Abstract
Oxygen sensing and redox signaling significantly affect bacterial physiology and host-pathogen interaction. Here we show that a Staphylococcus aureus two-component system, AirSR (anaerobic iron-sulfur cluster-containing redox sensor regulator, formerly YhcSR), responds to oxidation signals (O2, H2O2, NO, etc) by using a redox-active [2Fe-2S] cluster in the sensor kinase AirS. Mutagenesis studies demonstrate that the [2Fe-2S] cluster is essential for the kinase activity of AirS. We have also discovered that a homolog of IscS (SA1450) in S. aureus is active as a cysteine desulfurase, which enables the in vitro reconstitution of the [2Fe-2S] cluster in AirS. Phosphorylation assays show that the oxidized AirS with a [2Fe-2S]2+ cluster is the fully active form of the kinase but not the apo-AirS nor the reduced AirS possessing a [2Fe-2S]+ cluster. Over-oxidation by prolonged exposure to O2 or contact with H2O2 or NO led to inactivation of AirS. Transcriptome analysis revealed that mutation of airR impacts the expression of ~355 genes under anaerobic conditions. Moreover, the mutant strain displayed increased resistance toward H2O2, vancomycin, norfloxacin, and ciprofloxacin under anaerobic conditions. Together, our results show that S. aureus AirSR is a redox-dependent global regulatory system that plays important roles in gene regulation using a redox active Fe-S cluster under O2-limited conditions.
INTRODUCTION
A fundamental challenge to the study of host-pathogen interaction is to understand the molecular basis of redox signaling that facilitates pathogens to sense and evade host immune responses. Staphylococcus aureus, a major human pathogen that is the most common source of bacterial infections in the community and hospital, causes a wide variety of diseases ranging from minor skin infections to life-threatening sepsis.1 The success of this bacterium in pathogenesis is largely attributed to the sophisticated regulatory network composed of multiple global transcriptional regulators (e.g., SigB, Rot, MgrA, SarA, and SarA homologues) and sixteen two-component systems (TCSs) (e.g., Agr, SrrAB, ArlRS, VraRS, HssRS, and SaeRS),2–4 which enable the bacterium to rapidly respond to changing host microenvironments. In particular, two-component systems such as quorum-sensing agr or virulence-determining sae often play decisive roles in S. aureus virulence and infectivity during pathogenesis.2,5 Therefore, understanding the molecular mechanisms of these TCSs is critical in combating infections caused by S. aureus. There is a long-held belief that each two-component system has specific chemical signals, either environmental or endogenous, that activate or inactivate the corresponding signal transduction pathway. Despite extensive research efforts, to date, the specific signals and molecular basis leading to activation/inactivation of most S. aureus TCSs have yet to be characterized.
It is known that reactive oxygen/nitrogen species (ROS/RNS) serve beneficial roles during immune response, particularly when macrophages and neutrophils generate a burst of oxidants (O2−, HO•, H2O2, HClO, NO, etc.) to kill invading pathogens.6,7 ROSs/RNSs, on the other hand, are also exploited by pathogenic bacteria as signals to adapt and evade the host immune system. Previous studies have demonstrated that several global transcriptional regulators in S. aureus, including MgrA, SarZ, and SarA, act as oxidation-sensing regulatory proteins to control global gene expression via the sole conserved Cys residue.8–10 There are two S. aureus TCSs known to be redox-responsive. One is the two-component system SrrAB (for staphylococcal respiratory response AB), which is homologous to the Bacillus subtilis ResDE that has been considered a major regulatory system for anaerobic gene regulation.11 How SrrAB is controlled at molecular level, however, still remains a mystery. The other TCS known to be redox-responsive is the [4Fe-4S]-containing two-component system NreABC.12 NreABC is a specialized TCS that only regulates a limited set of genes involved in anaerobic nitrate/nitrite uptake, but without affecting global gene expression including virulence gene expression.12
In this work we discovered that the S. aureus sensor kinase AirS (SA1667, formerly YhcS) contains a redox active Fe-S cluster capable of sensing both oxygen and redox signals. To determine the genuine type of the Fe-S cluster, we developed a native enzymatic reconstitution system to functionally assemble Fe-S clusters in S. aureus proteins. We used EPR, UV-Vis spectroscopy, and in vitro phosphorylation assay to address how the sensor kinase AirS responds to O2, H2O2, and NO via its [2Fe-2S] cluster. We demonstrated that AirS is an oxygen sensor and only the oxidized AirS with [2Fe-2S]2+ exhibits an efficient kinase activity. Prolonged exposure to oxygen or treatment with strong oxidants such as H2O2 and NO abolishes the kinase activity. Thus, we renamed this TCS as AirSR for anaerobic iron-sulfur cluster-containing redox sensor regulator. We performed transcriptome analysis and revealed that AirSR is a global regulator that affects the expression of ~355 genes under anaerobic conditions. This TCS impacts S. aureus susceptibility toward H2O2 and a series of antibiotics. The elucidation of the molecular mechanism of this redox-dependent TCS will help to further understand how S. aureus senses and responds to the host and environmental stimuli.
EXPERIMENTAL METHODS
Bacterial Strains, Plasmids, and Culture Conditions
The bacterial strains and plasmids used in this study are listed in Table S1. Unless otherwise mentioned, S. aureus strain Newman was used in the study. Aerobic cultures of S. aureus, grown in tryptic soy broth (TSB), were incubated in Falcon tubes with shaking at 250 rpm. The culture medium did not exceed 20% of the tube volume. Anaerobic cultures of S. aureus were incubated in a Model 10–140 Incubator (Quincy Lab Inc.) placed in an anaerobic chamber (Sheldon Manufacturing Inc., 0.3% H2, 99.7% N2, and O2 ≤ 0.005 ppm) at 37 °C without shaking. E. coli cultures were grown in LB medium. Whenever required, antibiotics were added to the culture medium (for E. coli, 100 μg/ml ampicillin; for S. aureus, 10 μg/ml nalidixic acid, or 5 μg/ml chloramphenicol).
Overexpression and Purification of AirS, AirSC79S/C81S, AirR and S. aureus IscS
The ORFs of these proteins (AirS, AirR, and IscS) were PCR-amplified from Newman chromosomal DNA with the primers indicated in Table S2. The PCR products were treated with T4 DNA polymerase in the presence of dCTP at room temperature for 30 min. The target vector pMCSG713 was digested with SspI, gel-purified, and then treated with T4 DNA polymerase in the presence of dGTP at 16 °C for 15 min. The T4 DNA polymerase-treated plasmid vector and PCR product were gel-purified, mixed, incubated at room temperature for 5 min, and then transformed into E. coli strain DH5a. The resulting plasmid was transformed again into BL21 star (DE3) and transformants were selected on LB agar plates with 100 μg/ml ampicillin. The BL21 star (DE3) strain carrying the plasmid was grown in LB to OD600 of 0.6 and then 1 mM of isopropyl β-D-1-thiogalactopyranoside (IPTG) was added. After an overnight induction at 16 °C, the cells were harvested and frozen at −80 °C. The expressed protein was purified from the frozen cells with a HisTrap column (GE Healthcare, Inc.) by following manufacturer’s recommendations. During purification, all buffers were cooled to 4 °C and supplemented with 4 mM dithiothreitol (DTT) before use. The purified protein was supplemented with 20% glycerol and stored at −80 °C. Stratagene’s QuikChange™ Site-Directed Mutagenesis Kit was used to construct the mutant protein AirSC79S/C81S. Primers for mutagenesis are listed in Table S2. The protein expression and purification methods are the same as described above.
