Targeting antigens to the lectinlike DC-ASGPR receptor on human DCs and in nonhuman primates results in the induction of antigen-specific IL-10–producing CD4+ T cells.
Abstract
Dendritic cells (DCs) can initiate and shape host immune responses toward either immunity or tolerance by their effects on antigen-specific CD4+ T cells. DC-asialoglycoprotein receptor (DC-ASGPR), a lectinlike receptor, is a known scavenger receptor. Here, we report that targeting antigens to human DCs via DC-ASGPR, but not lectin-like oxidized-LDL receptor, Dectin-1, or DC-specific ICAM-3–grabbing nonintegrin favors the generation of antigen-specific suppressive CD4+ T cells that produce interleukin 10 (IL-10). These findings apply to both self- and foreign antigens, as well as memory and naive CD4+ T cells. The generation of such IL-10–producing CD4+ T cells requires p38/extracellular signal-regulated kinase phosphorylation and IL-10 induction in DCs. We further demonstrate that immunization of nonhuman primates with antigens fused to anti–DC-ASGPR monoclonal antibody generates antigen-specific CD4+ T cells that produce IL-10 in vivo. This study provides a new strategy for the establishment of antigen-specific IL-10–producing suppressive T cells in vivo by targeting whole protein antigens to DCs via DC-ASGPR.
DCs are major APCs and can direct host immune responses toward either immunity or tolerance (Banchereau and Steinman, 1998; Steinman et al., 2003). As immune controllers, DCs can deliver differential signals to other immune cells through intercellular interactions and soluble factors (Banchereau and Steinman, 1998; Rissoan et al., 1999; Akira et al., 2001; Soares et al., 2007), resulting in different quality and quantity of host immune responses. In addition, different subsets of DCs display common and unique biological functions for controlling host immune responses (Caux et al., 1996; Maldonado-López et al., 1999; Pulendran et al., 1999; Banchereau et al., 2000; Shortman and Liu, 2002; Dudziak et al., 2007; Soares et al., 2007; Klechevsky et al., 2008).
DCs express pattern recognition receptors (Figdor et al., 2002; Geijtenbeek et al., 2004; Brown, 2006), most notably represented by Toll-like receptors (Akira et al., 2001) and lectinlike receptors (LLRs; Figdor et al., 2002; Geijtenbeek et al., 2004; Brown, 2006; Caparrós et al., 2006). Ligation of TLRs results in the activation of DCs, followed by cytokine and chemokine secretion that contribute to the outcomes of host immune responses. LLRs operate as constituents of the powerful antigen capture and uptake system (Delneste et al., 2002; Figdor et al., 2002; Geijtenbeek et al., 2004; Brown, 2006; Geijtenbeek and Gringhuis, 2009). Recent compelling evidence also indicates that individual LLRs expressed on DCs might possess unique functions (Delneste et al., 2002; Figdor et al., 2002; Brown, 2006; Geijtenbeek and Gringhuis, 2009), that can contribute to shaping the quality and quantity of host immune responses. For example, lectinlike oxidized-LDL receptor (LOX-1), Dectin-1, and DC-specific ICAM-3–grabbing nonintegrin (DC-SIGN) are capable of delivering intracellular signals, either by themselves or by combination with TLRs, that activate DCs and can result in altered T cell responses (Figdor et al., 2002; Delneste et al., 2002; Geijtenbeek et al., 2004; Smits et al., 2005; Brown, 2006; Caparrós et al., 2006; Dillon et al., 2006; Geijtenbeek and Gringhuis, 2009; Geurtsen et al., 2009). Certain features of LLRs—antigen capture, uptake, and signaling capacity—place them as key immune receptors that could determine the outcomes of host immune responses. Indeed, DCs activated via Dectin-1 result in polarized Th17 CD4+ T cell responses (LeibundGut-Landmann et al., 2007; Gringhuis et al., 2009). It was also reported that signals via Dectin-1 induce IL-10 in DCs (Rogers et al., 2005; Ni et al., 2010), and activation of DCs via Dectin-1 and TLR2 results in regulatory T cell responses (Dillon et al., 2006). DC-SIGN binding by different pathogens can lead to promotion of Th2 responses (Bergman et al., 2004; Caparrós et al., 2006; Geurtsen et al., 2009) and the induction of regulatory T cell differentiation (Smits et al., 2005; Geijtenbeek and Gringhuis, 2009).
DC-asialoglycoprotein receptor (DC-ASGPR) is a scavenger receptor carrying an immunoreceptor tyrosine-based activation motiflike motif (Valladeau et al., 2001), but the biological function of DC-ASGPR expressed on DCs has not been characterized. In this study, we demonstrate for the first time that DC-ASGPR has a novel function for generating antigen-specific IL-10–producing suppressive CD4+ T cells in vitro. Furthermore, antigens fused to anti–DC-ASGPR antibody can generate IL-10–producing antigen-specific T cells in macaques. This study provides a novel strategy for the establishment of antigen-specific IL-10–producing regulatory T cells in vivo.
RESULTS
DCs express LOX-1 and DC-ASGPR
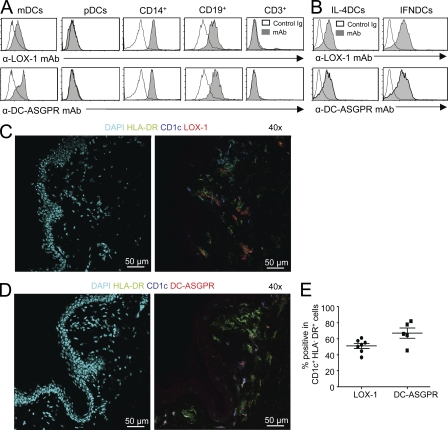
mAbs to human LOX-1 and DC-ASGPR were generated. Both anti–LOX-1 (IgG2a: clone 15C4) and anti–DC-ASGPR (IgG2a, clone 49C11) mAb bound to blood myeloid DCs (mDCs; Lin−HLA-DR+CD11c+CD123−), CD14+ monocytes, and CD19+ B cells, but not plasmacytoid DCs (pDCs; Lin−HLA-DR+CD11c−CD123+) or CD3+ T cells in peripheral blood mononuclear cells (PBMCs; Fig. 1 A). The entire population of monocyte-derived DCs cultured in vitro with GM-CSF and IL-4 (IL-4DCs) expressed both LOX-1 and DC-ASGPR in a similar level. IFNDCs generated with GM-CSF and IFN-α expressed slightly higher levels of both lectins than IL-4DCs did (Fig. 1 B). The specificity of mAbs was confirmed by staining 293F cells transfected with LOX-1 and DC-ASGPR expression vectors (unpublished data).
Figure 1.
DCs express LOX-1 and DC-ASGPR. (A) Expression of LOX-1 (top) and DC-ASGPR (bottom) on the surface of blood myeloid DCs (mDCs; Lin−HLA-DR+CD11c+CD123−), plasmacytoid DCs (pDCs; Lin−HLA-DR+CD11c−CD123+), CD14+ monocytes, CD19+ B cells, and CD3+ T cells. (B) Expression of LOX-1 (top) and DC-ASGPR (bottom) on the surface of monocyte-derived IFNDCs and IL-4DCs. Representative data from experiments using cells from six healthy donors are presented in A and B. Immunofluorescent staining of healthy human skin sections with DAPI, α-HLA-DR, α-CD1c and α-LOX-1 mAb (C), or α-DC-ASGPR (D) labeled with fluorescents. (E) Summary of data generated with skins from two healthy donors. Each dot represents data from one tissue section.
Human skin sections were stained with anti–LOX-1 and anti–DC-ASGPR mAbs. Confocal images show that both LOX-1 and DC-ASGPR are expressed mainly in the cells in dermis (Fig. 1, C and D). Approximately 50 and 70% of dermal CD1c+ DCs (CD1c+HLA-DR+) expressed LOX-1 and DC-ASGPR, respectively (Fig. 1 E). Detailed data for the expression of LOX-1 and DC-ASGPR in human skin sections are presented in Fig. S1. Neither DC-ASGPR nor LOX-1 was expressed on the surface of BDCA3+ DCs in the blood of healthy donors (unpublished data).
