Abstract
Studies of the processes leading to the construction of a bud and its separation from the mother cell in Saccharomyces cerevisiae have provided foundational paradigms for the mechanisms of polarity establishment, cytoskeletal organization, and cytokinesis. Here we review our current understanding of how these morphogenetic events occur and how they are controlled by the cell-cycle-regulatory cyclin-CDK system. In addition, defects in morphogenesis provide signals that feed back on the cyclin-CDK system, and we review what is known regarding regulation of cell-cycle progression in response to such defects, primarily acting through the kinase Swe1p. The bidirectional communication between morphogenesis and the cell cycle is crucial for successful proliferation, and its study has illuminated many elegant and often unexpected regulatory mechanisms. Despite considerable progress, however, many of the most puzzling mysteries in this field remain to be resolved.
IT has long been recognized that yeast cell shape is correlated with cell-cycle progression: indeed, arrest of proliferation with a uniform cell shape formed the basis of the landmark cdc screen of Hartwell et al. (1970). It follows that morphogenesis and the cell cycle are somehow coordinated, and numerous subsequent studies have established that the core cell-cycle machinery both regulates morphogenetic events and is in turn regulated by progression of (or defects in) cell morphogenesis. Here we review our imperfect understanding of this bidirectional communication.
Cell-Cycle Control of Morphogenesis
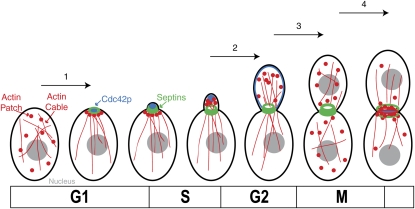
Early studies identified four major morphogenetic events of the cell cycle (Figure 1):
Polarization of the cytoskeleton and secretion in late G1, leading to bud emergence.
The apical-isotropic switch in early G2, a depolarization of growth within the bud leading to uniform bud expansion.
A breakdown of mother-bud asymmetry in growth, occurring in late mitosis. Before this, all growth is directed toward the bud; afterward it is evenly directed to both mother and bud.
Refocusing of growth toward the neck upon mitotic exit, leading to cytokinesis and cell separation.
Figure 1.
Morphogenetic events of the cell cycle. The four major morphogenetic events are (1) polarization in late G1, triggered by Cln1,2p-Cdc28p; (2) the apical-isotropic switch in early G2, triggered by Clb1,2p-Cdc28p; (3) breakdown of mother-bud asymmetry in late mitosis (trigger unknown); and (4) refocusing of growth toward the neck following mitotic exit, triggered by Clb-Cdc28p inactivation. Actin (red), septin (green), and Cdc42p (blue) localization during the cell cycle is indicated.
Events 1, 2, and 4 were associated with specific changes in the activity of the CDK Cdc28p (Lew and Reed 1993) (Figure 1); event 3 remains mysterious to this day.
Polarity establishment in G1
Bud emergence is dependent on G1 CDK activity and can be induced prematurely by premature CDK activation, indicating that CDK activation is the regulatory trigger for this event (Pringle and Hartwell 1981; Cross 1988; Nash et al. 1988; Richardson et al. 1989). There is considerable genetic redundancy in terms of specific cyclin requirements, but the major drivers for bud emergence appear to be the Cdc28p cyclins Cln1p and Cln2p, with some assistance from the Pho85p cyclins Pcl1p and Pcl2p (Measday et al. 1994; Moffat and Andrews 2004). To inform a discussion of how cyclinCDK complexes may promote bud emergence, we must first briefly summarize what is known regarding the molecular underpinnings of this process.
Events leading to bud emergence:
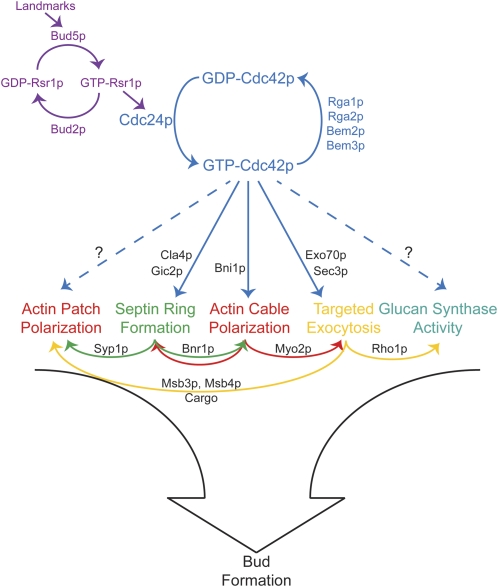
A series of seminal studies from John Pringle and colleagues (reviewed in Pringle et al. 1995) identified most of the key regulators of cell polarity in yeast and led to a hierarchical model for polarity establishment in which “bud-site selection” machinery recruits the master regulator Cdc42p, which then orients the cytoskeleton for bud growth (Figure 2).
Figure 2.
Polarity establishment. Bud-site selection (purple): prelocalized landmark proteins promote local GTP loading of Rsr1p, which recruits Cdc24p. In establishing polarity (blue), Cdc24p locally activates Cdc42p, employing positive feedback to generate and dynamically maintain a patch of highly concentrated GTP-Cdc42p at the cell cortex. During downstream events, localized GTP-Cdc42p employs various effectors to promote septin ring assembly (green), actin cable polarization (red), local exocytosis (yellow), and possibly also glucan synthesis and actin patch clustering. The downstream events also influence each other (only some of these links are shown) and together promote bud formation.
Bud-site selection:
At the top of the hierarchy is a set of “bud-site selection” proteins (reviewed in the YeastBook chapter by Bi and Park, in press). These define a machinery for properly placing and interpreting a set of guidepost or “landmark” proteins that are inherited by newborn cells at specific positions and influence subsequent bud placement. Many landmarks are integral plasma membrane proteins whose extracellular domains may interact with the cell wall to restrict their mobility, thereby preserving their initial localization (Halme et al. 1996; Roemer et al. 1996; Harkins et al. 2001; Kang et al. 2004a). The intracellular domains of the landmarks can interact with the GEF for the Ras-related Rsr1p GTPase (Kang et al. 2001, 2004b), and this is thought to result in localized accumulation of GTP-Rsr1p near the landmark. GTP-Rsr1p can interact with the Cdc42p-directed GEF, Cdc24p (Zheng et al. 1995), as well as with GDP-bound Cdc42p (Kozminski et al. 2003), connecting the bud-site selection landmarks to the next level of the hierarchy.
Polarization of Cdc42p:
At the next level (Figure 2) there is a set of “polarity establishment” proteins centered on the conserved Rho-family GTPase Cdc42p. Both Cdc42p and its GEF Cdc24p are absolutely required for polarized organization of the cytoskeleton and for bud emergence (Hartwell et al. 1974; Sloat et al. 1981; Adams and Pringle 1984; Adams et al. 1990). Cdc42p is concentrated in a patch at the presumptive bud site (Ziman et al. 1993; Richman et al. 2002) and then recruits and/or regulates a variety of “effector” proteins (Table 1) that bind specifically to GTP-Cdc42p and promote events in the next level of the hierarchy. It is universally assumed that localization of Cdc42p (and, in particular, GTP-Cdc42p) is critical to establish polarity, so the key question is: How does Cdc42p become localized to the presumptive bud site?
Table 1. CDK substrates with roles in morphogenesis.
| Protein | Function | CDK substratea | Phosphosite mutant phenotypeb | Reference | |
|---|---|---|---|---|---|
| Bud-site selection | Axl2p | Axial landmark | In vitro | Loog and Morgan (2005); Ubersax et al. (2003) | |
| Bud2p | GAP for Rsr1p | In vivo | Holt et al. (2009) | ||
| Bud3p | Axial bud-site selection | In vitro, in vivo | Holt et al. (2009); Ubersax et al. (2003) | ||
| Bud4p | Axial bud-site selection | In vitro, in vivo | Holt et al. (2009); Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Bud8p | Bipolar landmark | In vitro, in vivo | Holt et al. (2009); Ubersax et al. (2003) | ||
| Rax2p | Bipolar bud-site selection | In vitro | Ubersax et al. (2003) | ||
| Polarization of Cdc42p | Bem1p | Scaffold | In vitro | Vacuole fusion defects | Han et al. (2005); Loog and Morgan (2005); Ubersax et al. (2003) |
| Cdc24p | Cdc42p GEF | In vitro | None | Gulli et al. (2000); McCusker et al. (2007); Moffat and Andrews (2004); Wai et al. (2009) | |
| Bem2p | Cdc42p/Rho1p GAP | In vivo | Holt et al. (2009) | ||
| Bem3p | Cdc42p GAP | In vivo, in vitro | Polarity defect, toxic upon overexpression | Holt et al. (2009); Knaus et al. (2007); Loog and Morgan (2005); Ubersax et al. (2003) | |
| Rga1p | Cdc42p GAP | In vivo, in vitro | Holt et al. (2009); Ubersax et al. (2003) | ||
| Rga2p | Cdc42p GAP | In vivo, in vitro | Polarity defect, toxic upon overexpression | Holt et al. (2009); McCusker et al. (2007); Sopko et al. (2007); Ubersax et al. (2003) | |
| Cdc42p effectors | Boi1p | Secretion | In vitro, in vivo | Growth defect | Holt et al. (2009); McCusker et al. (2007); Ubersax et al. (2003) |
| Boi2p | Secretion | In vivo | Holt et al. (2009); McCusker et al. (2007) | ||
| Gic1p | Actin and septin organization | In vitro | Ubersax et al. (2003) | ||
| Gic2p | Actin and septin organization | In vivo | Holt et al. (2009); Jaquenoud et al. (1998) | ||
| Cla4p | p21-activated kinase (PAK) | In vivo | Holt et al. (2009) | ||
| Ste20p | p21-activated kinase (PAK) | In vitro, in vivo | None | Holt et al. (2009); Oda et al. (1999); Oehlen and Cross (1998); Wu et al. (1998) | |
| Bni1p | Formin | In vitro | Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Sec3p | Exocyst component | In vitro | Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Polarity regulators | Msb1p | Polarity | In vitro | Loog and Morgan (2005); Ubersax et al. (2003) | |
| Msb2p | Mucin | In vitro | Ubersax et al. (2003) | ||
| Actin regulators | Bnr1p | Formin | In vitro | Ubersax et al. (2003) | |
| Bud6p | Formin regulator | In vivo, in vitro | Holt et al. (2009); Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Spa2p | Formin regulator | In vivo, in vitro | Holt et al. (2009); Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Pea2p | Formin regulator | In vivo | Holt et al. (2009) | ||
| Septins and regulators | Cdc3p | Septin | In vivo, in vitro | Ring disassembly defect | Holt et al. (2009); Tang and Reed (2002) |
| Shs1p | Septin | In vivo, in vitro | Gin4p-binding defect | Dephoure et al. (2005); Egelhofer et al. (2008); Holt et al. (2009) | |
| Bni4p | Chitin synthase 3 and PP1 regulator | In vivo, in vitro | Reduced neck localization, toxic upon overexpression | Holt et al. (2009); Zou et al. (2009) | |
| Growth regulators | Dnf2p | Lipid flippase | In vitro | Loog and Morgan (2005); Ubersax et al. (2003) | |
| Sac7p | Rho1p GAP | In vitro, in vivo | Holt et al. (2009); Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Skg6p | Polarized growth | In vitro, in vivo | Holt et al. (2009); Loog and Morgan (2005); Ubersax et al. (2003) | ||
| Tos2p | Polarized growth | In vitro | Ubersax et al. (2003) | ||
| Cytokinesis | Chs2p | Chitin synthase 2 | In vitro, in vivo | ER retention defect | Holt et al. (2009); Loog and Morgan (2005); Teh et al. (2009); Ubersax et al. (2003) |
| Cyk3p | Chs2p regulator | In vitro | Ubersax et al. (2003) | ||
| Iqg1p | IQGAP | In vivo | Holt et al. (2009) | ||
| Rom2p | Rho1p GEF | In vitro, in vivo | Dephoure et al. (2005); Holt et al. (2009) | ||
| Tus1p | Rho1p GEF | In vitro, in vivo | Reduced Rho1p activation | Kono et al. (2008); Loog and Morgan (2005); Ubersax et al. (2003) | |
| Cell separation | Ace2p | Transcription factor | In vitro, in vivo | Nuclear localization | Holt et al. (2009); Loog and Morgan (2005); O’Conallain et al. (1999); Ubersax et al. (2003) |
| Cbk1p | NDR/LATS kinase | In vivo | Holt et al. (2009) | ||
| Mob2p | Cbk1p regulator | In vivo | Holt et al. (2009) | ||
| Kic1p | Cbk1p regulator | In vitro, in vivo | Holt et al. (2009); Ubersax et al. (2003) |
In vitro indicates that some CDK complex can phosphorylate the protein in vitro; in vivo indicates evidence for CDK-dependent phosphorylation of the protein in yeast cells.
If phosphorylation-site mutants of the protein have been made, the phenotype is reported; otherwise this column is left blank.
In principle, localization of Cdc42p could occur through interaction with a prelocalized anchoring structure such as a landmark protein. However, Cdc42p does not appear to interact with landmarks, and although localization studies have uncovered many examples of proteins that become localized through their interaction with GTP-Cdc42p, we know of no GTP-Cdc42p interactors that could act as anchors to localize Cdc42p itself. Moreover, polarization can occur at random sites presumed to lack prelocalized anchors (see Symmetry breaking). Thus, it is thought that Cdc42p can become clustered at a nascent polarization site and remain clustered, despite diffusion, without needing to be anchored to a stable structure. Fluorescence recovery after photobleaching (FRAP) experiments indicate that polarized GFP-Cdc42p exchanges in and out of the polarization site very quickly (t1/2 ∼4–5 s) (Wedlich-Soldner et al. 2004; Slaughter et al. 2009), arguing that the cluster of concentrated Cdc42p is very dynamic. How is such a dynamic cluster established and maintained?
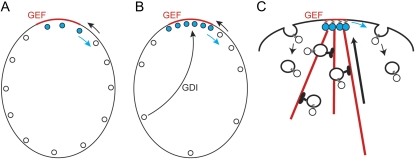
Localized GTP-Rsr1p in the vicinity of a landmark protein could recruit [and perhaps activate (Shimada et al. 2004)] the GEF Cdc24p from the cytoplasm, leading to local GTP loading of Cdc42p at the membrane. This would create a local patch of GTP-Cdc42p in a sea of GDP-Cdc42p at the plasma membrane. However, localized GEF activity would not, in itself, lead to local accumulation of GTP-Cdc42p to a concentration higher than that of the surrounding GDP-Cdc42p: inward diffusion of GDP-Cdc42p would provide a substrate for the GEF to generate more GTP-Cdc42p, but that would be balanced by outward diffusion of GTP-Cdc42p, so the overall Cdc42p concentration would not increase at the polarization site (Figure 3A). How, then, is the overall concentration of Cdc42p elevated at the presumptive bud site?
Figure 3.
Cdc42p localization. A localized GEF (red line along the cortex) can lead to local GTP loading of Cdc42p (blue circles). (A) Without further assistance, GDP-Cdc42p (open circles) diffusion into the patch is balanced by GTP-Cdc42p diffusion away from the patch, so the overall Cdc42p concentration is constant. (B) By reversibly extracting GDP-Cdc42p (and not GTP-Cdc42p) from the membrane, GDI selectively increases the mobility of GDP-Cdc42p, facilitating rapid GDP-Cdc42p diffusion through the cytoplasm into the GEF-containing patch. GTP-Cdc42p diffusion remains slow, so there is a net accumulation of Cdc42p at the cortex with high GEF activity (red). (C) Cdc42p could also become concentrated at the GEF-containing patch by vesicle traffic on actin cables. This model assumes that Cdc42p somehow becomes highly concentrated into the vesicles.
Cdc42p undergoes C-terminal prenylation that is critical for membrane association and function (Ziman et al. 1991, 1993). Yeast contain a single Rho-GDI homolog, Rdi1p, that can extract prenylated Cdc42p from the membrane (Masuda et al. 1994; Koch et al. 1997; Tcheperegine et al. 2005; Tiedje et al. 2008), and work on the human Cdc42p/GDI interaction suggests that GDI preferentially extracts GDP-bound (as opposed to GTP-bound) Cdc42p from membranes (Johnson et al. 2009). Because the cytoplasmic diffusion of Cdc42p-GDI complexes is expected to be fast and the yeast cell is small, the GDI could in principle “move” GDP-Cdc42p between outlying areas and the polarization site much faster than the rate at which GTP-Cdc42p diffuses at the plasma membrane (Figure 3B). Localized GEF activity would impart directionality to this process by locally converting the GDI-extractable (and therefore mobile) GDP-Cdc42p to the less extractable/mobile GTP-Cdc42p, causing accumulation of GTP-bound Cdc42p at the polarization site (Figure 3B). Mathematical modeling suggests that this mechanism would suffice to concentrate Cdc42p at the polarization site (Goryachev and Pokhilko 2008). Moreover, FRAP studies (Slaughter et al. 2009) indicate that GFP-Cdc42p exchange in and out of the polarization site is significantly slowed in rdi1Δ mutants (t1/2 ∼20 s vs. t1/2 ∼4–5 s in wild-type cells), supporting an important role for the GDI in concentrating the dynamic pool of Cdc42p.
As rdi1Δ mutants are viable (Masuda et al. 1994), and still manage to concentrate Cdc42p at the polarization site (Slaughter et al. 2009; Boulter et al. 2010), there must also be a GDI-independent route for concentrating Cdc42p. Some studies reported the presence of Cdc42p in cytoplasmic fractions even in rdi1Δ mutant cells (Koch et al. 1997; Tiedje et al. 2008), suggesting that there are other mechanisms that can extract prenylated Cdc42p from membranes. If such (currently undescribed) mechanisms were selective for GDP-Cdc42p, then (like the GDI) they too would promote Cdc42p concentration at the polarization site.
