Abstract
Oligomeric association of human small heat shock proteins HspB1, HspB5, HspB6 and HspB8 was analyzed by means of size-exclusion chromatography, analytical ultracentrifugation and chemical cross-linking. Wild-type HspB1 and Cys mutants of HspB5, HspB6 and HspB8 containing a single Cys residue in position homologous to that of Cys137 of human HspB1 were able to generate heterodimers cross-linked by disulfide bond. Cross-linked heterodimers between HspB1/HspB5, HspB1/HspB6 and HspB5/HspB6 were easily produced upon mixing, whereas formation of any heterodimers with participation of HspB8 was significantly less efficient. The size of heterooligomers formed by HspB1/HspB6 and HspB5/HspB6 was different from the size of the corresponding homooligomers. Disulfide cross-linked homodimers of small heat shock proteins were unable to participate in heterooligomer formation. Thus, monomers can be involved in subunit exchange leading to heterooligomer formation and restriction of flexibility induced by disulfide cross-linking prevents subunit exchange.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-011-0296-0) contains supplementary material, which is available to authorized users.
Keywords: Human small heat shock proteins, Heterooligomeric complexes, Subunit exchange, Chemical crosslinking
Introduction
More than 8,700 proteins expressed in all domains including archea, bacteria and eukarya and belonging to the family of the so-called small heat shock proteins (sHsp) are at present deposited in protein databases (Kriehuber et al. 2010). The monomers of small heat shock proteins have small molecular mass (12–43 kDa) and contain a conservative α-crystallin domain consisting of about 90 amino acid residues (Haslbeck et al. 2005; Vos et al. 2008; McHaourab et al. 2009). α-Crystallin domain is considered an unmistakable hallmark of this protein family (Kappe et al. 2010). This domain participates in formation of dimers of small heat shock proteins (Bagneris et al. 2009; Laganowsky et al. 2010; Baranova et al. 2011). In addition to the conservative α-crystallin domain, small heat shock proteins contain a variable in size and structure N-terminal domain and a short C-terminal extension, both of which are playing important roles in oligomerization of these proteins (McHaourab et al. 2009; Stamler et al. 2005; van Montfort et al. 2001). As a rule, the small heat shock proteins tend to form high molecular mass oligomers containing variable number of monomers (Kim et al. 1998; van Montfort et al. 2001; Stamler et al. 2005; Narberhaus 2002; Jehle et al. 2011). These oligomers display a very flexible quaternary structure (McHaourab et al. 2009; Haley et al. 2000; Jehle et al. 2011) easily exchanging their subunits (Studer and Narberhaus 2000; Sobott et al. 2002), thus making detailed investigation of their structure very complicated.
The human genome contains ten genes encoding for small heat shock proteins (Fontaine et al. 2003; Kappe et al. 2003). Some of these proteins (i.e. HspB2, HspB3, αA-crystallin (HspB4), HspB7, HspB9, HspB10) are tissues specific, whereas others (i.e. HspB1, αB-crystallin (HspB5), HspB6 and HspB8) are ubiquitously expressed in practically all human tissues (Taylor and Benjamin 2005). Some of the small heat shock proteins are very abundant. For instance, αB-crystallin (HspB5) comprises up to 3% of cardiac homogenate (Lutsch et al. 1997), and the concentration of HspB1 and HspB6 varies from 200 to 12,000 ng/mg of protein in different types of muscle (Kato et al. 1992, 1994; Sakuma et al. 1998). Very often, different small heat shock proteins are simultaneously expressed in the same tissue in high amounts, making possible formation of heterooligomeric complexes (Sugiyama et al. 2000; Suzuki et al. 1998). The data describing the interaction of different small heat shock proteins for formation of heterooligomeric complexes are constantly accumulating in the literature. Heterooligomeric complexes of ubiquitously expressed αA- and αB-crystallin (Datta and Rao 2000; Horwitz 2003), HspB1 and αA- (Bova et al. 2000) or αB-crystallin (Zantema et al. 1992), HspB6 and αB-crystallin (Kato et al. 1992, 1994) and HspB1 and HspB6 (Bukach et al. 2004, 2009) are few examples reported so far. It is also postulated that HspB8 is able to interact with practically all known small heat shock proteins (Fontaine et al. 2005; Sun et al. 2004).
Although formation of heterooligomeric complexes is universally recognized, many important questions remain unanswered. For instance, the subunit stoichiometry within the heterooligomeric complexes, the mechanism of subunit exchange, as well as the relative interaction preference of different small heat shock proteins to each other remain unclear. This paper is devoted to investigate the nature of heterooligomeric complexes formed by four ubiquitous human small heat shock proteins (HspB1, HspB5, HspB6 and HspB8) and mechanism of subunit exchange during the course of formation of heterooligomeric complexes.
Materials and methods
Expression and purification of recombinant proteins
Recombinant wild-type untagged human HspB1 and its 3D mutant with replacing Ser18, Ser78 and Ser82 with Asp residues, αB-crystallin (HspB5), HspB6 and HspB8 were expressed and purified as described earlier (Bukach et al. 2004; Kasakov et al. 2007; Mymrikov et al. 2010). The Cys mutants (see below) of all above-mentioned small heat shock proteins were obtained as described earlier (Mymrikov et al. 2010). Cys mutants were generated by replacing all endogenous Cys residues with Ser and insertion of a single Cys residue in position homologous to that of Cys137 of the wild-type HspB1. All proteins displayed single bands in sodium dodecyl sulphate (SDS)–gel electrophoresis (Laemmli 1970) and were stored frozen at −20°C in buffer B (20 mM Tris–acetate pH 7.6, 10 mM NaCl, 0.1 mM EDTA, 0.1 mM PMSF and 1 mM DTT). The protein concentration was determined spectrophotometrically using A0.1%280 equal to 1.775 for HspB1 (UniProtKB P04792), 0.693 for αB-crystallin (HspB5) (UniProtKB P02489), 0.582 for HspB6 (UniProtKB O14558) and 1.225 for HspB8 (UniProtKB Q9UJY1).
Preparation of reduced and oxidized samples of sHsp
Reduced samples were obtained by incubation of proteins at 37°C for 30 min in buffer B containing additionally freshly prepared 15 mM DTT. Thus, prepared samples were either used directly or subjected to different treatments. Oxidation of individual proteins (wild-type HspB1 and Cys mutants of all other sHsp) was carried out by overnight dialysis against buffer A (50 mM Tris–HCl, pH 7.4, containing 50 mM KCl, 1 mM MgCl2, 0.1 mM PMSF) at 37°C, which generated disulfide bond cross-linking for more than 70% of the protein amount (Mymrikov et al. 2010). For preparation of oxidized heterodimers (or heterooligomers), the wild-type HspB1 or Cys mutants of αB-crystallin, HspB6 and HspB8 were dialyzed overnight against buffer A at 15°C.
Formation of heterooligomeric complexes and subunit exchange
Reduced proteins were mixed pair-wise at the concentration equal to 0.5 or 1.0 mg/ml for each protein and incubated for 1 h at 42°C in buffer B. Similar procedure was used for the oxidized proteins, except that buffer A replaced buffer B. Earlier published results indicate that these conditions are sufficient for complete subunit exchange of different small heat shock proteins (Bukach et al. 2009). The subunit exchange was stopped by placing protein samples at cold (4°C), which made the subunit exchange negligibly slow (Bova et al. 1997; Bukach et al. 2009).
