Abstract
Fluorescence quenching groups are widely employed in biological detection, sensing, and imaging. To date, a relatively small number of such groups are in common use. Perhaps the most commonly used quencher, dabcyl, has limited efficiency with a broad range of fluorophores. Here we describe a molecular approach to improve the efficiency of quenchers by increasing their electronic complexity. Multiple pathway quenchers (MPQ) are designed to have multiple donor or acceptor groups in their structure, allowing for a multiplicity of conjugation pathways of varied length. This has the effect of broadening the absorption spectrum, which in turn can increase quenching efficiency and versatility. Six such MPQ derivatives are synthesized and tested for quenching efficiency in a DNA hybridization context. Duplexes placing quenchers and fluorophores within contact distance or beyond this distance are used to measure quenching via contact or FRET mechanisms. Results show that several of the quenchers are considerably more efficient than dabcyl at quenching a wider range of common fluorophores, and two quench fluorescein and TAMRA as well as or better than a Black Hole Quencher.
Introduction
Fluorescence quenchers are employed in a wide variety of fluorometric assays, particularly for detection of nucleic acids, reporting on enzymatic activity and detecting other molecules of interest with turn-on responses.1-11 However, for optimal performance, delicate matching of fluorophore and quencher is often needed, imposing limits on molecular designs and complicating applications.12-14 For example, in order to cover the emission spectrum from violet to near infrared, multiple different quenchers are needed, which can be an obstacle in assays designed to detect multiple analytes in one sample.14,15 4-(dimethylamino)-azobenzene-4’-carboxylic acid (Dabcyl), perhaps the most commonly used fluorescence quencher, is generally limited to quenching of fluorophores that emit in the violet to green region of the visible spectrum (approximately 390 nm to 520 nm).11,16 To cover fluorophores that emit further to the red, a variety of other quenchers have been developed, such as the Black Hole Quenchers (BHQ)16 and QSY quenchers17; however, coverage of a broad spectrum still requires the use of multiple quenchers. In the BHQ series, for example, a total of four different compounds are necessary to provide for quenching of all commonly used wavelengths in fluorescence assays.13 QSY quenchers are even more limited in quenching ability as a result of their narrow absorbance spectra.12,14 Recently, some non-fluorescent cyanine based quenchers have been reported that do have broadened spectral properties and provide for efficient quenching in caspase assays.8 However, these quenchers display strong spectral overlap only with near-IR emitting fluorophores and may not quench other fluorophores as effectively.
Most commonly, quenching of fluorophores occurs by one of two mechanisms: contact quenching and FRET quenching.12-14,18,19 In contact quenching, the fluorophore and quencher are in sufficiently close proximity to allow for direct electronic interaction of the excited state of the fluorophore with the quencher molecule.18-21 Consequently, absorbed light at the excitation wavelength will primarily be non-radiatively transferred as heat to the surrounding environment, with only a limited amount of energy released as fluorescence. When the distance between the fluorophore and quencher is increased, generally to the range of 20-100 Å, the alternative mechanism of FRET quenching is observed.12,14,18 In this mechanism, quenching efficiency is dependent upon the orientation of the fluorophore and quencher, the distance between them, and the spectral overlap of the emission spectrum of the fluorophore and the absorption spectrum of the quencher.12,14 To take advantage of these mechanisms it is desirable to design quenchers with broadened absorbance spectra to allow for a greater range of fluorophore emissions that can be quenched by the FRET mechanism, while maintaining strong contact quenching.12,14
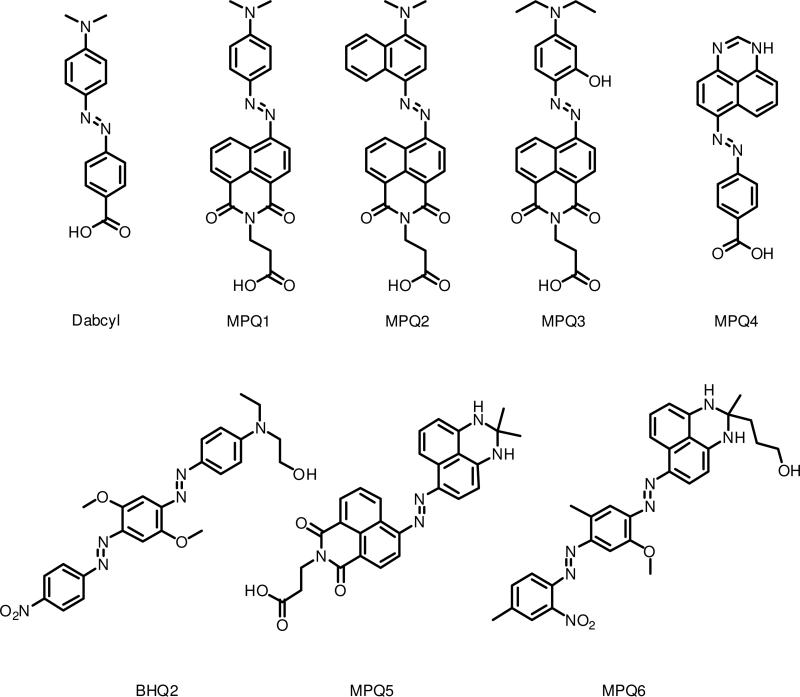
To address this issue, we have designed a novel set of quenchers that have multiple electronic conjugation pathways. By altering the existing scaffolds of common quenchers to increase the number of electron donor and acceptor groups, a series of Multiple Pathway Quenchers (MPQs) has been designed that provides for broader absorbance properties, in turn allowing for fluorescence quenching of a considerably wider range of fluorophores for each given quencher (Figure 1). Five new quenchers based on modifications to the dabcyl structure as well as one based upon modification of a Black Hole Quencher scaffold have been prepared and tested in a DNA context for quenching efficiency, for comparison with previous studies.3 We find that this strategy yields broadened absorption spectra and enhances efficiency over a wider range of wavelengths as compared with a common quencher such as dabcyl.
Figure 1.
Structures of Multiple Pathway Quenchers (MPQs) and commercially available quenchers used in this study.
Experimental Procedures
Chemicals and Reagents
Chemicals were purchased from Sigma-Aldrich and used without further purification. Solvents were purchased from Acros Organics and used as received. UltraMild DNA phosphoramidites and solid supports were purchased from Glen Research. BHQ2 phosphoramidite and 3’-Quasar 670 CPG were purchased from Biosearch Technologies. AlexaFluor 350 succinimidyl ester was purchased from Invitrogen. 5’-fluorophore modified oligonucleotides were purchased from Biosearch Technologies (FAM, TAMRA, Quasar 670) or Stanford University Protein and Nucleic Acid Facility (Cy3) and purified by HPLC.
Instrumentation
1H- and 13C-NMR spectra were recorded on a Varian 400 MHz NMR or a Varian 500 MHz NMR spectrometer and internally referenced to the residual solvent signal; J values are reported in Hz. Oligonucleotides sequences were synthesized on an ABI 394 DNA synthesizer using UltraMild reagents and phosphoramidites. ESI-MS analysis was performed by the Stanford University Mass Spectroscopy Facility. Analytical and semi-preparative high performance liquid chromatography was performed on a LC-CAD Shimadzu liquid chromatograph, equipped with a SPD-M10A VD diode array detector and a SCL 10A VP system controller using reverse phase C18 columns (Grace ProSphere C18-300 10 μ). DNA concentrations were determined on a Cary 100 Bio UV-Visible Spectrophotometer at 90 °C. Fluorescence measurements were performed on a Fluorolog 3 Jobin Yvon fluorophotospectrometer equipped with an external temperature controller. Oligonucleotide masses were determined by the Stanford University Protein and Nucleic Acid Facility using a Perspective Voyager-DE RP Biospectrometry MALDI-TOF mass-spectrometry instrument using a 3-hydroxypicolinic acid/diammonium hydrogen citrate matrix.
