Abstract
Synaptic vesicle recycling is in part mediated by clathrin-mediated endocytosis. This process involves the coordinated assembly of clathrin and adaptor proteins and the concomitant selection of cargo proteins. Here, we demonstrate that the endocytotic protein stonin 2 localizes to axonal vesicle clusters through its μ-homology domain. Interaction of this domain with synaptotagmin I is sufficient to recruit stonin 2 to the plasmalemma. The N-terminal domain of stonin 2 harbors multiple AP-2-interaction motifs that bind to the clathrin adaptor complex AP-2. These motifs with the consensus sequence WVxF are capable of binding to the α-adaptin ear domain and to μ2. Mutation of the tyrosine motif-binding pocket of μ2 abolishes recognition of the WVxF peptide, suggesting that association with stonin 2 renders AP-2 incompetent to sort tyrosine motif-containing cargo proteins. We hypothesize that stonin 2 may function as an AP-2-dependent sorting adaptor for synaptic vesicle recycling.
Synaptic vesicle (SV) recycling at least in part depends on clathrin/AP-2-mediated endocytosis (1, 2). Clathrin-coated pit formation is initiated by the assembly of clathrin and adaptor proteins onto phosphoinositide-rich membrane sites (reviewed in refs. 3–6). Adaptors such as AP-2 provide a physical link between clathrin and the membrane and sort membrane cargo proteins into nascent pits (4, 5). The sorting function of AP-2 at presynaptic sites may involve multiple interactions with accessory factors, tyrosine motif-containing cargo proteins (reviewed in refs. 1 and 2), and/or the presynaptic membrane protein synaptotagmin (7, 8). Possible regulators of AP-2 function at synapses are stonins, which have recently been identified as human homologues (9, 10) of Drosophila stoned B, a presynaptic protein implicated in neurotransmission and SV recycling (11, 12). Stoned B genetically (13) and biochemically interacts with synaptotagmin (14). Human stonin 2 (also termed hStnB; ref. 10) has two Eps15-binding NPF motifs (9), and its C-terminal (CT) μ-homology domain directly associates with synaptotagmin (9, 10), which is consistent with studies in Drosophila (13, 14).
We demonstrate here that stonin 2 colocalizes with synaptotagmin at axonal vesicle clusters and synaptotagmin, in turn, is sufficient to recruit stonin 2 to the plasmalemma. The N-terminal (NT) domain of stonin 2 harbors multiple AP-2-interaction motifs, which bind to the AP-2α ear domain and to the tyrosine motif-binding pocket of μ2. We hypothesize that stonin 2 may function as an AP-2-dependent sorting adaptor for SV recycling.
Materials and Methods
Antibodies. Polyclonal antibodies against human stonin 2 were raised by immunizing rabbits with GST-stonin 2 NT (amino acids 1–555) fusion protein.
Recombinant Proteins. To generate GST- or hemagglutinin (HA)-tagged fusion proteins, corresponding DNA fragments were PCR-amplified, cloned into pGEX4T or a pcDNA3 variant (pcHA2), and verified by PCR screening, restriction digest, and double-stranded DNA sequencing.
Immunoprecipitations. For information concerning immunoprecipitations, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Plasmid DNA and Site-Directed Mutagenesis. DNA manipulations were carried out by using either mouse stonin 2 (GenBank accession no. AK036612) or its corresponding human homologue as a template. Single, double, and triple mutants were produced by PCR with the QuikChange site-directed mutagenesis kit (Stratagene). The presence of the mutation was verified by double-stranded DNA sequencing.
Affinity Purification. Affinity purification by using rat brain homogenate was performed as described (8).
Transfection and Internalization Assay. Primary cortical neurons were transfected by calcium-phosphate precipitation. COS7 or N1E-115 cells were transiently transfected by using Lipofectamine 2000. For internalization assays, COS7 cells starved in serum-free medium were incubated with 20 μg/ml Alexa 488 transferrin for 15 min at 37°C. Cells were washed, fixed, and analyzed by indirect immunofluorescence microscopy. Confocal images were acquired on a Zeiss LSM system.
Miscellaneous. For SDS/PAGE, immunoblotting, in vitro transcription/translation (Promega), and indirect immunofluorescence microscopy, standard procedures were used. Subcellular fractionation experiments were carried out according to Maycox et al. (15). Rat brain cytosol was prepared as described (16).
