Abstract
Context:
Limited data suggest that testosterone is decreased during space flight, which could contribute to bone and muscle loss.
Objective:
The main objective was to assess testosterone and hormone status in long- and short-duration space flight and bed rest environments and to determine relationships with other physiological systems, including bone and muscle.
Design:
Blood and urine samples were collected before, during, and after long-duration space flight. Samples were also collected before and after 12- to 14-d missions and from participants in 30- to 90-d bed rest studies.
Setting:
Space flight studies were conducted on the International Space Station and before and after Space Shuttle missions. Bed rest studies were conducted in a clinical research center setting. Data from Skylab missions are also presented.
Participants:
All of the participants were male, and they included 15 long-duration and nine short-duration mission crew members and 30 bed rest subjects.
Main Outcome Measures:
Serum total, free, and bioavailable testosterone were measured along with serum and urinary cortisol, serum dehydroepiandrosterone, dehydroepiandrosterone sulfate, and SHBG.
Results:
Total, free, and bioavailable testosterone was not changed during long-duration space flight but were decreased (P < 0.01) on landing day after these flights and after short-duration space flight. There were no changes in other hormones measured. Testosterone concentrations dropped before and soon after bed rest, but bed rest itself had no effect on testosterone.
Conclusions:
There was no evidence for decrements in testosterone during long-duration space flight or bed rest.
The recent National Research Council review of the life sciences research programs at the National Aeronautics and Space Administration noted that “although exposure to microgravity per se triggers bone and muscle loss because of the reduction in mechanical loading forces, losses are likely exacerbated by additional factors in the space environment (e.g. altered nutrition, hormonal disruptions, psychological stress). Thus, the development of effective countermeasure strategies will require input from experts across multiple disciplines (e.g. basic bone and muscle biologists, cardiovascular physiologists, endocrinologists, exercise physiologists, nutritionists, biomechanists, behavioralists)” (1). Indeed, although the musculoskeletal systems have been studied in both actual space flight and ground simulations and the endocrine system has also been studied, the endocrine regulation of these systems has yet to be well characterized.
Testosterone, given its role in bone and muscle maintenance, is often considered either a potential mechanism for the musculoskeletal losses associated with space flight or a potential countermeasure (2, 3). These considerations are based on very limited flight data from humans and animal models that suggest that testosterone levels are reduced during space flight and on results from ground-based studies simulating space flight. To date, the only available human space flight data come from four crew members on a short-duration Space Shuttle flight conducted in the early 1990s. After 4–5 d of flight, in-flight serum, urinary, and salivary testosterone concentrations decreased by 50, 25, and 95%, respectively, compared with preflight levels (4). These data have been reported, and reviewed, in multiple papers (5–9). Studies of space-flown animals are more abundant, with many documenting decreases after landing (with no in-flight data available) in rats (9–12) and monkeys (13).
Because space flight experiments are not easily implemented, ground-based analogs of space flight are often used to study the effects of disuse on physiology. The most common of these analogs in humans is bed rest, in which subjects remain confined to either horizontal or −6 degree head-down-tilt bed rest for days to weeks to months. Studies of this type have generally shown no effect of bed rest on circulating total or free testosterone (3, 14–17). The rodent version of bed rest is suspension of the hindlimbs or tail. This has been shown to reduce circulating testosterone (2, 18, 19), and the administration of testosterone mitigates muscle and bone losses in these suspended rats (2).
Stress hormones have been studied widely in space flight, and the results have clearly shown that there is no clear effect of space flight on stress hormones in humans. Trends of increased levels of stress hormones during flight have been reported (20, 21), reflecting the increase in some subjects, on some missions, at some time points. During America's initial long-duration flights on Skylab in the early 1970s, eight of nine astronauts on 28-, 56-, and 84-d missions had increased urinary cortisol (22, 23).
The effects of weightlessness on endocrine function, especially with regard to gonadal hormones, remains poorly resolved. We report here testosterone, dehydroepiandrosterone (DHEA), and cortisol data from astronauts during long-duration International Space Station missions of 4–6 months and data from nine astronauts before and after short-duration Space Shuttle missions. We extend earlier findings by reporting testosterone analyzed both by RIA and liquid chromatography-tandem mass spectrometry (LC-MS/MS) sample analysis. We also report data from long- (60–90 d) and short (30 d)-duration bed rest studies. The long-duration study included two groups: untreated controls and subjects exposed to a vibration platform aimed at mitigating bone loss.
Subjects and Methods
We report here both ground-based and space flight studies, both types having involved many investigator teams and objectives. For the long-duration space flight study, the data presented here represent a subset of the objectives of the Nutritional Status Assessment experiment. The experiment was described in a previous paper in which vitamin K status data were reported (24). For the bed rest studies, the basic results (such as general nutritional results and results from other experiment teams, including bone, muscle, immune, and cardiovascular physiology) (25–29), and the vibration effects on bone (30) have been previously published. However, none of the data reported herein have been published. Details and citations from earlier studies are included below.
All protocols described herein were reviewed and approved by the National Aeronautics and Space Administration Johnson Space Center Committee for the Protection of Human Subjects (and often also by other local institutional review boards). Written informed consent was obtained from all subjects.
Study design, subjects, and sample collections
Space flight: long duration on the International Space Station
Participants (n = 15 male) in the long-duration study were astronauts on International Space Station expeditions 14–22 (missions of 48–215 d duration, flown between 2006 and 2010). Crew members averaged 46 ± 4 (mean ± sd) yr of age at the time of the flight, and their body mass index was 26 ± 2 kg/m2. Crew members were not screened for participation in this experiment specifically, but all had to meet all requirements for medical clearance for flight.