Synthetic Procedures
Methyl 3-(6-amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate (1)
0.196 g (0.81 mmol) of 4-nitronaphthalic anhydride23,24 and 0.107 g (1.5 eq, 1.21 mmol) of β-alanine were refluxed in 4 mL ethanol for 3 hours. The resulting orange suspension was cooled to room temperature, diluted with water, and filtered to provide 0.185 grams (73%) of the nitro intermediate. The intermediate was suspended in 3 mL methanol, and 0.7 mL concentrated HCl and 0.569 g (5.1 eq, 3 mmol) tin (II) chloride dihydrate was added and the solution was heated to reflux for 2 hours. Cooling to room temperature and dilution with water followed by filtration provided 0.125 g (71%) of a bright yellow-brown solid. 1H NMR (DMSO-d6, 400MHz): 8.61 (1H, d, J = 8.4), 8.41 (1H, d, J = 7.6), 8.18 (1H, d, J = 8.4), 7.64 (1H, dd, J = 7.6), 7.46 (2H, brs, NH), 6.83 (1H, d, J = 8.4), 4.24 (2H, t, J = 7.2), 3.57 (3H, s), 2.61 (2H, t, J = 7.2). 13C NMR (DMSO-d6, 100MHz): 32.3, 35.3, 51.5, 107.3, 108.2, 119.3, 121.6, 124.0, 129.4, 129.7, 131.0, 134.0, 152.8, 162.7, 163.7, 171.4. ESI MS: M + H+ = 299.58 (Calc 299.10).
General Procedure for MPQ methyl ester synthesis25
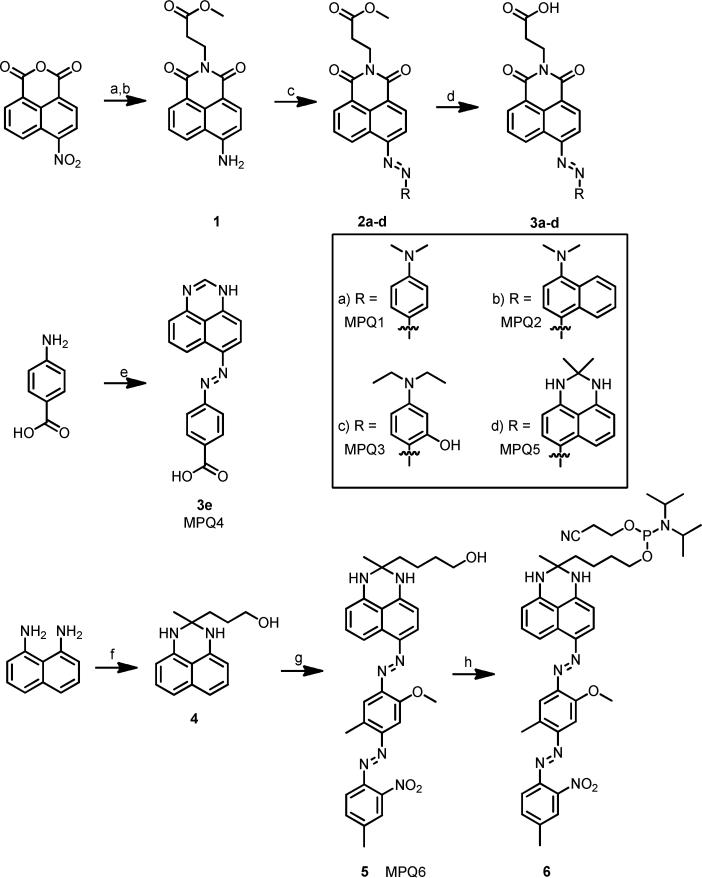
1.5 mL concentrated H2SO4 was cooled to 0 °C in an ice-water bath. 0.249 g (1.05 eq, 3.6 mmol) sodium nitrite was slowly added. The viscous suspension was heated to 65°C until complete dissolution occurred, then the solution was cooled to 0 °C. 1.02 g (3.04 mmol) of 1 was added in portions over thirty min. 2 mL Acetic acid was added to aid in solubility. After stirring at room temperature for 3 hours, a solution of 3.4 mmol coupling partner in 0.65 mL acetic acid was added dropwise at 0 °C. The resulting suspension was stirred for 1 hour, then diluted with saturated sodium acetate and stirred at 0 °C to 10°C for 3 hours. The resulting solution was filtered, washed with warm water, and crystallized in ethanol to afford the azo dye methyl ester 2.
MPQ1 Methyl Ester (2a)
(50% yield) 1H NMR (CDCl3, 400 MHz): 9.25 (1H, d, J = 7.2), 8.63 – 8.67 (2H, m), 8.04 (2H, d, J = 7.6), 7.96 (1H, d, J = 8.4), 7.83 (1H, dd, J = 7.2), 6.80 (2H, d, J = 7.6), 4.52 (2H, t, J = 7.4), 3.71 (3H, s), 3.17 (6H, s), 2.80 (2H, t, J = 7.4). 13C NMR (CDCl3, 100 MHz): 32.7, 36.2, 40.4, 51.9, 111.6, 112.3, 121.8, 122.3, 126.5, 126.8, 131.0, 131.6, 132.1, 144.7, 152.2, 153.5, 164.0, 164.4, 171.9. ESI MS: M + H+ = 431.42 (Calc 431.17).
MPQ2 Methyl Ester (2b)
(62% yield) 1H NMR (CDCl3, 400 MHz): 9.34 (1H, d, J = 8.8), 9.11 (1H, d, J = 8.0), 8.67 – 8.70 (2H, m), 8.22 (1H, d, J = 8.4), 8.10 – 8.14 (2H, m), 7.89 (1H, dd, J = 8.0), 7.69 (1H, dd, J = 8.0), 7.59 (1H, dd, J = 7.6), 7.11 (1H, d, J = 8.4), 4.54 (2H, t, J = 7.4), 3.71 (3H, s), 3.11 (6H, s), 2.81 (2H, t, J = 7.4). 13C NMR (CDCl3, 100 MHz): 32.7, 36.3, 44.8, 52.0, 112.8, 112.9, 114.0, 122.6, 123.8, 125.0, 125.6, 127.2. 127.6, 131.0, 131.7, 132.0, 143.3, 152.0, 156.4, 163.9, 164.3, 167.7, 171.9. ESI MS: M + H+ = 481.92 (Calc 481.19).
MPQ3 Methyl Ester (2c)
(52% yield) 1H NMR (CDCl3, 400 MHz): 8.59 – 8.63 (3H, m), 8.09 (1H, d, J = 8.4), 7.78 (1H, dd, J = 7.8), 7.38 (1H, d, J = 9.6), 6.53 (1H, dd, J = 9.6, 2.6), 5.97 (1H, d, J = 2.6), 4.50 (2H, t, J = 7.4), 3.70 (3H, s), 3.51 (4H, q, J = 7.2), 2.78 (2H, t, J = 7.4), 1.30 (6H, t, J = 7.2). 13C NMR (CDCl3, 100 MHz): 13.1, 32.7, 36.2, 45.6, 51.9, 98.1, 110.8, 111.6, 122.7, 126.9, 128.6, 131.6, 132.6, 154.9, 163.8, 171.9. ESI MS: M + H+ = 475.42 (Calc 475.20).
MPQ5 Methyl Ester (2d)
(20% yield) 1H NMR (CDCl3, 400 MHz): 9.34 (1H, d, J = 8.4), 8.66 – 8.68 (2H, m), 8.40 (d, 1H, J = 8.4), 8.19 (d, 1H, J = 8.4), 8.09 (d, 1H, J = 8.0), 7.84 (dd, 1H, J = 7.6), 7.50 (dd, 1H, J = 7.6), 6.62 (d, 1H, J = 7.2), 6.55 (d, 1H, J = 8.4), 4.53 (t, 2H, J = 7.2), 3.71 (s, 3H), 2.81 (t, 2H, J = 7.2), 1.61 (s, 6H). 13C NMR: (spectrum could not be obtained due to poor solubility). ESI MS: M + H+ = 508.42 (Calc 508.20).
