Abstract
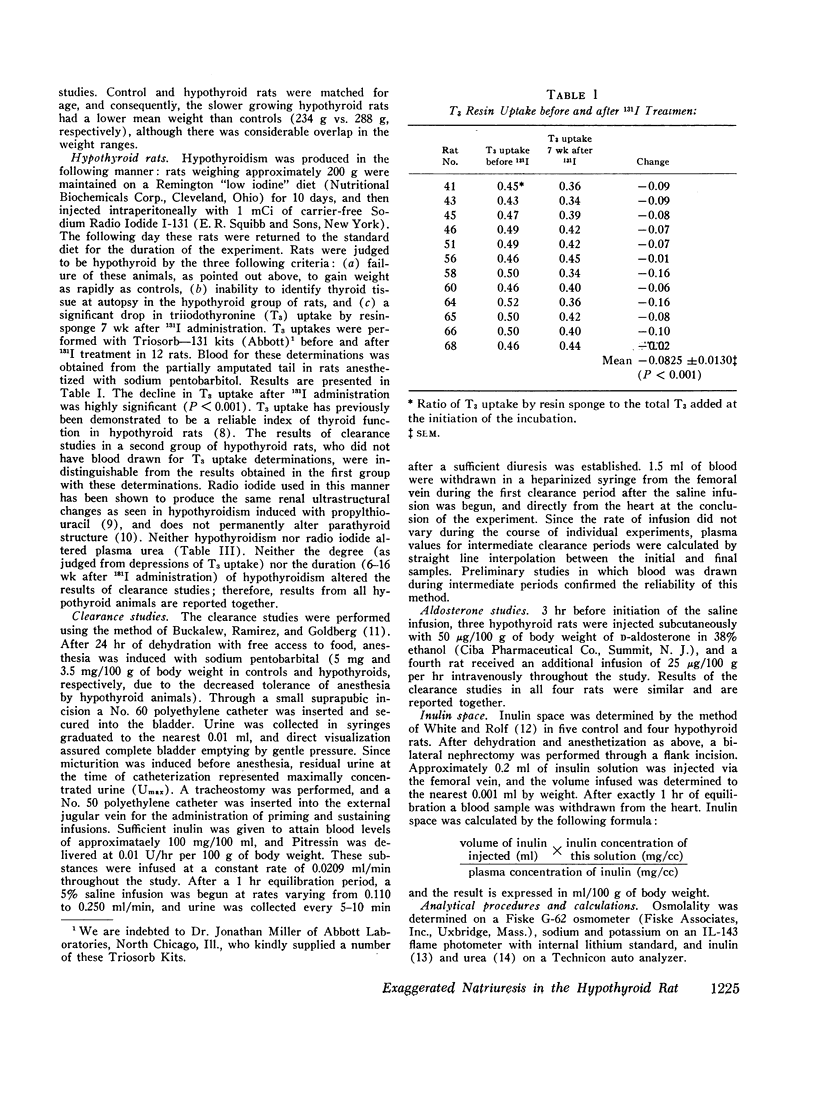
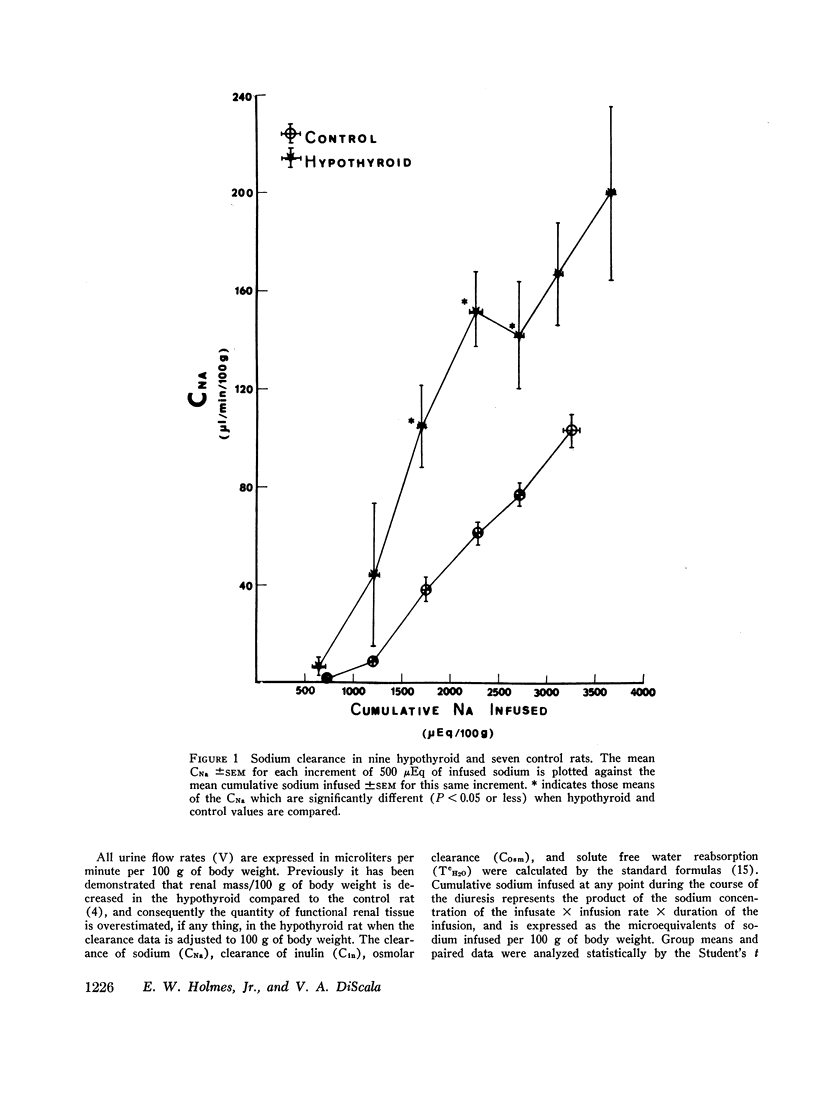
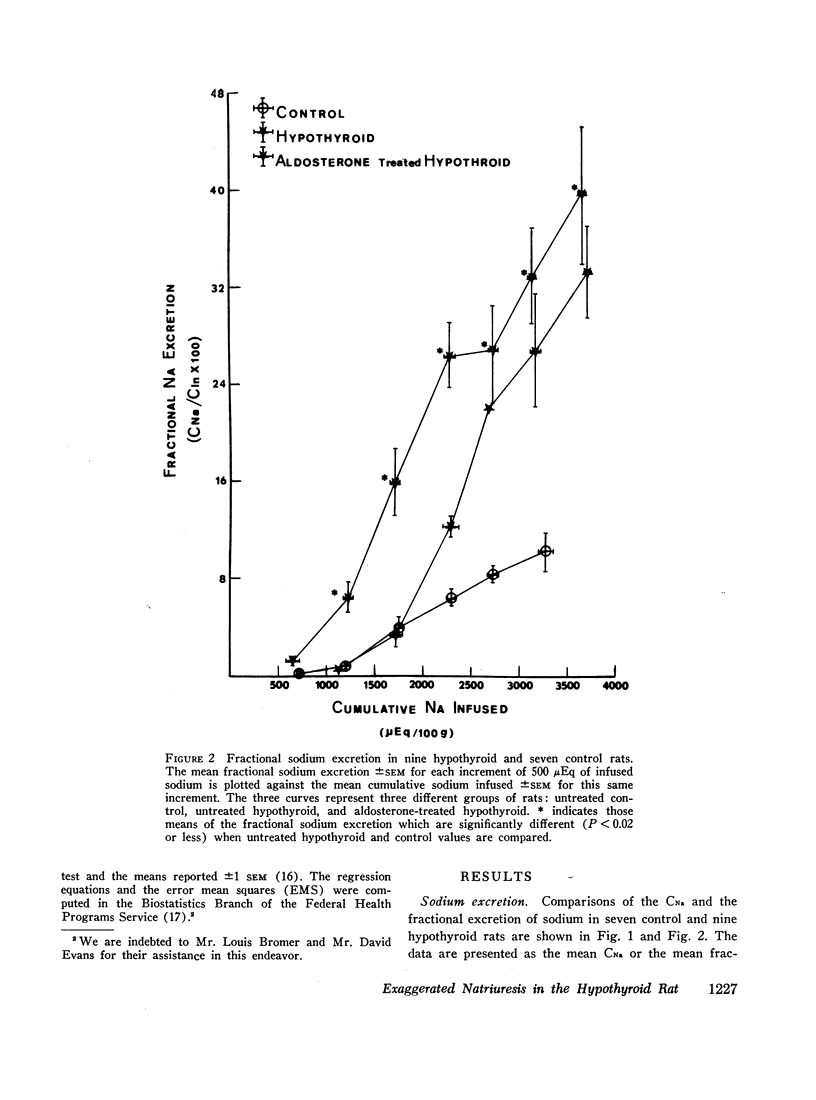
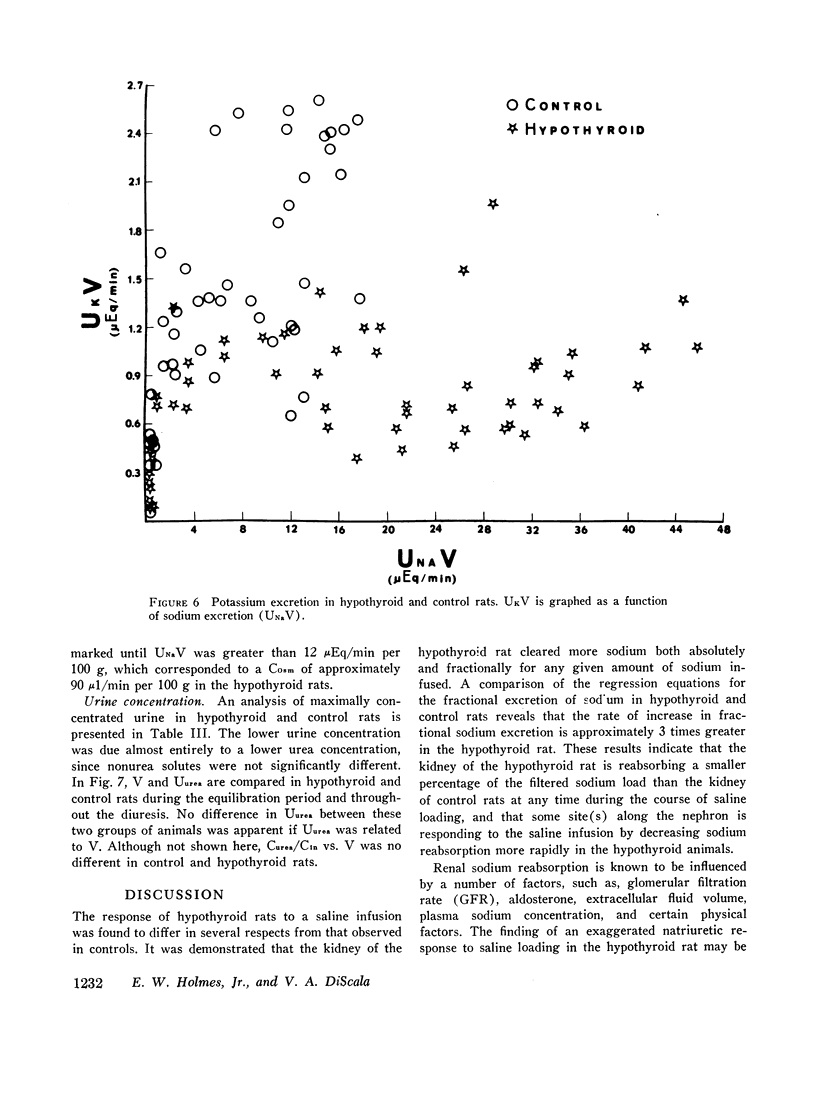
The exaggerated natriuresis of hypothyroid rats receiving a 5% saline infusion was studied to determine the mechanism and the site within the nephron responsible for this increase in sodium excretion. Sodium clearance (CNa) and fractional sodium excretion were both demonstrated to be greater in hypothyroid rats for any amount of sodium infused. The rate of increase in fractional sodium excretion in response to saline loading was 3.4 times greater in hypothyroid animals. At the conclusion of the diuresis some of the hypothyroid animals excreted greater than 45% of the filtered sodium load, while no control animal excreted more than 12% of the filtered sodium load.
The mean clearance of insulin during the saline diuresis was 36.6% lower (P < 0.001) in the hypothyroid rats. D-Aldosterone given to hypothyroid animals 3 hr before the experiment did not alter the magnitude or rate of increase in fractional sodium excretion. Inulin space determinations in nephrectomized rats revealed that extracellular fluid volume was contracted by 17.1% in the hypothyroid rats (P < 0.01). Plasma sodium was not significantly different in hypothyroid and control animals.
