Abstract
White adipose tissue (WAT) is perhaps the most plastic organ in the body, capable of regeneration following surgical removal and massive expansion or contraction in response to altered energy balance. Research conducted for over 70 years has investigated adipose tissue plasticity on a cellular level, spurred on by the increasing burden that obesity and associated diseases are placing on public health globally. This work has identified committed preadipocytes in the stromal vascular fraction of adipose tissue and led to our current understanding that adipogenesis is important not only for WAT expansion, but also for maintenance of adipocyte numbers under normal metabolic states. At the turn of the millenium, studies investigating preadipocyte differentiation collided with developments in stem cell research, leading to the discovery of multipotent stem cells within WAT. Such adipose tissue-derived stem cells (ASCs) are capable of differentiating into numerous cell types of both mesodermal and nonmesodermal origin, leading to their extensive investigation from a therapeutic and tissue engineering perspective. However, the insights gained through studying ASCs have also contributed to more-recent progress in attempts to better characterize committed preadipocytes in adipose tissue. Thus, ASC research has gone back to its roots, thereby expanding our knowledge of preadipocyte commitment and adipose tissue biology.
Keywords: adipocytes, bone marrow, signal transduction, preadipocytes
FROM HIPPOCRATES TO ADIPOSE TISSUE-DERIVED STEM CELLS: A BRIEF HISTORY OF PREADIPOCYTES AND ADIPOSE TISSUE STEM CELLS
White adipose tissue (WAT) is generally viewed negatively, cast under the shadow of obesity and its association with ill health. Such stigmatization distracts from a fascinating aspect of WAT biology: its enormous plasticity as an organ. Indeed, WAT is capable of massive expansion and contraction in response to chronic alterations in energy balance, accounting for as little as 5% body mass in extremely lean athletes (1) or as much as 60% body mass in morbidly obese individuals (2). Moreover in rodents, rabbits, and humans, WAT can regenerate following lipectomy (3–6). This striking degree of plasticity is unique among organs in adults. On a cellular level, WAT expansion is driven by both hypertrophy and hyperplasia of adipocytes (7–13). Even in nonexpanding WAT, adipocytes renew frequently to compensate for adipocyte death, with approximately 10% of adipocytes renewed annually (14, 15). These data, which indicate that committed adipocyte progenitors (preadipocytes) exist within WAT, are the product of decades of research motivated largely by our desire to understand adipose tissue in the context of obesity and related diseases.
Preadipocytes exist in adipose tissue
Though the notion of obesity as a disease has existed since at least the time of Hippocrates (16), investigation into the origins of the adipocyte and the individual contributions of adipocyte hypertrophy and hyperplasia to adipose bulk began in earnest only after the appearance of the stem cell concept, as related to blood cells, in the early 1900s (17). In the 1940s, histologic observations of the appearance of adipocytes in chambers implanted in the ears of live rabbits suggested that de novo fat formation was possible (18). Closer inspection revealed that new adipocytes formed only along the vasculature (18) (Fig. 1), extending earlier reports linking vascularization and fat formation (19) and foreshadowing our current realization that some preadipocytes reside in a perivascular niche (20–25). Subsequent studies confirmed the existence of a mitotically active cell population in WAT (7), and that WAT expansion is associated with increases in total DNA content and adipocyte number (26). Following the development of methods for separating WAT into adipocyte and stromal vascular fractions (SVFs) (27), the source of these new adipocytes was determined by Hollenberg and Vost (28): using tritiated thymidine to monitor DNA synthesis in WAT, they detected temporal progression of radioactivity from the SVF to mature adipocytes, thereby inferring the presence of an adipocyte precursor cell in the SVF (28). Shortly thereafter, studies reported growth of fibroblast-like cells from the SVF that are capable of adipogenesis (29–31). This led to the designation of these stromal vascular cells (SVCs) as “primary” preadipocytes, setting the stage for extensive characterization of the mechanisms regulating adipogenesis and WAT expansion.
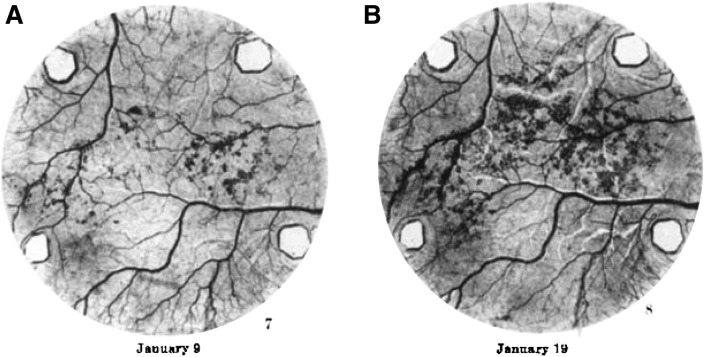
Fig. 1.
New adipocyte formation along the vasculature. Transparent chambers were implanted into the ears of live rabbits, allowing visualization of de novo adipocyte formation during tissue revascularization; adipocytes appear as dark outgrowths around the vasculature. The authors of this study (18) observed that “Frequently the fat cells appeared first in close proximity to a blood vessel ... the first fat cells in the right ear (A) appeared beside a vein, later spreading to the tissue between the blood vessels ... Frequently the new fat was first seen in one or two localized regions of the table area and subsequently it gradually spread out from those foci into adjoining regions (B).” This material is reproduced from (18) with permission from John Wiley and Sons, Inc.
Expanding knowledge of adipogenesis
The ability to isolate SVCs from WAT of distinct depots and under diverse conditions, such as with aging or obesity, forms the basis for our understanding of how these factors impact adipogenesis. For example, in humans, SVCs from visceral WAT are less proliferative and adipogenic and more susceptible to apoptosis than are SVCs from subcutaneous depots (32–34), whereas in rodents, SVCs of epididymal WAT show the poorest potential for adipogenesis or proliferation (35–40). Similar differences occur with aging, which is associated with decreased proliferation (35, 37, 38) and adipogenesis (41–43) of SVCs; the latter perhaps accounts for observed increases in preadipocyte number with age (44). In contrast, SVC proliferation is enhanced in massively obese humans (45), although obesity may only be associated with impaired SVC adipogenesis in older individuals (32, 46). Ultimately, such studies of SVC adipogenesis have highlighted how preadipocyte function might impact whole WAT biology in normal and disease states. However, SVCs are a heterogeneous cell population that, in addition to preadipocytes, contains fibroblasts, leukocytes, epithelial cells, endothelial cells, and other cells comprising the vasculature and nerve tissue (40, 47–49) (Fig. 2). SVCs also have practical limitations, such as inconsistent adipogenic differentiation and senescence during prolonged culture. To overcome these drawbacks, several clonal preadipocyte lines have been established, most notably the murine 3T3-L1 preadipocyte line (50–52). The use of these cell lines has facilitated extensive investigation of adipogenesis on a molecular level, leading to detailed characterization of extracellular modulators, intracellular signaling pathways, and, notably, transcriptional mechanisms that impact and underlie adipocyte differentiation. These developments have been reviewed extensively elsewhere (53–57) and continue to be refined and extended to this day, especially through global profiling of epigenetic modifications and transcription factor binding sites using DNase hypersensitivity, ChIP-chip or ChIP-seq analysis (58–61). Thus, the study of both SVCs and preadipocyte lines has made an enormous contribution to our current understanding of adipogenesis and WAT biology.
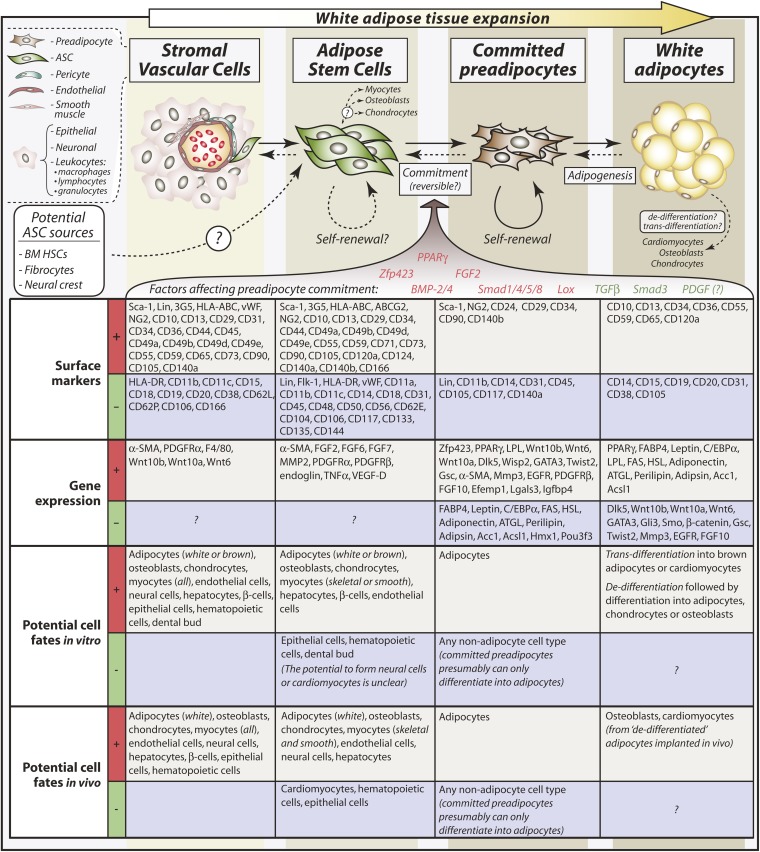
Fig. 2.
Relationships between SVCs, ASCs, committed preadipocytes, and mature adipocytes. Typical surface markers and other genes expressed or absent in each cell type are shown. More-extensive lists of surface markers and cell fates are provided, with citations, in Tables 1 (or see supplementary Table I) and 2, respectively. Comparisons of surface markers or other genes differentially expressed between the different cell types reveal potential mechanisms of preadipocyte commitment. Factors that positively regulate preadipocyte commitment are shown in red, and negative regulators are shown in green; effects of PDGF on preadipocyte commitment remain uncertain. Arrows shown as dotted lines designate pathways that are not yet firmly established. For example, whether committed preadipocytes continuously derive from ASCs or merely replenish through autologous self-renewal remains incompletely understood. Equally, whether ASCs or committed preadipocytes can revert to pericytes or ASCs, respectively, requires further investigation. Although mature adipocytes can give rise to other cell types in experimental conditions, the relevance of such trans-differentiation to physiological contexts is not clear. Finally, formation of adipocytes from BM HSCs, fibrocytes, or the neural crest has been reported, so it is possible that ASCs derive from some of these cell types; however, these findings remain controversial.
Adipose tissue enters the world of multipotent stem cells
This progress in our knowledge of preadipocyte differentiation paralleled that made in the field of stem cell biology. Although the term “stem cell” was first used in 1868, it was not applied in its modern context until the early 1900s, when it became used to describe the putative common progenitor for the hematopoietic system (17). Definitive evidence for existence of a common hematopoietic stem cell (HSC) emerged in the 1960s (62–64), followed by extensive HSC characterization based on cell surface markers (65). Importantly, HSCs are capable of both self-renewal and multilineage differentiation in vivo (66), two properties that are considered definitive of a true stem cell population (67). In addition to HSCs, bone marrow (BM) stroma also contains nonhematopoietic progenitor cells, the existence of which was first inferred through observations of donor cell-derived osteogenesis following BM transplants (68). These stromal cells can be separated from HSCs based on the inability of the latter to adhere in culture (as reviewed in Ref. 69); thereby isolated, these stromal cells display at least some capacity for self-renewal (70–72) and differentiation into mesenchymal cell types, such as osteoblasts, chondrocytes, adipocytes, and myoblasts, both in vitro and in vivo (69, 72–74). Based on these properties, such cells have been designated mesenchymal stem cells (MSCs). Related approaches have resulted in the identification of neural crest stem cells (75), as well as MSCs in numerous other tissues (76).
At the turn of the millennium, these parallel developments in our understanding of preadipocyte differentiation and stem cell biology collided, with the discovery of multipotent stem cells in WAT (77–79). The past decade has since witnessed an explosion in research focusing on such adipose tissue-derived stem cells (herein referred to as ASCs, but given various names in other studies3), largely fueled by interest in their potential for tissue engineering and regenerative medicine (80). Consequently, there is now a wealth of information regarding ASC multipotency, both in vitro and in vivo (Table 2). However, before discussing this, it is necessary to address a fundamental and still-unresolved question: what exactly is an ASC?
TABLE 2.
Multipotency of ASCs in vitro and in vivo
DEFINING THE ASC
The rapid expansion in ASC research has often generated conflicting findings, which is partly attributable to inconsistency and uncertainty in defining the ASC itself. Whereas some studies describe whole, unsorted SVC populations as “ASCs” (100–102), others define ASCs as a functionally distinct SVC subpopulation (49, 103–105); in yet other cases, it is not clear whether the ASCs investigated are whole SVCs or a subpopulation of these. Moreover, SVCs have been analyzed as freshly isolated cells (106, 107) or after different durations in culture (103, 108), with some studies drawing comparisons between these conditions (22, 109). Even naming of the ASC has varied hugely. These inconsistencies complicate our ability to draw meaningful conclusions across multiple studies, underscoring the need to better define exactly what the ASC is. Many insights are provided through extensive functional analyses of ASC multipotency, as discussed elsewhere. Additionally, ASCs have been characterized through global analyses of gene expression (108, 110, 111) and protein secretion (112), and by more-focused approaches, such as assessment of epigenetics (113), circadian genes (114), or electrophysiological properties (115). However, by far the most widespread analyses are of cell surface markers.