Results
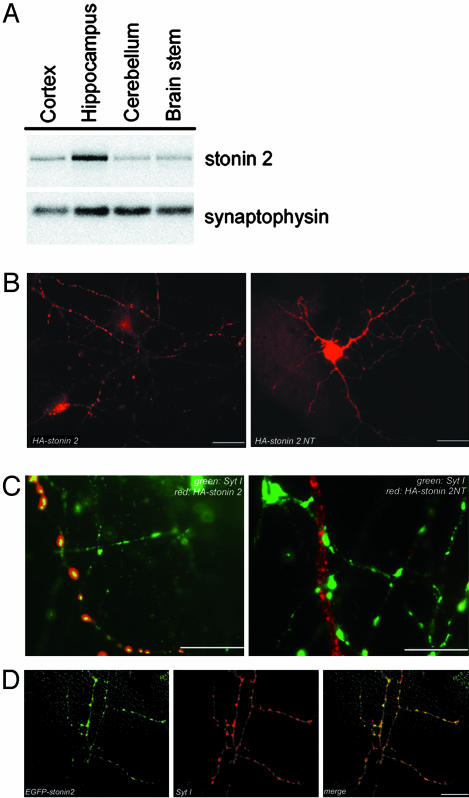
Stonin 2 Is Enriched in the Hippocampus Region of the Brain, Where It Colocalizes with Synaptotagmin I at Axonal Vesicle Clusters. Human homologues of the Drosophila presynaptic protein stoned B have recently been identified by us (10) and others (9). These reports have yielded inconclusive data regarding the expression pattern and subcellular distribution of mammalian stonin 2. We therefore decided to first reinvestigate the localization and tissue distribution of mammalian stonin 2. In agreement with our results reported earlier (10), antisera specific for stonin 2 (Fig. 6B, which is published as supporting information on the PNAS web site) revealed a strong enrichment of stonin 2 immunoreactivity in brain (Fig. 6A), and, within the brain, stonin 2 was most highly expressed in the hippocampus, a brain region implicated in synaptic plasticity. Synaptophysin, an SV marker, was equally distributed in all brain regions tested (Fig. 1A). Moreover, stonin 2 was concentrated in clathrin-coated vesicles (15) isolated from lysed nerve terminals (Fig. 6C), which was consistent with its proposed function in clathrin-mediated endocytosis at the synapse (9, 10).
Fig. 1.
Stonin 2 is enriched in the hippocampus and localizes to axonal vesicle clusters. (A) Western blot of homogenates (50 μg of protein) from different brain regions. (B–D) Localization of stonin 2 in primary neurons. Cortical neurons were transfected with plasmids encoding HA- or enhanced GFP (EGFP)-tagged stonin 2 or a truncation mutant lacking its CT μ-homology domain (stonin 2 NT). Eight days in vitro neurons were analyzed by immunofluorescence microscopy for the distribution of EGFP-stonin 2, HA-tagged stonin 2, HA-tagged stonin 2 NT, and synaptotagmin I. Low (B)- and high (C)-magnification views illustrating the colocalization of HA-stonin 2 but not HA-stonin 2 NT (red; Alexa 594) with synaptotagmin I (green; Alexa 488) at axonal vesicle clusters. (D) Colocalization of EGFP-stonin 2 (green) with synaptotagmin I (red; Alexa 594). (Scale bar, 20 μm.)
HA-tagged stonin 2 exhibited a punctate distribution in transfected primary neurons reminiscent of axonal vesicle clusters (Fig. 1B). Costaining with antibodies against the SV protein synaptotagmin I showed that HA-stonin 2 was concentrated in the vicinity of SV clusters (Fig. 1C). By contrast, a stonin 2 truncation mutant lacking its synaptotagmin-binding μ-homology domain, HA-stonin 2 NT, appeared randomly distributed throughout the neuronal cytoplasm and did not colocalize with synaptotagmin I (Fig. 1 B and C). Similar results were seen for enhanced GFP stonin 2 (Fig. 1D).
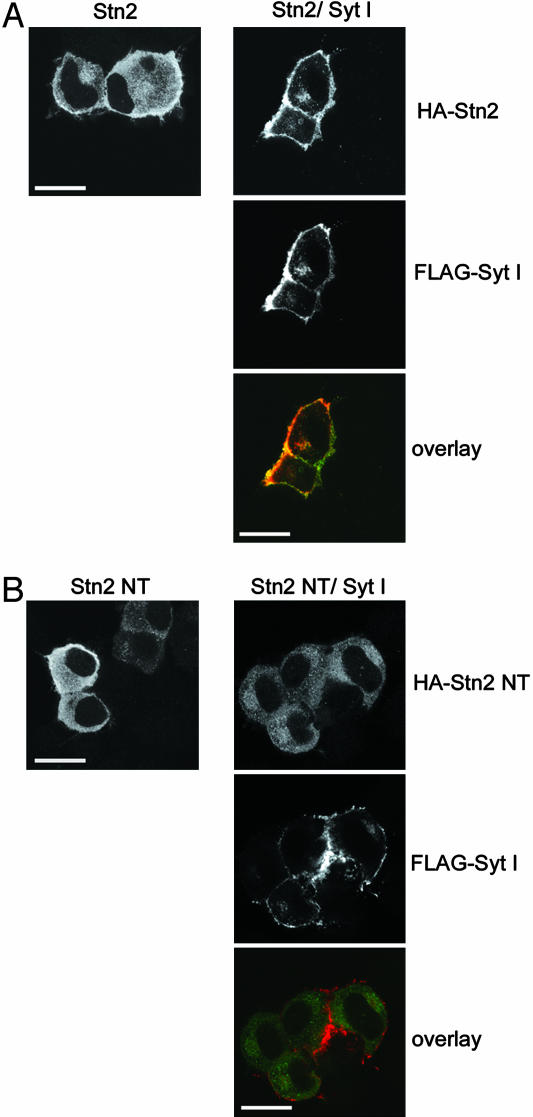
Overexpressed Synaptotagmin I Recruits Stonin 2 to the Plasma Membrane in Transfected Neuroblastoma Cells. The differential distribution of stonin 2 and a truncation mutant lacking the μ-homology domain (stonin 2 NT) in neurons suggests that synaptotagmin might play a direct role in targeting stonin 2 to the membrane. To test this possibility, we transfected HA-tagged stonin 2 (Fig. 2A) or the HA-stonin 2 NT truncation mutant (Fig. 2B), either alone or together with FLAG-tagged synaptotagmin I into undifferentiated N1E-115 neuroblastoma cells, and analyzed their intracellular distribution by confocal immunofluorescence microscopy. When expressed alone, HA-stonin 2 and HA-stonin 2 NT were homogenously distributed throughout the cell. However, on coexpression of FLAG-tagged synaptotagmin, I stonin 2 was effectively recruited to the plasmalemma where it colocalized with synaptotagmin I (Fig. 2 A). Both proteins were also present albeit to a minor extent at internal, presumably endocytotic organelles. The presence or absence of synaptotagmin I had no effect on the cytoplasmic distribution of the stonin 2 NT truncation mutant lacking the synaptotagmin-binding μ-homology domain (Fig. 2B).
