INTRODUCTION
Most eukaryotic cells contain and synthesize sterols. Whereas cholesterol is the unique sterol of vertebrates and ergosterol the major sterol of most fungi and some unicellular algae, most higher plants contain a complex mixture in which cholesterol is a minor component and 24-ethyl sterols (sitosterol and stigmasterol) account for more than 60% and 24-methyl sterols account for less than 40% (Table 1). Configuration at C-24 is 100% R in sitosterol. In contrast 24-methyl sterols are a mixture of 24-epimers where campesterol (24R-methyl cholesterol) and dihydro-brassicasterol (24S-methyl cholesterol) are in variable proportions (Benveniste, 1986; Nes, 1977). Important differences may occur in some plant families, for instance many plants belonging to the order of caryophillales contain large amounts of Δ7-sterols, spinach (Spinacea oleracea) and Chenopodium rubrum contain almost only Δ7-sterols such as spinasterol or 5α–stigmast-7-ene-3β–ol (Adler and Salt, 1987). Likewise, changes in the side chain structure may occur in some plant families. For instance sterols with a double bond at position C-25(27) occur often in cucurbitaceae and verbenaceae (Akihisa et al., 1987). The sterol composition of brassicaceae to which Arabidopsis belongs, presents a small difference to most plant species for an additional sterol, brassicasterol (ergosta-5,22E-diene-3β–ol), is found in the 24-methyl sterol fraction (Itoh et al., 1973; Schaeffer et al., 2001). It is noteworthy that insects, which constitute more than 80% of animal species, do not synthesize sterols. They use sterols from their diet. For instance, phytophageous insects are able to demethylate sitosterol into cholesterol, which is finally used to make ecdysteroids involved during embryonic and larval development (Svoboda and Weirich, 1995).
Table 1.
Sterol profile of Arabidopsis thalianaa

Sterols have multiple functions. First of all they are membrane constituents. They accumulate in the plasma membrane where they are present in concentrations similar to those of phospholipids (Hartmann-Bouillon and Benveniste, 1978). Several data obtained with model systems show that sterols in condensing the membrane bilayer regulate membrane fluidity and permeability (Bloch, 1983; Demel and De Kruyff, 1976). In addition to this role played by the bulk of sterols, a regulatory function has been assigned to sterols in various systems : i) covalent binding of cholesterol to HEDGEHOG is involved in embryonic development of vertebrates (Kip Guy, 2000) ; ii) interaction of cholesterol with caveolin induces the formation of membrane microdomains (caveolae), which may be signaling centers for multiple pathways (Karpen et al., 2001) ; iii) transport of plant sterols by elicitins from Phytophtora spp leads to a hypersensitive like response in tobacco (Mikes et al., 1998). These processes trigger signaling pathways involved in cell division, development or resistance to pathogens. Finally sterols are precursors of compounds with a high physiological activity such as brassinosteroids, an important class of hormones involved in higher plant growth and development (Li et al., 1996), ecdysteroids in invertebrates (Svoboda and Weirich, 1995), antheridiol and oogoniols, sexual pheromones of aquatic fungi belonging to the saprolegniale class (Brunt and Silver, 1991). Therefore, sterols, in addition to their own biological activity, are important interfaces of plant insect interactions.
In spite of this recent knowledge, several important questions remain open especially in plants : i) higher plants synthesize phospholipids containing polyunsaturated fatty acids and bulky 24-ethyl sterols, which are both incorporated into membranes in which they should interact (Schuler et al., 1991). Is the biosynthesis of both class of compounds co-regulated and, if so, how does such a coregulation work ? ii) Considering the regulatory processes enumerated above, what is the role of sterols in signal reception and transduction ? iii) It is well established that brassinosteroid biosynthesis constitutes one branch deriving from phytosterol biosynthesis. Whereas sterols are present usually in plants at concentrations in the range of several mg per g of dry weight, a few ng per g of dry weight of brassinosteroids are generally found. Therefore a very small fraction of the phytosterols synthesized by the plant will be used for brassinosteroid biosynthesis. This leads to the existence of a tight control at the junction point between both pathways.
In order to answer these questions, a strategy of gain or loss of function has been applied recently to the sterol biosynthetic pathway. The use in the past of potent and specific inhibitors gave unvaluable informations (Benveniste and Rahier, 1992; Schmitt et al., 1981) which in some case were not reliable because of side effects of inhibitors. Recently the impressive development of molecular genetics, the availability of DNA tagged mutants, multiple possibilities offered by stable transformation in sense and antisense orientation and gene silencing, assorted with the sequencing of the Arabidopsis genome led to an important breakthrough in the field of sterol metabolism and functions.
Before entering into this field, a short recall of the state of the art of the sterol biosynthesis is needed. Sterol biosynthesis is complex and involves at least 25 steps from isopentenyl diphosphate, the commited precursor of all isoprenoids, to end pathway sterols. The biosynthetic scheme of Figure 1 is common to most higher plants and also applies to Arabidopsis. From isopentenyl diphosphate to 2,3-oxidosqualene (OS) the biosynthetic pathway is the same in eukaryotes, however profound differences exist downstream of OS (Figure 2). Whereas OS is cyclized to lanosterol in nonphotosynthetic eukaryotes, it is cyclized to cycloartenol, an isomer of lanosterol possessing a cyclopropane ring in place of the Δ8 double bond, in photosynthetic eukaryotes. However some exceptions to this rule will be discussed below. As end pathway plant sterols do not contain a cyclopropane ring, an enzyme capable of opening it exist in photosynthetic eukaryotes. A third difference consists in the presence in higher plant sterols (sitosterol, stigmasterol…) of an extra 24-ethyl group, resulting from two methylation steps situated between cycloartenol and end pathway sterols. Sterols from vertebrates are not alkylated at C-24, whereas sterols from most fungi possess only one methyl at C-24. Finally the passage from cycloartenol to end pathway sterols involves three demethylation steps at positions C4 and C14 of the sterol skeleton. The order and the position of these steps strongly differ in non photosynthetic and photosynthetic eukaryotes.
Fig. 1.
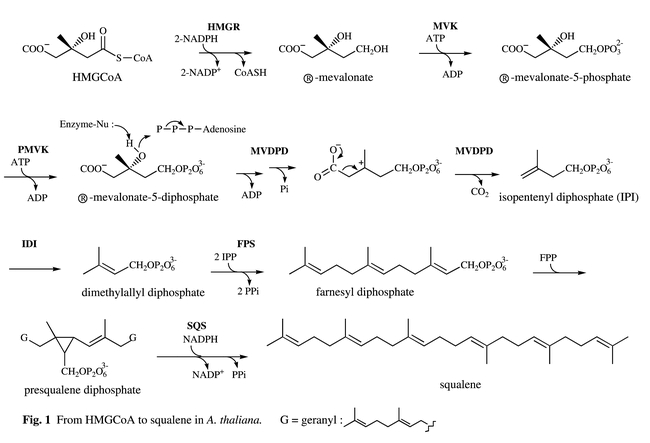
Biosynthetic pathway leading to plant sterols. From mevalonate to squalene.HMGR = (S)-3-hydroxy-3-methyl-glutaryl-CoA reductase ; MVK = mevalonate kinase ; PMVK : 5-phosphomevalonate kinase ; MVDPD = mevalonate diphosphate decarboxylase ; IDI = isopentenyl diphosphate-dimethylallyl diphosphate isomerase ; FPS = farnesyl diphosphate synthase ; SDS = squalene synthetase.
Fig. 2.
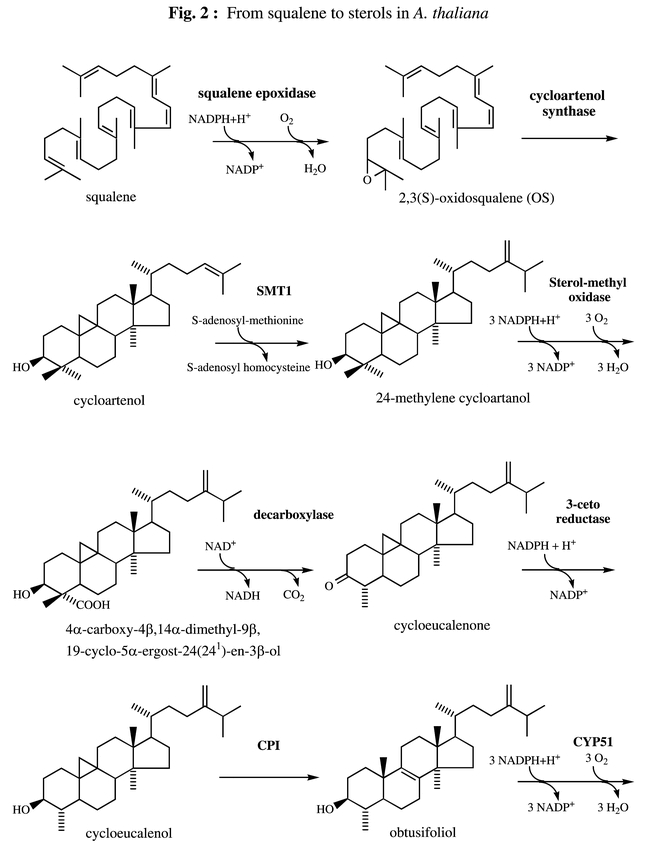
Biosynthetic pathway leading to plant sterols. From squalene to end pathway sterols.SMT1, SMT2 = sterol-methyltransferases ; FACKEL = Δ8,14-sterol-Δ14-reductase ; STE1/DWARF7/BUL1 = Δ7-sterol-C5(6)-desaturase ; DWARF5 = Δ5,7-sterol-Δ7-reductase ; DWARF1/DIM = Δ5-sterol-Δ24-reductase (isomerase) ; CPI = cyclopropyl sterol isomerase ; CYP51 = obtusifoliol-14α-demethylase.
Fig. 2a.
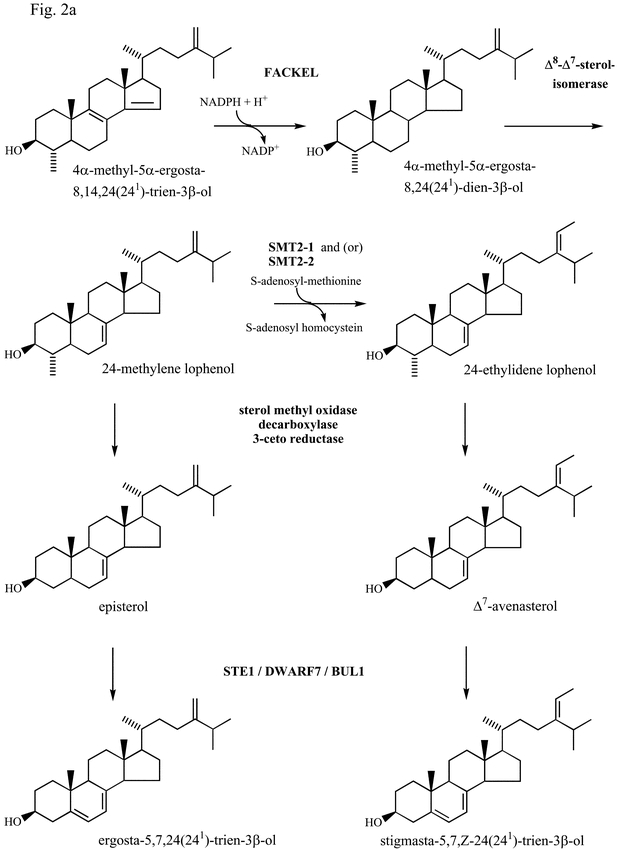
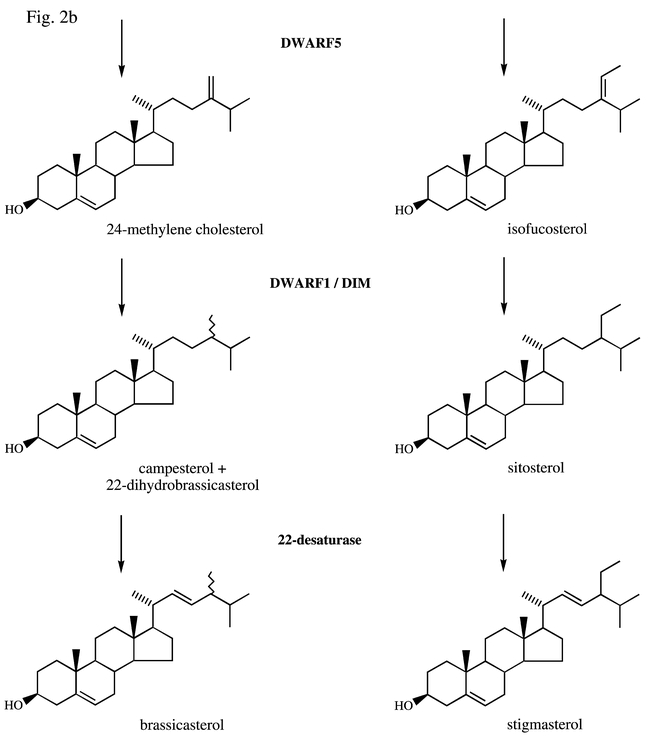
Fig. 2b.
In the present review we will present most significant progresses occurring between mevalonic acid and stigmasterol. As mevalonate is the precursor of sterols in most eukaryotes and especially in higher plants, we shall not consider here the non mevalonate (2-C-methyl-D-erythritol 4-phosphate) pathway leading to isoprenoids (Rohmer et al., 1993) which will be treated separately. Because genetical data obtained in recent years repeatetly show that brassinosteroid and campesterol biosynthesis are intimately linked, this chapter on sterol metabolism will include a few considerations concerning brassinosteroid biosynthesis. A specific chapter is devoted to brassinosteroid biosynthesis.
FROM HMGCoA TO SQUALENE
3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGR, EC 1.1.1.34) catalyzes the two-step reduction of (S)-3-hydroxy-3-methylglutaryl coenzyme A (S-HMG-CoA) into R-mevalonate (Fig 1) and is generally considered to be a regulatory enzyme in sterol biosynthesis. All HMGR genes from plants isolated so far code for proteins having the same general organization, a highly conserved membrane-spanning domain consisting of two hydrophobic amino-acid sequences that are separated from the globally hydrophilic and highly conserved catalytic domain by a poorly conserved linker region. It appears that plant HMGR isozymes are encoded by a small family of genes and a large body of evidence has been presented that these genes and their products are placed under developmental or environmental control (Bach, 1987; Choi et al., 1994; Enjuto et al., 1994). An Arabidopsis cDNA encoding HMGR has been cloned for the first time (Learned and Fink, 1989) by functional complementation of a yeast strain (JRY 2394) which was defective in mevalonic acid synthesis (Basson et al., 1988). Later on Arabidopsis was shown to contain two genes HMG1 and HMG2 (At1g76490 and At2g17370) that encode HMGR. HMG1 mRNA is detected in all tissues, whereas the presence of HMG2 mRNA is restricted to young seedlings, roots and inflorescences. In vitro translation products of these genes were shown to be efficiently inserted into endoplamic reticulum-derived microsomal membranes (Enjuto et al., 1994). Similar results were obtained with full-length cDNAs (HMG1 and HMG2) from Raphanus sativus, another brassicaceae (Vollack et al., 1994). Attemps to express a full-length cDNA (HMG1) encoding an isozyme of radish HMGR in Escherichia coli resulted in the production of catalytically inactive enzyme, while expression of a truncated form comprising only the soluble domain led to the formation of a highly active enzyme (Ferrer et al., 1990). Bacterial expression of the catalytical domain of HMGR (isoform HMG1) from Arabidopsis, and its inactivation by phosphorylation at S577 by Brassica oleracea 3-hydroxy-3-methylglutaryl-CoA reductase kinase were also reported (Dale et al., 1995). The use of an alternative promoter in the Arabidopsis HMG1 gene was shown to generate an mRNA that encodes a novel HMGR isoform (HMG1L) with an extended N-terminal region (Lumbreras et al., 1995). Results of this study support the conclusion that the ER is the only cell compartment for the primary targeting of HMGR in Arabidopsis and that the three HMGR isoforms are cotranslationally inserted into the ER membrane, where they behave as integral membrane proteins (Lumbreras et al., 1995). Despite the house keeping role that has been ascribed to HMG1, both the quantitative and qualitative features of its expression are modulated in response to a variety of cellular and environmental signals and especially to light (Korth et al., 2000). More precisely, light-mediated regulation of HMG1 expression has been shown to be an organ-autonomous response and indicated that the high levels of HMG CoA reductase expression detected in immature leaves may be primarily attributed to the dark-induced expression of HMG1, and that HMG1 is expressed at low levels throughout the plant in response to light (Learned and Connolly, 1997). Finally HMGR is a rate-limiting enzyme for higher plant sterol biosynthesis (Chappell et al., 1995; Schaller et al., 1995; Schaller et al., 1993) ; its regulation is complex and occurs at transcriptional, translational and post-translational (Sugden et al., 1999) levels. Enzymological studies of the plant enzyme are still needed to better understand features of post-translational regulation. Recent crystal structures of the catalytic portion of human (Istvan et al., 2000) and Pseudomonas mevalonii (Bochar et al., 1999), HMGRs have given precious clues to better understand some aspects of Arabidopsis HMGR regulation.
Mevalonate kinase (MVK), the enzyme that catalyzes the phosphorylation of R-mevalonate by ATP to produce R-mevalonate 5-phosphate and ADP, is considered as a potential regulatory enzyme of the isoprenoid biosynthetic pathway. Mevalonate kinase has recently been very much studied in animals because mutations in the gene encoding this enzyme have been shown to be responsible of several pathologies such as the periodic fever syndrome, mevalonic aciduria and hyperimmunoglobulinaemia (Houten et al., 2001). In contrast few studies have been reported in plants. Using an Arabidopsis cDNA library constructed in the yeast expression vector pFL61 (Minet et al., 1992), a cDNA of 1.64-kb encoding a protein of 378 amino acid residues with significant similarity to yeast and mammalian enzymes, was isolated and characterized by genetic complementation of the erg12-1 mutation in yeast (Riou et al., 1994). Recently molecular cloning and expression analysis of the mevalonate kinase gene from Arabidopsis (AT5g27450) have been reported (Lluch et al., 2000). The expression pattern of the MVK gene suggests that the role of the encoded MVK is the production of a general pool of mevalonate 5-phosphate for the synthesis of different classes of isoprenoids involved in both basic and specialized plant cell functions. Overexpression of both yeast or Arabidopsis cDNAs in the yeast had no significant effect on ergosterol accumulation (Riou et al., 1994). DNA hybridization blots and Arabidopsis complete genome sequencing show that only one locus exists in the Arabidopsis genome. The single gene from Arabidopsis contains five exons and four introns (Lluch et al., 2000).
cDNAs or genes encoding 5-phosphomevalonate kinase (PMVK, EC 2.7.4.2), the enzyme which catalyzes the phosphorylation of R-mevalonate 5-phosphate to R-mevalonate 5-diphosphate, has still not been isolated and characterized in higher plants. However complete sequencing of Arabidopsis genome has revealed the existence of a gene (At1g31910) whose encoded protein has 30% identity with the yeast PMVK (ERG8) (Tsay and Robinson, 1991). Therefore, with the availability of the erg8-1 yeast mutant, cloning by complementation seems feasible. Interestingly, so far, two nonorthologous genes encoding PMVK have been described, the Saccharomyces cerevisiae ERG8 gene and the human PMVK gene (Houten and Waterham, 2001). Molecular cloning of human phosphomevalonate kinase allowed the identification of a consensus peroxisomal targeting sequence suggesting that the conversion of mevalonate to farnesyl diphosphate occurs in parallel in the peroxisomes of mammalian cells (Biardi and Krisans, 1996).
Mevalonate diphosphate decarboxylase (MVDPD, EC 4.1.1.33) catalyzes the ATP-dependent conversion of mevalonate diphosphate into isopentenyl diphosphate (IPP). In Saccharomyces cerevisiae this enzyme is encoded by the ERG19 gene (Servouse et al., 1984). Sequence comparison with MVDPD amino acid sequence of S. cerevisiae identified an EST clone corresponding to a cDNA that may encode Arabidopsis MVDPD. The predicted amino acid sequence presents about 55% identity with the yeast, and human MVPDs. When expressed in yeast, the Arabidopsis cDNA complemented an ERG19 deleted strain. However the wild type sterol content was not fully restored, suggesting that the Arabidopsis MVPD activity may not be optimal in yeast (Cordier et al., 1999). Complete sequencing of the Arabidopsis genome revealed the existence of two genes (At2g38700 and AT3g54250). Using a two-hybrid assay, it was shown that the S. cerevisiae MVDPD forms homodimers in vivo and a single substitution impairs dimerization. The Arabidopsis MVDPD forms also homodimers in vivo. Heterodimer formation between the plant and the wild-type yeast enzymes was detected by these assays (Cordier et al., 1999). Crystal structure would be an attractive goal in order to better understand the intricate mechanism of action of this enzyme.
