Abstract
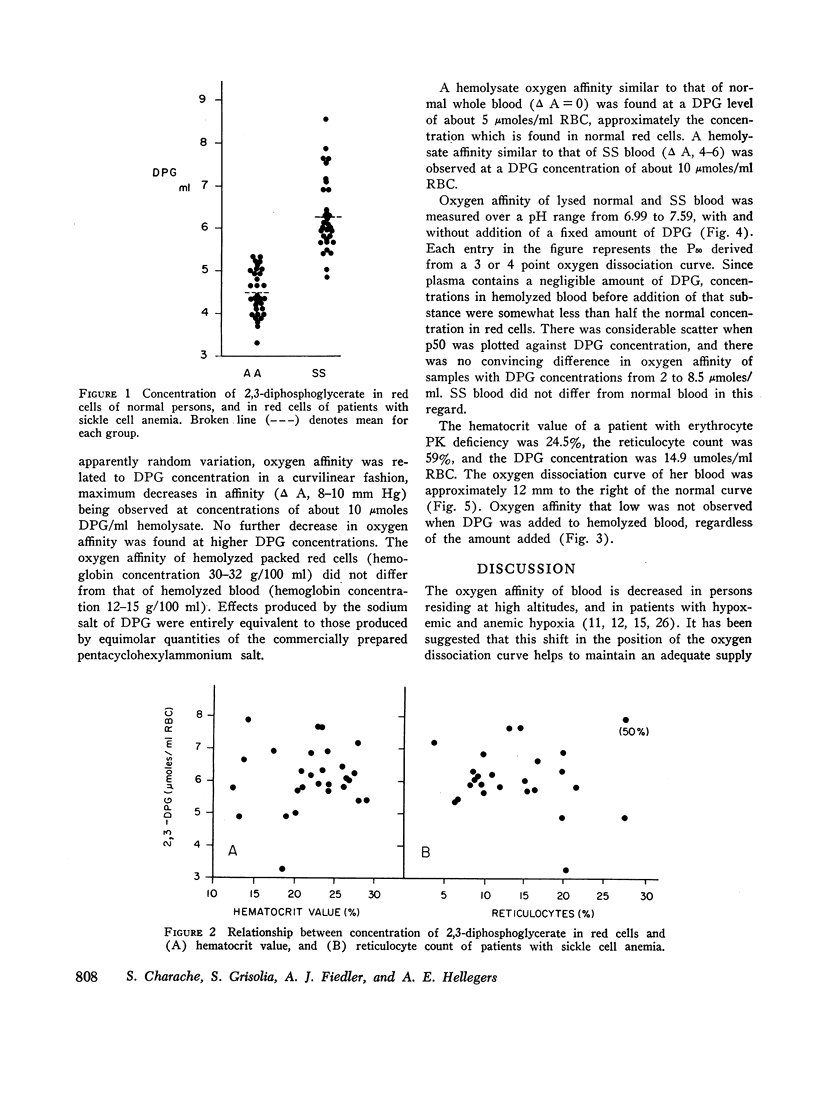
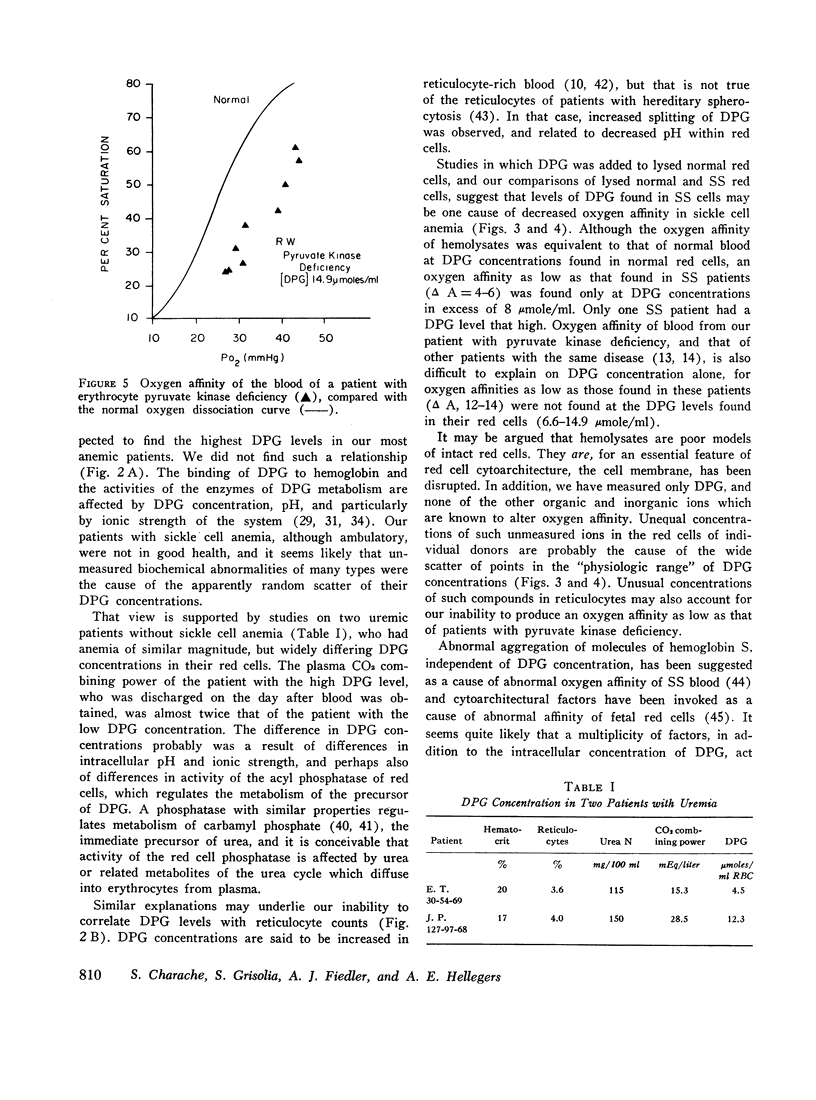
Blood of patients with sickle cell anemia (SS) exhibits decreased affinity for oxygen, although the oxygen affinity of hemoglobin S is the same as that of hemoglobin A. SS red cells contain more 2,3-diphosphoglycerate (DPG) than normal erythrocytes. The oxygen affinity of hemolyzed red cells is decreased by added DPG, and hemolysates prepared from SS red cells do not differ from normal hemolysates in this regard. Reduction of oxygen affinity to the levels found in intact SS red cells required DPG concentrations in excess of those found in most SS patients. The same was true of oxygen affinity of patients with pyruvate kinase deficiency. Other organic phosphates, as well as inorganic ions, are known to alter the oxygen affinity of dilute solutions of hemoglobin. These substances, the state of aggregation of hemoglobin molecules, and cytoarchitectural factors probably play roles in determining oxygen affinity of both normal and SS red cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., WYMAN J., Jr Equilibre de l'hémoglobine de drépanocytose avec l'oxygène. Rev Hematol. 1954;9(2):155–157. [PubMed] [Google Scholar]
- Akerblom O., de Verdier C. H., Garby L., Högman C. Restoration of defective oxygen-transport function of stored red blood cells by addition of inosine. Scand J Clin Lab Invest. 1968;21(3):245–248. doi: 10.3109/00365516809076991. [DOI] [PubMed] [Google Scholar]
- BATTAGLIA F. C., BEHRMAN R. E., HELLEGERS A. E., BATTAGLIA J. D. INTRACELLULAR HYDROGEN ION CONCENTRATION CHANGES DURING ACUTE RESPIRATORY ACIDOSIS AND ALKALOSIS. J Pediatr. 1965 Apr;66:737–742. doi: 10.1016/s0022-3476(65)80008-7. [DOI] [PubMed] [Google Scholar]
- BECKLAKE M. R., GRIFFITHS S. B., McGREGOR M., GOLDMAN H. I., SCHREVE J. P. Oxygen dissociation curves in sickle cell anemia and in subjects with the sickle cell trait. J Clin Invest. 1955 May;34(5):751–755. doi: 10.1172/JCI103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNSTEIN R. E. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959 Sep;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Enoki Y. The interaction of hemoglobin and its subunits with 2,3-diphosphoglycerate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1102–1106. doi: 10.1073/pnas.61.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967 Sep;70(3):480–488. [PubMed] [Google Scholar]