Fig. 2.
Overexpressed synaptotagmin I recruits stonin 2 to the plasma membrane in transfected N1E-115 neuroblastoma cells. N1E-115 neuroblastoma cells were transfected with plasmids encoding HA-tagged stonin 2 (HA-stn2) (A) or a truncation mutant lacking its CT μ-homology domain (HA-stn2 NT) (B), either alone or together with FLAG-tagged synaptotagmin I (Syt I). Cells were analyzed by confocal immunofluorescence microscopy for the distribution of HA-stonin 2, or HA-stonin 2 NT (green; Alexa 488) and FLAG-synaptotagmin I (red; Alexa 594). (Scale bar, 20 μm.)
These combined data suggest that stonin 2 is a clathrin-coated vesicle-associated axonal protein targeted to SV clusters by its direct interaction with synaptotagmin.
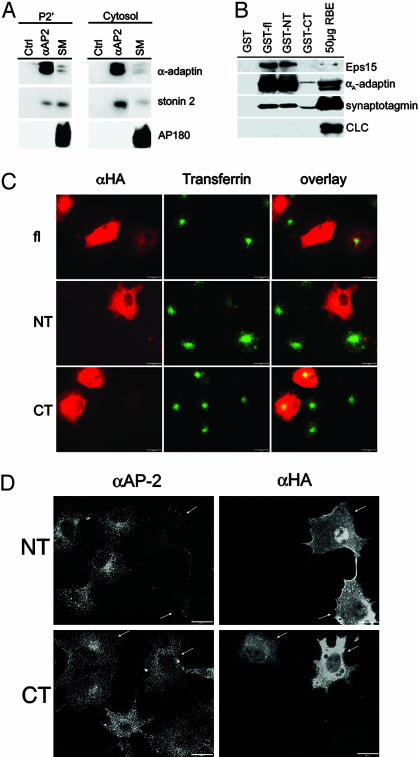
Overexpression of Stonin 2, a Major AP-2-Binding Partner in Brain, Inhibits Clathrin-Mediated Internalization of Transferrin Through Sequestration of AP-2. Because the subcellular distribution of stonin 2 paralleled that of the endocytotic clathrin adaptor complex AP-2 (ref. 10 and Fig. 6C), we analyzed whether the two proteins might interact with each other in brain. To this aim, we immunoprecipitated AP-2 from cytosolic or washed synaptosomal membrane fractions (P2′) and analyzed the immunoprecipitates for the presence of stonin 2. Significant amounts of stonin 2 coprecipitated with AP-2 from both soluble and membrane fractions. Quantitative analysis revealed that stonin 2 was preferentially associated with AP-2 in the cytoplasm (≈50–60% of the total stonin 2 in cytosol) and to a lesser extent (≈15% of the total stonin 2 in P2′) at the membrane (Fig. 3A). AP180, a monomeric endocytotic accessory protein was not detected in either immunoprecipitate under these conditions. To obtain further insights into the mechanism by which stonin 2 interacts with AP-2, we performed affinity chromatography by using GST-tagged full-length stonin 2 or its NT or CT domains. The NT domain harbors two NPF motifs that could mediate an indirect association with AP-2 by means of the EH domain-containing endocytotic adaptor protein Eps15 (9). The CT part is largely comprised of the μ-homology domain. Immunoblot analysis of the affinity-purified material revealed efficient interactions of full-length stonin 2 or its NT domain with AP-2, Eps15 (Fig. 3B), and intersectin (identified by matrix-assisted laser desorption ionization-MS analysis; data not shown), and a much weaker association of the CT μ-homology domain with AP-2 (10). Synaptotagmin I, which directly binds to both the CT μ-homology domain of stonin 2 (10) and to AP-2 (7, 8), was present in all stonin 2 affinity-purified samples (Fig. 3B). Quantitative immunoblot analysis revealed a striking preference of the GST-NT fusion protein for AP-2 over the related AP-1 or AP-3 adaptor complexes (Fig. 7A, which is published as supporting information on the PNAS web site). These combined data suggest that stonin 2 binds to AP-2 mainly through its NT domain.