General Procedure for Methyl Ester Hydrolysis
500 mg MPQ methyl ester was dissolved in anhydrous THF (at a concentration of 0.085 M). To this solution, 2.02 equiv potassium trimethylsilanolate was added, and the resulting solution stirred overnight at room temperature, at which point TLC indicated consumption of starting material. The solvent was removed in vacuo and the resulting solid dissolved in a minimal amount of water. 1 M hydrochloric acid was added to precipitate the free carboxylic acid, which was filtered, washed with water, and dried. The product 3 was used for DNA conjugation without further purification. All free acids were found to be poorly soluble in common deuterated solvents.
MPQ1 (3a)
(80% yield) 1H NMR (CDCl3, 500 MHz): 9.26 – 9.35 (1H, m), 8.64 – 8.96 (2H, m), 7.96 – 8.05 (3H, m), 7.84 – 7.91 (1H, m), 6.80 (2H, d, J = 9.0), 4.54 (2H, t, J = 7.0), 3.18 (s, 6H), 2.87 (2H, t, J = 7.0). NMR indicates some degradation to naphthalic anhydride during prolonged storage. 13C NMR: (spectrum could not be obtained due to poor solubility). ESI MS: M + H+ = 417.33 (Calc 417.16).
MPQ2 (3b)
(36% yield) 1H NMR (CDCl3, 500 MHz): 9.45 (1H, d, J = 8.5), 9.12 (1H, d, J = 7.5), 8.71 – 8.75 (2H, m), 8.24 (1H, d, J = 8.0), 8.14 – 8.18 (2H, m), 7.96 (1H, dd, J = 8.0), 7.73 (1H, dd, J = 7.0, 1.5), 7.63 (1H, dd, J = 7.0, 1.5), 7.14 (1H, dd, J = 8.5), 4.59 (2H, t, J = 7.0), 3.17 (6H, s), 2.91 (2H, t, J = 7.0). 13C NMR: (spectrum could not be obtained due to poor solubility). NMR and ESI-MS indicate that this compound degrades to the naphthalic anhydride during prolonged storage. ESI MS: M + H+ = 467.50 (Calc 467.17).
MPQ3 (3c)
(80% yield) 1H NMR (CDCl3, 500 MHz): 8.62 – 8.66 (3H, m), 8.12 (1H, dd, J = 8.5, 2.5), 7.80 (1H, dd, J = 7.0), 7.41 (1H, d, J = 9.5), 6.55 (1H, dd, J = 9.5, 2.5), 5.99 (1H, d, J = 2.5), 4.51 (2H, t, J = 7), 3.52 (4H, q, J = 7), 2.79 (2H, t, J = 7), 1.31 (6H, t, J = 7). 13C NMR: (spectrum could not be obtained due to poor solubility). ESI MS: M + H+ = 461.33 (Calc 461.18)
MPQ5 (3d)
(87% yield) 1H and 13C NMR data could not be obtained as a result of very poor solubility in common NMR solvents. ESI MS: M + H+ = 494.42 (Calc 494.18).
MPQ4 (3e)
A solution of 0.270 g (1.05 eq, 3.90 mmol) NaNO2 in 2 mL H2O was added dropwise to a mixture of 0.510 g (1.0 eq, 3.72 mmol) 4-aminobenzoic acid in 12 mL 1M HCl at 0 °C. After 30 minutes, a solution of 0.657 g (1.05 eq, 3.90 mmol) of 1-H-perimidine in 9 mL 1M HCl was added and the resulting suspension stirred at 0 °C for 2 hours. The suspension was diluted with a solution of 2.15 g NaOAc in 15 mL H2O, stirred at room temperature for 2 hours, then filtered and washed with warm water to give a dark purple solid. Crystallization from ethanol afforded 0.916 g (78%). A complex 1H NMR spectrum results from tautomerization in the perimidine scaffold (see Supporting Information). 13C NMR data could not be obtained as a result of poor solubility in common NMR solvents. ESI MS: M + H+ = 317.25 (Calc 317.10).
3-(2-methyl-2,3-dihydro-1H-perimidin-2-yl)propan-1-ol (4)
6.19 g (39 mmol) 1,8-diaminonaphthalene was added to a solution of 4.35 mL (1.1 eq, 43 mmol) 5-hydroxy-2-pentanone and 0.089 g (0.01 eq, 0.4 mmol) p-toluenesulfonic acid monohydrate in 15 mL ethanol. The solution was stirred at 60 °C for 90 minutes, whereupon the solution had solidified. The resulting solid was cooled to room temperature, filtered, washed with ethanol, and crystallized from aqueous ethanol to yield a pale gray solid (4.11 g, 43%). 1H (DMSO-d6, 400 MHz): 7.07 (2H, dd, J = 7.8), 6.83 (2H, d, J = 8.4), 6.34 (2H, d, J = 7.2), 6.29 (2H, brs, NH), 4.37 (1H, t, OH), 3.30 (2H, m), 1.53 – 1.62 (4H, m), 1.29 (3H, s). 13C NMR (DMSO-d6, 100 MHz): 26.5, 27.1, 36.9, 61.2, 65.5, 103.6, 111.5, 114.0, 127.0, 134.2, 141.8. ESI MS: M + H+ = 243.50 (Calc 243.15).
MPQ6 (5)
0.291 g (1.20 mmol) of 4 was dissolved in a solution of 20 mL 1:1 THF:acetone stirred at 0°C. 0.600 g (1.2 eq, 1.44 mmol) Fast Corinth V was added in portions to give a dark blue solution, which was stirred at 0 °C for 1 hour. The reaction was diluted with water and extracted 3 times with CH2Cl2. The combined organic extracts were washed once with water, once with brine, dried (MgSO4), filtered and the solvent removed in vacuo. Column chromatography (5% MeOH in CH2Cl2 containing 0.5% Et3N) afforded 0.094 g (14%) of a dark purple solid. 1H NMR (CDCl3, 400 MHz): 8.19 (1H, d, J = 8.0), 7.84 (1H, d, J = 8.8), 7.66 – 7.69 (3H, m), 7.40 – 7.47 (3H, m), 6.53 – 6.56 (2H, m), 4.02 (3H, s), 3.66 (2H, t, J = 6.0), 2.74 (3H, s), 2.50 (3H, s), 1.88 – 1.92 (2H, m), 1.75 – 1.80 (2H, m), 1.52 (3H, s). 13C NMR (CDCl3, 100 MHz): 17.0, 21.3, 27.2, 27.6, 29.8, 38.6, 56.3, 62.7, 99.0, 112.8, 119.2, 124.4, 133.6, 141.3, 143.6, 147.4. ESI MS: M + H+ = 554.42 (Calc 554.25).
MPQ6 Phosphoramidite (6)
0.164 g (0.296 mmol) MPQ6 was dissolved in 2.5 mL anhydrous acetonitrile, 0.15 mL (3 eq, 0.861 mmol) N,N-diisopropylethlyamine was added and the solution was stirred at room temperature under argon. 0.10 mL (1.5 eq, 0.448 mmol) 2-cyanoethyl N,N-diisopropylchlorophosphoramidite was added and the resulting solution was stirred at room temperature under argon for 2 hours. The solution was concentrated and purified by column chromatography (2:3 EtOAc:Hexanes → 2:1 EtOAc:Hexanes, containing 0.5% Et3N) to yield 0.036 g product (13%). 31P NMR displayed two peaks at 148.61 ppm and 148.55 ppm, and the product was used for DNA synthesis without further characterization.
