Abstract
Salmonella enterica is a major cause of morbidity worldwide and mortality in children and immunocompromised individuals in sub-Saharan Africa. Outer membrane proteins of Salmonella are of significance because they are at the interface between the pathogen and the host, they can contribute to adherence, colonization, and virulence, and they are frequently targets of antibody-mediated immunity. In this study, the properties of SadA, a purported trimeric autotransporter adhesin of Salmonella enterica serovar Typhimurium, were examined. We demonstrated that SadA is exposed on the Salmonella cell surface in vitro and in vivo during infection of mice. Expression of SadA resulted in cell aggregation, biofilm formation, and increased adhesion to human intestinal Caco-2 epithelial cells. Immunization of mice with folded, full-length, purified SadA elicited an IgG response which provided limited protection against bacterial challenge. When anti-SadA IgG titers were enhanced by administering alum-precipitated protein, a modest additional protection was afforded. Therefore, despite SadA having pleiotropic functions, it is not a dominant, protective antigen for antibody-mediated protection against Salmonella.
INTRODUCTION
Nontyphoidal strains of Salmonella (NTS) such as Salmonella enterica serovar Typhimurium are a major cause of human gastrointestinal infections and invasive disease. While NTS cause almost 100 million cases of gastroenteritis globally every year (54), invasive disease is a huge, yet underrecognized problem in regions such as sub-Saharan Africa, where it typically manifests as bacteremia and meningitis in young children (30) and HIV-infected individuals (28). Invasive disease is associated with high case-fatality rates and often occurs in the complete absence of gastrointestinal symptoms. Evidence points to a lack of specific antibody as a main contributing factor to severe infection and bacteremia (27, 53), making vaccine development a promising preventive option. Furthermore, antibiotic resistance in these bacteria is an increasing problem (29), and development of new intervention and preventative strategies is urgently required. Outer membrane proteins are part of the interface between pathogen and host, are targets of antibody, and are therefore potential candidates for vaccine development (14, 52). An improved knowledge of the surface-expressed virulence factors of S. Typhimurium is essential to understand bacterial virulence and to identify novel vaccine targets on the bacterium.
The S. Typhimurium protein SadA belongs to the family of trimeric autotransporter adhesins (TAAs), a subset of type V secreted proteins that form a stable trimer on the bacterial cell surface (12, 32, 38). TAAs, like all type V secreted proteins, contain an N-terminal sec-dependent signal sequence that allows the protein to cross the inner membrane, an internal passenger domain, and a C-terminal translocation domain (18, 34, 36, 37). TAAs are distinguished from conventional autotransporters (ATs) by having a small 70- to 100-amino-acid (aa) translocation domain, composed of four β-strands, which forms a homotrimer resulting in a full-sized β-barrel pore in the outer membrane (71). This pore is essential for transport of the passenger domain to the cell surface (61, 71). The β-barrel is inserted into the outer membrane via the β-barrel assembly machinery (BAM) complex, and thereafter, the passenger domain is secreted to the cell surface (44, 48).
The passenger domains of TAAs share common features. Structural determinations of TAA proteins such as YadA, Hia, and BadA have revealed three distinct regions within the passenger domain: an N-terminal head, a neck, and a stalk (56, 72, 81). The stalk is an α-helical region which spans the pore formed by the translocation unit in a manner analogous to the classical ATs. The neck region connects the stalk to the head domain. The YadA head structure contains single-stranded, left-handed helices, with the interface between them being formed by periodically occurring, conserved sequence motifs (56). These motifs can be found in the predicted head structures of many TAAs, and variations in this region are largely responsible for the functional variation observed for these proteins (56, 81).
Where characterized, TAAs are uniformly associated with adhesion (50), but they may also have other virulence properties. The exemplar TAA is YadA from Yersinia enterocolitica, which mediates adhesion to epithelial cells and extracellular matrix (ECM) molecules and is also crucial for intestinal colonization and serum resistance (3, 22, 73). Other characterized trimeric autotransporters include NadA and NhhA from Neisseria meningitidis, which facilitate binding and invasion of epithelial cells (11, 62); BadA from Bartonella henselae, which plays a role in binding to ECM proteins (40, 60); Hia and Hsf from Haemophilus influenzae, which enable high-affinity binding to respiratory epithelial cells (13, 46); and UspA1 and UspA2 from Moraxella catarrhalis, which mediate binding to human epithelial cells (47). Recently, a trimeric autotransporter from uropathogenic Escherichia coli, UpaG, which is required for binding to bladder epithelial cells and the ECM proteins fibronectin and laminin, has been described (10, 75). It also promotes autoaggregation and biofilm formation (75). SadA has not been established as playing a role in S. Typhimurium infection. We investigated the cellular localization of SadA, its functions, and the role that antibody to SadA may play in controlling S. Typhimurium infection.
MATERIALS AND METHODS
Cells and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All bacterial strains were routinely cultured in Luria-Bertani (LB) broth or M63 minimal medium (58) with shaking or on agar at 37°C supplemented with kanamycin (50 μg/ml), ampicillin (100 μg/ml), and/or 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) where appropriate. The semiadherent macrophage-like cell line J774 and the colorectal carcinoma cell line Caco-2 were cultured in Dulbecco's modified Eagle's medium (DMEM), as previously described (51, 69).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristic | Reference or source |
|---|---|---|
| Strains | ||
| S. enterica | ||
| SL1344 | Wild-type Salmonella enterica serovar Typhimurium | 80 |
| SL1344 sadA::aph | Part of sadA gene replaced with kanamycin resistance cassette | This study |
| SL1344 ΔsadA | SL1344 sadA::aph with aph cassette resolved | This study |
| SL3261 | Attenuated Salmonella enterica serovar Typhimurium SL1344 ΔaroA | 39 |
| SL3261 sadA::aph | Part of sadA gene replaced with kanamycin resistance cassette | This study |
| SL3261 ΔsadA | SL3261 sadA::aph with aph cassette resolved | This study |
| SL3261 galE::aph | The galE gene replaced with kanamycin resistance cassette | This study |
| SL3261 ΔsadAgalE::aph | The galE gene replaced with kanamycin resistance cassette | This study |
| E. coli | ||
| M15(pREP4) | For regulated high-level expression with pQE vectors | Qiagen |
| HB101 | Nonaggregating E. coli laboratory strain | 9 |
| BL21 | E. coli DE3 protein expression strain | Invitrogen |
| Plasmids | ||
| pET22b | IPTG-inducible expression vector | Invitrogen |
| pDR01 | sadA gene in the NdeI-EcoRI sites of pET22b | This study |
| pQE60 | IPTG-inducible expression vector | Qiagen |
| pQE60 NdeI | pQE60 with added NdeI restriction site in the multiple-cloning site and previously existing NdeI site removed | This study |
| pDR03 | pQE60NdeI carrying sadA gene in the NdeI-HindIII site | This study |
Molecular biology techniques and strain construction.
Plasmid DNA isolation, genomic DNA isolation, PCR product purification, and agarose gel extractions were performed with the relevant kits from Qiagen according to the manufacturer's instructions. All restriction enzymes (New England BioLabs) and T4 DNA ligases (Promega) were used in accordance with the manufacturer's specifications. PCR experiments were performed using Phusion high-fidelity DNA polymerase (F530-L; New England BioLabs) or 1× ReddyMix PCR master mix (Ab-0575; Thermo Scientific). DNA sequencing was carried out at the Functional Genomics and Proteomics Laboratory of the University of Birmingham. To obtain RNA, either bacterial cultures were harvested in the presence of Qiagen RNAprotect bacterial reagent or spleen sections were homogenized using a Qiagen QIAshredder. Subsequently, RNA was isolated using Qiagen's RNeasy minikit and RNase-Free DNase set in accordance with the manufacturer's instructions. RNA was quantified using NanoDrop prior to cDNA synthesis using SuperScript II reverse transcriptase and random hexamers (Invitrogen) and prior to reverse transcriptase (RT)-PCR using Taq polymerase and primers D3 and RT1. Primers used in the study are listed in Table S1 in the supplemental material.