An alternative proposed mechanism for concentrating Cdc42p involves vesicular traffic (Marco et al. 2007; Slaughter et al. 2009). GTP-Cdc42p orients actin cables (see below), which deliver secretory vesicles. If GTP-Cdc42p were sufficiently concentrated on such vesicles, then vesicle-mediated Cdc42p delivery could promote concentration of Cdc42p at the polarization site. As the Cdc42p diffuses away, endocytosis could remove the Cdc42p from the plasma membrane and deliver it to endosomes, maintaining a dynamically polarized Cdc42p localization by vesicle-mediated recycling (Figure 3C). Treatment of cells with Latrunculin A to depolymerize actin and block vesicle recycling resulted in slightly slower FRAP recovery of GFP-Cdc42p at the polarization site (t1/2 ∼5–6 s vs. t1/2 ∼4–5 s in untreated cells), which has been interpreted as support for the idea that vesicle recycling assists Cdc42p polarization (Slaughter et al. 2009).
It is not known whether vesicles carry sufficient Cdc42p to enable polarization by the vesicle recycling mechanism. Mathematical modeling indicates that effective Cdc42p polarization by a vesicle recycling mechanism would require that the Cdc42p be actively endocytosed and that it diffuse very slowly in the plasma membrane (Layton et al. 2011). At present, there is no evidence for active internalization of Cdc42p, and current estimates of the Cdc42p diffusion constant (Marco et al. 2007) are an order of magnitude higher than the values required for the model to develop robust polarity (Layton et al. 2011), so the viability of Cdc42p vesicular recycling as a way to concentrate Cdc42p at the pre-bud site remains unclear.
Symmetry breaking:
The preceding discussion assumed that Cdc42p concentration was triggered by prior localization of its GEF to a site demarcated by a landmark protein. However, elimination of RSR1 randomizes the location of bud emergence (Bender and Pringle 1989), yet rsr1 cells still pick one and only one (randomly located) bud site with apparently normal timing and efficiency. This process is sometimes called “symmetry breaking.”
Symmetry-breaking behavior suggests that there is a positive feedback loop or amplification mechanism that allows a stochastic fluctuation in polarity factor concentration at some random site to promote accumulation of more polarity factors at that site (Turing 1952). Polarization of Cdc42p in rsr1Δ cells does not require polymerized actin or microtubules (Irazoqui et al. 2003), suggesting that Cdc42p symmetry breaking requires neither the upstream nor the downstream levels of the hierarchy and that the polarity establishment machinery itself contains a positive feedback loop.
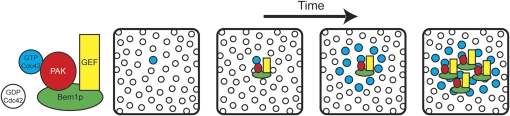
A proposed mechanism for the positive feedback is that stochastically arising GTP-Cdc42p can recruit the GEF Cdc24p to generate more GTP-Cdc42p in its vicinity, thereby growing a cluster of GTP-Cdc42p (Figure 4). This model was derived from the observation that polarization of rsr1Δ mutants requires the scaffold protein Bem1p (Irazoqui et al. 2003), which appears to function by bringing together the GEF Cdc24p and a p21-activated kinase (PAK)-family kinase (either Cla4p or Ste20p) (Kozubowski et al. 2008). The PAKs are effectors of Cdc42p: they bind to GTP-Cdc42p and that interaction relieves autoinhibition to activate the kinase (Bagrodia and Cerione 1999). Thus, GTP-Cdc42p at the membrane can (via PAK interaction) recruit a PAK-Bem1p-GEF complex that can then [via GEF activity, which may be stimulated by Bem1p interaction (Shimada et al. 2004)] convert neighboring GDP-Cdc42p to GTP-Cdc42p. This new GTP-Cdc42p can then recruit more GEF-containing complexes in a positive feedback loop (Figure 4). Support for this model comes from the striking observation that Bem1p function in symmetry breaking can be bypassed by introducing an artificial GEF-PAK fusion protein (Kozubowski et al. 2008).
Figure 4.
Model for symmetry breaking. Bem1p (green) mediates formation of a complex containing a PAK (red) and the Cdc42p-directed GEF (yellow). This complex enables GTP-Cdc42p at the plasma membrane (blue) to initiate positive feedback by binding the PAK, so that the associated GEF exchanges GDP for GTP on neighboring Cdc42p (GDP-Cdc42p: open circles). Thus, a stochastic GTP loading of Cdc42p can lead to amplification of a cluster of GTP-Cdc42p. Panels depict a patch of cortex as seen from inside the cell. Reprinted from Kozubowski, L., K. Saito, J. M. Johnson, A. S. Howell, T. R. Zyla et al., 2008 Symmetry-Breaking Polarization Driven by a Cdc42p GEF-PAK Complex, Curr. Biol. 18:22 1719–1726, with permission from Elsevier.
As discussed above for the Rsr1p-localized GEF, this Bem1p-mediated positive feedback loop could generate a local cluster of GTP-Cdc42p in a sea of GDP-Cdc42p, but other mechanisms would be needed to concentrate the GTP-Cdc42p to a level higher than that of the surrounding GDP-Cdc42p. Mathematical modeling suggests that, in combination with the GDI, Bem1p-mediated positive feedback would suffice to explain symmetry-breaking behavior (Goryachev and Pokhilko 2008). However, it is worth noting that the model works only within a limited parameter space, and we do not have sufficiently detailed knowledge of the relevant concentrations and rate constants in cells to know whether or not the parameter estimates are realistic.
Like the landmark Rsr1p pathway, the Bem1p positive feedback loop relies on localized GEF activity to concentrate GTP-Cdc42p. Thus, if Cdc42p were loaded with GTP in some other way (bypassing the GEF), these mechanisms would not be able to concentrate Cdc42p. Experimentally, this situation is approximated using the Cdc42pG12V or Cdc42pQ61L mutants, which bind to GTP upon initial folding and then cannot hydrolyze the GTP, so they remain GTP-bound and bypass the GEF. When endogenous Cdc42p was inactivated by a temperature-sensitive (ts) mutation and replaced by near-endogenous levels of Cdc42pQ61L, the cells failed to polarize (Irazoqui et al. 2003). Thus, GTP hydrolysis by Cdc42p appears to be essential for polarity establishment, consistent with the idea that localized GTP loading of Cdc42p by the GEF (which can occur only once the initially bound GTP is hydrolyzed) is needed to concentrate GTP-Cdc42p at the polarization site.
Unlike near-endogenous levels of Cdc42pQ61L, overexpression of Cdc42pQ61L or Cdc42pG12V does lead to concentration of the mutant protein, as well as clustering of cortical actin patches, at discrete sites (Gulli et al. 2000; Irazoqui et al. 2003; Wedlich-Soldner et al. 2003). Under these circumstances, Cdc42pQ61L polarization depends on F-actin and the type V myosin Myo2p responsible for vesicle delivery along actin cables (Wedlich-Soldner et al. 2003). On the basis of these findings, a proposed mechanism for symmetry breaking is that vesicle-mediated delivery of Cdc42pQ61L combined with Cdc42pQ61L-mediated orientation of actin cables constitutes a positive feedback loop for concentrating Cdc42pQ61L (Wedlich-Soldner et al. 2003).
Cdc42pQ61L polarization often produces more than one polarization site and results in cell death by lysis (Gulli et al. 2000; Wedlich-Soldner et al. 2003). These features raise the concern that this overexpression system is a pathological manifestation of cells attempting to cope with weak points in the cell wall, rather than an informative mimic of the normal polarization process.
In summary, polarity establishment involves the concentration of GTP-Cdc42p at the presumptive bud site on the plasma membrane. In wild-type cells, this is probably initiated by localized recruitment of the GEF Cdc24p by GTP-Rsr1p to a site defined by a previously deposited landmark protein. However, in the absence of Rsr1p, Cdc42p nevertheless becomes concentrated at an apparently random site. This symmetry breaking is presumably initiated by stochastic local fluctuations in polarity protein concentrations and subsequently amplified by positive feedback. A feedback loop involving a complex between Bem1p, Cdc24p, and a PAK that would generate a cluster of GTP-Cdc42p has been proposed (Figure 4). Two mechanisms (one mediated by the GDI Rdi1p and the other by vesicle recycling) that could then allow the concentration of GTP-Cdc42p in the cluster to rise above that of the surrounding GDP-Cdc42p have also been proposed (Figure 3), but their importance remains uncertain, and the existence of as-yet-uncharacterized mechanisms seems likely.
Polarization of the cytoskeleton and growth:
Once a polarization site with concentrated GTP-Cdc42p is established, actin cables are oriented toward the site, actin patches [which are sites of endocytosis (Kaksonen et al. 2003)] cluster around the site, a ring of septin filaments is assembled around the site, and exocytosis is targeted toward the site (Figure 2). Cell-wall glucan synthesis must also be activated at the polarization site, perhaps via localized activation of the glucan synthase regulator Rho1p (Abe et al. 2003). Targeted secretion, combined with localized cell-wall synthesis, then promotes bud emergence (Pruyne et al. 2004b).
To a significant degree, the downstream events initiated by Cdc42p are independent of each other: actin polarization can occur in the absence of organized septins (Adams and Pringle 1984), and septin rings can form in the absence of polymerized actin (Ayscough et al. 1997). Targeted secretion and even bud emergence can occur without septin rings (Hartwell 1971; Haarer and Pringle 1987) or actin cables (Sahin et al. 2008; Yamamoto et al. 2010). However, subsequent bud growth requires actin cables (Yamamoto et al. 2010), and proper shaping of the bud requires neck-localized septins (Gladfelter et al. 2005).
In mutants lacking actin cables, the small size and ovoid geometry of the unbudded yeast cell may enable bud emergence through chance encounters between secretory vesicles undergoing Brownian motion and the Cdc42p patch, which may promote local fusion via the exocyst (Figure 2). However, once the Cdc42p patch is separated from the bulk of the cell by a narrow bud neck, actin-mediated transport of vesicles through the neck would be needed to promote efficient secretory vesicle fusion at the bud tip. In addition, the septin collar at the neck somehow promotes expansion of the bud base so that it bulges out from the neck.
The ability of downstream events to occur independently suggests that Cdc42p is a master regulator of the micromanaging variety, separately promoting several parallel pathways required for harmonious bud growth. Supporting this view, specific cdc42 alleles have been isolated that impair targeted exocytosis without overt effects on actin or septins (Adamo et al. 2001), whereas other alleles impair septin organization without overt effects on actin or secretion (Gladfelter et al. 2002; Caviston et al. 2003). It is thought that different pathways are carried out by subsets of Cdc42p effectors (Table 1). Bni1p plays a prominent role in oriented actin cable assembly (Evangelista et al. 1997, 2002; Sagot et al. 2002). The PAKs (Longtine et al. 2000; Weiss et al. 2000; Gladfelter et al. 2004; Versele and Thorner 2004) and the Gic1p and Gic2p proteins (Iwase et al. 2006) aid in septin ring assembly. The exocyst components Sec3p and Exo70p (Zhang et al. 2001; Baek et al. 2010; Wu et al. 2010) and the scaffold proteins Boi1p and Boi2p (Adamo et al. 2001) promote targeted secretion. However, effectors are not restricted to one pathway (Gladfelter et al. 2001), and the detailed mechanisms by which the effectors operate remain largely unknown.
Although different Cdc42p outputs can occur individually when other outputs are blocked, there are also many interconnections among these downstream outputs. In some cases, direct mechanistic links have been identified: septin rings recruit the formin Bnr1p, which nucleates actin cable formation in mother cells (Pruyne et al. 2004a). Septins also recruit the endocytic actin patch initiator protein Syp1p, promoting patch clustering at the mother-bud neck (Qiu et al. 2008; Stimpson et al. 2009). In other cases, the evidence is less direct. Actin perturbations can impair septin ring assembly (Kadota et al. 2004; Kozubowski et al. 2005; Iwase et al. 2006), perhaps suggesting that some septin-organizing factors are delivered by actin cables. And perturbations of vesicle traffic can affect actin polarity (Gao et al. 2003) and the localization of Cdc42p (Wedlich-Soldner et al. 2004; Irazoqui et al. 2005; Zajac et al. 2005; Yamamoto et al. 2010), although the basis for these effects remains unclear.
CDK-mediated regulation of polarity establishment:
The above tour through polarity establishment indicates that CDK-mediated regulation of bud emergence could occur at multiple levels. A variety of fixed-cell synchrony experiments indicated that unpolarized cells become polarized ∼10–15 min before bud emergence (Haarer and Pringle 1987; Ford and Pringle 1991; Kim et al. 1991; Lew and Reed 1993; Ziman et al. 1993; Ayscough et al. 1997), and this timing was confirmed by live-cell filming (Howell et al. 2009). As G1 CDK activation (a.k.a. START) occurs ∼15–20 min before bud emergence (Lew and Reed 1993; Di Talia et al. 2007), these studies suggested that G1 CDK activity might promote concentration of Cdc42p (and the rest of the polarity establishment machinery) at the presumptive bud site. Below, we summarize the evidence for CDK involvement at different steps in polarity establishment and discuss possible mechanisms.
Not all studies on polarity establishment fit easily with the view that CDK triggers polarization. In particular, some studies suggested that the poorly understood protein Spa2p could polarize before G1 CDK activation (Snyder et al. 1991; Padmashree and Surana 2001). Moreover, in some strain backgrounds, cells arrested without (or with only a little) G1 CDK activity can polarize their growth and produce projections (Madden and Snyder 1992; Lew and Reed 1993). These findings suggest that cytoskeletal polarization is possible without (much) CDK input, although it does not lead to bud emergence.
One way to reconcile the apparently contradictory results on the role of G1 CDK in promoting polarization would be to posit that a small amount of CDK activity suffices to promote polarization of Cdc42p, actin, and secretion, but a larger amount of CDK activity is needed to promote both septin ring assembly and actual bud emergence. Thus, depending on the specific strain and CDK manipulation, CDK inhibition may block all polarization or only septin ring assembly and bud emergence.
Bud-site selection:
Before polarity establishment in G1, Rsr1p is localized all over the plasma membrane (Michelitch and Chant 1996; Park et al. 2002), while its GEF Bud5p is concentrated near the various landmark proteins (Kang et al. 2001; Marston et al. 2001) and its GAP Bud2p is delocalized (Park et al. 1999; Marston et al. 2001). This pattern suggests that GTP-Rsr1p would be concentrated near the landmarks. However, the Cdc42p-directed GEF Cdc24p, which directly binds to GTP-Rsr1p (Park et al. 1997), does not concentrate at that site in early G1. In diploids, Cdc24p is diffusely localized in the cytoplasm in early G1, whereas in haploids it is concentrated in the nucleus, due to interaction with Far1p, to prepare for potential mating (although if the haploid-specific FAR1 is deleted, then Cdc24p is diffusely localized in haploids as well) (Nern and Arkowitz 2000; Shimada et al. 2000). Activation of the G1 CDK promotes Cdc24p localization to the pre-bud site, even in cdc42 mutants where any feedback pathways would be inoperative (Gulli et al. 2000).
Perhaps the simplest way to interpret these observations is that CDK activation promotes GTP loading of Rsr1p by its prelocalized GEF, thereby enabling interaction of the localized GTP-Rsr1p with Cdc24p. Interestingly, in late G1, both Rsr1p and its regulators become concentrated at the polarization site (Park et al. 1999, 2002; Kang et al. 2001; Marston et al. 2001), consistent with the idea that they are somehow regulated by the CDK. However, that behavior could also reflect regulation of bud-site selection proteins downstream of Cdc42p localization. Bud2p is a putative CDK target (Holt et al. 2009), but the significance of that phosphorylation is untested.
An alternative interpretation of the localization data is that a localized pool of GTP-Rsr1p exists throughout G1, but that Cdc24p can bind to GTP-Rsr1p effectively only following CDK activation in late G1, either because some masking factor is removed or because phosphorylation of Cdc24p itself or a cofactor enhances its binding affinity for GTP-Rsr1p. Cdc24p is a CDK substrate in vitro (Moffat and Andrews 2004; McCusker et al. 2007), but mutation of 6 putative CDK target sites (Gulli et al. 2000) or up to 35 phosphorylation sites mapped by mass spectrometry (Wai et al. 2009) did not appear to affect Cdc24p localization or function. Thus, CDK activation probably promotes Rsr1p-Cdc24p interaction in vivo, leading to Cdc24p localization, but the relevant substrates and underlying mechanism remain unclear.
Polarization of Cdc42p:
Cells arrested in G1 due to lack of the G1 cyclins Cln1p-3p failed to polarize Cdc24p, Cdc42p, Bem1p, or the effectors Gic2p and Bni1p (Gulli et al. 2000; Jaquenoud and Peter 2000; Wedlich-Soldner et al. 2004). Induction of Cln2p in the arrested cells led to polarization of all of those factors, even in the absence of polymerized actin. These findings suggested that G1 CDK activity acts at the level of Cdc42p regulators to promote polarization (Cdc42p is not itself known to be phosphorylated). Such regulation could involve a change in Cdc42p-directed GEF or GAP activity leading to an increase in GTP-Cdc42p and triggering a localization feedback loop (Figure 4).
As mentioned above, the GEF Cdc24p is a CDK substrate in vitro, but as yet genetic analyses have not uncovered any role for that phosphorylation, so attention has turned to the Cdc42p-directed GAPs. The yeast genome encodes 11 proteins with Rho-GAP domains. Genetic analyses suggested that three of these (Bem3p, Rga1p, and Rga2p) might be Cdc42p-specific and that their GAP domains catalyze GTP hydrolysis by Cdc42p in vitro (Bender and Pringle 1991; Zheng et al. 1993, 1994; Stevenson et al. 1995; Chen et al. 1996; Gladfelter et al. 2002; Smith et al. 2002). A fourth Rho-GAP (Bem2p) with genetic links to Cdc42p was initially thought to be selective for Rho1p (Zheng et al. 1993), but was later shown to act on Cdc42p as well, at least in vitro (Marquitz et al. 2002). All of these GAPs are probably CDK substrates (Ubersax et al. 2003; Holt et al. 2009) (Table 1). Biochemical assays suggest that two other Rho-GAPs (Rgd2p and Lrg1p) may act on Cdc42p as well (Roumanie et al. 2001).