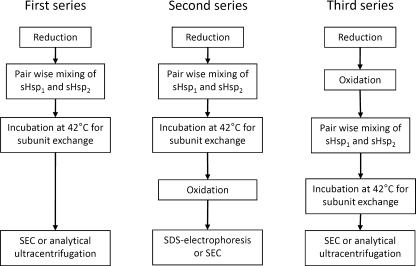
In the first series of experiments, reduced wild-type sHsp or its Cys mutants were pair-wise mixed together, subjected to subunit exchange and afterwards analyzed by means of size-exclusion chromatography (SEC) and analytical ultracentrifugation (Fig. 1). In the second series of experiments, reduced Cys mutants of the small heat shock proteins and the wild-type HspB1 were pair-wise mixed together, subjected to subunit exchange at 42°C and oxidized for formation of disulfide cross-links by dialysis overnight against buffer A at 15°C. The samples thus obtained were analyzed by means of 10–20% gradient SDS–gel electrophoresis (Treweek et al. 2005) or size-exclusion chromatography (Fig. 1). In the third series of experiments, Cys mutants of small heat shock proteins and the wild-type HspB1 were firstly oxidized by overnight dialysis against buffer A at 37°C for formation of cross-linked homodimers. Thus, obtained samples of oxidized proteins were mixed pair-wise, incubated at 42°C for 1 h for subunit exchange and afterwards analyzed by means of size-exclusion chromatography and analytical ultracentrifugation (Fig. 1).
Fig. 1.
The scheme of three series of experiments performed with different small heat shock proteins and their Cys mutants. sHsp1 and sHsp2 are two different small heat shock proteins or their Cys mutants; mild oxidation was used for cross-linking of two neighbouring subunits via disulfide bond formed by Cys residues located in the β7 strand of α-crystallin domain
Size-exclusion chromatography
Reduced or oxidized samples of sHsp were subjected to size-exclusion chromatography performed on Superdex 200 HR 10/30 column equilibrated with buffer C (20 mM Tris–acetate pH 7.6, 150 mM NaCl, 0.1 mM EDTA, 0.1 mM PMSF) in the presence or in the absence of 15 mM β-ME. The sample (200 μl) loaded on the column contained 180 μg of analyzed proteins. The column was run at the flow rate of 0.5 ml/min, and the fractions (400 μl) were collected and analyzed by SDS–gel electrophoresis using either 15% (Laemmli 1970) or 10–20% gradient (Treweek et al. 2005) polyacrylamide gel. The column was calibrated by using the following protein markers with Stokes radii and molecular masses indicated in parenthesis: thyroglobulin (85 Å, 669 kDa), ferritin (61 Å, 440 kDa), catalase (52 Å, 240 kDa), rabbit skeletal muscle glyceraldehyde-3-phosphate dehydrogenase (42 Å, 144 kDa), bovine serum albumin (35 Å, 68 kDa), ovalbumin (26.8 Å, 43 kDa), chymotrypsin (22.54 Å, 25 kDa) and ribonuclease (17.7 Å, 13.7 kDa).
Analytical ultracentrifugation
Samples of isolated sHsp (each 0.5 mg/ml) or their mixture (0.5 mg/ml of each) were incubated either at 4°C for 1 h (negligible slow subunit exchange) or at 42°C for 1 h (nearly complete subunit exchange) and then dialyzed overnight at 4°C against phosphate buffer saline (50 mM phosphate, pH 7.5, 150 mM NaCl) supplemented or not with 2.0 mM DTT. Sedimentation velocity experiments were performed in a Spinco model E analytical ultracentrifuge (Beckman) equipped with absorbance optics, scanner and computer on-line. A six-hole AnJ-Ti rotor and 12-mm double sector cells were used. The protein samples (400 μl) were subjected to ultracentrifugation at 48,000 rpm at 4°C. Absorbance at 280 nm was recorded simultaneously every 1.5 min in all cells. Sedimentation coefficients were estimated from differential distribution of sedimentation coefficient [C(S20,W) versus S20,W] using SEDFIT program (Schuck 2000).
Electrophoretic methods
Protein composition of samples was analyzed by SDS–gel electrophoresis using either homogeneous 15% (Laemmli 1970) or gradient 10–20% (Treweek et al. 2005) polyacrylamide gels, under both reducing and non-reducing conditions. In order to separate homodimers of HspB1, homodimers of Cys mutant of HspB8 and heterodimer formed by these proteins, SDS electrophoresis was performed on 10% polyacrylamide gels containing 6 M urea. After staining and destaining, the gels were subjected to the quantitative densitometry and integral optical density of each protein band was determined.
For estimation of the quantity of disulfide cross-linked heterodimers, we performed quantitative densitometry of all bands corresponding to cross-linked dimers (both homo- and heterodimers) (lane 2 on Fig. 2). Calculating the ratio of the integral optical density of the band corresponding to heterodimers to the sum of integrated optical densities of homo- and heterodimers, we estimated the portion of small heats shock proteins involved in heterodimer formation.
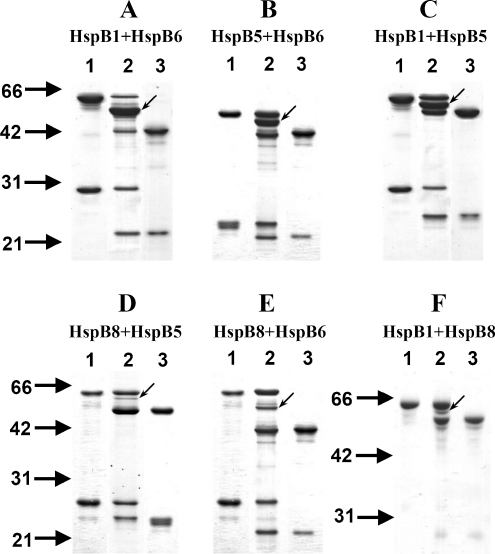
Fig. 2.
Disulfide cross-linking of the wild-type HspB1 and Cys mutant of human small heat shock proteins. Non-reducing SDS–gel electrophoresis of individual oxidized proteins is presented on lanes 1 and 3 of each panel. The protein composition of the oxidized mixture of two corresponding proteins is presented on lane 2 of each panel. a HspB1 + HspB6; b HspB5 + HspB6; c HspB1 + HspB5; d HspB8 + HspB5; e HspB8 + HspB6; f HspB1 + HspB8. Light arrows indicate position of cross-linked heterodimers. The positions of molecular mass standards (in kilodaltons) are indicated by heavy arrows
Results
Heterooligomeric complexes formed by Cys mutants of small heat shock proteins and HspB1
The data from literature indicate that the β7 strand of α-crystallin domain is directly involved in the formation of intersubunits contacts inside of sHsp dimers (Baranova et al. 2011; Bagneris et al. 2009; Laganowsky et al. 2010). Cys137 of human HspB1 is located in the central part of β7 strand. Moreover, two Cys137 residues of neighboring HspB1 monomers are close enough (less than 8 Å) (Berengian et al. 1999) to be easily oxidized into a disulfide bond generating a cross-linked dimer (Zavialov et al. 1998). Earlier, we obtained Cys mutants of HspB5, HspB6 and HspB8 lacking endogenous Cys residue and containing a single Cys in position homologous to that of Cys137 of HspB1 (Mymrikov et al. 2010). The structure and properties of these Cys mutants were practically indistinguishable from the corresponding properties of the wild-type proteins (Mymrikov et al. 2010); therefore, these mutants were considered to be suitable for investigation of heterooligomeric complexes formed by different small heat shock proteins.