For the long-duration crew members, blood and two consecutive 24-h urine samples were collected about 180 and 45 d before launch and again on landing day [return (R+0 d)]. In addition to the pre- and postflight collections, these crew members provided up to five blood and 24-h urine collections during space flight at about flight day (designated FD) 15, FD30, FD60, FD120, and FD180. Because flight durations varied [mission length was 151 ± 56 d (range 48–215)], not all crews had five in-flight sessions. Blood samples were collected using standard phlebotomy techniques. For in-flight blood collections, samples were collected into evacuated serum gel separator tubes. The tubes were centrifuged at 3800 rpm (1500 × g) for 30 min and then placed in a −80 C freezer (with typical temperature of −96 C) on the International Space Station. The samples were returned to Earth within 6–12 months of collection, either in a specially designed cooler that was capable of maintaining the samples at below −30 C until landing or in a powered −96 C freezer on the Space Shuttle. Within hours of landing, samples were inventoried, transferred to dry ice, and transported to Houston (Texas), where they were stored in a −80 C freezer until analysis. Samples collected 10 d before launch were processed identically (allowed to clot for 20–30 min, centrifuged, frozen in the tube, and thawed alongside flight samples when they returned to Earth). Except for samples collected on R+0 (landing day), all blood samples were collected after an 8-h fast in the morning, typically between 0700 and 0900 h. Urine collected before and after flight was collected into single-void urine containers (Cole-Parmer, Niles, IL). Samples were stored with ice packs or refrigerated until they were processed, within 24 h of collection. A pooled 24-h aliquot was prepared and stored frozen at −80 C until analysis. In-flight urine voids were collected into urine collection devices containing 1 ml of a LiCl solution as a volume marker. After being voided into the device, the sample was thoroughly mixed, and an aliquot was taken with a syringe and then frozen until return to Earth about 6–12 months later. After landing, the in-flight urine samples were analyzed for lithium concentration to determine void volume and subsequently to prepare 24-h pools, as previously described (24, 31).
During flight, crews use a once-weekly food frequency questionnaire to recall food items consumed in the past week. This food frequency questionnaire has been validated in ground-based models and during space flight (32, 33). The energy intake is reported as the percent of the energy requirement (using the World Health Organization equation for energy requirements).
Space flight: short duration on the Space Shuttle
In a separate experiment, crew members from two Space Shuttle missions were studied, with blood samples collected about 10 d before flight and again immediately after flight. These were nine male crew members on Space Shuttle missions STS-121 and STS-115, which were 12- to 13-d missions. Postflight blood collections were conducted within 2–3 h of landing.
Space flight: long duration on Skylab
We also report here urinary testosterone (measured by RIA) from nine crew members on Skylab missions of the early 1970s. These missions and related findings have been described in detail elsewhere (23). The urinary testosterone data from three of the crew members has been previously published (34), and we report here the combined data from all nine crew members.
Bed rest
Results from multiple bed rest studies are reported here, from a series of 60- or 90-d, −6 degree head-down-tilt bed rest studies with (n = 7 male) or without (n = 23 male) a vibration countermeasure on a vibration plate placed at the feet during bed rest. A 30-d bed rest study (n = 8) was also conducted in which subjects were not exposed to exercise or vibration. The details of the vibration protocol are described elsewhere (30). The bed rest studies were performed at the University of Texas Medical Branch in Galveston's Institute for Translational Sciences-Clinical Research Center. Diets were controlled and matched for all subjects. Details about these studies have been published (25, 28, 35).
The bed rest data collections are designated as pre-bed rest (BR−10 and BR−3, that is, 10 or 3 d before starting the bed rest protocol while subjects are ambulatory but residing in the Clinical Research Center), after 28 and 60 d of continuous bed rest (BR28 and BR60), after 90 d of continuous bed rest (BR+0; blood samples are collected just before reambulation), and 5 d after reambulation (BR+5), again while subjects are ambulatory but residing in the Clinical Research Center.
Biochemical analyses
Testosterone analyses were completed using traditional RIA techniques, and a subset of the samples were also analyzed using LC-MS/MS techniques (details are included in online Supplemental Material, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). For the RIA method, analyses were conducted using the Siemens Coat-A-Count RIA (Siemens Healthcare Diagnostics, Deerfield, IL). Within-assay coefficients of variation (CV) for both methods were less than 10%. Between-assay CV for total testosterone were less than 12%; for free testosterone they were less than 10% for most concentrations and less than 20% at the lower end of the curve.
DHEA and DHEA sulfate (DHEAS) were determined in serum using commercially available kits (IBL International GmbH, Hamburg, Germany). The within-assay CV was 11%. Cortisol was determined in serum and urine using the DiaSorin GammaCoat RIA kit (Stillwater, MN). Urine samples were extracted using methylene chloride, according to manufacturer guidelines. Within- and between-assay CV were less than 10%.
Statistical analysis
A repeated-measures, one-way ANOVA was performed on long-duration space flight data. A Student's t test was performed on the short-duration space flight data to compare the preflight and postflight data. A repeated-measures, two-way ANOVA was performed on the bed rest studies, with time and countermeasure (vibration) as variables. The 60- to 90-d bed rest control and vibration subjects were analyzed and compared. The 30-d bed rest subjects were analyzed separately. If overall significant differences were detected, a post hoc Bonferroni t test was performed to establish differences between time points or between treatments. Pearson correlation coefficients were determined to examine the relationships between testosterone and the measurements estimating muscle and bone mass after long-duration spaceflight. Statistical analyses were performed using Sigma Stat 3.11 (Systat Software, Inc., San Jose, CA).
Results
Long-duration crew members consumed an average of 80 ± 18% (mean ± sd; range was 60–114%) of their World Health Organization-estimated requirement (36) for energy intake over the course of the mission.
During long-duration space flight, total serum testosterone did not change relative to before flight, but it decreased significantly on landing day (R+0) when measured either by LC-MS/MS or RIA (Table 1). Free testosterone, when measured by either method, was also unchanged during flight but decreased on landing day. Free testosterone was lower when determined by RIA compared with the calculated value based on bioavailable testosterone measured by LC-MS/MS, but free testosterone determination by the two methods was proportionally similar (r = 0.37, P < 0.001). This is similar to what others have found (37). Bioavailable testosterone, which includes free testosterone and albumin-bound testosterone, was similarly unchanged during flight but decreased on landing day (P < 0.01). SHBG, DHEA, DHEAS, and serum and urinary cortisol did not change during or after long-duration space flight. In-flight total serum testosterone was positively correlated with serum cortisol (r = 0.20, P < 0.05).
