Abstract
5-Lipoxygenase catalyzes leukotriene generation from arachidonic acid. The gene that encodes 5-lipoxygenase, Alox5, has been identified in genome-wide association and mouse Quantitative Trait Locus studies as a candidate gene for obesity and low bone mass. Thus, we tested the hypothesis that Alox5−/− mice would exhibit metabolic and skeletal changes when challenged by a high-fat diet (HFD). On a regular diet, Alox5−/− mice did not differ in total body weight, percent fat mass, or bone mineral density compared with wild-type (WT) controls (P < 0.05). However, when placed on a HFD, Alox5−/− gained more fat mass and lost greater areal bone mass vs. WT (P < 0.05). Microarchitectural analyses revealed that on a HFD, WT showed increases in cortical area (P < 0.01) and trabecular thickness (P < 0.01), whereas Alox5−/− showed no change in cortical parameters but a decrease in trabecular number (P < 0.05) and bone volume fraction compared with WT controls (P < 0.05). By histomorphometry, a HFD did not change bone formation rates of either strain but produced an increase in osteoclast number per bone perimeter in Alox5−/− mice (P < 0.03). In vitro, osteoclastogenesis of marrow stromal cells was enhanced in mutant but not WT mice fed a HFD. Gene expression for Rankl, Pparg, and Cox-2 was greater in the femur of Alox5−/− than WT mice on a HFD (P < 0.01), but these increases were suppressed in the Alox5−/− mice after 8 wk of treatment with celecoxib, a cyclooxygenase-2 inhibitor. In sum, there is a strong gene by environmental interaction for bone mass when mice lacking the Alox5 gene are fed a HFD.
Obesity has become epidemic in proportion and is likely caused by a combination of environmental factors, including high-fat diets (HFD) and their interactions with genetic determinants in susceptible individuals (1). Previously, obesity was considered to be protective for the skeleton, in part because of the increased gravitational load on long bones and vertebrae (2). Indeed, studies have shown that body mass index is directly correlated with bone mineral density (BMD). However, accumulating evidence indicates that certain types of obesity might be associated with reduced bone mass and increased fracture risk both in childhood and adult populations (2–6). These data and genome-wide association studies reveal an overlap in candidate genes for BMD and body weight implying that the rising incidence of both osteoporosis and obesity might be causally linked. However, the mechanisms whereby body weight and a HFD affect skeletal turnover and the development of osteoporosis have not been determined.
Fatty acid metabolites function as lipid mediators and can have a profound effect on metabolic homeostasis, particularly with the chronic ingestion of high dietary fat (7). Arachidonic acid is a major component of fatty acids and also generates lipid mediators, such as leukotrienes and prostaglandins (PG), which can mediate inflammatory responses (7, 8). There is evidence from human studies of a strong association between tissue arachidonic acid levels and the prevalence of obesity (9–11). Alox5 is the gene that encodes the enzyme 5-lipoxygenase (5-LO), and 5-LO catalyzes the first step of biosynthesis of leukotriene from arachidonic acid (12–14). Whole-genome association and candidate gene studies have shown that genetic variations in the Alox5 gene are associated with atherosclerosis and myocardial infarction risk (8, 15). In addition, polymorphisms in the Alox5 gene have been shown to influence body composition, such as adiposity and bone mass (16). These lines of evidence suggest that there is an association between changes in 5-LO and the risk of several chronic diseases, including obesity, atherosclerosis, and osteoporosis. However, the underlying mechanisms have remained largely undefined.
A growing body of evidence highlights the critical roles of peroxisome proliferator-activated receptor-γ (Pparg) in the pathogenesis of osteoporosis (17, 18). Pparg is a member of the PPAR family of transcriptional factors and nuclear receptors, which possesses a variety of roles in cell differentiation, lipid and glucose metabolism, inflammation, and neoplasm development (19–21). Alternative splicing generates Pparg isoforms, including Pparg1 and Pparg2. Pparg1 is expressed in most tissues, whereas expression of Pparg2 is limited to adipocytes, where it has been shown to have a critical role in adipogenesis (19–21). Several lines of evidence have also demonstrated the suppressive effect of Pparg on osteoblastogenesis (22, 23), indicating that Pparg is a molecular switch for the determination of cell fate of mesenchymal stem cells. In line with this tenet, Pparg2 expression is enhanced in the bone marrow with aging, and this in turn is associated with reduced bone mass and increased marrow adiposity (23, 24). Furthermore, activation of Pparg by thiazolidinediones has been implicated in decreased bone mass and increased fracture risk in human (25, 26).
Ligands for Pparg include a synthetic class of compounds, thiazolidinediones, as well as naturally occurring substances, such as fatty acids and metabolites of arachidonic acids (17, 18). 15-Deoxy-Δ12,14-PGJ2 (15d-PGJ2) is one such endogenous Pparg ligand, which is derived from arachidonic acid (27, 28). The synthesis of 15d-PGJ2 from arachidonic acid is catalyzed by the constitutive and inducible cyclooxygenase (Cox) (Cox-1 and Cox-2, respectively), resulting in the synthesis of labile PGG2 and PGH2. These PG are then converted into stable PG, such as PGD2. PGD2 is nonenzymatically converted into PGJ2, which is further converted to the 15d-PGJ2 (see figure 5 below).
Recently, inhibition of 5-LO by a 5-LO inhibitor has been shown to increase Cox-2 expression and PGE2 production in cardiomyocytes (29) and enhance Pparg expression in breast cancer cell lines (30). Thus, the lack of 5-LO may switch the metabolic pathway of arachidonic acid from leukotrienes toward PG, which is further enhanced in the presence of excess arachidonic acid particularly with a HFD. Based on these findings, we hypothesized that deletion of the 5-LO enzyme would result in enhanced Pparg activity by enhancing the conversion of arachidonic acid into the PG pathway through up-regulation of Cox-2 activity. Indeed, in this study, we demonstrate that Alox5−/− mice gain more weight and exhibit significant bone loss compared with control mice when both are fed a HFD. This was accompanied by increased bone resorption and the enhanced expression of Cox-2, Rankl, and Pparg2. These changes were reversed by treatment with a Cox-2 inhibitor pointing to the central role of 5-LO in modulating bone turnover with a HFD.