For Bem3p (Knaus et al. 2007) and Rga2p (Sopko et al. 2007), mutation of putative or mapped phosphorylation sites revealed that overexpression of nonphosphorylatable mutants is more toxic to cells than overexpression of the wild-type proteins. Toxicity was associated with accumulation of depolarized cells and was abolished by mutations impairing GAP activity, suggesting that high levels of nonphosphorylatable GAPs can block polarity establishment, perhaps because they are resistant to phosphorylation-mediated inhibition. This suggests the attractive hypothesis that high GAP activity keeps GTP-Cdc42p levels low in early G1 and that CDK activation promotes polarization by phosphorylating GAPs to reduce total GAP activity (Knaus et al. 2007; Sopko et al. 2007).
As yet, biochemical evidence that phosphorylation inhibits GAP activity is lacking. Moreover, combined deletion of BEM3 and RGA2 does not overtly accelerate polarization, so inhibition of these two GAPs is not sufficient to trigger polarization. Replacement of BEM3 or RGA2 with nonphosphorylatable versions expressed at endogenous levels does not overtly delay polarization, but it remains possible that parallel regulation of several GAPs triggers polarization or that combined regulation of both the GEF and the GAPs constitutes redundant pathways to promote polarization.
An alternative to GEF/GAP regulation is that CDK activation regulates the capacity for positive feedback. We do not know whether polarization is accompanied by a rise in GTP-Cdc42p levels within the cells or whether the GTP-Cdc42p is simply redistributed from a delocalized to a localized pool. In principle, enabling a localized positive feedback pathway would be sufficient to promote polarization even if GEF and GAP activities were unchanged. For example, CDK could promote assembly of the PAK-Bem1p-GEF complex to enable the positive feedback loop illustrated in Figure 4. Like Cdc24p, Bem1p and the PAKs Ste20p and Cla4p are CDK substrates, but mutation of putative or mapped phosphorylation sites has thus far failed to reveal any role for those phosphorylations in polarization (Oda et al. 1999; Ubersax et al. 2003; Han et al. 2005).
In summary, CDK activity is thought to promote Cdc42p polarization, and many polarity establishment proteins are probably direct CDK substrates (Enserink and Kolodner 2010), but genetic analysis has thus far failed to demonstrate the significance of those phosphorylations. Either the CDK acts in a complex and highly redundant manner or key substrates remain to be identified.
Polarization of the cytoskeleton and growth:
The Rho1p GTPase is not necessary for polarity establishment but is crucial for cell-wall biosynthesis and bud growth. GTP-Rho1p is concentrated at the polarization site (Abe et al. 2003) and activates the glucan synthases critical for new cell-wall deposition (Drgonova et al. 1996; Qadota et al. 1996), as well as several other effectors. In a very elegant study, Kono et al. (2008) showed that Rho1p GTP loading is cell-cycle-regulated, peaking at around the time of bud emergence. Rho1p activation results from Cln2p-CDK-mediated phosphorylation of the Rho1p-GEF Tus1p (Kono et al. 2008). Phosphorylation-site mutants of Tus1p abolished CDK-mediated accumulation of GTP-Rho1p, but the mutant cells nevertheless survived, implying that sufficient Rho1p function was still provided. One attractive possibility is that, in the mutant cells, the attempt to engage in polarized growth with insufficient glucan synthesis caused transient cell-wall defects detected by the “cell integrity pathway” (Levin 2005), which led to compensatory activation of the stress-responsive Rho1p-GEF Rom2p (Gray et al. 1997; Kono et al. 2008). The Kono et al. (2008) study provides the clearest instance of a downstream event directly regulated by the G1 CDK.
As mentioned above, in some strain backgrounds, cells arrested in G1 by Cln1p-3p depletion or cdc28-ts temperature shift do polarize their actin cytoskeleton and exhibit polarized growth to make projections (Madden and Snyder 1992; Lew and Reed 1993), although polarization may be delayed relative to wild-type controls. However, such cells do not assemble septin rings and they do not make buds. There is strong genetic evidence that Cln1p and Cln2p in particular are needed to promote proper septin ring assembly (Benton et al. 1993; Cvrckova et al. 1995; Gladfelter et al. 2005) and that some septins are direct CDK targets (Tang and Reed 2002; Egelhofer et al. 2008), although phosphosite mutants of individual septins did not have any obvious effect on septin ring assembly. In contrast to the inconclusive findings from S. cerevisiae, analogous work in the related Candida albicans provided strong evidence that CDK-mediated septin phosphorylation directly impacts septin organization and hyphal growth (Sinha et al. 2007; Gonzalez-Novo et al. 2008). Thus, it seems highly likely that CDKs directly regulate septin assembly as well as indirectly promote septin organization through Cdc42p polarization.
Is CDK-mediated septin regulation sufficient to explain why cdk-ts cells make projections rather than buds? As septins are dispensable for bud emergence, there may be other targets of the CDK that promote budding itself. However, the difference between projection formation and bud formation is subtle and morphological, and it has been shown that improperly organized septins can lead to the formation of aberrantly shaped “buds” that resemble projections (Gladfelter et al. 2005).
In summary, considerable evidence supports the hypothesis that G1 CDK triggers polarization of Cdc42p and other polarity establishment proteins. Additional evidence suggests further links between the CDK and downstream events, including Rho1p activation and septin organization. However, despite the identification of numerous CDK substrates with roles in polarity establishment (Table 1), we are not yet in a position to state that any given set of phosphorylations can explain any specific step in polarity establishment.
Apical-Isotropic Switch in G2
Following bud emergence, most growth and new cell-wall deposition is targeted to the tip of the bud, but at some point this “apical” growth mode switches to a uniform or “isotropic” mode of growth (Farkas et al. 1974; Lew and Reed 1993). After the apical-isotropic switch, growth is still directed toward the bud (and the mother cell does not grow significantly), but it is now distributed diffusely within the bud. The proteins that were highly polarized in late G1 (Cdc42p, etc.) remain polarized during apical growth but become distributed around much of the bud cortex after the switch. The apical-isotropic switch is dependent on G2 CDK activity (primarily Clb2p, assisted by Clb1p) and can be induced prematurely by Clb1p or Clb2p overexpression, suggesting that Clb1p,2p-CDK activation is the regulatory trigger for this event (Lew and Reed 1993). Compared to polarity establishment, much less research has gone into understanding the basis for this depolarizing switch, but at least three interesting ideas have been put forward for how it might be triggered.
Reversal of Cln-CDK-promoted polarization:
Clb1p,2p-CDK activity represses the transcription of a set of promoters that includes those for CLN1 and CLN2 (Amon et al. 1993). Thus, if polarity-promoting G1-CDK substrates need to be continuously phosphorylated and cannot be phosphorylated by G2-CDK, then the apical-isotropic switch could simply reflect the reversal of G1-CDK-targeted phosphorylations following G1 cyclin repression. In support of this idea, overexpression of CLN1 or CLN2 from the GAL1 promoter leads to prolonged apical growth in otherwise wild-type cells (Lew and Reed 1993). However, it is possible that the overexpressed G1 cyclins compete with endogenous Clb2p for access to the CDK and that the continued apical growth stems from absence of sufficient G2 CDK, rather than from the presence of sufficient G1 CDK. Consistent with that possibility, an intriguing study reported that the continued apical growth of cells overexpressing CLN1 was dependent on the G2-CDK inhibitor Swe1p (Ahn et al. 2001). Moreover, inactivation of temperature-sensitive cdc28 alleles in G2 leads to a return to apical growth (Lew and Reed 1993), which is difficult to explain if G1-Cdc28p activity is continuously required to promote such growth (especially as the same cdc28 alleles effectively block G1-CDK-induced budding). Thus, on balance it appears that G2 CDK activity does more than simply inactivate G1 CDK.
Lipid-mediated GAP activation:
The lipid composition of many eukaryotic plasma membranes is highly asymmetric, with phosphatidylserine (PS) and phosphatidylethanolamine (PE) enriched in the inner leaflet and phosphatidylcholine and sphingolipids enriched in the outer leaflet. Using a probe for PE in the outer leaflet, Saito et al. (2007) found that the probe was readily detectable at the polarization site during apical growth, but not detectable during isotropic growth. Moreover, lipid “flippases” thought to translocate PS and PE from the outer to the inner leaflet also displayed a polarized localization during apical growth, and mutations in the genes encoding the flippases led to persistent external PE staining, polarized Cdc42p, and continued apical growth at low temperatures, resulting in elongated buds (Saito et al. 2007). These findings suggested that lipid flipping at the bud tip might trigger the apical-isotropic switch.
Cells in which the apical-isotropic switch is impaired would be expected to display elongated buds, so this phenotype was consistent with the idea that lipid flipping might be important for triggering the apical-isotropic switch. However, a large majority of elongated-bud mutants turn out to affect the timing of Clb2p-CDK activation (generally via effects on a septin-dependent Swe1p-regulatory pathway, as discussed below) (Barral et al. 1999; Edgington et al. 1999; Longtine et al. 2000; Thomas et al. 2003), rather than affecting the apical-isotropic switch per se. Saito et al. (2007) circumvented this issue by focusing on cells that had already undergone nuclear division, and therefore must have activated Clb-CDK. However, this approach does not guarantee that the phenotype did not arise from insufficient active Clb2p-CDK, as in some cases partial CDK inhibition blocks the apical-isotropic switch without blocking nuclear division (Lew and Reed 1993).
If Clb2p-CDK does trigger the apical-isotropic switch by activating lipid flippases, then how would flipping lipids affect polarized growth? Using in vitro GAP assays, Saito et al. (2007) showed that Rga1p and Rga2p GAP activity could be stimulated by PS or PE. They suggested that Clb2p-CDK-stimulated flipping of PS and PE to the inner leaflet at the bud tip would activate these GAPs to clear the local GTP-Cdc42p, terminating polar growth. This is an intriguing hypothesis worthy of further investigation. But it cannot be the whole story because flippase mutants exhibit only a delayed apical-isotropic switch at low temperatures.
Dissociation of GEF-PAK complexes:
As discussed above (Figure 4), the GEF Cdc24p can form complexes with Bem1p and a PAK, and such complexes are important for polarity establishment. In these complexes the Cdc24p becomes heavily phosphorylated by the PAK (Gulli et al. 2000; Bose et al. 2001). On the basis of a variety of observations, Gulli et al. (2000) suggested that Cdc24p phosphorylation might cause it to dissociate from Bem1p, terminating polarized growth. This hypothesis does not address why the inhibitory effects of Cdc24p phosphorylation would be manifested only in G2 or how this pathway might be regulated by the G2 CDK. In addition, later studies found that phosphorylated Cdc24p could still bind to Bem1p (Bose et al. 2001) and that neither fusion of Bem1p to Cdc24p (to prevent their separation) (Kozubowski et al. 2008) nor mutation of 35 mapped phosphorylation sites on Cdc24p (which greatly reduced Cdc24p phosphorylation) (Wai et al. 2009) affected the apical-isotropic switch. However, the idea that GEF inhibition may be involved in triggering depolarization in G2 remains attractive, and although fusion of Bem1p to Cdc24p had no effect, fusion of Cla4p to Cdc24p did lead to the development of elongated buds (Kozubowski et al. 2008). Thus, it remains possible that the G2 CDK somehow disrupts the Cdc24p-Bem1p-Cla4p complex to trigger the apical-isotropic switch. As with the lipid flippase pathway above, this pathway (if it exists) can be only part of the story, as only 11% of cells containing the Cdc24p-Cla4p fusion exhibited elongated buds (Kozubowski et al. 2008).
In summary, it seems likely that the apical-isotropic switch is actively triggered by the G2 CDK and is not a passive consequence of diminished G1 CDK activity. Depolarization may involve regulated lipid translocation and GAP activation, disassembly of a GEF-containing complex, or both, leading to diminished GTP-Cdc42p levels. However, both of these hypotheses remain tentative, and other mechanisms may well be important.
Breakdown in mother-bud asymmetry
Even after the apical-isotropic switch, growth remains restricted to the bud for most of G2/M. This mother-bud asymmetry requires polymerized actin, myosin V (Karpova et al. 2000), and an intact septin collar at the mother-bud neck (Barral et al. 2000). The asymmetry is most easily visualized by looking at the distribution of cortical actin patches, which are abundant in the bud and almost absent in the mother (Adams and Pringle 1984; Amberg 1998). Actin patches represent sites of endocytosis at a late stage where the plasma membrane is in the process of invaginating (Kaksonen et al. 2006). Markers of an earlier step of endocytosis (Ede1p or clathrin) are not as highly asymmetric (Newpher et al. 2005; Stimpson et al. 2009), and it was recently suggested that endocytic patches wait until they fill up with cargo before they initiate actin polymerization and invagination (Layton et al. 2011). In buds, where directed secretion delivers many proteins (e.g., v-SNAREs) to the plasma membrane that subsequently become endocytic cargo, the clathrin patches fill with cargo rapidly and convert to actin patches; in mothers, where there is little secretion, the clathrin patches must wait much longer to collect sufficient cargo, so conversion to actin patches is rare (Layton et al. 2011). In this way, the actin patch distribution reflects the polarization of secretion.
For a brief time prior to cytokinesis, the actin-patch distribution becomes symmetric between mother and bud, presumably reflecting a breakdown in the mother-bud asymmetry of secretion described above. Cell-cycle arrest by DNA checkpoints or the spindle assembly checkpoint results in the accumulation of cells with actin patches distributed between mother and bud (Jacobs et al. 1988). Similarly, cells expressing nondegradable mitotic cyclins arrest with symmetrically distributed actin patches (Lew and Reed 1993). However, these treatments do not accelerate the switch to symmetric actin patches (Lew and Reed 1993), suggesting that the switch is not simply a response to some threshold level of CDK activity. Thus, the breakdown in mother-bud asymmetry is not clearly linked to a change in CDK activity, and the regulatory trigger for this morphogenetic event remains enigmatic.
Cytokinesis
In S. cerevisiae, cytokinesis occurs at the mother-bud neck (see the YeastBook chapter by Bi and Park, in press). Below we first briefly summarize the series of events leading to cell separation and then discuss what is known regarding how these events are regulated by the cell cycle.
Events leading to cell separation:
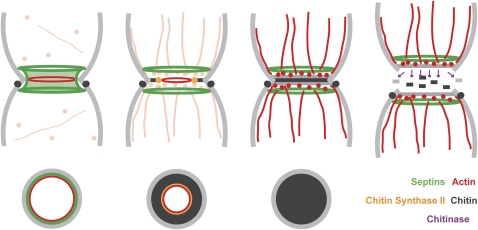
Cytokinesis involves the assembly and constriction of an actomyosin ring, which guides deposition of a chitinous primary septum, which is followed shortly by deposition of a glucan- and mannan-rich secondary septum on either side. The actual separation of mother and daughter involves the action of chitinase, which degrades the primary septum, as well as some glucanases. These processes are summarized in Figure 5.
Figure 5.
Cytokinesis and cell separation. Sequential panels showing (from left to right): (Left) In late anaphase, actin cables and patches are dispersed and an actomyosin ring (red) forms in the center of the neck, recruited to and maintained at that site by the septin collar (green). (Second from left) Upon CDK inactivation, the septin collar splits to form two rings, and the actomyosin ring constricts, guiding a chitin synthase and its regulators (yellow) to deposit a primary septum (black). At around this time the actin cables and patches reorient toward the neck. (Third from left) Upon completion of the primary septum, mother and bud deposit a secondary septum (gray) on either side. (Right) Daughter cells synthesize and secrete chitinase (purple arrows), which degrades the primary septum (black) and several glucanases, which presumably degrade the edge cell wall connecting mother and bud (gray), allowing cell separation. (Bottom) A cross section through the central plane. The mother cell also contains a ring of chitinous cell wall (black) surrounding the neck, which is synthesized in late G1/S phase as a bud first emerges by a distinct chitin synthase. This ring remains as a bud scar following cell separation.
Actomyosin-ring formation:
The actomyosin ring contains actin, the type II myosin heavy chain Myo1p, the IQGAP homolog Iqg1p, and light chains that bind Myo1p and Iqg1p. Localization of these proteins to the mother-bud neck relies on the septins, which form a collar tethering various proteins to that site (Epp and Chant 1997; Bi et al. 1998; Lippincott and Li 1998).
Myo1p is a classical two-headed non-muscle myosin with a long coiled-coil tail that has a pronounced kink region in which there are two independent “targeting domains” (Fang et al. 2010). One of these binds to the septin-binding protein Bni5p and targets Myo1p to the neck from late G1 until anaphase. This first targeting mechanism is largely dispensable for actomyosin-ring formation and may reflect earlier roles for Myo1p. The second targeting domain promotes neck localization in anaphase/telophase and largely suffices for actomyosin-ring formation and constriction (Fang et al. 2010).
Iqg1p contains a calponin-homology domain that interacts with F-actin, and several light-chain-binding IQ motifs (Epp and Chant 1997; Lippincott and Li 1998; Shannon and Li 1999). Iqg1p targeting to the neck requires the light chain Mlc1p (Boyne et al. 2000; Shannon and Li 2000; Luo et al. 2004). Iqg1p is synthesized during G2/M, becomes localized to the neck in anaphase, and is targeted for degradation by the anaphase-promoting complex (APC) ubiquitin ligase following cytokinesis to promote orderly disassembly of the constricted actomyosin ring (Ko et al. 2007; Tully et al. 2009).
Actin recruitment to the ring requires both Myo1p and Iqg1p, as well as one or the other of the formins Bni1p and Bnr1p (Bi et al. 1998; Lippincott and Li 1998; Vallen et al. 2000; Tolliday et al. 2002). It is thought that Rho1p-GTP activates the formins to produce the neck-ring actin filaments at this stage and that Rho1p and its GEF Tus1p are also targeted to the neck in anaphase (Tolliday et al. 2002; Yoshida et al. 2006, 2009).