- Bunn H. F., May M. H., Kocholaty W. F., Shields C. E. Hemoglobin function in stored blood. J Clin Invest. 1969 Feb;48(2):311–321. doi: 10.1172/JCI105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales M., Grisolia S. Influence of ionic strength on apparent reaction mechanism of phosphoglycerate mutase. Biochemistry. 1966 Oct;5(10):3116–3122. doi: 10.1021/bi00874a006. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Hermann E. The interaction of organic and inorganic phosphates with hemoglobin. Arch Biochem Biophys. 1969 Apr;131(1):180–184. doi: 10.1016/0003-9861(69)90119-2. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M., Oski F. A., Gottlieb A. J. Oxygen-hemoglobulin dissociation curves: effect of inherited enzyme defects of the red cell. Science. 1969 Aug 8;165(3893):601–602. doi: 10.1126/science.165.3893.601. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Brewer G. J., Grover R. F. Role of red cell 2,3-diphosphoglycerate in the adaptation of man to altitude. J Lab Clin Med. 1969 Apr;73(4):603–609. [PubMed] [Google Scholar]
- Eaton J. W., Brewer G. J. The relationship between red cell 2,3-diphosphoglycerate and levels of hemoglobin in the human. Proc Natl Acad Sci U S A. 1968 Oct;61(2):756–760. doi: 10.1073/pnas.61.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. J., Novy M. J., Walters C. L., Metcalfe J. Improved oxygen release: an adaptation of mature red cells to hypoxia. J Clin Invest. 1968 Aug;47(8):1851–1857. doi: 10.1172/JCI105875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K., Duc G. Effect of iodoacetate and fluoride on the position of the haemoglobin oxygen dissociation curve of human whole blood. Nature. 1968 Aug 31;219(5157):936–938. doi: 10.1038/219936a0. [DOI] [PubMed] [Google Scholar]
- Forstner J. F., Fortier N. L., Lionetti F. J. Factors affecting the synthesis of 2,3-diphosphoglycerate in hemolysates of human erythrocytes. Biochim Biophys Acta. 1968 Jun 24;158(3):317–328. doi: 10.1016/0304-4165(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Grisolia S. Enzyme regulation by substrate; rapid inactivation of glutamate dehydrogenase by carbamyl phosphate. Biochem Biophys Res Commun. 1968 Jul 11;32(1):56–59. doi: 10.1016/0006-291x(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Moore K., Luque J., Grady H. Automatic procedure for the microestimation of 2,3-diphosphoglycerate. Anal Biochem. 1969 Oct 1;31(1):235–245. doi: 10.1016/0003-2697(69)90262-0. [DOI] [PubMed] [Google Scholar]
