Abstract
Many philosophical and contemplative traditions teach that “living in the moment” increases happiness. However, the default mode of humans appears to be that of mind-wandering, which correlates with unhappiness, and with activation in a network of brain areas associated with self-referential processing. We investigated brain activity in experienced meditators and matched meditation-naive controls as they performed several different meditations (Concentration, Loving-Kindness, Choiceless Awareness). We found that the main nodes of the default-mode network (medial prefrontal and posterior cingulate cortices) were relatively deactivated in experienced meditators across all meditation types. Furthermore, functional connectivity analysis revealed stronger coupling in experienced meditators between the posterior cingulate, dorsal anterior cingulate, and dorsolateral prefrontal cortices (regions previously implicated in self-monitoring and cognitive control), both at baseline and during meditation. Our findings demonstrate differences in the default-mode network that are consistent with decreased mind-wandering. As such, these provide a unique understanding of possible neural mechanisms of meditation.
Keywords: mindfulness, task-positive network, attention
Mind-wandering is not only a common activity present in roughly 50% of our awake life, but is also associated with lower levels of happiness (1). Moreover, mind-wandering is known to correlate with neural activity in a network of brain areas that support self-referential processing, known as the default-mode network (DMN) (2–7). This network has been associated with processes ranging from attentional lapses to anxiety to clinical disorders, such as attention-deficit hyperactivity disorder (ADHD) and Alzheimer's Disease (6, 8, 9). Given the interrelationship between the DMN, mind-wandering, and unhappiness, a question arises: Is it possible to change this default mode into one that is more present-centered, and possibly happier?
One potential way to reduce DMN activity is through the practice of mindfulness meditation. Mindfulness, a core element of diverse forms of meditation, is thought to include two complementary components: (i) maintaining attention on the immediate experience, and (ii) maintaining an attitude of acceptance toward this experience (10). Specific types of mindfulness meditation have been taught in a standardized fashion for decades as a mainstay of mindfulness training in community and clinical settings [e.g., through traditional teacher- or retreat-led mindfulness meditation practice, mindfulness-based stress reduction (MBSR), mindfulness-based cognitive therapy, and mindfulness-based relapse prevention] (11–15). In the present investigation, we used three standard and commonly used meditation practices: Concentration, Loving-Kindness, and Choiceless Awareness. Through focused attention on a single object of awareness (typically the breath), Concentration meditation is intended to help individuals retrain their minds from habitually engaging in self-related preoccupations (such as thinking about the past or future, or reacting to stressful stimuli) to more present moment awareness (11). Loving-Kindness meditation is hypothesized to foster acceptance, both of oneself and others, as well as to increase concentration. This type of meditation is practiced through directed well-wishing, typically by repetition of phrases such as “may (I/someone else) be happy” (11). Choiceless Awareness is hypothesized to broaden the scope of mindfulness to all aspects of experience, whether during formal meditation practice or everyday life, via directly attending to whatever arises in one's conscious field of awareness at any moment (11, 16). During such training, meditators learn to clearly identify when self-related thoughts, emotions, and body sensations are occurring, and to differentiate identification of these from identifying with them (e.g., awareness that anger is present vs. “I am angry”). That is, meditators practice noticing when they are identifying with an object, and when this occurs, to “let go” and bring their attention back to the present moment. Across these practices, one common aim is to reverse the habit of mind-wandering, which has been defined as “thinking about something other than what [one is] currently doing” (1). In other words, the meditator's task is to remain aware from moment to moment, and self-identification is included in the off-task category of mind-wandering. Importantly, this information-processing task, common to all three of these meditation techniques, is a training of attention away from self-reference and mind-wandering, and potentially away from default-mode processing.
Clinically, mindfulness training has shown benefit for the treatment of pain (13), substance-use disorders (15, 17), anxiety disorders (18), and depression (14), and also helps to increase psychological well-being in nonclinical populations (19). These outcomes have been associated with changes in basic psychological processes, such as improved attentional focus (20, 21), improved cognitive flexibility (22), reduced affective reactivity (23, 24), and modification or shifts away from a distorted or exaggerated view of oneself (18, 25). However, direct links between the meditative practices that are part of mindfulness training and changes in neurobiology remain elusive. Investigation of the brain activation patterns during specific meditation practices may help to identify potential neural mechanisms of mindfulness training.