Materials and Methods
Animal husbandry
Alox5−/− mice on a C57BL/6J background and C57BL/6J mice [wild-type (WT)] were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained at 25 C on a 14-h dark, 10-h light cycle on a standard regular chow. For HFD challenge test, mice were fed either a control diet (10% fat by kcal) containing 0.3 g of arachidonic acid or a HFD (45% fat by kcal) containing 3.0 g of arachidonic acid from weaning (3 wk of age) for 13 wk unless otherwise mentioned (Research Diets, Inc., New Brunswick, NJ). All the animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Maine Medical Center Research Institute.
RNA isolation and quantitative real-time PCR
Total RNA from femurs was isolated using TRIzol extraction (Invitrogen, Carlsbad, CA) and QIAGEN (Valencia, CA) RNeasy purification kit according to the manufacturer's instructions. Genomic DNA was removed from the RNA samples using the deoxyribonuclease treatment and removal reagents (Ambion, Inc., Austin, TX). RNA integrity was confirmed by 1% agarose gel electrophoresis; 1.5 μg of the total RNA were then reverse transcribed using the SuperScript III First-Strand Synthesis SuperMix kit (Invitrogen) according to the manufacturer's protocols. Quantitative real-time PCR was performed using the iQ SYBR Green Supermix and Bio-Rad iQ5 multicolor Real-Time PCR detection system (Bio-Rad, Hercules, CA). Relative expression of mRNA was determined after normalization to GAPDH levels unless otherwise mentioned in the manuscript. Cox-2 primer pair was purchased from SABiosciences (Frederick, MD). Primer sequences used in this experiment are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Evaluation of body composition
Body weights were measured using a standard balance in grams. Dual-energy x-ray absorptiometry (DXA) scanning was done by the PIXImus (GE-Lunar, Madison, WI) as previously described (31). The PIXImus was used to assess whole-body areal BMD (aBMD), areal bone mineral content (aBMC), and body composition in Alox5−/− and Alox5+/+ mice at 8 and 16 wk of age. A phantom standard provided by the manufacturer was assessed each day for instrument calibration. BMC by PIXImus is highly correlated with mineral content of hydroxyapatite standard of known density (r2 = 0.997) (32).
Peripheral quantitative computed tomography (pQCT)
pQCT was performed on the femurs of female and male Alox5−/− and Alox5+/+ mice at 16 wk of age as previously described (31). Isolated femur lengths were measured with digital calipers (Stoelting, Wood Dale, IL), and then femurs were measured for density using the SA Plus densitometer (Orthometrics, White Plains, NY). Calibration of the SA Plus instrument was accomplished with a manufacturer-supplied phantom and with hydroxyapatite standards of known density (50–1000 mg/mm3) with cylindrical dimensions (2.4 mm diameter × 24 mm length) that approximate mouse femurs. Accuracy of linear measures was checked with defined thickness aluminum foils. The bone scans were analyzed with thresholds of 710 and 570 mg/cm3, using Orthometrics software version 5.50 yielding cortical bone areas (BA) that were consistent with histomorphometrically derived periosteal values. Mineral content was determined with thresholds of 220 and 400 mg/cm3, selected so that mineral from most partial voxels (0.07 mm) would be included in the analysis. Precision of the SA Plus for repeated measurement of a single femur was found to be within 1.2–1.4%. Cortical thickness (Ct.Th.) was obtained at the midshaft scan (33).
Microcomputed tomography (μCT)
Microarchitecture of the trabecular bone and midshaft cortical bone of the femur, and of the trabecular bone in the lumbar 5 (L5) vertebral body, was analyzed by μCT (resolution 10 μm, VivaCT-40; Scanco Medical AG, Bassersdorf, Switzerland). Bones were scanned at energy level of 55 kVp and intensity of 145 μA. The VivaCT-40 is calibrated weekly using a phantom provided by Scanco Medical AG. Trabecular bone volume fraction and microarchitecture were evaluated in the secondary spongiosa, starting approximately at 0.6 mm proximal to the distal femoral growth plate and extending proximally 1.5 mm. Approximately 230 consecutive slices were made at a 10.5-μm interval at the distal end of the growth plate and extending in a proximal direction, and 180 contiguous slices were selected for analysis. Measurements included bone volume/total volume, trabecular number (Tb.N.), trabecular thickness (Tb.Th.), and trabecular spacing. Scans for the cortical region were measured at the midpoint of each femur, with an isotropic pixel size of 21 μm and slice thickness of 21 μm, and used to calculate the average BA, total cross-sectional area, BA/total cross-sectional area, and Ct.Th. For midshaft analysis, the cortical shell was contoured by user-defined threshold and iterated across the 50 slices. For L5 vertebrae, slices were acquired for the entire vertebral body, and trabecular bone was evaluated in a region approximately 0.3 mm below the cranial and above the caudal growth plate regions with a 10.5-μm isotropic voxel size. All scans were analyzed using manufacturer software (version 4.05; Scanco Medical AG).
Histomorphometry
Histomorphometry was performed as previously described (31, 33). Briefly, tibias were collected at 16 wk of age, fixed in 70% ethyl alcohol, dissected, and embedded in methyl methacrylate. Longitudinal sections, 5 μm thick, were cut on a Micron microtome (Richard-Allan Scientific, Kalamazoo, MI) and stained with 0.1% toluidine blue (pH 6.4). Histomorphometric measurements were performed and analyzed by Osteomeasure software (Osteometrics, Atlanta, GA). The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (34).
Evaluation of marrow adiposity
Digital images of the proximal tibia were taken from the slides prepared for histomorphometry. Number of adipocytes was counted manually in four predetermined and fixed regions in the bone. 1) Just below the growth plate from the endosteal surface of the left cortical bone to midshaft. 2) Just below the growth plate from the endosteal surface of the right cortical bone to midshaft. 3) Distal (down the midshaft) from regions 1 and 2 and spanning the entire medullary canal from endosteum to endosteum. 4) A fixed region above the growth plate in the center of the secondary center of ossification. One bone from each of five mice was counted and the mean and sd were calculated.
In vitro osteoclastogenesis
Bone marrow cells were harvested from femurs and tibias. Four hundred and fifty thousand (4.5 × 105) cells were plated per well on 96-well plates in αMEM supplemented with 10% fetal calf serum, 1% penicillin-streptomycin 1000 U (Invitrogen), 30 ng/ml macrophage colony-stimulating factor (PeproTech, Inc., Rocky Hills, NJ), and 50 ng/ml soluble receptor activator of nuclear factor-κB ligand (RANKL) (PeproTech, Inc.). The culture medium was changed at d 3 and 6. At d 7, the culture was terminated, and cells were then fixed with 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA). Osteoclasts were identified by tartrate-resistant acid phosphatase staining kit from Sigma (St. Louis, MO). The number of cells containing three or more nuclei was counted as multinucleated osteoclasts.
