Abstract
An asteroid impact at the end of the Cretaceous caused mass extinction, but extinction mechanisms are not well-understood. The collapse of sea surface to sea floor carbon isotope gradients has been interpreted as reflecting a global collapse of primary productivity (Strangelove Ocean) or export productivity (Living Ocean), which caused mass extinction higher in the marine food chain. Phytoplankton-dependent benthic foraminifera on the deep-sea floor, however, did not suffer significant extinction, suggesting that export productivity persisted at a level sufficient to support their populations. We compare benthic foraminiferal records with benthic and bulk stable carbon isotope records from the Pacific, Southeast Atlantic, and Southern Oceans. We conclude that end-Cretaceous decrease in export productivity was moderate, regional, and insufficient to explain marine mass extinction. A transient episode of surface ocean acidification may have been the main cause of extinction of calcifying plankton and ammonites, and recovery of productivity may have been as fast in the oceans as on land.
Keywords: carbon cycle, Cretaceous/Paleogene boundary, pelagic ecosystems, cysts, inhibition of photosynthesis
At the Cretaceous/Paleogene (K/Pg) boundary (∼65.5 Ma) a large asteroid impacted the Yucatan Peninsula (Mexico), triggering severe but selective extinctions (1). Proposed causes of mass extinction resulting from the impact at different timescales include global darkness due to emission of dust and aerosols, ozone destruction, global cooling or warming, and ocean acidification (1–3). Light levels sufficiently low to prevent photosynthesis for longer than the life cycle of oceanic phytoplankton (weeks to months), have commonly been seen as the prime cause of collapse of oceanic primary productivity and the subsequent mass extinction at higher levels of the marine food chain (e.g., ammonites, large predatory fish, mosasaurs) (2). A collapse in oceanic surface-bottom gradient in carbon isotope values (i.e., the difference in carbon isotope values in shells of benthic and planktic organisms) persisted for hundreds of thousands to a few million years (4–6), and has been interpreted as reflecting global collapse of primary productivity (Strangelove Ocean) (4) or export productivity (Living Ocean) (5, 6). Recovery of oceanic productivity was argued to have been much slower in the oceans than on land (7). Neither deep-sea benthic foraminifera nor deep-sea benthic ostracodes, however, suffered significant extinction (8–11), although these depend upon phytoplankton for their food (12) and should have suffered severe extinction if their food supply had been cut off for 105 to 106 years (13).
There is considerable evidence that there was no long-term, global collapse of primary productivity (5). Extinction in calcareous nannoplankton was severe, although geographically variable (14) and followed by low-diversity blooms. Extinction in other photosynthesizers, such as the related noncalcifying haptophytes (15), which may have been dominant photosynthesizers (16), the siliceous diatoms (17, 18), and the organic-walled and calcareous dinoflagellates (19, 20) was much less severe (21). Algal biomarkers indicate a rapid recovery of primary productivity (22). At least regionally, dinoflagellates (19, 20) and heterotroph and mixotroph plankton such as planktic foraminifera (23) and radiolarians (17) flourished after the K/Pg extinction, and benthic foraminifera indicated a high food flux (9) (Fig. 1). Postextinction planktic foraminiferal and nannoplankton assemblages indicate eutrophic conditions, with oligotrophic assemblages evolving later (24).
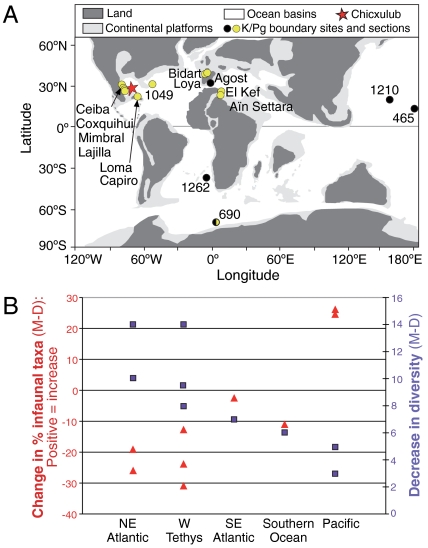
Fig. 1.