DNA Conjugation
TAMRA-, Fluorescein- and Quasar 670-conjugated DNA sequences were synthesized using their respective 3’ CPGs, cleaved, and purified by HPLC using a gradient elution of 0.05 M TEAA and acetonitrile. AlexaFluor 350 succinimidyl ester and ATTO 590 succinimidyl ester were reacted with 3’-amino modified DNA. Cy3 modified DNA was synthesized using Cy3 Phosphoramidite (Glen Research) with 5’ to 3’ synthesis. Quencher labeled oligonucleotides were synthesized with a 5’-amino-modifier 5 appended to the 5’-terminus. The monomethoxy-trityl protecting group was removed on the synthesizer using alternating 10 s cycles of deprotection reagent (3 % trichloroacetic acid in DCM) and DCM washes for 3 min. The solid support was added to a suspension containing 35 mM quencher-COOH, 35 mM 1-hydroxy-7-azabenzotriazole (HOAt), 35 mM 2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) and 150 mM diisopropylethylamine (DIPEA) in 750 L anhydrous DMF. Solutions were shaken at room temperature and protected from light for 16 h, then rinsed 4 × 500 L DMF and 4 × 500 L AcCN. The labeled DNA was cleaved from the solid support using UltraMild conditions (0.05M K2CO3 in MeOH) at room temperature for 4 h, filtered to remove CPG, diluted with water and purified by reverse phase HPLC.
Fluorescence Experiments
A solution of 30 nM of fluorescently labeled 20mer was created in a total volume of 800 L hybridization buffer (10 mM MgCl2, 70 mM Tris•Borate, pH 7.55) at 37 °C. The fluorescence of the solution was taken in triplicate (using published excitation and emission values with excitation and emission slits of 2 nm and 5 nm, respectively) and averaged. A concentrated solution of quencher labeled DNA was added to bring the final volume to 150 nM. The oligonucleotides were allowed to hybridize until the fluorescence emission remained constant, then the fluorescence spectrum was taken in triplicate and averaged. Quenching efficiency was calculated at the emission maxima by dividing the quenched fluorescence by the initial fluorescence, multiplying by 100, then subtracting from 100.14 All experiments were performed in triplicate and averaged.
Results
Quencher design
The new quencher structures are given in Figure 1. These were designed with the standard diazo dye framework analogous to the common dabcyl quencher, but with larger conjugated multifunctional donors or acceptors. The purpose of the design was to provide multiple conjugated pathways from donor to acceptor groups, thus potentially broadening the absorption spectra of the dyes. The first five (MPQ1-5) were built on the standard azobenzene framework, while the sixth (MPQ6) was designed as a multi-pathway variant of the bisazobenzene framework common to BHQ quenchers.
Synthesis
As shown in Scheme 1, reaction of β-alanine with 4-nitronaphthalic anhydride and subsequent reduction of the nitro group easily afforded 1 which could be diazotized to allow for formation of MPQs by reaction with a variety of anilines. Similar derivatives of these compounds were reported earlier for use as disperse dyes, and the properties of the compounds synthesized are consistent with those previously reported.25-26 Hydrolysis of the methyl ester afforded a carboxylic acid moiety that could easily be attached to amino-modified DNA by HATU/HOAt coupling. DNA synthesis utilized UltraMild protecting groups to avoid hydrolysis of the naphthalimide core under regular ammonia or ammonia/methylamine cleavage/deprotection. MPQ6 was easily prepared by the addition of dihydroperimidine 4 to Fast Corinth V salt in a cold solution of 1:1 THF:Acetone, and readily converted to a phosphoramidite for DNA coupling, again with UltraMild conditions. DNA sequences were selected to maintain consistency with previous oligonucleotide-based experiments for comparing fluorescence quenchers.14 For comparison with the new quenchers, we prepared analogous DNA conjugates of dabcyl and of BHQ2, possibly the two most widely used quenchers. BHQ2 was selected instead of BHQ1 based upon the better performance of BHQ2 against a variety of fluorophores in previous studies.14 For testing the quenchers we prepared DNA conjugates of common fluorescent dyes with varied emission properties, emitting from the blue to the far red (442 nm to 662 nm).
Scheme 1.
Synthesis of Multiple Pathway Quenchers. a) β-alanine, EtOH, (73%). b) SnCl2, MeOH, HCl, (71%). c) i) NaNO2, H2SO4 ii) aniline derivative iii) NaOAc (20-62%). d) KOTMS, THF (36-87%). e) i) NaNO2, HCl ii) 1H-perimidine, NaOAc. f) 5-hydroxy-2-pentanone, pTsOH (43%). g) Fast Corinth V, 1:1 THF:acetone, 0°C (14%). h) 2-cyanoethyl N,N-diisopropylchlorophosphoramidite, DIPEA.
Spectral Properties
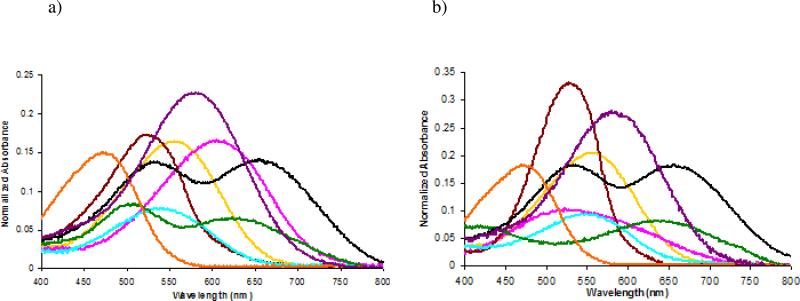
Absorption data for the six MPQs conjugated to DNAs are given in Figure 2 and Table 1. The data reveal that, as designed, the MPQ dyes have considerably broadened absorbance spectra compared to their parent quencher molecules (dabcyl and BHQ). Save for MPQ3, all other quenchers containing the naphthalimide core show significantly increased Δλ1/2 max values when compared to dabcyl, validating the design strategy. This is particularly the case for MPQ2 and MPQ5, whose Δλ1/2 max values are considerably greater and even further red-shifted than the other dyes.
Figure 2.
Absorption spectra (normalized to absorbance at 260nm) of quenchers on (a) 20mer and (b) 15mer oligonucleotide sequences. MPQ1 ( ), MPQ2 (
), MPQ2 ( ), MPQ3 (
), MPQ3 ( ), MPQ4 (
), MPQ4 ( ), MPQ5 (
), MPQ5 ( ), MPQ6 (
), MPQ6 ( ), Dabcyl (
), Dabcyl ( ), BHQ2 (
), BHQ2 ( ).
).
Table 1.
Spectral properties of quenchers conjugated at the 5’-terminus of 20mer and 15mer oligonucleotides.
| 20mer | 15mer | |||
|---|---|---|---|---|
| Quencher | λmax | Δλ(half max) | λmax | Δλ(half max) |
| MPQ1 | 557 nm | 481 - 618 (137 nm) | 560 nm | 481 - 619 (138 nm) |
| MPQ2 | 614 nm | 522 - 676 (154 nm) | 524 nm | 446 - 640 (194 nm) |
| MPQ3 | 521 nm | 461 - 574 (113 nm) | 529 nm | 478 - 571 (93 nm) |
| MPQ4 | 539 nm | 467 - 606 (139 nm) | 551 nm | 469 - 611 (142 nm) |
| MPQ5 | 503 nm, 623 nm | 447 - 689 (242 nm) | 646 nm | 524 - 723 (199 nm) |
| MPQ6 | 536 nm, 656 nm | 463 - 731 (268 nm) | 537 nm, 657 nm | 463 - 736 (273 nm) |
| Dabcyl | 472 nm | 405 - 520 (115 nm) | 472 nm | 405 - 519 (114 nm) |
| BHQ2 | 580 nm | 503 - 649 (146 nm) | 581 nm | 507 - 652 (145 nm) |
For the naphthalimide derivatives, where the electron acceptor has been altered to incorporate a second conjugation pathway, we noted some differences in spectral properties between the 20mer and 15mer quencher-oligonucleotide conjugates. As anticipated, the parent quencher dabcyl shows negligible change in spectral properties, maintaining an absorbance maxima of 472 nm and a Δλ1/2 max of 115 nm in both sequences. Both MPQ1 and MPQ3, however, display slight red-shifts upon moving from the 20mer oligonucleotide to a different 15mer sequence context. MPQ2, in contrast, shows a considerable blue shift and significant broadening on going from the 20mer to the 15mer DNA.