The sadA gene was amplified by PCR from S. enterica SL1344 using primers D5 and D6 (see Table S1 in the supplemental material). The PCR product containing the full-length sadA gene was digested with NdeI and EcoRI and ligated to the pET22b (Novagen) plasmid digested with the same enzymes to generate pDR01. The pDR03 plasmid contained the full-length sadA gene, amplified with primers D5 and D7 and cloned into a modified pQE60 vector (pQE60NdeI; see Table 1 for details) between the restriction sites NdeI and HindIII.
The sadA mutant strains were generated using the one-step gene inactivation method described for E. coli and S. Typhimurium (16, 21). Briefly, the kanamycin resistance cassette was amplified using primers D1 and D2, which possessed 40 bp that were identical to regions within sadA. The resulting DNA was transformed into S. enterica SL1344 carrying the pKD46 plasmid encoding the λ red recombinase. Disruption of sadA was confirmed by PCR using primers k1, k2, and kt (16) in combination with primers D3 (upstream check primer) and D4 (downstream check primer) and subsequent DNA sequencing. The mutation was then P22 transduced into S. enterica SL1344 and S. enterica SL3261, generating the strains S. enterica SL1344 sadA::aph and SL3261 sadA::aph, respectively. These latter strains were used to create S. enterica SL1344 ΔsadA and SL3261 ΔsadA by removing the kanamycin cassette as previously described (16).
Protein localization and Western blotting.
Cellular fractions were prepared as described previously (59). Cellular fractions and purified proteins were electrophoresed on 10% SDS-PAGE gels and stained with Coomassie blue or transferred to a polyvinylidene difluoride (PVDF) membrane for Western blotting as previously described (76). Western blotting was performed with a 1:1,000 dilution of the anti-SadA antibody and a 1:10,000 anti-rabbit dilution of IgG alkaline phosphatase before detection with NBP-BCIP (nitroblue tetrazolium chloride–5-bromo-4-chloro-3′-indolylphosphate) (Sigma) as the substrate. Proteins were localized by immunofluorescence as described previously (76). Briefly, 13-mm glass coverslips coated with 1 mg/ml poly l-lysine were overlaid with LB-cultured bacteria. Subsequently, coverslips were incubated with phosphate-buffered saline (PBS) containing 4% formalin, followed by a PBS-bovine serum albumin (PBS-BSA) blocking buffer. SadA was detected with anti-SadA antibody (1:100 in PBS-BSA) and, subsequently, goat anti-rabbit (GAR) Alexa Fluor 488 secondary antibody (1:100 in PBS-BSA) before visualization on a Leica DMRE fluorescence microscope-DC200 digital camera system. Captured images are representative of randomly selected fields of view.
Purification of full-length SadA protein.
E. coli BL21(pDR01) was grown with IPTG induction until an optical density at 600 nm (OD600) of 1 was reached. Cells were pelleted by centrifugation, washed, and resuspended in 50 mM HEPES, 150 mM sodium chloride, 1 mM phenylmethylsulfonyl fluoride (PMSF), pH 7.4. Cells were lysed by sonication, and the cell debris and inclusion bodies were pelleted by centrifugation at 20,000 × g. The pellet was resuspended in 50 mM HEPES, 150 mM NaCl, 8 M urea, 1 mM PMSF, pH 7.4, and incubated at room temperature for 1 h. Subsequently, insoluble material was removed by centrifugation at 50,000 × g, and the supernatant was recovered. This SadA-containing solution was run on SDS-PAGE gels, bands corresponding to SadA were excised, and gel slices were homogenized in 0.5 ml of 50 mM HEPES, 0.1% SDS, pH 7.4. SadA was electroeluted in a Novagen elution system per the manufacturer's instructions. The eluted protein was diluted to 10 ml in 50 mM HEPES, 0.1% SDS, pH 7.4, and then dialyzed three times against 5 liters of 50 mM HEPES, 150 mM NaCl, 0.5% Elugent detergent (Calbiochem). Refolding by dialysis was performed over 3 days. The concentration of the protein was estimated using a tannin assay. BSA made up in the final refolding buffer was used as the standard. Tryptophan fluorescence readings were taken at 280-nm excitation, with emission scanned over 300 to 450 nm, with a 0.5-mm slit width, an ∼1-nm bandwidth, a 1-s response time, and 1-nm increments using a QuantaMaster instrument (Photon Technologies International). These purified SadA protein preparations were tested with an endotoxin assay (Sigma E-Toxate kit) to measure levels of lipopolysaccharide (LPS). Antibodies against SadA were raised in New Zealand White rabbits using the purified refolded protein described above.
Autoaggregation and biofilm assays.
Autoaggregation was observed as described previously (77). The rate of autoaggregation was determined as the mean decrease in optical density over time. Biofilm formation on polystyrene surfaces was studied using 96-well microtiter plates as described previously (65). All the experiments were performed in triplicate on three separate days. Flow chamber experiments were performed as previously described (77), with the exception that the media also contained BacLight green bacterial stain (Invitrogen) to visualize the biofilms. Biofilm development was monitored at 18 h and 24 h after inoculation. All experiments were performed in triplicate.
Adherence and invasion/intracellular survival assays.
Tissue culture assays were performed as previously described (77). Briefly, overnight bacterial cultures were washed twice in 1× PBS (Invitrogen) and resuspended in serum-free culture medium (∼108 CFU/ml). Plates (24-well) were seeded with 105 J774 cells or 106 Caco-2 cells each well. For adherence assays, the washed cells were infected with 1 ml of bacterial cultures and incubated at 37°C in a 5% CO2 atmosphere for 2 h. Subsequently, the cells were washed several times with 1× Hanks balanced salt solution (HBSS) to remove the nonadherent bacteria prior to disruption with 1× Dulbecco's PBS (DPBS), 1% (vol/vol) Triton X-100 solution. Serial dilution of this solution onto LB agar plates supplemented with suitable antibiotics was used to enumerate CFU. To estimate the number of intracellular/invasive bacteria, the infected cells were washed several times with HBSS and then treated with 100 μg/ml gentamicin for 2 h at 37°C and 5% CO2 to kill the extracellular bacteria. The number of CFU was calculated as described above. The results were the means for three individual experiments conducted in triplicate on three separate days. Leaf infection and confocal fluorescence microscopy were performed as previously described (6). Briefly, supermarket salad leaves were affixed to a 30-mm petri dish base and immersed in 4 ml of the appropriate bacterial culture prior to incubation at 20°C for 1 h, with no agitation. Nonadherent bacteria were removed by washing, and the numbers of leaf-adherent bacteria were quantified by plating on agar plates. A two-tailed, paired Student t test was used to assess significance, using a P of <0.05 as a cutoff. ECM binding assays were performed as previously described (76), with all ECM molecules (collagen I, III, and IV, fibronectin, elastin, and laminin) purchased from Sigma.
Serum bactericidal assay.
