Abstract
Dietary selenium (]Se), mainly through its incorporation into selenoproteins, plays an important role in inflammation and immunity. Adequate levels of Se are important for initiating immunity, but they are also involved in regulating excessive immune responses and chronic inflammation. Evidence has emerged regarding roles for individual selenoproteins in regulating inflammation and immunity, and this has provided important insight into mechanisms by which Se influences these processes. Se deficiency has long been recognized to negatively impact immune cells during activation, differentiation, and proliferation. This is related to increased oxidative stress, but additional functions such as protein folding and calcium flux may also be impaired in immune cells under Se deficient conditions. Supplementing diets with above-adequate levels of Se can also impinge on immune cell function, with some types of inflammation and immunity particularly affected and sexually dimorphic effects of Se levels in some cases. In this comprehensivearticle, the roles of Se and individual selenoproteins in regulating immune cell signaling and function are discussed. Particular emphasis is given to how Se and selenoproteins are linked to redox signaling, oxidative burst, calcium flux, and the subsequent effector functions of immune cells. Data obtained from cell culture and animal models are reviewed and compared with those involving human physiology and pathophysiology, including the effects of Se levels on inflammatory or immune-related diseases including anti-viral immunity, autoimmunity, sepsis, allergic asthma, and chronic inflammatory disorders. Finally, the benefits and potential adverse effects of intervention with Se supplementation for various inflammatory or immune disorders are discussed. Antioxid. Redox Signal. 16, 705–743.
-
VII. Se and Immune Cell Effector Functions
IX. Can Se Supplementation Be Targeted to the Immune System?
I. Introduction
Selenium (Se) is an essential micronutrient that is important for various aspects of human health, including proper thyroid hormone metabolism, cardiovascular health, prevention of neurodegeneration and cancer, and optimal immune responses. Very low (deplete) or very high (toxic) levels of Se intake can be detrimental or possibly fatal. Extreme deficiency or toxicity is not commonly found in humans, but selenosis has been reported in cases of miscalculated supplement formulations, suicides, accidental overdose, or intentional poisoning (150, 177, 238). That said, less overt changes in Se status within an individual may still affect inflammation and immune responses. The biological effects of Se are mainly exerted through its incorporation into selenoproteins, and selenoproteins are involved in the activation, proliferation, and differentiation of cells that drive innate and adaptive immune responses. Dietary Se and selenoproteins are not only important for initiating or enhancing immunity, but they are also involved in immunoregulation, which is crucial for preventing excessive responses that may lead to autoimmunity or chronic inflammation. It should be noted that most studies in the literature involve modifications to dietary Se, and insights into mechanisms often are not clear, but roles for individual selenoproteins and mechanisms are discussed when data are available.
On a cellular level, dietary Se may influence various leukocytic effector functions including adherence, migration, phagocytosis, and cytokine secretion. Several members of the selenoprotein family regulate or are regulated by cellular redox tone, which is a crucial modulator of immune cell signaling and function. There are also important links between selenoproteins and calcium (Ca2+) flux, which is regulated by and regulates the oxidative burst required for optimal immune cell activation. New insights have been gained into specific roles for individual selenoproteins in modulating immune receptor-mediated signaling pathways linked to Ca2+ flux and oxidative burst, inducing cytokine production, migration, and other cellular processes. This article will describe redox-based mechanisms that affect these cellular processes during inflammation and immunity, and how Se and selenoproteins are involved in those processes. The impact of Se on immune-related human physiology and pathophysiology is also discussed, with emphasis placed on disorders related to immunity and chronic inflammation. It should be noted that health issues such as hypertension and cardiovascular diseases have been extensively covered in other reviews (141, 183, 245), and are not included in this article. Finally, issues are raised as to how Se supplementation may be best utilized to enhance or modulate certain types of inflammation and immune responses.