As reported earlier, mild oxidation of the wild-type HspB1 (or its 3D mutant mimicking phosphorylation) or of the Cys mutants of HspB5, HspB6 and HspB8 was accompanied by their cross-linking via disulfide bond formed by two neighbouring Cys residues located in the β7 strand of α-crystallin domain (lanes 1 and 3 on each panel of Fig. 2). As a rule, more than 70% of isolated small heat shock proteins generated cross-linked homodimers (see Fig. 2). When the equimolar protein mixture, containing either two Cys mutants or a Cys mutant and the wild-type HspB1, was subjected to mild oxidation, a new band (marked by arrow on Fig. 2) emerged on the unreduced SDS–gel. This band had an apparent molecular mass of intermediate value between the molecular masses of individual cross-linked homodimers of sHsp subjected to oxidation and corresponds to the cross-linked heterodimer. Indeed, sample reduction prior to electrophoresis was accompanied by disappearance of the cross-linked heterodimer band and accumulation of the bands corresponding to the monomers of the analyzed small heat shock proteins (Online Resource 1). The intensity of heterodimer band was especially high for the following pairs of sHsp: HspB1 + HspB6 (panel A on Fig. 2), HspB5 + HspB6 (panel B on Fig. 2) and HspB1 + HspB5 (panel C on Fig. 2). In all these cases, the intensity of the band corresponding to the heterodimer was larger or equal to the intensity of the bands corresponding to homodimers. Quantitative densitometry of the gels indicates that in these pairs of sHsp, no less than 35% of proteins were involved in formation of heterodimers. On the contrary, in all pairs containing HspB8 (Fig. 2d–f), the quantity of heterodimers was significantly lower, and only 15–25% of sHsp were involved in formation of heterodimers. The data presented here indicate that HspB1, HspB5 and HspB6 effectively interact with each other, easily forming heterodimers. At the same time, Cys mutant of HspB8 was less effective in formation of heterodimers than any other sHsp. It is worthwhile to mention that if oxidation was performed at high temperature (37°C), then the quantity of heterodimer formed was increased and at the same time the difference between different pairs of small heat shock proteins became less pronounced.
Interaction of the wild-type HspB1, HspB5 and HspB6 and of their Cys mutants
Since HspB8 was less effective in heterooligomer formation according to our results, we excluded it from further investigation and focused on the association of three other sHsp, namely HspB1, HspB5 and HspB6.
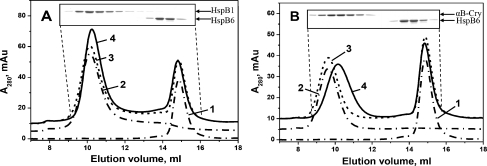
Isolated reduced wild-type small heat shock proteins or their pair-wise mixtures were incubated for 1 h at 42°C to achieve complete subunit exchange. In the control sample, the same mixture was incubated for 1 h at 4°C to prevent any subunit exchange. Thus, obtained samples were subjected to the size-exclusion chromatography and analytical ultracentrifugation. Isolated HspB1 and isolated HspB6 were eluted on the size-exclusion chromatography as symmetrical peaks with apparent molecular masses of about 660 kDa (10.2 ml) and 55 kDa (14.9 ml), respectively (Fig. 3a). If the mixture of HspB1 and HspB6 was pre-incubated at 42°C, then two new peaks with apparent molecular masses of about 100–150 kDa (13.6 ml) and 250–300 kDa (11.8 ml) appeared on the elution profile (Fig. 3a). According to the data of the SDS–gel electrophoresis, both these peaks contained equimolar amounts of HspB1 and HspB6 (insert on Fig. 3a). These data agree with our earlier published results (Bukach et al. 2009) and indicate that HspB1 and HspB6 form two types of heterooligomeric complexes.
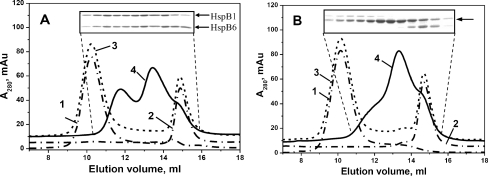
Fig. 3.
Size-exclusion chromatography of isolated human HspB1, HspB6 and their heterooligomeric complexes. a wild-type HspB1 + wild-type HspB6; b wild-type HspB1 + Cys mutant of HspB6 subjected to oxidation after subunits exchange. Elution profiles of isolated proteins are presented by dash-dotted curves 1 and 2, algebraic sum of elution profiles of isolated proteins (or elution profile of the protein mixture incubated at 4°C) is presented as a dashed curve 3 and elution profile of the mixture of two proteins pre-incubated for 1 h at 42°C is presented by solid curve 4. The inserts represent the SDS–gel electrophoresis of the fractions collected from elution profile indicated by curve 4. SDS–gel electrophoresis was run either in the presence (a) or in the absence (b) of reducing agents. Positions of small heat shock proteins on (a) are indicated by arrows. The single arrow on (b) indicates position of HspB1–HspB6 heterodimer
Similar experiments were performed with the wild-type HspB1 and Cys mutant of HspB6 (Fig. 3b). As mentioned earlier, the chromatographic behaviour of Cys mutant of HspB6 was indistinguishable from that of the wild-type protein, and the isolated Cys mutant of HspB6 preserved the peak shape and the apparent molecular mass of 55 kDa (elution volume 14.9 ml), characteristic to the wild-type protein (compare curve 2 from Fig. 3a with curve 2 from Fig. 3b). Incubation of Cys mutant of HspB6 with the wild-type HspB1 at 4°C followed by oxidation and formation of disulfide bridge was not accompanied by subunit exchange and formation of heterooligomers, nor did it produce any changes in the quaternary structure of these proteins (Fig. 3b). If in the same setup, pre-incubation of the above-mentioned protein occurred at 42°C instead of 4°C, then we were able to record heterooligomer formation (Fig. 3b). In this case, the main peak eluted at ~13.6 ml with apparent molecular mass of about 100–150 kDa contained predominantly cross-linked heterodimers of HspB1 and HspB6 (insert on Fig. 3b), whereas two shoulders preceding and following this peak also contained either cross-linked homodimer of HspB1 (the shoulder preceding the main peak) or cross-linked homodimer of HspB6 (the shoulder following the main peak). Overall, the heterodimer HspB1–HspB6 is predominant within the oligomeric mixture generated by these two small heat shock proteins.
Elution profiles of isolated αB-crystallin (HspB5) and isolated HspB6 and their mixture incubated at 4°C revealed two peaks with apparent molecular masses of 660 kDa (10.2 ml) and 55 kDa (14.9 ml) (corresponding to the homooligomers of HspB5 and HspB6, respectively (Fig. 4a)). If the mixture of HspB5 and HspB6 was pre-incubated at 42°C instead of 4°C, then the peaks of isolated homooligomers became significantly diminished, and a new peak with apparent molecular mass of 350 kDa (11.6 ml) appeared on the elution profile (Fig. 4a). This indicates generation of heterooligomeric complex having quaternary structure different from that of the individual HspB5 or HspB6 oligomers. SDS–polyacrylamide gel electrophoresis (PAGE) analysis of heterooligomer subunit composition indicated that the 350-kDa peak contained both HspB5 and HspB6 in the molar ratio close to 2:1 (insert on Fig. 4a).
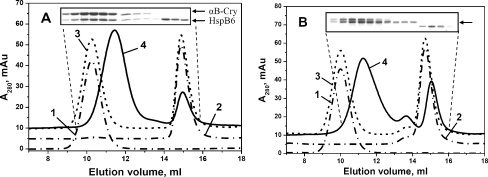
Fig. 4.