Fig. 3.
The N-terminal domain of stonin 2 binds to AP-2 in vitro and in living cells. (A) Immunoblot analysis of anti-AP-2 or control (Ctrl) immunoprecipitates from cytosolic or membrane (P2′) fractions. SM, starting material (80 μg of protein). (B) Affinity purification using full-length (amino acids 1–898), NT (amino acids 1–555), or CT (amino acids 557–898) domains of stonin 2 fused to GST. Bound material was separated by SDS/PAGE and analyzed by immunoblotting against AP-2α, Eps15, synaptotagmin I, and clathrin light chains (CLC). (C) Internalization of Alexa 488-labeled transferrin into COS7 cells overexpressing HA-tagged stonin 2 (amino acids 1–898) or its NT (amino acids 1–557) or CT (amino acids 557–898) domains. (D) AP-2 distribution in COS7 cells overexpressing NT (amino acids 1–557) or CT (amino acids 557–898) domains of HA-tagged stonin 2. (Scale bar, 20 μm.)
To investigate the functional significance of the AP-2/stonin 2 interaction for clathrin-mediated endocytosis, we transfected COS7 cells, which do not express stonin 2 endogenously (data not shown), with full-length stonin 2, or its NT or CT domains. Twenty-four hours posttransfection, cells were assayed for their ability to internalize extracellularly added, fluorescently labeled transferrin. Cells overexpressing full-length stonin 2 (9) or its NT domain showed a strongly reduced ability to endocytose transferrin. By contrast, cells expressing the stonin 2 CT domain internalized transferrin normally (Fig. 2C).
To determine how overexpression of stonin 2 or its NT domain might affect clathrin-mediated transferrin uptake, we analyzed the subcellular distribution of AP-2 in transfected COS7 cells. In cells overexpressing full-length stonin 2 (data not shown) or its NT domain, AP-2 became mislocalized to the cytoplasm and was no longer detectable at plasmalemmal-coated pits (Fig. 3D). No formation of intracellular aggregates or patches was detectable. Similar results were seen for the peripheral pool of plasmalemma-associated clathrin (not shown). Expression of the CT domain had no obvious effects on the localization of AP-2 (Fig. 3D). Hence, stonin 2 overexpression may inhibit receptor-mediated endocytosis by sequestering AP-2 from plasmalemmal coated pits through interactions with its NT domain.
Stonin 2 Interacts with AP-2 in Vitro and in Living Cells Through WVxF Motifs. To characterize the precise molecular mechanism by which stonin 2 or its NT domain interacts with AP-2, we performed site-directed mutagenesis experiments. To our surprise, we found that the ability of overexpressed stonin 2 to inhibit transferrin internalization (Fig. 7B) or to interact with AP-2 in living cells (Fig. 7C) was not affected by mutational inactivation of its two NPF motifs that avidly bind to Eps15 (ref. 9 and Fig. 7C). We thus reasoned that the N-terminal domain of stonin 2 may contain NPF motif-independent AP-2-binding sites. We therefore performed affinity chromatography experiments from rat brain lysates by using various truncated forms of the protein fused to GST. Fragments comprising amino acids 1–555, 1–203, or 204–555 from stonin 2 (Fig. 8A, which is published as supporting information on the PNAS web site) or amino acids 1–200 or 1–33 from stonin 1, but not residues 16–200, were able to pull down AP-2 (Fig. 8B). These data suggested that stonins associate with AP-2 independent of EH domain-containing proteins such as Eps15 or intersectin.