Previous studies have examined individuals using meditation techniques from different traditions (e.g., Tibetan Buddhism, Zen Buddhism, Vipassana, MBSR, and so forth), and employed a wide variety of experimental methods, ranging from performance of different types of meditation, to introduction of emotionally charged sounds during meditation, to assessment of functional connectivity (16, 26–30). However, given the methodological differences and, in some cases, difficulty in finding appropriately matched controls, no consensus has emerged as to what the neural correlates of meditation are or how they may underlie the behavioral changes that have been observed after mindfulness training.
We hypothesized that the DMN would be an important locus of change following meditation training, based on recently reported links between mind-wandering and increased activation in regions of the DMN (2, 8), and the observation that the task of mindfulness meditation is to maintain attention on an object of awareness and to redirect one's attention to this object when it has strayed (requiring both attention and cognitive control). Specifically, we predicted that brain activation during mindfulness meditation in experienced meditators compared with their matched controls would involve: (i) relatively reduced recruitment of the DMN, and (ii) relatively increased connectivity between DMN and brain structures that are implicated in monitoring for conflict, as well as cognitive control, such as the dorsal anterior cingulate (dACC) and dorsolateral prefrontal cortices (dlPFC), respectively (31, 32). To test these predictions, we used functional MRI to assess brain activation during both a resting state and a meditation period in experienced mindfulness meditation practitioners and controls. To determine common neural activation patterns across meditations, we scanned participants during periods of Concentration, Loving-Kindness, and Choiceless Awareness meditation.
Results
Self-Report.
As expected, experienced meditators reported less mind-wandering during meditation relative to controls [F(1,22) = 7.93, P = 0.010]. This finding was apparent for Concentration (controls = 4.9 ± 2.9, meditators = 3.2 ± 1.3), Loving-Kindness (controls = 5.0 ± 2.8, meditators = 3.2 ± 1.3), and Choiceless Awareness meditation (controls = 6.0 ± 3.1, meditators = 3.4 ± 1.5). Across groups, there was also an effect of time [F(1,22) = 5.01, P = 0.036], such that reported mind-wandering was greater during the second run of each meditation condition (Time 1 = 4.08 ± 1.9, Time 2 = 4.48 ± 2.16). Both meditators and controls reported being able to follow the instructions to a high degree for the Concentration (controls = 7.5 ± 2.3, meditators = 8.1 ± 1.2), Loving-Kindness (controls = 7.5 ± 2.6, meditators = 7.8 ± 1.5), and Choiceless Awareness meditation conditions (controls = 8.5 ± 2.0, meditators = 7.9 ± 1.3).
General Linear Modeling Results.
To test the hypothesis that meditators would show differential changes in brain activation during meditation relative to controls, we first performed a between-groups whole-brain contrast analysis collapsing across the three meditation conditions. We found relatively less activation in meditators compared with controls in the posterior cingulate cortex/precuneus (PCC), a primary node of the DMN (7), as well as the superior, middle, and medial temporal gyri and uncus (Fig. 1, Fig. S1, and Table S1). We found a similar pattern in the medial prefrontal cortex (mPFC), another primary node of the DMN, although it did not survive whole-brain correction for significance (cluster size k = 33, threshold k = 43) (Fig. 1 and Table S1).
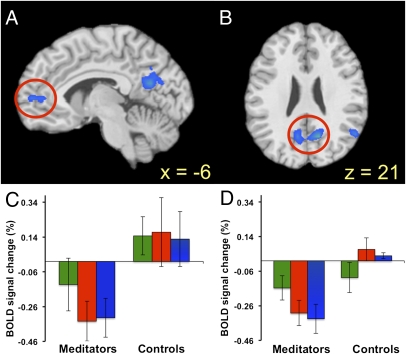
Fig. 1.
Experienced meditators demonstrate decreased DMN activation during meditation. Brain activation in meditators > controls is shown, collapsed across all meditations (relative to baseline). (A and B) Activations in the left mPFC and PCC. (C and D) Average percent signal change (± SD) during individual meditation conditions in the mPFC and PCC, respectively: Choiceless Awareness (green bars), Loving-Kindness (red), and Concentration (blue) meditations. Note that decreased activation in PCC in meditators is common across different meditation types. n = 12 per group.