Table 1.
Hormone concentrations before, during, and after long-duration space flight
| L−180 | L−45 | L−10 | FD15 | FD30 | FD60 | FD120 | FD180 | R+0 | R+30 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total testosterone (nmol/liter) | ||||||||||
| LC-MS/MSa | 22 ± 6 (n = 15) b | 22 ± 8 (n = 15)b | 25 ± 14 (n = 13)b | 24 ± 11 (n = 15)b | 25 ± 12 (n = 15)b | 23 ± 11 (n = 14)b | 24 ± 11 (n = 12)b | 23 ± 9 (n = 10)b | 15 ± 7 (n = 15)c | 22 ± 9 (n = 15)b |
| RIAa | 19 ± 5 (n = 12)b | 19 ± 6 (n = 12)b | 19 ± 11b (n = 10)b | 20 ± 9 (n = 12)b | 22 ± 9 (n = 12)b | 21 ± 8 (n = 11)b | 20 ± 7 (n = 9)b | 20 ± 7 (n = 7)b | 13 ± 6 (n = 12)c | 19 ± 7 (n = 12)b |
| Free testosterone (pmol/liter) | ||||||||||
| LC-MS/MSa | 253 ± 87 (n = 15)b | 245 ± 79 (n = 15)b | 237 ± 70 (n = 13)b | 213 ± 69 (n = 15)b | 224 ± 101 (n = 14)b | 224 ± 60 (n = 14)b | 213 ± 67 (n = 12)b | 217 ± 78 (n = 10)b | 126 ± 52 (n = 15)c | 180 ± 55 (n = 15)b,c |
| RIAa | 40 ± 9 (n = 12)b | 40 ± 13 (n = 12)b | 32 ± 12 (n = 10)b | 33 ± 11 (n = 12)b | 35 ± 12 (n = 12)b | 36 ± 11 (n = 11)b | 34 ± 13 (n = 9)b | 37 ± 8 (n = 7)b | 23 ± 10 (n = 12)c | 35 ± 16 (n = 12)b |
| Bioavailable testosterone (nmol/liter)a | 4 ± 1 (n = 15)b | 4 ± 1 (n = 15)b | 4 ± 1 (n = 13)b | 3 ± 1 (n = 15)b | 4 ± 2 (n = 15)b | 4 ± 1 (n = 14)b | 3 ± 1 (n = 12)b | 4 ± 1 (n = 10)b | 2 ± 1 (n = 14)c | 3 ± 1 (n = 15)b,c |
| SHBG (nmol/liter) | 53 ± 29 (n = 15) | 51 ± 23 (n = 15) | 58 ± 36 (n = 13) | 65 ± 40 (n = 15) | 69 ± 52 (n = 15) | 57 ± 38 (n = 14) | 65 ± 35 (n = 12) | 63 ± 36 (n = 10 | 59 ± 33 (n = 14) | 64 ± 27 (n = 15) |
| DHEA (nmol/liter) | 36 ± 19 (n = 11) | 35 ± 10 (n = 12) | 33 ± 14 (n = 10) | 36 ± 12 (n = 12) | 39 ± 13 (n = 12) | 37 ± 12 (n = 11) | 49 ± 18 (n = 9) | 43 ± 21 (n = 7) | 44 ± 16 (n = 12) | 30 ± 12 (n = 12) |
| DHEAS (μmol/liter) | 5 ± 2 (n = 11) | 5 ± 2 (n = 12) | 4 ± 1 (n = 10) | 5 ± 2 (n = 12) | 5 ± 2 (n = 12) | 5 ± 2 (n = 11) | 5 ± 2 (n = 9) | 5 ± 2 (n = 7) | 5 ± 2 (n = 12) | 5 ± 2 (n = 12) |
| Serum cortisol (nmol/liter) | 484 ± 110 (n = 12) | 531 ± 95 (n = 13) | 503 ± 208 (n = 11) | 499 ± 109 (n = 13) | 559 ± 189 (n = 13) | 526 ± 184 (n = 12) | 558 ± 88 (n = 10) | 535 ± 134 (n = 8) | 615 ± 282 (n = 13) | 399 ± 143 (n = 13) |
| Urinary cortisol (nmol/d)d | 171 ± 48 (n = 13) | 193 ± 77 (n = 13) | N/D | 213 ± 122 (n = 13) | 202 ± 95 (n = 13) | 183 ± 76 (n = 12) | 243 ± 125 (n = 9) | 231 ± 119 (n = 7) | 248 ± 111 (n = 13) | 149 ± 47 (n = 13) |
Data are means ± sd. All variables except urinary cortisol were measured in serum. Bioavailable testosterone (measured by LC-MS/MS) included free testosterone and albumin-bound testosterone. Free testosterone (LC-MS/MS) and SHBG were calculated using a mathematical model described in detail in the online Supplemental Material. Urinary cortisol was not significantly different after a Bonferroni post hoc comparison of time points. L−180, 180 d before flight (launch); N/D, not determined.
Different letters in each row (b and c) were significantly different when determined by a post hoc Bonferroni t test.
Significant main effect of time, P < 0.001.
Significant main effect of time, P < 0.01.
After 12–13 d of space flight on the Space Shuttle, serum total testosterone was significantly decreased about 30% when measured using an RIA method (P < 0.05) and tended to decrease on landing day when measured by LC-MS/MS (Table 2, P = 0.07). When measured by either method, free testosterone decreased after space flight (P < 0.05), and bioavailable testosterone decreased as well (P < 0.05). Serum cortisol was negatively correlated with total serum testosterone on landing day and before flight (r = −0.69, P < 0.05; r = −0.73, P < 0.01, respectively). On Skylab missions, urinary testosterone was unchanged or increased during flight relative to before flight (Fig. 1).
Table 2.
