Abstract
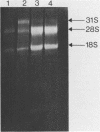
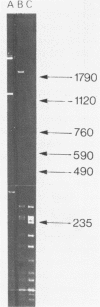
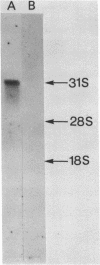
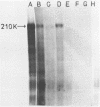
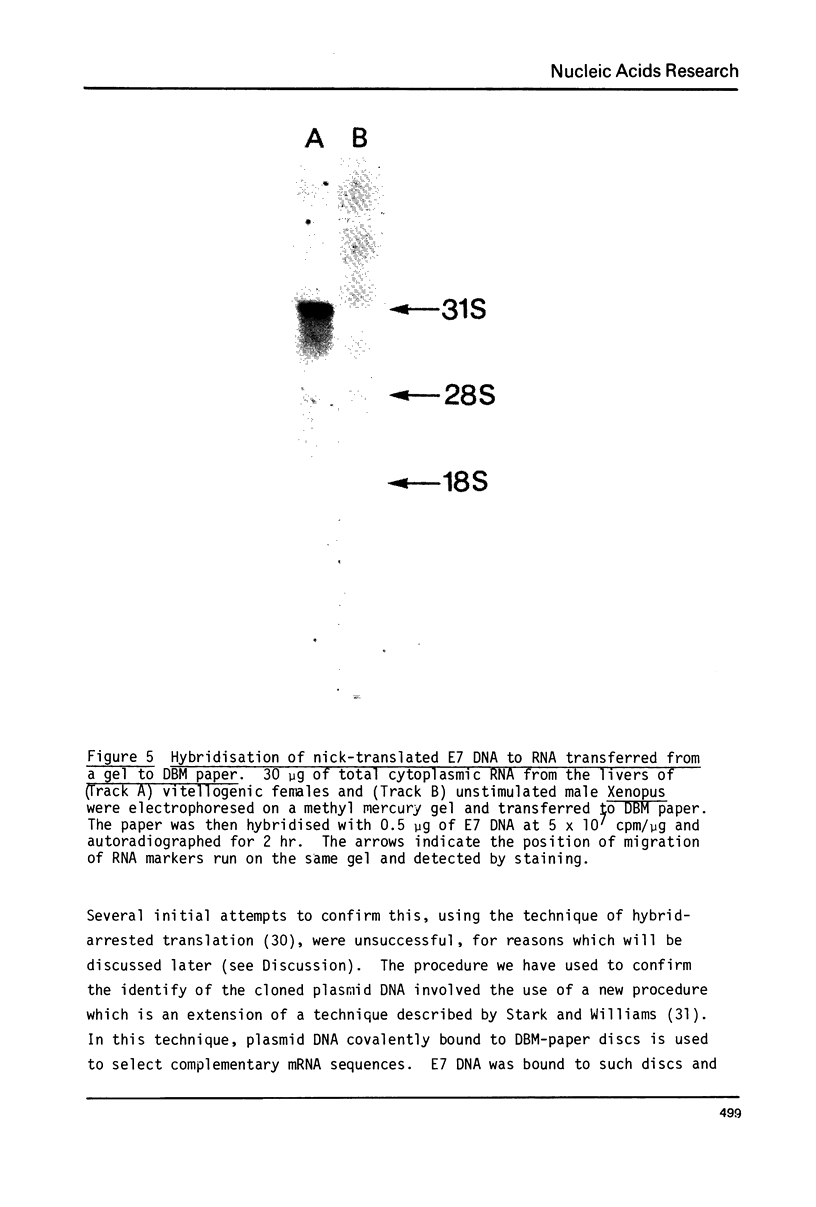
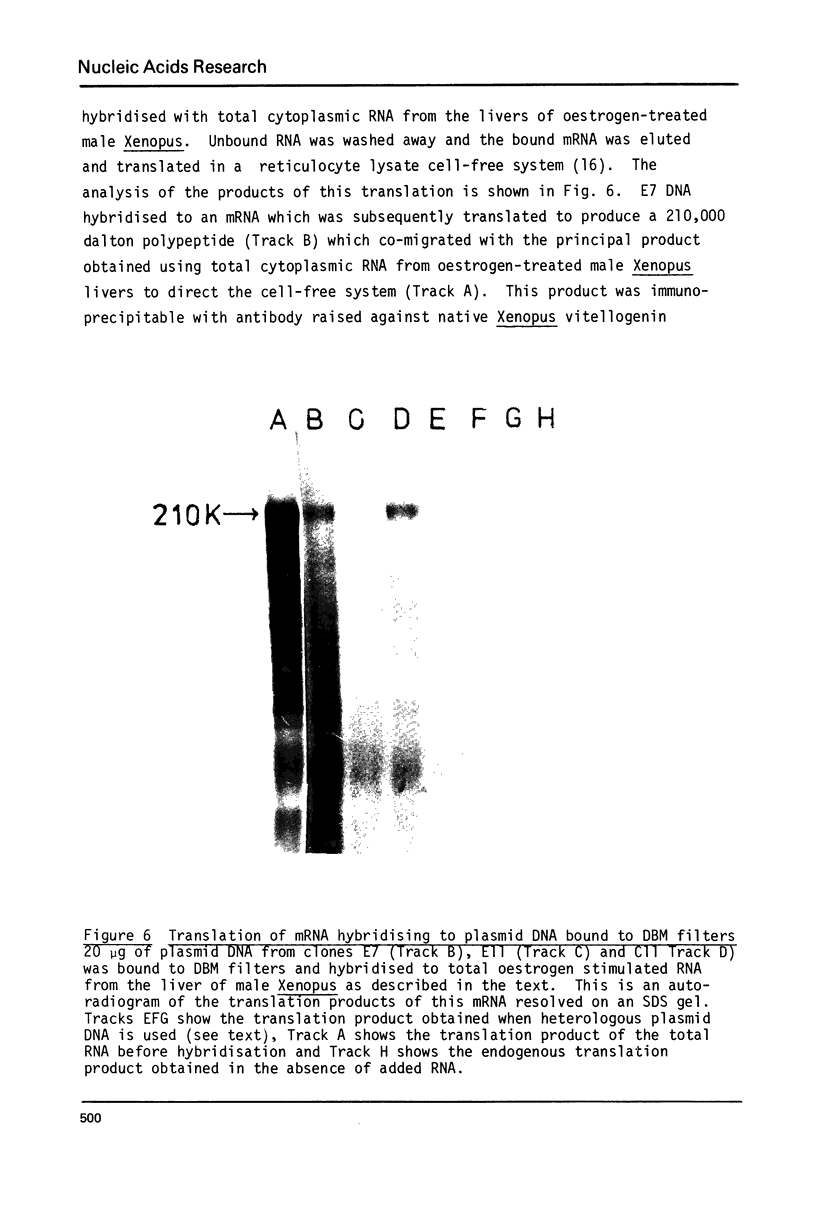
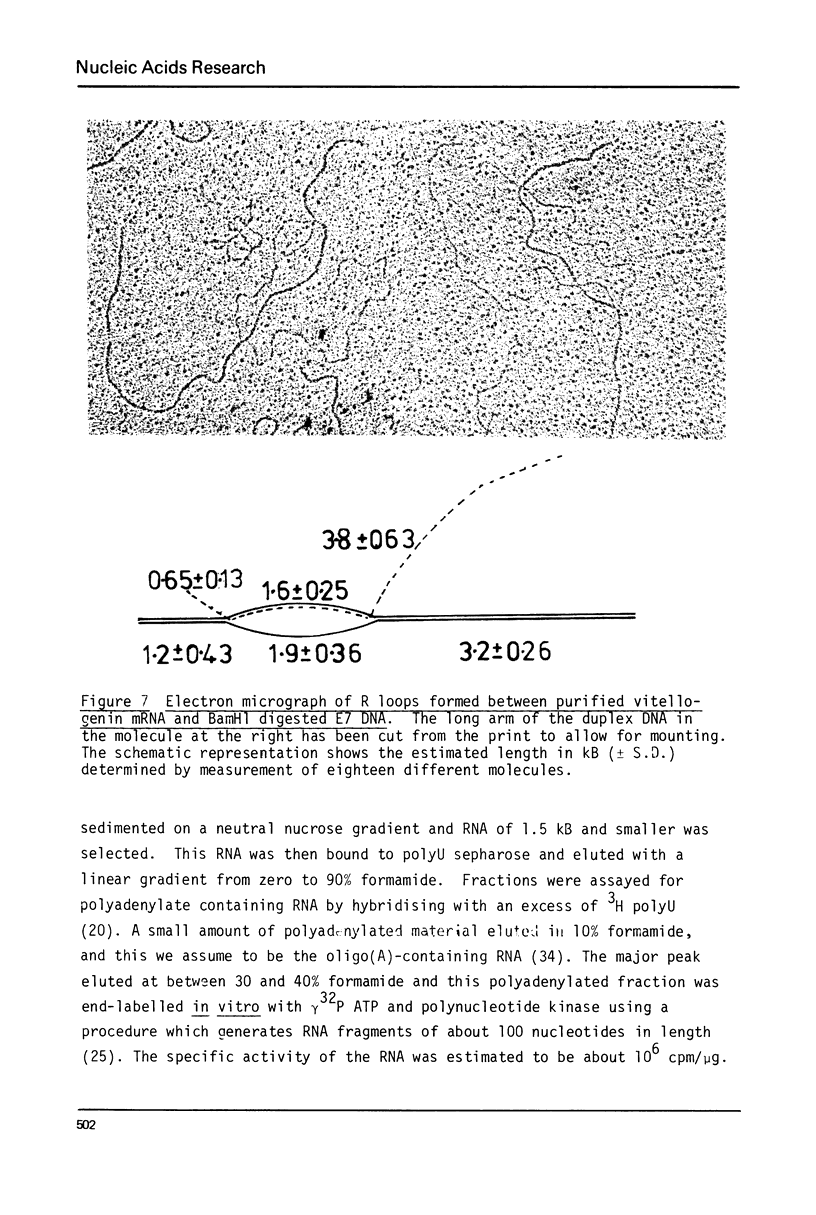
A 1700 nucleotide DNA sequence derived from Xenopus vitellogenin mRNA has been cloned in the bacterial plasmid pBR322. The identity of the cloned sequence was verified in two ways. Firstly, the plasmid DNA was shown to hybridise to an RNA of the correct size (6,700 nucleotides). This was shown by in situ hybridisation to electrophoretically