Isopentenyl diphosphate (IPP) : dimethylallyl diphosphate (DMAPP) isomerase (IDI, EC 5.3.3.2) catalyzes the conversion of IPP to its electrophilic isomer DMAPP, which is then used to prime the prenyltransferase for chain elongation with IPP. Two Arabidopsis cDNAs (IDI1 and IDI2) encoding isopentenyl diphosphate isomerase (IDI) were isolated by complementation of an IPP isomerase mutant strain of S. cerevisiae (Campbell et al., 1997). Both cDNAs encode enzymes with an amino terminus that may function as a transit peptide for localization in plastids. Total sequencing of the Arabidopsis genome and DNA blot analysis confirm that IDI1 and IDI2 are derived from two genes (AT5g16440, AT3g02780) in Arabidopsis. This seems to be a general feature in most higher plants examined so far and suggests that a single gene would encode plastid and cytosolic IDI in plants (Cunningham and Gantt, 2000). The intracellular localization of isoprenoid synthesis enzymes has been reconsidered in mammalian cells and IDI has been shown to be localized predominantly in peroxisomes and not in the cytosol (Olivier and Krisans, 2000).
Farnesyl diphosphate synthase (FPS, EC 2.5.1.1) catalyzes the condensation of one molecule of dimethylallyldiphosphate (DMAPP) with two molecules of isopentenyl diphosphate (IPP) to give farnesyl diphosphate (FPP). Farnesyl diphosphate is a very important compound in the plant (especially Solanaceae) cell economy because it is located at a branch point that leads to the formation of sterols and sesquiterpenoid phytoalexins. FPS is encoded by the gene ERG 20 in yeast. Disruption of the yeast ERG 20 gene is lethal (Blanchard and Karst, 1993). A cDNA encoding Arabidopsis FPS1 was subsequently isolated by complementation of the erg 20 deletion yeast mutant (Delourme et al., 1994) with the cDNA expression library cloned into pFL61 (Minet et al., 1992). This cDNA encodes a polypeptide of 343 amino acids residues presenting about 50% identity with yeast, rat and human FPSs. A second cDNA corresponding to the FPS2 was isolated by RT-PCR, it codes for a protein having 342 amino acid residues. FPS1 and FPS2 share an overall amino acid identity of 90.6%. The FPS1 gene (AT5g47770) consists of 12 exons and 11 introns whereas the FPS2 gene (AT4g17190) consists of 11 exons and 10 introns. In both genes, introns are located at equivalent positions relative to the coding sequences (Cunillera et al., 1996). FPS1 mRNA accumulates preferentially in roots and inflorescences whereas FPS2 mRNA is predominantly expressed in inflorescences. Later on it has been shown that the FPS1 gene generates a novel mRNA that encodes a mitochondrial farnesyl diphosphate synthase isoform (FPS1L) (Cunillera et al., 1997). The FPS1L mRNA accumulated preferentially in inflorescences whereas the previously reported FPS1 mRNA (FPS1S mRNA) is predominantly expressed in roots and inflorescences. Translation of the FPS1L mRNA from the upstream ATG start codon generates a novel FPS1 isoform (FPS1L) with an NH2-terminal extension of 41 amino-acid residues, which has all the characteristics of a mitochondrial transit peptide. The functionality of this extension as a mitochodrial transit peptide was demonstrated by in vitro import experiments using purified plant mitochondria (Cunillera et al., 1997). Spatial and temporal patterns of the β-glucuronidase (GUS) reporter gene expression directed by 5′ regions of the Arabidopsis FPS1 and FPS2 genes have been studied. The highest levels of FPS2 : GUS activity were detected in flowers, especially in pollen grains. Overall, the pattern of expression of FPS2 :GUS suggests a role for FPS2 in the synthesis of particular isoprenoids with specialized functions. In contrast, the FPS1S :GUS gene is widely expressed in all plant tissues throughout development, thus supporting a role for FPS1S in the synthesis of isoprenoids (for instance sterols) serving basic plant cell functions (Cunillera et al., 2000). Recently cDNA sequences encoding FPS have been cloned from several plant species such as Lupinus albus (Attucci et al., 1995), Zea mays (Li and Larkins, 1996), Artemisia annua (Matsushita et al., 1996), Hevea brasiliensis (Adiwilaga and Kush, 1996), and Parthenium argentatum (Pan et al., 1996). According to these studies, it seems that plant FPS would be encoded by multigene families like other key enzymes of the isoprenoid biosynthesis such as HMGR, IDI and several others (vide infra). The biological significance of the occurrence of similar isoforms in plants will be discussed in a conclusive paragraph. Nevertheless, FPSs are considered to play a key role in isoprenoid biosynthesis. It has been shown that in mammals FPS is a highly regulated enzyme involved in the control of the sterol biosynthetic pathway (Clarke et al., 1987). Relevant to this role are recent studies showing that : i) farnesol induces cell death and stimulation of HMGR in tobacco cv Bright Yellow-2 cells (Hemmerlin and Bach, 2000) ; ii) farnesol is utilized for isoprenoid biosynthesis in plant cells via FPP formed by successive monophosphorylation reactions (Thai et al., 1999).
Squalene synthase (SQS ; EC 2.5.1.21) catalyzes the reductive condensation of two molecules of FPP to squalene in the presence of NADPH and Mg2+, via the intermediate presqualene diphosphate (Fig. 1). In contrast to preceding enzymes, SQS catalyzes the first committed step in sterol formation and for this reason may be highly regulated (Kennedy and Bard, 2001; Radisky and Poulter, 2000). A SQS probe from Mus musculus was successfully used to isolate two overlapping Arabidopsis cDNA clones coding for a protein of 410 amino acids with about 40% identity with mammalian and yeast squalene synthetases (Nakashima et al., 1995). The Arabidopsis SQS enzyme was expressed in Escherichia coli. A cell-free extract of E. coli transformed with a recombinant plasmid containing the Arabidopsis SQS cDNA efficiently converted [14C]farnesyl diphosphate into squalene in the presence of NADPH and Mg2+. This study revealed that both the structures and reaction mechanisms of squalene synthases were markedly conserved between plants and mammals. Later on it was shown that Arabidopsis SQS is encoded by a small gene family of two genes : SQS1 (AT4g34640) and SQS2 (AT4g34650), which are organized in a tandem array (Kribii et al., 1997). SQS1 and SQS2 have an identical organization regarding intron positions and exon sizes and encode SQS isoforms showing a high level of sequence conservation (79% identity). Remarkably the SQS1 isoform is unable to complement the SQS-defective (gene disrupted) Saccharomyces cerevisiae strain 5302, although SQS activity was detected in the microsomal fraction of the transformed yeast strain. However a chimeric SQS resulting from the replacement of the 66 C-terminal residues of the Arabidopsis enzyme by the 111 C-terminal residues of the Schizosaccharomyces pombe enzyme was able to confer ergosterol prototrophy to strain 5302. This and other expriments indicated that the C-terminal region of the enzyme is involved in the channeling of squalene through the yeast sterol pathway (Kribii et al., 1997). Squalene synthase cDNAs were isolated also in Glycyrrhiza glabra, Nicotiana tabacum, Nicotiana benthamiana, Orizum sativa, Zea may, Solanum tuberosum and Glycine max (Devarenne et al., 1998; Hata et al., 1997; Hayashi et al., 1996; Yoshioka et al., 1999). Treatment of tobacco cell suspensions cultures with a fungal elicitor dramatically reduced SQS enzymatic activity. Analysis of SQS mRNA levels in elicitor-treated cells indicated that the suppression of SQS enzyme activity may result from a post-transcriptional modification of SQS (Devarenne et al., 1998). Squalestatin S1, a potent inhibitor of SQS in mammalian cells, has been shown to strongly inhibit sterol biosynthesis in tobacco BY-2 cells. Conversely enzymatic activity and mRNA levels of HMGR were several-fold increased (Hartmann et al., 2000). Finally a crystal structure of human squalene synthase has been recently reported and should be useful in the design of new inhibitors of potential therapeutic importance (Pandit et al., 2000).
FROM SQUALENE TO STEROLS
Squalene epoxidase (EC 1.14.99.7) catalyzes the conversion of squalene to 2,3(S)-oxidosqualene (OS) (Fig 2) which is the first oxygenation step in the sterol biosynthetic pathway. This microsomal-bound enzyme has been thoroughly studied in animal cells (Yamamoto and Bloch, 1970). Rat squalene epoxidase requires FAD, NADPH-cytochrome P450 reductase, NADPH and a supernatant protein factor (SPF). Rat squalene epoxidase cDNA was isolated by selecting S. cerevisiae transformants expressing rat cDNAs in the presence of terbinafin, a potent fungicide and inhibitor of fungal squalene epoxidase (Sakakibara et al., 1995). Rat squalene epoxidase, as deduced from the nucleotide sequence, contains 573 amino acids (Sakakibara et al., 1995). The amino acid sequence reveals one potential transmembrane domain and a FAD binding domain, suggesting that the squalene epoxidase is a flavo-protein. This deduced sequence is 30% identical to ERG1, the gene encoding squalene epoxidase in S. cerevisiae (Jandrositz et al., 1991). Rat squalene epoxidase expressed in E. coli was shown to catalyze the in vitro conversion of squalene to 2,3(S)-oxidosqualene, thus confirming the polypeptide as a functional squalene epoxidase (Sakakibara et al., 1995). The gene encoding the supernatant protein factor (SPF) that promotes the squalene epoxidation catalyzed by rat liver microsomes has been recently cloned (Shibata et al., 2001). The encoded protein of 403 amino acids belongs to a family of cytosolic lipid-binding/transfer proteins. Only a few studies concerning squalene epoxidase have been reported in plants. The sequencing of the Arabidopsis genome has revealed 6 genomic clones (AT1g58440, AT2g22830, AT4g37760, AT5g24140, AT5g24150 and AT5g24160) whose deduced polypeptidic sequences possess significant identities with the rat liver enzyme. The amino acid sequences reveal one potential transmembrane region and a FAD binding domain suggesting the existence in Arabidopsis of a family of genes encoding squalene epoxidase. However no data dealing with the function of theses genes has been reported so far. Sequences of 3 Arabidopsis and 2 Brassica napus cDNAs encoding squalene epoxidase homologues have been reported (Schäfer et al., 1999). Comparison of cDNA and genomic sequences indicate that the 3′ splice site of an intron in these genes has undergone junctional sliding (Schäfer et al., 1999), a phenomenon having interesting evolutionary significance.
2,3(S)-oxidosqualene-cycloartenol cyclases (cycloartenol synthase).
The 2,3(S)-oxidosqualene (OS) cyclases compose a family of biocatalysts that convert (S)-2,3-oxidosqualene (OS) to polycyclic triterpenes. The mammalian (Abe and Prestwich, 1995) and fungal (Shi et al., 1994) OS cyclase (EC 5.4.99.7) produces lanosterol, precursor of cholesterol in vertebrates and of ergosterol in most fungi. The higher plant OS cyclase (EC 5.4.99.8) catalyzes the formation of cycloartenol, precursor of phytosterols (Benveniste, 1986; Nes, 1977). This latter enzyme must break 11 bonds and form 11 new ones to form cycloartenol, which is a pentacyclic cyclopropyl triterpene containing 9 chiral centers. In addition to cycloartenol synthase, higher plants contain other OS-cyclases converting OS into a vast array of pentacyclic triterpenoids (oleanane, ursane, lupane, baccharane, friedelane derivatives). The stereoelectronic factors that understate the catalytic mechanism of these reactions have been discussed elsewhere (Abe et al., 1993). The formation of lanosterol and cycloartenol is initiated in the chair-boat-chair-like conformation of OS whereas the formation of amyrins and related pentacyclic triterpenes is initiated in the chair-chair-chair-like conformation of OS. In all cases the proton-initiated cyclization is postulated to proceed through the following events : i) a series of antiperiplanar 1,2 additions ; 1,2 rearrangements and 1,2 eliminations ; ii) the formation of conformationally rigid and partially cyclized carbocationic intermediates ; iii) stabilization of the developing carbocationic centers on the substrate by negative point charges at the active site of the enzyme (Johnson et al., 1987). As suggested by several authors (Feil et al., 1996; Shi et al., 1994) the electron density required for carbocationic stabilization could arise from anionic and (or) aromatic residues. These cyclization intermediates could be stabilized by cation-Π interactions (Dougherty, 1996). The fact that OS mimics possessing positively charged nitrogen atoms in place of potential carbocation behave as potent inhibitors is consistent with the above views (Taton et al., 1992). Most prokaryotic organisms do not synthesize sterols, but many of them synthesize hopanoids that are derived from pentacyclic triterpenic hydrocarbons which originate from squalene cyclisation owing to squalene-hopene cyclases (Wendt et al., 1997). A 2,3-OS-lanosterol cyclase (lanosterol synthase) gene (ERG7) from S. cerevisiae was isolated and encoded a 83-kDa protein (Corey et al., 1996; Shi et al., 1994). Later on a splendid approach led to the isolation of an Arabidopsis cDNA (ATCYC) encoding a cycloartenol synthase (Corey et al., 1993). This was achieved by functional expression in a yeast mutant lacking lanosterol synthase (GL7) by the use of a chromatographic screen. This cDNA contained a 2277-bp open reading frame encoding a 86-kD protein which was about 35% identical with S. cerevisiae lanosterol synthase and remarkably 46% identity with a Rattus norvegicus lanosterol synthase (Abe and Prestwich, 1995) showing that the plant cycloartenol synthase has more identity with a mammalian than a fungal lanosterol synthase. A single mutation (adenine1362guanine) occurring on the Arabidopsis cycloartenol synthase gene has been shown to confer to cycloartenol synthase the ability to form 25% lanosterol and 21% parkeol in addition to cycloartenol. This mutation leads to a I454V change in the peptidic sequence (Hart et al., 1999). A yeast mutant (erg7) defective in lanosterol synthase was transformed with a plasmid harbouring the cycloartenol synthase cDNA. The transformed yeast was still auxotrophic to ergosterol because cycloartenol is not usable by yeast to make sterols. Erg7 did not need any more ergosterol if transformed with a cycloartenol synthase cDNA possessing the A1362G mutation (Hart et al., 1999). Likewise, a tyrosine-to-threonine mutation was shown to convert the Arabidopsis cycloartenol synthase synthase to an OS cyclase that forms lanosterol as its major product (Herrera et al., 2000). Such experiments have far-reaching evolutionary implications. The corresponding single gene (At2g07050) consists of 18 exons and 17 introns. The Arabidopsis genome sequencing program reveals the presence of a gene (AT3g45130) whose deduced polypeptide sequence possessed 62% identity with the cycloartenol synthase protein and appeared closest to cycloartenol synthase in a phylogenetic tree containing many of the triterpene synthases already known (Fig. 4). The function of the protein encoded by this gene is still unknown.
Fig. 4.
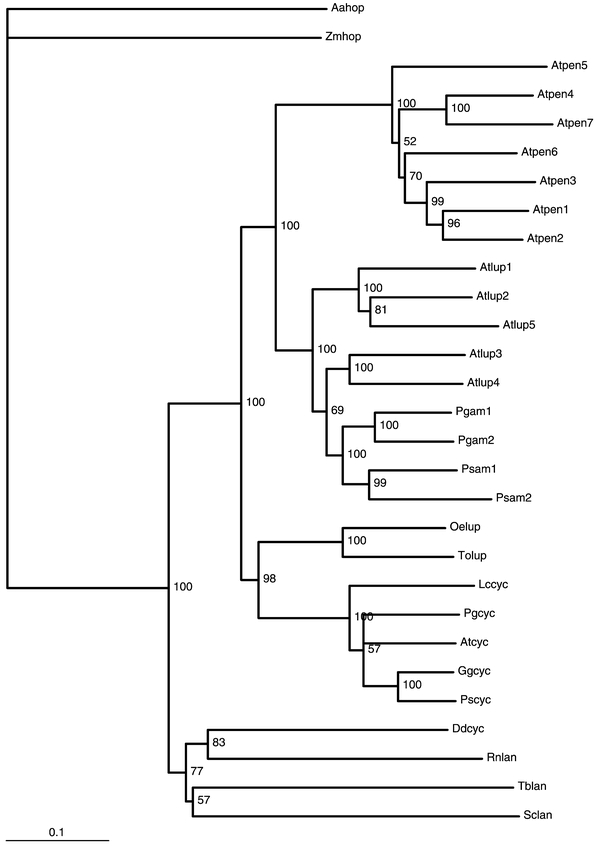
Phylogenetic tree constructed for several plant, animal, fungal and bacterial triterpene synthases. AAHOP and ZMHOP : Alicyclobacillus acidocaldarius and Zymomonas mobilis hopene synthases (Wendt et al., 1997), RNLAN, SCLAN and TBLAN : Rattus norvegicus (Abe and Prestwich, 1995), Saccharomyces cerevisiae (Shi et al., 1994) and Trypanosoma cruzi (Joubert et al., 2001) lanosterol synthases. ATCYC, DDCYC, PSCYC, GGCYC, PGCYC, LCCYC : Arabidopsis thaliana (Corey et al., 1993), Dictyostelium discoidum (Godzina et al., 2000), Pisum sativum, Glycyrrhiza glabra, Panax ginseng (Kushiro et al., 1998), Luffa cylindrica cycloartenol synthases (Kushiro et al., 1999). ATLUP1 to 5 : Arabidopsis thaliana triterpene synthases which cyclize 2,3-oxidosqualene into various pentacyclic triterpenes (Herrera et al., 1998 ; Husselstein-Muller et al., 2001 ; Kushiro et al., 2000). PGAM1 and PGAM2 : Panax Ginseng β-amyrin synthase (Kushiro et al., 1998). PSAM1 and PSAM2 : Pisum sativum β-amyrin synthase and multifunctional triterpene synthase respectively. OELUP and TOLUP : Olea europeae and Taraxacum officinalis lupeol synthases (Kushiro et al., 1999) ; ATPEN1 to 7 ; Arabidopsis thaliana putative triterpene synthases. Accession Numbers for ATLUP1-5, ATPEN1-7, and ATCYC are given in Table 1. The phylogenetic tree has been rooted with AAHOP as outgroup. The numbers at the nodes of the dendogram are bootstrap which indicate the frequencies of occurrence of partitions found in the tree. The phylogenetic tree was developed with the aligned sequences from GCG files (version 10.1 of UNIX) and the PAUP program according to Doyle and Gaut (2000). The phylogenetic tree was drawn with TreeView.
In recent years a breakthrough has been achieved in the field of triterpene synthases. A cDNA encoding β-amyrin synthase (PGAM) has been characterized in Panax ginseng. Expression of the cDNA in yeast led to formation of a polypeptide capable of converting OS into β-amyrin (Kushiro et al., 1998). A cDNA encoding a lupeol synthase (ATLUP1) has been identified in Arabidopsis after expression in S. cerevisiae (Herrera et al., 1998; Husselstein-Muller et al., 2001). The protein (ATLUP1) encoded by this cDNA was shown to convert OS into lupeol, minor triter-penoid compounds were also identified (Herrera et al.,1998). Another cDNA (ATLUP2) was subsequently cloned from Arabidopsis and expressed in S. cerevisiae (Husselstein-Muller et al., 2001; Kushiro et al., 2000). The transformed S. cerevisiae was shown to cyclize OS into α-, β-amyrins and lupeol (Husselstein-Muller et al., 2001). During parallell experiments performed in another laboratory, taraxasterol, Ψ-taraxasterol, bauerenol, multiflorenol, butyrospermol and tirrucalla-7,21-dienol were identified in addition to α- and β-amyrins and lupeol (Kushiro et al., 2000). Therefore the polypeptide (ATLUP2) encoded by this gene is a novel multifunctional triterpene synthase. Shuffling domain mutagenesis has been done on ATLUP1 and PGAM in order to investigate the region important for protein specificity. The second quarter from the N-terminus of the protein was shown to have a crucial importance in determining the enzymatic process toward lupeol or amyrin (Kushiro et al., 1999). The genome sequencing project has revealed a rather complex situation. ATLUP1 and ATLUP2 are representatives of a small family of five genes (ATLUP1-5), in addition seven genes (ATPEN1-7) whose deduced polypeptide sequences have about 55% identity with the ATLUP family and which could encode triterpene synthases have been also identified (Husselstein-Muller et al., 2001). All genes encoding demonstrated or postulated triterpene synthases have been gathered in the Table 2. Each genomic sequence is labeled with a code and is also characterized by the number of exons and introns. In order to obtain information on evolutionary relationships, a phylogenetic tree was constructed using a heuristic search (Doyle and Gaut, 2000) (Fig. 4). In this tree the prokaryotic hopane synthases from Alicyclobacillus (AAHOP) was considered as reference taxa. According to this tree, the seven pentacyclic triterpene synthases from Arabidopsis (ATPEN1to 7) form a group of genes clearly distinct from the five lupeol synthases (ATLUP1 to 5). Interestingly, the ATLUP subfamily also encompasses the two β-amyrin synthases of Panax ginseng (PGAM) and of Pisum sativum (PSAM) and is clearly distinct from the lupeol synthases from Olea europaea (OELUP) and Taraxacum officinalis (TOLUP) (Kushiro et al., 1999).
