Abstract
Candida albicans is a major fungal pathogen in humans. In C. albicans, secreted aspartyl protease 2 (Sap2) is the most highly expressed secreted aspartic protease in vitro and is a virulence factor. Recent research links the small GTPase Rhb1 to C. albicans target of rapamycin (TOR) signaling in response to nitrogen availability. The results of this study show that Rhb1 is related to cell growth through the control of SAP2 expression when protein is the major nitrogen source. This process involves various components of the TOR signaling pathway, including Tor1 kinase and its downstream effectors. TOR signaling not only controls SAP2 transcription but also affects Sap2 protein levels, possibly through general amino acid control. DNA microarray analysis identifies other target genes downstream of Rhb1 in addition to SAP2. These findings provide new insight into nutrients, Rhb1-TOR signaling, and expression of C. albicans virulence factor.
INTRODUCTION
Candida albicans is a major opportunistic fungal pathogen in humans, and it inhabits the skin and mucosal surfaces of healthy people (12). In immunocompromised patients such as those undergoing organ transplantation or chemotherapy or those with AIDS, C. albicans is responsible for a number of life-threatening infections with considerable morbidity and mortality (54). Several virulence factors contribute to C. albicans infection, including the expression of adhesins, yeast-to-hypha morphogenesis, phenotypic switching, and secreted hydrolytic activities (13).
Secreted aspartyl proteases (Saps) encoded by 10 members of the SAP gene family, SAP1 to SAP10 (50), are among the hydrolytic factors of C. albicans virulence. These genes are differentially expressed during various stages of C. albicans-host interaction, and each Sap protein possesses unique enzymatic characteristics and substrate specificities (50). Sap2 is the most highly expressed secreted protease in vitro and is capable of digesting human albumin, hemoglobin, keratin, and secreted immunoglobulin A (33). Sap2 is believed to allow C. albicans to destroy host barriers through the degradation of human proteins, followed by deep penetration into tissues or the bloodstream. In addition, Sap2 digests extracellular proteins into oligopeptides that can be taken up by oligopeptide transporters encoded by the OPT gene family (59). Therefore, Sap2 may also be critical for cell growth in the human host and may enable the use of host proteins as a nitrogen source. A C. albicans strain that lacks SAP2 loses its virulence in a murine model of infection (34). However, using URA3 as a marker during the construction of C. albicans mutants can make it difficult to interpret the resulting phenotypes (4, 11, 16, 37, 65). When URA3 assessment is eliminated from strain construction, Sap1 to Sap6 do not seem to be required for C. albicans invasion into reconstituted human epithelia (39). Sap1 to -6 played limited roles in C. albicans virulence and the host immune response in a murine model of disseminated infection (17). These studies showed that the roles of Sap2 and other Sap proteins in vivo still need to be clarified.
In fungi, the target of rapamycin (TOR) signaling pathway plays a key role in controlling different cellular processes in response to multiple environmental cues, including nutrient availability and stress (55, 61). The central component of this pathway is Tor, a serine/threonine protein kinase. Saccharomyces cerevisiae contains 2 TOR genes encoding 2 closely related kinases, Tor1 and Tor2. In association with a subset of proteins, including Kog1, Lst8, and Tco89, Tor1 or Tor2 forms TOR complex 1 (TORC1) (15, 41, 58, 79). Tor2 can also associate with another subset of proteins (e.g., Avo1, Avo2, Lst8, and Bit2) to form TOR complex 2 (TORC2) (24, 41, 58, 79). Although TORC1 and TORC2 share some functions, each complex controls distinct cell processes in response to environmental signals (41, 61).
In S. cerevisiae, TORC1 is rapamycin sensitive (61). Genetic analysis reveals that TORC1 functionally interacts with genes involved in actin polarization and membrane trafficking (2). TORC1 is also involved in nitrogen catabolite repression (NCR) through its regulation of 2 GATA-type transcriptional factors, Gat1 and Gln3 (7, 9, 18, 27, 35, 45, 46, 61, 62). In the presence of good nitrogen sources, Gln3 and Gat1 are restricted to the cytosol, leading to inactivation of the NCR genes. Similar expression patterns of NCR genes during nitrogen deprivation also appear in cells treated with rapamycin (14). The TORC2 protein complex regulates the polarization of the actin cytoskeleton in a rapamycin-insensitive manner (41). TORC2 is also involved in controlling cell wall integrity and receptor endocytosis (23). In the fission yeast Schizosaccharomyces pombe, loss of Tor2 function activates genes that are also induced during nitrogen starvation (49). Only 1 TOR gene, TOR1, has been identified in C. albicans (20). A recent study suggests that the C. albicans Tor1 kinase is involved in cell-cell adhesion and biofilm formation in nutrient-poor Spider medium but not under other tested conditions (6). Cell adhesion and biofilm formation are mediated by controlling the expression of the adhesin genes ALS1, ALS3, and HWP1 through Tor1 (6). In C. albicans, Gln3 and Gat1 are also regulate nitrogen metabolism and may function downstream of Tor1 kinase (40).
Recent research links the small GTPase Rhb1 to TOR signaling in C. albicans (72). Rhb1 was first identified in mammals and functions as a positive regulator of mammalian Tor (mTOR) kinases (42). In humans, GTP binding activates the homolog of C. albicans Rhb1 (named Rheb), and Rheb-GTP in turn stimulates the kinase activity of mammalian TORC1 (mTORC1) to trigger downstream signaling cascades (42). In fission yeast, rhb1-null mutants show arrested cell growth and division with a terminal phenotype similar to that caused by nitrogen starvation (44). Rhb1 depletion induces the expression of fnx1+ and mei2+, which are normally induced by nitrogen starvation (44). In C. albicans, Rhb1-TOR is involved in nitrogen starvation-induced morphogenesis, likely by controlling the expression of MEP2 (72). Mep2 is a permease and ammonium sensor (10, 21). The expression of MEP2 is upregulated in nitrogen-limited conditions and is controlled by C. albicans Gln3 and Gat1 (21). In addition, gln3 and gat1 deletion mutants exhibit reduced sensitivity to rapamycin (40), suggesting that these transcriptional regulators may also be involved in TOR signaling in C. albicans. In the presence of protein and low nitrogen availability, Gln3 and Gat1 control the expression of SAP2 by regulating the Stp1 transcription factor (22). These studies suggested that Rhb1 and Tor1 kinase may also be related to SAP2 expression.
This study investigated the roles of Rhb1 and TOR signaling in SAP2 expression. In addition to SAP2, other target genes of Rhb1 were identified using DNA microarray analysis. The results highlight an important link between protein utilization and Rhb1-TOR signaling in C. albicans.
MATERIALS AND METHODS
Strains and growth conditions.
Table 1 lists the C. albicans strains used in this study. All strains were routinely grown at 30°C in YPD medium (10 g/liter yeast extract, 20 g/liter peptone, 20 g/liter glucose) and synthetic defined (SD) medium (6.7 g/liter yeast nitrogen base without amino acids, 20 g/liter glucose, 0.79 g/liter complete supplement mixture [CSM] of amino acids; MP Biochemicals, Solon, OH). To determine cell growth and the expression levels of SAP2 and its gene product, a single colony from each strain was grown overnight at 30°C in YPD medium, washed, and subcultured at an optical density at 600 nm (OD600) of approximately 0.5 in YCB-BSA-YE (0.01% or 0.05%) medium (11.7 g/liter Difco yeast carbon base, 0.1 g/liter bovine serum albumin [BSA], 0.01 g/liter or 0.05 g/liter yeast extract, adjusted to pH 4.4 with HCl) (19) and subsequently grown at 30°C for different times.
Table 1.
C. albicans strains used in this study
| Strain | Parent strain | Genotype or characteristic(s) | Reference |
|---|---|---|---|
| SC5314 | Wild type | 26 | |
| RHB1 mutants | |||
| CCT-D1 | SC5314 | rhb1Δ::FRT/rhb1Δ::FRT | 72 |
| CCT-D2 | SC5314 | rhb1Δ::FRT/rhb1Δ::FRT | 72 |
| CCT-RD1 | CCT-D1 | rhb1Δ::RHB1/rhb1Δ::RHB1 | 72 |
| CCT-OE1 | SC5314 | ADH1/adh1Δ::RHB1-SAT1 | 72 |
| CCT-OE2 | SC5314 | ADH1/adh1Δ::RHB1-SAT1 | 72 |
| GLN3, GAT1, STP1 mutants | |||
| GLN3M4A | GLN3M3A | gln3Δ::FRT/gln3Δ::FRT | 21 |
| GLN3M4B | GLN3M3B | gln3Δ::FRT/gln3Δ::FRT | 21 |
| GAT1M4A | GAT1M3A | gat1Δ::FRT/gat1Δ::FRT | 21 |
| GAT1M4B | GAT1M3A | gat1Δ::FRT/gat1Δ::FRT | 21 |
| Δgln3GAT1M4A | Δgln3GAT1M3A | gln3Δ::FRT/gln3Δ::FRT | 21 |
| gat1Δ::FRT/gat1Δ::FRT | |||
| Δgln3GAT1M4B | Δgln3GAT1M3B | gln3Δ::FRT/gln3Δ::FRT | 21 |
| gat1Δ::FRT/gat1Δ::FRT | |||
| STP1M4A | STP1M3A | stp1Δ::FRT/stp1Δ::FRT | 21 |
| CHIA-D1 | SC5314 | stp1Δ::FRT/stp1Δ::FRT | This study |
| CHIA-D2 | SC5314 | stp1Δ::FRT/stp1Δ::FRT | This study |
| TOR1-FRB mutant | |||
| JRB12 | SC5314 | TOR1S1984I/TOR1 | 20 |
| TOR1-FRB RHB1 double mutants | |||
| JRB12RHB1D1 | JRB12 rhb1Δ::FRT/rhb1Δ::FRT | This study | |
| JRB12RHB1D2 | JRB12 rhb1Δ::FRT/rhb1Δ::FRT | This study | |
| SAP2 mutants | |||
| SAP2MS4A | sap2-1Δ::FRT/sap2-2Δ::FRT | 67 | |
| SAP2ex4A | SAP2MS4A | sap2-1Δ::FRT/sap2-2Δ::FRT | 67 |
| ADH1/adh1::Ptet-SAP2-1 | |||
| TetGFP-1 | SC5314 | ADH1/adh1::Ptet-GFP | This study |
| Yeast one-hybrid assay | |||
| COP1 | CAI8 | CAI8 ade2::hisG/ade2::hisG::[pCR-OPlacZ] | 31 |
| CCR1 | CAI8 | CAI8 ade2::hisG/ade2::hisG::[pCR-lacZ] | 31 |
| OC1 | COP1 | COP1 RPS1/RPS1::[CIp-LexA-F1] | 31 |
| CC1 | CCR1 | CCR1 RPS1/RPS1::[CIp-LexA-F1] | 31 |
| OG21 | COP1 | COP1 RPS1/RPS1::[CIp-LexA-F-Gcn4-21] | 31 |
| CG21 | CCR1 | CCR1 RPS1/RPS1::[CIp-LexA-F-Gcn4-21] | 31 |
| COP-Stp1LexA | COP1 | COP1 RPS1/RPS1::[CIp-Stp1-LexA] | This study |
| RHB1DCOP-Stp1LexA | COP-Stp1LexA | COP-Stp1LexA rhb1Δ::FRT/rhb1Δ::FRT | This study |
| RHB1DCCR-Stp1LexA | CCR-Stp1LexA | CCR-Stp1LexA rhb1Δ::FRT/rhb1Δ::FRT | This study |
| OERHB1COP-Stp1LexA | COP-Stp1LexA | COP-Stp1LexA ADH1/adh1Δ::RHB1-SAT1 | This study |
| OERHB1CCR-Stp1LexA | CCR-Stp1LexA | CCR-Stp1LexA ADH1/adh1Δ::RHB1-SAT1 | This study |
| COP-LexAGat1 | COP1 | COP1 RPS1/RPS1::[CIp-LexA-F-Gat1] | This study |
| CCR-LexAGat1 | CCR1 | CCR1 RPS1/RPS1::[CIp-LexA-F-Gat1] | This study |
| RHB1DCOP-LexAGat1 | COP-LexAGat1 | COP-LexAGat1 rhb1Δ::FRT/rhb1Δ::FRT | This study |
| RHB1DCCR-LexAGat1 | CCR-LexAGat1 | CCR-LexAGat1 rhb1Δ::FRT/rhb1Δ::FRT | This study |
| OERHB1-COP-LexAGat1 | COP-LexAGat1 | COP-LexAGat1 ADH1/adh1Δ::RHB1-SAT1 | This study |
| OERHB1-CCR-LexAGat1 | CCR-LexAGat1 | CCR-LexAGat1 ADH1/adh1Δ::RHB1-SAT1 | This study |
Cells were grown in SD medium overnight to determine the effect of rapamycin on SAP2 expression. The overnight culture was diluted in fresh SD medium to an OD600 ∼ 0.5. Each cell culture was then incubated at 30°C to reach mid-log phase (OD600 ∼ 3.5) and divided into 6 individual tubes. Three of the tubes contained 0.2 μg/ml rapamycin (from a 100 μg/ml stock dissolved in methanol), and the others contained equal volumes of methanol (vehicle control [VC]). Cells were incubated at 30°C for 30, 60, or 120 min and collected by centrifugation for RNA preparation.