Given the precedents from other systems, it was expected that the actomyosin ring would consist of actin filaments aligned and cross-linked by bipolar myosin filaments via interactions between actin and the myosin motor domains. Remarkably, however, the Myo1p motor domain is dispensable for actomyosin-ring formation, and even (largely) for its constriction (Lord et al. 2005; Fang et al. 2010). Thus, it appears that the Myo1p tail (which is not thought to bind actin) promotes actin recruitment indirectly, presumably by affecting Iqg1p interaction with actin (Fang et al. 2010).
Splitting of the septin collar:
Upon bud emergence, the initial septin ring spreads to form an hourglass-shaped collar at the neck, which persists until mitotic exit and then abruptly splits into two discrete rings (Kim et al. 1991; Lippincott et al. 2001). Ring splitting involves dramatic changes in septin organization and dynamics (Caviston et al. 2003; Dobbelaere et al. 2003; Vrabioiu and Mitchison 2006). It seems likely that ring splitting is necessary for the invagination of the cleavage furrow, but this has not been directly tested as no mutations are known that specifically block the process.
Cleavage-furrow ingression and primary-septum deposition:
Coincident with or immediately after septin-ring splitting, the actomyosin ring constricts and the cleavage furrow ingresses, centripetally depositing a primary septum composed of chitin in its wake (Figure 5).
The primary septum is deposited by chitin synthase 2 (Chs2p), an integral membrane protein that polymerizes chitin from the precursor UDP-N-acetyl-glucosamine and extrudes it through the plasma membrane. Chs2p is synthesized in G2/M and accumulates in the endoplasmic reticulum until mitotic exit, when it rapidly traverses the secretory pathway and is delivered to a ring of plasma membrane at the bud neck (Chuang and Schekman 1996; Zhang et al. 2006). Targeting of Chs2p depends on the septins, and in mutant cells where septins assemble in aberrant patches away from the neck, Chs2p is targeted to those patches and synthesizes chitin ectopically (Roh et al. 2002). Following primary-septum deposition, Chs2p is removed from the neck by endocytosis and transferred to the vacuole for degradation (Chuang and Schekman 1996).
Several proteins colocalize with Chs2p during primary-septum formation, including Hof1p, Cyk3p, and Inn1p (Lippincott and Li 1998; Korinek et al. 2000; Vallen et al. 2000; Sanchez-Diaz et al. 2008; Nishihama et al. 2009). These proteins interact with one another, and Inn1p and Cyk3p appear to activate Chs2p (Jendretzki et al. 2009; Nishihama et al. 2009; Meitinger et al. 2010).
The actomyosin ring constricts together with the cleavage furrow as the primary septum forms. Cells lacking the myosin motor domain constrict the ring a little more slowly (Lord et al. 2005; Fang et al. 2010), suggesting that myosin-mediated contractility normally contributes modestly to this process. Consistent with contractile activity, in cells that cannot form a primary septum (e.g., chs2 or inn1 mutants) the actomyosin ring appears to pull itself off the membrane and collapse to a dot on one side or disassemble asymmetrically (Verplank and Li 2005?; Nishihama et al. 2009). However, cleavage (although a bit slower) is largely normal in cells lacking the myosin motor domain, suggesting that the primary force for constriction derives from centripetal deposition of the rigid septum.
Mutant cells with impaired actomyosin rings often display misoriented, wavy, or branched primary septa, supporting the hypothesis that the main role of the actomyosin ring is to guide the primary septum so that it precisely bisects the neck (Fang et al. 2010; R. Nishihama and J. R. Pringle, personal communication). Interestingly, mutations that impair different aspects of actomyosin-ring formation have effects of quite different severity on the overall process of cytokinesis: lack of an actin ring leads to mild defects, lack of myosin to more severe defects, and lack of Iqg1p to a complete block in cytokinesis (although this can be overcome by extra Cyk3p or Inn1p) (Shannon and Li 1999; Nishihama et al. 2009; Fang et al. 2010). Thus, significant primary-septum guidance can be provided by Iqg1p and Myo1p in the absence of an actin ring.
Secondary-septum deposition:
Immediately after primary-septum completion, cells deposit secondary septa on each side of the chitin plate. The secondary septum is similar in composition to the bulk of the yeast cell wall and contains glucans (polymers of glucose) and mannan (a heterogeneous set of heavily glycosylated cell-wall proteins bearing abundant mannose sugars) (planned YeastBook chapter by Orlean and Strahl). As for cell-wall deposition during bud growth, secondary-septum deposition is thought to involve directed secretion and Rho1p-mediated activation of glucan synthases. Actin cables are oriented toward the neck, and actin patches cluster at the neck during this process. Cdc42p and many other polarity-establishment proteins are also concentrated at the neck during this process, but almost all temperature-sensitive cdc24 and cdc42 alleles complete cytokinesis and cell separation and arrest as unbudded cells in the next cell cycle at restrictive temperature (Adams et al. 1990; Adamo et al. 2001; D. J. Lew, unpublished results), suggesting that Cdc42p and Cdc24p are completely dispensable for cytokinesis. The mechanisms responsible for redirecting actin and vesicle traffic to the neck remain mysterious.
Secondary-septum formation normally begins only when the primary septum is complete, but can proceed in the absence of an actomyosin ring or a primary septum. In such cells, secondary-septum deposition is quite exuberant, filling the neck with large amounts of disorganized cell-wall material that can trap pockets of cytoplasm (Schmidt et al. 2002; Rancati et al. 2008; Nishihama et al. 2009). These observations suggest that the primary septum may initially restrict deposition of the secondary septum and subsequently guide that process to the correct location.
Cell separation:
Upon completion of primary- and secondary-septum formation, mother and daughter cells are connected by a trilaminar cell wall. Daughter cells then synthesize and secrete a chitinase, Cts1p, to degrade the primary septum (Kuranda and Robbins 1991) (see chapter by Weiss, in press). At least three glucanases, Dse2p, Dse4p, and Egt2p, are also made by daughters at this time (Colman-Lerner et al. 2001), presumably to enable degradation of the outer cell wall that attaches mother and daughter (Figure 5), allowing cell separation.
CDK-mediated regulation of cell separation:
During mitotic exit, APC-mediated degradation of cyclins inactivates the CDK. This process involves a signaling pathway called the mitotic-exit network (MEN), which is activated when the anaphase spindle elongates through the mother-bud neck and results in the release of the phosphatase Cdc14p from the nucleolus (Yeong et al. 2002). Cdc14p contributes to CDK inactivation and dephosphorylates many CDK substrates (Stegmeier and Amon 2004). When CDK inactivation is prevented using MEN pathway mutants or nondegradable cyclin mutants, the actomyosin ring forms but all other aspects of cytokinesis are blocked (Lew and Reed 1993; Corbett et al. 2006; Yoshida et al. 2006).
Interestingly, the terminal MEN kinase Mob1p-Dbf2p relocates to the mother-bud neck during cytokinesis, and this is apparently triggered by CDK inactivation (Frenz et al. 2000; Xu et al. 2000; Luca et al. 2001; Hwa Lim et al. 2003). Thus, individual cytokinetic events could be triggered by MEN activity itself, instead of being triggered by the ensuing CDK inactivation. As MEN activity is needed for CDK inactivation and CDK inactivation promotes MEN component localization, it is not a straightforward process to tease apart which of these processes is the specific trigger for a given event. Thus, the most incisive findings come from experiments in which strains are manipulated so that CDK inactivation is uncoupled from MEN activity. Below, we discuss what is known regarding the regulation of the specific events leading to cell separation.
Actomyosin-ring formation:
Recruitment of the ring component Iqg1p to the neck appears to be regulated simply by Iqg1p abundance because overexpression of Iqg1p leads to premature neck localization of Iqg1p (Epp and Chant 1997). Interestingly, premature Iqg1p localization is often accompanied by premature actin ring formation (Epp and Chant 1997), suggesting that Iqg1p suffices for some level of actin-ring assembly.
Another pathway important for actin-ring formation is mediated by the Polo-family kinase Cdc5p (Yoshida et al. 2006). Like Iqg1p, Cdc5p accumulates in G2/M due to regulated transcription and is degraded following mitotic exit by the APC (Shirayama et al. 1998). Cdc5p phosphorylates the Rho1p GEFs Tus1p and Rom2p (after priming phosphorylations at CDK target sites), and mutations that reduce Cdc5p-mediated phosphorylation impair actin-ring formation, whereas phosphomimetic mutations at some Cdc5p target sites on Tus1p can partially bypass the actin-ring defect in cdc5 mutants (Yoshida et al. 2006). Phosphorylation of Tus1p appears to promote its localization to the neck, where it assists in Rho1p recruitment and GTP loading. Rho1p GTP loading spikes at around the time of cytokinesis (Kono et al. 2008), and the Rho1p-GTP is thought to promote actin-ring formation by stimulating formin-mediated actin polymerization at the neck (Tolliday et al. 2002; Yoshida et al. 2006).
Splitting of the septin collar:
Splitting of the septin collar is blocked by inactivation of the upstream MEN pathway regulator Tem1p (a GTPase), even when other mutations allow Cdc14p release, CDK inactivation, and mitotic exit (Lippincott et al. 2001). Inactivation of the downstream MEN pathway kinase Dbf2p also blocks splitting of the septin collar, but in this context CDK inactivation can trigger septin splitting (Meitinger et al. 2010). Thus, it appears that a combination of CDK inactivation and MEN components upstream of Dbf2p triggers this event, although the mechanism remains unknown.
Cleavage-furrow ingression and primary-septum deposition
Traffic of Chs2p from the ER to the plasma membrane requires CDK inactivation and can be triggered by CDK inactivation even in the absence of MEN activity (in cdc15 mutants) (Zhang et al. 2006). However, neck targeting of Chs2p following release from the ER is not as robust in MEN pathway mutants (Meitinger et al. 2010). Chs2p is a CDK substrate (Loog and Morgan 2005; Holt et al. 2009), and phosphomimetic mutations in consensus CDK target sites block Chs2p ER exit, whereas nonphosphorylatable mutants permit Chs2p ER exit regardless of CDK status (Teh et al. 2009). Thus, CDK-mediated Chs2p phosphorylation blocks Chs2p exit from the ER, and CDK inactivation relieves that block, allowing Chs2p delivery to the neck.
Despite some Chs2p localization, inactivation of MEN components impairs furrow ingression even when CDK inactivation is triggered (Lippincott et al. 2001; Luca et al. 2001; Meitinger et al. 2010). Localization of the Chs2p activators Inn1p and Cyk3p to the neck is MEN-regulated, and Inn1p [as well as its binding partner Hof1p (Vallen et al. 2000; Blondel et al. 2005; Corbett et al. 2006)] undergoes MEN-dependent phosphorylation (Nishihama et al. 2009). However, it is unclear which MEN components are responsible for regulating furrow ingression, and the functional significance of MEN-stimulated phosphorylations of Chs2p regulators has not yet been tested. In summary, it seems likely that MEN-mediated phosphorylations of Chs2p regulators (and perhaps of Chs2p itself) trigger furrow ingression, once CDK inactivation has enabled Chs2p exit from the ER and delivery to the neck.
Secondary-septum deposition:
Redirection of the actin cytoskeleton (Lew and Reed 1993) and secretory pathway (Verplank and Li 2005) to the neck requires CDK inactivation. CDK inactivation can apparently trigger relocation of the exocyst component Sec3p to the neck even when MEN activity is blocked (Verplank and Li 2005), suggesting that MEN pathway activity impacts this process primarily by aiding in CDK inactivation. How CDK inactivation promotes redirection of actin and secretion to the neck remains unknown.
Cell separation:
Synthesis of chitinase and glucanases is directed by a daughter-specific transcription program that is initiated by concentration of the transcription factor Ace2p into the bud-localized nucleus immediately after nuclear division. Asymmetric Ace2p distribution is controlled by the kinase Cbk1p, which itself is regulated by the “RAM” network, and the phosphatase Cdc14p, activated by the MEN (Weiss et al. 2002; Nelson et al. 2003; Brace et al. 2011) (see the YeastBook chapter by Weiss, in press).
In summary, many aspects of cytokinesis are triggered by CDK inactivation or MEN pathway activity, but, although candidate CDK and MEN substrates exist, the detailed mechanisms have not yet been elucidated.
Control of Cdc28p by the Morphogenesis Checkpoint
Successful progression through the cell cycle requires that certain events be executed in a specific order. For example, chromosomal DNA must be replicated before the chromosomes can be segregated, and chromosomes must be segregated before the cell divides. In the normal course of events, these processes are triggered in the proper order by the sequential activation and inactivation of cyclin-CDK complexes. However, stochastic or environmental factors can occasionally derail a key process, potentially throwing off the correct order of events. Checkpoint controls are surveillance pathways that can detect such problems and restore order by delaying subsequent cell-cycle progression (Hartwell and Weinert 1989).
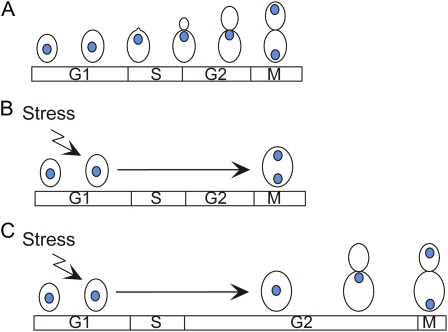
In budding yeast, the morphogenesis checkpoint delays nuclear division until a bud has been formed (reviewed in Lew 2003; Keaton and Lew 2006) (Figure 6). The existence of this checkpoint was first suggested by the observation that environmental stresses, genetic manipulations, or drug treatments that delayed bud formation also caused a delay in nuclear division (Lew and Reed 1995; McMillan et al. 1998). The delay in nuclear division was dependent on the CDK-inhibitory kinase Swe1p (Sia et al. 1996). Swe1p is homologous to Wee1-family kinases in other organisms and phosphorylates tyrosine 19 of Cdc28p (Booher et al. 1993). Below we summarize what has been learned regarding Swe1p action and its regulation during the unperturbed cell cycle and then address the question of what processes are monitored by the checkpoint and how that sensing takes place.
Figure 6.
The morphogenesis checkpoint. (A) During an unperturbed cell cycle, bud formation is coincident with DNA replication, and by the time of nuclear division, a bud is ready to receive the daughter nucleus. (B) Stresses can temporarily halt bud formation, and if the cell cycle continued unabated, cells would become binucleate. (C) In reality, delays in bud formation trigger compensatory G2 delays in the cell cycle through the morphogenesis checkpoint. Reprinted from Lew, D.J., 2003 The morphogenesis checkpoint: How yeast cells watch their figures, Curr. Opin. Cell Biol., 15:6 648–653, with permission from Elsevier.
Regulation of Cdc28p tyrosine phosphorylation during the cell cycle
Cdc28p phosphorylation in unperturbed cells:
Given the precedent from Schizosaccharomyces pombe, where Cdc2 tyrosine 15 phosphorylation inhibits the mitotic CDK and enforces a long G2 delay in every cycle, it was quite a surprise when early studies indicated that CDK tyrosine phosphorylation had no discernible effect on the S. cerevisiae cell cycle, even in the face of treatments that triggered arrest via the DNA replication or spindle assembly checkpoints (Amon et al. 1992; Sorger and Murray 1992). Some (Lim et al. 1996; Harvey and Kellogg 2003; Rahal and Amon 2008), but not all (McNulty and Lew 2005), subsequent studies found that Swe1p did have a small effect on the timing of spindle assembly. Why is the effect of Swe1p so minor?
Cdc28p tyrosine phosphorylation occurs only in G2, although Swe1p is synthesized during late G1 as part of a large set of periodically expressed genes (Lim et al. 1996; Sia et al. 1996). However, at that time, the predominant G1 CDK (Cln1-3p-Cdc28p) complexes are not recognized by Swe1p (Booher et al. 1993). Later Clb-CDK complexes are all Swe1p substrates, but the S-phase Clb5p-Cdc28p complexes are poorer substrates than the M-phase Clb2p-Cdc28p complexes and are initially protected from phosphorylation by binding of the CDK inhibitor Sic1p (Keaton et al. 2007). Even once Sic1p is degraded, Cdc28p tyrosine phosphorylation does not accumulate because S-phase CDK complexes are excellent substrates of the Cdc25-related phosphatase Mih1p (Keaton et al. 2007), which is present throughout the cell cycle (Keaton et al. 2008; Pal et al. 2008). These features account for the lack of Cdc28p phosphorylation in S phase even though Swe1p is abundant at that time.
In G2, cells no longer make Swe1p and begin to degrade it (Sia et al. 1998), so Swe1p abundance decreases as the mitotic Clb2p-Cdc28p complexes [which are excellent Swe1p substrates (Keaton et al. 2007)] accumulate. The combination of Swe1p degradation and Mih1p-mediated dephosphorylation of Cdc28p explains why Swe1p does not greatly delay the cell cycle.
Swe1p degradation during the unperturbed cell cycle:
Swe1p degradation is cell-cycle-regulated in unstressed cells. In early G1, any residual Swe1p left over from the previous cycle is degraded slowly [t1/2 ∼90 min (Sia et al. 1998)], probably via ubiquitination by the APC (Thornton and Toczyski 2003). In G2/M, Swe1p is degraded more rapidly (t1/2 ∼14 min) in a manner that requires both Clb1p,2p-Cdc28p (Sia et al. 1998) and the Polo-family kinase Cdc5p (Sakchaisri et al. 2004). Both of these kinases phosphorylate Swe1p at multiple sites, and mutation of 18 Cdc28p target sites (Harvey et al. 2005) or up to 20 Cdc5p target sites (Sakchaisri et al. 2004) significantly retards Swe1p degradation. Phosphorylation by Cdc28p primes Swe1p for subsequent phosphorylation by Cdc5p (Asano et al. 2005). The ubiquitin ligase responsible for Swe1p degradation was initially identified as SCFMet30 (Kaiser et al. 1998), although subsequent studies indicated that Met30p was not required for Swe1p degradation in a strain lacking Met4p (a transcription factor also targeted by SCFMet30) (McMillan et al. 2002). This finding indicates that Swe1p can be degraded by other pathways, but it remains possible that in wild-type cells SCFMet30 is a major contributor. In mammalian cells, Wee1 degradation involves sequential Wee1 phosphorylation by cyclin B-CDK1 and by the Polo-family kinase Plk1, and these phosphorylations generate a phosphodegron recognized by the SCFMet30 homolog SCFβTrCP (Watanabe et al. 2004, 2005). Thus, it is attractive to speculate (although it has yet to be proved) that the multisite phosphorylation of Swe1p in yeast similarly creates phosphodegrons recognized by SCFMet30 or another ubiquitin ligase.
