Abstract
The lack of efficacy for antipsychotics with respect to negative symptoms and cognitive deficits is a significant obstacle for the treatment of schizophrenia. Developing new drugs to target these symptoms requires appropriate neural biomarkers that can be investigated in model organisms, be used to track treatment response, and provide insight into pathophysiological disease mechanisms. A growing body of evidence indicates that neural oscillations in the gamma frequency range (30–80 Hz) are disturbed in schizophrenia. Gamma synchrony has been shown to mediate a host of sensory and cognitive functions, including perceptual encoding, selective attention, salience, and working memory – neurocognitive processes that are dysfunctional in schizophrenia and largely refractory to treatment. This review summarizes the current state of clinical literature with respect to gamma band responses (GBRs) in schizophrenia, focusing on resting and auditory paradigms. Next, preclinical studies of schizophrenia that have investigated gamma band activity are reviewed to gain insight into neural mechanisms associated with these deficits. We conclude that abnormalities in gamma synchrony are ubiquitous in schizophrenia and likely reflect an elevation in baseline cortical gamma synchrony (‘noise’) coupled with reduced stimulus-evoked GBRs (‘signal’). Such a model likely reflects hippocampal and cortical dysfunction, as well as reduced glutamatergic signaling with downstream GABAergic deficits, but is probably less influenced by dopaminergic abnormalities implicated in schizophrenia. Finally, we propose that analogous signal-to-noise deficits in the flow of cortical information in preclinical models are useful targets for the development of new drugs that target the treatment-resistant symptoms of schizophrenia.
Keywords: schizophrenia, gamma oscillations, electrophysiology, endophenotype, animal models, GABA
1.1 Introduction
Schizophrenia is a debilitating neuropsychiatric illness with a prevalence of 1–2%. Core impairments include positive symptoms (hallucinations, delusions), negative symptoms (flat affect, impoverished speech, social deficits, anhedonia, avolition), and cognitive deficits (attention, working memory, executive function). Additionally, there are significant abnormalities in sensory and perceptual processing. Numerous antipsychotic medications are available and are characterized by a strong correlation between dose and dopamine D2 receptor affinity. This led to the “dopamine hypothesis,” which attributes disease pathogenesis to excess mesolimbic dopamine signaling. Whereas these drugs are effective in treating positive symptoms, they have little efficacy for negative and cognitive symptoms. These “treatment-resistant symptoms” are frequently associated, suggesting a potential pathophysiological link, and the severity of these deficits are the best predictors of long-term outcome (Barr et al., 2010; Green, 1996; Harvey et al., 1998; Milev et al., 2005; Park et al., 1999; Siegel et al., 2006).
Developing new therapies to target treatment-resistant symptoms requires identification of neural endophenotypes associated with these deficits (Braff and Light, 2005). Emerging evidence from EEG/MEG studies indicates that abnormal gamma range (30–80 Hz) synchrony may be such a biomarker, reflecting core pathophysiological features of schizophrenia including cognitive and perceptual abnormalities. Gamma oscillatory activity is thought to be a fundamental mechanism that integrates neural networks within and across brain structures, facilitating coherent sensory registration. In schizophrenia, gamma abnormalities have been reported in a variety of contexts, including in sensory-driven, cognitive, and resting-state paradigms. As reviewed below, these deficits are present at first-episode psychosis (Symond et al., 2005), in unmedicated patients (Gallinat et al., 2004), and, to a lesser degree, in unaffected relatives (Leicht et al., 2010a), suggesting that abnormal gamma synchrony is a heritable feature of schizophrenia. Gamma-band responses (GBRs) have been associated with symptom scales and cognitive performance, indicating that these measures are likely related to disease pathophysiology. Finally, abnormal GBRs have been reported in preclinical disease models, providing potential targets for treatment development.
2.0 Gamma Oscillations: Neurophysiology, Cognitive Correlates
2.1 Overview
Information processing is achieved in part by the coordinated firing of distinct neural populations (Buzsáki, 2006). Such synchrony is thought to be an emergent property of neural networks, generated by the temporal coordination between synaptic transmission and firing of individual neuronal populations. Hans Berger first described a dominant oscillation of ∼10 Hz, which he termed alpha (Berger, 1929). Berger and others coined terms still used today to designate brain activity within specific frequency bands: delta (0–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (>30 Hz). Distinct frequency bands have been associated with unique cognitive processes and behavioral states (Basar et al., 2001)
2.2 Function
Gamma oscillations have been shown to mediate cognitive and perceptual processes in mammalian brains (Herrmann et al., 2010). Gray and Singer demonstrated that gamma synchronization within cortical columns of the cat visual cortex act as a mechanism for perceptual ‘feature binding’ – the ability to integrate different sensory features of a stimulus into a single coherent neural representation (Gray et al., 1989). Similar processes have been demonstrated in humans, leading to a more general hypothesis that the early-evoked (e.g. phase-locked) cortical GBRs reflect initial stages of sensory perception (Joliot et al., 1994; Singer, 1999). Relationships between gamma oscillations and other cognitive processes have been reported, including selective attention (Fries et al., 2001; Tiitinen et al., 1993), stimulus salience (Roye et al., 2010), associative and perceptual learning (Miltner et al., 1999), sensorimotor integration (Murthy and Fetz, 1992), and object representation (Tallon-Baudry and Bertrand, 1999). Such findings, observed across human and animal studies, demonstrate a phylogenetically conserved role for gamma synchrony in coordinating distinct aspects of sensory information among distributed brain regions.
In addition to basic sensory processes, gamma responses play a role in higher cognitive functions. Several studies demonstrated a role for gamma activity in the encoding, maintenance, and subsequent recall of information during short- and long-term memory tasks (Sederberg et al., 2007; Tallon-Baudry et al., 1998). Other groups have shown that gamma synchrony is associated with social cognition (Williams, Whitford, Nagy, et al., 2009) and language function (Benasich et al., 2008; Meyer et al., 2005). Finally, some have argued that gamma activity plays a role in subjective consciousness (Llinas et al., 1998; Singer, 1998).
Physiologically, gamma oscillations have been associated with thalamo-cortical arousal (Griskova et al., 2007) and have been shown to couple local neuronal assemblies to enable synchronization (Canolty et al., 2006; Fries et al., 2007). Such synchrony tends to occur over relatively short distances, such as within a single cortical column, whereas longer-range functional neural coupling occurs at lower (e.g., beta-range) frequencies (Kopell et al., 2000). Finally, gamma activity has been shown to be important for gating of synaptic plasticity with high temporal precision (Uhlhaas et al., 2008; Wespatat et al., 2004)
2.3 Generators and Modulators
Gamma oscillations are found in virtually all mammalian brain structures, at both cortical and subcortical locations. Specific structures (e.g., thalamus, hippocampus, and cortex) contribute prominently to scalp recorded activity (Basar and Bullock, 1992). Gamma oscillations time- and phase-locked to a stimulus (i.e., “evoked”, see below) have been observed in auditory, visual, and somatosensory paradigms, usually around 40 Hz and within the first 100 ms post-stimulus (Tallon-Baudry and Bertrand, 1999). This transient 40-Hz evoked response is thought to be generated either in underlying primary sensory cortices (Pantev et al., 1991; Tallon et al., 1995) or via thalamo-cortical loops (Ribary et al., 1991), or by both structures in concert (Mulert et al., 2010). Later gamma activity tends to be time- but not phase-locked (i.e., “induced”) and is much more variable, likely reflecting widespread top-down changes in cortical network connectivity (Tallon-Baudry and Bertrand, 1999)
At the local circuit level, gamma activity is generated through feedback inhibition on pyramidal neurons by synaptically and electrically connected networks of fast-spiking interneurons expressing the calcium binding protein parvalbumin (PV+) (Tamas et al., 2000). Recent optogenetic studies have shown that activation of PV+ interneurons is both necessary and sufficient to generate gamma oscillations in the mammalian cortex, which in turn regulates cortical information processing (Cardin et al., 2009; Sohal et al., 2009). These studies have demonstrated than an increase in the excitatory input to these fast-spiking interneuron via selective, optogenetic stimulation leads to an increase in gamma activity. Such an elevation in gamma synchrony was not observed when selectively driving pyramidal cell excitatory input (Cardin et al., 2009). While the precise network and synaptic mechanisms underlying these effects remain unclear (Tiesinga and Sejnowski, 2009), phasic inhibition mediated by fast-spiking interneurons appears to be to the driving force behind gamma oscillations (Bartos et al., 2007), as opposed to tonic GABA(A) agonist application. Gamma activity can be pharmacologically modulated by directly or indirectly altering the kinetics of GABAergic signaling, as detailed in (Whittington et al., 2000). Further details regarding the generation of cortical rhythms are described in a recent review (Wang, 2010).
2.4 Recording Paradigms
Gamma activity is generally investigated in one of three paradigms: (1) at rest, (2) during “bottom-up” sensory stimulation, or (3) “top-down” cognitively driven tasks. Such oscillations can be detected at various levels within the brain, ranging from macroscopic scalp recordings using electroencephalography (EEG) and magnetoencephalography (MEG), to acute brain slice recordings, to the mescoscopic level with single/multiunit recordings, to the microscopic level with intracellular/synaptic resolution. [FIGURE 1]
Figure 1.