Fe-S Cluster Assembly
Aerobically purified AirS was degassed and transferred into a glove box (0.3% H2, 99.7% N2 and O2 ≤ 0.005 ppm) to be pre-equilibrated. Reconstitution buffer (10 mM Tris-HCl, pH 7.4, 100 mM NaCl) was anaerobically equilibrated overnight in the glove box. A mixture of 20 μM AirS, 0.2 mM ammonium ferrous sulfate, 2 mM L-cysteine, and 2.5 mM DTT was prepared followed by addition of 1 μM S. aureus IscS to initiate the reaction. The reaction was incubated overnight at room temperature and terminated by removing low-molecule-weight compounds with PD-10 column (GE Healthcare). For oxidation by O2, the reconstituted product (40 μM, 120 μl) was exposed to air and UV-Vis spectra were measured after O2 exposure at different time points (0, 0.5, 1, 2, 3 h). For H2O2 treatment, the reconstituted AirS (40 μM, 120 μl) was treated with 80 μM or 2 mM H2O2 for 2 min before UV-Vis spectra were acquired. For NO treatment, the reconstituted AirS (40 μM, 120 μl) was mixed with 40 μM or 160 μM S-nitrosoglutathione for 2 min before UV-Vis measurement. For EPR, the following five samples were prepared: 40 μM AirS (aerobically purified and anaerobically equilibrated), 40 μM aerobically purified AirS (anaerobically equilibrated) with 5 mM sodium dithionite, 40 μM reconstituted AirS, 40 μM reconstituted AirS with 5 mM sodium dithionite, and 40 μM reconstituted AirS with 160 μM S-nitrosoglutathione. These samples were placed into EPR tubes and immediately frozen in liquid nitrogen.
Autophosphorylation of AirS and Phosphorylation of AirR by AirS
For autophosphorylation of AirS, the phosphorylation assays were performed in 10 μl of phosphorylation buffer (10 mM Tris-HCl, pH 7.4, 50 mM KCl, 5 mM MgCl2, and 10% glycerol) containing AirS (5 μM). γ-32P-ATP (80 μCi) was added to initiate the reaction. For phosphorylation of AirR by AirS, AirR (20 μM) was added 5 min after the autophosphorylation reaction of AirS was initiated. The reactions stayed at room temperature for a designated time period and then were stopped by the addition of 2 × SDS loading buffer. Samples were analyzed by 13% SDS- PAGE followed by autoradiography.
35S Transfer Assay
Sulfur transfer reactions using S. aureus IscS were performed at room temperature for 5 min with 40 μM IscS in a 20 μl solution that contained 50 mM sodium phosphate buffer (pH 7.4) and 10 mM MgCl2. Reactions were started by the addition of 35S-labeled L-cysteine (Perkin Elmer) (0.1 mM). To investigate the effects of the reducing (DTT) and alkylating agent (iodoacetamide), reactions were performed in the presence of 2 mM DTT and after treating IscS with 0.5 mM iodoacetamide (room temperature for 30 min), respectively. Reactions were quenched by 2 × SDS-PAGE loading dye. Samples were analyzed by 13 % SDS-PAGE followed by autoradiography.
EPR Measurements
EPR spectra were recorded on several different spectrometers. To obtain both the overall greatest signal-to-noise ratio (S/N) and the best dispersion of g values, a 35 GHz (Q-band) continuous wave (CW) spectrometer was employed. This spectrometer uses an immersion Dewar containing superfluid helium (2 K) and has been described elsewhere.14 Under these conditions, EPR spectra are recorded under “passage” conditions and exhibit absorption, rather than first derivative appearance.15 To obtain the most reliable quantification of EPR signals from AirS, a standard CW X-band EPR spectrometer (Bruker ESP300 with Oxford Instruments cryostat and temperature controller) was employed. Optimal S/N for AirS samples resulted from use of a temperature of 17 – 18 K, which is typical for Fe-S systems.16–18 Other experimental parameters were typically: microwave power, 10 mW; field modulation amplitude, 13 G; time constant, 160 ms; scan time, 120 s.
In contrast to e.g., Type II copper EPR signals, for which aqueous Cu(EDTA) is an appropriate standard,19 it is difficult to find a suitable quantification standard for an Fe-S EPR signal, as the relaxation properties of such systems differ from a simple paramagnet such as Cu(II).20 We have available to us samples of reduced [2Fe-2S] Fd’s isolated from Aquifex aeolicus (Aae Fd1) by Meyer and co-workers,21 for which the concentration (2 – 3 mM) has been reasonably well determined via biochemical methods. We have previously used Aae Fd1 as an approximate concentration standard for the EPR-active Fe-S cluster in spore photoproduct lyase (SPL),22 and use it again here for this purpose.
For quantification at X-band, a baseline EPR spectrum recorded under identical conditions for aerobic AirS was subtracted from the AirS spectrum of interest. This helped to remove the sextet signal from trace amounts of adventitious aqueous Mn2+. Aerobically isolated AirS also exhibits a weak, isotropic EPR signal at g ≈ 2.00 (335 mT at X-band; 1.25 T at Q-band), which was not fully removed in the subtractions and is of unknown origin.
EPR simulations used the program QPOWA,23 as modified by J. Telser. Double integration of the X-band spectra was used for correlation of signal intensity to concentration, as described elsewhere.19
Iron Quantification in AirS
The iron quantification assay was performed as previously described.24 Briefly, to each protein sample (80 μl) was added 100 μl of solution A (2.25 % KMnO4 in 0.6N HCl). The resulting mixture was incubated at 37 °C for 2 h, followed by addition of 20 μl solution B containing 6.5 mM Ferrozine, 13.1 mM neocuproine, 2 M ascorbic acid, and 5 M ammonium acetate. After 2 h at room temperature, the absorbance at 562 nm and 750 nm was measured. Fe concentration was obtained by fitting a standard curve. This quantification assay was repeated three times with similar results obtained.