Measurements of serum N-terminal propeptide of type I procollagen (P1NP) and C-telopeptide fragments of collagen type I (CTx) levels
Serum P1NP and CTx levels were measured using commercially available kits from IDS (Fountain Hills, AZ) according to the manufacturer's instructions. The assay sensitivities were 0.7 and 2 ng/ml for P1NP and CTx, respectively. The intraassay variations were 6.3 and 6.9%, respectively, for both assays. All measurements were performed in duplicate.
Celecoxib, a Cox-2 inhibitor, treatment on B6 and Alox5−/− mice
B6 and Alox5−/− were fed either 10% fat by kcal diet (control diet) or 45% fat by kcal diet (HFD) supplemented with 1000 ppm celecoxib, a Cox-2 inhibitor (Research Diets, Inc.) for 8 wk starting at 3 wk old. Mice were harvested at 11 wk old; μCT and gene expression were performed and analyzed for the effect of celecoxib on the skeletal phenotype. All procedures were approved by the Institutional Animal Care and Use Committee of the Maine Medical Center Research Institute.
Statistical analyses
All data are expressed as the mean ± sem. Results were analyzed for statistically differences using Student's t test or one-way ANOVA followed by Bonferroni multiple comparison post hoc test. A P value of less than 0.05 was considered statistically significant.
Results
Alox5−/− mice exhibit a low cortical bone mass phenotype
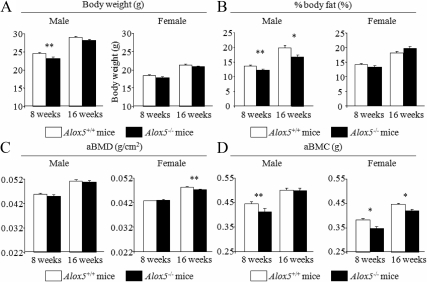
To investigate the in vivo consequences of the deletion of Alox5 gene on skeletal mass and adiposity, we first examined the body composition of Alox5−/− mice on a C57BL/6J (B6) background fed a regular chow diet and compared them with WT controls. Body weight of Alox5−/− male mice was lower than controls at 8 wk of age, but at 16 wk, body weight of male mice was comparable between the two genotypes (Fig. 1A). In females, body weight of Alox5−/− mice did not differ from WT mice both at 8 and 16 wk (Fig. 1A). Percent body fat was significantly decreased in Alox5−/− male mice compared with controls in male, but in females, there was no difference between Alox5−/− and controls (Fig. 1B).
Fig. 1.
Body composition of Alox5−/− mice. Alox5+/+ mice and Alox5−/− mice were fed on a standard chow, and body weight (A), % body fat (B), areal whole-body BMD (aBMD) (C), and aBMC (D) were analyzed by DXA at 8 and 16 wk of age. Values are expressed as the mean ± sem (n = 20–36). *, P < 0.01; **, P < 0.05.
With respect to the skeletal compartment, in male mice areal whole-body BMD (aBMD) was not different by genotype, both at 8 and 16 wk of age, whereas BMC was decreased in Alox5−/− mice compared with controls at 8 wk of age (P < 0.05) (Figs. 1, C and D). In female mice, Alox5−/− mice showed a significant reduction in aBMD at 16 wk of age, which is accompanied by a decrease in aBMC (Fig. 1, C and D). To evaluate skeletal microarchitecture, we performed pQCT and μCT analyses. pQCT analysis at 16 wk of age revealed a cortical bone phenotype such that Alox5−/− mice exhibited smaller cortical bones with a reduced periosteal circumference compared with WT controls (P < 0.01 and P < 0.05 for female and male vs. WT) (Table 1). The reduction in periosteal circumference was greater in Alox5−/− females than males (Table 1). The length of the femur was not different between Alox5−/− and control mice (Table 1). μCT analysis confirmed that microarchitecture parameters of trabecular bone were comparable between Alox5−/− and control mice both in males and females (Table 2). Taken together, Alox5−/− mice showed a moderate reduction in bone mass, which is partially explained by the reduced size and thickness of the cortical bones.
Table 1.
Cortical bone phenotype of midshaft of the femur by pQCT
| Male Alox5+/+ (n = 24) | Male Alox5−/− (n = 16) | Female Alox5+/+ (n = 14) | Female Alox5−/− (n = 20) | |
|---|---|---|---|---|
| Femur length (mm) | 15.89 ± 0.07 | 15.88 ± 0.08 | 15.57 ± 0.08 | 15.36 ± 0.09 |
| Cortical thickness (mm) | 0.199 ± 0.002 | 0.191 ± 0.004a | 0.187 ± 0.002 | 0.179 ± 0.003 |
| Periosteal circumference (mm) | 5.37 ± 0.05 | 5.19 ± 0.07b | 4.86 ± 0.03 | 4.75 ± 0.02c |
| Endosteal circumference (mm) | 4.12 ± 0.05 | 3.99 ± 0.06 | 3.69 ± 0.03 | 3.62 ± 0.03 |
Values are expressed as the mean ± sem.
P = 0.07 vs. male Alox5+/+.
P < 0.05 vs. male Alox5+/+.
P < 0.01 vs. female Alox5+/+.
Table 2.
Trabecular bone phenotype of distal femur by microCT
| Male Alox5+/+ (n = 15) | Male Alox5−/− (n = 8) | Female Alox5+/+ (n = 16) | Female Alox5−/− (n = 19) | |
|---|---|---|---|---|
| Bone volume/total volume (%) | 25.23 ± 0.62 | 24.22 ± 1.29 | 7.67 ± 0.49 | 8.50 ± 0.39 |
| Tb.N. (1/mm) | 5.62 ± 0.08 | 5.68 ± 0.06 | 3.92 ± 0.08 | 4.06 ± 0.08 |
| Tb.Th. (μm) | 59.87 ± 1.22 | 57.61 ± 1.31 | 47.18 ± 0.69 | 47.86 ± 0.79 |
| Trabecular spacing (μm) | 165.04 ± 2.81 | 162.29 ± 2.87 | 253.50 ± 5.43 | 245.05 ± 4.82 |
Values are expressed as the mean ± sem.