Beauty is only skin deep: cell surface markers in ASC characterization
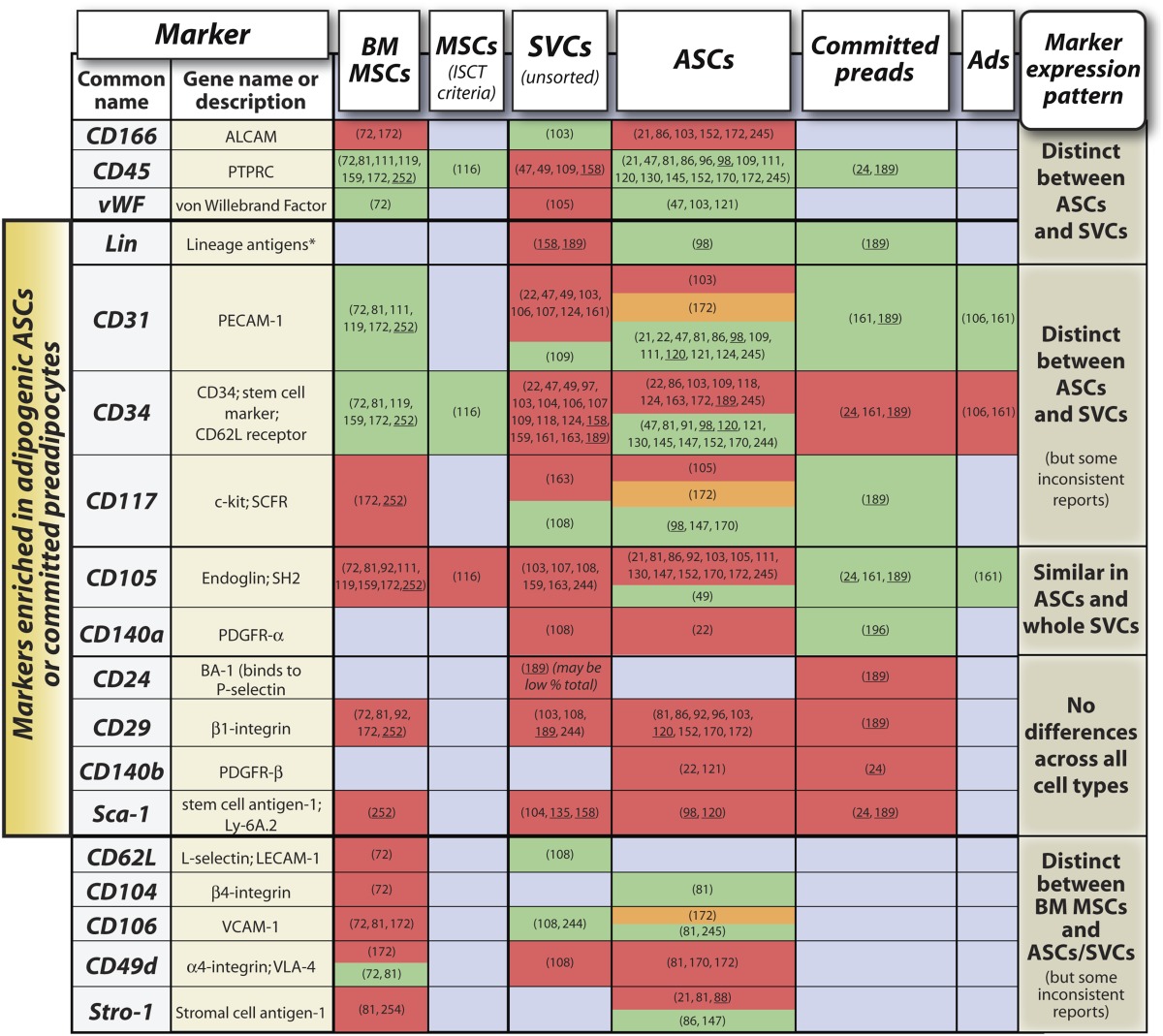
Extensive study of HSCs has established the utility of using cell surface markers to characterize genuine stem cells within a heterogenous cell population (65, 66). This is also true for BM MSCs, which are defined by the International Society for Cellular Therapy as being CD73+, CD90+, CD105+, CD45–, CD34–, CD14–, or CD11b–, CD79–, or CD19–, and devoid of human leukocyte antigen-DR (Table 1, supplementary Fig. I) (116). Similarly, numerous studies have characterized whole SVCs or ASCs based on surface marker expression (Table 1 and supplementary Fig. I), and several researchers have suggested definitive marker profiles for ASCs. For example, Yoshimura et al. designate ASCs as a CD34+/CD31–/CD45–/CD90+/CD105–/CD146– subpopulation of SVCs (49); Lin et al. suggest that ASCs are CD34+/CD31–/CD104b– and negative for α-smooth muscle actin (α-SMA) (117); and Quirici et al. used positive selection for CD34 and CD271 (L-NGFR) to identify ASCs based on their capacity for proliferation and multilineage differentiation (118). However, the heterogeneity and evolution of surface phenotypes in long-term culture bring into question the ability to reliably identify a “phenotypic fingerprint” of a stromal stem cell based on surface markers alone: like beauty, many of the most attractive surface markers fade with time as cells are maintained and passaged in vitro (21, 49, 88, 103, 119, 120). Species-specific differences in surface marker expression could also account for discrepancies between studies based on murine or human cells. Finally, most of the markers are not directly related to the stem cell itself and are often expressed on nonprogenitor lineages. Perhaps because of these drawbacks, agreement for a definitive ASC marker profile remains to be established. Therefore, in Table 1, we present a concise list of surface markers differentially expressed between these cell types in an attempt to find some consensus on marker profiles typical of ASCs; a more comprehensive overview is shown in the supplement (see supplementary Table I). Such an overview allows direct comparison of marker expression between BM MSCs, whole WAT SVCs, and purified ASCs, which we feel can provide further insights into ASC biology. For example, numerous markers are expressed or absent in both BM MSCs and ASCs, suggesting much similarity between these cell types. In contrast, several markers distinguish BM MSCs from ASCs, including CD34, CD104, CD106, and possibly CD49d (Table 1). Equally, differences between ASCs and whole SVCs potentially exist in expression of CD11a, CD14, CD31, CD34, CD45, CD117, CD146, and von Willebrand factor (Table 1 and supplementary Table I), which might facilitate isolation of ASCs from whole SVC populations. Indeed, the use of surface markers has been pivotal to recent progress in localizing ASCs within WAT.
TABLE 1.
Surface marker expression in BM MSCs, SVCs, ASCs, committed preadipocytes (preads), and mature adipocytes (Ads)
ASCs as perivascular cells
Increasing evidence suggests that MSCs from multiple human tissues, including WAT, are perivascular cells (pericytes) (21, 22, 121). Defined anatomically in vivo based on their location surrounding the endothelia (122), pericytes can be distinguished from endothelial and smooth muscle cells by their extensive cytoplasmic branching, abundant rough endoplasmic reticulum, moderate amounts of lysosome-like bodies, and abundance of micropinocytotic vesicles (122). Functionally, pericytes can modulate both endothelial cell proliferation and vessel contractility (121). Using immunostaining, Crisan et al. identified pericytes in multiple organs as CD146+, NG2+ CD140b+, α-SMA+, CD144–, vWF–, CD34–, and CD31– (121), consistent with other studies (22, 49, 123, 124). Pericytes thereby isolated using fluorescence-activated cell sorting (FACS) were found to be multipotent, even after long-term passaging in culture, and to express MSC markers such as CD44, CD73, CD90, and CD105 (Table 1) (121). Of two initial studies focusing on the perivascular nature of ASCs specifically, one reported findings very similar to those of Crisan et al. (121) whereas another found that CD34+/CD31– ASCs are also perivascular (22, 23). Further evidence comes from more-recent work based on α-SMA expression. In transgenic mice that express green fluorescent protein (GFP) from the α-SMA promoter, α-SMA-expressing cells in WAT are exclusively perivascular, as assessed by tissue localization and coexpression of platelet-derived growth factor receptor-β (PDGFR-β; also known as CD140b) (25). Moreover, the α-SMA+ cells are multipotent in vitro, whereas α-SMA– SVCs are not (25), indicating that ASCs exist within the α-SMA+ population (Fig. 2). Thus, findings from several studies strongly suggest that ASCs are perivascular.
MULTIPOTENCY OF ASCS
In addition to facilitating ASC localization, surface marker expression can also distinguish between functionally distinct SVC subpopulations, and thereby has implications for one of the most important aspects of ASC biology: their capacity to differentiate into multiple cell types. The earliest studies reported in vitro differentiation of human ASCs into cells of mesenchymal origin, such as adipocytes, osteoblasts, chondrocytes, and myocytes (Table 2). Later studies have also used murine ASCs, and have extended this in vitro repertoire to include cells of nonmesodermal origin (Table 2). Generally, these analyses of ASC multipotency are based on morphology or expression of markers characteristic for the various differentiated cell types; in some cases, terminal differentiation is also assessed functionally, for example by contractility of ASC-derived myocytes (125–127), or myelin production from ASC-derived Schwann cells (128). Further research has focused on the ability of ASCs to engraft in host tissues and give rise to these diverse cell types in vivo, often in the context of tissue engineering and regenerative medicine (Table 2). In many cases, differentiation or manipulation in vitro is a prerequisite for ASC engraftment or differentiation in vivo, although this may not be required for all cell fates or tissue types (Table 2). These issues, as related to specific cell or tissue types, are discussed further below.
Adipocytes, osteoblasts, and chondrocytes
Given their adipose origin, it is not surprising that ASCs are capable of forming adipose tissue in vivo (129–131). However, as found for BM MSCs and some committed preadipocyte lines (132–134), this requires the ASCs to be seeded onto polymeric three-dimensional (3-D) scaffolds after adipogenic induction in vitro (129–131). Moreover, at least one study suggests that donor ASCs may contribute to adipose tissue regeneration by stimulating differentiation of host-derived adipocytes (131). More recently, a population of Sca-1+/CD45–/Mac-1– progenitor cells (called “ScaPCs” for simplicity) with potential for brown adipogenesis has been identified in WAT and skeletal muscle (135). These ScaPC-derived brown adipocytes express traditional brown adipocyte markers such as uncoupling protein-1 (UCP-1) and PRDM16, a transcriptional regulator of brown fat determination that also impacts the thermogenic program of WAT (136–138). In contrast, so-called “brite” (“brown-in-white”) adipocytes that arise in WAT under some conditions express UCP-1 but not PRDM16 (139). Thus, it is unclear whether ScaPCs contribute to formation of brite adipocytes or give rise to a distinct population of brown-like adipocytes in WAT.
ASCs can also form bone and cartilage in vivo (Table 2), with osteogenic capacity similar to that of BM MSCs (74). However, as with adipogenesis, this requires preseeding onto 3-D scaffolds (93, 120, 129, 140, 141) and/or prior differentiation in vitro (85, 129, 142, 143), for example by transfecting the ASCs for stable expression of bone morphogenetic protein-2 (BMP-2) (141) or BMP-4 (120). A related study found that undifferentiated ASCs on 3-D scaffolds could form osteoid in vivo, but this required scaffold-derived BMP-2 release at the site of transplantation (143). Thus, exogenous stimuli such as BMP-2 or -4 seem to be required for effective osteogenesis or chondrogenesis by ASCs in vivo. It should be noted, however, that such exogenous BMP expression also promotes osteoblastogenesis and chondrogenesis by host-derived cells (120), suggesting that the ASC may simply be serving as a gene carrier in these studies. Nevertheless, undifferentiated ASCs can reportedly heal calvarial defects in mice even without exogeneous osteogenic factors (93, 102, 144). In such cases, we postulate that calvarial trauma may induce release of endogenous osteogenic factors, thereby circumventing the need for exogenous stimuli.
Myocytes
Skeletal muscle.
In vitro myogenesis of ASCs can be stimulated by coculture with primary myoblasts or myoblast cell lines, with which the ASCs can also fuse (104, 145). Moreover, unsorted or CD34– SVCs are capable of incorporation into damaged skeletal muscle fibers in vivo, thereby contributing to skeletal muscle regeneration (100, 104, 146, 147). However, other studies suggest that ASCs have only limited capacity to form skeletal muscle in vivo (120). This discrepancy probably results from differences in ASC isolation and culture techniques: although long-term passages of CD34– SVCs or pericytes may form myofibers in dystrophic mice (104, 121, 147), ASC myogenic capacity is reportedly lost after expansion in culture and is restricted to CD34–/Sca-1– cells from freshly isolated SVF (104). Given that ASCs probably express CD34 and Sca-1 (Table 1), it is unclear whether they are genuinely myogenic in vivo.
Cardiac muscle.
Whole SVC populations can form spontaneously beating cardiomyocytes in vitro after treatment with 5-azacytidine (125) or interleukins (126), and can also express some cardiomyocyte markers in vivo after injection into damaged myocardium (148). However, these observations are based on unsorted murine adipose SVCs, rather than purified ASC subpopulations. One study suggests that cells isolated from human WAT can form spontaneously contractile cells after exposure to rat cardiomyocyte extracts (149); however, it is unclear whether the cells used in this study were whole SVCs or a purified subpopulation (Table 2). In contrast, ASCs purified from WAT are unable to undergo 5-azacytidine-induced cardiomyogenesis (150, 151) and only form spontaneously contractile cells through coculture with neonatal rat cardiomyocytes (151, 152). Additionally, the ability of ASCs to repair damaged myocardium after myocardial infarction may be a result of stimulation of neovascularization by ASCs, rather than formation of ASC-derived cardiomyocytes (153, 154). Thus, no studies have convincingly demonstrated that purified WAT ASCs are capable of forming functional cardiomyocytes in vivo. However, SVCs from brown adipose tissue (BAT) may be better suited to this purpose. Our lab has demonstrated that BAT SVCs have intrinsic cardiomyogenic potential that is enriched in subpopulations expressing Sca-1 or c-kit (155). Moreover, this cardiomyogenic potential is enhanced by treatment with Wnt5a, a noncanonical Wnt ligand, or with DKK1, an inhibitor of canonical Wnt signaling (155). Interestingly, precursors from BAT show both greater cardiomyogenic potential in vitro and ability to promote recovery from myocardial infarction than ASCs from WAT (126, 156). Thus, BAT ASCs may prove more useful than WAT ASCs for treatment of cardiac dysfunction.
Smooth muscle.
In addition to skeletal and cardiac myocytes, ASCs can differentiate into smooth muscle, as assessed by marker gene expression and contractility (88, 127, 157). Smooth myocytes thereby derived from human ASCs in vitro are also capable of maintaining smooth muscle function in vivo, as evidenced by their use in tissue engineering of bladder smooth muscle (157).
Hematopoietic and endothelial cells
Two studies have suggested that ASCs can form hematopoietic cells in vivo (158, 159), albeit without the self-renewal capacity characteristic of genuine HSCs (66). However, each of these reports used whole SVCs, which include cells expressing the hematopoietic marker CD45 (Table 1); in contrast, MSCs and ASCs are CD45– (Table 1 and see Ref. 116). Although the CD45– fraction of SVCs may be capable of limited hematopoiesis and endothelial activity (160), most evidence suggests that CD45– ASCs are incapable of hematopoiesis in vitro or in vivo (120, 161).
Related studies have investigated endothelial cell differentiation of ASCs. CD34+ subsets of SVCs (CD34+/CD31– and CD34+/CD90+) are capable of generating endothelial cells in vitro, with formation of tube-like structures and upregulation of the endothelial progenitor cell marker Flk-1 sometimes apparent (22, 97, 109, 162, 163). In contrast, CD34– SVCs are unable to form endothelial cells (98, 163). In vivo, both unsorted SVCs and purified ASCs can promote neovascularization of host tissues (97, 109). This effect may occur largely through ASC/SVC-derived release of factors such as VEGF, thereby stimulating angiogenesis by host endothelial cells (22, 98, 109, 154). However, donor ASCs may also form functional vasculature in vivo (97, 109) that can reportedly connect with host blood vessels (107).
Ectodermal cells
Neural cells.