RESULTS
Aerobically Purified AirS Contains a [2Fe-2S]2+ Cluster
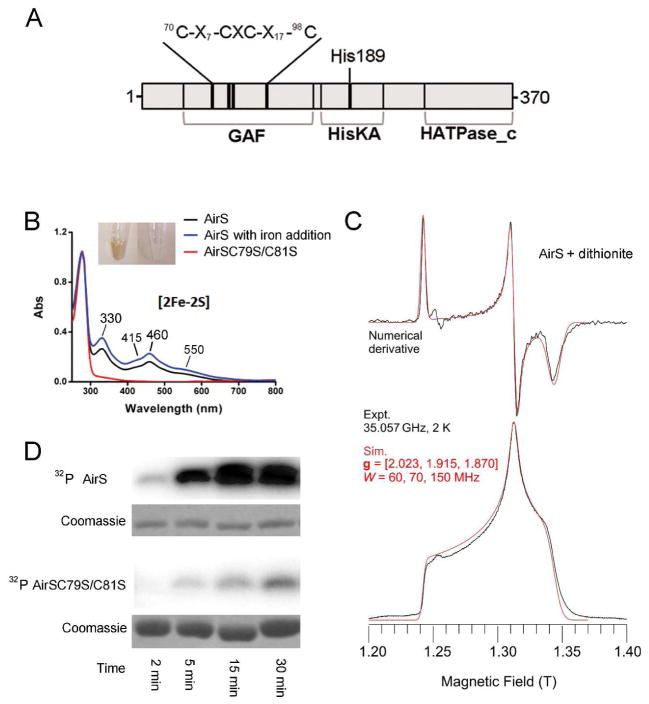
The AirS protein is a putative histidine sensor kinase consisting of two domains, the N-terminal sensory domain and C-terminal histidine-kinase domain. The protein sequence of the N-terminal sensory domain reveals a likely Fe-S cluster-binding motif composed of four conserved cysteine residues C-X7-CXC-X17-C (where X is any amino acid) (Figure 1A), which is reminiscent of the [2Fe-2S] type (C-X2-CXC-X5-C) SoxR25 and the [2Fe-2S] type (C-X8-C-X2-C-X25-C) ferredoxins (Fds).26 Intriguingly, the full-length sensor kinase AirS, expressed and aerobically purified from Escherichia coli, was yellow-brown in color, a characteristic feature of proteins that contain Fe-S clusters, while mutation of the middle two Cys-79 and Cys-81 to Ser residues renders the AirS protein colorless (Figure 1B, inset), implying that these Cys residues are required for the binding of the Fe-S cluster by the protein. Moreover, the wild-type AirS displayed UV-Vis absorbance at 330, 415, 460, and 550 (nm) (Figure 1B), which resembles the UV-Vis pattern typically observed for [2Fe-2S] clusters.27 This aerobically purified AirS is sub-stoichiometric in the Fe-S cluster content, as when we induced the expression of AirS in the E. coli culture supplemented with 1 mM FeCl2, the purified AirS protein displayed enhanced UV-Vis absorbance (Figure 1B), indicating that iron supplementation in the culture increased the iron content of the expressed AirS. On the other hand, the Cys to Ser mutant protein AirSC79S/C81S exhibited no peak at these wavelengths, showing the absence of the Fe-S cluster (Figure 1B). To further confirm the presence of a [2Fe-2S] cluster, EPR spectroscopy measurements were performed. AirS aerobically purified from E. coli was EPR silent, while upon reduction by sodium dithionite, AirS became EPR visible and developed a characteristic [2Fe-2S]+ EPR pattern (g = [2.02, 1.92 and 1.87]) (Figures 1C and S1),28 in line with a one-electron reduction of the EPR silent [2Fe-2S]2+ to the EPR visible [2Fe-2S]+.29
Figure 1.
S. aureus AirS is a Fe-S cluster-containing sensor kinase. (A) Domain organization of AirS (370 aa). The four cysteine residues (Cys-71, Cys-79, Cys-81, and Cys-99) involved in binding the Fe-S cluster are located in the N-terminal half. The remaining portion of the protein contains a typical histidine protein kinase domain, composed of the proposed autophosphorylation site (His-189), HisKA (Histidine kinase A), and HATPase_c (HK-like ATPases). (B) UV-visible spectra of AirS and its mutant AirSC79S/C81S. Note the characteristic [2Fe-2S]2+ absorbance peaks at 330, 415, 460, and 550 (nm). (Inset) The wild-type AirS is yellow-brown (left panel) while its mutant AirSC79S/C81S is colorless (right panel), indicating that Cys-79 and Cys-81 are required for Fe-S cluster binding. (C) Q-band EPR spectrum of AirS isolated from E. coli after dithionite treatment (Na2S2O4, 5 mM) to generate an EPR active [2Fe-2S]+ cluster. The g values are 2.023, 1.915, and 1.870. EPR simulations were performed using the program QPOW, as described in Experimental Methods. (D) Fe-S cluster is crucial for the kinase activity of AirS. The wild-type AirS displayed a much higher autokinase activity than its mutant AirSC79S/C81S.
To investigate whether the Fe-S cluster is essential for the kinase activity of AirS, we compared the autokinase activities of AirS and its mutant AirSC79S/C81S by incubating the protein with γ-32P-ATP. As shown in Figure 1D, the wild-type AirS exhibited high efficiency in autophosphorylation, which reached a maximum within 15 min, while its AirSC79S/C81S mutant was relatively inert towards phosphorylation, suggesting that the Fe-S cluster is critical for AirS kinase activity. Collectively, these data demonstrate that S. aureus AirS is a histidine sensor kinase with a [2Fe-2S] cluster and that the Fe-S cluster is essential for its kinase activity.
S. aureus IscS (SA1450) Can Reconstitute [2Fe-2S]+ in AirS
Prokaryotes have evolved three different conserved systems (Isc, Nif, and Suf) for the in vivo biogenesis of Fe-S clusters.29 Previous studies have shown that these Fe-S clusters can be assembled anaerobically by mixing an iron source, a reducing agent, L-Cys (sulfur donor), and a cysteine desulfurase (e.g., NifS/IscS) in vitro.30 Azotobacter vinelandii NifS/IscS is the prototype of cysteine desulfurase,30 which has been widely used in in vitro Fe-S cluster assembly.31 We were concerned that a non-native cysteine desulfurase might not appropriately reconstitute S. aureus Fe-S clusters. Hence, we proceeded to identify the S. aureus cysteine desulfurase for in vitro S. aureus Fe-S cluster assembly. To date, no homolog of cysteine desulfurase in S. aureus has ever been characterized, nor has assembly of any S. aureus Fe-S cluster protein been achieved using these enzymes. A BLAST search revealed a S. aureus homolog (SA1450) of the A. vinelandii NifS that shows the highest homology (35% identity, 55% homology), whereas the other homolog (SA0776) shows a relatively low homology (22% identity, 44% homology). S. aureus IscS (SA1450) contains a conserved pyridoxal 5′-phosphate-binding site at Lys-201 and an active Cys-324 residue required for desulfurating L-Cys to form an enzyme-bound cysteinyl persulfide.29
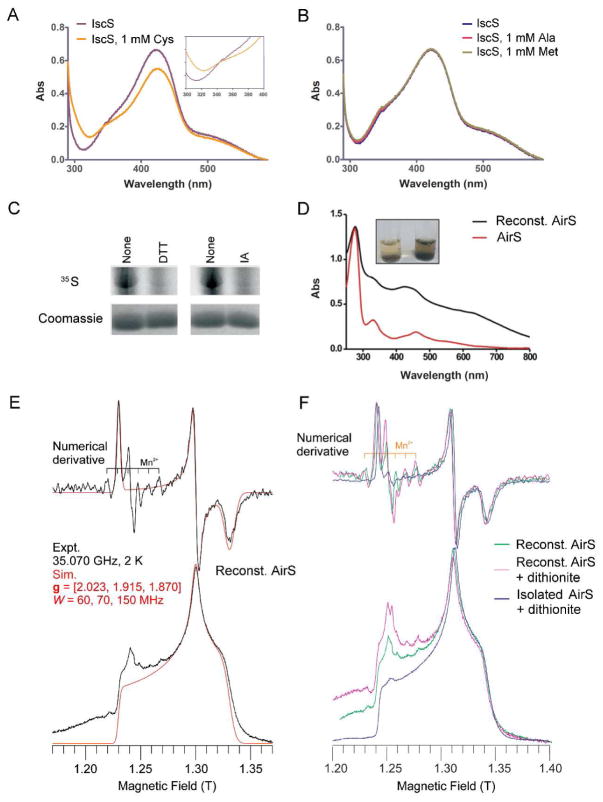
S. aureus IscS (SA1450), heterologously expressed and purified from E. coli, displayed a yellow color, and UV-Vis of this S. aureus IscS revealed a major absorbance peak at 420 nm (Figure 2A), which confirmed that the purified S. aureus IscS is a pyridoxal 5′-phosphate-bound protein.30 Furthermore, the addition of L-Cys (Figure 2A), but not L-Ala nor L-Met (Figure 2B), led to decreased absorbance at 420 nm and a concomitant increase in absorbance at 310 nm, which strongly suggests cysteine desulfurase activity of IscS.30 To further assess the ability of S. aureus IscS to desulfurate L-Cys to generate persulfide, we performed an in vitro sulfur-transfer assay using 35S-L-Cys. As shown in Figure 2C, S. aureus IscS is able to acquire 35S from 35S-L-Cys, whereas this reaction was greatly inhibited by either an excess of reducing agent DTT or alkylating the active Cys residue in IscS with iodoacetamide (IA). These results confirm the formation of the IscS-bound persulfide intermediate as well as the cysteine desulfurase activity of S. aureus IscS.30
Figure 2.
