Abstract
Wolbachia are maternally transmitted symbiotic bacteria that can spread within insect populations because of their unique ability to manipulate host reproduction. When introduced to nonnative mosquito hosts, Wolbachia induce resistance to a number of human pathogens, including dengue virus (DENV), Plasmodium, and filarial nematodes, but the molecular mechanism involved is unclear. In this study, we have deciphered how Wolbachia infection affects the Aedes aegypti host in inducing resistance to DENV. The microarray assay indicates that transcripts of genes with functions related to immunity and reduction-oxidation (redox) reactions are up-regulated in Ae. aegypti infected with Wolbachia. Infection with this bacterium leads to induction of oxidative stress and an increased level of reactive oxygen species in its mosquito host. Reactive oxygen species elevation is linked to the activation of the Toll pathway, which is essential in mediating the expression of antioxidants to counterbalance oxidative stress. This immune pathway also is responsible for activation of antimicrobial peptides—defensins and cecropins. We provide evidence that these antimicrobial peptides are involved in inhibition of DENV proliferation in Wolbachia-infected mosquitoes. Utilization of transgenic Ae. aegypti and the RNAi depletion approach has been instrumental in proving the role of defensins and cecropins in the resistance of Wolbachia-infected Ae. aegypti to DENV. These results indicate that a symbiotic bacterium can manipulate the host defense system to facilitate its own persistent infection, resulting in a compromise of the mosquito's ability to host human pathogens. Our discoveries will aid in the development of control strategies for mosquito-transmitted diseases.
Keywords: antiviral resistance, innate immunity, population replacement
Diseases transmitted by hematophagous arthropod vectors, such as mosquito-borne malaria and dengue fever, cause 1.5 million human deaths every year (1). The insufficiency of currently available control strategies, such as vaccines, drugs, and pesticides, has led to a dramatic increase in occurrence of vector-borne diseases (2, 3). Presently, two-fifths of the world's population are at risk from dengue fever, and 50–100 million individuals are infected each year (2). Significant efforts have been devoted to developing novel strategies for dengue control. One such approach involves making the mosquito host inhospitable to dengue virus (DENV) through population replacement, with the goal of impeding or halting disease transmission.
Wolbachia (Rickettsiales) species are reproductive parasites or endosymbionts of arthropods and nematodes. A unique feature of Wolbachia biology in many arthropods is their ability to spread through host populations by means of a reproductive mechanism referred to as “sperm–egg cytoplasmic incompatibility” (CI) (4). CI results in early embryonic death when a Wolbachia-infected male mates with a female that is uninfected or is harboring a different Wolbachia type. Because an uninfected male can mate successfully with an infected female, CI provides a reproductive advantage to a Wolbachia-infected female over an uninfected one.
Wolbachia have been explored as a potential tool in the control of mosquito-borne diseases because of their unique ability to invade host populations rapidly (5). Wolbachia are found in many mosquito species in nature but surprisingly not in Anopheles malaria vectors or the major dengue vector, Aedes aegypti. Although a transient somatic infection was made recently in Anopheles gambiae (6, 7), Ae. aegypti has been stably transfected by several Wolbachia strains (8, 9). Previously, we established the Ae. aegypti WB1 line carrying the Wolbachia strain wAlbB by means of the microinjection transfer of this bacterium from Aedes albopictus to the wild-type Ae. aegypti Waco strain (8). This WB1 Ae. aegypti strain shows strong resistance to DENV compared with the parental Waco strain (10). The wAlbB strain inhibited viral replication in the mosquito midgut as well as its dissemination throughout the mosquito thorax and head, dramatically reducing the mosquito's potential for viral transmission. Resistance to several arboviruses was observed in Ae. aegypti infected with the Wolbachia strain wMelPop-CLA (11), and in Culex quinquefasciatus infected with wPip (12). Development of Plasmodium also was inhibited in An. gambiae mosquitoes carrying the somatic infection with wMelPOP and wAlbB Wolbachia strains (6, 7). These recent discoveries of Wolbachia-mediated effects in vector mosquitoes have a great potential for use in population-replacement strategies (5).
Although the molecular mechanism underlying Wolbachia-mediated resistance to pathogens is not understood completely, it appears to be associated with boosted mosquito innate immunity (6, 7, 10, 11, 13). A number of immune genes, such as defensins, cecropins, and several Toll pathway genes, were up-regulated by Wolbachia in Ae. aegypti (10, 11, 13). In agreement with these observations, activation of the Toll pathway by means of RNAi depletion of cactus, an inhibitor of REL1, has been shown to block proliferation of DENV in Ae. aegypti (14).
Here, we have deciphered the molecular mechanism underlying Wolbachia-mediated resistance to DENV in this mosquito. Microarray and real-time PCR analyses revealed that the Toll pathway and antioxidant genes were induced in Ae. aegypti infected with the Wolbachia wAlbB strain. This bacterium elevated the expression of NADPH oxidase and of reactive oxygen species (ROS). In turn, ROS up-regulation resulted in activation of the Toll pathway, which mediated both the antioxidant and production of antimicrobial peptides—cecropins and defensins. We used transgenic Ae. aegypti mosquitoes with altered immunity and RNAi depletion of immune factors to demonstrate the involvement of these antimicrobial peptides in anti-dengue responses.
Results
Wolbachia Induces the Toll Pathway and Antioxidant Genes in Ae. Aegypti.