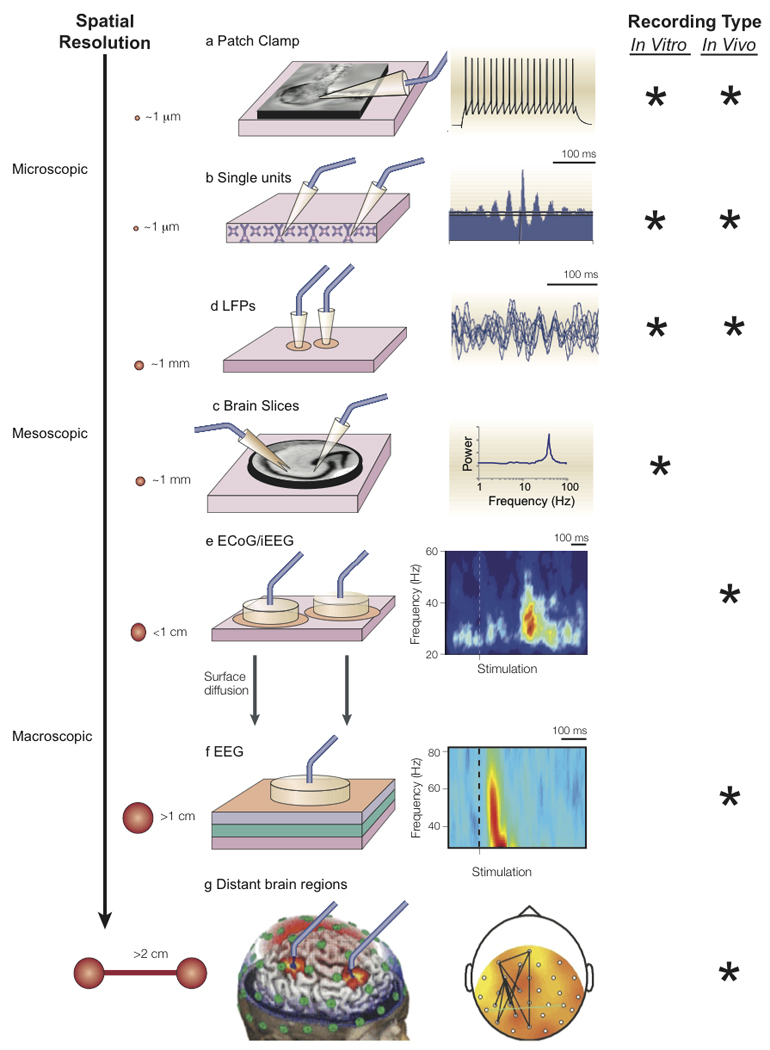
Gamma oscillations can be detected at various levels of investigation. A.) At the microscopic level, patterns of oscillatory activity can be detected from intracellular current and membrane potential recordings using patch clamp B.) Inter-neural phase synchrony can be assessed using extracellular recordings from high impedance microelectrodes. When high-pass filtered (>500 Hz), single/multi-unit activity can be detected. C.) When low-pass filtered, such activity gives rise to the local field potential (1–500 Hz), reflecting the summation of synaptic potentials oriented in parallel. D.) In preclinical studies, neuronal synchrony can be investigated in acute, ex vivo brain slices kept alive for several hours by artificial cerebrospinal fluid. Intracellular and extracellular signals can be recorded from these slices, as well as population averages of synchronously activated neurons using voltage sensitive dye imaging (Carlson and Coulter, 2008). Depending on the brain region and slice preparation, gamma oscillations can be spontaneously recorded (e.g., in auditory cortex), can be pharmacologically induced such as with carbachol or kainate, or can be induced using tetanic stimuli (Whittington et al., 2000). E.) At an intermediate level, gamma oscillations can be recorded from a more spatially localized region of the brain using subdural electrocorticography (‘ECoG’) grids or intracortical electrodes. These approaches record the synchronous synaptic potentials of regional pyramidal cells oriented in parallel. F.) At the macroscopic level, electroencephalography (EEG) and magnetoencephalography (MEG) measure electromagnetic brain activity. Whereas the EEG signal results from the extracellular volume conducted currents triggered mainly by the spatial summation of underlying cortical postsynaptic potentials, MEG is thought to arise from the intracellular branch of this process – from the currents flowing from the dendritic tree to the soma (Lewine and Orrison, 1995). Although gamma activity can be examined at the sensor (e.g., scalp) level, many studies also use source localization methods to examine gamma activity in source (i.e., brain) rather than sensor space. G.) Finally, gamma synchrony can be assessed between distant brain regions or scalp electrodes using phase-coupling methods. Figure adapted with permission from (Varela et al., 2001)
2.5 Recording Measures
A variety of signal processing methods are used to investigate oscillatory activity. The main approach is to decompose a neural time series into amplitude and phase information at a given frequency. Applying time-frequency transforms, one can investigate changes in frequency-specific measures during a given task with millisecond precision.
2.5.1 Power Measures
Power reflects the amplitude of an oscillation.1 For a stationary signal, in which the EEG/MEG does not change over time, the Fast-Fourier Transform (FFT) is used to spectrally decompose the time-invariant signal into component frequencies. The “power spectrum” yielded by FFT analysis is used for resting-state tasks.
The analysis of non-stationary neural activity requires signal-processing methods that compute changes in oscillatory activity at a particular frequency across time. See (Ho et al., 2008; Roach and Mathalon, 2008; van Vugt et al., 2007) for recent reviews. Compared to a baseline period, changes in oscillatory power elicited by a repeated stimulus have been termed “event-related oscillations”, “event-related spectral perturbation” (ERSP), or “event-related synchronization/desynchronization”.
Oscillatory responses can be categorized by their phase- and temporal-relationship to repeated trials of a sensory or cognitive event (Galambos, 1992; Tallon-Baudry et al., 1998). Oscillations directly in phase with a stimulus (i.e., phase- and time-locked) are called evoked oscillations. Oscillatory activity that is in-phase with a stimulus averages across trials to produce an evoked-response.2 Induced oscillatory activity is modulated by a stimulus but is not strictly phase-locked to event onset (i.e., time- but not phase-locked). In the time domain, such oscillations tend to average out and thus require different single-trial signal processing methods for identification. Finally, total power refers to the sum of evoked and induced power and is typically represented as difference from or a percentage change from pre-stimulus baseline power at each frequency.3
2.5.2 Phase Measures
Time-frequency transforms also provide measures of the phase of oscillations, allowing for investigation of “phase-synchrony.” Phase-synchrony is independent of oscillatory amplitude and is therefore thought to be a more direct measure of the synchronization of neural signals.4 Phase-synchrony can be investigated in several contexts, depending on recording methodologies. Unfortunately, a lack of standardization in the terms applied to phase measures as well as the fact that there are several ways to compute “phase synchrony” has caused confusion in the clinical literature and sometimes led to an inappropriate comparison across studies (Roach et al., 2008). See Figure 2 for a description of various methods for computing phase synchrony. Briefly, the “phase locking factor (PLF)” (i.e., intertrial coherence, ITC) describes the similarity in phase at a given point in time across trials at a single electrode site. The “phase locking index (PLI)” (also called “phase synchronicity”) describes the precise phase-relationships across multiple electrodes at a given time with respect to a repeated external stimulus. Finally, “phase coherence” describes the similarity between two continuous signals irrespective of an external stimulus. These measures are all unitless, ranging from 0 to 1. [FIGURE 2]
Figure 2.
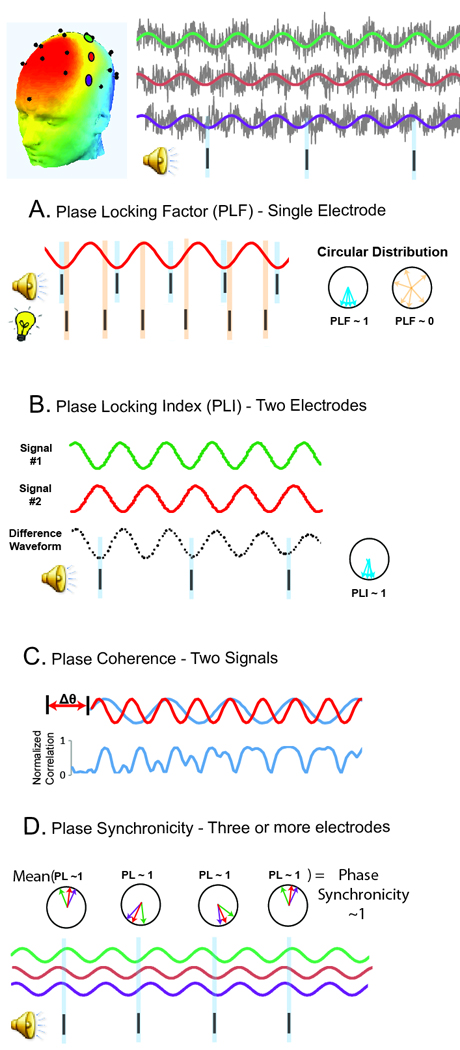
Gamma “phase synchrony” can be measured using several different methods. A.) Phase-dependence between a series of repeated events (e.g., onset of an external stimulus) and a continuous time series (e.g., EEG/MEG) is referred to as the “phase-locking factor (PLF)”. If the phase measures across trials tend to align in the same direction at a given point in time, the PLF will approach 1, indicating a strong phase relationship between the signals. When measuring the phase-relationship between repeated sensory stimuli and EEG/MEG activity at a single electrode or source site, this measure has been termed “intertrial phase coherence (ITPC)” or “intertrial coherence (ITC)” (Light et al., 2006). We, and others, prefer to call this PLF, since the term “coherence” implies multiple neuronal signals are being compared, whereas the PLF only depends on a single electrode site (Roach and Mathalon, 2008). An important feature of PLF is that this measure does not depend on the actual value of the phase-angles themselves; rather, PLF is simply a measure of the consistency of the phase-angles across trials. As such, PLF and evoked power are related measures (whereas event-related PLF and total power are independent). B.) Whereas PLF assesses phase synchrony at a single site, functional connectivity between brain regions with respect to a repeated stimulus is frequently assessed via the “phase-locking index (PLI)”. Synchrony between two areas is computed by assessing the consistency across trials in the difference in the phase values in those areas. Like PLF, the PLI is a normalized measure, and varies between 0 (independent signals) and 1 (fixed phase-lag between the two signals). C.) The term “phase synchronicity” has been used to refer to the precise phase-relationships across 3 or more EEG/MEG electrodes at a given time with respect to an external stimulus. PLF and PLI are very different measures of neuronal synchrony. PLF assesses the temporal consistency of oscillatory phase across trials at a single site, while PLI provides a spatial measure of synchrony. Finally, both measures are highly sensitive to trial numbers, which may be problematic if experimental groups differ in number of artifact free trials or a paradigm with few trials is employed. D.) The phase-dependence between two continuous time series (e.g., two EEG electrodes) irrespective to a repeated discrete event is called “phase-coherence,” or “phase coupling.” Such measures require calculating Pearson correlation coefficients between the two frequency-filtered time series, after unit normalization to yield a measure of pure phase synchrony (independent of amplitude). To account for possible conduction delays between two electrodes/regions, phase-coherence is usually computed after discrete phase shifts and then corrected for multiple comparisons (Dzirasa et al., 2009; Varela et al., 2001). Finally, phase coherence can be assessed between two sites at different frequencies, for example, “cross frequency phase-coupling (CFPC)” between theta and gamma oscillations (Sigurdsson et al., 2010). A recent review describes such calculation of such measures and their functional role in cortical information processing (Canolty and Knight, 2010).
3.0 Gamma Oscillations in Clinical Studies of Schizophrenia
This section reviews clinical evidence for gamma abnormalities in schizophrenia, focusing on the experimental paradigms that are most directly translatable to model organisms – resting, passive sensory, and a few cognitive paradigms.
3.1 Baseline Gamma Oscillations
Baseline oscillatory activity can be investigated in two different contexts. First, in “default-mode” or “resting-state” paradigms, EEG/MEG is acquired while the subject lies still without engaging in a task. Second, during tasks with a repetitive time-locked event, “pre-stimulus” baseline oscillatory activity can be extracted to examine the receptiveness of neural networks to the next stimulus.
In resting-state paradigms, several studies reported elevated high-frequency EEG activity in schizophrenia (Davis, 1942; Fenton et al., 1980; FINLEY, 1944; Giannitrapani and Kayton, 1974; Itil et al., 1972; Itil et al., 1974; Kennard and Schwartzman, 1957; Rodin et al., 1968), although they tended to investigate activity below 40 Hz. For example, in a large (n=100/group) clinical study, schizophrenia patients demonstrated increased 24–33 Hz activity (Itil et al., 1972) which was stable over three months (Itil et al., 1974). Several smaller studies did not detect group differences in resting gamma activity. However, three did report elevations in high beta (20–30 Hz) power, interpreted as reflecting “cortical noise” (Brockhaus-Dumke et al., 2008; Kissler et al., 2000; Krishnan et al., 2005), although another did not (Miyauchi et al., 1990). A larger EEG study observed increased 20–50 Hz power in schizophrenia subjects and their relatives (Venables et al., 2009), although a similar MEG study employing source space projections found opposite results (Rutter et al., 2009). These mixed results may reflect differences in sample size, imaging modality (EEG/MEG), or recording site (scalp vs. source-space projections).