We next examined between-group differences in each meditation condition. During the Concentration meditation condition, there was relatively less activation in meditators in the PCC and left angular gyrus (Fig. 1D, Fig. S1B, and Table S1) compared with controls. During Loving-Kindness meditation, there was relatively less activation in meditators compared with controls in the PCC, inferior parietal lobule, and inferior temporal gyrus extending into the hippocampal formations, amygdala, and uncus (Fig. 1D, Fig. S1C, and Table S1). During Choiceless Awareness, there was relatively less activation observed in meditators compared with controls in the superior and medial temporal gyrus (Fig. S1D and Table S1).
Functional Connectivity Results.
To test the hypothesis that meditators coactivate different brain regions compared with controls when nodes of the DMN become activated, we next performed functional connectivity analyses during both baseline and meditation periods, using a priori-defined DMN seed regions from the mPFC and PCC [Montreal Neurological Institute (MNI) coordinates −6, 52, −2 and −8, −56, 26, respectively] (7).
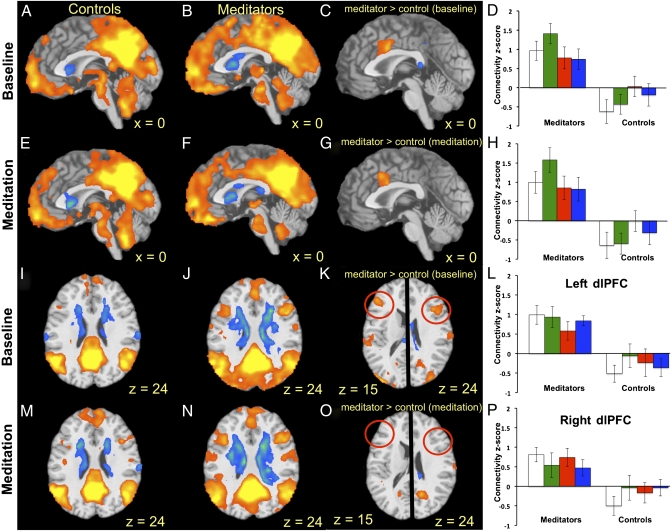
Using the PCC as the seed region, across all meditation conditions we found significant differences in connectivity patterns with several regions, notably the dACC, (Fig. 2 E and H, Fig. S1F, and Table S2). This pattern of differential between-group connectivity was also found during the resting-state baseline period, suggesting a stable pattern of connectivity regardless of task (resting-state baseline vs. meditation) (Fig. 3, Fig. S1E, and Table S2). We found a similar connectivity pattern between the PCC and dlPFC at baseline (Fig. 2 I–L) that was not significantly different between groups during meditation because of a relatively lower strength of anticorrelations in controls (Fig. 2 M–P).
Fig. 2.
Experienced meditators demonstrate coactivation of PCC, dACC, and dlPFC at baseline and during meditation. Functional connectivity with the PCC seed region collapsed across all meditation conditions, is shown in (A and I) controls at baseline; (B and J) meditators at baseline; (C and K) meditators > controls at baseline; (E and M) controls during meditation; (F and N) meditators during meditation; (G and O) meditators > controls during meditation. Connectivity z-scores (± SD) are shown (D) for dACC cluster from C; (H) for dACC cluster from G; (L) for left dlPFC cluster from K; and (P) for right dlPFC cluster from K. Baseline (white bars), Choiceless Awareness (green bars), Loving-Kindness (red bars), and Concentration (blue bars) meditation conditions are shown separately for meditators (Left) and controls (Right). n = 12/group. FWE-corrected, P < 0.05.
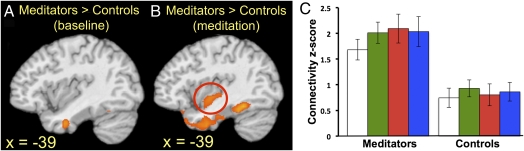
Fig. 3.