Altering the electron donating group also provides for some interesting and broadened spectral properties. As seen with MPQ4, a considerable red shift is observed in the absorbance maximum while an almost 35 nm increase in Δλ1/2 max is observed compared to dabcyl. Much like MPQ1 - 3, this quencher also shows a change when moved from the 20mer to a 15mer, with the Δλ1/2 max increasing with the absorbance maximum again shifting to the red.
MPQ5 and MPQ6, incorporating the dihydroperimidine moiety, displayed the most distinct spectral properties. Whereas all other quenchers possess a single broadened absorbance maximum, MPQ5 and MPQ6 display two maxima. MPQ5, like the other quenchers, exhibits a slightly altered absorbance spectrum as the sequence of the oligonucleotide changes, while MPQ6, like BHQ2, maintains approximately the same spectrum on the two DNAs. For these last two quenchers the formation of a red-shifted absorbance maximum comes with a moderate cost to the extinction coefficient. When compared to MPQ1, MPQ5 clearly displays a lower extinction coefficient at the Δλmax, both on DNA and in ethanol (see Supporting Information). Comparison of BHQ2 and MPQ6 yields the same result, indicating that the addition of the new conjugation pathway may reduce the extinction coefficient of each individual peak.
The most obvious benefit of the added conjugation is observed in a comparison of MPQ6 with its parent BHQ1. While BHQ1 displays an absorption maximum at 534 nm and generally quenches fluorophores that emit in the 100 nm range from 480 nm to 580 nm,16 MPQ6 displays considerably broadened properties. MPQ6 shares one conjugation pathway with BHQ1, giving rise to an absorbance maximum at 537 nm, but the alternative conjugation pathway inherent to the dihydroperimidine scaffold adds an absorption at 656 nm as well, extending the range of quenching available to the dye greatly (vide infra). Thus MPQ6 has a Δλ1/2 max ranging from 465 nm to 735 nm (270 nm total), considerably greater than BHQ2 (146 nm) and in fact greater than the quenching ranges of BHQ1 and BHQ2 combined.
Fluorescence experiments confirmed that these quenchers are completely non-emissive, as excitation of solutions containing the DNA-labeled quenchers at 100 nM concentration showed no measurable emission above that of buffer (see Supporting Information). Extinction coefficients were determined at the absorbance maximum of each compound in ethanol and can be found in the Supporting Information; note that they differ slightly from the absorbance maxima found when the quenchers are placed on DNA in Figure 2 and Table 1.
Quenching Efficiency
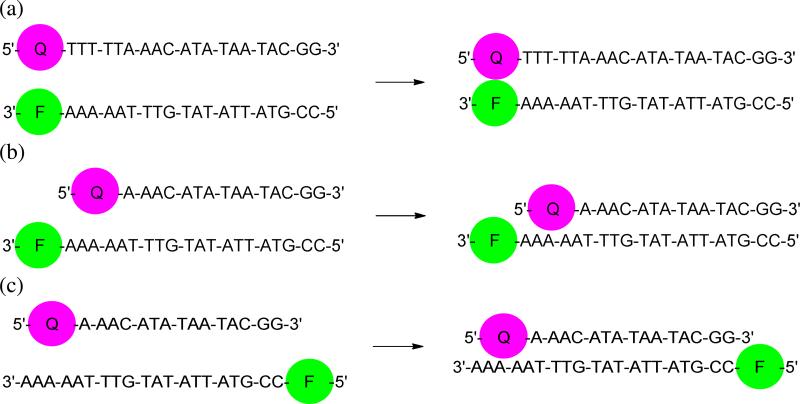
Quenching efficiency was determined by use of DNA hybridization as a means of controlling fluorophore-quencher distance (Figure 3).14 As such, fluorophores were attached to the 3’ end of the sequence 5’-CCG-TAT-TAT-ATG-TTT-AAA-AA-3’ and quenchers were attached to the 5’ end of complementary 15mer or 20mer sequences. Use of a 20mer context (placing dye and quencher immediately adjacent to one another at the duplex terminus) allowed for analysis of contact quenching. The selected 15mer sequence had been previously reported to allow for examination of FRET quenching,14 but the observation of ground state complex formation between quencher labeled 15mers and fluorophore containing 20mers indicates a strong degree of contact quenching as well, consistent with other quencher studies and indicating a mixed mechanism of quenching for the sequences shown in Figure 3b (see Supporting Information).27 To allow for analysis of quenching entirely by FRET, 5’ fluorophore labeled sequenches were used (see Figure 3c), separating the quencher and fluorophore by 15 bp of duplex structure. Contact quenching data would be most relevant for molecular applications where dye and quencher are in very close proximity, whereas FRET data may be most predictive of applications in which the two are held at some distance. Since FRET efficiency depends on spectral overlap, one expects that FRET quenching performance would be more strongly dependent on absorption characteristics than contact quenching, providing a better means of examining the newly created quenchers.
Figure 3.
DNA hybridization formats used for determining (a) contact quenching and (b) mixed mechanism quenching and (c) FRET quenching efficiencies of MP quenchers.
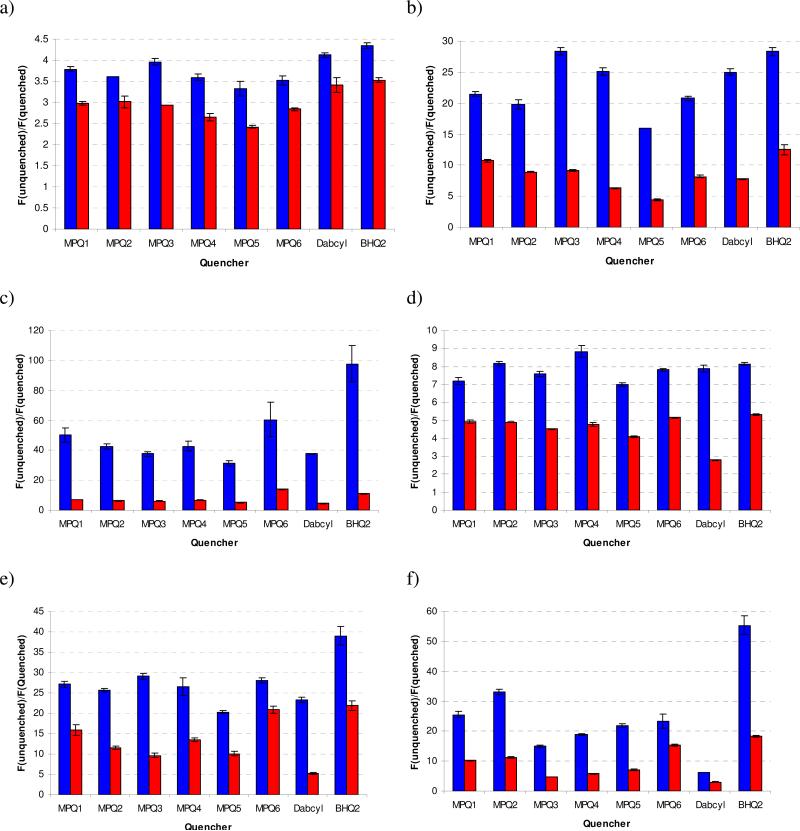
Quenching efficiencies were measured as fraction (percent) loss of emission intensity upon hybridization to the fluorophore of interest, as displayed in Table 2 (contact quenching), Table 3 (mixed mechanism quenching) and Table 4 (FRET quenching). We also compared the fluorescence ratios (fluorescence in the unquenched state divided by fluorescence in the quenched state) of the quenchers against each fluorophore (Fig. 3), which better illustrates the differences among the dyes, and which is often the most important factor in a fluorometric assay.
Table 2.