HFD causes loss of trabecular bone mass in Alox5−/− mice, whereas cortical bone mass is increased in C57BL/6J mice fed a HFD
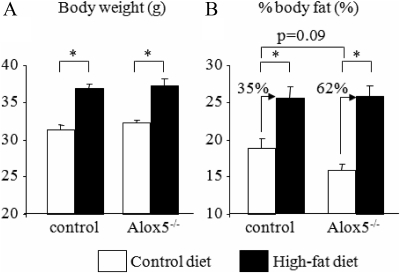
Next, we asked how dietary fat affects skeletal mass and adiposity in Alox5−/− mice. Because male Alox5−/− mice exhibited a more obvious phenotype in adiposity than female mice, i.e. reduced % body fat, we used male mice for this study. Male null and WT control mice were fed a 10% fat by kcal diet containing 0.3 g of arachidonic acid (control diet) or a 45% fat by kcal diet containing 3.0 g of arachidonic acid (HFD) for 13 wk after weaning (3 wk of age). We then analyzed body composition and skeletal phenotypes using DXA and μCT in both strains at 16 wk of age. HFD caused body weight gain both in control and Alox5−/− mice, but there was no difference in the weight gain between controls and Alox5−/− mice (Fig. 2A). Interestingly, Alox5−/− mice showed reduced % body fat on the regular diet compared with controls on a regular diet, but on a HFD, % body fat was not different between these two groups (Fig. 2B). However, the increase in % body fat between control diet and a HFD in Alox5−/− mice was much greater than that in control mice (62% difference in Alox5−/− vs. 35% difference in control mice) (Fig. 2B). Thus, the lack of Alox5 in mice resulted in a relatively greater increase in fat mass compared with WT mice fed a HFD.
Fig. 2.
Alox5−/− mice gained more adiposity than control mice. Alox5−/− mice were fed a control diet (10% fat by kcal) or a HFD (45% fat by kcal) from 3 wk of age for 13 wk. At 16 wk of age, body weight (A) and % body fat (B) were analyzed by DXA. Age and gender-matched C57BL/6J mice were used as control mice. Values are expressed as the mean ± sem (n = 19–35). *, P < 0.0001.
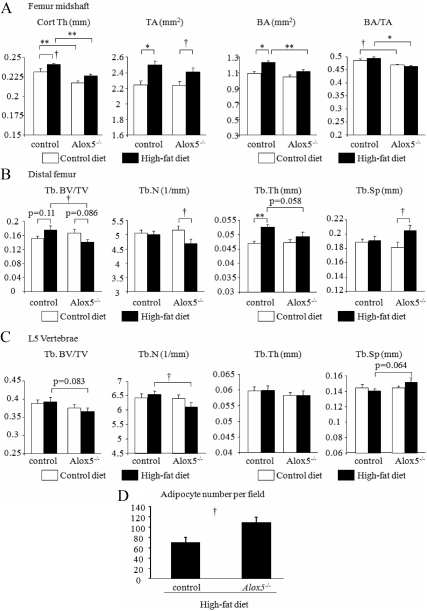
With respect to the skeletal phenotype, a HFD resulted in a significantly increased total femoral area (i.e. in the midshaft) both in WT and Alox5−/− mice (P < 0.05) (Fig. 3A). That increase in WT on a HFD (P < 0.001) was associated with significantly enhanced Ct.Th. (P < 0.05 vs. WT on a regular diet), whereas Ct.Th. did not differ in the Alox5−/− mice on either diet. Trabecular bone microarchitecture also showed differences between control and Alox5−/− mice on a HFD. In WT mice, a HFD did not significantly change femoral bone volume fraction, but there was a marked increase in femoral Tb.Th. (P < 0.01 WT on a HFD vs. WT on a regular diet), whereas the HFD reduced trabecular bone volume in Alox5−/− mice compared with WT on a HFD (P < 0.05), and this was associated with a significant decrease in Tb.N. (P < 0.05 vs. Alox5−/− and WT on a regular diet) (Fig. 3B). A similar skeletal phenotype was also observed in the L5 vertebrae (Fig. 3C). Interestingly, marrow adiposity was significantly increased in Alox5−/− mice compared with control mice on HFD (P < 0.05) (Fig. 3D). Taken together, Alox5−/− mice demonstrated a unique phenotype such that these mice gain adiposity but lose bone mass in response to a HFD compared with control mice on the same diet.
Fig. 3.
HFD induced bone loss in Alox5−/− mice but not in control mice. Alox5−/− mice were fed a control diet (10% fat by kcal) or HFD (45% fat by kcal) from 3 wk of age for 13 wk. At 16 wk of age, femur and vertebrae (L5) were collected, and skeletal microarchitecture was analyzed using μCT analysis. Cortical bone compartment was analyzed at the levels of midshaft of the femur (n = 9–10) (A). Trabecular bone compartment was assessed at the distal femur (n = 9–10) (B) and L5 vertebrae (n = 9–10) (C). The number of marrow adipocytes was counted at 16 wk of age (n = 5) (D). TA, Total cross-sectional area; BV/TV, bone volume/total volume; Tb.Sp, trabecular spacing. Age and gender-matched C57BL/6J mice were used as control mice. Values are expressed as the mean ± sem. *, P < 0.001; **, P < 0.01; †, P < 0.05.
HFD increases bone resorption in Alox5−/− mice
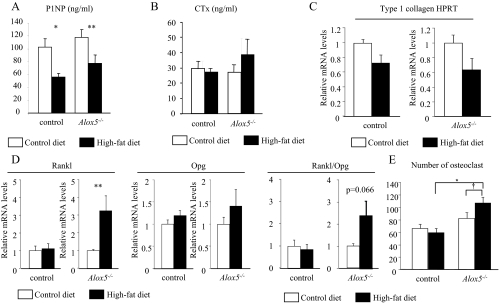
To further investigate the mechanisms whereby a HFD decreased bone mass in Alox5−/− mice, we performed static and dynamic histomorphometric analyses of the proximal tibia from both strains. There were no significant differences in the number of osteoblasts (N.Ob)/bone surface (BS) or the N.Ob/bone perimeter (BPm) on the regular (i.e. control diet) or the HFD in the WT mice (Table 3). In Alox5−/− mice, the HFD caused an increase in N.Ob/BPm (P < 0.05) (Table 3). However, bone formation rate and mineral apposition rate did not differ between mutants and controls either on a regular or HFD (Supplemental Table 2). Interestingly, serum P1NP was significantly decreased on a HFD both in control and Alox5−/− mice (P < 0.01 and P < 0.05 control diet vs. HFD, respectively) (Fig. 4A) whereas in the WT mice osteocalcin levels fell by 40% on a HFD (P < 0.05) but were unchanged in the Alox5−/− fed a HFD (Supplemental Fig. 1). Expression of type I collagen was decreased in the femur both in control and Alox5−/− mice on a HFD (Fig. 4C).