In addition to mesodermal fates, in vitro differentiation of ASCs into neural cell types has long been reported (Table 2). However, this is largely based on morphology or limited expression of typical neural markers, such as nestin, NeuN or GFAP (81, 91, 95, 164); although ASC-derived Schwann-like cells can produce myelin structures during in vitro coculture (128), few reports have demonstrated any responsiveness to neurotransmitters or electrophysiological activity (84, 94). Indeed, it has recently been suggested that murine ASCs are unable to form functional neurons (165). Nevertheless, an earlier study found that human ASCs can home to injured brain regions and improve ischemia-induced neurological defects (91), and more-recent reports demonstrate the potential for murine ASCs to enhance neuronal regeneration (166, 167). Thus, ASCs may hold promise for the treatment of neural defects.
Epithelial cells and dental bud.
SVCs lacking CD34 can form epithelial cells in vitro in response to all-trans retinoic acid (47). A related study shows that ASCs can differentiate in vitro into cells resembling retinal pigment epithelial cells, with the ability to form pigmented granula (168). Moreover, CD34– SVCs can engraft into retinas in a model of diabetic retinopathy (169), suggesting another potential therapeutic application of these cells. Another ectodermal fate was recently reported by Ferro et al., who show that CD34− SVCs form dental bud-like structures under prolonged 3-D culture in vitro (170). However, as mentioned above and discussed later, ASCs are probably CD34+; hence, no studies have conclusively shown that ASCs can form epithelial cells (Fig. 2).
Endodermal cells
Hepatocytes.
ASCs can undergo hepatic differentiation in vitro, as assessed by morphology, gene expression, and functional assays such as LDL uptake, urea production, and glycogen storage (87, 171–173), and this may be enhanced in a CD105+ subpopulation of SVCs (172). These ASC-derived hepatocytes can also engraft into livers and reconstitute some hepatocyte functions in immunodeficient mice, often after induction of liver injury (87, 173–175). Although prior hepatic differentiation in vitro enhances hepatic ASC engraftment in vivo (173), undifferentiated ASCs are also capable of hepatic engraftment (87, 174). Thus, prior in vitro manipulation is not absolutely required for ASCs to differentiate into some cell types in vivo (Table 2). However, it is not clear whether the hepatocytes derived from ASCs in vivo are generated through genuine hepatic differentiation or simply by fusion of the ASCs with host cells in the liver, as reported for BM MSCs (176, 177). Nevertheless, human ASCs are currently under investigation as a treatment for liver injury (175).
Pancreatic islet cells.
Finally, ASCs may be able to differentiate into pancreatic cell types, with specific culture conditions promoting expression of insulin, glucagon, and other pancreatic genes in vitro (178). ASCs can also engraft into the pancreas, acquire a β-cell phenotype, and restore pancreatic function in vivo in diabetic animals, albeit only after prior transduction for exogenous Pdx1 expression (101, 179). Thus, while it is unlikely that unmodified ASCs are similarly capable of functional islet cell formation, they may be useful therapeutically in this context.
Multipotency of mature adipocytes
While most researchers currently agree that multipotent ASCs are derived from the SVF of adipose tissue, more-controversial studies report that mature adipocytes also possess multidifferentiation potential. Adipocytes isolated from murine or human WAT can be “de-differentiated” through ceiling culture, whereby the floating, lipid-laden adipocytes adhere to the top of culture vessels that are filled with culture medium; subsequently, the adipocytes lose lipid, adopt a fibroblast-like morphology, and begin to undergo cell division (180–182). Importantly, these studies appear to exclude the possibility that the dedifferentiated adipocytes are simply derived from residual SVCs, which have been noted to persist among the floating adipocyte layer following collagenase digestion of WAT (7). As for ASCs, the proliferative, dedifferentiated adipocytes can form adipocytes, osteoblasts, chondrocytes, and cardiomyocytes in vitro (180–182). They also exhibit some capacity for in vivo osteoblastogenesis or cardiomyogenesis following subcutaneous implantation into NOD/SCID mice (182) or injection into damaged myocardium (183), respectively. Additionally, these dedifferentiated adipocytes express cell surface markers similar to those of preadipocytes and ASCs (Table 1 and supplementary Table I), including CD13, CD29, CD44, CD90, CD105, and CD49d, but not CD31 (181). Thus, murine and human adipocytes can dedifferentiate into ASC-like cells, at least in vitro (Fig. 2). Mature adipocytes may also be able to directly differentiate into other cell types through so-called ‘trans-differentiation’ (Fig. 2). For example, treatment of lipid-laden mature adipocytes with inhibitors of BMP or canonical Wnt signaling promotes their differentiation into cardiomyocytes in vitro (183). White adipocytes may also trans-differentiate into PRDM16-expressing brown-like adipocytes following chronic cold exposure or treatment with β3-adrenoreceptor agonists (184). These observations challenge the notion that adipogenesis is irreversible and suggest that ASCs may not be the only multipotent cells in adipose tissue.
Multidifferentiation potential of ASCs: nature or nurture?
The ability to differentiate ASCs into numerous cell types clearly holds enormous therapeutic potential. However, what this tells us about the role of ASCs in more biological contexts is less certain. Though SVCs or ASCs are often capable of tissue engraftment or differentiation into some cell types in vivo, in many cases it is unclear whether these donor-derived cells can actually perform or maintain cell type-specific functions in this context, as reported for whole blood reconstitution by HSCs (66). Morever, despite reported maintenance of ASC multipotency after extended passaging in vitro (21, 121, 147, 185), whether ASCs are capable of true self-renewal in vivo remains poorly understood. Thus, whether ASCs are bona fide stem cells is still a matter of debate.
THE ASC MEETS PREADIPOCYTE COMMITMENT
While the stem cell nature of ASCs remains controversial, the fact that WAT contains committed preadipocytes stands unrefuted. However, the great advances in our knowledge of terminal adipocyte differentiation (53–57) have occurred against a backdrop of relative ignorance regarding both the identity of committed preadipocytes and mechanisms regulating preadipocyte commitment (186). Given that both ASCs and committed preadipocytes are subpopulations of WAT SVCs, it is perhaps unsurprising that progress in our understanding of ASC biology has also shed light on preadipocyte commitment.
Preadipocyte commitment: scratching the surface
Among the many surface markers investigated in ASCs, perhaps the most informative with regard to preadipocyte commitment is CD34. The CD34 family (CD34, podocalyxin, and endoglycan) comprises single-pass transmembrane glycoproteins of the sialomucin family that can potentially impact cell adhesion, proliferation, migration, and differentiation (187). CD34 expression is most commonly noted on hematopoietic progenitor cells and vascular tissue, but has also been observed on various other cell types (187). Although CD34 expression is a classic marker of an active self-renewing HSC population (188), BM MSCs (72, 116, 119), embryonic stem cell-derived MSCs (Paul Krebsbach, personal communication) and pericytes (23, 121) are thought to be CD34–. Findings for CD34 expression in ASCs have been inconsistent (Table 1), possibly due to progressive downregulation of CD34 in culture (88, 103, 119, 120) or the relatively weak expression of CD34 in Lin– stroma. Nevertheless, CD34 appears to distinguish between different functional subgroups of ASCs, especially with regard to adipogenesis: although CD34– SVCs can undergo adipogenesis (47, 121, 123), CD34+ cells make up approximately 82% of the SVF (106) and are consistently more adipogenic in vitro than CD34– populations after negative selection for CD31 (124, 161, 189). Similarly, exclusion of CD34+ cells in muscle regeneration studies inhibits ectopic adipose formation both in vitro and in vivo (190), indicating that CD34– cells are incapable of adipogenesis. Finally, adipocytes isolated from WAT are noted as CD34+ (106). Thus, CD34+/CD31– appears to define a population of robustly adipogenic ASCs in vitro and to distinguish adipogenic progenitors in vivo. Further refinement of the adipogenic ASC population has been made based on CD105 expression. One study found that in whole SVCs, cells expressing CD105 are more adipogenic than unsorted SVCs (172). In contrast, within the CD34+/CD31– subpopulation, the ability to undergo adipogenesis is restricted to CD105– cells (161).
Based on these observations, one might predict committed preadipocytes to also be CD34+/CD31–/CD105–. In 2008, an elegant study by Rodeheffer, Birsoy, and Friedman showed this to indeed be the case (189). In an approach reminiscent of some studies of ASC multipotency, they used FACS to isolate distinct subpopulations of SVCs, first assessing the multipotency of each population in vitro. This revealed that in vitro formation of adipocytes, chondrocytes, osteoblasts, or myocytes is restricted to Lin–/CD34+/CD29+ SVCs, which strongly supports independent assertions that ASCs are CD34+ (22, 109, 118, 161). Further separation of the Lin–/CD34+/CD29+ SVCs based on Sca-1 and CD24 revealed a striking result: although Lin–/CD29+/CD34+/Sca-1+/CD105–/CD117– SVCs are capable of in vitro adipogenesis regardless of CD24 status, only the CD24+ population can reconstitute WAT in lipodystrophic mice (189). Importantly, this WAT reconstitution was associated with decreased serum glucose and insulin and was maintained in the host mice for at least 12 weeks. Thus, Lin–/CD29+/CD34+/Sca-1+/CD105–/CD117–/CD24+ SVCs are capable of long-term reconstitution of functional WAT in vivo, strongly suggesting that these markers are expressed on committed preadipocytes (Table 1 and Fig. 2). However, given that this SVC subpopulation is also capable of osteoblastogenesis, chondrogenesis, and myogenesis in vitro (189), additional factors must further distinguish committed preadipocytes from multipotent cells within this subpopulation.
In a related study, Tang et al. used lineage tracing to identify committed preadipocytes based on expression of PPARγ (24), a transcription factor that is essential for adipogenesis and adipocyte function (53, 54, 191–193). Perhaps unsurprisingly, they found that SVCs with expression of PPARγ (PPARγ+) are more adipogenic in vitro than PPARγ– SVCs, and are also capable of adipocyte formation in vivo (24). In a clever twist, their system was also made doxycycline-sensitive, which allows the formation of PPARγ-expressing progenitors in WAT to be temporally assessed. This revealed that these PPARγ+ SVCs proliferate in vivo. Therefore, these findings suggest that among whole WAT SVCs, committed preadipocytes are distinguished by their expression of PPARγ and that these preadipocytes self-renew through proliferation (Fig. 2). FACS analysis of surface markers revealed that the PPARγ+ SVCs express Sca-1 and CD34, but not CD105, CD45, Ter-119, or Mac-1 (also called CD11b). This pattern is consistent with the aforementioned findings for adipogenic ASCs and committed preadipocytes (Table 1). Importantly, SVCs isolated based on these markers, but independently of PPARγ-expression, were also capable of in vivo adipogenesis (24). These data suggest that, in addition to being Sca-1+, CD34+, CD105–, and CD24+, committed preadipocytes can be distinguished among whole WAT SVCs by their expression of PPARγ (Fig. 2).
Committed preadipocytes are perivascular cells
Decades of histological studies have suggested a close association between the vasculature and adipocyte progenitors (18, 20, 194) (Fig. 1); hence, the possibility that preadipocytes derive from pericytes has long been proposed (20). The more-recent progress in ASC characterization further supports this possibility. As discussed above, CD34+/CD31− ASCs are capable of both endothelial and adipocyte differentiation (22, 109, 124, 161, 189). This suggests that adipocytes and vascular endothelial cells may share a common origin and is therefore consistent with the notion of preadipocytes as perivascular cells. However, the 2008 study by Tang et al. was the first to provide convincing evidence of this. They found that PPARγ-expressing SVCs (i.e., committed preadipocytes) also express the pericyte markers α-SMA, PDGFR-β (CD140b), and NG2 (chondroitin sulfate proteoglycan) (Fig. 2), and that these cells localize around tube-like structures in WAT (24). Additional lineage tracing revealed that adipose tissue derives from PDGFR-β-expressing cells, and that PDGFR-β+ SVCs isolated from WAT can form adipocytes in vivo (24). Thus, like ASCs, committed preadipocytes appear to be perivascular in WAT. Though this perivascular nature has also been noted for brown adipocyte progenitors (195), pericytes are PPARγ– in the liver and skeletal and cardiac muscles, kidney, retina, pancreas, spleen, and lung (24). This observation suggests, quite logically, that pericytic preadipocytes may exist only in adipose tissues.
Finally, it is worth noting that PPARγ or PDGFR-β alone is not sufficient to identify committed preadipocytes: PPARγ-expressing cells from aged muscle cannot undergo adipogenesis in vitro and, whereas PDGFR-β+ SVCs from WAT can form adipocytes in vivo, those from muscle, kidney, or heart cannot (24). These observations suggest that additional mechanisms are involved in commitment of preadipocytes around the vasculature of WAT.
Below the surface: transcriptional regulators of ASCs and committed preadipocytes
Although the transcriptional regulation of adipogenesis is extremely well characterized (53, 54, 56, 57), how transcription factors impact ASC biology or preadipocyte commitment remains poorly understood. This is especially true for ASCs. One earlier microarray analysis was limited to angiogenic and extracellular matrix genes (108), whereas another focused on gene expression during adipogenesis or osteoblastogenesis (110). A more-recent microarray study compared transcriptional profiles of human ASCs, BM MSCs, and stem cells from umbilical cord, identifying gene expression signatures distinct to each stem cell type (111). However, these studies have not been followed by any comprehensive mechanistic analysis. In contrast, progress has recently been made for committed preadipocytes. Work from the Spiegelman lab identified both PPARγ2 and zinc-finger protein 423 (Zfp423) as transcription factors that are enriched in adipogenic fibroblast cell lines, relative to nonadipogenic cells (196). Enrichment of PPARγ2 supports the notion of PPARγ as a marker of preadipocytes (24); however, Zfp423 had not previously been reported in this context. Although Zfp423 is not upregulated during adipogenesis, exogenous expression of Zfp423 in otherwise nonadipogenic cells is sufficient to specifically upregulate PPARγ2 and markedly increases the adipogenic potential of these cells (196). Conversely, in vitro adipogenesis is impaired in 3T3-L1 preadipocytes or mouse embryonic fibroblasts with stable knockdown or total knockout of Zfp423, respectively. These observations identify Zfp423 as a transcriptional regulator of preadipocyte commitment (Fig. 2).
More-recent work identifies the related transcription factor Zfp467 as another potential regulator of preadipocyte commitment (197). Like Zfp423, exogenous expression of Zfp467 sensitizes cells to adipogenic stimuli and upregulates expression of PPARγ and adiponectin, but, unlike Zfp423, exogenous Zfp467 also enhances expression of C/EBPα (197), another transcription factor that plays a key role in adipogenesis (53, 54, 56, 57). Conversely, knockdown of Zfp467 decreases expression of these adipocyte genes and impairs adipogenesis, similar to the findings for Zfp423. Thus, Zfp467 may also affect preadipocyte commitment on a transcriptional level.