Table 2.
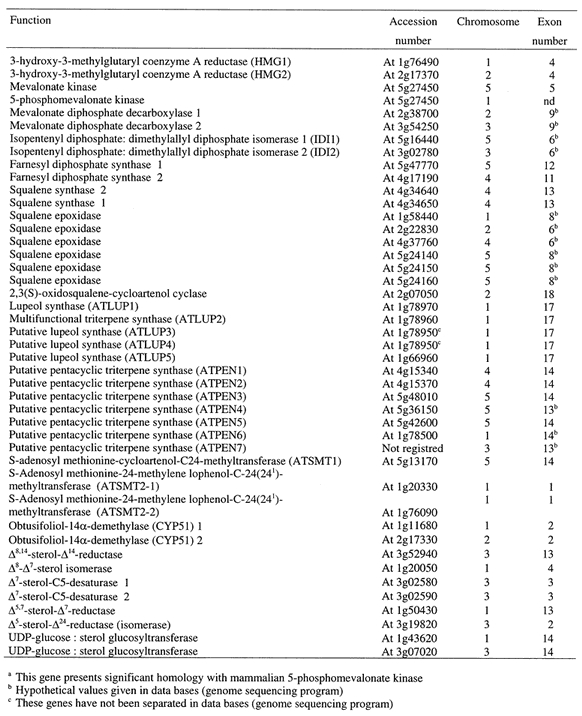
Summary of genomic sequences found in databases and corresponding to enzymes of sterol biosynthesis and metabolism
S-Adenosylmethionine-sterol-C-methyltransferases.
Sterols from fungi and higher plants differ from vertebrate sterols by the presence of an extra alkylgroup at C-24 (Nes, 2000). Whereas most fungi sterols possess a methyl group at C-24, higher plants contain both 24-methyl- and 24-ethyl sterols. This alkylation of the side chain is catalyzed by S-adenosylmethionine (AdoMet)-sterol-C-methyltransferases (SMTs). In S. cerevisiae, the SMT converts zymosterol into fecosterol (Moore and Gaylor, 1969). In higher plants (and therefore in Arabidopsis), the presence of 24-ethylsterols results from two distinct methyl-transfers from AdoMet (Nes, 2000; Rahier et al., 1984). According to the chemical structures of intermediates of plant sterol biosynthesis and substrate specificity studies, it is generally assumed that cycloartenol (Fig2) is the substrate of the first methylation reaction, resulting in 24-methylene cycloartanol (Wojciechowski et al., 1973), whereas 24-methylene lophenol is the preferred substrate for the second methylation, yielding 24-ethylidene lophenol (Fonteneau et al., 1977). Because the chemical structures of 24-methylene cyclartanol and 24-methylene lophenol are very different, it has been suggested that the two methylation ractions would be catalyzed by two different enzymes (Rahier et al., 1986). However, since no plant SMT has been purified so far, the hypothesis of an unique plant SMT catalyzing both alkylations (Nes et al., 1991) should also be considered. The first SMT gene (ERG6) cloned was from S. cerevisiae (Gaber et al., 1989; Hardwick and Pelham, 1994). Later on complete identification was performed by enzymatic assays on the protein derived from ERG6 after expression in E. coli. According to these studies the best substrate of the recombinant enzyme was zymosterol which was methylated to give fecosterol in agreement with previous enzymatic assays performed on microsomes from WT S. cerevisiae (Venkatramesh et al., 1996). A SMT cDNA of 1411 bp has been cloned from an Arabidopsis cDNA library, the deduced amino acid sequence of this cDNA showed three conserved regions found in AdoMet-dependent methyltransferases (Kagan and Clarke, 1994) and 38% identity with ERG6. This cDNA was used to transform a wild-type S. cerevisiae as well as the yeast null mutant erg6, which is deficient in the SMT-zymosterol-C-24 methyltransferase. In both cases, several 24-ethyl and 24-ethylidene sterols were synthetized, indicating that the Arabidopsis cDNA encodes a plant SMT capable of performing two sequencial methylations at C24 and C24-1 of the yeast sterols (Husselstein et al., 1996). Microsomes from erg6 expressing the Arabidopsis SMT were shown to possess AdoMet-dependent sterol-C-methyltransferase activity. Delipidated preparations of these microsomes converted cycloartenol into 24-methylene cycloartanol and 24-methylene lophenol into 24-ethylidene lophenol. The catalytic efficiency of the expressed SMT was 17-times higher with 24-methylene lophenol than with cycloartenol. This result provides evidence that the Arabidopsis cDNA encodes a 24-methylene-lophenol-C-241-methyltransferase catalyzing the second methylation step of plant sterol biosynthesis (Bouvier-Navé et al., 1997). This cDNA was named ATSMT2-1. A second cDNA (ATSMT2-2) of 1249 bp was cloned and was shown to encode a protein of 359 amino acids, 82% identical with the protein deduced from ATSMT2-1. Meanwhile two cDNAs from Nicotiana tabacum (NTSMT2-1 and NTSMT2-2) and one from Oriza sativa (OSSMT2) were characterized and were shown to be about 80% identical to the Arabidopsis cDNAs. The cDNA NTSMT2-1 was expressed in erg6 and the corresponding protein was shown to convert 24-methylene lophenol into 24-ethylidene lophenol as efficiently as ATSMT2-1 (Bouvier-Navé et al., 1997). Meanwhile cDNAs from Glycine max (Shi et al., 1995; Shi et al., 1996), Ricinus communis (Bouvier-Navé et al., 1997), Zea mays (Grebenok et al., 1997), N. tabacum and O. sativa (Bouvier-Navé et al., 1998) and Arabidopsis (Diener et al., 2000; Schaeffer et al., 2001) were isolated and characterized. The proteins encoded by these cDNAs are about 80% identical in all possible combinations, but have only 40% identity with SMT2 and 48% identity with ERG6. They constitute therefore a group of cDNAs (SMT1) distinct of the group formed by SMT2. SMT1 from Z. mays (Grebenok et al., 1997), from Arabidopsis (Diener et al., 2000) and from N. tabacum (Bouvier-Navé et al., 1998) were shown to partially restore the abillity of erg6 to make ergosterol. The G.max SMT1 was expressed in E. coli and the recombinant protein was shown to alkylate lanosterol (an unnatural substrate in plants) to give eburicol (Shi et al., 1996). In the presence of AdoMet, delipidated microsomes from erg6 transformed with tobacco SMT1 efficiently converted cycloartenol into 24-methylene cycloartanol, but did not produce any 24-ethylidene lophenol upon incubation with 24-methylene lophenol. This demonstrated that cDNA NTSMT1 (and most probably the other plant SMT of the same group such as ATSMT1) encoded a cycloartenol-C24 methyltransferase (Bouvier-Navé et al., 1998). Sequence alignment of SMT1 and SMT2 reveal three highly conserved domains. One of them (LDXGCGXGGPXRXI) corresponds to the G-rich consensus motif described for all AdoMet-dependent methyltransferases (Kagan and Clarke, 1994). A second (IEATCHAP) and a third (YEW/F/YGWGXSFHF) motives are believed to be typical of methyltransferases acting on a sterol substrate (Bouvier-Navé et al., 1997; Nes, 2000). Whereas SMT2 possess a hydrophobic domain of approximately 25 amino acids at the N-terminal position, SMT1 are devoid of such a hydrophobic domain (Bouvier-Navé et al., 1997). As confirmed by the Arabidopsis sequencing project and the above results, the Arabidopsis genome contains three distinct genes (At5g13710, At1g20330, At1g76090) encoding sterol-C24 methyltransferases(ATSMT1, ATSMT21, ATSMT22 respectively). smt1 mutations, which lack SMT1, were isolated from a transposon Ac transgenic line (Diener et al., 2000). The smt1 plants have pleiotropic defects: poor growth and fertility, root sensitivity to Ca2+ ions, and a loss of proper embryo morphogenesis. smt1 was shown to have an altered sterol content. It accumulates cholesterol, has much less 24-ethyl sterols, but its campesterol content is similar to this in control plants, reflecting that methylation of endogenous sterols is still active even in the absence of any functional SMT1 present. Actually, if SMT1 were the only enzyme responsible in planta for the first methylation reaction of cycloartenol to produce 24-methylene cycloartanol, smt1 should be completely deficient for C-24 alkylation. Because the majority of sterols in smt1 plants are alkylated, SMT1 cannot be the only enzyme in Arabidopsis capable of the first C-24 methylation step (Diener et al., 2000). One explanation for the presence of 24-methylated sterols in smt1 is that in the absence of SMT1, these methylations would be performed by SMT21 or SMT22 (that do not ordinarily perform this reaction in the wild type because of their preference for 24-methylene lophenol) even though at a much slower rate. The expression of SMT2 and SMT1 was also studied in plants (Schaeffer et al., 2000; Schaeffer et al., 2001; Schaller et al., 1998). The expression of ATSMT21 was modulated in 35S : :SMT21 Arabidopsis in order to study its physiological function. Plants overexpressing the transgene accumulated sitosterol at the expense of campesterol. These plants displayed a reduced stature and growth that could be restored by brassinosteroid treatment. Plants showing co-suppression of SMT21 were characterized by a high campesterol content and a depletion in sitosterol. Pleiotropic effects on development such as reduced growth, increased branching, and low fertility of high campesterol plants were not modified by exogenous brassinosteroids, indicative of specific sterol requirements to promote normal development. Thus ATSMT21 has a crucial role in balancing the ratio of campesterol to sitosterol in order to satisfy both growth requirements and membrane integrity (Schaeffer et al., 2001).
4,4-dimethyl sterol and 4α-methyl sterol 4-demethylation.
The passage of 24-methylene cyclartanol to end-pathway sterols involves removal of two methyls at position 4 and one methyl at position 14. The steps involved in these operations are depicted in Fig.2. The enzymatic system operating in the removal of the two methyls at C-4 in plants presents profound differences with the fungal or mammalian systems (Pascal et al., 1990; Pascal et al., 1993). In contrast to animals and fungi where the two C-4-methyl group are sequentially removed, a series of results have unequivocally established that two distinct oxidative systems are involved in the removal of the first and the second C4-methyl of phytosterol precursors. The first will act on 24-methylene cycloartanol whereas the second will operate on 24-methylene and 24-ethylidene lophenol (Fig 2). In the case of the first enzymatic system it has been shown that the oxidative conversion of 24-methylene cycloartanol to cycloeucalenol exhibits similar cofactor requirements, and inhibitor sensitivity as do corresponding animal systems. The demethylation is specific for the C-4α methyl group and is initiated by the C-4 methyl oxidase, which converts the methyl group to the alcohol, the aldehyde and finally to the carboxylic acid. This sequential oxidation was shown to require NADH, oxygen and cytochrome b5 as an electron carrier between the terminal oxidase and NADH. It is not inhibited by CO but is susceptible to cyanide, indicating that a cytochrome P-450 is not involved. In the next reaction, the carboxyl group is removed by a second enzyme, the C4 decarboxylase acting on 4α-carboxy-4β,14α-dimethyl-9β,19-cyclo-5α–ergost-24(241)-en-3β–ol and resulting in formation of cycloeucalenone possessing a keto group at C-3 (Rondet et al., 1999). A third enzyme, 3-keto reductase, then reduces the keto group of cycloeucalenone to give cycloeucalenol (Pascal et al., 1994). This process is repeated with the second methyl group but the substrate is then 24-methylene or 24-ethylidene lophenol and the product is then episterol or Δ7-avenasterol, respectively. Cloning and characterization of ERG25, the S. cerevisiae gene encoding C4-methyl oxidase has been reported (Bard et al., 1996). This gene encodes a 309-amino acid polypeptide showing a C-terminal endoplasmic reticulum (ER) retrieval signal KKXX and three histidine-rich clusters found in eukaryotic membrane bound fatty acid desaturases (Shanklin et al., 1994). A human homologue of ERG25 was cloned and sequenced. It encodes a polypeptide of 293 amino acids with an identity of 38% to ERG25 and contains histidine clusters (Li and Kaplan, 1996). Very recently the functional identification of sterol-4α-methyl oxidase cDNAs from A. thaliana was achieved by complementation of a yeast erg25 mutant lacking sterol-4α-methyl oxidation (Darnet el al., 2001).
Cycloeucalenol obtusifoliol isomerase.
One of the most striking event occurring during sterol biosynthesis is the opening of the cyclopropane ring of cycleucalenol to give obtusifoliol, a step that is restricted to the plant kingdom and catalyzed by an enzyme, the cycloeucalenol-obtusifoliol isomerase or cyclopropyl sterol isomerase (CPI), the catalytic mechanism of which has been thoroughly studied (Heintz and Benveniste, 1974; Rahier et al., 1989) (Fig. 2). Photosynthetic eukaryotes and certain non photosynthetic protists such as Acantamoeba polyphaga (Raederstorff and Rohmer, 1987) or Dictyostelium discoidum (Godzina et al., 2000; Nes et al., 1990) use CPI (EC 5.5.1.9) to convert pentacyclic cyclo-propyl sterols to tetracyclic end-pathway sterols. Arabidopsis CPI was cloned by functional complementation of a S. cerevisiae mutant (Lovato et al., 2000). Expression of an Arabidopsis cycloartenol synthase cDNA in a S. cerevisiae lanosterol mutant (erg7) provided a sterol auxotroph because cycloartenol is not usable by yeast to make ergosterol. This yeast strain was transformed with an Arabidopsis expression library constructed in a yeast vector (pFL61) and sterol prototrophs were selected. A strain accumulating biosynthetic ergosterol was obtained. The novel phenotype was conferred by an Arabidopsis cDNA (CPI) that encoded a 36-kDa protein of 280 amino acids. It was assumed that the S. cerevisiae sterol C4 demethylase complex (Erg25p, Erg26p, and Erg27p) that normally metabolize lanosterol would have sufficiently broad substrate specificity to accept its isomer cycloartenol. Thus cycloartenol would be demethylated at C4 (at least partially) to give small amounts of 31-nor-cycloartenol which was shown to be readily transformed into 31-nor lanosterol (Heintz and Benveniste, 1974), which could then enter the normal yeast sterol pathway (Lovato et al., 2000). CPI is encoded by a unique gene (At 5g50375) possessing 8 exons.
Obtusifoliol-14α-demethylase.
In animals and fungi, the 14α–methyl group is the first of the C14 and C4 methyls to be removed; however in higher plants, the 14α–methyl is removed after one C-4 methyl has disappeared (Fig 2). The mechanism of the 14α–methyl removal involves two oxidation steps leading to an alcohol, then an aldehyde at C-29 and a further oxidative step involving a deformylation leading to formic acid and the sterol product with a typical 8,14-diene (Aoyama et al., 1987). The three steps are catalyzed by a single enzyme, which has been shown to be a cytochrome P-450 (Trzaskos et al., 1986). Lanosterol is the substrate of this enzyme in S. cerevisiae and in mammals (Aoyama et al., 1987; Shyadehi et al., 1996), eburicol would be the preferred substrate in filamentous fungi (Delye et al., 1998), while obtusifoliol is the substrate in higher plants (Taton and Rahier, 1991). Much attention has been payed to the 14α–demethylase since the enzyme is the target of a vast array of compounds having antimycotic effect and used in agriculture (Benveniste and Rahier, 1992) and medicine (Vanden Bossche and Janssen, 1992). These compounds, which are derivatives of pyridine, pyrimidine, imidazole, triazole…interact by their nitrogen sp2 doublet with the protohemic iron of cytochrome P-450 and hamper the access to oxygen. As shown above, the plant pathway differs from fungal and animal pathways downstream of 2(3)-oxidosqualene. No lanosterol is present in plants to serve as substrate for the 14-demethylation step. It is obtusifoliol that is the substrate for 14-demethylation. A thorough enzymological study has defined the stuctural requirements of this enzyme, which was shown to be remarkably specific for the structure of obtusifoliol and which does not use lanosterol (Taton and Rahier, 1991). A panoply of azole derivatives were shown to inhibit this enzyme ; some of them, developed as herbicides, were shown to be specific to obtusifoliol-14-demethylase (OBT14DM) (Benveniste and Rahier, 1992; Salmon et al., 1992). Tobacco calli resistant to phytotoxic azole derivatives have been isolated in mutagenesis experiments. The mechanism of resistance consists of an overproduction of sterols, which is not due to OBT14DM overproduction or modification but to an increase of HMGR enzymatic activity (Gondet et al., 1992; Schaller et al., 1994). cDNAs encoding lanosterol, eburicol and obtusifoliol 14-demethylases have been isolated from mammals (Aoyama et al., 1994), fungi (Kalb et al., 1986) and plants (Bak et al., 1997; Cabello-Hurtado et al., 1999) respectively. They shared a remarkable amino acid identity ranging from 38% to 65% though they belong to widely diverging species. Therefore they were classified in the same family, which is CYP51 (Nelson et al., 1996). A functional Sorghum bicolor OBT14DM was cloned and expressed at high levels in E. coli. The recombinant enzyme was shown to catalyze efficiently the 14α–demethylation of obtusifoliol (Bak et al., 1997). Likewise a Triticum vulgare OBT14DM was expressed in a S. cerevisiae mutant (erg11) defective in lanosterol 14-demethylase. Appropriate engineering of the TvOBT14DM cDNA and of the recipient erg11 mutant allowed to optimize expression (Cabello-Hurtado et al., 1999). Experimental structural informations on the active sites of the fungal, plant, and mammalian CYP51 would greatly facilitate developing more efficient antifungal drugs. However all forms of CYP51 were membrane-bound microsomal enzymes which complicates structural studies of this protein by X-ray crystallography. A soluble CYP51 ortholog has been discovered recently in Mycobacterium tuberculosis (Bellamine et al., 1999). It has been shown to demethylate lanosterol and obtusifoliol and to be inhibited by azole antifungals. E. coli-expressed MTCYP51 has been recently crystallized in the presence of fluconazole and a crystal structure at 2.2 A has been reported (Podust et al., 2001). This new structure provides a basis for rational design of more efficacious antifungal agents or new herbicides (Salmon et al., 1992; Grausem et al., 1995) as well as insight into the molecular mechanism of P450 catalysis. Arabidopsis genome sequencing has revealed the existence of two genes (At2g17330, At1g11680) encoding proteins having 65–75 % identity with an already characterized OBT14DM from Sorghum bicolor (Bak et al., 1997). Both genes possess two exons and an intron. The cDNA (ATCYP51) corresponding to At1g11680 has been cloned and has been shown to be able to sustain growth of the erg11 mutant suggesting ATCYP51 to be a functional sterol 14α–demethylase. Arabidopsis plants were transformed with AtCYP51 in antisense orientation. The resulting transgenic plants showed a semi-dwarf phenotype in the early growth stage. Their obtusifoliol content increased while campesterol and campestanol levels were unchanged (Kushiro et al., 2001). As their 24-ethyl sterols content was not reported, it is still not possible to draw conclusions as to the link between growth reduction and sterol biosynthesis inhibition in these transgenic plants.
Δ8,14-sterol-Δ14-reductase.