S. aureus IscS (SA1450) mediates AirS Fe-S cluster reconstitution. (A and B) UV-Vis absorbance spectra of S. aureus IscS showing a major peak at 420 nm. Spectroscopic analysis was performed in a 0.1-ml cuvette containing purified S. aureus IscS (30 μM) before and after adding L-Cys or L-Ala or L-Met. (Inset) Perturbation of the spectrum of IscS upon addition of L-Cys. (C) Effect of reducing (DTT) and alkylating (iodoacetamide, IA) agents on the IscS-based sulfur transfer reaction. The reaction was carried out at room temperature using 35S-labeled L-Cys and IscS (lane 1), modification of IscS with 10 mM DTT (left panel), and modification with 1 mM iodoacetamide (right panel). All reactions were analyzed by 13% SDS-PAGE followed by autoradiography. Coomassie blue staining was used to ensure the quality of the loaded protein. (D) Absorbance spectra of AirS before and after IscS reconstitution. Reconstitution was performed in an anaerobic chamber described in Experimental Methods. (Inset) Development of a green-brown color before and after IscS reconstitution. (E) Q-band EPR spectrum of the reconstituted AirS. The g values are 2.023, 1.915, and 1.870. (F) Overlay of Q-band EPR spectra of AirS directly isolated from E. coli after dithionite reduction (g = [2.023, 1.915, 1.870]) and reconstituted AirS before and after dithionite reduction (g = [2.023, 1.915, 1.870]). Very weak signals from exogenous Mn2+ and a radical (both centered at g = 2.00) are also seen (E and F). Mn2+ is a minor contaminant in the Fe2+ salts used for cluster reconstitution; the origin of the radical is unknown, but may be a consequence of dithionite reduction.
To demonstrate that S. aureus IscS is capable of transferring sulfur from L-Cys to assemble the Fe-S cluster in AirS, we combined aerobically purified AirS, L-Cys (sulfur donor), IscS (cysteine desulfurase), ammonium ferrous sulfate (iron source), and DTT (reducing agent) in a one-pot reaction under anaerobic conditions. After overnight incubation at room temperature, the reconstituted AirS, which was purified by removing excess of small molecules with a desalting column, developed a green-brown color. Compared with AirS before reconstitution, UV-Vis of the reconstituted AirS showed overall enhanced UV absorbance (Figure 2D), indicating an increased ratio of Fe-S cluster-bound AirS versus apo-AirS. We performed EPR analysis on this anaerobically reconstituted AirS, which exhibited an EPR signal at g = [2.02, 1.92, 1.87] (Figure 2E). This is identical to that of the aerobically purified AirS after dithionite reduction (g = [2.02, 1.92, 1.87]) and consistent with the characteristic feature of the reduced [2Fe-2S]+. Addition of dithionite did not alter the EPR pattern of the reconstituted AirS (Figure 2F), further supporting the incorporation of the reduced [2Fe-2S]+ into AirS during the Fe-S cluster assembly. To examine the efficiency of the reconstitution reaction, we employed a Ferrozine-based iron analysis method to quantify the iron content in the AirS protein before and after reconstitution as described in Experimental Methods.24 As a result, aerobically purified AirS (40 μM) contained only one equivalent of Fe (40 μM), suggesting that only 50 % of the isolated AirS possesses [2Fe-2S]. Nevertheless, the protein (40 μM), after anaerobic reconstitution, was found to contain up to 90 μM of iron (2.3 Fe per protein). Moreover, quantitative comparison of the X-band EPR spectra at 17 K of Aae Fd1 with both as isolated and reconstituted, dithionite treated AirS suggested that the isolated AirS (40 μM) contains roughly 15–25 μM of [2Fe-2S] (38–62% occupancy) and that the reconstituted AirS (13 μM) possesses nearly 10–15 μM of [2Fe-2S] (77–115% occupancy) (Figure S2). These results convincingly demonstrate that the [2Fe-2S] cluster can be nearly quantitatively reassembled in AirS by the IscS-mediated reconstitution reaction. Taken together, our results demonstrate that S. aureus IscS is a functional cysteine desulfurase, with which we were able to anaerobically reconstitute [2Fe-2S]+-bound AirS in vitro.
AirS Responds to O2, H2O2, and NO via the [2Fe-2S] cluster
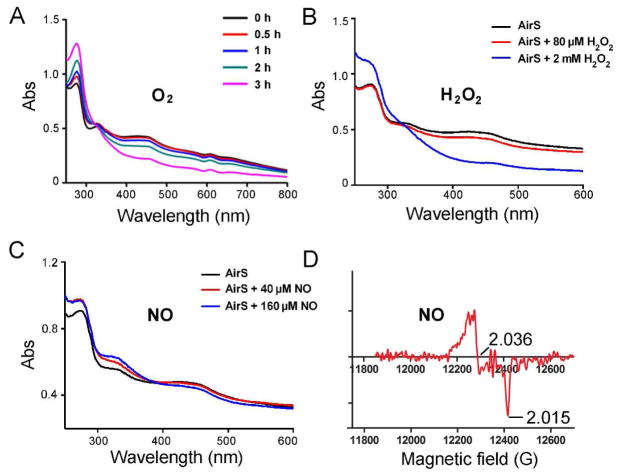
In E. coli, FNR (4Fe-4S) and SoxR (2Fe-2S) are prototypes of the Fe-S cluster-containing sensors responsive to oxidation.32,33 To establish whether AirS is capable of reacting with these agents, the reconstituted AirS was independently exposed to O2, H2O2, or NO. Since the purified AirS after dithionite reduction displayed absorbance patterns similar (but weaker) to the reconstituted protein under these conditions, herein we only show the results of the reconstituted protein (Figure 3). The reconstituted protein (40 μM, 120 μl) produced in the anaerobic chamber was exposed to air and the oxygen-induced changes in the UV-Vis spectra were monitored over time. At the initial time points (30 min and 1 h), we observed an absorbance increase in < 300 nm region, but only a slight absorbance decrease in the 300 nm to 700 nm region (Figure 3A). At the subsequent time points (2 h and 3 h), there was a significant reduction of the absorbance in the 300 nm to 700 nm region (Figure 3A), probably due to the degradation of the Fe-S cluster.