CFU counting.
To determine the cell numbers of each strain grown in YCB–BSA–YE (0.01%) medium, the CFU were counted after various incubation periods. Sterile phosphate-buffered saline (PBS) buffer was used for the serial dilution of the cell. Each cell was placed on ice before being plated onto YPD agar plates. CFU were counted after incubation at 30°C for 36 h.
Plasmid and strain constructions.
The SAT1 flipper method (60) was used to generate the C. albicans STP1 null mutant. The primers used in this study are listed in Table S3 in the supplemental material. A 420-bp ApaI-XhoI DNA fragment carrying the upstream flanking sequence of STP1 was obtained from the C. albicans SC5314 genome with PCR amplification using primers CaSTP1-5′-1 and CaSTP1-5′-2. A 416-bp SacII-SacI DNA fragment containing the downstream flanking sequence of STP1 was similarly amplified using primers CaSTP1-3′-1 and CaSTP1-3′-2. Both fragments were independently digested and cloned into the pSFS2A plasmid (60) to generate the pSFS2dSTP1 plasmid. The DNA fragment carrying the flanking regions of STP1 and the SAT1 flipper cassette was isolated from pSFS2dSTP1 by digestion with ApaI and SacI. This fragment was purified and transformed into the C. albicans SC5314 strain (60). Transformants were selected for nourseothricin resistance, and resistant colonies were verified by PCR. To induce the MAL2p-regulated excision of the SAT1-FLIP cassette, cells were grown overnight with shaking in YPM medium (10 g/liter yeast extract, 20 g/liter peptone, 20 g/liter maltose) and subsequently grown on YPM agar plates. The second round of deletion cassette integration and excision used a heterozygous mutant and yielded 2 independent homozygous stp1Δ constructs: CHIA-D1 and CHIA-D2. The SAT1 flipper method was also used to construct the RHB1 deletion in the C. albicans JRB12 strain containing the rapamycin-resistant TOR1S1984I allele (20). The ApaI-SacI DNA fragment containing 5′- and 3′-flanking regions of RHB1 and the entire SAT1 flipper cassette was isolated from pSFSdRHB1 (72). The DNA fragment was then transformed into JRB12 for integration into the RHB1 locus by homologous recombination. Transformants were selected for nourseothricin resistance and grown for 3 days in YCB medium containing BSA (23.4 g/liter yeast carbon base, 4 g/liter BSA, pH 4.0) to induce SAP2p-regulated SAT1-FLIP cassette excision (60). The second round of gene deletion used C. albicans heterozygous RHB1 mutants, yielding 2 independent constructs: JRB12RHB1D1 and JRB12RHB1D2.
LexA-Gat1 and Stp1-LexA fusions were constructed to test the transcriptional activity of Gat1 and Stp1 on the wild-type and rhb1-deleted backgrounds. Briefly, the DNA fragment containing the entire GAT1 gene was PCR amplified from the SC5314 genome by the use of primer pair CaGAT1-5′ and CaGAT1-3′. The fragment was purified, digested with MluI and PstI, and cloned into the plasmid pCIplexA-F1 (31, 64) to generate pCIpLexA-F-Gat1. The plasmid was linearized with StuI, and the lexA-GAT1 fusion fragment was introduced into the RPS1 locus in COP1 and CCR1 strains (31) to generate COP-LexAGat1 and CCR-LexAGat1. Because the Csy1, Ptr3, and Ssy5 (SPS) sensor can proteolytically cleave the N terminus region of immature Stp1, LexA was fused to the C terminus of Stp1. The DNA fragment containing lexA with the MluI and PstI restriction sites was amplified by PCR from the pCIpLexA-F-Gat1 plasmid by the use of primer pair LexA and MluI-F and primer pair LexA and PstI-R. The fragment was purified, digested with MluI and PstI, and cloned into pCIpLexA-F-Gat1 to replace GAT1 and generate the plasmid pCIpLexA-F-LexA. The DNA fragment containing the entire STP1 gene was PCR amplified from the SC5314 genome by the use of primer pair CaSTP1-5′-3 and CaSTP1-3′-3. This DNA fragment was purified, digested with HindIII and MluI, and cloned into pCIpLexA-F-LexA. The fragment replaced the region downstream of lexA, generating pCIpStp1-LexA. The plasmid was then linearized with StuI, and the STP1-lexA fusion was introduced into the RPS1 locus in the COP1 and CCR1 strains to generate COP-Stp1LexA and CCR-Stp1LexA. Finally, the RHB1 deletion was constructed in the COP-LexAGat1, CCR-LexAGat1, COP-Stp1LexA, and CCR-Stp1LexA strains as described above, yielding RHB1DCOP-LexAGat1, RHB1DCCR-LexAGat1, RHB1D-COP-Stp1LexA, and RHB1DCCR-Stp1-LexA, respectively (Table 1). To overexpress RHB1 in COP-LexAGat1, CCR-LexAGat1, COP-Stp1LexA, and CCR-Stp1LexA, a KpnI-SacII fragment containing PADH1-RHB1 was obtained from pADH1OERHB1 (72) and transformed into the ADH1 locus of each strain; this generated OERHB1-COP-LexAGat1, OERHB1-CCR-LexAGat1, OERHB1-COP-Stp1LexA, and OERHB1-CCR-Stp1LexA, respectively.
To construct the strain harboring expression of the tetracycline-induced green fluorescent protein-encoding gene (GFP), a DNA fragment containing a Ptet-GFP-SAT1 cassette was obtained from pNIM1 (56) with SacII and KpnI digestion. The fragment was then transformed into 1 allele of the ADH1 locus of SC5314 to generate TetGFP.
RNA isolation, reverse transcription-PCR (RT-PCR), and real-time quantitative PCR.
Total RNA isolation and cDNA synthesis were performed as described previously (72). For PCRs, 1 μl of cDNA served as the template for 1 cycle of 94°C for 10 min, followed by different numbers of cycles of 94°C for 30 s and 56 to 60°C for 30 s as indicated and 1 cycle of 72°C for 5 min. The PCR products were analyzed on 1.2% agarose gels and visualized by staining with SYBR Safe (Invitrogen, Carlsbad, CA).
Quantitative PCR was performed using MicroAMP optical 96-well reaction plates (catalog no. N801-0560; Applied Biosystems, Framingham, MA) and MicroAMP optical adhesive film (catalog no. 4311971; Applied Biosystems), the SYBR green-based detection assay, and a 7500 real-time PCR system (Applied Biosystems). Each 20-μl reaction mixture consisted of 1 μl of cDNA, 0.3 μl of 20 μM of sense and antisense primers, 8.4 μl of water, and 10 μl of Power SYBR green PCR Master Mix (catalog no. 4367659; Applied Biosystems). The reaction conditions were 1 cycle of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Each sample was amplified in triplicate, and each experiment was performed 3 times. Data analysis was performed with Real-Time PCR System Sequence Detection software version 1.4 (Applied Biosystems). An average threshold cycle (CT) value was obtained and normalized to the average CT value of EFB1. The comparative CT method was used to quantify gene expression, and the relative expression was determined to be 2−ΔΔCT (36).
Protein preparation and Western blotting.
Whole-protein lysate was prepared by suspending cells in 1% Nonidet P-40 (NP-40) extraction buffer (50 mM NaH2PO4 [pH 8.0], 150 mM NaCl, 1% NP-40) containing a 100-fold-diluted protease inhibitor cocktail (catalog no. P-8215; Sigma-Aldrich, St. Louis, MO) and 200 mM phenylmethylsulfonyl fluoride. Cells were broken using a vortex procedure with acid-washed glass beads for 30 s and immediately placed on ice for 30 s. This step was repeated 10 times. Soluble proteins were collected by centrifugation (13,300 × g) for 10 min at 4°C and quantified with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). To detect secreted Sap2 and BSA in the medium, 20 μl of the supernatant was loaded onto a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and proteins were visualized with Coomassie blue staining. For Western blot analysis, proteins were subjected to 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Pall, Port Washington, NY) by the use of Towbin transfer buffer (24 mM Tris base, 192 mM glycine, 20% methanol) and a TE77 semidry transfer unit (GE Healthcare Bio-Sciences, Piscataway, NJ). Sap2 was detected with mouse monoclonal anti-Sap2 (catalog no. M166; Takara Bio, Shiga, Japan) (1:2,000 dilution). GFP was detected with JL-8 anti-GFP mouse monoclonal antibody (catalog no. 632380; Clontech, Palo Alto, CA) (1:2,000 dilution), and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) (1:10,000 dilution) was used as the secondary antibody. The chemiluminescence (ECL) was detected with Western Lighting Plus ECL reagents (Perkin-Elmer, Waltham, MA) and exposed to X-ray films (Fujifilm, Tokyo, Japan).
One-hybrid assay.
