Abstract
Stomata are formed by pairs of surrounding guard cells and perform important roles in photosynthesis, transpiration and innate immunity of terrestrial plants. Ionic solutes in the cytosol of guard cells are important for cell turgor and volume change. Consequently, trans-membrane flux of ions such as K+, Cl−, and malate2− through K+ channels and anion channels of guard cells are a direct driving force for turgor change, while the opening of calcium permeable channels can serve as a trigger of cytosolic free calcium concentration elevations or oscillations, which play second messenger roles. In plants, heterotrimeric G proteins have fewer members than in animals, but they are well investigated and found to regulate these channels and to play fundamental roles in guard cell function. This mini-review focuses on the recent understanding of G-protein regulation of ion channels on the plasma membrane of guard cells and their participation in stomatal movements.
Key words: guard cell, heterotrimeric G protein, ion channel, arabidopsis thaliana, stomata, plasma membrane, patch clamp
Heterotrimeric G proteins, composed of Gα, Gβ and Gγ subunits, are key elements of cellular signal transduction networks. In plant species, fewer members of G proteins are present than in animals. For example, only one Gα subunit (GPA1), one Gβ subunit (AGB1) and two Gγ subunits (AGG1 and AGG2) are reported in Arabidopsis while 23 Gα, 5 Gβ and 12 Gγ subunits have been identified in human.1 All three kinds of subunits are expressed in guard cells. Ubiquitous expression of GPA1 throughout plant was ascertained by northern and promoter::GUS analyses and RT-PCR results also indicate guard cell expression.2–4 AGB1 is ubiquitously expressed throughout the plant and its promoter::GUS transgenic lines show strong expression in guard cells.5–7 For Gγ subunits, RNA blots show AGG1 and AGG2 expression throughout the plant, however, reporter gene analysis shows guard cell expression of AGG2 but not AGG1.7–9 The guard cell expression of G protein subunits implies the function of G protein in guard cell signaling and stomatal movement regulation.
Stomata are microscopic pores in the epidermis of terrestrial plants, which serve as the mouths of plants for gas change since through them CO2 enters leaves for photosynthesis and water vapor is lost as transpiration.10–13 In addition, stomatal movements induced by pathogen and pathogen/microbe-associated molecular patterns (PAMPs or MAMPs) are a component of the plant innate immunity system.14–16 Biotic and abiotic stresses (e.g. water deficiency, cold, pathogens) and their induced phytohormone changes (e.g. abscisic acid [ABA], ethylene) have been widely investigated in stomatal movement regulation, and stomatal apertures are directly regulated by volume change of the surrounding guard cell pairs. The accumulation/release of ionic solutes through ion channels on the guard-cell plasma membrane together with malate production/metabolism induces water influx/efflux driving increase/decrease of cell turgor and volume which co-operates with the radial reinforcement of the guard cell walls to widen/shrink stomatal aperture.10,17 Given that mature guard cells lack plasmodesmata with neighboring cells, all ion uptake and efflux must pass through ion channels and ion transporters on the plasma membrane.
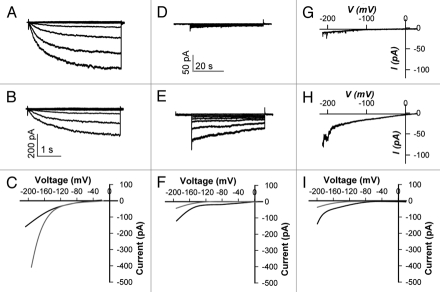
In Arabidopsis guard cells, the model cell type for cell signaling of the model plant species, all three kinds of ion channels (K+ channels, anion channels and Ca2+-permeable channels) have been investigated and found to be regulated by heterotrimeric G proteins.10,17 Their ion channel activities can be measured in intact guard cells, guard cell protoplasts, or cell membrane patches using the patch clamp technique.15,18,19 Patch clamping can be used to measure ion fluxes in whole cells or even through a single ion channel.20,21 The patch clamp technique under the whole-cell recording configuration can measure the currents through hyperpolarization-activated inward K+ channels which account for K+ accumulation during stomatal opening, and the depolarizationactivated outward K+ channels which, together with R-type and S-type anion channels, mediate solute removal during stomatal closure. Besides these ionic fluxes which directly elicit changes in turgor, Ca2+-permeable channels which participate in Ca2+ signaling are also regulated by G proteins. For better visualization of the currents through K+, anion and Ca2+permeable channels, real current traces and their idealized current/voltage relationships are indicated in Figure 1. The G-protein regulation of inward and outward K+ channels, S-type anion channels, and Ca2+-permeable channels and their significance for stomatal movements will be discussed below, and the genes encoding them which have been explored up to now also will be discussed.