Figure 3.
The S. aureus AirS Fe-S cluster responds to O2, H2O2, and NO. (A) Anaerobically reconstituted AirS was purified through a desalting column and exposed to O2. UV-visible spectra were recorded before and after air exposure at various time intervals as indicated. (B) Effect of H2O2 on AirS. Reconstituted AirS (40 μM) was treated with H2O2 (80 μM and 2 mM) followed by UV-visible analysis. (C) UV-visible spectra of AirS (40 μM) before and after NO treatment (S-nitrosoglutathione (40 μM and 160 μM) was used as the NO donor). (D) Q-band EPR spectrum (g = 2.036) indicated the formation of a dinitrosyl-iron-dithiol (DNIC) complex.
The reconstituted AirS (40 μM, 120 μl) was also treated with H2O2 (80 μM and 2 mM) under anaerobic conditions for 2 min before UV-Vis measurement. While treatment of 80 μM H2O2 (2 eq) resulted in a slight decrease in the absorbance at the 300 nm to 600 nm region, a dramatic decrease in the same absorbance region was observed when 2 mM H2O2 was used (Figure 3B), suggesting a high sensitivity of the Fe-S cluster in AirS towards millimolar H2O2. To examine whether NO can be a ligand of the AirS Fe-S cluster, the reconstituted AirS (40 μM, 120 μl) was treated with an efficient NO-releasing compound S-nitrosoglutathione (40 μM and 160 μM) anaerobically for 2 min before UV-Vis and EPR measurements. As shown in Figure 3C, treatment of 40 μM S-nitrosoglutathione (1 eq) caused a significant change of the UV-Vis absorbance pattern of AirS, while adding an increased amount of NO-donor (160 μM, 4 eq) greatly altered the UV-Vis pattern with a significant absorbance increase in the 280 nm to 400 nm region and a concomitant reduction of absorbance in the 400 nm to 600 nm region. Further, the EPR spectrum of the NO-treated AirS exhibited a signal at g = 2.036 (Figure 3D), consistent with the formation of a protein-bound dinitrosyl-iron-dithiol (DNIC) complex.34
AirS is Fully Active Only in Its Oxidized Form ([2Fe-2S]2+)
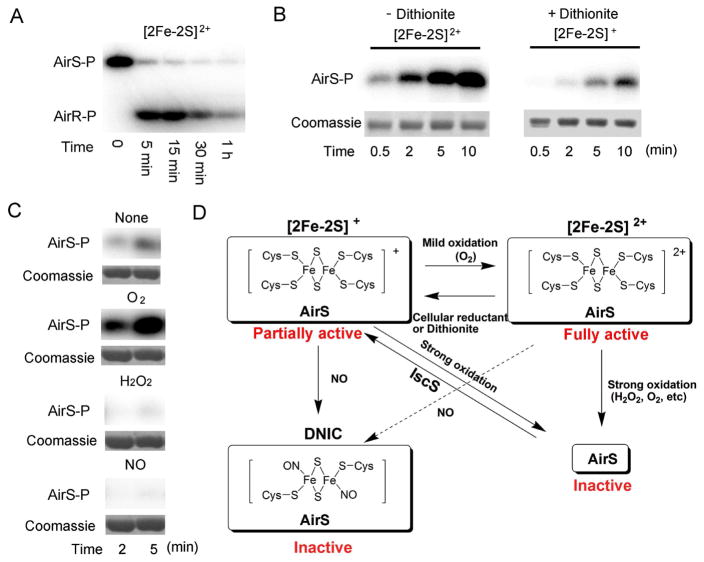
In a typical TCS, the activated sensor kinase is subject to autophosphorylation of its conserved His residue. A subsequent transfer of this phosphate group to the conserved Asp residue of its cognate response regulator turns on the downstream response. Since the aerobically purified AirS with an oxidized [2Fe-2S]2+ cluster exhibited a remarkable autokinase activity (Figure 1D), we wondered whether this oxidized form of AirS was capable of phosphorylating its cognate partner AirR. The oxidized AirS purified from E. coli was treated with γ-32P-ATP at room temperature for 10 min followed by the addition of AirR. As shown in Figure 4A, AirS underwent rapid autophosphorylation. Upon addition of AirR, 32P was rapidly transferred from AirS to AirR, which reached maximum phosphorylation within 5 min. The Asp-phosphorylation of AirR was transient, and the signal corresponding to the phosphorylated AirR diminished after 30 min.
Figure 4.
The kinase activity of AirS depends on the redox status of the Fe-S cluster. (A) Phospho-transfer reaction between the aerobically purified AirS from E. coli and its cognate response regulator AirR. AirS (5 μM) was phosphorylated with γ-32P-ATP, followed by addition of AirR (10 μM). (B) Autophosphorylation of AirS with and without dithionite reduction. Purified AirS (from E. coli) was reduced by dithionite (5 mM) at room temperature for 10 min followed by phosphorylation. Aliquots of the reaction mixture were quenched by 2× SDS loading buffer at various time intervals. (C) Effects of O2, H2O2, and NO on the kinase activity of AirS. Anaerobically reconstituted AirS was treated with O2 (air, 3 min), H2O2 (3 min), or NO (S-nitrosoglutathione, 3 min). (D) A hypothetical model depicting AirS as an intricate redox sensor. The AirS/[2Fe-2S]+ (partially active) can be oxidized by O2 to yield AirS/[2Fe-2S]2+ (fully active), which can be inactivated through either reduction by cellular reductants to AirS/[2Fe-2S]+, or further oxidation by strong oxidants (H2O2, O2, etc) to yield apo-AirS (inactive). S. aureus IscS can convert apo-AirS back to AirS/[2Fe-2S]+. The AirS Fe-S cluster can also form a DNIC complex with NO, thus inactivating its kinase activity. All the experiments in (A) and (B) were performed under aerobic conditions, whereas all the experiments in (C) were done inside an anaerobic chamber.
Given that the Fe-S cluster in AirS is redox active, we were prompted to study how the redox status of the Fe-S cluster impacts the kinase activity of AirS. We tested the autokinase activity of aerobically purified AirS in the absence or presence of 5 mM dithionite. As shown in Figure 4B, AirS exhibited a high kinase activity in the absence of dithionite, whereas its kinase activity was drastically reduced after dithionite reduction. Since aerobically purified AirS with the ERP-silent [2Fe-2S]2+ can be reduced by dithionite to the EPR-visible, reduced form of [2Fe-2S]+ (Figure 1), we conclude that AirS is fully active in its oxidized [2Fe-2S]2+ form, but not in its reduced [2Fe-2S]+ form.