Antigens delivered to DCs via DC-ASGPR or LOX-1 result in antigen-specific CD4+ T cell responses
Recombinant mAbs carrying mouse variable region chimeras with human κ chain and human IgG4 carrying two site mutations (S228P and L235E; Reddy et al., 2000) were made, and then fused to hemagglutinin 1 (HA1 subunit of influenza virus A/PR/8/34, H1N1; anti–LOX-1-HA1, anti–DC-ASGPR-HA1, and control IgG4-HA1) and prostate-specific antigen (PSA; anti–LOX-1-PSA, anti–DC-ASGPR-PSA, and control IgG4-PSA; Fig. S2 A). Binding assays show that both anti–LOX-1-HA1 and anti–DC-ASGPR-HA1 bind to DCs in similar levels, and the binding was greater than with IgG4-HA1 (Fig. S2 B). Anti–LOX-1-PSA and anti–DC-ASGPR-PSA also bound to DCs greater than IgG4-PSA (Fig. S2 C). DCs loaded with 1 µg/ml anti–LOX-1-HA1 or anti–DC-ASGPR-HA1 induced similar, but greater CD4+ T cell proliferation than did DCs loaded with IgG4-HA1 or unloaded-DCs (Fig. S2 D). Anti–LOX-1-PSA and anti–DC-ASGPR-PSA also induced greater naive CD4+ T cell proliferation than did IgG4-PSA (Fig. S2 E). Although both anti–LOX-1 and anti–DC-ASGPR constructs were more efficient than control IgG4 constructs at inducing CD4+ T cell proliferation, the level of CD4+ T cell proliferation was variable in different experiments. This was largely dependent on the numbers of T cells in the culture, maturation stages of monocyte-derived IFNDCs, amounts of recombinant constructs loaded to DCs, and cells from different healthy donors. Collectively, our data demonstrate that antigens (HA1 as a foreign antigen and PSA as a self-antigen) delivered to DCs via DC-ASGPR and LOX-1 resulted in enhanced CD4+ T cell responses.
To test the ability of recombinant fusion proteins to direct antigen-specific CD4+ T cell responses, CD4+ T cells expanded with anti–DC-ASGPR-HA1 and anti–LOX-1-HA1 were restimulated at day 7 with clusters of HA1 peptides for measuring intracellular IFN-γ expression (Fig. S2 F). Individual peptides in cluster 4 that had been selected as stimulating maximal IFN-γ expression were further tested, and three peptides, HA1250-266, HA1256-272, and HA1262-278, were selected (Fig. S2 G). HA1280-296 was used as a control. In the same experiment, DCs loaded with 1 µg/ml IgG4-HA1 did not result in significant numbers of HA1-specific CD4+ T cell responses (unpublished data). The frequency of total HA1-specific IFN-γ–expressing CD4+ T cells in the blood was ∼0.4% of total CD4+ T cells (Fig. S2 H). Thus, our data demonstrate that targeting HA1 to DCs via LOX-1 or DC-ASGPR is an efficient way to expand HA1-specific CD4+ T cells, although not all the proliferating cells are HA1-specific CD4+ T cells.
DC-ASGPR favors the generation of IL-10–producing CD4+ T cells
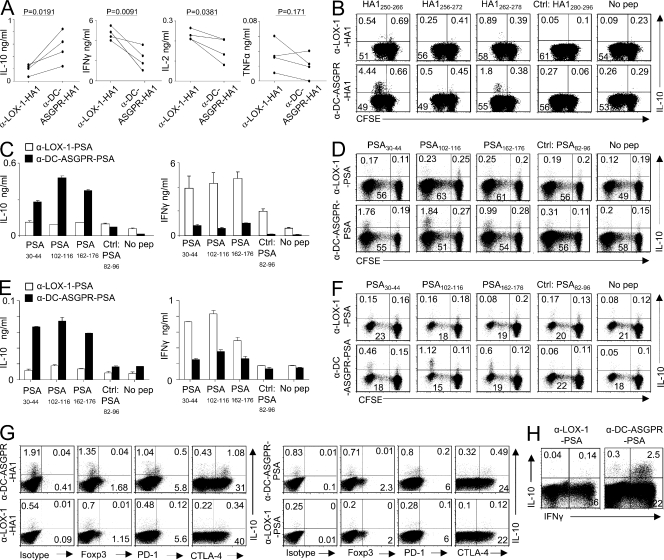
We observed that HA1250-266-specific CD4+ T cells expanded with anti–DC-ASGPR-HA1 secreted greater amounts of IL-10, but lower amounts of IFN-γ and IL-2 when compared with those expanded with anti–LOX-1-HA1 (Fig. 2 A). This increased amount of IL-10 was caused by the enhanced expansion of IL-10–producing CD4+ T cells (Fig. 2 B). CD4+ T cells expanded with either anti–DC-ASGPR-HA1 or anti–LOX-1-HA1 secreted low amounts of IL-4 and IL-5 (<20 pg/ml). We further confirmed at a single-cell level that anti–DC-ASGPR-HA1 resulted in greater numbers of IL-10–producing, but lower numbers of IFN-γ–, TNF-, and IL-2–producing HA1-specific CD4+ T cells compared with those expanded with anti–LOX-1-HA1 (Fig. S3 A). In the same experiment, we also tested the frequency of HA1-specific CD4+ T cells expanded by DCs loaded with HA1 peptide pool. Fig. S3 (A and B) shows that peptide pool was less efficient than anti–DC-ASGPR-HA1 and anti–LOX-1-HA1 at expanding IL-10– and IFN-γ–producing HA1-specific CD4+ T cells, respectively. Peptide pool alone did not result in significant numbers of either TNF+ or IL-2+ CD4+ T cell responses after 7 d of culture (unpublished data). However, we observed that DCs loaded with combinations of peptide pools and anti–DC-ASGPR resulted in enhanced HA1-specific IL-10–producing CD4+ T cell responses (Fig. S3 C). Conversely, combinations of peptide pools and anti–LOX-1 resulted in enhanced IFN-γ–producing CD4+ T cell responses. Neither IL-4– nor IL-5–producing HA1-specific CD4+ T cells were detected. DCs loaded with either anti–DC-ASGPR or anti–LOX-1 mAb alone did not result in HA1- or PSA-specific CD4+ T cell responses (unpublished data).
Figure 2.
Antigen targeting to DCs via DC-ASGPR favors the generation of antigen-specific IL-10–producing CD4+ T cells. IFNDCs (5 × 103) were loaded with 1 µg/ml α-DC-ASGPR-HA1 or α-LOX-1-HA1, and then co-cultured with CFSE-labeled autologous total CD4+ T cells (1–2 × 105) for 7 d. (A) CD4+ T cells were restimulated with peptide HA1250-266 for 48h, and cytokine levels in the culture supernatants were measured. Each line represents the data from a single experiment. P-values were tested by Student’s t test. (B) CD4+ T cells were restimulated with indicated peptides and stained for intracellular IL-10. HA1280-296 is a negative control. Peptides tested in B had been selected in Fig. S2 (F and G). Four independent experiments showed similar results. (C) Naive CD4+ T cells (1–2 × 105) were co-cultured with IFNDCs (5 × 103) loaded with 1 µg/ml recombinant fusion proteins for 7 d. CD4+ T cells were restimulated with peptides indicated for 48 h. IL-10 and IFN-γ levels in culture supernatants were assessed. Error bars represent mean ± SEM of triplicate assay. Three independent experiments with 59 PSA-derived peptides showed similar results. (D) Frequency of PSA-specific IL-10–producing CD4+ T cells elicited by recombinant fusion proteins. Four independent experiments showed similar results. (E) Experiments in C were performed with blood mDCs (Lin−HLA-DR+CD11c+CD123−). (F) Experiments in D were performed with blood mDCs. In both E and F, two independent experiments showed similar results. (G) Expression levels of Foxp3, PD-1, and CTLA-4 on HA1250-266-specific (left) and PSA30-44-specific CD4+ T cells producing IL-10 (right). (H) After 7 d of co-culture of purified naive CD4+ T cells and DCs loaded with either α-LOX-1-PSA or α-DC-ASGPR-PSA, CD4+ T cells were stained for intracellular IFN-γ and IL-10 during restimulation with 50 ng/ml PMA and 1 µg/ml ionomycin. Three independent experiments showed similar results (G and H).
