Cells regulate physiological processes through compartmentalization of molecular-scale signaling and through coordinated interaction with neighboring cells. To unravel the complex interplay of these events on these small-length scales, researchers track or selectively modify cell biology at the single-cell and single-molecule levels by inserting foreign molecules (e.g., dyes, drugs, DNA, RNA, proteins, peptides, and amino acids) by microinjection, transfection, chemical modification, or electroporation. Single-cell electroporation (SCE) is an emerging noninvasive technique for cell-specific insertion of small molecules by electro-kinetic force and diffusion across electric field-induced membrane pores at the end of a small electrode. These electrodes are based on micropipets (micropipet electrode; ME) (1), solid carbon fibers (2), electrolyte-filled capillaries (3), or chip-based microfabricated electrode arrays (4).
The ME-based (or patchclamp-based) microelectroporation method is ideal for adherent cells because (i) the ME can indent the cell and create membrane tension that lowers the voltage needed for electroporation (5), thus reducing electrically induced cell damage; (ii) the volume (often expensive or rare) of inserted molecules is as small as the ME tip (<1.0 μL); and (iii) there are no toxic by-products from electrode reactions (6). There are, however, significant impediments to wide-scale use of ME-based SCE. First, it is difficult to manually regulate the approach of the pipet toward the cell using only a microscope or resistance increases arising from pipet contact with the cell membrane (1). Second, SCE efficiency is hampered by variable ME resistances arising from inconsistencies in ME fabrication. Variable ME resistances result in inconsistent applied membrane voltages when ME input voltages are constant, making it difficult to consistently achieve pore-forming transmembrane voltages of 0.2–1.0 V (7–9). Thus, in this study, we developed a new method of ME-based SCE with 40-nm precision feedback control of ME approach and a method to prescribe the applied membrane potential (Vm), a new electrical parameter for increased SCE efficiency.
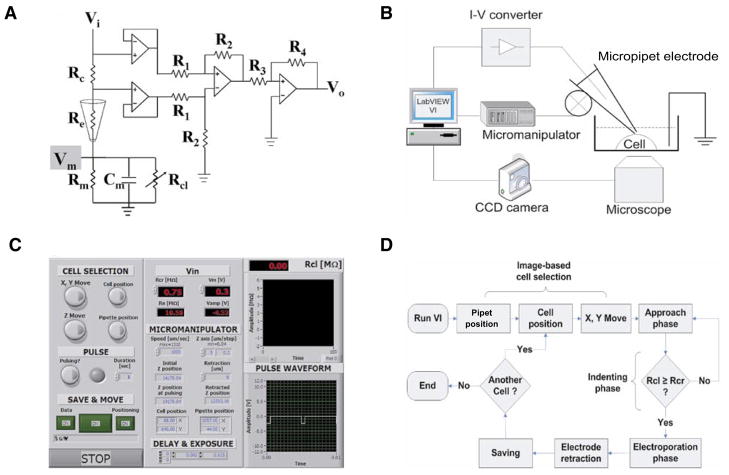
The three principal design goals for automated SCE (aSCE) were (i) image-based cell selection; (ii) feedback control of the ME position by real-time measurement of changes in cleft resistance (Rcl, see Equation 2); and (iii) automation of pulse timing and amplitude. To accomplish these design goals, we integrated a computer-controlled micromanipulator (MP-285; Sutter Instruments, Novato, CA, USA), a high-performance cooled charge-coupled device (CCD) digital imaging camera (Sensicam-ER; Cooke Corporation, Romulus, MI, USA), an A/D board (National Instruments, Austin, TX, USA) (Figure 1B), and a modified current-to-voltage-converting circuit (Figure 1A) with an IX71 microscope (Olympus, Lehigh, PA, USA).
Figure 1. Components of automated single-cell electroporation (aSCE).
