Abstract
Expression controls of the carbon acquisition system in marine diatoms in response to environmental factors are an essential issue to understand the changes in marine primary productivity. A pyrenoidal β-carbonic anhydrase, PtCA1, is one of the most important candidates to investigate the control mechanisms of the CO2 acquisition system in the marine diatom Phaeodactylum tricornutum. A detailed functional assay was carried out on the putative core regulatory region of the ptca1 promoter using a β-glucuronidase reporter in P. tricornutum cells under changing CO2 conditions. A set of loss-of-function assays led to the identification of three CO2-responsive elements, TGACGT, ACGTCA, and TGACGC, at a region −86 to −42 relative to the transcription start site. Treatment with a cyclic (c)AMP analog, dibutyryl cAMP, revealed these three elements to be under the control of cAMP; thus, we designated them, from 5′ to 3′, as CO2-cAMP-Responsive Element1 (CCRE1), CCRE2, and CCRE3. Because the sequence TGACGT is known to be a typical target of human Activating Transcription Factor6 (ATF6), we searched for genes containing a basic zipper (bZIP) region homologous to that of ATF6 in the genome of P. tricornutum. Gel-shift assays using CCRE pentamers as labeled probes showed that at least one candidate of bZIP proteins, PtbZIP11, bound specifically to CCREs. A series of gain-of-function assays with CCREs fused to a minimal promoter strongly suggested that the alternative combination of CCRE1/2 or CCRE2/3 at proper distances from the minimal promoter is required as a potential target of PtbZIP11 for an effective CO2 response of the ptca1 gene.
Marine diatoms are the most successful group of algae in the ocean and are responsible for about one-fifth of global carbon fixation (Tréguer et al., 1995; Falkowski et al., 2000). The maximum CO2 concentration dissolved in seawater under the present atmosphere at 20°C is calculated to be below 15 μm, which is far lower than the Km[CO2] of the carbon fixation enzyme Rubisco in diatoms (Badger et al., 1998). This implies that CO2 in seawater would not support sufficient primary production; instead, an alternative inorganic carbon source is additionally required for it. It has been well established that many microalgae including marine diatoms possess CO2-concentrating mechanisms (CCMs), which actively take up both CO2 and HCO3− from the surrounding media and efficiently supply CO2 from accumulated HCO3− to Rubisco (Kaplan et al., 1980; Colman and Rotatore, 1995; Matsuda and Colman, 1995; Johnston and Raven, 1996; Matsuda et al., 2001; Moroney and Ynalvez, 2007; Spalding, 2008; Trimborn et al., 2008). Besides the CCMs, it has been reported in one species of marine diatom that a C4-like metabolism also operates in the CO2 acquisition system (Reinfelder et al., 2004). However, general occurrences of a C4-type system in diatoms are still in question (Roberts et al., 2007; Matsuda et al., 2011).
The activity of CCMs is suppressed under high-CO2 conditions and induced (or derepressed) under normal air (and/or further lower CO2) conditions in a few hours (Kaplan et al., 1980; Miller et al., 1990; Colman and Rotatore, 1995; Matsuda and Colman, 1995; Johnston and Raven, 1996; Matsuda et al., 2001; Kucho et al., 2003; Vance and Spalding, 2005). This indicates that algal cells can perceive changes in ambient CO2 concentration as a signal and acclimate to new CO2 conditions in a short period. Mechanisms of acclimation to CO2 and its relationship with other abiotic signals in major oceanic primary producers such as diatoms are a critical matter in order to understand the fate of aquatic primary productivity. Notwithstanding, molecular level studies of them are still in their infancy.
In contrast, mechanisms of CCM regulation by CO2 have been well studied in cyanobacteria. In Synechocystis sp. PCC 6803, the cytoplasmic-membrane protein (cmp)ABCD operon, which encodes Bicarbonate Transporter1, is induced by binding of Cytoplasmic-membrane protein Regulator (CmpR) to the promoter under low-CO2 conditions (Omata et al., 2001; Nishimura et al., 2008), but in contrast to this, other CO2-responsive genes (e.g. sodium bicarbonate transporter (sbt)A/sbtB, NAD(P)H dehydrogenase (ndh)F3/ndhD3/CO2 hydration protein Y/open reading frame133, and modified NDH-1 (mnh)D1/mnhD2) are repressed by binding of CO2-concentrating mechanism Regulator (CcmR)/NAD(P)H dehydrogenase Regulator (NdhR) to their promoters under high-CO2 conditions (Wang et al., 2004; Price et al., 2008). Similar functions of CcmR/NdhR were also observed in the marine cyanobacterium Synechococcus sp. PCC7002 (Price et al., 2008).
On the other hand, knowledge about the regulations of putative CCM components is relatively limited in eukaryotic algae. In the green alga Chlamydomonas reinhardtii, it is known that the transcriptional regulation of a periplasmic carbonic anhydrase (CA), Cah1 (Fujiwara et al., 1990), and mitochondrial CA, mtCA, are highly responsive to ambient CO2 concentration (Eriksson et al., 1998). The transcriptional activity of a promoter region of Cah1 is known to be under bidirectional controls in response to CO2, being ensured by enhancer and silencer regions, which reside back to back on the Cah1 promoter. This enhancer region is further found to possess two critical elements designated as enhancer-element consensus (Kucho et al., 2003), which is the target of a class of transcription factors, enhancer-element consensus-binding proteins (Yoshioka et al., 2004). Although the upstream signal cascade toward this promoter is not known in C. reinhardtii, a zinc finger protein, CCM1/Cia5, has been found to govern the up-regulation of most low-CO2-inducible genes and thus is considered to be a master regulator of the transcriptional response (Fukuzawa et al., 2001; Xiang et al., 2001).
The CCM and controls on expression of the genes involved in response to changes in ambient [CO2] are also observed in many marine eukaryotic photoautotrophs (Johnston and Raven, 1996; Badger et al., 1998; Sültemeyer 1998; Thoms et al., 2001; Matsuda et al., 2001; Colman et al., 2002). In marine diatoms, physiological evidence has shown that active uptake systems for CO2 and HCO3− operate in both centric and pennate species (Rotatore and Colman, 1992; Burkhardt et al., 2001; Matsuda et al., 2001, 2011; Chen et al., 2006; Trimborn et al., 2008). Since both the capacity to take up dissolved inorganic carbon (DIC) and a cell’s affinity for DIC were shown to be induced upon transferring cells of the marine diatom Phaeodactylum tricornutum from high CO2 to air (Matsuda et al., 2001), DIC uptake and internal CO2 flow toward Rubisco seem to be strengthened under CO2 limitation. As one of the putative key components for this acclimation process, chloroplastic CAs have been isolated and genes have been cloned (Satoh et al., 2001; Harada and Matsuda, 2005).
Similarly to CCMs in green algae and cyanobacteria, CAs at close proximity to Rubisco must play a crucial role in marine diatoms to create an efficient flux of CO2 to Rubisco under CO2-limiting conditions. Two β-type CAs, PtCA1 and PtCA2, found as chloroplast-localized enzymes in P. tricornutum, were localized in the pyrenoid, a large protein body in the stroma, and it is known that the C-terminal amphipathic helices of PtCAs play a role in the formation of a protein complex in the pyrenoid (Tanaka et al., 2005; Kitao and Matsuda, 2009; Tachibana et al., 2011). Transcription of the ptca1 and ptca2 genes is regulated by CO2, and the response of ptca1 to changes in [CO2] has been shown to be stronger than that of ptca2 (Harada et al., 2005, 2006).
The function of PtCA1 has yet to be determined, but this enzyme is one of the most interesting candidates for elucidating the molecular mechanisms of the CCM and acclimation to CO2 in diatoms. In previous studies, the promoter region of ptca1 (Pptca1) was isolated, and it was shown that the core regulatory region is composed of two putative cAMP-response elements (CRE1 and CRE2) and a putative p300-binding element (p300be; Harada et al., 2006); additionally, a putative SKiNhead-1 (Skn-1)-binding element was found downstream of the p300be (in this article). CRE and p300be were related to cAMP response elements in mammalian systems (Montminy et al., 1986; Deutsch et al., 1988; Rikitake and Moran 1992; Tanaka et al., 1997), and Skn-1 was activated by binding with cAMP-responsive element-binding protein 1 in the nematode Caenorhabditis elegans (Walker et al., 2000). It was indeed shown in P. tricornutum that transcription of ptca1 was repressed even under CO2-limiting conditions in the presence of the cAMP analog, dibutyryl cAMP (dbcAMP; Harada et al., 2006). This repressive effect of dbcAMP was negated by impairing the sequences around CREs and p300be (Harada et al., 2006), thus strongly suggesting that the core regulatory region of Pptca1 is under the repressive control of cAMP as a second messenger for CO2 signaling, but critical cis-elements for this process have yet to be determined in the Pptca1 promoter.