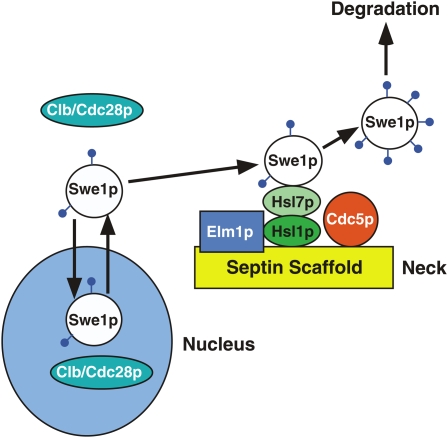
Swe1p degradation is coupled to localization at the mother-bud neck:
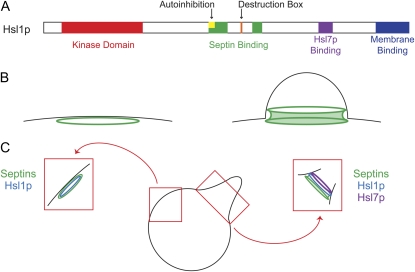
Swe1p degradation is exquisitely regulated by subcellular localization (Figure 7). Swe1p shuttles in and out of the nucleus, and nuclear export is required for effective Swe1p degradation in G2/M (Keaton et al. 2008). Having exited the nucleus, Swe1p accumulates at the bud side of the mother-bud neck (Longtine et al. 2000). Neck targeting requires interaction of Swe1p with Hsl7p, which is also concentrated at the neck (McMillan et al. 1999; Shulewitz et al. 1999; Longtine et al. 2000). Hsl7p is a protein methyltransferase, although that activity appears to be dispensable for Swe1p regulation (Theesfeld et al. 2003). Hsl7p itself is targeted to the neck by interaction with Hsl1p, a neck-localized protein kinase (Barral et al. 1999; Shulewitz et al. 1999; Longtine et al. 2000). Small mutations that abrogate the direct interactions between Hsl1p and Hsl7p (Cid et al. 2001) or Hsl7p and Swe1p (McMillan et al. 2002) prevent Swe1p neck targeting and also block Swe1p degradation, suggesting that neck localization is critical for Swe1p degradation.
Figure 7.
Swe1p degradation pathway. Swe1p shuttles in and out of the nucleus and can be recruited to the mother-bud neck by a hierarchy of interactions involving septins, Hsl1p, and Hsl7p. Hsl1p is activated by Elm1p, another neck-localized kinase. At the neck, Swe1p is phosphorylated at multiple sites by Cdc5p, which is thought to target Swe1p for degradation. Phosphorylation of Swe1p by Clb-Cdc28p (which may occur in the nucleus, in the cytoplasm, or at the neck) primes Swe1p for subsequent phosphorylation by Cdc5p.
Multisite phosphorylation of Swe1p is rapidly reversed upon Cdc28p inhibition (Harvey et al. 2005). Thus, there appear to be very active (although currently uncharacterized) Swe1p-directed phosphatases that would presumably antagonize Swe1p degradation. Like Swe1p, the Clb2p-Cdc28p complex (Bailly et al. 2003) and the Cdc5p kinase (Sakchaisri et al. 2004) are also concentrated at the mother-bud neck. It is attractive to speculate that neck localization serves to co-concentrate Swe1p with the kinases that target it for degradation, thereby overcoming the barrier provided by Swe1p-directed phosphatases. This hypothesis remains to be rigorously tested.
Effect of Swe1p phosphorylation on its activity:
In addition to slowing Swe1p degradation, mutation of 18 CDK consensus target sites on Swe1p generated a protein with significantly reduced CDK-inhibitory activity (Harvey et al. 2005). The simplest interpretation of this result is that Cdc28p-mediated Swe1p phosphorylation activates Swe1p to inhibit Clb-Cdc28p. This would constitute a negative feedback loop whereby Cdc28p promotes its own inhibition. As Cdc28p-mediated Swe1p phosphorylation also targets Swe1p for degradation (a double-negative feedback loop with the same consequence as a positive feedback loop in Cdc28p activation), the combined feedbacks would create a rather confusing scenario.
In the well-studied Xenopus egg extract system, it is clear that CDK-mediated Wee1 phosphorylation inhibits Wee1 (rather than activates it) (Dunphy 1994). Analysis of Wee1 phosphorylation-site mutants indicated that multisite phosphorylation targeted two inhibitory sites and at least three “decoy” sites (Kim et al. 2005; Kim and Ferrell 2007). Phosphorylation of the decoy sites, which were preferentially targeted by the CDK, did not affect Wee1 activity. Rather, the decoys delayed phosphorylation of the inhibitory sites. These findings suggested that, when there is little CDK activity, Wee1 undergoes repeated phosphorylation and dephosphorylation at decoy sites and that the inhibitory sites are phosphorylated only when there is high CDK activity. This arrangement is thought to introduce ultrasensitivity to Wee1 regulation by the CDK (Kim and Ferrell 2007). Conceivably, Swe1p phosphorylation may involve a large number of decoy sites; in that case, mutational removal of the decoys may enhance the targeting of less-preferred inhibitory sites, resulting in less active Swe1p (as observed for the 18-site mutant).
Distinguishing between the different hypotheses on the role of Swe1p phosphorylation may not be trivial: Swe1p is phosphorylated at many nonconsensus sites (Harvey et al. 2005), and phosphosite mutants carry the risk of altering Swe1p activity for reasons unrelated to phosphorylation.
In the case of Cdc5p-targeted Swe1p phosphorylation, the nonphosphorylatable mutants enhance the potency of Swe1p, as expected for mutants that increase Swe1p abundance (Sakchaisri et al. 2004). Because there is not a great correlation between the abundance and potency of mutants affecting different clusters of target sites, it may be that some phosphorylations act to inhibit Swe1p whereas others target Swe1p for degradation. Analysis of a mathematical model for the morphogenesis checkpoint (Ciliberto et al. 2003) suggested that for a robust checkpoint it would be useful to inhibit Swe1p (a rapid event) prior to its degradation (a slower event). The possibility that a subset of Swe1p phosphorylations (catalyzed by either Cdc28p or Cdc5p) inhibits Swe1p activity merits further investigation.
In addition to the kinases discussed above, the PAK Cla4p can phosphorylate Swe1p in vitro (Sakchaisri et al. 2004). Because of overlapping site specificity, it has been difficult to discern the role of that phosphorylation in vivo.
Regulation of Mih1p:
A variety of studies on Cdc25 phosphatases in other systems have revealed complex regulation of Cdc25 abundance, nuclear localization, and activity (Perry and Kornbluth 2007; Lindqvist et al. 2009). In contrast, we know very little about how the Cdc25 homolog Mih1p is regulated. Mih1p abundance does not vary through the cell cycle, but Mih1p phosphorylation undergoes dramatic changes, with hyperphosphorylation predominating in interphase and dephosphorylation accompanying mitosis (Pal et al. 2008). Mih1p appears to be mostly cytoplasmic (although not detectably excluded from the nucleus) for almost the entire cell cycle, except for a brief interval during mitotic exit when it becomes heavily concentrated in the nucleus (Keaton et al. 2008). These behaviors differ markedly from what has been seen in other systems, and their significance remains to be determined.
Regulation of Cdc28p tyrosine phosphorylation in response to stress
Many perturbations lead to Swe1p-dependent delays in nuclear division. External stresses shown to act in this manner include hyperosmotic shock (Lew and Reed 1995; Sia et al. 1998; Alexander et al. 2001), exposure to high concentrations of ethanol (Kubota et al. 2004), nutrient depletion (Uesono et al. 2004), and even physical constraint of cells in microfabricated chambers (Suzuki et al. 2004). In all of these cases, the stresses lead to depolarization of the actin cytoskeleton. Mutations impairing actin organization also trigger Swe1p-dependent delays, as does treatment with Latrunculin A or B to cause actin depolymerization (McMillan et al. 1998).
Mutations affecting septin organization also lead to Swe1p-mediated cell-cycle delays (Barral et al. 1999; Longtine et al. 2000). In these cases, the actin cytoskeleton is unaffected, and the most obvious consequence of Swe1p activity is a delay in the apical-isotropic switch, leading to the development of elongated buds.
In some strains, agents that slow DNA replication (e.g., hydroxyurea) also promote Swe1p-dependent bud elongation (Jiang and Kang 2003; Liu and Wang 2006), perhaps suggesting that replication stress activates Swe1p. However, in other strain backgrounds it appears that DNA checkpoint proteins, including Rad53p, prevent Swe1p from causing bud elongation in response to replication stress (Enserink et al. 2006). The role of Swe1p, if any, following replication stress remains mysterious.
Swe1p is stabilized upon disruption of the actin cytoskeleton:
Pulse-chase analysis demonstrated that Swe1p becomes dramatically more stable in mutants that abolish polarity establishment (cdc24) or impair actin cables (tpm1) (Sia et al. 1998). Swe1p is also stabilized following hyperosmotic shock (Sia et al. 1998), which causes rapid actin cable disruption (Chowdhury et al. 1992). Stabilization of Swe1p leads to a delay in Clb2p-Cdc28p activation, which in turn delays the transcriptional repression of SWE1 in a feedback loop that enhances Swe1p accumulation (Sia et al. 1996, 1998). Although Swe1p degradation has not been examined in similar detail following other perturbations, it seems likely that stress-induced stabilization and accumulation of Swe1p also occur in those cases [particularly following septin perturbations, which would disrupt the Swe1p degradation pathway (Figure 7)].
Parallel regulatory pathways combine with Swe1p stabilization to delay nuclear division:
Swe1p stabilization is not sufficient, on its own, to explain stress-induced cell-cycle delays. Deletion of HSL1 or HSL7 (McMillan et al. 1999) or mutation of Swe1p degradation motifs (McMillan et al. 2002) stabilizes Swe1p but leads to only a minimal cell-cycle delay. In contrast, nuclear division is delayed by ∼45 min in septin mutants (Barral et al. 1999), by ∼2 h in cdc24 mutants (Lew and Reed 1995), and by at least 12 h upon treatment with high doses of Latrunculin (McMillan et al. 1998). These findings indicate that additional pathways (possibly stress-specific) must contribute to the cell-cycle arrest.
Treatment of cells with Latrunculin leads to activation of the “cell integrity” MAPK pathway culminating in Slt2p phosphorylation (Harrison et al. 2001). Slt2p is also activated in response to mutations that impair septin organization (R. Nishihama and J. R. Pringle, personal communication). Swe1p-mediated cell-cycle arrest in response to Latrunculin requires Slt2p and its upstream kinases, although not the transcription factors known to act downstream of Slt2p. Slt2p acts in parallel with Swe1p stabilization, and the requirement for Slt2p can be bypassed by deleting Mih1p (Harrison et al. 2001). These findings suggest that stress-induced Slt2p activity may inhibit Mih1p, giving the stabilized Swe1p unopposed access to Cdc28p.
Swe1p stabilization and full Mih1p inhibition together would suffice to arrest the cell cycle for a long time (McMillan et al. 1999). Nevertheless, at least in the case of Latrunculin, an additional pathway involving the GAP Bem2p is required for arrest (Marquitz et al. 2002). Surprisingly, Bem2p is not required for either Swe1p stabilization or Slt2p activation, yet cell-cycle arrest is ineffective in bem2 mutants. The GAP activity of Bem2p appears to be dispensable for checkpoint arrest (Marquitz et al. 2002), and Bem2p’s mode of action remains completely mysterious.
In summary, many stresses cause stabilization and accumulation of Swe1p, but this alone has little effect on the cell cycle because Mih1p counteracts Swe1p action. In at least some cases, stresses also activate the MAPK Slt2p, which probably inhibits Mih1p. These studies suggest that the delay caused by a given perturbation is due to a combination of at least two pathways, which may themselves be responsive to distinct defects. A third pathway involving Bem2p is also required in some cases. Thus, the checkpoint may be a “coincidence detector,” calibrating the delay that it produces to a combination of stimuli that separately stabilize Swe1p, inhibit Mih1p, and/or regulate Bem2p.
What does the morphogenesis checkpoint monitor?
If checkpoint controls are surveillance pathways that evolved to protect cells from certain types of chance errors or stress-induced mistakes that can derail cell-cycle progress, then what are the errors/mistakes monitored by the morphogenesis checkpoint?
To be useful (i.e., evolutionarily adaptive), a checkpoint must detect errors that occur in the natural environment of yeast, and the delay provided by the checkpoint must be beneficial (e.g., by allowing time for error correction). The observation that an experimentally induced unnatural perturbation can lead to Swe1p-mediated delay does not necessarily mean that the checkpoint evolved to monitor that specific defect. Given the variety of stresses and mutations that can engage Swe1p to delay Clb2p-Cdc28p activation, a number of proposals have been put forward regarding the nature of the cell-cycle event (or defect) monitored by the checkpoint. These ideas, and the key arguments for or against them, are summarized below. They are not mutually exclusive, and it is possible that several different “sensors” promote Swe1p-dependent cell-cycle delays in different circumstances.
Septin organization:
The observation that mutations affecting septin organization lead to Swe1p-mediated cell-cycle delays is consistent with the proposal that the checkpoint evolved to monitor septin organization (Barral et al. 1999). It is currently unknown whether any physiological stresses actually perturb septin organization or whether Swe1p-mediated delays would be beneficial if they did. Nevertheless, the localization of Swe1p and its regulators Hsl1p and Hsl7p to the septin collar is striking and surely not accidental. One possibility is that the septin “organization” monitored by the checkpoint is the switch from a septin ring (in unbudded cells) to a septin collar (in budded cells) (Theesfeld et al. 2003). In that way, septins might provide a path to detect whether or not a bud has been formed.
Actin organization:
Many physiological stresses that occur frequently in the yeast’s natural environment (e.g., changing temperature, osmolarity, nutrient level, or ethanol concentration) cause a transient depolarization of the actin cytoskeleton (Chowdhury et al. 1992; Lillie and Brown 1994; Kubota et al. 2004; Uesono et al. 2004). This is thought to represent an adaptive response that allows the cell to adjust to the altered environment before engaging in polarized growth (Delley and Hall 1999; Keaton and Lew 2006). Actin depolarization delays bud formation. If Clb2p-Cdc28p (which triggers the depolarizing apical-isotropic switch) were to be activated during a stress-induced depolarized period, then that might terminate bud growth before a mature bud had time to form. Moreover, nuclear division might occur in the absence of a bud large enough to receive the daughter nucleus (Figure 6). Thus, common environmental stresses perturb actin organization, and a compensatory Swe1p-mediated delay in Clb2p-Cdc28p activation would seem to have obvious adaptive value.
How would cells “know” that actin was depolarized? This is entirely unclear, and it has been suggested that, rather than monitoring actin per se, the checkpoint assesses whether or not a bud has been formed or whether a critical bud size has been attained.
Bud size:
The most direct way to ensure that a suitable bud has been formed before allowing nuclear division would be for Swe1p to restrain Clb1,2p-Cdc28p activation until the bud had reached a critical size. This would be pleasingly analogous to the situation in S. pombe, where Wee1 is thought to restrain CDK activation until the cell has reached a critical length (Moseley et al. 2009). The critical bud size hypothesis (Harvey and Kellogg 2003) was supported by the observation that Latrunculin treatment of cells with small buds caused cell-cycle arrest, whereas Latrunculin treatment of cells with large buds did not. However, subsequent work showed that this difference was due to cell-cycle position, not bud size per se (McNulty and Lew 2005). Moreover, inactivation of the type V myosin Myo2p halted bud growth (like Latrunculin) but did not cause cell-cycle arrest in either small-budded or large-budded cells (McNulty and Lew 2005). Thus, it appears that whether or not budded cells arrest depends on the type of actin perturbation rather than bud size.
Bud emergence:
Unlike the situation in budded cells, where some actin perturbations cause arrest but others do not, all perturbations (including Myo2p inactivation) that delay bud emergence also delay nuclear division. This includes a mutation (bed1 or mnn10) that delays budding by affecting protein glycosylation without overtly perturbing actin organization (Mondesert and Reed 1996; Theesfeld et al. 2003). Thus, it seems possible that the checkpoint monitors bud emergence and delays Clb1,2p-Cdc28p activation when there is no bud. As described above, bud emergence is accompanied by a change in septin organization, and this provides one avenue by which a cell might “know” whether or not it had begun to grow a bud (Theesfeld et al. 2003).
An awkward observation for the view that the checkpoint arrests the cell cycle until a bud has formed is that some conditions that block bud emergence do not completely arrest the cell cycle; rather, they delay but eventually allow nuclear division. For example, inactivation of Cdc24p or Cdc42p blocks bud emergence and septin ring formation, but only delays nuclear division for ∼2 h (Lew and Reed 1995). And whereas high doses of Latrunculin cause effectively permanent cell-cycle arrest, lower doses cause only a transient delay even though they still block budding (McMillan et al. 1998). In these cases, deleting MIH1 blocks nuclear division (Sia et al. 1996; McMillan et al. 1998). Thus, one could view the checkpoint as having two parallel branches: a “Swe1p branch” that monitors bud emergence and stabilizes Swe1p until a bud has formed and a “Mih1p branch” that monitors something else (perhaps related to the degree of actin disruption?) and inhibits Mih1p to a variable extent. Presumably, the degree/duration of Mih1p inhibition would be properly calibrated to respond to physiological stresses, but would provide inappropriate delays when confronted with unnatural perturbations like cdc24 mutants or specific Latrunculin doses.