Location map and changes in benthic foraminiferal diversity and infaunal morphogroups across the K/Pg boundary. Location of sections and drill sites discussed in the text. (A) Yellow circles indicate a decrease in food flux to the sea floor as estimated from benthic foraminiferal evidence, black circles an increase, and half black circles indicate no significant change. See SI Materials and Methods for construction of map. (B) Blue squares show the decrease in diversity from uppermost Maastrichtian (Cretaceous) to lowermost Danian (Paleogene) for locations shown in A, red triangles show the change in the percentage infaunal taxa, a productivity indicator; data sources are given in SI Materials and Methods.
According to ecological theory, one would expect productivity in terms of biomass (though not biodiversity) to recover as soon as environmentally possible after the asteroid impact, probably with large opportunistic blooms reflecting nutrient availability and environmental instability (5). The collapse in vertical δ13C gradient has been argued to represent only a slight increase (from 90 to 95%) in the fraction of total organic production remineralized in the upper 200 m of the oceans (5), but others invoked catastrophic decline of the organic flux to the sea floor (6). A regionally variable, moderate decrease in export productivity agrees with deep-sea benthic foraminiferal evidence (9, 25) (Fig. 1) and geochemical export productivity proxies (26), but a global collapse of export productivity for several millions of years (4) is in strong disagreement with foraminiferal and geochemical evidence.
We attempt to reconcile records of benthic foraminiferal assemblage change with bulk carbon isotope records (reflecting calcification by calcareous nannoplankton in the upper few hundred meters of the ocean, e.g., ref. 27) and bottom (benthic foraminiferal) records obtained on the same samples from four sites (Fig. 1).
Results and Discussion
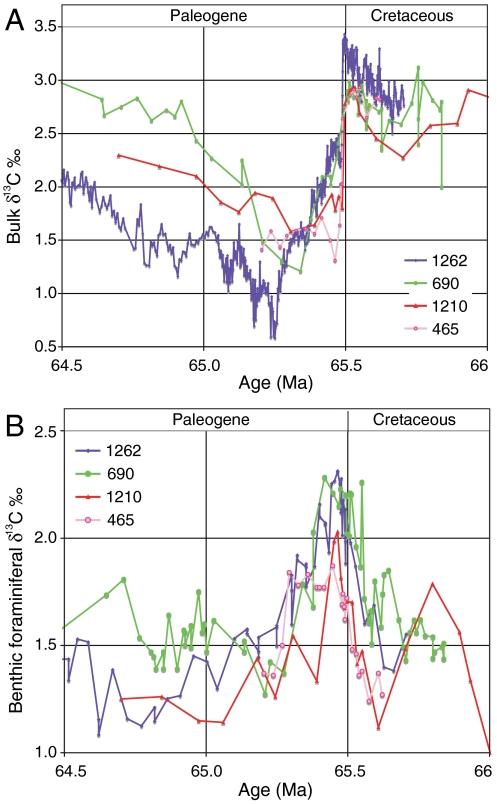
Bulk δ13C values increased in the latest 200 kyr of the Cretaceous (Fig. 2), reaching a maximum just before the K/Pg boundary (65.5 Ma). All sites show a sharp decrease in bulk carbonate δ13C values at the boundary as reported earlier (1, 3–7), but the pattern of change varies geographically. Across the K/Pg boundary, benthic foraminiferal δ13C values show an increase of about 0.8‰ at all sites, with Southeast Atlantic and Southern Ocean values reaching maximum values (∼2.25‰), higher than Pacific samples. The gradient between benthic and planktic values collapsed, at some locations even reversed (Fig. 3, Fig. 4).
Fig. 2.
Bulk (A) and benthic foraminiferal (B) carbon isotope records across the K/Pg boundary at sites in the Pacific, Southeast Atlantic, and Southern Ocean. See Fig. 1 for location.