Experienced meditators demonstrate coactivation of mPFC, insula, and temporal lobes during meditation. Differential functional connectivity with mPFC seed region and left posterior insula is shown in meditators > controls: (A) at baseline and (B) during meditation. (C) Connectivity z-scores (±SD) are shown for left posterior insula. Choiceless Awareness (green bars), Loving-Kindness (red), and Concentration (blue) meditation conditions are shown separately. For each color, baseline condition is displayed on the left and the meditation period on right. n = 12/group. FWE-corrected, P < 0.05.
Using the mPFC as the seed region, we found increased connectivity with the fusiform gyrus, inferior temporal and parahippocampal gyri, and left posterior insula (among other regions) in meditators relative to controls during meditation (Fig. 3, Fig. S1H, and Table S3). A subset of those regions showed the same relatively increased connectivity in meditators during the baseline period as well (Fig. S1G and Table S3).
Discussion
As predicted, across all mindfulness meditation conditions, the two primary nodes of the DMN (the PCC and mPFC) were less active in meditators than controls. We also observed meditation-specific regional differences in activation patterns, such as deactivation in the amygdala during Loving-Kindness. Finally, using DMN seed regions, we observed distinct functional connectivity patterns in meditators that differed from controls, and which were consistent across resting-state baseline and meditation conditions. These results suggest that the neural mechanisms underlying mindfulness training are associated with differential activation and connectivity of the DMN. As meditators also reported significantly less mind-wandering, which has been previously associated with activity in the DMN, these results support the hypothesis that alterations in the DMN are related to reduction in mind-wandering. Finally, the consistency of connectivity across both meditation and baseline periods suggests that meditation practice may transform the resting-state experience into one that resembles a meditative state, and as such, is a more present-centered default mode.
We deliberately restricted our meditation sample to very experienced meditators from a single practice tradition (mindfulness/insight meditation). This approach was intended to reduce heterogeneity in meditation practices. Additional strengths of the study include the use of three standardized meditation techniques that are taught within this tradition, and the utilization of control subjects that were case-matched for a number of demographic parameters. This kind of matching increases the likelihood of yielding results that are both valid and generalizable to individuals in the Western hemisphere. Furthermore, because experienced meditators train to be mindfully aware all of the time, and thus may be activating similar brain regions during both resting-state and meditation, general linear modeling (GLM) analyses may be limited because of their dependence upon a relative change from baseline. Therefore, we employed functional connectivity as a complementary analytic technique within a single dataset. This convergent analysis directly addresses the limitations of baseline conditions in previous studies.
This study has several limitations. Most importantly, the sample size is moderately small, which typically limits the ability to detect small differences between conditions but increases the chances of false or inflated positive findings. Notwithstanding, we found whole-brain corrected, between-group differences, although these warrant replication before definitive conclusions may be drawn. Additionally, the use of meditation periods that were several minutes in length provides ecological validity to the meditation tasks as it approximates meditators’ usual practice more than shorter blocks and allows them to “sink into” deeper states of meditation, and at the same time optimizes functional connectivity analysis. However, this process de-optimizes GLM analysis. Finally, postrun recall of behavioral performance is limited by reporting bias. In addition, meditators may have differential awareness of the degree to which their minds wandered. Further studies are required to better correlate temporal patterns of neural activation with first-person reports.
From a theoretical perspective, the view of meditation as consisting of training away from mind-wandering and self-identification gave rise to several predictions that were confirmed by our data. First, given the primacy of the DMN in self-referential processing (33) and mind-wandering (2, 8), our primary prediction was that the DMN would be the main “target” of meditation practice, and that alterations in classic DMN activity would be found in experienced meditators relative to controls. Indeed, although not consistently, prior work has suggested alterations in DMN following brief meditation training and in experienced meditators (18, 25, 34). For example, consistent with previous reports of PCC activation during “selfing” tasks (33), Pagnoni et al. showed relative activation in the PCC in Zen meditators plus controls when viewing words vs. scrambled nonword letters when meditating, although no between-group differences were found (34). Furthermore, Farb et al. reported that individuals who had received 8 wk of MBSR demonstrated relative deactivation of the PCC when performing a task in which they engaged in awareness of thoughts, feelings, and body sensations when reading personality trait adjectives, compared with determining what the words meant to them personally (25). However, to date no studies have reported alterations in DMN activation or functional connectivity during meditation itself. Clarifying this prior work, our data are unique in that they provide direct evidence for this prediction, as meditators showed relatively decreased activation in the mPFC and PCC, the two primary nodes of the DMN during meditation. This finding is especially salient as meditators reported significantly less mind-wandering during meditation periods relative to controls. Taken together, and inasmuch as activity in DMN regions reflects self-referential processing and mind-wandering, the current data suggest that meditators are engaged in these processes less than their control counterparts.