Contact quenching efficiencies of fluorophore/quencher pairs. Results are provided as the average of three experiments with standard deviation shown. See Fig. 3a for structural context.
| Alexa 350 | FAM | Cy 3 | TAMRA | Atto 590 | Quasar 670 | |
|---|---|---|---|---|---|---|
| Quencher | λem = 442nm | λem = 517nm | λem = 563nm | λem = 580nm | λem = 624nm | λem = 662nm |
| MPQ1 | 73.6 ± 0.7% | 95.4 ± 0.2% | 98.0 ± 0.4% | 86.0 ± 0.7% | 96.3 ± 0.2% | 96.1 ± 0.3% |
| MPQ2 | 72.4 ± 0.1% | 94.9 ± 0.4% | 97.6 ± 0.2% | 87.8 ± 0.4% | 96.1 ± 0.1% | 97.0 ± 0.2% |
| MPQ3 | 74.7 ± 0.9% | 96.5 ± 0.2% | 97.3 ± 0.2% | 86.8 ± 0.5% | 96.6 ± 0.2% | 93.3 ± 0.4% |
| MPQ4 | 72.1 ± 1.2% | 96.0 ± 0.2% | 97.6 ± 0.3% | 88.8 ± 1.0% | 96.2 ± 0.7% | 94.7 ± 0.2% |
| MPQ5 | 69.7 ± 3.5% | 93.7 ± 0.1% | 96.8 ± 0.4% | 85.7 ± 0.4% | 95.1 ± 0.2% | 95.4 ± 0.3% |
| MPQ6 | 71.6 ± 1.8% | 95.2 ± 0.2% | 98.2 ± 0.6% | 87.2 ± 0.2% | 96.4 ± 0.2% | 95.6 ± 0.8% |
| Dabcyl | 75.7 ± 0.7% | 96.0 ± 0.2% | 97.4 ± 0.1% | 87.3 ± 0.6% | 95.7 ± 0.3% | 83.9 ± 0.4% |
| BHQ2 | 77.0 ± 0.7% | 96.5 ± 0.2% | 98.9 ± 0.3% | 87.8 ± 0.2% | 97.4 ± 0.3% | 98.2 ± 0.2% |
Table 3.
Mixed mechanism quenching efficiencies of fluorophore/quencher pairs. Results are provided as the average of three experiments with standard deviation shown. See Fig. 3b for structural context.
| Alexa 350 | FAM | Cy 3 | TAMRA | Atto 590 | Quasar 670 | |
|---|---|---|---|---|---|---|
| Quencher | λem = 442nm | λem = 517nm | λem = 563nm | λem = 580nm | λem = 624nm | λem = 662nm |
| MPQ1 | 66.4 ± 0.9% | 90.7 ± 0.4% | 85.6 ± 0.4% | 79.7 ± 0.7% | 93.6 ± 1.0% | 90.2 ± 0.5% |
| MPQ2 | 66.6 ± 3.1% | 89.1 ± 0.3% | 84.0 ± 0.7% | 79.6 ± 0.2% | 91.3 ± 0.8% | 91.1 ± 0.3% |
| MPQ3 | 66.0 ± 0.1% | 89.1 ± 0.4% | 83.5 ± 1.0% | 77.9 ± 0.3% | 89.5 ± 1.1% | 78.7 ± 0.5% |
| MPQ4 | 62.2 ± 2.5% | 84.1 ± 0.2% | 85.1 ± 0.6% | 79.1 ± 0.9% | 92.6 ± 0.6% | 82.8 ± 1.3% |
| MPQ5 | 58.7 ± 1.2% | 77.4 ± 1.8% | 80.2 ± 0.3% | 75.5 ± 0.6% | 90.0 ± 1.1% | 85.9 ± 0.7% |
| MPQ6 | 64.8 ± 0.8% | 87.8 ± 0.8% | 92.9 ± 0.4% | 80.6 ± 0.3% | 95.2 ± 0.4% | 93.5 ± 0.3% |
| Dabcyl | 70.4 ± 3.2% | 87.1 ± 0.3% | 77.8 ± 0.9% | 64.3 ± 0.7% | 80.7 ± 1.7% | 66.5 ± 3.7% |
| BHQ2 | 71.6 ± 0.8% | 91.9 ± 1.1% | 90.8 ± 0.6% | 81.2 ± 0.3% | 95.4 ± 0.5% | 94.6 ± 0.1% |
Table 4.
FRET quenching efficiences of fluorophore/quencher pairs. Results are provided as the average of three experiments with standard deviation shown. See Fig. 3c for structural context.
| FAM | Cy 3 | TAMRA | Atto 590 | Quasar 670 | |
|---|---|---|---|---|---|
| Quencher | λem = 517nm | λem = 563nm | λem = 580nm | λem = 624nm | λem = 662nm |
| MPQ1 | 53.1 ± 1.4% | 62.4 ± 0.7% | 77.1 ± 0.5% | 45.6 ± 1.5% | 28.9 ± 0.8% |
| MPQ2 | 50.3 ± 0.2% | 60.7 ± 2.2% | 74.9 ± 1.4% | 43.9 ± 0.8% | 33.5 ± 1.2% |
| MPQ3 | 60.2 ± 1.3% | 59.6 ± 0.9% | 74.1 ± 0.4% | 29.1 ± 0.9% | 22.2 ± 0.5% |
| MPQ4 | 47.3 ± 1.4% | 56.9 ± 1.9% | 73.7 ± 0.6% | 33.4 ± 2.1% | 25.6 ± 2.9% |
| MPQ6 | 50.4 ± 0.9% | 64.4 ± 1.2% | 78.6 ± 0.4% | 57.6 ± 0.6% | 43.0 ± 0.5% |
| Dabcyl | 41.3 ± 0.9% | 52.1 ± 2.8% | 71.1 ± 0.8% | 26.8 ± 1.9% | 22.5 ± 3.1% |
| BHQ2 | 54.3 ± 1.0% | 63.5 ± 1.8% | 80.4 ± 1.2% | 60.0 ± 1.3% | 40.4 ± 2.8% |
| No Quencher | 29.2 ± 0.7% | 53.2 ± 1.2% | 71.4 ± 1.1% | 23.3 ± 1.4% | 23.9 ± 1.5% |
Contact and Mixed Mechanism Quenching
As anticipated, quenching of AlexaFluor 350 (λem = 442 nm), is poor for the MPQ series (Figure 3a), with dabcyl having a greater quenching efficiency (75.7% contact, 70.4% FRET) than those prepared and tested. This is consistent with the fact that dabcyl is the most blue-shifted quencher of those assayed and has the greatest spectral overlap with AlexaFluor 350. However, the quenchers of the MPQ series (particularly MPQ3) nearly equal dabcyl in contact quenching of this dye. BHQ2 is slightly superior to either of these quenchers in performance with this blue coumarin fluorophore.
Figure 3.
Fluorescence quenching ratios for contact ( ) and mixed mechanism (
) and mixed mechanism ( ) quenching with (a) AlexaFluor 350, (b) Fluorescein, (c) Cy 3, (d) TAMRA, (e) ATTO 590 and (f) Quasar 670.
) quenching with (a) AlexaFluor 350, (b) Fluorescein, (c) Cy 3, (d) TAMRA, (e) ATTO 590 and (f) Quasar 670.
The data shows enhanced performance of the red-shifted MPQ quenchers as the emission wavelength of the fluorophore increases. For fluorescein (λem = 517 nm, Figure 3b), it is readily observed that MPQ3 is as efficient as even BHQ2 in contact quenching, while four of the novel quenchers (MPQ1, MPQ2, MPQ3 and MPQ6) all display superior quenching abilities in a mixed mechanism context. At the emission of Cy3 (λem = 563 nm), only MPQ5 performs less efficiently than dabcyl in terms of contact quenching, with all others displaying superior performance to the common quencher. These results begin to show the red-shifted properties of the new quenchers created, as the parent dabcyl is no longer able to efficiently quench Cy3 emission, while the new dyes display considerably better quenching properties. Moreover, MPQ6 is particularly intriguing, as it is equals (contact quenching) or even surpasses (mixed mechanism quenching) BHQ2 in quenching of Cy3 emission.