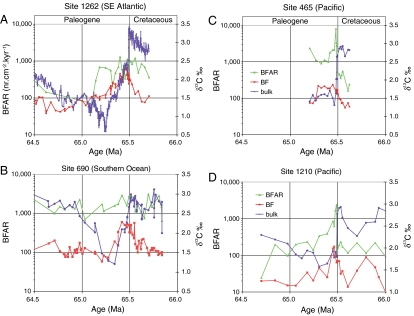
Fig. 3.
BFAR (nr·cm-2·kyr-1), indicative of food supply to the sea floor, as compared to benthic (BF) and bulk carbon isotope records for sites in the Southeast Atlantic (A), Southern Ocean (B), and Pacific Ocean (C and D). See Fig. 1 for location.
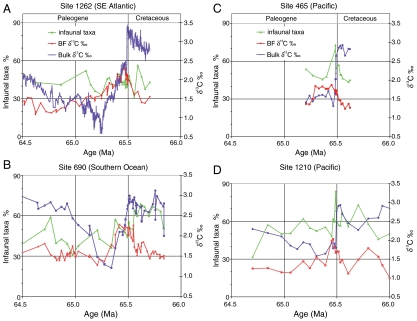
Fig. 4.
Relative abundance of infaunal benthic foraminifera, indicative of food supply to the sea floor, as compared to benthic (BF) and bulk carbon isotope records for sites in the Southeast Atlantic (A), Southern Ocean (B), and Pacific Ocean (C and D). See Fig. 1 for location.
The benthic faunal records also show geographically different patterns (Table S1, Table S2), with Pacific sites characterized by a sharp peak in benthic foraminiferal accumulation rates (BFARs) (Fig. 3) and high percentages of infaunal taxa in the earliest Paleocene (Fig. 4). Southern Ocean site 690 shows relatively little difference in BFAR, with overall declining values of infaunal taxa. Southeast Atlantic site 1262 shows declining values in both proxies, with a further decline about 400 kyr after the boundary (Fig. 3, Fig. 4). In general, the benthic foraminiferal patterns agree with biogenic Ba records (26). There is thus disagreement between benthic foraminiferal and geochemical proxies and stable isotope records from the same samples: Where the vertical carbon isotope gradient collapsed (suggesting decreased export productivity), benthic foraminifera and biogenic Ba records either show little change (Southeast Atlantic, Southern Ocean), or an increase (Pacific).
It has been argued that benthic foraminifera survived because they are adapted to food-starved environments (4). We argue that it is precisely because the deep-sea is food starved that food supply is the most important ecologically limiting variable (12), and a long-term global collapse of the food supply would have caused massive extinction. We argue that the collapsed carbon isotope gradient (∼500 kyr, Fig. 2) does not reflect a severe global collapse in export productivity. Extinction of fecal pellet producing zooplankton does not have to lead to a collapse in export productivity: Flocs of organic matter (marine snow) are transported to the sea floor more efficiently in the absence of zooplankton (28), because zooplankton’s activity disaggregates these particles.
The bulk carbon isotope records probably represent complex, multiple signals (29). The rapid and sharp decrease coincident with the K/Pg boundary may well represent a moderate decrease in export productivity (5). Another part of the decrease reflects the extinction of the carriers of the isotopic record, calcareous nannoplankton, rather than a change in δ13C of dissolved inorganic carbon (DIC) in the oceans (29). Benthic δ13C records are based on the same species throughout, but the bulk records before and after extinction are measured on different components because of the mass extinction of pelagic calcifiers (Fig. 2) (1, 5, 6, 14). Postextinction values in part show the isotopically light signature of calcareous dinocysts (Table S3), similar to the isotopic signature of the living Thoracosphaera heimi (30). Small biserial and triserial planktic foraminifera are abundant after the boundary and their fragments contribute to the bulk signal, and generally have a light isotopic signature, as does the living triserial planktic Gallitellia vivans (31). Planktic foraminiferal δ13C values declined because smaller forms are generally isotopically lighter because of metabolic effects, and postextinction, nonsymbiont bearing species were isotopically lighter than preextinction symbiont bearers (32). In addition, the effect of the solubility pump on δ13C of DIC, working in the opposite way as the biological pump, became more pronounced during decreased export productivity (33). Finally, the long-term (several 105 years) bulk carbon isotope record is influenced by orbital scale variability (34), in part linked to hyperthermal events with associated carbon isotope excursions, one of which might have occurred about 250 kyr after the K/Pg boundary (35), coeval with the minimum bulk value at site 1262 (Fig. 2). A discussion of hyperthermal events and variability in long-term δ13C records is outside the range of this paper, but we conclude that the bulk carbon isotopic record cannot be explained by the effect of the K/Pg extinction on vertical gradients in DIC only.