Figure 1.
Current traces and idealized current/voltage relationships of wild type guard cell plasma membrane ion channels involved in G-protein regulation (A–C), ABA inhibition of whole-cell inward K+ currents. (A) indicates inward K+ currents of wild type guard cell protoplasts in response to hyperpolarizing voltages under control conditions [Scale bar is shown in (B)]; (B) indicates inward K+ currents of wild type guard cell protoplasts with ABA treatment; (C) indicates the idealized current/voltage relationship of inward K+ currents for control (gray) and ABA treatments (black). (D–F), ABA activation of slow anion currents. (D) indicates anion currents of wild type under control condition and (E) shows current after ABA treatment; (F) indicates the idealized current/voltage relationship of anion currents for control (gray) and ABA treatments (black). (G–I), ABA activation of currents through Ca2+-permeable channels. (G) indicates currents through Ca2+-permeable channels of wild type under control condition and (H) shows current after ABA treatments; (I) indicates the idealized current/voltage relationship of currents through Ca2+-permeable channels for control (gray) and ABA treatments (black).
G-protein Regulation of Guard Cell Ion Channels During Stomatal Movements
Evidence suggesting that G proteins regulate stomatal movements and ion channel activities was first obtained in the early 1990s using electrophysiological and pharamacological methods applied mainly to guard cells of the broad bean, Vica faba. With the sequencing of the Arabidopsis genome and identification of G protein encoding genes, Arabidopsis quickly became the model system for the study of G protein function in plants. The acquisition and characterization of mutants lacking functional heterotrimeric G proteins facilitated direct examination of the roles of heterotrimeric G proteins in the regulation of ion channels and stomatal movements.
ABA is the best studied regulator of stomatal movements. ABA inhibits stomatal opening and promotes stomatal closure, reducing transpirational water loss; in addition, guard cell ABA signaling is one of the best-defined cellular signaling networks in plants.13,22,23 Most of the roles of G proteins in regulation of ion channels during stomatal opening are associated with ABA signaling. Electrophysiological experiments using G protein modulators, phytohormones and G protein mutants have greatly contributed to our understanding of the G-protein signaling network of stomatal movements.
G-protein regulation of K+ channels.
Guard cell plasma membrane K+ channels mediate K+ uptake/release and thus control changes in guard cell turgor change.24,25 Since G proteins can be constitutively activated by GTPγS or cholera toxin, and inactivated by binding to GDPβS or pertussis toxin, a combination of pharmacological and electrophysiological methods was once widely used to study G-protein regulation of guard cell ion channels and stomatal movements. G protein regulation of ion channels was first demonstrated in Vicia faba guard cells, in which the inward-rectifying K+ channels were found to be activated by GDPβS and inhibited by GTPγS, cholera and pertussis toxins.26 That finding was supported by further single-channel recordings from isolated membrane patches, showing the G-protein regulation of ion channels can occur via a membrane delimited mechanism.27 Besides electrophysiology, light-induced stomatal opening could be promoted by microinjection of GTPγS into guard cells.28 These early studies strongly suggested the involvement of heterotrimeric G proteins in the regulation of ion channels and stomatal movements.