The studies from the Spiegelman and Graff labs further investigated gene expression signatures of committed preadipocytes using transcriptional profiling. Thus, Tang et al. found that PPARγ+ SVCs are transcriptionally distinct from both adipocytes and PPARγ– SVCs; specifically, they found that Gsc, Twist2, Mmp3, Egfr, and Fgf10 are enriched in the PPARγ+ SVCs (24) (Fig. 2). Speigelman's Zfp423 study transcriptionally profiled adipogenic and nonadipogenic fibroblasts, revealing numerous differentially expressed genes. Examples include PdgfrA (aka CD140a; Table 1), Hmx1, and Pou3f3, which are enriched in nonadipogenic cells; and Efemp1, Lgals3, Igfbp4, and Lpl, which are enriched in adipogenic cells (Fig. 2) (196). Clearly, future investigation of these genes, in both murine and human cells, may further improve our understanding of the mechanisms underlying preadipocyte commitment.
Mechanisms of preadipocyte commitment
With our increased knowledge of surface markers, perivascular localization, and gene expression, the identity of both ASCs and committed preadipocytes is coming into clearer focus. But further light is shed on ASC biology and preadipocyte commitment by drawing comparisons across these studies. For example, are Zfp423, Zfp467, or other putative marker genes of committed preadipocytes also enriched in adipogenic ASCs or in the Lin–/CD29+/CD34+/Sca-1+/CD105–/CD117–/CD24+ population identified by Rodeheffer, Birsoy, and Friedman? And do SVCs that express these preadipocyte markers localize perivascularly in WAT, as reported for PPARγ-expressing SVCs? It is worth noting that not all pericytes in WAT express PPARγ (24), which indicates that only a subset of pericytes are committed preadipocytes. This could explain disparate findings in other studies, which variously describe pericytes as CD34− (49, 121, 124) or CD34+ (22). Given that CD34 expression is variable in ASCs (Table 1) but that committed preadipocytes are CD34+, it is possible that increased expression of CD34 may facilitate the commitment of preadipocytes (CD34+) from perivascular ASCs (CD34–; Fig. 2), possibly by promoting migration of CD34– ASCs from the tunica media to the adventitia of blood vessels (23).
Comparing reported surface markers of preadipocytes with those of ASCs (Table 1) reveals many other possibilities. If committed preadipocytes are Lin–/CD29+/CD34+/Sca-1+/CD105–/CD117–/CD24+, what does this tell us about the formation of these cells from the heterogenous SVC population, presumably via perivascular ASCs (Fig. 2)? The Lin (lineage) antigens comprise CD2, CD3, CD4, CD5, CD8, NK1.1, B220, Ter119, and Gr-1 (Table 1). Whole SVF contains some Lin+ cells, whereas ASCs are reportedly Lin– (98). This suggests that lack of Lin expression distinguishes both ASCs and committed preadipocytes from the general SVC pool. A similar pattern exists for the endothelial cell marker CD31 (Table 1). Interestingly, endothelial cells have been shown to suppress adipogenesis of human SVCs by secreting Wnt ligands (198), which are well-established inhibitors of both white and brown adipocyte differentiation (55, 199–203). Therefore, we postulate that negative selection for CD31 may enhance ASC adipogenesis by removing endothelial cells and thereby reducing the anti-adipogenic effects of Wnt ligands. In contrast to Lin and CD31, both CD29 (aka β1-integrin) and Sca-1 are expressed in BM MSCs, whole SVCs, and ASCs (Table 1), demonstrating that neither is restricted to committed preadipocytes. However, the number of adipocyte progenitors in BM is significantly reduced in Sca-1-null mice (204). Thus, unlike many other markers, the Sca-1 antigen itself has been implicated as a regulator of preadipocyte commitment, at least in mice.
Among all the reported surface markers, only CD105 and CD140a appear to be differentially expressed, consistently, between ASCs and committed preadipocytes (Table 1). The enhanced adipogenic capacity of CD105– ASCs (161) suggests that downregulation of CD105 may be a functional requirement for preadipocyte commitment. A potential mechanism becomes apparent when the function of this protein is considered: CD105, also called endoglin, forms part of the TGF-β receptor complex. Numerous studies demonstrate that TGF-β inhibits adipogenesis, probably through activation of the transcriptional regulator Smad3 (205–207). Moreover, the cell surface availability of TGF-β receptors decreases during adipogenesis in vitro (207). Therefore, we postulate that downregulation of CD105 may enhance ASC adipogenesis and facilitate preadipocyte commitment by decreasing TGF-β signaling in these cells. Similarly, CD140a is better known as PDGFR-α; hence, given that PDGF suppresses adipogenesis and PPARγ activity (208–211), decreased PDGFR-α expression in committed preadipocytes (Table 1) may be obligatory for preadipocyte commitment (Fig. 2). However, other studies find that PDGFR-α expression is enriched in more-adipogenic subpopulations of some cell types, including MSCs derived from mouse embryonic stem cells (212) or skeletal muscle (213). This is similar to the aforementioned finding that PDGFR-β-expressing SVCs are adipogenic (24). Finally, a recent study shows that in mice, expression of PDGFA is enriched by 100-fold in preadipocytes relative to SVCs (214). These observations question the notion of PDGF as an inhibitor of adipogenesis and underscore the need for further investigation of PDGF ligands and receptors in regulating preadipocyte commitment.
Although TGF-β inhibits adipogenesis, other members of the TGF-β superfamily, namely BMP-2, BMP-4, and BMP-7, stimulate commitment toward the white or brown adipocyte lineages. This observation is based largely on studies in the C3H10T1/2 MSC line, which can differentiate into adipocytes, osteoblasts, chondrocytes, and myocytes (215, 216). For example, BMP-7 pretreatment induces expression of UCP-1 both in C3H10T1/2 MSCs and in brown preadipocytes, and BMP-7-treated C3H10T1/2 MSCs have an enhanced capacity to form BAT after implantation into athymic mice (217). Compellingly, both total BAT mass and UCP-1 expression are reduced in perinatal BMP-7 knockout mice, whereas adenoviral expression of BMP-7 in wild-type mice increases BAT mass and whole-body thermogenesis (217). Thus, BMP-7 can augment BAT function both by driving commitment of MSCs to the brown adipocyte lineage and by enhancing adipogenesis of committed brown preadipocytes. Although BMP-2 and -4 also stimulate lipid accumulation in brown preadipocytes, they do not induce UCP-1 expression in these cells (217). Indeed, these BMPs are more strongly implicated as positive regulators of white preadipocyte commitment (Fig. 2). For example, treatment of C3H10T/12 MSCs with BMP-4, prior to adipogenic induction, upregulates expression of PPARγ and dose-dependently enhances subsequent adipogenesis, both in vitro after exposure to adipogenic stimuli and in vivo after implantation into athymic mice (218). BMP-2 similarly enhances C3H10T1/2 adipogenic commitment, albeit less potently than BMP-4 (219–221). It is worth noting that effects of BMP-2 on cell fate are dose-dependent, with lower concentrations favoring adipogenesis but higher concentrations favoring osteoblast or chondrocyte differentiation (219). This could explain why exogenous BMP-2 can promote osteoblastogenesis or chondrogenesis by ASCs, as mentioned above. Mechanistically, BMP-2 and -4 promote preadipocyte commitment by signaling through type 1a and type 2 BMP receptors (BMPr1a and BMPr2) to phosphorylate and activate Smads 1, 5, and 8, which each function as heterodimers with Smad4 (221). Indeed, BMP-2 stimulates binding of Smad1/4 heterodimers to the promoter of the PPARγ2 gene (222), and RNAi-mediated knockdown of Smad4 prevents BMP-2-induced preadipocyte commitment (221).
Mechanisms through which Smads might exert these effects are also coming into focus. For example, Zfp423 contains a Smad binding domain and is a BMP-dependent transcriptional coactivator of Smad proteins (223). As such, expression of Zfp423 sensitizes NIH3T3 cells to the proadipogenic effects of BMP-2 or BMP-4, but only when the Smad binding domain is intact (196). Another zinc finger protein, Schnurri-2, also binds directly to Smad1/4 heterodimers in response to BMP-2 stimulation. This Schnurri-2/Smad1/Smad4 complex thereby acts synergistically with C/EBPα to activate PPARγ expression downstream of BMP-2 (222). In addition to PPARγ, another transcriptional target of Smad1/4 that is important for preadipocyte commitment is lysyl oxidase (Lox). Both BMP-2 and BMP-4 increase Lox expression in a Smad4-dependent manner, and knockdown of Lox impairs BMP-2-induced commitment of C3H10T1/2 MSCs (221). Lox knockdown also blocks adipogenesis of murine embryonic fibroblasts, even in the absence of ectopic BMPs (221). These observations identify Lox as another positive regulator of preadipocyte commitment (Fig. 2) and indicate that endogenous BMP activity is also important for this process. Indeed, expression of BMP-4, BMPr2, BMPr1a, and Smads 1, 4, 5, and 8 is elevated in a subline of C3H10T1/2 MSCs that has increased adipogenic potential (220), and exogenous expression of BMPr1a promotes commitment of MSCs even in the absence of ectopic BMP-2 or -4 (221). These observations underscore the importance of BMP2/4, Smad1/4/5/8, and Lox as positive regulators of white preadipocyte commitment (Fig. 2), at least in mice. This suggests that expression of these factors may be enriched in adipogenic ASC subpopulations or in the Lin–/CD29+/CD34+/Sca-1+/CD105–/CD117–/CD24+ population of committed preadipocytes. Future studies should explore these possibilities and further investigate whether these mechanisms also impact preadipocyte commitment in humans.
Fibroblast growth factor (FGF) may be another positive regulator of preadipocyte commitment. Microarray analysis reveals that FGF2, FGF6, and FGF7 are among the most highly expressed genes in human ASCs (108) (Fig. 2). Interestingly, culture of rat BM MSCs or human ASCs in the presence of FGF2 prior to adipogenic induction upregulates expression of PPARγ and enhances subsequent adipogenesis (224, 225). Indeed, exogenous FGF2 facilitates in vivo WAT formation by isolated human SVCs (226). These observations are reminiscent of effects of BMP-2 and BMP-4 and support the possibility that FGF may promote commitment from ASCs to preadipocytes (Fig. 2).
A final marker that must be considered is CD24. To our knowledge, no studies have reported CD24 expression status in ASCs (Table 1). Given that only a small population of SVCs express CD24 (189), it is likely that ASCs studied thus far are largely CD24–. This could explain the relatively poor adipogenic capacity of ASCs, which require prior adipogenic induction to form adipocytes in vivo (129–131). The unique ability of CD24 expression status to distinguish between SVC adipogenic capacity in vivo clearly warrants further investigation. For example, does CD24 status also distinguish between other preadipocyte markers, such as PPARγ or Zfp423? And are all of the adipogenic Lin–/CD29+/CD34+/Sca-1+/ CD105–/CD117– SVCs perivascular, or are there differences in localization of the CD24+ and CD24– subpopulations? Knowledge of the function of CD24 may provide further clues. CD24 is a GPI-linked sialoglycoprotein that was initially characterized as related to B lymphocytes and hematopoietic lineages (227, 228). In relation to adult stem cells, positive selection for CD24 facilitated regeneration of a mouse mammary gland in vivo from a single cell (229), and CD24 has recently gained prominence as a marker of cancer stem cells. Here, CD24 expression has been linked to metastatic progression, thereby making CD24 a putative therapeutic target (230). The relationship between CD24 and metastasis probably relates to the ability of CD24 to facilitate interactions with the vasculature. This ability of CD24 to alter adhesive properties may also explain why putative stem cells expressing CD24 have the ability to engraft and reconstitute diverse organ systems, whereas those lacking CD24 do not (231). However, current knowledge provides no clues to the mechanisms through which CD24 might impact adipogenesis. Clearly, further exploration of the relationship between CD24 expression, stem cell engraftment, and preadipocyte commitment is warranted.
Compared with SVCs and ASCs, very little is known about surface marker expression on committed preadipocytes (Table 1 and supplementary Table I). But even less is known about these cell types on a global level. Future studies should attempt global characterization of these cells, from both murine models and humans, for example through RNA sequencing, proteomics, and large-scale epigenetic analyses. This information would allow far more extensive comparisons to be drawn between SVCs, ASCs, and committed preadipocytes, which almost certainly will provide many additional insights into the mechanisms that underlie preadipocyte commitment.
Beyond adipose tissue: potential origins of ASCs
The notion of ASCs and committed preadipocytes as perivascular cells raises the possibility that precursors for these cells might derive from outside adipose tissue. One study found that fibrocytes, a subset of peripheral blood mononuclear cells, can undergo adipogenesis in vitro and can form adipocytes in vivo after reimplantation into SCID mice (232). Several other studies have transplanted BM from GFP-expressing donors into wild-type recipients to investigate whether BM-derived cells might home through the vasculature to form adipocytes in WAT. For example, following such a transplant, Klemm and colleagues detected GFP+ multilocular adipocytes in WAT, the presence of which is enhanced by high-fat diet feeding or treatment with rosiglitazone, a PPARγ agonist (233). Based on these observations, the authors concluded that adipocytes can indeed derive from BM in adult mice. Because there is no evidence that BM MSCs enter the circulation, these authors postulated that the GFP+ multilocular adipocytes may derive from donor HSCs. In a subsequent study, they addressed this possibility by transplanting BM that had been selected for MSCs or HSCs based on CD45 expression (CD45– or CD45+, respectively). They found that donor-derived adipocytes are only detectable after transplantation of CD45+ BM (234). This suggests that CD45+ HSCs can give rise to adipocytes, a possibility that is supported by one independent study (235). In additional lineage tracing experiments using mice in which LacZ expression is restricted to the myeloid lineage, LacZ+ adipocytes were detectable in WAT (234). This observation further suggests that adipocytes can derive from myeloid progenitor cells even beyond the context of BM transplantation. However, these findings remain controversial. For example, Koh et al. also detected GFP+ cells in WAT following BM transplantation; however, they found that these GFP+ cells do not express the adipocyte markers perilipin or adipophilin, whether under conditions of normal diet, high-fat diet, rosiglitazone treatment, during WAT regrowth following lipectomy, or even in a parabiosis model between GFP+ and GFP– mice (236). Instead, the GFP+ cells in WAT express macrophage markers, such as CD11b and CD45 (236). An independent study also showed that the majority of BM-derived GFP+ cells in WAT are CD45+, and that ASCs isolated from these WAT depots and differentiated into adipocytes in vitro do not express GFP (237). These findings suggest that the donor BM-derived, multilocular cells that engraft in WAT are not bona fide adipocytes. Indeed, data overwhelmingly establish ASCs and committed preadipocytes as CD45–/CD11b– (Table 1 and supplementary Table I; Fig. 2), which is inconsistent with HSCs as adipocyte progenitors. However, the lineage tracing study by Majka et al. identified a population of CD45–/CD11b–SVCs in WAT that are also myeloid-derived (234). This supports the possibility that BM-derived myeloid progenitor cells might engraft in WAT and subsequently decrease their expression of CD45 and CD11b as they become ASCs or committed preadipocytes (Fig. 2). Nevertheless, in light of the observation that committed preadipocytes are a self-renewing population in WAT (24), the relative contribution of such nonresident cell types to WAT expansion remains to be firmly established.