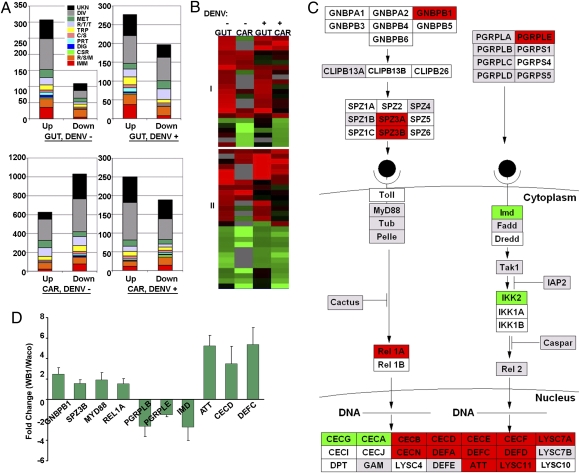
To determine the physiological responses of the mosquito to Wolbachia infection, we conducted microarray assays to compare transcript repertoires in the mosquito midgut and remaining carcass tissues of 7-d-old Wolbachia-infected Ae. aegypti WB1 females with those of the uninfected Waco strain, before blood feeding. Similar microarray assays were conducted using Wolbachia-infected mosquitoes collected 12 d after additional infection with DENV serotype 2. This time point was chosen because it marks inhibition of DENV replication by Wolbachia infection (10). We classified differentially expressed genes that were identified in the two series of microarray assays. The most notable Wolbachia-responsive transcripts were related to immunity and the redox/stress/mitochondrion (R/S/M) groups (Fig. 1A). Except for genes belonging to unknown or diverse functions, the immunity and R/S/M groups were the two most highly regulated functional groups in the midgut and comprised the largest number of differently expressed genes in the carcass. Genes within these two groups represented 21.9% (midgut) and 14.4% (carcass) of all of the regulated genes with predicted functions in the non–blood-fed mosquitoes, and 20.2% (midgut) and 13.9% (carcass) in the dengue-infected mosquitoes. Transcripts of 23 immune genes and 27 R/S/M genes exhibited similar patterns in both tissues (midgut and carcass) and with two treatments (with and without dengue infection, Fig. 1B). These genes included defensin C (DEFC), attacin (ATT), Cu superoxide dismutase (CuSOD2), 13 cytochrome P450 genes, two putative NADH dehydrogenase genes, and three heat-shock proteins. Importantly, genes belonging to the Toll pathway—Gram-negative binding protein B1 (GNBPB1) and Relish-like protein 1A (REL1A)—were up-regulated in the midguts of Wolbachia-infected mosquitoes, both with and without DENV (Fig. 1C and Fig. S1). The effect of Wolbachia on the induction of the Toll pathway was confirmed further using real-time PCR. Transcripts of the antimicrobial peptide genes ATT, cecropin D (CECD), and DEFC were highly elevated in Wolbachia-infected Aedes mosquitoes (Fig. 1D). Likewise, the components of the Toll pathway—GNBPB1, Spaetzle 3 (SPZ3), myeloid differentiation primary response gene 88 (MYD88), and REL1A—also were up-regulated (Fig. 1D). In contrast, genes representing the immune deficiency (IMD) pathway—IMD, peptidoglycan recognition protein LB (PGRP-LB), and peptidoglycan recognition protein LE (PGRPLE)—were down-regulated.
Fig. 1.
Wolbachia-regulated genes in Ae. aegypti. (A) Functional classification of differentially expressed genes in the midgut (GUT) and carcass (CAR) of 7-d-old mosquitoes before a blood meal (DENV−) and 12 d after infection with DENV serotype 2 (DENV+). The graph shows the functional class distributions in real numbers of genes that are regulated by Wolbachia (“Up” indicates induced, and “Down” indicates repressed). The Wolbachia-regulated gene expression data are presented in Tables S1, S2, S3, and S4. Functional group abbreviations: C/S, cytoskeletal and structural; CSR, chemosensory reception; DIG, blood and sugar food digestive; DIV, diverse functions; IMM, immunity; MET, metabolism; PRT, proteolysis; R/S/M, redox, stress, and mitochondrion; R/T/T, replication, transcription, and translation; TRP, transport; UNK, unknown functions. (B) Cluster analysis of 23 immune genes (I) and 27 R/S/M genes (II) that were regulated in midguts and carcasses of Wolbachia-infected female mosquitoes in at least two of four combinations: midgut with (GUT+) or without (GUT−) DENV infection; carcass with (CAR+) or without (CAR−) DENV infection. All genes presented in the cluster are listed in Tables S5 and S6. (C) Regulation of putative Toll signaling pathway genes by Wolbachia in the mosquito midgut 12 d after feeding on DENV-infected blood. Red, green, and gray colors indicate Wolbachia-responsive up-, down- and nonregulated genes, respectively. White indicates unfound or filtered signal. The pathway was built with GenMAPP software based on the immunogenomics prediction (49, 50). CECB, Cecropin B; CECE, Cecropin E; CECF, Cecropin F; CECG, Cecropin G; CECI, Cecropin I; CECJ, Cecropin J; CECN, Cecropin N; CLIPB13A, Clip-domain serine protease 13A; CLIPB13B, Clip-domain serine protease 13B; CLIPB26, Clip-domain serine protease 26; DEFD, Defensin D; DEFE, Defensin E; DPT, Diptericin; Dredd, Death-related ced-3/Nedd2-like protein; Fadd, Fas-Associated Death Domain; GAM, Gambicin; GNBPA1, Gram-negative binding protein A1; GNBPA2, Gram-negative binding protein A2; GNBPB3, Gram-negative binding protein B3; GNBPB4, Gram-negative binding protein B4; GNBPB5, Gram-negative binding protein B5; GNBPB6, Gram-negative binding protein B6; IAP2, Inhibitor of apoptosis 2; IKK1A, I-Kappa-B Kinase 1 A; IKK1B, I-Kappa-B Kinase 1 B; IKK2, I-Kappa-B Kinase 2; LYSC4, C-Type lysozyme 4; LYSC7A, C-Type Lysozyme 7A; LYSC7B, C-Type Lysozyme 7B; LYSC10, C-Type lysozyme 10; LYSC11, C-Type lysozyme 11; Pelle, PGRPLA, Peptidoglycan Recognition Protein (Long) A; PGRPS1, Peptidoglycan Recognition Protein (Short) 1; PGRPLC, Peptidoglycan Recognition Protein (Long) C; PGRPS4, Peptidoglycan Recognition Protein (Short) 4; PGRPLD, Peptidoglycan Recognition Protein (Long) D; PGRPS5, Peptidoglycan Recognition Protein (Short) 5; Rel2, Relish-like protein 2; SPZ2, Spaetzle-likecytokine 2; SPZ4, Spaetzle-like cytokine 4; SPZ5, Spaetzle-like cytokine 5; SPZ1C, Spaetzle-like cytokine 1C; SPZ6, Spaetzle-like cytokine 6; Tak1, TGF-Beta-Activated Kinase-1; Tub, Tube. (D) Real-time PCR validation of Wolbachia-regulated genes identified in the microarray assays. Data are shown as fold change in WB1 midguts compared with Waco midguts 12 d after feeding on DENV-infected blood.
Moreover, we observed a number of antioxidant genes being induced by Wolbachia in our microarray analysis, including glutathione peroxidase (AAEL012069 and AAEL008397), CuZnSOD (AAEL006271 and AAEL011498), manganese superoxide dismutase (MnSOD; AAEL004823 and AAEL005108), thioredoxin peroxidase (AAEL013528 and AAEL014548), and glutathione peroxidase (AAEL008397) (Table S7). Transcripts of both ferritin heavy chain (AAEL007385) and light chain (AAEL004335) also were up-regulated by Wolbachia in midguts of 7-d-old WB1 mosquitoes before blood feeding or at 12 d postinfection. Such a dramatic induction of antioxidant genes suggests that ROS levels within host cells are elevated because of Wolbachia infection.