4α,14α-dimethyl-5α-ergosta-8,14,24(241)-trien-3β–ol is the product of the C14 demethylation of obtusifoliol in plants. The following step is the hydrogenation by NADPH of the Δ14 double bond to give fecosterol (4α–methyl-5α–ergosta-8,24(241)-dien-3β–ol) (Fig. 2). The product of the C14 demethylase reaction in S. cerevisiae, 4,4-dimethyl-cholesta-8,14,24-trienol, is reduced at the C-14 position to form 4,4-dimethylcholesta-8,24-dienol by the action of the product of the ERG24 gene (Lorenz and Parks, 1992; Marcireau et al., 1992). ERG24 encoded a protein of 438 amino acids. The C14 reductase is inhibited by Fenpropidine and Fenpropimorph, two fungicides used in agriculture (Baloch and Mercer, 1987). By chromosomal gene disruption a S. cerevisiae defective in 14-reductase has been constructed. This strain was shown to grow in aerobiosis if it beared an additional mutation allowing sterol uptake. In this last growth condition, the cells required a sparking ergosterol supplementation and accumulated 5α-ergosta-8,14-dienol as the end-product of the sterol pathway (Marcireau et al., 1992). Most interestingly it has been shown that the human lamin B receptor exhibited sterol C-14-reductase activity in S. cerevisiae (Silve et al., 1998). In fact, the sterol C14 reduction step and ergosterol prototrophy were restored in lamin B receptor-producing erg24 transformants which lack endogenous C14-reductase. C14 reductase was studied in plants. Rubus fruticosus suspension cell cultures were highly susceptible to A25822B, an azasterol antimycotic agent and accumulated 5α–stigmasta-8,14-dien-3β–ol and 5α–stigmasta-8,14, Z-24(241)-trien-3–ol at the expense of Δ5-end pathway sterols (Schmitt et al., 1980). An enzymatic assay for 8,14-sterol 14-reductase was devised allowing to characterize this enzyme and to study its inhibition by analogues of a presumptive carbo-cationic intermediate of the reduction reaction (Taton et al., 1989). Cloning of the gene (FACKEL) encoding this enzyme was performed recently through the isolation of dwarf mutants (EMS and TDNA insertion mutants). FACK-EL (At 3g52940) was isolated and was shown to encode a predicted integral membrane protein of 369 amino acids with eight to nine transmembrane segments related to the vertebrate lamin receptor and several sterol C-14 reduc-tases including S. cerevisiae sterol C-14 reductase (Jang et al., 2000; Schrick et al., 2000). The Arabidopsis protein possessed a signature motif (LLXSGYWGXXRH) of sterol reductases. Functional evidence that FACKEL encoded a sterol C-14 reductase was provided by complementation of erg24 (Schrick et al., 2000). GC/MS analysis confirmed that fk mutations lead to accumulation of Δ8,14-sterol intermediates in the biosynthetic pathway preceding the C-14 reductase step. The sterol profile of fk calli was similar to that of plant cells treated with A25822B (Schmitt et al., 1980; Schaller et al., 1994). The fk mutation resulted also in reduction of brassinosteroid (BRs) content (Jang et al., 2000). However, unlike other BR-deficient mutants, the defect of hypotyl elongation could not be overcome by exogenous BRs (Jang et al., 2000; Schrick et al., 2000). Thorough microscopical and cytological observations indicated that mutations in the FACKEL gene affect body organization of the Arabidopsis seedlings and that FACKEL was required for cell division and expansion and was involved in proper organization of the embryo. These results indicated a novel role for sterols in the embryogenesis of plants (Clouse, 2000).
Δ8-Δ7-sterol isomerase.
When the 14α–methyl group is removed and the 14 double bond is reduced, the resulting Δ8-sterols are isomerized to Δ7-sterols in mammals, fungi, and higher plants. This process is catalyzed by a Δ8-Δ7-isomerase. The reaction involves two steps : first a protonation of the 8 double bond leading to an intermediate bearing a carbocation at C8 then the elimination of a proton at C7 leads to the Δ7-sterol (Akhtar et al., 1970; Goad et al., 1969). From enzymatic assays and biogenetic considerations it appears that zymosterol, fecosterol and 4α–methyl-5α–stigmasta-8, Z-24(241)-dien-3β–ol are the substrates of this enzyme in vertebrates (Yamaga and Gaylor, 1978), S. cerevisiae (Yabuzaki et al., 1979) and higher plants (Taton et al., 1987) respectively. A mutant (erg2) defective in Δ8-Δ7-sterol isomerase was described for S. cerevisiae (Barton et al., 1974). The erg2 mutation leads to the accumulation of sterols containing the Δ8-double bond. ERG2, the gene encoding the Δ8-Δ7-sterol isomerase (SI) was cloned by functional complementation of erg2 (Arthington et al., 1991). Later, SI-encoding genes were isolated from the rice blast fungus Magnaporthe grisea and the maize pathogen Ustilago maydis (Keon et al., 1994). Genetic and biochemical evidence showed that ERG2 would encode an enzyme catalyzing conversion of Δ8-sterols to Δ7-sterols. Recently a S. cerevisiae mutant defective in the internalization step of endocytosis was shown to contain defects in ERG2 leading to a lack of Δ8-Δ7-isomerase activity (Munn et al., 1999). A murine SI encoding cDNA has been cloned by functional complementation of the corresponding deficiency (erg2) in S. cerevisiae. The amino acid sequence deduced from the cDNA open reading frame was shown to be highly similar to human emopamil-binding protein (EBP), a protein which is the target for neuroprotective drugs (Silve et al., 1996). A yeast strain in which the SI coding sequence has been replaced by that of human EBP or its murine homologue, recovered the ability to convert Δ8-sterols to Δ7-sterols both in vivo and in vitro. Interestingly, the amino acid sequence deduced from the murine (SI) or human (EBP) failed to reveal any striking similarity with S. cerevisiae Erg2p (13% identity). Mutations in the gene encoding Homo sapiens SI were shown to cause X-linked dominant Conradi-Hunermann syndrome (Braverman et al., 1999). Alanine scanning mutagenesis was used to identify residues in the four putative transmembrane α-helices of human EBP that are required for Δ8-Δ7-sterol isomerase catalytical activity and binding of inhibitors. Out of 64 ala-nine mutants of EBP mutants H77A, E81A, T126A, N194A, W197A, contained less than 10% end-pathway 5,7-sterols. Therefore amino acids H77, E81, T126, N194 and W197 should play an important role in sterol Δ8-Δ7 isomerization (Moebius et al., 1999). An Arabidopsis Δ8-Δ7-sterol isomerase cDNA has been isolated by functional complementation of the corresponding S. cerevisiae sterol mutant (erg2). The full length Arabidopsis cDNA that complement the erg2 mutation contained an open reading frame encoding a protein of 223 amino acids sharing 35% amino acid identity to the Mus musculus SI and the H. sapiens EBP and very low identity (less than 15%) with Erg2p. The sigma ligands, haloperidol, ifenprodil and verapamil inhibited the production of ergosterol in wild-type S. cerevisiae and in the erg2 mutant complemented with the Arabidopsis SI (Grebenok et al., 1998). The Arabidopsis protein contained three transmembrane segments and presented several structural and biochemical similarities with the mammalian EBP. It is encoded by an unique gene (At1g20050) possessing 4 exons and 3 introns.
Δ7-sterol-C5(6)-desaturase.
Δ7-Sterol products of the Δ8-Δ7-sterol isomerase are transformed into Δ5-sterols through two reactions : the first one involves a desatura-tion step at C5 leading to a Δ5,7-sterol, then the Δ7-double bond of Δ5,7-sterols is reduced to give Δ5-sterols (Fig. 2). Insertion of the 5,6-double bond has been shown to involve stereospecific removal of the 5 (4-proR in MVA) and 6 (5-proS in MVA) hydrogen atoms during ergosterol biosynthesis in S. cerevisiae (Bimpson et al., 1969). The same stereospecificity is seen during the insertion of the 5,6-double bond during sterol biosynthesis in rat liver, higher plants and Ochromonas malhamensis (Goodwin, 1979). The close similarity of fatty acid desaturation in rat liver microsomes to the introduction of the sterol 5,6-double bond has been pointed out (Reddy et al., 1977). Both systems require NADH and molecular oxygen, and involve a cyanide-sensitive factor ; they both abstract cis-oriented hydrogens, occur in the microsomes and are inhibited by cyanide and thiol-blocking agents, but not by carbon monoxide. Further evidence of this came witth the demonstration of the participation of cytochrome b5 in the conversion of cholest-7-en-3-ol into cholesta-5,7-dien-3-ol (Osumi et al., 1979). The characterization of a Δ7-sterol C5(6)-desaturase from Zea mays has been reported (Taton and Rahier, 1996). This desaturase presents features similar to those reported for rat liver and yeast. It is strongly inhibited by cyanide, is sensitive to 1,10-phenanthroline and to salicylhydroxamic acid, but is insensitive to carbon monoxide, thereby suggesting the involvment of a metal ion, presumably iron, in an enzyme-bound form in the desaturing system (Taton and Rahier, 1996). More recently the ERG3 gene from S. cerevisiae has been cloned by complementation of an erg3 mutant defective in C5-sterol desaturase. The functional gene contained an open reading frame of 365 amino acids. Gene disruption demonstrated that ERG3 is not essential for cell viability (Arthington et al., 1991). A nuclear and recessive mutant of Arabidopsis affected in sterol biosynthesis (ste1) has been isolated and identified. This mutant accumulated Δ7-sterols (24R)-24-ethyl-5α-cholest-7-en-3-ol, and (24ξ)-24-methyl-5-cholest-7-en-3β-ol at the expense of the normally occurring campesterol and sitosterol. It has been suggested that ste1 is defective in the sterol-C5-desat-urase (Gachotte et al., 1995). To check this hypothesis ste1 was transformed with ERG3 encoding a Δ7-sterol-C5-desaturase from yeast, which resulted in partial recovery of wild-type sterol composition. To definitely identify ste1, homologous complementation was necessary. To this end an Arabidopsis cDNA encoding a Δ7-sterol-C5-desat-urase was isolated and characterized by functional complementation of erg3. This was achieved by transformation of erg3 with an Arabidopsis cDNA library inserted in a yeast vector (pFL61). Transformants were screened for cycloheximide resistance and resistant clones were analyzed to determine their sterol profile. The presence of ergosterol was detected in one clone in which a plasmid containing a cDNA of 1141bp was found. The 1141 cDNA allowed the deduction of an open reading frame of 843 bp encoding a 281 amino acid polypeptide (Gachotte et al., 1996). Three histidine-rich motifs (HX3H, HX2HH and HX2HH) and three transmembrane segment were found in the Arabidopsis polypeptide and are also present in Erg3p as well as in Nicotiana tabacum (Husselstein et al., 1999) and Homo sapiens (Husselstein et al., 1999; Matsushima et al., 1996; Nishi et al., 2000) orthologues. Histidine-rich motifs are also characteristic of many membrane-bound fatty acid desaturases from higher plants (Shanklin et al., 1994). Overexpression of the Arabidopsis desaturase cDNA driven by a 35S promoter in transgenic ste1 plants led to full complementation of the mutant. This result demonstrated that STE1 was the impaired component in the desaturation system. The ste1-derived open reading frame has been cloned by RT-PCR. Alignment of the wild-type with the ste1 derived protein sequences revealed a single amino acid substitution : T114 in the wild-type is changed to I114 in Ste1p. The presence of this mutation in the mutant STE1 genomic sequence demonstrated that the change of the T114 to I was necessary and sufficient to create the leaky allele ste1 (Husselstein et al., 1999). The role of 15 residues in the reaction catalyzed by Arabidopsis Δ7-sterol-C5(6)-desaturase (5-DES) was investigated using site-directed mutagenesis and expression of the mutated enzymes in an erg3 S. cerevisiae strain defective in 5-DES. One group of mutants was affected in the eight invariant histidine residues from three histidine-rich motifs. Replacement of these residues by leucine completely eliminated the desaturase enzymatic activity both in vivo and in vitro (Taton et al., 2000) in agreement with the hypothesis that the function of histidine-rich motifs would be to provide the ligands for a presumed catalytic Fe center, as previously proposed (Shanklin et al., 1994). Another group of mutants was affected in residue 114 based on previous observations indicating that mutant T114I (ste1) was deficient in 5-DES. It was shown that the enzyme T114I had an 8-fold higher Km and 10-fold reduced catalytic efficiency. Conversely, the functionally conservative substituted mutant enzyme T114S displayed a 28-fold higher Vmax value and an 8-fold higher Km value than the wild-type enzyme. The data suggested that T114 strongly affected the reactivity of the desaturase and gave clues to get engineered 5-DES with much higher performances (Taton et al., 2000). Deuterated 7-cholestenol analogues were used as mechanistic probes for wild-type and mutated Δ7-sterol-C5(6)-desaturase. Deuterium kinetic isotopic effects for the removal of 6α- and 5α-hydrogens were measured. The observed pattern of isotope effects allowed Rahier (2000) to propose a mechanism for the desaturation of cholest-7-en-3β-ol. The Arabidopsis 5-DES considered above is encoded by one gene (At3g02580). Sequencing of Arabidopsis genome has revealed the existence of a second gene (At3g02590), the deduced protein sequence of it having 80% identity with Ste1p. Both genes possess three exons and two introns. Screening of about 50,000 M2 seeds of an EMS mutant population allowed the isolation of 43 dwarf mutants. Two of them (dwf7-1 and dwf7-2) were biochemically complemented by brassinolide (BR) and early BR biosynthetic precursors. Furthermore, results from feeding studies with 13C-labeled MVA and mevastatin and from sterol analysis suggested that the defective step was specifically the Δ7-sterol C5(6) desaturation. Sequencing revealed a premature stop codon in exon1 (dwf7-2) and in exon 3 (dwf7-1) of the the 5-DES gene confirming that dwf7-1 and dwf7-2 were null alleles of STE1. This work showed for the first time that the reduction of BR biosynthesis in dwf7 was due to a shortage of substrate sterols (campesterol) and was the direct cause of the dwarf phenotype (Choe et al., 1999b). An extremely dwarf mutant (bul1-1) of Arabidopsis was recently isolated and shown to contain as much as 90% of Δ7-sterols and less than 2% of Δ5-sterols. It was therefore defective in the Δ7-sterol-C5-desaturation step leading to brassinosteroid biosynthesis. Cellular characterization and rescue experiments with brassinosteroids demonstrated the involvment of the 5-DES in brassinosteroid-dependent plant growth processes (Catterou et al., 2001a). Indirect immunofluorescence of α-tubulin in bul1-1 revealed a total lack of the parallel microtubule organization that is typical of elongating cells in the wild-type. Rescue experiments with brassi-nosteroids and subsequent molecular analyses suggested that a brassinosteroid-responsive pathway exists, which allows microtubule nucleation/organization and cell elongation without activation of tubulin gene expression (Catterou et al., 2001b).
Δ5,7-sterol Δ7-reductase (Δ7-SR) catalyzes the reduction of the Δ7-double bond of the Δ5,7-sterols into Δ5-sterols. This step occurs only in vertebrates and higher plants. A microsomal preparation from seedlings of Zea mays catalyzed the NADPH dependent reduction of the Δ 7-bond of Δ5,7-cholestadienol (Taton and Rahier, 1991). Novel 6-aza-B-homosteroids were shown to strongly inhibit (Δ7-SR) and to behave as analogues of a high energy carbocationic intermediate occurring during the reaction (Rahier and Taton, 1996). The gene encoding the Δ7-SR was cloned by transformation of wild-type S. cerevisiae with a cDNA library of Arabidopsis constructed in the yeast vector pFL61 and the subsequent selection of cells producing Δ5-sterols at the expense of ergosterol (a Δ5,7-sterol) by nystatin selection. These cells were selected due to their resistance to nystatin, a polyene fungicide that is highly toxic to ergosterol producing cells. This clever strategy allowed recovery of a plasmid bearing a 1290-bp cDNA open reading frame, which encoded a protein of 430 amino acids that had significant sequence identity (32%) with Arabidopsis sterol Δ14-reductase and the previously reported LLXSGWWGXXRH signature of sterol reductases. Cholesta-5,7-dien-3β-ol was shown to be reduced in vitro to cholesterol by an enzymic preparation from a yeast strain expressing the Arabidopsis (Δ7-SR) (Lecain et al., 1996). Screening of a population of dwarf mutants led to a series of mutants (dwf5) presenting a defect in the gene encoding the Δ7-SR. dwf5 plants display the characteristic dwarf phenotype typical of BR mutants. This phenotype included small, round, dark-green leaves, and short stems, pedicels, and petioles. dwf5 mutants were biochemically complemented with exogenous BRs. Metabolite tracing with 13C-labeled precursors in dwf5 and sterol analysis suggested a deficiency in a Δ7-SR activity. In particular sterol analysis showed that Δ5,7-campestenol (a possible substrate of Δ7-SR) accumulated. Surprisingly, the major compound analyzed was shown to be 5α-ergost-7-en 3β-ol. To explain this result authors suggested that a Δ5-reductase (det2) acting downstream of campesterol could reduce the Δ5 double bond of Δ5,7-sterols which accumulated in dwf5. Deficiency in Δ7-SR activity was verified by DNA sequencing showing that all six independent alleles contained loss-of-function mutations in the Δ7-SR gene. The dwf5 plant could be restored to wild type by ectopic overexpression of the wild-type copy of the gene (Choe et al., 2000). This gene (At1g50430) was shown to contain 13 exons and 12 introns. The Smith-Lemli-Opitz syndrome (SLOS) is an inborn disorder of sterol metabolism. All patients suffer from mental retardation. The SLOS gene has been shown to be a Δ7-SR (EC 1.3.1.21) required for the de novo cholesterol biosynthesis. The protein encoded by the Homo sapiens Δ7-SR has 35% identity with the Arabidopsis Δ7-SR (Moebius et al., 1998). Results showed also that defects in the Δ7-SR gene (several missense, nonsense, and splice site mutations) caused the SLOS (Fitzky et al., 1998).
Δ5-sterol Δ24-reductase(isomerase) catalyzes the reduction of the Δ24 double bond of the side chain of sterols. This reaction uses a substrate possessing one double bond in animals and in higher plants. In animals this substrate is desmosterol, a Δ24(25) sterol, whereas in higher plants, substrates are 24-methylene cholesterol and isofucosterol (Fig. 2) and Δ24(241)-sterols are probably isomerized in Δ24(25)-sterols prior to be reduced. However, reduction of the Δ24 double bond proceeds on sterols possessing two conjugated double bonds such as ergosta-5,7,22,24-tetraen-3β-ol in S. cerevisiae and other organisms producing ergosterol. The ERG4 gene provides the enzyme that reduces the double bond at C24(241), the final step in ergosterol biosynthesis. Disruption of ERG4 led to a mutant (erg4) which was able to grow similarly to the wild-type showing that the C24 reductase was not essential for viability (Lai et al., 1994). As discussed earlier (Lees et al., 1995), the sterols accumulated in erg4 mutants (e.g. ergosta-5,7,22,24-tetraen-3β-ol) are similar in structure to ergosterol and could fulfil functions normally attributed to ergosterol. The diminuto mutant (dim) was initially isolated as a slowly growing dwarf Arabidopsis mutant with greatly reduced fertility (Klahre et al., 1998). The DIM gene was cloned, and genomic analysis and gene expression studies strongly suggested that dim did not produce any DIM protein. The DIM gene (AT3g19820), also referred to as DWF1 (Choe et al., 1999b) or CBB1 (Kauschmann et al., 1996), is composed of two exons and one intron. Both the mutant phenotype and gene expression could be rescued by the addition of exogenous BRs. Analysis of endogenous sterols demonstrated that dim accumulated 24-methylene cholesterol but was deficient in campesterol, an earlier precursor of BR and in BRs as well. Feeding experiments using deuterium-labeled 24-methyl-enecholesterol and 24-methyl desmosterol confirmed that DIM/DWF1 is involved in both the isomerization and reduction of the 24(241) bond and encoded a sterol C24(241) reductase isomerase (S24REDISO). Transient expression of a green fluorescent protein-DIM/DWF1 fusion protein and biochemical experiments showed that DIM/DWF1 is an integral membrane protein that most probably is associated with the ER (Klahre et al., 1998). Several dwf1 alleles were also characterized. 7 of 10 dwf1 mutations were shown to directly affect a flavin adenin dinucleotide binding domain conserved in various oxidoreductases (Choe et al., 1999b). The proteic sequence of S24REDISO from Arabidopsis was shown to have 41% identity with an Homo sapiens ortholog (seladin-1) but no significant identity with ERG4. No ortholog of S24REDI has been reported in the S. cerevisiae genome. Thus the C24 reduction step seems to be performed by completely different enzymatic systems in higher plants and animals on one hand and in S. cerevisiae (and probably most fungi) on the other hand. The human DIM/DWARF1 homolog seladin-1 has been shown to confer resistance to Alzheimer's disease-associated neurodegeneration and oxidative stress (Greeve et al., 2000). This exciting result would attribute a novel role of cholesterol in neurodegenerative diseases. However the involvment of seladin-1 in cholesterol biosynthesis has not been demonstrated so far. Finally mutations in the 3β-hydroxysterol Δ24-reductase gene were shown to cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis (Waterham et al., 2001).
