Abstract
Honey bee (Apis mellifera) colonies are declining, and a number of stressors have been identified that affect, alone or in combination, the health of honey bees. The ectoparasitic mite Varroa destructor, honey bee viruses that are often closely associated with the mite, and pesticides used to control the mite population form a complex system of stressors that may affect honey bee health in different ways. During an acaricide treatment using Apistan (plastic strips coated with tau-fluvalinate), we analyzed the infection dynamics of deformed wing virus (DWV), sacbrood virus (SBV), and black queen cell virus (BQCV) in adult bees, mite-infested pupae, their associated Varroa mites, and uninfested pupae, comparing these to similar samples from untreated control colonies. Titers of DWV increased initially with the onset of the acaricide application and then slightly decreased progressively coinciding with the removal of the Varroa mite infestation. This initial increase in DWV titers suggests a physiological effect of tau-fluvalinate on the host's susceptibility to viral infection. DWV titers in adult bees and uninfested pupae remained higher in treated colonies than in untreated colonies. The titers of SBV and BQCV did not show any direct relationship with mite infestation and showed a variety of possible effects of the acaricide treatment. The results indicate that other factors besides Varroa mite infestation may be important to the development and maintenance of damaging DWV titers in colonies. Possible biochemical explanations for the observed synergistic effects between tau-fluvalinate and virus infections are discussed.
INTRODUCTION
Recent declines in honey bee abundance in the United States and Europe have led to an increased gap between supply and demand in pollination services, causing pressure both economically in agricultural crop production (18) and ecologically on natural plant biodiversity (4). Today, no clear explanation for these declines exists. However, a variety of stressors affecting honey bee health, such as viruses (13), microsporidia (23), and pesticides (51), have been identified as potential causes of such colony losses.
The ectoparasitic mite Varroa destructor is a well-known pest and has become the most significant economic threat to apiculture on a global scale (30). By feeding on hemolymph, the mite is a significant stressor on honey bee health, causing a variety of physical and physiological effects for both individual bees and the colony (30, 41). In addition, the indirect effects of Varroa, primarily caused by the viral infections vectored by the mite, are the most devastating and can lead to severe disease and mortality at the individual or colony level (2, 5, 10, 15, 33, 40, 44). These viruses usually persist as covert infections in the colony without the vectoring capacity of Varroa (37). Besides Varroa-mediated transmission, viruses can also be transmitted horizontally within the colony through trophallaxis, contact, feces, and salivary gland secretions and vertically from infected queens to their progeny (10, 12, 14, 54). There are over 18 identified and characterized honey bee viruses, many of which are suggested to be associated with mite infestation to various degrees (2, 9, 12, 33, 34, 40, 44, 49). Most notably, deformed wing virus (DWV) has become the most prevalent virus associated with Varroa mite infestation (15, 20), causing wing deformities in developing pupae that result in flightless adults that die shortly after emerging (2, 35).
Exponential mite population growth can lead to increased virus transmission, causing overt viral infections ultimately resulting in colony mortality within 2 to 3 years (5, 33). In order to prevent colony mortality, management strategies are implemented by beekeepers to reduce the mite population within the colony and thereby limit the transmission opportunities for potentially lethal virus infections. A common Varroa control treatment is Apistan (plastic strips coated with tau-fluvalinate), a wax-soluble, nonvolatile, potent pyrethroid acaricide with 98% to 100% efficacy against Varroa mite infestation (41, 52) except where fluvalinate resistance is present (25, 52). Although tau-fluvalinate had previously been considered to have low toxicity for bees (38), recent studies have shown it to be a stressor on colony health, particularly affecting the development and performance of queens (22). In addition, tau-fluvalinate has been found to act synergistically with sublethal doses of other Varroa-targeted pesticides administered to honey bee colonies (28). Recently, it was demonstrated that the neonicotinoid pesticide imidacloprid interacted synergistically with Nosema sp., a honey bee pathogen that is otherwise relatively benign in infectivity (1). If pesticides are an additional stress to honey bee colonies and can influence host susceptibility to the infectivity of certain pathogens, what effect might be seen on the susceptibility of honey bees to virus infections when using a pesticide against their virus vector, the Varroa mite?
The relationship between Varroa and honey bee viruses has been well documented in laboratory studies and field surveys (3, 6, 9, 34, 35, 37, 49, 50), and the outcomes of virus infections and Varroa mite population growth rates and transmission dynamics have been modeled (33, 47). It has also been demonstrated that remedial Varroa control treatment can reverse virus titer accumulation in colonies (3, 34). However, these studies looked at long-term virus titers after the completion of the acaricide treatment. The present study investigates the dynamics of virus infections during the acaricide treatment, aiming to identify any effects of tau-fluvalinate (Apistan) treatment on virus titers in adult bees, Varroa-infested and uninfested pupae, and Varroa mites.
MATERIALS AND METHODS
Experimental design.
In July 2008, nine single-brood-chamber honey bee colonies were established from splits containing equal proportions of adult bees, brood, honey, pollen, and mite infestations in Uppsala, Sweden. Colonies with high mite infestations were chosen under the assumption that higher mite levels would cause higher viral titers and, therefore, that a decrease in titers due to changing mite infestation would be more easily recognized. On 8 August 2008, six of the colonies were treated with Apistan (plastic strips coated with tau-fluvalinate), while the remaining three colonies served as an untreated control group.
Data and sample collection.
An initial set of measurements and samples were taken from the colonies prior to the acaricide treatment, while subsequent sampling continued approximately once a week for the duration of the treatment, giving a total of six data sets per colony. Population estimations of the adult bees and brood were made using the Liebefeld estimation method (27). The phoretic Varroa mite infestation rates were determined by washing samples of approximately 200 bees with soapy water to dislodge the mites (16). The brood Varroa mite infestation rates were determined by opening 100 randomly selected pupal cells (17). Samples of adult bees, mite-infested pupae, the mites infesting these pupae, and uninfested pupae were collected and stored at −20°C until subsequent virus analysis (11).