- HELLEGERS A. E., MESCHIA G., PRYSTOWSKY H., WOLKOFF A. S., BARRON D. H. A comparison of the oxygen dissociation curves of the bloods of maternal and fetal goats at various pHs. Q J Exp Physiol Cogn Med Sci. 1959 Jul;44:215–221. doi: 10.1113/expphysiol.1959.sp001393. [DOI] [PubMed] [Google Scholar]
- HELLEGERS A. E., SCHRUEFER J. J. Nomograms and empirical equations relating oxygen tension, percentage saturation, and pH in maternal and fetal blood. Am J Obstet Gynecol. 1961 Feb;81:377–384. doi: 10.1016/s0002-9378(16)36380-3. [DOI] [PubMed] [Google Scholar]
- JOYCE B. K., GRISOLIA S. The purification and properties of muscle diphosphoglycerate mutase. J Biol Chem. 1959 Jun;234(6):1330–1334. [PubMed] [Google Scholar]
- KENNEDY A. C., VALTIS D. J. The oxygen dissociation curve in anemia of various types. J Clin Invest. 1954 Oct;33(10):1372–1381. doi: 10.1172/JCI103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J., Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968 Dec;47(12):2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NECHTMAN C. M., HUISMAN T. H. COMPARATIVE STUDIES OF OXYGEN EQUILIBRIA OF HUMAN ADULT AND CORD BLOOD RED CELL HEMOLYSATES AND SUSPENSIONS. Clin Chim Acta. 1964 Aug;10:165–174. doi: 10.1016/0009-8981(64)90161-5. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Gottlieb A. J., Delivoria-Papadopoulos M., Miller W. W. Red-cell 2,3-diphosphoglycerate levels in subjects with chronic hypoxemia. N Engl J Med. 1969 May 22;280(21):1165–1166. doi: 10.1056/NEJM196905222802108. [DOI] [PubMed] [Google Scholar]
- Rose Z. B. The purification and properties of diphosphoglycerate mutase from human erythrocytes. J Biol Chem. 1968 Sep 25;243(18):4810–4820. [PubMed] [Google Scholar]
- Seeds A. E., Hellegers A. E., Battaglia F. C. Oxygen dissociation curves of lysed maternal and fetal erythrocytes. Am J Obstet Gynecol. 1969 Jan 1;103(1):68–70. doi: 10.1016/s0002-9378(16)34342-3. [DOI] [PubMed] [Google Scholar]
- Valeri C. R., Fortier N. L. Red-cell 2,3-diphosphoglycerate and creatine levels in patients with red-cell mass deficits or with cardiopulmonary insufficiency. N Engl J Med. 1969 Dec 25;281(26):1452–1455. doi: 10.1056/NEJM196912252812605. [DOI] [PubMed] [Google Scholar]