To investigate effects of oxidative stresses on the activity of AirS, we treated the anaerobically reconstituted AirS with O2, H2O2, or NO, and performed the phosphorylation assay under anaerobic conditions. Without any treatment, we observed only a minimal autokinase activity (Figure 4C), in line with the findings that S. aureus IscS reconstitutes the reduced [2Fe-2S]+ into AirS and that the reduced AirS is inactive. A brief exposure of the reduced AirS to atmospheric oxygen (3 min) significantly enhanced the autophosphorylation level of AirS (Figure 4C), which is comparable to that of the aerobically isolated AirS without any treatment, suggesting that the limited exposure to oxygen activates AirS by oxidizing [2Fe-2S]+ to [2Fe-2S]2+. By contrast, when we extended the oxygen exposure to 1 h in the absence of any reducing agent, we did not observe any autokinase activity of AirS, indicating that extended exposure to oxygen may have caused over-oxidation and loss of the Fe-S cluster, thus eliminating the AirS activity (Figure S3). When AirS was treated anaerobically with H2O2 for 2 min, the autokinase activity of AirS was completely abolished, showing that AirS activity is particularly susceptible to H2O2. To examine whether the formation of the protein-bound dinitrosyl-iron-dithiol (DNIC) complex activates or inactivates AirS, we incubated AirS anaerobically with S- nitrosoglutathione for 2 min followed by reaction with γ-32P-ATP: no phosphorylation of AirS was observed (Figure 4C), demonstrating that the formation of DNIC with NO is detrimental to the activity of AirS. Cumulatively, these results suggest that AirS is fully active only in its oxidized form [2Fe-2S]2+, either reduction or further oxidation impairs the kinase activity (Figure 4D). The redox-dependent kinase activity of AirS (Figure 4D) allows this TCS to rapidly sense and respond to oxygen and various oxidative stresses.
AirSR Acts as a Global Regulatory System under Oxygen-Limited Conditions
To investigate the role of AirSR in S. aureus gene expression, we compared the global gene expression profiles of a ΔairR mutant, a bursa aurealis transposon insertion mutant obtained from the Phoenix library (Figure S4),35 and the parental strain Newman by microarray analysis. Because the oxidation status of AirS is critical for its function, we performed the analysis under both aerobic and anaerobic conditions (see Experimental Methods). As a result, when cells were grown aerobically, no difference was observed in the microarray analysis. However, when cells were grown inside an anaerobic chamber, a total of 67 (mid-exponential growth phase) or 355 genes (stationary phase) were differentially expressed in the airR mutant (Tables S1 and S2). AirSR globally impacts S. aureus gene regulation such that these differentially expressed genes include ones encoding master regulatory systems such as the quorum sensing Agr (RNAIII, AgrA, and AgrD), the virulence TCS SaeRS, and stress-associated factors (RsbU and RsbW), as well as key virulence factors such as capsular polysaccharide biosynthesis protein (Cap5A), protein A (Spa), and γ-hemolysin (HlgC) (Table 1). To validate the microarray results, we carried out quantitative RT-PCR on three selected genes (downregulated cap5A, RNAIII, and upregulated spa) that are involved in virulence. Consistent with the microarray analysis, qRT-PCR analysis revealed that all these genes exhibited significantly altered transcriptional expression in the airR mutant compared with the wild-type Newman (Table 1). In addition, electrophoretic mobility shift assays (EMSAs) established that the response regulator AirR is capable of binding to the promoter regions of agr, saeP3, and cap5A, suggesting that AirSR might control the expression of these genes via a direct DNA-binding mechanism (Figure S5). Taken together, these data demonstrate that AirSR is a key regulatory system that affects the transcription of more than 350 genes under anaerobic conditions.
Table 1.
Selected genes differentially expressed in the S. aureus ΔairR mutant strain compared to Newman under anaerobic conditions
| Gene name (synonym) | Function/similarity | Transcriptome analysis | qRT-PCR | ||
|---|---|---|---|---|---|
| ΔairR/Newman expression ratio | Δ airR/Newman expression ratio | ||||
| Mid-log | Stationary | Mid-log | Stationary | ||
| NWMN_0095 cap5A | capsular polysaccharide biosynthesis protein Cap5A | 4.92 | 45.71 | 2.81±0.53 | 5.76±0.08 |
| NWMN_2624 RNAIII | delta-hemolysin precursor | 3.57 | 3.39 | 16.99±0.09 | 10.99±0.07 |
| NWMN_1946 agrA | accessory gene regulator protein A | 1.96 | |||
| NWMN_1944 agrD | accessory gene regulator protein D | 2.63 | |||
| NWMN_0674 saeS | sensor histidine kinase SaeS | 3.00 | |||
| NWMN_0675 saeR | DNA-binding response regulator SaeR | 2.27 | |||
| NWMN_1971 rsbW | serine-protein kinase RsbW | 2.77 | |||
| NWMN_1973 rsbU | sigma-B regulation protein | 1.87 | |||
| NWMN_0055 spa | Immunoglobulin G binding protein A precursor | −2.20 | −3.96 | −18.00±0.12 | −9.00±0.08 |
| NWMN_2319 hlgC | gamma-hemolysin component C precursor | −2.97 | −2.08 | ||
AirSR Mediates Drug Resistance under Anaerobic Conditions
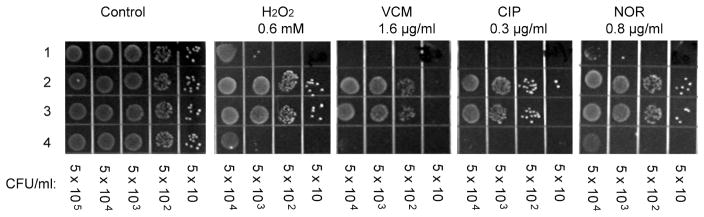
The biochemical properties of AirS are highly indicative of its sensory role towards oxidative stress. We performed plate sensitivity assays to examine the impact of AirSR on the susceptibility of S. aureus towards chemical agents that potentially exert oxidative stress. Since microarray analysis results showed that AirSR impacts global gene expression under oxygen-limited conditions, we used the plate assays inside an anaerobic chamber. As shown in Figure 5, compared with the wild-type strain, the airR mutant strain was more resistant to H2O2, vancomycin, ciprofloxacin, and norfloxacin. These phenotypes were complemented by introducing the entire airSR operon into the airR mutant via an integration plasmid pCL55. Previous studies have shown that antibiotics such as vancomycin, ciprofloxacin, and norfloxacin induce oxidative stress inside bacteria.8,36,37 AirSR may respond to these antibiotics through sensing the generated ROS, although the detailed mechanism of how AirSR affects the S. aureus antibiotic susceptibility remains to be determined. Overall, these results further support the in vivo oxidation sensing role of AirSR in S. aureus.
Figure 5.
Plate sensitivity assay under anaerobic conditions. Four S. aureus strains were treated with H2O2 and different antibiotics. Row 1, wild-type Newman; row 2, ΔairR mutant; row 3, Δ airR mutant with pCL55; row 4, ΔairR mutant complemented by airSR in pCL55. The control plate had no antibiotics. VCM, vancomycin; CIP, ciprofloxacin; NOR, norfloxacin.
DISCUSSION
Except a few examples such as the quorum-sensing agr, little is known regarding the molecular signals and the sensing mechanisms of S. aureus TCSs. Here we show that S. aureus AirSR is a redox-responsive regulatory system that contains a redox active [2Fe-2S] cluster which responds to O2, H2O2, and NO. The oxidized AirS with a [2Fe-2S]2+ cluster exhibits the optimal kinase activity. Both reduced and over-oxidized AirS lose this activity. We have also established by transcriptome analysis that AirSR is a global regulatory system that functions in an oxygen-dependent manner. However, the exact mechanism connecting the redox status of the Fe-S cluster with the AirSR function in gene regulation has yet to be defined.