RNA extraction.
Bulk samples of 30 adult bees were placed with 5 ml nuclease-free water in a plastic mesh bag, flash frozen with liquid nitrogen, and homogenized with a pestle. Bulk samples of 5 infested pupae were separated from their mites and homogenized in 2.5 ml RLT buffer (Qiagen) with a micropestle. The corresponding 5 mites were pooled and homogenized mechanically in 0.5 ml RLT buffer with glass beads using the FastPrep system (Thermo Electron Corporation Sweden AB). Bulk samples of 5 uninfested pupae were homogenized similarly to the infested pupae. Total RNA was extracted by a Qiacube automated extraction robot (Qiagen) from 100 μl of each crude extract using the RNEasy protocol for plant tissues (Qiagen) and stored as two 50-μl aliquots at −80°C.
RT-qPCR assays.
To determine which viruses were present in the colonies at the start of the experiment, adult bees, infested pupae, and uninfested pupae collected from the first sampling date were screened by reverse transcription-quantitative PCR (RT-qPCR) for 10 honey bee viruses: acute bee paralysis virus (ABPV), black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), DWV, Israeli acute paralysis virus (IAPV), Kashmir bee virus (KBV), sacbrood virus (SBV), slow bee paralysis virus (SBPV), Varroa destructor virus 1 (VDV-1), and Varroa destructor macula-like virus (VdMLV) (see Table S1 in the supplemental material). Only those viruses detected in the initial screen, DWV, BQCV, and SBV, were analyzed in the remaining samples. DWV, as mentioned above, is widespread and has a well-characterized association with Varroa mites, while BQCV and SBV are both virulent viruses at the individual level affecting honey bee brood, but less virulent at the colony level and with unclear associations with Varroa mites. Each sample was also assayed for Apis mellifera or Varroa β-actin mRNA, using intron-spanning primers (see Table S1 in the supplemental material). β-Actin is a common internal reference RNA (32), used here to normalize the RT-qPCR data for differences between samples in the quantity and quality of the RNA. All assays had excellent linearity (r2) (see Table S1 in the supplemental material) between log10[template] and cycle number, covering the full range of experimental titers, as well as highly acceptable PCR efficiencies (E) (see Table S1 in the supplemental material).
The amounts of DWV, BQCV, SBV, and β-actin in each sample were determined using the Bio-Rad iScript One-Step RT-qPCR Kit with SYBR Green as the detection chemistry, 96-well optical qPCR plates, and the Bio-Rad Chromo4 thermocycler. The assays were performed in 20-μl volumes containing 3.0 μl RNA, 0.2 μl of each primer, and 0.4 μl iScript. All primers were designed to have the same optimal annealing temperature so that all four assays for each sample can be run concurrently in the same plate, together with five positive controls and one negative H2O control for each assay. The positive controls were obtained from 10-fold serial dilutions of a purified PCR product of known concentration and covered 6 orders of magnitude. The following reaction conditions were used: 10 min at 50°C for cDNA synthesis plus 5 min at 95°C for reverse transcription inactivation and Taq polymerase activation, followed by 35 cycles of 10 s at 95°C for denaturation and 30 s at 58°C for primer annealing, extension, and data collection. The amplification reaction was followed by a melting curve analysis to determine the specificity of the amplification products by incubating them for 60 s at 95°C and 60 s at 55°C and then reading the fluorescence at 0.5°C increments from 55°C to 95°C. Nonspecific amplification products melt and lose fluorescence at a distinctly lower melting temperature (Tm) than the specific “true” reaction products (8).
RT-qPCR data conversion.
The RT-qPCR data were first screened for the presence of specific target PCR product, as determined by the melting curve analyses. The quantification cycle (Cq) values (8) of all confirmed target amplifications were calculated with Bio-Rad Opticon Monitor software version 3.1 following a reaction baseline subtraction using the Global Minimum Trend option and with the fluorescence threshold set uniformly at 0.05 for all plates. All Cq values coincided with the logarithmic phase of the amplifications. For each target RNA, the Cq values of the external dilution standards of all RT-qPCR runs were pooled into a single linear regression analysis of Cq value onto log10[template]. These regression equations were then used to estimate the absolute amounts of virus and β-actin RNA in each reaction. These were subsequently converted to estimated amounts per bee through the different reaction and extraction dilution factors. The regression slopes were also used to calculate the amplification efficiencies (E) of the different assays as follows: Eassay = 10−1/slope (39).
Data transformations.
The virus titers were exponentially distributed, spanning between 4 and 7 orders of magnitude, depending on the virus. This broad titer distribution is a logical consequence of the largely exponential nature of virus replication and proliferation (7). A log transformation was therefore used to render the data suitable for parametric analyses (45). Since it is not possible to log transform zero values (i.e., instances of no virus amplification), a constant value is usually added to the data to allow the inclusion of zero values in the analyses (45). For these experiments, this constant value was determined by assuming a hypothetical Cq value of 41 for the virus assays and converting these to theoretical titers as indicated above.
Data normalization.
The data set was partitioned into 12 subsets (3 viruses × 4 sample types) for data normalization and statistical analysis. This was a necessary consequence of the separate β-actin titer adjustments for the different sample types and the distinct data and error distributions of the three viruses (Fig. 1). For each subset, the virus titer and mite infestation rate data for each colony were normalized to the average pretreatment value for all colonies to avoid statistically significant effects purely due to natural, preexisting differences between the colonies.