These enhanced IL-10 responses were also observed when PSA was delivered to either IFNDCs (Fig. 2, C and D) or blood mDCs (Fig. 2, E and F) via DC-ASGPR. Conversely, PSA-specific CD4+ T cells induced with anti–LOX-1-PSA secreted higher IFN-γ levels than those induced with anti–DC-ASGPR-PSA (Fig. 2, C and E). In the same experiment, DCs loaded with PSA peptide pool did not result in significant numbers of IL-10–producing PSA-specific CD4+ T cells (unpublished data). Neither HA1-specific (Fig. 2 G, top left) nor PSA-specific IL-10–producing CD4+ T cells elicited by IFNDCs loaded with anti–DC-ASGPR constructs (Fig. 2 G, top right) expressed Foxp3. However, >60% of IL-10–producing CD4+ T cells elicited with anti–DC-ASGPR constructs expressed CTLA-4. A small fraction (∼30%) of them also expressed PD-1. IL-10–producing CD4+ T cells expanded with anti–LOX-1 constructs did not express Foxp3 either (Fig. 2 G, bottom left). Fig. 2 H shows that the majority of IL-10–producing CD4+ T cells also expressed IFN-γ when stimulated with PMA/ionomycin, suggesting their Th1 origin (Macián et al., 2002; Saraiva et al., 2009). Fig. S4 (A and B) further demonstrates that targeting HA1 and PSA to DCs via DC-ASGPR result in enhanced HA1- (Fig. S4 A) and PSA-specific (Fig. S4 B) IL-10–producing CD4+ T cell responses when compared with targeting antigens to DCs via LOX-1. Collectively, we conclude that both foreign (HA1) and self-antigens (PSA) delivered to DCs via DC-ASGPR generate higher IL-10–producing, but lower IFN-γ–producing CD4+ T cell responses than did the same antigens delivered to DCs via LOX-1. In addition, targeting antigens to DCs via DC-ASGPR and LOX-1 is far more efficient than loading antigen-derived peptide pools to DCs at eliciting antigen-specific CD4+ T cell responses.
Functional specialization of DC-ASGPR for the generation of IL-10–producing CD4+ T cells
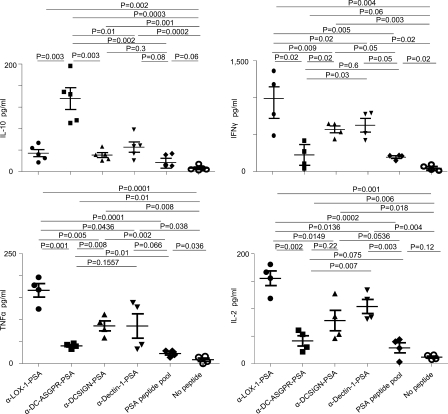
Both Dectin-1 and DC-SIGN can contribute to the enhanced regulatory T cell responses (Smits et al., 2005; Dillon et al., 2006; Geijtenbeek and Gringhuis, 2009) in the presence of signals via other TLRs and in the models using pathogens. Thus, we tested whether Dectin-1 and DC-SIGN could also favor the generation of IL-10–producing CD4+ T cells. Anti–Dectin-1 (clone 15E2; Ni et al., 2010) and anti–DC-SIGN mAbs (IgG2a: clone 16E7) were generated. Recombinant anti–Dectin-1 and anti–DC-SIGN mAbs were fused to PSA as for anti–DC-ASGPR-PSA and anti–LOX-1-PSA. Both anti–Dectin-1-PSA and anti–DC-SIGN-PSA bound to DC surface, similar to anti–DC-ASGPR-PSA (unpublished data). DCs were loaded with 1 µg/ml PSA fusion proteins or pooled PSA-derived peptides (total 59 peptides of 15-mers overlapping 11 aa, 10 µM), and then incubated with autologous naive CD4+ T cells for 7 d. CD4+ T cells were restimulated with PSA-derived peptides (PSA102-116 or PSA82-96 as a control peptide), which were preselected by testing peptide clusters and individual peptides in the selected clusters. We observed that CD4+ T cells induced with anti–DC-ASGPR-PSA secreted greater amounts of IL-10, but less IFN-γ, TNF, and IL-2 when compared with those induced with anti–LOX-1-PSA, anti–Dectin-1-PSA, and anti–DC-SIGN-PSA (Fig. 3). The amount of IFN-γ secreted from CD4+ T cells induced with anti–DC-ASGPR-PSA was comparable to that secreted from CD4+ T cells induced with peptide pool. However, anti–DC-ASGPR-PSA was more efficient than PSA peptide pool at inducing PSA-specific IL-10–producing CD4+ T cell responses. Interestingly, anti–LOX-1-PSA was more efficient than others at inducing IFN-γ–producing CD4+ T cell responses, although this needs to be further studied. CD4+ T cells induced with anti–LOX-1-PSA also secreted the greatest amount of IL-2 and TNF. Similar to the HA1-specific CD4+ T cell responses (Fig. S3), targeting PSA to DCs via LOX-1, DC-SIGN, or Dectin-1 are more efficient than loading PSA peptide pool to DCs at inducing IFN-γ–producing CD4+ T cell responses. In addition, CD4+ T cells induced with PSA peptide pool or any of those recombinant construct did not secrete significant amounts of IL-4 or IL-5 (unpublished data). Most notably, anti–DC-ASGPR-PSA resulted in the greatest level of IL-10–producing PSA-specific CD4+ T cell responses. Thus, we conclude that DC-ASGPR possesses a specialized function for generating antigen-specific IL-10–producing CD4+ T cells.
Figure 3.
DC-ASGPR ligation generates antigen-specific IL-10–producing CD4+ T cells. IFNDCs (5 × 103) were loaded with 1 µg/ml recombinant fusion proteins of PSA, pooled peptides (10 µM), or none overnight. CFSE-labeled autologous naive CD4+ T cells (1–2 × 105) were co-cultured with primed DCs for 7 d. CD4+ T cells were restimulated with 1 µM PSA102-116. After 48 h, IL-10, IFN-γ, TNF, and IL-2 in the culture supernatants were assessed. Each dot represents the data from independent experiments. P-values were calculated by Student’s t test.
Upon loading with anti–DC-ASGPR-HA1, DCs were more efficient than monocytes and B cells in generating IL-10–producing CD4+ T cells (Fig. S5, A and B). DCs were also more potent than monocytes or B cells in expanding IFN-γ–producing CD4+ T cells when they were loaded with anti–LOX-1-HA1 (Fig. S5, C and D). HA1- and PSA-derived peptides characterized in this study and their corresponding HLA class II types are summarized in Table S1. The majority of HA1- and PSA-derived peptides in Table S1 have not been previously described. Thus, targeting antigens to DCs is an efficient way to elicit high magnitudes and broad repertoires of antigen-specific CD4+ T cells.
Suppressive function of IL-10–producing CD4+ T cells
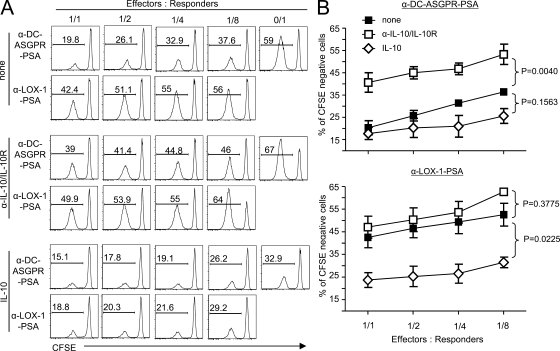
We next tested the suppressive function of IL-10–producing CD4+ T cells generated with anti–DC-ASGPR constructs in both allogeneic and autologous systems. Differentiated Qtracker 565low CD4+ T cells were sorted from co-cultures of autologous naive T cells and IFNDCs loaded with anti–DC-ASGPR-PSA or anti–LOX-1-PSA, and then re-stained with Qtracker 565. CFSE-labeled allogeneic naive CD4+ T cells (responders) were mixed with increasing numbers of the Qtracker 565+CD4+ T cells (effectors), and then co-cultured with anti–DC-ASGPR-PSA–loaded IFNDCs for 5 d. Proliferation of responders was assessed by measuring CFSE dilution. The CD4+ T cells induced with anti–DC-ASGPR-PSA, but not those induced with anti–LOX-1-PSA, significantly inhibited proliferation of responders, and this was dependent on the numbers of effector cells (Fig. 4 A, top). Neutralizing IL-10 partially reverted the inhibition of naive allogeneic CD4+ T cell proliferation (Fig. 4 A, middle). Exogenous IL-10 (100 pg/ml) added to the co-cultures resulted in significantly decreased proliferation of allogeneic CD4+ T cells (Fig. 4 A, bottom). Data from three experiments repeated in Fig. 4 A are summarized in Fig. 4 B. Collectively, IL-10 secreted from effector CD4+ T cells induced with anti–DC-ASGPR-PSA is mainly responsible for the inhibition of allogeneic CD4+ T cell proliferation.
Figure 4.
CD4+ T cells generated with α-DC-ASGPR-PSA suppress allogeneic naive CD4+ T cell proliferation in an IL-10–dependent manner. (A) IFNDCs were loaded with 1 µg/ml α-DC-ASGPR-PSA or α-LOX-1-PSA, and then co-cultured with Qtracker 565–labeled purified autologous naive CD4+ T cells for 7 d. Qtracker 565low CD4+ T cells were sorted as effector cells. Different numbers of effector cells were added into co-cultures of autologous DCs (5 × 103) loaded with 1 µg/ml α-DC-ASGPR-PSA and newly purified CFSE-labeled allogeneic naive CD4+ T cells as responders (105). Proliferation of allogeneic CD4+ T cells was assessed by measuring CFSE dilution. Two independent experiments with triplicate assay were performed in the presence and absence of 10 µg/ml α–IL-10/IL-10R antibodies or 100 pg/ml IL-10. (B) Summarized data from three independent experiments. Error bars represent SD. Statistical significance was tested by ANOVA.