Arabidopsis genes encoding Gα and Gβ subunits of the G protein were identified even before the Arabidiopsis genome was completely sequenced.5,29 Completion of whole genome sequencing, together with the development of guard cell protoplast isolation and patch clamping techniques for Arabidopsis, resulted in this species becoming the model plant system for study of G protein regulation of ion channels at molecular level. T-DNA insertional mutants lacking functional genes for heterotrimeric G proteins could be used directly for the study of the involvement of heterotrimeric G proteins in regulation of guard cell ion channels and G protein effects on stomatal movements.1 In guard cells of null mutants of the sole Gα gene, GPA1, ABA inhibition of inward K+ channels was abolished, consistent with the observation that light-induced stomatal opening is hyposensitive to inhibition by ABA in these gpa1 mutants.4 Interestingly, consistent ABA hyposensitive phenotypes were observed in agb1, and gpa1abg1 mutants: without ABA, there is no alteration in K+ currents and with ABA, inhibition of inward K+ currents and light-induced stomatal opening is impaired.30 Similar experiments have also been conducted in mutants of the two identified Gγ genes, agg1 and agg2,8,9 unexpectedly, neither single mutants nor double mutants of these two Gγ subunits showed similar phenotypes to that of gpa1 or agb1; rather, these mutants showed wild-type ABA inhibition of inward K+ channels and stomatal opening.31 So, it is reasonable to speculate that there exist unidentified, additional Arabidopsis Gγ(s) which work(s) in guard cells together with GPA1 and AGB1. Besides the phytohormone ABA, loss of function of GPA1 also blocks flg22 inhibition of inward K+ channels and stomatal opening, indicating that plant G proteins are common elements for crosstalk of ABA and elicitors in guard cells.15
To date, nine genes encoding K+-channels have been identified in Arabidopsis: KAT1, KAT2, AKT1, AKT5, SPIK, AKT2/3, AtKC1, SKOR and GORK.10,32,33 In guard cells, the inward K+ channel members or subunits KAT1, KAT2, AKT1, AKT2/3, AtKC1, and the outward K+ channel, GORK, are expressed.32–34 Evidence accumulating from heterologous expression and functional analysis of heteromeric inward K+ subunits (e.g. AtKC1 and AKT1) indicates that functional inward K+ channels are heteromers,35 and since ABA could not totally inhibit the inward K+ currents, further work is needed to clarify which kind of specific inward K+ channels are regulated by G proteins. Furthermore, even though there is no significant difference before and after ABA application in outward K+ currents of guard cells in wild type and the mutants of G-protein subunits,4,30,31 a gradual inhibitory effect of flg22 on outward K+ channels observed in wild type guard cells was absent from gpa1 mutants, indicating the involvement of G-protein in regulation of outward K+ channels and thereby stomatal closing.15
G-protein regulation of anion channels.
Anion channels on the plasma membrane of guard cells which mediate anion efflux during stomatal closure are categorized as R-type (rapid) and S-type (slow) according to their electrophysiological characteristics.11 Since the S-type channels could be activated by a large range of voltages and exhibit slow deactivation allowing export of a large amount of anions, they are a major component of the membrane depolarization mechanisms that drive stomatal closure (Fig. 1).36 ABA activates both S-type and R-type anion channels;36 G-proteins involvement in ABA regulation of S-type channels has been observed, while R-type channels have not yet been assessed for such regulation.4,30 gpa1 and agb1 mutants show reduced ABA activation of outward anion channels under strong cytosolic pH buffer, however, under weak pH buffering, ABA activation of outward anion channels is identical in wild type and in mutants of gpa1 or agb1.4,30 These results demonstrate the existence of two parallel pathways that mediate ABA activation of S-type anion channels: one through cytosolic pH and another dependent on G proteins. The genes encoding anion channels have been identified recently; SLAC1 encodes S-type anion channels and R-type channels are likely encoded by AtALMT12, but other reports showed the existence of R-type anion currents in atalmt12, so its function needs further confirmation.37–39 GPA1 and AGB1 mediate ABA activation of S-type anion channels implies indirect or direct regulation of SLAC1 by G proteins.
G-protein regulation of Ca2+-permeable channels.