Wolbachia Infection Induces Oxidative Stress in Ae. Aegypti.
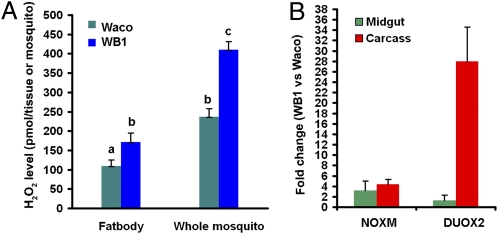
To confirm that infection with Wolbachia induces oxidative stress in Ae. aegypti, we compared the levels of hydrogen peroxide (H2O2) in WB1 and Waco mosquitoes. The level of H2O2 was significantly higher in both fat bodies (Student's t test, P < 0.05) and whole bodies (Student's t test, P < 0.001) of WB1 mosquitoes than in those of Waco mosquitoes (Fig. 2A). Similar to the oxidative burst in vertebrates, insects generate ROS through the enzymatic activity of NADPH oxidase (NOX) and dual oxidase (DUOX) (15, 16). Therefore, we compared transcript levels of genes encoding two NOX enzymes (NOX5 and NOXM) and two DUOX enzymes (DUOX1 and DUOX2) in WB1 and control Waco females using real-time PCR. There was a 3.1-fold and 4.4-fold increase in NOXM transcript abundance in the midgut and carcass, respectively, in WB1 females compared with Waco controls. Remarkably, we observed a 28.0-fold increase in the DUOX2 transcript in the carcass but only a 1.3-fold increase in the midgut of WB1 mosquitoes compared with Waco controls (Fig. 2B). No significant change in transcript levels of either NOX5 or DUOX1 was found as a result of Wolbachia infection. These results support the suggestion that NADPH oxidase-dependent ROS are induced in Ae. aegypti as a result of infection with Wolbachia.
Fig. 2.
Wolbachia induces ROS formation in the mosquito Ae. aegypti. (A) Comparison of H2O2 levels in the fat body and whole mosquito in 7-d-old WB1 and Waco mosquitoes before a blood meal. The data shown are means of six replicates, with five fat bodies or three whole mosquitoes for each. The level of H2O2 was significantly higher in both fat bodies and whole bodies of WB1 mosquitoes than in those of Waco mosquitoes. Statistical significance is represented by letters above each column, with different letters signifying distinct statistical groups. Student's t test: a vs. b, P < 0.05; b vs. c, P < 0.001. (B) Fold changes in expression of NADPH oxidase (NOXM) and dual oxidase (DUOX2) in the midguts and carcasses of WB1 and Waco mosquitoes. Real-time PCR analysis was performed using midguts and carcasses of 7-d-old, non–blood-fed, female mosquitoes.
Increased Level of ROS Is Sufficient to Activate the Toll Pathway in Ae. Aegypti.
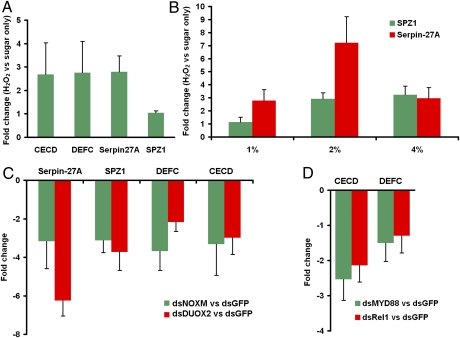
To test whether a high level of ROS could activate the Toll pathway in Ae. aegypti, we fed 2-d-old Waco female mosquitoes with a sugar solution containing 1% (vol/vol) H2O2 for 5 d and then measured the expression of two Toll pathway marker genes, Serpin-27A (Spn27A) and spermatogenic leucine zipper 1 (SPZ1) (17), in mosquito midguts. The Spn27A transcript level increased by 2.8-fold as a result of H2O2 treatment (Fig. 3A), indicating that H2O2 alone could activate the Toll pathway. We observed no up-regulation of SPZ1 in the midgut of Waco mosquitoes in response to 1% (vol/vol) H2O2 treatment, suggesting that SPZ1 is not as sensitive as Spn27A to increased ROS. Consistently, Spn27A but not SPZ1 was induced in the WB1 females. To understand better the mechanism of ROS-mediated activation of the Toll pathway, we measured the expression of SPZ1 and Spn27A in fat bodies of Waco mosquitoes after treating with H2O2 at three different doses: 1%, 2%, and 4% (vol/vol). Spn27A was induced by H2O2 at all three doses; the highest fold increase (7.2-fold) was seen with 2% (vol/vol) H2O2 treatment. SPZ1 was induced only with 2% and 4% (vol/vol) H2O2 treatments (Fig. 3B). In addition, we measured the expression of two antimicrobial peptide genes, CECD and DEFC, that are strongly induced by Wolbachia in the WB1 mosquitoes. Both were up-regulated by more than 2.5-fold in midguts of Waco mosquitoes as a result of H2O2 treatment (Fig. 3A). Thus, an increase in H2O2 in uninfected Waco mosquitoes is sufficient to activate the Toll pathway and induce CECD and DEFC, similar to observations in the WB1 mosquitoes as a result of Wolbachia infection.
Fig. 3.
Wolbachia-induced ROS-dependent activation of the Toll pathway. (A) Fold changes in expression of Toll pathway-related genes in midguts of Waco females after H2O2 treatment. Two-day-old Waco females were fed with a sugar solution containing 1% H2O2 (vol/vol) for 5 d. The total RNA of midguts was extracted to measure the fold change in gene expression compared with a control group fed only a sugar solution. (B) Fold changes in the expression of Toll pathway marker genes in fat bodies of Waco females after H2O2 treatment at three different doses—1%, 2%, and 4% (vol/vol)—compared with a control group fed a sugar solution only. (C) Fold changes in the expression of Toll pathway-related genes in WB1 females after silencing of NOXM and DUOX2. Three days after injection with dsRNA, midguts were collected to extract total RNA and measure the fold change in gene expression compared with that in the control group injected with GFP dsRNA. (D) Fold changes in the expression of CECD and DEFC after silencing of the Toll pathway in WB1. Three days after injection with MYD88 or REL1 dsRNA, midguts were collected to extract the total RNA and measure the fold change in gene expression compared with that in the control group injected with GFP dsRNA.
