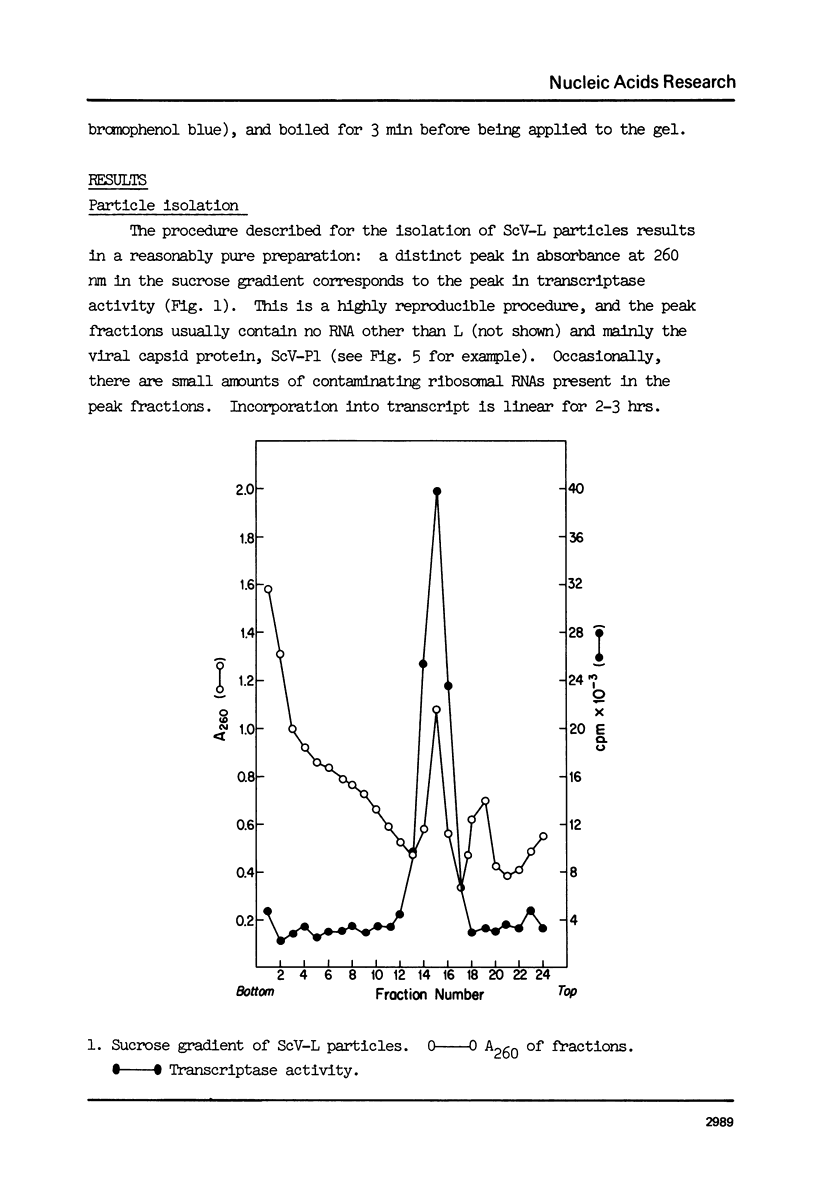
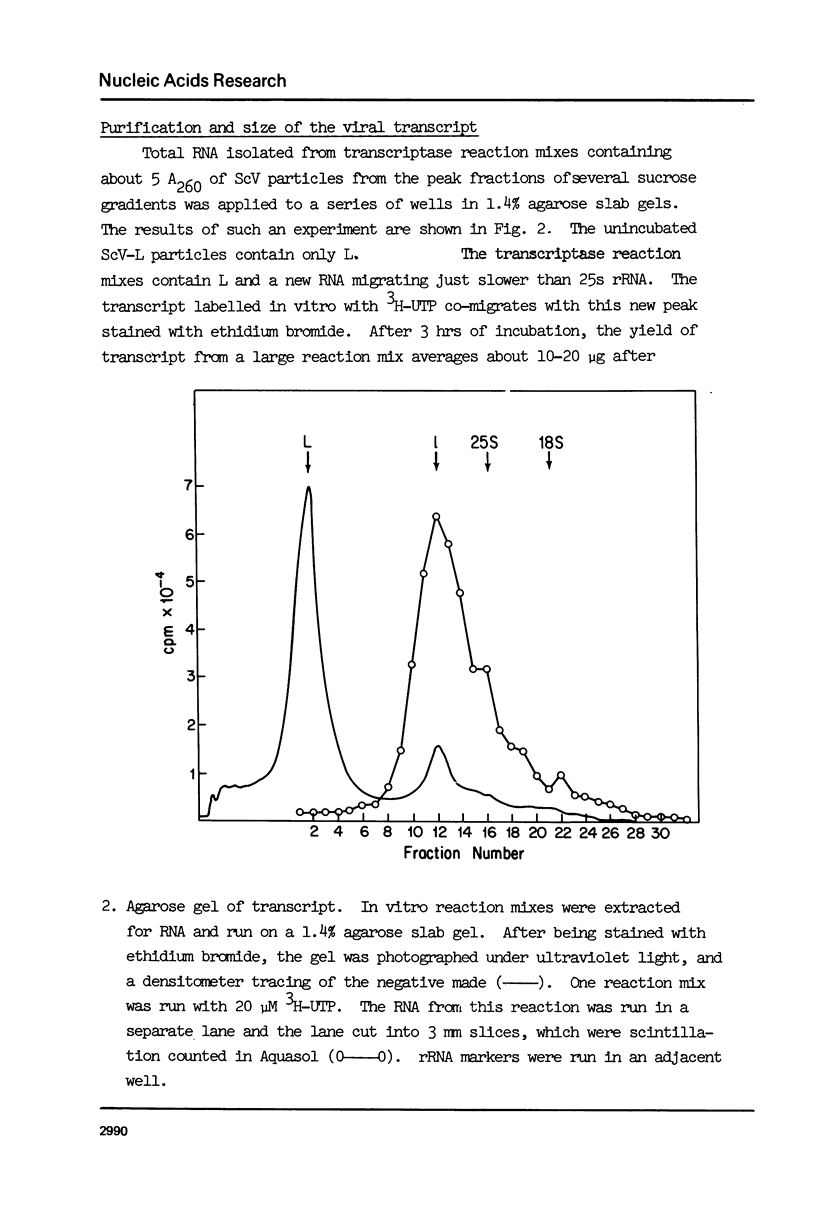
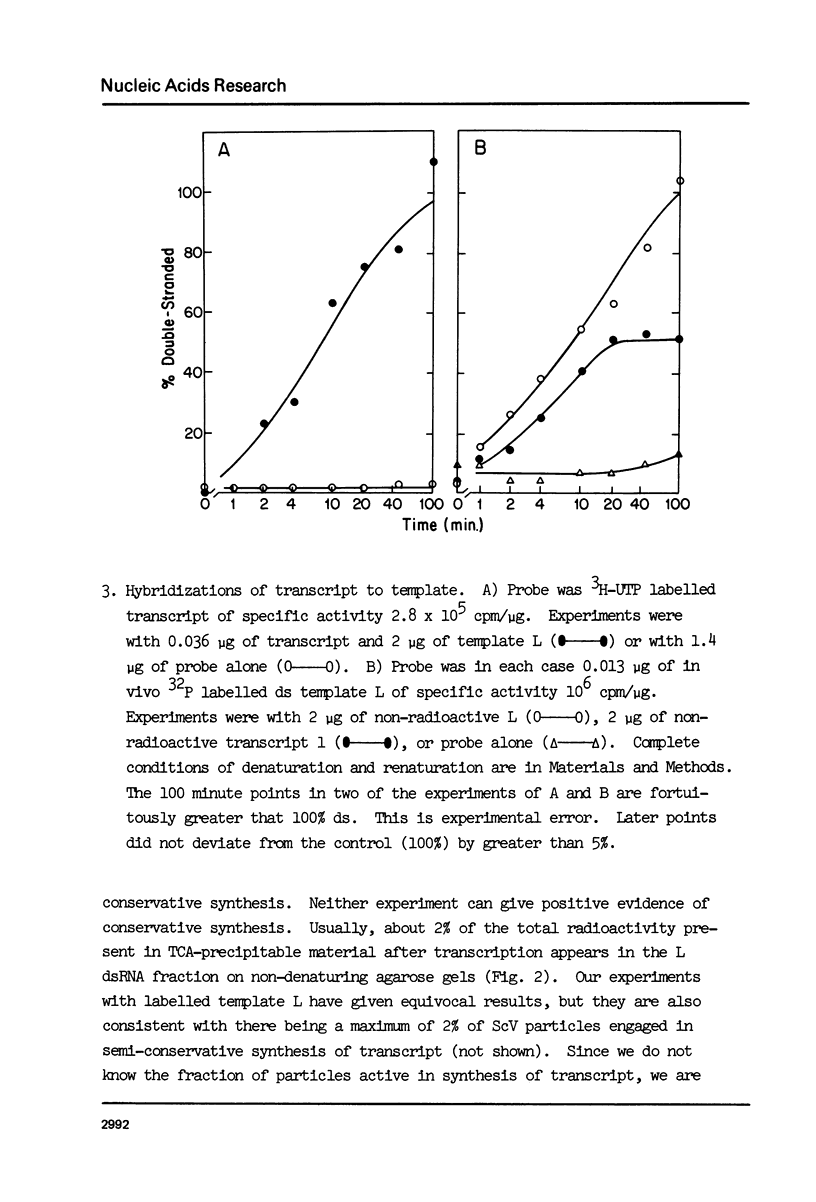
Abstract
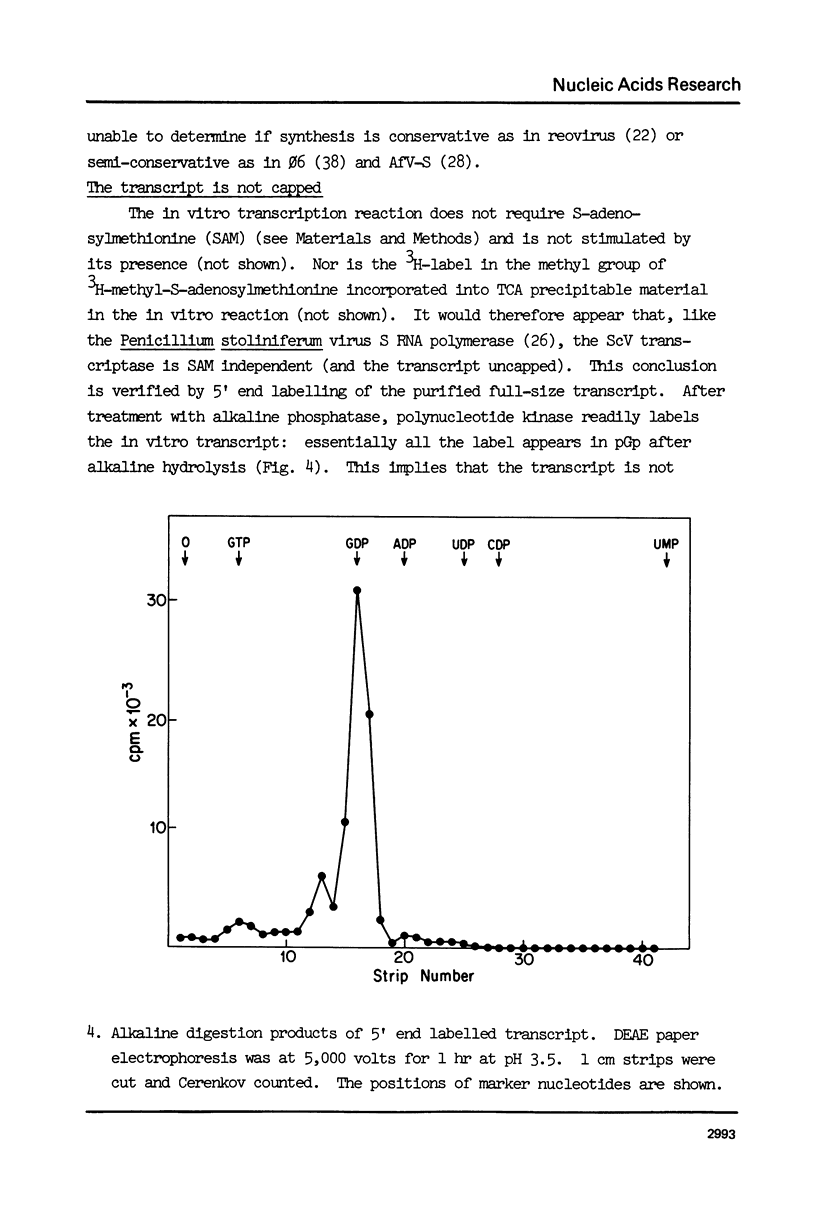
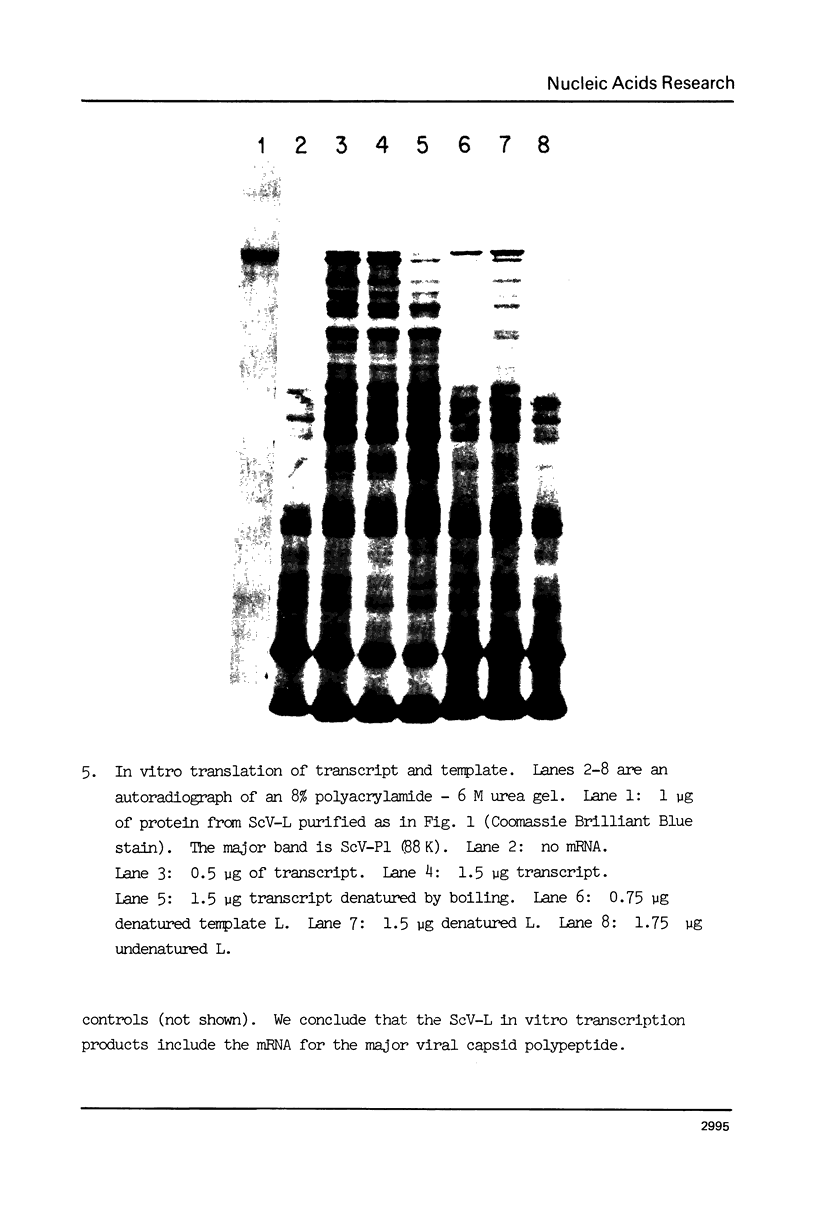
ScV-L is a simple double-stranded RNA virus of yeast, consisting of a 4.8 kilobase pair double-stranded RNA (L) encapsidated in isometric particles composed mainly of one polypeptide (ScV-Pl) of 88,000 daltons. L encodes ScV-Pl. There is a capsid-associated RNA polymerase that synthesizes in vitro predominantly single-stranded RNA. We show that this polymerase activity is a transcriptase, at the least one product of which is the mRNA for ScV-Pl. The transcript, like its template, is uncapped.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Kane W. Relatedness of the double-stranded RNAs present in yeast virus-like particles. J Virol. 