Several groups have examined “pre-stimulus” baseline gamma activity in schizophrenia. The issue of baseline gamma differences is important, given that most studies examining group differences in post-stimulus activity employ some form of baseline correction (Urbach and Kutas, 2006). Two very large studies reported elevated pre-stimulus gamma power in schizophrenia patients during auditory paradigms (Hong et al., 2008; Winterer et al., 2004), in accordance with our preliminary data (Turetsky and Siegel, 2007). Two smaller studies found no group differences in prestimulus GBRs, but did report elevated baseline beta power in schizophrenia (Brockhaus-Dumke et al., 2008) or elevated gamma power with outliers included (Reinhart et al. personal communiction).
3.2 Stimulus Evoked
3.2.1 Auditory
Auditory abnormalities in schizophrenia have been observed when examining both basic (listening to tone) and higher-order auditory processes (performing a cognitive auditory task) (Turetsky et al., 2009).
Auditory Single Stimulus
Paradigms involving simple auditory stimuli have investigated early (<150 ms) phase-locked GBRs, thought to reflect perceptual encoding (Pantev et al., 1991). The largest studies have observed gamma deficits in schizophrenia (Basar-Eroglu et al., 2009; Hall et al., 2010; Hall et al., 2009; Hirano et al., 2008; Krishnan et al., 2009; Lee et al., 2001; Leicht et al., 2010a; Leicht et al., 2010b; Lenz et al., 2010; Roach and Mathalon, 2008; Teale et al., 2008), in accordance with our preliminary findings (Turetsky and Siegel, 2007). A recent study found similar deficits in first-degree unaffected relatives (Leicht et al., 2010a). Gamma abnormalities are generally detected across all recording sites, although deficits are most pronounced at electrodes Cz and Fz or bilateral auditory cortices in sensor space (Leicht et al., 2010b). A twin study estimated the heritability of early evoked-gamma power and phase-locking at h2 = 0.65 and 0.63, respectively (Hall et al., 2009).
Not all studies using transient stimuli have observed group differences in early phase-locked gamma activity (Blumenfeld and Clementz, 2001; Brenner, Krishnan, et al., 2009; Brockhaus-Dumke et al., 2008; Gallinat et al., 2004; Spencer, Niznikiewicz, et al., 2008). Negative results may reflect smaller sample sizes (<20/group), where findings were consistent in direction but not significant (Brenner, Kieffaber, et al., 2009; Clementz et al., 1997; Gallinat et al., 2004). Some of these studies also failed to observe other replicated neurophysiological biomarkers of schizophrenia, such as reduced P50/N100 peak amplitudes (Brenner et al., 2003; Brockhaus-Dumke et al., 2008; Johannesen et al., 2008; Spencer, Niznikiewicz, et al., 2008). A lack of findings in these studies may also be due to suboptimal time-frequency transform methods (Roach and Mathalon, 2008), lack of baseline correction, differences in trial numbers, or problems associated with examining multidetermined neural activity using scalp electrodes (Edgar et al., 2003).
Fewer studies have investigated late (>200 ms) gamma activity following auditory stimulation in schizophrenia. In oddball paradigms, three groups reported reduced late gamma activity in schizophrenia (Gallinat et al., 2004; Gordon et al., 2001; Haig et al., 2000). A fourth study found elevated late gamma power in schizophrenia patients, but did not baseline correct post-stimulus gamma activity like the other studies, which was also elevated (Hong et al., 2008). Finally, several studies investigated gamma phase-synchronicity (GPS) in schizophrenia. Schizophrenia patients have repeatedly shown deficits in GPS, particularly at early time points (<150 ms) and in the left hemisphere (Lee et al., 2003; Slewa-Younan et al., 2004; Symond et al., 2005; Williams, Whitford, Gordon, et al., 2009). A recent study investigated non-baseline corrected absolute values of GPS and found elevations in first-episode patients (Flynn et al., 2008). The authors suggested that diminished task-related GBRs occurred in the context of elevated ongoing gamma activity, a finding that was mirrored in a visual backward masking study (Williams, Whitford, Nagy, et al., 2009).
Auditory Steady State
The auditory steady-state response (ASSR), elicited to amplitude modulated tones or repetitive click trains, has enabled investigation of bottom-up neural synchronization. In normal subjects, maximal responses occur to stimuli with repetition rates around 40 Hz (Galambos et al., 1981). Schizophrenia patients have shown consistent gamma ASSR deficits, as reviewed by (Brenner, Krishnan, et al., 2009). Several groups reported reduced 40+ Hz ASSR power and/or phase-locking in schizophrenia (Brenner et al., 2003; Hamm et al., 2011; Krishnan et al., 2009; Kwon et al., 1999; Light et al., 2006; Maharajh et al.; Spencer et al., 2009; Spencer, Salisbury, et al., 2008; Teale et al., 2008; Vierling-Claassen et al., 2008), but not for 20 or 30 Hz click trains (Kwon et al., 1999).
3.2.2 Visual
See Brenner et al. (2009b) and Spencer et al. (2008) for reviews on visual GBRs in schizophrenia. Using visual Gestalt stimuli to assess perceptual ‘feature binding’, multiple studies reported reduced early gamma phase synchrony in schizophrenia (Spencer et al., 2003; Spencer et al., 2004; Uhlhaas et al., 2006). Three studies employing visual backward masking paradigms also found reduced baseline-corrected gamma responses in schizophrenia (Green et al., 2003; Williams, Whitford, Nagy, et al., 2009; Wynn et al., 2005). Finally, schizophrenia patients showed reduced responses to steady-state visual stimuli at gamma-frequencies (Brenner, Krishnan, et al., 2009; Krishnan et al., 2005), although contrary findings have been reported (Riecansky et al., 2010).
3.2.2 Other Stimuli
Clinical studies have investigated GBRs elicited by other types of sensory and motor events. For example, early evoked-gamma reductions in schizophrenia have been observed during proprioceptive stimulation (Arnfred et al., 2010) and transcranial magnetic stimulation (Ferrarelli et al., 2008). In a button press paradigm, schizophrenia patients had reduced GBRs directly preceding the motor response (“corollary discharge”) (Ford et al., 2007). These findings suggest that aberrant stimulus-evoked GBRs are a generalized impairment in schizophrenia likely reflecting widespread deficits in cortical gamma synchronization.
3.3 Task Driven
For a review of cognitively-evoked GBRs in schizophrenia, see (Haenschel and Linden, 2010). During working memory paradigms, multiple studies found that individuals with schizophrenia fail to mount a robust gamma response as a function of tasks demands (Barr et al., 2010; Basar-Eroglu et al., 2007; Gonzalez-Hernandez et al., 2003; Haenschel et al., 2009). Several of these report elevated levels of baseline gamma activity in schizophrenia despite a lack of task-specific activation (Barr et al., 2010; Basar-Eroglu et al., 2007; Gonzalez-Hernandez et al., 2003). Multiple groups found reduced gamma power in schizophrenia during selective attention paradigms (Basar-Eroglu et al., 2009; Cho et al., 2006; Kissler et al., 2000; Minzenberg et al.). These findings suggest greater absolute magnitude of gamma activity in schizophrenia coupled with reduced task-specific activations relative to healthy controls, although at least one study has reported contrary results (Pachou et al., 2008).
3.4 Association with Symptoms
Several studies reported associations between gamma activity and symptoms, although identifying such relationships is often difficult.5 Using an auditory paradigm, Haig et al. (2000) found that reduced GBRs predicted higher PANSS scores, although others did not replicate this finding (Hall et al., 2009; Leicht et al., 2010b). Hamm et al. (2011) recently found a significant inverse correlation between the gamma ASSR response and negative symptoms. Associations have been reported between deficits in gamma-power, reaction time, and illness duration (Gallinat et al., 2004; Hall et al., 2010; Reinhart et al.). Additional studies demonstrate that gamma abnormalities are associated with ‘treatment resistant’ symptoms, including impaired working memory (Light et al., 2006; Winterer et al., 2004), perceptual abnormalities (Johannesen et al., 2008), disorganization (Gordon et al., 2001), and psychomotor poverty (Gordon et al., 2001; Lee et al., 2003). In cognitive and visual paradigms, reduced GBRs have been associated with severity of negative symptoms (Uhlhaas et al., 2006), deficits in social cognition (Williams, Whitford, Nagy, et al., 2009), and disorganization (Cho et al., 2006). Finally, a recent clinical trial demonstrated that pharmacologic enhancement of frontal gamma activity was associated with improved cognitive function in schizophrenia (Lewis et al., 2008).
Associations between gamma alterations and positive symptoms have also been reported, although they tend to be opposite in direction to what might be predicted. In particular, multiple studies found that patients with larger (i.e., more “normal”) early auditory or visual GBRs have more severe positive symptoms (Hirano et al., 2008; Lee et al., 2003; Spencer et al., 2004; Spencer et al., 2009; Spencer, Niznikiewicz, et al., 2008; Uhlhaas et al., 2006). One study reported that gamma activity was significantly elevated during somatosensory hallucinations (Baldeweg et al., 1998).
3.6 Synopsis
Previous sections have demonstrated widespread gamma abnormalities in schizophrenia. Early, evoked abnormalities are most consistent for PLF measures, which are independent of (potentially opposing) power measures and avoid baseline correction issues (Hall et al., 2009; Spencer et al., 2003; Uhlhaas et al., 2006). It is unclear whether the same neural circuit abnormalities contribute to the gamma abnormalities observed across sensory-evoked, cognitive, and resting-state paradigms. Future studies are needed to compare these different gamma measures in the same cohort of subjects. Deficits in sensory-evoked GBRs have been associated with cognitive impairments, suggesting a connection (Light et al., 2006). Likewise, it has been suggested that higher-order cognitive deficits result from impairments in early sensory processing (Haenschel and Linden, 2010).
4.0 Gamma Oscillations in Preclinical Studies of Schizophrenia
As in humans, gamma activity has been associated with a wide range of cognitive and sensory processes across species. For example, cats (Gray et al., 1989; Lakatos et al., 2004), rats (Sukov and Barth, 2001) and mice (Ehrlichman et al., 2009; Lazarewicz et al., 2010; Nase et al., 2003) show a peak in phase-locked gamma activity (∼40 Hz) within the first 100 ms of auditory or visual stimulation, following a time course similar to that observed in humans (Pantev et al., 1991; Shibata et al., 1999). Indeed, similar evoked GBRs have been observed in insects during sensory stimulation, suggesting that gamma synchrony is a phylogenetically conserved neural coding mechanism (MacLeod and Laurent, 1996). Similar to the human studies, gamma oscillations have been shown to mediate cognitive processes in mice including selective attention (Lakatos et al., 2004), memory (Dzirasa et al., 2009), sensory perception (Cardin et al., 2009), and cortical information processing (Sohal et al., 2009). This section reviews preclinical studies of schizophrenia that have investigated gamma synchrony.