Wolbachia-Induced ROS Activate the Toll Pathway in WB1 Mosquitoes.
To determine whether Wolbachia-induced ROS are required to activate the Toll pathway in WB1 mosquitoes, we silenced two Wolbachia-induced oxidases, NOXM and DUOX2, using their dsRNA in WB1 females, and then measured the impact of their silencing on the Toll pathway. We consistently found that silencing both oxidases deactivated the Toll pathway in WB1 mosquitoes, as indicated by a dramatic decrease in the expression of Spn27A and SPZ1 (Fig. 3C). Inhibiting ROS production by silencing NOXM and DUOX2 in WB1 also suppressed the expression of CECD and DEFC (Fig. 3C). To confirm that Wolbachia infection induced the expression of CECD and DEFC in WB1 by activating the Toll pathway, we injected dsRNAs of two genes encoding components of the Toll pathway, MYD88 and REL1A, to turn off this pathway and then tested the impact of this treatment on CECD and DEFC expression. This silencing of the Toll pathway caused a significant down-regulation of CECD and DEFC (Fig. 3D). Taken together, our results indicate that Wolbachia-induced ROS are required to maintain the activated Toll pathway and induce its downstream expression of CECD and DEFC.
Activation of the Toll Pathway Is Essential for Antioxidant Expression.
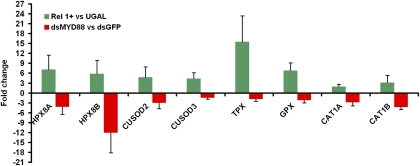
Considering that Wolbachia-induced ROS activated the Toll pathway, and antioxidants were up-regulated by Wolbachia infection in our microarray assays, we hypothesized that the Toll pathway controlled the expression of host antioxidants to combat damaging effects of ROS. Therefore, we used transgenic Ae. aegypti with fat-body–specific ectopic REL1 expression (REL1+) to determine whether activation of the Toll pathway is sufficient to induce the expression of antioxidants. Because REL1 in transgenic mosquitoes is driven by the female fat-body–specific vitellogenin (Vg) promoter, the Toll pathway is activated after mosquitoes consume a blood meal (17). We therefore collected WB1 mosquito fat bodies 24 h after a blood meal, a peak time for Vg gene expression, and, using real-time PCR, measured the expression of eight antioxidants and compared their expression with that in wild-type UGAL mosquitoes at the same time point (Fig. 4). The eight antioxidants were selected because they are induced by Wolbachia infection in WB1 mosquitoes, as shown by the microarray assay (Table S7). We found that the expression of all eight antioxidants was strongly induced when the Toll pathway was activated in the REL1+ transgenic mosquitoes. In particular, we saw a 15.5-fold increase in thiol peroxidase (TPX), a 7.5-fold increase in peroxinectin (HPX8A), a 6.8-fold increase in glutathione peroxidase (GPX), and a 4.7-fold increase in CuSOD2 (Fig. 4).
Fig. 4.
The Toll pathway controls the expression of antioxidant proteins. Fat bodies of REL1-overexpressing transgenic mosquitoes (Rel1+) were collected 24 h after a blood meal, and the expression of eight antioxidants was measured using real-time PCR and compared with that of wild-type UGAL mosquitoes. Midguts of WB1 females injected with MYD88 dsRNA were collected to measure the fold changes in gene expression compared with that of the control group injected with GFP dsRNA. For all experiments, the gene expression data were normalized with RPS6. The primer sequences are listed in Tables S8 and S9. Error bars indicate SE.
To confirm further that the Toll pathway is required to induce the expression of these antioxidants, we injected MYD88 dsRNA to knock down the Toll pathway in WB1 mosquitoes and then measured the change in expression of the same eight antioxidants in midguts, comparing the results with those for a control injection of GFP dsRNA. Silencing of the Toll pathway caused a more than twofold decrease in six of the eight antioxidants (Fig. 4). Strikingly, there were 12.0-fold, 4.2-fold, and 3.0-fold decreases in the expression of HPA8B, catalase (CAT1B), and CuSOD2, respectively. Thus, the Toll pathway was shown to regulate the expression of antioxidants in the mosquito.
Knockdown of DEFC and CECD Compromise Resistance to DENV in Ae. aegypti WB1 Mosquitoes.
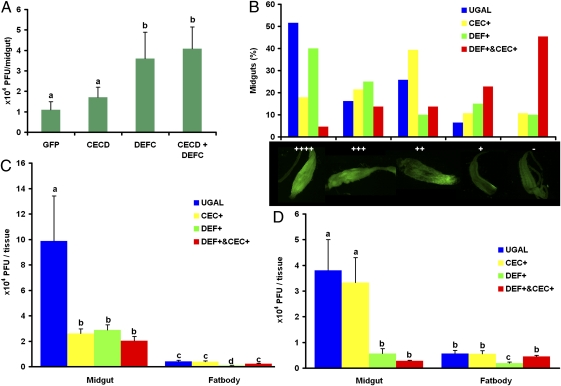
We previously reported that the Toll pathway controls dengue infection in Ae. aegypti (14), but which downstream effectors restrict DENV has remained unclear. Because DEFC and CECD are strongly induced through the Toll pathway in WB1 mosquitoes, we investigated whether they play a role as antidengue effectors. We applied RNAi depletion of DEFC and CECD individually or in combination in WB1 mosquitoes and then used plaque assays to measure the titers of DENV serotype 2 in mosquito midguts 7 d after viral infection. Viral titers were increased significantly by individual knockdown of DEFC and by double knockdown of DEFC and CECD compared with the effects of injecting GFP dsRNA (Mann–Whitney u test, P < 0.05; Fig. 5A). A trend toward an increase in viral titers as a result of single knockdown of CECD was observed also, but the effect was not statistically significant.
Fig. 5.