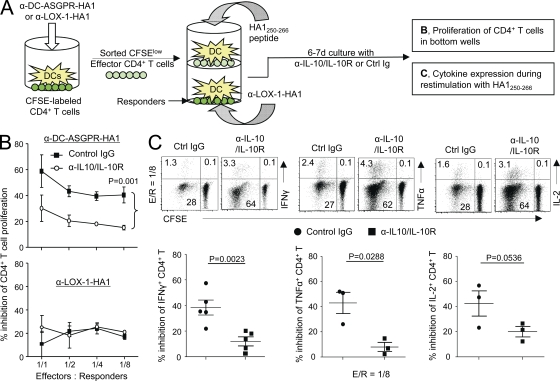
The suppressive function of these IL-10–producing CD4+ T cells was also confirmed in an autologous system using trans-well plates (Fig. 5 A). CD4+ T cells generated with anti–DC-ASGPR-HA1 (Fig. 5 B, top) were more efficient (∼60 and ∼40% at 1:1 and 1:8 effector/responder ratios, respectively) than those generated with anti–LOX-1-HA1 (Fig. 5 B, bottom) at inhibiting autologous CD4+ T cell proliferation. The inhibition of autologous CD4+ T cell proliferation was significantly recovered by the addition of anti–IL-10 and anti–IL-10R antibodies (Fig. 5 B, top). In addition, neutralizing IL-10 secreted from effector CD4+ T cells induced with anti–DC-ASGPR-HA1 resulted in enhanced IFN-γ, IL-2, and TNF expression by HA1250-266-specific CD4+ T cells (Fig. 5 C, top). Summarized data from independent experiments are summarized in Fig. 5 C (bottom). Collectively, we conclude that CD4+ T cells generated with anti–DC-ASGPR-HA1 can suppress proliferation as well as cytokine, IFN-γ, IL-2, and TNF expression in responding cells. This suppression was largely dependent on IL-10 secreted by effector cells.
Figure 5.
CD4+ T cells generated with α-DC-ASGPR-HA1 suppress HA1-specific IFN-γ–, TNF-, and IL-2–producing CD4+ T cell responses. (A) Experimental scheme for B and C. (B) FACS-sorted CFSElow effector cells generated with α-DC-ASGPR-HA1 (top) or α–LOX-1-HA1 (bottom) were co-cultured with IFNDCs (5 × 103) loaded with HA1250-266 in the upper wells. Newly purified and CFSE-labeled CD4+ T cells (1–2 × 105) and IFNDCs (5 × 103) loaded with α–LOX-1-HA1 were co-cultured in the lower wells of trans-well plates. On day 6, proliferation of CD4+ T cells in the lower wells were assessed by measuring CFSE dilution. α–IL-10/IL-10R or control IgG was added. Statistical significance was tested by ANOVA. Three independent experiments showed similar results. Error bars represent SD. (C) Frequency of IFN-γ–, TNF-, and IL-2–expressing CD4+ T cells was measured. Each dot in lower panels represents data from independent experiments. P-values were acquired by Student’s t test.
Mechanisms of DC-ASGPR–mediated generation of antigen-specific IL-10–producing CD4+ T cells
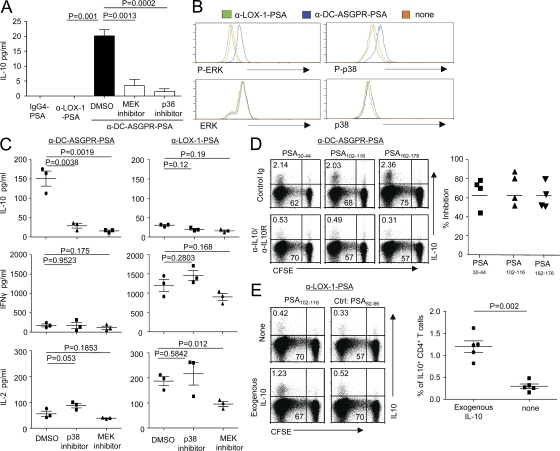
IL-12 (Saraiva et al., 2009), IL-10 (Barrat et al., 2002; Groux et al., 1997), inducible T cell co-stimulator ligand (ICOSL; Ito et al., 2007), and IL-27 (Awasthi et al., 2007) are known to contribute to the generation of IL-10–producing CD4+ T cells. Interestingly, DCs loaded with anti–DC-ASGPR-PSA, but not IgG4-PSA or anti–LOX-1-PSA, secreted IL-10 (Fig. 6 A). This IL-10 induction was dependent on anti–DC-ASGPR mAb (Fig. S6 A) and inhibited by MEK inhibitor (PD0325901), which inhibits extracellular signal-regulated kinase (ERK) activation, and p38 inhibitor (SB203580; Fig. 6 A). In parallel, DCs exposed to anti–DC-ASGPR-PSA displayed enhanced phosphorylation of ERK and p38 (Fig. 6 B). Anti–DC-ASGPR-PSA did not induce IL-12, IL-27, or ICOSL expression (unpublished data).
Figure 6.
DC-ASGPR ligation induces IL-10 from DCs dependent on ERK/p38 contributing to the generation of antigen-specific IL-10–producing CD4+ T cells. (A) IFNDCs (105) were incubated with indicated inhibitors for 1 h, washed, and loaded with 1 µg/ml IgG4-PSA, α-LOX-1-PSA, or α-DC-ASGPR-PSA. After 24 h, culture supernatants were harvested and IL-10 production was assessed. Error bars indicate the mean ± SEM of two independent experiments with triplicate assay. (B) After 15 min of loading IFNDCs with 1 µg/ml recombinant proteins, α-DC-ASGPR-PSA, or α-LOX-1-PSA, cells were stained with indicated antibodies. Representative data from four independent experiments are presented. (C) 5 × 103 IFNDCs were treated with 2.5 µM PD0325901 (MEK inhibitor) or SB203580 (p38 inhibitor) for 1 h and washed thoroughly. DCs were loaded with 1 µg/ml α-DC-ASGPR-PSA or α-LOX-1-PSA. CFSE-labeled autologous naive CD4+ T cells (1–2 × 105) were co-cultured for 7 d. CD4+ T cells were then restimulated with 1 µM PSA30-44 for 48 h. IL-10, IFN-γ, and IL-2 in culture supernatants were assessed. Each dot represents the data generated with a single experiment. Background values acquired with control peptide (PSA82-96) were substracted. (D) Purified naive CD4+ T cells (1–2 × 105) were co-cultured with α-DC-ASGPR-PSA–loaded IFNDCs in the presence of control IgG or α–IL-10/IL-10R antibodies for 7 d. Cells were then restimulated with indicated peptides (1 µM) and stained for intracellular IL-10. Summary of the data from four independent experiments are presented on the right. (E) Naive CD4+ T cells (1–2 × 105) were co-cultured for 7 d with IFNDCs (5 × 103) loaded with 1 µg/ml α-LOX-1-PSA in the presence or absence of 20 pg/ml IL-10. Cells were then restimulated with indicated peptides (1 µM) and stained for intracellular IL-10. Summary of the data from five independent experiments are presented in right panel. P-values were calculated with Student’s t test.