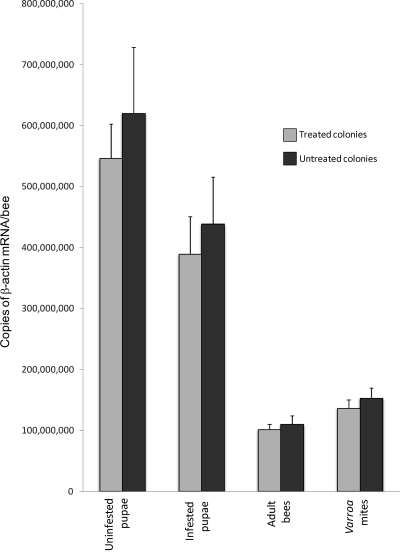
Fig 1.
β-Actin titers in uninfested pupae, infested pupae, adult bees, and Varroa mites from treated colonies and untreated colonies. The values are averaged over all sampling dates and colonies within each treatment category. The error bars denote standard errors.
Statistical analysis.
The effect of acaricide treatment on the normalized virus titers in each of the four sample types (adults, infested pupae, uninfested pupae, and Varroa mites) was analyzed by mixed-effects models as implemented by SAS 9.1 for Windows and included the normalized adult and pupal mite infestation rates as independent explanatory variables. The models included a linear repeated-measures factor to account for the bias inherent in sampling the same colonies repeatedly over time, with the covariance structure selected based on the Aikaike's information criteria (AIC) value (31). Assumptions of normality were verified by analysis of residuals and equality of variance (31).
Correlation analysis was used to assess the relationships across the 12 data subsets, i.e., between the three viruses in each sample type, between the different sample types for each virus, or between the virus titers and the mite infestation rates in pupae or adults, using the original, prenormalized data. The correlations involving the mite infestation rates included only data from the pretreated and untreated colonies to avoid biasing the natural global correlation between virus titers and the mite infestation rates by the rapid artificial removal of the mite population from the treated colonies.
RESULTS
Mite and bee population development.
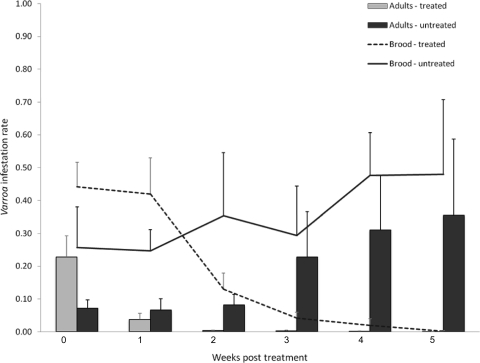
Although the colonies were randomly allocated to the treated and untreated groups, the initial mite infestation rates were higher in the treated group than in the untreated group for both the brood and adult infestation rates (Fig. 2). The acaricide treatment significantly reduced the mite infestation rates (df in parentheses) in the brood [F(10,35) = 15.04; P < 0.0001] and on adult bees [F(10,35) = 8.27; P < 0.0001] but did not eliminate mite infestation completely (Fig. 2). A very low adult mite infestation rate persisted in all the treated colonies after the second week of treatment, even though mites were no longer detectable in the brood in all but one of the acaricide-treated colonies after the third week of treatment (Fig. 2). The numbers of adult bees and brood in the untreated colonies were significantly reduced during the study to nearly half their initial numbers compared to the treated colonies, whose populations remained relatively constant [adult bees, F(10,33) = 7.09, P < 0.0001; brood, F(10,33) = 2.22, P = 0.0413]. Clinical symptoms of DWV were observed with increasing frequency in the untreated colonies, while in the treated colonies, clinical DWV symptoms decreased. The presence of clinically deformed bees, which have very short life expectancy, was likely the cause of the reduced adult bee population in the untreated group, also considering that the mite infestation rates of adult bees significantly affected the numbers of adult bees [F(1, 33) = 5.38; P = 0.0267]. All untreated colonies died before or during the following winter, while all treated colonies survived.
Fig 2.
Colony level mite infestation rates for adult bees represented in a histogram for the treated and untreated colonies, along with a line graph indicating the brood mite infestation rates for treated and untreated colonies through the duration of the experiment. Samples were taken weekly, with the first sample taken just prior to administering the acaricide treatment to the treated group, identified as week 0. Treated colonies at weeks 4 and 5 are represented by only 1 colony where mites were still present. The error bars denote standard errors.
Assays.
There were clear differences between the four sample types (infested pupae, uninfested pupae, adults, and mites) in the total estimated levels of β-actin mRNA per bee or mite, covering a 6-fold range, although there were no differences between treated and untreated colonies in this regard (Fig. 1) (t tests, P > 0.05). The amount of virus in each sample was therefore adjusted to the average β-actin levels for that particular sample type, effectively using β-actin as a molecular marker for the quantity and quality of the RNA sample. These values were then multiplied by the different experimental dilution factors to arrive at the estimated number of virus genome equivalents per sample. A negative result worth noting is the apparent absence of all viruses other than DWV, BQCV, and SBV, including the absence of VDV-1, a close relative of DWV that is specifically associated with V. destructor (15).
Virus titer analyses.
The statistical effects of the acaricide treatment on the normalized virus titers over the 6-week sampling period, and how much of this is due to the variable effect of treatment with time or can be explained by the mite infestation rates, are summarized in Table 1. The most significant effects of acaricide treatment are seen in titers in adult bees and mites for DWV, in titers in infested pupae for BQCV, and in titers in both infested and uninfested pupae for SBV. The mite infestation rates (both adult and pupal) affected only the DWV titers of adult bees (Table 1).
Table 1.