AirSR was formerly known as YhcSR28 because of its homology to Bacillus subtilis TCS YhcYZ with unknown function. Since in this study, we identified that YhcSR is a global regulator that senses oxidative stress using an iron-sulfur cluster under oxygen-limited conditions, we renamed the TCS AirSR. Previously, AirSR was suggested as an essential TCS for S. aureus growth in vitro.38 When antisense airS RNA was overexpressed in a clinical isolate S. aureus WCUH29, no bacterial growth was observed.28 However, the existence of both ΔairS and ΔairR transposon mutants in the Phoenix library indicates that the AirSR system might be nonessential, at least in the strain Newman background (Figure S4).28 The microarray results (Tables S1 and S2) also imply that the transposon insertion disrupts the function of AirR. When we repeated the antisense RNA experiment with the strain Newman, no growth defects were observed (Figure S6). Our results suggest that AirSR is nonessential in the Newman strain background.
The N-terminus of AirS contains a GAF (cGMP-Adenylyl cyclase-FhlA) domain, which is reminiscent of the sensory domains of Mycobacterium tuberculosis TCSs DosS-R and DosT-R. Both TCSs are believed to be involved in gene regulation during the entry of Mtb into a dormant state, which is associated with O2-starvation and CO/NO exposure.39 The sensory domains of DosS and DosT consist of tandem GAF modules housing a pentacoordinate heme cofactor that is capable of binding various ligands such as O2, NO, and CO. Unlike DosS and DosT, AirS contains a sole GAF domain that binds a [2Fe-2S] cluster. Our current study with AirS is a rare demonstration of how a non-heme iron-bound GAF sensory domain acts as a redox sensor to participate in bacterial signal transduction.
The [4Fe-4S] cluster is a motif employed by the FNR type transcriptional regulators to sense oxygen in various bacteria.40,41 Along with AirS, E. coli SoxR (superoxide-responsive regulator) represents a transcriptional regulatory system that has a genuine [2Fe-2S]2+ as its functional motif.25,42 In order to confirm that [2Fe-2S] is a physiologically relevant form, we identified and used the endogenous S. aureus cysteine desulfurase IscS. We established that IscS is a pyridoxal 5′-phosphate-containing enzyme capable of assembling the AirS Fe-S cluster anaerobically. Subsequent EPR analysis of the reconstituted AirS established [2Fe-2S]+ as the genuine type of Fe-S cluster bound to AirS.
Aerobically purified AirS with an oxidized [2Fe-2S]2+ is EPR-silent but shows high kinase activity. The reduced form of AirS with an EPR visible [2Fe-2S]+ loses its kinase activity. The Fe-S cluster in AirS is also particularly sensitive to H2O2 and NO treatments; oxidation by these ROS/RNS species inactivates the kinase activity of AirS. Collectively, S. aureus AirSR represents the first TCS known to utilize [2Fe-2S] as a sensory motif to respond to oxygen and various ROSs/RNSs.
Given the redox sensing role of AirS [2Fe-2S], it is intriguing to ask whether oxidation of [2Fe-2S] by RNS and ROS can be reversed in order to return this TCS system to the pre-stimulus state. Indeed, in addition to its role in the de novo synthesis of Fe-S clusters, cysteine desulfurase, IscS, has also been shown to be able to repair both ROS- and RNS-modified Fe-S clusters.43 In particular, IscS can efficiently repair the NO-modified [2Fe-2S] cluster and the putative dinitrosyl iron-sulfur complex can be recycled for the reassembly of iron-sulfur clusters in proteins.44 Besides IscS, another study provided both in vitro and in vivo evidence that S. aureus ScdA, a di-iron protein that protects the bacterium from damage caused by oxidative stress, can also repair Fe-S clusters after damage by both H2O2 and NO.45 Together, we speculate that mild oxidative or nitrosative modification of AirS [2Fe-2S] can be reversible, which could be readily repaired by either S. aureus IscS or ScdA.
Previous studies with S. aureus have shown that the TCS NreABC (for anaerobic nitrogen regulation) contains a [4Fe-4S]2+ in the sensory PAS (Per-ARNT-Sim) domain of NreB.12 Transcriptome analysis revealed that mutation of nreABC in S. aureus SA113 only influences the expression of 37 genes that are mainly involved in nitrate/nitrite reduction and fermentation.12 Our transcriptome analysis showed that mutation of airR in S. aureus Newman grown under anaerobic conditions affects the expression of 67 genes in mid-exponential phase (Table S3), and 355 genes in stationary phase (Table S4). These genes regulate functions that are far more diverse, ranging from virulence, transcriptional regulation, stress response, protein synthesis, DNA replication, metabolism, cell wall synthesis, to a number of uncharacterized functions.
Many bactericidal antibiotics are known to stimulate the production of hydroxyl radicals in bacteria.8,36,37 According to our plate sensitivity assays, the airR mutant exhibits enhanced resistances toward H2O2, vancomycin, ciprofloxacin, and norfloxacin compared with the wild-type Newman. Given that AirS contains a redox-responsive Fe-S cluster, it is very likely that the reactive oxygen species generated from the antibiotic treatment are sensed by AirS through its [2Fe-2S] cluster. This mechanism has also been shown with other transcriptional regulators.8,9,12,37,46 Furthermore, microarray data revealed that AirSR modulates the expression of a number of oxidative stress-associated genes, suggesting a regulatory role of AirSR in S. aureus viability under oxidative stress.
CONCLUSIONS
Metal cofactors are typically used in oxygen and NO sensing in biology.47–52 Our study reveals S. aureus AirSR as a redox-responsive regulatory system that senses oxygen as well as diverse environmental/endogenous redox signals through its unique [2Fe-2S] cluster, and has a global impact on bacterial gene expression under oxygen-limited conditions. The [2Fe-2S] cluster is redox active and critical for its kinase activity. The oxidized AirS with [2Fe-2S]2+ is fully active, while the reduced AirS shows minimal kinase activity. A brief oxidation of AirS with H2O2 or NO destroys the Fe-S cluster and abolishes the kinase activity. Transcriptome analysis revealed that AirSR impacts the expression of ~355 genes under anaerobic conditions. Moreover, the mutant strain with inactivated AirSR displayed increased resistances toward H2O2, vancomycin, norfloxacin, and ciprofloxacin under anaerobic conditions. The intricate correlation between the oxidation sensing of AirS through the Fe-S cluster and the function of AirSR in gene regulation remains an object of further study.
Supplementary Material
Acknowledgments
We thank Drs. O. Schneewind and D. Missiakas at the University of Chicago for providing transposon mutants. We also thank S. F. Reichard, M.A. for editing the manuscript. This work was financially supported by NIH NIAID AI074658 from the National Institute of Allergy and Infectious Diseases (to C.H.), a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease Award (to C.H.), NIH NIGMS P50GM081892, a Scientist Development Grant 0835158N from the American Heart Association (to T.B.), AI077564 from the National Institute of Allergy and Infectious Diseases (to T.B). F.S. is a Scholar of the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust.