A one-hybrid assay was performed as described previously (64). Briefly, cells were grown overnight in YPD broth at 30°C, washed, subcultured in fresh YCB–BSA–0.01% YE medium, and grown for 5.5 h to reach mid-log phase. The supernatant was removed by centrifugation (1,500 × g), and the cell pellets were suspended in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4 · 7H2O, and 4 μl/ml 98% β-mercaptoethanol; pH 7.0). Cells were then lysed by adding 15 μl of 0.1% SDS and 30 μl of chloroform and vortexing for 15 s. The suspensions were incubated for 30 min at 37°C in 0.2 ml of potassium phosphate buffer (pH 7.0) containing 4 mg of o-nitrophenyl-β-d-galactopyranoside/ml. The reactions were terminated with 0.4 ml of 1 M Na2CO3 when the suspension turned yellow. The supernatants from the reaction mixture were collected by centrifugation, and the optical density was measured at 420 and 550 nm. β-Galactosidase activity was calculated in Miller units as follows: 1 Miller unit = 1,000 × [(OD420) − (1.75 × OD550)]/(T × V × OD600), where T is the duration of the reaction (in minutes), V is the volume of the supernatant used in the assay (in milliliters), OD600 is the cell density at the beginning of the reaction, OD420 is the absorbance derived from o-nitrophenyl-β-d-galactopyranoside and light scattering from cell debris, and OD550 indicates light scattering from cell debris. Analysis was performed in triplicate based on 3 independent experiments.
Cell susceptibility to rapamycin.
After growing overnight in YPD broth, cells were collected, washed, and suspended in sterile water. Tenfold serial dilutions of the cells were prepared at a concentration of approximately 2 × 107 to 2 × 103 cells/ml. Diluted cells (5 μl) were spotted onto YPD plates containing 0.01-μg/ml rapamycin (catalog no. 553210; Merck KGaK, Darmstadt, Germany) (prepared as a 100 μg/ml stock by dissolution in methanol) or YPD plates made with the same volume of methanol (as a control). Cell viability was recorded after incubation at 30°C for 2 days. This assay was performed independently 3 times.
Analysis of Sap2 gene and protein levels.
The SAP2ex4A strain was used to determine whether rapamycin affects Sap2 protein levels (67). SAP2ex4A cells were grown overnight in YPD broth and collected by centrifugation. The cell pellets were washed and subcultured in fresh YCB–BSA–YE (0.01%) containing 30 mg/ml doxycycline to maintain the basal level of SAP2 expression. The cells were grown at 30°C for 6 h (to reach OD600 = 2.5 to 3), and 5 ml of cell culture was then transferred into 50-ml conical tubes containing 0.2 mg/ml rapamycin (prepared from a stock of 100 μg/ml dissolved in 100% methanol) or an equal volume of methanol (0.2% [vehicle control]). The conical tubes were also filled with 1.8× CSM (MP Biochemicals) (prepared from a 20× stock) to determine the effects of amino acid availability. The cells were incubated at 30°C for different times and collected by centrifugation. The cell pellets and supernatants were used for RNA or protein isolation.
To correlate amino acid availability with intracellular Sap2 protein levels, SAP2ex4A cells were grown in YPD at 30°C overnight, washed, and subcultured in SD medium containing 30 mg/ml doxycycline at OD600 = 0.5. The cells were grown at 30°C for 4 h (to reach OD600 = 3.5 to 4.0), and 5 ml of cell culture treated with doxycycline was transferred into 50-ml conical tubes containing 0.2 mg/ml rapamycin or an equal volume of methanol (0.2% [vehicle control]). The cells were then incubated at 30°C for different times and collected by centrifugation. The resulting cell pellets were used for protein isolation.
Analysis of GFP protein levels.
The TetGFP strain carries a Ptet-GFP fusion that is similar to the construct in the SAP2ex4A strain, except the latter contains a Ptet-SAP2 fusion. The SAP2ex4A cell was incubated in fresh YCB—BSA—YE (0.01%) containing 30 mg/ml doxycycline. The growth conditions were the same as those described for analysis of Sap2 gene and protein levels. GFP protein levels were analyzed as described for protein preparation and detected by Western blotting.
Proteolytic assay.
Cells were grown in YPD overnight and collected by centrifugation. The cell pellets were washed and resuspended in sterile water to approximately 5 × 104 cells/μl. The resulting cell suspension (5 μl) was spotted onto YCB—BSA—YE (0.01%) plates (with 1.5% agar) containing 0.01 mg/ml rapamycin. The size of the clear zone surrounding the cells was measured after incubation at 37°C for 4 days.
DNA microarray fabrication and sample preparation.
An Operon 70-mer probe set (Array-Ready Oligo Sets; AROS V1.2), containing 6,266 probes, and the C. albicans AROS upgrade set (V1.1), containing 1,659 probes, was used to construct DNA microarrays. These 70-mer oligonucleotide sets contain 7,925 optimized probes that represent the entire genome of C. albicans and more than 10 different controls. These oligonucleotide sets have been successfully used in studies on C. albicans biology and pathogenesis (1, 6, 84). The probes were resuspended in 3× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) to a final concentration of 40 μM.
The printing of the C. albicans microarrays was carried out at the DNA Microarray Core Laboratory of the Institute of Plant and Microbial Biology, Academia Sinica, Taiwan. The oligonucleotides were spotted onto UltraGAPS coated slides (Corning, New York, NY) using an OmniGrid 100 microarrayer (Genomic Solutions, Ann Arbor, MI). The 1st, 50th, and 100th chip of each batch (100 chips per batch) were selected and scanned with an Axon GenePix 4000B array scanner (Axon Instruments, Inc., Union City, CA) to ensure the quality of the microarray printing. The quality of the test microarrays was determined visually.
For DNA microarray analysis, a single colony of either the SC5314 (wild-type) strain or the CCT-D1 (rhb1-deleted) strain was inoculated into SD medium and grown overnight at 30°C. These cultures were then subcultured in 40 ml of SD medium and grown at 30°C for 5.5 h until they reached the mid-log phase (OD600 ∼ 4.0). The cells were then collected by centrifugation. Total RNA was extracted with an RNeasy kit (catalog no. 74106; Qiagen, Valencia, CA) and treated with Turbo DNA-free DNase (Ambion, Austin, TX) at 37°C for 1 h. After purification, the quality and quantity of total RNA were determined using a model 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) or agarose gel electrophoresis and an ND-1000 spectrophotometer (NanoDrop Technologies). cDNA synthesis, aRNA synthesis, and Cy dye coupling were conducted using an Amino Allyl MessageAmp II aRNA amplification kit (catalog no. AM1753; Ambion). First-strand cDNA was prepared using total RNA and ArrayScript reverse transcriptase. After processing, DNA polymerase and RNase H were used for synthesis of the second-strand cDNA. To synthesize amino allyl UTP (aaUTP)-incorporated aRNA (amino allyl-modified aRNA), in vitro transcription was performed with T7 RNA polymerase and purified cDNA was used as a template. Cy dye coupling was performed after aRNA purification. Cy dye-coupled aRNA was purified by ultrafiltration using an RNeasy MinElute Cleanup kit (Qiagen). For biological replications, samples were prepared from 5 different batches each of wild-type and rhb1-deleted mutant cells. Dye swap experiments produced 5 sets of data for Cy5-labeled wild-type cells (Amersham Cy5 monoreactive dye pack [catalog no. PA25001]; GE Healthcare) and Cy3-labeled rhb1-deleted (CCT-D1) mutant cells (Amersham Cy3 monoreactive dye pack [catalog no. PA23001]; GE Healthcare). Another 5 sets were labeled by dye swapping (Cy3-labeled wild type versus Cy5-labeled rhb1-deleted mutant). Slides were prehybridized with 1% BSA, washed, and hybridized with Cy3- and Cy5-labeled aRNA in a Corning microarray hybridization chamber (catalog no. 2551; Corning). Hybridization was performed in a water bath at 65°C for 16 h. After hybridization, the slides were washed several times using SSC buffers with different stringencies. Slides were dried and scanned using an Axon GenePix 4000B scanner (Axon Instruments, Inc.) with wavelengths of 635 nm and 532 nm.
DNA microarray data analysis.
The microarray data were first processed with Lowess normalization to remove intensity-based variation across channels. The following linear model was then fitted to each spot on the DNA microarrays: yijkl = straini + dyej + arrayk + Σijkl, where straini represents the ith strain for i = 1, 2, dyej represents the jth dye effect for j = 1, 2, arrayk represents the overall array effect and is a random effect, and Σijkl represents the residual term for random variation, which is not considered in this model. yijkl is the intensity to be modeled. Genes with significant strain effects were selected using a false-discovery rate of 0.05, which is equivalent to a P value of ≤0.0148. The results showed that 1,028 genes were significantly changed. Among these genes, 36 were upregulated in the rhb1-deleted strain with more than a 1.5-fold change, whereas 77 were downregulated in the rhb1-deleted strain with more than a 1.5-fold change. These 113 genes were selected for further analysis (see Tables S1 and S2 in the supplemental material). The analysis was conducted with JMP Genomics software (SAS Institute Inc., Cary, NC) and the R package qvalue (69).
Statistical analysis.
The statistically significant threshold was dependent on the P value from quantitative real-time PCR, and the yeast one-hybrid assay was analyzed with Student's t test (Microsoft Office Excel 2007; Microsoft, Redmond, WA) using at least 3 individual experiments.
Microarray data accession number.
The microarray data are available at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE29128.
RESULTS
Rhb1 regulates cell growth in the presence of BSA by controlling Sap2 expression.
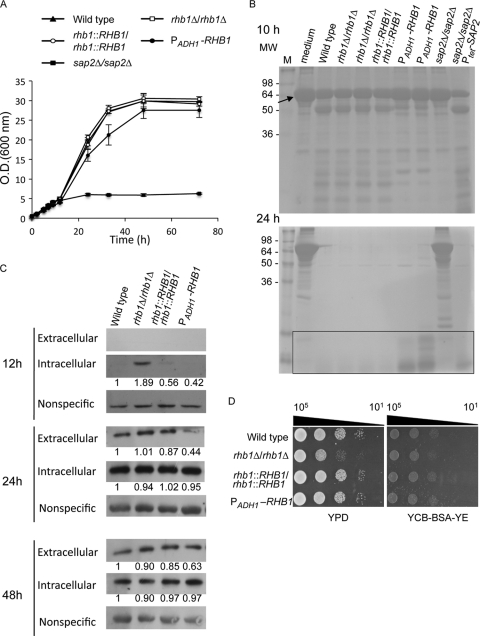
Sap2 is the major secreted protease of C. albicans in vitro (32, 81). SAP2 expression is induced in medium in which protein is the nitrogen source and is repressed in the presence of a more favorable nitrogen source, such as ammonium (22). Previous research showed that Rhb1 is involved in filamentation mediated by nitrogen starvation (72). This implies that Rhb1 may play a role in cell growth in medium where protein is the major source of nitrogen. To test this hypothesis, C. albicans cells were grown in yeast carbon base (YCB) medium supplemented with bovine serum albumin (BSA) (as the nitrogen source) and a limited amount (0.01%) of yeast extract (YE) to reduce overall incubation time (22). Figure 1A depicts the growth rate of C. albicans. The rhb1-deleted strain showed a growth rate similar to those of the wild-type and RHB1-reconstituted strains. Cells in which RHB1 expression was driven by the ADH1 promoter and RHB1 was overexpressed (72) grew slower than wild-type cells. However, cells overexpressing RHB1 exhibited an optical density similar to that of the wild type after prolonged incubation (Fig. 1A). The sap2-deleted mutant was used as a control, as it did not grow in YCB–BSA–YE (0.01%) medium (Fig. 1A) (21). The numbers of CFU were also counted (see Fig. S1 in the supplemental material) to avoid cell size or morphology variations that might have led to inaccurate OD600 data for Fig. 1A. The pattern of cell growth derived from CFU counting was similar to that represented in Fig. 1A. However, the slow growth of the strain overexpressing RHB1 might have been caused by other nitrogen sources in the medium. The concentration of yeast extract in the medium was increased to 0.05% to avoid this possibility. This diminished the slow growth of the strain overexpressing RHB1 (see Fig. S2 in the supplemental material) and largely restored the defect of the sap2-deleted strain (see Fig. S2 in the supplemental material). These results indicate that the slow growth of the strain overexpressing RHB1 and the growth defect of sap2-deleted strain are related to BSA utilization.