The serum bactericidal assays were performed as described previously (53). Killing of S. enterica SL1344 and SL1344 ΔsadA and E. coli strains expressing SadA was investigated with normal serum obtained from healthy African and European individuals. Cultures of bacteria in the log growth phase were prepared in 1× PBS, and 10 μl of each sample was added to 90 μl of freshly thawed, undiluted serum to give a final bacterial concentration of 106 CFU/ml. The mixtures were incubated at 37°C with gentle rocking (20 rpm), and 10-μl samples were withdrawn at 45, 90, and 180 min. Serial dilutions of the samples were plated onto LB agar plates to determine the viable bacterial counts.
In vivo assessment of virulence and immune response.
Caenorhabditis elegans strain Bristol N2 was cultured using standard methods, and survival assays were performed as described previously (1, 2). The worms were grown on nematode growth medium (NGM) agar plates and fed with E. coli strain OP50. Larval stage 4 (L-4) C. elegans worms were picked and transferred to assay plates. The plates were incubated at 25°C and scored daily for survival. Death was defined as a failure to respond to mechanical stimulus. A minimum of 100 worms were used on three independent occasions. A Kaplan-Meier estimate was used to determine the probability of C. elegans survival to the next day. Survival curves were generated by plotting probability of survival against time and were then compared using the log rank test to establish whether the difference between two curves was statistically significant.
Mice were infected intraperitoneally (i.p.) with ∼5 × 105 CFU and sacrificed at the described times postinfection. For oral infection, mice were given 5 × 106 bacteria by oral gavage and sacrificed 4 or 6 days later. After the mice were sacrificed, their blood, spleens, and livers were harvested and the bacterial counts in their organs were measured by homogenizing the tissues and plating them onto LB agar as described previously (14). In some experiments, mice were immunized via the i.p. route with 10 μg purified SadA protein with or without alum precipitation as described previously (15). After immunization, for the prescribed times, mice were bled and subsequently challenged with ∼5 × 105 CFU S. enterica SL1344 i.p. and sacrificed 2 days later. Organs and blood were isolated at the time of sacrifice, and numbers of bacteria were enumerated as described above. Serum was isolated from blood by centrifugation and assessed by enzyme-linked immunosorbent assay (ELISA) for SadA-specific IgM and total IgG titers. MaxiSorp 96-well plates (Nunc) were coated with 5 μg/ml of purified SadA in carbonate coating buffer. After washing, plates were blocked for 1 h at 37°C with 1% BSA in PBS. Subsequently, serum samples were added in blocking buffer and serially diluted and incubation was continued for 1 h at 37°C. Secondary goat anti-mouse IgM or IgG conjugated to alkaline phosphatase (Southern Biotech) was diluted in blocking buffer and added for 1 h at 37°C. A signal was detected using the SIGMAFAST p-nitrophenyl phosphate system, and plates were read at 405 nm. In some cases, bacteria were administered after opsonization. Before being used for opsonization, all sera were heated to 56°C for 20 min to inactivate complement. The heat-inactivated sera and organisms were mixed (1:200) with gentle agitation for 30 min at room temperature, the bacterial concentration was adjusted to the concentration used for infection (∼5 × 105 CFU), and the bacterial suspension was administered via the i.p. route; bacteria were cultured to ensure no loss of viability.
RESULTS
Comparative sequence analysis of SadA positional orthologues.
Analysis of the S. enterica SL1344 genome identified sadA, a 4,383-bp gene that encodes a TAA protein. The 1,461-aa protein is a typical TAA, possessing all of the expected features of this family. Analysis of the amino acid sequence revealed a putative signal sequence possessing a conserved N-terminal extended signal peptide region (ESPR), which is common to many autotransporter proteins (4, 17, 19, 49), and is predicted to be cleaved after amino acid 50. The analysis also revealed a 1,333-aa passenger domain and a C-terminal translocation unit indicated by the Pfam:YadA (PF03895) domain. The translocator domain is only 78 aa, which is consistent with other TAAs that form a homotrimer to make a full-sized β-barrel. The passenger regions of TAAs have three conserved structural features: the head, neck, and stalk (45, 50). The head structure is composed of multiple Pfam:Hep_Hag (PF05658) motifs, while the neck consists of a single Pfam:HIM (PF05662) motif. The stalk is composed of multiple small repeats that are not conserved between TAAs and therefore does not have a Pfam identifier (50). SadA contains these motifs (Fig. 1), suggesting that it has an N-terminal head followed by a long region of alternating neck and stalk segments similar to other long TAAs, such as BadA from Bartonella henselae (72).
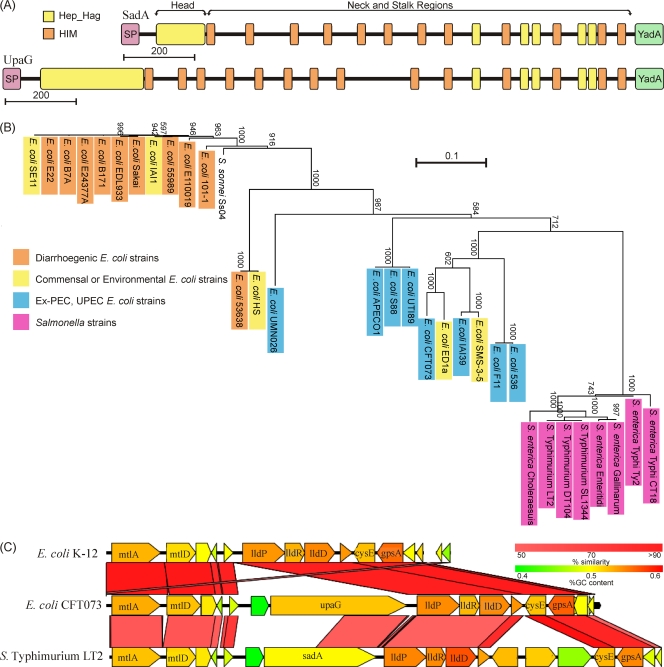
Fig. 1.
In silico analysis of SadA protein. (A) Schematic diagram comparing SadA and UpaG proteins. Structural domains are shown as colored boxes, and Pfam motifs are shown as follows: signal peptide (SP), pink; head, yellow; neck, orange; stalk, black; and translocation domain, green. (B) Unrooted phylogram of full-length SadA orthologues from 32 genomes. Genomes are color coded according to pathogenesis groups. Bootstrap values from 1,000 replicates are shown. (C) Genomic region containing sadA in S. Typhimurium LT2 and alignment to the orthologue's region in E. coli CFT073 and E. coli K-12.
To find orthologues of SadA, the full protein sequence was used to probe the available sequenced bacterial genomes using BLASTP. Sequences identified by this method were then screened to determine if they had the same domain organization as SadA, as well as sharing over 50% similarity. This analysis identified orthologues of SadA in E. coli (including Shigella sonnei), Citrobacter youngae, and S. enterica genomes. In each case, the sadA gene was found to be in the same genomic location adjacent to the lld locus, suggesting that sadA was acquired prior to the divergence of these species (Fig. 1C). A multiple alignment of the full-length proteins revealed that the proteins from Salmonella were very well conserved, sharing over 98% similarity with each other, whereas the proteins from E. coli were more variable (Fig. 1B). A phylogenetic tree was created from the multiple alignments identifying that the sequences grouped according to species and pathotypes (10). SadA orthologues from intestinal E. coli isolates were more conserved than proteins from extraintestinal, uropathogenic, and environmental E. coli strains (Fig. 1B).
One of the positional orthologues identified was the E. coli CFT073 UpaG (75). However, UpaG and SadA differ substantially in protein sequence. UpaG is 317 aa longer than SadA, due to extra amino acids in the N-terminal head, stalk, and neck domains of the protein. The head domain in particular differed in sequence from the analogous region in UpaG. Although the full-length protein sequences shared 56.8% similarity, the head domain shared only 30.4% similarity. In addition to this, the UpaG head sequence is over twice the length of the SadA head region (Fig. 1A).