Hyperpolarizationactivated Ca2+-permeable channels on the plasma membrane of guard cells trigger Ca2+cyt elevation, and such elevation inhibits inward K+ channels and activates both S-type and R-type anion channels, facilitating net solute removal during stomatal closure.12,40–42 ABA promotes the production of reactive oxygen species (ROS) such as H2O2 which activate plasma membrane Ca2+ channels resulting in an increase in Ca2+cyt levels and stomatal closure.42 Since ABA promotes Ca2+cyt elevation and Ca2+cyt elevation in turn activates S-type anion channels,4,12,30 it is reasonable to hypothesize that G proteins are involved in ABA regulation of Ca2+-permeable channels and Ca2+cyt generation. This hypothesis was directly supported by recent electrophysiology experiments.43 In that study, ABA activation of plasma membrane Ca2+-permeable channels and ROS elevation in guard cells was minimal in gpa1 mutants, while H2O2 activation of these channels and of ROS elevation could be observed in guard cells of both wild type and gpa1. These data suggest that G proteins function upstream of ROS production but downstream of ABA perception in guard cells.43 To date, the specific genes encoding guard-cell voltage-dependent Ca2+-channels have not been identified. Three gene families, comprising 41 genes total have been proposed as the best candidates: glutamate receptor family (20 members), cyclic nucleotide gated channel family (CNGCs, 20 members) and the two pore calcium channel, TPC1.32 TPC1 was identified as vacuolar Ca2+ activated channel, but its function still needs further confirmation.44 Ca2+ currents could be activated by cAMP, and heterologous expression and functional analyses of CNGC2 showing CNGC members mediate Ca2+ influx in response to Ca2+ needed stresses, but Ca2+ current could also be seen without cAMP indicating the existence of other voltage-dependent Ca2+-permeable channels.45–49 The uncertainty of molecular identification of the Ca2+ channels that mediate ABA activation of guard cell Ca2+ currents make it impossible to address which gene(s) encode(s) the Ca2+ channels targeted by G-proteins.
Conclusion and Perspectives
To summarize, with the aid of the patch clamp technique and G protein subunit mutants, accumulating evidence firmly supports the G protein regulation of K+, anion and Ca2+ channels. With the collective assistance of ion channel cloning and in vivo functional analyses, soon we may know the specific molecular mechanisms and channel(s) targeted by the G protein.
Acknowledgments
The author thanks Sarah M. Assmann for critically reading of the manuscript, and research in the author's laboratory is supported by Qilu Scholar Program and Independent Innovation Foundation from Shandong University.
References
- 1.Assmann SM. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002;14:S355–S373. doi: 10.1105/tpc.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss CA, Huang H, Ma H. Immunolocalization of the G protein alpha subunit encoded by the GPA1 gene in Arabidopsis. Plant Cell. 1993;5:1513–1528. doi: 10.1105/tpc.5.11.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Weiss CA, Ma H. Regulated expression of the Arabidopsis G protein alpha subunit gene GPA1. Int J Plant Sci. 1994;155:3–14. [Google Scholar]
- 4.Wang XQ, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- 5.Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H. Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1) Proc Natl Acad Sci USA. 1994;91:9554–9558. doi: 10.1073/pnas.91.20.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson DJ, Botella JR. Expression analysis and subcellular localization of the Arabidopsis thaliana G-protein beta-subunit AGB1. Plant Cell Rep. 2007;26:1469–1480. doi: 10.1007/s00299-007-0356-1. [DOI] [PubMed] [Google Scholar]
- 7.Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, et al. Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell. 2007;19:1235–1250. doi: 10.1105/tpc.107.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason MG, Botella JR. Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim Biophys Acta. 2001;1520:147–153. doi: 10.1016/s0167-4781(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 9.Mason MG, Botella JR. Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc Natl Acad Sci USA. 2000;97:14784–14788. doi: 10.1073/pnas.97.26.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 12.Kim T-H, Bohmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya B, Assmann S. Hormone interactions in stomatal function. Plant Mol Biol. 2009;69:451–462. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- 14.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, He SY, Assmann SM. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008;56:984–996. doi: 10.1111/j.1365-313X.2008.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng W, Melotto M, He SY. Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr Opin Biotechnol. 2010;21:599–603. doi: 10.1016/j.copbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilson SE, Assmann SM. The α-subunit of the Arabidopsis heterotrimeric G protein, GPA1, is a regulator of transpiration efficiency. Plant Physiol. 2010;152:2067–2077. doi: 10.1104/pp.109.148262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forestier C, Bouteau F, Leonhardt N, Vavasseur A. Pharmacological properties of slow anion currents in intact guard cells of Arabidopsis. Application of the discontinuous single-electrode voltage-clamp to different species. Pflügers Arch. 1998;436:920–927. doi: 10.1007/s004240050724. [DOI] [PubMed] [Google Scholar]