Other studies have also challenged the notion that adipocytes derive exclusively from mesoderm. For example, neuroepithelial cells derived from mouse embryonic stem cells or mouse embryos are capable of adipogenesis in vitro (212, 238). Using lineage tracing based on expression of Sox10, a neural crest marker, Billon et al. further identified a subset of neural crest-derived adipocytes around the salivary gland and ears; however, they found that subcutaneous or peri-ovarial adipocytes do not derive from the neural crest (238). A separate lineage-tracing study based on expression of Wnt1, another neural crest marker, also found no evidence that ASCs from BAT or WAT are of neural crest origin (165). Thus, although a small subset of adipocytes in craniofacial WAT depots may derive from neural crest (Fig. 2), this is not the case for ASCs and adipocytes in most other adipose depots.
ASCs and preadipocytes in health and disease
As discussed above, decades of research have shown that factors such as WAT depot, aging, or obesity can dramatically impact adipogenesis in whole SVC populations. Whether these factors affect ASC multipotency or specific preadipocyte subpopulations is only beginning to be established. Although aging clearly impairs the adipogenic capacity of SVCs (above), it does not affect ASC function (172, 239). In contrast, obesity does appear to impact ASC biology. For example, the proportion of adipogenic CD34+/CD31– SVCs in subcutaneous WAT (scWAT) positively correlates with body mass index in nonobese individuals (97, 161), suggesting that nutritional signals might stimulate preadipocyte commitment. In contrast, the number of committed preadipocytes in scWAT declines in obesity (240). Thus, obesity may exhaust the capacity for preadipocyte self-renewal, consistent with the hypothesis that impaired adipose tissue expandability contributes to obesity-associated metabolic dysregulation (241). This possibility is especially interesting in light of recent findings about thiazolidinediones (TZDs), synthetic PPARγ ligands that have been used as anti-diabetic drugs; chronic TZD treatment reportedly decreases the adipogenic capacity of ASCs, thereby exhausting the pool of committed preadipocytes in WAT (242). Other studies have investigated the effects of obesity on more-distinct SVC subpopulations. For example, brown adipogenesis by ScaPCs is enhanced in obesity-resistant mice (135). Thus, differences in propensity for brown adipogenesis might impact obesity susceptibility.
Depot-specific differences in ASCs are also emerging. For example, ASCs isolated from visceral WAT are more osteogenic than those from scWAT (243), but have a lesser potential for brown adipogenesis than scWAT ASCs (135). These differences could be explained by an increased proportion of committed preadipocytes in scWAT. Indeed, one report finds that SVCs from scWAT contain more CD34+ cells than SVCs from visceral WAT (97); however, this has not been observed in all studies (106, 244). Even the precise location of scWAT can impact resident ASC biology, with abdominal scWAT containing a greater percentage of ASCs than that from the hip/thigh; however, neither ASC proliferation, osteogenesis, or chondrogenesis differs between these scWAT depots (245). Similarly, WAT depot reportedly does not affect ASC myogenesis (104, 244) or secretion of angiogenic or proinflammatory proteins (244). Transcriptional profiling has revealed further depot-specific differences in preadipocyte gene expression (246, 247). These differences are maintained in clonal preadipocyte cultures over 40 population doublings (247), which suggests that epigenetic modifications may contribute to depot-specific variation in preadipocyte phenotypes. A comparison of these transcriptional profiles with putative markers of committed preadipocytes is clearly warranted.
BROADER QUESTIONS
Although the past decade has witnessed enormous advances in our knowledge of both ASCs and committed preadipocytes, numerous questions remain. A major unresolved issue is whether ASCs are genuine stem cells, which remains questionable owing to lack of conclusive evidence for their ability to undergo self-renewal or to uniformly reconstitute all mesodermal lineages in vivo. Similar questions have been raised for BM MSCs, despite their ability to home from the BM to various extramedullary mesenchymal tissues (69, 73). ASCs isolated ex vivo can also engraft into multiple extramedullary tissues after host injection (248, 249); however, whether this is also true for ASCs within WAT remains unknown. It would be far more informative to transplant whole fat pads from luciferase-expressing mice to WT recipients, followed by analysis of luciferase activity in nonadipose tissues. This would reveal whether ASCs are capable of moving from their resident tissue to other tissue types, as evident for BM MSCs or HSCs.
But even this approach has drawbacks. Numerous studies have shown that tissue engraftment or differentiation of implanted ASCs/SVCs into other cell types in vivo requires specific conditions, such as prior tissue injury (87, 91, 97, 157, 174, 250, 251) or use of immunodeficient mice as recipients (e.g., SCID mice) (87, 120, 173). Indeed, even SVC subpopulations containing committed preadipocytes can only reconstitute WAT under conditions that favor new WAT formation, such as genetic lipodystrophy or high-fat diet (189). Therefore, migration of ASCs from WAT and subsequent engraftment in other tissues might require some type of injury or other intervention posttransplant. These observations highlight the importance of the host tissue when assessing in vivo ASC multipotency: how do local tissue environments impact multipotent cell fate to promote the differentiation of cell types specific to those tissues? And how do these mechanisms vary under different physiological or pathophysiological conditions? The importance of host tissue environment also highlights a drawback to current analyses of ASC differentiation in vivo, in that the ability or inability of ASCs to form certain cell/tissue types under particular experimental conditions may not accurately reflect their actual multilineage capacity in different physiological or pathophysiological contexts. More-refined lineage tracing, for example based on Zfp423 or multiple other markers, would further facilitate the identification of ASC-derived cells in vivo.
In addition to these questions about terminal fates of ASCs, there is also uncertainty about their relationship to preadipocytes. Although preadipocytes are clearly a distinct subpopulation of SVCs, differences between ASCs and preadipocytes are less marked (Table 1; Fig. 2). This raises an important question: what are the functional distinctions between ASCs and committed preadipocytes in vivo? In Fig. 2, we present ASCs as a potentially self-renewing intermediate between pericytic SVCs and committed preadipocytes, implying that ASCs might contribute to preadipocyte formation and, thereby, WAT expansion. But are ASCs really required to replenish the population of committed preadipocytes? As mentioned above, the observations of Graff and colleagues suggest that most preadipocyte commitment occurs prenatally or just after birth, and that committed preadipocytes self-renew to replenish those lost during adipogenesis (24) (Fig. 2). These observations suggest that preadipocytes do not derive from ASCs postnatally, which brings into question the role of ASCs in the context of WAT biology. More importantly, this line of reasoning relies on the assumption that ASCs are a genuine, functionally distinct cell type; without definitive proof that, in vivo, ASCs are genuine stem cells, it remains possible that ASCs and committed preadipocytes are essentially the same cell, with multipotency and other distinctions only apparent in experimental contexts. This brings into question not only the purpose of ASCs, but also their very existence.
PERSPECTIVES AND CONCLUSIONS
The past century has witnessed huge strides in our understanding of stem cell biology, WAT expansion, and preadipocyte differentiation, which laid the foundation for the identification of adipose tissue-derived stem cells at the turn of the millenium. Research over the past decade has since established the utility of ASCs for tissue engineering and regenerative medicine, which may shift our perceptions: rather than being a cause of ill health, adipose tissue might also harbor potential cures. Despite this promise, much remains to be known about ASCs from a more physiological perspective. Are ASCs truly self-renewing, multipotent stem cells? Are they even a discrete cell type at all? While these important questions have yet to be answered, ASC research has contributed to more-recent progress in the characterization of committed preadipocytes. Viewed in this context, our knowledge of the ASC provides valuable insights into the mechanisms underlying preadipocyte commitment. Exploration of ASCs has thereby fed back into research at the root of their discovery, echoing the words of T.S. Elliot:
We shall not cease from exploration, and the end of all our exploring will be to arrive where we started and know the place for the first time.
But because our exploration has not yet ended, knowledge of both ASC biology and preadipocyte commitment will surely continue expanding in the future.
Supplementary Material
Footnotes
Abbreviations:
- ASC
- adipose tissue-derived stem cell
- BAT
- brown adipose tissue
- BM
- bone marrow
- BMP
- bone morphogenetic protein
- FACS
- fluorescence-activated cell sorting
- FGF
- fibroblast growth factor
- GFP
- green fluorescent protein
- HSC
- hematopoietic stem cell
- MSC
- mesenchymal stem cell
- PDGFR
- platelet-derived growth factor receptor
- PLA
- processed lipoaspirate cells
- α-SMA
- alpha-smooth muscle actin
- SVC
- stromal vascular cell
- scWAT
- subcutaneous WAT
- SVF
- stromal vascular fraction of WAT
- TGF-β
- transforming growth factor-beta
- TZD
- thiazolidinedione
- WAT
- white adipose tissue
This work was supported by Grants DK-51563, DK-62876, and DK-92759 from the National Institutes of Health (OAM), and by a Postdoctoral Research Fellowship from the Royal Commission for the Exhibition of 1851 (United Kingdom) (WPC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
The cells described herein as ASCs have had many other aliases. In 2001, Zuk et al. published the first report of multipotent cells in adipose tissue, naming these processed lipoaspirate cells (PLAs) based on their method of isolation (78); hence, numerous later studies also refer to these cells as PLAs (81–85). At around the same time, Gimble and colleagues identified adipose tissue-derived stromal cells that were capable of both adipogenic and osteogenic induction (77, 79, 86), a name now applied to more broadly multipotent cells from adipose tissue (87–89) and sometimes abbreviated as ATSCs (90–92) or ASCs (22). Other subtle variations of this name include adipose-derived adult stromal (ADAS) cells (47, 93, 94), adipose-derived adult stem (ADAS) cells (95, 96), ASCs (97), adipose-derived stromal\stem cells (21), adipose tissue-derived cells (ATDCs) (98, 99), or simply MSCs (87, 92). Many of these variations are still used today, with combinations often used within a single study (87, 89, 95, 97). Although these different names may sometimes be used meaningfully to distinguish specific cell characteristics, such as method of isolation, such variability has undoubtedly cast confusion over this field of research. Thus, for consistency in the present manuscript, we refer to these adipose-derived stem/stromal cells as ASCs, as advocated by a previous review (80).
REFERENCES
- 1.Fleck S. J. 1983. Body composition of elite American athletes. Am. J. Sports Med. 11: 398–403. [DOI] [PubMed] [Google Scholar]
- 2.Ortega F. J., Mayas D., Moreno-Navarrete J. M., Catalan V., Gomez-Ambrosi J., Esteve E., Rodriguez-Hermosa J. I., Ruiz B., Ricart W., Peral B., et al. 2010. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring). 18: 13–20. [DOI] [PubMed] [Google Scholar]
- 3.Larson K. A., Anderson D. B. 1978. The effects of lipectomy on remaining adipose tissue depots in the Sprague Dawley rat. Growth. 42: 469–477. [PubMed] [Google Scholar]
- 4.Reyne Y., Nougues J., Vezinhet A. 1983. Adipose tissue regeneration in 6-month-old and adult rabbits following lipectomy. Proc. Soc. Exp. Biol. Med. 174: 258–264. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez T. L., Kittelson J. M., Law C. K., Ketch L. L., Stob N. R., Lindstrom R. C., Scherzinger A., Stamm E. R., Eckel R. H. 2011. Fat redistribution following suction lipectomy: defense of body fat and patterns of restoration. Obesity (Silver Spring). 19: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 6.Faust I. M., Johnson P. R., Hirsch J. 1977. Adipose tissue regeneration following lipectomy. Science. 197: 391–393. [DOI] [PubMed] [Google Scholar]
- 7.Hellman B., Hellerstrom C. 1961. Cell renewal in the white and brown fat tissue of the rat. Acta Pathol. Microbiol. Scand. 51: 347–353. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch J., Han P. W. 1969. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J. Lipid Res. 10: 77–82. [PubMed] [Google Scholar]
- 9.Lemonnier D. 1972. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J. Clin. Invest. 51: 2907–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Häger A., Sjostrm L., Arvidsson B., Bjorntorp P., Smith U. 1977. Body fat and adipose tissue cellularity in infants: a longitudinal study. Metabolism. 26: 607–614. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand H. A., Masoro E. J., Yu B. P. 1978. Increasing adipocyte number as the basis for perirenal depot growth in adult rats. Science. 201: 1234–1235. [DOI] [PubMed] [Google Scholar]
- 12.Faust I. M., Johnson P. R., Stern J. S., Hirsch J. 1978. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am. J. Physiol. 235: E279–E286. [DOI] [PubMed] [Google Scholar]
- 13.Knittle J. L., Timmers K., Ginsberg-Fellner F., Brown R. E., Katz D. P. 1979. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J. Clin. Invest. 63: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spalding K. L., Arner E., Westermark P. O., Bernard S., Buchholz B. A., Bergmann O., Blomqvist L., Hoffstedt J., Naslund E., Britton T., et al. 2008. Dynamics of fat cell turnover in humans. Nature. 453: 783–787. [DOI] [PubMed] [Google Scholar]
- 15.Rigamonti A., Brennand K., Lau F., Cowan C. A. 2011. Rapid cellular turnover in adipose tissue. PLoS ONE. 6: e17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslam D. 2007. Obesity: a medical history. Obes. Rev. 8 (Suppl.): 31–36. [DOI] [PubMed] [Google Scholar]
- 17.Ramalho-Santos M., Willenbring H. 2007. On the origin of the term “stem cell.” Cell Stem Cell. 1: 35–38. [DOI] [PubMed] [Google Scholar]
- 18.Clark E. R., Clark E. L. 1940. Microscopic studies of the new formation of fat in living adult rabbits. Am. J. Anat. 67: 255–285. [Google Scholar]
- 19.Coats J. 1883. A Manual of Pathology. H. C. Lea's Sons and Co., Philadelphia. 123.