Hormone concentrations before and after short-duration (Space Shuttle) space flight
| Before | After | |
|---|---|---|
| Total testosterone (nmol/liter) | ||
| LC-MS/MS | 21 ± 10 | 13 ± 4 |
| RIAa | 18 ± 6 | 12 ± 3 |
| Free testosterone (pmol/liter) | ||
| LC-MS/MSa | 235 ± 93 | 123 ± 82 |
| RIAa | 38 ± 11 | 19 ± 9 |
| Bioavailable testosterone (nmol/liter) | 4 ± 2 | 2 ± 1 |
| Cortisol (serum nmol/liter) | 523 ± 105 | 494 ± 261 |
| SHBG (nmol/liter) | 47 ± 25 | 54 ± 18 |
Data are means ± sd. All variables were measured in serum. Bioavailable testosterone (measured by LC-MS/MS) included free testosterone and albumin-bound testosterone. Free testosterone (LC-MS/MS) and SHBG were calculated using a mathematical model described in detail in the online Supplemental Material.
Significant main effect of time, P < 0.05 (n = 9 male Space Shuttle crew members from STS-121 and STS-115).
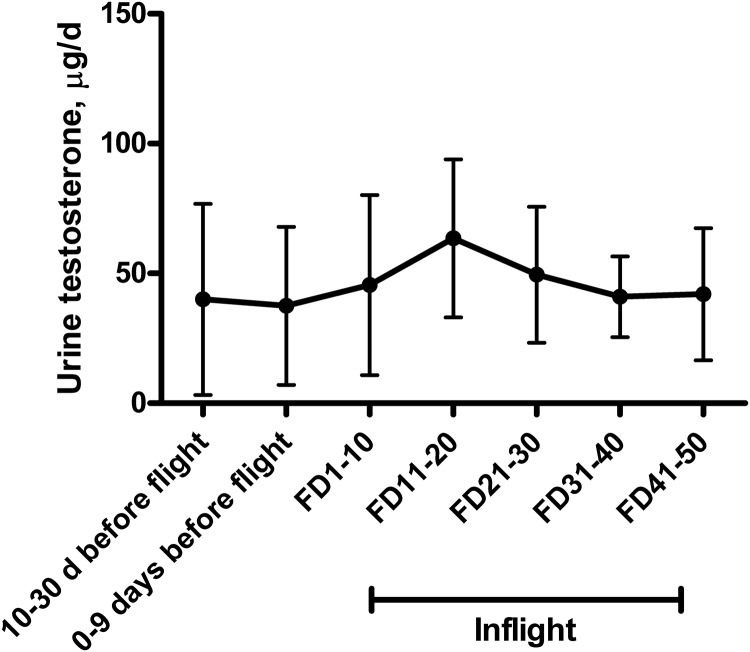
Fig. 1.
Urinary testosterone in Skylab crew members before and during flight on Skylab missions 2, 3, and 4 in 1973–1974 (n = 9 for all time points except FD31–40 and FD41–50, where n = 6). Data are mean ± sd and are adapted from elsewhere (34). Urinary testosterone excretion on FD11–20 was higher than on FD1–10 (P < 0.05) but not different from other time points.
During the 60- to 90-d bed rest studies, total serum testosterone was consistently lower on BR−3 than on BR−10, and it was consistently lower on BR+5 than on BR+0, the day the subjects got out of bed. Free testosterone was lower on BR+5 than on BR−10 (Table 3). DHEA and DHEAS were not changed during or after bed rest. Serum and urinary cortisol were both elevated on BR+0 relative to before or during bed rest.
Table 3.
Hormone concentrations before, during, and after 30, 60, or 90 d bed rest in subjects with and without a vibration countermeasure
| BR −10 | BR −3 | BR 28 | BR 60 | BR +0 | BR +5 | |
|---|---|---|---|---|---|---|
| Total testosterone (RIA) (nmol/liter)a | ||||||
| Control (60–90 d) | 20 ± 7 (n = 15)b,c | 17 ± 6c (n = 15)c,d | 17 ± 5 (n = 15)b,c,d | 18 ± 4 (n = 10)b,c,d | 17 ± 5 (n = 12)c | 15 ± 6 (n = 12)d |
| Vibration (60–90 d) | 20 ± 2 (n = 7)b,c | 16 ± 3 (n = 7)c | 18 ± 4 (n = 7)b,c,d | 18 ± 4 (n = 7)b,c,d | 19 ± 3 (n = 7)c | 15 ± 5 (n = 7)d |
| Control (30 d) | 18 ± 7 (n = 8) | 17 ± 5 (n = 8) | 18 ± 6 (n = 8) | 16 ± 6 (n = 8) | ||
| Total testosterone (LC-MS/MS) (nmol/liter) | ||||||
| Control (30 d) | 21 ± 8 (n = 8) | 18 ± 6 (n = 8) | 18 ± 6 (n = 8) | 19 ± 7 (n = 8) | ||
| Free testosterone (RIA) (pmol/liter)e | ||||||
| Control (60–90 d) | 55 ± 27 (n = 15)b | 45 ± 22 (n = 15)b,d | 49 ± 14a,b (n = 15)b,d | 46 ± 8 (n = 10)b,d | 41 ± 11 (n = 12)b,d | 38 ± 10 (n = 12)d |
| Vibration (60–90 d) | 54 ± 17 (n = 7)b | 55 ± 15 (n = 7)b,d | 50 ± 11a,b (n = 7)b,d | 48 ± 11 (n = 7)b,d | 47 ± 16 (n = 7)b,d | 45 ± 8 (n = 7)d |
| Control (30 d) | 54 ± 18 (n = 8) | 50 ± 17 (n = 8) | 48 ± 13 (n = 8) | 42 ± 10 (n = 8) | 54 ± 18 (n = 8) | 50 ± 17 (n = 8) |
| Free testosterone (LC-MS/MS) (pmol/liter) | ||||||
| Control (30 d) | 375 ± 136 (n = 8) | 350 ± 126 (n = 8) | 315 ± 81 (n = 8) | 283 ± 100 (n = 8) | ||
| Bioavailable testosterone (nmol/liter)1 | ||||||
| Control (30 d) | 7 ± 2 (n = 8)b | 5 ± 1 (n = 8)d | 5 ± 1b (n = 8)d | 5 ± 2 (n = 8)d | ||
| SHBG (nmol/liter) | ||||||
| Control (30 d) | 30 ± 11 (n = 8) | 26 ± 7 (n = 8) | 29 ± 8 (n = 8) | 31 ± 11 (n = 8) | ||
| DHEA (nmol/liter) | ||||||
| Control (60–90 d) | 99 ± 43 (n = 15) | 101 ± 40 (n = 15) | 97 ± 43 (n = 15) | 110 ± 38 (n = 10) | 107 ± 37 (n = 12) | 97 ± 37 (n = 12) |
| Vibration (60–90 d) | 78 ± 20 (n = 7) | 73 ± 22 (n = 7) | 74 ± 26 (n = 7) | 83 ± 17 (n = 7) | 67 ± 22 (n = 7) | 58 ± 17 (n = 7) |
| DHEAS (μmol/liter) | ||||||
| Control (60–90 d) | 8 ± 3 (n = 15) | 8 ± 3 (n = 15) | 8 ± 2 (n = 15) | 8 ± 2 (n = 10) | 8 ± 2 (n = 12) | 8 ± 3 (n = 12) |
| Vibration (60–90 d) | 5.8 ± 2.2 (n = 7) | 6.2 ± 2.3 (n = 7) | 6.5 ± 3.1 (n = 7) | 6.9 ± 3.3 (n = 7) | 6.7 ± 3.1 (n = 7) | 6.4 ± 3.1 (n = 7) |
| Serum cortisol (nmol/liter) | ||||||
| Control (60–90 d) | 567 ± 120 (n = 15) | 537 ± 121 (n = 15) | 563 ± 125 (n = 15) | 582 ± 118 (n = 10) | 616 ± 108 (n = 12) | 569 ± 84 (n = 12) |
| Vibration (60–90 d) | 555 ± 91 (n = 7) | 514 ± 72 (n = 7) | 488 ± 90 (n = 7) | 534 ± 103 (n = 7) | 481 ± 80 (n = 7) | 435 ± 87 (n = 7) |
| Control (30 d)e | 469 ± 138 (n = 8)b | 504 ± 172 (n = 8)b,d | 640 ± 112 (n = 8)d | 553 ± 99 (n = 8)b,d | ||
| Urinary cortisol (nmol/d)a | ||||||