Results of mixed-effects model analysesa
| Parameter | Uninfested pupae |
Infested pupae |
Adult bees |
Varroa mites |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | F | df | P | F | df | P | |
| DWV | ||||||||||||
| Treatment | 0.03 | 1,7 | 0.8711 | 0.76 | 1,7 | 0.4127 | 20.43 | 1,7 | 0.0027c | 9.32 | 1,7 | 0.0185b |
| Date | 3.52 | 4,26 | 0.0199b | 2.50 | 4,14 | 0.0902 | 9.75 | 4,26 | <0.0001d | 1.50 | 4,14 | 0.2561 |
| Treat × Date | 1.81 | 4,26 | 0.1567 | 0.80 | 4,14 | 0.5464 | 6.03 | 4,26 | 0.0014c | 3.25 | 4,14 | 0.0440b |
| Mites ad | 1.07 | 1,26 | 0.3096 | 1.03 | 1,14 | 0.3267 | 6.60 | 1,26 | 0.0163b | 1.58 | 1,14 | 0.2292 |
| Mites pu | 1.07 | 1,26 | 0.3108 | 0.07 | 1,14 | 0.7982 | 6.73 | 1,26 | 0.0154b | 0.05 | 1,14 | 0.8227 |
| BQCV | ||||||||||||
| Treatment | 0.03 | 1,7 | 0.2119 | 0.10 | 1,7 | 0.7583 | 2.36 | 1,7 | 0.1684 | 0.00 | 1,7 | 0.9974 |
| Date | 3.52 | 4,26 | 0.3354 | 6.35 | 4,14 | 0.0039c | 2.38 | 4,26 | 0.0775 | 1.38 | 4,14 | 0.9800 |
| Treat × Date | 1.81 | 4,26 | 0.2033 | 9.61 | 4,14 | 0.0006d | 1.40 | 4,26 | 0.2611 | 0.10 | 4,14 | 0.2914 |
| Mites ad | 1.07 | 1,26 | 0.4890 | 1.21 | 1,14 | 0.2898 | 0.47 | 1,26 | 0.4994 | 0.00 | 1,14 | 0.9709 |
| Mites pu | 1.07 | 1,26 | 0.1296 | 0.05 | 1,14 | 0.8338 | 0.09 | 1,26 | 0.7725 | 0.03 | 1,14 | 0.8715 |
| SBV | ||||||||||||
| Treatment | 0.03 | 1,7 | 0.3597 | 0.16 | 1,7 | 0.6973 | 1.05 | 1,7 | 0.3394 | |||
| Date | 3.52 | 4,26 | 0.0002d | 4.30 | 4,14 | 0.0178b | 4.20 | 4,26 | 0.0094c | |||
| Treat × Date | 1.81 | 4,26 | 0.0001d | 6.69 | 4,14 | 0.0031c | 2.52 | 4,26 | 0.0655 | |||
| Mites ad | 1.07 | 1,26 | 0.2551 | 0.23 | 1,14 | 0.6402 | 0.62 | 1,26 | 0.4386 | |||
| Mites pu | 1.07 | 1,26 | 0.2059 | 0.22 | 1,12 | 0.6490 | 2.79 | 1,26 | 0.1067 | |||
Effects of the acaricide treatment (Treatment), sampling date (Date), and their interactions (Treat × Date), as well as the effects of two independent variables, the adult and pupal mite infestation rates (Mites ad and Mites pu, respectively) on the DWV, BQCV, and SBV titers in uninfested pupae, mite-infested pupae, adult bees, and the Varroa mites found in the infested pupae. F values with associated P values of <0.05, <0.01, or <0.001 are in boldface.
P < 0.05.
P < 0.01.
P < 0.001.
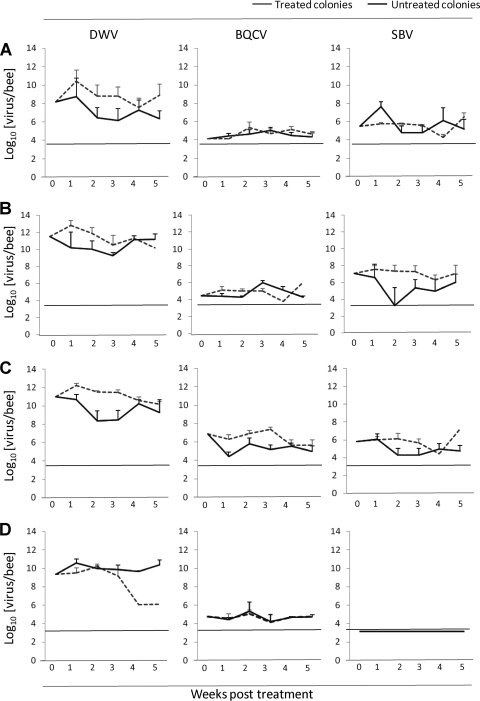
Relative to pretreatment values, an initial increase in the average DWV titers in the treated colonies was observed in mite-infested and uninfested pupae and adult bees, which was not observed in the untreated colonies. The untreated colonies experienced a decrease in DWV titers relative to the initial titers (Fig. 3, DWV) in mite-infested pupae and adult bees that did not rise above the titers in the treated colonies until the 4th week of treatment. The DWV titers in uninfested pupae in the untreated colonies were never higher than in the treated colonies. The most pronounced treatment effect is the reduction of DWV titers in mite samples from the treated group by the 4th and 5th weeks of treatment (Fig. 3). Although this is based on a single colony, since mites were undetectable in all other treated colonies by week 4, this reduction with treatment is a true observation for that colony and not an artifact of using group means. The significance in the mixed-effects model analysis for this drastic reduction in the mite DWV titer is reflected in both the treatment and treatment-date effects.
Fig 3.
Pretreatment-normalized DWV, BQCV, and SBV titers of uninfested pupae (A), infested pupae (B), adult bees (C), or Varroa mites (D) in treated colonies and untreated colonies for the duration of the acaricide treatment. Week 0 represents the pretreatment sample, and the titers in infested pupae and Varroa mites in treated colonies at weeks 4 and 5 are represented by only 1 colony where mites were still present. The titers are given on a log10 scale, and the dotted lines indicate the limit of detection of the RT-qPCR assays. The error bars denote standard errors.