Size-exclusion chromatography of isolated human HspB5, HspB6 and their heterooligomeric complexes. a wild-type HspB5 + wild-type HspB6; b Cys mutant of HspB5 + Cys mutant of HspB6 subjected to oxidation after subunits exchange. Elution profiles of isolated proteins are presented by dash-dotted curves 1 and 2, algebraic sum of elution profiles of isolated proteins (or elution profile of the protein mixture incubated at 4°C) is presented as a dashed curve 3 and elution profile of the mixture of two proteins pre-incubated for 1 h at 42°C is presented by solid curve 4. The inserts represent the SDS–gel electrophoresis of the fractions collected from elution profile indicated by curve 4. SDS–gel electrophoresis was run either in the presence (a) or in the absence (b) of reducing agents. Positions of small heat shock proteins on (a) are indicated by arrows. The single arrow on (b) indicates position of HspB5–HspB6 heterodimer
We performed similar experiments with Cys mutants of HspB5 and HspB6. Under conditions that prevented subunit exchange, we detected only two peaks with apparent molecular mass of 660 and 55 kDa corresponding to homooligomers of these proteins (Fig. 4b, curve 3). On the contrary, when mixture of HspB5 and HspB6 was pre-incubated at 42°C followed by Cys cross-linking, a new peak with apparent molecular mass of 350 kDa (11.6 ml) emerged in the elution profile (Fig. 4b, curve 4). Analysis of the protein composition of this main peak revealed that it predominantly contained cross-linked homodimers of HspB5 and cross-linked heterodimers HspB5–HspB6, but it did not contain cross-linked homodimers of HspB6, which eluted as a peak with apparent molecular mass of 55 kDa (insert on Fig. 4b). Thus, homooligomers of HspB6 are not a part of large heterooligomeric complexes formed by HspB5 and HspB6. It seems that heterooligomers of HspB5–HspB6 consist of homodimers of HspB5 and heterodimers HspB5–HspB6; however, they are lacking homodimers of HspB6.
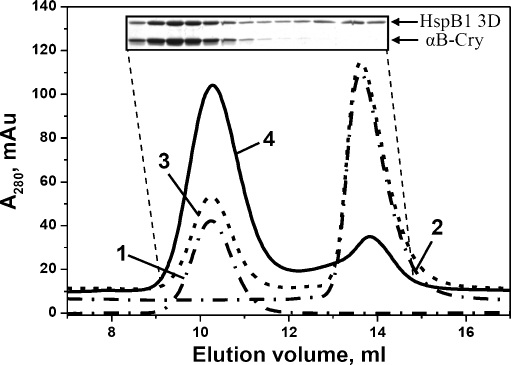
It is well known that both the wild-type HspB1 and HspB5 form large homooligomers of various sizes, with apparent molecular masses of more than 600 kDa (McHaourab et al. 2009; Horwitz 2003; Bukach et al. 2009), which cannot be separated from each other by means of size-exclusion chromatography. In order to prevent formation of large homooligomers that interfere with analysis of heterooligomers, we used a three-aspartate mutant of HspB1 (S18D, S78D, S82D) or “3D” that mimicks the MAPKAP2-phosphorylated species of HspB1.This mutant forms only small-size oligomers (Rogalla et al. 1999; Hayes et al. 2009). Indeed, in our experiments, isolated 3D mutant of HspB1 eluted from size-exclusion column in the form of well-resolved symmetrical peaks with apparent molecular masses of 100 kDa (~13.6 ml), typical for small oligomers (Fig. 5). As described above, the HspB5 eluted as a large homooligomer of ~600 kDa (10.2 ml) (Fig. 5). Upon co-incubation of 3D mutant of HspB1 and HspB5 at 42°C, the 3D mutant peak shifted almost entirely to a ~660-kDa peak (elution at 10.2 ml), which contained both HspB5 and 3D mutant of HspB1, as judged by SDS–PAGE. Consequently, the 3D mutant of HspB1 and HspB5 interact with each other and form a heterocomplex of size similar or identical to that of the HspB5 homooligomer.
Fig. 5.
Size-exclusion chromatography of isolated human HspB5, 3D mutant of HspB1 and their heterooligomeric complexes. Elution profiles of isolated proteins are presented by dash-dotted curves 1 and 2, algebraic sum of elution profiles of isolated proteins (or elution profile of the protein mixture incubated at 4°C) is presented as a dashed curve 3 and elution profile of the mixture of two proteins pre-incubated for 1 h at 42°C is presented by solid curve 4. The insert represent the SDS–gel electrophoresis of the fractions collected from elution profile indicated by curve 4. SDS–gel electrophoresis was run in the presence of reducing agents. Positions of small heat shock proteins are indicated by arrows
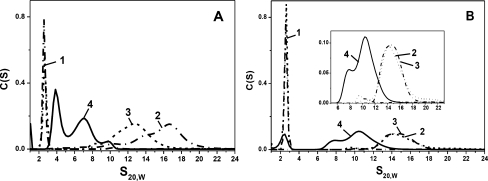
Continuing our investigation, we have also analyzed oligomer formation by wild-type HpsB1, HpsB5 and HpsB6 using analytical ultracentrifugation. Four types of samples were analyzed in each case, corresponding to isolated small heat shock proteins incubated for 1 h at 42°C and to equimolar mixtures of the two small heat shock proteins incubated for 1 h at either 4°C or at 42°C. Results showed that isolated HspB6 was sedimented in the form of a sharp peak with sedimentation coefficient of about 2.6 S (Fig.6a, b curve 1), while isolated HspB1 incubated for 1 h at 42°C was sedimented in the form of a broad asymmetric peak with sedimentation coefficients in the range of 12–20 S (Fig. 6a, curve 2). Unheated mixture of HspB1 and HspB6 displayed two peaks. One sharp and symmetrical peak with sedimentation coefficient 2.6 S coincided with the peak of isolated HspB6 (Fig. 6a, curve 3), while other asymmetric peak with sedimentation coefficients in the range of 10–15 S partially overlapped with the peak of isolated HspB1 (Fig. 6a, curve 3 and data not shown). The shift of the HspB1 peak maximum to higher sedimentation coefficients upon heating seems to be due to the changes in the oligomeric structure of HspB1, as supported by literature (Lelj-Garolla and Mauk 2006). Finally, if the heated mixture of HspB1 and HspB6 was subjected to ultracentrifugation, the peaks corresponding to isolated HspB6 (2.6 S) and HspB1 (10–18 S) completely disappeared and two new peaks with sedimentation coefficients of about 3.9 and 7.0 S emerged on the sedimentogram (Fig. 6a, curve 4). These peaks probably correspond to the two types of heterooligomeric complexes detected on the size-exclusion chromatography (Fig. 3a, curve 4).
Fig. 6.
Analysis of interaction of the wild type HspB1 and HspB6 (a) and HspB5 and HspB6 (b) by means of analytical ultracentrifugation. Distribution of sedimentation coefficients of isolated HspB6 (dash-dotted curve 1), of isolated HspB1 and HspB5 (dash-dotted curves 2 on (a, b), respectively), of the unheated mixture of two small heat shock proteins (dashed curve 3) and of the heated mixture of two corresponding small heat shock proteins (solid curve 4) is presented on each panel. Insert on (b) represents enlarge part of sedimentogram in the range of 6–22 S
Similar results were obtained in the case of HspB5 and HspB6. As already mentioned, isolated HspB6 was sedimented in the form of sharp symmetrical peak with sedimentation coefficient 2.6 S (Fig. 6a, b, curve 1), whereas isolated HspB5 was sedimented as a very broad asymmetric peak with sedimentation coefficients in the range 13–17 S (Fig. 6b, curve 2). On the sedimentogram of the unheated mixture of HspB5 and HspB6, we detected two peaks overlapping with the peaks corresponding to isolated HspB5 and HspB6 (Fig. 6b, curve 3). This means that at low temperature, subunits of these two small heat shock proteins were not exchanged. At the same time, if the heated mixture of HspB5 and HspB6 was subjected to ultracentrifugation, then the peaks corresponding to isolated proteins were significantly diminished and two new poorly resolved peaks with sedimentation coefficients of about 7.9 and 10.4 S appeared on sedimentogram (Fig. 6b, curve 4). Thus, in good agreement with the data of size-exclusion chromatography, we found that HspB5 and HspB6 interact with each other and the size of heterooligomeric complex formed by these two small heat shock proteins is smaller than the size of the complex formed by isolated HspB5.