Table 3.
Histomorphometric analysis at 16 wk
| Alox5+/+ control diet | Alox5+/+ high-fat diet | Alox5−/− control diet | Alox5−/− high-fat diet | |
|---|---|---|---|---|
| ObS/BS | 9.64 ± 1.07 | 12.11 ± 0.98 | 12.06 ± 1.32 | 15.93 ± 1.09 |
| N.Ob/BPm | 10.68 ± 0.96 | 12.80 ± 1.07 | 12.95 ± 1.33 | 17.15 ± 1.20a |
| OcS/BS | 5.02 ± 0.65 | 3.71 ± 0.32 | 3.46 ± 0.37 | 3.95 ± 0.44 |
| N.Oc/BPm | 1.70 ± 0.21 | 1.21 ± 0.12 | 1.03 ± 0.07 | 1.40 ± 0.12b |
Values are expressed as the mean ± sem (n = 5). ObS, Osteoblast surface; OcS, osteoclast surface.
P < 0.05 vs. Alox5−/− control diet.
P = 0.028 vs. Alox5−/− control diet.
Fig. 4.
HFD increases N.Oc in Alox5−/− mice. Alox5−/− mice were fed a control diet (10% fat by kcal) or a HFD (45% fat by kcal) from 3 wk of age for 8 or 13 wk. A and B, Serum was collected at 16 wk of age, and serum concentration of P1NP (A) and CTx (B) was measured using ELISA (n = 5–10). C and D, RNA was collected from the femur at 11 wk of age, and the expression of type I collagen (n = 4) (C), Rankl, and Opg (n = 5–7) (D) was analyzed by real-time RT-PCR. HPRT expression was used as an internal control for the quantification of type I collagen expression. E, Osteoclastogenesis was induced using the BMSC isolated at 11 wk of age. The number of multinucleated cells (three or more than three nuclei) was calculated (n = 10). Age and gender-matched C57BL/6J mice were used as control mice. Values are expressed as the mean ± sem. *, P < 0.001; **, P < 0.01; †, P < 0.05.
In respect to bone resorption parameters, such as number of osteoclasts (N.Oc) and osteoclast surface/BS, WT mice showed a nonsignificant decrease in N.Oc/BPm on a HFD, whereas the N.Oc/BPm was increased 40% in Alox5−/− mice on a HFD compared with control diet (P = 0.028) (Table 3). Consistent with the alteration in N.Oc, serum markers for bone resorption, such as CTx, showed a nonsignificant increase in Alox5−/− on a HFD, whereas CTx levels were similar in WT mice on a regular and HFD (Fig. 4B).
To examine the underlying mechanisms of increased N.Oc in HFD-fed Alox5−/− mice, we first induced osteoclastogenesis from bone marrow stromal cells (BMSC) of WT (P < 0.01) and Alox5−/− mice fed either a regular or a HFD. As shown in Fig. 4E, BMSC from Alox5−/− fed a HFD demonstrated increased in vitro osteoclastogenesis, whereas osteoclastogenesis of BMSC from WT mice was comparable for a regular and HFD, suggesting that high fat enhances the osteoclast progenitor pool in the bone marrow of Alox5−/− mice (P < 0.01 vs. WT and P < 0.05 vs. Alox5−/− regular diet). Second, to investigate the possibility that increased N.Oc is caused by the indirect activation of osteoclastogenesis by osteoblasts, we analyzed the expression of Rankl and Opg in the femur of Alox5 null mice. Expression of Rankl and the ratio of Rankl expression over Opg expression were both enhanced in Alox5−/− mice on a HFD compared with those on a regular diet, whereas these parameters were unchanged in WT mice (P < 0.01) (Fig. 4D). These lines of evidence imply that HFD-induced bone loss in Alox5−/− mice is in part explained by enhanced bone resorption due to both noncell and cell autonomous increases in osteoclastogenesis.
The lack of Alox5 increases the skeletal expression of Cox-2 and Pparg and is magnified on a HFD
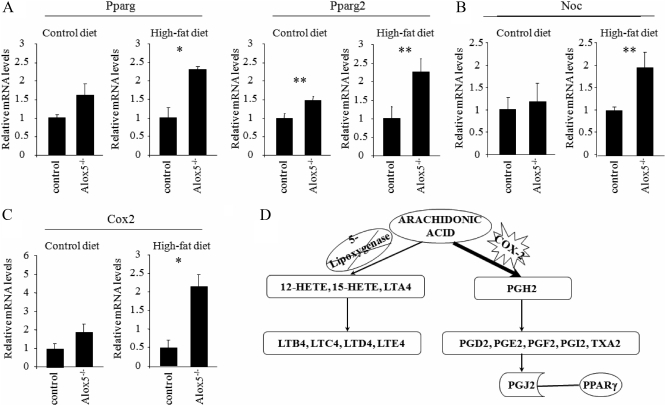
To test the hypothesis that in the absence of 5-LO, Pparg activity was enhanced, we first analyzed Pparg expression in whole femurs of Alox5−/− and control mice. Expression of Pparg in Alox5−/− mice was increased compared with control mice on a HFD (P < 0.01) (Fig. 5A). In addition, one of the downstream target of Pparg, Nocturnin, which is a positive regulator for adipogenesis and negative regulator for osteoblastogenesis (35), was increased in Alox5−/− mice compared with controls on a HFD (P < 0.05) (Fig. 5B).
Fig. 5.
HFD enhanced the expression of Pparg2 and Cox-2 in the femur of Alox5−/− mice. A–C, Alox5−/− mice were fed on a control diet (10% fat by kcal) or a HFD (45% fat by kcal) from 3 wk of age for 8 wk. RNA was collected from the femur at 11 wk of age, and the expression of Pparg, Pparg2 (A), Nocturnin (Noc) (B), and Cox-2 (C) was analyzed by real-time RT-PCR (n ≥ 4). D, Schematic model of the influence of the lack of Alox5 gene on Cox-2 pathway. The lack of Alox5 gene may enhance the Cox-2 expression, resulting in the Pparg activation probably due to the increased conversion of arachidonic acid into PG. HETE, Hydroxyeicosatetraenoic acid; LT, leukotriene. Age and gender-matched C57BL/6J mice were used as control mice. Values are expressed as the mean ± sem. *, P < 0.01; **, P < 0.05.