4.1 Neurotransmitter Disruption
4.1.1 Glutamate – Acute Models
Considerable evidence implicates reduced NMDA-receptor mediated signalling as the core pathophysiologic deficit of schizophrenia (e.g., the “Glutamate Hypothesis”) (Coyle, 2006; Goff and Coyle, 2001). Preclinical studies have increasingly employed pharmacologic NMDAR blockade as a disease model, as this model shows deficits consistent with schizophrenia (Olney et al., 1999). Several studies demonstrated that NMDAR antagonists (including ketamine, MK-801, and PCP) produce a dose-dependent increase in baseline gamma power using in vivo LFP and EEG recordings in awake rodents (Ehrlichman et al., 2009; Hakami et al., 2009; Lazarewicz et al., 2010; Leung, 1985; Ma and Leung, 2000, 2007; Pinault, 2008). Consistent with this finding, ketamine increases baseline gamma power in healthy human subjects (Hong et al., 2010). LFP recordings in awake rodents demonstrate ketamine-induced baseline gamma elevations throughout the cortex as well as in subcortical structures including amygdala, hippocampus, and thalamus (Hakami et al., 2009; Hunt et al., 2009; Leung, 1985). Behaviorally, this increase in gamma power is associated with locomotor hyperactivity and deficits in prepulse inhibition (Hakami et al., 2009; Leung, 1985; Ma and Leung, 2000, 2007). Mechanistically, it has been proposed that the effect of NMDAR antagonists on gamma oscillations (and their psychomimetic properties) is due to reduced excitation of PV+ interneurons (Lisman et al., 2008), as discussed below.
Acute brain slice preparations have also been used to investigate gamma synchrony in pharmacologic models of schizophrenia. Such rhythms are typically measured in the hippocampus, and after being induced by bath application of carbachol (Fisahn et al 1998) or kainate (Hajos et al 2000), modulation of this rhythm by NMDAR antagonists is then investigated. Such paradigms have demonstrated strikingly divergent results from the in vivo studies described above. Whereas in vivo studies demonstrated consistent brain-region independent increases in gamma activity with ketamine, slice studies reported increased gamma power only in auditory cortex, with no change in prelimbic cortex, somatosensory association cortex, medial orbital cortex, insula, and perirhinal cortex. Multiple studies have reported reduced gamma activity in entorhinal cortex following ketamine with no change in the hippocampus (Cunningham et al., 2006; Roopun et al., 2008). Using tetanic stimulation instead of carbachol/kainate to generate oscillatory activity, ketamine has been shown to reduce hippocampal gamma responses (Doheny et al., 2000; Faulkner et al., 1998). Several factors could explain the large discrepancies between findings from ex vivo and in vivo studies. Brain slices typically sever thalamo-cortical connections, which are important generators of gamma synchrony (Ribary et al., 1991; Whittington et al., 2000). Likewise, ex vivo gamma oscillations induced by carbachol, kainate, and tetanic stimulation have very different characteristics from each other. In light of this, it is unclear which, if any of the ex vivo measures, bears direct physiological relevance to those recorded in clinical paradigms at scalp electrodes (Wojtowicz et al., 2009).
4.1.1 Glutamate – Chronic Models
NMDAR antagonists produce acute receptor hypofunction and therefore fail to reflect chronic, developmental disruption in glutamatergic signalling that may underlie schizophrenia pathogenesis. In recent years, transgenic mice have been generated to model NMDAR disruption associated with schizophrenia.
The schizophrenia susceptibility genes neuregulin-1 (NRG1) and its receptor ErbB4 are highly expressed in interneurons where they negatively regulate NMDAR signalling (Banerjee et al., 2010; Hahn et al., 2006). In acute WT hippocampal rodent slices, bath application of Nrg-1 selectively increased the power of kainate- but not carbachol-induced gamma oscillations (Fisahn et al., 2009). The effect of Nrg-1 bath application on gamma was absent in ErbB4−/− mice, which showed a 60% reduction in kainate-indued gamma power as well as a reduction PV+ immunoreactivity (Fisahn et al., 2009).
To model the effects of constitutively reduced NMDAR signalling, Mohn et al. generated a line of NMDA-NR1neo−/− mice which express 5–10% of the obligatory NMDAR1 subunit (Mohn et al., 1999). These mice show electrophysiological, behavioral, and molecular deficits consistent with schizophrenia (Dzirasa et al., 2009; Halene et al., 2009; Mohn et al., 1999). Similar to acute administration of NMDAR antagonists, NR1−/−mice show a marked increase in baseline gamma power (Dzirasa et al., 2009, supplement) as well as a reduction in stimulus-evoked gamma band responses (unpublished observation), mirroring findings in schizophrenia. During a working memory task, NR1−/− demonstrate reduced gamma-theta cross frequency phase-coupling between hippocampus and prefrontal cortex, consistent with deficits in long range neuronal synchronization observed in schizophrenia (Dzirasa et al., 2009; Meyer-Lindenberg et al., 2005).
4.1.2 Dopamine
Dopaminergic agents like amphetamine are known to induce a schizophrenia-like psychosis, but fail to reproduce negative symptoms or cognitive deficits to the same degree as observed in the disease. In rodent cortex and hippocampus, activation of dopaminergic neurotransmission with acute d-amphetamine, apomorphine, or methamphetamine had no significant effect on baseline gamma activity (Ehrlichman et al., 2009; Ma and Leung, 2000; Pinault, 2008) or auditory evoked gamma power (Ehrlichman et al., 2009). Likewise, dopamine-transporter knockout (DAT1-ko) mice showed no difference in cortical gamma power (Dzirasa et al., 2009, supplement). Surprisingly, DAT1-ko mice did show extremely elevated hippocampal-PFC gamma phase coherence compared with WT controls (Dzirasa et al., 2009). In hippocampal slices, dopamine reduces pharmacologically induced gamma oscillations but augments stimulus-evoked gamma synchrony (Weiss et al., 2003; Wojtowicz et al., 2009), again in contrast to results from in vivo recordings.
Several clinical studies investigated the effect of polymorphisms in the dopamine system on gamma-band activity. Schizophrenia-associated polymorphisms in catechol-O-methyltransferase (COMT), which regulates dopamine degradation, have no effect on resting or auditory evoked gamma activity (Demiralp et al., 2007; Venables et al., 2009). Conversely, mutations in dopamine receptor D4 (DRD4) and DAT1 observed in schizophrenia were associated with elevated evoked GBRs, opposite to the findings in schizophrenia (Demiralp et al., 2007; Venables et al., 2009). These results suggest that dopaminergic dysregulation in schizophrenia likely does not contribute to resting state or stimulus evoked gamma abnormalities. Given the relevance of dopamine to cognitive function, particularly in the prefrontal cortex, future studies should investigate the effect of schizophrenia-associated alterations in DA neurotransmission on cognitively-evoked gamma activity.
4.1.3 GABA
The GABAergic system, in particular parvalbumin-expressing basket and chandelier cells, is integrally involved in the generation of high-frequency oscillations in the mammalian brain (Cardin et al., 2009; Sohal et al., 2009). A wealth of evidence implicates disturbances in GABAergic circuitry in schizophrenia, notably a downregulation of PV and GAD67 (1 of 2 enzymes responsible for GABA synthesis), which likely contributes to core symptoms (Guidotti et al., 2005; Lewis et al., 2005).6
Several preclinical models of schizophrenia demonstrate similar disruptions in PV+ interneurons and/or GAD67, including models of redox dysregulation (Steullet et al., 2010), prenatal exposure to methylazoxymethanol acetate (MAM) (Lodge et al., 2009), reelin haploinsufficiency (Ammassari-Teule et al., 2009), ketamine exposure (Behrens et al., 2007), altered NRG1/ErbB4 signaling (Neddens and Buonanno, 2010), polymorphisms in DISC1 (Ayhan et al., 2010), neonatal lesions of the ventral hippocampus (Francois et al., 2009), prenatal inflammatory exposure (Jenkins et al., 2009), isolation rearing (Harte et al., 2007), chronic methionine exposure (Tremolizzo et al., 2005), the amygdala activation model (Berretta et al., 2004), lysophosphatidic acid 1 (LPA1) receptor-deficient mice (Cunningham et al., 2006), and constitutive NMDAR1 downregulation (Sisti et al., 2008). Although many of these models do not involve a primary perturbation in the GABAergic system, the fact that they share similar downstream disruptions in this neurotransmitter system suggests that PV cell-dysfunction may be a final common pathway connecting multiple schizophrenia genetic and environmental risk factors.
There has been a strong effort recently to connect putative pathogenic disease mechanisms (i.e., NMDA-hypofunction) to downstream GABAergic dysfunction (i.e., loss of PV and GAD67) and associated electrophysiological and neurocognitive deficits (Behrens et al., 2007; Belforte et al., 2010; Gonzalez-Burgos et al., 2010; Lisman et al., 2008; Rotaru et al., 2011). There have been some reports that NMDA-receptors on PV interneurons are more sensitive to pharmacologic blockade than receptors expressed on excitatory pyramidal cells, particularly in the hippocampus and entorhinal cortex (Grunze et al., 1996; Jones and Buhl, 1993; Middleton et al., 2008). However, other studies have shown that the NMDAR contribution to excitatory signaling in PV+ cells is much less than that on principle cells, such as in the prefrontal cortex (Rotaru et al., 2011; Wang and Gao, 2009). As such, it is currently unclear whether the loss of PV and GAD67 expression is a direct, or indirect, effect of NMDAR antagonism.
Nevertheless, the increase in gamma power associated with NMDAR antagonists is likely associated with reduced GABA release onto pyramidal neurons, as a number of studies have demonstrated elevated pyramidal cell activity following NMDAR blockade (Belforte et al., 2010; Jackson et al., 2004; Santana et al., 2011). In accordance with this theory, administration of the GABAA agonist muscimol reversed the baseline gamma power elevation (Ma and Leung, 2000) and loss of GAD67, PV expression (Behrens et al., 2007) induced by NMDAR antagonists in rodents. Computational models have suggested that an extended inhibitory postsynaptic current (IPCS) decay time constant could account for the observed decreased 40 Hz evoked gamma activity (Vierling-Claassen et al., 2008). The authors suggested that the alternations in the GABA transporter GAT1 and GAD67 in fast-spiking interneurons which synapse onto the axon initial segment of pyramidal cells observed in patients with Sz (Lewis et al., 2005) may account for extended IPSC decay times and thus decreased 40 Hz evoked activity in patients.
Two recent studies have directly investigated the effect of reduced NMDAR signaling in PV+ interneurons by cell-type selective knockdown of NR1, the obligatory NMDAR subunit. Early postnatal NR1 deletion from cortico-limbic interneurons caused a loss of PV and GAD67 immunoreactivity coupled with behavioral deficits analogous to the treatment resistant symptoms of schizophrenia, including impaired social interactions, decreased reward seeking behavior (i.e., anhedonia), and reduced working memory (Belforte et al., 2010). Although gamma activity was not assessed, the authors reported elevated baseline firing rates in cortical pyramidal neurons as well as decreased cross-correlation with nearby neurons. In a second study, selective deletion of NR1 from PV+ interneurons significantly increased hippocampal gamma power while disrupting cross-frequency synchrony and caused deficits in spatial and working memory (Korotkova et al., 2010). These findings further strengthen the association between NMDAR signaling, fast-spiking neurons, neuronal synchrony, and cognitive function.