Sterol-Δ22-desaturase.
Ergosterol from S. cerevisiae, most fungi and several algae, and stigmasterol : (24S)-24-ethyl-cholesta-5, E-22-dien-3β-ol from most higher plants possess a double bond at C-22. Δ22-sterols are not present in vertebrates. This double bond results from a desaturation step. This step is the antepenultimate during ergosterol biosynthesis, it is probably the last step of plant sterol biosynthesis. The substrate of Δ22-desaturase is ergosta-5,7,24(241)-trien-3β-ol in yeast, which is transformed into ergosta-5,7,22,24(241)-tetraen-3-ol by yeast microsomes. This reaction has been shown to involve molecular oxygen, NADPH, and is inhibited by CO and metyrapone. Thus it involves a cytchrome P-450 species (Hata et al., 1983). The gene (ERG5) encoding this reaction has been recently cloned by complementation of an erg5 mutant (Skaggs et al., 1996). The deduced amino acid sequence (538 amino acid residues) presented general features common to most cytochrome P-450 open reading frames and exhibits the highest homology with some animal cytochrome P-450 proteins of the family 2. Surprisingly, the highest homology was not with CYP51A1 (lanosterol-14-demethylase) (Skaggs et al., 1996). Thus ERG5 is the first member of the CYP61 family. Enzymatic properties and inhibition by azole antifungal agents of CYP61 have been studied. Results revealed CYP61 to have a similar affinity to azole drugs (fluconazole, keto-conazole) when compared with data available for CYP51 (Kelly et al., 1997). The results of gene disruption demonstrated that ERG5 is not essential for cell viability suggesting that the Δ22 would not be essential. Most biosynthetic studies show that sitosterol would be the substrate for a C-22 desaturase leading to stigmasterol (Benveniste, 1986; Nes, 1977). Very little is known about this step in higher plants, since the in vitro conversion of sitosterol to stigmasterol has never been shown enzymatically. Arabidopsis is remarkable among higher plants since it was shown to contain brassicasterol (ergosta-5, E-22-dien-3-ol) in addition to stigmasterol, indicating that both 24-methyl and 24-ethyl sterols could be substrates of the C-22 desaturase. No cDNA encoding this proteins from Arabidopsis (or any other higher plant) has been reported so far.
STEROL METABOLISM
Previous studies performed with a tobacco mutant sterov overproducing sterols have shown that the free sterol content of this mutant remained close to this of wild-type plants and that the excess of sterols was converted into steryl esters (mostly steryl palmitate, oleate, linoleate and linolenate) which accumulated in lipid droplets (Gondet et al., 1994; Schaller et al., 1994). Such a result stressed the importance of sterol metabolization in order to maintain a level of free sterols in membranes compatible with their vital functions. At least three pathways are universally involved in sterol metabolization in plants and especially in Arabidopsis : i) sterol acylation ; ii) sterol glycosylation ; iii) oxydative conversion of sterols into brassinosteroids. Other pathways also exist such as formation of ecdysteroids, steroidal alkaloids, cardiotonic steroidal glucosides etc…but they belong to the secondary metabolism in specific plant families and therefore would not be relevant of the general plant metabolism.
Sterol esterification.
Steryl esters are present in all plants (Dyas and Goad, 1993) and are most often localized in the cytoplam of plant cells. They accumulate also as lipid bodies in the elaioplasts of the tapetum in developing Brassica napus anthers (Hernandez-Pinzon et al., 1999; Wu et al., 1999). In most cases sterols are esterified by fatty acids but in some plants (Oriza sativa) sterols are esterified by phenylpropanoids and especially by ferulic acid (Akihisa et al., 2000). A large number of studies have established that phytosterol and phytostanol fatty acid esters have serum cholesterol-lowering properties and are inhibitors of cholesterol absorption in human small intestine (Gylling et al., 1999; Jones, 1999; Normén et al., 2000). Triterpene and phytosteryl ferulates from rice bran have been shown to have anti-inflammatory effects against 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice (Akihisa et al., 2000). Two enzymatic systems are involved in sterol esterification : In mammals cellular cholesterol is esterified by a fatty acid CoA derivative and this step is catalyzed by an acylCoA cholesterol acyltransferase (ACAT) (Farese Jr, 1998; Lin et al., 1999; Yang et al., 1997), whereas blood cholesterol is esterified by a fatty acid from lecithin using a lecithin cholesterol acyltransferase (LCAT) (Jonas, 2000; Peelman et al., 1999). In S. cerevisiae ergosterol is esterified by two polypeptides encoded by two ACAT related genes (Yang et al., 1996). Deletion of both ACAT genes resulted in a viable cell with undetectable esterified sterol (Yang et al., 1996). ACAT and LCAT are completely different proteins and their aminoacid sequences have no significant identity. The enzymatic system operating in higher plants is still not clearly defined. The reaction seems to be associated with the microsomal fraction that is able to convert phytosterols into steryl esters (Bouvier-Navé and Benveniste, 1995). However, several acyl donors : triacylglycerol (Zimowski and Wojciechowski, 1981), diacylglycerol (Garcia and Mudd, 1978), and lecithin (Bouvier-Navé and Benveniste, 1995; Garcia and Mudd, 1978) have been reported so far. Still, no cDNA encoding a sterol acyltransferase has been characterized. A polypeptide from Arabidopsis possessing 25% identity with the human ACAT1 was shown to be in fact a diacylglycerol acylCoA acyltransferase (DGAT) (Bouvier-Navé et al., 2000). The Arabidopsis genome sequencing program has revealed the existence of several candidate genes encoding proteins having significant identity (higher than 25%) with human LCAT.
Sterol glycosylation.
Extensive reviews on sterol glycosylation have been published (Ullmann et al., 1993; Wojciechowski, 1991). This reaction consists of the reaction of free sterols with UDP-glucose to give sterylglyco-sides and UDP in the presence of a UDP-glucose :sterol glucosyltransferase. This reaction has been shown to be associated with membranes in higher plants and more precisely with the plasma membrane in Zea mays (Hartmann-Bouillon and Benveniste, 1978). Such a reaction plays an important role in eukaryotic organisms. It is obvious that the attachment of a glycosyl moiety to the sterol backbone alters the physical properties of this lipid. Such changes have been studied with artificial membranes (Grunwald, 1975; Webb et al., 1995). To evaluate the impact of these changes in the living cell a genetic approach would be needed. For this purpose isolation and characterization of sterol glycosyltransferases from eukaryotes have been undertaken. A purified enzyme (Warnecke and Heinz, 1994) has been used for the cloning of a corresponding cDNA from oat. Amino acid sequences derived from the amino terminus of the purified protein and from peptides of a trypsin digestion were used to construct oligonucleotide primers for PCR experiments. Screening of oat and Arabidopsis cDNA libraries with amplified labeled DNA fragments resulted in the isolation of sterol glucosyltransferase-specific cDNAs with inserts lenghts of ca. 2.3 kb for both plants. These cDNAs encode polypeptides of 608 (oat) and 637 (Arabidopsis) amino acid residues with molecular masses of 66kDa and 69kDa, respectively (Warnecke et al., 1997). Genes from S. cerevisiae, Pichia pastoris, Candida albicans, Dyctiostelium discoidum (Warnecke et al., 1999) and cDNAs from oat (Avena sativa) and Arabidopsis (Warnecke et al., 1997) have been cloned and were expressed in E. coli. In vitro enzyme assays with cell-free extracts of transgenic E. coli strains showed that the genes encoded UDP-glucose :sterol glucosyltransferases which can use different sterols such as cholesterol, sitosterol, and ergosterol as sterol acceptors. The genes from Arabidopsis (At3g07020 and At1g43620) are composed of 14 exons and 13 introns.
Conversion of campesterol into brassinosteroids will be developed in another chapter of this book.
DISCUSSION
Since the publication of a review dealing with cloning of cDNAs or genes encoding enzymes of sterol biosynthesis from plants (Bach and Benveniste, 1997), impressive progress has been in that field, due to important advances achieved in the following three directions : i) cloning of most cDNAs and genes encoding enzymes of sterol biosynthesis and metabolism ; ii) isolation and characterization of mutants defective in several key steps of these pathways ; iii) total sequencing of the Arabidopsis genome. The main informations already obtained or expected concern : j) gene organization and expression ; jj) mechanism of action of enzymes ; jjj) functions of sterols.
Organization and expression of genes
A general picture concerning regulation of plant sterol biosynthesis is still far from being available. Whereas important work has already been done on the first steps of isoprenoid synthesis : HMGCoA synthase and reductase (Alex et al., 2000; Lumbreras et al., 1995), farnesyl diphosphate synthase (Cunillera et al., 2000) and squalene synthase (Devarenne et al., 1998), such a study is still in its infancy in the post-squalene block of steps. Some interesting informations arise from Table 2 which gathers data concerning the organization of most of genes encoding sterol biosynthesis and metabolism in Arabidopsis. According to Table 2, genes are scattered among the five chromosomes and no block of genes corresponding to segments of the biosynthetic pathway are detectable. The number of genes present for a given enzymatic step is variable. As far as Arabidopsis is concerned, most enzymes listed on Table 2 are encoded by one gene (MVK, PMVK, OS-cycloartenol cyclase, SMT1, Δ8,14-sterol-Δ14-reductase, Δ8-Δ7-sterol isomerase, Δ7-SR, Δ5-sterol-Δ24-reductase (isomerase) and UDP-glucose : sterol glucosyltransferase) or by two genes (HMGR, MVDPD, IDI, FPS, SQS, SMT2, CYP51, 5-DES). Exceptions are squalene epoxidase (6 genes) and the triterpene synthases (12 genes). Gene multiplicity seems to be frequent in the plant kingdom. According to a recent study performed on HMGR, FPS and SQS, one gene would be expressed in all parts of the plant and would have a « house keeping function ≫ while a paralogue would be expressed selectively in some organs or tissues (Cunillera et al., 2000). As such genes are situated at the beginning of the sterol pathway, it could be suggested that paralogous genes may be involved in the synthesis of different isoprenoids other than sterols. This explanation does not apply to genes encoding enzymes situated downstream of cycloartenol that are involved only in sterol synthesis. However, it is reasonable to assume that one gene should be involved in the main pathway, whereas its paralogue could be linked to a parallel pathway. This could occur if the substrate specificity of the protein encoded by the second gene was different from that of the enzyme encoded by the first one. Such a situation is not unlikely in sterol synthesis, where several minor parallel pathways may exist beside the main pathway. The number of exons (one to eighteen) is extremely variable and does not seem to be typical of a defined class of enzyme. For instance, group of genes encoding enzymes having similar mechanism of action such as IDI, FPS and SQS on one hand or methyltransferases on the other hand contain different number of exons. As only a few genes encoding sterol biosynthesis enzymes have been sequenced in other plants than Arabidopsis, it is still difficult to hypothesize on whether the exon-intron pattern is conserved in orthologous genes or not. Likewise, no phylogenetic rules arise from comparison of exon number. Important points remain to be solved : i) how is the genetic machinery involved in sterol synthesis informed when the steady-state level of sterols is reached in cellular membranes ? ; ii) how is this information transmitted from membranes to the genetic machinery ? ; iii) what is the type of regulation involved : transcriptional, translational or post-translational ? ; iv) if transcription constitutes a major site of regulation, what are the cis and trans regulatory elements involved ? To answer this last question, regulation studies should include isolation and careful analysis of gene promoter sequences in order to disclose regulatory elements and their corresponding binding proteins.
Mechanisms of enzymatic catalysis
The possibility to express cDNAs encoding enzymes of sterol biosynthesis and metabolism in heterologous systems (S. cerevisiae mutants, E. coli, and Sodoptera frugiperda cultured cells) has opened new avenues to perform structural studies on enzymes as well as studies of their catalytical mechanism and inhibition by tailor-made inhibitors (transition-state analogues or suicide inhibitors). However only few enzymological data have been reported on plant enzymes so far. Recently site-directed mutagenesis of recombinant Arabidopsis 5-DES has given precious clues to do deeper studies on the catalytical mechanism of this enzyme (Rahier, 2001; Taton et al., 2000). As shown above, crystal structures have been successfully got in the case of human (Istvan et al., 2000) and Pseudomonas mevalonii (Bochar et al., 1999) HMGRs, human squalene synthetase (Pandit et al., 2000), Alicyclobacillus acidocaldarius hopene synthase (Wendt et al., 1999), and Mycobacterium tuberculosis lanosterol 14-demethylase (Podust et al., 2001). It is expected that crystal structures of Arabidopsis enzymes of sterol biosynthesis will be obtained in the next future.
Functions of sterols
A breakthrough on sterol biosynthesis and function was possible due to the isolation and biochemical and molecular characterization of Arabidopsis sterol mutants. Here we consider only mutations affecting enzymes of sterol biosynthesis leading to the synthesis of the so-called end-pathway sterols (campesterol, sitosterol and stigmasterol). We are not considering the steps involved during the conversion of e.g. campesterol into brassinolide. A major insight obtained from these mutants was that most 〈 sterol 〉 mutants had a dwarf phenotype and that a wild-type size could be at least partially recovered following treatment of the mutant with exogenous brassinosteroids (BRs). The extent of recovery was highly variable and dependent of the gene affected by the mutation. For instance, dwarf1, dwarf5 and dwarf7 mutants defective in the conversion of 24-methylene cholesterol to campesterol (Choe et al., 1999b), in the Δ7-reductase (Choe et al., 2000) and the Δ7-sterol-C5(6)-desaturase (Choe et al., 1999a) respectively, were shown to be totally rescued by BRs. In contrast, the phenotype of smt1 plants which are defective in the sterol methyltransferase involved in the first methylation occuring during sterol biosynthesis is poorly rescued by exogenous brassinosteroids (Diener et al., 2000). Likewise the phenotype of fackel which is defective in the Δ8,14-sterol C-14 reductase was not rescued by feeding with BRs (Jang et al., 2000; Schrick et al., 2000). Although a complete sterol profile (including 24-ethyl sterols in addition of 24-methyl sterols) was not reported in the articles dealing with dwarf1, dwarf5, and dwarf7, it is possible to suggest that sterols which accumulate in these mutants could fulfil most of the functions attributed to sterols in the cell economy and in development. The fact that some plants belonging to the order of caryophyllales contain 100% of Δ7-sterols (Adler and Salt, 1987) and have a sterol profile similar to dwarf7 is in agreement with this suggestion. In contrast sterols which are present in fackel (Δ8,14-sterols) (Schrick et al., 2000), in smt1 (Diener et al., 2000), and in transgenic plants where SMT2 is silenced (Schaeffer et al., 2001) should not satisfy membrane integrity. As Arabidopsis mutants fackel and smt1 have defects associated with body organization of the seedling, it was suggested that plant sterols, in addition of their structural role in membranes, may be key signaling molecules influencing position-dependent cell-fate during embryonic development (Clouse, 2000; Jang et al., 2000; Schrick et al., 2000). A role in embryonic development was already proposed for cholesterol in mammalian cells (Kip Guy, 2000). In this context S. cerevisiae sterol mutants (erg2) (Munn et al., 1999) and erg6 (Hardwick and Pelham, 1994) were shown to present defects in membrane dynamics. In addition, membrane receptors such as the lamin B receptor (Silve et al., 1998) or emopamil binding proteins (Moebius et al., 1999; Silve et al., 1996) were shown to catalyze Δ8,14-sterol-14-reduction and Δ8-Δ7-sterol isomerisation respectively. These observations stress the importance of accurately determining the cellular localization of enzymes of sterol biosynthesis. This goal could be reached using enzymes of sterol biosynthesis fused to the green fluorescent protein and suitable fluorescent antibodies against markers for components of the endomembrane system. Such techniques could also apply to detect the formation of multienzyme complexes (metabolons) which could be independently regulated (Cunillera et al., 1996).
Acknowledgments
I am indebted to B. Bastian for kindly typing the manuscript.
Footnotes
Citation: Benveniste P. (2002) Sterol Metabolism. The Arabidopsis Book 1:e0004. doi:10.1199/tab.0004
elocation-id: e0004
Published on: March 27, 2002
Fig. 3.
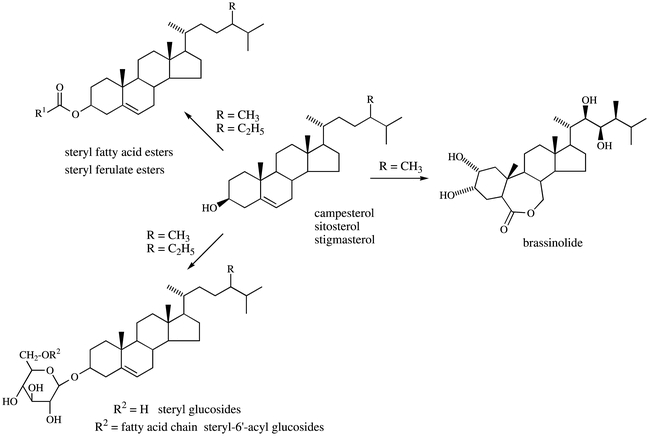
Plant sterol metabolism.