separated RNA and also by the formation of "R-loops" with purified vitellogenin mRNA. Then, using a novel procedure in which plasmid DNA covalently bound to diazotised paper is used to select complementary mRNA sequences, the cloned sequence was shown to hybridise to an mRNA which directed the synthesis of vitellogenin when translated in a reticulocyte lysate cell-free system.
Full text
PDF






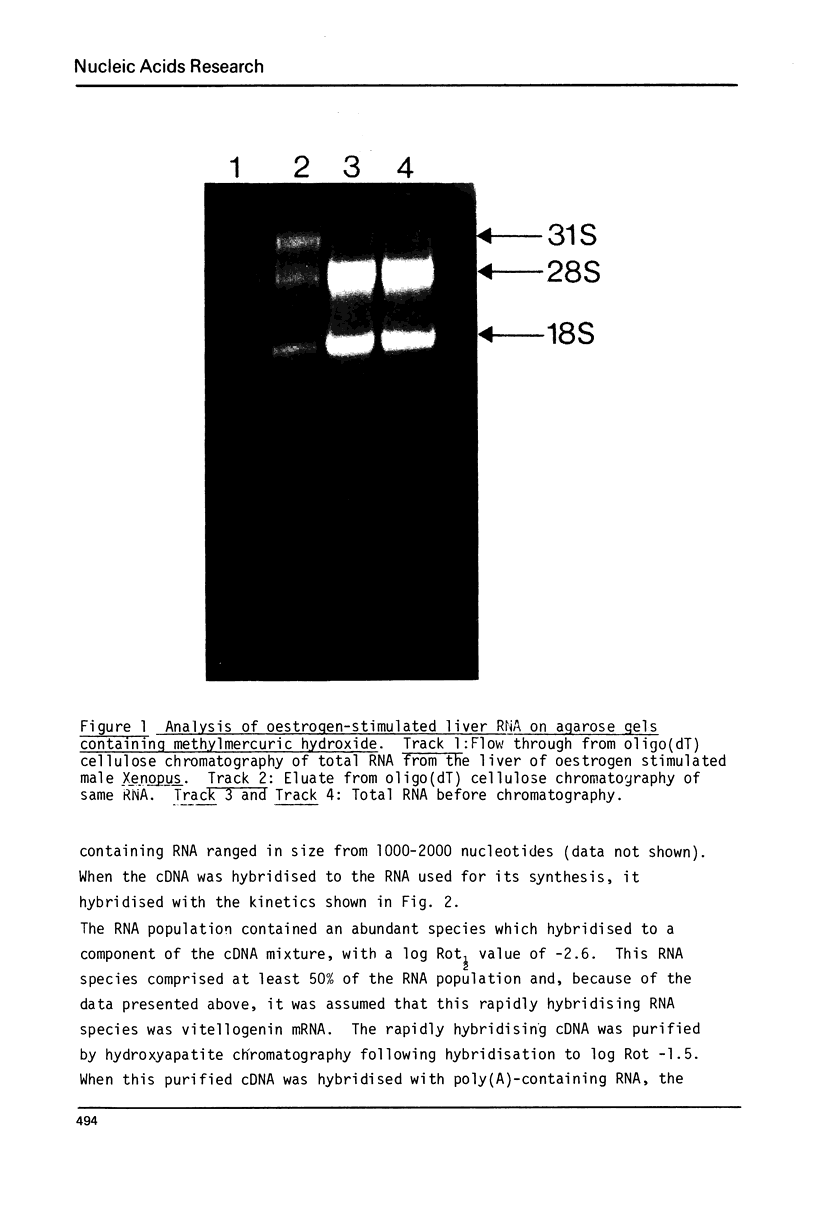
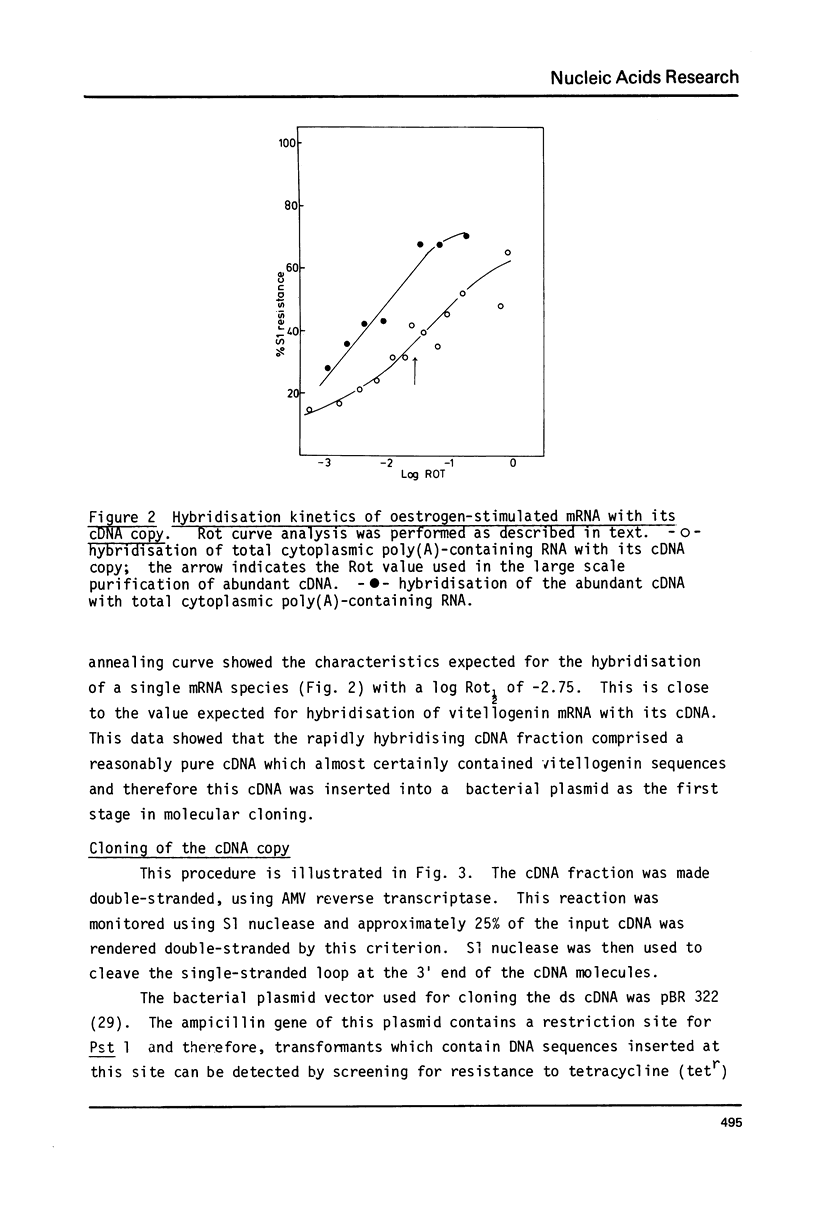
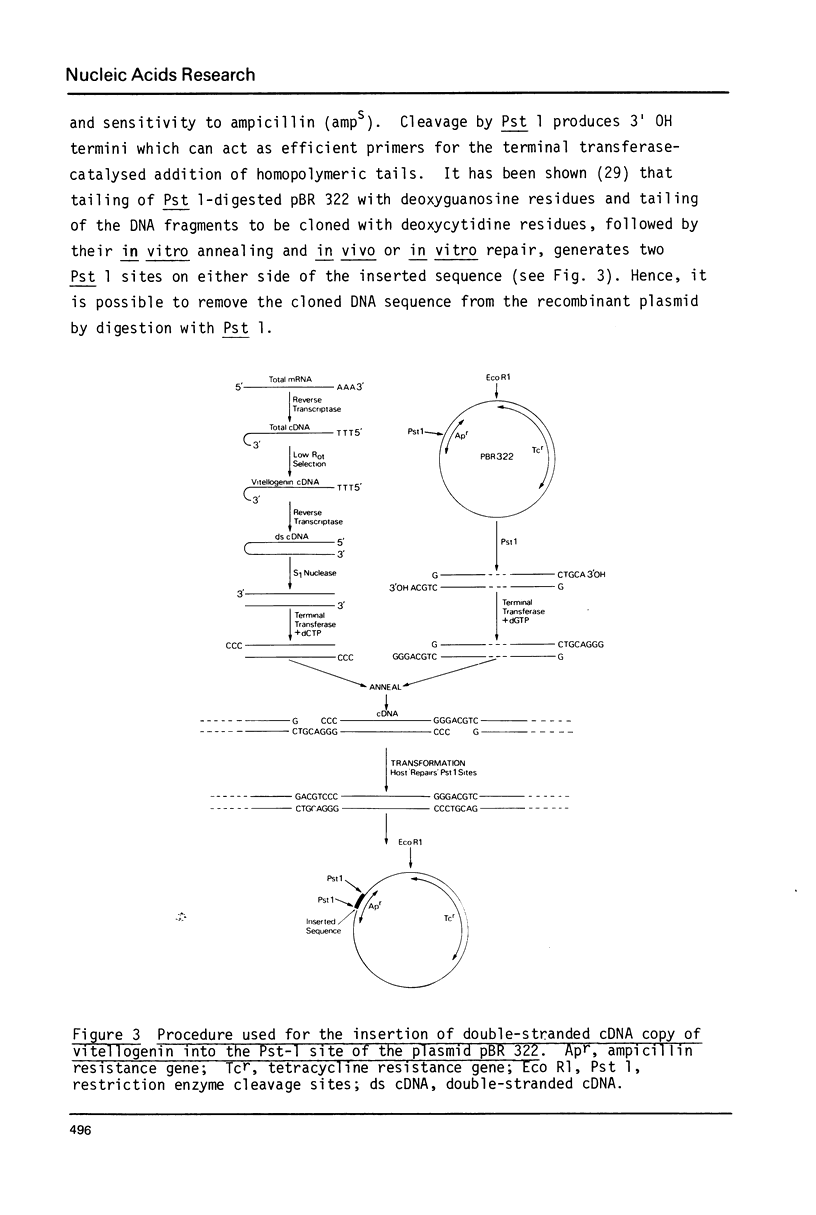

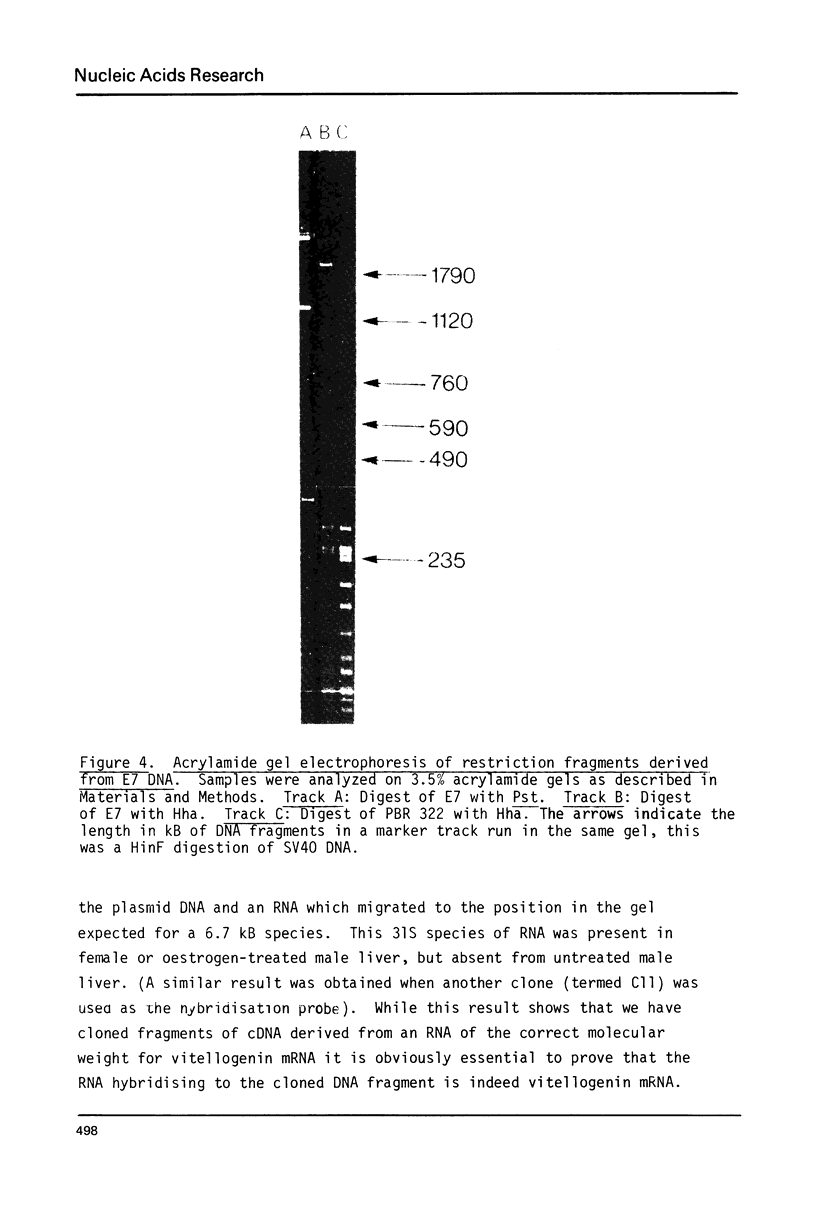