- 20.Iyama K., Ohzono K., Usuku G. 1979. Electron microscopical studies on the genesis of white adipocytes: differentiation of immature pericytes into adipocytes in transplanted preadipose tissue. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 31: 143–155. [DOI] [PubMed] [Google Scholar]
- 21.Zannettino A. C., Paton S., Arthur A., Khor F., Itescu S., Gimble J. M., Gronthos S. 2008. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J. Cell. Physiol. 214: 413–421. [DOI] [PubMed] [Google Scholar]
- 22.Traktuev D. O., Merfeld-Clauss S., Li J., Kolonin M., Arap W., Pasqualini R., Johnstone B. H., March K. L. 2008. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 102: 77–85. [DOI] [PubMed] [Google Scholar]
- 23.Lin G., Garcia M., Ning H., Banie L., Guo Y. L., Lue T. F., Lin C. S. 2008. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 17: 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. 2008. White fat progenitor cells reside in the adipose vasculature. Science. 322: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai X., Lin Y., Hauschka P., Grottkau B. E. 2011. Adipose stem cells originate from perivascular cells. Biol. Cell. 103: 435–447. [DOI] [PubMed] [Google Scholar]
- 26.Peckham S. C., Entenman C., Carroll H. W. 1962. The influence of a hypercaloric diet on gross body and adipose tissue composition in the rat. J. Nutr. 77: 187–197. [DOI] [PubMed] [Google Scholar]
- 27.Rodbell M. 1964. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 239: 375–380. [PubMed] [Google Scholar]
- 28.Hollenberg C. H., Vost A. 1969. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J. Clin. Invest. 47: 2485–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng C. W., Poznanski W. J., Borowiecki M., Reimer G. 1971. Differences in growth in vitro of adipose cells from normal and obese patients. Nature. 231: 445. [DOI] [PubMed] [Google Scholar]
- 30.Poznanski W. J., Waheed I., Van R. 1973. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Lab. Invest. 29: 570–576. [PubMed] [Google Scholar]
- 31.Van R. L., Bayliss C. E., Roncari D. A. 1976. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J. Clin. Invest. 58: 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauner H., Wabitsch M., Pfeiffer E. F. 1988. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm. Metab. Res. Suppl. 19: 35–39. [PubMed] [Google Scholar]
- 33.Maslowska M. H., Sniderman A. D., MacLean L. D., Cianflone K. 1993. Regional differences in triacylglycerol synthesis in adipose tissue and in cultured preadipocytes. J. Lipid Res. 34: 219–228. [PubMed] [Google Scholar]
- 34.Niesler C. U., Siddle K., Prins J. B. 1998. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 47: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 35.Djian P., Roncari A. K., Hollenberg C. H. 1983. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J. Clin. Invest. 72: 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djian P., Roncari D. A., Hollenberg C. H. 1985. Adipocyte precursor clones vary in capacity for differentiation. Metabolism. 34: 880–883. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Kirkland J. L., Hollenberg C. H. 1989. Varying capacities for replication of rat adipocyte precursor clones and adipose tissue growth. J. Clin. Invest. 83: 1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkland J. L., Hollenberg C. H., Gillon W. S. 1990. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am. J. Physiol. 258: C206–C210. [DOI] [PubMed] [Google Scholar]
- 39.Sztalryd C., Faust I. M. 1990. Depot-specific features of adipocyte progenitors revealed by primary cultures plated at low density. Int. J. Obes. 14(Suppl.3): 165–175. [PubMed] [Google Scholar]
- 40.Grégoire F., Todoroff G., Hauser N., Remacle C. 1990. The stroma-vascular fraction of rat inguinal and epididymal adipose tissue and the adipoconversion of fat cell precursors in primary culture. Biol. Cell. 69: 215–222. [DOI] [PubMed] [Google Scholar]
- 41.Hauner H., Entenmann G., Wabitsch M., Gaillard D., Ailhaud G., Negrel R., Pfeiffer E. F. 1989. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Invest. 84: 1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkland J. L., Hollenberg C. H., Gillon W. S. 1993. Ageing, differentiation, and gene expression in rat epididymal preadipocytes. Biochem. Cell Biol. 71: 556–561. [DOI] [PubMed] [Google Scholar]
- 43.Carraro R., Li Z. H., Johnson J. E., Jr, Gregerman R. I. 1992. Adipocytes of old rats produce a decreased amount of differentiation factor for preadipocytes derived from adipose tissue islets. J. Gerontol. 47: B198–B201. [DOI] [PubMed] [Google Scholar]
- 44.Kirkland J. L., Hollenberg C. H., Kindler S., Gillon W. S. 1994. Effects of age and anatomic site on preadipocyte number in rat fat depots. J. Gerontol. 49: B31–B35. [DOI] [PubMed] [Google Scholar]
- 45.Roncari D. A., Lau D. C., Kindler S. 1981. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism. 30: 425–427. [DOI] [PubMed] [Google Scholar]
- 46.Grégoire F. M., Johnson P. R., Greenwood M. R. 1995. Comparison of the adipoconversion of preadipocytes derived from lean and obese Zucker rats in serum-free cultures. Int. J. Obes. Relat. Metab. Disord. 19: 664–670. [PubMed] [Google Scholar]
- 47.Brzoska M., Geiger H., Gauer S., Baer P. 2005. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem. Biophys. Res. Commun. 330: 142–150. [DOI] [PubMed] [Google Scholar]
- 48.Avram A. S., Avram M. M., James W. D. 2005. Subcutaneous fat in normal and diseased states. 2. Anatomy and physiology of white and brown adipose tissue. J. Am. Acad. Dermatol. 53: 671–683. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura K., Shigeura T., Matsumoto D., Sato T., Takaki Y., Aiba-Kojima E., Sato K., Inoue K., Nagase T., Koshima I., et al. 2006. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 208: 64–76. [DOI] [PubMed] [Google Scholar]
- 50.Green H., Kehinde O. 1975. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 5: 19–27. [DOI] [PubMed] [Google Scholar]
- 51.Green H., Kehinde O. 1976. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 7: 105–113. [DOI] [PubMed] [Google Scholar]
- 52.Green H., Meuth M. 1974. An established pre-adipose cell line and its differentiation in culture. Cell. 3: 127–133. [DOI] [PubMed] [Google Scholar]
- 53.Farmer S. R. 2006. Transcriptional control of adipocyte formation. Cell Metab. 4: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen E. D., MacDougald O. A. 2006. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7: 885–896. [DOI] [PubMed] [Google Scholar]
- 55.Christodoulides C., Lagathu C., Sethi J. K., Vidal-Puig A. 2009. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 20: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefterova M. I., Lazar M. A. 2009. New developments in adipogenesis. Trends Endocrinol. Metab. 20: 107–114. [DOI] [PubMed] [Google Scholar]
- 57.Lowe C. E., O'Rahilly S., Rochford J. J. 2011. Adipogenesis at a glance. J. Cell Sci. 124: 2681–2686. [DOI] [PubMed] [Google Scholar]
- 58.Eguchi J., Yan Q. W., Schones D. E., Kamal M., Hsu C. H., Zhang M. Q., Crawford G. E., Rosen E. D. 2008. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metab. 7: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nielsen R., Pedersen T. A., Hagenbeek D., Moulos P., Siersbaek R., Megens E., Denissov S., Borgesen M., Francoijs K. J., Mandrup S., et al. 2008. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 22: 2953–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steger D. J., Grant G. R., Schupp M., Tomaru T., Lefterova M. I., Schug J., Manduchi E., Stoeckert C. J., Jr, Lazar M. A. 2010. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 24: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siersbæk R., Nielsen R., John S., Sung M. H., Baek S., Loft A., Hager G. L., Mandrup S. 2011. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 30: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker A. J., McCulloch C. E., Till J. E. 1963. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 197: 452–454. [DOI] [PubMed] [Google Scholar]
- 63.Till J. E., McCulloch E. A. 1961. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14: 213–222. [PubMed] [Google Scholar]
- 64.Till J. E., McCulloch E. A., Siminovitch L. 1964. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc. Natl. Acad. Sci. USA. 51: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weissman I. L. 2000. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 100: 157–168. [DOI] [PubMed] [Google Scholar]
- 66.Spangrude G. J., Heimfeld S., Weissman I. L. 1988. Purification and characterization of mouse hematopoietic stem cells. Science. 241: 58–62. [DOI] [PubMed] [Google Scholar]
- 67.Morrison S. J., Shah N. M., Anderson D. J. 1997. Regulatory mechanisms in stem cell biology. Cell. 88: 287–298. [DOI] [PubMed] [Google Scholar]
- 68.Amsel S., Dell E. S. 1972. Bone formation by hemopoietic tissue: separation of preosteoblast from hemopoietic stem cell function in the rat. Blood. 39: 267–273. [PubMed] [Google Scholar]
- 69.Prockop D. J. 1997. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 276: 71–74. [DOI] [PubMed] [Google Scholar]
- 70.Friedenstein A. J., Chailakhyan R. K., Gerasimov U. V. 1987. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 20: 263–272. [DOI] [PubMed] [Google Scholar]
- 71.Kuznetsov S. A., Krebsbach P. H., Satomura K., Kerr J., Riminucci M., Benayahu D., Robey P. G. 1997. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J. Bone Miner. Res. 12: 1335–1347. [DOI] [PubMed] [Google Scholar]
- 72.Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 73.Pereira R. F., Halford K. W., O'Hara M. D., Leeper D. B., Sokolov B. P., Pollard M. D., Bagasra O., Prockop D. J. 1995. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA. 92: 4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krebsbach P. H., Kuznetsov S. A., Satomura K., Emmons R. V., Rowe D. W., Robey P. G. 1997. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 63: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 75.Morrison S. J., White P. M., Zock C., Anderson D. J. 1999. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 96: 737–749. [DOI] [PubMed] [Google Scholar]
- 76.da Silva Meirelles L., Chagastelles P. C., Nardi N. B. 2006. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119: 2204–2213. [DOI] [PubMed] [Google Scholar]
- 77.Halvorsen Y. C., Wilkison W. O., Gimble J. M. 2000. Adipose-derived stromal cells–their utility and potential in bone formation. Int. J. Obes. Relat. Metab. Disord. 24 (Suppl.): 41–44. [DOI] [PubMed] [Google Scholar]
- 78.Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., Benhaim P., Lorenz H. P., Hedrick M. H. 2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 79.Halvorsen Y. D., Franklin D., Bond A. L., Hitt D. C., Auchter C., Boskey A. L., Paschalis E. P., Wilkison W. O., Gimble J. M. 2001. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 7: 729–741. [DOI] [PubMed] [Google Scholar]
- 80.Gimble J. M., Katz A. J., Bunnell B. A. 2007. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuk P. A., Zhu M., Ashjian P., De Ugarte D. A., Huang J. I., Mizuno H., Alfonso Z. C., Fraser J. K., Benhaim P., Hedrick M. H. 2002. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Ugarte D. A., Morizono K., Elbarbary A., Alfonso Z., Zuk P. A., Zhu M., Dragoo J. L., Ashjian P., Thomas B., Benhaim P., et al. 2003. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 174: 101–109. [DOI] [PubMed] [Google Scholar]
- 83.Dragoo J. L., Choi J. Y., Lieberman J. R., Huang J., Zuk P. A., Zhang J., Hedrick M. H., Benhaim P. 2003. Bone induction by BMP-2 transduced stem cells derived from human fat. J. Orthop. Res. 21: 622–629. [DOI] [PubMed] [Google Scholar]
- 84.Ashjian P. H., Elbarbary A. S., Edmonds B., DeUgarte D., Zhu M., Zuk P. A., Lorenz H. P., Benhaim P., Hedrick M. H. 2003. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast. Reconstr. Surg. 111: 1922–1931. [DOI] [PubMed] [Google Scholar]
- 85.Lin Y., Luo E., Chen X., Liu L., Qiao J., Yan Z., Li Z., Tang W., Zheng X., Tian W. 2005. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J. Cell. Mol. Med. 9: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gronthos S., Franklin D. M., Leddy H. A., Robey P. G., Storms R. W., Gimble J. M. 2001. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 189: 54–63. [DOI] [PubMed] [Google Scholar]
- 87.Seo M. J., Suh S. Y., Bae Y. C., Jung J. S. 2005. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 328: 258–264. [DOI] [PubMed] [Google Scholar]
- 88.Ning H., Lin G., Lue T. F., Lin C. S. 2006. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 74: 510–518. [DOI] [PubMed] [Google Scholar]
- 89.Hwa Cho H., Bae Y. C., Jung J. S. 2006. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 24: 2744–2752. [DOI] [PubMed] [Google Scholar]
- 90.Winter A., Breit S., Parsch D., Benz K., Steck E., Hauner H., Weber R. M., Ewerbeck V., Richter W. 2003. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 48: 418–429. [DOI] [PubMed] [Google Scholar]
- 91.Kang S. K., Lee D. H., Bae Y. C., Kim H. K., Baik S. Y., Jung J. S. 2003. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp. Neurol. 183: 355–366. [DOI] [PubMed] [Google Scholar]
- 92.Lee R. H., Kim B., Choi I., Kim H., Choi H. S., Suh K., Bae Y. C., Jung J. S. 2004. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 14: 311–324. [DOI] [PubMed] [Google Scholar]
- 93.Cowan C. M., Shi Y. Y., Aalami O. O., Chou Y. F., Mari C., Thomas R., Quarto N., Contag C. H., Wu B., Longaker M. T. 2004. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 22: 560–567. [DOI] [PubMed] [Google Scholar]
- 94.Safford K. M., Safford S. D., Gimble J. M., Shetty A. K., Rice H. E. 2004. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp. Neurol. 187: 319–328. [DOI] [PubMed] [Google Scholar]
- 95.Safford K. M., Hicok K. C., Safford S. D., Halvorsen Y. D., Wilkison W. O., Gimble J. M., Rice H. E. 2002. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 294: 371–379. [DOI] [PubMed] [Google Scholar]
- 96.Aust L., Devlin B., Foster S. J., Halvorsen Y. D., Hicok K., du Laney T., Sen A., Willingmyre G. D., Gimble J. M. 2004. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 6: 7–14. [DOI] [PubMed] [Google Scholar]
- 97.Miranville A., Heeschen C., Sengenes C., Curat C. A., Busse R., Bouloumie A. 2004. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 110: 349–355. [DOI] [PubMed] [Google Scholar]
- 98.Nakagami H., Maeda K., Morishita R., Iguchi S., Nishikawa T., Takami Y., Kikuchi Y., Saito Y., Tamai K., Ogihara T., et al. 2005. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 25: 2542–2547. [DOI] [PubMed] [Google Scholar]
- 99.Toyoda M., Matsubara Y., Lin K., Sugimachi K., Furue M. 2009. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem. Funct. 27: 440–447. [DOI] [PubMed] [Google Scholar]
- 100.Kang Y., Park C., Kim D., Seong C. M., Kwon K., Choi C. 2010. Unsorted human adipose tissue-derived stem cells promote angiogenesis and myogenesis in murine ischemic hindlimb model. Microvasc. Res. 80: 310–316. [DOI] [PubMed] [Google Scholar]
- 101.Kajiyama H., Hamazaki T. S., Tokuhara M., Masui S., Okabayashi K., Ohnuma K., Yabe S., Yasuda K., Ishiura S., Okochi H., et al. 2010. Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int. J. Dev. Biol. 54: 699–705. [DOI] [PubMed] [Google Scholar]
- 102.Levi B., James A. W., Nelson E. R., Vistnes D., Wu B., Lee M., Gupta A., Longaker M. T. 2010. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 5: e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitchell J. B., McIntosh K., Zvonic S., Garrett S., Floyd Z. E., Kloster A., Di Halvorsen Y., Storms R. W., Goh B., Kilroy G., et al. 2006. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 24: 376–385. [DOI] [PubMed] [Google Scholar]