A second prediction that emerged from the view of mindfulness as a task of monitoring and letting go of self-referential thought to keep present-focused attention, was that experienced meditators would be more likely to activate “task-positive” brain regions, such as those implicated in conflict monitoring, working memory, and cognitive control (8, 35–37). However, as noted above, we believe that this may be because of the dependence of GLM analysis on activity during baseline. Our baseline-independent functional connectivity analyses directly addressed this confound. We found that relative to controls, meditators showed increased connectivity between PCC and task-positive regions, during resting-state baseline and all meditation conditions, including those involved in conflict monitoring, cognitive control, and working memory (dACC and dlPFC) (8, 32, 35, 38). These findings suggest that meditators may be on-task regardless of condition, which also provides a possible explanation for the relative paucity of between-group differences that were observed with GLM analyses. Importantly, this increased connectivity with the dACC and dlPFC was not seen using the mPFC as the seed region, which is consistent with the purported role of the mPFC in integrating information gathered from the internal and external environment and relaying it to the PCC, rather than being directly involved in self-related processing (33, 39, 40). Interestingly, a study using independent component analysis to assess functional connectivity during a “mindful awareness” scan after an 8-wk MBSR course was recently reported (41). Similar to our mPFC seed-region results, the authors found increased connectivity between the mPFC and primary interoceptive awareness regions, including the posterior insula. However, the authors did not find increased connectivity with other DMN regions, such as the PCC. Several possible explanations for this difference include: (i) the use of different analytic tools (independent component analysis vs. a PCC seed region for connectivity analysis); (ii) the brief duration of meditation training (8 wk); and (iii) the specific emphasis on mindful awareness of sounds in the task instructions, among others.
Although direct links between white-matter tract integrity (e.g., diffusion tensor imaging), brain volume, and functional connectivity are just beginning to be established, several recent studies of meditation using these measures may support our findings. For example, Tang et al. showed improved white-matter tract integrity in the ventral anterior cingulate and dACC after just 11 h of Integrative Body-Mind Training meditation (42). In addition, Luders et al. found increased white-matter integrity in the dACC, among others in experienced meditators compared with controls (43). Regarding gray-matter density, in an exploratory analysis of individuals who had received MBSR, Holzel et al. found increased gray-matter concentration in the PCC (44). Furthermore, Luders et al. found increased gray-matter concentration in the inferior temporal gyrus in experienced meditators (45). Taken together, these studies of neuronal integrity and brain concentration may corroborate our findings, as these regions were shown to have increased connectivity in the present study.
The findings from this study support the default-mode interference hypothesis, which states that the DMN can persist or reemerge during goal-directed tasks “to such an extent that it competes with task-specific neural processing and creates the context for periodic attentional intrusions/lapses and cyclical deficits in performance” (46). This hypothesis has been built from observations of decreased activity in the task-positive network and increased activity in the DMN during mindlessness (2, 8), and has been further supported by the demonstration that stimulant (nicotine) administration enhances attention by deactivating areas of the DMN, such as the PCC (47). More importantly, pathological states have shown altered DMN connectivity and anticorrelations with the task-positive network (48). However, to our knowledge, no studies have shown convergence of the two networks, in states of well-being or otherwise. With reduced self-reported mind wandering, decreased mPFC and PCC activation during meditation, and increased connectivity patterns between DMN and self-control regions of the brain, our data provide corollary support for the interference hypothesis. Moreover, our functional connectivity data speculatively suggest that meditation practice may couple primary nodes of these networks in a potentially beneficial way. One possibility is that PCC is temporally linked to self-control regions such that when regions of the DMN emerge to “interfere” with a task, control regions may coactivate to monitor and dampen this process. This coactivation of monitoring/control regions along with nodes of the DMN may, over time, become a new “default mode” that can be observed during meditation as well as during the resting state.