Roles of defensins and cecropins in the inhibition of DENV proliferation. Viral infection was detected by plaque assay in C6/36 cells (A, C, and D) or by immunofluorescence staining of the DENV-2 envelope protein (B). (A) DENV loads were measured in the midguts of mosquitoes after RNAi depletion of CECD, DEFC, a double depletion (CECD + DEFC), or in GFP dsRNA-treated control mosquitoes. a vs. b, P < 0.05 (Mann\x{2013}Whitney U test). (B) Midgut infection rates were measured at 7 d after viral infection in transgenic Ae. aegypti with fat body ectopic expression of either defensin A (DEF+) or cecropin A (CEC+) and in their hybrid strain (DEF+ and CEC+). Midgut infection levels were recorded as uninfected (−) or infected in one of four increasing levels (+, ++, +++, ++++). There were significant differences in the levels of midgut DENV infection in the hybrid strain and UGAL controls (χ2 test, P < 0.0001) and in levels in CECA transgenic mosquitoes and UGAL controls (χ2 test, P < 0.05). DENV loads were measured in the midguts and fat bodies of three transgenic mosquitoes and the wild-type UGAL strain 10 d after infection with DENV-2 during the first blood feeding (C) or 10 d after infection with DENV-2 during the second blood feeding (the first blood meal was blood without DENV to activate the transgenes) (D). For all figures, error bars represent SE. Statistical significance is represented by letters above each column, with different letters signifying distinct statistical groups. For C and D, P < 0.05 for a vs b, b vs c, and c vs d in Student's t test.
Fat-Body–Specific Ectopic Expression of Defensin A and Cecropin A Inhibit Proliferation of DENV in Transgenic Ae. Aegypti.
We used transgenic Ae. aegypti mosquito lines with Vg-driven fat body ectopic expression of either defensin A (DEFA+) or cecropin A (CECA+) (18, 19) to study a general role for defensins and cecropins in antidengue defense. In addition, we took advantage of the DEFA/CECA hybrid line produced by crossing these two transgenic mosquito lines, which expressed both DEFA and CECA (19). In the first experiment, 7-d-old Ae. aegypti female mosquitoes, the three transgenic lines, and the wild-type UGAL strain were fed dengue-infected blood, and the level of viral infection was measured in their midguts by indirect fluorescence assay (IFA) 7 d post viral infection. There were significant differences in the midgut infections between the DEFA/CECA hybrid strain and UGAL control mosquitoes (χ2 test, P < 0.0001; Fig. 5B) and between CECA+ transgenic and UGAL control mosquitoes (χ2 test, P < 0.05; Fig. 5A). Strong inhibition of DENV proliferation was observed in the CECA+ and DEFA/CECA transgenic mosquitoes compared with UGAL controls 7 d post viral infection; 45.5% of the DEFA/CECA hybrids and 10.7% of the CECA+ transgenic mosquitoes were negative for DENV envelope protein in the midgut, compared with 0% of the UGAL mosquitoes. Of the DEFA+ transgenic mosquitoes, 10% also were negative for DENV, but this effect was not statistically significant (Fig. 5B).
We conducted another comparison in a similar manner, instead measuring the viral titers in both the midgut and fat body by plaque assay 10 d after viral infection. The DENV titers in the midgut were significantly lower in all three transgenic mosquito lines than in the UGAL wild-type mosquitoes (Student's t test, P < 0.05; Fig. 5C). A significantly lower viral titer also was observed in the fat bodies of DEFA+ transgenic mosquitoes compared with the other three groups (Student's t test, P < 0.05; Fig. 5C).
CECD and DEFC were induced by Wolbachia infection before viral invasion in the WB1 mosquitoes, whereas the CECA and DEFA transgenes were induced in fat bodies after DENV entry with a blood meal into the midgut of the transgenic mosquitoes. Immune factors produced in the preinvasion and postinvasion phase have different effects on pathogens (20). To mimic the effect of CECD and DEFC on DENV in WB mosquitoes more closely, we fed 7-d-old transgenic and control female mosquitoes with uninfected blood to induce the expression of DEFA and CECA in their fat bodies. Five days later, female mosquitoes were fed with DENV-containing blood. Ten days after viral infection, mosquito midguts were dissected to measure viral titer. Again, we observed a significantly lower viral titer in the midguts of both the DEFA/CECA hybrid and DEFA+ transgenic mosquitoes than in the midguts of the UGAL controls (Student's t test, P < 0.05; Fig. 5D). Similarly, a significant inhibition of DENV was observed only in the fat bodies of the DEFA+ transgenic mosquitoes (Student's t test, P < 0.05; Fig. 5D). This result was consistent with the RNAi knockdown assay (Fig. 5A) showing that defensin had a stronger inhibitory effect against DENV than cecropin. Thus, we demonstrated that antimicrobial peptides (defensins and cecropins) induced by Wolbachia infection in Ae. aegypti are downstream effectors that inhibit DENV proliferation. Although both defensins and cecropins contributed to viral inhibition, utilization of the DEFA/CECA transgenic mosquitoes clearly indicated that up-regulation of both these antimicrobial peptides produces a synergistic inhibitory effect against DENV.
Discussion
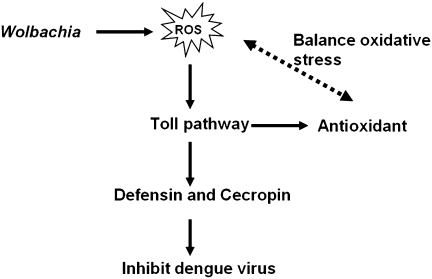
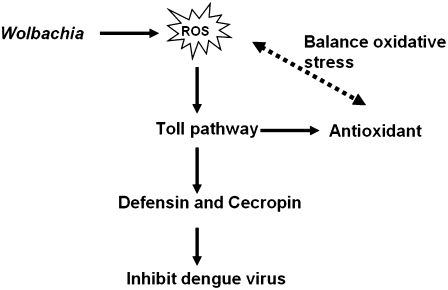
In this study, we deciphered how Wolbachia infection affects the mosquito host in inducing resistance to DENV. Our microarray assay indicates that transcripts of genes with functions related to immunity and R/S/M are up-regulated in Ae. aegypti infected with Wolbachia. Infection with this bacterium leads to induction of oxidative stress and an increased level of ROS in its mosquito host. ROS elevation is linked to the activation of the Toll pathway, which is essential in mediating the expression of antioxidants to counterbalance oxidative stress. This immune pathway also is responsible for activation of antimicrobial peptides, e.g., defensins and cecropins. We provide evidence that these antimicrobial peptides are involved in inhibition of DENV proliferation in Wolbachia-infected mosquitoes. Utilization of transgenic Ae. aegypti and the RNAi depletion approach have been instrumental in proving the role of defensins and cecropins in resistance to DENV in Wolbachia-infected Ae. aegypti (Fig. 6).
Fig. 6.
A current model of Wolbachia-mediated resistance to DENV in Ae. aegypti.