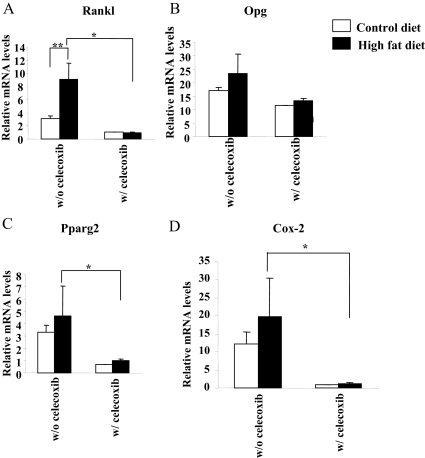
Previous studies revealed that 15d-PGJ2, which is derived from the sequential conversion from arachidonic acid through the COX pathway, is the endogenous ligand for Pparg (28). Because the expression of Cox-2 in the femur is inducible, unlike Cox-1, we tested whether Cox-2 expression was altered in the absence of 5-LO and with a HFD. As shown in Fig. 5C, Cox-2 expression was more than 4-fold enhanced in Alox5−/− mice fed a HFD (P < 0.01), implying that the Cox-2 pathway might be activated in Alox5−/− mice fed a HFD, resulting in activation of Pparg (Fig. 5D). To test whether activation of the Cox-2 pathway was responsible for the increase in osteoclastogenesis, we treated young male Alox5−/− mice on a regular or HFD with and without a Cox-2 inhibitor, celecoxib, for 8 wk and examined gene expression profiles in the femur. In the absence of celecoxib, Alox5−/− on a HFD had a nearly 4-fold increase in Rankl gene expression (P < 0.05) compared with mice on a control diet. This increase was totally suppressed by administration of the Cox-2 inhibitor (P < 0.002) (Fig. 6A), whereas no significant changes in Opg were noted (Fig. 6B). Similarly, Pparg2 expression was markedly suppressed in the mutant mice on a HFD by treatment with celecoxib (P < 0.011) (Fig. 6C). Not surprisingly, treatment with a Cox-2 inhibitor resulted in near total absence of Cox-2 gene expression in the Alox5 null mice on either a control or HFD (Fig. 6D).
Fig. 6.
Celecoxib treatment significantly suppressed Rankl, Pparg2, and Cox-2 expression in the femur of Alox5−/− mice fed a HFD. A–D, Alox5−/− mice were fed a control diet (10% fat by kcal) or a HFD (45% fat by kcal) supplemented with 1000 ppm celecoxib from 3 wk of age for 8 wk. RNA was collected from the femur at 11 wk of age, and the expression of Rankl (A), Opg (B), Pparg2 (C), and Cox-2 (D) was analyzed by real-time RT-PCR (n ≥ 5). Values are expressed as the mean ± sem. *, P < 0.02; **, P < 0.05.
Discussion
In this study, we demonstrated an important gene by environmental interaction in a critical pathway for the generation of fatty acids, PG, and chemokines. On a regular diet, global deletion of the Alox5 gene was associated with minimal differences in body weight, areal bone mass, and trabecular bone volume compared with control mice. However, when control and mutant mice were fed a 45% fat by kcal diet for 13 wk, control mice increased cortical and Tb.Th., whereas there was either no change or loss of bone mass in Alox5−/− mice. In addition, these changes were associated with increased bone resorption due to enhanced Rankl and Pparg2 expression. Furthermore, the higher expression of Cox-2 suggested that absence of 5-LO activity was associated with a shift of arachidonic acid metabolism into the PG pathway with subsequent activation of Pparg (Fig. 5D).
There is emerging evidence that obesity may impact both skeletal accrual and bone maintenance, although the effects may differ by skeletal compartment. However, the mechanisms whereby obesity or increased dietary fat intake affects bone remodeling require further clarification.
For example, Goulding et al. (4) demonstrated that in pubertal children, BMD and high body weight were both significant risk factors for fractures. Gilsanz and co-workers (6) have shown that increased body fat was negatively associated with trabecular bone mass but positively associated with Ct.Th. as measured by QCT. We previously noted that in the Framingham cohort, polymorphisms in the Pparg gene were associated with lower bone mass but only when a HFD was consumed (36). Furthermore, in experimental animals, a diet high in saturated fat was associated with bone loss due to increased bone resorption (37).
Two mechanisms have been proposed for HFD-induced bone loss. The first is indirect and related to the release of leptin from enlarging adipocytes during the weight gain associated with high intakes of dietary fat. High leptin levels act on the hypothalamus, and/or brain stem, to stimulate sympathetic nervous system activation, which in turn leads to suppression of bone formation and an increase in bone resorption through the β2 adrenergic receptor (38, 39). However, we measured serum leptin levels in our study and found that although leptin increased in both strains on a HFD, there were no significant differences between WT control and Alox5−/− mice (Supplemental Fig. 2). The second hypothesis relates to fatty acid metabolism. High levels of fatty acids and their metabolites in the circulation may have an endocrine effect on bone cell function by suppressing bone formation (40). In addition, these compounds may locally increase oxidative stress and generate inflammatory cytokines that uncouple resorption from formation by enhancing osteoclastogenesis (41).
In this study, we identified another possible mechanism that may be operative in mice and potentially in some individuals, i.e. changes in the Alox5/Cox-2/Pparg signaling network. Polymorphisms in the Alox5 gene are common and have been associated with a number of metabolic and cardiovascular disorders (8, 15, 16). Hence, our observations may have clinical significance particularly in individuals who have partially reduced 5-LO function. In Alox5−/− mice, it is likely that conversion of arachidonic acid toward PG synthesis was enhanced due to the increased expression of Cox-2. PGE2 is one such PG which has a profound role in skeletal metabolism. PGE2 was initially found to stimulate bone resorption, but accumulating evidence also demonstrates that PGE2 can enhance bone formation (42). Interestingly, continuous PGE2 administration results in bone loss in rats, whereas intermittent administration enhances bone mass (43). The effect of other PG on skeletal metabolism is not as well described, but PGD2 has been shown to have positive effects on osteogenesis in vitro (44) and can increase bone mass in ovariectomized rat models (45). Similarly, agonists and antagonists for the PG receptors can have significant effects on bone formation and have been studied as a means to enhance fracture healing. Further studies are needed to clarify the involvement of other types of PG in the skeletal phenotype of Alox5−/− mice placed on a HFD.