SadA is surface expressed in S. enterica SL1344.
SadA was overexpressed in E. coli BL21 pDR01, purified, and refolded as described in Materials and Methods (Fig. 2A). The fluorescence spectrum of the refolded protein was typical of proteins containing multiple tryptophans, with a broad band at half height. The λmax was found at 336 nm, which indicates that the tryptophans are in a folded conformation (Fig. 2B). The refolded material was analyzed by mass spectrometry identification analysis and was identified as SadA, and analysis by silver staining confirmed that the sample was >99% pure and had no detectable LPS. An endotoxin detection assay also verified that the sample had no detectable LPS (<0.05 endotoxin units [EU] in 40 μg protein). This purified protein was used for infection studies (see below) and to raise polyclonal antibodies to SadA.
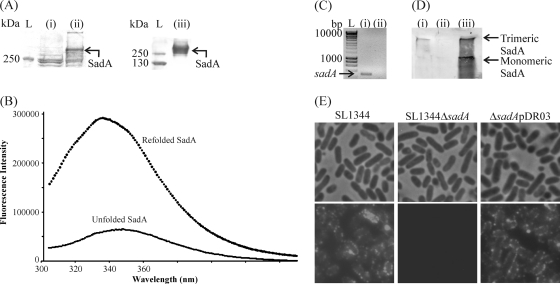
Fig. 2.
Purification, expression, and localization of SadA. (A) Stained SDS-PAGE gel of total cell protein extracts from E. coli BL21(pET22b) (i) and E. coli BL21(pDR01) (SadA+) (ii) and the protein sample after electroelution and purification (iii). L, PageRuler Plus protein ladder. (B) Tryptophan fluorescence emission of refolded SadA. An excitation wavelength of 280 nm was used, and emission was scanned from 300 to 450 nm with 1-nm increments. (C) RT-PCR gel using sadA specific primers of wild-type S. enterica SL1344 (i) and isogenic sadA mutant (ii) grown in LB at 37°C. L, HyperLadder I (Bioline). (D) Western immunoblot analysis using SadA-specific antibody from outer membrane preparations of wild-type S. enterica SL1344 (i), mutant S. enterica SL1344 ΔsadA (ii), and complemented S. enterica ΔsadA(pDR03) (iii). (E) Localization of SadA on the cell surface. S. enterica SL1344, S. enterica SL1344 ΔsadA, and S. enterica ΔsadA(pDR03) were probed with anti-SadA antibody followed by GAR Alexa Fluor 488. Phase-contrast and fluorescence micrographs demonstrate labeling of the wild-type and complemented strains but not the mutant strain. Two control experiments were performed in which SL1344 was treated with secondary antibody only or a different primary antibody followed by the secondary antibody to rule out binding to immunoglobulins. Neither control had any labeling (not shown). Scale bar, 0.5 μm.
As SadA belongs to the TAA family, it was expected to adopt a 426-kDa homotrimeric conformation associated with the outer membrane, with the passenger domain displayed on the cell surface. The protein expressed in E. coli, and the purified refolded form, are ca. 400 kDa, indicating that SadA adopts a native form when expressed in E. coli. To confirm that SadA was expressed and surface localized in S. enterica SL1344, a number of assays were undertaken. First, RT-PCRs were used to detect sadA expression in S. enterica SL1344 grown at 37°C in LB; notably, no transcript was detected for the isogenic sadA null mutant (Fig. 2C). Next, the presence of SadA in the outer membrane was confirmed using the anti-SadA specific antibody; thus, a band of ca. 400 kDa was detected in outer membrane preparations of wild-type S. enterica SL1344 but not in an isogenic sadA mutant grown in LB at 37°C. Outer membrane localization of SadA was restored by introducing plasmid pDR03 into S. enterica SL1344 ΔsadA (Fig. 2D); in addition to the trimeric form, a lower band, consistent with monomeric SadA, was also detected when SadA was overexpressed. Finally, the surface localization of SadA was confirmed by immunofluorescence microscopy (Fig. 2E). S. enterica SL1344 displayed abundant surface labeling, whereas the S. enterica SL1344 ΔsadA mutant strain was devoid of any labeling. The labeling was restored in the complemented mutant S. enterica SL1344 ΔsadA(pDR03). Interestingly, the labeling is punctate in both the wild type and the complemented mutant and in E. coli pDR01 strains (data not shown), suggesting that localization is a phenomenon of spatial expression and not a product of variations in O-antigen length. Punctate labeling has been observed previously for the prototypical TAA, YadA (31), though other TAAs seem to have a variety of surface expression patterns, including polar (75) and whole-cell labeling (31). As some TAAs are known to bind immunoglobulins (23), two further control experiments were performed. First, the wild-type strain was incubated with the fluorescently labeled secondary antibody only, and second, the wild-type strain was incubated with a different primary antibody (anti-Pet) prior to the addition of fluorescently labeled secondary antibody. In both controls, no labeling was detected (data not shown).
Expression of SadA promotes biofilm formation and autoaggregation.
Many of the TAAs characterized previously promote biofilm formation and autoaggregation (23, 75). To determine whether SadA also shared these phenotypic properties, wild-type S. enterica SL1344, the ΔsadA mutant strain, and the complemented mutant strain [S. enterica SL1344 ΔsadA(pDR03)] were tested for their ability to promote biofilm formation and aggregation. No statistically significant difference was seen in aggregation between the S. enterica SL1344 wild type, the ΔsadA mutant, and the complemented strain (Fig. 3A). Similarly, in the polystyrene biofilm assay, no difference in biofilm formation was seen between S. enterica SL1344, the ΔsadA isogenic mutant, and the complemented strain S. enterica SL1344 ΔsadA(pDR03) overexpressing SadA (Fig. 3B).
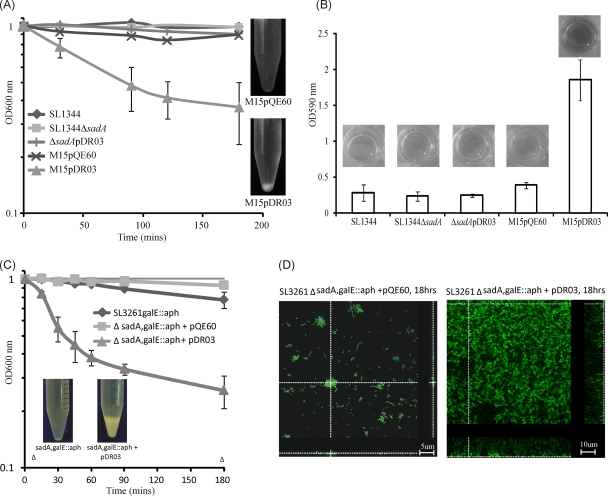
Fig. 3.
Autoaggregation and biofilm formation mediated by SadA. (A) Settling profiles of S. enterica SL1344, S. enterica SL1344 ΔsadA, S. enterica SL1344 ΔsadA(pDR03), E. coli M15(pQE60), and E. coli M15(pDR03). Images show settling from static liquid suspensions in the SadA-expressing E. coli M15 strain. (B) Biofilm formation of the five strains described for panel A in polystyrene plates. All strains were cultured under conditions suitable for inducing sadA expression from pDR03. The data are the average of experiments with 8 replicate samples, performed on three separate occasions. (C) Settling profiles of an S. enterica galE sadA double mutant and the double mutant expressing SadA (pDR03). (D) Flow cell biofilms demonstrating the spatial distribution of the double mutant S. enterica SL3261 ΔsadA galE::aph with either pQE60 or pDR03 after 18 h of growth. Shown are representative horizontal sections within each biofilm and vertical sections representing the yz plane and xz plane at the positions indicated by the white dotted lines.