To test the role of IL-10 secreted from DCs in the induction of IL-10–producing antigen-specific CD4+ T cells, DCs were first treated with either the MEK inhibitor or p38 inhibitor, and residual inhibitors were washed out. DCs were then loaded with either anti–DC-ASGPR-PSA or anti–LOX-1-PSA, and then co-cultured with naive CD4+ T cells for 7 d. Treatment of DCs with the inhibitors resulted in significantly decreased IL-10 secretion from CD4+ T cells induced with anti–DC-ASGPR-PSA (Fig. 6 C, left). Treatment of DCs with MEK inhibitor resulted in slightly decreased IL-2 secretion from CD4+ T cells induced with anti–LOX-1-PSA, but p38 inhibitor did not significantly alter cytokine secretion (Fig. 6 C, right). Thus, the decreased IL-10–producing CD4+ T cell responses by the inhibitors are not caused by the general reduction of CD4+ T cell responses induced by anti–DC-ASGPR-PSA. Indeed, blocking IL-10 in co-cultures of naive CD4+ T cells and DCs loaded with anti–DC-ASGPR-PSA resulted in a significantly decreased PSA-specific IL-10–producing CD4+ T cell responses (Fig. 6 D), but not IFN-γ– or IL-2-producing CD4+ T cell responses (not depicted). Conversely, exogenous IL-10 added to the co-culture of anti–LOX-1-PSA-loaded DCs and naive CD4+ T cells could enhance PSA-specific IL-10–producing CD4+ T cell responses (Fig. 6 E). We also confirmed that IL-10 induction in DCs is mediated by anti–DC-ASGPR mAb (Fig. S6 A). In addition, DCs loaded with combinations of PSA peptide pool and anti–DC-ASGPR mAb can induce IL-10–producing CD4+ T cell responses, although anti–DC-ASGPR-PSA was more efficient than combinations of PSA peptide pool and anti–DC-ASGPR mAb (Fig. S6 B). Furthermore, Fig. S6 C demonstrates that anti–DC-ASGPR-HA1 could prevail over anti–LOX-1-HA1 to elicit IL-10– and IFN-γ–producing HA1-specific CD4+ T cell responses. It is also important to note that naive CD4+ T cells co-cultured with DCs loaded with anti–DC-ASGPR-PSA do not express IL-10 unless they are restimulated with PSA-derived peptides. This rules out the contribution of autocrine IL-10 production by T cells in the induction of IL-10–producing CD4+ T cells. We also demonstrate that the outcomes of antigen targeting to DC-ASGPR or LOX-1 using our recombinant mAbs were not significantly affected by Fcγ receptors expressed on DCs (Fig. S7). Collectively, we conclude that anti–DC-ASGPR mAb induces ERK/p38-phosphorylation and IL-10 expression in DCs, which contribute to the induction of antigen-specific IL-10–producing CD4+ T cells.
In vivo establishment of antigen-specific IL-10–producing CD4+ T cells
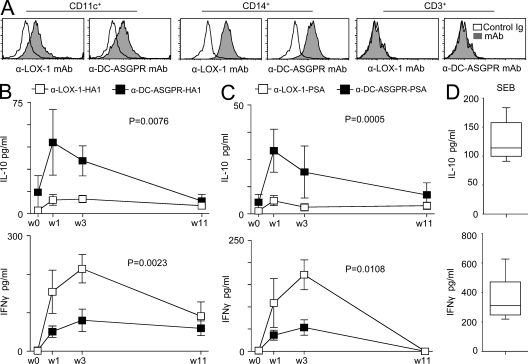
We next assessed whether the anti–DC-ASGPR–based constructs would induce high IL-10–producing antigen-specific CD4+ T cells in vivo using nonhuman primates. DC-ASGPR is not expressed in mouse. As observed with human DCs, both anti–LOX-1 and anti–DC-ASGPR mAbs bound to CD11c+ and CD14+ cells in PBMCs of cynomolgus macaques (Fig. 7 A). All animals (total 12 animals: 6 animals per group) were preimmunized with live influenza viruses (H1N1, PR8). Sera from all animals displayed HA1-specific IgG (unpublished data). 4 mo after priming, animals were immunized i.d. with either anti–LOX-1-HA1 (right arm) and anti–LOX-1-PSA (left arm), or anti–DC-ASGPR-HA1 (right arm) and anti–DC-ASGPR-PSA (left arm). After three immunizations at 40-d intervals with the same recombinant fusion proteins, blood was collected as indicated in Fig. 7, B and C. PBMCs from animals immunized with anti–DC-ASGPR-HA1 secreted higher levels of IL-10 in response to the HA1 peptide pool when compared with those immunized with anti–LOX-1-HA1 (Fig. 7 B, top). Conversely, PBMCs from animals immunized with anti–LOX-1-HA1 secreted significantly higher levels of IFN-γ than animals immunized with anti–DC-ASGPR-HA1 (Fig. 7 B, bottom). The same findings were made with animals that were primed and boosted twice with PSA fusion proteins. PSA-specific IL-10–producing cellular responses were preferentially mounted in animals immunized with anti–DC-ASGPR-PSA (Fig. 7 C, top). Consistent with our in vitro data (Figs. 2 and 3), animals immunized with anti–LOX-1-PSA mounted higher PSA-specific IFN-γ–producing cellular responses than animals immunized with anti–DC-ASGPR-PSA (Fig. 7 C, bottom). For both HA1 and PSA, the peak of IL-10–producing cellular responses was obtained at week one, but the peak of IFN-γ–producing cellular responses was obtained at week three. Levels of IL-10 and IFN-γ secreted from Staphylococcal enterotoxin B–stimulated PBMCs are presented in Fig. 7 D. Collectively, targeting antigens to in vivo DCs via DC-ASGPR can establish antigen-specific IL-10–producing T cells in vivo.
Figure 7.
Immunization with α-DC-ASGPR-HA1 and α-DC-ASGPR-PSA generates antigen-specific IL-10–producing T cell responses in nonhuman primates. (A) Expression levels of LOX-1 and DC-ASGPR in PBMCs of cynomolgus macaques. (B and C) 12 animals primed and boosted i.d. with live influenza viruses (H1N1, A/PR8/38) were divided into two groups (6 animals/group). One group of animals was immunized i.d. with 250 µg α-LOX-1-HA1 (right arm) and 250 µg α-LOX-1-PSA (left arm). The other group was immunized i.d. with 250 µg α-DC-ASGPR-HA1 (right arm) and 250 µg α-DC-ASGPR-PSA (left arm). After the second boosting with the recombinant fusion proteins, PBMCs (2 × 105 cells/200 µl of medium/well in 96-well plates) were restimulated with 25 µM peptide pools of HA1 (B) or PSA (C), respectively. Cytokines in the culture supernatants were measured by ELISA. Peptide pools of HIVgag were controls. Values presented in B and D are after substraction of control values. Statistical significance was tested by ANOVA. (D) 2 × 105/200 µl PBMCs were stimulated with 0.1 µg/ml Staphylococcal enterotoxin B for 48h. IL-10 and IFN-γ levels in culture supernatants were measured.
DISCUSSION
DCs induce and control immune responses. The complexity of DC-mediated regulation of host immune responses has been mainly explained by the functional plasticity driven by external stimuli, as well as by the presence of different subsets of DCs. In this study, we demonstrated that DC-ASGPR expressed on DCs has a novel function to generate antigen-specific IL-10–producing CD4+ T cells both in vitro and in vivo.
DC-ASGPR was first cloned by Valladeau et al. (2001), and it has been known as a scavenger receptor. We further demonstrate that DC-ASGPR can deliver intracellular signals to activate DCs. As results of activation, DCs secrete IL-10 and chemokines (MCP-1 and MIP-1α; unpublished data). DC-ASGPR–mediated IL-10 induction in DCs requires ERK/p38 phosphorylation (Saraiva et al., 2009). This IL-10 contributes to the generation of IL-10–producing CD4+ T cells, as previously described (Groux et al., 1997; Barrat et al., 2002). Furthermore, differences in IL-10 production by DCs correlate with ERK activation (Kaiser et al., 2009). Ligation of DC-ASGPR did not induce IL-12, IL-27, or ICOSL expression in DCs. We have previously described that ligation of Dectin-1 with anti–Dectin-1 mAb delivers signals through Syk and induces human DCs to secrete significant amount of IL-10, as well as IL-1β and IL-6 (Ni et al., 2010). However, activation of DCs via Dectin-1 did not result in polarized IL-10–producing CD4+ T cell responses. Indeed, DCs activated via Dectin-1 result in polarized Th17 responses (LeibundGut-Landmann et al., 2007; Saijo et al., 2007; Gringhuis et al., 2009). Cross-linking of DC-SIGN with anti–DC-SIGN mAbs or anti–DC-SIGN fusion proteins was able to activate DCs only when DCs were co-activated with TLR2 ligands, resulting in MIP-1α and minimal level of IL-10 secretion (unpublished data). Subsequently, DCs loaded with anti–DC-SIGN fusion proteins did not favor the generation of IL-10–producing CD4+ T cells. Thus, the altered T cell responses (Bergman et al., 2004; Caparrós et al., 2006; Geurtsen et al., 2009) by pathogens recognized by DC-SIGN may not be solely dependent on signal via DC-SIGN. Pathogens and pathogen-derived components could also bind to and trigger other receptors that are not yet characterized. In support of this, signals via DC-SIGN induce IL-10 only when DCs are activated via DC-SIGN in the presence of other Toll-like receptor– or cytokine-mediated signals (Caparrós et al., 2006; Gringhuis et al., 2007; Geurtsen et al., 2009). Notably, targeting antigens to DCs via LOX-1 resulted in significantly greater level of IFN-γ–producing CD4+ T cell responses than loading peptide antigens to DC or targeting the same antigens to DCs via any other molecule tested in this study. Collectively, we conclude that DC-ASGPR possesses a unique function that favors immune responses toward IL-10–producing regulatory CD4+ T cell responses. Our data represent a new biological function for DC-ASGPR to induce antigen-specific IL-10–producing CD4+ T cells. In addition, our data show that individual LLRs expressed on DCs possess both common and unique functions that contribute to host immune responses in different ways. Thus, it is now possible to postulate that delivering antigens to the same type of DCs, but via different LLRs, can evoke different quantity and quality of antigen-specific CD4+ T cell responses.