4.1.4 Other Neurotransmitter Systems
Disturbances in other neurotransmitter systems have been linked schizophrenia, including acetylcholine (ACh), serotonin, and cannabinoids. While beyond the scope of this review, schizophrenia-like gamma abnormalities have been demonstrated following blockade of muscarinic ACh receptors (Rodriguez et al., 2004) and blockade of α7-nicotinic ACh receptors (Song et al., 2005), which localize heavily on hippocampal GABAergic interneurons (Ji and Dani, 2000). In rats, gamma activity is modulated by cannabinoid agonists (Hajos et al., 2008) and serotonin, likely through 5-HT1A and 5-HT2A receptors (Puig et al., 2010; Wojtowicz et al., 2009).
4.2 Other Preclinical Disease Models
Gamma synchrony has been investigated in other models of schizophrenia that do not involve strict neurotransmitter system disruption. A recent study investigated in vivo synchrony between prefrontal cortex and hippocampus (PFC-HC) during a working memory task in Df(16)A+/− mice. These mice model the human 22q11.2 microdeletion, which is strongly linked with schizophrenia (Sigurdsson et al., 2010). While the study focused on theta activity, gamma phase synchrony was qualitatively reduced (Fig 2c, Fig 3a). The authors reported the gamma difference as non-significant after Bonferroni post-hoc correction for tests across all frequencies. In the prenatal MAM rat model of schizophrenia, Lodge et al. (2006) reported a reduction in in vivo PFC and HC gamma power during a reversal-learning paradigm, and this gamma reduction predicted worse task performance. Baseline gamma activity, however, was not altered with MAM exposure. LPA1-receptor deficient mice, another strain proposed as an animal model of schizophrenia, showed erratic ex-vivo kainate induced gamma oscillations in entrorhial cortex (Cunningham et al., 2006). Vohs et al. investigated auditory evoked gamma activity in the neonatal ventral hippocampal lesion (NVHL) rat model of schizophrenia using a paired click paradigm (Vohs et al., 2009). Although NVHL rats showed no difference in baseline-corrected total gamma power following either stimulus, these rats showed significantly reduced gamma PLF, highly consistent with clinical findings. Although the authors did not test for differences in spontaneous gamma activity, they note in the discussion that NVHL rats take significantly longer to return to pre-stimulus levels of neuronal activity, suggestive of baseline elevations. Finally, Steullet et al. investigated hippocampal gamma synchrony in GCLM−/− mice, which are susceptible to elevated redox dysregulation due to impaired glutathione synthesis (Steullet et al., 2010). Kainate-induced gamma oscillations were weaker in brain slices from transgenic mice in ventral, but not dorsal, hippocampus.
Figure 3.
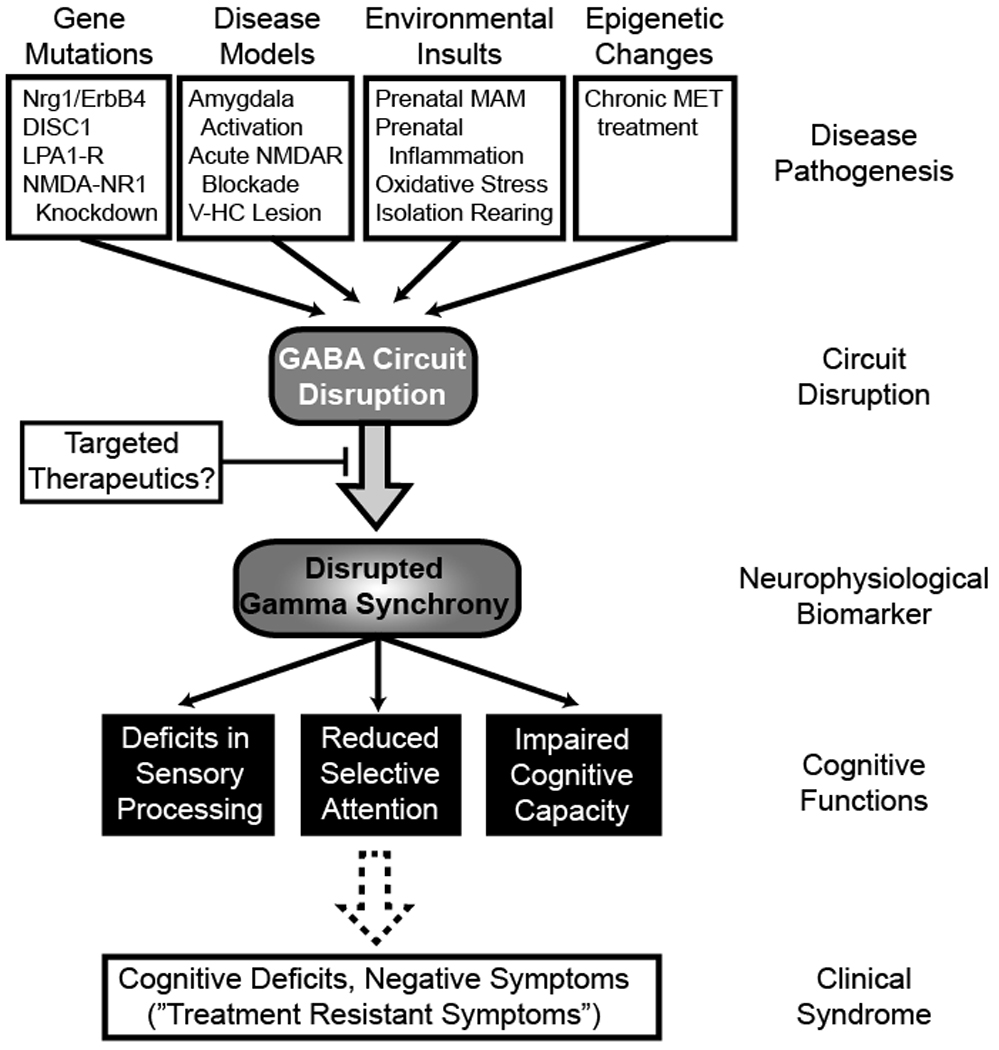
Gamma oscillatory abnormalities represent an intermediate phenotype in schizophrenia that likely reflect pathophysiological deficits and contribute to core symptoms. Despite distinct pathogenic insults, several schizophrenia models converge on GABAergic circuit deficits, particular the disruption of fast-spiking parvalbumin-expressing interneurons. Dysfunctional fast-spiking interneuron activity appears to be a final common pathway in schizophrenia that could explain gamma abnormalities. This loss-of-inhibition phenotype could account for both elevated baseline and reduced stimulus-evoked endophenotypes. A lack of precise PV+ cell firing during task or stimulus-specific activation would prevent robust high-frequency synchronization of cortical pyramidal neurons, thereby leading to a reduced evoked gamma response (i.e., decreased PLF). Likewise, appropriate inhibitory tone mediated in part by PV+ cells would be required to restore neuronal oscillatory activity to an appropriate pre-stimulus resting state. Reduced activity of a subpopulation of interneurons could delay the ability of a circuit to return to this resting state, leading to the elevation in baseline gamma activity observed in clinical and preclinical settings.
4.3 Translational Potential of Gamma Synchrony
Emerging evidence indicates that gamma oscillations are disrupted in preclinical models of schizophrenia, often similar in pattern to that observed in schizophrenia. Importantly, such aberrant neuronal synchrony arises from selective perturbation of neurotransmitter signaling and/or gene expression relevant to schizophrenia, providing key insights into the pathophysiological bases of disease-related neuronal deficits. Altered GBRs may reflect disruptions of a final common GABAergic pathway downstream of etiologically diverse pathogenic insults that have been linked to schizophrenia, a pathway which may provide targets for pathophysiologically based therapeutic strategies (Lewis and Gonzalez-Burgos, 2006; Whittington et al., 2000).
The overarching hypothesis of this review is that gamma abnormalities are a biomarker for the treatment resistant symptoms of schizophrenia and can be used as a rationale target for therapeutic development. Preliminary evidence from a recent clinical trial bolstered the validity of this approach (Lewis et al., 2008). The identification of similar gamma-band abnormalities in preclinical models of schizophrenia provides a promising opportunity for drug development. Therapeutics that substantially increase gamma signal-to-noise during sensory processing tasks (e.g., reduce elevated background, pre-stimulus gamma-band “noise” and increase stimulus-evoked GBRs) may improve perceptual abnormalities in schizophrenia. Likewise, compounds that elevate gamma power during cognitive paradigms may help alleviate these deficits in schizophrenia. Although beyond the scope of this review, it is hypothesized that compounds which positively modulate selective GABA-receptor subtypes (Guidotti et al., 2005; Lewis and Gonzalez-Burgos; Lewis et al., 2004) or cholinergic signaling (Gray and Roth, 2007) are promising candidates for restoring gamma abnormalities in schizophrenia and associated neuronal impairments.
4.4 Caveats and Future Studies
While the translational potential of gamma oscillations in preclinical studies of schizophrenia is strong, there are a number of important caveats to consider. As with any other preclinical study of a complex human disease, one must evaluate the validity of the animal model before extrapolating to the clinical population (Chadman et al., 2009).
Construct validity indicates that the etiologic cause of phenotypic deficits in the preclinical model has been established as a risk factor (genetic, environmental, etc.) in the clinical population. As such, knockout mouse models for schizophrenia risk genes generally demonstrate excellent construct validity. However, one must be careful that the genetic change is similar to that observed in the patient population. Likewise, several groups (including ours) have employed acute NMDAR-blockade as animal models of schizophrenia, despite the fact that these models fail to reflect the neurodevelopmental consequences associated with constitutive NMDAR dysfunction.
Face validity indicates that a mouse model recapitulates core phenotypic (and endophenotypic) deficits associated with the clinical disorder. Models of elevated dopaminergic signaling do not demonstrate gamma abnormalities, indicating a lack of face validity for certain aspects of schizophrenia. When assessing the face validity of a mouse model, it is important to consider that phenotypic/endophenotypic indices are measured as similarly as possible between humans and mice. Since the gamma abnormalities in schizophrenia have been detected using scalp EEG/MEG recordings, similar skull recordings or those using large macroelectrodes performed in rodents are the most directly comparable human EEG/MEG studies (Gandal et al., 2010; Gandal et al., 2008; Umbricht et al., 2004). More localized LFP or unit recordings, which are extremely important for mechanistic understanding of neural information processing, likely have less face validity for human findings, given that such paradigms are difficult to perform in a clinical setting. In addition, questions remain about the face validity for ex vivo brain slice paradigms, given that these recordings are nearly impossible to perform in humans and tend not to replicate findings from unanesthetized in vivo recordings.
Finally, predictive validity indicates that an intervention which is effective in the preclinical setting translates successfully to the clinical population (and vice versa). This stipulation further demonstrates the pitfalls of acute NMDAR-blockade models. For example, pre-administration of GABAA agonists and benzodiazepines ameliorate several neuronal deficits in acute NMDAR-blockade models (Behrens et al., 2007; Castner et al., 2010; Ma and Leung, 2007), despite the fact that these drugs do not improve, or sometimes even worsen, schizophrenia symptoms (Buchanan et al., 2010; Menzies et al., 2007).