Although the findings discussed above support the view that the Swe1p branch of the checkpoint responds to bud emergence, that cannot be the whole story because some treatments (notably osmotic shock and actin disruption) appear to stabilize Swe1p even in budded cells (McMillan et al. 1998; Sia et al. 1998).
Specific stresses:
The hypothesis that the checkpoint monitors a common outcome of many stresses (such as actin perturbation or a delay in bud emergence) provides a simple and parsimonious explanation for many observations. However, each physiological stress elicits a specific response as well, which varies from stress to stress. Thus, another way for the checkpoint to operate would be to have many stress-specific pathways feed into common outputs regulating Swe1p and Mih1p. In the case of hyperosmotic shock, there is some evidence to support this idea, as discussed below.
Sensing morphogenesis defects or stresses
If cells do monitor septins, actin, or budding, how exactly do they do it? Or, if stresses signal to Swe1p/Mih1p directly, how does that work? As mentioned above, there is genetic evidence that the “cell integrity” MAPK Slt2p, which is activated in response to a plethora of cell-membrane/cell-wall stressors (Levin 2005), can inhibit Mih1p (Harrison et al. 2001). However, the mechanism of Mih1p inhibition (if indeed it occurs) remains unknown. In contrast, studies on Swe1p regulation have converged on the checkpoint kinase Hsl1p as a key transducer of information regarding septin status, cell shape, and osmotic shock.
Checkpoint kinase Hsl1p:
Hsl1p is a member of a fungal-specific kinase subfamily related to the MARK/PAR1 kinases (Rubenstein and Schmidt 2007). The founding member of the family is S. pombe Nim1, which directly phosphorylates and inhibits Wee1 (Coleman et al. 1993; Parker et al. 1993). S. cerevisiae cells have three Nim1-related kinases (Hsl1p, Gin4p, and Kcc4p), which appear to have distinct roles such that only Hsl1p directly regulates Swe1p (Longtine et al. 2000). Hsl1p undergoes extensive autophosphorylation (Barral et al. 1999; McMillan et al. 1999) and phosphorylates Hsl7p (McMillan et al. 1999; Shulewitz et al. 1999), but it does not appear to phosphorylate Swe1p (Cid et al. 2001). This observation, combined with localization studies, suggested that a primary (if not the only) role of Hsl1p in Swe1p regulation is to recruit Swe1p (via the bridging action of Hsl7p) to the septin collar (Figure 7).
Hsl1p contains an N-terminal kinase domain and a large C-terminal regulatory domain (Figure 8A). Dissection of the nonkinase domain revealed that it contains degradation motifs recognized by the APC (Burton and Solomon 2000), septin-binding regions (Hanrahan and Snyder 2003; Crutchley et al. 2009), an Hsl7p-binding region (Crutchley et al. 2009), and an acidic phospholipid-binding domain at the C terminus (Moravcevic et al. 2010). The C-terminal domain localizes to the entire plasma membrane when expressed on its own (Moravcevic et al. 2010) and is critical for bud-neck localization of full-length Hsl1p (Crutchley et al. 2009).
Figure 8.
Hsl1p: a checkpoint sensory kinase. (A) Domain organization of Hsl1p. (B) Septins form a ring in unbudded cells adjacent to a locally flat plasma membrane (left), which is converted to a collar adjacent to a locally more tubular plasma membrane upon bud emergence (right). (C) When shmoo-shaped yeast are released into the cell cycle but prevented from budding (due to actin depolymerization), septins form rings (green) in either locally flat (left) or locally tubular (right) plasma membrane geometries. Both rings recruit Hsl1p, but only those inside the shmoo recruit Hsl7p (purple), suggesting that Hsl1p can respond to local membrane geometry.
The ability of Hsl1p to recruit Hsl7p to the septin collar is correlated with Hsl1p activity, and kinase-dead mutants of Hsl1p are greatly impaired in Hsl7p recruitment even though in vitro binding of Hsl1p to Hsl7p does not require the kinase domain (Cid et al. 2001; Theesfeld et al. 2003; Crutchley et al. 2009). One explanation for these observations is that activation of Hsl1p involves a conformational change that unmasks the Hsl7p-binding site. Alternatively, phosphorylation of Hsl1p or Hsl7p may promote stronger interaction and hence effective neck localization of Hsl7p.
Hsl1p regulation by septins:
Hsl1p kinase activity (at least as assessed by monitoring its autophosphorylation) depends on the presence of assembled septins (Barral et al. 1999). Hsl1p kinase activity is stimulated by another septin-localized kinase, Elm1p (Blacketer et al. 1993; Thomas et al. 2003), which is thought to act by phosphorylating Thr273 in the T-loop of the Hsl1p kinase domain (Szkotnicki et al. 2008). However, T273E phosphomimetic mutants that bypass the need for Elm1p do not bypass the need for assembled septins, indicating that septins play an additional role (Szkotnicki et al. 2008). Septins are thought to bind Hsl1p directly (Hanrahan and Snyder 2003), and a region that overlaps the septin-binding region was identified as an autoinhibitory domain (Crutchley et al. 2009) (Figure 8A). Hsl1p-“activated” mutants lacking the putative autoinhibitory domain remain autophosphorylated even in the absence of assembled septins, suggesting that binding of Hsl1p to assembled septins activates the kinase by a relief-of-autoinhibition mechanism (Crutchley et al. 2009). However, the activated mutants cannot downregulate Swe1p in the absence of assembled septins, consistent with the idea that active Hsl1p serves primarily to localize Swe1p.
The dual need for septins to recruit the upstream kinase Elm1p and to relieve autoinhibition would make Hsl1p activity heavily dependent on assembled septins. However, septin interaction is not sufficient to activate Hsl1p because, in unbudded cells treated with Latrunculin, Hsl1p appears to be inactive (as judged by autophosphorylation) even though it is localized to the septin ring (Theesfeld et al. 2003). In contrast, in budded cells, Latrunculin treatment does not inhibit Hsl1p. These findings suggest that Hsl1p activation might require a specific septin organization that occurs only in the septin collar of budded cells.
Hsl1p regulation in response to cell shape:
In wild-type cells, Hsl1p appears at the septin collar immediately after bud emergence and localizes Hsl7p to that site (Theesfeld et al. 2003). In mnn10 mutants, bud emergence is delayed, and there is a significant interval in which cells contain a septin ring but no bud. Although Hsl1p was recruited to the septin ring in these cells, it did not become autophosphorylated or recruit Hsl7p to the ring until just after eventual bud emergence (Theesfeld et al. 2003). This temporal correlation suggested that Hsl1p is normally activated by bud emergence.
The septin ring in an unbudded cell is apposed to a locally “flat” plasma membrane, whereas the septin collar in a budded cell contacts a locally more “tubular” plasma membrane (Figure 8B). In an attempt to determine whether this geometric difference was sufficient to account for the difference in Hsl1p’s ability to recruit Hsl7p, Theesfeld et al. (2003) generated shmoo-shaped cells by exposure to mating pheromone and then withdrew the pheromone but added Latrunculin to prevent bud formation. In the resulting cell population, some cells formed septin rings within the “tubular” shmoo projection while others formed septin rings elsewhere (in locally “flat” regions). Strikingly, Hsl1p did recruit Hsl7p to the ring in those cells with septin rings in the projection, but not in those with rings elsewhere (Figure 8C) (Theesfeld et al. 2003). This result supports the hypothesis that the local geometry of the plasma membrane somehow controls Hsl1p activation. One possibility is that septins reorganize from a ring to a collar in a manner triggered by the change in plasma membrane geometry and that this septin reorganization promotes Hsl1p activation.
Hsl1p regulation in response to osmotic shock:
Osmotic stress activates a MAPK pathway culminating in Hog1p phosphorylation, and active Hog1p can phosphorylate Hsl1p directly in a manner that displaces Hsl7p from the neck (Clotet et al. 2006). This stress-specific pathway may explain why osmotic shock (unlike Latrunculin treatment) promotes a Swe1p-mediated delay of nuclear division even in cells that have formed quite large buds (Alexander et al. 2001).
In summary, stresses that activate the morphogenesis checkpoint do so through at least two separate branches. One branch stabilizes Swe1p, and the other is thought to inhibit Mih1p. Studies on Swe1p stabilization suggest that the checkpoint kinase Hsl1p is a key regulator whose activity is responsive to septin organization, to the cell shape change that accompanies bud emergence, and to the osmotic-shock-responsive Hog1p MAPK. The Mih1p-regulatory pathway appears to respond to actin disruption through the cell-integrity MAPK Slt2p. Both branches must act to produce a significant cell-cycle delay, perhaps suggesting that the checkpoint responds to combinations of specific perturbations.
Perspectives and Open Questions
In the late 1980s and 1990s, general principles of cell-cycle control were elucidated at a rapid clip, revealing a central regulatory cell-cycle “clock” centered around the cyclin-CDK system that triggered downstream events at the proper time, as well as the existence of surveillance pathways that provided feedback to the clock about the success with which its instructions were being followed. There was considerable optimism that the triggering of cell-cycle events would rapidly be understood in terms of the paradigm “cyclin-CDK phosphorylates substrates X1, X2, … (hopefully a small number) to promote a specific downstream event.” In addition, there was optimism that checkpoint control pathways would rapidly be elucidated and would follow the paradigm “a sensor detects a given cell-cycle event and regulates a signaling cascade that stalls the cyclin-CDK clock at a specific point.” The simplicity of these paradigms makes them very seductive, and they retain a powerful influence on our expectations. However, at least in the case of morphogenesis, large gaps remain in our understanding of both the path from the cyclin-CDK system to morphogenetic outputs and the path from morphogenesis defects to CDK regulation.
In terms of how changes in CDK activity promote specific events, there has been no lack of putative CDK substrates relevant to polarity or cytokinesis. However, definitive analysis has in many cases been hampered by the discovery that CDK substrates are phosphorylated at a large number of sites, which need not all conform to the expected consensus. If mutation of multiple sites renders a protein inactive, then there is a significant possibility that the mutations affect protein structure or activity, rather than simply eliminating phosphorylation, hindering unambiguous interpretation. If mutation of the mapped sites has little effect, how are we to interpret that result? It could be that the multisite phosphorylation is “accidental” or “recreational” (i.e., not selected for some regulatory function but simply a harmless by-product of proximity to a relatively promiscuous kinase). However, it is hard to be sure that all relevant sites on the target have been identified, leaving open the possibility that phosphorylation on some unknown site is a critical regulatory event. This is exacerbated by the suspicion that in some cases mutation of mapped “preferred” sites may simply shift phosphorylation to other nearby sites with similar eventual effect. And it is always possible that the phosphorylation plays an important role that is masked by redundant pathways. For all of these reasons, it has been difficult to forge clear-cut connections, and the mechanisms underlying cell-cycle control of morphogenesis remain to be fully worked out. Nevertheless, as reviewed above, there has been considerable progress in understanding the molecular basis for polarity establishment and cytokinesis and, with greater understanding, has come the ability to manipulate these processes in novel ways [e.g., with unnatural fusion proteins that can yield mechanistic insight (Kozubowski et al. 2008; Sanchez-Diaz et al. 2008; Howell et al. 2009; Nishihama et al. 2009)]. We hope that new studies inspired by this progress will reveal how these processes are regulated during the cell cycle.
In terms of how morphogenesis defects regulate the CDK clock, it quickly became clear that a key player was the Swe1p kinase and that many perturbations caused Swe1p-dependent cell-cycle delays. But the very abundance of conditions that cause such delays has made it difficult to come up with a unifying hypothesis that accounts for all of the observed effects, and it seems unlikely that the checkpoint monitors only one event. Rather, it may well be that multiple “sensors” conduct surveillance on many aspects of morphogenesis, including cell shape (is there a bud?), cytoskeletal integrity, and membrane or cell-wall stress (although it is hard to know exactly what these vague phrases mean), and that the duration of Swe1p-dependent delay is calibrated in some combinatorial manner by the signals from each of these parallel sensors. Our effort must focus on elucidating how each individual sensor conducts its surveillance.
In addition to the remaining questions outlined above, studies of morphogenesis and the cell cycle may reveal entirely new levels of coordination. For example, how does a cell know when to stop constructing a primary septum? Is there a “closure signal” that indicates that mother and bud have been separated? Also, how is secondary-septum formation linked to primary-septum formation? Why does secondary-septum deposition become so exaggerated when primary-septum formation fails? And how can cells adjust to drastic defects in septum formation yet retain integrity and not lyse when septum-degrading enzymes are unleashed? In S. pombe, there appears to be a “cytokinesis checkpoint” that delays the subsequent cell cycle in G2 if cytokinesis is impaired (Liu et al. 2000). Does something similar occur in S. cerevisiae? If so, what does that pathway monitor? This field remains full of promise to provide fundamental insights into how cells successfully coordinate the complex and dangerous process of morphogenesis.
Acknowledgments
We thank Erfei Bi, Ryuichi Nishihama, Masayuki Onishi, John Pringle, and members of the Lew laboratory for comments on the manuscript. Work in our laboratory was supported by National Institutes of Health grants GM53050 and GM62300 to D.J.L.
