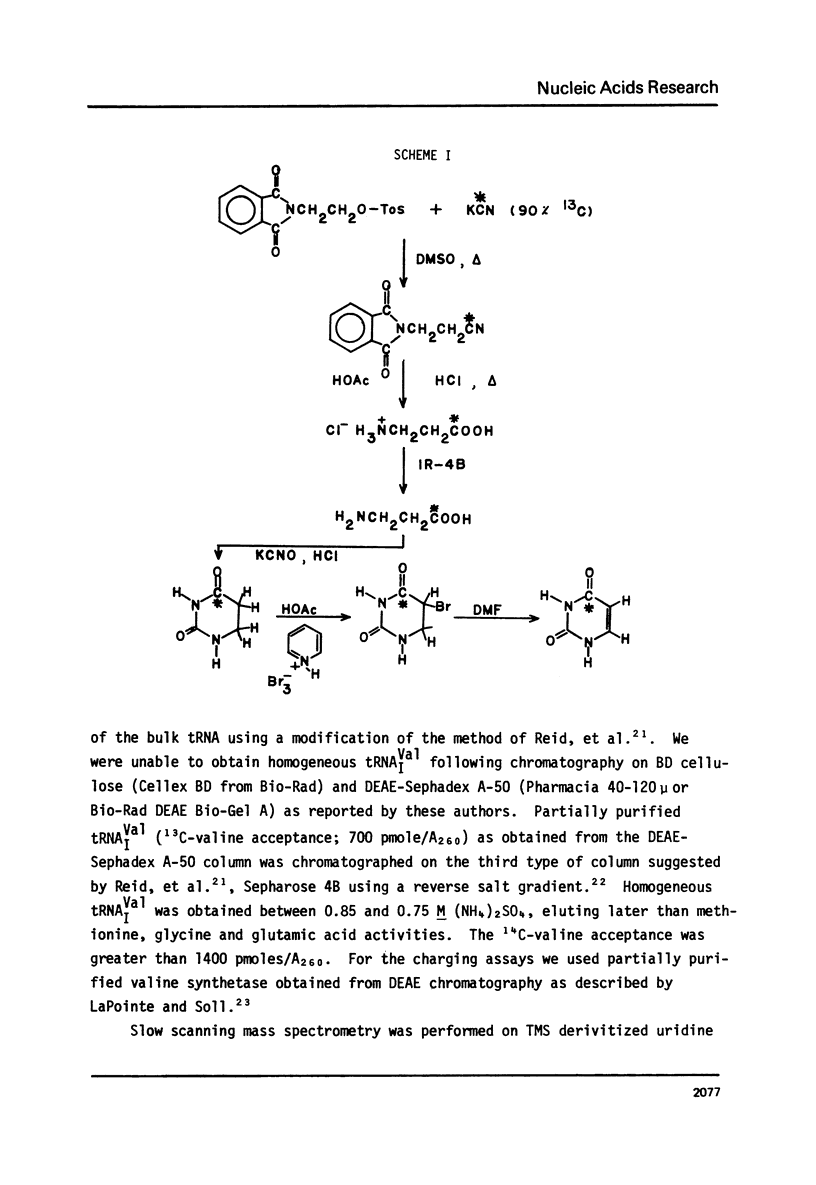
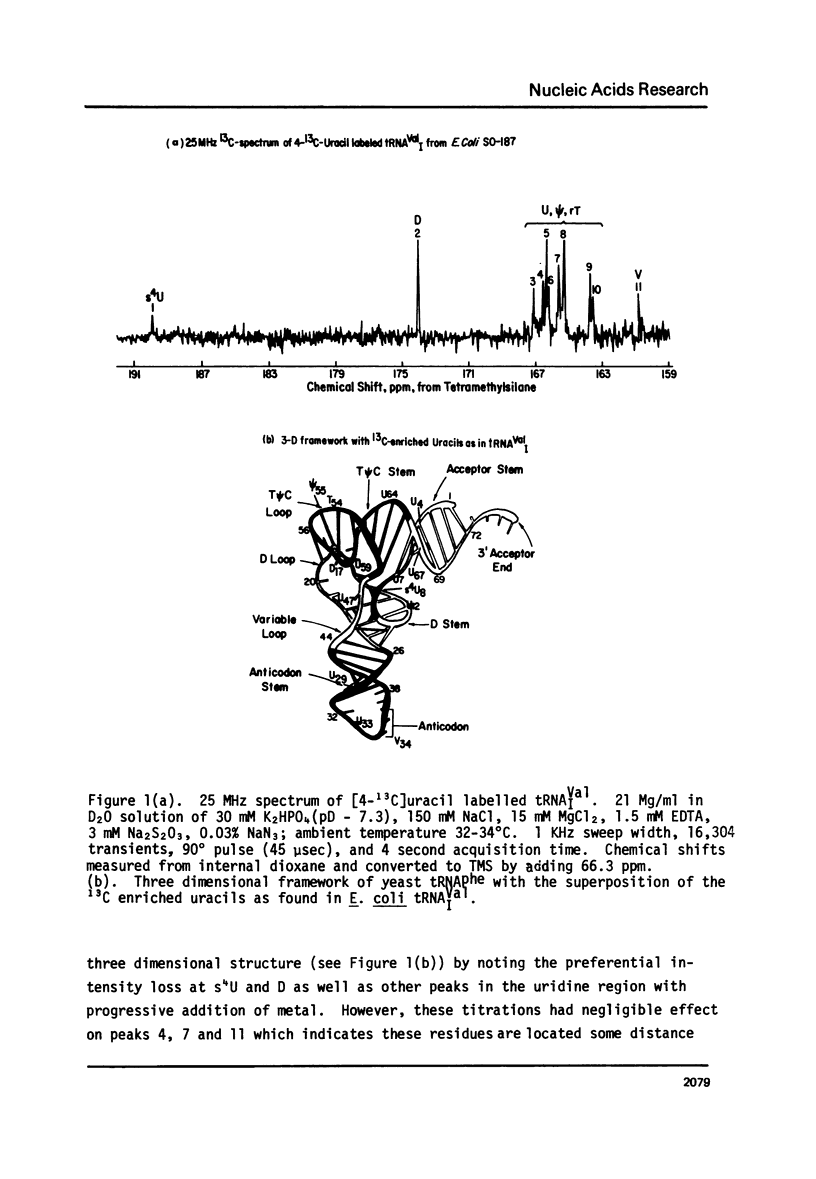
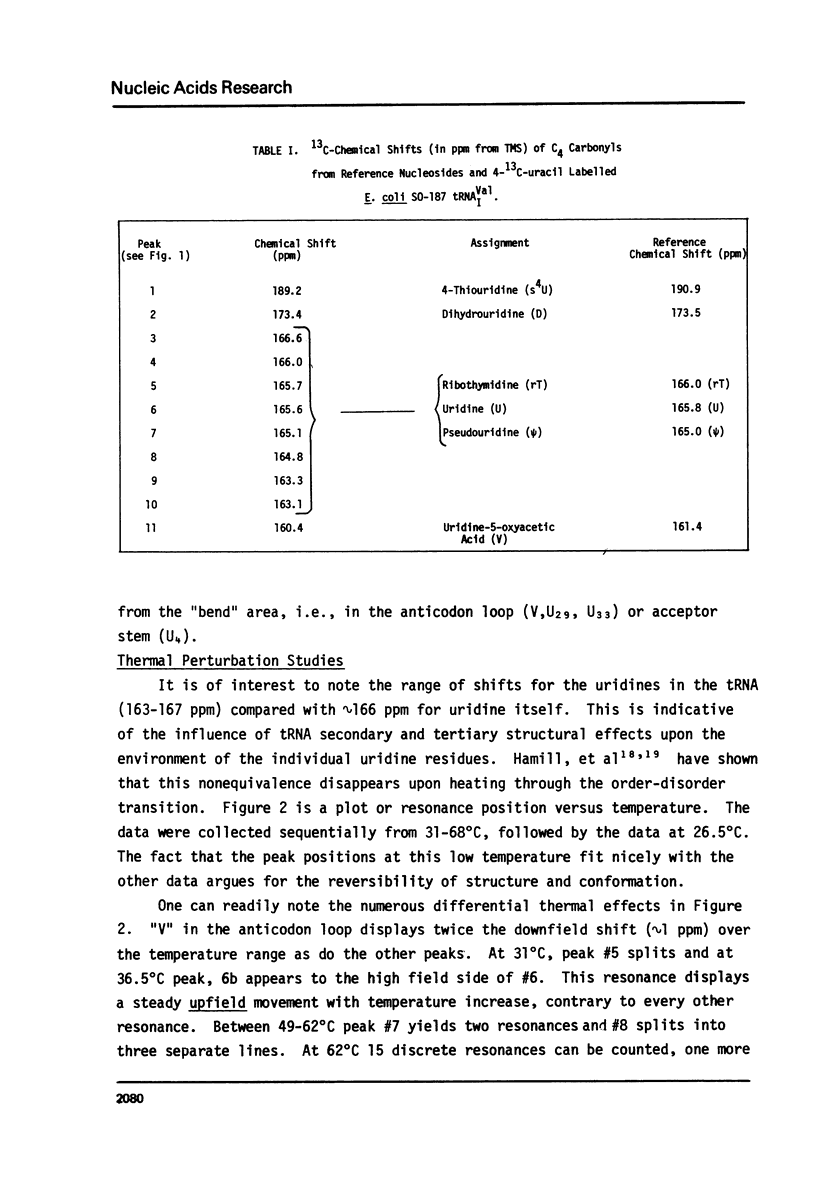
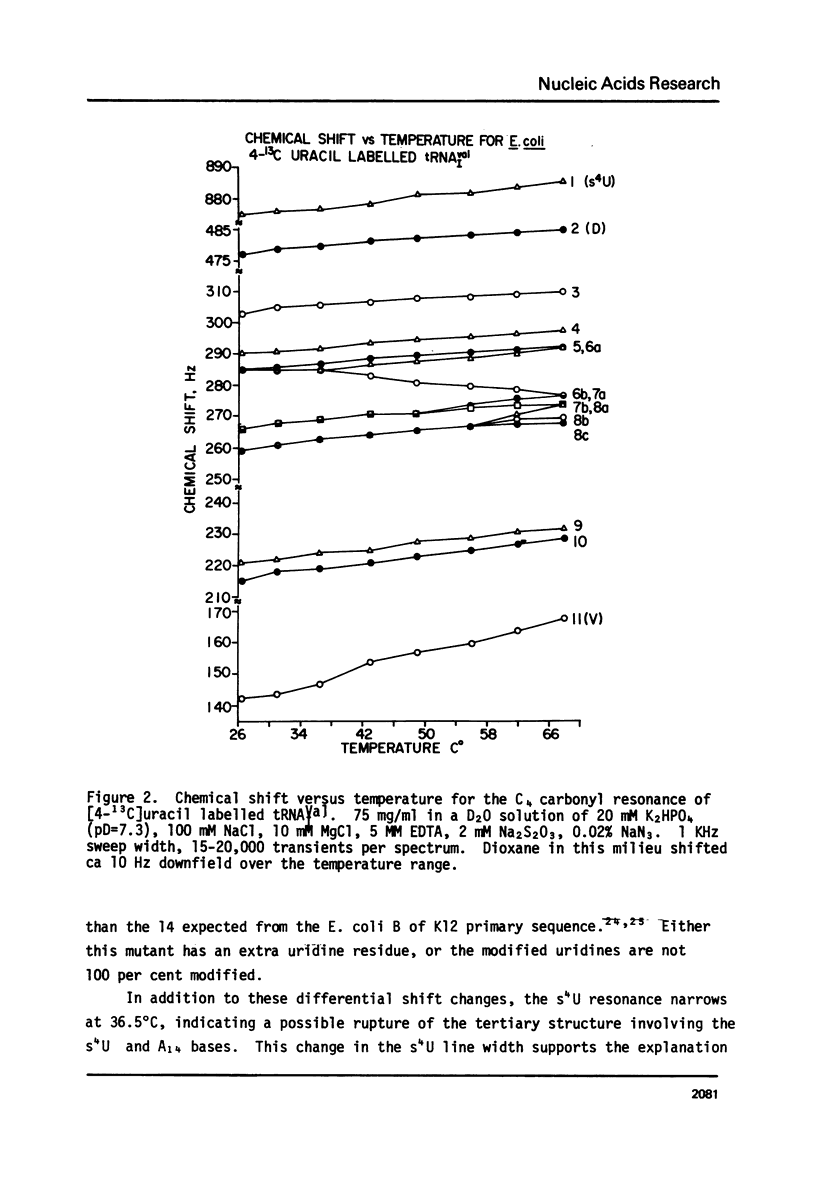
Abstract
In this paper we describe carbon-13 nuclear magnetic resonance results on 13C-enriched purified transfer RNAI(VAL) from from E. coli SO-187, a uracil requiring auxotroph. The organism was grown on uracil 90% 13C-enriched at the carbonyl C4 position. Transfer RNAI(Val) was purified from bulk tRNA by sequential chromatography on columns of BD cellulose, DEAE-Sephadex A-50 and reverse gradient sepharose 4B. Dihydrouridine, 4-thiouridine, and uridine 5-oxyacetic acid located at discrete positions in the polymer backbone were tentatively assigned in the highly resolved 25 MHz 13C-spectra. Chemical shift versus temperature plots reveal differential thermal perturbation of the ordered solution structure, evident in the large dispersion (ca 3-4 ppm) of the uridine C4 resonances. Over the range 26-68 degrees C, V in the anticodon displays the largest downfield shift. Whereas several uridine residues rapidly shift downfield between 50-68 degrees, one moves upfield beginning at 37 degrees. The results are qualitatively compared with proton NMR analysis of the three dimensional structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Fujiwara