxelwise analysis of multi-subject diffusion data with Tract-Based Spatial Statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Strangman G, Goldstein R, Rauch SL, Stein J. Near-infrared spectroscopy and imaging for investigating stroke rehabilitation: test-retest reliability and review of the literature. Arch Phys Med Rehabil. 2006;87(12 Suppl 2):S12–S19. doi: 10.1016/j.apmr.2006.07.269. [DOI] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, et al. Corticomotor organisation and motor function in multiple sclerosis. J Neurol. 2005;252:765–771. doi: 10.1007/s00415-005-0728-9. [DOI] [PubMed] [Google Scholar]; Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. NeuroImage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breathholding reduces group variance during a cognitive task. Hum Brain Mapp. 2007;28:59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- Vernieri F, Rosato N, Pauria F, Tibuzzi F, Passarellia F, Rossinim PM. Near infrared spectroscopy and transcranial Doppler in monohemispheric stroke. Eur Neurol. 1999;41:159–162. doi: 10.1159/000008041. [DOI] [PubMed] [Google Scholar]
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2(6):e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]