5.0 Conclusion
5.1 Synthesis: A Model of Gamma-Band Activity
Based on a comprehensive review of the literature, we suggest that there is reduced gamma signal-to-noise during cortical information processing in schizophrenia, a hypothesis previously suggested (Flynn et al., 2008; Kissler et al., 2000; Krishnan et al., 2005; Tseng et al., 2008; Williams, Whitford, Nagy, et al., 2009; Winterer et al., 2004; Winterer et al., 2000). There is strong evidence that pre-stimulus baseline gamma activity is elevated and that task-driven ‘evoked’ gamma-band responses are reduced in schizophrenia. Baseline elevations are most evident with pre-stimulus measurements, suggesting an impairment in the ability to rapidly return to a resting state prior to the next stimulus. Indeed, previous studies have reported a relationship between pre-stimulus power and the amplitude of an ensuing evoked response (Basar et al., 1987). This pattern suggests that the ability to attribute salience to incoming sensory stimuli is impaired in schizophrenia, as is the ability to appropriately engage frontal neuronal networks during complex cognitive tasks. Physiologically, such findings could be explained by a dysfunction in inhibitory interneuron networks, which maintain appropriate resting ‘inhibitory tone’ but are also necessary for task-specific up-regulation of gamma synchrony, as recently demonstrated (Sohal et al., 2009).
5.2 Specificity
Although this review has focused on gamma oscillatory responses, it is important to note that abnormalities in other frequency bands (especially theta), have been well documented in the disorder (Edgar et al., 2008; Lisman and Buzsaki, 2008; Uhlhaas et al., 2008; Uhlhaas and Singer, 2010). Given the interdependence of neuroonal oscillatory activity in different frequencies (e.g., gamma-theta cross frequency coupling), it may be difficult to parse out the selective contribution of deficits within a single frequency range without considering others bands (Canolty and Knight, 2010; Varela et al., 2001).
5.3 Gamma Synchrony as a Common Endophenotype
Gamma alterations are not specific to schizophrenia. For example, similar deficits in evoked GBRs have been reported in preclinical and clinical studies of autism (Gandal et al., 2010) as well as in other neuropsychiatric diseases (Herrmann and Demiralp, 2005). Interestingly, several of these neuropsychiatric diseases have significant pathophysiological overlap. For example, schizophrenia and autism share common epidemiological characteristics (highly heritable, neurodevelopmental origin, 1–2% population prevalence), common core phenotypic deficits (e.g., social withdrawal, language problems, cognitive deficits), common neurological comorbidities (e.g., epilepsy, ADHD, sleep disruption), common endophenotypes (e.g., attentional deficits, deficits in sensory processing and prepulse inhibition), common genetic insults (e.g., mutations in NRXN1, DISC1, 22q11.2), common environmental etiologies (e.g., prenatal infection, oxidative stress), common approved but deficient treatments (e.g., risperidone), and common molecular disruptions (e.g., GABAergic interneurons) (Ching et al., 2010; Eagleson et al., 2010; Kilpinen et al., 2008; Levy et al., 2009; Ousley et al., 2007; Qin et al., 2005; Siegel and Ralph, 2011). It is our hypothesis that similar abnormalities in gamma synchrony across disorders reflect common neuronal circuit insults leading to shared downstream phenotypic deficits. In light of this, we speculate that gamma synchrony is an endophenotype for a common constellation of neuronal deficits independent of diagnostic categorization. Targeting gamma-band deficits (and the circuit insults they index) with novel therapeutic approaches could thus have implications for a broad group of patients, similar to the approach recently proposed as the “future of psychiatric research” (Akil et al., 2010).
Table 1.
Several studies have investigated the early, evoked auditory gamma-band response (e.g., evoked-power or phase-locking factor) to simple tones or clicks in schizophrenia, which is associated with perceptual encoding of sensory information (Pantev et al., 1991). Of the published studies, 10/16 found significantly reduced early, evoked GBRs in schizophrenia, while 13/16 studies found qualitative and/or quantitative reductions in these measures. Study characteristics are detailed above, using the following abbreviations: interstimulus interval (ISI, seconds), baseline correction (B.C.), non-significant (N.S.), qualitative but non-significant reduction in the schizophrenia group ( “(↓)” ), continuous performance task (CPT), regional field activity (REFA), primary auditory cortex (A1), right hemisphere (RH), left hemisphere (LH), dorsal anterior cingulate cortex (dACC) and low-resolution brain electromagnetic tomography (LORETA). References: (1) Clementz et al., 1997; (2) Leicht et al., 2010a; (3) Hall et al., 2009; (4) Galliant et al., 2004; (5) Roach and Mathalon, 2008; (6) Spencer et al., 2008a; (7) Hall et al., 2010; (8) Lenz et al., 2010; (9) Hirano et al., 2010; (10) Blumenfeld et al., 2001; (11) Basar-Eroglu et al., 2009; (12) Brenner et al., 2009a; (13) Krishnan et al., 2009; (14) Brockhaus-Dumke et al., 2007; (15) Teale et al., 2008; (16) Leicht et al., 2010b.
| Modality, # sensors |
Paradigm (Stimulus) | Subject Type | Gamma Measure (Method) | SC2 Effect (P) |
Sites Looked | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration | ISI (sec) |
Trials | # SCZ | # CTL | Freq (Hz) | Time (ms) |
B.C. | Site with Effect | |||
| MEG, 148 | Paired click (white noise) | Chronic, Meds | Power, Evoked Buttorworth, REFA) | (↓) N.S. |
All | 1 | |||||
| 0.1 ms | 8–10s | 200 | 10 | 10 | 24–48 | Max | |||||
| EEG, 27 | Pure tone (800, 1300 Hz) | Chronic, Meds | Power, Evoked (Wavelet) | ↓ <0.02 |
Cz, Fz; Loreta | 2 | |||||
| 32–48 | 20–80 | Yes | Cz, Fz; A1, dACC | ||||||||
| PLF (Wavelet) | ↓ 0.004 |
Cz, Fz; Loreta | |||||||||
| 250 ms | 2.5–7.5 | 30 | 90 | 90 | 32–48 | 20–80 | Yes | Cz, Fz; A1, dACC |
|||
| EEG, 16 | Oddball (1kHz, non-target) | Chronic, Meds | Power, Evoked (Wavelet) | ↓ 0.001 |
Cz, Fz | 3 | |||||
| 35–46 | 20–80 | Yes | Cz, Fz | ||||||||
| PLF (Wavelet) | ↓ 0.001 |
Cz, Fz | |||||||||
| 20 ms | 1.8–2.2 | 400 | 30 | 147 | 35–46 | 20–80 | Yes | Cz, Fz | |||
| EEG, 32 | Oddball (1 kHz target) | Chronic, Unmeditated |
Power, Evoked (Wavelet) | N.S. | All | 4 | |||||
| 40 Hz | 20–100 | yes | |||||||||
| Power, Evoked (Wavelet) | ↓ 0.02 |
All | |||||||||
| 1 ms | 1.5–4.6 | 55 | 15 | 15 | 40HZ | 220–350 | yes | RH, Anteror | |||
| EEG, 26 | Oddball (500 Hz non- target) |
Chronic, Meds | Power, Evoked (Wavelet) | ↓ 0.031 |
All | 5 | |||||
| 50 ms | 1.25 | 210 | 21 | 22 | 35–50 | 20–60 | ? | All | |||
| EEG, 60 | Oddball (1kHz. non-target) | Chronic, Meds | PLF (Wavelet) | (↓) N.S. |
Fronto-Contral electrode sites |
6 | |||||
| 34–54 | 30–74 | Yes | |||||||||
| Power, Evoked (Wavelet) | (↓) N.S. |
Fronto-Control electrode sites |
|||||||||
| 70 ms | 1.2 | 180 | 23 | 21 | 34–54 | 30–74 | Yes | ||||
| EEG, 60 | Paired click | Chronic, Meds | Power, Evoked (Wavelet) | ↓ 0.001 |
All | 7 | |||||
| 5 ms | 10 | 160 | 51 | 34 | 35–48 | −100–400 | Yes | Widely distributed | |||
| EEG, 20 | Oddball (1 kHz non-target) | Chronic, Meds | Power, Evoked (Wavelet) | ↓ 0.01 |
Fronto-Central | 8 | |||||
| 500 ms | 1.8–2.2 | 480 | 30–80 | 50–150 | Yes | Fronto-Central | |||||
| Oddball (2 kHz target) | Power, Evoked (Wavelet) | ↓ 0.03 |
Fronto-Central | ||||||||
| 500 ms | 1.8–2.2 | 480 | 18 | 19 | 30–80 | 50–150 | Yes | Fronto-Contral | |||
| MEG, 37 | Puro tone (2 kHz) | Chronic Mode | Power, Evoked (Wavelet) | ↓ 0.03 |
Temporal channels (10) |
9 | |||||
| 200 ms | 1–2 | 220 | 20 | 23 | 20–45 | 0–50 | Yes | LH | |||
| MEG, 148 | Paired click | Chronic, Meds | Power, Evoked (FFT) | N.S | Spatial decomposition of channels |
10 | |||||
| 0.04 | 9 | 120 | 20 | 20 | 20–50 | 0–200 | No | ||||
| EEG, 4 | Auditory CPT (−1440 Hz) | Chronic, Meds | Power, Evoked (FFT) | ↓ 0.01 |
Fz, Cz, Pz, Oz | 11 | |||||
| 300 | 1 | 28 | 10 | 10 | 28–48 | 0–200 | No | Vertex | |||
| EEG, 29 | Oddball (paired click non- target) |
Chronic, Meds | Power, Evoked (Wavelet) | (↓) N.S. |
Cz | 12 | |||||
| 3 | 7-11 | 130 | 21 | 22 | 30–50 | 32–74 | No | ||||
| EEG, 29 | Pure tone (1000 Hz) | Chronic, Meds | PLF (FIR, Hilbert) | N.S. | Cz | 13 | |||||
| 1000 | 0.9 | 90 | 21 | 21 | 40 | 0–300 ms | No | ||||
| EEG, 32 | Paired click | Chronic, Meds |
PLF (Wavelet) | N.S | Cz | 14 | |||||
| 1 | 10 | 96 | 32 | 32 | 33–60 Hz | 25–125 | Yes | ||||
| MEG, 248 | Pure tone (1000 Hz) | Chronic, Meds | PLF (Wavelet) | ↓ <0.06 |
LH, RH sources | 15 | |||||
| 500 | 3.5 | 200 | 14 | 13 | 40 | 20–100 | No | LH > RH | |||
| EEG, 27 | Pure tone (800, 1300 Hz) | Chronic, Meds | Power, Evoked (Wavelet) | ↓ 0.004 |
Cz | 16 | |||||
| 32–48 | 20–80 | Cz | |||||||||
| PLF (Wavelet) |
↓ 0 002 |
Cz | |||||||||
| 250 | 2.5–7.5 | 60 | 17 | 17 | 32–48 | 20–80 | Cz | ||||
Acknowledgments
The authors would like to thank Greg Carlson, Chang-Gyu Hahn, and Bruce Turetsky for helpful discussions regarding the content of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Power typically has units of µV2 or is converted into decibels (e.g., 10*log[µV2/Hz]) to approximate a Gaussian distribution.