Delivering both self- (PSA) and foreign antigens (HA1) to DCs via DC-ASGPR results in antigen-specific IL-10–producing CD4+ T cell responses. In addition, antigens delivered to DCs via DC-ASGPR could polarize both naive and memory CD4+ T cells toward IL-10–producing suppressive cells. The majority of HA1-specific CD4+ T cell responses observed in this study were caused by the activation of preexisting memory CD4+ T cells. Although fractions of both PSA- and HA1-specific IL-10–producing CD4+ T cells expressed CTLA-4 and PD-1 to a lesser extent, suppressive function of those effector cells were mainly dependent on IL-10. IL-10–mediated suppression of CD4+ T cell proliferation and IFN-γ expression did not require large numbers of IL-10–secreting effector cells, a finding in line with previous studies (Bacchetta et al., 1994; Groux et al., 1997; Levings et al., 2001; Barrat et al., 2002; Roncarolo and Battaglia, 2007). A minute amount of IL-10 secreted by regulatory T cells can efficiently suppress proliferation and IFN-γ expression by responding cells. To further determine whether those IL-10–producing cells were from Th1, cells were restimulated with PMA/ionomycin; our data showed that the majority of IL-10–producing cells can also express IFN-γ (Macián et al., 2002; Saraiva et al., 2009). IL-10–producing CD4+ T cells of Th1 origin plays crucial roles in limiting host immune pathology (Jankovic et al., 2007; O’Garra and Vieira, 2007; Roncarolo and Battaglia, 2007; Trinchieri, 2007; Gabrysová et al., 2009).
The nature of cytokines produced by CD4+ T cells is a crucial element that directs the quantity and quality of immune responses. IL-10–producing CD4+ T cells are major effectors limiting immune pathology in autoimmune and inflammatory diseases (Moore et al., 2001; Anderson et al., 2007; Jankovic et al., 2007; Roncarolo and Battaglia, 2007; Trinchieri, 2007; Gabrysová et al., 2009; Häringer et al., 2009; Saraiva and O’Garra, 2010). Administration of IL-10 in clinical trials has so far shown limited efficacy (Moore et al., 2001; Schreiber et al., 2000; Baumgart and Sandborn, 2007). Although immunizations of soluble antigens have been shown to induce IL-10 in mouse in vivo models (Gabrysová et al., 2009), their use in human disease is limited as result of potential induction of inflammatory effector cytokines with deleterious side effects, which led to interruption of such trials for multiple sclerosis (MS; Kappos et al., 2000). Recent studies have demonstrated that in vitro expanded regulatory T cells, particularly alloantigen-specific regulatory T cells (Sagoo et al., 2011), have clinical efficacy at preventing graft rejection in humanized mice (Feng et al., 2011; Sagoo et al., 2011). However, adoptive transfer of in vitro–generated regulatory T cells has not been successful yet in patients (Roncarolo and Battaglia, 2007). Thus, a strategy for in vivo establishment of IL-10–producing antigen-specific CD4+ T cells has long been demanded for the management of such diseases. This study provides a new concept for the establishment of antigen-specific IL-10–producing regulatory T cells in vivo by targeting whole protein antigens to DCs via DC-ASGPR. Furthermore, DC-ASGPR appears to be a universal target for designing vaccines against autoimmune diseases where autoantigens are well defined, such as type 1 diabetes and multiple sclerosis. Therapeutic efficacy of such vaccines needs to be tested in clinical trials. Beyond the ability of generating antigen-specific regulatory T cells, IL-10 produced through anti–DC-ASGPR engagement may be expected to have potential positive effects on antigen-specific antibody production, and this will need to be examined carefully in future studies.
MATERIALS AND METHODS
Cells.
Monocyte-derived DCs, IFNDCs, and IL-4DCs, were generated as previously described (Rogers et al., 2005; Ni et al., 2010). Total CD4+ T cells were purified using CD4+ T cell enrichment kits (StemCell) and naive CD4+ T cells (CD45RA+CD45RO−; purity > 99.8%) from healthy male donors were sorted by FACSAria (BD). Naive CD4+ T cells were used for assessing PSA-specific T cell responses. Total CD4+ T cells were used for testing HA1-specific CD4+ T cell responses. Blood mDCs (Lin−HLA-DR+CD11c+CD123−) were sorted (purity > 98%). CD14+ monocytes and CD19+ B cells were purified by using StemCell enrichment kits. All work related to human subjects was in compliance with Institutional Review Board protocols approved by the Baylor Research Institute Review Board.
Monoclonal antibodies (mAbs) to lectins.
Mouse mAbs specific for human DC-ASGPR, LOX-1, DC-SIGN, and Dectin-1 were generated as previously described (Rogers et al., 2005; Ni et al., 2010).
Other antibodies, reagents, and peptides.
Fluorescent-labeled anti-CD3, anti-CD4, anti-CD11c, anti-CD14, anti-CD19, anti-CD123, anti-CTLA-4, anti-CD1c, anti-HLA-DR, anti-PD-1, and anti-Foxp3 antibodies were purchased from BioLegend. Anti-IFN-γ and anti-IL-10 mAbs were purchased from BD. Anti−LOX-1 mAb for immunofluorescent staining was purchased from Abcam (MA). IL-10 (R&D Systems), anti-IL-10 (BIIR) and anti-IL-10R antibodies (R&D Systems) were used. Anti-CD16 (BD), anti-CD32 (BD), and anti-CD64 (R&D Systems) antibodies were used. GM-CSF, IL-4 and IFN-α were purchased from the pharmacy at Baylor University Medical Center (TX). CFSE (Invitrogen), PD0325901 (Selleck Chemicals) and SB203580 (EMD) were used. Peptide libraries were purchased from Mimotopes.
Recombinant mAbs and fusion proteins of antigens.
Total RNA from hybridomas was used for cDNA synthesis and PCR amplification (BD) using the 5′ primers provided by the kits and the Ig constant-specific 3′ primers as previously described (Rogers et al., 2005; Ni et al., 2010). For constructing the full-length chimeric antibodies, variable region-specific primers with flanking restriction sites were used to amplify DNA from either selected clonal TA plasmids or original cDNA. Enzyme-digested PCR fragments were cloned into a modified pIRES2-DsRed2 vector (BD) with the constant-coding region of either mutated hIgG4H (S228P and L235E) or the wild-type hIgK that were previously engineered in the vector. The Flu HA1 fragment containing bp 52–993 of Influenza A virus (A/PR8/34, H1N1) hemagglutinin (gi/21693168/gb/AF389118.1/; with A619C and C959T changes) was engineered with a flanking NheI site followed by 5′-GATACAACAGAACCTGCAACACCTACAACACCTGTAACAACA-3′ at the 5′ end and 6xHis tag coding sequence followed by stop codon and NotI site at the 3′ end, which was fused downstream of and in-frame with the H chain coding region at the NheI site by NheI-Not I swamping. Stable CHO-S cells were made and grown in GlutaMAX and HT media (Invitrogen) and recombinant proteins were purified by protein A column chromatography. Similarly, recombinant antibody-PSA fusion proteins (rAb.PSA) were encoded by inserting gi/34784812/ prostate-specific antigen residues 101–832 with proximal sequence 5′-GCTAGCGATACAACAGAACCTGCAACACCTACAACACCTGTAACAACACCGACAACAACACTTCTAGCGC-3′ (NheI site and Clostridium thermocellum CipA-derived spacer) and a distal NotI site into the same H chain vector. Recombinant antibody proteins were expressed in stable CHO-S transfectants and purified as described for hFc fusion proteins. Endotoxin levels were <0.2 U/ml.
CD4+ T cell responses.