II. Bioactive Forms of Se and Their Effects
Dietary Se is essential in trace amounts and is attained through a wide variety of food sources including grains, vegetables, seafood, meats, dairy products, and nuts (68). The major form of Se ingested by humans is selenomethionine (Se-Met), although other forms of Se are present in foods. Dietary Se may exert some of its biological effects through small-molecular-weight selenocompounds. For example, both selenite and Se-Met may be metabolized into methylated Se compounds, some of which have cancer chemopreventive effects (113). One example is the inhibition histone deacetylase (HDAC) activity in diffuse large B-cell lymphoma cell lines by methylseleninic acid and the toxic effects this may exert in chemoprevention (125). In addition, some studies have used the selenoorganic compound, ebselen, to show that macrophage and dendritic cell functions are affected by this small-molecular-weight selenocompound (156, 229). However, there are very few studies that investigate the effects of selenocompounds on inflammation or immunity, and most of the data regarding the biological activity of Se is related to its incorporation into selenoproteins. Thus, this article will mainly focus on the role of selenoproteins in exerting the effects of dietary Se on inflammatory and immune responses. Se is often referred to as an antioxidant, mainly due to the role of certain selenoproteins in detoxifying hydrogen peroxidase or reversing the effects of oxidized lipids or methionine residues. In addition, certain selenoproteins are crucial for regenerating reduced forms of thioredoxin to maintain balanced levels of reduced/oxidized molecules within cells (cellular redox tone) as described in greater detail next. However, there are selenoproteins that are not directly involved in antioxidant functions, and these need to be considered when determining how alterations in Se intake affect cellullar processes or health outcomes.
III. Incorporation of Dietary Se into Selenoproteins
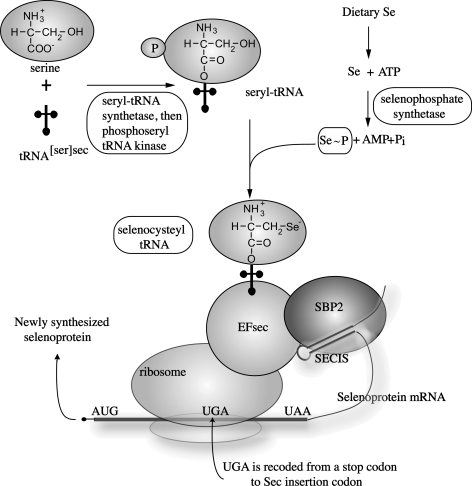
Within all cell-types there exists a complex selenoprotein biosynthesis pathway (Fig. 1), into which dietary Se is ultimately shuttled (5). Selenoproteins contain the 21st amino acid, selenocysteine (Sec), which is co-translationally inserted during protein synthesis. Selenoprotein biosynthesis is initiated by the charging of serine (Ser) onto a dedicated tRNA (tRNASec) to generate Ser-tRNASec. The seryl residue of Ser-tRNASec is phosphorylated by phosphoseryl-tRNA[Ser]Sec kinase (Pstk), and is then converted to selenocysteyl-tRNASec (Sec-tRNASec) using monoselenophosphate (Se-p) as a donor of Se. The Sec-tRNASec is used to transfer Sec into nascent selenoproteins co-translationally through a mechanism that requires dedicated cis elements present in the selenoprotein mRNA (e.g., selenocysteine insertion sequence, SECIS) and protein factors that act in trans including SECIS-binding protein-2 (SBP2) and a Sec-specific translational elongation factor (EFsec) and others (239). This results in recoding UGA from a stop codon to a Sec-insertion codon, and the resulting protein contains the Sec amino acid, which is utilized by selenoproteins for various biological processes.
FIG. 1.
Selenoprotein synthesis. The process is initiated by the charging of serine (Ser) onto a dedicated tRNA (tRNA[ser]Sec) to generate Ser-tRNASec. The seryl residue of Ser-tRNASec is enzymatically phosphorylated, and then is converted to Sec-tRNASec using monoselenophosphate as a donor of Se. The Sec-tRNASec is used to transfer Sec into nascent selenoproteins co-translationally through a mechanism that requires several dedicated cis elements present in the selenoprotein mRNA (SECIS element) and protein factors that act in trans including SBP2 and EFsec and others. This results in recoding UGA from a stop codon to a Sec-insertion codon and the resulting protein contains the Sec amino acid, which is utilized by selenoproteins for various biological processes. Sec-tRNASec, selenocysteyl-tRNASec; Se, selenium; SBP2, SECIS-binding protein 2; SECIS, selenocysteine insertion sequence; EFSec, selenocysteine-specific translation elongation factor.
IV. The Selenoprotein Family
A. An overview of selenoproteins
In humans, there are a total of 25 human genes encoding selenoproteins (134), 24 of which exist as Sec-containing proteins in mice and rats. Selenoprotein expression is essential for life as demonstrated by the generation of mice lacking Sec-tRNASec required for translation of all selenoproteins, which was embryonic lethal (22). However, knockout mouse models of individual selenoprotein genes generated to date suggest that only some are embryonic lethal (Gpx4, Txnrd1, and Txnrd2) or severely impair fertility (Dio3 and SelP). Although broadly classified as antioxidants, selenoproteins actually exhibit a wide range of tissue distribution, cellular locations, and functions (Table 1). Functions for several selenoproteins remain unclear or altogether unknown. However, the pace of discovery is quickening regarding selenoprotein functions and as more biological roles are identified, the effects of Se levels on physiological or pathophysiological processes will be better understood.