As facultative symbionts, Wolbachia can be transferred artificially to novel host species and form stable associations. Within their new host Ae. aegypti, Wolbachia elicit a strong immune response (10, 11, 13). Such responses have been observed in An. gambiae with transient somatic Wolbachia infections (6, 7). In addition to DENV, Wolbachia have been shown to induce resistance to several other pathogens—Plasmodium, Chikungunya, and filarial nematodes—in Ae. aegypti (10, 11, 13) and to Plasmodium in An. gambiae (6, 7). However, the vector competence of mosquito species with naturally occurring Wolbachia is not significantly affected by these bacteria. For example, the Wolbachia wAlbB strain lacks the ability to induce resistance to DENV in the naturally infected Ae. albopictus (10). Previous studies reported that Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides in Ae. albopictus (21). Thus, resistance to pathogens in Wolbachia-infected Ae. aegypti and An. gambiae mosquitoes is likely a consequence of the elevated host immunity to Wolbachia, which is aimed at counterbalancing the adverse effects of this newly acquired parasite.
Despite numerous immune responses mounted against pathogens, Wolbachia-uninfected Ae. aegypti and An. gambiae are highly competent vectors for DENV and Plasmodium, respectively (14, 22-24). The Wolbachia-boosted immunity differs from pathogen-induced immune responses in that immune factors are induced by Wolbachia before pathogen invasion, whereas the immune factors induced by pathogens occur after pathogen invasion. It was reported that knockdown of the inhibitor protein Cactus of the Toll pathway before infection renders mosquitoes totally refractory to the Plasmodium parasites, whereas Cactus depletion after infection has no effect on oocyst development (20). Because Wolbachia boost Toll pathway activity before DENV invasion, Wolbachia can play more crucial roles in killing invading virus than the late activation of Toll pathway by DENV (14).
Wolbachia infection of Ae. aegypti leads to a high elevation of transcripts of genes encoding NOXM and DUOX2 enzymes, which are involved in production of ROS (15, 16). We also observed an increased H2O2 level in WB1 relative to Waco mosquitoes. This increase is similar to that seen in closely related parasitic Rickettsia bacteria, in which H2O2 represents a major oxidant and causes cell injury. High concentrations of ROS damage cellular components, creating a state of oxidative stress in a host (25). Such a negative effect of ROS on an organism is controlled through an antioxidant defense system. We found that a number of antioxidant genes were up-regulated in the presence of Wolbachia in WB1 mosquitoes. Previous studies also have shown induction of ROS and expression of antioxidant proteins in an Ae. albopictus cell line infected by Wolbachia (26). It is likely that the existence of an antioxidant regulatory feedback plays an important role in counteracting a potential damaging effect of ROS on the infected host cells and permitting Wolbachia to maintain a persistent infection within their newly acquired host, Ae. aegypti.
In Ae. aegypti, Wolbachia infection activates the Toll pathway, and our data suggest that ROS mediate this activation. In both Wolbachia-infected WB1 and H2O2-treated Waco mosquitoes, the levels of transcripts Spn27A, DEFC, and CECD were elevated, whereas RNAi depletion of either NOXM or DUOX2 resulted in a significant decrease of these transcripts in WB1 mosquitoes. In vertebrates, multiple links exist between ROS and NF-ĸB signaling pathways (27, 28). ROS mediate activation of NF-ĸB, which is central to immunity, inflammation, and cell survival (27, 29). Exposure to H2O2 or the other oxidants triggers nuclear translocation of NF-ĸB in certain cell types (30, 31). The other side of the cross-talk between ROS and the NF-ĸB pathway is the activation of antioxidant gene expression, which mediates protection from ROS (27). A similar up-regulation of the antioxidant genes—MnSOD, CuZnSOD, ferritin heavy and light chains, GPX, and catalase—is observed in WB1 mosquitoes. We further show that the Toll pathway is essential for this antioxidant gene expression. Eight antioxidant genes are strongly up-regulated in transgenic Ae. aegypti with ectopic expression of REL1, the Toll pathway NF-ĸB factor. Furthermore, silencing the Toll pathway in WB1 mosquitoes by means of RNAi MYD88 depletion results in down-regulation of antioxidant genes. Taken together, our data suggest that Wolbachia-induced oxidative stress activates the Toll pathway, which then elevates the expression of mosquito antioxidants to counteract damaging effects of ROS.
Insects use powerful immune mechanisms—siRNA, JAK-STAT, Toll, IMD, and autophagy—to regulate viral infections (32–36). Insect RNA viruses, including DENV, are targets of the RNAi antiviral pathway (22, 32, 36). Involvement of other immune pathways in antiviral defense appears to be virus specific, but their precise modes of action in response to each specific viral infection are not clear (35). Interestingly, viral infections by DENV and Drosophila X virus induce genes of the Toll pathway (14, 34), whereas DENV and Drosophila C virus activate the JAK-STAT pathway in Ae. aegypti and Drosophila, respectively (37, 38). Activation of both these pathways likely is related to cellular signaling from virus-damaged cells and proliferation and function of hemocytes (34, 37). Similar mechanisms also have been suggested to be active in the IMD-mediated antiviral response against cricket paralysis virus (33). Although up-regulation of antimicrobial peptides has been documented in all these studies, their role in antiviral defense has remained unclear. In our current study, we have demonstrated that Aedes defensins and cecropins are strongly induced by the Wolbachia-mediated Toll pathway and confer resistance to DENV in the Ae. aegypti WB mosquitoes. RNAi depletion of either DEFC or CECD compromises Wolbachia-induced resistance to DENV in WB1 mosquitoes. Moreover, ectopic expression of DEFA and CECA inhibits proliferation of DENV in transgenic Ae. aegypti. The antiviral activity of defensins was reported first in vertebrates, and defensins were thought to target enveloped viruses by disrupting the viral envelope membrane in a manner similar to defensin antibacterial action (39). Recent results indicate that human defensins inhibit infections of both enveloped and nonenveloped viruses through multiple mechanisms: preventing viral membrane attachment and cellular entry, disrupting the viral envelope, and blocking viral nuclear import (40). The exact mechanism of action of the mosquito antimicrobial peptides (cecropins and defensins) on DENV proliferation in the mosquito host remains to be determined.
In transgenic Aedes mosquitoes, CECA and DEFA are produced in the fat body under the control of the Vg promoter and are secreted to the hemolymph (18, 19). Thus, their antiviral effect is extracellular. Interestingly, the fat-body–specific expression of DEFA and CECA affects DENV loads in the midgut, indicating their humoral effect on midgut virus proliferation. Our previous study showed that Wolbachia infection inhibits DENV replication in the Aedes midgut (10). However, the sites of Wolbachia-elicited antimicrobial peptides in the mosquito have yet to be determined. A synergistic antidengue activity of CECA and DEFA was observed when these antimicrobial peptides were coexpressed in the DEF/CEC hybrid transgenic mosquitoes. This synergistic effect is similar to the previously reported strong anti-Plasmodium activity through cooperative action of CECA and DEFA in the DEF/CEC hybrid transgenic mosquitoes (19). Given the activation of numerous antimicrobial peptides in Wolbachia-infected mosquitoes, it is likely that these peptides act synergistically in limiting DENV proliferation, as has been demonstrated using the DEF/CEC transgenic Ae. aegypti (19).