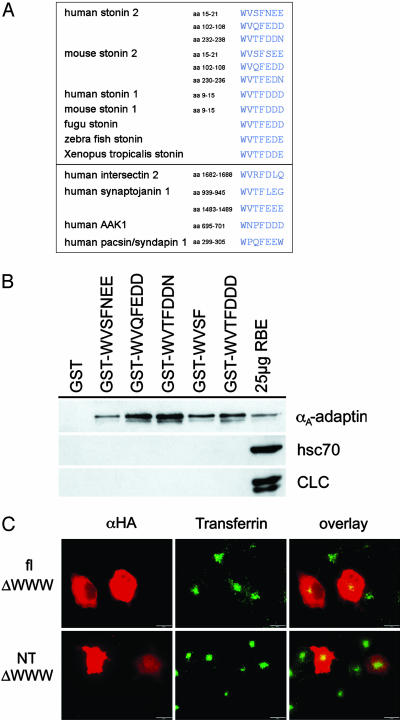
To identify potential AP-2-binding motifs within stonins, we performed multiple sequence alignments of the various AP-2-binding fragments described above. This analysis revealed the presence of a common motif with the consensus sequence WVxF, which in most cases, was followed by several acidic amino acids. This motif is present once in human or mouse stonin 1 and three times in the N-terminal domain of stonin 2. WVxF motifs were also identified in stonins from other species, and in the endocytotic proteins adaptor-associated kinase 1 (AAK1), pacsin/syndapin, and synaptojanin 1, a protein involved in vesicle uncoating (ref. 17 and Fig. 4A).
Fig. 4.
Stonin 2 interacts with AP-2 through WVxF motifs. (A) Putative AP-2-binding motifs found in stonins and other endocytotic proteins. (B) Stonin-derived peptides interact with AP-2. Material affinity-purified from rat brain by using GST-fused peptides encompassing putative AP-2-binding motifs (see A) was analyzed by immunoblotting for AP-2α, heat shock cognate protein 70, and clathrin (CLC). (C) Transferrin internalization in COS7 cells overexpressing ΔWWW mutant, HA-stonin 2, or its N-terminal domain. (Scale bar, 20 μm.)
Affinity chromatography experiments from rat brain using various GST-fused WVxF-containing peptides derived from stonins 1 or 2 indicated that all peptides were capable of binding to AP-2, and that a WVxF-tetrapeptide is sufficient for this interaction (Fig. 4B). Neither heat shock cognate protein 70 nor clathrin associated with any of these peptides. The interaction of the GST-fused peptides with AP-2 was apparently direct, because it could also be seen with AP-2 from clathrin coat protein fractions (data not shown). Moreover, AP-2 subunits represent the major proteins retained by GST-stonin 2 NT domain (Fig. 8A) or GST-WVxF affinity matrices (Fig. 9B, which is published as supporting information on the PNAS web site).
Alanine-scan mutagenesis of a WVTF tetrapeptide suggested that the conserved tryptophane and phenylalanine residues were both required for binding to AP-2, whereas the valine and threonine residues in the +1 and +2 positions were modulatory (Fig. 9A). A peptide in which the essential tryptophane and phenylalanine residues had been mutated to alanines displayed no detectable AP-2-binding activity under any condition tested. Consistent with this mutational analysis, the addition of increasing concentrations of a synthetic WVxF peptide, but not its inactive AVxA mutant, inhibited the association of AP-2 with the NT domain of stonin 2 fused to GST (Fig. 9B).
Mutational Inactivation of all Three WVxF Motifs Within Stonin 2 Abolishes Its Ability to Interact with AP-2 and to Inhibit Transferrin Internalization. To study the physiological importance of the WVxF motifs within stonin 2 for its ability to inhibit clathrin-mediated endocytosis of transferrin, we prepared a triple-point mutant of stonin 2 in which all three putative WVxF motifs had been mutationally inactivated (ΔWWW). This ΔWWW mutant had selectively lost the ability to interact with AP-2 in affinity chromatography experiments, yet was still capable of binding to Eps15 (Fig. 9C). Mutant forms of full-length stonin 2 or its NT domain expressed in COS7 cells had not only lost their ability to associate with AP-2 but also to inhibit transferrin internalization (Fig. 4C).
