Abstract
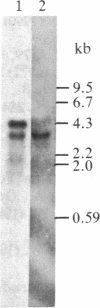
A cDNA for human ceruloplasmin (EC 1.16.3.1) was identified in a human liver cDNA library by screening with two mixtures of synthetic oligodeoxyribonucleotides that were complementary to two regions of ceruloplasmin mRNA as predicted from the amino acid sequence of plasma ceruloplasmin. The resulting clone (phCP1) contained DNA coding for amino acid residues 202-1046 of the protein, followed by a stop codon, a 3' untranslated region of 123 base pairs, and a poly(A) tail. To isolate cDNAs encoding the 5' end of ceruloplasmin mRNA, a cDNA library was constructed in lambda gt10. The cDNA for this library was synthesized by reverse transcription of human liver poly(A)+ RNA, using random oligonucleotides as primers. When this cDNA library was screened by using a 5' fragment of phCP1 as a hybridization probe, several positive clones were identified. One of these clones (lambda hCP1) contained DNA coding for a probable signal peptide of 19 amino acid residues followed by DNA coding for residues 1-380 of plasma ceruloplasmin. Blot hybridization analysis showed that ceruloplasmin mRNA from human liver and the human hepatoma cell line HepG2 is 3700 nucleotides in size. Liver contained an additional mRNA species that is like ceruloplasmin mRNA and is 4500 nucleotides in size. Comparison of the complete nucleotide sequences of human ceruloplasmin cDNA and human clotting factor VIII cDNA showed regions of sequence homology, suggesting that these two proteins have evolved from a common ancestor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrell B. G., Bankier A. T., Drouin J. A different genetic code in human mitochondria. Nature. 1979 Nov 8;282(5735):189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- CURZON G. Some properties of coupled iron-caeruloplasmin oxidation systems. Biochem J. 1961 Jun;79:656–663. doi: 10.1042/bj0790656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church W. R., Jernigan R. L., Toole J., Hewick R. M., Knopf J., Knutson G. J., Nesheim M. E., Mann K. G., Fass D. N. Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6934–6937. doi: 10.1073/pnas.81.22.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Edgell C. J., Louie G. V., Zoller M. J., Brayer G. D., MacGillivray R. T. Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa. J Biol Chem. 1985 Nov 5;260(25):13666–13676. [PubMed] [Google Scholar]
- Cooper E. H., Ward A. M. Acute phase reactant proteins as aids to monitoring disease. Invest Cell Pathol. 1979 Oct-Dec;2(4):293–301. [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Putnam F. W. Internal duplication and evolution of human ceruloplasmin. Proc Natl Acad Sci U S A. 1981 May;78(5):2805–2809. doi: 10.1073/pnas.78.5.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. R., Campbell R. M., MacGillivray R. T. Blood coagulation factor X mRNA encodes a single polypeptide chain containing a prepro leader sequence. Nucleic Acids Res. 1984 Jun 11;12(11):4481–4492. doi: 10.1093/nar/12.11.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. R., Hay C. W., MacGillivray R. T. Characterization of an almost full-length cDNA coding for human blood coagulation factor X. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3591–3595. doi: 10.1073/pnas.82.11.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Karn J. Methods for cDNA cloning and sequencing tobacco mosaic virus RNA. Gene. 1984 Sep;29(3):331–342. doi: 10.1016/0378-1119(84)90062-3. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kingston I. B., Kingston B. L., Putnam F. W. Chemical evidence that proteolytic cleavage causes the heterogeneity present in human ceruloplasmin preparations. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5377–5381. doi: 10.1073/pnas.74.12.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- MacGillivray R. T., Davie E. W. Characterization of bovine prothrombin mRNA and its translation product. Biochemistry. 1984 Apr 10;23(8):1626–1634. doi: 10.1021/bi00303a007. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G., Lawler C. M., Vehar G. A., Church W. R. Coagulation Factor V contains copper ion. J Biol Chem. 1984 Nov 10;259(21):12949–12951. [PubMed] [Google Scholar]
- McKee D. J., Frieden E. Binding of transition metal ions by ceruloplasmin (ferroxidase). Biochemistry. 1971 Oct 12;10(21):3880–3883. doi: 10.1021/bi00797a013. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Markham A. F., Orkin S. H. Isolation of a cDNA clone for human antithrombin III. J Biol Chem. 1983 Jul 10;258(13):8389–8394. [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak R. H., Candido E. P. Locus encoding a family of small heat shock genes in Caenorhabditis elegans: two genes duplicated to form a 3.8-kilobase inverted repeat. Mol Cell Biol. 1985 Jun;5(6):1268–1278. doi: 10.1128/mcb.5.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén L., Björk I. Reinvestigation of some physicochemical and chemical properties of human ceruloplasmin (ferroxidase). Biochemistry. 1976 Aug 10;15(16):3411–3417. doi: 10.1021/bi00661a003. [DOI] [PubMed] [Google Scholar]
- Rydén L. Model of the active site in the blue oxidases based on the ceruloplasmin-plastocyanin homology. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6767–6771. doi: 10.1073/pnas.79.22.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEINBERG I. H., GITLIN D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson's disease). Science. 1952 Oct 31;116(3018):484–485. doi: 10.1126/science.116.3018.484. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Bauman R. A., Ortel T. L., Dwulet F. E., Wang C. C., Putnam F. W. Internal triplication in the structure of human ceruloplasmin. Proc Natl Acad Sci U S A. 1983 Jan;80(1):115–119. doi: 10.1073/pnas.80.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Ortel T. L., Putnam F. W. Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc Natl Acad Sci U S A. 1984 Jan;81(2):390–394. doi: 10.1073/pnas.81.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Young S. N., Curzon G. A method for obtaining linear reciprocal plots with caeruloplasmin and its application in a study of the kinetic parameters of caeruloplasmin substrates. Biochem J. 1972 Sep;129(2):273–283. doi: 10.1042/bj1290273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences are not uniformly hydrophobic. J Mol Biol. 1982 Aug 15;159(3):537–541. doi: 10.1016/0022-2836(82)90300-x. [DOI] [PubMed] [Google Scholar]