BQCV and SBV titers were consistently higher in adult bees of the acaricide-treated colonies than in those of the untreated control colonies (Fig. 3). However, relative to the pretreatment titers, the fluctuations in both BQCV and SBV titers in the different samples with increasing treatment duration appear random rather than progressive (Fig. 3), although they were large enough to render most of the corresponding treatment-date interactions significant (Table 1).
In all samples, the DWV titers were several orders of magnitude higher than the BQCV and SBV titers. While DWV titers in infested pupae were 2 to 3 orders of magnitude higher than in uninfested pupae, there was no difference between infested and uninfested pupae for the BQCV or SBV titers (Fig. 3). DWV titers for adult bees were slightly lower than for the mite-infested pupae but considerably higher than for the uninfested pupae (Fig. 3, DWV). The BQCV and SBV titers in adult bees were both slightly higher than for either infested or uninfested pupae (Fig. 3, BQCV and SBV).
Correlation analyses.
The relationships between the 12 data subsets were assessed through correlation analyses of the original prenormalized data. The titers of the three viruses within each sample type were generally not correlated, i.e., the viruses appear to act independently of each other (Table 2). The only exception is in the infested pupae, where SBV varies positively with both DWV and BQCV. The DWV titers in infested pupae and their mites were highly correlated, but not the BQCV or the SBV titers, suggesting that during pupal infestation, Varroa has a more active relationship with DWV than with BQCV or SBV (Table 2). This observation is indirectly supported by the significant correlation between infested and uninfested pupae for the BQCV and SBV titers, which appear to be unaffected by mite infestation, but not for the DWV titers, where mite infestation has a large impact (Fig. 2 and Table 2).
Table 2.
Correlation analyses across all colonies and sampling dates relating log10[titer] of DWV, BQCV, and SBV in uninfested pupae, mite-infested pupae, adult bees, and Varroa mites found in infested pupae to each other and to colony level adult and pupal mite infestation rates
| Parameter |
R valuea |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uninfested pupae |
Infested pupae |
Adult mites |
Varroa mites |
Mite infestation, adults | |||||||||
| DWV | BQCV | SBV | DWV | BQCV | SBV | DWV | BQCV | SBV | DWV | BQCV | SBV | ||
| Uninfested Pupae | |||||||||||||
| DWV | |||||||||||||
| BQCV | −0.10 | ||||||||||||
| SBV | 0.31 | −0.05 | |||||||||||
| Infested pupae | |||||||||||||
| DWV | 0.29 | −0.02 | 0.34 | ||||||||||
| BQCV | −0.05 | 0.49c | 0.45b | 0.12 | |||||||||
| SBV | 0.31 | 0.11 | 0.79c | 0.64c | 0.42b | ||||||||
| Adult bees | |||||||||||||
| DWV | 0.35b | −0.03 | 0.28 | 0.64c | 0.05 | 0.50c | |||||||
| BQCV | −0.02 | 0.19 | 0.07 | 0.22 | 0.20 | 0.30 | 0.33 | ||||||
| SBV | 0.29 | −0.08 | 0.21 | 0.31 | 0.22 | 0.44b | 0.29 | 0.13 | |||||
| Varroa mites | |||||||||||||
| DWV | 0.17 | −0.04 | 0.20 | 0.68c | 0.04 | 0.38 | 0.48b | 0.21 | 0.09 | ||||
| BQCV | −0.13 | −0.23 | −0.13 | −0.17 | −0.22 | −0.16 | −0.20 | −0.09 | −0.05 | −0.30 | |||
| SBV | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Mite infestation | |||||||||||||
| Adults | −0.16 | 0.29 | 0.01 | 0.51 | 0.28 | 0.19 | 0.41 | 0.22 | 0.13 | 0.52 | −0.28 | 0.00 | |
| Pupae | −0.08 | 0.33 | 0.09 | 0.66b | 0.00 | 0.33 | 0.59b | 0.21 | 0.09 | 0.71c | −0.27 | 0.00 | 0.85c |
The correlations involving exclusively adults and/or uninfested pupae have 52 degrees of freedom, while those involving infested pupae and/or Varroa mites have 40 degrees of freedom. The correlations involving the mite infestation rates have 16 degrees of freedom. Correlations with associated P values of <0.01 or <0.001 are in boldface.
P < 0.01.
P < 0.001.
Of special interest are the relationships between the virus titers in adult bees and those in the pupae and mites, since the pupae will become part of the adult population, while the adults can influence the pupal titers indirectly through oral transmission to the larvae. For DWV, all these correlations were positive and highly significant, but this is true for only one of the SBV correlations (between adults and infested pupae) and not at all for any of the BQCV correlations (Table 2).
There was excellent correspondence between the two mite infestation rate estimates. Of the viruses, only the DWV titers are correlated with the mite infestation rates in infested pupae, their mites, and adult bees. These correlations for the pupal infestation rates are superior to those for the adult infestation rates (Table 2), which is largely due to the more even distribution of the pupal infestation rate data across the correlation. There was no relationship between the mite infestation rates and the BQCV or SBV titers in any of the samples (Table 2).
DISCUSSION
Viral dynamics during acaricide treatment.
Given the strong influence of Varroa infestation and Varroa-vectored transmission on DWV titers in honeybee colonies (6, 33, 42, 48, 53), the expectation before this study was that removal of the mites using an acaricide treatment should result in a progressive reduction of DWV titers throughout the colony with a trend similar to the mite infestation rate, as was previously shown (34). On the contrary, our results showed a significant increase in DWV titers coinciding with the onset of the tau-fluvalinate treatment application in adult honey bees, mite-infested pupae, and uninfested pupae. A slight progressive decrease in DWV titers in adult bees and pupae was seen in treated colonies after this initial increase. However, the DWV titers in adult bees and uninfested pupae never fell below those in the untreated colonies during the treatment period. The DWV titers in mite-infested pupae eventually dropped below the titers in the untreated colonies, but only in the final week of the treatment. These results illustrate the short-term colony level changes in virus titers during the actual acaricide treatment rather than the long-term dynamics of virus titers in either treated or untreated colonies.