The perception of CO2 signals via cAMP has been reported in cyanobacteria, a pathogenic fungus, and mammals. The activity of soluble adenylyl cyclase (sAC) in rat testis is regulated by HCO3−pH, and it functions as a HCO3− sensor (Chen et al., 2000). In the pathogenic fungus Candida albicans, the activity of sAC was related directly to the regulation of filamentation by CO2/HCO3− (Klengel et al., 2005). The activity of CyaB1, one of the sACs in Anabaena PCC7120, is also regulated by HCO3− (Cann et al., 2003). In the cyanobacterium Synechocystis PCC6803, it was shown that another sAC, Cya1, is regulated directly by CO2 rather than HCO3− (Hammer et al., 2006). However, there are no data yet to support the relationship between sACs and CCM regulation in cyanobacteria, while it is strongly suggested that a product of the photorespiratory pathway, 2-phosphoglycolate, directly enhances the binding of CmpR to the promoter of the cmpABCD operon to ensure the CO2 response of this promoter (Nishimura et al., 2008). In eukaryotic algae, besides the above-described information from C. reinhardtii and P. tricornutum, little is known about the sensing mechanism for ambient CO2 concentrations, the subsequent signal transduction pathways, and transcriptional controls of promoters in response to changes in CO2 concentration.
In this study, critical cis-elements required for the CO2-cAMP response of the ptca1 promoter were identified, and transcription factors that may target the responsive elements were investigated.
RESULTS
Determination of Critical CO2-Responsive Elements in the Core Regulatory Region of Pptca1
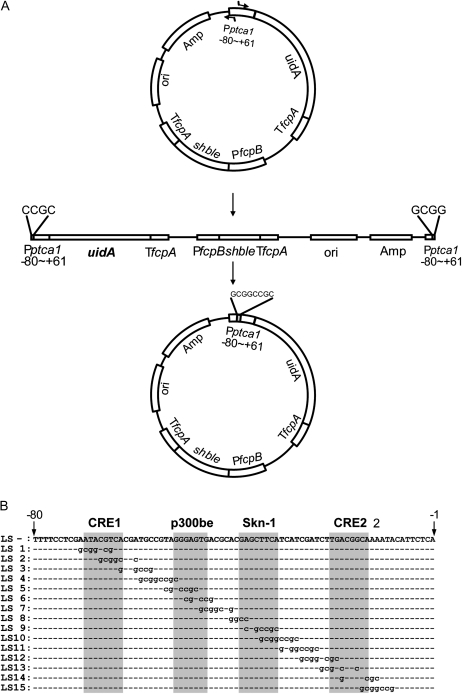
Our previous study has shown that the core regulatory region governing the CO2 response of Pptca1 resided −80 bp downstream relative to the transcription start site. To determine the precise CO2-responsive elements of Pptca1, a linker scan and the following one-base replacement assays were carried out on this core region. A series of linker-scan substitutions by the NotI restriction site (Fig. 1A) were introduced into the putative core regulatory region (−80 to +61 relative to the transcription start site) of Pptca1 (Fig. 1B). These fragments were ligated to the β-glucuronidase gene, uidA, and were transformed into P. tricornutum by microparticle bombardment. As shown in the previous study (Harada and Matsuda, 2005), transcript levels of uidA closely reflected the levels of GUS activity in P. tricornutum. The results in Figure 2 show the apparent loss of the CO2 response in the manipulated promoters LS1 to LS3 and LS5 to LS7. In contrast, other linker-scan promoters exhibited a clear CO2 response equivalent to that observed in the intact promoter (Fig. 2, LS-). Since the impaired sites of LS1 to LS3 and LS5 to LS7 corresponded, respectively, to regions around CRE1 and p300be, it was likely that these two elements are required for the CO2 response, as was assumed previously (Harada et al., 2005), while other sequences including the Skn-1 and the CRE2 elements were not required for the CO2 response of Pptca1. Levels of GUS activities expressed in transformants varied significantly depending upon clones. This is presumably due to position effects and the copy number of the promoter::uidA constructs integrating into different parts of the genome. However, GUS activity ratios (air to 5% CO2) were relatively stable, which enabled us to assess the CO2 response with some accuracy (Fig. 2). GUS activity ratios are thus presented for the GUS promoter assays hereafter.
Figure 1.
Linker-scan constructs. A, The promoter region of ptca1 from −80 to +61 relative to the transcription start site was packaged into the transformation vector pPha-T1 upstream of the GUS reporter gene uidA. The NotI restriction site (gcggccgc) was introduced between −71 and −8 relative to the transcription start site by inverse PCR on pPha-T1 with Pptca1 (−80 to +61) as the template. The resulting PCR products were self-ligated. B, The position of the introduced NotI site was slid toward downstream by 4 bp from LS1 to LS15. CRE1 and CRE2, p300be, and a putative Skn-1 site are indicated at the top of the sequence and highlighted in gray.
Figure 2.
GUS reporter assay of P. tricornutum cells transformed with intact Pptca1 and the linker-scan constructs. Schematic drawings of native Pptca1 (−80 to +61; LS-) and the linker-scan constructs 1 to 15 (LS1–LS15) are indicated at the left. GUS activity per total protein in lysates was determined in three clones of each transformant grown in 5% CO2 (white bars) or air (black bars). Values of each bar are means ± sd from three separate cultures. Ratios of GUS activity in air-grown cells to that in 5% CO2-grown cells are shown to the right of the bars. Asterisks indicate ratios below 2.0. PNP, p-Nitrophenol.
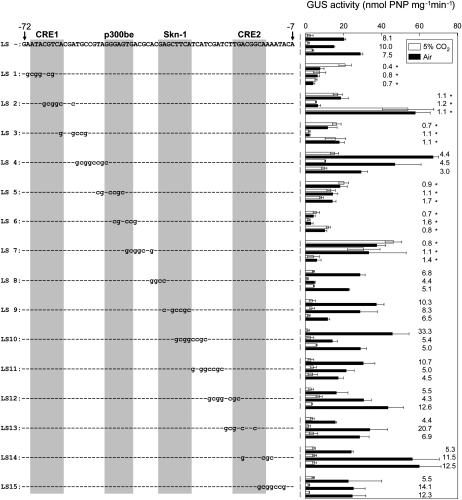
We followed these initial results by determining the precise CO2-responsive elements around the putative CRE1 and p300be elements. Single-nucleotide exchanges within purines or pyrimidines (A for C or T for G and vice versa) in regions around CRE1 (Fig. 3A) and p300be (Fig. 3B) revealed that the sequences 5′-ACGTCA-3′ (−68 to −63) and 5′-GTGACGC-3′ (−47 to −41), which did not completely overlap with CRE1 and p300be, were critical for the CO2 response (Fig. 3). This implied that the originally described CO2-responsive elements, CRE1 and p300be, were not adequately defined. Raw activity data for Figure 3 are available in Supplemental Figure S4.
Figure 3.
One-base replacements introduced to regions around the putative CRE1 and putative p300be elements, and effects on GUS activity. A, One-base replacements introduced to regions around CRE1 (−71 to −60) are shown in the left half. GUS reporter assays performed on transformants with each construct of Pptca1::uidA are shown in the right half. B, One-base replacements introduced to regions around p300be (−52 to −42) are shown in the left half. GUS reporter assays done on transformants with each construct of Pptca1::uidA are shown in the right half. GUS activities in lysates of each transformant grown in 5% CO2 (white bars) are expressed as relative values to those in air-grown cells (black bars). GUS reporter assays were performed on two to three clones obtained from each transformant. Values of each bar are means ± sd obtained from three separate cultures. Ratios of GUS activity in air-grown cells to that in 5% CO2-grown cells are shown to the right of the bars. Asterisks indicate ratios below 2.0.
Search for Further Upstream Elements That May Work Cooperatively with Elements around CRE1 and p300be
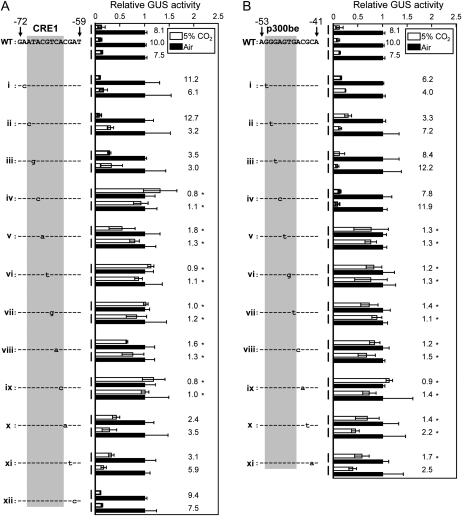
The intact promoter, which has both ACGTCA and GTGACGC motifs, showed a clear CO2 response (Fig. 4A, i). On the other hand, impairment of either one or both of these motifs by NotI insertion resulted in the loss of CO2 response (Fig. 4A, ii–iv). Interestingly, however, addition of the upstream sequence of Pptca1 (−1,292 to −81) to the region upstream of the short promoter sequence (−80 to +61), in which one of two critical CO2-responsive motifs was impaired by the NotI insertion, conferred a clear CO2 response on Pptca1 (Fig. 4A, vi and vii). However, such synergistic effects on the CO2 response did not occur when both of these motifs were impaired by NotI insertion (Fig. 4A, viii). These results indicated the occurrence of additional critical elements upstream of −80 relative to the transcription start site, which could work cooperatively with the two newly found motifs.