Fig 1.
Rhb1 regulates cell growth in medium with BSA as the major nitrogen source by controlling C. albicans Sap2 expression. (A) Cells were grown in YPD broth overnight, washed twice with sterile water, and subcultured in YCB–BSA–YE (0.01%) medium (initial cell density, OD600 = 0.5) at 30°C. The C. albicans strains used in this experiment were SC5314 (wild type; filled triangles), CCT-D1 (rhb1 deleted; open squares), CCT-RD1 (RHB1 reconstituted; open circles), CCT-OE1 (overexpressing RHB1; filled circles), and SAP2MS4A (sap2 deleted; filled squares). The doubling times for wild-type, rhb1-deleted, and RHB1-reconstituted strains were approximately 5.7 ± 0.2 h, 5.8 ± 0.02 h, and 5.7 ± 0.1 h, respectively. The doubling time for the strain overexpressing RHB1 in YCB–BSA–YE (0.01%) was approximately 6.1 ± 0.4 h. (B) BSA degradation by C. albicans after 10 and 24 h of incubation. Each cell supernatant (20 μl) was analyzed by 12% SDS-PAGE, and proteins were visualized by Coomassie blue staining. Data represent the results of only 1 experiment, although experiments were performed at least 3 times with identical results. The rectangular box indicates peptides or low-molecular-weight proteins derived from BSA degradation. The arrow indicates the intact BSA (66 kDa). M, protein molecular marker; MW, molecular weight. (C) Expression of extracellular and intracellular Sap2 in different C. albicans strains. Cells were grown as described in panel A for 12, 24, and 48 h, and Sap2 was detected by Western blotting. The nonspecific band served as the loading control and was used to normalize the intracellular Sap2 levels indicated by the fold change values. (D) Comparison of levels of cell growth on YPD and YCB–BSA–YE (0.01%) agar plates. Cells were grown overnight in YPD, washed, serially diluted 10-fold, and spotted onto agar plates. Plates were photographed after incubation at 30°C for 1 day. Data are representative of at least 3 independent experiments with identical results. Cell numbers are shown at the top of the panels.
To further correlate cell growth with BSA utilization, supernatants of cells grown in YCB–BSA–YE (0.01%) were analyzed with SDS-PAGE. After 24 h of incubation, BSA was completely digested by the wild-type, rhb1-deleted, and RHB1-reconstituted strains (Fig. 1B). However, 2 independent strains overexpressing RHB1 showed lower BSA utilization efficiency. The strains overexpressing RHB1 degraded BSA slowly after 10 h of incubation compared to the wild type (Fig. 1B), and the molecular weight of degraded protein was relatively high. Even after 24 h of incubation, peptides or low-molecular-weight proteins derived from BSA degradation were still apparent in the strains overexpressing RHB1 (Fig. 1B). The low efficiency of BSA degradation seen with RHB1 overexpression confirms the low growth rate of the same strain (Fig. 1A). The sap2-deleted mutant and a strain in which SAP2 is regulated by a tetracycline-inducible Ptet promoter (65) were used as controls. The sap2-deleted mutant slightly degraded BSA, and the degradation was substantially slower (Fig. 1B). However, protein degradation became apparent in the SAP2-induced strain after the addition of doxycycline (a tetracycline antibiotic) (Fig. 1B). Western blotting using BSA antibody was performed to determine whether the lower-molecular-weight bands represented BSA or other proteins. The lower-molecular-weight bands were derived from BSA degradation (see Fig. S3 in the supplemental material). These results suggest that the protein utilization of the strain overexpressing RHB1 is not as efficient as that of the other strains. Thus, the Rhb1 level may be correlated with the secretion or expression of secreted proteases.
Because Sap2 is the major secreted protease in vitro, the experiments in this study tested whether lower Sap2 expression levels result in delay of growth of the strain overexpressing RHB1. The expression of Sap2 was detected by Western blot analysis performed with Sap2 monoclonal antibody following previously described procedures (21). The rhb1-deleted mutant was the only mutant in which intracellular Sap2 was easily detected in cells after 12 h of incubation, as shown in Fig. 1C. The levels of extracellular Sap2 protein in other tested strains were too low to be detected. After 24 h of incubation, the expression levels of intracellular Sap2 seemed to reach saturation and were indistinguishable among the different samples. Nevertheless, the level of extracellular Sap2 in the strain overexpressing RHB1 was much lower than in the others. The level of extracellular Sap2 in the rhb1-deleted strain was slightly higher than in the wild-type and RHB1-reconstituted strain. After prolonged incubation (48 h), the level of the protein remaining in the medium was relatively low. Thus, Sap2 might not be required and was proteolyzed, further reducing the intracellular level of Sap2 compared to that seen in cells after 24 h of incubation. The different extracellular Sap2 levels among the strains were still apparent after 12 h but were almost indistinguishable after 24 h of incubation. Although the levels of Sap2 protein were higher in the rhb1-deleted mutant than in the wild type (Fig. 1C), the wild-type and rhb1-deleted strains showed similar growth rates in YCB–BSA–YE (0.01%) liquid medium (Fig. 1A). A possible explanation is that the rhb1-deleted mutant may exhibit a growth delay phenotype when growing in a nutrient-rich environment. To test this possibility, cells were grown in YPD-rich medium and compared to cells grown in a medium in which protein is the major nitrogen source. Equal numbers of cells were serially diluted and spotted onto YPD and YCB–BSA–YE (0.01%) agar plates. The rhb1-deleted mutant showed a slight delay in cell growth on YPD but not on YCB–BSA–YE (0.01%) agar plates (Fig. 1D). This suggests that high levels of Sap2 protein expression (Fig. 1B and C) may somehow compensate for the growth delay of the rhb1-deleted mutant in YCB–BSA–YE (0.01%) medium (Fig. 1A and D). The spot assay somehow masked the minor growth delay of strain overexpressing RHB1 shown in the liquid medium (Fig. 1A). It is possible that the spot assay is not sensitive enough to detect a minor delay in cell growth. Overall, these results indicate that Rhb1 is involved in the expression of Sap2, which is critical for cell growth in media with protein as the major nitrogen source.
The TOR signaling pathway is also involved in regulating SAP2 expression.
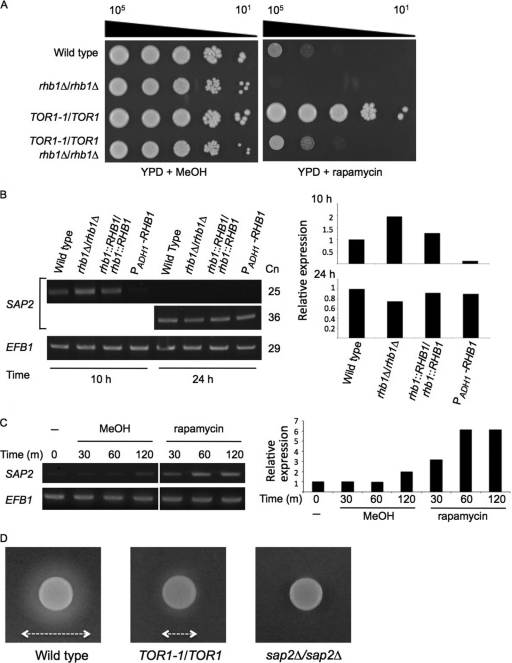
Rapamycin is an inhibitor of Tor kinase and forms a complex with FKBP12, a 12-kDa protein (28). S. cerevisiae cells treated with rapamycin express a phenotype that mimics that of cells undergoing nitrogen starvation (14). Previous research showed that mutants lacking Rhb1 are hypersensitive to rapamycin, suggesting that Rhb1 plays a role in TOR signaling (72). Figure 2A illustrates the close relationship among Rhb1, rapamycin, and Tor1. The rhb1-deleted mutant was rapamycin sensitive whereas the TOR1-1/TOR1 mutant was rapamycin resistant compared with the wild type (Fig. 2A). The C. albicans TOR1-1/TOR1 strain carries a TOR1-1 allele with a point mutation (TOR1S1984I) in the FKBP12 rapamycin-binding (FRB) domain of the Tor1 kinase (20). The TOR1-1/TOR1 strain is resistant to rapamycin, because the FKBP12-rapamycin complex cannot bind to Tor1 kinase with this mutation (20). Two independent constructs of the rhb1 deletion on the TOR1S1984I mutant background were not as strongly resistant to rapamycin as TOR1-1/TOR1 (Fig. 2A). In fact, rhb1 deletion in the TOR1-1/TOR1 background showed slightly enhanced resistance to rapamycin compared with the rhb1-deleted mutant. These results suggest that deletion of RHB1 compensates for the defect in rapamycin binding to the mutant FRB domain of the Tor1 kinase. Rapamycin and the rhb1 deletion combined have an additional effect on the Tor1 kinase, thus reinforcing rapamycin susceptibility.
Fig 2.
Tor1 kinase regulates SAP2 expression and affects Sap2 protease secretion. (A) Epistatic interaction between Rhb1 and Tor1 kinase. Tenfold serial dilutions of each strain were prepared and spotted onto YPD with 0.01-μg/ml rapamycin or with 0.1% methanol (MeOH [the vehicle control]). Plates were incubated at 30°C for 2 days. Data are representative of at least 3 independent experiments with identical results. Cell numbers are shown at the top of the panels. (B) SAP2 expression in different C. albicans strains. Cells were grown as described in the Fig. 1A legend for 10 and 24 h, and SAP2 expression was analyzed with RT-PCR. EFB1 served as an endogenous control. Cn, number of cycles in the PCRs. The semiquantitative levels of SAP2 induction were calculated and plotted. (C) Induction of SAP2 expression by rapamycin treatment. The SC5314 strain was incubated in SD medium at 30°C until reaching mid-log phase. The cell pellets were washed and subsequently subcultured in SD (−), SD containing 0.2% methanol (MeOH [the vehicle control]), or SD containing 0.2-μg/ml rapamycin. After cells were grown for the indicated times, the level of SAP2 expression was detected with RT-PCR. EFB1 served as an endogenous control. The semiquantitative levels of SAP2 induction were calculated and plotted. m, minutes. (D) Assay for Sap2 secretion by BSA degradation. Approximately 2.5 × 105 cells of the SC5314, JRB12 (TOR1-1/TOR1), and SAP2MS4A (sap2Δ/sap2Δ) strains were spotted on YCB–BSA–YE (0.01%) agar plates containing rapamycin (0.01 μg/ml) and incubated at 37°C for 4 days. The double arrows indicate the diameter of the proteolytic zone.