Previous investigations have demonstrated that the function of type V secreted molecules can be obscured in in vitro assays by occlusion of the functional portion of the protein by other surface structures, such as capsular polysaccharide or smooth lipopolysaccharide (LPS) (33, 63, 67, 68, 74). To determine if this was the case for SadA, we took two different approaches. First, SadA was expressed in E. coli M15 lacking smooth LPS, and second, we introduced a galE null mutation into S. enterica SL3261 and its sadA mutant derivatives, resulting in strains defective in O-antigen production. In contrast to wild-type S. enterica SL1344, E. coli M15(pDR03) demonstrated significant levels of autoaggregation (OD600 = 0.3/h), whereas the strain carrying the empty vector (pQE60NdeI) did not settle (OD600 = 0.1/h) (Fig. 3A). Similarly, the S. enterica SL3261 ΔsadA ΔgalE double mutant strain did not aggregate (OD600 = 0.1/h), but the mutant expressing SadA did aggregate (OD600 = 0.45/h) (Fig. 3C). These data clearly demonstrate the capacity of SadA to induce autoaggregation.
To determine whether the LPS could be blocking the capacity of SadA to mediate biofilm formation, E. coli M15 was tested in the polystyrene biofilm assay. Compared to that of the empty vector control, biofilm formation was significantly increased when SadA was expressed from E. coli M15(pDR03) (P < 0.001) (Fig. 3B). Furthermore, under continuous-flow conditions, SadA mediated a strong biofilm-forming phenotype in S. enterica lacking long O-antigen side chains; this phenotype was absent from an isogenic mutant lacking SadA expression (Fig. 3D). This verifies that SadA can induce biofilm formation phenotypes in S. enterica.
SadA increases E. coli K-12 adherence to and invasion of intestinal epithelial cells.
All characterized TAAs have been found to have a role in adhesion. To determine if the SadA protein is also involved in adhesion, the strains described above were tested for their abilities to adhere to or invade two epithelial cell lines: the human intestinal Caco-2 cells and murine J774 macrophages. No difference was detected between the abilities of S. enterica SL1344 and S. enterica SL1344 sadA::aph to adhere to or invade Caco-2 cells. However, when SadA was expressed in E. coli M15, the ability of these bacteria to adhere to (P < 0.05) and invade (P < 0.01) Caco-2 cells was significantly increased (Fig. 4). In fact, E. coli M15(pDR03) was five times more adherent and over 100 times more invasive than the empty vector control E. coli M15(pQE60). No difference in adhesion to or invasion of J774 macrophages was observed for any of the strains tested (data not shown). This suggests that SadA can promote bacterial adherence to intestinal cells but that in Salmonella, other factors can compensate for the loss of sadA.
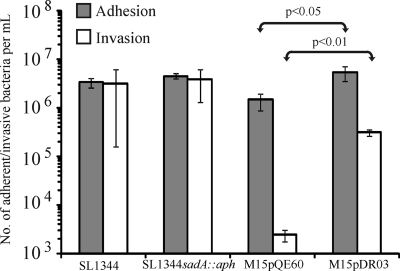
Fig. 4.
Adherence and invasion of S. enterica SL1344, S. enterica SL1344 sadA::aph, E. coli M15(pQE60), and E. coli M15(pDR03) (SadA+) to intestinal Caco-2. The E. coli strain expressing SadA had increased adhesion (P < 0.05) and invasion (P < 0.01) over the E. coli strain with the empty plasmid.
Many TAAs promote binding to extracellular matrix (ECM) molecules (50, 62, 73), including the SadA homologue UpaG, which binds to fibronectin and laminin (75). To determine if expression of SadA also conferred this phenotype, the S. enterica SL1344 wild type and the ΔsadA mutant, as well as E. coli overexpressing SadA, were tested for their ability to adhere to ECM molecules as previously performed (70). Our studies were unable to detect any SadA-mediated adhesion to ECM molecules collagen I, collagen III, collagen IV, elastin, fibronectin, and laminin (data not shown).
Finally, ingestion of salad leaves contaminated with S. enterica strains has been associated with outbreaks of disease. Specific adherence of S. enterica has been demonstrated (6, 7, 66). Thus, we investigated the ability of SadA to mediate binding of S. enterica SL1344, the ΔsadA isogenic mutant, and E. coli strains expressing SadA to rocket leaves. While strains were capable of binding to the leaves, there was no significant difference between those strains expressing SadA and those which did not produce SadA (see Fig. S1 in the supplemental material).
SadA does not promote serum resistance.
YadA, the prototypical TAA, and other members of the type V secretion family have been shown to confer the ability to resist complement-mediated killing in serum (23, 35). Such serum resistance is an important phenotype of invasive salmonellae, as it enables the bacteria to survive in the blood (53). S. enterica SL1344, the isogenic ΔsadA mutant strain, and the complemented version [S. enterica ΔsadA(pDR03)] were tested for survival in human serum derived from healthy individuals. No difference in serum resistance between the Salmonella strains was observed, all being equally resistant. SadA was also unable to confer serum resistance to E. coli, with both E. coli M15(pQE60) and M15(pDR03) strains remaining serum sensitive (see Fig. S2 in the supplemental material).
Evaluation of the role of SadA in the virulence of Salmonella Typhimurium.
As SadA had been shown to be involved in adhesion, aggregation, and biofilm formation, we wanted to test its role in virulence. A common model organism used to study Salmonella infection is C. elegans, a hermaphroditic worm, which has been used to identify Salmonella virulence factors in the past (1). C. elegans N2 wild-type worms were infected with either S. enterica SL1344 or S. enterica SL1344 sadA::aph, and the relative survival rates were compared. No significant difference in survival rate was observed (see Fig. S3 in the supplemental material).
Since the worm model showed no attenuation of virulence, we assessed the virulence of a sadA mutant in two murine models of infection. First, the S. enterica sadA::aph mutation was transferred to the S. enterica SL3261 strain using P22 transduction. This allowed any potential role for virulence to be assessed over a longer period when CD4 T cells are required for the resolution of infection. Wild-type C57BL/6 mice were infected i.p. with S. enterica SL3261 or the isogenic sadA::aph mutant. The mutant strain exhibited no attenuation compared to the parental S. enterica SL3261 in the liver or spleen at all time points (see Fig. S3 in the supplemental material).
As SadA mediates binding and invasion of intestinal epithelial cells, but had no apparent role in disease when administered by the i.p. route, we hypothesized that SadA may play a role in the establishment of S. Typhimurium infection in the intestine. To test this, mice were orally challenged with S. enterica SL1344 or the isogenic sadA::aph mutant. No significant difference was observed between the numbers of wild-type and mutant bacteria present in either the spleen or liver at 4 of 6 days postinfection (see Fig. S3 in the supplemental material). These data suggest that SadA is not required for the development or maintenance of either oral or systemic Salmonella infection in mice.
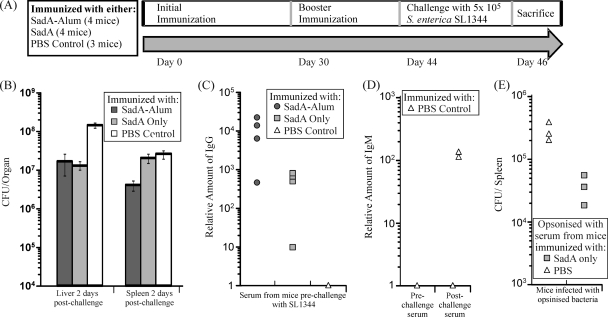
Immunization with alum-precipitated SadA induces marked IgG titers and provides limited protection against S. Typhimurium infection.