Figure 4.
CO2 response of Pptca1 with an introduced NotI site within putative CO2-responsive elements. A, Two different lengths of Pptca1 (−80 to +61 and −1,292 to +61 relative to the transcription start site) were ligated to the uidA reporter gene in the pPha-T1 vector, and fragments of newly identified CO2-responsive elements, ACGTCA and/or GTGACGC, were substituted with the NotI restriction site. GUS activities in lysates of each transformant grown in 5% CO2 (white bars) are expressed as relative values to those in air-grown cells (black bars). GUS reporter assays were made on two to three clones obtained from each transformation. Values of each bar are means ± sd of three separate cultures. Ratios of GUS activity in air-grown cells to that in 5% CO2-grown cells are shown to the right of the bars. B, The upstream truncated fragments (−484 to +61, −225 to +61, −115 to +61, and −80 to +61 relative to the transcription start site) of Pptca1 were ligated to the uidA reporter gene in the pPha-T1 vector, and either the ACGTCA or GTGACGT motif was disrupted by the NotI restriction site. The intact promoter of each length is also shown as a control. GUS activities in lysates of each transformant grown in 5% CO2 (white bars) are expressed as relative values to those in air-grown cells (black bars). GUS reporter assays were made on two to three clones obtained from each transformation. Values of each bar are means ± sd obtained from three separate cultures. Ratios of GUS activity in air-grown cells to that in 5% CO2-grown cells are shown to the right of the bars.
In order to detect the upstream elements, which were anticipated from the results of Figure 4A to work cooperatively with the two elements, the long Pptca1 constructs with singly mutated motifs (Fig. 4A, vi and vii) were truncated from upstream at positions −484, −225, −115, and −80 relative to the transcription start site and promoter activity was compared with those of intact promoters of each length (Fig. 4B). All transformants with these truncated constructs exhibited a clear CO2 response, with GUS activity ratios between 1.8 and 7.0 (Fig. 4B, ii, iii, v, vi, viii, and ix), except that uidA was totally derepressed when truncations were made to −80 (Fig. 4A, xi and xii). Results of GUS assays on cells containing the intact promoters of each length are also shown as controls (Fig. 4B, i, iv, vii, and x). These results clearly demonstrated the occurrence of the additional CO2-responsive element, which works with either of two downstream elements, at the region −115 to −80 relative to the transcription start site of Pptca1. Raw activity data for Figure 4 are available in Supplemental Figure S4.
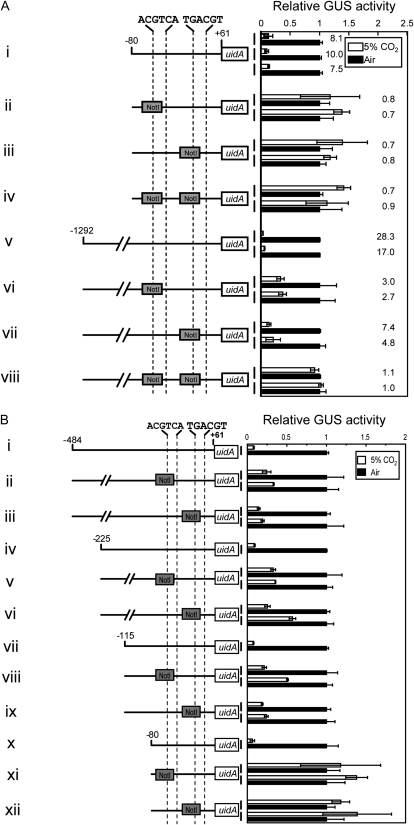
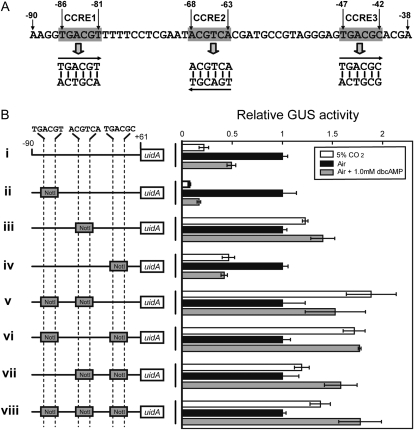
Role of Three Inverted Sequences, 5′-TGACGT/C-3′ in Responsiveness to CO2 and cAMP
The primary sequence between −115 and −80 relative to the transcription start site revealed a specific motif sequence at −86 to −81 (Fig. 5A). This sequence, 5′-TGACGT-3′, was highly homologous to one of the critical elements, 5′-TGACGC-3′, at −47 to −42 (Fig. 5A). These two motifs are of complementary relationship with the other critical element, 5′-ACGTCA-3′, at −68 to −63 (Fig. 5A). When either the −86 to −81 or the −47 to −42 sequence relative to the transcription start site was replaced by the NotI sequence, the CO2 response of the promoter (−90 to +61) was well conserved (Fig. 5B, ii and iv). Furthermore, treatment with dbcAMP repressed these mutated promoters even under the air condition, mimicking the response under 5% CO2 (Fig. 5B, ii and iv). However, impairment of the −68 to −63 sequence relative to the transcription start site or impairments of more than two motifs of these three inverted sequences resulted in full derepression of these manipulated promoters under 5% CO2 (Fig. 5B, iii and v–viii). dbcAMP treatment also became completely ineffective in regulating these manipulated promoters; that is, the uidA reporter was derepressed independently of either CO2 or dbcAMP (Fig. 5B, iii and v–viii). According to these results, three inverted elements were designated, from 5′ to 3′, as CO2-cAMP-Responsive Element1 (CCRE1), CCRE2, and CCRE3 (Fig. 5A), and it was strongly suggested that CCRE2 is the most important element for the CO2-cAMP response, as a single impairment of this sequence totally abolished the function of Pptca1 (Fig. 5B, iii). Raw activity data for Figure 5 are available in Supplemental Figure S4.
Figure 5.
Primary structure of the putative core regulatory region of Pptca1, and confirmation of the function of three inverted repeats in the CO2 and cAMP responsiveness of Pptca1. A, The three inverted sequences, TGACGT/C, newly found in Pptca1. B, The core regulatory region (−90 to +61 relative to the transcription start site) of Pptca1 was ligated to the uidA reporter gene in the pPha-T1 vector, and parts or all three inverted sequences were substituted with the NotI restriction site with different combinations (left half). GUS activities in lysates of each transformant grown in 5% CO2 (white bars) and acclimated to air in the presence of dbcAMP (gray bars) are expressed as relative values to those in air-grown cells (black bars). Values of each bar are means ± sd obtained from three separate cultures. Ratios of GUS activity in air-grown cells to that in 5% CO2-grown cells are shown to the right of the bars.
Search for Putative Transcription Factors That May Target CCREs
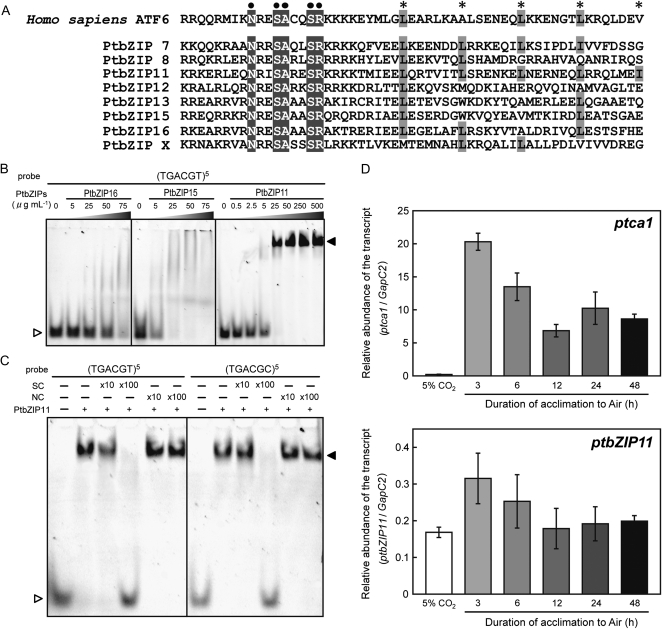
The CCRE1 element was identical to the cis-element known to be the typical target sequence of human Activating Transcription Factor6 (ATF6; Wang et al., 2000). This prompted us to search for homologs of human ATF6 in the genome database of P. tricornutum. Candidates were screened on the basis of homology of the DNA-binding site, NxxSAxxSR, which is well conserved in the mammalian ATF6 family (Vinson et al., 2002), and the putative Leu-zipper motif, whose primary structure is constituted of a heptad repeat of Leu residues (Fig. 6A). As a result, eight genes were found as candidates encoding basic zipper (bZIP) proteins that may target CCREs in Pptca1 (Fig. 6A). Seven of these bZIP sequences, PtbZIP7, -8, -11, -12, -13, -15, and -16, were recently described and grouped by Rayko et al. (2010), although PtbZIPx had not previously been reported. The critical amino acid sequence for the bZIP domain deduced from these eight genes revealed significant similarity (Fig. 6A), although the sequence similarity of other regions of the open reading frames of these putative proteins was minimal (Supplemental Fig. S1). These eight candidates were expressed in Escherichia coli BL21(DE3), and PtbZIP11, -15, and -16 were successfully expressed as soluble proteins, whereas the other candidates were found in insoluble fractions (Supplemental Fig. S2). For PtbZIP13, we could not detect induction in the E. coli system (Supplemental Fig. S2), so it was not studied further.