Literature Cited
- Abe M., Qadota H., Hirata A., Ohya Y., 2003. Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J. Cell Biol. 162: 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo J. E., Moskow J. J., Gladfelter A. S., Viterbo D., Lew D. J., et al. , 2001. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155: 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E. M., Pringle J. R., 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98: 934–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E. M., Johnson D. I., Longnecker R. M., Sloat B. F., Pringle J. R., 1990. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S. H., Tobe B. T., Fitz Gerald J. N., Anderson S. L., Acurio A., et al. , 2001. Enhanced cell polarity in mutants of the budding yeast cyclin-dependent kinase Cdc28p. Mol. Biol. Cell 12: 3589–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. R., Tyers M., Perret M., Craig B. M., Fang K. S., et al. , 2001. Regulation of cell cycle progression by swe1p and hog1p following hypertonic stress. Mol. Biol. Cell 12: 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg D. C., 1998. Three-dimensional imaging of the yeast actin cytoskeleton through the budding cell cycle. Mol. Biol. Cell 9: 3259–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A., Surana U., Muroff I., Nasmyth K., 1992. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature 355: 368–371 [DOI] [PubMed] [Google Scholar]
- Amon A., Tyers M., Futcher B., Nasmyth K., 1993. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell 74: 993–1007 [DOI] [PubMed] [Google Scholar]
- Asano S., Park J. E., Sakchaisri K., Yu L. R., Song S., et al. , 2005. Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J. 24: 2194–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K. R., Stryker J., Pokala N., Sanders M., Crews P., et al. , 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137: 399–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K., Knodler A., Lee S. H., Zhang X., Orlando K., et al. , 2010. Structure-function study of the N-terminal domain of exocyst subunit Sec3. J. Biol. Chem. 285: 10424–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S., Cerione R. A., 1999. Pak to the future. Trends Cell Biol. 9: 350–355 [DOI] [PubMed] [Google Scholar]
- Bailly E., Cabantous S., Sondaz D., Bernadac A., Simon M. N., 2003. Differential cellular localization among mitotic cyclins from Saccharomyces cerevisiae: a new role for the axial budding protein Bud3 in targeting Clb2 to the mother-bud neck. J. Cell Sci. 116: 4119–4130 [DOI] [PubMed] [Google Scholar]
- Barral Y., Parra M., Bidlingmaier S., Snyder M., 1999. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 13: 176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y., Mermall V., Mooseker M. S., Snyder M., 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5: 841–851 [DOI] [PubMed] [Google Scholar]
- Bender A., Pringle J. R., 1989. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. USA 86: 9976–9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A., Pringle J. R., 1991. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton B. K., Tinkelenberg A. H., Jean D., Plump S. D., Cross F. R., 1993. Genetic analysis of Cln/Cdc28 regulation of cell morphogenesis in budding yeast. EMBO J. 12: 5267–5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Park H-O., 2012. Cell polarization and cytokinesis. Genetics (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Maddox P., Lew D. J., Salmon E. D., McMillan J. N., et al. , 1998. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142: 1301–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacketer M. J., Koehler C. M., Coats S. G., Myers A. M., Madaule P., 1993. Regulation of dimorphism in Saccharomyces cerevisiae: involvement of the novel protein kinase homolog Elm1p and protein phosphatase 2A. Mol. Cell. Biol. 13: 5567–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel M., Bach S., Bamps S., Dobbelaere J., Wiget P., et al. , 2005. Degradation of Hof1 by SCF(Grr1) is important for actomyosin contraction during cytokinesis in yeast. EMBO J. 24: 1440–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R. N., Deshaies R. J., Kirschner M. W., 1993. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12: 3417–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose I., Irazoqui J. E., Moskow J. J., Bardes E. S., Zyla T. R., et al. , 2001. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276: 7176–7186 [DOI] [PubMed] [Google Scholar]
- Boulter E., Garcia-Mata R., Guilluy C., Dubash A., Rossi G., et al. , 2010. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI. Nat. Cell Biol. 12: 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne J. R., Yosuf H. M., Bieganowski P., Brenner C., Price C., 2000. Yeast myosin light chain, Mlc1p, interacts with both IQGAP and class II myosin to effect cytokinesis. J. Cell Sci. 113: 4533–4543 [DOI] [PubMed] [Google Scholar]
- Brace J., Hsu J., Weiss E. L., 2011. Mitotic exit control of the Saccharomyces cerevisiae Ndr/LATS kinase Cbk1 regulates daughter cell separation after cytokinesis. Mol. Cell. Biol. 31: 721–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J. L., Solomon M. J., 2000. Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex. Mol. Cell. Biol. 20: 4614–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston J. P., Longtine M., Pringle J. R., Bi E., 2003. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14: 4051–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. C., Zheng L., Chan C. S., 1996. The LIM domain-containing Dbm1 GTPase-activating protein is required for normal cellular morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 1376–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Smith K. W., Gustin M. C., 1992. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol. 118: 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J. S., Schekman R. W., 1996. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J. Cell Biol. 135: 597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid V. J., Shulewitz M. J., McDonald K. L., Thorner J., 2001. Dynamic localization of the Swe1 regulator Hsl7 during the Saccharomyces cerevisiae cell cycle. Mol. Biol. Cell 12: 1645–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto A., Novak B., Tyson J. J., 2003. Mathematical model of the morphogenesis checkpoint in budding yeast. J. Cell Biol. 163: 1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotet J., Escote X., Adrover M. A., Yaakov G., Gari E., et al. , 2006. Phosphorylation of Hsl1 by Hog1 leads to a G(2) arrest essential for cell survival at high osmolarity. EMBO J. 25: 2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T. R., Tang Z., Dunphy W. G., 1993. Negative regulation of the Wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell 72: 919–929 [DOI] [PubMed] [Google Scholar]
- Colman-Lerner A., Chin T. E., Brent R., 2001. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107: 739–750 [DOI] [PubMed] [Google Scholar]
- Corbett M., Xiong Y., Boyne J. R., Wright D. J., Munro E., et al. , 2006. IQGAP and mitotic exit network (MEN) proteins are required for cytokinesis and re-polarization of the actin cytoskeleton in the budding yeast, Saccharomyces cerevisiae. Eur. J. Cell Biol. 85: 1201–1215 [DOI] [PubMed] [Google Scholar]
- Cross F., 1988. DAF1, a mutant gene affecting size control, pheromone arrest and cell cycle kinetics of S. cerevisiae. Mol. Cell. Biol. 8: 4675–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutchley J., King K. M., Keaton M. A., Szkotnicki L., Orlando D. A., et al. , 2009. Molecular dissection of the checkpoint kinase Hsl1p. Mol. Biol. Cell 20: 1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrckova F., De Virgilio C., Manser E., Pringle J. R., Nasmyth K., 1995. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9: 1817–1830 [DOI] [PubMed] [Google Scholar]
- Delley P. A., Hall M. N., 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147: 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N., Howson R. W., Blethrow J. D., Shokat K. M., O’Shea E. K., 2005. Combining chemical genetics and proteomics to identify protein kinase substrates. Proc. Natl. Acad. Sci. USA 102: 17940–17945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Talia S., Skotheim J. M., Bean J. M., Siggia E. D., Cross F. R., 2007. The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature 448: 947–951 [DOI] [PubMed] [Google Scholar]
- Dobbelaere J., Gentry M. S., Hallberg R. L., Barral Y., 2003. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 4: 345–357 [DOI] [PubMed] [Google Scholar]
- Drgonova J., Drgon T., Tanaka K., Kollar R., Chen G. C., et al. , 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272: 277–279 [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., 1994. The decision to enter mitosis. Trends Cell Biol. 4: 202–207 [DOI] [PubMed] [Google Scholar]
- Edgington N. P., Blacketer M. J., Bierwagen T. A., Myers A. M., 1999. Control of Saccharomyces cerevisiae filamentous growth by cyclin-dependent kinase Cdc28. Mol. Cell. Biol. 19: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhofer T. A., Villen J., McCusker D., Gygi S. P., Kellogg D. R., 2008. The septins function in G1 pathways that influence the pattern of cell growth in budding yeast. PLoS ONE 3: e2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink J. M., Kolodner R. D., 2010. An overview of Cdk1-controlled targets and processes. Cell Div. 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink J. M., Smolka M. B., Zhou H., Kolodner R. D., 2006. Checkpoint proteins control morphogenetic events during DNA replication stress in Saccharomyces cerevisiae. J. Cell Biol. 175: 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp J. A., Chant J., 1997. An IQGAP-related protein controls actin-ring formation and cytokinesis in yeast. Curr. Biol. 7: 921–929 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Blundell K., Longtine M. S., Chow C. J., Adames N., et al. , 1997. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276: 118–122 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A., 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4: 260–269 [DOI] [PubMed] [Google Scholar]
- Fang X., Luo J., Nishihama R., Wloka C., Dravis C., et al. , 2010. Biphasic targeting and cleavage furrow ingression directed by the tail of a myosin II. J. Cell Biol. 191: 1333–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V., Kovarik J., Kosinova A., Bauer S., 1974. Autoradiographic study of mannan incorporation into the growing cell walls of Saccharomyces cerevisiae. J. Bacteriol. 117: 265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S. K., Pringle J. R., 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev. Genet. 12: 281–292 [DOI] [PubMed] [Google Scholar]
- Frenz L. M., Lee S. E., Fesquet D., Johnston L. H., 2000. The budding yeast Dbf2 protein kinase localises to the centrosome and moves to the bud neck in late mitosis. J. Cell Sci. 113: 3399–3408 [DOI] [PubMed] [Google Scholar]
- Gao X. D., Albert S., Tcheperegine S. E., Burd C. G., Gallwitz D., et al. , 2003. The GAP activity of Msb3p and Msb4p for the Rab GTPase Sec4p is required for efficient exocytosis and actin organization. J. Cell Biol. 162: 635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Moskow J. J., Zyla T. R., Lew D. J., 2001. Isolation and characterization of effector-loop mutants of CDC42 in yeast. Mol. Biol. Cell 12: 1239–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Bose I., Zyla T. R., Bardes E. S., Lew D. J., 2002. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol. 156: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Zyla T. R., Lew D. J., 2004. Genetic interactions among regulators of septin organization. Eukaryot. Cell 3: 847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Kozubowski L., Zyla T. R., Lew D. J., 2005. Interplay between septin organization, cell cycle and cell shape in yeast. J. Cell Sci. 118: 1617–1628 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Novo A., Correa-Bordes J., Labrador L., Sanchez M., Vazquez de Aldana C. R., et al. , 2008. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol. Biol. Cell 19: 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryachev A. B., Pokhilko A. V., 2008. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 582: 1437–1443 [DOI] [PubMed] [Google Scholar]
- Gray J. V., Ogas J. P., Kamada Y., Stone M., Levin D. E., et al. , 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16: 4924–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli M. P., Jaquenoud M., Shimada Y., Niederhauser G., Wiget P., et al. , 2000. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6: 1155–1167 [DOI] [PubMed] [Google Scholar]
- Haarer B. K., Pringle J. R., 1987. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother-bud neck. Mol. Cell. Biol. 7: 3678–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A., Michelitch M., Mitchell E. L., Chant J., 1996. Bud10p directs axial cell polarization in budding yeast and resembles a transmembrane receptor. Curr. Biol. 6: 570–579 [DOI] [PubMed] [Google Scholar]
- Han B. K., Bogomolnaya L. M., Totten J. M., Blank H. M., Dangott L. J., et al. , 2005. Bem1p, a scaffold signaling protein, mediates cyclin-dependent control of vacuolar homeostasis in Saccharomyces cerevisiae. Genes Dev. 19: 2606–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan J., Snyder M., 2003. Cytoskeletal activation of a checkpoint kinase. Mol. Cell 12: 663–673 [DOI] [PubMed] [Google Scholar]
- Harkins H. A., Page N., Schenkman L. R., De Virgilio C., Shaw S., et al. , 2001. Bud8p and Bud9p, proteins that may mark the sites for bipolar budding in yeast. Mol. Biol. Cell 12: 2497–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. C., Bardes E. S., Ohya Y., Lew D. J., 2001. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 3: 417–420 [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., 1971. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69: 265–276 [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A., 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634 [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Reid B., 1970. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl. Acad. Sci. USA 66: 352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J., 1974. Genetic control of the cell division cycle in yeast. Science 183: 46–51 [DOI] [PubMed] [Google Scholar]
- Harvey S. L., Kellogg D. R., 2003. Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr. Biol. 13: 264–275 [DOI] [PubMed] [Google Scholar]
- Harvey S. L., Charlet A., Haas W., Gygi S. P., Kellogg D. R., 2005. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122: 407–420 [DOI] [PubMed] [Google Scholar]
- Holt L. J., Tuch B. B., Villen J., Johnson A. D., Gygi S. P., et al. , 2009. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325: 1682–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A. S., Savage N. S., Johnson S. A., Bose I., Wagner A. W., et al. , 2009. Singularity in polarization: rewiring yeast cells to make two buds. Cell 139: 731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa Lim H., Yeong F. M., Surana U., 2003. Inactivation of mitotic kinase triggers translocation of MEN components to mother-daughter neck in yeast. Mol. Biol. Cell 14: 4734–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui J. E., Gladfelter A. S., Lew D. J., 2003. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5: 1062–1070 [DOI] [PubMed] [Google Scholar]
- Irazoqui J. E., Howell A. S., Theesfeld C. L., Lew D. J., 2005. Opposing roles for actin in Cdc42p polarization. Mol. Biol. Cell 16: 1296–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M., Luo J., Nagaraj S., Longtine M., Kim H. B., et al. , 2006. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol. Biol. Cell 17: 1110–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C. W., Adams A. E. M., Szaniszlo P. J., Pringle J. R., 1988. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 107: 1409–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquenoud M., Peter M., 2000. Gic2p may link activated Cdc42p to components involved in actin polarization, including Bni1p and Bud6p (Aip3p). Mol. Cell. Biol. 20: 6244–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquenoud M., Gulli M. P., Peter K., Peter M., 1998. The Cdc42p effector Gic2p is targeted for ubiquitin-dependent degradation by the SCFGrr1 complex. EMBO J. 17: 5360–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendretzki A., Ciklic I., Rodicio R., Schmitz H. P., Heinisch J. J., 2009. Cyk3 acts in actomyosin ring independent cytokinesis by recruiting Inn1 to the yeast bud neck. Mol. Genet. Genomics 282: 437–451 [DOI] [PubMed] [Google Scholar]
- Jiang Y. W., Kang C. M., 2003. Induction of S. cerevisiae filamentous differentiation by slowed DNA synthesis involves Mec1, Rad53 and Swe1 checkpoint proteins. Mol. Biol. Cell 14: 5116–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Erickson J. W., Cerione R. A., 2009. New insights into how the Rho guanine nucleotide dissociation inhibitor regulates the interaction of Cdc42 with membranes. J. Biol. Chem. 284: 23860–23871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota J., Yamamoto T., Yoshiuchi S., Bi E., Tanaka K., 2004. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 15: 5329–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P., Sia R. A. L., Bardes E. G. S., Lew D. J., Reed S. I., 1998. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 12: 2587–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G., 2003. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115: 475–487 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G., 2006. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 7: 404–414 [DOI] [PubMed] [Google Scholar]
- Kang P. J., Sanson A., Lee B., Park H. O., 2001. A GDP/GTP exchange factor involved in linking a spatial landmark to cell polarity. Science 292: 1376–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Angerman E., Nakashima K., Pringle J. R., Park H. O., 2004a Interactions among Rax1p, Rax2p, Bud8p, and Bud9p in marking cortical sites for bipolar bud-site selection in yeast. Mol. Biol. Cell 15: 5145–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Lee B., Park H. O., 2004b Specific residues of the GDP/GTP exchange factor Bud5p are involved in establishment of the cell type-specific budding pattern in yeast. J. Biol. Chem. 279: 27980–27985 [DOI] [PubMed] [Google Scholar]
- Karpova T. S., Reck-Peterson S. L., Elkind N. B., Mooseker M. S., Novick P. J., et al. , 2000. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell 11: 1727–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaton M. A., Lew D. J., 2006. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr. Opin. Microbiol. 9: 540–546 [DOI] [PubMed] [Google Scholar]
- Keaton M. A., Bardes E. S., Marquitz A. R., Freel C. D., Zyla T. R., et al. , 2007. Differential susceptibility of yeast S and M phase CDK complexes to inhibitory tyrosine phosphorylation. Curr. Biol. 17: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaton M. A., Szkotnicki L., Marquitz A. R., Harrison J., Zyla T. R., et al. , 2008. Nucleocytoplasmic trafficking of G2/M regulators in yeast. Mol. Biol. Cell 19: 4006–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. B., Haarer B. K., Pringle J. R., 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J. Cell Biol. 112: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Ferrell J. E., Jr, 2007. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 128: 1133–1145 [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Song E. J., Lee K. J., Ferrell J. E., Jr, 2005. Multisite M-phase phosphorylation of Xenopus Wee1A. Mol. Cell. Biol. 25: 10580–10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus M., Pelli-Gulli M. P., van Drogen F., Springer S., Jaquenoud M., et al. , 2007. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J. 26: 4501–4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko N., Nishihama R., Tully G. H., Ostapenko D., Solomon M. J., et al. , 2007. Identification of yeast IQGAP (Iqg1p) as an anaphase-promoting-complex substrate and its role in actomyosin-ring-independent cytokinesis. Mol. Biol. Cell 18: 5139–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Tanaka K., Masuda T., Yamochi W., Nonaka H., et al. , 1997. Association of the Rho family small GTP-binding proteins with Rho GDP dissociation inhibitor (Rho GDI) in Saccharomyces cerevisiae. Oncogene 15: 417–422 [DOI] [PubMed] [Google Scholar]
- Kono K., Nogami S., Abe M., Nishizawa M., Morishita S., et al. , 2008. G1/S cyclin-dependent kinase regulates small GTPase Rho1p through phosphorylation of RhoGEF Tus1p in Saccharomyces cerevisiae. Mol. Biol. Cell 19: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek W. S., Bi E., Epp J. A., Wang L., Ho J., et al. , 2000. Cyk3, a novel SH3-domain protein, affects cytokinesis in yeast. Curr. Biol. 10: 947–950 [DOI] [PubMed] [Google Scholar]
- Kozminski K. G., Beven L., Angerman E., Tong A. H., Boone C., et al. , 2003. Interaction between a Ras and a Rho GTPase couples selection of a growth site to the development of cell polarity in yeast. Mol. Biol. Cell 14: 4958–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L., Larson J. R., Tatchell K., 2005. Role of the septin ring in the asymmetric localization of proteins at the mother-bud neck in Saccharomyces cerevisiae. Mol. Biol. Cell 16: 3455–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L., Saito K., Johnson J. M., Howell A. S., Zyla T. R., et al. , 2008. Symmetry-breaking polarization driven by a Cdc42p GEF-PAK complex. Curr. Biol. 18: 1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S., Takeo I., Kume K., Kanai M., Shitamukai A., et al. , 2004. Effect of ethanol on cell growth of budding yeast: genes that are important for cell growth in the presence of ethanol. Biosci. Biotechnol. Biochem. 68: 968–972 [DOI] [PubMed] [Google Scholar]
- Kuranda M. J., Robbins P. W., 1991. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 266: 19758–19767 [PubMed] [Google Scholar]
- Layton A. T., Savage N. S., Howell A. S., Carroll S. Y., Drubin D. G., et al. , 2011. Modeling vesicle traffic reveals unexpected consequences for cdc42p-mediated polarity establishment. Curr. Biol. 21: 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., 2003. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 15: 648–653 [DOI] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I., 1993. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 120: 1305–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I., 1995. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J. Cell Biol. 129: 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S. H., Brown S. S., 1994. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125: 825–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. H., Goh P.-Y., Surana U., 1996. Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol. Cell. Biol. 16: 6385–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A., Rodriguez-Bravo V., Medema R. H., 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J., Li R., 1998. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol. 140: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J., Shannon K. B., Shou W., Deshaies R. J., Li R., 2001. The Tem1 small GTPase controls actomyosin and septin dynamics during cytokinesis. J. Cell Sci. 114: 1379–1386 [DOI] [PubMed] [Google Scholar]