As emission shifts further red to TAMRA (λem = 580 nm), all MPQ quenchers display markedly greater quenching efficiency than dabcyl (Figure 3d). We note that TAMRA (unique among the fluorophores tested here as it is poorly quenched in general14) is relatively poorly quenched by all quenchers including dabcyl and BHQ2, an effect observed previously. Moreover, MPQ4 is as good at contact quenching of TAMRA as even BHQ2, while all but MPQ3 and MPQ5 are approximately as efficient in mixed mechanism quenching as BHQ2. Again, MPQ6 offers promising results as it displays comparable quenching properties to BHQ2. At the emission of Atto 590 (λem = 624 nm), all quenchers still perform markedly better than the parent dabcyl (Figure 3e), with MPQ5 again serving as the single exception. Although not operating by an entirely FRET mechanism, the advantages of the red-shifted absorbance properties become clearer as the relatively small five-base increase in length between quencher and fluorophore greatly affects the quenching ability of dabcyl with considerably smaller effect on the ability of the MPQs to quench the emission of Atto 590. Quasar 670 (a Cy5 variant, λem = 662 nm) was the furthest-red emitting fluorophore tested. At this wavelength the limits of certain MPQs begin to become evident, although contact quenching overall remains highly efficient (up to 97%; see Tables 2, 3 and Figure 3f).
FRET Quenching
To properly assess quenching based entirely on a FRET mechanism, hybridization of 5’-quencher labeled sequenches to 5’-fluorophore labeled sequences was carried out (Figure 3c).27 As a result of the poor spectral overlap of all new quenchers with the emission of the short-wavelength AlexaFluor 350 (λem = 442 nm), FRET quenching of this fluorophore was not examined. Similarly, with the poor quenching efficiency of MPQ5, particularly in mixed mechanism quenching studies (Table 2) and the low extinction coefficient of this compound relative to the others synthesized, the quencher was not used in FRET quenching studies.
In general terms, the FRET results (Table 4) confirm the expanded quenching properties of the new dyes, with all new quenchers performing better than dabcyl for all fluorophores tested. MPQ1 and MPQ2 were even capable of quenching Atto 590 to a moderate degree, while the parent dabcyl showed, as expected, negligible quenching of the red-emitting dye, indicating the ability to red-shift the absorbance properties and quenching abilities. MPQ6 again provides superior results, as it was able to quench all fluorophores as well as or even better than BHQ2 in an entirely FRET context. Whereas BHQ1 (the parent compound of MPQ6) is a poor FRET quencher of red-emitting fluorophores such as Quasar 670 and Atto 590 as a result of negligible spectral overlap,27 incorporation of the dihydroperimidine subunit in MPQ6 gives rise to a greatly broadened absorbance spectrum and results in a compound with FRET quenching efficiency better than BHQ2, as in the case of Quasar 670.
FRET quenching studies were complicated by the inherent nucleobase quenching by the 3’-dG on the quencher-containing strand. To account for this, an oligonucleotide sequence lacking a 5’ quencher was synthesized and served as a control for quenching due only to the 3’-dG (Table 4). Although such quenching was negligible for fluorescein, Atto 590 and Quasar 670, the 3’-dG was found to strongly quench both Cy3 and TAMRA emission (Table 4). Quenching of TAMRA by guanosine is well known and unsurprising,28, 29 but quenching of Cy3 in this context was unexpected, as previous studies had indicated an activation of Cy3 fluorescence by guanosine.14,28
Discussion
Overall, the data show that several MPQ dyes outperform their parent dye (dabcyl) in performance in a contact quenching format with all of the fluorophores tested except the most blue-shifted fluorophore (Alexa 350). As for quenching by the FRET mechanism, which is most relevant to the present electronic design concept, all of the MPQ quenchers perform better than dabcyl with all of the fluorophores.. This confirms that broadening of the absorbance spectrum of the quencher can confer superior performance. While most of the new dyes do not exceed the quenching ability of the advanced BHQ2 compound, a number of MPQ quenchers equal this compound's performance with specific fluorescent labels, and thus could be considered as serious alternatives to this compound.
The chief intent of the molecular design of MPQs was to broaden the efficient quenching range of the parent quenchers. The data show that this approach is successful: several of the MPQs give >95% contact quenching and excellent FRET quenching of fluorophores as far to the blue as fluorescein and as far red as Quasar 670, while the parent dabcyl compound quenches none of the five fluorophores significantly in the FRET mode and only one better than 95% in the contact mode. Thus we conclude that incorporation of the naphthalimide core does result in the desired increase in generality and a greater range of fluorescence quenching beyond that of dabcyl. Moreover, incorporation of the dihydroperimidine moiety into the BHQ1 scaffold affords a new quencher (MPQ6) that also displays quenching efficiency over a wide wavelength range.
All five quenchers derived from the dabcyl structure (MPQs 1 – 5) display significantly enhanced quenching of all fluorophores emitting beyond fluorescein, in both contact and FRET quenching. As such, they should be considered as excellent alternatives to dabcyl in any assay that relies on quenching of a fluorophore emitting above approximately 520 nm and even as far as 624 nm, as shown by the ability of MPQ1 and MPQ2 to effectively quench the emission of Atto 590 in the FRET mode. MPQ3 appears to be an excellent quencher specifically for fluorescein, as it performed better than other quenchers for this fluorophore in both contact and FRET quenching. Further, MPQ6 uniquely serves as a competitive alternative to BHQ2 for both contact and FRET quenching of all fluorophores used in this study, particularly those emitting between approximately 560 and 670 nm. As the FRET quenching of this compound is approximately equal to or greater than BHQ2 for Fluorescein, Cy3, TAMRA, Atto 590 and Quasar 670, it may have its greatest utility in FRET based fluorescence assays.
To our knowledge, the only previous similar approach in quencher design (i.e. taking advantage of increased electronic complexity) employed an azulene dimer, but was limited to quenching only in the near-IR region with limited efficiency.30 An alternative design utilizing an azaphthalocyanine structure displayed a broadened absorbance spectrum, but has only been fully assayed to date in quenching of only FAM and Cy5.27 Experiments further displayed the abilitiy of the azophthalocyanine dye to quench a larger set of fluorophores (ranging in emission from 517nm to 703nm), but only in a contact quenching setting, without exploration of FRET quenching of the expanded set of fluorophores.31 The use of the perimidine scaffold has been shown before to proffer a red-shift to fluorescent dyes,32 but the current work is the first case of the use of perimidine for altering the properties of fluorescence quenchers.
One interesting aspect of the spectral properties of some the new Multiple Pathway Quenchers is their environmental sensitivity. MPQs 1-5 undergo moderate to significant changes upon a change in nearly DNA sequence. For all but MPQ4, this is likely the result of the incorporation of the naphthalimide core, which is well known to be environmentally sensitive itself.22,33 In addition, the 4-aminonaphthalimide core has been employed in DNA sensors due to its inherent environmental sensitivity, providing a further explanation for the changes observed here.34 As use of the 4-aminonaphthalimide core has shown environmental dependence in different DNA settings, it is likely that such is the same explanation for the derived quenchers, but a further exploration is necessary to allow for an accurate conclusion of the mechanism creating the different absorbance spectra for MPQs 1-5. It seems possible, however, that one might in the future take advantage of this property in the design of “smart quenchers”, where the environment of the quencher could be part of the design, either blue-shifting or red-shifting the absorbance spectrum in response to structure or changes in environment. Further investigation is needed to explore this possibility.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (GM068122 and GM067201).