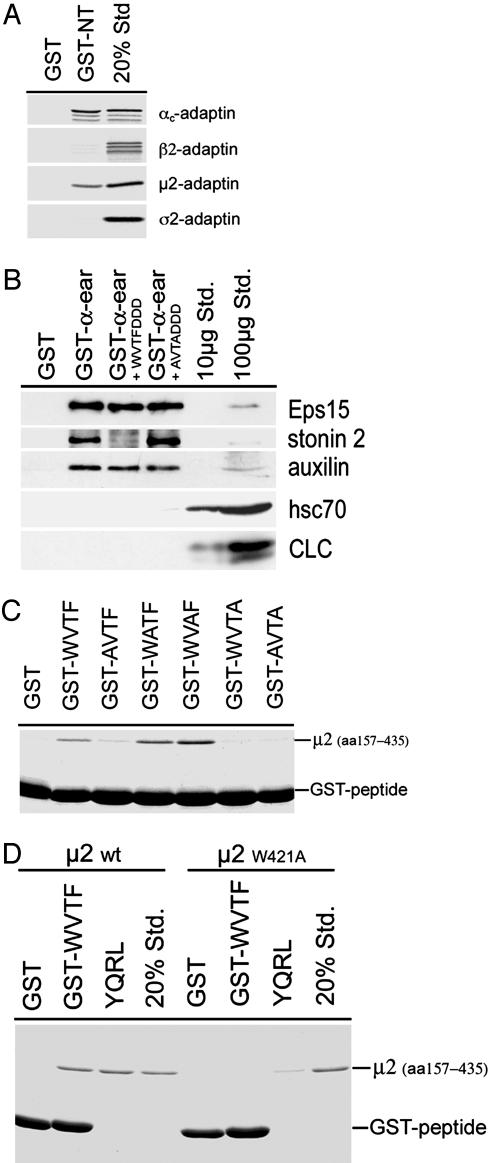
Stonin 2 Binds to AP-2 Through the Ear Domain of α-Adaptin and μ2. Finally, we wanted to determine the binding site(s) for WVxF motifs within the AP-2 heterotetramer. To this aim, 35S-radiolabeled individually translated subunits of AP-2 synthesized in vitro were incubated with GST or the GST-stonin 2 NT domain fusion protein. Samples were washed and bound proteins detected by autoradiography. To our surprise, both α- and μ2-adaptins were capable of interacting with the stonin 2 NT domain fusion protein but not with GST (Fig. 5A). The interaction with both subunits was specific because in vitro-translated α and recombinantly expressed, purified μ2 (amino acids 157–435) were able to bind to the GST-WVTF peptide but not to an inactive alanine mutant (GST-AVTA; Fig. 9D). As seen for other endocytotic proteins, the association of stonin 2 with AP-2α appeared to involve interactions with the α-ear domain. Stonin 2 was specifically retained on a GST-α-ear domain fusion protein and this interaction could be competed by the synthetic WVxF peptide but not its alanine mutant (Fig. 5B). Surprisingly, the addition of the WVxF peptide did not affect the ability of the α-ear to bind to Eps15 or auxilin, two accessory proteins that associate with AP-2 by means of DxF motifs (18). This finding suggests that WVxF motifs may use a surface within the α-ear domain distinct from the DxF-binding site.
Fig. 5.
Stonin 2 directly binds to the α-adaptin ear and to the tyrosine motif-binding domain of μ2. (A) 35S-labeled AP-2 subunits synthesized by coupled transcription/translation in vitro were incubated with GST-stonin 2NT or GST. Affinity-purified material was analyzed by SDS/PAGE and PhosphorImager analysis. (B) Affinity purification from rat brain by using the α-ear domain of AP-2 fused to GST in the presence or absence of the indicated peptides (500 μM). Samples were analyzed by immunoblotting for Eps15, stonin 2, auxilin, heat shock cognate protein 70, and CLCs. (C) Binding of purified μ2 (amino acids 157–435) to GST-WVxF or single-point mutants thereof. Samples were separated by SDS/PAGE and were analyzed by Coomassie blue staining. (D) Purified wild-type μ2 (amino acids 157–435) or a W421A single-point mutant was incubated with GST-WVSF peptide or GST alone. Binding of μ2 (amino acids 157–435) to an immobilized peptide harboring the tyrosine-based sorting signal of TGN38 (YQRL) was assayed as a control.
The WVxF motif bears similarity to tyrosine-based endocytosis signals found in a variety of plasma membrane receptors undergoing clathrin-mediated internalization. Consistent with this idea, mutational inactivation of the tryptophane or the large hydrophobic phenylalanine residues within this motif abolishes its ability to bind to μ2-adaptin (Fig. 5C). To test whether the WVxF peptide indeed associates with the tyrosine motif-binding site of μ2, we made use of the structural information available from x-ray crystallographic data (19). Mutation of a conserved tryptophane residue, W421 within μ2, diminishes the ability of the protein to recognize tyrosine-based endocytosis signals both in vitro and in living cells (20). Mutant μ2 (amino acids 157–435 harboring the tyrosine motif-binding site; ref. 19) W421A not only displayed a reduced ability to bind to an immobilized peptide comprising the tyrosine-based endocytosis motif of TGN38 but also to interact with the GST-fused WVxF peptide (Fig. 5D).
In summary, our data indicate that stonins interact with AP-2 through WVxF motifs that are capable of associating with the ear domain of α-adaptin and with the tyrosine motif-binding pocket of μ2.
Discussion
In this study, we have characterized mammalian stonin 2 and its interactions with synaptotagmin and the endocytotic adaptor complex AP-2. We provide evidence that stonin 2, like its Drosophila counterpart, is a brain-enriched protein most highly expressed in the hippocampus where it colocalizes with synaptotagmin I at axonal vesicle clusters. We also show that stonin 2 regulates clathrin-mediated endocytosis by associating with AP-2 through WVxF motifs. The WVxF tetrapetide sequence is also present in other endocytotic proteins including stonin 1, and synaptojanin 1, an inositol polyphosphate phosphatase implicated in clathrin coat removal (17, 21). Most surprisingly, we find that stonin 2 through its WVxF motifs directly associates with the α-adaptin ear domain and with μ2. WVxF binding to μ2 appears to involve the binding site for tyrosine-based endocytosis motifs, suggesting that stonin 2 might be capable of regulating the sorting function of AP-2.