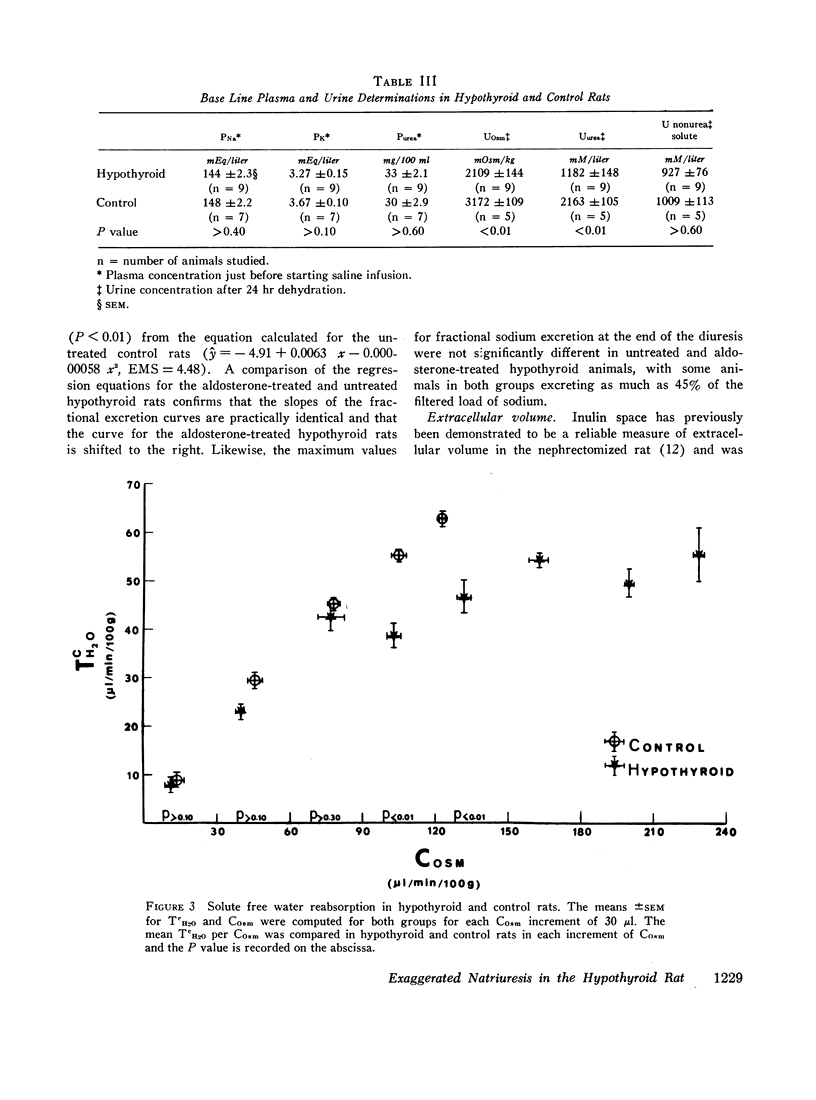
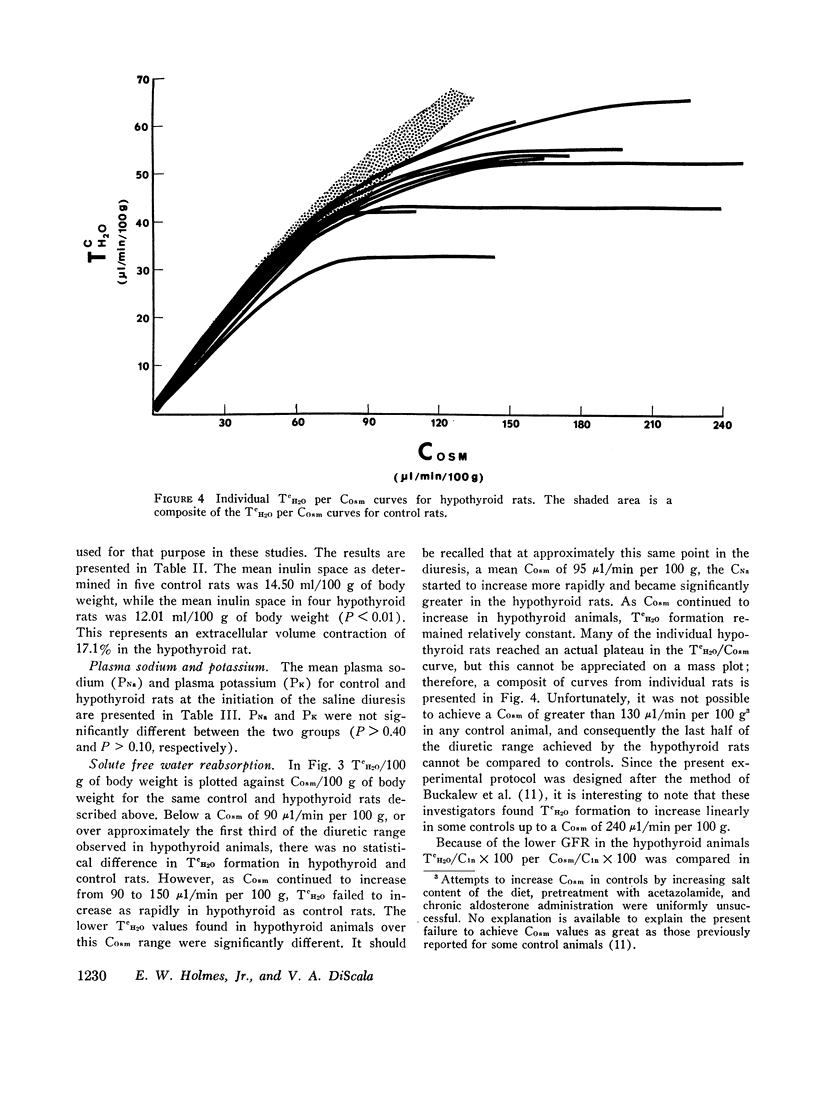
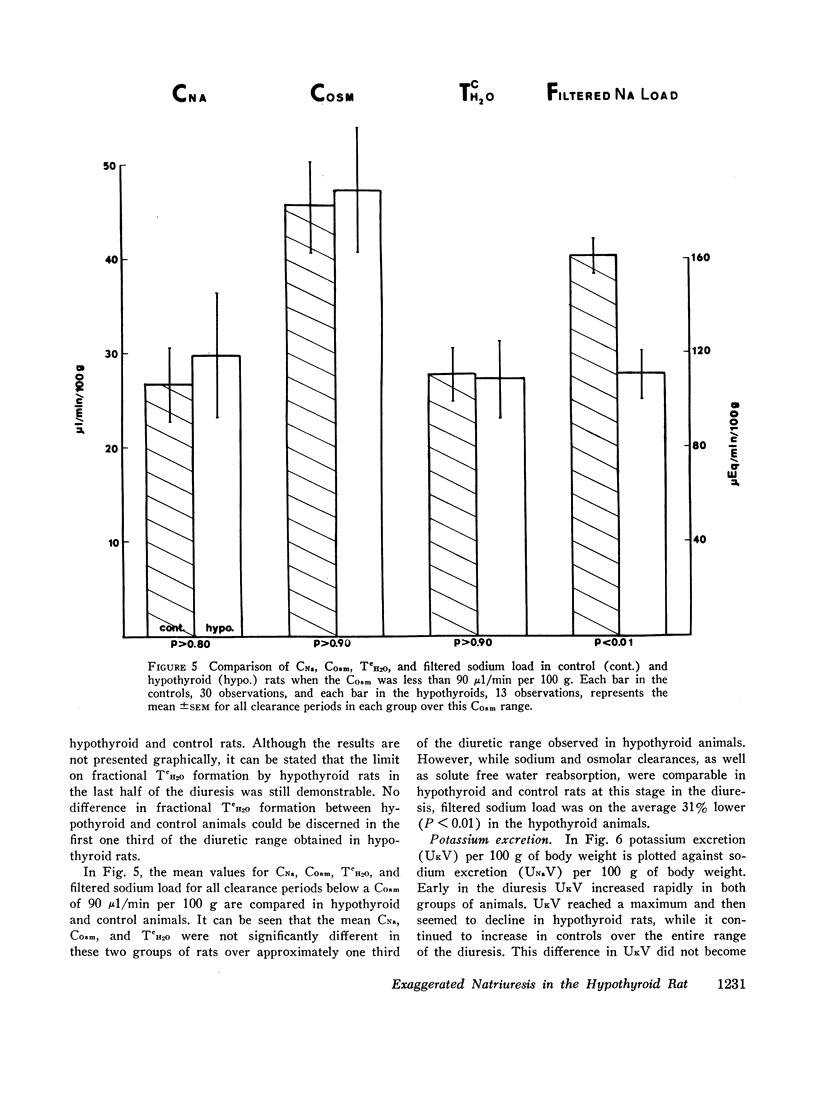
A limit on solute free water reabsorption (TeH2O) per osmolar clearance (COsm) was demonstrated in the hypothyroid rats when these animals excreted greater than 12% of the filtered osmotic load. The limit on TeH2O formation was associated with an acceleration in the rate of sodium excretion and a decline in the rate of potassium excretion. Early in the diuresis when COsm, CNa, and TeH2O were comparable in hypothyroid and control rats, the filtered sodium load was 31% lower (P < 0.01) in the hypothyroid animals.