Table 1.
Summary of Selenoproteins
| Selenoprotein | Abbreviation(s) | Function and significance |
|---|---|---|
| Cytosolic glutathione peroxidase | GPX1 | GPX1 knockout is more suseptible to oxidative challenge. Overexpression of GPX1 increases risk of diabetes. |
| Gastrointestinal glutathione peroxidase | GPX2 | GPX1/GPX2 double knockout mice develop intestinal cancer, one allele of GPX2 added back confers protection. |
| Plasma glutathione peroxidase | GPX3 | Important for cardiovascular protection, perhaps through modulation of nitrous oxide levels; antioxidant in thyroid gland. |
| Phosholipid hydroperoxide glutathione peroxidase | GPX4 | Genetic deletion is embryonic lethal; GPX4 acts as crucial antioxidant, and sensor of oxidative stress and proapoptotic signals. |
| Olfactory glutathione peroxidase | GPX6 | Importance unknown. |
| Thioredoxin reductase type I | TXNRD1, TrxR1, TR1 | Localized to cytoplasm and nucleus. Genetic deletion is embryonic lethal. |
| Thioredoxin reductase type II | TXNRD2, TrxR2, TR3 | Localized to mitochondria. Genetic deletion is embryonic lethal. |
| Thioredoxin reductase type III | TXNRD3, TRxR3, TR2, TGR | Testes-specific expression. |
| Deiodinase type I | D1, DIO1 | Important for systemic active thyroid hormone levels. |
| Deiodinase type II | D2, DIO2 | Important for local active thyroid hormone levels. |
| Deiodinase type III | D3, DIO3 | Inactivates thyroid hormone. |
| Selenoprotein H | SELH | Nuclear localization, involved in transcription. Essential for viability and antioxidant defense in Drosophila. |
| Selenoprotein I | SELI, hEPT1 | Possibly involved in phospholipid biosynthesis. |
| Selenoprotein K | SELK | Transmembrane protein localized to endoplasmic reticulum and involved in calcium flux in immune cells. |
| Selenoprotein M, Selenoprotein 15 | SELM, SEP15 | Thiol-disulfide oxidoreductases localized to endoplasmic reticulum. Possibly involved in protein-folding. |
| Selenoprotein N | SELN, SEPN1, SepN | Potential role in early muscle formation; involved in RyR-related calcium mobilization from ER; mutations lead to multiminicore disease and other myopathies. |
| Selenoprotein O | SELO | Contains a Cys-X-X-Sec motif suggestive of redox function, but importance remains unknown. |
| Selenoprotein P | SELP, SEPP | Selenium transport to brain and testes—SELP knockout leads to neurological problems and male sterility. SELP also functions as intracellular antioxidant in phagocytes. |
| Selenoprotein R | SELR, MsrB1 | Functions as a methionine sulfoxide reductase and SELR knockouts show mild damage to oxidative insult. |
| Selenoprotein S | SELS, SEPS1, SELENOS, VIMP | Transmembrane protein found in plasma membrane and endoplasmic reticulum. May be involved in ER stress. |
| Selenoprotein T | SELT | Endoplasmic reticulum protein involved in calcium mobilization. |
| Selenoprotein V | SELV | Testes-specific expression. |
| Selenoprotein W | SELW, SEPW1 | Putative antioxidant role, perhaps important in muscle growth. |
| Selenophosphate synthetase | SPS2 | Involved in synthesis of all selenoproteins, including itself. |
B. Selenoprotein functions
1. Glutathione peroxidases
The glutathione peroxidase (GPX) selenoenzymes in humans consist of eight isoforms, but only six (GPX1-6) contain Sec. The first selenoprotein identified in mammals was GPX1 (cellular GPX) (208). Other members of this subfamily include GPX2 (intestinal GPX), GPX3 (plasma GPX), and GPX4 (phospholipid GPX). GPX1 and GPX4 are expressed in most tissues, whereas GPX2 is expressed mainly at the epithelium of the gastrointestinal tract, and GPX3 is synthesized predominately in kidney, heart, and thyroid gland. Of these four GPX enzymes, only GPX3 is secreted for circulation or for use in plasma, in extracellular spaces, or by neighboring cells. In fact, GPX3 accounts for 20%–40% of total plasma Se in humans (131). GPX6 is a selenoprotein in humans (in mice, Gpx6 contains cysteine (Cys) instead of Sec) that is localized to olfactory epithelium and embryonic tissues (134). The GPX enzymes utilize Se at their active sites to detoxify reactive oxygen species (ROS) including hydrogen peroxide (H2O2) and phospholipid hydroperoxide. GPX1 and 4 are among the most abundant selenoproteins in several immune cells and tissues (36, 104).