What caused the marine mass extinction if we are correct in our argument that severe collapse in productivity (in terms of biomass) did not occur? Noncalcifying phytoplankton (dinoflagellates, diatoms, haptophytes) did not suffer severe faunal turnover, suggesting that darkness may have contributed, but may not have been the most important cause of extinction, in agreement with arguments that not sufficient fine dust was generated by the impact to cause prolonged, severe darkness (36). Patterns of nannoplankton extinction as well as changes in the benthic foraminiferal assemblages confirm that phytoplankton extinction may have been less severe at high southern latitudes (14, 24) (Figs. 1, 3, and 4), possibly because the impact occurred during southern hemisphere winter when photosynthesizers would have been hibernating because of seasonal darkness. Changes in overall composition of oceanic phytoplankton, due in part to the extinction of calcareous nannoplankton and replacement by other photosynthesizers, could have severely affected higher levels of the food chain, even if primary productivity was high (37).
We speculate that rapid ocean acidification could have been a major causal factor (2, 3). Acidification probably was not caused by high atmospheric CO2 levels (38), but nitric acid may have been generated by N2-oxidation due to heating of the atmosphere by the impactor (with N-deposition contributing to nutrients for blooms), and sulfuric acid by an impact on gypsum-containing sediment (1, 3). Fragile, thin-walled foraminifera in the clay interval deposited during about 10,000 y following the extinction are preserved in pristine condition, but this observation does not preclude a transient (months to years) event of severe acidification of surface waters. A severe but short, rapidly buffered period of acidification of surface waters may well explain the extreme severity of extinction of short-lived, open-ocean unicellular calcifiers (1–6, 14), and possibly Caribbean rudists (39). There is insufficient evidence that the extinction of planktic foraminifera and calcareous nannoplankton was caused by their lack of resting cysts: non-cyst-forming groups such as radiolarians (17) and noncalcifying haptophytes (15) survived as well. Calcifying dinoflagellates survived and bloomed after the extinction (19, 20), but their modern relatives have noncalcifying life stages. Hermatypic corals did not suffer extreme extinction (40), but these organisms could have survived short-term, ocean acidification (41), which was rapidly buffered in shallow waters.
Transient severe acidification could have been a factor in the differential extinction of ammonites and nautiloids. Both groups form aragonitic shells, but the planktivorous ammonites lived within the uppermost few hundred meters of the oceans, whereas nautiloids live deeper and form large lecitrophic eggs in protective egg capsules (42), in contrast to the small floating egg masses of ammonites. Nautiloids would therefore have been less affected by acidification. Extinction of such an important group of large invertebrates may have reverberated through the food chain, specifically affecting top-level predators such as large, active fish (43) and mosasaurs. Removal of the top-predators in its turn may have had cascading effects on the lower levels of the food chain (44). Calcifying organisms in coastal waters, from which living planktic foraminifera and nannoplankton evolved after the extinction, may have been protected from the short-term surface water acidification if the coastal regions became eutrophic (45), because of destruction of flora and resulting increase in nutrient-rich runoff.
Finally, rapid acidification could have been a cause of decreased export productivity in some regions because of its effects on the pelagic calcifiers, leading to increased remineralization (5), and thus to low oxygen conditions in eutrophic regions (46), as, e.g., observed in the Danish K/Pg sections (3, 19, 22).