(A) Modified current-to-voltage (I–V) converter circuit. Vi, dc/pulse input voltage; Vo, output voltage; Rc, measurement resistance (100 kΩ); Re, electrode resistance (10–20 MΩ); Rm, membrane resistance; Cm, membrane capacitance; Rcl, cleft resistance; R1 and R2, difference amplifier resistance (100 Ωand 1 kΩ, respectively); R3 and R4, amplifier resistance (10 and 470 Ω, respectively). (B) aSCE experimental setup. aSCE consists of micropipet electrode (ME), micromanipulator, charge-coupled device (CCD) camera, I–V converter, computer, and microscope. (C) Features and options in the graphic-user interface. Vm and Rcr are controllable, and options such as saving data and images, micromanipulator speed, camera exposure time, and retraction length are displayed on the front panel. (D) aSCE protocol. After image capture, a cell position for microelectroporation was selected by image-based cell selection. The ME (attached to computer-controlled motorized micromanipulator) was moved to the selected position (x- and y-axis movement). During approach phases, the electrode was moved incrementally in the z direction until it touched and indented the cell membrane (indenting phase). Approach was stopped when the cleft resistance reached the critical resistance value, Rcl (approximately 0.75 Ω). The electroporation phase was then initiated using preselected membrane voltage (Vm) (square-wave pulse frequency, 200 Hz; duration, 1 s; duty ratio, 10%), which induced membrane pore formation and the transport of molecules into the cell via electrophoresis and diffusion. Upon completion of electroporation, the ME was retracted, and data was saved.
| [Eq. 1] |
| [Eq. 2] |
| [Eq. 3] |
A LabVIEW-based software program coordinated ME position, cell imaging, electrical monitoring and pulsing, and data acquisition (Figure 1C).
The phase image of the cell was projected on the computer screen followed by coarse positioning of the ME tip into the field of view. Image-based cell selection was initiated by computer registration of mouse clicks at starting x and y coordinates of the ME tip and destination coordinates of the target cell (Figure 1D). These coordinates defined a vector along which the micromanipulator moved and placed the ME tip directly over the cell to be electroporated. The calibration of distance for a 20× objective was 3.13 pixel/μm.
A circuit was devised by which cleft resistance (Rcl) could be used to control and terminate the z-axis approach phase of ME movement toward the target cell (Figure 1A). In the circuit diagram, parameters (R1, R2, R3, R4, Vi, Rc) were chosen such that the total gain, G, was 470. By measuring the initial output voltage (Vo,ini), Re was calculated (Equation 1) and was used to monitor Rcl (Equation 2). During the approach and indenting phase, Vi was maintained at 1 V in order to dynamically calculate Rcl (Equation 2), which was used for feedback control of pipet approach by the micro-manipulator (0.04 μm/step maximum resolution).
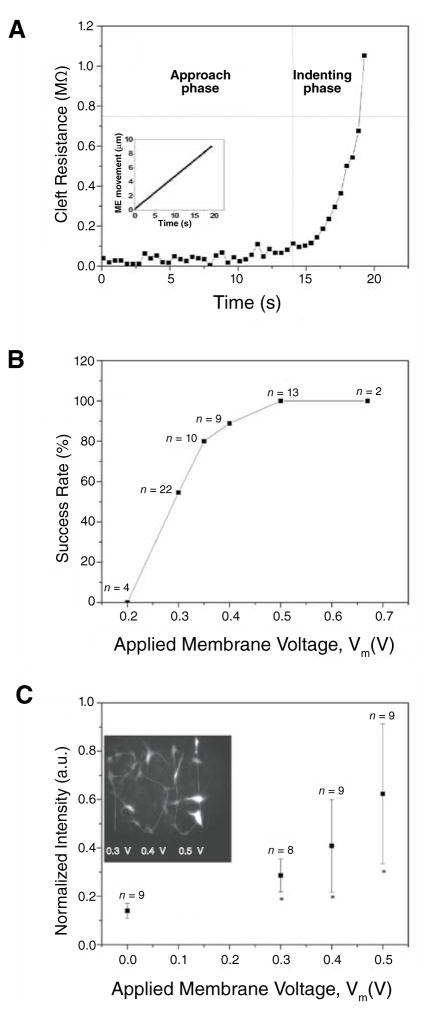
When the ME touched and indented a cell, Rcl increased sharply (Figure 2A). The indenting phase of pipet movement was terminated when Rcl reached a critical resistance value (Rcr) of 0.75 MΩ (determined from control experiments). was then calculated from Equation 3, using Vm > 0.2 V, and applied as dc square-pulses at the circuit input to initiate electroporation. It is important to note that when the same is used for all pipets, Vm can be highly variable because Re varies from pipet to pipet. Our system can accommodate pipets with variable Re by dynamically adjusting . Also, when pulses are based on Vm, which is a physiologically relevant pore-forming potential, electroporation efficiency is higher than in experiments where pulse amplitude does not account for variable Re (unpublished observations).