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A. Q., Dolphin P. J., Lazier C. B., Munday K. A., Akhtar M. Chemical composition of an oestrogen-induced calcium-binding glycolipophosphoprotein in Xenopus laevis. Biochem J. 1971 Mar;122(1):107–113. doi: 10.1042/bj1220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem. 1977 Dec 10;252(23):8428–8434. [PubMed] [Google Scholar]
- Berridge M. V., Farmer S. R., Green C. D., Henshaw E. C., Tata J. R. Characterization of polysomes from Xenopus liver synthesizing vitellogenin and translation of vitellogenin and albumin messenger RNA's in vitro. Eur J Biochem. 1976 Feb 2;62(1):161–171. doi: 10.1111/j.1432-1033.1976.tb10109.x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Enea V., Vovis G. F., Zinder N. D. Genetic studies with heteroduplex DNA of bacteriophage fl. Asymmetric segregation, base correction and implications for the mechanism of genetic recombination. J Mol Biol. 1975 Aug 15;96(3):495–509. doi: 10.1016/0022-2836(75)90175-8. [DOI] [PubMed] [Google Scholar]
- Green C. D., Tata J. R. Direct induction by estradiol on vitellogenin synthesis in organ cultures of male Xenopus laevis liver. Cell. 1976 Jan;7(1):131–139. doi: 10.1016/0092-8674(76)90263-4. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Redshaw M. R., Follett B. K. The crystalline yolk-platelet proteins and their soluble plasma precursor in an amphibian, Xenopus laevis. Biochem J. 1971 Oct;124(4):759–766. doi: 10.1042/bj1240759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Wahli W., Weber R. Quantitation of vitellogenin messenger RNA in the liver of male Xenopus toads during primary and secondary stimulation by estrogen. Cell. 1977 May;11(1):213–221. doi: 10.1016/0092-8674(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Baker H. J. Purification and characterization of Xenopus laevis vitellogenin messenger RNA. J Biol Chem. 1977 Aug 10;252(15):5244–5250. [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Wahli W., Wyler T., Weber R., Ryffel G. U. Electron-microscopic demonstration of terminal and internal initiation sites for cDNA synthesis on vitellogenin mRNA. Eur J Biochem. 1978 May;86(1):225–234. doi: 10.1111/j.1432-1033.1978.tb12303.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Studies on amphibian yolk. IX. Xenopus vitellogenin. Biochim Biophys Acta. 1970 Jul 21;215(1):176–183. doi: 10.1016/0304-4165(70)90400-9. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Penman S. The messenger RNA sequences in growing and resting mouse fibroblasts. Cell. 1975 Oct;6(2):197–206. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]