| Control (60–90 d) | 165 ± 63 (n = 15)b | 200 ± 60 (n = 15)b | 210 ± 58 (n = 15)b | 205 ± 60 (n = 10)b | 289 ± 101 (n = 12)d | 190 ± 77 (n = 12)b |
| Vibration (60–90 d) | 279 ± 165 (n = 7)b | 202 ± 69 (n = 7)b | 210 ± 31 (n = 7)b | 275 ± 80 (n = 7)b | 311 ± 75 (n = 7)d | 205 ± 100 (n = 7)b |
Data are means ± sd. The bed rest data collections are designated as BR−10 and BR−3, that is, 10 or 3 d before starting the bed rest protocol; after 28 and 60 d of continuous bed rest (BR28 and BR60), after 90 d of continuous bed rest (BR+0; the blood collections occurred just before reambulation), and again 5 d after reambulation (BR+5). For subjects in the 30-d bed rest, samples were collected after 30 d of bed rest but are shown above as BR28 for ease in combining studies into one table. All variables were measured in serum. Free testosterone (LC-MS/MS) and SHBG were calculated using a mathematical model described in detail in the online Supplemental Material. Different letters in each row (b, c, d) were significantly different when determined by a post hoc Bonferroni t test.
P < 0.001.
P < 0.05.
The long-duration space flight testosterone data were evaluated to determine whether a new piece of exercise equipment, an advanced resistive exercise device (aRED; replacing an interim resistive exercise device, capable only of much smaller loading), had any impact on serum testosterone concentration. There was no difference in testosterone between crew members who used the aRED (n = 9) and those who used only the interim device because the aRED was not yet available (n = 6). All crew members had also had access to a cycle ergometer and treadmill. There was also no correlation between in-flight serum testosterone concentrations and the number of hours before blood collection that the crew member completed resistance (18 ± 3 h before) or aerobic exercise (18 ± 4 h before). We also examined the potential effect that female crew member presence could have on in-flight serum testosterone in the male crew members and found no differences between days when female crew members were aboard the International Space Station and days when no female crew members were aboard (data not shown).
Total testosterone (in-flight mean and change in testosterone at R+0 relative to before flight) was compared with the postflight changes in dual-energy x-ray absorptiometry measurements including lean body mass, total body mass, whole-body bone mineral density, and bone mineral content, and there were no significant correlations (data not shown).
Discussion
The data reported here document the lack of an effect of long-duration space flight on circulating testosterone during flight. No matter what form of testosterone (total, free, or bioavailable) was measured, or with which method, there was clearly no change during long-duration space flight. There was, however, a consistent decrease in all of these forms of testosterone on landing day. Landing day is different from the other time points in that the crew members may not have fasted, and a number of rapid physiological changes occur as the body readjusts to gravity. Consistent with the long-duration flight data, evidence is presented here for decreased testosterone after short-duration missions on landing day relative to 10 d before flight. Interestingly, serum total testosterone was negatively correlated with serum cortisol before and after landing in the short-duration crew members, suggesting that stress may be part of the response on landing day that affects testosterone. However, among long-duration crew members, serum cortisol was positively correlated with total testosterone but only during space flight. These possible relationships between stress responses and testosterone warrant further investigation.
The bed rest analog data reported here corroborate those from earlier published studies, showing no effect of bed rest on circulating testosterone concentrations (3, 14–16). Consistent decreases were observed after the subjects had been in the bed rest facility for 7 d (while they were still ambulatory), and then another decrease occurred 5 d after reambulation. The pre-bed rest change is likely related to stress and decreased ambulation while subjects were in the bed rest facility, and the post-bed rest change to fluid shifts during and after bed rest. No changes in testosterone occurred during bed rest. The footplate vibration protocol also had no effect on testosterone.