Figure 2. Electroporation degree and efficiency.
(A) Cleft resistance (Rcl) versus time; Rcl was stable during approach phase, then sharply increased when micropipet electrode (ME) touched and indented the cell membrane. Indentation improves pipet sealing by the membrane. Horizontal dashed line indicates Rcr. Inset: ME movement as a function of time. (B) Electroporation success rate versus applied membrane voltage (Vm). Success was achieved when cell fluorescence was 2 sd above cellular intrinsic fluorescence; success ratio increased as Vm increased; 90% success rate was achieved with Vm = 0.4 V. (C) Inserted BODIPY FL-GTP intensity versus applied Vm; cells were selected individually using Vm of 0.3, 0.4, and 0.5 V for cells marked by P, S, and U, respectively. Inserted dye intensity was highly dependent on Vm. Cell viability was confirmed by calcein staining, phase microscopy, and absence of electroporation-induced vesicles, dye leakage, or membrane bleb formation. * Indicates significant difference from average cell intrinsic fluorescence as determined by analysis of variance (ANOVA) and a Student’s t-test (P < 0.01). a.u., arbitrary units.
To find the optimal Vm and test system repeatability, we used the aSCE system to insert BODIPY® FL-GTP (100 μM, negatively charged; Invitrogen, Carlsbad, CA, USA) into bovine aortic endothelial cells (BAECs) with Rcr = 0.75 MΩ, Re = 10.4 ± 0.41 MΩ, and Vm of 0.2, 0.3, 0.4, 0.5, and 0.67 V. Cells (n > 20) were electroporated one by one using image-based cell selection. The average time for SCE was 40 s/cell. Success rate (when electroporated cell fluorescence intensity was 2 standard deviations above the average intrinsic cell fluorescence intensity) was sharply increased when Vm > 0.3 V; when Vm = 0.4 V, nearly 90% of cells were successively electroporated (Figure 2B). To demonstrate image-based cell selection and assess the relationship between inserted dye and Vm, cells were selected individually as shown in Figure 2C using Vm of 0.3, 0.4, and 0.5 V for cells marked by P, S, and U, respectively. The electroporated-BODIPY FL-GTP intensity increased with increasing Vm suggesting that Vm can be used to ensure and predetermine dye uptake. It is important to note that increases in pulse amplitude increase both membrane pore size (10), number (11), and electrokinetic forces. Because the pipet indents the membrane and seals the tip, most of the voltage drop is across the membrane and nearly equal to Vm. Thus, the ability of Vm of 0.4 V to generate pores large enough for electrokinetic-induced BODIPY FL-GTP dye insertion is consistent with previous studies that demonstrated a pore-inducing transmembrane voltage threshold of 0.2 V (7).
Viability of electroporated cells was also assessed. Using protocols and voltages identical to the previous BODIPY-FL experiments (n = 15 for each voltage), cells were incubated for 2.5 h and then stained with calcein red-orange AM (Invitrogen). In order to assist in finding the cells after the incubation period, small scratches were made on the coverslips using a diamond-tipped marker. We found that nearly all the cells were stained positively with calcein, indicating that cell membranes were intact. Upon visual inspection of the cells under 20× phase microscopy, small vacuoles appeared in about 20% of cells that were electroporated with Vm = 0.67 V. Vm of 0.5 V or less did not induce any vacuole formation. Cell damage with Vm of 0.67 V, which corresponded to a Vi of 10 V and Re of 10 MΩ, is consistent with previous findings (1). Additional indications of cell viability include retention of dyes in the minutes following electroporation and cell motility after 2.5 h incubation.