1978 Jun;26(3):762–772. doi: 10.1128/jvi.26.3.762-772.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Keitz B. The 5' ends of yeast killer factor RNAs are pppGp. Nucleic Acids Res. 1976 Oct;3(10):2427–2436. doi: 10.1093/nar/3.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K. W. Replication of double-stranded RNA in particles of Penicillium stoloniferum virus S. Nucleic Acids Res. 1975 Oct;2(10):1889–1902. doi: 10.1093/nar/2.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Morgan D. H. Ribonucleic acid synthesis by isolated viruses of Penicillium stoloniferum. J Gen Virol. 1974 Aug;24(2):307–317. doi: 10.1099/0022-1317-24-2-307. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. S. Virus-like particles and double stranded RNA from killer and non-killer strains of Saccharomyces cerevisiae. Microbios. 1978;21(85-86):161–176. [PubMed] [Google Scholar]
- Hastie N. D., Brennan V., Bruenn J. A. No homology between double-stranded RNA and nuclear DNA of yeast. J Virol. 1978 Dec;28(3):1002–1005. doi: 10.1128/jvi.28.3.1002-1005.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W. A., West K., Gallagher J. F. Importance of initiation factor preparations in the translation of reovirus and globin mRNAs lacking a 5'-terminal 7-methylguanosine. J Biol Chem. 1977 Dec 10;252(23):8489–8497. [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Yeast virus-like particles possess a capsid-associated single-stranded RNA polymerase. Nature. 1977 Aug 4;268(5619):464–466. doi: 10.1038/268464a0. [DOI] [PubMed] [Google Scholar]
- Hollings M. Mycoviruses: viruses that infect fungi. Adv Virus Res. 1978;22:1–53. doi: 10.1016/s0065-3527(08)60771-x. [DOI] [PubMed] [Google Scholar]