2. Thioredoxin reductases
The thioredoxin reductase (TXNRD) enzymes are another well-characterized subfamily of selenoproteins that perform an essential redox role by regenerating reduced thioredoxin (TXN or TRX) within cells (147, 251). TXN is a small redox active protein distributed ubiquitously in various mammalian tissues and cells that serves to reduce oxidized moities (e.g., Cys–Cys disulfide bonds), and the TXN/TXNRD system is one of the most important mechanisms for regulating cellular redox balance (108). TXNRDs include cytoplasmic/nuclear TXNRD1 (also called TR1 or TRXR1) that reduces TXN1, mitochondrial TXNRD2 (also called TR3 or TRXR2) that reduces TXN2, and testes-specific thioredoxin-glutathione reductase (also called TXNRD3, TR2, TRXR3, or TGR). The essential roles of Txnrd1 and 2 during development are evident by studies in mice demonstrating that genetic deletion of either is embryonic lethal (44, 117). TXNRD1 is particularly important for maintaining redox tone in immune cells through regeneration of reduced cytosolic TXN1. Txnrd1 is the most abundant selenoprotein in mouse macrophages and is upregulated by activation with lipopolysaccharide (LPS) (35).
3. Deiodinases
The iodothyronine deiodinase family is central for thyroid hormone regulation and consists of three enzymes: types 1, 2, and 3 (DIO1, 2, and 3) (219). Thyroid hormone action is initiated by the activation of thyroxine or 3,3′,5, 5′-tetraiodothyronine (T4) prohormone to liothyronine or 3,3′,5-triiodothyronine (T3), which is carried out by DIO1 or DIO2. T4 and T3 are irreversibly inactivated in a reaction catalyzed by DIO3. All three deiodinases are expressed in a number of fetal and adult tissues, with minimal expression detected in immune cells. However, levels of active thyroid hormone may affect systemic Se available for selenoprotein sythesis in a variety of tissues, including those involved in immune responses (172). In this sense, the DIO enzymes may have important indirect roles in inflammation and immunity.
4. Selenoprotein P
Selenoprotein P (SELP or SEPP1) is unique in that it contains multiple Sec residues (up to 10 per SELP and Selp molecule in humans and rodents, respectively). SELP has been shown to play an important role in the transport of Se through the plasma to certain tissues, with the testes and brain particularly dependent on SELP for adequate Se levels (32, 101, 212). SELP is synthesized in several different tissues, but hepatically derived SELP serves as a key Se transporter. Hepatic SELP is secreted into plasma, which then influences whole-body Se homeostasis (222). Interestingly, studies in mice have shown that expression of liver SELP is higher in women compared with men (220). There is mounting evidence that SELP not only transports Se but also performs crucial antioxidant functions, which are particularly important for certain immune functions as discussed in greater detail next.
5. Selenoproteins K and S
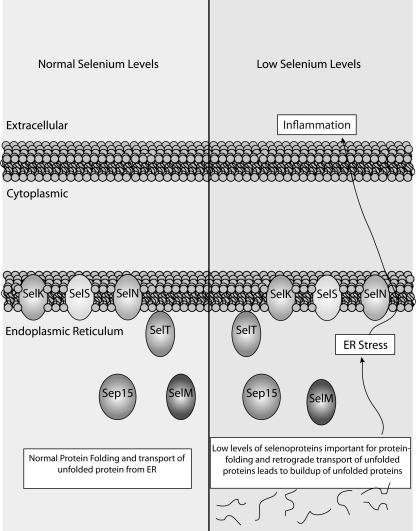
Two selenoproteins related to inflammation and immunity include the endoplasmic reticulum (ER) transmembrane proteins, SELK and SELS. Both of these proteins have been proposed to play a role in protecting cells during conditions that lead to ER stress. For SELS, this appears to be related to its role in retrograde translocation of misfolded proteins from the ER (80). However, the link between SELK and ER stress has only been demonstrated in the HepG2 cell line with no defined in vivo role for SELK in modulating this process (60). Our laboratory recently revealed the requirement of SELK in promoting Ca2+ flux during the activation of several types of immune cells (256). This role is independent of ER stress and affects Ca2+-dependent effector functions of T cells, neutrophils, and macrophages. In addition, Selk is particularly sensitive to Se status in human peripheral leukocytes (192), which further suggests that this selenoprotein may have a special role in immune cells separate from potential ER stress-related functions. Specific functions for SELK and SELS and their relationship to redox signaling during inflammation and immunity are discussed ingreater detail next.