- Liu H., Wang Y., 2006. The function and regulation of budding yeast Swe1 in response to interrupted DNA synthesis. Mol. Biol. Cell 17: 2746–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang H., Balasubramanian M. K., 2000. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J. Cell Sci. 113: 1223–1230 [DOI] [PubMed] [Google Scholar]
- Longtine M. S., Theesfeld C. L., McMillan J. N., Weaver E., Pringle J. R., et al. , 2000. Septin-dependent assembly of a cell-cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20: 4049–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loog M., Morgan D. O., 2005. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434: 104–108 [DOI] [PubMed] [Google Scholar]
- Lord M., Laves E., Pollard T. D., 2005. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol. Biol. Cell 16: 5346–5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F. C., Mody M., Kurischko C., Roof D. M., Giddings T. H., et al. , 2001. Saccharomyces cerevisiae Mob1p is required for cytokinesis and mitotic exit. Mol. Cell. Biol. 21: 6972–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Vallen E. A., Dravis C., Tcheperegine S. E., Drees B., et al. , 2004. Identification and functional analysis of the essential and regulatory light chains of the only type II myosin Myo1p in Saccharomyces cerevisiae. J. Cell Biol. 165: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden K., Snyder M., 1992. Specification of sites for polarized growth in Saccharomyces cerevisiae and the influence of external factors on site selection. Mol. Biol. Cell 3: 1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E., Wedlich-Soldner R., Li R., Altschuler S. J., Wu L. F., 2007. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell 129: 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz A. R., Harrison J. C., Bose I., Zyla T. R., McMillan J. N., et al. , 2002. The Rho-GAP Bem2p plays a GAP-independent role in the morphogenesis checkpoint. EMBO J. 21: 4012–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A. L., Chen T., Yang M. C., Belhumeur P., Chant J., 2001. A localized GTPase exchange factor, Bud5, determines the orientation of division axes in yeast. Curr. Biol. 11: 803–807 [DOI] [PubMed] [Google Scholar]
- Masuda T., Tanaka K., Nonaka H., Yamochi W., Maeda A., et al. , 1994. Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J. Biol. Chem. 269: 19713–19718 [PubMed] [Google Scholar]
- McCusker D., Denison C., Anderson S., Egelhofer T. A., Yates J. R., III, et al. , 2007. Cdk1 coordinates cell-surface growth with the cell cycle. Nat. Cell Biol. 9: 506–515 [DOI] [PubMed] [Google Scholar]
- McMillan J. N., Sia R. A. L., Lew D. J., 1998. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 142: 1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Longtine M. S., Sia R. A. L., Theesfeld C. L., Bardes E. S. G., et al. , 1999. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol. 19: 6929–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Theesfeld C. L., Harrison J. C., Bardes E. S., Lew D. J., 2002. Determinants of Swe1p degradation in Saccharomyces cerevisiae. Mol. Biol. Cell 13: 3560–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty J. J., Lew D. J., 2005. Swe1p responds to cytoskeletal perturbation, not bud size, in S. cerevisiae. Curr. Biol. 15: 2190–2198 [DOI] [PubMed] [Google Scholar]
- Measday V., Moore L., Ogas J., Tyers M., Andrews B., 1994. The PCL2 (ORFD)-PHO85 cyclin-dependent kinase complex: a cell cycle regulator in yeast. Science 266: 1391–1395 [DOI] [PubMed] [Google Scholar]
- Meitinger F., Petrova B., Lombardi I. M., Bertazzi D. T., Hub B., et al. , 2010. Targeted localization of Inn1, Cyk3 and Chs2 by the mitotic-exit network regulates cytokinesis in budding yeast. J. Cell Sci. 123: 1851–1861 [DOI] [PubMed] [Google Scholar]
- Michelitch M., Chant J., 1996. A mechanism of Bud1p GTPase action suggested by mutational analysis and immunolocalization. Curr. Biol. 6: 446–454 [DOI] [PubMed] [Google Scholar]
- Moffat J., Andrews B., 2004. Late-G1 cyclin-CDK activity is essential for control of cell morphogenesis in budding yeast. Nat. Cell Biol. 6: 59–66 [DOI] [PubMed] [Google Scholar]
- Mondesert G., Reed S. I., 1996. BED1, a gene encoding a galactosyltransferase homologue, is required for polarized growth and efficient bud emergence in Saccharomyces cerevisiae. J. Cell Biol. 132: 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravcevic K., Mendrola J. M., Schmitz K. R., Wang Y. H., Slochower D., et al. , 2010. Kinase associated-1 domains drive MARK/PAR1 kinases to membrane targets by binding acidic phospholipids. Cell 143: 966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. B., Mayeux A., Paoletti A., Nurse P., 2009. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 459: 857–860 [DOI] [PubMed] [Google Scholar]
- Nash R., Tokiwa G., Anand S., Erickson K., Futcher A. B., 1988. The WHI1+ gene of S. cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 7: 4335–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., Kurischko C., Horecka J., Mody M., Nair P., et al. , 2003. RAM: a conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol. Biol. Cell 14: 3782–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A., Arkowitz R. A., 2000. Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J. Cell Biol. 148: 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher T. M., Smith R. P., Lemmon V., Lemmon S. K., 2005. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev. Cell 9: 87–98 [DOI] [PubMed] [Google Scholar]
- Nishihama R., Schreiter J. H., Onishi M., Vallen E. A., Hanna J., et al. , 2009. Role of Inn1 and its interactions with Hof1 and Cyk3 in promoting cleavage furrow and septum formation in S. cerevisiae. J. Cell Biol. 185: 995–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Conallain C., Doolin M. T., Taggart C., Thornton F., Butler G., 1999. Regulated nuclear localisation of the yeast transcription factor Ace2p controls expression of chitinase (CTS1) in Saccharomyces cerevisiae. Mol. Gen. Genet. 262: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y., Huang K., Cross F. R., Cowburn D., Chait B. T., 1999. Accurate quantitation of protein expression and site-specific phosphorylation. Proc. Natl. Acad. Sci. USA 96: 6591–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlen L. J., Cross F. R., 1998. Potential regulation of Ste20 function by the Cln1-Cdc28 and Cln2-Cdc28 cyclin-dependent protein kinases. J. Biol. Chem. 273: 25089–25097 [DOI] [PubMed] [Google Scholar]
- Padmashree C. G., Surana U., 2001. Cdc28-Clb mitotic kinase negatively regulates bud site assembly in the budding yeast. J. Cell Sci. 114: 207–218 [DOI] [PubMed] [Google Scholar]
- Pal G., Paraz M. T., Kellogg D. R., 2008. Regulation of Mih1/Cdc25 by protein phosphatase 2A and casein kinase 1. J. Cell Biol. 180: 931–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Bi E., Pringle J. R., Herskowitz I., 1997. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc. Natl. Acad. Sci. USA 94: 4463–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Sanson A., Herskowitz I., 1999. Localization of Bud2p, a GTPase-activating protein necessary for programming cell polarity in yeast to the presumptive bud site. Genes Dev. 13: 1912–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Kang P. J., Rachfal A. W., 2002. Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J. Biol. Chem. 277: 26721–26724 [DOI] [PubMed] [Google Scholar]
- Parker L. L., Walter S. A., Young P. G., Piwnica-Worms H., 1993. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature 363: 736–738 [DOI] [PubMed] [Google Scholar]
- Perry J. A., Kornbluth S., 2007. Cdc25 and Wee1: Analogous opposites? Cell Div. 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R., Hartwell L. H., 1981. The Saccharomyces cerevisiae cell cycle, pp. 97–142 The Molecular Biology of the Yeast Saccharomyces, edited by Strathern J. D., Jones E. W., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Pringle J. R., Bi E., Harkins H. A., Zahner J. E., De Virgilio C., et al. , 1995. Establishment of cell polarity in yeast. Cold Spring Harb. Symp. Quant. Biol. 60: 729–744 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Gao L., Bi E., Bretscher A., 2004a Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell 15: 4971–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A., 2004b Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20: 559–591 [DOI] [PubMed] [Google Scholar]
- Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., et al. , 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272: 279–281 [DOI] [PubMed] [Google Scholar]
- Qiu W., Neo S. P., Yu X., Cai M., 2008. A novel septin-associated protein, Syp1p, is required for normal cell cycle-dependent septin cytoskeleton dynamics in yeast. Genetics 180: 1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal R., Amon A., 2008. Mitotic CDKs control the metaphase-anaphase transition and trigger spindle elongation. Genes Dev. 22: 1534–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancati G., Pavelka N., Fleharty B., Noll A., Trimble R., et al. , 2008. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell 135: 879–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H. E., Wittenberg C., Cross F., Reed S. I., 1989. An essential G1 function for cyclin-like proteins in yeast. Cell 59: 1127–1133 [DOI] [PubMed] [Google Scholar]
- Richman T. J., Sawyer M. M., Johnson D. I., 2002. Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot. Cell 1: 458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T., Madden K., Chang J., Snyder M., 1996. Selection of axial growth sites in yeast requires Axl2p, a novel plasma membrane glycoprotein. Genes Dev. 10: 777–793 [DOI] [PubMed] [Google Scholar]
- Roh D. H., Bowers B., Schmidt M., Cabib E., 2002. The septation apparatus, an autonomous system in budding yeast. Mol. Biol. Cell 13: 2747–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumanie O., Weinachter C., Larrieu I., Crouzet M., Doignon F., 2001. Functional characterization of the Bag7, Lrg1 and Rgd2 RhoGAP proteins from Saccharomyces cerevisiae. FEBS Lett. 506: 149–156 [DOI] [PubMed] [Google Scholar]
- Rubenstein E. M., Schmidt M. C., 2007. Mechanisms regulating the protein kinases of Saccharomyces cerevisiae. Eukaryot. Cell 6: 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D., 2002. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4: 42–50 [DOI] [PubMed] [Google Scholar]
- Sahin A., Daignan-Fornier B., Sagot I., 2008. Polarized growth in the absence of F-actin in Saccharomyces cerevisiae exiting quiescence. PLoS ONE 3: e2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Fujimura-Kamada K., Hanamatsu H., Kato U., Umeda M., et al. , 2007. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev. Cell 13: 743–751 [DOI] [PubMed] [Google Scholar]
- Sakchaisri K., Asano S., Yu L. R., Shulewitz M. J., Park C. J., et al. , 2004. Coupling morphogenesis to mitotic entry. Proc. Natl. Acad. Sci. USA 101: 4124–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Diaz A., Marchesi V., Murray S., Jones R., Pereira G., et al. , 2008. Inn1 couples contraction of the actomyosin ring to membrane ingression during cytokinesis in budding yeast. Nat. Cell Biol. 10: 395–406 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Bowers B., Varma A., Roh D. H., Cabib E., 2002. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115: 293–302 [DOI] [PubMed] [Google Scholar]
- Shannon K. B., Li R., 1999. The multiple roles of Cyk1p in the assembly and function of the actomyosin ring in budding yeast. Mol. Biol. Cell 10: 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K. B., Li R., 2000. A myosin light chain mediates the localization of the budding yeast IQGAP-like protein during contractile ring formation. Curr. Biol. 10: 727–730 [DOI] [PubMed] [Google Scholar]
- Shimada Y., Gulli M. P., Peter M., 2000. Nuclear sequestration of the exchange factor Cdc24 by Far1 regulates cell polarity during yeast mating. Nat. Cell Biol. 2: 117–124 [DOI] [PubMed] [Google Scholar]
- Shimada Y., Wiget P., Gulli M. P., Bi E., Peter M., 2004. The nucleotide exchange factor Cdc24p may be regulated by auto-inhibition. EMBO J. 23: 1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M., Zachariae W., Ciosk R., Nasmyth K., 1998. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17: 1336–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulewitz M. J., Inouye C. J., Thorner J., 1999. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 7123–7137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia R. A. L., Herald H. A., Lew D. J., 1996. Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol. Biol. Cell 7: 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia R. A. L., Bardes E. S. G., Lew D. J., 1998. Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J. 17: 6678–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I., Wang Y. M., Philp R., Li C. R., Yap W. H., et al. , 2007. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev. Cell 13: 421–432 [DOI] [PubMed] [Google Scholar]
- Slaughter B. D., Das A., Schwartz J. W., Rubinstein B., Li R., 2009. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev. Cell 17: 823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloat B. F., Adams A. E. M., Pringle J. R., 1981. Roles of the CDC24 gene product in cellular morphogenesis during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 89: 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Givan S. A., Cullen P., Sprague G. F., Jr, 2002. GTPase-activating proteins for Cdc42. Eukaryot. Cell 1: 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Gehrung S., Page B. D., 1991. Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae. J. Cell Biol. 114: 515–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R., Huang D., Smith J. C., Figeys D., Andrews B. J., 2007. Activation of the Cdc42p GTPase by cyclin-dependent protein kinases in budding yeast. EMBO J. 26: 4487–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Murray A. W., 1992. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34CDC28. Nature 355: 365–368 [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Amon A., 2004. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38: 203–232 [DOI] [PubMed] [Google Scholar]
- Stevenson B. J., Ferguson B., De Virgilio C., Bi E., Pringle J. R., et al. , 1995. Mutation of RGA1, which encodes a putative GTPase-activating protein for the polarity-establishment protein Cdc42p, activates the pheromone-response pathway in the yeast Saccharomyces cerevisiae. Genes Dev. 9: 2949–2963 [DOI] [PubMed] [Google Scholar]
- Stimpson H. E., Toret C. P., Cheng A. T., Pauly B. S., Drubin D. G., 2009. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol. Biol. Cell 20: 4640–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Asada Y., Watanabe D., Ohya Y., 2004. Cell shape and growth of budding yeast cells in restrictive microenvironments. Yeast 21: 983–989 [DOI] [PubMed] [Google Scholar]
- Szkotnicki L., Crutchley J. M., Zyla T. R., Bardes E. S., Lew D. J., 2008. The checkpoint kinase Hsl1p is activated by Elm1p-dependent phosphorylation. Mol. Biol. Cell 19: 4675–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. S., Reed S. I., 2002. Phosphorylation of the septin cdc3 in g1 by the cdc28 kinase is essential for efficient septin ring disassembly. Cell Cycle 1: 42–49 [PubMed] [Google Scholar]
- Tcheperegine S. E., Gao X. D., Bi E., 2005. Regulation of cell polarity by interactions of Msb3 and Msb4 with Cdc42 and polarisome components. Mol. Cell. Biol. 25: 8567–8580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh E. M., Chai C. C., Yeong F. M., 2009. Retention of Chs2p in the ER requires N-terminal CDK1-phosphorylation sites. Cell Cycle 8: 2964–2974 [PubMed] [Google Scholar]
- Theesfeld C. L., Zyla T. R., Bardes E. G., Lew D. J., 2003. A monitor for bud emergence in the yeast morphogenesis checkpoint. Mol. Biol. Cell 14: 3280–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. L., Blacketer M. J., Edgington N. P., Myers A. M., 2003. Assembly interdependence among the S. cerevisiae bud neck ring proteins Elm1p, Hsl1p and Cdc12p. Yeast 20: 813–826 [DOI] [PubMed] [Google Scholar]
- Thornton B. R., Toczyski D. P., 2003. Securin and B-cyclin/CDK are the only essential targets of the APC. Nat. Cell Biol. 5: 1090–1094 [DOI] [PubMed] [Google Scholar]
- Tiedje C., Sakwa I., Just U., Hofken T., 2008. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol. Biol. Cell 19: 2885–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday N., VerPlank L., Li R., 2002. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12: 1864–1870 [DOI] [PubMed] [Google Scholar]
- Tully G. H., Nishihama R., Pringle J. R., Morgan D. O., 2009. The anaphase-promoting complex promotes actomyosin-ring disassembly during cytokinesis in yeast. Mol. Biol. Cell 20: 1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing A., 1952. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 237: 37–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., et al. , 2003. Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864 [DOI] [PubMed] [Google Scholar]
- Uesono Y., Ashe M. P., Toh E. A., 2004. Simultaneous yet independent regulation of actin cytoskeletal organization and translation initiation by glucose in Saccharomyces cerevisiae. Mol. Biol. Cell 15: 1544–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen E. A., Caviston J., Bi E., 2000. Roles of Hof1p, Bni1p, Bnr1p, and Myo1p in cytokinesis in Saccharomyces cerevisiae. Mol. Biol. Cell 11: 593–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VerPlank L., Li R., 2005. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol. Biol. Cell 16: 2529–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele M., Thorner J., 2004. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J. Cell Biol. 164: 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu A. M., Mitchison T. J., 2006. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature 443: 466–469 [DOI] [PubMed] [Google Scholar]
- Wai S. C., Gerber S. A., Li R., 2009. Multisite phosphorylation of the guanine nucleotide exchange factor Cdc24 during yeast cell polarization. PLoS ONE 4: e6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Nishihara Y., Taniguchi M., Hunter T., et al. , 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA 101: 4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Iwasaki J., Shiina M., Ogata K., et al. , 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA 102: 11663–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R., Altschuler S., Wu L., Li R., 2003. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299: 1231–1235 [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R., Wai S. C., Schmidt T., Li R., 2004. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 166: 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. L., 2012. Separation of mother and daughter cells. Genetics (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. L., Bishop A. C., Shokat K. M., Drubin D. G., 2000. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat. Cell Biol. 2: 677–685 [DOI] [PubMed] [Google Scholar]
- Weiss E. L., Kurischko C., Zhang C., Shokat K., Drubin D. G., et al. , 2002. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J. Cell Biol. 158: 885–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Leeuw T., Leberer E., Thomas D. Y., Whiteway M., 1998. Cell cycle- and Cln2p-Cdc28p-dependent phosphorylation of the yeast Ste20p protein kinase. J. Biol. Chem. 273: 28107–28115 [DOI] [PubMed] [Google Scholar]
- Wu H., Turner C., Gardner J., Temple B., Brennwald P., 2010. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol. Biol. Cell 21: 430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Huang H. K., Kaiser P., Latterich M., Hunter T., 2000. Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast. Curr. Biol. 10: 329–332 [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Mochida J., Kadota J., Takeda M., Bi E., et al. , 2010. Initial polarized bud growth by endocytic recycling in the absence of actin cable-dependent vesicle transport in yeast. Mol. Biol. Cell 21: 1237–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong F. M., Lim H. H., Surana U., 2002. MEN, destruction and separation: mechanistic links between mitotic exit and cytokinesis in budding yeast. Bioessays 24: 659–666 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kono K., Lowery D. M., Bartolini S., Yaffe M. B., et al. , 2006. Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science 313: 108–111 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Bartolini S., Pellman D., 2009. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 23: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A., Sun X., Zhang J., Guo W., 2005. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol. Biol. Cell 16: 1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Kashimshetty R., Ng K. E., Tan H. B., Yeong F. M., 2006. Exit from mitosis triggers Chs2p transport from the endoplasmic reticulum to mother-daughter neck via the secretory pathway in budding yeast. J. Cell Biol. 174: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bi E., Novick P., Du L., Kozminski K. G., et al. , 2001. Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276: 46745–46750 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Hart M. J., Shinjo K., Evans T., Bender A., et al. , 1993. Biochemical comparisons of the Saccharomyces cerevisiae Bem2 and Bem3 proteins. Delineation of a limit Cdc42 GTPase-activating protein domain. J. Biol. Chem. 268: 24629–24634 [PubMed] [Google Scholar]
- Zheng Y., Cerione R., Bender A., 1994. Control of the yeast bud-site assembly GTPase Cdc42. Catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3. J. Biol. Chem. 269: 2369–2372 [PubMed] [Google Scholar]
- Zheng Y., Bender A., Cerione R. A., 1995. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J. Biol. Chem. 270: 626–630 [DOI] [PubMed] [Google Scholar]
- Ziman M., O’Brien J. M., Ouellette L. A., Church W. R., Johnson D. I., 1991. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11: 3537–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M., Preuss D., Mulholland J., O’Brien J. M., Botstein D., et al. , 1993. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4: 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Friesen H., Larson J., Huang D., Cox M., et al. , 2009. Regulation of cell polarity through phosphorylation of Bni4 by Pho85 G1 cyclin-dependent kinases in Saccharomyces cerevisiae. Mol. Biol. Cell 20: 3239–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]