The only previously published in-flight data from human space flight were from four astronauts on Space Shuttle mission STS-55, which flew in 1993, and from three astronauts on Skylab 4, an 84-d mission (34). On the Shuttle mission, after 4 or 5 d of flight, circulating testosterone levels were decreased relative to preflight levels, when measured in serum, saliva, and urine. Serum cortisol, cortisol biorhythms, and DHEAS concentrations in these four astronauts were unchanged during flight (38, 39). A significant confounding issue is that these crew members were only consuming about 60–85% of their basal metabolic energy requirements during the flight (40). Estimates of space flight energy requirements typically use an activity factor of 1.7 (that is, 1.7 × basal metabolic rate) (41). This is based on data documenting that total energy requirements are unchanged during flight (42) or in some cases are even increased with heavy exercise (43), relative to before flight. Even if lower estimates of activity were used, the result would reveal significant energy deficit in crew members on the STS-55 mission, especially during the days of sample collections (40). Indeed, energy intake, which was very carefully documented on these missions, was below even basal requirements. Energy deficits, both short and long term, are associated with lower circulating testosterone (free and total) (44–46). Thus, the discrepancy between the long-duration data presented here and the earlier reports of effects observed during the first week of flight could be explained simply by inadequate energy intake.
The previously published Skylab data include urinary testosterone from three crew members, and excretion was increased at two in-flight data points before flight (34). We have extended those findings and report here urinary testosterone data from all three Skylab missions (Fig. 1). Plasma data from these Skylab missions (n = 9) are reported to have “show(n) a trend toward lower values after the mission” (34). Although we do not have urinary testosterone data from the International Space Station missions, these reports from the 1970s confirm our findings.
Bed rest studies have generally shown no effect of bed rest on circulating total or free testosterone (3, 14–16) in sedentary subjects, as confirmed here. Bed rest subjects ideally, and as in the studies we report here, are required to consume energy at a level to maintain body mass. If energy deficits are indeed part of the observed decrease in testosterone during the Shuttle flights previously reported, this may also explain the difference between those flight data and bed rest study data, reported herein and elsewhere. Although we showed an intermittent decrease in total and free testosterone in bed rest subjects with or without an artificial gravity (i.e. centrifugation) protocol (47), we had combined the two pre-bed rest collection sessions. When we analyzed these sessions separately because of our results in the present study, it turned out that testosterone concentrations were indeed higher only at the first data collection point (BR−10) than during or after bed rest (data not shown).
One criticism of sedentary bed rest studies as an analog for space flight is that astronauts are not sedentary, especially on long-duration missions, when they exercise extensively. Wade et al. (15) reported that bed rest subjects with intensive exercise protocols had lower nonfasting circulating plasma testosterone concentrations than nonexercising bed-rested controls. In this 4-wk study, they reported a small loss of (nonfasting) postbreakfast body mass (15, 48) and reported that caloric and liquid intakes were designed to maintain body mass. Despite the exercise, which was described as including an expenditure of 214 or 446 kcal/d (five times a week), actual intakes in the exercise groups were only 155 or 212 kcal/d greater than those of the nonexercise group (48). Clearly the interrelationship of energy balance, exercise, stress response, and endocrine function warrants further evaluation to better understand the adaptive responses to space flight (and bed rest).
In studies of rats with sample collections after space flight, serum (11) and urinary (10) testosterone have generally been decreased relative to the preflight period (12) and as reviewed by Tou et al. (49). Unfortunately, in-flight biological samples are typically not available, given the difficulties with collection procedures in the microgravity environment. These postflight conclusions are consistent with what we report here for landing day after a short-duration space flight. Hind-limb suspension studies have been used as a ground-based analog for rodents but not without another unique set of inherent difficulties and conflicting results. Differences in age, suspension duration, food intake, stress, and failure to surgically prevent testicular movement into the peritoneal cavity all contribute.
In summary, testosterone and related hormones are unchanged by real or simulated weightlessness, apart from transient effects after flight. Nonetheless, as we contemplate space exploration beyond low Earth orbit, endocrine data will be important for understanding human adaptation in this unique environment and potentially for helping to counteract the negative effects of space flight on the human body.
Supplementary Material
Acknowledgments
We thank the astronauts who participated in the flight studies and the volunteers who participated in the bed rest studies. Space flight and bed rest studies are complex and complicated and require teams of individuals to ensure that all details are captured, implemented, and documented according to plan. Although we cannot thank each individual, we thank and recognize the Human Research Program, the Human Health and Countermeasures Element, the International Space Station Medical Project, the Flight Analogs Project, and the staffs at the University of Texas Medical Branch in Galveston's Institute for Translational Sciences-Clinical Research Center. The Nutritional Biochemistry Laboratory was responsible for protocol coordination, sample collection and processing, and most of the analyses presented herein. We also thank Jane Krauhs for editorial assistance.
This work was funded by the National Aeronautics and Space Administration Human Research Program and specifically the Human Health and Countermeasures Element, the International Space Station Medical Project, the Flight Analogs Project, and the Nonexercise Physiological Countermeasures Project. Bed rest studies were conducted at the University of Texas Medical Branch in Galveston's Institute for Translational Sciences-Clinical Research Center and were supported in part by Grant 1UL1RR029876-01 from the National Center for Research Resources, National Institutes of Health. Support was also provided in part by Grant WB 0931 from the German Aerospace Center (Germany).
Disclosure summary: None of the authors had any conflicts of interest.
Footnotes
- aRED
- Advanced resistive exercise device
- CV
- coefficient of variation
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- FD
- flight day
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- R
- return.