The level of Sap2 is altered in the rhb1-deleted strain and the strain overexpressing RHB1 when protein is the major nitrogen source (Fig. 1B and C). Thus, it is possible that both Rhb1 and Tor1 kinase are involved in regulating SAP2 gene expression. Total RNA was isolated and analyzed by reverse transcription-PCR (RT-PCR). After 10 h of growth in YCB–BSA–YE (0.01%) medium, the expression level of the SAP2 transcript was slightly higher in the rhb1-deleted strain than in the wild-type and RHB1-reconstituted strains (Fig. 2B). However, the expression of SAP2 transcripts was nearly undetectable in the strain overexpressing RHB1 after 10 h (Fig. 2B). Expression of SAP2 reached similar levels in all strains tested after 24 h. To determine the role of Tor1 kinase in SAP2 expression, the experiments in this study used synthetic defined (SD) medium, which contains no protein but has ammonium, a favorable nitrogen source, and a complete supplement mixture (CSM) of amino acids. The cultures were also treated with and without rapamycin. As expected, rapamycin induced SAP2 expression at a level greater than that seen with vehicle-treated control cells (Fig. 2C). The extracellular proteolytic activity of Sap2 was also assessed (38) by spotting the cells onto YCB–BSA–YE (0.01%) agar plates containing rapamycin. A high concentration of cells (∼2.5 × 105 cells/spot) was used for each tested strain to compensate for the possible reduction in cell growth caused by rapamycin. The zones of proteolysis surrounding the cell spots represent the relative levels of protease secretion (38). The wild-type strain produced a larger proteolytic zone than the TOR1-1/TOR1 strain (Fig. 2D). The sap2-deleted strain served as a negative control and did not show a clear proteolytic zone (Fig. 2D). These results indicate that Tor1 kinase is involved in SAP2 expression and somehow affects the secretion of Sap2.
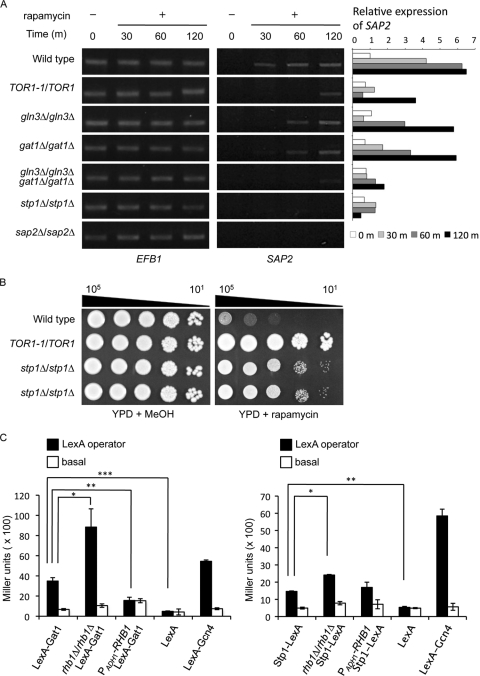
To further correlate the TOR signaling pathway with SAP2 expression, transcription factors that may be downstream of the Tor1 kinase were also examined during rapamycin treatment. In S. cerevisiae, Gln3 and Gat1 are the downstream effectors of TOR signaling in regulating nitrogen catabolism (7). In C. albicans, Gln3 and Gat1 activate the Stp1 transcription factor, which in turn controls SAP2 expression (22). A time course analysis of SAP2 expression was performed using RT-PCR. SAP2 was activated with rapamycin treatment in wild-type cells, whereas a relatively low level of SAP2 was expressed in the TOR1-1/TOR1 strain (Fig. 3A). After rapamycin treatment, cells lacking GLN3 or GAT1 showed a delay and a decrease in SAP2 expression compared with wild-type cells. Moreover, the gln3Δ/gat1Δ double mutant showed an even lower level of SAP2 expression than the gln3Δ and gat1Δ single mutant strains (Fig. 3A). With rapamycin treatment, deletion of STP1 also decreased SAP2 expression (Fig. 3A). The sap2-deleted strain served as a negative control. This suggests that SAP2 expression controlled by TOR signaling involves Gln3 or Gat1 or both and is further activated by Stp1. To further determine the function of Stp1 in TOR signaling, this study examined the susceptibility of the stp1-deleted mutants to rapamycin. As with the gln3- and gat1-deleted mutants, the stp1-deleted mutants were resistant to rapamycin (Fig. 3B) (40). The S. cerevisiae stp1-deleted mutant is rapamycin sensitive (66), suggesting that the Stp1 proteins in the two budding yeasts may function differently.
Fig 3.
Gln3, Gat1, and Stp1 regulate SAP2 expression downstream of the Tor1 kinase. (A) The gln3-, gat1-, and stp1-deleted strains attenuate rapamycin-induced SAP2 expression. Cells were grown in YPD medium at 30°C until reaching the mid-log phase. The cell pellets were washed and subcultured in YPD with and without 0.2 μg/ml of rapamycin. After cells were grown for the indicated times, the level of SAP2 expression was detected with RT-PCR. The strains used in this experiment include SC5314 (wild type), JRB12 (TOR1-1/TOR1), CCT-D1 (rhb1Δ/rhb1Δ), STP1M4A (stp1Δ/stp1Δ), GLN3M4A (gln3Δ/gln3Δ), GAT1M4A (gat1Δ/gat1Δ), Δgln3GAT1M4A (gln3Δ/gln3Δ gat1Δ/gat1Δ), and SAP2MS4A (sap2Δ/sap2Δ). EFB1 served as an endogenous control. The semiquantitative levels of SAP2 induction were calculated and plotted. The sample of wild type (without rapamycin treatment) from time zero was used as the calibrator. m, minutes. (B) Stp1 is involved in the TOR signaling pathway. SC5314, JRB12 (TOR1-1/TOR1), STP1M4A, and CHIA-D1 (the last 2 are independent stp1Δ/stp1Δ mutants as shown in the third and fourth lanes from the top, respectively) were incubated in YPD overnight. The cell pellets were washed, serially diluted, spotted onto YPD agar plates with 0.01 μg/ml rapamycin or 0.1% methanol (MeOH [the vehicle control]), and incubated at 30°C for 2 days. Data are representative of 3 independent experiments with identical results. Cell numbers are shown at the top of the panels. (C) Yeast one-hybrid analysis demonstrated that Rhb1 affects the transcriptional activity of Gat1 and Stp1. Strains with and without the LexA operator upstream of the basal promoter of the lacZ reporter gene are labeled LexA operator (CCR1 background) and basal (COP1 background), respectively. COP1 and CCR1 were constructed in the rhb1-deleted (rhb1Δ/rhb1Δ) or RHB1-overexpressing (PADH1-RHB1) background. Cells were incubated in YCB–BSA–YE (0.01%) medium at 30°C for 5.5 h, and the LacZ activity modulated by Stp1-LexA or LexA-Gat1 binding to the LexA operator was measured with a liquid β-galactosidase assay. β-Galactosidase data are shown in Miller units and represent averages of the results of 3 independent experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005. The error bars show standard deviations.
The transcriptional activity of Gat1 and Stp1 was assessed with a one-hybrid analysis (64) using cells grown in YCB–BSA–YE (0.01%) medium (Fig. 3C). LexA-Gcn4 and LexA constructs served as positive and negative controls, respectively (31). Compared to wild-type cells, Gat1 and Stp1 showed relatively higher transcriptional activity in cells on the rhb1-deleted background. However, the transcriptional activity of Gat1 (but not Stp1) was substantially lower than that seen with the wild type in the RHB1 overexpression background (Fig. 3C). These results suggest that Rhb1 and Tor1 are involved in the activity of Gln3 and Gat1 in transcriptional regulation.
Tor1 and amino acid regulation of Sap2 protein level.
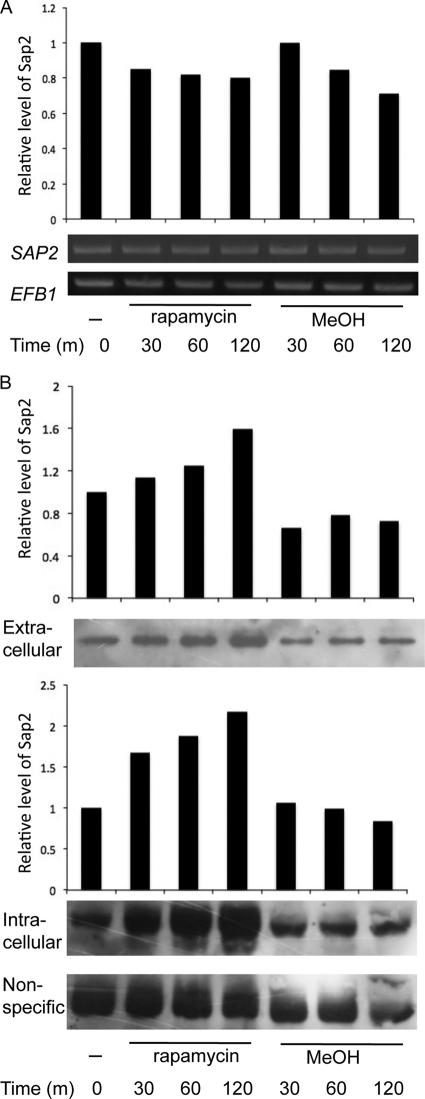
Because Tor1 is involved in SAP2 transcription and somehow affects Sap2 secretion (Fig. 2B and C), we hypothesized that Tor1 may affect Sap2 directly at the protein level. This possibility was tested using a strain in which SAP2 is regulated by the Ptet promoter (Fig. 1B). In this strain, SAP2 expression is likely to be activated by doxycycline independently of TOR signaling. When doxycycline was added, cells showed almost equal levels of SAP2 transcription throughout all examined time points, in both the presence and absence of rapamycin (Fig. 4A). However, the levels of both extracellular and intracellular Sap2 in the presence of rapamycin were higher than those in control cells without rapamycin treatment (Fig. 4B). A strain carrying a Ptet-GFP fusion was also tested to rule out the possibility that the rapamycin-related effect on the level of Sap2 protein was due to the construction of the Ptet promoter. GFP was expressed at similar levels under the same growth conditions (see Fig. S4 in the supplemental material). The data suggest that TOR signaling is involved in regulating SAP2 transcription and further affects the expression of Sap2 protein.
Fig 4.