The initial increase of DWV titers in adult bees and pupae of the acaricide-treated colonies coincides with the most potent chemical effect of the treatment and could therefore potentially be a consequence of debilitating direct effects of tau-fluvalinate on honey bee physiology and/or immune system responses, causing increased host susceptibility to DWV infection. The subsequent decrease in DWV titers after the initial increase is at least partly due to the removal of Varroa-mediated transmission, as implied by the strong correlation between DWV titers and Varroa infestation rates in both adults and pupae. However, it may also be an effect of the adaptation of the host immune system to the presence of the tau-fluvalinate.
Although the primary effect of the acaricide treatment is the removal of mites from the colony, tau-fluvalinate is a potent chemical pesticide that can have distinct physiological effects on exposed bees (22). Synergistic interactions between chemicals and infectious pathogens causing reduced fitness in honey bee colonies have been demonstrated before (1). At the cellular and biochemical levels, fluvalinate interferes with the voltage-gated sodium ion transport channels (36, 41). These channels regulate the osmotic potential of the cell and are therefore also frequently a target for virus infections that use osmotic pressure to burst the cell to release newly formed virus particles (26, 29, 46). This could be a possible explanation for the initial increase in DWV titers seen in the bees. The sharp reduction in DWV titers in those mites surviving the fluvalinate treatment could have a similar origin. A variable proportion of Varroa mites are themselves also hosts for DWV (53), and such mites have highly elevated DWV titers compared to those mites that do not replicate DWV (21). DWV-replicating mites may therefore suffer enhanced mortality due to synergistic interactions between fluvalinate and DWV at the sodium transport channels so that only mites that do not replicate DWV, with drastically reduced DWV titers, have a chance of surviving the fluvalinate treatment. These are speculative arguments, but certainly plausible. Mechanisms behind the possible direct effects of the tau-fluvalinate treatment on host immunity and pathogen virulence in both bees and mites, as well as those of other chemical treatments administered to honey bee colonies, need further investigation.
With respect to BQCV and SBV infection, the acaricide treatment seemed to have a random influence rather than a progressive one. Although for these viruses there are also slight increase in titers following the initial treatment application in most sample types, it is much less pronounced than for DWV. In adult bees, BQCV and SBV titers remained relatively constant in colonies with the tau-fluvalinate acaricide treatment, while those in the untreated colonies decreased, something also seen for the SBV titers in infested pupae. The fluctuations in BQCV and SBV titers over time and between the treated and untreated colonies were large enough to produce statistically significant treatment-date effects for infested and uninfested pupae in the mixed-effects model analyses, but it is unclear how these can be interpreted, since they did not show a clear progressive increase or decrease throughout the experiment.
Varroa-virus relationships.
The strong positive correlation between DWV titers in infested pupae, adult bees, and Varroa mites and the colony pupal mite infestation; the statistically significant explanatory effect of colony mite infestation rates on DWV titers in adult bees; and the elevated DWV titers in infested pupae compared to uninfested pupae are all observations that complement numerous prior reports demonstrating the active relationship between DWV and Varroa (2, 6, 15, 21, 53). However, the unexpected observation in these experiments was that DWV titers remained high in our treated colonies even after the removal of the mites. Notably, DWV titers also remained high in the uninfested pupae throughout the experiment, even though these pupae did not have any contact with Varroa mites. These observations suggest that high-titer DWV infections may be able to persist for a considerable time after the removal of Varroa through alternative transmission routes, such as oral or vertical transmission. This supports observations by Highfield et al. (24) that winter colony mortality due to DWV infections is partly independent of Varroa mite infestation levels. The second unexpected observation was a significant initial increase in the DWV titer immediately following acaricide application in both adults and pupae, which subsequently decreased progressively. Highfield et al. (24) also implied the existence of an additional, unknown factor interacting with DWV that contributed to colony mortality. Perhaps the results of the present study offer a hint as to the nature of this unknown factor, considering that the colonies of the Highfield et al. study (24) were treated with acaricides before the winter and before their experiment, began.
The incidental observation that clinical symptoms, i.e., deformed bees, disappeared in the treated colonies while those in the untreated colonies increased, even though there was very little net change in DWV titer between treated and untreated colonies over the study period, reflects the very particular circumstances that lead to wing deformities. Although deformed bees usually have higher DWV titers than nondeformed bees, it is not the titers alone that result in deformities but rather how and when the virus is transmitted. While DWV is directly responsible for these wing deformities (35), they only appear in natural circumstances through Varroa-mediated DWV transmission during the pupal stage (6, 21, 53). Varroa-mediated DWV transmission among adult bees also results in high DWV titers but does not result in deformities (10, 19, 35, 48). In addition, deformed bees are expelled more rapidly from stronger colonies and may therefore also simply be more easily detected in the untreated colonies than in the treated ones. During an acaricide treatment, the adult bee population is increasingly derived from uninfested pupae (with lower DWV titers) as the pupal mite infestation rate drops, resulting in a rapid decrease in deformed emerging bees, even with a similar overall colony level DWV titer. The gradual decrease in DWV titers following mite treatment is due to a progressive turnover of the bee population with increasingly healthier bees rather than an instant cleansing with the removal of the mite vector alone. Just as it takes more than one season to build up to lethal DWV infection levels with Varroa mite infestation, it takes more than 5 weeks to bring them back down again through the reverse process of eliminating the mite. A decrease in DWV titers may therefore be more pronounced over a longer period than was covered by this study.