At the skeletal level, a HFD after weaning induces changes in both bone resorption and bone formation (36, 46). As we noted previously, C57BL/6J mice exposed to a 45% fat diet (by kcal) for 13 wk during the pubertal and postpubertal growth phases show either no change or a modest increase in aBMD (36). This may be related to a marked reduction in both bone resorption and bone formation, leading to some slowing in age-related bone loss. In addition, an increase in overall percent body fat can also lead to increased cortical bone mass, presumably due to the loading effect of greater weight on the periosteum. On the other hand, in Alox5−/− mice, N.Oc was significantly increased, and this was accompanied by a marked increase in skeletal Rankl expression. Furthermore, in vitro, bone marrow cells isolated from Alox5−/− mice on a HFD cultured with macrophage colony-stimulating factor and soluble RANKL showed a greater propensity to form multinucleated Trap5b positive cells than marrow cells from control mice.
To further investigate the mechanism of bone loss with a HFD in Alox5−/− mice, we looked more carefully at the Pparg signaling pathways. Several lines of evidence led us in that direction. First, Pparg expression is increased in the skeleton and adipose tissue of mice on a HFD. Second, our earlier studies have shown that in congenic mice with a gain of function polymorphism in Pparg, there was low bone mass and high bone turnover (47). Third, inhibition of 5-LO has been shown in vitro to activate the Pparg pathway through increased expression of Cox-2 (30). Indeed, we showed that in Alox5−/− mice, but not C57BL/6J mice on a regular diet, there is a modest increase in Pparg2 expression in bone marrow. That difference is markedly enhanced when both strains were placed on a HFD but was suppressed when Alox5−/− mice were placed on a HFD and treated with a selective Cox-2 inhibitor.
Increased Pparg activity has been strongly associated with impaired bone formation and a lineage shift of mesenchymal stem cells from preosteoblasts to adipocytes (17, 18). The most frequent example of this change is noted in mice treated with rosiglitazone (36). Indeed, we observed an increase in marrow adipocytes in the Alox5−/− but not the control mice exposed to the HFD. Interestingly, bone formation markers were reduced in both strains but did not differ by genotype in response to high-fat feeding. Recently, Wan et al. (48) demonstrated that conditional deletion of Pparg in the hematopoietic lineage led to markedly reduced bone resorption and an osteopetrotic phenotype by suppressing c-Fos. Moreover, the Wan group also found that Pparg activation results in high bone resorption due to changes in the expression of estrogen receptor-related receptor alpha and peroxisome proliferator-activated receptor gamma coactivator 1-beta, key enzymes in mitochondrial function (49). Those findings coupled with our data that in vitro osteoclast formation and skeletal Rankl expression is markedly enhanced in Alox5−/− on a HFD suggest there may be both cell autonomous and noncell autonomous effects from high dietary fat. Notwithstanding, it is likely that the bone loss induced by a HFD in Alox5−/− mice can be attributed to changes in Pparg activation. Whether this is a direct result of changes in PG production cannot be ascertained from this study. Nor can we exclude the possibility that sympathetic activity is higher in the mutant mice fed a HFD as some form of a compensatory mechanism related to their diets.
In conclusion, deletion of the Alox5 gene leading to global loss of function in 5-LO results in a strain-specific response to high-fat feeding that includes greater weight gain and bone loss due to enhanced Pparg activity. Our study once again demonstrates the importance of gene by environmental interactions and supports the current tenet that under certain circumstances, a HFD and the resultant obesity may cause significant bone loss.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Health Grant NIH AR 56404 (to C.J.R. and M.C.H.). Additional support was provided by the Maine Medical Center Research Institute Bioinformatics Core Facility as supported by NIH P20RR18789 (DM Wojchowski, PI) and the Small Animal Imaging Core Facility as supported by NIH P20RR01555 and P30RR030927 (Robert Friesel, PI).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMC
- Areal bone mineral content
- aBMD
- areal BMD
- BA
- bone area
- BMD
- bone mineral density
- BMSC
- bone marrow stromal cell
- BPm
- bone perimeter
- BS
- bone surface
- Cox
- cyclooxygenase
- μCT
- microcomputed tomography
- Ct.Th.
- cortical thickness
- CTx
- C-telopeptide fragments of collagen type I
- 15d-PGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- DXA
- dual-energy x-ray absorptiometry
- HFD
- high-fat diet
- L5
- lumbar 5
- 5-LO
- 5-lipoxygenase
- N.Ob
- number of osteoblast
- N.Oc
- number of osteoclast
- PG
- prostaglandin
- P1NP
- N-terminal propeptide of type I procollagen
- Pparg
- peroxisome proliferator-activated receptor-γ
- pQCT
- peripheral quantitative computed tomography
- RANKL
- receptor activator of nuclear factor-κB ligand
- Tb.N.
- trabecular number
- Tb.Th.
- trabecular thickness
- WT
- wild type.