Tor1 kinase is involved in Sap2 level regulation. (A) The SAP2ex4A strain carrying doxycycline-inducible SAP2 was first grown to an OD600 of 2.5 to 3 in YCB–BSA–YE (0.01%) medium containing 30 μg/ml doxycycline at 30°C for 6 h. The cultures were then equally divided into tubes containing 0.2 μg/ml rapamycin or 0.2% methanol (MeOH [the vehicle control]). The cells were incubated and collected at the indicated times. The expression of SAP2 was examined with RT-PCR. EFB1 served as an endogenous control and was used for the semiquantitation of SAP2 expression. m, minutes. (B) Protein level of Sap2 induced by rapamycin. The levels of intracellular and extracellular Sap2 were detected in the SAP2ex4A strain grown as described for panel A. Whole-cell extract (20 μg) or 20 μl of supernatant from each sample was analyzed with SDS-PAGE and immunoblotted using monoclonal anti-Sap2. The nonspecific band served as the loading control. The semiquantitative levels of Sap2 protein were calculated and plotted. m, minutes.
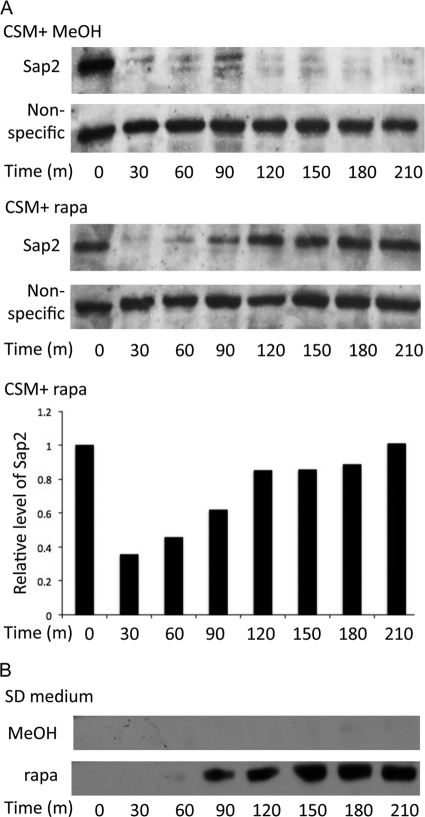
This discussion and previous research (22) indicate that nitrogen depletion and the presence of protein function as signals for SAP2 activation in C. albicans through Rhb1-TOR signaling. This study further determined whether the Sap2 protein level is also affected by the availability of amino acids, another possible source of nitrogen. We detected Sap2 in response to treatment with amino acids and rapamycin (Fig. 5). After 3.5 h of incubation with doxycycline, the intracellular levels of Sap2 were significantly abated in the strain in which SAP2 was regulated by the Ptet promoter when a combination of amino acids (CSM) was added to the nitrogen-poor YCB–BSA–YE (0.01%) medium (Fig. 5A). The reduction of Sap2 protein levels by a high concentration of amino acids did not occur in the presence of rapamycin (Fig. 5A). In the amino acid-containing SD medium, Sap2 was not detected even when SAP2 transcription was turned on with doxycycline (Fig. 5B). In contrast, Sap2 was first detected after cells were treated with rapamycin for 1 h (Fig. 5B). These results indicate that amino acid starvation is also a signal for Sap2 protein expression through TOR signaling.
Fig 5.
Amino acid availability plays a role in the protein level regulation of Sap2.A. Reduction of Sap2 protein levels induced by a high concentration of amino acid did not occur in the presence of rapamycin. The SAP2ex4A strain carrying doxycycline-inducible SAP2 was first grown to mid-log phase in YCB–BSA–YE (0.01%) medium containing 30 μg/ml doxycycline at 30°C (Fig. 4). The cultures were then equally divided into tubes containing 1.8× CSM plus 0.2 μg/ml rapamycin (rapa) or 1.8× CSM plus 0.2% methanol (MeOH [the vehicle control]). The cells were incubated and collected at the indicated times. Intracellular protein samples (20 μg) were analyzed with SDS-PAGE and immunoblotted using monoclonal anti-Sap2. Time zero indicates a sample without rapamycin or methanol. The nonspecific band served as the loading control. The semiquantitative levels of Sap2 protein induced by rapamycin were calculated and plotted. m, minutes. (B) Sap2 protein level in SD medium with and without rapamycin. The SAP2ex4A strain was incubated in SD containing 30 μg/ml doxycycline at 30°C until reaching mid-log phase. The cell pellets were washed and resuspended in fresh SD medium with 30 μg/ml doxycycline. The cultures were equally divided into tubes containing 0.2 μg/ml rapamycin (rapa) or 0.2% methanol (MeOH [the vehicle control]). The cultures were grown and collected at the indicated times. Each sample (20 μg) was analyzed with SDS-PAGE and immunoblotted using monoclonal anti-Sap2. Time zero indicates a sample without rapamycin or methanol.
Identification of Rhb1-regulated genes in C. albicans.
This study also investigated possible target genes of C. albicans Rhb1 with DNA microarray analysis. Both wild-type and rhb1-deleted strains were grown in SD medium to mid-log phase, and total RNA was isolated. Samples were prepared and labeled with Cy3 or Cy5 fluorescent dye before data analysis. Using a P value of ≤0.0148 as the cutoff, expression of 109, 17, and 6 gene transcripts changed at least 1.5-, 2-, and 3-fold, respectively, in the rhb1-deleted mutant compared with levels in the wild type (Fig. 6A). The following discussion reviews the highlights of the 113 genes, and the details are listed in Tables S1 and S2 in the supplemental material. In addition to SAP2, the expression of genes such as MEP2, GAP2, RBT1, OPT1, and ALS3 was upregulated in the rhb1 mutant. MEP2 encodes an ammonium sensor and transporter (10), and its regulation by Rhb1 is consistent with previous findings (72). The GAP2 and OPT1 gene products are a general amino acid permease and an oligopeptide transporter, respectively (47). The RBT1 gene product is predicted to be a cell wall protein (6). ALS3 encodes an adhesin that plays a complementary role in C. albicans biofilm formation (52, 53). Tor1 kinase also regulates RBT1 and ALS3 (6), and GAP1, HIP1, and EFG1 are among the downregulated genes in the rhb1-deleted mutant. GAP1 encodes a general amino acid permease, and EFG1 encodes a transcription factor controlling C. albicans morphogenesis (52, 53). HIP1 encodes an uncharacterized protein regulated by Gcn2 and Gcn4 (70), and its sequence shows the best hit with S. cerevisiae GAP1. Real-time quantitative PCR verified the expression patterns of GAP1, GAP2, and HIP1 (Fig. 6B).
Fig 6.
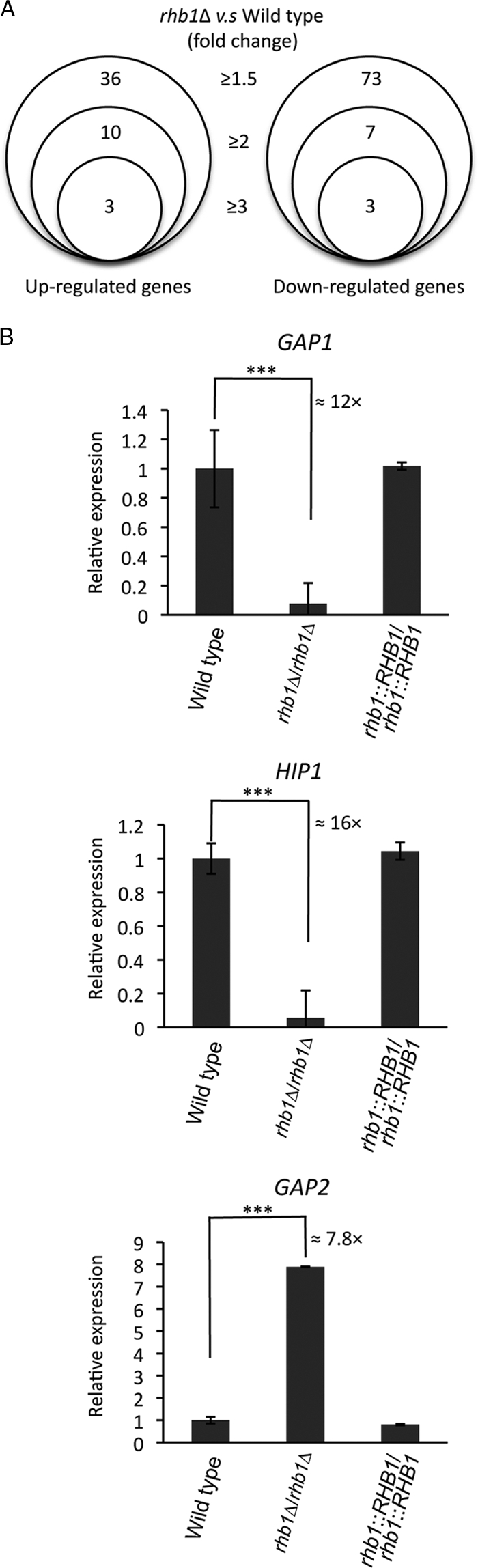
Identification of Rhb1 target genes by the use of DNA microarray analysis. (A) Summary of the fold change thresholds of the differentially expressed genes in the rhb1-deleted mutant (CCT-D1) compared with the wild type (SC5314). RNAs were collected after both strains were grown to mid-log phase in SD medium at 30°C. (B) Relative expression levels of three general amino acid permease genes: GAP1, HIP1, and GAP2. Quantitative real-time PCR was performed for GAP1, HIP1, and GAP2 in the wild-type (SC5314), CCT-D1 (rhb1Δ/rhb1Δ), and CCT-RD1 (RHB1-reconstituted) strains. Cells were grown in SD medium for 4 h to mid-log phase under the same conditions used in the microarray experiment. The threshold cycle (CT) value of each gene was derived from the average of the results of 3 experiments. The ΔCT value was determined by subtracting the average ΔCT of endogenous control EFB1 from the average CT of the gene tested. The ΔΔCT of each gene was calculated by subtracting the ΔCT value of the corresponding calibration value (wild-type sample). The average ΔΔCT and standard deviation values were determined from experiments performed in triplicate. The relative expression level of each gene is 2−ΔΔCT. ***, P ≤ 0.005. The error bars show the standard deviation.
DISCUSSION
The Ras superfamily of small GTPases is generally classified into 5 families, including Ras, Rho, Rab, Arf, and Ran (3). Of the Ras members, Ras homolog enriched in brain (Rheb) is a novel and unique small G protein (3). Most of the information on Rheb has been gathered from mammal studies. Translationally controlled tumor protein (TCTP; also known as p23) and tuberous sclerosis complex 2 (Tsc2) act as a guanine nucleotide exchange factor (GEF) and a GTPase-activating protein (GAP), respectively, to regulate Rheb (25, 57). Rheb is likely a positive regulator of the mTOR kinase and activates mTORC1 signaling (42).
The functions of Rhb1 (the homolog of Rheb) in fungi are not well characterized. In S. cerevisiae, Rhb1 functions in the uptake of arginine (75). However, no homolog of mammalian Tsc2 has been found in the genome of S. cerevisiae (3). Whether environmental signals can be conveyed from Rhb1 to TORC1 remains unknown. Rhb1 regulates amino acid uptake, mating, cell growth, cell cycle progression, and stress response in S. pombe (44, 73, 74, 76). Mutations in TSC2 cause defects in the uptake of arginine and leucine (77, 80) and lead to a delayed response in nitrogen starvation-mediated G1 arrest (48, 78). In fission yeast, TORC1 controls cell proliferation in response to nutrient signals and acts downstream of Rhb1 (51, 76).