Figure 6.
Structures, binding property to CCRE, and expression profile of putative human ATF6 superfamily transcription factors in P. tricornutum. A, Putative bZIP regions of eight candidates, which may belong to the human ATF6 superfamily (PtbZIP7, -8, -11, -12, -13, -15, -16, and -x), were aligned with that of the human ATF6 protein. The well-conserved DNA-binding sequences, which may target the CCRE motif, are indicated by black dots and highlighted. The putative repeats of Leu in the ZIP region are indicated by asterisks and highlighted. B, A concentration of 125 μm CCRE1 pentamer labeled by Cy5 was mixed with different concentrations of PtbZIP11, -15, and -16. The reactant was separated on 6% polyacrylamide gels, and bands were detected by fluorography. C, A concentration of 125 μm labeled CCRE1 or CCRE3 pentamer was mixed with 25 μg mL−1 PtbZIP11 in the absence or presence of competitors. A specific competitor (SC), which is the nonlabeled CCRE1 or CCRE3 pentamer, or a nonspecific competitor (NC), which is 5′-CGATTCAAATGGAGAGTGGGTGAGCAAGGGCGAG-3′, was applied at concentrations 10 or 100 times more than the labeled probe. White and black arrowheads indicate free probe and shifted bands, respectively. D, qPCR of the ptca1 and ptbZIP11 transcripts was carried out on total RNAs extracted from cells grown in 5% CO2, air, and cells acclimated from 5% CO2 to air for 3, 6, 12, 24, and 48 h. Values are expressed as relative abundance to the amount of Cytoplasmic Glyceraldehyde-3-Phosphate Dehydrogenase2 (GapC2) transcript.
Gel-Shift Assay of the CCRE Sequences by PtbZIP11, -15, and -16
PtbZIP11, -15, and -16 were purified by nickel-Sepharose column chromatography (Supplemental Fig. S3), and the binding capacity of these candidates to a pentameric repeat of the TGACGT motif, (TGACGT)5, was examined. When 5 to 75 μg mL−1 PtbZIP15 and -16 was mixed with 125 μm (TGACGT)5, which was labeled by Cy5, the band of free probe started to disappear (Fig. 6B). However, in these assays, clear shifted bands were not detected; instead, some smeared signals were apparent at the top part of the gels (Fig. 6B). In contrast, a band shift accompanied by a disappearance of free probe was clearly observed by mixing more than 5 μg mL−1 PtbZIP11 with 125 μm (TGACGT)5 (Fig. 6B).
In order to confirm the binding specificity between CCRE motifs and PtbZIP11, additional gel-shift assays were carried out using nonlabeled competitor sequences. When 25 μg mL−1 PtbZIP11 was mixed with 125 μg mL−1 of either of the two types of CCRE pentamers, (TGACGT/C)5, clear band shifts were observed (Fig. 6C). However, these shifted bands began to disappear with the addition of nonlabeled specific competitors for (TGACGT)5 or (TGACGC)5 at 10-fold higher concentrations for the labeled probe, and band shifts were completely abolished by the addition of 100-fold specific competitors (Fig. 6C). In sharp contrast, the addition of nonspecific competitor to concentrations 100-fold higher than the labeled probe did not inhibit the band shifts of either (TGACGT)5 or (TGACGC)5 (Fig. 6C).
Expression Profiles of ptbZIP11 during Acclimation from High CO2 to Air
Since the mode of regulation of Pptca1 was demonstrated previously to be repression under high-CO2 conditions mediated by cAMP and derepression under normal air conditions (Harada et al., 2006), PtbZIP11 is most likely to act as a repressor of transcription. The expression of ptbZIP11 was analyzed during cell acclimation from 5% CO2 to air by quantitative (q)PCR and was related to that of ptca1 (Fig. 6D). It was apparent that ptbZIP11 mRNA constitutively accumulated independently of ambient CO2 concentrations (Fig. 6D), whereas that of ptca1 was completely repressed in 5% CO2 but was fully derepressed under air conditions (Fig. 6D). Transcript levels of ptbZIP11 were temporarily increased upon transfer of high-CO2 cells to air, but levels under high CO2 were equivalent to the basal air levels in cells acclimated for 12, 24, and 48 h (Fig. 6D). The relative expression level of ptbZIP11 was about 2 orders of magnitude lower than that of ptca1 in air-grown cells (Fig. 6D).
A Gain-of-Function Assay Using the CCRE Elements
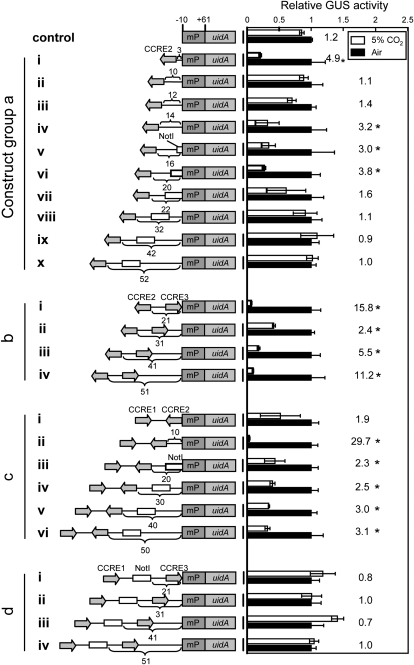
In order to understand the topology of the putative transcription factors bound on the newly found CCRE elements to the basal transcriptional complex, a range of CCRE sequences were ligated to the minimal promoter (mP) of Pptca1 (−10 to +61), and GUS reporter assays were carried out to assess the activity of the promoter. The distance between CCRE2 and mP was changed from 3 to 52 bp (Fig. 7, construct group a). The mP region alone drove GUS expression constitutively (Fig. 7, control). Interestingly, when CCRE2 was positioned immediately upstream (−3 bp) of mP, it conferred a clear CO2 response on it (Fig. 7, construct group a, i). However, by extending the interval to 10 or 12 bp, CCRE2 became inert in conferring the CO2 response (Fig. 7, construct group a, ii and iii), while the function was recovered again when the interval was further extended to between 14 and 22 bp (Fig. 7, construct group a, iv–vii). At distances greater than 32 bp, the effect of the single CCRE2 was lost completely (Fig. 7, construct group a, viii–x).
Figure 7.
Gain-of-function assay using CCREs at altered distances from the mP region of Pptca1. The CCRE2 sequence was fused to mP of Pptca1 (−10 to +61 relative to the transcription start site) at 10 different distances from 3 to 52 bp from mP (left half). GUS activities in lysates from each transformant grown in 5% CO2 (white bars) are expressed as relative values to those in air-grown cells (black bars). Values of each bar are means ± sd obtained from three separate cultures. Ratios of GUS activity in air-grown cells to that in 5% CO2-grown cells are shown to the right of the bars. Asterisks indicate ratios that exceed 2.0.
In contrast to the results for construct group a, vii to x, in which promoters partially or totally lost CO2 responsiveness, CCRE2 at intervals 21, 31, 41, and 51 from mP showed a clear CO2 response when CCRE3 was added downstream of CCRE2 (Fig. 7, construct group b, i–iv). Similarly, the presence of CCRE1 upstream of CCRE2 also increased the repressive activity of CCRE2 at longer intervals from mP (Fig. 7, construct group c). Notwithstanding, the promoters completely lost their CO2 response in the absence of CCRE2 at any interval, even in the presence of both CCRE1 and CCRE3 (Fig. 7, construct group d). Raw activity data for Figure 7 are available in Supplemental Figure S4.