Finally, the findings from this study have several clinical implications, as a number of pathological conditions have been linked to dysfunction within areas of the DMN (for a review see ref. 6). For example, ADHD is characterized by attentional lapses. The majority of research on the pathophysiology of ADHD has centered on frontal-striatal circuitry (49), but recent studies have begun to explore other mechanisms, including activity in and connectivity with nodes of the DMN (9). In particular, Castellanos et al. found decreases in correlations between the PCC and dACC in individuals with ADHD (9). Individuals who have undergone mindfulness training, during which they try to minimize attentional lapses, may be an interesting contrast to those with ADHD. Indeed, mindfulness training has shown preliminary efficacy in treating this disorder, but how it affects brain function in individuals with ADHD remains unknown (50). Our data raise the intriguing possibility that mindfulness may help to enhance PCC–dACC connectivity in individuals with ADHD, which may correlate with reduced attentional lapses. Another pathological condition that has been connected to DMN activity is Alzheimer's disease. Sustained neuronal activity has recently been linked to increased amyloid-β deposition (51). Results from our study suggest that meditation may decrease DMN activity in a relatively specific manner, using simple instructions and at low cost. As such, meditation may also bring with it the advantage of being accessible to many individuals, regardless of educational and economic background. Of course, prospective studies will be crucial in demonstrating this effect experimentally, and determining if meditation can delay the onset of Alzheimer's disease. Regardless of potential clinical implications, our findings demonstrate group differences in the DMN that are consistent with a decrease in mind-wandering in experienced meditators, and provide a basis for a new understanding of the neural bases of mindfulness meditation practice.
Methods
Subjects.
Twelve right-handed individuals with > 10 y and an average of 10,565 ± 5,148 h of mindfulness meditation experience, and 13 healthy volunteers were recruited to participate. Right-handed meditation-naive controls were case-control matched for country of origin (United States), primary language (English), sex, age, race, education, and employment status. One control participant did not follow directions and was removed before any analyses were performed. With the exception of a single mismatch in sex and age, respectively, all participants were well-matched (e.g., within 3 y of age of their match; see Table S4). All participants gave informed consent in accordance with the procedures of the Yale University Human Investigation Committee.
Task.
Just before scanning, all participants were introduced to three standard mindfulness meditation instructions: (i) Concentration: “Please pay attention to the physical sensation of the breath wherever you feel it most strongly in the body. Follow the natural and spontaneous movement of the breath, not trying to change it in any way. Just pay attention to it. If you find that your attention has wandered to something else, gently but firmly bring it back to the physical sensation of the breath.” (ii) Loving-Kindness: “Please think of a time when you genuinely wished someone well (pause). Using this feeling as a focus, silently wish all beings well, by repeating a few short phrases of your choosing over and over. For example: May all beings be happy, may all beings be healthy, may all beings be safe from harm.” (iii) Choiceless Awareness: “Please pay attention to whatever comes into your awareness, whether it is a thought, emotion, or body sensation. Just follow it until something else comes into your awareness, not trying to hold onto it or change it in any way. When something else comes into your awareness, just pay attention to it until the next thing comes along” (11). Participants practiced each meditation type outside of the scanner and confirmed that they understood and could follow the instructions before proceeding. Each run began with a 2-min resting-state baseline period (“please close your eyes and don't think of anything in particular”), which is consistent with standard resting-state induction procedures (3, 9, 36). This state was followed by a 30-s recorded meditation instruction (as above), and a 4.5-min meditation period. Every subject performed each meditation twice. Meditation conditions were presented in a random order, but the second instance of each meditation was blocked (i.e., AABBCC). After each run, participants were asked to rate how well they were able to follow the instructions and how much their mind wandered during each meditation period on a scale of 0 to 10.
Statistical Analysis of Self-Report Data.
We performed multivariate ANOVA using SPSS 18 (SPSS, Inc.). All tests of significance are reported as two-tailed and means are reported with ± SD.
Imaging Data Acquisition.