References
- 1. McCarthy MI. 2010. Genomics, type 2 diabetes, and obesity. N Engl J Med 363:2339–2350 [DOI] [PubMed] [Google Scholar]
- 2. Kawai M, Rosen CJ, Bone 2010. adiposity and bone accrual-still an established paradigm? Nat Rev Endocrinol 6:63–64 [DOI] [PubMed] [Google Scholar]
- 3. von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. 2007. Associations between the metabolic syndrome and bone health in older men and women: the rancho Bernardo study. Osteoporos Int 18:1337–1344 [DOI] [PubMed] [Google Scholar]
- 4. Goulding A, Taylor RW, Grant AM, Jones S, Taylor BJ, Williams SM. 2009. Relationships of appendicular LMI and total body LMI to bone mass and physical activity levels in a birth cohort of New Zealand five-year olds. Bone 45:455–459 [DOI] [PubMed] [Google Scholar]
- 5. Dimitri P, Wales JK, Bishop N. 2010. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J Bone Miner Res 25:527–536 [DOI] [PubMed] [Google Scholar]
- 6. Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. 2007. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab 92:143–147 [DOI] [PubMed] [Google Scholar]
- 7. Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. 2010. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol 6:71–82 [DOI] [PubMed] [Google Scholar]
- 8. Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. 2004. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med 350:29–37 [DOI] [PubMed] [Google Scholar]
- 9. Williams ES, Baylin A, Campos H. 2007. Adipose tissue arachidonic acid and the metabolic syndrome in Costa Rican adults. Clin Nutr 26:474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garaulet M, Pérez-Llamas F, Pérez-Ayala M, Martínez P, de Medina FS, Tebar FJ, Zamora S. 2001. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 74:585–591 [DOI] [PubMed] [Google Scholar]
- 11. Decsi T, Molnár D, Koletzko B. 1996. Long-chain polyunsaturated fatty acids in plasma lipids of obese children. Lipids 31:305–311 [DOI] [PubMed] [Google Scholar]
- 12. Samuelsson B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220:568–575 [DOI] [PubMed] [Google Scholar]
- 13. Peters-Golden M, Henderson WR., Jr 2007. Leukotrienes. N Engl J Med 357:1841–1854 [DOI] [PubMed] [Google Scholar]
- 14. Rådmark O, Samuelsson B. 2010. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun 396:105–110 [DOI] [PubMed] [Google Scholar]
- 15. Allayee H, Baylin A, Hartiala J, Wijesuriya H, Mehrabian M, Lusis AJ, Campos H. 2008. Nutrigenetic association of the 5-lipoxygenase gene with myocardial infarction. Am J Clin Nutr 88:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehrabian M, Allayee H, Stockton J, Lum PY, Drake TA, Castellani LW, Suh M, Armour C, Edwards S, Lamb J, Lusis AJ, Schadt EE. 2005. Integrating genotypic and expression data in a segregating mouse population to identify 5-lipoxygenase as a susceptibility gene for obesity and bone traits. Nat Genet 37:1224–1233 [DOI] [PubMed] [Google Scholar]
- 17. Kawai M, Sousa KM, MacDougald OA, Rosen CJ. 2010. The many facets of PPARγ: novel insights for the skeleton. Am J Physiol Endocrinol Metab 299:E3–E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawai M, Rosen CJ. 2010. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol 6:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tontonoz P, Spiegelman BM. 2008. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312 [DOI] [PubMed] [Google Scholar]
- 20. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. 1994. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8:1224–1234 [DOI] [PubMed] [Google Scholar]
- 21. Rosen ED, MacDougald OA. 2006. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896 [DOI] [PubMed] [Google Scholar]
- 22. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. 2004. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. 2004. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lecka-Czernik B, Rosen CJ, Kawai M. 2010. Skeletal aging and the adipocyte program: new insights from an “old” molecule. Cell Cycle 9:3648–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. 2010. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95:592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. 2007. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92:1305–1310 [DOI] [PubMed] [Google Scholar]
- 27. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 1995. 15-Deoxy-δ 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83:803–812 [DOI] [PubMed] [Google Scholar]
- 28. Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. 2003. Biosynthesis of 15-deoxy- δ 12,14-PGJ2 and the ligation of PPARγ. J Clin Invest 112:945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwak HJ, Park KM, Choi HE, Lim HJ, Park JH, Park HY. 2010. The cardioprotective effects of zileuton, a 5-lipoxygenase inhibitor, are mediated by COX-2 via activation of PKCδ. Cell Signal 22:80–87 [DOI] [PubMed] [Google Scholar]
- 30. Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, Das R, Jett M, Mulshine JL. 2001. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J 15:2007–2009 [DOI] [PubMed] [Google Scholar]
- 31. DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. 2008. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology 149:2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delahunty KM, Shultz KL, Gronowicz GA, Koczon-Jaremko B, Adamo ML, Horton LG, Lorenzo J, Donahue LR, Ackert-Bicknell C, Kream BE, Beamer WG, Rosen CJ. 2006. Congenic mice provide in vivo evidence for a genetic locus that modulates serum insulin-like growth factor-I and bone acquisition. Endocrinology 147:3915–3923 [DOI] [PubMed] [Google Scholar]
- 33. Beamer WG, Shultz KL, Ackert-Bicknell CL, Horton LG, Delahunty KM, Coombs HF, 3rd, Donahue LR, Canalis E, Rosen CJ. 2007. Genetic dissection of mouse distal chromosome 1 reveals three linked BMD QTLs with sex-dependent regulation of bone phenotypes. J Bone Miner Res 22:1187–1196 [DOI] [PubMed] [Google Scholar]
- 34. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 35. Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ. 2010. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-γ nuclear translocation. Proc Natl Acad Sci USA 107:10508–10513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ackert-Bicknell CL, Demissie S, Marín de Evsikova C, Hsu YH, DeMambro VE, Karasik D, Cupples LA, Ordovas JM, Tucker KL, Cho K, Canalis E, Paigen B, Churchill GA, Forejt J, Beamer WG, Ferrari S, Bouxsein ML, Kiel DP, Rosen CJ. 2008. PPARG by dietary fat interaction influences bone mass in mice and humans. J Bone Miner Res 23:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao JJ, Gregoire BR, Gao H. 2009. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 44:1097–1104 [DOI] [PubMed] [Google Scholar]
- 38. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 39. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- 40. Kawai M, Devlin MJ, Rosen CJ. 2009. Fat targets for skeletal health. Nat Rev Rheumatol 5:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manolagas SC. 2010. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blackwell KA, Raisz LG, Pilbeam CC. 2010. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab 21:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tian XY, Zhang Q, Zhao R, Setterberg RB, Zeng QQ, Iturria SJ, Ma YF, Jee WS. 2008. Continuous PGE2 leads to net bone loss while intermittent PGE2 leads to net bone gain in lumbar vertebral bodies of adult female rats. Bone 42:914–920 [DOI] [PubMed] [Google Scholar]
- 44. Koshihara Y, Kawamura M. 1989. Prostaglandin D2 stimulates calcification of human osteoblastic cells. Biochem Biophys Res Commun 159:1206–1212 [DOI] [PubMed] [Google Scholar]
- 45. Takagi T, Yamamoto T, Asano S, Tamaki H. 1993. Effect of prostaglandin D2 on the femoral bone mineral density in ovariectomized rats. Calcif Tissue Int 52:442–446 [DOI] [PubMed] [Google Scholar]
- 46. Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. 2009. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology 150:1330–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M. 2004. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35:1046–1058 [DOI] [PubMed] [Google Scholar]
- 48. Wan Y, Chong LW, Evans RM. 2007. PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13:1496–1503 [DOI] [PubMed] [Google Scholar]
- 49. Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. 2010. PGC1β mediates PPARγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11:503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.