Previous research identified Tsc2 and Rhb1 in C. albicans and linked Rhb1 to the Tor1 kinase (72). Rhb1 and Tor1 are involved in nitrogen starvation-induced morphogenesis by controlling MEP2 and cell wall integrity (6, 72). The results of the current study also indicate that the Rhb1-TOR signaling pathway controls the expression of the C. albicans virulence factor Sap2. In medium containing protein as the major nitrogen source, RHB1 overexpression attenuates the expression of Sap2 before 24 h but is otherwise normal (Fig. 1C). In the absence of RHB1, cells express Sap2 before other strains but otherwise function as normal (Fig. 1C). Although intrinsic growth defects appeared in the rhb1-deleted strain (Fig. 1D), a higher level of Sap2 may somehow compensate for this growth defect and result in a similar growth rate in the YCB–BSA–YE (0.01%) medium (Fig. 1A).
Rapamycin treatment also activated SAP2 in nitrogen-rich and no-protein conditions, which are generally unfavorable for SAP2 transcription (Fig. 2B). Rapamycin also enhanced the expression of Sap2 (Fig. 4B). The increased expression of SAP2 and its gene product somehow produced highly efficient Sap2 secretion (Fig. 1C and 2C). This result agrees with previous research showing that Sap synthesis and secretion are tightly coupled (30). Although the mechanisms of this process remain unclear, almost all S. cerevisiae mutants lacking vacuolar protein sorting (VPS) genes show susceptibility to rapamycin (82). Researchers recently reported that C. albicans mutants lacking VPS1 and VPS4 were defective in Sap2 secretion in an extracellular environment (8, 38). Moreover, we also tested a vps4-deleted strain and a strain in which VPS1 was repressed by doxycycline and found that both were hypersensitive to rapamycin (data not shown). The mechanisms of VPS-mediated Sap2 secretion and Rhb1-TOR signaling are under investigation in our laboratory.
The transcription factors Gln3 and Gat1 regulate another transcriptional factor, Stp1, which in turn controls SAP2 (22). This report links the Rhb1-TOR signaling pathway with this Stp1-mediated SAP2 regulation (Fig. 3A and B). The Csy1, Ptr3, and Ssy5 (SPS) sensor complex likely regulates the activities of C. albicans Stp1 and another related transcriptional factor, Stp2 (47). In the presence of amino acids, Stp1 and Stp2 undergo proteolytic cleavage by the activated SPS complex. Under these conditions, processed Stp1 and Stp2 lack N-terminal inhibitory domains and are translocated from the cytosol to the nucleus. Processed Stp1 regulates protein utilization genes such as SAP2 and OPT1, whereas processed Stp2 regulates genes related to amino acid utilization, including the amino acid permeases Gap1 and Gap2 (47). Expression of some other genes (e.g., an oligopeptide transporter [OPT3]) can be induced by either processed Stp1 or Stp2. DNA microarray analysis revealed that OPT1, GAP2, and SAP2 were upregulated after deletion of RHB1, whereas GAP1 and HIP1 were downregulated (see Tables S1 and S2 in the supplemental material). These permeases may be responsible for assimilating extracellular amino acids or oligopeptides generated from protein degradation by proteases such as Sap2 (47). Based on the pattern of differential expression in the rhb1-deleted mutant, GAP1, GAP2, and the potential amino acid permease HIP1 may function in a different manner. Previous research suggested that the control of GAP1 and GAP2 expression occurs through Stp2 acting downstream of SPS (47). This notion is consistent with the expression of GAP2 in YPD and M199 (pH 8.0) upon rapamycin treatment (83). However, the observation that GAP1 is upregulated in response to rapamycin (data not shown) conflicts with data from the rhb1-deleted strain (Fig. 6B). This suggests that another factor(s) downstream or between Rhb1 and Tor1 kinase may be involved, particularly in controlling SPS-regulated genes. The divergent expression patterns of GAP1 also suggest that Rhb1 and rapamycin affect Tor1 kinase differently. Rhb1 and the FKBP12-rapamycin complex may target different regions of the C. albicans Tor1 kinase. This notion is supported by the observations that human Rheb interacts with the N terminus of mTOR (43) and that FKBP12-rapamycin interacts with the FRB domain of C. albicans Tor1 (20). In the absence of RHB1, the binding defect of the FKBP12-rapamycin complex due to the mutant FRB domain of Tor1 kinase (TOR1S1984I) was partially restored (Fig. 2A). One possible explanation is that Rhb1 targeting may alter the structure of Tor1 kinase, which in turn may change the binding of the FKBP12-rapamycin complex to Tor1 kinase. However, it is also possible that Rhb1 regulates GAP1 in an unknown, rapamycin-independent manner.
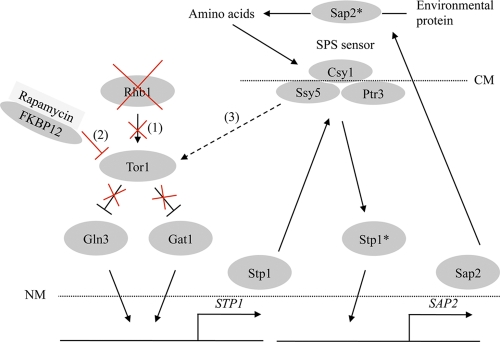
These results indicate that the Rhb1-TOR signaling pathway controls protein utilization through Stp1 and suggest that this pathway may function during amino acid utilization through Stp2. Nevertheless, there may be another regulatory route that is independent of TOR but controlled by the Rhb1 small GTPase. Figure 7 provides a simple model for exploring the interplay between Rhb1-TOR signaling and the downstream effectors in controlling SAP2 expression. Rapamycin inactivation of Tor in S. cerevisiae effectively inhibits translation initiation and leads to G1 arrest (5). Although these molecular mechanisms have not been thoroughly defined, the Sap2 protein level was enhanced by rapamycin treatment (Fig. 4B) but was largely reduced after the addition of amino acids (Fig. 5A). This suggests that the inhibition of Tor1 kinase targeted by rapamycin-FKBP12 complex reduced Sap2 protein expression or prevented sorting of newly synthesized Sap2 for subsequent degradation, as is the case for certain amino acid permeases, for example, Gap1 of S. cerevisiae (63). In other words, high concentrations of environmental amino acids might promote degradation of Sap2 or inhibit Sap2 expression via the general amino acid control (GAAC) pathway. This is because Sap2 is no longer essential for cell growth under these conditions. These results also reveal that a nutrient-rich growing environment has an effect opposite that of rapamycin, which inhibits Tor1 kinase. Similar effects were also observed regarding the activity of mTOR and amino acid availability. The regulation of the intracellular Sap2 protein level by TOR signaling is part of the cellular response to amino acid availability. Starvation resulting from amino acid deficiency activates the GAAC pathway in S. cerevisiae (29), and the GAAC is integrated with the TOR signaling pathway (68). The GAAC pathway is poorly understood in C. albicans. Although Gcn4 regulates the expression of amino acid synthetic genes in response to amino acid starvation, regulation of Gcn4 mRNA does not seem to occur (71). The C. albicans ortholog of Gcn2 also has limited involvement in the cellular response to amino acid starvation. This is likely because Gcn2 inactivation only partially attenuates cell growth under conditions of amino acid starvation (70). However, the regulation of amino acid biosynthetic genes is still dependent on Gcn4 (70). The DNA microarray analysis in this study confirms that several amino acid biosynthetic and Gcn4 target genes were differentially expressed in the rhb1-deleted mutant (see Tables S1 and S2 in the supplemental material). This suggests that GAAC regulation in C. albicans may occur through the Rhb1-TOR signaling pathway. Future research on this topic will investigate the details of C. albicans GCN-like regulation mediated by Rhb1 and TOR signaling.
Fig 7.
Model depicting Rhb1-TOR signaling in the regulation of SAP2 expression. In the absence of Rhb1 (“1”) or in cells treated with rapamycin (“2”), TOR signaling directly or indirectly affects SAP2 expression by transmitting the signal from Gln3 and Gat1 to activate STP1 expression. The SPS complex processes Stp1, and the active form of Stp1 in turn triggers SAP2 expression. Sap2 is processed before secretion into the environment. Enriched extracellular amino acids from extracellular proteins digested by Sap2 or other protease may also transmit a signal to activate the Tor1 kinase (“3”) (which may or may not occur through the SPS sensor) to inhibit SAP2 expression (and thus the Sap2 protein level) and other SPS-responsive genes. CM, cell membrane; NM, nuclear membrane. An asterisk indicates the active form of a particular protein, and a dashed line represents a hypothetical correlation.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Alistair J. Brown (University of Aberdeen, United Kingdom), Maris Cardenas (Duke University), Samuel Lee (University of New Mexico Health Science Center), and Joachim Morschhäuser (Universität Würzburg, Germany) for providing plasmids and strains. We also thank Yi-Ta Chen (National Tsing Hua University, Taiwan), Shu-Jen Chou, and Shu-Hsing Wu (DNA Microarray Core Laboratory, Institute of Plant and Microbial Biology, Academia Sinica, Taiwan) for the technical assistance in the fabrication of DNA microarray of C. albicans.
This work was supported by grants NSC98-2311-B-007-010-MY3 and NSC99-2627-B-007-007 (to C.-Y.L.) and NSC97-2118-M-007-004-MY2 (to W.-P.H.) from the National Science Council (Taiwan).