DISCUSSION
In our previous study, a repressive effect of both CO2 and cAMP to Pptca1 was clearly demonstrated both in qPCR assays of the endogenous ptca1 transcript and in Pptca1::uidA reporter assays (Harada et al., 2006). This previous study also identified CRE1 and CRE2 and a putative p300be occurring downstream of −70 in Pptca1. Partial deletion of one of the CREs or the putative p300be resulted in derepression of Pptca1 in high CO2 or in the presence of dbcAMP in air. In this study, the newly found elements, CCRE1 to CCRE3, were clearly shown to be CO2-responsive elements that repressively control the transcription of ptca1 in high-CO2 conditions via the second messenger cAMP. We have further demonstrated that CCRE2 clearly overlapped with CRE1, but CCRE3 is new, since it did not match the putative p300be, which turned out no longer to be important. Skn-1 and CRE2 are also found to not be functional for CO2 response in this study. The occurrence of CCRE1 was overlooked in the previous study due to an apparently sufficient repressive effect with only CCRE2 and CCRE3 in response to high CO2 or dbcAMP. However, it was clearly shown in this study that CCRE1 complemented the absence of CCRE3.
CCRE1 to CCRE3 occurred with intervals of 12 to 15 nucleotides, which implies that each CCRE region locates by about two pitch turns of double-stranded DNA; thus, the plane of a base pair hybrid, which is targeted by a transcription factor, presumably faces to one side of the double helical structure of DNA in CCREs. CCRE2 is likely the most critical component among CCREs, due to its requirement for the CO2 response of Pptca1 (Fig. 5B).
It is interesting that CCRE1 and CCRE3 were nearly perfect inverted sequences of the CCRE2 element (Fig. 5A), strongly suggesting the pivotal requirement for CCRE2 to work cooperatively with CCRE1 and/or CCRE3. Synergistic effects between CRE-binding protein (CREB) and other transcription factors, which target other cis-elements, have frequently been observed in cAMP-responsive transcriptional controls in mammalian cells (Hyman et al., 1989; Nitsch et al., 1993; Roesler et al., 1995). However, to our knowledge, the repression of a promoter by alternative combinations of three cAMP-responsive elements has not been observed previously.
In this study, eight genes presumably encoding putative transcription factors were retrieved in the genome database of P. tricornutum, based on the homology of bZIP domains to that of the human ATF6 family (Fig. 6A). These proteins showed a striking similarity in their putative DNA-binding domains, but sequences other than bZIP domains showed little similarity to each other or to any known sequences (Supplemental Fig. S1). It is thus suggested from the primary structure that these putative bZIP proteins could be categorized to the ATF6 superfamily, but their interaction partners and functions are likely to be unique to diatoms.
Structures and functions of ATF family proteins have been studied mostly in mammalian cells. In general, transcription-activating domains reside at their N termini, although the domain structures of the N-terminal regions are totally different depending on each ATF subclass (Rehfuss et al., 1991; Nomura et al., 1993; Ribeiro et al., 1994; van Dam et al., 1995; Haze et al., 1999; Hai and Hartman, 2001; Hashimoto et al., 2002). Most mammalian ATF family proteins have a bZIP domain at their C terminus, but ATF6, exceptionally, has a bZIP region within the central part of the sequence (Haze et al., 1999; Hai and Hartman, 2001). Human ATF6 is a transmembrane protein localized on the endoplasmic reticulum membrane and is transferred to the nucleus in response to endoplasmic reticulum stresses by proteolysis of both sides of the transmembrane domain by the proteases, S1P and S2P (Haze et al., 1999). PtbZIPs reported in this study also contained more central bZIP domains (Supplemental Fig. S1), although none of them possessed transmembrane regions, suggesting that their initial functional location in cells would also be unique to diatoms.
Recently, putative bZIP sequences were analyzed by Rayko et al. (2010) within the genomes of the two model diatoms, P. tricornutum and Thalassiosira pseudonana. Diatom bZIP sequences were divided into six groups and constituted a stramenopile-specific family, which showed no notable homology with those from other species (Rayko et al., 2010). ATF family candidates found in this study mainly belong to group 1 to 3 diatom bZIPs, although none of them (except PtbZIP7 and PtbZIPx) contained Per-Arnt-Sim domains. PtbZIPx could not be categorized into any of the subgroups reported by Rayko et al. (2010), but this putative transcription factor possesses a Per-Arnt-Sim domain overlapping with the bZIP domain (data not shown), strongly suggesting its relation to group 2 diatom bZIPs.
ATF family proteins form homodimers or heterodimers via their Leu-zipper domain upon binding to cis-elements (Hai and Curran, 1991; Liu et al., 2006). On the other hand, the number of Leu or Ile residues in putative Leu-zipper regions in PtbZIPs was variable, and some of the proteins, PtbZIP8, -12, -13, -15, and -x, may not even be able to form a dimer due to the lack of enough Leu repeats (Fig. 6A). Nonetheless, their DNA-binding motif is well conserved, strongly suggesting their function as transcription factors that are closely related to the bZIP family.
The binding and specificity of PtbZIP15 and -16 to (TGACGT)5 were not clear (Fig. 6B), although an apparent decrease in free probe strongly suggested the affinity of these proteins to the element (Fig. 6B). Thus, PtbZIP11 is so far the only candidate transcription factor that directly binds to CCREs. However, from the results shown in Figure 6B, there is a possibility that other directly binding transcription factors besides PtbZIP11, which target CCREs with different affinities and may recruit different binding partners to CCRE regions, also participate in the regulatory system.
The constitutive expression under changing CO2 conditions of the ptbZIP11 transcription factor gene (Fig. 6D) strongly suggested that the function of PtbZIP11 is activated under high-CO2 conditions by posttranslational processes in response to the increment of cytosolic cAMP. This is also the case for human ATF6, which is known to be expressed constitutively and whose function is regulated at the posttranslational level by proteolytic digestion (Haze et al., 1999). Accordingly, upon exposure of P. tricornutum cells to limited-CO2 conditions, these repressive factors could be quickly deactivated by the reverse processes of the posttranscriptional modification to enable the derepression of Pptca1 within 3 h (Fig. 6D).
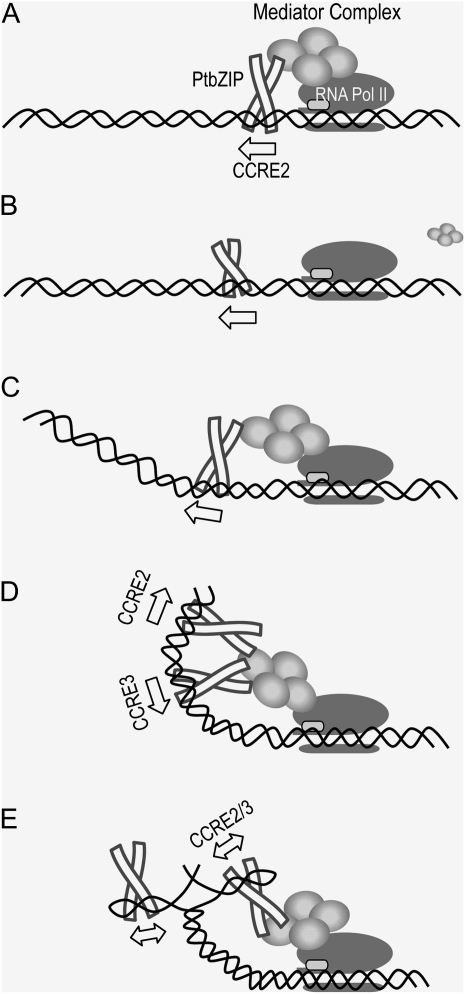
The data from the gain-of-function assays strongly suggest that spatial configurations between transcription factors on CCRE2 and mP regions on the double helical strands of DNA critically control the affinity of the basal transcriptional complex on the mP region, and that CCRE1 or CCRE3 reveals a function to ensure this regulation only in the presence of CCRE2. In other words, when CCRE2 is close enough to the mP region, PtbZIPs on CCRE2 would stably immobilize the basal transcription complex or stabilize the repressor on mP and repress transcription (Fig. 8A), presumably mediated by some other transcription factors, which is conceptually depicted in Figure 8, as a mediator complex. But this system probably requires specific topology of these complexes on the DNA strands, which would be why repression was lost when CCRE2 was located on the twisted face of the DNA strand at relatively longer distances from mP (10–12 bp and more than 32 bp; Fig. 8B). The construct shown in Figure 7 (construct a, x) has the same distance between CCRE2 and mP as that in the intact Pptca1, but construct a, x, exhibited no repression. It is thus probable that, at distances greater than 10 to 12 bp, the DNA strand downstream of CCRE2 would need to bend to interact with the basal transcriptional complex, as demonstrated previously in NeP1 binding to the F1 silencer element of the chicken lysozyme gene (Arnold et al., 1996; Fig. 8C), and CCRE1 or CCRE3 may work with CCRE2 by stabilizing this DNA bending (Fig. 8D). This assumption is supported by the gain-of-function assays using construct groups b to d in Figure 7, which show that the repressive function of CCRE2 at locations longer than 32 bp from mP under high CO2 was resumed when CCRE2 was combined with either CCRE1 or CCRE3.
Figure 8.