- Holm C. A., Oliver S. G., Newman A. M., Holland L. E., McLaughlin C. S., Wagner E. K., Warner R. C. The molecular weight of yeast P1 double-stranded RNA. J Biol Chem. 1978 Nov 25;253(22):8332–8336. [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Kane W. P., Pietras D. F., Bruenn J. A. Evolution of defective-interfering double-stranded RNAs of the yeast killer virus. J Virol. 1979 Nov;32(2):692–696. doi: 10.1128/jvi.32.2.692-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nash C. H., Douthart R. J., Ellis L. F., Van Frank R. M., Burnett J. P., Lemke P. A. On the mycophage of Penicillium chrysogenum. Can J Microbiol. 1973 Jan;19(1):97–103. doi: 10.1139/m73-014. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Ratti G., Buck K. W. RNA polymerase activity in double-stranded ribonucleic acid virus particles from Aspergillus foetidus. Biochem Biophys Res Commun. 1975 Sep 16;66(2):706–711. doi: 10.1016/0006-291x(75)90567-7. [DOI] [PubMed] [Google Scholar]
- Ratti G., Buck K. W. Semi-conservative transcription in particles of a double-stranded RNA mycovirus. Nucleic Acids Res. 1978 Oct;5(10):3843–3854. doi: 10.1093/nar/5.10.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Somers J. M. Isolation of Suppressive Sensitive Mutants from Killer and Neutral Strains of SACCHAROMYCES CEREVISIAE. Genetics. 1973 Aug;74(4):571–579. doi: 10.1093/genetics/74.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Cuppels D. A., Lane L. C., Vidaver A. K. Semiconservative synthesis of single-stranded RNA by bacteriophage phi 6 RNA polymerase. J Virol. 1980 Feb;33(2):769–773. doi: 10.1128/jvi.33.2.769-773.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. The killer double-stranded RNA plasmids of yeast. Plasmid. 1979 Jul;2(3):303–322. doi: 10.1016/0147-619x(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]