Interaction of oxidized Cys mutants of HspB4, HspB6 and wild-type HspB1
As stated earlier, the detailed mechanism of subunit exchange of different small heat shock proteins remains not fully understood, and the question whether monomers or dimers of small heat shock proteins are exchangeable remains still unanswered. Trying to elucidate the mechanism of subunit exchange, we analyzed the interaction abilities of oxidized Cys mutants of HspB4, HspB5 and oxidized wild-type HspB1.
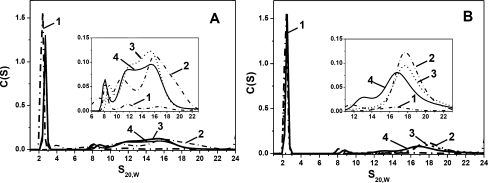
In good agreement with the earlier published data (Mymrikov et al. 2010; Zavialov et al. 1998), we found that oxidation of the wild-type HspB1 and of the Cys mutant of HspB6 did not affect their quaternary structure and that both proteins were eluted as symmetrical peaks with apparent molecular masses indistinguishable from those of reduced wild-type proteins (Fig. 7a). As mentioned earlier, oxidation performed overnight at 37°C was accompanied by more than 70% cross-linking of isolated proteins. Equimolar quantities of thus obtained oxidized wild-type HspB1 and oxidized Cys mutant of HspB6 were mixed together and incubated in the absence of reducers for 1 h at 42°C to promote exchange of their subunits. Afterwards, the samples were subjected to the size-exclusion chromatography (Fig. 7) and analytical ultracentrifugation (Fig. 8). The data of Fig. 7a indicate that the oxidized samples of HspB1 and Cys mutant of HspB6 were unable to exchange their subunits due to the fact that each protein was eluted separately in positions exactly corresponding to those of isolated proteins. This means that cross-linked dimers are unable to participate in the subunit exchange that was characteristic for reduced wild-type proteins (see Fig. 3a).
Fig. 7.
Size-exclusion chromatography of oxidized wild-type HspB1 and oxidized Cys mutant of HspB6 (a) and of oxidized Cys mutants of HspB5 and HspB6 (b). Elution profiles of isolated proteins are presented by dash-dotted curves 1 and 2, algebraic sum of elution profiles of isolated proteins is presented as a dashed curve 3 and elution profile of the mixture of two oxidized proteins pre-incubated for 1 h at 42°C is presented by solid curve 4. The inserts represent the protein composition of the fractions collected from elution profile indicated by curve 4. Positions of human small heat shock proteins are indicated by arrows
Fig. 8.
Analysis of interaction of oxidized wild-type HspB1 and oxidized Cys mutant of HspB6 (a) and oxidized Cys mutants of HspB5 and HspB6 (b) by means of analytical ultracentrifugation. Distribution of sedimentation coefficients of isolated oxidized Cys mutant of HspB6 (dash-dotted curve 1), of isolated oxidized HspB1 and oxidized Cys mutant of HspB5 (dash-dotted curves 2 on (a, b), respectively), of the unheated mixture of two oxidized small heat shock proteins (dashed curve 3) and of the heated mixture of two oxidized small heat shock proteins (solid curve 4) is presented on each panel. Inserts represent enlarged part of sedimentation profiles in the range of 6–22 S (a) or 12–22 S (b)
Similar results were obtained in the case of oxidized mutants of HspB5 and HspB6. Isolated oxidized samples of Cys mutant of HspB5 and HspB6 were eluted in the form of symmetrical peaks with apparent molecular mass of about 660 kDa (10.2 ml) and 55 kDa (14.9 ml), respectively (Fig. 7b). Oxidized samples of Cys mutants of HspB5 and HspB6 were mixed together and after heating for 1 h at 42°C were loaded on the Superdex 200 column (Fig. 7b). The position and the amplitude of the peak corresponding to oxidized HspB6 remain unchanged, whereas position of the peak corresponding to oxidized HspB5 was slightly shifted to the larger elution volumes (Fig. 7b); however, HspB6 was not detected in this peak (insert on Fig. 7b). This might indicate that oxidized HspB6 only weakly interacts with oxidized HspB5, and due to this weak interaction, the peak of oxidized HspB5 was slightly retarded on the elution profile. However, this type of interaction is very weak, and oxidized proteins cannot form stable heterooligomers.
Analytical ultracentrifugation confirmed the data obtained by size-exclusion chromatography. Isolated oxidized Cys mutant of HspB6 and wild-type HspB1 were sedimented as a sharp symmetrical peak with sedimentation coefficient 2.6 S and as a very broad peak in the range of 8–22 S, respectively (Fig. 8a). Sedimentation profiles of the mixture of two oxidized proteins pre-incubated either at 4°C or at 42°C were practically identical. Independent of the temperature of pre-incubation, the sedimentation profile revealed the same two peaks as described above with sedimentation coefficients of about 2.6 S and a broad peak with sedimentation coefficients in the range of 8–22 S (Fig. 8a). In good agreement with the data obtained by SEC, the data of analytical ultracentrifugation indicate that cross-linked dimers of the wild-type HspB1 and of the Cys mutant of HspB6 cannot form heterooligomers.
Analogous results were obtained with oxidized Cys mutants of HspB5 and HspB6 (Fig. 8b). In this case, isolated oxidized Cys mutant of HspB6 sedimented in the form of sharp peak with sedimentation coefficient of 2.6 S, whereas oxidized Cys mutant of HspB5 was sedimented as a broad peak with sedimentation coefficients in the range of 16–21 S (Fig. 8b). Sedimentation of the mixture of two oxidized proteins pre-incubated at 4°C revealed the same two peaks as above (Fig. 8b). Pre-incubation of the mixture of two oxidized proteins at 42°C also did not significantly affect the sedimentation profile. Position and the amplitude of the peak corresponding to HspB6 remained unchanged, whereas position of the peak corresponding to HspB5 was slightly shifted towards smaller S values (Fig. 8b). Thus, the fact that the data of ultracentrifugation are in a good agreement with the results obtained by size-exclusion chromatography indicates that the cross-linked dimers are much less effective in formation of heterooligomers than non-cross-linked reduced HspB5 and HspB6.
Discussion
The wild-type HspB1 contains the single Cys137 located in the middle of the β7 strand, i.e. at the interface between two HspB1 neighboring monomers. Therefore, upon oxidation, two monomers of HspB1 could be easily cross-linked via disulfide bond, modification that does not affect the protein structure and properties (Zavialov et al. 1998). Earlier, we obtained the so-called Cys mutants of three other human small heat shock proteins (HspB5, HspB6 and HspB8). These proteins contain only one Cys residue located in a position homologous to that of Cys137 of HspB1, while the rest of native Cys residues are substituted with Ser. The resulting mutations do not affect the structure and properties of the mentioned small heat shock proteins (Mymrikov et al. 2010). These mutants seem to be useful for analyzing subunit exchange between different human small heat shock proteins.