Speculative scheme showing repression models of Pptca1 mediated by CCREs and the PtbZIP protein complex under high CO2. A, In the gain-of-function analysis in Figure 7, direct harboring of a single CCRE2 to mP conferred a repression in high CO2 (Fig. 7, construct a, i). The PtbZIP complex anchors into CCRE2 and immobilizes the basal transcription complex, presumably mediated by some repressive complex depicted as a mediator. B, Extension of the distance between mP and the single CCRE2 abolishes the repressive interaction (Fig. 7, construct a, ii and iii), presumably due to changes in the relative configuration between the PtbZIP complex and the basal transcription complex. C, The PtbZIP complex regains repression (Fig. 7, construct a, iv–vi) when it is turned back to the proper face of the DNA strand. The DNA strand needs to be bent at longer distances between CCRE2 and mP, which would be ensured by the strength of interaction between the PtbZIP complex, the putative repressive mediator, and the basal transcription complex. D and E, In the endogenous Pptca1, CCRE2 cooperates with either CCRE1 or CCRE3, which would ensure a stronger interaction with the basal transcription complex to be immobilized. Multiple PtbZIPs may strengthen the interaction (D), or a partial unfolding of the DNA strand at CCRE1/2 or CCRE2/3 may make the angle of the CCRE-PtbZIP complex pliable and ensure the interaction with the basal transcription complex (E). In the cartoons, the case of CCRE2 and CCRE3 is depicted.
CCREs are almost identical sequences that align in inverted directions within 50 bp of Pptca1 (Fig. 5A). The functions of CCREs are likely specific for their directionality, because CCRE3 could not repress Pptca1 even when the element was attached immediately upstream of mP in the absence of CCRE2 (Fig. 7, construct d, i). It is probable that the repression complex formed on CCREs would be asymmetric, whose function is thus highly dependent on the direction of the CCRE sequence. Such a direction-dependent complex should show its asymmetric property strongly when the location is artificially approximated to mP. In the intact Pptca1, CCREs need to work with alternative combinations of CCRE1/2 or CCRE2/3. Combination did not work between CCRE1 and CCRE3; thus, the partners probably need to be complementary with each other. According to the structural model of the CREB-CRE complex, a bZIP dimer occupies one CRE element (Schumacher et al., 2000). This is also presumed to be the case for PtbZIP11. Each of the two CCRE combinations would thus be expected to form two complexes of CCRE-PtbZIP11 on Pptca1 (Fig. 8D). Alternatively, it is also possible that these combinations of CCREs form stem loops on each DNA strand when DNA is single stranded (Fig. 8E). However, epigenetic properties enabling such structural controls of Pptca1 upon transcriptional switching still remain to be explored.
CONCLUSION
In this study, the precise sequences of CCREs in Pptca1 and a possible transcription factor targeting them, PtbZIP11, were determined. Presumed topological models of Pptca1 required for CO2-cAMP-responsive transcription were proposed in this study, and the model suggested that a repeated number and directionality of CCREs (i.e. distances between CCREs and the relative location of CCREs from mP) are the critical factors in controlling Pptca1 activity. Our preliminary study further found that other CO2-responsive genes also possessed CCREs in their promoters but have different numbers and arrangements, and these promoters behave differently from CO2 compared with that of Pptca1 (Y. Matsuda, unpublished data). The model we propose in this study thus potentially provides an elemental example to understand the mechanisms of structural control of a promoter region, which lead to switching of transcriptional activity in response to changes in ambient CO2 in marine diatoms.
MATERIALS AND METHODS
Cells and Culture Conditions
The marine diatom Phaeodactylum tricornutum (UTEX 642) was obtained from the University of Texas Culture Collection and was grown in artificial seawater, which was supplemented with half-strength Guillard’s “f” solution (F/2ASW; Guillard and Ryther, 1962; Harrison et al., 1980) under continuous illumination of 50 μmol m−2 s−1 photosynthetic photon flux density under constant aeration with 5% CO2 or ambient air at 20°C. In acclimation experiments, 5% CO2-grown cells were harvested by centrifugation at 3,500g for 5 min under room temperature, washed twice with CO2-free F/2ASW, transferred to F/2ASW aerated with air, and allowed to acclimate for 1 to 2 d. In some experiments, 1.0 mm dbcAMP (Sigma-Aldrich) was added during the acclimation to air.
Preparation of Transformation Constructs
A series of upstream truncated constructs of Pptca1 (−1,292 to +61, −484 to +61, −225 to +61, −115 to +61, −90 to +61, and −80 to +61 relative to the transcription start site) were amplified by PCR using Pptca1 (−1,292 to +61) as a template. These constructs were phosphorylated and inserted into the blunt-ended site of transformation vector pFcpApGUS (Harada et al., 2005), which is a derivative of the general P. tricornutum transformation vector pPha-T1 equipped with a bleomycin-resistant gene cassette (Zaslavskaia et al., 2000).
A set of linker scan constructs with a NotI restriction site as the linker was synthesized by a modified inverse PCR method using the vector plasmid pFcpApGUS containing Pptca1 (−80 to +61) as a template (Fig. 1A). Primer sets were designed to generate a NotI restriction site (gcggccgc) at desired locations by self-ligation (Fig. 1A). Fifteen linker-scan constructs −80 downstream from the transcription start site were generated (Fig. 1B, LS- and LS1–LS15).
In some experiments, NotI substitutions were introduced into −67 to −60 and/or −47 to −40 relative to the transcription start site using the aforementioned vector plasmid pFcpApGUS containing upstream truncated Pptca1s of five different lengths (−1,292 to +61, −484 to +61, −225 to +61, −115 to +61, and −80 to +61) as templates. In some experiments, NotI substitutions were introduced into −87 to −80, −69 to −62, and/or −48 to −41 relative to the transcription start site using the vector plasmid pFcpApGUS containing upstream truncated Pptca1 (−90 to +61) as a template.
To generate one-base replacement constructs, PCR was carried out targeting the sequences −71 to −60 and −52 to −42 using the vector pFcpApGUS containing Pptca1 (−80 to +61). To generate gain-of-function constructs for modeling Pptca1 structure, LS7 was used as a template, and the distance between ACGTCA (−68 to −63) and mP was altered by a modified inverse PCR method. Primer sets were designed to alter the distance between ACGTCA and mP from 3 to 52 bp by self-ligation. Primer sequences used to generate these transformation constructs are summarized in Supplemental Table S7.
Transformation of P. tricornutum
Air-grown cells of P. tricornutum were harvested at midlogarithmic phase (optical density at 730 nm = 0.3–0.4). Approximately 5 × 107 cells were spotted as a plaque of 2.5 cm diameter on the surface of the F/2ASW agar plate. Five hundred micrograms of tungsten microcarrier (Bio-Rad; M17; approximately 1.1 μm particle size) was coated with 1.0 μg of plasmid DNA containing 1.0 m CaCl2 and 16 mm spermidine. The PDS-1000/He Biolistic Particle Delivery System (Bio-Rad) was used for microprojectile bombardment of the microcarrier. The bombardment was done at 10.7 MPa to the cells in the chamber under negative pressure of 91.4 kPa with a target distance of 6 cm. Bombarded cells were maintained on the plate for 1 d in the dark and were then suspended in 2 mL of F/2ASW. This cell suspension was plated on agar plates containing 100 μg mL−1 Zeocin (Takara Bio) in F/2ASW and allowed to form colonies for 3 to 4 weeks under continuous illumination.
Screening of GUS-Expressing Transformants
Zeocin-resistant clones were suspended in 60 μL of GUS extraction buffer (50 mm sodium phosphate [pH 7.0], 10 mm β-mercaptoethanol, 0.1% [w/v] sodium lauryl sarcosine, and 0.1% [v/v] Triton X-100) containing 1 mm 5-bromo-4-chloro-3-indolyl-β-d-GlcA cyclohexyl ammonium salt (Sigma-Aldrich) on 96-well plates and were incubated overnight at 37°C. Transformants expressing GUS, which displayed the dark-blue-colored chromogenic reaction in the presence of 5-bromo-4-chloro-3-indolyl-β-d-GlcA cyclohexyl ammonium salt, were inoculated and used for further experiments.
Quantification of GUS Activity
Cells at different acclimation stages were harvested, disrupted by sonicator (Ultrasonic disruptor model UD-201; TOMY Seiko Co. Ltd.) in 0.5 to 1.0 mL of GUS extraction buffer (50 mm sodium phosphate [pH 7.0], 10 mm β-mercaptoethanol, and 0.1% [v/v] Triton X-100] in an ice bath, and then centrifuged at 15,000g for 5 min at 4°C. Lysate (18 μL) was added to 882 μL of GUS reaction buffer (10 mm p-nitrophenyl β-d-glucuronide in the GUS extraction buffer) and incubated at 37°C. The reaction was terminated by the addition of 0.5 m Na2CO3 every 10 min after starting the reaction, and the absorbance of p-nitrophenol released from the substrate was measured at 405 nm. Protein concentrations in lysate were measured by the Bradford method (Bio-Rad) according to the manufacturer’s protocol.