F. G., Schmidt C. F., Loeppky R. N. Utilization of an Escherichia coli mutant for carbon-13 enrichment of tRNA for NMR studies. Nucleic Acids Res. 1975 Sep;2(9):1503–1512. doi: 10.1093/nar/2.9.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital S., Elson D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- Hamill W. D., Jr, Grant D. M., Horton W. J., Lundquist R., Dickman S. Letter: Magnetic resonance spectroscopy on carbon-13 labeled uracil in transfer ribonucleic acid. J Am Chem Soc. 1976 Mar 3;98(5):1276–1273. doi: 10.1021/ja00421a047. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R. E., Azhderian E., Reid B. R. Paramagnetic ion effects on the nuclear magnetic resonance spectrum of transfer ribonucleic acid: assignment of the 15--48 tertiary resonance. Biochemistry. 1979 Sep 4;18(18):4012–4017. doi: 10.1021/bi00585a026. [DOI] [PubMed] [Google Scholar]
- Hurd R. E., Reid B. R. Nuclear magnetic resonance studies on the tertiary folding of transfer ribonucleic acid: assignment of the 7-methylguanosine resonance. Biochemistry. 1979 Sep 4;18(18):4017–4024. doi: 10.1021/bi00585a027. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Kimura F., Harada F., Nishimura S. Primary sequence of tRNA-Val-1 from Escherichia coli B. II. Isolation of large fragments by limited digestion with RNases, and overlapping of fragments to reduce the total primary sequence. Biochemistry. 1971 Aug 17;10(17):3277–3283. doi: 10.1021/bi00793a018. [DOI] [PubMed] [Google Scholar]