Footnotes
Supporting Information Available
NMR spectra of quencher dyes and intermediates, HPLC purity and MALDI-TOF of quencher and fluorophore substituted DNAs, and additional spectral data. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Franzini RM, Kool ET. Efficient nucleic acid detection by templated reductive quencher release. J. Am. Chem. Soc. 2009;131(44):16021–16023. doi: 10.1021/ja904138v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franzini RM, Kool ET. Two successive reactions on a DNA template: a strategy for improving background fluorescence and specificity in nucleic acid detection. Chem. Eur. J. 2011;17(7):2168–2175. doi: 10.1002/chem.201002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 1996;14(3):303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama S, Yan L, Sintim HO. Junction probes – sequence specific detection of nucleic acids via template enhanced hybridization processes. J. Am. Chem. Soc. 2008;130(38):12560–12561. doi: 10.1021/ja803146f. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Wang F, Mamon H, Kulke MH, Harris L, Maher E, Wang L, Makrigiorgos GM. Antiprimer quenching-based real-time PCR and its application to the analysis of clinical cancer samples. Clinical Chem. 2006;52(4):624–633. doi: 10.1373/clinchem.2005.063321. [DOI] [PubMed] [Google Scholar]
- 6.Dai N, Guo J, Teo YN, Kool ET. Protease probes built from DNA: multispectral fluorescent DNA-peptide conjugates as caspase chemosensors. Angew. Chem. Int. Ed. 2011;50(22):5105–5109. doi: 10.1002/anie.201007805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai N, Teo YN, Kool ET. DNA-polyfluorophore excimers as sensitive reporters for esterases and lipases. Chem. Commun. 2010;46(8):1221–1223. doi: 10.1039/b926338a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng X, Chen H, Draney DR, Volcheck W, Schutz-Geschwender A, Olive DM. A nonfluorescent, broad-range quencher dye for Förster resonance energy transfer assays. Anal. Biochemistry. 2009;388:220–228. doi: 10.1016/j.ab.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Matayoshi ED, Wang GT, Kraft GA, Erickson J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science. 1990;247:954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- 10.Blum G, Mullins SR, Keren K, Fonovic M, Jedeszko C, Rice MJ, Sloane BF, Bogyo M. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat. Chem. Biol. 2005;1(4):203–209. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- 11.Tung C. Fluorescent peptide probes for in vivo diagnostic imaging. Peptide Science. 2004;76(5):391–403. doi: 10.1002/bip.20139. [DOI] [PubMed] [Google Scholar]
- 12.Marras SE. Selection of fluorophore and quencher pairs for fluorescent nucleic acid hybridization probes. In: Didenko VV, editor. Methods in Molecular Biology, Vol 335, Fluorescent Energy Transfer Nucleic Acid Probes: Designs and Protocols. Humana Press Inc.; Totowa, NJ: 2006. [DOI] [PubMed] [Google Scholar]
- 13.Johansson MK. Choosing reporter-quencher pairs for efficient quenching through formation of intramolecular dimers. In: Didenko VV, editor. Methods of Molecular Biology, Vol 335, Fluorescent Energy Transfer Nucleic Acid Probes: Designs and Protocols. Humana Press Inc.; Totowa, NJ: 2006. [DOI] [PubMed] [Google Scholar]
- 14.Marras SAE, Kramer FR, Tyagi S. Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes. Nucleic Acids Res. 2002;30:e122. doi: 10.1093/nar/gnf121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicens MC, Sen A, Vanderlaan A, Drake TJ, Tan W. Investigation of molecular beacon aptamer-based bioassay for platelet-derived growth factor detection. ChemBioChem. 2005;6(5):900–907. doi: 10.1002/cbic.200400308. [DOI] [PubMed] [Google Scholar]
- 16.Cook RM, Lyttle M, Dick D. Dark quenchers for donor-acceptor energy transfer. 2006 March 28; U.S. Patent 7,019,129.
- 17.Haugland RP, Singer VL, Yue SL. Xanthene dyes and their application as luminescence quenching compounds. 2002 March 4; U.S. Patent 6,399,392.
- 18.Tyagi S, Bratu DP, Kramer FR. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 1998;16:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 19.Johansson MK, Fidder H, Dick D, Cook RM. Intramolecular dimers: a new strategy to fluorescence quenching in dual-labeled oligonucleotide probes. J. Am. Chem. Soc. 2002;124(24):6950–6956. doi: 10.1021/ja025678o. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz JR. Principles of Fluorescence Spectroscopy. Kluwer Academic/Plenum Publishers; New York, NY: 1999. [Google Scholar]
- 21.Bernacchi S, Mely Y. Exciton interaction in molecular beacons: a sensitive sensor for short range modifications of the nucleic acid structure. Nucleic Acids Res. 2001;29:e62. doi: 10.1093/nar/29.13.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan D, Brown RG, Hepworth JD, Alexiou MS, Tyman JHP. The synthesis and fluorescence of novel N-substituted-1,8-naphthylimides. J. Heterocylic Chem. 2008;45(2):397–404. [Google Scholar]
- 23.Cava MP, Merkel KE, Schlessinger RH. Pleiadene systems – II: on the mechanism of acepleiadylene formation – a vinylogous elimination in the acenaphthene series. Tetrahedron. 1965;21(11):3059–3064. [Google Scholar]
- 24.Jones LA, Joyner CT, Kim HK, Kyff RA. Acenaphthane I. The preparation of derivatives of 4,5-diamino naphthalic anhydride. Can. J. Chem. 1970;48(20):3132–3135. [Google Scholar]
- 25.Gharanjing K, Arami M, Rouhani S, Bahrami H, Movassagh B, Mahmoodi NM. Synthesis and characterization of novel monazo N-ester-1,8-naphthalimide disperse dyestuffs. J. Chin. Chem. Soc. 2007;54(10):1021–1028. [Google Scholar]
- 26.Wojciechowski K. Spectral properties of disperse dyes, derivatives of N-methylnaphthalimidoazobenzene. Dyes and Pigments. 1990;12:273–286. [Google Scholar]
- 27.Kopecky K, Novakova V, Miletin M, Kučera R, Zimcik P. Solid-phase synthesis of azaphthalocyanine-oligonucleotide conjugates and their evaluation as new dark quenchers of fluorescence. Bioconj. Chem. 2010;21:1872–1879. doi: 10.1021/bc100226x. [DOI] [PubMed] [Google Scholar]
- 28.Torimura M, Kurata S, Yamada K, Yokomaku T, Kamagata Y, Kanagawa T, Kurane R. Fluorescence-quenching phenomenon by photoinduced electron transfer between a fluorescent dye and a nucleotide base. Anal. Sci. 2001;17:155–160. doi: 10.2116/analsci.17.155. [DOI] [PubMed] [Google Scholar]
- 29.Xiao SJ, Hu PP, Li YF, Huang CZ, Huang, Huang T, Xiao GF. Aptamer-mediated turn-on fluorescence assay for prion protein based on guanine quenched fluophor. Talanta. 2009;79(5):1283–1286. doi: 10.1016/j.talanta.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 30.Pham W, Weissleder R, Tung CH. An azulene dimer as a near-infrared quencher. Angew. Chem. Int. Ed. Engl. 2002;41(19):3659–3662. doi: 10.1002/1521-3773(20021004)41:19<3659::AID-ANIE3659>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Kopecky K, Novakova V, Miletin M, Kučera R, Zimcik P. Synthesis of new azaphthalocyanine dark quencher and evaluation of its quenching efficiency with different fluorophores. Tetrahedron. 2011;67:5956–5963. [Google Scholar]
- 32.Bello KA, Corns SN, Griffiths J. Near-infrared-absorbing squaraine dyes containing 2,3-dihydroperimidine terminal groups. J. Chem. Soc., Chem. Commun. 1993;5:452–454. [Google Scholar]
- 33.Loving G, Imperiali B. A versatile amino acid analogue of the solvatochromic fluorophore 4-N,N-dimethylamino-1,8-naphthalimide: a powerful tool for the study of dynamic protein interactions. J. Am. Chem. Soc. 2008;130(41):13630–13638. doi: 10.1021/ja804754y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu E, Peng X, Song F, Fan J. A novel fluorescent sensor for triplex DNA. Bioorg. Med. Chem. Lett. 2005;15(2):255–257. doi: 10.1016/j.bmcl.2004.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.