Footnotes
Supporting Information. Supporting results (Figures S1- S6), bacterial strains and plasmids (Table S1), primers (Table S2), and microarray results (Tables S3 and S4). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lowy FD. N Engl J Med. 1998;339:520. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Novick RP. Mol Microbiol. 2003;48:1429. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 3.Stauff DL, Skaar EP. Contrib Microbiol. 2009;16:120. doi: 10.1159/000219376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung AL, Nishina KA, Trotonda MP, Tamber S. Int J Biochem Cell Biol. 2008;40:355. doi: 10.1016/j.biocel.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR, Whitney AR, Otto M, DeLeo FR. J Infect Dis. 2009;199:1698. doi: 10.1086/598967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson BC, Chang CJ. Nat Chem Biol. 2011;7:504. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson BC, Huynh C, Chang CJ. J Am Chem Soc. 2010;132:5906. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PR, Bae T, Williams WA, Duguid EM, Rice PA, Schneewind O, He C. Nat Chem Biol. 2006;2:591. doi: 10.1038/nchembio820. [DOI] [PubMed] [Google Scholar]
- 9.Chen PR, Nishida S, Poor CB, Cheng A, Bae T, Kuechenmeister L, Dunman PM, Missiakas D, He C. Mol Microbiol. 2009;71:198. doi: 10.1111/j.1365-2958.2008.06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballal A, Manna AC. J Bacteriol. 2010;192:336. doi: 10.1128/JB.01202-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarwood JM, McCormick JK, Schlievert PM. J Bacteriol. 2001;183:1113. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlag S, Fuchs S, Nerz C, Gaupp R, Engelmann S, Liebeke M, Lalk M, Hecker M, Gotz F. J Bacteriol. 2008;190:7847. doi: 10.1128/JB.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly MI, Zhou M, Millard CS, Clancy S, Stols L, Eschenfeldt WH, Collart FR, Joachimiak A. Protein Expr Purif. 2006;47:446. doi: 10.1016/j.pep.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werst MM, Davoust CE, Hoffman BM. J Am Chem Soc. 1991;113:1533. [Google Scholar]
- 15.Mailer C, Taylor CPS. Biochim Biophys Acta. 1973;322:195. doi: 10.1016/0005-2795(73)90293-6. [DOI] [PubMed] [Google Scholar]
- 16.Beinert H. FASEB Journal. 1990;4:2483. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- 17.Hagen WR, Albracht SPJ. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1982;702:61. doi: 10.1016/0167-4838(82)90027-9. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 19.Hakemian AS, Kondapalli KC, Telser J, Hoffman BM, Stemmler TL, Rosenzweig AC. Biochemistry. 2008;47:6793. doi: 10.1021/bi800598h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertrand P, Gayda JP, Fee JA, Kuila D, Cammack R. Biochim Biophys Acta. 1987;916:24. doi: 10.1016/0167-4838(87)90206-8. [DOI] [PubMed] [Google Scholar]
- 21.Meyer J, Clay MD, Johnson MK, Stubna A, Münck E, Higgins C, Wittung-Stafshede P. Biochemistry. 2002;41:3096. doi: 10.1021/bi015981m. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Lin G, Liu D, Dria KJ, Telser J, Li L. J Am Chem Soc. 2011;133:10434. doi: 10.1021/ja110196d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belford RL, Nilges MJ. EPR Symposium, 21st Rocky Mountain Conference; Denver, Colorado. August,1979. [Google Scholar]
- 24.Fish WW. Methods Enzymol. 1988;158:357. doi: 10.1016/0076-6879(88)58067-9. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe S, Kita A, Kobayashi K, Miki K. Proc Natl Acad Sci U S A. 2008;105:4121. doi: 10.1073/pnas.0709188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer J. FEBS Lett. 2001;509:1. doi: 10.1016/s0014-5793(01)03049-6. [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo E, Demple B. EMBO J. 1994;13:138. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagen WR, Albracht SP. Biochim Biophys Acta. 1982;702:61. doi: 10.1016/0167-4838(82)90027-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, White RH, Cash VL, Jack RF, Dean DR. Proc Natl Acad Sci U S A. 1993;90:2754. doi: 10.1073/pnas.90.7.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mihara H, Esaki N. Appl Microbiol Biotechnol. 2002;60:12. doi: 10.1007/s00253-002-1107-4. [DOI] [PubMed] [Google Scholar]
- 32.Green J, Paget MS. Nat Rev Microbiol. 2004;2:954. doi: 10.1038/nrmicro1022. [DOI] [PubMed] [Google Scholar]
- 33.Imlay JA. Annu Rev Biochem. 2008;77:755. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H, Demple B. Proc Natl Acad Sci U S A. 2000;97:5146. doi: 10.1073/pnas.97.10.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, Missiakas DM. Proc Natl Acad Sci U S A. 2004;101:12312. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. Cell. 2007;130:797. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Hu J, Chen PR, Lan L, Li Z, Hicks LM, Dinner AR, He C. Proc Natl Acad Sci U S A. 2008;105:13586. doi: 10.1073/pnas.0803391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J, Zheng L, Landwehr C, Yang J, Ji Y. J Bacteriol. 2005;187:7876. doi: 10.1128/JB.187.22.7876-7880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green J, Crack JC, Thomson AJ, LeBrun NE. Curr Opin Microbiol. 2009;12:145. doi: 10.1016/j.mib.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Kiley PJ, Beinert H. Curr Opin Microbiol. 2003;6:181. doi: 10.1016/s1369-5274(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 41.Kiley PJ, Beinert H. FEMS Microbiol Rev. 1998;22:341. doi: 10.1111/j.1574-6976.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 42.Pomposiello PJ, Demple B. Trends Biotechnol. 2001;19:109. doi: 10.1016/s0167-7799(00)01542-0. [DOI] [PubMed] [Google Scholar]
- 43.Djaman O, Outten FW, Imlay JA. J Biol Chem. 2004;279:44590. doi: 10.1074/jbc.M406487200. [DOI] [PubMed] [Google Scholar]
- 44.Yang W, Rogers PA, Ding H. J Biol Chem. 2002;277:12868. doi: 10.1074/jbc.M109485200. [DOI] [PubMed] [Google Scholar]
- 45.Overton TW, Justino MC, Li Y, Baptista JM, Melo AM, Cole JA, Saraiva LM. J Bacteriol. 2008;190:2004. doi: 10.1128/JB.01733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lan L, Murray TS, Kazmierczak BI, He C. Mol Microbiol. 2010;75:76. doi: 10.1111/j.1365-2958.2009.06955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischhacker AS, Kiley PJ. Curr Opin Chem Biol. 2011;15:335. doi: 10.1016/j.cbpa.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong W, Hao B, Mansy SS, Gonzalez G, Gilles-Gonzalez MA, Chan MK. Proc Natl Acad Sci U S A. 1998;95:15177. doi: 10.1073/pnas.95.26.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong W, Hao B, Chan MK. Biochemistry. 2000;39:3955. doi: 10.1021/bi992346w. [DOI] [PubMed] [Google Scholar]
- 50.Marvin KA, Reinking JL, Lee AJ, Pardee K, Krause HM, Burstyn JN. Biochemistry. 2009;48:7056. doi: 10.1021/bi900697c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Dufour YS, Carlson HK, Donohue TJ, Marletta MA, Ruby EG. Proc Natl Acad Sci U S A. 2010;107:8375. doi: 10.1073/pnas.1003571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erbil WK, Price MS, Wemmer DE, Marletta MA. Proc Natl Acad Sci U S A. 2009;106:19753. doi: 10.1073/pnas.0911645106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.