Stonins thus join a growing number of endocytotic accessory proteins that regulate coat dynamics and/or cargo protein sorting (1–6). Whereas many of these endocytotic adaptor proteins (1, 6) have been shown to bind to phosphoinositides no lipid binding activity has been reported for stonins. Consistent with this idea, residues contributing to phosphatidylinositol (4, 5)-bisphosphate binding in μ2-adaptin (22, 23) are not conserved in the μ-homology domain of stonins (9, 10). Instead, this domain interacts with members of the synaptotagmin family (9, 10, 13, 14), perhaps serving as a means to recruit stonin 2 to sites of exoendocytotic vesicle cycling. Our data suggest that the μ-homology domain of stonin 2 plays an important role in targeting the protein to the plasma membrane and to axonal vesicle clusters, presumably by means of direct interaction with synaptotagmin (see Fig. 2). Interactions between stonin's μ-homology domain may also contribute to its proposed role in sorting SV cargo proteins during endocytosis at the synapse (discussed below).
Despite of the fact that the WVxF motif bears similarity to the previously identified DxF, DPW, or FxDxF sequences (1, 24), peptide competition experiments (see Fig. 5B) suggest that WVxF motifs may use a distinct interface for binding to the α-adaptin ear domain. This result could allow multiple peptide ligands to accommodate the α-adaptin ear domain simultaneously or to increase the avidity of the interaction. Moreover, WVxF motifs within stonin 2 in addition to associating with the α-ear domain of AP-2 can bind to the tyrosine-based sorting motif-binding site within μ2-adaptin. Interestingly, some membrane proteins internalized via the clathrin/AP-2 pathway such as the neonatal Fc receptor, a transcytotic plasma membrane protein, have been found to make use of a tryptophane-based endocytosis signal for internalization (25). It is therefore tempting to speculate that tryptophane-containing sorting signals could be decoded by mechanisms, similar to the recognition of WVxF motifs described here. The observed multiplicity of interactions between stonin 2 and AP-2 might also help to ensure conformational flexibility as coat proteins, in particular the AP-2 complex (23), have been proposed to undergo drastic conformational changes during coated pit assembly.
Our data further support the proposal that stonins, like their Drosophila homologue stoned B, are endocytotic proteins involved in clathrin-mediated endocytosis at synapses. Consistent with this idea, endogenous stonin 2 cofractionates with AP-2 in clathrin-coated vesicles and a major fraction of stonin 2 is complexed to AP-2 in the brain. Like some other endocytotic proteins including AP180, amphiphysin 1, and epsin 1 (1–6), stonin 2 is expressed predominantly in brain. We were also unable to detect the protein in a variety of nonneuronal cell lines (unpublished data), a fact that has prevented us from analyzing stonin 2 function further by short interfering RNA-mediated knockdown.
Given its enrichment in brain, one might speculate that stonin 2 could play a particularly important role in triggered endocytosis at synapses. In contrast to the constitutive internalization of plasmalemmal receptors seen in nonneuronal cells, the endocytotic recycling of SVs is a highly regulated compensatory event tightly coupled to the exocytotic insertion of SV proteins and lipids into the presynaptic membrane (26). By interacting with a variety of endocytotic proteins including AP-2, Eps15, and intersectin (refs. 9 and 10 and this study), stonin 2 could restrict endocytotic activity in time and space, or contribute to the specific retrieval of SV cargo proteins including synaptotagmin. This possibility is supported by genetic and biochemical data in Drosophila, which indicate that stoned B regulates SV recycling kinetics and the fidelity of SV protein sorting by interacting with synaptotagmin (12, 14). An intriguing possibility would be that stonin 2 by preventing recognition of constitutively internalized tyrosine motif-containing plasmalemmal receptors by AP-2μ may contribute to the specific endocytotic retrieval of SV proteins from the presynaptic plasmalemma. In agreement with this proposal, it has been observed that SV cargo proteins are segregated from the transferrin receptor during clathrin-mediated endocytosis in neuroendocrine cells but not in transfected fibroblasts (27). As soon as genetic models to study stonin 2 function in vivo will become available, these possibilities can be put to the test.
Supplementary Material
Acknowledgments
We thank Drs. Reinhard Jahn (Max Planck Insitute for Biophysical Chemistry, Göttingen, Germany), Pietro De Camilli (Yale University School of Medicine, New Haven, CT), and Peter Schu (Department of Biochemistry, University of Göttingen) for their kind gift of reagents. cDNA clones encoding mouse stonins 1 (GenBank accession no. AK014958) and 2 (GenBank accession no. AK036612) were obtained from the RIKEN Genomic Sciences Center (Yokohama City, Japan). This study was supported by Deutsche Forschungsgemeinschaft Grants SFB523 and SFB366 (to V.H.). N.J. and M.K.D. are recipients of a Lichtenberg Scholarship from the State of Niedersachsen, Germany.