Expression and Purification of PtbZIPs
Open reading frames of ptbZIPs were inserted into pET-21a vector designed to fuse the His hexamer to the C termini of products. Primer sets for the amplification of ptbZIPs are shown in Supplemental Table S6. PtbZIPs were expressed in Escherichia coli strain BL21(DE3) in 200 mL of Luria-Bertani medium supplemented with 1.0 mg mL−1 ampicillin and 0.5 mm isopropyl-1-thio-β-d-galactopyranoside. The E. coli cells were lysed by sonication in 10 mL of binding buffer (50 mm Tris-HCl [pH 8.0], 500 mm NaCl, and 5 mm imidazole). Crude lysates were applied to a 1-mL nickel-Sepharose column (Ni-Sepharose 6 Fast Flow; GE Healthcare Biosciences) repeatedly three times at 4°C, then washed with 12 mL of binding buffer (50 mm Tris-HCl [pH 8.0], 500 mm NaCl, and 5 mm imidazole) and 12 mL of washing buffer (50 mm Tris-HCl [pH 8.0], 500 mm NaCl, and 50 mm imidazole). After confirming the absorbance of eluate at 280 nm to be below 0.05, 12 mL of elution buffer (50 mm Tris-HCl [pH 8.0], 500 mm NaCl, and 300 mm imidazole) was applied to the column, and the eluates were collected for every 1 mL. The eluates were dialyzed with 1.2 L of imidazole-deprived buffer (50 mm Tris-HCl [pH 8.0] and 500 mm NaCl) using cellulose membranes (Viskase Companies). Concentrations of protein in eluate were determined by the Bradford method (Bio-Rad).
Gel-Shift Assay
As probes, pentamers of double-stranded 5′-TGACGT-3′ or 5′-TGACGC-3′ were labeled with Cy5 at their 5′ termini. A concentration of 2.5 mm labeled probe was added to 100 mL of annealing buffer (10 mm Tris-HCl [pH 7.5], 50 mm NaCl, and 5 mm EDTA), incubated at 96°C for 10 min, and then gradually cooled to 30°C for 45 min. The labeled probes at a final concentration of 125 μm were mixed with different concentrations of purified PtbZIPs in binding buffer (30 mm Tris-HCl [pH 7.5], 0.6 mm EDTA, 0.6 mm dithiothreitol, 50 mm KCl, 1 mm MgCl2, and 12% glycerol) and incubated for 30 min at 20°C. In some experiments, 1.25 or 12.5 mm nonlabeled DNA probes, which have either the same sequence to labeled probes or noncompetitive sequence (5′-CGATTCAAATGGAGAGTGGGTGAGCAAGGGCGAG-3′) to the labeled probe, were added in the binding buffer. Samples were mixed with 3 μL of 50% glycerol, separated on a nondenaturing 6% polyacrylamide gel in 0.5× Tris-borate/EDTA, and Cy5 signal was observed by a fluorescence imager (Molecular Imager FX; Bio-Rad).
Quantification of ptbZIP11 Transcript
Wild-type cells of P. tricornutum grown in 5% CO2 or acclimated to air were harvested at midlogarithmic phase. Cells were pelleted by centrifugation and immediately frozen in liquid nitrogen. Total RNA was extracted from 5% CO2-grown cells or cells acclimated to air for 3, 6, 12, 24, and 48 h using the RNeasy Plant Mini kit (Qiagen) according to the manufacturer’s protocol. One microgram of total RNA was reverse transcribed with oligo(dT)20 primer (Toyobo) and reverse transcriptase (Revertra Ace; Toyobo) to generate single-stranded cDNAs. The amount of PCR products was standardized by known amounts of template using plasmids containing full-length ptbZIP11 (accession no. AB573061), ptca1 (accession no. AF414191), or cytosolic glyceraldehyde-3-phosphate dehydrogenase gene (GapC2; accession no. AF063805), which were amplified respectively with the following sets of forward and reverse primers: PtbZIP7RTF (5′-CTTGCAAGCGATTGTGGGTC-3′) and PtbZIP7RTR (5′-CCAGGCCATGGGATTAAAGTTC-3′); PtCA1RTF (5′-TCACAATTCCTAGCAGAAAATCATCG-3′) and PtCA1RTR (5′-ACGCATCCAATGTACAAGTACTTGGG-3′); GapC2F (5′-TTTTTCGCCTTTCTAAACATCAGTT-3′) and GapC2R (5′-TACTCGGGCGTCAAGAAGG-3′). qPCR was carried out using the Smart Cycler Thermal Cycler System (Cepheid) and SYBR Premix Ex Taq (Takara Bio) under PCR conditions as follows; for ptbZIP11 and ptca1, heating at 95°C for 10 s, followed by 45 cycles of denaturing at 95°C for 5 s, annealing at 63°C for 20 s, and polymerase reaction at 72°C for 10 s; for GapC2, annealing temperature was 60°C. The amounts of cDNAs of ptbZIP11 and ptca1 were normalized as ratios to that of GapC2.
Sequence data from this article can be found in the GenBank/EMBL database under accession numbers as follows: ptbZIP7 (AB573067), ptbZIP8 (AB573064), ptbZIP11 (AB573066), ptbZIP12 (AB573065), ptbZIP13 (AB573061), ptbZIP15 (AB573063), ptbZIP16 (AB573062), ptbZIPx (AB648938), ptca1 (AF414191), uidA (S69414), and Homo sapiens ATF6 (AAB64434).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sequence alignment of human ATF6 and eight PtbZIPs.
Supplemental Figure S2. Confirmation of the expression of PtbZIPs in E. coli strain BL21(DE3).
Supplemental Figure S3. Purification of PtbZIP11, -15, and -16 on a nickel-Sepharose column.
Supplemental Figure S4. Original activity data of GUS assays in Figures 3, 4, 5, and 7.
Supplemental Table S1. Primer sequences used to synthesize upstream truncated constructs.
Supplemental Table S2. Primer sequences used to synthesize linker-scan constructs.
Supplemental Table S3. Primer sequences used to synthesize one-base replacement constructs around CRE1.
Supplemental Table S4. Primer sequences used to synthesize one-base replacement constructs around p300be.
Supplemental Table S5. Primer sequences used for the insertion of the NotI sequence into CCREs.
Supplemental Table S6. Primer sequences for the amplification of ptbZIPs.
Supplemental Table S7. Primer sequences used to synthesize Pptca1 modeling constructs.
Acknowledgments
We thank Prof. Chris Bowler for critical reading of the manuscript, Mr. Atsushi Tanaka, Mr. Keiji Goto, and Ms. Nobuko Higashiuchi for their technical assistance, and Ms. Miyabi Inoue for her skillful secretarial aid.