As SadA is surface expressed on the bacterium, we tested whether immunization with purified SadA protein could reduce bacterial colonization after infection. Mice were immunized according to the schedule shown in Fig. 5A, being immunized twice (day 0 and day 30) with PBS, purified SadA, or alum-precipitated SadA (SadA-Alum) before i.p. challenge with S. enterica SL1344 14 days after the second immunization. Mice were sacrificed 2 days postchallenge, and the bacterial numbers in spleens and livers were recorded. In addition, serum samples were taken before and after the challenge with the bacteria. Mice immunized with purified SadA-Alum had 6.5-fold lower numbers of bacteria in the spleen (P < 0.05) and 10-fold fewer bacteria in the liver (P < 0.01) than the PBS control. Mice immunized with purified unadjuvanted SadA had 10-fold fewer bacterial numbers in the liver (P < 0.01) than the control mice but no significant differences in splenic bacterial numbers (Fig. 5B).
Fig. 5.
SadA induces an antibody response in mice. (A) Experimental design of challenge experiment. (B) Numbers of CFU recovered from the livers and spleens of mice 2 days after challenge. Mice dosed with SadA-Alum had lower levels of S. enterica in both the spleen and liver than the control, whereas mice dosed with SadA only had lower bacterial numbers in the liver. (C) Sera taken from mice prior to challenge with S. enterica SL1344 were tested for relative amounts of SadA-specific IgG. SadA-specific IgG was found in mice dosed with SadA and SadA-Alum. (D) SadA-specific IgM was found in serum from PBS control mice collected postchallenge but not in serum collected prechallenge, indicating SadA expression in vivo during S. enterica SL1344 infection. (E) Numbers of CFU recovered from the spleens of mice infected with opsonized S. enterica SL1344. Bacteria opsonized with the serum from SadA-immunized mice had a reduction in bacterial numbers compared to bacteria opsonized with the PBS control serum.
Assessment of antibody titers in mice prior to infection showed that SadA-immunized mice induced marked IgG titers against SadA and that the group that had the highest IgG titers had the lowest bacterial burdens. While alum-precipitating SadA augmented the IgG response, it was surprising to see a potent anti-SadA IgG response in mice that had received the protein without adjuvant (Fig. 5C). Finally, IgM titers to SadA were examined in serum taken pre- and postchallenge from the PBS control mice. As expected, no IgM response was seen in mice prechallenge; however, two of the three control mice had a SadA-specific IgM response 2 days postchallenge with S. enterica SL1344, indicating that SadA was being expressed in vivo during infection (Fig. 5D).
To prove the protective effect of anti-SadA antibody, and to rule out the influence of nonspecific effects or the effects of innate immunity, bacteria were opsonized before mice were infected. After 5 days, mice which were challenged with opsonized bacteria demonstrated a marginal but significant reduction in bacterial numbers compared to mice challenged with nonopsonized bacteria (Fig. 5E). The reduction in bacterial numbers was similar to that observed for mice immunized with SadA. Taken together, the data indicate that SadA is expressed in vivo during infection and can elicit a rapid immunological response to itself but that preexisting IgG antibody to SadA provokes only a modest protective effect against challenge in this host.
DISCUSSION
Proteins that use the type V secretion system represent an increasingly important family of secreted virulence factors in Gram-negative bacteria, not only for their contribution to virulence but also as targets for vaccine development (8, 79). Two type V, or autotransporter (AT), proteins have previously been characterized in S. Typhimurium: MisL and ShdA. These AT proteins have a role in S. Typhimurium intestinal colonization and persistence via binding to various ECM molecules (20, 41–43). In this study, we characterize SadA, the first trimeric autotransporter adhesin (TAA) to be characterized in detail in S. enterica. We demonstrate that SadA possesses functional properties associated with biofilm formation, aggregation, and adhesion to intestinal epithelial cells. Loss of SadA from the bacterium does not affect the course of infection, and while antibody to SadA can reduce bacterial colonization, this effect is modest.
Although SadA is a clear positional orthologue of the previously characterized TAA UpaG from E. coli CFT073, this study has revealed that they have differing functions. SadA is similar to UpaG in that it promotes biofilm formation and bacterial cell-cell aggregation, but nonetheless, it differs from UpaG in adhesion phenotypes. In contrast to the findings for UpaG, we were unable to detect SadA-mediated binding to any ECM molecules (75). Despite the lack of ECM binding, SadA does promote significant adhesion and invasion of Caco-2 epithelial cells, whereas UpaG was reported to increase binding to T24 epithelial cell lines, albeit very slightly (75). These differences are most likely due to sequence differences in the passenger domain. The passenger domains of UpaG and SadA varied significantly (46.2% identity) in contrast to the translocator (79.7% identity) and signal sequences (72.5% identity). Further analysis of the passenger domain revealed that the motifs in the predicted head region were more variable than motifs in the stalk and neck structures. The head is responsible for the adhesion phenotype in other TAAs (50), and thus, it is not surprising that SadA and UpaG have differing adhesion phenotypes.
In contrast to the high sequence variability between SadA and positional orthologues in E. coli strains, the orthologues of SadA in S. enterica strains are highly conserved (over 97% amino acid similarity). This is even more pronounced in the SadA orthologues from the four sequenced S. Typhimurium strains which share 100% amino acid identity. Similarly, the majority of SadA orthologues in E. coli strains from intestinal isolates are also highly conserved. The fact that the protein is also in the same genomic location in all strains (including in C. youngae) suggests that the gene has been maintained since before Salmonella and E. coli diverged. These facts suggest that SadA has an important function for the bacterium. The conserved nature of the protein also infers that either SadA is not under immunological pressure despite surface localization or that the amino acid sequence must remain highly conserved to maintain the functionality of the protein.
SadA was expressed in S. enterica SL1344 at low levels in vitro. Interestingly, SadA is likely to be surface exposed in vivo since IgM antibody to it was detectable within 2 days of infection. Our ongoing experiments indicate that SadA expression may be increased at 42°C, the avian body temperature (data not shown). As Salmonella is also highly associated with birds (25), it may be that SadA plays a more significant role in avian colonization.
SadA-mediated bacterial cell aggregation and biofilm formation. These phenotypes have been implicated in persistence and pathogenicity for many bacterial pathogens (5, 57, 64) and are also associated with many AT and TAA proteins (78), although no structural domains for these conserved functions have yet been defined. Many other AT and TAA proteins that do mediate aggregation and biofilm formation have other more specific virulence functions, such as adhesion to epithelial cells (75, 77) or extracellular matrix molecules (76), serum resistance (23), and toxin and esterase activity. Likewise, SadA has a further, more specific function, promoting adhesion to and invasion of human intestinal epithelial cells (Caco-2) when expressed in an E. coli background. Initial attachment and invasion of human intestinal epithelial cells are vital for the initiation of infection (55). As SadA can mediate adhesion and invasion of Caco-2 cells when expressed in E. coli K-12, it may play a role in the initial attachment and colonization of Salmonella in the host. Previous studies with TAA proteins that bind epithelial cells have suggested that this is due to the ability of the proteins to bind ECM components (75). As we could not detect any SadA-mediated binding to ECM molecules, it is likely that SadA has other binding targets on the epithelial cell surface or binds to ECM molecules not tested in this study.