- Komoroski R. A., Allerhand A. Natural-abundance carbon-13 Fourier-transform nuclear magnetic resonance spectra and spin lattice relaxation times of unfractionated yeast transfer-FNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1804–1808. doi: 10.1073/pnas.69.7.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoroski R. A., Allerhand A. Observation of resonances from some minor bases in the natural-abundance carbon-13 nuclear magnetic resonance spectrum of unfractionated yeast transfer ribonucleic acid. Evidence for fast internal motion of the dihydrouracil rings. Biochemistry. 1974 Jan 15;13(2):369–372. doi: 10.1021/bi00699a023. [DOI] [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification and properties. J Biol Chem. 1972 Aug 25;247(16):4966–4974. [PubMed] [Google Scholar]
- Reid B. R., McCollum L., Ribeiro N. S., Abbate J., Hurd R. E. Identification of tertiary base pair resonances in the nuclear magnetic resonance spectra of transfer ribonucleic acid. Biochemistry. 1979 Sep 4;18(18):3996–4005. doi: 10.1021/bi00585a024. [DOI] [PubMed] [Google Scholar]
- Reid B. R., Ribeiro N. S., McCollum L., Abbate J., Hurd R. E. High-resolution nuclear magnetic resonance determination of transfer RNA tertiary base pairs in solution. 1. Species containing a small variable loop. Biochemistry. 1977 May 17;16(10):2086–2094. doi: 10.1021/bi00629a006. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Tarr C. E., Vosman F., Berendsen H. J. Similarity of the crystal and solution structure of yeast tRNAPhe. Nature. 1976 Jul 29;262(5567):363–369. doi: 10.1038/262363a0. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Tarr C. E., Vosman F., Reid B. R. A nuclear magnetic resonance study of secondary and tertiary structure in yeast tRNAPhe. Biochemistry. 1977 Nov 29;16(24):5261–5273. doi: 10.1021/bi00643a016. [DOI] [PubMed] [Google Scholar]
- Tompson J. G., Agris P. F. Production of specific site probes of tRNA structure by enrichment with carbon 13 at particular locations. Nucleic Acids Res. 1979 Oct 10;7(3):765–779. doi: 10.1093/nar/7.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompson J. G., Hayashi F., Paukstelis J. V., Loeppky R. N., Agris P. F. Complete nuclear magnetic resonance signal assignments and initial structural studies of [13C]methyl-enriched transfer ribonucleic acid. Biochemistry. 1979 May 15;18(10):2079–2085. doi: 10.1021/bi00577a037. [DOI] [PubMed] [Google Scholar]