Although our results indicate that ROS function as signaling molecules to activate the Toll pathway-mediated antiviral defense, this function may represent one of multiple mechanisms underlying Wolbachia-mediated viral interference. ROS also may function as final effectors of antiviral immunity. A 28-fold increase in the DUOX2 transcript in WB1 mosquitoes provides a rationale for characterizing the role of ROS generated by this enzyme. Furthermore, we cannot exclude the possibility that the other physiological changes caused by Wolbachia also may contribute to viral interference. Wolbachia may perturb the host metabolic network that provides DENV the energy and macromolecule subunits necessary for replication (41). Future studies should include the identification of each key factor and the determination of their associations and relative contributions to the overall resistance effect.
In conclusion, we have shown that Wolbachia can induce ROS-dependent activation of the Toll pathway to control DENV. The ability of Wolbachia to boost immunity and block DENV proliferation in a newly acquired host, Ae. aegypti, makes it a potential “mosquito vaccine” that could be used efficiently to prevent pathogen transmission. More detailed understanding of molecular mechanisms of Wolbachia effects on DENV proliferation in Ae. aegypti is of paramount importance for refining the utilization of a Wolbachia/Aedes system approach for the future control of DENV transmission.
Materials and Methods
Mosquito Rearing and Cell-Culture Maintenance.
The Wolbachia-uninfected Ae. aegypti Waco strain and Wolbachia-infected Ae. aegypti WB1 strain were maintained in sugar solution at 27 °C and 85% humidity, with a 12-h/12-h light/dark cycle, according to standard rearing procedures. The same conditions were used to maintain transgenic Ae. aegypti lines and the wild-type Rockefeller/UGAL strain. We used the following transgenic Ae. aegypti lines: Vg-REL1-A (Rel1+), Vg-DefA (DEF+), Vg-CecA (CEC+), and Vg-CecA/Vg-DefA (DEF+ and CEC+) (17–19). Female mosquitoes, 3–5 d after eclosion, were fed on the blood of anesthetized white mice to initiate egg development. This study was carried out in strict accordance with the recommendations set out in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by Michigan State University and the University of California, Riverside Institutional Animal Care and Use Committees, and all efforts were made to minimize suffering.
The Ae. albopictus cell line C6/36 was grown in Eagle's minimal essential medium (MEM) with 10% (vol/vol) heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 100 μM nonessential amino acids at 32 °C and 5% (vol/vol) CO2.
DENV Infection in Mosquitoes.
The New Guinea C strain of DENV-2 was propagated in C6/36 cells, according to standard procedures (42). In short, 180-μL aliquots of virus stock were used to infect 25-cm2 flasks of C6/36 cells at 80% confluence with a multiplicity of infection of 1.0 virus particle per cell. Infected cells were incubated at 32 °C and 5% (vol/vol) CO2 for 7 d. Cells were harvested with a cell scraper and lysed to release the virus particles by repeated freezing and thawing using dry ice and a 37 °C water bath. The virus suspension was mixed 1:1 with commercial ox defibrinated blood (Colorado Serum Company). The blood meal was maintained at 37 °C for 30 min before 7-d-old mosquitoes were fed.
Microarray Analysis.
Seven-day-old WB1 (treatment) and Waco (reference) mosquitoes before blood feeding and 19-d-old WB1 (treatment) and Waco (reference) mosquitoes at 12 d postinfection were dissected to collect the midguts and remaining carcass tissues. Four biological replicates, with 80 tissue samples for each, were used for each treatment or reference group. Total RNA was extracted using RNeasy Mini Kit (QIAGEN), according to the manufacturer's instruction. A 4 × 44K microarray (Agilent) was designed based on the V1.2 version annotation of the Ae. aegypti full genome sequence. A microarray assay was conducted as reported previously (43). Briefly, 3 μg total RNA was used for probe synthesis of cy3- and cy5-labeled cRNA. Hybridizations were carried out using an Agilent Technologies In Situ Hybridization kit (Agilent Technologies) at 65 °C, according to the manufacturer's instructions. Hybridization intensities were determined using an Axon GenePix 4000B scanner, and images were analyzed using GenePix software. The expression data were processed and analyzed as described previously (43).
H2O2 Assay.
Fat bodies and whole bodies of 7-d-old Waco and WB1 mosquitoes were collected in 1× PBS containing 2 mg/mL of the catalase inhibitor 3-amino-1,2,4-trizole (44). After homogenization, samples were filtrated through a 10K molecular weight cutoff spin filter (Corning Spin-XUF; Corning Incorporated Life Sciences). The elution from each experimental group then was collected and tested using a Hydrogen Peroxide Assay Kit (BioVision). The fluorescence intensity was detected with Excitation/Emission = 550/590 using a fluorescence multidetector microplate reader (BMG Labtech), according to the manufacturer's instructions. The values were normalized by the total amount of proteins in the sample, as determined by the Piece bicinchoninic acid protein assay (Thermo Scientific). Six biological replicates, with five fat bodies or three whole bodies for each, were used for each treatment.
Effect of Exogenous H2O2 on Mosquito Toll Pathway.
Two-day-old Waco mosquitoes were fed with either the 10% (wt/wt) sugar meal containing 1%, 2% or 4% (vol/vol) H2O2 or the 10% (wt/wt) sugar meal only (control). Five days later, mosquito midguts and fat bodies were dissected to extract the total RNA. The expression of the Toll pathway-related genes— Spn27A, SPZ1, CECD, and DEFC—were measured using real-time PCR.
RNAi-Mediated Gene Silencing.
The dsRNA was synthesized in vitro using the Megascript T7 high-yield transcription kit (Ambion). The primers designed to amplify Ae. Aegypti MYD88, REL1, DEFC, CECD, NOXM, NOX5, DUOX1, and DUOX2 are listed in Tables S8 and S9. An RNAi-based assay was conducted according to standard methodology (45). Approximately 69 nl dsRNA (4 μg/μL) in water was injected into the thorax of CO2-anesthetized 2-d-old female mosquitoes using a nano-injector, as previously described (46). To measure gene expression, the midguts of mosquitoes were dissected 3 d after injection and used for RNA extraction.