6. Other selenoprotein family members
What defines members of the selenoprotein family is the incorporated Sec residue, but how the different selenoproteins functionally utilize Sec is quite diverse. Some biological functions include transcriptional regulation (SelH), phospholipid synthesis (SELI), protein-folding (SELM and SEP15), methionine sulfoxide reduction (SELR), and the biosynthesis of selenoproteins (SPS2). Most of these functions are necessary for proper functioning of most tissues and cell types, including those involved in immune responses. Functions for several selenoprotein family members remain unclear or unknown.
C. The hierarchy of selenoprotein expression
With moderate Se deficiency, it has been suggested that expression of nonessential selenoproteins are preferentially lost, whereas essential selenoproteins are maintained (160). In addition, under Se deficient conditions, not all tissues are equivalently supplied with the limited amounts Se (221). Tissues such as the thyroid gland and brain maintain Se levels during deficiency, and tissues such as those of the immune system exhibit a more rapid decline in bioavailable Se leading to lower selenoprotein synthesis. These concepts are often referred to as “the hierarchy of selenoprotein synthesis” and should be carefully considered when investigating the effects of low Se status on immune responses or other aspects of human health. In the same manner, increasing dietary Se leads to more bioavailable Sec-tRNASec required for translation of all selenoproteins, but expression of some selenoproteins are increased at higher levels than others. This is likely due to differences in stability of the individual selenoprotein mRNAs, which leads to higher representation within the total selenoprotein mRNA pool. However, there are bound to be other factors that give certain selenoprotein mRNAs more access to increased Sec-tRNASec during Se supplementation. In fact, genotype and other metabollic factors are likely to influence how Se supplementation affects increased synthesis of different selenoproteins in different tissues (43).
V. Selenoprotein Expression in Immune Tissues and Cells
A. Tissue and cellular distribution under physiological conditions
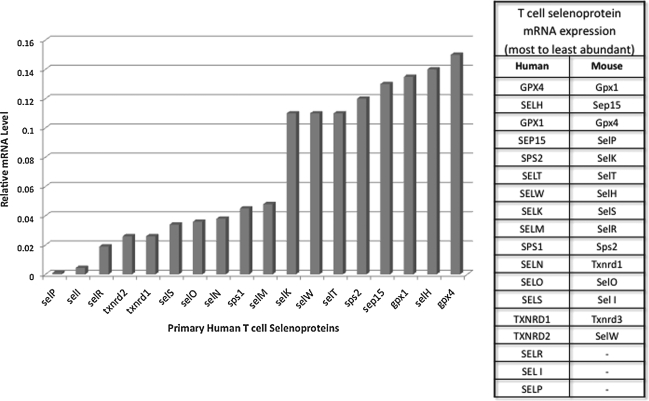
Immune cells express many, but not all, members of the selenoprotein family. Within cells of the immune system, selenoproteins within immune cells perform antioxidant functions, carry out protein folding, promote certain cell signaling events during activation, or serve to carry out as of yet undefined functions. The most abundant selenoprotein mRNAs in mouse spleen include Gpx1 and 4, Selw, Selk, and Sep15 (104). When T cells from mouse spleen were analyzed separately from other splenocytes, the most abundant transcripts included Gpx1 and 4, Sep15, Selp, and Selk (36). Our analyses of human peripheral blood T cells suggest not only some similarities between human and mouse T cells, but some differences as well (Fig. 2). For example, in both species, there is a high abundance of GPX1 and 4, SEP15 and SELT mRNAs, but Selp mRNA is much more abundant in mouse compared with human T cells. In mouse macrophages, Gpx1 and 4, Sep15, Selp, Selk, Selr, and Txnrd1 are the most abundant mRNAs detected (35). Thus, T cells and macrophages from mice are quite similar in patterns of selenoprotein expression. These studies collectively indicate that several selenoprotein mRNAs such as DIO1, 2, and 3, as well as GPX2 and SELV are not detectable in lymphoid tissues or cells, and several are expressed at very low levels. In this sense, immune cells do not differ greatly from most other cell types in their patterns of selenoprotein expression, with redox-regulating and protein-folding selenoproteins expressed at the highest levels. One exception to this notion is SELK, which appears not to exhibit oxidoreductase properties found in antioxidant or protein-folding selenoenzymes. Selk protein is expressed at particularly high levels in mouse immune tissues (256), thus suggesting an important role for this selenoprotein in the immune system and this is further discussed next. It would be valuable to conduct similar comparisons of different tissues for other selenoproteins at the level of protein expression. It is also important to note that the hierarchal control of selenoprotein expression in T cells may be influenced by both dietary Se levels and the activation or differentiation state of the cells. Selenoproteins expressed at low levels in naive T cells may be increased in expression levels on activation or may be retained at high levels in memory T cells. These types of changes may reveal important roles of some of the selenoproteins in Figure 2 expressed at relatively low levels.