Although SadA is expressed in S. Typhimurium in vitro, no difference in cell aggregation, biofilm formation, or adhesion to Caco-2 epithelial cells was detected between the wild type and sadA mutant strain. Large bacterial surface structures, such as capsule, LPS, or fimbriae, have been shown to block the function of AT proteins such as antigen 43 and EhaA (33, 63, 77). We demonstrated that by removing the O antigen from S. Typhimurium, we could restore the aggregation and biofilm formation phenotypes of SadA. This suggests that large surface structures can block the function of SadA and expression of surface factors needs to be carefully orchestrated in the bacterium for TAAs such as SadA to play a role in colonization and infection. Thus, SadA may also be important under particular conditions where LPS production is reduced, such as during macrophage infection (24).
No difference in virulence between the S. Typhimurium wild-type and sadA mutant strains was seen in any of the assays performed. This may be due to functional redundancy with other expressed outer membrane proteins which compensate for the deletion of sadA. This is also seen for other AT proteins characterized in S. Typhimurium. Although shown to be critical for maintenance of fecal shedding, there is no effect on virulence when MisL and ShdA are deleted (20, 43). Indeed, the roles of MisL, ShdA, and SadA may be compensatory; loss of one AT may not reduce virulence, but loss of all three may severely attenuate S. enterica. Studies to test such functional redundancy are under way in our laboratory.
A surprising finding from this study was that SadA alone was sufficient to drive a switched IgG response to itself in the absence of exogenous adjuvant. This coupled to the finding that during infection in naive mice, anti-SadA IgM was detectable within 48 h, suggests that this trimeric autotransporter is likely to be recognized by selective B cell subsets, such as B1b cells that are known to recognize Salmonella proteins such as OmpD (26). Nevertheless, IgG antibody to SadA afforded limited protection of only a 10-fold reduction even when given twice as an alum-precipitated protein. Recent studies have shown that antibodies targeting the LPS of S. Typhimurium inhibit complement-mediated killing in immunocompromised individuals (52) despite being an immunodominant target of the immune response. Therefore, collectively, the data from this study and the described studies indicate that identifying the most effective targets of antibody-mediated protection is likely to be a complex process and may involve targeting multiple antigens in parallel.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by project grants from the BBRSC and MRC to I.R.H. T.J.W. is a recipient of the Fellowship Bridging Award through the Wellcome Trust Value in People scheme.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Aballay A., Ausubel F. M. 2001. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl. Acad. Sci. U.S.A. 98:2735–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey A. M., et al. 2010. RamA, a member of the AraC/XylS family, influences both virulence and efflux in Salmonella enterica serovar Typhimurium. J. Bacteriol. 192:1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balligand G., Laroche Y., Cornelis G. 1985. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect. Immun. 48:782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 5. Berge A., Kihlberg B. M., Sjoholm A. G., Bjorck L. 1997. Streptococcal protein H forms soluble complement-activating complexes with IgG, but inhibits complement activation by IgG-coated targets. J. Biol. Chem. 272:20774–20781 [DOI] [PubMed] [Google Scholar]
- 6. Berger C. N., et al. 2009. Interaction of Salmonella enterica with basil and other salad leaves. ISME J. 3:261–265 [DOI] [PubMed] [Google Scholar]
- 7. Berger C. N., et al. 2009. Interaction of enteroaggregative Escherichia coli with salad leaves. Environ. Microbiol. Rep. 1:234–239 [DOI] [PubMed] [Google Scholar]
- 8. Bowe F., et al. 2004. Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin. Infect. Immun. 72:4052–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyer H. W., Roulland-Dussoix D. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459–472 [DOI] [PubMed] [Google Scholar]
- 10. Chaudhuri R. R., et al. 2010. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One 5:e8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comanducci M., et al. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cotter S. E., Surana N. K., St. Geme J. W., III 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 13:199–205 [DOI] [PubMed] [Google Scholar]
- 13. Cotter S. E., Yeo H. J., Juehne T., St. Geme J. W., III 2005. Architecture and adhesive activity of the Haemophilus influenzae Hsf adhesin. J. Bacteriol. 187:4656–4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cunningham A. F., et al. 2007. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 178:6200–6207 [DOI] [PubMed] [Google Scholar]
- 15. Cunningham A. F., Serre K., Mohr E., Khan M., Toellner K. M. 2004. Loss of CD154 impairs the Th2 extrafollicular plasma cell response but not early T cell proliferation and interleukin-4 induction. Immunology 113:187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desvaux M., et al. 2006. The unusual extended signal peptide region of the type V secretion system is phylogenetically restricted. FEMS Microbiol. Lett. 264:22–30 [DOI] [PubMed] [Google Scholar]
- 18. Desvaux M., Parham N. J., Henderson I. R. 2004. Type V protein secretion: simplicity gone awry? Curr. Issues Mol. Biol. 6:111–124 [PubMed] [Google Scholar]
- 19. Desvaux M., et al. 2007. A conserved extended signal peptide region directs posttranslational protein translocation via a novel mechanism. Microbiology 153:59–70 [DOI] [PubMed] [Google Scholar]
- 20. Dorsey C. W., Laarakker M. C., Humphries A. D., Weening E. H., Baumler A. J. 2005. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 57:196–211 [DOI] [PubMed] [Google Scholar]
- 21. Eaves D. J., et al. 2004. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 48:4012–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El Tahir Y., Kuusela P., Skurnik M. 2000. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol. Microbiol. 37:192–206 [DOI] [PubMed] [Google Scholar]
- 23. El Tahir Y., Skurnik M. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209–218 [DOI] [PubMed] [Google Scholar]
- 24. Eriksson S., Lucchini S., Thompson A., Rhen M., Hinton J. C. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103–118 [DOI] [PubMed] [Google Scholar]
- 25. Foley S. L., Lynne A. M., Nayak R. 2008. Salmonella challenges: prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 86(14 Suppl):E149–E162 [DOI] [PubMed] [Google Scholar]
- 26. Gil-Cruz C., et al. 2009. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. U. S. A. 106:9803–9808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gondwe E. N., et al. 2010. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc. Natl. Acad. Sci. U. S. A. 107:3070–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gordon M. A. 2008. Salmonella infections in immunocompromised adults. J. Infection 56:413–422 [DOI] [PubMed] [Google Scholar]
- 29. Gordon M. A., et al. 2008. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin. Infect. Dis. 46:963–969 [DOI] [PubMed] [Google Scholar]
- 30. Graham S. M., et al. 2000. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr. Infect. Dis. J. 19:1189–1196 [DOI] [PubMed] [Google Scholar]
- 31. Grosskinsky U., et al. 2007. A conserved glycine residue of trimeric autotransporter domains plays a key role in Yersinia adhesin A autotransport. J. Bacteriol. 189:9011–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hartmann M. D., et al. 2009. A coiled-coil motif that sequesters ions to the hydrophobic core. Proc. Natl. Acad. Sci. U. S. A. 106:16950–16955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hasman H., Chakraborty T., Klemm P. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henderson I. R., Cappello R., Nataro J. P. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529–532 [DOI] [PubMed] [Google Scholar]