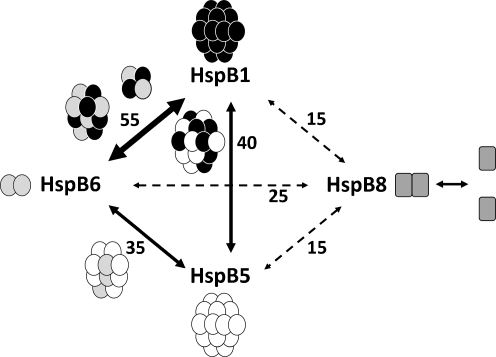
Pair-wise mixtures of different sHsp were heated for 1 h at 42°C to ensure rapid subunit exchange. Effective subunit exchange can also be achieved after incubation at 37°C; however, longer incubation (3 h) was needed for completion of this process (Bukach et al. 2009). After formation of heterooligomeric complexes, they were subjected to mild oxidation leading to the formation of disulfide bond between two neighboring monomers. The results obtained are schematically represented in Fig. 9 and indicate that three human small heat shock proteins, namely HspB1, HspB5 and HspB6, effectively exchange their subunits and easily form disulfide cross-linked heterodimers. This exchange was especially effective in the case of HspB1/HspB6 complex, for which about 55% of proteins were involved in formation of heterodimers, followed by the HspB1/HspB5 and HspB5/HspB6 complexes, for which about 40% of proteins participated in formation of heterodimers. Our results agree with the data from literature indicating that HspB1/HspB5 (Zantema et al. 1992), HspB5/HspB6 (Kato et al. 1992) and HspB1/HspB6 (Bukach et al. 2004, 2009) effectively form heterooligomeric complexes.
Fig. 9.
Schematic representation of pair-wise monomer exchange of different human small heat shock proteins. High probability of subunit exchange is shown by solid thick line, lower probability of subunit exchange is indicated by solid thin lines and weak probability of exchange is indicated by dashed lines. The numbers at the lines indicate the average percentage of corresponding proteins involved in heterodimer formation. The composition and the size of heterooligomeric complexes formed by HspB1, HspB5 and HspB6 are schematically shown at the arrows connecting the corresponding proteins
At the same time, the data of disulfide cross-linking (Fig. 2) indicate that the probability of subunit exchange in all pairs containing HspB8 was rather small and also that in all pairs containing this protein less than 25% of proteins was involved in formation of heterodimers (Fig. 2). Detailed investigation of HspB8 performed earlier (Sun et al. 2004; Fontaine et al. 2005, 2006) showed that this protein interacted with practically all human small heat shock proteins, including HspB1, HspB5 and HspB6. Our data confirmed that under certain conditions HspB8 indeed interacted with all above-mentioned proteins; however, HspB8 was much less effective in formation of heterodimers than any other small heat shock protein. This can be due to certain peculiarities of HspB8 structure. This protein is lacking the properly folded β2 strand of the α-crystallin domain and the conservative I-X-I (V) tripeptide in the C-terminal extension; in addition, it belongs to the group of intrinsically disordered proteins (Kasakov et al. 2007, 2009). Therefore, it is possible that HspB8 occurs as a monomer–dimer mixture at equilibrium with weak affinity for other human small heat shock proteins. It is worthwhile to mention that expression of different small heat shock protein is tissue-specific and is also dependent on the physiological stage. However, on the average, the levels of HspB1 and HspB5 expression are comparable, exceeding by up two times the level of HspB6 and by up to four times the levels of HspB8, respectively (Kato et al. 1992, 1994; Louapre et al. 2005; Verschuure et al. 2003; Huey et al. 2004). This fact together with the low probability of subunit exchange makes participation of HspB8 in the formation of heterooligomeric complexes with other small heat shock proteins rather improbable. HspB8 seems to have unique binding properties as, despite its weak affinity for other small heat shock proteins, it forms tight complexes with Bag3 (Bcl2-associated anthanogene) and seems to be involved in the regulation of degradation of partially denatured proteins in autophagosomes (Carra et al. 2008; Fuchs et al. 2010; Vos et al. 2011; Shemetov and Gusev 2011).
As already mentioned, molecular mechanisms underlying heterooligomer formation remain until now poorly investigated. By using Cys mutants of sHsp, we found that the β7 strand of crystallin domain plays important role in formation of homo- and heterodimers interfaces (Fig. 2). Therefore, the engineering the Cys residue located in the centre of this strand can be successfully used in obtaining of the cross-linked homo- (Mymrikov et al. 2010) and heterodimers of different small heat shock proteins.
In order to delineate the mechanism of subunit exchange, we analyzed the oligomeric state of heterooligomers formed by different small heat shock proteins. Interaction of HspB1 with HspB5 was reported earlier (Zantema et al. 1992); however, the structure and composition of these complexes were not analyzed in detail. By using size-exclusion chromatography and ultracentrifugation, we found that the size of heterooligomeric complexes formed by HspB5 and 3D mutant of HspB1 was identical to that of homooligomers of HspB5 (Fig. 5). Thus, the subunits of 3D mutant of HspB1 could replace certain subunits of HspB5 within the large oligomers formed by HspB5, a replacement that did not affect significantly the overall size of these oligomers.
On the contrary, subunit exchange between HspB5 and HspB6 was accompanied by formation of a new type of oligomers. These oligomers, having apparent molecular mass of 350 kDa, were smaller than homooligomers of HspB5 (apparent molecular mass about 660 kDa) and larger than homooligomers of HspB6 (apparent molecular mass 55 kDa) (Fig. 4a). Formation of a new type of complex was also confirmed by means of analytical ultracentrifugation (Fig. 6b). This means that the subunit exchange between these two proteins leads to formation of a new type of oligomers. Uniquely, HspB5/HspB6 heterooligomers were built of homodimers of HspB5 that alternated with heterodimers HspB5–HspB6, however lacking homodimers of HspB6. Although interaction of HspB5 and HspB6 was reported earlier (Kato et al. 1992), to our knowledge, formation of this new type of heterooligomers was not described earlier.
Finally, subunit exchange of HspB1 and HspB6 leads to formation of at least two different heterooligomeric complexes, each containing equimolar quantities of each protein and having apparent molecular masses of 100–150 and 250–300 kDa, respectively (Fig. 3). These findings also confirmed by analytical ultracentrifugation (Fig. 6a) agree well with our earlier published results (Bukach et al. 2004, 2009). Heterooligomeric complexes formed by these proteins contain HspB1–HspB6 heterodimers and variable quantities of homodimers of HspB1 and HspB6. Summing up, we might conclude that HspB1, HspB5 and HspB6 easily participate to formation of different types of heterooligomeric complexes. The size of these complexes is often different from the size of the homooligomeric complexes formed by individual small heat shock proteins.
We analyzed heterooligomeric complexes formed by two different small heat shock proteins; however, the probability of existence of heterooligomeric complexes composed of more than two different species of small heat shock proteins cannot be excluded. These complexes can be formed by different human sHsp, which are simultaneously and at high extent expressed in the same human tissue (for instance, by HspB1, HspB5 and HspB6). The composition, structure and properties of these hypothetical complexes remain unknown and deserve further investigations.
Finding that the small heat shock proteins are able to form heterooligomers, we tried to analyze the mechanism of subunit exchange. We determined that reduced Cys mutants of all small heat shock proteins behave like the wild-type proteins and easily forms heterooligomers of the same size and composition as the wild-type proteins (data not shown). However, formation of heterooligomers was completely blocked if, before mixing, Cys mutants of HspB5, HspB6 and the wild-type HspB1 were subjected to mild oxidation to cross-link the monomers by disulfide bond (Figs. 7 and 8). It is well-accepted that the oligomers of small heat shock proteins usually contain an even number of subunits and that the dimer is the main unit of exchange between different sHsp homooligomers (Sobott et al. 2002; Benesch et al. 2008). If this conclusion is universal and applicable for heterooligomers, then it will be difficult to explain why in our hands the cross-linked homodimers of small heat shock proteins are unable to participate in subunit exchange and to form heterooligomers. In addition, if only the dimers were the exchangeable subunits, then it is impossible to explain formation of heterodimers cross-linked by disulfide bond (Fig. 2). Therefore, we should postulate that both monomers and dimers are able to be involved in subunit exchange and in formation of the heterooligomers. Recently published data agree with this suggestion and indicate that depending on conditions (temperature and pH value) large oligomers of αB-crystallin (HspB5) can dissociate forming both monomers and dimers, which later on can be reassociated forming polydisperse large oligomers (Baldwin et al. 2011a, b).