BQCV fails to replicate after injection into adult bees, but it is able to multiply after injection into pupae (40), and SBV has been detected in mites and their saliva (43), so a potential for Varroa-mediated transmission of BQCV and SBV exists. In this study, however, mite infestation had no effect or explanatory influence on BQCV titers in pupae or adult bees, and none of the correlations involving BQCV titers in infested pupae, their mites, or the mite infestation rates were significant. The mere detection of BQCV in the Varroa samples is inconclusive with regard to the ability of Varroa to transmit BQCV, since RT-qPCR detection does not distinguish between infectious particles and fragmented noninfectious BQCV RNA. The same conclusion, with the same arguments, can be reached for SBV, which, moreover, could not even be detected in any of the mites examined in this study. However, the probability of successful transmission depends on the amount of virus transmitted, and the BQCV and SBV titers were very low in all sample types. Although it is safe to conclude that in these experiments Varroa-mediated BQCV or SBV transmission did not occur, we cannot rule out the possibility that such transmission could occur with higher titers of these viruses. Our results are consistent with other studies that have been unable to demonstrate an epidemiological relationship between the Varroa mites and BQCV or SBV (19, 40, 43, 49, 50).
DWV, BQCV, and SBV each react differently to Varroa mite infestation, despite similarities in particle and genome structures (12). Therefore, knowledge of virus epidemiology and, more specifically, virus-vector interactions is important in order to implement effective techniques to manage different virus infections. This complex system of stressors, including Varroa mites, the viruses associated with Varroa, and the variety of harsh treatments used to control Varroa, needs further investigation, especially for implementing effective honey bee health control strategies. One obvious line of research resulting from these experiments includes measuring the effects of tau-fluvalinate on virus titers in bees directly, using Varroa-free colonies, to better differentiate between the effects of Varroa transmission and acaricide treatment on colony DWV titers. Such experiments are currently being planned.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to four anonymous reviewers for improving the manuscript. We thank Ulf Olsson for consultation on data transformation and mixed-model analyses.
This work was financially supported by the Swedish Board of Agriculture and the EU-funded 7th Framework Project BEE DOC, Grant Agreement 244956.
Footnotes
Published ahead of print 21 October 2011
Supplemental material for this article may be found at http://aem.asm.org.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Alaux C, et al. 2010. Interactions between Nosema microspores and a neonicotinoid weaken honey bees (Apis mellifera). Environ. Microbiol. 12:774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey L, Ball BV. 1991. Honey bee pathology, 2nd ed. Academic Press, London, United Kingdom [Google Scholar]
- 3. Berthoud H, Imdorf A, Haueter M, Radloff S, Neumann P. 2010. Virus infections and winter losses of honey bee colonies (Apis mellifera). J. Apic. Res. 49:60–65 [Google Scholar]
- 4. Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect pollinated plants in Britain and the Netherlands. Science 313:351–354 [DOI] [PubMed] [Google Scholar]
- 5. Boecking O, Genersch E. 2008. Varroosis—the ongoing crisis in bee keeping. J. Consum. Protect. Food Safety 3:221–228 [Google Scholar]
- 6. Bowen-Walker PL, Martin SJ, Gunn A. 1999. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 73:101–106 [DOI] [PubMed] [Google Scholar]
- 7. Brunetto MR, Colombatto P, Bonino F. 2009. Bio-mathematical models of viral dynamics to tailor antiviral therapy in chronic viral hepatitis. World. J. Gastroenterol. 15:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bustin SA, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 9. Carreck NL, Ball BV, Martin SJ. 2010. Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. J. Apic. Res. 49:93–94 [Google Scholar]
- 10. Chen YP, Evans JD, Feldlaufer MF. 2006. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 92:152–159 [DOI] [PubMed] [Google Scholar]
- 11. Chen YP, Evans JD, Hamilton M, Feldlaufer M. 2007. The influence of RNA integrity on the detection of honey bee viruses: molecular assessment of different sample storage methods. J. Apic. Res. 46:81–87 [Google Scholar]
- 12. Chen YP, Siede R. 2007. Honey bee viruses. Adv. Virus Res. 70:33–80 [DOI] [PubMed] [Google Scholar]
- 13. Cox-Foster DL, et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287 [DOI] [PubMed] [Google Scholar]
- 14. de Miranda JR, Fries I. 2008. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 98:184–189 [DOI] [PubMed] [Google Scholar]
- 15. de Miranda JR, Genersch E. 2010. Deformed wing virus. J. Invertebr. Pathol. 103(Suppl. 1):S48–S61 [DOI] [PubMed] [Google Scholar]
- 16. Fries I, Aarhus A, Hansen H, Korpela S. 1991. Comparisons of diagnostic methods for detection of Varroa jacobsoni in honey bee (Apis mellifera) colonies at low infestation levels. Exp. Appl. Acarol. 10:279–287 [Google Scholar]
- 17. Fries I, Aarhus A, Hansen H, Korpela S. 1991. Development of early infestations of Varroa jacobsoni in honey bee (Apis mellifera) colonies in cold climates. Exp. Appl. Acarol. 11:205–214 [Google Scholar]
- 18. Gallai N, Salles JM, Settele J, Vaissieres BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68:810–821 [Google Scholar]
- 19. Gauthier L, et al. 2007. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie 38:426–435 [Google Scholar]