- 20.Neher E, Sakmann B, Steinbach JH. The extracellular patch clamp: A method for resolving currents through individual open channels in biological membranes. Pflügers Arch. 1978;375:219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- 21.Kornreich BG. The patch clamp technique: Principles and technical considerations. J Vet Cardiol. 2007;9:25–37. doi: 10.1016/j.jvc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Assmann SM, Albert R. Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol. 2006;4:e312. doi: 10.1371/journal.pbio.0040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebaudy A, Very A, Sentenac H. K+ channel activity in plants: genes, regulations and functions. FEBS Lett. 2007;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Cherel I. Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot. 2004;55:337–351. doi: 10.1093/jxb/erh028. [DOI] [PubMed] [Google Scholar]
- 26.Fairley-Grenot K, Assmann SM. Evidence for G-protein regulation of inward K+ channel current in guard cells of fava bean. Plant Cell. 1991;3:1037–1044. doi: 10.1105/tpc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu WH, Assmann SM. A membrane-delimited pathway of G-protein regulation of the guard-cell inward K+ channel. Proc Natl Acad Sci USA. 1994;91:6310–6314. doi: 10.1073/pnas.91.14.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Tucker EB, Crain RC, Lee Y. Stomatal opening is induced in epidermal peels of Commelina communis L. by GTP analogs or pertussis toxin. Plant Physiol. 1993;102:95–100. doi: 10.1104/pp.102.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Yanofsky MF, Meyerowitz EM. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA. 1990;87:3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan LM, Zhang W, Chen JG, Taylor JP, Jones AM, Assmann SM. Abscisic acid regulation of guard-cell K+ and anion channels in Gβ-and RGS-deficient Arabidopsis lines. Proc Natl Acad Sci USA. 2008;105:8476–8481. doi: 10.1073/pnas.0800980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trusov Y, Zhang W, Assmann SM, Botella JR. Gγ1 + Gγ2 not equal to Gβ: heterotrimeric G protein Gγ-deficient mutants do not recapitulate all phenotypes of Gβ-deficient mutants. Plant Physiol. 2008;147:636–649. doi: 10.1104/pp.108.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Very AA, Sentenac H. Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 2002;7:168–175. doi: 10.1016/s1360-1385(02)02262-8. [DOI] [PubMed] [Google Scholar]
- 33.Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H. Five-group distribution of the shaker-like K+ channel family in higher plants. J Mol Evol. 2003;56:418–434. doi: 10.1007/s00239-002-2413-2. [DOI] [PubMed] [Google Scholar]
- 34.Ward JM, Mäser P, Schroeder JI. Plant ion channels: Gene families, physiology, and functional genomics analyses. Annu Rev Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, et al. Heteromeric AtKC1·AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem. 2009;284:21288–21295. doi: 10.1074/jbc.M109.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roelfsema MR, Levchenko V, Hedrich R. ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J. 2004;37:578–588. doi: 10.1111/j.1365-313x.2003.01985.x. [DOI] [PubMed] [Google Scholar]
- 37.Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signaling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, et al. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010;63:1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, et al. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 2010;51:354–365. doi: 10.1093/pcp/pcq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton DW, Hills A, Kohler B, Blatt MR. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetherington AM, Brownlee C. The generation of Ca2+ signals in plants. Annu Rev Plant Biol. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- 42.Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Jeon BW, Assmann SM. Heterotrimeric G-protein regulation of ROS signaling and calcium currents in Arabidopsis guard cells. J Exp Bot. 2011;62:2371–2379. doi: 10.1093/jxb/erq424. [DOI] [PubMed] [Google Scholar]
- 44.Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 45.Ranf S, Wunnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, et al. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 2008;53:287–299. doi: 10.1111/j.1365-313X.2007.03342.x. [DOI] [PubMed] [Google Scholar]
- 46.Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, et al. Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan CW, Schorrak LM, Smith RK, Jr, Bent AF, Sussman MR. A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol. 2003;132:728–731. doi: 10.1104/pp.102.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng Q, Mercier RW, Yao W, Berkowitz GA. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemtiri-Chlieh F, Berkowitz GA. Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J Biol Chem. 2004;279:35306–35312. doi: 10.1074/jbc.M400311200. [DOI] [PubMed] [Google Scholar]