The rapid onset of the acidification event due to an impact (more rapid than even anthropogenic acidification) may have led to a transient, severe acidification of the surface ocean followed by rapid buffering, but leading to the massive extinction of the short-lived pelagic calcifyers while providing for the survival of deep-sea benthos including ostracodes (10). This pattern of extinction contrasts with the much slower acidification at the end of the Paleocene, when deep-sea benthos suffered severe extinction but calcifying plankton survived (47).
We conclude that it was not a collapse of primary or export productivity that caused the marine mass extinction after the asteroid impact at the K/Pg boundary. The oceans may have been relatively eutrophic, supporting plankton blooms while oceanic productivity in terms of biomass, but not in terms of diversity, recovered rapidly, as proposed for terrestrial productivity (7, 48). Mass extinction in the oceans may have been caused mainly by a transient period of extreme acidification of the surface oceans. Study of the effects of the end-Cretaceous extinction thus may assist in evaluation of the effects of extreme and rapid acidification, having more severe effects on calcifying plankton in the surface waters and less on deep-sea benthos than slower acidification.
Materials and Methods
Samples were provided by the integrated Ocean Drilling Program. Sediments were dried, then soaked in warm water with detergent, and wet-sieved over a 63-μm sieve. Benthic foraminifera were picked from the >63-μm size fraction, with taxonomy and assignment to infaunal and epifaunal groups and calculation of BFAR according to refs. 9, 12, and 25 (SI Materials and Methods, Table S1, Table S2). We use the percentage of infaunal taxa and the BFAR as independent proxies for food flux to the sea floor (12, 25) (SI Materials and Methods). Isotope analyses were performed at the University of Santa Cruz, Yale University, and the University of Michigan, and age models were developed following ref. 34 (SI Materials and Methods, Table S3).
Supplementary Material
Acknowledgments.
We are grateful to three anonymous reviewers for valuable comments. Funding from the Spanish Ministry of Science and Innovation Grant CGL2007-63724/BTE and CGL2009-07101-E (to L.A.), National Science Foundation Grant OCE-720049 (to E.T.). Samples were provided by the Integrated Ocean Drilling Program.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110601109/-/DCSupplemental.
References
- 1.Schulte P, et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science. 2010;327:1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling W, Claeys PA. In: Geological and Biological Effects of Impact Events. Buffetaut E, Koeberl C, editors. Berlin: Springer; 2001. pp. 33–140. [Google Scholar]
- 3.Kring DA. The Chicxulub impact event and its environmental consequences at the Cretaceous-Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;255:4–21. [Google Scholar]
- 4.Hsü KJ, McKenzie J. Broecker WS, Sundquist ET, editors. (AGU Geophysical Monographs).The Carbon Cycle and Atmospheric CO2: Natural Variations, Archean to Present. 1985;Vol 32:487–492. [Google Scholar]
- 5.D’Hondt S, Donaghay P, Zachos JC, Luttenberg D, Lindinger M. Organic carbon fluxes and ecological recovery from the Cretaceous-Tertiary mass extinction. Science. 1998;282:276–279. doi: 10.1126/science.282.5387.276. [DOI] [PubMed] [Google Scholar]
- 6.Coxall HK, d’Hondt S, Zachos JC. Pelagic evolution and environmental recovery after the Cretaceous-Paleogene mass extinction. Geology. 2006;34:297–300. [Google Scholar]
- 7.Beerling DJ, et al. Evidence for the recovery of terrestrial ecosystems ahead of marine primary production following a biotic crisis at the Cretaceous-Tertiary boundary. J Geol Soc London. 2001;158:737–740. [Google Scholar]