References
- Arnold R, Burcin M, Kaiser B, Muller M, Renkawitz R. (1996) DNA bending by the silencer protein NeP1 is modulated by TR and RXR. Nucleic Acids Res 24: 2640–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. (1998) The diversity and co-evolution of Rubisco, plastids, pyrenoids and chloroplast-based CO2-concentrating mechanisms in the algae. Can J Bot 76: 1052–1071 [Google Scholar]
- Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D. (2001) CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46: 1378–1391 [Google Scholar]
- Cann MJ, Hammer A, Zhou J, Kanacher T. (2003) A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J Biol Chem 278: 35033–35038 [DOI] [PubMed] [Google Scholar]
- Chen X, Qiu CE, Shao JZ. (2006) Evidence for K+-dependent HCO3− utilization in the marine diatom Phaeodactylum tricornutum. Plant Physiol 141: 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. (2000) Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628 [DOI] [PubMed] [Google Scholar]
- Colman B, Huertas E, Bhatti S, Dason JS. (2002) The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct Plant Biol 29: 261–270 [DOI] [PubMed] [Google Scholar]
- Colman B, Rotatore C. (1995) Photosynthetic inorganic carbon uptake and accumulation in two marine diatoms. Plant Cell Environ 18: 919–924 [Google Scholar]
- Deutsch PJ, Hoeffler JP, Jameson JL, Lin JC, Habener JF. (1988) Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem 263: 18466–18472 [PubMed] [Google Scholar]
- Eriksson M, Villand P, Gardeström P, Samuelsson G. (1998) Induction and regulation of expression of a low-CO2-induced mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 116: 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski P, Scholes RJ, Boyle E, Canadell J, Canfield D, Elser J, Gruber N, Hibbard K, Högberg P, Linder S, et al. (2000) The global carbon cycle: a test of our knowledge of earth as a system. Science 290: 291–296 [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Fukuzawa H, Tachiki A, Miyachi S. (1990) Structure and differential expression of two genes encoding carbonic anhydrase in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 87: 9779–9783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H, Miura K, Ishizaki K, Kucho KI, Saito T, Kohinata T, Ohyama K. (2001) Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci USA 98: 5347–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL, Ryther JH. (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8: 229–239 [DOI] [PubMed] [Google Scholar]
- Hai T, Curran T. (1991) Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA 88: 3720–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Hartman MG. (2001) The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273: 1–11 [DOI] [PubMed] [Google Scholar]
- Hammer A, Hodgson DRW, Cann MJ. (2006) Regulation of prokaryotic adenylyl cyclases by CO2. Biochem J 396: 215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Matsuda Y. (2005) Identification and characterization of a new carbonic anhydrase in the marine diatom Phaeodactylum tricornutum. Can J Bot 83: 909–916 [Google Scholar]
- Harada H, Nakajima K, Sakaue K, Matsuda Y. (2006) CO2 sensing at ocean surface mediated by cAMP in a marine diatom. Plant Physiol 142: 1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Nakatsuma D, Ishida M, Matsuda Y. (2005) Regulation of the expression of intracellular β-carbonic anhydrase in response to CO2 and light in the marine diatom Phaeodactylum tricornutum. Plant Physiol 139: 1041–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Waters RE, Taylor FJR. (1980) A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16: 28–35 [Google Scholar]
- Hashimoto Y, Zhang C, Kawauchi J, Imoto I, Adachi MT, Inazawa J, Amagasa T, Hai T, Kitajima S. (2002) An alternatively spliced isoform of transcriptional repressor ATF3 and its induction by stress stimuli. Nucleic Acids Res 30: 2398–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10: 3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Comb M, Pearlberg J, Goodman HM. (1989) An AP-2 element acts synergistically with the cyclic AMP- and phorbol ester-inducible enhancer of the human proenkephalin gene. Mol Cell Biol 9: 321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AM, Raven JA. (1996) Inorganic carbon accumulation by the marine diatom Phaeodactylum tricornutum. Eur J Phycol 31: 285–290 [Google Scholar]
- Kaplan A, Badger MR, Berry JA. (1980) Photosynthesis and intracellular inorganic carbon pool in the blue green alga Anabaena variabilis: response to external CO2 concentration. Planta 149: 219–226 [DOI] [PubMed] [Google Scholar]
- Kitao Y, Matsuda Y. (2009) Formation of macromolecular complexes of carbonic anhydrases in the chloroplast of a marine diatom by the action of the C-terminal helix. Biochem J 419: 681–688 [DOI] [PubMed] [Google Scholar]
- Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schröppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, et al. (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15: 2021–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucho K, Yoshioka S, Taniguchi F, Ohyama K, Fukuzawa H. (2003) Cis-acting elements and DNA-binding proteins involved in CO2-responsive transcriptional activation of Cah1 encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 133: 783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Deng X, Shyu YJ, Li JJ, Taparowsky EJ, Hu CD. (2006) Mutual regulation of c-Jun and ATF2 by transcriptional activation and subcellular localization. EMBO J 25: 1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Colman B. (1995) Induction of CO2 and bicarbonate transport in the green alga Chlorella ellipsoidea: time course of induction of the two systems. Plant Physiol 108: 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Hara T, Colman B. (2001) Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom, Phaeodactylum tricornutum. Plant Cell Environ 24: 611–620 [Google Scholar]
- Matsuda Y, Nakajima K, Tachibana M. (2011) Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynth Res 109: 191–203 [DOI] [PubMed] [Google Scholar]
- Miller AG, Espie GS, Canvin DT. (1990) Physiological aspects of CO2 and HCO3− transport by cyanobacteria: a review. Can J Bot 68: 1291–1302 [Google Scholar]
- Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. (1986) Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA 83: 6682–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Ynalvez RA. (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6: 1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Takahashi Y, Yamaguchi O, Suzuki H, Maeda S, Omata T. (2008) Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol Microbiol 68: 98–109 [DOI] [PubMed] [Google Scholar]
- Nitsch D, Boshart M, Schütz G. (1993) Activation of the tyrosine aminotransferase gene is dependent on synergy between liver-specific and hormone-responsive elements. Proc Natl Acad Sci USA 90: 5479–5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N, Zu YL, Maekawa T, Tabata S, Akiyama T, Ishii S. (1993) Isolation and characterization of a novel member of the gene family encoding the cAMP response element-binding protein CRE-BP1. J Biol Chem 268: 4259–4266 [PubMed] [Google Scholar]
- Omata T, Gohta S, Takahashi Y, Harano Y, Maeda S. (2001) Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J Bacteriol 183: 1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59: 1441–1461 [DOI] [PubMed] [Google Scholar]
- Rayko E, Maumus F, Maheswari U, Jabbari K, Bowler C. (2010) Transcription factor families inferred from genome sequences of photosynthetic stramenopiles. New Phytol 188: 52–66 [DOI] [PubMed] [Google Scholar]
- Rehfuss RP, Walton KM, Loriaux MM, Goodman RH. (1991) The cAMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem 266: 18431–18434 [PubMed] [Google Scholar]
- Reinfelder JR, Milligan AJ, Morel FMM. (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135: 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A, Brown A, Lee KAW. (1994) An in vivo assay for members of the cAMP response element-binding protein family of transcription factors. J Biol Chem 269: 31124–31128 [PubMed] [Google Scholar]
- Rikitake Y, Moran E. (1992) DNA-binding properties of the E1A-associated 300-kilodalton protein. Mol Cell Biol 12: 2826–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K, Granum E, Leegood RC, Raven JA. (2007) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 145: 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler WJ, Graham JG, Kolen R, Klemm DJ, McFie PJ. (1995) The cAMP response element binding protein synergizes with other transcription factors to mediate cAMP responsiveness. J Biol Chem 270: 8225–8232 [DOI] [PubMed] [Google Scholar]
- Rotatore C, Colman B. (1992) Active uptake of CO2 by the diatom Navicula pelliculosa. J Exp Bot 43: 571–576 [Google Scholar]
- Satoh D, Hiraoka Y, Colman B, Matsuda Y. (2001) Physiological and molecular biological characterization of intracellular carbonic anhydrase from the marine diatom Phaeodactylum tricornutum. Plant Physiol 126: 1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Goodman RH, Brennan RG. (2000) The structure of a CREB bZIP.somatostatin CRE complex reveals the basis for selective dimerization and divalent cation-enhanced DNA binding. J Biol Chem 275: 35242–35247 [DOI] [PubMed] [Google Scholar]
- Spalding MH. (2008) Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot 59: 1463–1473 [DOI] [PubMed] [Google Scholar]
- Sültemeyer D. (1998) Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis. Can J Bot 76: 962–972 [Google Scholar]
- Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C, Matsuda Y. (2011) Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth Res 109: 205–221 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakatsuma D, Harada H, Ishida M, Matsuda Y. (2005) Localization of soluble β-carbonic anhydrase in the marine diatom Phaeodactylum tricornutum: sorting to the chloroplast and cluster formation on the girdle lamellae. Plant Physiol 138: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Naruse I, Maekawa T, Masuya H, Shiroishi T, Ishii S. (1997) Abnormal skeletal patterning in embryos lacking a single Cbp allele: a partial similarity with Rubinstein-Taybi syndrome. Proc Natl Acad Sci USA 94: 10215–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms S, Pahlow M, Gladrow DAW. (2001) Model of the carbon concentrating mechanism in chloroplasts of eukaryotic algae. J Theor Biol 208: 295–313 [DOI] [PubMed] [Google Scholar]
- Tréguer P, Nelson DM, Van Bennekom AJ, Demaster DJ, Leynaert A, Quéguiner B. (1995) The silica balance in the world ocean: a reestimate. Science 268: 375–379 [DOI] [PubMed] [Google Scholar]
- Trimborn S, Lundholm N, Thoms S, Richter KU, Krock B, Hansen PJ, Rost B. (2008) Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in seawater carbonate chemistry. Physiol Plant 133: 92–105 [DOI] [PubMed] [Google Scholar]
- Vance P, Spalding MH. (2005) Growth, photosynthesis and gene expression in Chlamydomonas over a range of CO2 concentrations and CO2/O2 ratios: CO2 regulates multiple acclimation states. Can J Bot 83: 796–809 [Google Scholar]
- van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. (1995) ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J 14: 1798–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C, Myakishev M, Acharya A, Mir AA, Moll JR, Bonovich M. (2002) Classification of human B-ZIP proteins based on dimerization properties. Mol Cell Biol 22: 6321–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, See R, Batchelder C, Kophengnavong T, Gronniger JT, Shi Y, Blackwell TK. (2000) A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap’n’Collar-related basic leucine zipper proteins. J Biol Chem 275: 22166–22171 [DOI] [PubMed] [Google Scholar]
- Wang HL, Postier BL, Burnap RL. (2004) Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J Biol Chem 279: 5739–5751 [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. (2000) Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 275: 27013–27020 [DOI] [PubMed] [Google Scholar]
- Yoshioka S, Taniguchi F, Miura K, Inoue T, Yamano T, Fukuzawa H. (2004) The novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1, encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Cell 16: 1466–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Zhang J, Weeks DP. (2001) The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 98: 5341–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE. (2000) Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol 36: 379–386 [Google Scholar]