- 35. Henderson I. R., Czeczulin J., Eslava C., Noriega F., Nataro J. P. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587–5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala'Aldeen D. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henderson I. R., Navarro-Garcia F., Nataro J. P. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370–378 [DOI] [PubMed] [Google Scholar]
- 38. Hernandez Alvarez B., et al. 2008. A new expression system for protein crystallization using trimeric coiled-coil adaptors. Protein Eng. Des. Sel. 21:11–18 [DOI] [PubMed] [Google Scholar]
- 39. Hoiseth S. K., Stocker B. A. D. 1981. Aromatic-dependent Salmonella Typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239 [DOI] [PubMed] [Google Scholar]
- 40. Kaiser P. O., et al. 2008. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell. Microbiol. 10:2223–2234 [DOI] [PubMed] [Google Scholar]
- 41. Kingsley R. A., et al. 2004. Fibronectin binding to the Salmonella enterica serotype Typhimurium ShdA autotransporter protein is inhibited by a monoclonal antibody recognizing the A3 repeat. J. Bacteriol. 186:4931–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kingsley R. A., Santos R. L., Keestra A. M., Adams L. G., Baumler A. J. 2002. Salmonella enterica serotype Typhimurium ShdA is an outer membrane fibronectin-binding protein that is expressed in the intestine. Mol. Microbiol. 43:895–905 [DOI] [PubMed] [Google Scholar]
- 43. Kingsley R. A., van Amsterdam K., Kramer N., Baumler A. J. 2000. The shdA gene is restricted to serotypes of Salmonella enterica subspecies I and contributes to efficient and prolonged fecal shedding. Infect. Immun. 68:2720–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knowles T. J., Scott-Tucker A., Overduin M., Henderson I. R. 2009. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat. Rev. Microbiol. 7:206–214 [DOI] [PubMed] [Google Scholar]
- 45. Koretke K. K., Szczesny P., Gruber M., Lupas A. N. 2006. Model structure of the prototypical non-fimbrial adhesin YadA of Yersinia enterocolitica. J. Struct. Biol. 155:154–161 [DOI] [PubMed] [Google Scholar]
- 46. Laarmann S., Cutter D., Juehne T., Barenkamp S. J., St. Geme J. W. 2002. The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Mol. Microbiol. 46:731–743 [DOI] [PubMed] [Google Scholar]
- 47. Lafontaine E. R., et al. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lehr U., et al. 2010. C-terminal amino acid residues of the trimeric autotransporter adhesin YadA of Yersinia enterocolitica are decisive for its recognition and assembly by BamA. Mol. Microbiol. 78:932–946 [DOI] [PubMed] [Google Scholar]
- 49. Leyton D. L., et al. 2010. The unusual extended signal peptide region is not required for secretion and function of an Escherichia coli autotransporter. FEMS Microbiol. Lett. 311:133–139 [DOI] [PubMed] [Google Scholar]
- 50. Linke D., Riess T., Autenrieth I. B., Lupas A., Kempf V. A. J. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14:264–270 [DOI] [PubMed] [Google Scholar]
- 51. Ma H. S., Croudace J. E., Lammas D. A., May R. C. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16:2156–2160 [DOI] [PubMed] [Google Scholar]
- 52. MacLennan C. A., et al. 2010. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacLennan C. A., et al. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118:1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Majowicz S. E., et al. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889 [DOI] [PubMed] [Google Scholar]
- 55. Monack D. M., Mueller A., Falkow S. 2004. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2:747–765 [DOI] [PubMed] [Google Scholar]
- 56. Nummelin H., et al. 2003. The collagen-binding domain structure is a novel left-handed parallel beta-roll. EMBO J. 23:701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ochiai K., Kuritaochiai T., Kamino Y., Ikeda T. 1993. Effect of coaggregation on the pathogenicity of oral bacteria. J. Med. Microbiol. 39:183–190 [DOI] [PubMed] [Google Scholar]
- 58. Ojha A., et al. 2005. GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123:861–873 [DOI] [PubMed] [Google Scholar]
- 59. Parham N. J., et al. 2004. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 230:73–83 [DOI] [PubMed] [Google Scholar]
- 60. Riess T., et al. 2004. Bartonella adhesin A mediates a proangiogenic host cell response. J. Exp. Med. 200:1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roggenkamp A., et al. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 185:3735–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scarselli M., et al. 2006. Neisseria meningitidis NhhA is a multifunctional trimeric autotransporter adhesin. Mol. Microbiol. 61:631–644 [DOI] [PubMed] [Google Scholar]
- 63. Schembri M. A., Dalsgaard D., Klemm P. 2004. Capsule shields the function of short bacterial adhesins. J. Bacteriol. 186:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schembri M. A., Kjaergaard K., Klemm P. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253–267 [DOI] [PubMed] [Google Scholar]
- 65. Schembri M. A., Klemm P. 2001. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect. Immun. 69:1322–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shaw R. K., et al. 2008. Enterohemorrhagic Escherichia coli exploits EspA filaments for attachment to salad leaves. Appl. Environ. Microbiol. 74:2908–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sherlock O., Schembri M. A., Reisner A., Klemm P. 2004. Novel roles for the AIDA adhesin from diarrheagenic Escherichia coli: cell aggregation and biofilm formation. J. Bacteriol. 186:8058–8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sherlock O., Vejborg R. M., Klemm P. 2005. The TibA adhesin/invasin from enterotoxigenic Escherichia coli is self recognizing and induces bacterial aggregation and biofilm formation. Infect. Immun. 73:1954–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smollett K., Shaw R. K., Garmendia J., Knutton S., Frankel G. 2006. Function and distribution of EspG2, a type III secretion system effector of enteropathogenic Escherichia coli. Microbes Infect. 8:2220–2227 [DOI] [PubMed] [Google Scholar]
- 70. Sokurenko E. V., McMackin V. A., Hasty D. L. 1995. Bacterial adhesion measured by growth of adherent organisms. Methods Enzymol. 253:519–528 [DOI] [PubMed] [Google Scholar]
- 71. Surana N. K., Cutter D., Barenkamp S. J., St. Geme J. W. 2004. The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J. Biol. Chem. 279:14679–14685 [DOI] [PubMed] [Google Scholar]
- 72. Szczesny P., et al. 2008. Structure of the head of the Bartonella adhesin BadA. PLoS Pathog. 4:e1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tertti R., Skurnik M., Vartio T., Kuusela P. 1992. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect. Immun. 60:3021–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ulett G. C., Webb R. I., Schembri M. A. 2006. Antigen-43-mediated autoaggregation impairs motility in Escherichia coli. Microbiology 152:2101–2110 [DOI] [PubMed] [Google Scholar]
- 75. Valle J., et al. 2008. UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli. J. Bacteriol. 190:4147–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wells T. J., et al. 2009. The Escherichia coli O157:H7 EhaB autotransporter protein binds to laminin and collagen I and induces a serum IgA response in O157:H7 challenged cattle. Environ. Microbiol. 11:1803–1814 [DOI] [PubMed] [Google Scholar]
- 77. Wells T. J., et al. 2008. EhaA is a novel autotransporter protein of enterohemorrhagic Escherichia coli O157:H7 that contributes to adhesion and biofilm formation. Environ. Microbiol. 10:589–604 [DOI] [PubMed] [Google Scholar]
- 78. Wells T. J., Totsika M., Schembri M. A. 2010. Autotransporters of Escherichia coli: a sequence-based characterization. Microbiology 156(Part 8):2459–2469 [DOI] [PubMed] [Google Scholar]
- 79. Wells T. J., Tree J. J., Ulett G. C., Schembri M. A. 2007. Autotransporter proteins: novel targets at the bacterial cell surface. FEMS Microbiol. Lett. 274:163–172 [DOI] [PubMed] [Google Scholar]
- 80. Wray C., Sojka W. J. 1978. Experimental Salmonella Typhimurium infection in calves. Res. Vet. Sci. 25:139–143 [PubMed] [Google Scholar]
- 81. Yeo H. J., et al. 2004. Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J. 23:1245–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.