References
- Abe I., Prestwich G. D. Molecular cloning, characterization, and functional expression of rat oxidosqualene cyclase cDNA. Proc. Natl. Acad. Sci. USA. 1995;921(1):9274–9278. doi: 10.1073/pnas.92.20.9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe I., Rohmer M., Prestwich G. D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993;931(1):2189–2206. [Google Scholar]
- Adiwilaga K., Kush A. Cloning and characterization of cDNA encoding farnesyl diphosphate synthase from rubber tree (Hevea brasiliensis). Plant Mol. Biol. 1996;301(1):935–946. doi: 10.1007/BF00020805. [DOI] [PubMed] [Google Scholar]
- Adler J. H., Salt T. A. Phytosterol structure and composition in the chemosystematics of the caryophyllales. 1987;1(1):119–121. In The metabolism, structure and function of plant, P. K. Stumpf, J. B. Mudd and W. D. Nes, eds. (New York: Plenum Publishing Corporation), pp. [Google Scholar]
- Akhtar M., Rahimtula A. D., Wilton D. C. The stereochemistry of hydrogen elimination from C-7 in cholesterol and ergosterol biosynthesis. Biochem. J. 1970;1171(1):539–542. doi: 10.1042/bj1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akihisa T., Shimizu N., Ghosh P., Thakur S., Rosenstein F. U., Tamura T., Matsumoto T. Sterols of the cucurbitaceae. Phytochemistry. 1987;261(1):1693–1700. [Google Scholar]
- Akihisa T., Yasukawa K., Yamaura M., Ukiya M., Kimura Y., Shimizu N., Arai K. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J. Agric. Food Chem. 2000;481(1):2313–2319. doi: 10.1021/jf000135o. [DOI] [PubMed] [Google Scholar]
- Alex D., Bach T. J., Chye M. L. Expression of Brassica juncea 3-hydroxy-3-methylglutaryl CoA synthase is developmentally regulated and stress-responsive. Plant J. 2000;221(1):415–426. doi: 10.1046/j.1365-313x.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Funae Y., Noshiro M., Horiuchi T., Yoshida Y. Occurrence of a P450 showing high homology to yeast lanosterol 14-demethylase (P45014DM) in the rat liver. Biochem. Biophys. Res. Commun. 1994;2011(1):1320–1326. doi: 10.1006/bbrc.1994.1848. [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Yoshida Y., Sonoda Y., Sato Y. Metabolism of 32-hydroxy-24,25-dihydrolanosterol by purified cytochrome P-45014DM from yeast. J. Biol. Chem. 1987;2621(1):1239–1243. [PubMed] [Google Scholar]
- Arthington B. A., Bennett L. G., Skatrud P. L., Guynn C. J., Barbuch R. J., Ulbright C. E., Bard M. Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene. 1991;1021(1):39–44. doi: 10.1016/0378-1119(91)90535-j. [DOI] [PubMed] [Google Scholar]
- Attucci S., Aitken S. M., Gulick P. J., Ibrahim R. K. Farnesyl pyrophosphate synthase from white lupin : molecular cloning, expression, and purification of the expressed protein. Arch. Biochem. Biophys. 1995;3211(1):493–500. doi: 10.1006/abbi.1995.1422. [DOI] [PubMed] [Google Scholar]
- Bach T. J., Benveniste P. Cloning of cDNAs or genes encoding enzymes of sterol biosynthesis from plants and other eukaryotes : heterologous expression and commentation analysis of mutations for functional characterization. Prog. Lipid Res. 1997;361(1):197–226. doi: 10.1016/s0163-7827(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Bak S., Kahn R. A., Olsen C. E., Halkier B. A. Cloning and expression in Escherichia coli of the obtusifoliol 14α-demethylase of Sorghum bicolor (L.) Moench, a cytochrome P450 orthologous to the sterol 14α-demethylases (CYP51) from fungi and mammals. Plant J. 1997;111(1):191–201. doi: 10.1046/j.1365-313x.1997.11020191.x. [DOI] [PubMed] [Google Scholar]
- Baloch R. I., Mercer E. I. Inhibition of sterol Δ8-Δ7-isomerase and Δ14-reductase by fenpropimorph, tridemorph and fenpropidin in cell-free enzyme systems from Saccharomyces cerevisiae. Phytochemistry. 1987;261(1):663–668. [Google Scholar]
- Bard M., Bruner D. A., Pierson C. A., Lees N. D., Biermann B., Frye L., Koegel C., Barbuch R. Cloning and characterization of ERG25, the Saccharomyces cerevisiae gene encoding C-4 sterol methyl oxidase. Proc. Natl. Acad. Sci. USA. 1996;931(1):186–190. doi: 10.1073/pnas.93.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. H. R., Corrie J. E. T., Widdowson D. A. Biosynthesis of terpenes and steroids. Part IX. The sterols of some mutant yeasts and their relationship to the biosynthesis of ergosterol. JCS Perkin I. 1974;41(1):1326–1333. [PubMed] [Google Scholar]
- Basson M. E., Thorsness M., Finer-Moore J., Stroud R. M., Rine J. Structural and functional conservation between yeast and Human 3-hydroxy-3-methylglutaryl coen-zyle A reductases, the rate-limiting enzyme of sterol biosynthesis. Mol. Cell Biol. 1988;81(1):3797–3808. doi: 10.1128/mcb.8.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamine A., Mangla A. T., Nes W. D., Waterman M. R. Characterization and catalytic properties of the sterol 14 α-demethylase from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 1999;961(1):8937–8942. doi: 10.1073/pnas.96.16.8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste P. Sterol biosynthesis. Ann. Rev. Plant Physiol. 1986;371(1):275–307. [Google Scholar]
- Benveniste P., Rahier A. Target sites of sterol biosynthesis inhibitors in plants. 1992;1(1):207–225. In Target sites of fungicide action, W. Koeller, ed.: CRC Press), pp. [Google Scholar]
- Biardi L., Krisans S. K. Compartmentation of cholesterol biosynthesis. Conversion of mevalonate to farnesyl diphosphate occurs in the peroxisomes. J. Biol. Chem. 1996;2711(1):1784–1788. doi: 10.1074/jbc.271.3.1784. [DOI] [PubMed] [Google Scholar]
- Bimpson T., Goad L. J., Goodwin T. W. The stereochemistry of hydrogen elimination at C-6, C-22, and C-23 during ergosterol biosynthesis by Aspergillus fumigatus Fres. Chem. Communic. 1969;0111(1):297–298. [Google Scholar]
- Blanchard L., Karst F. Characterization of a lysine-to-glutamic acid mutation in a conservative sequence of farnesyl diphosphate synthase from Saccharomyces cerevisiae. Gene. 1993;1251(1):185–189. doi: 10.1016/0378-1119(93)90326-x. [DOI] [PubMed] [Google Scholar]
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;141(1):47–91. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Bochar D. A., Stauffacher C. V., Rodwell V. W. Investigation of the conserved lysines of syrian hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochemistry. 1999;381(1):15848–15852. doi: 10.1021/bi9914311. [DOI] [PubMed] [Google Scholar]
- Braverman N., Lin P., Moebius F. F., Obie C., Moser A., Glossmann H., Wilcox W. R., Rimoin D. L., Smith M., Kratz L., Kelley R. I., Valle D. Mutations in the gene encoding 3beta-hydroxysteroid-delta 8, delta 7-isomerase cause X-linked dominant Conradi-Hunermann syndrome. Nat. Genet. 1999;221(1):291–294. doi: 10.1038/10357. [DOI] [PubMed] [Google Scholar]
- Brunt S. A., Silver J. C. Molecular cloning and characterization of two distinct hsp85 sequences from the steroid responsive fungus Achlya ambisexualis. Curr. Genet. 1991;191(1):383–388. doi: 10.1007/BF00309599. [DOI] [PubMed] [Google Scholar]
- Cabello-Hurtado F., Taton M., Forthoffer N., Bak S., Kahn R., Rahier A., Werck-Reichhart D. Optimized expression and catalytical properties of a wheat obtusifoliol 14 α-demethylase (CYP51) expressed in yeast. Complementation of erg11 yeast mutants by wheat CYP51. Eur. J. Biochem. 1999;2621(1):435–446. doi: 10.1046/j.1432-1327.1999.00376.x. [DOI] [PubMed] [Google Scholar]
- Campbell M., Hahn F. M., Poulter C. D., Leustek T. Analysis of the isopentenyl diphosphate isomerase gene family from Arabidopsis thaliana. Plant Mol. Biol. 1997;361(1):323–328. doi: 10.1023/a:1005935516274. [DOI] [PubMed] [Google Scholar]
- Catterou M., Dubois F., Schaller H., Aubanelle L., Vilcot B., Sangwan-Norreel B. S., Sangwan R. S. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana, I. Molecular, cellular and physiological characterization of the Arabidopsis bull mutant, defective in the delta 7-sterol-C5-desaturation step leading to brassinosteroid biosynthesis. Planta. 2001a;2121(1):659–672. doi: 10.1007/s004250000466. [DOI] [PubMed] [Google Scholar]
- Catterou M., Dubois F., Schaller H., Aubanelle L., Vilcot B., Sangwan-Norreel B. S., Sangwan R. S. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana, II. Effects of brassinosteroids on micro-tubules and cell elongation in the bul1 mutant. Planta. 2001b;2121(1):673–683. doi: 10.1007/s004250000467. [DOI] [PubMed] [Google Scholar]
- Chappell J., Wolf F., Proulx J., Cuellar R., Saunders C. Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol. 1995;1091(1):1337–1343. doi: 10.1104/pp.109.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S., Dilkes B. P., Gregory B. D., Ross A. S., Yuan H., Noguchi T., Fujioka S., Takatsuto S., Tanaka A., Yoshida S., Tax F. E., Feldmann K. A. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylene-cholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999b;1191(1):897–907. doi: 10.1104/pp.119.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S., Noguchi T., Fujioka S., Takatsuto S., Tissier C. P., Gregory B. D., Ross A. S., Tanaka A., Yoshida S., Tax F. E., Feldmann K. A. wThe arabidopsis dwf7/ste1 mutant is defective in the Δ7-sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell. 1999a;111(1):207–221. [PMC free article] [PubMed] [Google Scholar]
- Choe S., Tanaka A., Noguchi T., Fujioka S., Takatsuto S., Ross A. S., Tax F. E., Yoshida S., Feldmann K. A. Lesions in the sterol Δ7-reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 2000;211(1):431–443. doi: 10.1046/j.1365-313x.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- Choi D., Bostock R. M., Avdiushko S., Hildebrand D. F. Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants : methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc. Natl. Acad. Sci. USA. 1994;911(1):2329–2333. doi: 10.1073/pnas.91.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. F., Tanaka R. D., Svenson K., Wamsley M., Fogelman A. M., Edwards P. A. Molecular cloning and sequence of a cholesterol-repressible enzyme related to prenyltransferase in the isoprene biosynthetic pathway. Mol. Cell. Biol. 1987;71(1):3138–3146. doi: 10.1128/mcb.7.9.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse S. D. Plant development : a role for sterols in embryogenesis. Curr. Biol. 2000;101(1):601–604. doi: 10.1016/s0960-9822(00)00639-4. [DOI] [PubMed] [Google Scholar]
- Cordier H., Karst F., Bergès T. Heterologous expression in Saccharomyces cerevisiae of an Arabidopsis thaliana cDNA encoding mevalonate diphosphate decarboxylase. Plant Mol. Biol. 1999;391(1):953–967. doi: 10.1023/a:1006181720100. [DOI] [PubMed] [Google Scholar]
- Cordier H., Lacombe C., Karst F., Bergès T. The Saccharomyces cerevisiae mevalonate diphosphate decarboxylase (Erg19p) forms homodimers in vivo, and a single substitution in a structurally conserved region impairs dimerization. Curr. Microbiol. 1999;381(1):290–294. doi: 10.1007/pl00006804. [DOI] [PubMed] [Google Scholar]
- Corey E. J., Matsuda S. P. T., Baker C. H., Ting A. Y., Cheng H. Molecular cloning of a Schizosaccharomyces pombe cDNA encoding lanosterol synthase and investigation of conserved tryptophan residues. Biochem. Biophys. Res. Commun. 1996;2191(1):327–331. doi: 10.1006/bbrc.1996.0232. [DOI] [PubMed] [Google Scholar]
- Corey E. J., Natsuda S. P. T., Bartel B. Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen. Proc Natl Acad Sci USA. 1993;901(1):11628–11632. doi: 10.1073/pnas.90.24.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunillera N., Arro M., Delourme D., Karst F., Boronat A., Ferrer A. Arabidopsis thaliana contains two differentially expressed farnesyl-diphosphate synthase genes. J. Biol. Chem. 1996;2711(1):7774–7780. doi: 10.1074/jbc.271.13.7774. [DOI] [PubMed] [Google Scholar]
- Cunillera N., Boronat A., Ferrer A. The Arabidopsis thaliana FPS1 gene generates a novel mRNA that encodes a mitochondrial farnesyl-diphosphate synthase isoform. J. Biol. Chem. 1997;2721(1):15381–15388. doi: 10.1074/jbc.272.24.15381. [DOI] [PubMed] [Google Scholar]
- Cunillera N., Boronat A., Ferrer A. Spatial and temporal patterns of GUS expression directed by 5′ regions of the Arabidopsis thaliana farnesyl diphosphate synthase genes FPS1 and FPS2. Plant Mol. Biol. 2000;441(1):747–758. doi: 10.1023/a:1026588708849. [DOI] [PubMed] [Google Scholar]
- Cunningham F. X., Gantt E. Identification of multi-gene families encoding isopentenyl diphosphate isomerase in plants by heterologous complementation in Escherichia coli. Plant Cell Physiol. 2000;411(1):119–123. doi: 10.1093/pcp/41.1.119. [DOI] [PubMed] [Google Scholar]
- Dale S., Arró M., Becerra B., Morrice N. G., Boronat A., Hardie D. G., Ferrer A. Bacterial expression of the catalytic domain of 3-hydroxy-3-methylglutaryl-CoA reductase (isoform HMGR1) from Arabidopsis thaliana, and its inactivation by phosphorylation at Ser577 by Brassica oleracea 3-hydroxy-3-methylglutaryl-CoA reductase kinase. Eur. J. Biochem. 1995;2331(1):506–513. doi: 10.1111/j.1432-1033.1995.506_2.x. [DOI] [PubMed] [Google Scholar]
- Darnet S., Bard M., Rahier A. Functional identification of sterol-4α-methyl oxidase cDNAs from Arabidopsis thaliana by complementation of a yeast erg25 mutant lacking sterol-4α-methyl oxidation. FEBS Letters. 2001;5081(1):39–43. doi: 10.1016/s0014-5793(01)03002-2. [DOI] [PubMed] [Google Scholar]
- Delourme D., Lacroute F., Karst F. Cloning of an Arabidopsis thaliana cDNA coding for farnesyl diphosphate synthase by functional complementation in yeast. Plant Mol. Biol. 1994;261(1):1867–1873. doi: 10.1007/BF00019499. [DOI] [PubMed] [Google Scholar]
- Delye C., Bousset L., Corio-Costet M. F. PCR cloning and detection of point mutations in the eburicol 14alpha-demethylase (CYP51) gene from Erysiphe graminis f. sp. hordei, a “recalcitrant” fungus. Curr. Genet. 1998;341(1):399–403. doi: 10.1007/s002940050413. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim. Biophys. Acta. 1976;4571(1):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Devarenne T. P., Shin D. H., Back K., Yin S., Chappell J. Molecular characterization of tobacco squalene synthase and regulation in response to fungal elicitor. Arch. Biochem. Biophys. 1998;3491(1):205–215. doi: 10.1006/abbi.1997.0463. [DOI] [PubMed] [Google Scholar]
- Diener A. C., Li H., Zhou W. X., Whoriskey W. J., Nes W. D., Fink G. R. STEROL METHYLTRANSFERASE 1 Controls the level of cholesterol in plants. Plant Cell. 2000;121(1):853–870. doi: 10.1105/tpc.12.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D. A. Cation-pi interactions in chemistry and biology : a new view of benzene, Phe, Tyr and Trp. Science. 1996;32461(1):163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Gaut B. S. Evolution of genes and taxa : a primer. Plant Mol. Biol. 2000;421(1):1–23. [PubMed] [Google Scholar]
- Dyas L., Goad L. J. Steryl fatty acyl esters in plants. Phytochemistry. 1993;341(1):17–29. [Google Scholar]
- Enjuto M., Balcells L., Campos N., Caelles C., Arró M., Boronat A. Arabidopsis thaliana contains two differentially expressed 3-hydroxy-3-methylglutaryl-CoA reductase genes, which encode microsomal forms of the enzyme. Proc. Natl. Acad. Sci. USA. 1994;911(1):927–931. doi: 10.1073/pnas.91.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Jr Acyl CoA:cholesterol acyltransferase genes and knockout mice. Curr. Op. Lipidol. 1998;91(1):119–123. doi: 10.1097/00041433-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Feil C., Süssmuth R., Jung G., Poralla K. Site-directed mutagenesis of putative active-site residues in squalene-hopene cyclase. Eur. J. Biochem. 1996;2421(1):51–55. doi: 10.1111/j.1432-1033.1996.0051r.x. [DOI] [PubMed] [Google Scholar]
- Ferrer A., Aparicio C., Nogués N., Wettstein A., Bach T. J., Boronat A. Expression of catalytically active radish 3-hydroxy-3-methylglutaryl coenzyme A reductase in Escherichia coli. FEBS Lett. 1990;2661(1):67–71. doi: 10.1016/0014-5793(90)81508-l. [DOI] [PubMed] [Google Scholar]
- Fitzky B. U., Witsch-Baumgartner M., Erdel M., Lee J. N., Paik Y. K., Glossmann H., Utermann G., Moebius F. F. Mutations in the Δ7-sterol reductase gene in patients with the smith-lemli-opitz syndrome. Proc. Natl. Acad. Sci. USA. 1998;951(1):8181–8186. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau P., Hartmann M. A., Benveniste P. A 24-methylene lophenol C-28 methyl transferase from suspension cultures of bramble cells. Plant Sci. Lett. 1977;101(1):147–155. [Google Scholar]
- Gaber R. F., Copple D. M., Kennedy B. K., Vidal M., Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol. Cell Biol. 1989;91(1):3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachotte D., Husselstein T., Bard M., Lacroute F., Benveniste P. Isolation and characterization of an Arabidopsis thaliana cDNA encoding a Δ7-sterol-C-5-desaturase by functional complementation of a defective yeast mutant. Plant J. 1996;91(1):391–398. doi: 10.1046/j.1365-313x.1996.09030391.x. [DOI] [PubMed] [Google Scholar]
- Gachotte D., Meens R., Benveniste P. An Arabidopsis mutant deficient in sterol biosynthesis : heterologous complementation by ERG3 encoding a Δ7-sterol-C-5-desaturase from yeats. Plant J. 1995;81(1):407–416. doi: 10.1046/j.1365-313x.1995.08030407.x. [DOI] [PubMed] [Google Scholar]
- Garcia R. E., Mudd J. B. Partial characterization of steryl ester biosynthesis in spinach leaves. Plant Physiol. 1978;611(1):357–360. doi: 10.1104/pp.61.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Gibbons G. F., Bolger L. M., Rees H. H., Goodwin T. W. Incorporation of (2-14C, (5r)-5-3H1) mevalonic acid into cholesterol by a rat liver homogenate and into beta-sitosterol and 28-isofucosterol by larix decidua leaves. Biochem. J. 1969;1141(1):885–892. doi: 10.1042/bj1140885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godzina S. M., Lovato M. A., Meyer M. M., Foster K. A., Wilson W. K., Gu W., De Hostos E. L., Matsuda S. P. T. Cloning and characterization of the Dictyostelium discoideum cycloartenol synthase cDNA. Lipids. 2000;351(1):249–255. doi: 10.1007/s11745-000-0520-3. [DOI] [PubMed] [Google Scholar]
- Gondet L., Bronner R., Benveniste P. Regulation of sterol content in membranes by subcellular compartmentation of sterylesters accumulation in a sterol overproduction tobacco mutant. Plant Physiol. 1994;1051(1):509–518. doi: 10.1104/pp.105.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondet L., Weber T., Maillot-Vernier P., Benveniste M., Bach T. J. Regulatory role of microsomal 3-hydroxy-3-methyl-glutaryl-Coenzyme A reductase in a tobacco mutant that overproduces sterols. Biochem. Biophys. Res. Commun. 1992;1861(1):878–893. doi: 10.1016/0006-291x(92)90829-a. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W. Biosynthesis of terpenoids. Ann. Rev. Plant Physiol. 1979;301(1):369–404. [Google Scholar]
- Grausem B., Chaubet N., Gigot C., Loper J. C., Benveniste P. Functional expression of Saccharomyces cerevisiae CYP51A1 encoding lanosterol-14-demethylase in tobacco results in bypass of endogenous sterol biosynthetic pathway and resistant to an obtusifoliol-14-demethylase herbicide inhibitor. Plant J. 1995;71(1):761–770. doi: 10.1046/j.1365-313x.1995.07050761.x. [DOI] [PubMed] [Google Scholar]