- 8.Culver SJ. Benthic foraminifera across the Cretaceous/Tertiary (K/T) boundary: A review. Mar Micropaleontol. 2003;47:177–226. [Google Scholar]
- 9.Alegret L, Thomas E. Food supply to the seafloor in the Pacific Ocean after the Cretaceous/Paleogene boundary event. Mar Micropaleontol. 2009;73:105–116. [Google Scholar]
- 10.Boomer I. Late Cretaceous and Cenozoic bathyal ostracoda from the Central Pacific (DSDP site 463) Mar Micropaleontol. 1999;37:131–147. [Google Scholar]
- 11.Elewa AMT. Paleobiography of Maastrichtian to early Eocene Ostracoda of North and West Africa and the Middle East. Micropaleontology. 2002;48:391–398. [Google Scholar]
- 12.Jorissen FJ, Fontanier C, Thomas E. In: Proxies in Late Cenozoic Paleoceanography. Hillaire-Marcel C, de Vernal A, editors. Amsterdam: Elsevier; 2007. pp. 263–326. [Google Scholar]
- 13.Levinton JS. Trophic groups and the end-Cretaceous extinction: Did deposit feeders have it made in the shade? Paleobiology. 1996;22:104–112. [Google Scholar]
- 14.Jiang S, Bralower TJ, Patzkowsky ME, Kump LR, Schueth JD. Geographic controls on nannoplankton extinction across the Cretaceous/Paleogene boundary. Nat Geosci. 2010;3:280–285. [Google Scholar]
- 15.Medlin LK, Daez AG, Young JR. A molecular clock for coccolithophores and implications for selectivity of phytoplankton extinctions across the K/T boundary. Mar Micropaleontol. 2008;67:69–86. [Google Scholar]
- 16.Lou H, et al. Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. Proc Natl Acad Sci USA. 2009;106:12803–12808. doi: 10.1073/pnas.0905841106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollis CJ, Strong CP, Rodgers KA, Rogers KM. Paleoenvironmental changes across the Cretaceous/Tertiary boundary at Flaxbourne River and Woodside Creek, eastern Marlborough, New Zealand. NZ J Geol Geophys. 2003;46:177–197. [Google Scholar]
- 18.Harwood DM. Upper Cretaceous and lower Paleocene diatom and silicoflagellate biostratigraphy of Seymour Island, eastern Antarctic Peninsula. Geol Soc Am Mem. 1988;169:55–129. [Google Scholar]
- 19.Brinkhuis H, Bujak JP, Smit J, Versteegh GJM, Visscher H. Dinoflagellate-based sea surface temperature reconstructions across the Cretaceous-Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 1998;141:67–83. [Google Scholar]
- 20.Hildebrand-Habel T, Streng M. Calcareous dinoflagellate associations and Maastrichtian-Tertiary climatic changes in a high latitude core (ODP Hole 689B, Maud Rise, Weddell Sea) Palaeogeogr Palaeoclimatol Palaeoecol. 2003;197:293–321. [Google Scholar]
- 21.Ribeiro T, et al. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat Commun. 2011;2:311. doi: 10.1038/ncomms1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sepulveda J, Wendler JE, Summons RE, Hinrichs KU. Rapid resurgence of marine productivity after the Cretaceous-Paleogene mass extinction. Science. 2010;326:129–132. doi: 10.1126/science.1176233. [DOI] [PubMed] [Google Scholar]
- 23.Coccioni R, Luciani V. In: Biological Processes Associated with Impact Events. Cockell C, Koeberl C, Gilmour I, editors. Berlin: Springer; 2006. pp. 79–196. [Google Scholar]
- 24.Fuqua LM, Bralower TJ, Arthur MA, Patzkowsky ME. Evolution of calcareous nannoplankton and the recovery of marine food webs after the Cretaceous-Tertiary mass extinction. Palaios. 2008;23:185–194. [Google Scholar]
- 25.Alegret L, Thomas E. Paleoenvironments across the Cretaceous/Tertiary boundary in the central North Pacific (DSDP Site 465), the Gulf of Mexico and the Tethys: The benthic foraminiferal record. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;224:53–82. [Google Scholar]
- 26.Hull PM, Norris RD. Diverse patterns of ocean export productivity change across the Cretaceous-Paleogene boundary: New insights from biogenic barium. Paleoceanography. 2011;26:PA3205. [Google Scholar]