Footnotes
Published ahead of print 22 December 2011
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Alonso-Monge R, et al. 2010. The Sko1 protein represses the yeast-to-hypha transition and regulates the oxidative stress response in Candida albicans. Fungal Genet. Biol. 47:587–601 [DOI] [PubMed] [Google Scholar]
- 2. Aronova S, Wedaman K, Anderson S, Yates J, III, Powers T. 2007. Probing the membrane environment of the TOR kinases reveals functional interactions between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Mol. Biol. Cell 18:2779–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aspuria PJ, Tamanoi F. 2004. The Rheb family of GTP-binding proteins. Cell Signal. 16:1105–1112 [DOI] [PubMed] [Google Scholar]
- 4. Bain JM, Stubberfield C, Gow NA. 2001. Ura-status-dependent adhesion of Candida albicans mutants. FEMS Microbiol. Lett. 204:323–328 [DOI] [PubMed] [Google Scholar]
- 5. Barbet NC, et al. 1996. TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 7:25–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bastidas RJ, Heitman J, Cardenas ME. 2009. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 5:e1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck T, Hall MN. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689–692 [DOI] [PubMed] [Google Scholar]
- 8. Bernardo SM, Khalique Z, Kot J, Jones JK, Lee SA. 2008. Candida albicans VPS1 contributes to protease secretion, filamentation, and biofilm formation. Fungal Genet. Biol. 45:861–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bertram PG, et al. 2000. Tripartite regulation of Gln3p by TOR, Ure2p, and phosphatases. J. Biol. Chem. 275:35727–35733 [DOI] [PubMed] [Google Scholar]
- 10. Biswas K, Morschhauser J. 2005. The Mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Mol. Microbiol. 56:649–669 [DOI] [PubMed] [Google Scholar]
- 11. Brand A, MacCallum DM, Brown AJ, Gow NA, Odds FC. 2004. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot. Cell 3:900–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calderone RA. 2002. Candida and candidiasis. ASM Press, Washington, DC [Google Scholar]
- 13. Calderone RA, Fonzi WA. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327–335 [DOI] [PubMed] [Google Scholar]
- 14. Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J. 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13:3271–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen EJ, Kaiser CA. 2003. LST8 negatively regulates amino acid biosynthesis as a component of the TOR pathway. J. Cell Biol. 161:333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng S, et al. 2003. Evaluation of the roles of four Candida albicans genes in virulence by using gene disruption strains that express URA3 from the native locus. Infect. Immun. 71:6101–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Correia A, et al. 2010. Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect. Immun. 78:4839–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox KH, et al. 2000. Saccharomyces cerevisiae GATA sequences function as TATA elements during nitrogen catabolite repression and when Gln3p is excluded from the nucleus by overproduction of Ure2p. J. Biol. Chem. 275:17611–17618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crandall M, Edwards JE., Jr 1987. Segregation of proteinase-negative mutants from heterozygous Candida albicans. J. Gen. Microbiol. 133:2817–2824 [DOI] [PubMed] [Google Scholar]
- 20. Cruz MC, et al. 2001. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob. Agents Chemother. 45:3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dabas N, Morschhauser J. 2007. Control of ammonium permease expression and filamentous growth by the GATA transcription factors GLN3 and GAT1 in Candida albicans. Eukaryot. Cell 6:875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dabas N, Morschhauser J. 2008. A transcription factor regulatory cascade controls secreted aspartic protease expression in Candida albicans. Mol. Microbiol. 69:586–602 [DOI] [PubMed] [Google Scholar]
- 23. deHart AK, Schnell JD, Allen DA, Tsai JY, Hicke L. 2003. Receptor internalization in yeast requires the Tor2-Rho1 signaling pathway. Mol. Biol. Cell 14:4676–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fadri M, Daquinag A, Wang S, Xue T, Kunz J. 2005. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol. Biol. Cell 16:1883–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garami A, et al. 2003. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11:1457–1466 [DOI] [PubMed] [Google Scholar]
- 26. Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 27. Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. 1999. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc. Natl. Acad. Sci. U. S. A. 96:14866–14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heitman J, Movva NR, Hall MN. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253:905–909 [DOI] [PubMed] [Google Scholar]
- 29. Hinnebusch AG. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. J. Biol. Chem. 272:21661–21664 [DOI] [PubMed] [Google Scholar]
- 30. Homma M, Chibana H, Tanaka K. 1993. Induction of extracellular proteinase in Candida albicans. J. Gen. Microbiol. 139(Pt. 6):1187–1193 [DOI] [PubMed] [Google Scholar]
- 31. Hsu PC, Yang CY, Lan CY. 2011. Candida albicans Hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot. Cell 10:207–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hube B, Monod M, Schofield DA, Brown AJ, Gow NA. 1994. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol. Microbiol. 14:87–99 [DOI] [PubMed] [Google Scholar]
- 33. Hube B, Ruchel R, Monod M, Sanglard D, Odds FC. 1998. Functional aspects of secreted Candida proteinases. Adv. Exp. Med. Biol. 436:339–344 [DOI] [PubMed] [Google Scholar]
- 34. Hube B, et al. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuruvilla FG, Shamji AF, Schreiber SL. 2001. Carbon- and nitrogen-quality signaling to translation are mediated by distinct GATA-type transcription factors. Proc. Natl. Acad. Sci. U. S. A. 98:7283–7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lan CY, et al. 2004. Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53:1451–1469 [DOI] [PubMed] [Google Scholar]
- 37. Lay J, et al. 1998. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect. Immun. 66:5301–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SA, et al. 2009. Candida albicans VPS4 is required for secretion of aspartyl proteases and in vivo virulence. Mycopathologia 167:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lermann U, Morschhauser J. 2008. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 154:3281–3295 [DOI] [PubMed] [Google Scholar]
- 40. Liao WL, Ramon AM, Fonzi WA. 2008. GLN3 encodes a global regulator of nitrogen metabolism and virulence of C. albicans. Fungal Genet. Biol. 45:514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loewith R, et al. 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10:457–468 [DOI] [PubMed] [Google Scholar]
- 42. Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. 2005. Rheb binds and regulates the mTOR kinase. Curr. Biol. 15:702–713 [DOI] [PubMed] [Google Scholar]
- 43. Long X, Ortiz-Vega S, Lin Y, Avruch J. 2005. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J. Biol. Chem. 280:23433–23436 [DOI] [PubMed] [Google Scholar]
- 44. Mach KE, Furge KA, Albright CF. 2000. Loss of Rhb1, a Rheb-related GTPase in fission yeast, causes growth arrest with a terminal phenotype similar to that caused by nitrogen starvation. Genetics 155:611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Magasanik B. 2005. The transduction of the nitrogen regulation signal in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 102:16537–16538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Magasanik B, Kaiser CA. 2002. Nitrogen regulation in Saccharomyces cerevisiae. Gene 290:1–18 [DOI] [PubMed] [Google Scholar]
- 47. Martínez P, Ljungdahl PO. 2005. Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol. Cell. Biol. 25:9435–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsumoto S, Bandyopadhyay A, Kwiatkowski DJ, Maitra U, Matsumoto T. 2002. Role of the Tsc1-Tsc2 complex in signaling and transport across the cell membrane in the fission yeast Schizosaccharomyces pombe. Genetics 161:1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M. 2007. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol. Cell. Biol. 27:3154–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naglik JR, Challacombe SJ, Hube B. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 67:400–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakashima A, Sato T, Tamanoi F. 2010. Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J. Cell Sci. 123:777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nobile CJ, et al. 2006. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nobile CJ, Nett JE, Andes DR, Mitchell AP. 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 5:1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Odds FC. 1988. Candida and candidosis. Baillière Tindall, London, United Kingdom [Google Scholar]
- 55. Otsubo Y, Yamamato M. 2008. TOR signaling in fission yeast. Crit. Rev. Biochem. Mol. Biol. 43:277–283 [DOI] [PubMed] [Google Scholar]
- 56. Park YN, Morschhauser J. 2005. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot. Cell 4:1328–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rehmann H, et al. 2008. Biochemical characterisation of TCTP questions its function as a guanine nucleotide exchange factor for Rheb. FEBS Lett. 582:3005–3010 [DOI] [PubMed] [Google Scholar]
- 58. Reinke A, et al. 2004. TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. J. Biol. Chem. 279:14752–14762 [DOI] [PubMed] [Google Scholar]
- 59. Reuss O, Morschhauser J. 2006. A family of oligopeptide transporters is required for growth of Candida albicans on proteins. Mol. Microbiol. 60:795–812 [DOI] [PubMed] [Google Scholar]
- 60. Reuss O, Vik A, Kolter R, Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 61. Rohde JR, Bastidas R, Puria R, Cardenas ME. 2008. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr. Opin. Microbiol. 11:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rohde JR, et al. 2004. TOR controls transcriptional and translational programs via Sap-Sit4 protein phosphatase signaling effectors. Mol. Cell. Biol. 24:8332–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rubio-Texeira M, Kaiser CA. 2006. Amino acids regulate retrieval of the yeast general amino acid permease from the vacuolar targeting pathway. Mol. Biol. Cell 17:3031–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Russell CL, Brown AJ. 2005. Expression of one-hybrid fusions with Staphylococcus aureus lexA in Candida albicans confirms that Nrg1 is a transcriptional repressor and that Gcn4 is a transcriptional activator. Fungal Genet. Biol. 42:676–683 [DOI] [PubMed] [Google Scholar]
- 65. Sharkey LL, Liao WL, Ghosh AK, Fonzi WA. 2005. Flanking direct repeats of hisG alter URA3 marker expression at the HWP1 locus of Candida albicans. Microbiology 151:1061–1071 [DOI] [PubMed] [Google Scholar]
- 66. Shin CS, Kim SY, Huh WK. 2009. TORC1 controls degradation of the transcription factor Stp1, a key effector of the SPS amino-acid-sensing pathway in Saccharomyces cerevisiae. J. Cell Sci. 122:2089–2099 [DOI] [PubMed] [Google Scholar]
- 67. Staib P, et al. 2008. Tetracycline-inducible expression of individual secreted aspartic proteases in Candida albicans allows isoenzyme-specific inhibitor screening. Antimicrob. Agents Chemother. 52:146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Staschke KA, et al. 2010. Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J. Biol. Chem. 285:16893–16911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U. S. A. 100:9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tournu H, et al. 2005. Global role of the protein kinase Gcn2 in the human pathogen Candida albicans. Eukaryot. Cell 4:1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tripathi G, et al. 2002. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 21:5448–5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tsao CC, Chen YT, Lan CY. 2009. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet. Biol. 46:126–136 [DOI] [PubMed] [Google Scholar]
- 73. Urano J, et al. 2005. Identification of novel single amino acid changes that result in hyperactivation of the unique GTPase, Rheb, in fission yeast. Mol. Microbiol. 58:1074–1086 [DOI] [PubMed] [Google Scholar]
- 74. Urano J, et al. 2007. Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 104:3514–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Urano J, Tabancay AP, Yang W, Tamanoi F. 2000. The Saccharomyces cerevisiae Rheb G-protein is involved in regulating canavanine resistance and arginine uptake. J. Biol. Chem. 275:11198–11206 [DOI] [PubMed] [Google Scholar]
- 76. Uritani M, et al. 2006. Fission yeast Tor2 links nitrogen signals to cell proliferation and acts downstream of the Rheb GTPase. Genes Cells 11:1367–1379 [DOI] [PubMed] [Google Scholar]
- 77. van Slegtenhorst M, Carr E, Stoyanova R, Kruger WD, Henske EP. 2004. Tsc1+ and tsc2+ regulate arginine uptake and metabolism in Schizosaccharomyces pombe. J. Biol. Chem. 279:12706–12713 [DOI] [PubMed] [Google Scholar]
- 78. van Slegtenhorst M, Mustafa A, Henske EP. 2005. Pas1, a G1 cyclin, regulates amino acid uptake and rescues a delay in G1 arrest in Tsc1 and Tsc2 mutants in Schizosaccharomyces pombe. Hum. Mol. Genet. 14:2851–2858 [DOI] [PubMed] [Google Scholar]
- 79. Wedaman KP, et al. 2003. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell 14:1204–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weisman R, Roitburg I, Nahari T, Kupiec M. 2005. Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 169:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. White TC, Agabian N. 1995. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J. Bacteriol. 177:5215–5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xie MW, et al. 2005. Insights into TOR function and rapamycin response: chemical genomic profiling by using a high-density cell array method. Proc. Natl. Acad. Sci. U. S. A. 102:7215–7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zacchi LF, Gomez-Raja J, Davis DA. 2010. Mds3 regulates morphogenesis in Candida albicans through the TOR pathway. Mol. Cell. Biol. 30:3695–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhao X, Oh SH, Yeater KM, Hoyer LL. 2005. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology 151:1619–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.