Functional and structural data were acquired on a 3T TRIO Siemens MRI scanner (Siemens Healthcare) located at Yale's Magnetic Resonance Research Center. A high-resolution, 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted sequence was used to acquire anatomical images [TR = 2,530 ms; echo time (TE) = 3.66 ms; Flip angle = 7°; Field of view = 256 × 256 mm; Matrix = 256 × 256; 176 1-mm slices]. Blood oxygen level-dependent (BOLD) functional images were acquired with a T2*-sensitive echo-planar image (EPI) gradient-echo pulse sequence (TR = 2,000 ms; TE = 25 ms; Flip angle: 85°; Field of view = 220× 220 mm; Matrix = 64 × 64; and 32 4-mm slices). Each functional run consisted of 210 volumes, including an initial rest period of 10 s (to achieve signal stability) that was removed from the data before preprocessing.
Imaging Data Processing.
Functional images were subjected to standard preprocessing using SPM5 (Wellcome Department of Cognitive Neurology) following our prior published methods (e.g., ref. 38), which included the following steps: slice scan-time correction to the middle slice of each volume; a two-pass realignment of all functional images, first to the first image of the first functional scan, and then to an interim computed mean image; coregistration of the anatomical image and the average of these realigned functional images; coregistration of all functional images using the parameters obtained from coregistration of the mean image; application of the SPM Unified Segmentation process to the anatomical scan, using prior information from the International Consortium for Brain Mapping Tissue Probabilistic Atlas and estimation of nonlinear warping parameters (52); warping the functional images to the MNI template space, followed by smoothing of functional images using a 6-mm isometric Gaussian kernel.
GLM Data Analysis.
First-level robust regression was performed on each participant's preprocessed images, using the standard GLM but with iteratively reweighted least squares using the bisquare weighting function for robustness (38, 53), as implemented in MATLAB 7.3 (Mathworks; robust.m), using scripts created by the authors (H.K. and J.W.). Motion parameters and high-pass filter parameters were added as additional regressors of no interest. Activity during each meditation epoch was estimated as percentage of signal change from resting baseline. Next, a second-level, random-effects analysis was performed to estimate group activity during each meditation epoch, and to compare activity between groups, using NeuroElf (NeuroElf.net). Results are familywise error (FWE)-corrected for multiple comparisons at P < 0.05, unless otherwise indicated.
Functional Connectivity Analysis: Region-of-Interest Definition.
To assess the connectivity of brain regions with the DMN, we defined two regions of interest (ROIs) in the mPFC and PCC (MNI coordinates −6, 52, −2 and −8, −56, 26, respectively), based on DMN coordinates reported previously (e.g., ref. 7). Given that these were located very close to the midplane (x = 0) we combined right and left mPFC and PCC respectively by selecting all voxels within a sphere of 10-mm radius around coordinates projected orthogonally onto the midplane (x = 0) of the brain.
Definition of Temporal Segments of Interest.
To determine differences in network connectivity, we defined three temporal epochs of 50 volumes/100 s each, as follows: (i) resting-state baseline (“please close your eyes and don't think of anything in particular”; the epoch before the instruction to meditate; volumes 6 through 55); (ii) an initial meditation phase (immediately following the instruction; volumes 76–125); and (iii) a later meditation phase (at the end of each of the meditation sessions; volumes 158–207). For each of these segments, seed-correlations were then computed.
ROI Time-Course Preparation.
For each of the six meditation sessions (three types with one repetition each), the average time course of the ROIs was extracted for the three different 50-volume/100-s segments. To ensure that maps representing the covariance (correlation) between regions and other brain areas were as unbiased as possible toward spurious positive correlation, the average time course of all white-matter voxels was also extracted. White matter is typically considered to not show any BOLD-related changes, so that any signal variation in these areas is usually attributed to noise components. Therefore, the ROI time courses were orthogonalized against this white-matter time course.
Generation of First-Level Seed-Correlation Maps.