These findings indicate that diminished thyroid hormone activity decreases renal sodium reabsorptive capacity. Indirect evidence suggests that the distal and possibly the proximal tubules are the sites of this diminished sodium reabsorption in hypothyroid animals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barger A. C. Renal hemodynamic factors in congestive heart failure. Ann N Y Acad Sci. 1966 Nov 22;139(2):276–284. doi: 10.1111/j.1749-6632.1966.tb41202.x. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Berliner R. W. Relationship between extracellular volume and fluid reabsorption by the rat nephron. Am J Physiol. 1969 Jul;217(1):6–12. doi: 10.1152/ajplegacy.1969.217.1.6. [DOI] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Ramirez M. A., Goldberg M. Free water reabsorption during solute diuresis in normal and potassium-depleted rats. Am J Physiol. 1967 Feb;212(2):381–386. doi: 10.1152/ajplegacy.1967.212.2.381. [DOI] [PubMed] [Google Scholar]
- Cortney M. A. Renal tubular transfer of water and electrolytes in adrenalectomized rats. Am J Physiol. 1969 Mar;216(3):589–598. doi: 10.1152/ajplegacy.1969.216.3.589. [DOI] [PubMed] [Google Scholar]
- FREGLY M. J., BRIMHALL R. L., GALINDO O. J. Effect of the antithyroid drug propylthiouracil on the sodium balance of rats. Endocrinology. 1962 Nov;71:693–700. doi: 10.1210/endo-71-5-693. [DOI] [PubMed] [Google Scholar]
- FREGLY M. J. Increased water exchange in rats treated with antithyroid drugs. J Pharmacol Exp Ther. 1961 Oct;134:69–76. [PubMed] [Google Scholar]
- Fjeldbo W., Stamey T. A. Adapted method for determination of inulin in serum and urine with an AutoAnalyzer. J Lab Clin Med. 1968 Aug;72(2):353–358. [PubMed] [Google Scholar]
- Fregly M. J., Cade J. R., Waters I. W., Straw J. A., Taylor R. E., Jr Secretion of aldosterone by adrenal glands of propylthiouracil-treated rats. Endocrinology. 1965 Nov;77(5):777–784. doi: 10.1210/endo-77-5-777. [DOI] [PubMed] [Google Scholar]
- GIEBISCH G., KLOSE R. M., WINDHAGER E. E. MICROPUNCTURE STUDY OF HYPERTONIC SODIUM CHLORIDE LOADING IN THE RAT. Am J Physiol. 1964 Apr;206:687–693. doi: 10.1152/ajplegacy.1964.206.4.687. [DOI] [PubMed] [Google Scholar]
- GOLDBERG R. C., CHAIKOFF I. L. Histopathological changes induced in the normal thyroid and other tissues of the rat by internal radiation with various doses of radioactive iodine. Endocrinology. 1950 Jan;46(1):72–90. doi: 10.1210/endo-46-1-72. [DOI] [PubMed] [Google Scholar]
- GREEN K., MATTY A. J. Action of thyroxine on active transport in isolated membranes of Bufo bufo. Gen Comp Endocrinol. 1963 Jun;3:244–252. doi: 10.1016/0016-6480(63)90019-4. [DOI] [PubMed] [Google Scholar]
- Horster M., Thurau K. Micropuncture studies on the filtration rate of single superficial and juxtamedullary glomeruli in the rat kidney. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):162–181. doi: 10.1007/BF00362733. [DOI] [PubMed] [Google Scholar]
- JAHN H., REVILLE P., STEPHAN F. ACTION DE L'INSUFFISANCE THYROUIDIENNE CHRONIQUE SUR LA CONCENTRATION OSMOLAIRE TOTALE ET LA CONCENTRATION DE SODIUM DES URINES DU RAT AU COURS DE LA POLYURIE OSMOTIQUE PAR LE MANNITOL. Rev Fr Etud Clin Biol. 1964 Feb;9:181–187. [PubMed] [Google Scholar]