- Grebenok R. J., Galbraith D. W., Penna D. D. Characterization of Zea mays endosperm C-24 sterol methyl-transferase : one of two types of sterol methyltransferase in higher plants. Plant Mol. Biol. 1997;341(1):891–896. doi: 10.1023/a:1005818210641. [DOI] [PubMed] [Google Scholar]
- Grebenok R. J., Ohnmeiss T. E., Yamamoto A., Huntley E. D., Galbraith D. W., Della Penna D. Isolation and characterization of an Arabidopsis thaliana C-8,7 sterol isomerase : functional and structural similarities to mammalian C-8,7 sterol isomerase/emopamil-binding protein. Plant Mol. Biol. 1998;381(1):807–815. doi: 10.1023/a:1006028623875. [DOI] [PubMed] [Google Scholar]
- Greeve I., Hermans-Borgmeyer I., Brellinger C., Kasper D., Gomez-Isla T., Behl C., Levkau B., Nitsch R. M. The human DIMINUTO:DWARF1 homolog seladin-1 confers resistance to Alzheimer's disease-associated neurode-generation and oxidative stress. J. Neurosci. 2000;201(1):7345–7352. doi: 10.1523/JNEUROSCI.20-19-07345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Plant sterols. Annu. Rev. Plant Physiol. 1975;261(1):209–236. [Google Scholar]
- Gylling H., Puska P., Vartiainen E., Miettinen T. A. Serum sterols during stanol ester feeding in a mildly hypercholesterolemic population. J. Lipid Res. 1999;401(1):593–600. [PubMed] [Google Scholar]
- Hardwick K. G., Pelham H. R. SED6 is identical to ERG6, and encodes a putative methyltransferase required for ergosterol synthesis. Yeast. 1994;101(1):265–269. doi: 10.1002/yea.320100213. [DOI] [PubMed] [Google Scholar]
- Hart E. A., Hua L., Daer L. B., Wilson W. K., Pang J., Matsuda S. P. Directed evolution to investigate steric control of enzymatic oxidosqualene cyclization. An isoleucine-co-valine mutation in cycloartenol synthase allows lanosterol and parkeol biosynthesis. J. Am. Chem. Soc. 1999;1211(1):9887–9888. [Google Scholar]
- Hartmann M. A., Wentzinger L., Hemmerlin A., Bach T. J. Metabolism of farnesyl diphosphate in tobacco BY-2 cells treated with squalestatin. Biochem. Soc. Trans. 2000;281(1):794–796. [PubMed] [Google Scholar]
- Hartmann-Bouillon M. A., Benveniste P. Sterol biosynthetic capability of purified membrane fractions from maize coleoptiles. Phytochemistry. 1978;171(1):1037–1042. [Google Scholar]
- Hata S., Nishino T., Katsuki H., Aoyama Y., Yoshida Y. Two species of cytochrome P-450 involved in ergosterol biosynthesis of yeast. Biochem. Biophys. Res. Comm. 1983;1161(1):162–166. doi: 10.1016/0006-291x(83)90395-9. [DOI] [PubMed] [Google Scholar]
- Hata S., Sanmiya K., Kouchi H., Matsuoka M., Yamamoto N., Izui K. cDNA cloning of squalene synthase genes from mono- and dicotyledonous plants, and expression of the gene in rice. Plant Cell. Physiol. 1997;381(1):1409–1413. doi: 10.1093/oxfordjournals.pcp.a029137. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Hiraoka N., Ikeshiro Y. Molecular cloning and functional expression of cDNAs for Glycyrrhiza glabra squalene synthase. Biol. Pharm. Bull. 1996;191(1):1387–1389. doi: 10.1248/bpb.19.1387. [DOI] [PubMed] [Google Scholar]
- Heintz R., Benveniste P. Enzymatic cleavage of the 9β,19-cyclopropane ring of cyclopropyl sterols in brambe tissue cultures. J. Biol. Chem. 1974;2491(1):4267. [PubMed] [Google Scholar]
- Hemmerlin A., Bach T. J. Farnesol-induced cell death and stimulation of 3-hydroxy-3-methylglutarylcoenzyme A reductase activity in tobacco cv bright yellow-2 cells. Plant Physiol. 2000;1231(1):1257–1268. doi: 10.1104/pp.123.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pinzon I., Ross J. H., Barnes K. A., Damant A. P., Murphy D. J. Composition and role of tapetal lipid bodies in the biogenesis of the pollen coat of Brassica napus. Planta. 1999;2081(1):588–598. doi: 10.1007/s004250050597. [DOI] [PubMed] [Google Scholar]
- Herrera J. B. R., Bartel B., Wilson W. K., Matsuda S. P. T. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry. 1998;491(1):1905–1911. doi: 10.1016/s0031-9422(98)00366-5. [DOI] [PubMed] [Google Scholar]
- Herrera J. B. R., Wilson W. K., Matsuda S. P. T. A tyrosine-to threonine mutation converts cycloartenol synthase to an oxidosqualene cyclase that forms lanosterol as its major product. J. Am. Chem. Soc. 2000;1221(1):6765–6766. [Google Scholar]
- Houten S. M., Koster J., Romeijn G. J., Frenkel J., Di Rocco M., Caruso U., Landrieu P., Kelley R. I., Kuis W., Poll-The B. T., Gibson K. M., Wanders R. J., Waterham H. R. Organization of the mevalonate kinase (MVK) gene and identification of novel mutations causing mevalonic aciduria and hyperimmunoglobulinaemia D and periodic fever syndrome. Eur. J. Hum. Genet. 2001;91(1):253–259. doi: 10.1038/sj.ejhg.5200595. [DOI] [PubMed] [Google Scholar]
- Houten S. M., Waterham H. R. Nonorthologous gene displacement of phosphomevalonate kinase. Mol. Genet. Metab. 2001;721(1):273–276. doi: 10.1006/mgme.2000.3133. [DOI] [PubMed] [Google Scholar]
- Husselstein T., Gachotte D., Desprez T., Bard M., Benveniste P. Transformation of Saccharomyces cerevisiae with a cDNA encoding a sterol C-methyltransferase from Arabidopsis thaliana results in the synthesis of 24-ethyl sterols. FEBS Lett. 1996;3811(1):87–92. doi: 10.1016/0014-5793(96)00089-0. [DOI] [PubMed] [Google Scholar]
- Husselstein T., Schaller H., Gachotte D., Benveniste P. Δ7-sterol-C5-desaturase molecular characterization and functional expression of wild type and mutant alleles. Plant Mol. Biol. 1999;391(1):891–906. doi: 10.1023/a:1006113919172. [DOI] [PubMed] [Google Scholar]
- Husselstein-Muller T., Schaller H., Benveniste P. Molecular cloning and expression in yeast of 2,3-oxidosqualene-triterpenoid cyclases from Arabidopsis thaliana. Plant Mol. Biol. 2001;421(1):75–92. doi: 10.1023/a:1006476123930. [DOI] [PubMed] [Google Scholar]
- Istvan E. S., Palnitkar M., Buchanan S. K., Deisenhofer J. Crystal structure of the catalytic portion of human HMG-CoA reductase : insights into regulation of activity and catalysis. EMBO J. 2000;191(1):819–830. doi: 10.1093/emboj/19.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tamura T., Matsumoto T. Methylsterol compositions of 19 vegetable oils. J. Am. Oil Chem. Soc. 1973;501(1):300–303. doi: 10.1007/BF02641360. [DOI] [PubMed] [Google Scholar]
- Jandrositz A., Turnowsky F., Högenauer G. The gene encoding squalene epoxidase from Saccharomyces cerevisiae : cloning and characterization. Gene. 1991;1071(1):155–160. doi: 10.1016/0378-1119(91)90310-8. [DOI] [PubMed] [Google Scholar]
- Jang Y. C., Fujioka S., Tasaka M., Seto H., Takatsuto S., Ishii A., Aida M., Yoshida S., Sheen J. A critical role of sterols in embryonic patterning and meristem programming revealed by the fackel mutants of Arabidopsis thaliana. Genes & Dev. 2000;141(1):1485–1497. [PMC free article] [PubMed] [Google Scholar]
- Johnson W. S., Telfer S. J., Cheng S., Schubert U. J. Am. Chem. Soc. 1987;1091(1):2517–2518. [Google Scholar]
- Jonas A. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta. 2000;15291(1):245–256. doi: 10.1016/s1388-1981(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Jones P. J. Cholesterol-lowering action of plant sterols. Curr. Atheroscler. 1999;11(1):230–235. doi: 10.1007/s11883-999-0037-3. [DOI] [PubMed] [Google Scholar]
- Kagan R. M., Clarke S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch. Biochem. Biophys. 1994;3101(1):417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Loper J. C., Dey C. R., Woods C. W., Sutter T. R. Isolation of a cytochrome P-450 structural gene from Saccharomyces cerevisiae. Gene. 1986;451(1):237–245. doi: 10.1016/0378-1119(86)90021-1. [DOI] [PubMed] [Google Scholar]
- Karpen H. E., Bukowski J. T., Hughes T., Gratton J. P., Sessa W. C., Gailani M. R. The sonic hedgehog receptor patched associates with caveolin-1 in cholesterol-rich microdomains of the plasma membrane. J. Biol. Chem. 2001;2761(1):19503–19511. doi: 10.1074/jbc.M010832200. [DOI] [PubMed] [Google Scholar]
- Kauschmann A., Jessop A., Koncz C., Szekeres M., Willmitzer L., Altmann T. Genetic evidence for an essential role of brassinosteroids in plants development. Plant J. 1996;91(1):701–713. [Google Scholar]
- Kelly S. L., Lamb D. C., Baldwin B. C., Corran A. J., Kelly D. E. Characterization of Saccharomyces cerevisiae CYP61, sterol Δ22-desaturase, and inhibition by azole antifungal agents. J. Biol. Chem. 1997;2721(1):9986–9988. doi: 10.1074/jbc.272.15.9986. [DOI] [PubMed] [Google Scholar]
- Kennedy M. A., Bard M. Positive and negative regulation of squalene synthase (ERG9), an ergosterol biosynthetic gene, in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2001;15171(1):177–189. doi: 10.1016/s0167-4781(00)00246-3. [DOI] [PubMed] [Google Scholar]
- Keon J. P. R., James C. S., Court S., Baden-Daintree C., Bailey A. M., Burden R. S., Bard M., Hargreaves J. A. Isolation of the ERG2 gene, encoding sterol Δ8-Δ7-isomerase, from the rice blast fungus Magnaporthe grisea and its expression in the maize smut pathogen Ustilago maydis. Curr. Genet. 1994;251(1):531–537. doi: 10.1007/BF00351674. [DOI] [PubMed] [Google Scholar]
- Kip Guy R. Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc. Nat. Acad. Sci USA. 2000;971(1):7307–7312. doi: 10.1073/pnas.97.13.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U., Noguchi T., Fujioka S., Takatsuto S., Yokota T., Nomura T., Yoshida S., Chua N. H. The Arabidopsis DIMINUTO/DWARF1 Gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;101(1):1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth K. L., Jaggard D. A., Dixon R. A. Developmental and light-regulated post-translational control of 3-hydroxy-3-methylglutaryl-CoA reductase levels in potato. Plant J. 2000;231(1):507–516. doi: 10.1046/j.1365-313x.2000.00821.x. [DOI] [PubMed] [Google Scholar]
- Kribii R., Arró M., Del Arco A., Gonzalez V., Balcells L., Delourme D., Ferrer A., Karst F., Boronat A. Cloning and characterization of the Arabidopsis thaliana SQS1 gene encoding squalene synthase. Involvement of the C-terminal region of the enzyme in the channeling of squalene through the sterol pathway. Eur. J. Biochem. 1997;2491(1):61–69. doi: 10.1111/j.1432-1033.1997.00061.x. [DOI] [PubMed] [Google Scholar]
- Kushiro M., Nakano T., Sato K., Yamagishi K., Asami T., Nakano A., Takatsuto S., Fujioka S., Ebizuka Y., Yoshida S. Obtusifoliol 14α-demethylase (CYP51) antisense Arabidopsis shows slow growth and long life. Biochem. Biophys. Res. Communic. 2001;2851(1):98–104. doi: 10.1006/bbrc.2001.5122. [DOI] [PubMed] [Google Scholar]
- Kushiro T., Shibuya M., Ebizuka Y. β-amyrin synthase. Cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998;2561(1):238–244. doi: 10.1046/j.1432-1327.1998.2560238.x. [DOI] [PubMed] [Google Scholar]
- Kushiro T., Shibuya M., Ebizuka Y. Chimeric triterpene synthase. A possible model for multifunctional triterpene synthase. J. Am. Chem. Soc. 1999;1211(1):1208–1216. [Google Scholar]
- Kushiro T., Shibuya M., Ebizuka Y. Cryptic regiospecificity in deprotonation step of triterpene biosynthesis catalyzed by new members of lupeol synthase. Tetrahedron Lett. 1999;401(1):5553–5556. [Google Scholar]
- Kushiro T., Shibuya M., Masuda K., Ebizuka Y. A novel multifunctional triterpene synthase from Arabidopsis thaliana. Tetrahedron Lett. 2000;411(1):7705–7710. [Google Scholar]
- Lai M. H., Bard M., Pierson C. A., Alexander J. F., Goebl M., Carter G. T., Kirsch D. R. The identification of a gene family in the Saccharomyces cerevisiae ergosterol biosynthesis pathway. Gene. 1994;1401(1):41–49. doi: 10.1016/0378-1119(94)90728-5. [DOI] [PubMed] [Google Scholar]
- Learned R. M., Connolly E. L. Light modulates the spatial patterns of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in Arabidopsis thaliana. Plant J. 1997;111(1):499–511. doi: 10.1046/j.1365-313x.1997.11030499.x. [DOI] [PubMed] [Google Scholar]
- Learned R. M., Fink G. R. 3-hydroxy-3-methylglutarylcoenzyme A reductase from Arabidopsis thaliana is structurally distinct from the yeast and animal enzymes. Proc. Natl. Acad. Sci. USA. 1989;861(1):2779–2783. doi: 10.1073/pnas.86.8.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecain E., Chenivesse X., Spagnoli R., Pompon D. Cloning by metabolic interference in yeast and enzymatic characterization of Arabidopsis thaliana sterol Δ7-reductase. J. Biol. Chem. 1996;2711(1):10866–10873. doi: 10.1074/jbc.271.18.10866. [DOI] [PubMed] [Google Scholar]
- Lees N. D., Skaggs B., Kirsch D. R., Bard M. Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae. Lipids. 1995;301(1):221–226. doi: 10.1007/BF02537824. [DOI] [PubMed] [Google Scholar]
- Li C. P., Larkins B. A. Identification of a maize endosperm-specific cDNA encoding farnesyl pyrophosphate synthetase. Gene. 1996;1711(1):193–196. doi: 10.1016/0378-1119(95)00880-2. [DOI] [PubMed] [Google Scholar]
- Li J., Nagpal P., Vitart V., McMorris T. C., Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;2721(1):398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Li L., Kaplan J. Characterization of yeast methyl sterol oxidase (ERG25) and identification of a human homologue. J. Biol. Chem. 1996;2711(1):16927–16933. doi: 10.1074/jbc.271.28.16927. [DOI] [PubMed] [Google Scholar]
- Lin S., Cheng D., Liu M. S., Chen J., Chang T. Y. Human acyl-CoA : cholesterol acyltransferase-1 in the endoplasmic reticulum contains seven transmembrane domains. J. Biol. Chem. 1999;2741(1):23276–23285. doi: 10.1074/jbc.274.33.23276. [DOI] [PubMed] [Google Scholar]
- Lluch M. A., Masferrer A., Arró M., Boronat A., Ferrer A. Molecular cloning and expression analysis of the mevalonate kinase gene from Arabidopsis thaliana. Plant Mol. Biol. 2000;421(1):365–376. doi: 10.1023/a:1006325630792. [DOI] [PubMed] [Google Scholar]
- Lorenz R. T., Parks L. W. Cloning, sequencing and disruption of the gene encoding sterol C-14 reductase in Saccharomyces cerevisiae. DNA Cell. Biol. 1992;111(1):685–692. doi: 10.1089/dna.1992.11.685. [DOI] [PubMed] [Google Scholar]
- Lovato M. A., Hart E. A., Segura M. J. R., Giner J. L., Matsuda S. P. T. Functional cloning of an Arabidopsis thaliana cDNA encoding cycloeucalenol cycloisomerase. J. Biol. Chem. 2000;2751(1):13394–13397. doi: 10.1074/jbc.275.18.13394. [DOI] [PubMed] [Google Scholar]
- Lumbreras V., Campos N., Boronat A. The use of an alternative promoter in the Arabidopsis thaliana HMG1 gene generates an mRNA that encodes a novel 3-hydroxy-3-methyl-glutaryl coenzyme A reductase isoform with an extended N-terminal region. Plant J. 1995;81(1):541–549. doi: 10.1046/j.1365-313x.1995.8040541.x. [DOI] [PubMed] [Google Scholar]
- Marcireau C., Guyonnet D., Karst F. Construction and growth properties of a yeast strain defective in sterol 14-reductase. Curr. Genet. 1992;221(1):267–272. doi: 10.1007/BF00317919. [DOI] [PubMed] [Google Scholar]
- Matsushima M., Inazawa J., Takahashi E., Suzumori K., Nakamura Y. Molecular cloning and mapping of a human cDNA (SC5DL) encoding a protein homologous to fungal sterol-C5-desaturase. Cytogenet. Cell Genet. 1996;741(1):252–254. doi: 10.1159/000134427. [DOI] [PubMed] [Google Scholar]
- Matsushita Y., Kang W., Charlwood B. V. Cloning and analysis of a cDNA encoding farnesyl diphosphate synthase from Artemisia annua. Gene. 1996;1721(1):207–209. doi: 10.1016/0378-1119(96)00054-6. [DOI] [PubMed] [Google Scholar]
- Mikes V., Milat M. L., Ponchet M., Panabières F., Ricci P., Blein J. P. Elicitins, proteinaceous elicitors of plant defense, are a new class of sterol carrier proteins. Biochem. Biophys. Res. Commun. 1998;2451(1):133–139. doi: 10.1006/bbrc.1998.8341. [DOI] [PubMed] [Google Scholar]
- Minet M., Dufour M. E., Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992;21(1):417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Moebius F. F., Fitzky B. U., Lee J. N., Paik Y. K., Glossmann H. Molecular cloning and expression of the human Δ7-sterol reductase. Proc. Natl. Sci. USA. 1998;951(1):1899–1902. doi: 10.1073/pnas.95.4.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius F. F., Soellner K. E. M., Fiechtner B., Huck C. W., Bonn G., Glossmann H. Histidine77, glutamic acid81, glutamic acid123, threonine126, asparagine194 and tryptophan197 of the human emopamil binding protein are required for in vivo sterol Δ8-Δ7 isomerization. Biochemistry. 1999;381(1):1119–1127. doi: 10.1021/bi981804i. [DOI] [PubMed] [Google Scholar]
- Moore J. T., Gaylor J. L. Isolation and purification of an S-adenosylmethionine : Δ24-sterol methyltransferase from yeast. J. Biol. Chem. 1969;2441(1):6334–6340. [PubMed] [Google Scholar]
- Munn A. L., Heese-Peck A., Stevenson B. J., Pichler H., Riezman H. Specific sterols required for the internalization step of endocytosis in yeast. Mol. Biol. Cell. 1999;101(1):3943–3957. doi: 10.1091/mbc.10.11.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T., Inoue T., Oka A., Nishino T., Osumi T., Hata S. Cloning, expression, and characterization of cDNAs encoding Arabidopsis thaliana squalene synthase. Proc. Natl. Acad. Sci USA. 1995;921(1):2328–2332. doi: 10.1073/pnas.92.6.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Koymans L., Kamataki T., Stegeman J. J., Feyereisen R., Waxman D. J., Waterman M. R., Gotoh O., Coon M. J., Estabrook R. W., Gunsalus I. C., Nebert D. W. P450 superfamily : update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;61(1):1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Nes W. D. Sterol methyl transferase : enzymology and inhibition. Biochim. Biophys. Acta. 2000;15291(1):63–88. doi: 10.1016/s1388-1981(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Nes W. D., Janssen G. G., Bergenstråhle A. Structural requirements for transformation of substrates by the (S)-adenosyl-L-methionine : Δ24(25)-sterol methyl transferase. J. Biol. Chem. 1991;2661(1):15202–15212. [PubMed] [Google Scholar]
- Nes W. D., Norton R. A., Crumley F. G., Madigan S. J., Katz E. R. Sterol phylogenesis and algal evolution. Proc. Natl. Acad. Sci. USA. 1990;871(1):7565–7569. doi: 10.1073/pnas.87.19.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R. The biochemistry of plant sterols. Adv. Lipid Res. 1977;151(1):233–324. [Google Scholar]
- Nishi S., Nishino H., Ishibashi T. cDNA cloning of the mammalian sterol C5-desaturase and the expression in yeast mutant. Biochim. Biophys. Acta. 2000;14901(1):106–108. doi: 10.1016/s0167-4781(99)00248-1. [DOI] [PubMed] [Google Scholar]
- Normén L., Dutta P., Lia A., Andersson H. Soy sterol esters and β-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 2000;711(1):908–913. doi: 10.1093/ajcn/71.4.908. [DOI] [PubMed] [Google Scholar]
- Olivier L. M., Krisans S. K. Peroxisomal protein targeting and identification of peroxisomal targeting signals in cholesterol biosynthetic enzymes. Biochim. Biophys. Acta. 2000;15291(1):89–102. doi: 10.1016/s1388-1981(00)00139-6. [DOI] [PubMed] [Google Scholar]
- Osumi T., Nishino T., Katsuki H. Studies on the Δ 5-desaturation in ergosterol biosynthesis in yeast. J. Biochem. 1979;851(1):819–826. [PubMed] [Google Scholar]