5 × 103 IFNDCs loaded with 1–2 µg/ml recombinant proteins were co-cultured with 2 × 105 CFSE-labeled CD4+ T cells. Cell proliferation was tested by measuring CFSE dilution on day 6. CD4+ T cells were restimulated with peptides for 6 h in the presence of brefeldin A (BFA) for staining IFN-γ, IL-2, IL-4, IL-5, and TNF. For IL-10, cells were activated with peptide for 18-24 h and BFA was added for the final 6–8 h of restimulation. Cytokine levels in the culture supernatants were measured using the BeadLyte Luminex assay kit (Millipore) as per the manufacturer’s protocol. In suppression assays, effector cells were obtained by sorting Qtracker 565low CD4+ T cells (effectors) after 1 wk co-cultures of Qtracker 565 (InvivoGen)-labeled autologous naive CD4+ T cells and IFNDCs loaded with recombinant fusion proteins. FACS-sorted effector cells were restained with Qtracker 565 to separate them from responding cells, CFSE-labeled allogeneic naive CD4+ T cells. Mixtures of different ratios of effectors and responders were co-cultured with DCs loaded with 1 µg/ml anti–DC-ASGPR-PSA in the presence of 10 µg/ml anti–IL-10/IL-10R antibodies, 10 µg/ml control antibodies, or 100 pg/ml IL-10 for 5 d. Proliferation of responding cells was measured by CFSE dilution. Trans-well plates (Nunc) were used in autologous HA1-specific suppression assays. CFSElow CD4+ T cells (effectors) were sorted from co-cultures of total CD4+ T cells and IFNDCs loaded with anti–DC-ASGPR-HA1 or anti–LOX-1-HA1. Effectors in upper wells were activated with DCs loaded with 1–5 µM HA1-derived peptides. Newly purified and CFSE-labeled CD4+ T cells (responders) were co-cultured with IFNDCs loaded with anti–LOX-1-HA1 in lower wells. Anti–IL-10 and anti–IL-10R antibodies were added into lower wells.
Phospho-flow cytometry.
IFNDCs were loaded with 1 µg/ml recombinant fusion proteins in PBS for 15 min at 37°C. Cells were stained with primary antibody, rabbit anti-phospho-ERK, or anti-phospho-p38 mitogen-activated protein kinase antibodies (Cell Signaling Technology), and then stained with fluorescently labeled goat anti–rabbit IgG (Sigma-Aldrich).
Immunofluorescent staining of human skins.
Human abdominal skins were obtained from healthy donors who underwent cosmetic surgeries at Baylor University Medical Center, according to Institutional Review Board guidelines. Cryosections were fixed in cold acetone, dried, and blocked for nonspecific fluorescence with Fc Receptor Block and Background Buster (Innovex). Sections were incubated in either anti–LOX-1, anti–DC-ASGPR, or isotype antibodies labeled with Alexa Fluor 568 (Invitrogen). After blocking with normal mouse serum, sections were stained with anti–CD1c-Alexa Fluor 647, anti–HLA-DR-FITC, and then subsequently stained with DAPI (Invitrogen). Digital images were taken using an Olympus BX51 with a Planapo20/0.7 or Planapo40/0.95 objective, a Roper Coolsnap HQ camera and Metamorph software (Molecular Devices). Images were acquired using the same exposures for antibody and isotype staining and identical scaling was applied. Confocal images were taken with the Leica SP1 and Planapo63/1.32 objective.
Animals and immunization.
12 male cynomolgus macaques (Macaca fascicularis), weighing 3–6 kg, were maintained and handled in accordance with European guidelines for nonhuman primate care (EEC Directive N 86–609, November 24, 1986). All work related to animals was in compliance with Institutional Review Board protocols approved by the Institute of Emerging Diseases and Innovative Therapies, Commissariat á l’Energie Atomique. Animals were primed and boosted i.d. with 107 PFU live influenza viruses, and then divided into 2 groups (6 animals/group). One group was immunized and boosted twice i.d. with 250 µg/dose anti–LOX-1-HA1 (right arm) and anti–LOX-1-PSA (left arm). Another group was immunized i.d. with 250 µg/dose anti–DC-ASGPR-HA1 (right arm) and anti–DC-ASGPR-PSA (left arm). Boosting was performed at every 5–6 wk after each immunization. After the second boosting with recombinant vaccines, PBMCs were isolated from blood using Percoll gradients (GE Healthcare). PBMCs were then restimulated with 25 µM peptide pools of PSA and HA1, respectively, and supernatant was harvested after 36 and 72 h to measure the levels of IFN-γ and IL-10, respectively, using ELISA kits (U-CyTech).
Statistical analysis.
Bar graphs represent mean ± SD. Significance of difference between experimental variables was determined using the Student’s t test and analysis of variance (ANOVA).
Online supplemental material.
Fig. S1 shows that human skin dermal DCs express DC-ASGPR and LOX-1. Fig. S2 shows that antigen targeting to DCs via DC-ASGPR or LOX-1 results in antigen-specific CD4+ T cell responses. Fig. S3 demonstrates that combinations of HA1 peptide pool and anti–DC-ASGPR or anti–LOX-1 mAb are more efficient than HA1 peptide pool alone, but less efficient than anti–DC-ASGPR-HA1 and anti–LOX-1-HA1 at eliciting HA1-specific IL-10– and IFN-γ–producing CD4+ T cell responses, respectively. Fig. S4 demonstrates that both HA1 and PSA delivered to DCs via DC-ASGPR results in enhanced HA1- and PSA-specific IL-10–producing CD4+ T cell responses. Fig. S5 shows that DCs are more potent than other antigen-presenting cells in generating antigen-specific IL-10– and IFN-γ–producing CD4+ T cells. Fig. S6 demonstrates that anti–DC-ASGPR mAb alone induces DCs to secrete IL-10; that combinations of PSA peptide pool and anti–DC-ASGPR mAb are more efficient than peptide pool alone, but less efficient than anti–DC-ASGPR-PSA fusion construct at inducing IL-10–producing CD4+ T cells; and that anti–DC-ASGPR-HA1 could dominate over anti–LOX-1-HA1 for inducing HA1-specific IL-10–producing CD4+ T cells. Fig. S7 shows that HA1-specific IL-10– and IFN-γ–producing CD4+ T cell responses elicited by anti–DC-ASGPR-HA1 and anti–LOX-1-HA1, respectively, are not significantly affected by Fcγ receptors expressed on DCs. Table S1 summarizes the HA1 and PSA peptide epitopes and HLA class II types characterized in this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110399/DC1.
Acknowledgments
We thank the FACS Core (Elizabeth Kowalski and Sebastien Coquery at Baylor Institute for Immunology Research [BIIR]), Cell Processing Core (Lynnette Walters at BIIR), and Imaging Core (Sandra Clayton and Dr. Jean-Pierre Gorvel at BIIR). We thank Dr. Isabelle Mangeot (Commissariat á l’Energie Atomique, France), Shannon Lunt (BIIR), and Dr. Steven Phillips (BIIR) for their help in organizing animal samples. We thank Drs. Carson Harrod (BIIR), Maryna Eichelberger (Food and Drug Administration), and Jay A. Berzofsky (National Cancer Institute, National Institutes of Health) for critical reading of this manuscript. We also thank Dr. Michael Ramsay for supporting our program.