Abbreviations: SV, synaptic vesicle; RT, room temperature; NT, N-terminal; CT, C-terminal; HA, hemagglutinin.
References
- 1.Slepnev, V. I. & De Camilli, P. (2000) Nat. Rev. Neurosci. 1, 161–172. [DOI] [PubMed] [Google Scholar]
- 2.Jarousse, N. & Kelly, R. B. (2001) Curr. Opin. Cell Biol. 13, 461–469. [DOI] [PubMed] [Google Scholar]
- 3.Cremona, O. & De Camilli, P. (2001) J. Cell Sci. 114, 1041–1052. [DOI] [PubMed] [Google Scholar]
- 4.Marsh, M. & McMahon, H. T. (1999) Science 285, 215–220. [DOI] [PubMed] [Google Scholar]
- 5.Robinson, M. S. & Bonifacino, J. S. (2001) Curr. Opin. Cell Biol. 13, 444–453. [DOI] [PubMed] [Google Scholar]
- 6.Conner, S. D. & Schmid, S. L. (2003) J. Cell Biol. 162, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, J. Z., Davletov, B. A., Sudhof, T. C. & Anderson, R. G. (1994) Cell 78, 751–760. [DOI] [PubMed] [Google Scholar]
- 8.Haucke, V. & De Camilli, P. (1999) Science 285, 1268–1271. [DOI] [PubMed] [Google Scholar]
- 9.Martina, J. A., Bonangelino, C. J., Aguilar, R. C. & Bonifacino, J. S. (2001) J. Cell Biol. 153, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walther, K., Krauss, M., Diril, M. K., Lemke, S., Ricotta, D., Honing, S., Kaiser, S. & Haucke, V. (2001) EMBO Rep. 2, 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stimson, D. T., Estes, P. S., Smith, M., Kelly, L. E. & Ramaswami, M. (1998) J. Neurosci. 18, 9638–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fergestad, T., Davis, W. S. & Broadie, K. (1999) J. Neurosci. 19, 5847–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fergestad, T. & Broadie, K. (2001) J. Neurosci. 21, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips, A. M., Smith, M., Ramaswami, M. & Kelly, L. E. (2000) J. Neurosci. 20, 8254–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maycox, P. R., Link, E., Reetz, A., Morris, S. A. & Jahn, R. (1992) J. Cell Biol. 118, 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takei, K., Haucke, V., Slepnev, V., Farsad, K., Salazar, M., Chen, H. & De Camilli, P. (1998) Cell 94, 131–141. [DOI] [PubMed] [Google Scholar]
- 17.Cremona, O., Di Paolo, G., Wenk, M. R., Luthi, A., Kim, W. T., Takei, K., Daniell, L., Nemoto, Y., Shears, S. B., Flavell, R. A., et al. (1999) Cell 99, 179–188. [DOI] [PubMed] [Google Scholar]
- 18.Brett, T. J., Traub, L. M. & Fremont, D. H. (2002) Structure (London) 10, 797–809. [DOI] [PubMed] [Google Scholar]
- 19.Owen, D. J. & Evans, P. R. (1998) Science 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nesterov, A., Carter, R. E., Sorkina, T., Gill, G. N. & Sorkin, A. (1999) EMBO J. 18, 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris, T. W., Hartwieg, E., Horvitz, H. R. & Jorgensen, E. M. (2000) J. Cell Biol. 150, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohde, G., Wenzel, D. & Haucke, V. (2002) J. Cell Biol. 158, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins, B. M., McCoy, A. J., Kent, H. M., Evans, P. R. & Owen, D. J. (2002) Cell 109, 523–535. [DOI] [PubMed] [Google Scholar]
- 24.Owen, D. J., Vallis, Y., Noble, M. E., Hunter, J. B., Dafforn, T. R., Evans, P. R. & McMahon, H. T. (1999) Cell 97, 805–815. [DOI] [PubMed] [Google Scholar]
- 25.Wu, Z. & Simister, N. E. (2001) J. Biol. Chem. 276, 5240–5247. [DOI] [PubMed] [Google Scholar]
- 26.Gad, H., Low, P., Zotova, E., Brodin, L. & Shupliakov, O. (1998) Neuron 21, 607–616. [DOI] [PubMed] [Google Scholar]
- 27.Linstedt, A. D. & Kelly, R. B. (1991) Neuron 7, 309–317. [DOI] [PubMed] [Google Scholar]
- 28.Ritter, B., Philie, J., Girard, M., Tung, E. C., Blondeau, F. & McPherson, P. S. (2003) EMBO Rep. 4, 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jha, A., Agostinelli, N. R., Mishra, S. K., Keyel, P. A., Hawryluk, M. J. & Traub, L. M. (October 17, 2003) J. Biol. Chem. 10.1074/jbc.M305644200.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.