- LARAGH J. H., KELLY W. G. ALDOSTERONE: ITS BIOCHEMISTRY AND PHYSIOLOGY. Adv Metab Disord. 1964;15:217–262. doi: 10.1016/b978-1-4831-6748-0.50012-4. [DOI] [PubMed] [Google Scholar]
- MARSH W. H., FINGERHUT B., MILLER H. AUTOMATED AND MANUAL DIRECT METHODS FOR THE DETERMINATION OF BLOOD UREA. Clin Chem. 1965 Jun;11:624–627. [PubMed] [Google Scholar]
- MARUSIC E., TORRETTI J. SYNERGISTIC ACTION OF VASOPRESSIN AND THYROXINE ON WATER TRANSFER ON THE ISOLATED TOAD BLADDER. Nature. 1964 Jun 13;202:1118–1119. doi: 10.1038/2021118a0. [DOI] [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Microperfusion study of distal tubular potassium and sodium transfer in rat kidney. Am J Physiol. 1966 Sep;211(3):548–559. doi: 10.1152/ajplegacy.1966.211.3.548. [DOI] [PubMed] [Google Scholar]
- Martino J. A., Earley L. E. Demonstraton of a role of physical factors as determinants of the natriuretic response to volume expansion. J Clin Invest. 1967 Dec;46(12):1963–1978. doi: 10.1172/JCI105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSORIO J. A., ZADUNAISKY J. A. Clearances de inulina y diodrast y peso renal en ratas tratadas con I131 y polvo de tiroides. Rev Soc Argent Biol. 1956 Sep-Nov;32(6-8):195–203. [PubMed] [Google Scholar]
- Reville P., Stephan F. Détermination du gradient intrarénal de concentration de l'urée et du sodium chez des rats hypothyroïdiens et des rats surrénalectomisés. C R Seances Soc Biol Fil. 1967;161(1):174–179. [PubMed] [Google Scholar]
- Reville P., Stephan F. Etude comparative de la composition du plasma de rats hypothyroïdiens et dd rats surrénalectomisés. C R Seances Soc Biol Fil. 1968 Oct 19;162(3):754–759. [PubMed] [Google Scholar]
- Réville P., Stephan F., Jahn H. Etude comparative des effets de l'hyperthyroïdisme et de l'hypothyroïdisme sur le débit et la concentration des urines du rat. Arch Int Physiol Biochim. 1965 Jan;73(1):97–116. doi: 10.3109/13813456509079335. [DOI] [PubMed] [Google Scholar]
- STEPHAN F., JAHN H., METZ B. Action de l'insuffisance thyroïdienne sur l'élimination urinaire de l'eau, du sodium et du potassium chez le rat. C R Seances Soc Biol Fil. 1959;153(2):332–334. [PubMed] [Google Scholar]
- STEPHAN F., JAHN H., REVILLE P., URBAN M. [Effect of thyroid insufficiency on the concentration of urea, sodium and potassium in the urine of the rat]. C R Seances Soc Biol Fil. 1961;155:1555–1560. [PubMed] [Google Scholar]
- Schrier R. W., Fein R. L., McNeil J. S., Cirksena W. J. Influence of interstitial fluid volume expansion and plasma sodium concentration on the natriuretic response to volume expansion in dogs. Clin Sci. 1969 Jun;36(3):371–385. [PubMed] [Google Scholar]
- Sealey J. E., Kirshman J. D., Laragh J. H. Natriuretic activity in plasma and urine of salt-loaded man and sheep. J Clin Invest. 1969 Dec;48(12):2210–2224. doi: 10.1172/JCI106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. E., Jr, FREGLY M. J. RENAL RESPONSE OF PROPYLTHIOURACIL-TREATED RATS TO INJECTED MINERALOCORTICOIDS. Endocrinology. 1964 Jul;75:33–41. doi: 10.1210/endo-75-1-33. [DOI] [PubMed] [Google Scholar]
- WHITE H. L., ROLF D. Whole body and tissue inulin and sucrose spaces in the rat. Am J Physiol. 1957 Jan;188(1):151–155. doi: 10.1152/ajplegacy.1956.188.1.151. [DOI] [PubMed] [Google Scholar]