- 104.Di Rocco G., Iachininoto M. G., Tritarelli A., Straino S., Zacheo A., Germani A., Crea F., Capogrossi M. C. 2006. Myogenic potential of adipose-tissue-derived cells. J. Cell Sci. 119: 2945–2952. [DOI] [PubMed] [Google Scholar]
- 105.Basu J., Genheimer C. W., Guthrie K. I., Sangha N., Quinlan S. F., Bruce A. T., Reavis B., Halberstadt C., Ilagan R. M., Ludlow J. W. 2011. Expansion of the human adipose-derived stromal vascular cell fraction yields a population of smooth muscle-like cells with markedly distinct phenotypic and functional properties relative to mesenchymal stem cells. Tissue Eng. Part C Methods. 17: 843–860. [DOI] [PubMed] [Google Scholar]
- 106.Festy F., Hoareau L., Bes-Houtmann S., Pequin A. M., Gonthier M. P., Munstun A., Hoarau J. J., Cesari M., Roche R. 2005. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem. Cell Biol. 124: 113–121. [DOI] [PubMed] [Google Scholar]
- 107.Müller A. M., Mehrkens A., Schafer D. J., Jaquiery C., Guven S., Lehmicke M., Martinetti R., Farhadi I., Jakob M., Scherberich A., et al. 2010. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur. Cell. Mater. 19: 127–135. [DOI] [PubMed] [Google Scholar]
- 108.Katz A. J., Tholpady A., Tholpady S. S., Shang H., Ogle R. C. 2005. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 23: 412–423. [DOI] [PubMed] [Google Scholar]
- 109.Planat-Benard V., Silvestre J. S., Cousin B., Andre M., Nibbelink M., Tamarat R., Clergue M., Manneville C., Saillan-Barreau C., Duriez M., et al. 2004. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 109: 656–663. [DOI] [PubMed] [Google Scholar]
- 110.Scheideler M., Elabd C., Zaragosi L. E., Chiellini C., Hackl H., Sanchez-Cabo F., Yadav S., Duszka K., Friedl G., Papak C., et al. 2008. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics. 9: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jansen B. J., Gilissen C., Roelofs H., Schaap-Oziemlak A., Veltman J. A., Raymakers R. A., Jansen J. H., Kogler G., Figdor C. G., Torensma R., et al. 2010. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 19: 481–490. [DOI] [PubMed] [Google Scholar]
- 112.Salgado A. J., Reis R. L., Sousa N. J., Gimble J. M. 2010. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 5: 103–110. [DOI] [PubMed] [Google Scholar]
- 113.Boquest A. C., Noer A., Collas P. 2006. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2: 319–329. [DOI] [PubMed] [Google Scholar]
- 114.Gimble J. M., Floyd Z. E., Bunnell B. A. 2009. The 4th dimension and adult stem cells: Can timing be everything? J. Cell. Biochem. 107: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bai X., Ma J., Pan Z., Song Y. H., Freyberg S., Yan Y., Vykoukal D., Alt E. 2007. Electrophysiological properties of human adipose tissue-derived stem cells. Am. J. Physiol. Cell Physiol. 293: C1539–C1550. [DOI] [PubMed] [Google Scholar]
- 116.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 117.Lin C. S., Xin Z. C., Deng C. H., Ning H., Lin G., Lue T. F. 2010. Defining adipose tissue-derived stem cells in tissue and in culture. Histol. Histopathol. 25: 807–815. [DOI] [PubMed] [Google Scholar]
- 118.Quirici N., Scavullo C., de Girolamo L., Lopa S., Arrigoni E., Deliliers G. L., Brini A. T. 2010. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev. 19: 915–925. [DOI] [PubMed] [Google Scholar]
- 119.Conget P. A., Minguell J. J. 1999. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J. Cell. Physiol. 181: 67–73. [DOI] [PubMed] [Google Scholar]
- 120.Zheng B., Cao B., Li G., Huard J. 2006. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 12: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 121.Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., Andriolo G., Sun B., Zheng B., Zhang L., et al. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 3: 301–313. [DOI] [PubMed] [Google Scholar]
- 122.Rhodin J. A. 1968. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J. Ultrastruct. Res. 25: 452–500. [DOI] [PubMed] [Google Scholar]
- 123.Suga H., Matsumoto D., Eto H., Inoue K., Aoi N., Kato H., Araki J., Yoshimura K. 2009. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 18: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 124.Li H., Zimmerlin L., Marra K. G., Donnenberg V. S., Donnenberg A. D., Rubin J. P. 2011. Adipogenic potential of adipose stem cell subpopulations. Plast. Reconstr. Surg. 128: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rangappa S., Fen C., Lee E. H., Bongso A., Sim E. K. 2003. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg. 75: 775–779. [DOI] [PubMed] [Google Scholar]
- 126.Planat-Bénard V., Menard C., Andre M., Puceat M., Perez A., Garcia-Verdugo J. M., Penicaud L., Casteilla L. 2004. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94: 223–229. [DOI] [PubMed] [Google Scholar]
- 127.Rodríguez L. V., Alfonso Z., Zhang R., Leung J., Wu B., Ignarro L. J. 2006. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc. Natl. Acad. Sci. USA. 103: 12167–12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu Y., Liu L., Li Y., Zhou C., Xiong F., Liu Z., Gu R., Hou X., Zhang C. 2008. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 1239: 49–55. [DOI] [PubMed] [Google Scholar]
- 129.Lee J. A., Parrett B. M., Conejero J. A., Laser J., Chen J., Kogon A. J., Nanda D., Grant R. T., Breitbart A. S. 2003. Biological alchemy: engineering bone and fat from fat-derived stem cells. Ann. Plast. Surg. 50: 610–617. [DOI] [PubMed] [Google Scholar]
- 130.Choi Y. S., Cha S. M., Lee Y. Y., Kwon S. W., Park C. J., Kim M. 2006. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem. Biophys. Res. Commun. 345: 631–637. [DOI] [PubMed] [Google Scholar]
- 131.Mauney J. R., Nguyen T., Gillen K., Kirker-Head C., Gimble J. M., Kaplan D. L. 2007. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 28: 5280–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fischbach C., Spruss T., Weiser B., Neubauer M., Becker C., Hacker M., Gopferich A., Blunk T. 2004. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp. Cell Res. 300: 54–64. [DOI] [PubMed] [Google Scholar]
- 133.Choi Y. S., Park S. N., Suh H. 2005. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials. 26: 5855–5863. [DOI] [PubMed] [Google Scholar]
- 134.Neubauer M., Hacker M., Bauer-Kreisel P., Weiser B., Fischbach C., Schulz M. B., Goepferich A., Blunk T. 2005. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 11: 1840–1851. [DOI] [PubMed] [Google Scholar]
- 135.Schulz T. J., Huang T. L., Tran T. T., Zhang H., Townsend K. L., Shadrach J. L., Cerletti M., McDougall L. E., Giorgadze N., Tchkonia T., et al. 2011. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA. 108: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scime A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., et al. 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 454: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., Spiegelman B. M. 2007. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6: 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seale P., Conroe H. M., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., Spiegelman B. M. 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. 2010. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285: 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hicok K. C., Du Laney T. V., Zhou Y. S., Halvorsen Y. D., Hitt D. C., Cooper L. F., Gimble J. M. 2004. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 10: 371–380. [DOI] [PubMed] [Google Scholar]
- 141.Dragoo J. L., Lieberman J. R., Lee R. S., Deugarte D. A., Lee Y., Zuk P. A., Hedrick M. H., Benhaim P. 2005. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast. Reconstr. Surg. 115: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 142.Erickson G. R., Gimble J. M., Franklin D. M., Rice H. E., Awad H., Guilak F. 2002. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 290: 763–769. [DOI] [PubMed] [Google Scholar]
- 143.Jeon O., Rhie J. W., Kwon I. K., Kim J. H., Kim B. S., Lee S. H. 2008. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng. Part A. 14: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 144.Li X., Yao J., Wu L., Jing W., Tang W., Lin Y., Tian W., Liu L. 2010. Osteogenic induction of adipose-derived stromal cells: not a requirement for bone formation in vivo. Artif. Organs. 34: 46–54. [DOI] [PubMed] [Google Scholar]
- 145.Lee J. H., Kemp D. M. 2006. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem. Biophys. Res. Commun. 341: 882–888. [DOI] [PubMed] [Google Scholar]
- 146.Bacou F., el Andalousi R. B., Daussin P. A., Micallef J. P., Levin J. M., Chammas M., Casteilla L., Reyne Y., Nougues J. 2004. Transplantation of adipose tissue-derived stromal cells increases mass and functional capacity of damaged skeletal muscle. Cell Transplant. 13: 103–111. [DOI] [PubMed] [Google Scholar]
- 147.Rodriguez A. M., Pisani D., Dechesne C. A., Turc-Carel C., Kurzenne J. Y., Wdziekonski B., Villageois A., Bagnis C., Breittmayer J. P., Groux H., et al. 2005. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 201: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Strem B. M., Zhu M., Alfonso Z., Daniels E. J., Schreiber R., Beygui R., MacLellan W. R., Hedrick M. H., Fraser J. K. 2005. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 7: 282–291. [DOI] [PubMed] [Google Scholar]
- 149.Gaustad K. G., Boquest A. C., Anderson B. E., Gerdes A. M., Collas P. 2004. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem. Biophys. Res. Commun. 314: 420–427. [DOI] [PubMed] [Google Scholar]
- 150.Lee W. C., Sepulveda J. L., Rubin J. P., Marra K. G. 2009. Cardiomyogenic differentiation potential of human adipose precursor cells. Int. J. Cardiol. 133: 399–401. [DOI] [PubMed] [Google Scholar]
- 151.Choi Y. S., Dusting G. J., Stubbs S., Arunothayaraj S., Han X. L., Collas P., Morrison W. A., Dilley R. J. 2010. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 14: 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu Y., Liu T., Song K., Ning R., Ma X., Cui Z. 2009. ADSCs differentiated into cardiomyocytes in cardiac microenvironment. Mol. Cell. Biochem. 324: 117–129. [DOI] [PubMed] [Google Scholar]
- 153.Miyahara Y., Nagaya N., Kataoka M., Yanagawa B., Tanaka K., Hao H., Ishino K., Ishida H., Shimizu T., Kangawa K., et al. 2006. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 12: 459–465. [DOI] [PubMed] [Google Scholar]
- 154.Sadat S., Gehmert S., Song Y. H., Yen Y., Bai X., Gaiser S., Klein H., Alt E. 2007. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem. Biophys. Res. Commun. 363: 674–679. [DOI] [PubMed] [Google Scholar]
- 155.Palpant N. J., Yasuda S., MacDougald O., Metzger J. M. 2007. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J. Mol. Cell. Cardiol. 43: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamada Y., Wang X. D., Yokoyama S., Fukuda N., Takakura N. 2006. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem. Biophys. Res. Commun. 342: 662–670. [DOI] [PubMed] [Google Scholar]
- 157.Jack G. S., Zhang R., Lee M., Xu Y., Wu B. M., Rodriguez L. V. 2009. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 30: 3259–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cousin B., Andre M., Arnaud E., Penicaud L., Casteilla L. 2003. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem. Biophys. Res. Commun. 301: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 159.Corre J., Barreau C., Cousin B., Chavoin J. P., Caton D., Fournial G., Penicaud L., Casteilla L., Laharrague P. 2006. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J. Cell. Physiol. 208: 282–288. [DOI] [PubMed] [Google Scholar]
- 160.Miñana M. D., Carbonell-Uberos F., Mirabet V., Marin S., Encabo A. 2008. IFATS collection: identification of hemangioblasts in the adult human adipose tissue. Stem Cells. 26: 2696–2704. [DOI] [PubMed] [Google Scholar]
- 161.Sengenès C., Lolmede K., Zakaroff-Girard A., Busse R., Bouloumie A. 2005. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J. Cell. Physiol. 205: 114–122. [DOI] [PubMed] [Google Scholar]
- 162.Martínez-Estrada O. M., Munoz-Santos Y., Julve J., Reina M., Vilaro S. 2005. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc. Res. 65: 328–333. [DOI] [PubMed] [Google Scholar]
- 163.De Francesco F., Tirino V., Desiderio V., Ferraro G., D'Andrea F., Giuliano M., Libondi G., Pirozzi G., De Rosa A., Papaccio G. 2009. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS ONE. 4: e6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang L. Y., Liu X. M., Sun B., Hui G. Z., Fei J., Guo L. H. 2004. Adipose tissue-derived stromal cells express neuronal phenotypes. Chin. Med. J. (Engl.). 117: 425–429. [PubMed] [Google Scholar]
- 165.Wrage P. C., Tran T., To K., Keefer E. W., Ruhn K. A., Hong J., Hattangadi S., Trevino I., Tansey M. G. 2008. The neuro-glial properties of adipose-derived adult stromal (ADAS) cells are not regulated by Notch 1 and are not derived from neural crest lineage. PLoS ONE. 3: e1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nakada A., Fukuda S., Ichihara S., Sato T., Itoi S., Inada Y., Endo K., Nakamura T. 2009. Regeneration of central nervous tissue using a collagen scaffold and adipose-derived stromal cells. Cells Tissues Organs. 190: 326–335. [DOI] [PubMed] [Google Scholar]
- 167.di Summa P. G., Kingham P. J., Raffoul W., Wiberg M., Terenghi G., Kalbermatten D. F. 2010. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 63: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 168.Vossmerbaeumer U., Ohnesorge S., Kuehl S., Haapalahti M., Kluter H., Jonas J. B., Thierse H. J., Bieback K. 2009. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 11: 177–188. [DOI] [PubMed] [Google Scholar]
- 169.Yang Z., Li K., Yan X., Dong F., Zhao C. 2010. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch. Clin. Exp. Ophthalmol. 248: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 170.Ferro F., Spelat R., Falini G., Gallelli A., D'Aurizio F., Puppato E., Pandolfi M., Beltrami A. P., Cesselli D., Beltrami C. A., et al. 2011. Adipose tissue-derived stem cell in vitro differentiation in a three-dimensional dental bud structure. Am. J. Pathol. 178: 2299–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sgodda M., Aurich H., Kleist S., Aurich I., Konig S., Dollinger M. M., Fleig W. E., Christ B. 2007. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp. Cell Res. 313: 2875–2886. [DOI] [PubMed] [Google Scholar]
- 172.Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Quinn G., Okochi H., Ochiya T. 2007. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 46: 219–228. [DOI] [PubMed] [Google Scholar]
- 173.Aurich H., Sgodda M., Kaltwasser P., Vetter M., Weise A., Liehr T., Brulport M., Hengstler J. G., Dollinger M. M., Fleig W. E., et al. 2009. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 58: 570–581. [DOI] [PubMed] [Google Scholar]
- 174.Kim D. H., Je C. M., Sin J. Y., Jung J. S. 2003. Effect of partial hepatectomy on in vivo engraftment after intravenous administration of human adipose tissue stromal cells in mouse. Microsurgery. 23: 424–431. [DOI] [PubMed] [Google Scholar]
- 175.Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Osaki M., Kato T., Okochi H., Ochiya T. 2009. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J. Gastroenterol. Hepatol. 24: 70–77. [DOI] [PubMed] [Google Scholar]
- 176.Wang X., Willenbring H., Akkari Y., Torimaru Y., Foster M., Al-Dhalimy M., Lagasse E., Finegold M., Olson S., Grompe M. 2003. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 422: 897–901. [DOI] [PubMed] [Google Scholar]