It may be useful to investigate oscillations phase-locked with a reaction response, rather than a stimulus, as some studies suggested that decision making processes are correlated more strongly with reaction time than stimulus onset (Spencer, 2004).
It should be noted that baseline subtraction, although an important aspect of signal normalization, is potentially problematic when groups differ in background activity (e.g., cortical noise), which seems to be the case in schizophrenia.
This also reflects the fact that weak coupling between two neural signals first affects their phases, before modulating amplitudes Herrmann, C. S., Grigutsch, M., Busch, N. A., 2005. EEG oscillations and wavelet analysis. In: Handy, T. C., (Ed), Event-related potentials : a methods handbook. MIT Press, Cambridge, Mass., pp. xi, 404 p..
Behavioral measures are often highly variable and necessitate large sample sizes, while exploratory analyses require stringent correction for multiple comparisons
There is some controversy whether these cells are actually reduced in number or whether they simply have downregulated expression of the calcium-binding protein parvalbumin.
References
- Akil H, Brenner S, Kandel E, Kendler KS, King MC, Scolnick E, Watson JD, Zoghbi HY. Medicine. The future of psychiatric research: genomes and neural circuits. Science. 2010;327:1580–1581. doi: 10.1126/science.1188654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammassari-Teule M, Sgobio C, Biamonte F, Marrone C, Mercuri NB, Keller F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) 2009;204:511–521. doi: 10.1007/s00213-009-1483-x. [DOI] [PubMed] [Google Scholar]
- Arnfred SM, Morup M, Thalbitzer J, Jansson L, Parnas J. Attenuation of beta and gamma oscillations in schizophrenia spectrum patients following hand posture perturbation. Psychiatry Res. 2010 doi: 10.1016/j.psychres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeweg T, Spence S, Hirsch SR, Gruzelier J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–621. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Macdonald ML, Borgmann-Winter KE, Hahn CG. Neuregulin 1-erbB4 pathway in schizophrenia: From genes to an interactome. Brain Res Bull. 2010;83:132–139. doi: 10.1016/j.brainresbull.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Tran LC, Chen R, Fitzgerald PB, Daskalakis ZJ. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res. 2010;121:146–152. doi: 10.1016/j.schres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Bullock TH. Induced rhythms in the brain. Boston: Birkhäuser; 1992. [Google Scholar]
- Basar E, Rosen B, Basar-Eroglu C, Greitschus F. The associations between 40 Hz-EEG and the middle latency response of the auditory evoked potential. Int J Neurosci. 1987;33:103–117. doi: 10.3109/00207458708985933. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Schmiedt-Fehr C, Mathes B, Zimmermann J, Brand A. Are oscillatory brain responses generally reduced in schizophrenia during long sustained attentional processing? Int J Psychophysiol. 2009;71:75–83. doi: 10.1016/j.ijpsycho.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav Brain Res. 2008;195:215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Ueber das Electrocephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14:876–894. doi: 10.1002/hipo.20002. [DOI] [PubMed] [Google Scholar]
- Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clin Neurophysiol. 2001;112:1650–1659. doi: 10.1016/s1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. The use of neurophysiological endophenotypes to understand the genetic basis of schizophrenia. Dialogues Clin Neurosci. 2005;7:125–135. doi: 10.31887/DCNS.2005.7.2/dlbraff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O'Donnell BF, Hetrick WP. Event-related potential abnormalities in schizophrenia: a failure to "gate in" salient information? Schizophr Res. 2009;113:332–338. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O'Donnell BF. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr Res. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RS, Lieberman JA, Barch DM, JGC, Goff DE, Gold JM, Green MF, Jarksog LF, Javitt DC, DK, Kraus MS, McEvoy JP, Mesholam-Gately RI, Seidman LJ, Ball MP, McMahon R, Kern RS, Robinson J, Marder SR. A Randomized Clinical Trial of MK-0777 for the Treatment of Cognitive Impairments in People with Schizophrenia. Biol Psychiatry In Press. 2010 doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. New York: Oxford University Press, Oxford; 2006. [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Coulter DA. In vitro functional imaging in brain slices using fast voltage-sensitive dye imaging combined with whole-cell patch recording. Nat Protoc. 2008;3:249–255. doi: 10.1038/nprot.2007.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Arriza JL, Roberts JC, Mrzljak L, Christian EP, Williams GV. Reversal of ketamine-induced working memory impairments by the GABAAalpha2/3 agonist TPA023. Biol Psychiatry. 2010;67:998–1001. doi: 10.1016/j.biopsych.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, Austin C, Becker RE, Berry GT, Driscoll K, Engle EC, Friedman S, Gusella JF, Hisama FM, Irons MB, Lafiosca T, LeClair E, Miller DT, Neessen M, Picker JD, Rappaport L, Rooney CM, Sarco DP, Stoler JM, Walsh CA, Wolff RR, Zhang T, Nasir RH, Wu BL. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:937–947. doi: 10.1002/ajmg.b.31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PA. Comparative Study of the EEGs of Schizophrenic and Manic-Depressive Patients. Am J Psychiatry. 1942;99:210–217. [Google Scholar]
- Demiralp T, Herrmann CS, Erdal ME, Ergenoglu T, Keskin YH, Ergen M, Beydagi H. DRD4 and DAT1 polymorphisms modulate human gamma band responses. Cereb Cortex. 2007;17:1007–1019. doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- Doheny HC, Faulkner HJ, Gruzelier JH, Baldeweg T, Whittington MA. Pathway-specific habituation of induced gamma oscillations in the hippocampal slice. Neuroreport. 2000;11:2629–2633. doi: 10.1097/00001756-200008210-00005. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, Gainetdinov RR, Sameshima K, Caron MG, Nicolelis MA. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009;29:8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Hammock EAD, Levitt P. Interneuron Pathophysiologies: Paths to Neurodevelopmental Disorders. In: Pallas SL, editor. Developmental Plasticity of Inhibitory Circuitry. Boston, MA: Springer US; 2010. pp. 167–184. [Google Scholar]
- Edgar JC, Hanlon FM, Huang MX, Weisend MP, Thoma RJ, Carpenter B, Hoechstetter K, Canive JM, Miller GA. Superior temporal gyrus spectral abnormalities in schizophrenia. Psychophysiology. 2008;45:812–824. doi: 10.1111/j.1469-8986.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE, Canive JM. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psychol. 2003;65:1–20. doi: 10.1016/s0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, Turetsky BI, Siegel SJ. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Faulkner HJ, Traub RD, Whittington MA. Disruption of synchronous gamma oscillations in the rat hippocampal slice: a common mechanism of anaesthetic drug action. Br J Pharmacol. 1998;125:483–492. doi: 10.1038/sj.bjp.0702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton GW, Fenwick PB, Dollimore J, Dunn TL, Hirsch SR. EEG spectral analysis in schizophrenia. Br J Psychiatry. 1980;136:445–455. doi: 10.1192/bjp.136.5.445. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- Finley KH. ON THE OCCURRENCE OF RAPID FREQUENCY POTENTIAL CHANGES IN THE HUMAN ELECTROENCEPHALOGRAM. Am J Psychiatry. 1944;101:194–200. [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G, Alexander D, Harris A, Whitford T, Wong W, Galletly C, Silverstein S, Gordon E, Williams LM. Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophr Res. 2008;105:262–271. doi: 10.1016/j.schres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Galambos R. A comparison of certain gamma band (40-Hz) brain rhythms in cat and man. In: Basar E, Bullock TH, editors. Induced Rhythms in the Brain. Boston: Birkhauser; 1992. pp. 201–216. [Google Scholar]
- Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating Gamma Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biol Psychiatry In press. 2010 doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157:95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitrapani D, Kayton L. Schizophrenia and EEG spectral analysis. Electroencephalogr Clin Neurophysiol. 1974;36:377–386. doi: 10.1016/0013-4694(74)90187-4. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez JA, Cedeno I, Pita-Alcorta C, Galan L, Aubert E, Figueredo-Rodriguez P. Induced oscillations and the distributed cortical sources during the Wisconsin card sorting test performance in schizophrenic patients: new clues to neural connectivity. Int J Psychophysiol. 2003;48:11–24. doi: 10.1016/s0167-8760(03)00019-9. [DOI] [PubMed] [Google Scholar]
- Gordon E, Williams LM, Haig A, Wright J, Meares RA. Symptom profile and "gamma" processing in schizophrenia. Cognitive Neuropsychiatry. 2001;6:7–19. [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100–1119. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer B, Light GA, Braff DL. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- Griskova I, Morup M, Parnas J, Ruksenas O, Arnfred SM. The amplitude and phase precision of 40 Hz auditory steady-state response depend on the level of arousal. Exp Brain Res. 2007;183:133–138. doi: 10.1007/s00221-007-1111-0. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, Linden DE, Rodriguez E. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29:9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Linden D. Exploring intermediate phenotypes with EEG: Working memory dysfunction in schizophrenia. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding? Clin Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O'Brien TJ, Pinault D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, Blendy JA, Dow HC, Brodkin ES, Schneider F, Gur RC, Siegel SJ. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory Gating Event-Related Potentials and Oscillations in Schizophrenia Patients and Their Unaffected Relatives. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The Early Auditory Gamma-Band Response Is Heritable and a Putative Endophenotype of Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NA, Sponheim SR, Clementz BA. Abnormalities of Neuronal Oscillations and Temporal Integration to Low- and High-Frequency Auditory Stimulation in Schizophrenia. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, Hoblyn J, Davis KL. Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. Am J Psychiatry. 1998;155:1080–1086. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Frund I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev. 2010;34:981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Grigutsch M, Busch NA. EEG oscillations and wavelet analysis. In: Handy TC, editor. Event-related potentials : a methods handbook. Cambridge, Mass.: MIT Press; 2005. p. xi. 404 p. [Google Scholar]
- Hirano S, Hirano Y, Maekawa T, Obayashi C, Oribe N, Kuroki T, Kanba S, Onitsuka T. Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci. 2008;28:4897–4903. doi: 10.1523/JNEUROSCI.5031-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MH, Ombao H, Edgar JC, Canive JM, Miller GA. Time-frequency discriminant analysis of MEG signals. Neuroimage. 2008;40:174–186. doi: 10.1016/j.neuroimage.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MJ, Matulewicz P, Gottesmann C, Kasicki S. State-dependent changes in high-frequency oscillations recorded in the rat nucleus accumbens. Neuroscience. 2009;164:380–386. doi: 10.1016/j.neuroscience.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Itil TM, Saletu B, Davis S. EEG findings in chronic schizophrenics based on digital computer period analysis and analog power spectra. Biol Psychiatry. 1972;5:1–13. [PubMed] [Google Scholar]
- Itil TM, Saletu B, Davis S, Allen M. Stability studies in schizophrenics and normals using computer-analyzed EEG. Biol Psychiatry. 1974;8:321–335. [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Stenson G, Reynolds GP. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav Brain Res. 2009;205:355–359. doi: 10.1016/j.bbr.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Bodkins M, O'Donnell BF, Shekhar A, Hetrick WP. Perceptual anomalies in schizophrenia co-occur with selective impairments in the gamma frequency component of midlatency auditory ERPs. J Abnorm Psychol. 2008;117:106–118. doi: 10.1037/0021-843X.117.1.106. [DOI] [PubMed] [Google Scholar]