RNA Extraction, cDNA Synthesis, and Real-Time PCR Assays.
Total RNA was extracted from mosquito tissues using the RNeasy Mini Kit (QIAGEN Sciences), and the cDNA transcript was produced using QuantiTect Reverse Transcription Kit (QIAGEN Sciences). Real-time PCR was performed using the QuantiTect SYBR Green PCR Kit (QIAGEN Sciences) and the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The ribosomal protein S6 (RPS6) gene was used for normalization of cDNA templates. All primers used for real-time PCR are listed in Table S9.
IFA.
The viral antigen in mosquito midguts was detected using an IFA7 d postinfection. IFAs were performed as previously described (47). The midgut samples were dissected and fixed in 4% (vol/vol) paraformaldehyde overnight and washed with PBS. Then the samples were incubated in PBS, 0.1% (vol/vol) Triton X-100, and 1% (wt/wt) BSA at room temperature for 1 h. To detect the antigen, a mouse anti-Dengue complex monoclonal antibody (obtained from the Centers for Disease Control, Atlanta, GA) and a fluorescein-conjugated affinity-purified secondary antibody (Invitrogen) were used in all IFA assays. Specimens were examined under an Olympus IX71 fluorescence microscope (Olympus).
Mosquito Tissue Collections.
To measure the virus titer in mosquito tissues, transgenic mosquitoes [Vg-DefA (DEF+), Vg-CecA (CEC+), and Vg-CecA/Vg-DefA (CxD)] at 10 d postinfection were washed briefly in 70% (vol/vol) ethanol and then were rinsed in sterile distilled water. The midguts and fat bodies were dissected in sterile PBS, transferred to microcentrifuge tubes containing 150 μL MEM and then were homogenized using a Kontes pellet pestle motor in a sterile environment. In the knockdown experiment, 2-d-old WB1 mosquitoes were injected with the dsRNA of GFP, CECD, DEFC, or a mixture of CECD and DEFC. Five days later, mosquitoes were fed with the dengue-infected blood. Seven days after infection, the midguts were dissected and collected as described in the above.
Plaque Assays.
The DENV titers in the mosquitoes’ midgut and fat-body homogenates were measured using a plaque assay, as previously reported (48). The virus-containing homogenates were serially diluted and inoculated into C6/36 cells in 24-well plates. After incubation at 32 °C and 5% (vol/vol) CO2 for 5 d, the plates were assayed for plaque formation by peroxidase immunostaining, using mouse hyperimmune ascitic fluid (specific for DENV-2) and a goat anti-mouse HRP conjugate as the primary and secondary antibody, respectively.
Accession Numbers.
Genes mentioned in this study are listed in the Entrez Gene database: Cactus (ID no. 5565922), REL1A (ID no. 5569526), SPZ3B (ID no. 5565708), MYD88 (ID no. 5569574), PGRPLB (ID no. 5572949), PGRPLE (ID no. 5577250), IMD (ID no. 5572865), DEFC (ID no. 5579094), CECD (ID no. 5564826), attacin (ID no. 5578028), NOX5 (ID no. 5573122), NOXM (ID no. 5572965), DUOX1 (ID no. 5569378), DUOX2 (ID no. 5569376), Spn27A (ID no. 5566833), SPZ1 (ID no. 5577953), HPX8A (ID no. 5564683), HPX8B (ID no. 5564679 ), CUSOD2 (ID no. 5567695), CUSOD3 (ID no. 5574885), TPX (ID no. 5564123), GPX (ID no. 5570587), catalase (CAT1A and CAT1B, ID no. 5577893), and GNBPB1 (ID no. 5579192).
Supplementary Material
Acknowledgments
We thank the Arbovirus Diseases Branch at the Centers for Disease Control for providing the anti-DENV antibodies (mouse hyperimmune ascitic fluid) and Dr. Deborah McClellan for editorial assistance. This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Disease Grants R01AI080597 and R37AI24716. J.W. is supported in part by a fellowship from the China Scholarship Council.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 13.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116932108/-/DCSupplemental.
References
- 1.Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: Vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 4.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 5.Iturbe-Ormaetxe I, Walker T, O'Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011;12:508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambris Z, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 9.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 10.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti Toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian G, Shin SW, Cheon HM, Kokoza V, Raikhel AS. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2005;102:13568–13573. doi: 10.1073/pnas.0502815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokoza V, et al. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokoza V, et al. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2010;107:8111–8116. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Bourtzis K, Pettigrew MM, O'Neill SL. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol. 2000;9:635–639. doi: 10.1046/j.1365-2583.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 22.Blair CD. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011;6:265–277. doi: 10.2217/fmb.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez JL, Dimopoulos G. The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev Comp Immunol. 2010;34:625–629. doi: 10.1016/j.dci.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridell YW, Sánchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Brennan LJ, Keddie BA, Braig HR, Harris HL. The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS ONE. 2008;3:e2083. doi: 10.1371/journal.pone.0002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: Molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 29.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 32.Ding SW. RNA-based antiviral immunity. Nat Rev Immunol. 2010;10:632–644. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- 33.Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci USA. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabin LR, Hanna SL, Cherry S. Innate antiviral immunity in Drosophila. Curr Opin Immunol. 2010;22:4–9. doi: 10.1016/j.coi.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kemp C, Imler JL. Antiviral immunity in Drosophila. Curr Opin Immunol. 2009;21:3–9. doi: 10.1016/j.coi.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dostert C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 38.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding J, Chou YY, Chang TL. Defensins in viral infections. J Innate Immun. 2009;1:413–420. doi: 10.1159/000226256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo X, et al. Response of the mosquito protein interaction network to dengue infection. BMC Genomics. 2010;11:380. doi: 10.1186/1471-2164-11-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troyer JM, et al. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg. 2001;65:414–419. doi: 10.4269/ajtmh.2001.65.414. [DOI] [PubMed] [Google Scholar]
- 43.Nene V, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina-Cruz A, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 45.Dong Y, et al. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garver L, Dimopoulos G. Protocol for RNAi assays in adult mosquitoes (A. gambiae) J Vis Exp. 2007;(5):230. doi: 10.3791/230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das S, Garver L, Ramirez JR, Xi Z, Dimopoulos G. Protocol for dengue infections in mosquitoes (A. aegypti) and infection phenotype determination. J Vis Exp. 2007;(5):220. doi: 10.3791/220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waterhouse RM, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]