- 177.Vassilopoulos G., Wang P. R., Russell D. W. 2003. Transplanted bone marrow regenerates liver by cell fusion. Nature. 422: 901–904. [DOI] [PubMed] [Google Scholar]
- 178.Timper K., Seboek D., Eberhardt M., Linscheid P., Christ-Crain M., Keller U., Muller B., Zulewski H. 2006. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys. Res. Commun. 341: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 179.Lin G., Wang G., Liu G., Yang L. J., Chang L. J., Lue T. F., Lin C. S. 2009. Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev. 18: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jumabay M., Matsumoto T., Yokoyama S., Kano K., Kusumi Y., Masuko T., Mitsumata M., Saito S., Hirayama A., Mugishima H., et al. 2009. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J. Mol. Cell. Cardiol. 47: 565–575. [DOI] [PubMed] [Google Scholar]
- 181.Matsumoto T., Kano K., Kondo D., Fukuda N., Iribe Y., Tanaka N., Matsubara Y., Sakuma T., Satomi A., Otaki M., et al. 2008. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 215: 210–222. [DOI] [PubMed] [Google Scholar]
- 182.Justesen J., Pedersen S. B., Stenderup K., Kassem M. 2004. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 10: 381–391. [DOI] [PubMed] [Google Scholar]
- 183.Jumabay M., Zhang R., Yao Y., Goldhaber J. I., Bostrom K. I. 2010. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc. Res. 85: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S. 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 185.Guilak F., Lott K. E., Awad H. A., Cao Q., Hicok K. C., Fermor B., Gimble J. M. 2006. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J. Cell. Physiol. 206: 229–237. [DOI] [PubMed] [Google Scholar]
- 186.Gesta S., Tseng Y. H., Kahn C. R. 2007. Developmental origin of fat: tracking obesity to its source. Cell. 131: 242–256. [DOI] [PubMed] [Google Scholar]
- 187.Nielsen J. S., McNagny K. M. 2008. Novel functions of the CD34 family. J. Cell Sci. 121: 3683–3692. [DOI] [PubMed] [Google Scholar]
- 188.Wilson A., Oser G. M., Jaworski M., Blanco-Bose W. E., Laurenti E., Adolphe C., Essers M. A., Macdonald H. R., Trumpp A. 2007. Dormant and self-renewing hematopoietic stem cells and their niches. Ann. N. Y. Acad. Sci. 1106: 64–75. [DOI] [PubMed] [Google Scholar]
- 189.Rodeheffer M. S., Birsoy K., Friedman J. M. 2008. Identification of white adipocyte progenitor cells in vivo. Cell. 135: 240–249. [DOI] [PubMed] [Google Scholar]
- 190.Pisani D. F., Dechesne C. A., Sacconi S., Delplace S., Belmonte N., Cochet O., Clement N., Wdziekonski B., Villageois A. P., Butori C., et al. 2010. Isolation of a highly myogenic CD34-negative subset of human skeletal muscle cells free of adipogenic potential. Stem Cells. 28: 753–764. [DOI] [PubMed] [Google Scholar]
- 191.Tontonoz P., Hu E., Spiegelman B. M. 1994. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 79: 1147–1156. [DOI] [PubMed] [Google Scholar]
- 192.Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. 1999. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 4: 611–617. [DOI] [PubMed] [Google Scholar]
- 193.Tontonoz P., Spiegelman B. M. 2008. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77: 289–312. [DOI] [PubMed] [Google Scholar]
- 194.Cinti S., Cigolini M., Bosello O., Bjorntorp P. 1984. A morphological study of the adipocyte precursor. J. Submicrosc. Cytol. 16: 243–251. [PubMed] [Google Scholar]
- 195.Zingaretti M. C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. 2009. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23: 3113–3120. [DOI] [PubMed] [Google Scholar]
- 196.Gupta R. K., Arany Z., Seale P., Mepani R. J., Ye L., Conroe H. M., Roby Y. A., Kulaga H., Reed R. R., Spiegelman B. M. 2010. Transcriptional control of preadipocyte determination by Zfp423. Nature. 464: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Quach J. M., Walker E. C., Allan E., Solano M., Yokoyama A., Kato S., Sims N. A., Gillespie M. T., Martin T. J. 2011. Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. J. Biol. Chem. 286: 4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rajashekhar G., Traktuev D. O., Roell W. C., Johnstone B. H., Merfeld-Clauss S., Van Natta B., Rosen E. D., March K. L., Clauss M. 2008. IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells. 26: 2674–2681. [DOI] [PubMed] [Google Scholar]
- 199.Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., MacDougald O. A. 2000. Inhibition of adipogenesis by Wnt signaling. Science. 289: 950–953. [DOI] [PubMed] [Google Scholar]
- 200.Longo K. A., Wright W. S., Kang S., Gerin I., Chiang S. H., Lucas P. C., Opp M. R., MacDougald O. A. 2004. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 279: 35503–35509. [DOI] [PubMed] [Google Scholar]
- 201.Kang S., Bajnok L., Longo K. A., Petersen R. K., Hansen J. B., Kristiansen K., MacDougald O. A. 2005. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1alpha. Mol. Cell. Biol. 25: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wright W. S., Longo K. A., Dolinsky V. W., Gerin I., Kang S., Bennett C. N., Chiang S. H., Prestwich T. C., Gress C., Burant C. F., et al. 2007. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 56: 295–303. [DOI] [PubMed] [Google Scholar]
- 203.Cawthorn W. P., Bree A. J., Yao Y., Du B., Hemati N., Martinez-Santibanez G., MacDougald O. A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone Epub ahead of print. August 18, 2011; doi:10/1016/j.bone2011.08.010. [DOI] [PMC free article] [PubMed]
- 204.Bonyadi M., Waldman S. D., Liu D., Aubin J. E., Grynpas M. D., Stanford W. L. 2003. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. USA. 100: 5840–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Torti F. M., Torti S. V., Larrick J. W., Ringold G. M. 1989. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J. Cell Biol. 108: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Choy L., Derynck R. 2003. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278: 9609–9619. [DOI] [PubMed] [Google Scholar]
- 207.Choy L., Skillington J., Derynck R. 2000. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149: 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Artemenko Y., Gagnon A., Aubin D., Sorisky A. 2005. Anti-adipogenic effect of PDGF is reversed by PKC inhibition. J. Cell. Physiol. 204: 646–653. [DOI] [PubMed] [Google Scholar]
- 209.Dührsen U., Martinez T., Vohwinkel G., Ergun S., Sun L., McMahon G., Durig J., Hossfeld D. K., Fiedler W. 2001. Effects of vascular endothelial and platelet-derived growth factor receptor inhibitors on long-term cultures from normal human bone marrow. Growth Factors. 19: 1–17. [DOI] [PubMed] [Google Scholar]
- 210.Camp H. S., Tafuri S. R. 1997. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J. Biol. Chem. 272: 10811–10816. [DOI] [PubMed] [Google Scholar]
- 211.Krieger-Brauer H. I., Kather H. 1995. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem. J. 307: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takashima Y., Era T., Nakao K., Kondo S., Kasuga M., Smith A. G., Nishikawa S. 2007. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 129: 1377–1388. [DOI] [PubMed] [Google Scholar]
- 213.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. 2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12: 143–152. [DOI] [PubMed] [Google Scholar]
- 214.Festa E., Fretz J., Berry R., Schmidt B., Rodeheffer M., Horowitz M., Horsley V. 2011. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 146: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Taylor S. M., Jones P. A. 1979. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 17: 771–779. [DOI] [PubMed] [Google Scholar]
- 216.Katagiri T., Yamaguchi A., Ikeda T., Yoshiki S., Wozney J. M., Rosen V., Wang E. A., Tanaka H., Omura S., Suda T. 1990. The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem. Biophys. Res. Commun. 172: 295–299. [DOI] [PubMed] [Google Scholar]
- 217.Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y., et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 454: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tang Q. Q., Otto T. C., Lane M. D. 2004. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA. 101: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang E. A., Israel D. I., Kelly S., Luxenberg D. P. 1993. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 9: 57–71. [DOI] [PubMed] [Google Scholar]
- 220.Bowers R. R., Kim J. W., Otto T. C., Lane M. D. 2006. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc. Natl. Acad. Sci. USA. 103: 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huang H., Song T. J., Li X., Hu L., He Q., Liu M., Lane M. D., Tang Q. Q. 2009. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA. 106: 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jin W., Takagi T., Kanesashi S. N., Kurahashi T., Nomura T., Harada J., Ishii S. 2006. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev. Cell. 10: 461–471. [DOI] [PubMed] [Google Scholar]
- 223.Hata A., Seoane J., Lagna G., Montalvo E., Hemmati-Brivanlou A., Massague J. 2000. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell. 100: 229–240. [DOI] [PubMed] [Google Scholar]
- 224.Neubauer M., Fischbach C., Bauer-Kreisel P., Lieb E., Hacker M., Tessmar J., Schulz M. B., Goepferich A., Blunk T. 2004. Basic fibroblast growth factor enhances PPARgamma ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett. 577: 277–283. [DOI] [PubMed] [Google Scholar]
- 225.Kakudo N., Shimotsuma A., Kusumoto K. 2007. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 359: 239–244. [DOI] [PubMed] [Google Scholar]
- 226.Kimura Y., Ozeki M., Inamoto T., Tabata Y. 2003. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 24: 2513–2521. [DOI] [PubMed] [Google Scholar]
- 227.Pirruccello S. J., LeBien T. W. 1986. The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J. Immunol. 136: 3779–3784. [PubMed] [Google Scholar]
- 228.Hough M. R., Rosten P. M., Sexton T. L., Kay R., Humphries R. K. 1994. Mapping of CD24 and homologous sequences to multiple chromosomal loci. Genomics. 22: 154–161. [DOI] [PubMed] [Google Scholar]
- 229.Shackleton M., Vaillant F., Simpson K. J., Stingl J., Smyth G. K., Asselin-Labat M. L., Wu L., Lindeman G. J., Visvader J. E. 2006. Generation of a functional mammary gland from a single stem cell. Nature. 439: 84–88. [DOI] [PubMed] [Google Scholar]
- 230.Overdevest J. B., Thomas S., Kristiansen G., Hansel D. E., Smith S. C., Theodorescu D. 2011. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 71: 3802–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sagiv E., Arber N. 2008. The novel oncogene CD24 and its arising role in the carcinogenesis of the GI tract: from research to therapy. Expert Rev. Gastroenterol. Hepatol. 2: 125–133. [DOI] [PubMed] [Google Scholar]
- 232.Hong K. M., Burdick M. D., Phillips R. J., Heber D., Strieter R. M. 2005. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 19: 2029–2031. [DOI] [PubMed] [Google Scholar]
- 233.Crossno J. T., Jr, Majka S. M., Grazia T., Gill R. G., Klemm D. J. 2006. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest. 116: 3220–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Majka S. M., Fox K. E., Psilas J. C., Helm K. M., Childs C. R., Acosta A. S., Janssen R. C., Friedman J. E., Woessner B. T., Shade T. R., et al. 2010. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc. Natl. Acad. Sci. USA. 107: 14781–14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sera Y., LaRue A. C., Moussa O., Mehrotra M., Duncan J. D., Williams C. R., Nishimoto E., Schulte B. A., Watson P. M., Watson D. K., et al. 2009. Hematopoietic stem cell origin of adipocytes. Exp. Hematol. 37: 1108–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Koh Y. J., Kang S., Lee H. J., Choi T. S., Lee H. S., Cho C. H., Koh G. Y. 2007. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J. Clin. Invest. 117: 3684–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tomiyama K., Murase N., Stolz D. B., Toyokawa H., O'Donnell D. R., Smith D. M., Dudas J. R., Rubin J. P., Marra K. G. 2008. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells. 26: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Billon N., Iannarelli P., Monteiro M. C., Glavieux-Pardanaud C., Richardson W. D., Kessaris N., Dani C., Dupin E. 2007. The generation of adipocytes by the neural crest. Development. 134: 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mojallal A., Lequeux C., Shipkov C., Duclos A., Braye F., Rohrich R., Brown S., Damour O. 2011. Influence of age and body mass index on the yield and proliferation capacity of adipose-derived stem cells. Aesthetic Plast. Surg. 35: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 240.Tchoukalova Y., Koutsari C., Jensen M. 2007. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 50: 151–157. [DOI] [PubMed] [Google Scholar]
- 241.Virtue S., Vidal-Puig A. 2008. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 6: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tang W., Zeve D., Seo J., Jo A. Y., Graff J. M. 2011. Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 14: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Peptan I. A., Hong L., Mao J. J. 2006. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast. Reconstr. Surg. 117: 1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Baglioni S., Francalanci M., Squecco R., Lombardi A., Cantini G., Angeli R., Gelmini S., Guasti D., Benvenuti S., Annunziato F., et al. 2009. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 23: 3494–3505. [DOI] [PubMed] [Google Scholar]
- 245.Jurgens W. J., Oedayrajsingh-Varma M. J., Helder M. N., Zandiehdoulabi B., Schouten T. E., Kuik D. J., Ritt M. J., van Milligen F. J. 2008. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 332: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gesta S., Bluher M., Yamamoto Y., Norris A. W., Berndt J., Kralisch S., Boucher J., Lewis C., Kahn C. R. 2006. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA. 103: 6676–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tchkonia T., Lenburg M., Thomou T., Giorgadze N., Frampton G., Pirtskhalava T., Cartwright A., Cartwright M., Flanagan J., Karagiannides I., et al. 2007. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab. 292: E298–E307. [DOI] [PubMed] [Google Scholar]
- 248.Fang B., Li Y., Song Y., Li N., Cao Y., Wei X., Lin Q., Zhao R. C. 2010. Human adipose tissue-derived adult stem cells can lead to multiorgan engraftment. Transplant. Proc. 42: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 249.Meyerrose T. E., De Ugarte D. A., Hofling A. A., Herrbrich P. E., Cordonnier T. D., Shultz L. D., Eagon J. C., Wirthlin L., Sands M. S., Hedrick M. A., et al. 2007. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 25: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. 2000. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 251.Joe A. W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M. A., Rossi F. M. 2010. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sun S., Guo Z., Xiao X., Liu B., Liu X., Tang P. H., Mao N. 2003. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 21: 527–535. [DOI] [PubMed] [Google Scholar]
- 253.Peraldi P., Hotamisligil G. S., Buurman W. A., White M. F., Spiegelman B. M. 1996. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 271: 13018–13022. [DOI] [PubMed] [Google Scholar]
- 254.Simmons P. J., Torok-Storb B. 1991. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 78: 55–62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.