To assess connectivity and between-group differences, separate multiple linear regression models were computed for each of the segment-by-ROI pairs. The models contained the ROI time course as covariate of interest and the respective white-matter time course as covariate of no interest (to account for fluctuations most likely driven by global signal changes). For each of these models a z-map was computed, reflecting the z-score in each voxel, assessing the likelihood of signal changes being correlated to the seed under the null hypothesis. The two homonymous maps (stemming from the two segments of equal meditation technique; for example, early meditation for the two Loving-Kindness runs) were combined using Stouffer's z-method. The rationale behind this approach is that under the null hypothesis (no effect for simple tests and no differential effect for task-difference tests) this measure is normally distributed around 0, a prerequisite for subsequent second-level analyses.
Second-Level Random-Effects Statistical Analysis.
Using these correlation maps (the initial nine maps per subject, based on three meditation types and three parts of the time courses—baseline, early, and late meditation—which were condensed into six maps, whereas the early and late correlation maps were combined using the Stouffer z-method), we computed between-group differences for the three meditation types.
Supplementary Material
Acknowledgments
We thank the participants of this study for their interest and willingness to participate; Joseph Goldstein and Ginny Morgan for input regarding meditation instructions and the task paradigm; and Marc Potenza and Kathleen Carroll, the staff of the Yale Therapeutic Neuroscience Clinic, the Clinical and Affective Neuroscience Laboratory, and the MRI technicians in the Yale Magnetic Resonance Research Center for their contributions to this research. This study was funded by Grants K12-DA00167 and P50-DA09241 from the National Institute on Drug Abuse and the US Veterans Affairs New England Mental Illness Research, Education, and Clinical Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112029108/-/DCSupplemental.
References
- 1.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 2.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci USA. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. In: Kingstone A, Miller MB, editors. The Year in Cognitive Neuroscience 2008. Malden, MA: Blackwell Publishing; 2008. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- 7.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos FX, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop SR, et al. Mindfulness: A proposed operational definition. Clin Psychol. 2004;11:230–241. [Google Scholar]
- 11.Gunaratana H. Mindfulness in Plain English. Somerville, MA: Wisdom Publications; 2002. [Google Scholar]
- 12.Chiesa A. Vipassana meditation: Systematic review of current evidence. J Altern Complement Med. 2010;16(1):37–46. doi: 10.1089/acm.2009.0362. [DOI] [PubMed] [Google Scholar]
- 13.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 14.Teasdale JD, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 15.Bowen S, et al. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Subst Abus. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer JA, et al. Mindfulness training and stress reactivity in substance abuse: Results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30:306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldin P, Ramel W, Gross J. Mindfulness meditation training and self-referential processing in social anxiety disorder: Behavioral and neural effects. J Cogn Psychother. 2009;23:242–257. doi: 10.1891/0889-8391.23.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingston J, Chadwick P, Meron D, Skinner TC. A pilot randomized control trial investigating the effect of mindfulness practice on pain tolerance, psychological well-being, and physiological activity. J Psychosom Res. 2007;62:297–300. doi: 10.1016/j.jpsychores.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 21.Lutz A, et al. Mental training enhances attentional stability: Neural and behavioral evidence. J Neurosci. 2009;29:13418–13427. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn. 2009;18(1):176–186. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Farb NA, et al. Minding one's emotions: Mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farb NAS, et al. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna A, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull. 2010;82(1-2):46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: An fMRI investigation. Soc Neurosci. 2011;6(3):231–242. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- 28.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hölzel BK, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 30.Newberg AB, et al. Cerebral blood flow differences between long-term meditators and non-meditators. Conscious Cogn. 2010;19:899–905. doi: 10.1016/j.concog.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 31.van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- 32.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 33.Northoff G, et al. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Pagnoni G, Cekic M, Guo Y. “Thinking about not-thinking”: Neural correlates of conceptual processing during Zen meditation. PLoS ONE. 2008;3:e3083. doi: 10.1371/journal.pone.0003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6785):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 36.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kober H, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 41.Kilpatrick LA, et al. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. Neuroimage. 2011;56:290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Y-Y, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011;57:1308–1316. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hölzel BK, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Hahn B, et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greicius MD, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Zylowska L, et al. Mindfulness meditation training in adults and adolescents with ADHD: A feasibility study. J Atten Disord. 2008;11:737–746. doi: 10.1177/1087054707308502. [DOI] [PubMed] [Google Scholar]
- 51.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.