- Pan Z., Herickhoff L., Backhaus R. A. Cloning, characterization and heterologous expression of cDNAs for farnesyl diphosphate synthase from the guayule rubber plant reveals that this prenyltransferase occurs in rubber particles. Arch. Biochem. Biophys. 1996;3321(1):196–204. doi: 10.1006/abbi.1996.0333. [DOI] [PubMed] [Google Scholar]
- Pandit J., Danley D. E., Schulte G. K., Mazzalupo S., Pauly T. A., Hayward C. M., Hamanaka E. S., Thompson J. F., Harwood H. J., Jr Crystal structure of human squalene synthase. J. Biol. Chem. 2000;2751(1):30610–30617. doi: 10.1074/jbc.M004132200. [DOI] [PubMed] [Google Scholar]
- Pascal S., Taton M., Rahier A. Oxidative C4-demethylation of 24-methylene cycloartanol by a cyanide-sensitive enzymatic system from higher plant microsomes. Biochem. Biophys. Res. Commun. 1990;1721(1):98–106. doi: 10.1016/s0006-291x(05)80178-0. [DOI] [PubMed] [Google Scholar]
- Pascal S., Taton M., Rahier A. Plant sterol biosynthesis : Identification and characterization of two distinct microsomal oxidative enzymatic systems involved in sterol C4-demethylation. J. Biol. Chem. 1993;2681(1):11639–11654. [PubMed] [Google Scholar]
- Pascal S., Taton M., Rahier A. Plant sterol biosynthesis : identification of a NADPH dependent plant sterone reductase involved in the sterol-4-demethylation. Arch. Biochem. Biophys. 1994;3121(1):260–271. doi: 10.1006/abbi.1994.1308. [DOI] [PubMed] [Google Scholar]
- Peelman F., Vanloo B., Perez-Mendez O., Decout A., Verschelde J. L., Labeur C., Vinaimont N., Verhee A., Duverger N., Brasseur R., Vandekerckhove J., Tavernier J., Rosseneu M. Characterization of functional residues in the interfacial recognition domain of lecithin cholesterol acyltyransferase (LCAT). Protein Engineering. 1999;121(1):71–78. doi: 10.1093/protein/12.1.71. [DOI] [PubMed] [Google Scholar]
- Podust L. M., Poulos T. L., Waterman M. R. Crystal structure of cytochrome P450 14α-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. 2001;981(1):3068–3073. doi: 10.1073/pnas.061562898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky E. S., Poulter C. A. Squalene synthase : Steady-State, pre-steady-state, and isotope-trapping studies. Biochemistry. 2000;391(1):1748–1760. doi: 10.1021/bi9915014. [DOI] [PubMed] [Google Scholar]
- Raederstorff D., Rohmer M. Sterol biosynthesis via cycloartenol and other biochemical features related to photo-synthetic phyla in the amoeba Naegleria lovaniensis and Naegleria gruberi. Eur. J. Biochem. 1987;1641(1):427–434. doi: 10.1111/j.1432-1033.1987.tb11075.x. [DOI] [PubMed] [Google Scholar]
- Rahier A. Deuterated Δ7-cholestenol analogues as mechanistic probes for wild-type and mutated Δ7-sterol-C5(6)-desaturase. Biochemistry. 2001;401(1):256–267. doi: 10.1021/bi001696b. [DOI] [PubMed] [Google Scholar]
- Rahier A., Génot J. C., Schuber F., Benveniste P., Narula A. S. Inhibition of (S)-adenosyl-L-methionine sterol-C-24-methyltransferase by analogues of a carbocationic ion high energy intermediate - Structure activity relationships for C-25 heteroatoms (N, As, S) substituted triterpenoid derivatives. J. Biol. Chem. 1984;2591(1):15215–15223. [PubMed] [Google Scholar]
- Rahier A., Taton M. Sterol biosynthesis : strong inhibition of maize Δ5,7-sterol-Δ7-reductase by novel 6-aza-B-homosteroids and other analogs of a presumptive carbocationic intermediate of the reduction reaction. Biochemistry. 1996;351(1):7069. doi: 10.1021/bi9528154. [DOI] [PubMed] [Google Scholar]
- Rahier A., Taton M., Benveniste P. Cycloeucalenol-obtusifoliol isomerase. Structural requirements for substrates and inhibitors binding or transformation. Eur. J. Biochem. 1989;1811(1):615–626. doi: 10.1111/j.1432-1033.1989.tb14768.x. [DOI] [PubMed] [Google Scholar]
- Rahier A., Taton M., Bouvier-Navé P., Schmitt P., Benveniste P., Schuber F., Narula A., Anding C., Place P. Design of high energy intermediate analogues to study sterol biosynthesis in higher plants. Lipids. 1986;211(1):52–62. doi: 10.1007/BF02534303. [DOI] [PubMed] [Google Scholar]
- Reddy V. V. R., Kupfer D., Caspi E. J. J. Biol. Chem. 1977;2521(1):2797–2801. xxx. [PubMed] [Google Scholar]
- Riou C., Tourte Y., Lacroute F., Karst F. Isolation and characterization of a cDNA encoding Arabidopsis thaliana mevalonate kinase by genetic complementation in yeast. Gene. 1994;1481(1):293–297. doi: 10.1016/0378-1119(94)90701-3. [DOI] [PubMed] [Google Scholar]
- Rohmer M., Knani M., Simonin P., Sutter B., Sahm H. Isoprenoid biosynthesis in bacteria : a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993;2951(1):517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondet S., Taton M., Rahier A. Identification, characterization, and partial purification of 4α-carboxysterol-C3-dehydrogenase/C4-decarboxylase from Zea mays. Arch. Biochem. Biophys. 1999;3661(1):249–260. doi: 10.1006/abbi.1999.1218. [DOI] [PubMed] [Google Scholar]
- Sakakibara J., Watanabe R., Kanai Y., Ono T. Molecular cloning and expression of rat squalene epoxidase. J. Biol. Chem. 1995;2701(1):17–20. doi: 10.1074/jbc.270.1.17. [DOI] [PubMed] [Google Scholar]
- Salmon F., Taton M., Benveniste P., Rahier A. Plant sterol biosynthesis : novel potent and selective inhibitors of cytochrome P-450 dependent obtusifoliol 14α-methyl demethylase. Arch. Biochem. Biophys. 1992;2971(1):123–131. doi: 10.1016/0003-9861(92)90649-h. [DOI] [PubMed] [Google Scholar]
- Schaeffer A., Bouvier-Navé P., Benveniste P., Schaller H. Plant sterol-C24-methyl transferases : different profiles of tobacco transformed with SMT1 or SMT2. Lipids. 2000;351(1):263–269. doi: 10.1007/s11745-000-0522-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer A., Bronner R., Benveniste P., Schaller H. The ratio of campesterol to sitosterol with modulates growth in Arabidopsis is controlled by STEROL METHYL-TRANSFERASE 2-1. Plant J. 2001;251(1):605–615. doi: 10.1046/j.1365-313x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Schäfer U. A., Reed D. W., Hunter D. G., Yao K., Weninger A. M., Tsang E. W. T., Reaney M. J. T., MacKenzie S. L., Covello P. S. An example of intron junctional slid ing in the gene families encoding squalene monooxygenase homologues in Arabidopsis thaliana and Brassica napus. Plant Mol. Biol. 1999;391(1):721–728. doi: 10.1023/a:1006172120929. [DOI] [PubMed] [Google Scholar]
- Schaller H., Bouvier-Navé P., Benveniste P. Overexpression of an Arabidopsis thaliana (L.) Heynh. cDNA encoding a sterol-C241-methyltransferase in Nicotiana tabacum L. Modifies the ratio of 24-methyl cholesterol to sitosterol and is associated with growth reduction. Plant Physiol. 1998;1181(1):461–469. doi: 10.1104/pp.118.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Gondet L., Maillot-Vernier P., Benveniste P. Sterol overproduction is the biochemical basis of resistance to a triazole in calli from a tobacco mutant. Planta. 1994;1941(1):295–305. [Google Scholar]
- Schaller H., Grausem B., Benveniste P., Chye M. L., Tan C. T., Song Y. H., Chua N. H. Expression of the Hevea brasiliensis (H.B.K.) Müll. Arg. 3-hydroxy-3-methylglutaryl-Coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol. 1995;1091(1):761–770. doi: 10.1104/pp.109.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Maillot-Vernier P., Gondet L., Belliard G., Benveniste P. Biochemical characterization of tobacco mutants resistant to azole fungicides and herbicides. Biochem. Soc. Trans. 1993;211(1):1052–1057. doi: 10.1042/bst0211052. [DOI] [PubMed] [Google Scholar]
- Schmitt P., Narula A. S., Benveniste P., Rahier A. Manipulation by 25-azacycloartanol of the relative percentage of C10, C9 and C8 side-chain sterols in suspension cultures bramble cells. Phytochemistry. 1981;201(1):197–201. [Google Scholar]
- Schmitt P., Scheid F., Benveniste P. Accumulation of Δ8,14-sterols in suspension cultures of bramble cells cultured with an azasterol antimycotic agent (A 25822 B). Phytochemistry. 1980;191(1):525–530. [Google Scholar]
- Schrick K., Mayer U., Horrichs A., Kuhnt C., Bellini C., Dangl J., Schmidt J., Jürgens G. FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes & Develop. 2000;141(1):1471–1484. [PMC free article] [PubMed] [Google Scholar]
- Schuler I., Milon A., Nakatani Y., Ourisson G., Albrecht A. M., Benveniste P., Hartmann M. A. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 1991;881(1):6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servouse M., Mons N., Baillargeat J. L., Karst F. Isolation and characterization of yeast mutants blocked in mevalonic acid formation. Biochem. Biophys. Res. Commun. 1984;1231(1):424. doi: 10.1016/0006-291x(84)90247-x. [DOI] [PubMed] [Google Scholar]
- Shanklin J., Whittle E., Fox B. G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry. 1994;331(1):12787–12794. doi: 10.1021/bi00209a009. [DOI] [PubMed] [Google Scholar]
- Shi J., Dixon R. A., Gonzales R. A., Kjellborn P., Bhattacharyya M. K. Identification of cDNA clones encoding valosin-containing protein and other plant plasma membrane-associated proteins by a general immunoscreening strategy. Proc. Natl. Acad. Sci. USA. 1995;921(1):4457–4461. doi: 10.1073/pnas.92.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Gonzales R. A., Bhattacharyya M. K. Identification and characterization of an S-adenosyl-L-methionine : Δ24-sterol-C-methyltransferase cDNA from soybean (*). J. Biol. Chem. 1996;2711(1):9384–9389. doi: 10.1074/jbc.271.16.9384. [DOI] [PubMed] [Google Scholar]
- Shi Z., Buntel C. J., Griffin J. H. Isolation and characterization of the gene encoding 2,3-oxidosqualene-lanosterol cyclase from Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1994;911(1):7370–7324. doi: 10.1073/pnas.91.15.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Arita M., Misaki Y., Dohmae N., Takio K., Ono T., Inoue K., Arai H. Supernatant protein factor, which stimulates the conversion of squalene to lanosterol, is a cytosolic squalene transfer protein and enhances cholesterol biosynthesis. Proc. Nat. Acad. Sci. 2001;981(1):2244–2249. doi: 10.1073/pnas.041620398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyadehi A. Z., Lamb D. C., Kelly S. L., Kelly D. E., Schunck W. H., Wright J. N., Corina D., Akhtar M. The mechanism of the acyl-carbon bond cleavage reaction catalyzed by recombinant sterol 14α-demethylase of Candida albicans (Other names are : lanosterol 14α-demethylase, P-45014DM, and CYP51). J. Biol. Chem. 1996;2711(1):12445–12450. doi: 10.1074/jbc.271.21.12445. [DOI] [PubMed] [Google Scholar]
- Silve S., Dupuy P. H., Ferrara P., Loison G. Human lamin B receptor exhibits sterol C14-reductase activity in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1998;13921(1):233–244. doi: 10.1016/s0005-2760(98)00041-1. [DOI] [PubMed] [Google Scholar]
- Silve S., Dupuy P. H., Labit-Lebouteiller C., Kaghad M., Chalon P., Rahier A., Taton M., Lupker J., Shire D., Loison G. Emopamil-binding protein, a mammalian protein that binds a series of structurally diverse neuroprotective agents, exhibits Δ8-Δ7-sterol isomerase activity in yeast. J. Biol. Chem. 1996;2711(1):22434–22439. doi: 10.1074/jbc.271.37.22434. [DOI] [PubMed] [Google Scholar]
- Skaggs B. A., Alexander J. F., Pierson C. A., Schweitzer K. S., Chun K. T., Kocgcl C., Barbuch R., Bard M. Cloning and characterization of the Saccharomyces cerevisiae C-22 sterol desaturase gene, encoding a second cytochrome P-450 involved in ergosterol biosynthesis. Gene. 1996;1691(1):105–109. doi: 10.1016/0378-1119(95)00770-9. [DOI] [PubMed] [Google Scholar]
- Sugden C., Donaghy P. G., Halford N. G., Hardie D. G. Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol. 1999;1201(1):257–274. doi: 10.1104/pp.120.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda J. A., Weirich G. F. Sterol metabolism in the tobacco hornworm, Manduca sexta. Lipids. 1995;301(1):263–267. doi: 10.1007/BF02537831. [DOI] [PubMed] [Google Scholar]
- Taton M., Benveniste P., Rahier A. Comparative study of the inhibition of sterol biosynthesis in Rubus fruticosus suspension cultures and Zea mays seedlings by (N-(1,5,9-trimethyldecyl)-4α,10-dimethyl-8-aza-trans-decal-3β-ol and derivatives. Phytochemistry. 1987;261(1):385–392. [Google Scholar]
- Taton M., Benveniste P., Rahier A. Microsomal sterol Δ14-reductase in higher plants. Characterization and inhibition by analogues of a presumptive carbocationic intermediate of the reduction reaction. Eur. J. Biochem. 1989;1851(1):605–614. doi: 10.1111/j.1432-1033.1989.tb15156.x. [DOI] [PubMed] [Google Scholar]
- Taton M., Benveniste P., Rahier A., Johnson W. S., Liu H. T., Sudhakar A. R. Inhibition of 2,3-oxidosqualene cyclase. Biochemistry. 1992;311(1):7892–7898. doi: 10.1021/bi00149a021. [DOI] [PubMed] [Google Scholar]
- Taton M., Husselstein T., Benveniste P., Rahier A. Role of highly conserved residues in the reaction catalyzed by recombinant Δ7-sterol-C5(6)-desaturase studied by site-directed mutagenesis. Biochemistry. 2000;391(1):701–711. doi: 10.1021/bi991467t. [DOI] [PubMed] [Google Scholar]
- Taton M., Rahier A. Plant sterol biosynthesis : identification and characterization of higher plant Δ7-sterol-C5(6)-desaturase. Arch. Biochem. Biophys. 1996;3251(1):279–288. doi: 10.1006/abbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- Taton M., Rahier A. Properties and structural requirements for substrate specificity of cytochrome P450-dependent obtusifoliol 14α-demethylase from maize (Zea mays) seedlings. Biochem. J. 1991;2771(1):483. doi: 10.1042/bj2770483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai L., Rush J. S., Maul J. E., Devarenne T., Rodgers D. L., Chappell J., Waechter C. J. Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proc. Nat; Acad. Sci. 1999;961(1):13080–13085. doi: 10.1073/pnas.96.23.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskos J. M., Fischer R. T., Favata M. F. Mechanistic studies of lanosterol C-32 demethylation. J. Biol. Chem. 1986;2611(1):16937–16942. [PubMed] [Google Scholar]
- Tsay Y. H., Robinson G. W. Cloning and characterization of ERG8, an essential gene of Saccharomyces cerevisiae that encodes phosphomevalonate kinase. Mol. Cell. Biol. 1991;111(1):620–631. doi: 10.1128/mcb.11.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann P., Ury A., Rimmele D., Benveniste P., Bouvier-Navé P. UDP-glucose sterol-β-D-glucosyltransferase, a plasma membrane-bound enzyme of plants : enzymatic, properties and lipid dependence. Biochimie. 1993;751(1):713–723. doi: 10.1016/0300-9084(93)90102-x. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H., Janssen P. A. J. Target sites of sterol biosynthesis inhibitors : secondary activities on cytochrome P-450-dependent reactions. 1992;1(1):227–254. In Target sites of fungicide action, W. Köller, ed. (London: CRC Press), pp. [Google Scholar]
- Venkatramesh M., Guo D. A., Harman J. G., Nes W. D. Sterol specificity of the Saccharomyces cerevisiae ERG6 gene product expressed in Escherichia coli. Lipids. 1996;311(1):373–377. doi: 10.1007/BF02522922. [DOI] [PubMed] [Google Scholar]
- Vollack K. U., Dittrich B., Ferrer A., Boronat A., Bach T. J. Two radish genes for 3-hydroxy-3-methylglutaryl-CoA reductase isozymes complement mevalonate auxotrophy in a yeast mutant and yield membrane-bound active enzyme. J. Plant Physiol. 1994;1431(1):479–487. [Google Scholar]
- Warnecke D., Erdmann R., Fahl A., Hube B., Müller F., Zank T., Zähringer U., Heinz E. Cloning and functional expression of UGT genes encoding sterol glucosyl-transferases from Saccharomyces cerevisiae, Candida albicans, Pichia pastoris, and Dictyostelium discoideum. J. Biol. Chem. 1999;2741(1):13048–13059. doi: 10.1074/jbc.274.19.13048. [DOI] [PubMed] [Google Scholar]
- Warnecke D. C., Baltrusch M., Buck F., Wolter F. P., Heinz E. UDP-glucose:sterol glucosyltransferase : cloning and functional expression in Escherichia coli. Plant Mol. Biol. 1997;351(1):597–603. doi: 10.1023/a:1005806119807. [DOI] [PubMed] [Google Scholar]
- Warnecke D. C., Heinz E. Purification of a membrane-bound UDP-glucose:sterol β-D-glucosyltransferase based on its solubility in diethyl ether. Plant Physiol. 1994;1051(1):1067–1073. doi: 10.1104/pp.105.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham H. R., Koster J., Romeijn G. J., Hennekam R. C. M., Vreken P., Andersson H. C., FitzPatrick D. R., Kelley R. I., Wanders R. J. A. Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 2001;691(1):685–694. doi: 10.1086/323473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. S., Irving T. C., Steponkus P. L. Effects of plant sterols on the hydration and phase behavior of DOPE/DOPC mixtures. Biochim. Biophys. Acta. 1995;12391(1):226–238. doi: 10.1016/0005-2736(95)00147-u. [DOI] [PubMed] [Google Scholar]
- Wendt K. U., Lenhart A., Schulz G. E. The structure of the membrane protein squalene-hopene cyclase at 2.0 A resolution. J. Mol. Biol. 1999;2861(1):175–187. doi: 10.1006/jmbi.1998.2470. [DOI] [PubMed] [Google Scholar]
- Wendt K. U., Poralla K., Schulz G. E. Structure and function of a squalene cyclase. Science. 1997;2771(1):1811–1814. doi: 10.1126/science.277.5333.1811. [DOI] [PubMed] [Google Scholar]
- Wojciechowski Z. A. Biochemistry of phytosterol conjugates. 1991;1(1):361–395. In GW Patterson, WD Nes, eds, Physiology and Biochemistry of Sterols. American Oil Chemists' Society, Champaign, IL, pp. [Google Scholar]
- Wojciechowski Z. A., Goad L. J., Goodwin T. W. S-adenosyl-L-methionine-cycloartenol methyltransferase activity in cell-free systems from Trebouxia sp. and Scenedesmus obliquus. Biochem. J. 1973;1361(1):405–412. doi: 10.1042/bj1360405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. S. H., Moreau R. A., Whitaker B. D., Huang A. H. C. Steryl esters in the elaioplasts of the tapetum in developing Brassica anthers and their recovery on the pollen surface. Lipids. 1999;341(1):517–523. doi: 10.1007/s11745-999-0393-5. [DOI] [PubMed] [Google Scholar]
- Yabuzaki Y., Niohino T., Ariga N., Katsuki H. J. Biochem. 1979;851(1):1531–1544. doi: 10.1093/oxfordjournals.jbchem.a132483. [DOI] [PubMed] [Google Scholar]
- Yamaga N., Gaylor J. L. Characterization of the microsomal steroid-8-en isomerase of cholesterol biosynthesis. J. Lipid Res. 1978;191(1):375–382. [PubMed] [Google Scholar]
- Yamamoto S., Bloch K. J. Biol. Chem. 1970;2451(1):1670–1674. [PubMed] [Google Scholar]
- Yang H., Bard M., Bruner D. A., Gleeson A., Deckelbaum R. J., Aljinovic G., Pohl T. M., Rothstein R., Sturley S. L. Sterol esterification in yeast : a two-gene process. Science. 1996;2721(1):1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- Yang H., Cromley D., Wang H., Billheimer J. T., Sturley S. L. Functional expression of a cDNA to human acyl-coenzyme A-cholesterol acyltransferase in yeast. J. Biol. Chem. 1997;2721(1):3980–3985. doi: 10.1074/jbc.272.7.3980. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Yama N., Doka N. cDNA cloning of sesquiterpene cyclase and squalene synthase, and expression of the genes in potato tuber infected with Phytophthora infestans. Plant Cell. Physiol. 1999;401(1):993–998. doi: 10.1093/oxfordjournals.pcp.a029633. [DOI] [PubMed] [Google Scholar]
- Zimowski J., Wojciechowski Z. A. Partial purification and specificity of triacylglycerol sterol acyltransferase from Sinapis alba. Phytochemistry. 1981;201(1):1799–1803. [Google Scholar]