- Joliot M, Ribary U, Llinas R. Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc Natl Acad Sci U S A. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS, Buhl EH. Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci Lett. 1993;149:35–39. doi: 10.1016/0304-3940(93)90341-h. [DOI] [PubMed] [Google Scholar]
- Kennard MA, Schwartzman AE. A longitudinal study of electroencephalographic frequency patterns in mental hospital patients and normal controls. Electroencephalogr Clin Neurophysiol. 1957;9:263–274. doi: 10.1016/0013-4694(57)90059-7. [DOI] [PubMed] [Google Scholar]
- Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, Nieminen-von Wendt T, von Wendt L, Paunio T, Peltonen L. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- Kissler J, Muller MM, Fehr T, Rockstroh B, Elbert T. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin Neurophysiol. 2000;111:2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O'Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O'Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Szilagyi N, Pincze Z, Rajkai C, Ulbert I, Karmos G. Attention and arousal related modulation of spontaneous gamma-activity in the auditory cortex of the cat. Brain Res Cogn Brain Res. 2004;19:1–9. doi: 10.1016/j.cogbrainres.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Goldberg E, Gordon E. An integration of 40 Hz Gamma and phasic arousal: novelty and routinization processing in schizophrenia. Clin Neurophysiol. 2001;112:1499–1507. doi: 10.1016/s1388-2457(01)00584-3. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E. "Gamma (40 Hz) phase synchronicity" and symptom dimensions in schizophrenia. Cogn Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- Leicht G, Karch S, Karamatskos E, Giegling I, Moller HJ, Hegerl U, Pogarell O, Rujescu D, Mulert C. Alterations of the early auditory evoked gamma-band response in first-degree relatives of patients with schizophrenia: Hints to a new intermediate phenotype. J Psychiatr Res. 2010a doi: 10.1016/j.jpsychires.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Leicht G, Kirsch V, Giegling I, Karch S, Hantschk I, Moller HJ, Pogarell O, Hegerl U, Rujescu D, Mulert C. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol Psychiatry. 2010b;67:224–231. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Lenz D, Fischer S, Schadow J, Bogerts B, Herrmann CS. Altered evoked gamma-band responses as a neurophysiological marker of schizophrenia? Int J Psychophysiol. 2010 doi: 10.1016/j.ijpsycho.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Leung LW. Spectral analysis of hippocampal EEG in the freely moving rat: effects of centrally active drugs and relations to evoked potentials. Electroencephalogr Clin Neurophysiol. 1985;60:65–77. doi: 10.1016/0013-4694(85)90952-6. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW., Jr Spike and slow wave localization by magnetoencephalography. Neuroimaging Clin N Am. 1995;5:575–596. [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Leung LS. Relation between hippocampal gamma waves and behavioral disturbances induced by phencyclidine and methamphetamine. Behav Brain Res. 2000;111:1–11. doi: 10.1016/s0166-4328(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Ma J, Leung LS. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology (Berl) 2007;191:961–974. doi: 10.1007/s00213-006-0667-x. [DOI] [PubMed] [Google Scholar]
- MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- Maharajh K, Teale P, Rojas DC, Reite ML. Fluctuation of gamma-band phase synchronization within the auditory cortex in schizophrenia. Clin Neurophysiol. 121:542–548. doi: 10.1016/j.clinph.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Ooi C, Kamath S, Suckling J, McKenna P, Fletcher P, Bullmore E, Stephenson C. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry. 2007;64:156–167. doi: 10.1001/archpsyc.64.2.156. [DOI] [PubMed] [Google Scholar]
- Meyer P, Mecklinger A, Grunwald T, Fell J, Elger CE, Friederici AD. Language processing within the human medial temporal lobe. Hippocampus. 2005;15:451–459. doi: 10.1002/hipo.20070. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Middleton S, Jalics J, Kispersky T, Lebeau FE, Roopun AK, Kopell NJ, Whittington MA, Cunningham MO. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma Oscillatory Power is Impaired During Cognitive Control Independent of Medication Status in First-Episode Schizophrenia. Neuropsychopharmacology. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T, Tanaka K, Hagimoto H, Miura T, Kishimoto H, Matsushita M. Computerized EEG in schizophrenic patients. Biol Psychiatry. 1990;28:488–494. doi: 10.1016/0006-3223(90)90482-h. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Mulert C, Leicht G, Hepp P, Kirsch V, Karch S, Pogarell O, Reiser M, Hegerl U, Jager L, Moller HJ, McCarley RW. Single-trial coupling of the gamma-band response and the corresponding BOLD signal. Neuroimage. 2010;49:2238–2247. doi: 10.1016/j.neuroimage.2009.10.058. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nase G, Singer W, Monyer H, Engel AK. Features of neuronal synchrony in mouse visual cortex. J Neurophysiol. 2003;90:1115–1123. doi: 10.1152/jn.00480.2002. [DOI] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Ousley O, Rockers K, Dell ML, Coleman K, Cubells JF. A review of neurocognitive and behavioral profiles associated with 22q11 deletion syndrome: implications for clinical evaluation and treatment. Curr Psychiatry Rep. 2007;9:148–158. doi: 10.1007/s11920-007-0085-8. [DOI] [PubMed] [Google Scholar]
- Pachou E, Vourkas M, Simos P, Smit D, Stam CJ, Tsirka V, Micheloyannis S. Working memory in schizophrenia: an EEG study using power spectrum and coherence analysis to estimate cortical activation and network behavior. Brain Topogr. 2008;21:128–137. doi: 10.1007/s10548-008-0062-5. [DOI] [PubMed] [Google Scholar]
- Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci U S A. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: a 4-month follow-up study. Biol Psychiatry. 1999;46:392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005;331:23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RM, Mathalon DH, Roach BJ, Ford JM. Relationships between pre-stimulus gamma power and subsequent P300 and reaction time breakdown in schizophrenia. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Mogilner A, Llinas R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecansky I, Kasparek T, Rehulova J, Katina S, Prikryl R. Aberrant EEG responses to gamma-frequency visual stimulation in schizophrenia. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin E, Grisell J, Gottlieb J. [Some electrographic differences between chronic schizophrenic patients and normal subjects.] Recent Adv Biol Psychiatry. 1968;10:194–204. doi: 10.1007/978-1-4684-9072-5_15. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roye A, Schroger E, Jacobsen T, Gruber T. Is my mobile ringing? Evidence for rapid processing of a personally significant sound in humans. J Neurosci. 2010;30:7310–7313. doi: 10.1523/JNEUROSCI.1113-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter L, Carver FW, Holroyd T, Nadar SR, Mitchell-Francis J, Apud J, Weinberger DR, Coppola R. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Troyano-Rodriguez E, Mengod G, Celada P, Artigas F. Activation of Thalamocortical Networks by the N-methyl-D-aspartate Receptor Antagonist Phencyclidine: Reversal by Clozapine. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. Attention changes the peak latency of the visual gamma-band oscillation of the EEG. Neuroreport. 1999;10:1167–1170. doi: 10.1097/00001756-199904260-00002. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Irani F, Brensinger CM, Kohler CG, Bilker WB, Ragland JD, Kanes SJ, Gur RC, Gur RE. Prognostic variables at intake and long-term level of function in schizophrenia. Am J Psychiatry. 2006;163:433–441. doi: 10.1176/appi.ajp.163.3.433. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Ralph L. Demystifying schizophrenia for the general practitioner. Sudbury, Mass: Jones and Bartlett Publishers; 2011. [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Consciousness and the structure of neuronal representations. Philos Trans R Soc Lond B Biol Sci. 1998;353:1829–1840. doi: 10.1098/rstb.1998.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. 111–125. [DOI] [PubMed] [Google Scholar]
- Sisti J, Halene TB, Majewski-Tiedeken C, Jonak G, Christian EP, Siegel SJ. 63rd Annual Scientific Convention and Meeting of the Society of Biological Psychiatry. Washington, DC: Elsevier; 2008. Reduced NMDAR1 Expression Leads to a Reduction in Parvalbumin Positive Immunoreactive Cells in Frontal Cortex. [Google Scholar]
- Slewa-Younan S, Gordon E, Harris AW, Haig AR, Brown KJ, Flor-Henry P, Williams LM. Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2004;161:1595–1602. doi: 10.1176/appi.ajp.161.9.1595. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Murray TA, Kimura R, Wakui M, Ellsworth K, Javedan SP, Marxer-Miller S, Lukas RJ, Wu J. Role of alpha7-nicotinic acetylcholine receptors in tetanic stimulation-induced gamma oscillations in rat hippocampal slices. Neuropharmacology. 2005;48:869–880. doi: 10.1016/j.neuropharm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukov W, Barth DS. Cellular mechanisms of thalamically evoked gamma oscillations in auditory cortex. J Neurophysiol. 2001;85:1235–1245. doi: 10.1152/jn.2001.85.3.1235. [DOI] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. "Gamma synchrony" in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Tallon C, Bertrand O, Bouchet P, Pernier J. Gamma-range activity evoked by coherent visual stimuli in humans. Eur J Neurosci. 1995;7:1285–1291. doi: 10.1111/j.1460-9568.1995.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42:1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O'Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Siegel SJ. Persistent auditory-evoked gamma band oscillations in schizophrenia. Boca Raton, FL: American College of Neuropsychopharmacology; 2007. [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D'Adamo P, Lipp HP. Midlatency auditory event-related potentials in mice: comparison to midlatency auditory ERPs in humans. Brain Res. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Urbach TP, Kutas M. Interpreting event-related brain potential (ERP) distributions: implications of baseline potentials and variability with application to amplitude normalization by vector scaling. Biol Psychol. 2006;72:333–343. doi: 10.1016/j.biopsycho.2005.11.012. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. J Neurosci Methods. 2007;162:49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Hetrick WP, Kaiser ST, Berg S, Morzorati SL. Auditory sensory gating in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropsychobiology. 2009;60:12–22. doi: 10.1159/000234813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and Computational Principles of Cortical Rhythms in Cognition. Physiological Reviews. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Veh RW, Heinemann U. Dopamine depresses cholinergic oscillatory network activity in rat hippocampus. Eur J Neurosci. 2003;18:2573–2580. doi: 10.1046/j.1460-9568.2003.02970.x. [DOI] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci. 2004;24:9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- Williams LM, Whitford TJ, Gordon E, Gomes L, Brown KJ, Harris AW. Neural synchrony in patients with a first episode of schizophrenia: tracking relations with grey matter and symptom profile. J Psychiatry Neurosci. 2009;34:21–29. [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Whitford TJ, Nagy M, Flynn G, Harris AW, Silverstein SM, Gordon E. Emotion-elicited gamma synchrony in patients with first-episode schizophrenia: a neural correlate of social cognition outcomes. J Psychiatry Neurosci. 2009;34:303–313. [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann WM, Coppola R. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Wojtowicz AM, van den Boom L, Chakrabarty A, Maggio N, Haq RU, Behrens CJ, Heinemann U. Monoamines block kainate- and carbachol-induced gamma-oscillations but augment stimulus-induced gamma-oscillations in rat hippocampus in vitro. Hippocampus. 2009;19:273–288. doi: 10.1002/hipo.20508. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]