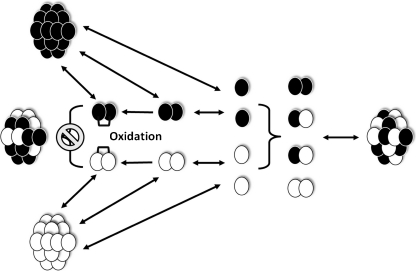
Summing up, we can propose the following mechanism describing heterooligomer formation of human small heat shock proteins (Fig. 10). The data from literature indicate that human sHsp have different oligomeric states. For instance, HspB1 and HspB5 predominantly form large heterogeneous oligomers, whereas HspB6 and HspB8 occur predominantly in the form of small oligomers or even monomers (HspB8). Homooligomers of sHsp can dissociate with accumulation of structurally stable dimers and less structurally stable monomers. Stabilization of dissociated monomers is achieved by formation of homo- or heterodimers, which can re-associate with each other forming heterooligomers of different size and different subunit composition. Homodimers that are cross-linked by disulfide bond have a restricted flexibility and therefore can re-associate into large homooligomers; however, they are unable to form heterooligomers, the formation of which requires significant structural flexibility. We suppose that the mechanism proposed can explain the formation of sHsp heterooligomers earlier described in the literature. For instance, depending on age, native α-crystallin occurs in the form of heterooligomer containing αA- (HspB4) and αB-crystallin (HspB5) in the molar ratio varying from approximately 3:1 up to 1:1 (Horwitz 2003); in addition, HspB2 and HspB3 form well-organized heterooligomeric complexes with an unusual stoichiometry of 3:1 (den Engelsman et al. 2009). Thus, these heterooligomeric complexes contain an even number of total subunits, however preserving an uneven number of individual subunits. This fact agrees with our suggestion that not only dimers but also monomers can be involved in the formation of heterooligomeric complexes. This suggestion is confirmed by recently published data indicating that phosphorylation and certain methods of renaturation lead to rearrangement of substructure of dimers, accompanied by formation of oligomers with uneven subunit number (Aquilina et al. 2004; Benesch et al. 2008). Thus, the monomers might be considered to be the exchangeable subunits involved in formation of sHsp heterooligomers.
Fig. 10.
Proposed mechanism of heterooligomers formation by different human small heat shock proteins. Homooligomers undergo dissociation leading to formation of dimers and monomers. Monomers can re-associate forming homo- or heterodimers, which can be used as building blocks for formation of large-size heterooligomers. Homodimers cross-linked via disulfide bond (brackets) became rigid and can re-associate forming homooligomers but cannot participate in formation of heterooligomers. See text for details
Electronic supplementary material
Reduction of disulfide cross-linked wild-type HspB1 and Cys mutants of human small heat shock proteins. Isolated small heat shock proteins (lanes 1 and 3) or their pair-wise mixture (lane 2) were subjected to subunit exchange followed by mild oxidation as described in “Material and methods”. The samples thus obtained were subjected to reduction and SDS–gel electrophoresis. a HspB1 + HspB6; b HspB5 + HspB6; c HspB1 + HspB5; d HspB8 + HspB5; e HspB8 + HspB6; f HspB8 + HspB1. Trace amounts of non-reduced disulfide cross-linked dimers are detected as a very faint bands with apparent molecular masses of 42–66 kDa. The positions of molecular mass standards (in kilodaltons) are indicated by arrows (PDF 233 kb)
Acknowledgements
This investigation was supported by Russian Foundation for Basic Science (grant 10-04-00026).
Glossary
- DTT
Dithiothreitol
- ME
Mercaptoethanol
- SEC
Size-exclusion chromatography
- sHsp
Small heat shock proteins
References
- Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279(27):28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol. 2009;392(5):1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Hilton GR, Lioe H, Bagneris C, Benesch JL, Kay LE (2011a) Quaternary Dynamics of alphaB-Crystallin as a Direct Consequence of Localised Tertiary Fluctuations in the C-Terminus. J Mol Biol. doi:10.1016/j.jmb.2011.07.017 [DOI] [PubMed]
- Baldwin AJ, Lioe H, Robinson CV, Kay LE, Benesch JL (2011b) alphaB-Crystallin Polydispersity Is a Consequence of Unbiased Quaternary Dynamics. J Mol Biol. doi:10.1016/j.jmb.2011.07.016 [DOI] [PubMed]
- Baranova EV, Weeks SD, Beelen S, Bukach OV, Gusev NB, Strelkov SV. Three-dimensional structure of alpha-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J Mol Biol. 2011;411(1):110–122. doi: 10.1016/j.jmb.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Benesch JL, Ayoub M, Robinson CV, Aquilina JA. Small heat shock protein activity is regulated by variable oligomeric substructure. J Biol Chem. 2008;283(42):28513–28517. doi: 10.1074/jbc.M804729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berengian AR, Parfenova M, McHaourab HS. Site-directed spin labeling study of subunit interactions in the alpha-crystallin domain of small heat-shock proteins. Comparison of the oligomer symmetry in alphaA-crystallin, HSP 27, and HSP 16.3. J Biol Chem. 1999;274(10):6305–6314. doi: 10.1074/jbc.274.10.6305. [DOI] [PubMed] [Google Scholar]
- Bova MP, Ding LL, Horwitz J, Fung BK. Subunit exchange of alphaA-crystallin. J Biol Chem. 1997;272(47):29511–29517. doi: 10.1074/jbc.272.47.29511. [DOI] [PubMed] [Google Scholar]
- Bova MP, McHaourab HS, Han Y, Fung BK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem. 2000;275(2):1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- Bukach OV, Seit-Nebi AS, Marston SB, Gusev NB. Some properties of human small heat shock protein Hsp20 (HspB6) Eur J Biochem. 2004;271(2):291–302. doi: 10.1046/j.1432-1033.2003.03928.x. [DOI] [PubMed] [Google Scholar]
- Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20) Biochim Biophys Acta. 2009;1794(3):486–495. doi: 10.1016/j.bbapap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Carra S, Seguin SJ, Landry J. HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4(2):237–239. doi: 10.4161/auto.5407. [DOI] [PubMed] [Google Scholar]
- Datta SA, Rao CM. Packing-induced conformational and functional changes in the subunits of alpha-crystallin. J Biol Chem. 2000;275(52):41004–41010. doi: 10.1074/jbc.M009322200. [DOI] [PubMed] [Google Scholar]
- Engelsman J, Boros S, Dankers PY, Kamps B, Vree Egberts WT, Bode CS, Lane LA, Aquilina JA, Benesch JL, Robinson CV, Jong WW, Boelens WC. The small heat-shock proteins HSPB2 and HSPB3 form well-defined heterooligomers in a unique 3 to 1 subunit ratio. J Mol Biol. 2009;393(5):1022–1032. doi: 10.1016/j.jmb.2009.08.052. [DOI] [PubMed] [Google Scholar]
- Fontaine JM, Rest JS, Welsh MJ, Benndorf R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones. 2003;8(1):62–69. doi: 10.1379/1466-1268(2003)8<62:TSODFP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine JM, Sun X, Benndorf R, Welsh MJ. Interactions of HSP22 (HSPB8) with HSP20, alphaB-crystallin, and HSPB3. Biochem Biophys Res Commun. 2005;337(3):1006–1011. doi: 10.1016/j.bbrc.2005.09.148. [DOI] [PubMed] [Google Scholar]
- Fontaine JM, Sun X, Hoppe AD, Simon S, Vicart P, Welsh MJ, Benndorf R. Abnormal small heat shock protein interactions involving neuropathy-associated HSP22 (HSPB8) mutants. FASEB J. 2006;20(12):2168–2170. doi: 10.1096/fj.06-5911fje. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, Landry J. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J. 2010;425(1):245–255. doi: 10.1042/BJ20090907. [DOI] [PubMed] [Google Scholar]