This study was funded by U19 AI057234, Baylor Health Care System Foundation, and Agence Nationale de Recherches sur le SIDA (ANRS) HIV Vaccine Network. G. Romain received a fellowship from the ANRS and Sidaction, Paris, France.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ANOVA
- analysis of variance
- DC-ASGPR
- DC-asialoglycoprotein receptor
- DC-SIGN
- DC-specific ICAM-3–grabbing nonintegrin
- ERK
- extracellular signal-regulated kinase
- HA
- hemagglutinin
- ICOSL
- inducible T cell co-stimulator ligand
- LLR
- lectinlike receptor
- LOX-1
- lectinlike oxidized-LDL receptor
- PSA
- prostate-specific antigen
References
- Akira S., Takeda K., Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680 10.1038/90609 [DOI] [PubMed] [Google Scholar]
- Anderson C.F., Oukka M., Kuchroo V.J., Sacks D. 2007. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297 10.1084/jem.20061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A., Carrier Y., Peron J.P., Bettelli E., Kamanaka M., Flavell R.A., Kuchroo V.K., Oukka M., Weiner H.L. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8:1380–1389 10.1038/ni1541 [DOI] [PubMed] [Google Scholar]
- Bacchetta R., Bigler M., Touraine J.L., Parkman R., Tovo P.A., Abrams J., de Waal Malefyt R., de Vries J.E., Roncarolo M.G. 1994. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J. Exp. Med. 179:493–502 10.1084/jem.179.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- Barrat F.J., Cua D.J., Boonstra A., Richards D.F., Crain C., Savelkoul H.F., de Waal-Malefyt R., Coffman R.L., Hawrylowicz C.M., O’Garra A. 2002. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195:603–616 10.1084/jem.20011629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart D.C., Sandborn W.J. 2007. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 369:1641–1657 10.1016/S0140-6736(07)60751-X [DOI] [PubMed] [Google Scholar]
- Bergman M.P., Engering A., Smits H.H., van Vliet S.J., van Bodegraven A.A., Wirth H.P., Kapsenberg M.L., Vandenbroucke-Grauls C.M., van Kooyk Y., Appelmelk B.J. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979–990 10.1084/jem.20041061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.D. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6:33–43 10.1038/nri1745 [DOI] [PubMed] [Google Scholar]
- Caparrós E., Munoz P., Sierra-Filardi E., Serrano-Gómez D., Puig-Kröger A., Rodríguez-Fernández J.L., Mellado M., Sancho J., Zubiaur M., Corbí A.L. 2006. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 107:3950–3958 10.1182/blood-2005-03-1252 [DOI] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 184:695–706 10.1084/jem.184.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delneste Y., Magistrelli G., Gauchat J., Haeuw J., Aubry J., Nakamura K., Kawakami-Honda N., Goetsch L., Sawamura T., Bonnefoy J., Jeannin P. 2002. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 17:353–362 10.1016/S1074-7613(02)00388-6 [DOI] [PubMed] [Google Scholar]
- Dillon S., Agrawal S., Banerjee K., Letterio J., Denning T.L., Oswald-Richter K., Kasprowicz D.J., Kellar K., Pare J., van Dyke T., et al. 2006. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116:916–928 10.1172/JCI27203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D., Kamphorst A.O., Heidkamp G.F., Buchholz V.R., Trumpfheller C., Yamazaki S., Cheong C., Liu K., Lee H.W., Park C.G., et al. 2007. Differential antigen processing by dendritic cell subsets in vivo. Science. 315:107–111 10.1126/science.1136080 [DOI] [PubMed] [Google Scholar]
- Feng G., Nadig S.N., Backdahl L., Beck S., Francis R.S., Schiopu A., Whatcott A., Wood K.J., Bushell A. 2011. Functional regulatory T cells produced by inhibiting cyclic nucleotide phosphodiesterase type 3 prevent allograft rejection. Sci. Transl. Med. 3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor C.G., van Kooyk Y., Adema G.J. 2002. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2:77–84 10.1038/nri723 [DOI] [PubMed] [Google Scholar]
- Gabrysová L., Nicolson K.S., Streeter H.B., Verhagen J., Sabatos-Peyton C.A., Morgan D.J., Wraith D.C. 2009. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J. Exp. Med. 206:1755–1767 10.1084/jem.20082118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Gringhuis S.I. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9:465–479 10.1038/nri2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Vliet S.J., Engering A., ’t Hart B.A., van Kooyk Y. 2004. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 22:33–54 10.1146/annurev.immunol.22.012703.104558 [DOI] [PubMed] [Google Scholar]
- Geurtsen J., Chedammi S., Mesters J., Cot M., Driessen N.N., Sambou T., Kakutani R., Ummels R., Maaskant J., Takata H., et al. 2009. Identification of mycobacterial alpha-glucan as a novel ligand for DC-SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. J. Immunol. 183:5221–5231 10.4049/jimmunol.0900768 [DOI] [PubMed] [Google Scholar]
- Gringhuis S.I., den Dunnen J., Litjens M., van Het Hof B., van Kooyk Y., Geijtenbeek T.B. 2007. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 26:605–616 10.1016/j.immuni.2007.03.012 [DOI] [PubMed] [Google Scholar]
- Gringhuis S.I., den Dunnen J., Litjens M., van der Vlist M., Wevers B., Bruijns S.C., Geijtenbeek T.B. 2009. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 10:203–213 10.1038/ni.1692 [DOI] [PubMed] [Google Scholar]
- Groux H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742 10.1038/39614 [DOI] [PubMed] [Google Scholar]
- Häringer B., Lozza L., Steckel B., Geginat J. 2009. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J. Exp. Med. 206:1009–1017 10.1084/jem.20082238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Yang M., Wang Y.H., Lande R., Gregorio J., Perng O.A., Qin X.F., Liu Y.J., Gilliet M. 2007. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204:105–115 10.1084/jem.20061660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. 2007. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser F., Cook D., Papoutsopoulou S., Rajsbaum R., Wu X., Yang H.T., Grant S., Ricciardi-Castagnoli P., Tsichlis P.N., Ley S.C., O’Garra A. 2009. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J. Exp. Med. 206:1863–1871 10.1084/jem.20091059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Comi G., Panitch H., Oger J., Antel J., Conlon P., Steinman L.; The Altered Peptide Ligand in Relapsing MS Study Group 2000. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat. Med. 6:1176–1182 10.1038/80525 [DOI] [PubMed] [Google Scholar]
- Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A.K., et al. 2008. Functional specializations of human epidermal Langerhans cells and CD14(+) dermal dendritic cells. Immunity. 29:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- Levings M.K., Sangregorio R., Galbiati F., Squadrone S., de Waal Malefyt R., Roncarolo M.G. 2001. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166:5530–5539 [DOI] [PubMed] [Google Scholar]
- Macián F., García-Cózar F., Im S.H., Horton H.F., Byrne M.C., Rao A. 2002. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 109:719–731 10.1016/S0092-8674(02)00767-5 [DOI] [PubMed] [Google Scholar]
- Maldonado-López R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. 1999. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592 10.1084/jem.189.3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- Ni L., Gayet I., Zurawski S., Duluc D., Flamar A.L., Li X.H., O’Bar A., Clayton S., Palucka A.K., Zurawski G., et al. 2010. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J. Immunol. 185:3504–3513 10.4049/jimmunol.1000999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Garra A., Vieira P. 2007. T(H)1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 7:425–428 10.1038/nri2097 [DOI] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 96:1036–1041 10.1073/pnas.96.3.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M.P., Kinney C.A., Chaikin M.A., Payne A., Fishman-Lobell J., Tsui P., Dal Monte P.R., Doyle M.L., Brigham-Burke M.R., Anderson D., et al. 2000. Elimination of Fc receptor-dependent effector functions of a modified IgG4 monoclonal antibody to human CD4. J. Immunol. 164:1925–1933 [DOI] [PubMed] [Google Scholar]
- Rissoan M.-C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.-J. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186 10.1126/science.283.5405.1183 [DOI] [PubMed] [Google Scholar]
- Rogers N.C., Slack E.C., Edwards A.D., Nolte M.A., Schulz O., Schweighoffer E., Williams D.L., Gordon S., Tybulewicz V.L., Brown G.D., Reis e Sousa C. 2005. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 22:507–517 10.1016/j.immuni.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Roncarolo M.G., Battaglia M. 2007. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 7:585–598 10.1038/nri2138 [DOI] [PubMed] [Google Scholar]
- Sagoo P., Ali N., Garg G., Nestle F.O., Lechler R.I., Lombardi G. 2011. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 3:83ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S., Fujikado N., Furuta T., Chung S.H., Kotaki H., Seki K., Sudo K., Akira S., Adachi Y., Ohno N., et al. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8:39–46 10.1038/ni1425 [DOI] [PubMed] [Google Scholar]
- Saraiva M., O’Garra A. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170–181 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- Saraiva M., Christensen J.R., Veldhoen M., Murphy T.L., Murphy K.M., O’Garra A. 2009. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 31:209–219 10.1016/j.immuni.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Fedorak R.N., Nielsen O.H., Wild G., Williams C.N., Nikolaus S., Jacyna M., Lashner B.A., Gangl A., Rutgeerts P., et al. 2000. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 119:1461–1472 10.1053/gast.2000.20196 [DOI] [PubMed] [Google Scholar]
- Shortman K., Liu Y.J. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161 10.1038/nri746 [DOI] [PubMed] [Google Scholar]
- Smits H.H., Engering A., van der Kleij D., de Jong E.C., Schipper K., van Capel T.M., Zaat B.A., Yazdanbakhsh M., Wierenga E.A., van Kooyk Y., Kapsenberg M.L. 2005. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 115:1260–1267 10.1016/j.jaci.2005.03.036 [DOI] [PubMed] [Google Scholar]
- Soares H., Waechter H., Glaichenhaus N., Mougneau E., Yagita H., Mizenina O., Dudziak D., Nussenzweig M.C., Steinman R.M. 2007. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 204:1095–1106 10.1084/jem.20070176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Hawiger D., Nussenzweig M.C. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- Trinchieri G. 2007. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 204:239–243 10.1084/jem.20070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J., Duvert-Frances V., Pin J.J., Kleijmeer M.J., Ait-Yahia S., Ravel O., Vincent C., Vega F., Jr, Helms A., Gorman D., et al. 2001. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J. Immunol. 167:5767–5774 [DOI] [PubMed] [Google Scholar]