- 20. Genersch E, Aubert M. 2010. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. doi:10.1051/vetres/2010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gisder S, Aumeier P, Genersch E. 2009. Deformed wing virus: replication and viral load in mites (Varroa destructor). J. Gen. Virol. 90:463–467 [DOI] [PubMed] [Google Scholar]
- 22. Haarmann T, Spivak M, Weaver D, Weaver B, Glenn T. 2002. Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J. Econ. Entomol. 95:28–35 [DOI] [PubMed] [Google Scholar]
- 23. Higes M, Garcia-Palencia P, Martin-Hernandez R, Meana A. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94:211–217 [DOI] [PubMed] [Google Scholar]
- 24. Highfield AC, et al. 2009. Deformed wing virus implications in overwintering honeybee colony losses. Appl. Environ. Microbiol. 75:7212–7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hillesheim E, Ritter W, Bassand D. 1996. First data on resistance mechanisms of Varroa jacobsoni (Oud.) against tau-fluvalinate. Exp. Appl. Acarol. 20:283–296 [Google Scholar]
- 26. Hoffmann H-H, Palese P, Shaw ML. 2008. Modulations of influenza virus replication by alteration of sodium ion transport and protein kinase C activity. Antiviral Res. 80:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imdorf A, Bühlmann G, Gerig L, Kilchenmann V, Wille H. 1987. Überprüfung der Schätzmethode zur Ermittlung der Brutfläche und der Anzahl Arbeiterinnen in freifliegenden Bienenvölkern. Apidologie 18:137–146 [Google Scholar]
- 28. Johnson RM, Pollock HS, Berenbaum MR. 2009. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 102:474–479 [DOI] [PubMed] [Google Scholar]
- 29. Kunzelmann K, et al. 2000. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc. Natl. Acad. Sci. U. S. A. 97:10282–10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Conte Y, Ellis M, Ritter W. 2010. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41:353–363 [Google Scholar]
- 31. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. 1996. SAS system for mixed models. SAS Publishing, Cary, NC [Google Scholar]
- 32. Lourenco AP, Mackert A, Cristino AD, Simoes ZLP. 2008. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 39:372–385 [Google Scholar]
- 33. Martin SJ. 2001. The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J. Appl. Ecol. 38:1082–1093 [Google Scholar]
- 34. Martin SJ, Ball BV, Carreck NL. 2010. Prevalence and persistence of deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. J. Apic. Res. 49:72–79 [Google Scholar]
- 35. Möckel N, Gisder S, Genersch E. 2011. Horizontal transmission of deformed wing virus: pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J. Gen. Virol. 92:370–377 [DOI] [PubMed] [Google Scholar]
- 36. Narahashi T. 1996. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 79:1–14 [DOI] [PubMed] [Google Scholar]
- 37. Nordström S, Fries I, Aarhus A, Hansen H, Korpela S. 1999. Virus infections in Nordic honey bee colonies with no, low or severe Varroa jacobsoni infestations. Apidologie 30:475–484 [Google Scholar]
- 38. Pettis JS, Wilson WT, Shimanuki H, Teel PD. 1991. Fluvalinate treatment of queen and worker honey bees (Apis mellifera L.) and effects on subsequent mortality, queen acceptance and supersedure. Apidologie 22:1–7 [Google Scholar]
- 39. Pfaffl M. 2001. A new mathematical model for relative quantification in real time RT PCR. Nucleic Acids Res. 29:2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ribière M, Ball BV, Aubert MFA. 2008. Natural history and geographical distribution of honey bee viruses, p 15–84 In Aubert M, et al. (ed), Virology and the honey bee. European Community, Luxembourg [Google Scholar]
- 41. Rosenkranz P, Aumeier P, Ziegelmann B. 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103(Suppl. 1):S96–S119 [DOI] [PubMed] [Google Scholar]
- 42. Santillán-Galícia MT, Carzaniga R, Ball BV, Alderson PG. 2008. Immunolocalization of deformed wing virus particles within the mite Varroa destructor. J. Gen. Virol. 89:1685–1689 [DOI] [PubMed] [Google Scholar]
- 43. Shen M, Cui L, Ostiguy N, Cox-Foster D. 2005. Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic Varroa mite. J. Gen. Virol. 86:2281–2289 [DOI] [PubMed] [Google Scholar]
- 44. Shen MQ, Yang XL, Cox-Foster D, Cui LW. 2005. The role of Varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 342:141–149 [DOI] [PubMed] [Google Scholar]
- 45. Snedecor GW, Cochran WG. 1980. Statistical Methods, 7th ed. Iowa State University Press, Ames, IA [Google Scholar]
- 46. Stauffer EK, Ziegler RJ. 1989. Loss of functional voltage-gated sodium channels in persistent mumps virus-infected PC12 cells. J. Gen. Virol. 70:749–754 [DOI] [PubMed] [Google Scholar]
- 47. Sumpter DJT, Martin SJ. 2004. The dynamics of virus epidemics in Varroa infested honey bee colonies. J. Anim. Ecol. 73:51–63 [Google Scholar]
- 48. Tentcheva D, et al. 2006. Comparative analysis of deformed wing virus (DWV) RNA in Apis mellifera and Varroa destructor. Apidologie 37:41–50 [Google Scholar]
- 49. Tentcheva D, et al. 2004. Prevalence and seasonal variation of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 70:7185–7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Todd JH, de Miranda JR, Ball BV. 2007. Incidence and molecular characterization of viruses found in dying New Zealand honey bee (Apis mellifera) colonies infested with Varroa destructor. Apidologie 38:354–367 [Google Scholar]
- 51. Tremolada P, Mazzoleni M, Saliu F, Colombo M, Vighi M. 2010. Field trials for evaluating the effects on honeybees of corn sown using Cruiser and Celest xl treated seeds. Bull. Environ. Contam. Toxicol. 85:229–234 [DOI] [PubMed] [Google Scholar]
- 52. Wallner K. 1999. Varroacides and their residues in bee products. Apidologie 30:235–248 [Google Scholar]
- 53. Yue C, Genersch E. 2005. RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 86:3419–3424 [DOI] [PubMed] [Google Scholar]
- 54. Yue C, Schröder M, Gisder S, Genersch E. 2007. Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). J. Gen. Virol. 88:2329–2336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.