- 27.Okada H, McIntyre A. Seasonal distribution of modern coccolithophores in the Western North Atlantic Ocean. Mar Biol. 1979;54:319–328. [Google Scholar]
- 28.Turner JT. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol. 2002;27:57–102. [Google Scholar]
- 29.Minoletti F, de Rafaelis M, Renard M, Gardin S, Young J. Changes in the pelagic fine fraction carbonate sedimentation during the Cretaceous-Paleocene transition: Contribution of the separation technique to the study of the Bidart section. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;216:119–137. [Google Scholar]
- 30.Zonneveld K. Potential use of stable oxygen isotope composition of Thoracosphaera heimii (Dinophyceae) for upper watercolumn (thermocline) temperature reconstruction. Mar Micropaleontol. 2004;50:307–317. [Google Scholar]
- 31.Kimoto K, Ishimura T, Tsunogai T, Ujie Y. The living triserial planktic foraminifer Gallitellia vivans (Cushman): Distribution, stable isotopes, and paleoecological implications. Mar Micropaleontol. 2009;71:71–79. [Google Scholar]
- 32.Birch H, Coxall HK, Pearson PN. Evolutionary ecology of early Paleocene planktonic foraminifera: Size, depth habitat and symbiosis. Paleobiology. in press.
- 33.Cameron DR, et al. A factorial analysis of the marine carbon cycle and ocean circulation controls on atmospheric CO2. Global Biogeochem Cycles. 2005;19:GB4027. [Google Scholar]
- 34.Westerhold T, et al. Astronomical calibration of Paleocene time. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;257:377–403. [Google Scholar]
- 35.Quillevere F, Norris RD, Kroon D, Wilson PA. Transient ocean warming and shifts in carbon reservoirs during the early Danian. Earth Planet Sci Lett. 2008;265:600–615. [Google Scholar]
- 36.Pope KO. Impact dust not the cause of the Cretaceous-Tertiary mass extinction. Geology. 2002;30:99–102. [Google Scholar]
- 37.Finkel Z, et al. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J Plankton Res. 2010;32:119–137. [Google Scholar]
- 38.Yancey TE, Guillemette RN. Carbonate accretionary lapilli in distal deposits of the Chicxulub impact event. Geol Soc Am Bull. 2008;120:1105–1118. [Google Scholar]
- 39.Steuber T, Mitchell SF, Buhl D, Gunter G, Kasper HU. Catastrophic extinction of Caribbean rudist bivalves at the Cretaceous-Tertiary Boundary. Geology. 2002;30:999–1002. [Google Scholar]
- 40.Kiessling W, Simpson C. On the potential for ocean acidification to be a general cause of ancient reef crises. Glob Change Biol. 2011;17:56–67. [Google Scholar]
- 41.Fine M, Tchernov D. Scleractinian coral species survive and recover from decalcification. Science. 2007;315:1811. doi: 10.1126/science.1137094. [DOI] [PubMed] [Google Scholar]
- 42.Landman NH, Tanabe K, Davis RA, editors. Ammonoid Paleobiology. New York: Plenum; 1996. p. 830. [Google Scholar]
- 43.Friedman M. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc R Soc B. 2011;277:1675–1683. doi: 10.1098/rspb.2009.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estes JV, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 45.Borges AV, Gypens N. Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnol Oceanogr. 2010;55:346–353. [Google Scholar]
- 46.Hofmann M, Schellnhuber H-J. Oceanic acidification affects marine carbon pump and triggers extended marine oxygen holes. Proc Natl Acad Sci USA. 2009;106:3017–3022. doi: 10.1073/pnas.0813384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas E. Cenozoic mass extinctions in the deep sea; what disturbs the largest habitat on Earth? Geol Soc Am Spec Pap. 2007;424:1–24. [Google Scholar]
- 48.Lomax B, Beerling D, Upchurch G, Jr, Otto-Bliesner B. Rapid (10 y) recovery of terrestrial productivity in a simulation study of the terminal Cretaceous impact event. Earth Planet Sci Lett. 2001;192:137–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.