References
- 1. Committee for the Decadal Survey on Biological and Physical Sciences in Space Studies Board 2010. Life and physical sciences research for a new era of space exploration: an interim report. Washington, DC: The National Academies Press; 1–46 [Google Scholar]
- 2. Wimalawansa SM, Chapa MT, Wei JN, Westlund KN, Quast MJ, Wimalawansa SJ. 1999. Reversal of weightlessness-induced musculoskeletal losses with androgens: quantification by MRI. J Appl Physiol 86:1841–1846 [DOI] [PubMed] [Google Scholar]
- 3. Zachwieja JJ, Smith SR, Lovejoy JC, Rood JC, Windhauser MM, Bray GA. 1999. Testosterone administration preserves protein balance but not muscle strength during 28 days of bed rest. J Clin Endocrinol Metab 84:207–212 [DOI] [PubMed] [Google Scholar]
- 4. Strollo F, Riondino G, Harris B, Strollo G, Casarosa E, Mangrossa N, Ferretti C, Luisi M. 1998. The effect of microgravity on testicular androgen secretion. Aviat Space Environ Med 69:133–136 [PubMed] [Google Scholar]
- 5. Strollo F. 1999. Hormonal changes in humans during spaceflight. Adv Space Biol Med 7:99–129 [DOI] [PubMed] [Google Scholar]
- 6. Strollo F, Boitani C, Basciani S, Pecorelli L, Palumbo D, Borgia L, Masini MA, More M, Strollo G, Spera G, Uva BM, Riondino G. 2005. The pituitary-testicular axis in microgravity: analogies with the aging male syndrome. J Endocrinol Invest 28:78–83 [PubMed] [Google Scholar]
- 7. Strollo F, Strollo G, More M, Bollanti L, Ciarmatori A, Longo E, Quintiliani R, Mambro A, Mangrossa N, Ferretti C. 1998. Hormonal adaptation to real and simulated microgravity. J Gravit Physiol 5:P89–P92 [PubMed] [Google Scholar]
- 8. Strollo F. 2000. Adaptation of the human endocrine system to microgravity in the context of integrative physiology and ageing. Pflugers Arch 441:R85–R90 [DOI] [PubMed] [Google Scholar]
- 9. Macho L, Kvetnansky R, Fickova M, Popova IA, Grigoriev A. 2001. Effects of exposure to space flight on endocrine regulations in experimental animals. Endocr Regul 35:101–114 [PubMed] [Google Scholar]
- 10. Ortiz RM, Wade CE, Morey-Holton E. 2000. Urinary excretion of LH and testosterone from male rats during exposure to increased gravity: post-spaceflight and centrifugation. Proc Soc Exp Biol Med 225:98–102 [DOI] [PubMed] [Google Scholar]
- 11. Vasques M, Lang C, Grindeland RE, Roy RR, Daunton N, Bigbee AJ, Wade CE. 1998. Comparison of hyper- and microgravity on rat muscle, organ weights and selected plasma constituents. Aviat Space Environ Med 69:A2–A8 [PubMed] [Google Scholar]
- 12. Amann RP, Deaver DR, Zirkin BR, Grills GS, Sapp WJ, Veeramachaneni DN, Clemens JW, Banerjee SD, Folmer J, Gruppi CM, et al. 1992. Effects of microgravity or simulated launch on testicular function in rats. J Appl Physiol 73:174S–185S [DOI] [PubMed] [Google Scholar]
- 13. Grindeland RE, Dotsenko MA, Mukku VR, Bigbee AJ, Bengtson SG. 2000. Rhesus monkey hormonal responses to microgravity. J Gravit Physiol 7:S143. [PubMed] [Google Scholar]
- 14. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. 1996. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270:E627–E633 [DOI] [PubMed] [Google Scholar]
- 15. Wade CE, Stanford KI, Stein TP, Greenleaf JE. 2005. Intensive exercise training suppresses testosterone during bed rest. J Appl Physiol 99:59–63 [DOI] [PubMed] [Google Scholar]
- 16. Murdaca G, Setti M, Brenci S, Fenoglio D, Lantieri P, Indiveri F, Puppo F. 2003. Modifications of immunological and neuro-endocrine parameters induced by antiorthostatic bed-rest in human healthy volunteers. Minerva Med 94:363–378 [PubMed] [Google Scholar]
- 17. Smorawiński J, Nazar K, Kaciuba-Uscilko H, Kamińska E, Cybulski G, Kodrzycka A, Bicz B, Greenleaf JE. 2001. Effects of 3-day bed rest on physiological responses to graded exercise in athletes and sedentary men. J Appl Physiol 91:249–257 [DOI] [PubMed] [Google Scholar]
- 18. Tash JS, Johnson DC, Enders GC. 2002. Long-term (6-wk) hindlimb suspension inhibits spermatogenesis in adult male rats. J Appl Physiol 92:1191–1198 [DOI] [PubMed] [Google Scholar]
- 19. Wimalawansa SM, Wimalawansa SJ. 1999. Simulated weightlessness-induced attenuation of testosterone production may be responsible for bone loss. Endocrine 10:253–260 [DOI] [PubMed] [Google Scholar]
- 20. Stein TP, Schluter MD, Moldawer LL. 1999. Endocrine relationships during human spaceflight. Am J Physiol Endocrinol Metab 276:E155–E162 [DOI] [PubMed] [Google Scholar]
- 21. Leach C, Alfrey C, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW, Krauhs JM. 1996. Regulation of body fluid compartments during short-term spaceflight. J Appl Physiol 81:105–116 [DOI] [PubMed] [Google Scholar]
- 22. Leach CS, Johnson PC, Rambaut PC. 1976. Metabolic and endocrine studies: the second manned Skylab mission. Aviat Space Environ Med 47:402–410 [PubMed] [Google Scholar]
- 23. Leach CS, Rambaut PC. 1977. Biochemical responses of the Skylab crewmen: an overview. In: Johnston RS, Dietlein LF, eds. Biomedical results from Skylab (NASA SP-377). Washington, DC: National Aeronautics and Space Administration; 204–216 [Google Scholar]
- 24. Zwart SR, Booth SL, Peterson JW, Wang Z, Smith SM. 2011. Vitamin K status in spaceflight and ground-based models of spaceflight. J Bone Miner Res 26:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zwart SR, Oliver SA, Fesperman JV, Kala G, Krauhs J, Ericson K, Smith SM. 2009. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat Space Environ Med 80:A15–A22 [DOI] [PubMed] [Google Scholar]