- Haley DA, Bova MP, Huang QL, McHaourab HS, Stewart PL. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J Mol Biol. 2000;298(2):261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nature Struct Mol Biol. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hayes D, Napoli V, Mazurkie A, Stafford WF, Graceffa P. Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. J Biol Chem. 2009;284(28):18801–18807. doi: 10.1074/jbc.M109.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76(2):145–153. doi: 10.1016/S0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Huey KA, Thresher JS, Brophy CM, Roy RR. Inactivity-induced modulation of Hsp20 and Hsp25 content in rat hindlimb muscles. Muscle Nerve. 2004;30(1):95–101. doi: 10.1002/mus.20063. [DOI] [PubMed] [Google Scholar]
- Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE. N-terminal domain of {alpha}B-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci U S A. 2011;108(16):6409–6414. doi: 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8(1):53–61. doi: 10.1379/1466-1268(2003)8<53:THGECS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Boelens WC, Jong WW. Why proteins without an alpha-crystallin domain should not be included in the human small heat shock protein family HSPB. Cell Stress Chaperones. 2010;15(4):457–461. doi: 10.1007/s12192-009-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov AS, Bukach OV, Seit-Nebi AS, Marston SB, Gusev NB. Effect of mutations in the beta5–beta7 loop on the structure and properties of human small heat shock protein HSP22 (HspB8, H11) FEBS J. 2007;274(21):5628–5642. doi: 10.1111/j.1742-4658.2007.06086.x. [DOI] [PubMed] [Google Scholar]
- Kato K, Shinohara H, Goto S, Inaguma Y, Morishita R, Asano T. Copurification of small heat shock protein with alpha B crystallin from human skeletal muscle. J Biol Chem. 1992;267(11):7718–7725. [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T. Purification and characterization of a 20-kDa protein that is highly homologous to alpha B crystallin. J Biol Chem. 1994;269(21):15302–15309. [PubMed] [Google Scholar]
- Kazakov AS, Markov DI, Gusev NB, Levitsky DI. Thermally induced structural changes of intrinsically disordered small heat shock protein Hsp22. Biophys Chem. 2009;145(2–3):79–85. doi: 10.1016/j.bpc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394(6693):595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24(10):3633–3642. doi: 10.1096/fj.10-156992. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Benesch JL, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19(5):1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelj-Garolla B, Mauk AG. Self-association and chaperone activity of Hsp27 are thermally activated. J Biol Chem. 2006;281(12):8169–8174. doi: 10.1074/jbc.M512553200. [DOI] [PubMed] [Google Scholar]
- Louapre P, Grongnet JF, Tanguay RM, David JC. Effects of hypoxia on stress proteins in the piglet heart at birth. Cell Stress Chaperones. 2005;10(1):17–23. doi: 10.1379/CSC-74R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsch G, Vetter R, Offhauss U, Wieske M, Grone HJ, Klemenz R, Schimke I, Stahl J, Benndorf R. Abundance and location of the small heat shock proteins HSP25 and alphaB-crystallin in rat and human heart. Circulation. 1997;96(10):3466–3476. doi: 10.1161/01.cir.96.10.3466. [DOI] [PubMed] [Google Scholar]
- McHaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48(18):3828–3837. doi: 10.1021/bi900212j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Bukach OV, Seit-Nebi AS, Gusev NB. The pivotal role of the beta 7 strand in the intersubunit contacts of different human small heat shock proteins. Cell Stress Chaperones. 2010;15(4):365–377. doi: 10.1007/s12192-009-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66(1):64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Totsuka T, Kato K. Pathological changes in levels of three small stress proteins, alphaB crystallin, HSP 27 and p20, in the hindlimb muscles of dy mouse. Biochim Biophys Acta. 1998;1406(2):162–168. doi: 10.1016/s0925-4439(97)00094-x. [DOI] [PubMed] [Google Scholar]
- Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J. 2000;78(3):1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemetov AA, Gusev NB. Biochemical characterization of small heat shock protein HspB8 (Hsp22)-Bag3 interaction. Arch Biochem Biophys. 2011;513(1):1–9. doi: 10.1016/j.abb.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Sobott F, Benesch JL, Vierling E, Robinson CV. Subunit exchange of multimeric protein complexes. Real-time monitoring of subunit exchange between small heat shock proteins by using electrospray mass spectrometry. J Biol Chem. 2002;277(41):38921–38929. doi: 10.1074/jbc.M206060200. [DOI] [PubMed] [Google Scholar]
- Stamler R, Kappe G, Boelens W, Slingsby C. Wrapping the alpha-crystallin domain fold in a chaperone assembly. J Mol Biol. 2005;353(1):68–79. doi: 10.1016/j.jmb.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Studer S, Narberhaus F. Chaperone activity and homo- and hetero-oligomer formation of bacterial small heat shock proteins. J Biol Chem. 2000;275(47):37212–37218. doi: 10.1074/jbc.M004701200. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MM, Tsui SK, Yoshida S, Ohno S. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem. 2000;275(2):1095–1104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R. Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem. 2004;279(4):2394–2402. doi: 10.1074/jbc.M311324200. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sugiyama Y, Hayashi Y, Nyu-i N, Yoshida M, Nonaka I, Ishiura S, Arahata K, Ohno S. MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J Cell Biol. 1998;140(5):1113–1124. doi: 10.1083/jcb.140.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol. 2005;38(3):433–444. doi: 10.1016/j.yjmcc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, Lindner RA, Walker MJ, Aquilina JA, Robinson CV, Horwitz J, Perng MD, Quinlan RA, Carver JA. R120G alphaB-crystallin promotes the unfolding of reduced alpha-lactalbumin and is inherently unstable. FEBS J. 2005;272(3):711–724. doi: 10.1111/j.1742-4658.2004.04507.x. [DOI] [PubMed] [Google Scholar]
- Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nature Struct Biol. 2001;8(12):1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC. Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol. 2003;82(10):523–530. doi: 10.1078/0171-9335-00337. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47(27):7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Zijlstra MP, Carra S, Sibon OC, Kampinga HH. Small heat shock proteins, protein degradation and protein aggregation diseases. Autophagy. 2011;7(1):101–103. doi: 10.4161/auto.7.1.13935. [DOI] [PubMed] [Google Scholar]
- Zantema A, Verlaan-De Vries M, Maasdam D, Bol S, Eb A. Heat shock protein 27 and alpha B-crystallin can form a complex, which dissociates by heat shock. J Biol Chem. 1992;267(18):12936–12941. [PubMed] [Google Scholar]
- Zavialov A, Benndorf R, Ehrnsperger M, Zav’yalov V, Dudich I, Buchner J, Gaestel M. The effect of the intersubunit disulfide bond on the structural and functional properties of the small heat shock protein Hsp25. Int J Biol Macromol. 1998;22(3–4):163–173. doi: 10.1016/S0141-8130(98)00014-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reduction of disulfide cross-linked wild-type HspB1 and Cys mutants of human small heat shock proteins. Isolated small heat shock proteins (lanes 1 and 3) or their pair-wise mixture (lane 2) were subjected to subunit exchange followed by mild oxidation as described in “Material and methods”. The samples thus obtained were subjected to reduction and SDS–gel electrophoresis. a HspB1 + HspB6; b HspB5 + HspB6; c HspB1 + HspB5; d HspB8 + HspB5; e HspB8 + HspB6; f HspB8 + HspB1. Trace amounts of non-reduced disulfide cross-linked dimers are detected as a very faint bands with apparent molecular masses of 42–66 kDa. The positions of molecular mass standards (in kilodaltons) are indicated by arrows (PDF 233 kb)