Modified patch-clamp MEs apply pore-forming transmembrane potentials (0.2–1.0 V) with low electrode applied voltages (<10 V), because the electric fields are concentrated at the ME tip near the membrane. Use of low applied potentials makes automation by a computer possible because the maximum voltage from an A/D board is 10 V. Thus, aSCE offers a new and convenient method to noninvasively insert foreign molecules into adherent cells that is cell-specific and highly reproducible. Our new aSCE system, along with a new method to define the pulse amplitude, simplify SCE and provide enhanced tools to differentially manipulate the genetic, metabolic, fluorescent, and synthetic contents of single, targeted adherent cells in a population.
Acknowledgments
This work was supported in part by a grant to P.J.B. from the National Heart Lung and Blood Institute (R01 HL 077542-01A1), by a National Science Foundation Career Award to P.J.B. (BES 0238910), and by a seed grant from the Center for Optical Technologies, Bethlehem, PA.
Footnotes
To purchase reprints of this article, contact: Reprints@BioTechniques.com
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
References
- 1.Rae JL, Levis RA. Single-cell electroporation. Pflugers Arch. 2002;443:664–670. doi: 10.1007/s00424-001-0753-1. [DOI] [PubMed] [Google Scholar]
- 2.Lundqvist JA, Sahlin F, Aberg MA, Stromberg A, Eriksson PS, Orwar O. Altering the biochemical state of individual cultured cells and organelles with ultramicroelectrodes. Proc Natl Acad Sci USA. 1998;95:10356–10360. doi: 10.1073/pnas.95.18.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolkrantz K, Farre C, Brederlau A, Karlsson RI, Brennan C, Eriksson PS, Weber SG, Sandberg M, Orwar O. Electroporation of single cells and tissues with an electrolyte-filled capillary. Anal Chem. 2001;73:4469–4477. doi: 10.1021/ac010403x. [DOI] [PubMed] [Google Scholar]
- 4.Khine M, Lau A, Ionescu-Zanetti C, Seo J, Lee LP. A single cell electroporation chip. Lab Chip. 2005;5:38–43. doi: 10.1039/b408352k. [DOI] [PubMed] [Google Scholar]
- 5.Needham D, Hochmuth RM. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys J. 1989;55:1001–1009. doi: 10.1016/S0006-3495(89)82898-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olofsson J, Nolkrantz K, Ryttsen F, Lambie BA, Weber SG, Orwar O. Single-cell electroporation. Curr Opin Biotechnol. 2003;14:29–34. doi: 10.1016/s0958-1669(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 7.Teissie J, Rols MP. An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophys J. 1993;65:409–413. doi: 10.1016/S0006-3495(93)81052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinosita K, Jr, Tsong TY. Voltage-induced conductance in human erythrocyte membranes. Biochim Biophys Acta. 1979;554:479–497. doi: 10.1016/0005-2736(79)90386-9. [DOI] [PubMed] [Google Scholar]
- 9.Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993;51:426–435. doi: 10.1002/jcb.2400510407. [DOI] [PubMed] [Google Scholar]
- 10.Frey W, White JA, Price RO, Blackmore PF, Joshi RP, Nuccitelli R, Beebe SJ, Schoenbach KH, Kolb JF. Plasma membrane voltage changes during nanosecond pulsed electric field exposure. Biophys J. 2006;90:3608–3615. doi: 10.1529/biophysj.105.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBruin KA, Krassowska W. Modeling electroporation in a single cell. I. Effects of field strength and rest potential. Biophys J. 1999;77:1213–1224. doi: 10.1016/S0006-3495(99)76973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]