- 26. Spector ER, Smith SM, Sibonga JD. 2009. Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med 80:A23–A28 [DOI] [PubMed] [Google Scholar]
- 27. Crucian BE, Stowe RP, Mehta SK, Yetman DL, Leal MJ, Quiriarte HD, Pierson DL, Sams CF. 2009. Immune status, latent viral reactivation, and stress during long-duration head-down bed rest. Aviat Space Environ Med 80:A37–A44 [DOI] [PubMed] [Google Scholar]
- 28. Meck JV, Dreyer SA, Warren LE. 2009. Long-duration head-down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med 80:A1–A8 [DOI] [PubMed] [Google Scholar]
- 29. Platts SH, Martin DS, Stenger MB, Perez SA, Ribeiro LC, Summers R, Meck JV. 2009. Cardiovascular adaptations to long-duration head-down bed rest. Aviat Space Environ Med 80:A29–A36 [DOI] [PubMed] [Google Scholar]
- 30. Holguin N, Muir J, Rubin C, Judex S. 2009. Short applications of very low-magnitude vibrations attenuate expansion of the intervertebral disc during extended bed rest. Spine J 9:470–477 [DOI] [PubMed] [Google Scholar]
- 31. Smith SM, Wastney ME, O'Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. 2005. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J Bone Miner Res 20:208–218 [DOI] [PubMed] [Google Scholar]
- 32. Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE. 2005. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J Nutr 135:437–443 [DOI] [PubMed] [Google Scholar]
- 33. Smith SM, Davis-Street JE, Rice BL, Nillen JL, Gillman PL, Block G. 2001. Nutritional status assessment in semiclosed environments: ground-based and space flight studies in humans. J Nutr 131:2053–2061 [DOI] [PubMed] [Google Scholar]
- 34. Endocrinology Panel, Space Science Board 1979. Endocrinology. In: Life beyond the Earth's environment: the biology of living organisms in space. Washington, DC: National Academy of Sciences; 45–68 [Google Scholar]
- 35. Inniss AM, Rice BL, Smith SM. 2009. Dietary support of long-duration head-down bed rest. Aviat Space Environ Med 80:A9–A14 [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization 1985. Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: World Health Organization; [PubMed] [Google Scholar]
- 37. Chen Y, Yazdanpanah M, Wang XY, Hoffman BR, Diamandis EP, Wong PY. 2010. Direct measurement of serum free testosterone by ultrafiltration followed by liquid chromatography tandem mass spectrometry. Clin Biochem 43:490–496 [DOI] [PubMed] [Google Scholar]
- 38. Strollo F, Strollo G, More M, Ferretti C, Mangrossa N, Casarosa E, Luisi M, Riondino G. 1997. Changes in human adrenal and gonadal function onboard Spacelab. J Gravit Physiol 4:P103–P104 [PubMed] [Google Scholar]
- 39. Strollo F, Norsk P, Roecker L, Strollo G, Morè M, Bollanti L, Riondino G, Scano A. 1998. Indirect evidence of CNS adrenergic pathways activation during spaceflight. Aviat Space Environ Med 69:777–780 [PubMed] [Google Scholar]
- 40. Heer M, Boerger A, Kamps N, Mika C, Korr C, Drummer C. 2000. Nutrient supply during recent European missions. Pflugers Arch 441:R8–R14 [DOI] [PubMed] [Google Scholar]
- 41. Smith SM, Zwart SR, Kloeris V, Heer M. 2009. Nutritional biochemistry of space flight. New York: Nova Science Publishers [Google Scholar]
- 42. Lane HW, Gretebeck RJ, Schoeller DA, Davis-Street J, Socki RA, Gibson EK. 1997. Comparison of ground-based and space flight energy expenditure and water turnover in middle-aged healthy male U.S. astronauts. Am J Clin Nutr 65:4–12 [DOI] [PubMed] [Google Scholar]
- 43. Stein TP, Leskiw MJ, Schluter MD, Hoyt RW, Lane HW, Gretebeck RE, LeBlanc AD. 1999. Energy expenditure and balance during spaceflight on the space shuttle. Am J Physiol Regul Integr Comp Physiol 276:R1739–R1748 [DOI] [PubMed] [Google Scholar]
- 44. Alemany JA, Nindl BC, Kellogg MD, Tharion WJ, Young AJ, Montain SJ. 2008. Effects of dietary protein content on IGF-I, testosterone, and body composition during 8 days of severe energy deficit and arduous physical activity. J Appl Physiol 105:58–64 [DOI] [PubMed] [Google Scholar]
- 45. Kyröläinen H, Karinkanta J, Santtila M, Koski H, Mäntysaari M, Pullinen T. 2008. Hormonal responses during a prolonged military field exercise with variable exercise intensity. Eur J Appl Physiol 102:539–546 [DOI] [PubMed] [Google Scholar]
- 46. Cangemi R, Friedmann AJ, Holloszy JO, Fontana L. 2010. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 9:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zwart SR, Crawford GE, Gillman PL, Kala G, Rodgers AS, Rogers A, Inniss AM, Rice BL, Ericson K, Coburn S, Bourbeau Y, Hudson E, Mathew G, DeKerlegand DE, Sams CF, Heer MA, Paloski WH, Smith SM. 2009. Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J Appl Physiol 107:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Greenleaf JE, Bernauer EM, Ertl AC, Trowbridge TS, Wade CE. 1989. Work capacity during 30 days of bed rest with isotonic and isokinetic exercise training. J Appl Physiol 67:1820–1826 [DOI] [PubMed] [Google Scholar]
- 49. Tou J, Ronca A, Grindeland R, Wade C. 2002. Models to study gravitational biology of Mammalian reproduction. Biol Reprod 67:1681–1687 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.