FIG. 2.
Comparison of the selenoprotein transcriptome in human and mouse T cells. Total RNA was extracted from T cells from a normal healthy volunteer and real-time polymerase chain reaction was performed with primers as previously described (240). Levels of each mRNA were normalized to the housekeeping mRNA, ubiquitin c, and the relative abundance compared with published results for mouse T cells (36). Results show some similarities between human and mouse T cells, with the most abundant mRNAs common to both species. One exception to this is SelP, which is much higher in relative abundance in mouse T cells compared with humans.
B. Selenoprotein expression in immune cells and tissues in response to Se changes
Similar to other cell types, immune cells respond to increased dietary Se by increasing expression of many selenoproteins, although not all selenoproteins are equivalently affected. T cells from mice fed diets with increasing Se content (from 0.08 to 1.0 ppm for 8 weeks) exhibited higher Gpx1 and Txnrd1 activity (102). Similar results were obtained in human studies involving Se supplementation (50 or 100 μg/day as sodium selenite) in which both GPX1 and GPX4 activity were increased in lymphocytes from supplemented individuals compared with nonsupplemented controls (29). In a recent study involving humans receiving enriched Se diets (50–200 μg/day Se-enriched yeast or 50 g/day Se-enriched onions) or placebos, higher Se diets increased mRNA levels for SELR, SELW, and SELS in peripheral blood mononuclear cells (PBMCs) (88). Se-enriched onions were more effective than Se-enriched yeast supplements in increasing all three selenoprotein mRNAs, thus emphasizing that the form of Se supplementation does influence the bioavailability of Se to the immune system.
Microarray studies have investigated the effects of Se deficiency on global gene expression and found that certain selenoprotein mRNAs in immune tissues are decreased more than others under Se deficient conditions. For example, the colon is lined with gut-associated lymphoid tissues, and mice fed moderately deficient (0.08 ppm Se) or adequate (0.15 ppm Se) Se diet for 6 weeks were analyzed for mRNA and Se deficiency caused a decrease in colonic Selw, Gpx1, Selh, and Selm mRNA (129). In addition, decreased in these tissues were mRNAs for inflammatory pathways, including tumor necrosis factor α (TNF-α) and interleukin 2 (IL-2). Interestingly, marginal Se deficiency (0.08 ppm Se) actually upregulated mRNA for Txnrd1 and Gpx2 in duodenum, thus suggesting that these mRNAs may be most abundant in the pool of selenoprotein mRNAs and first to become translated once the tissue is restored to Se-replete conditions (178). In humans who were supplemented with 100 μg/day Se as sodium selenite for 6 weeks, microarrays were used to analyze peripheral lymphocytes and the main pathways affected were those involving increased ribosomal protein and translation factor gene expression (192). The conclusions from these array data are that lower Se status in immune cells has a stronger effect on certain antioxidant selenoproteins (particularly Gpx1 expression) and decreases mRNAs involved in inflammatory signaling pathways. In contrast, higher Se status in immune cells increases the protein synthesis machinery, presumably for increased production of selenoproteins.
C. The selenoproteomic response during immune cell activation
Expression of important selenoproteins in immune cells may change during activation. Of course, major fluctuations in mRNA or protein levels do not alone indicate the importance of individual selenoproteins in the activation process, but discerning how the selenoproteome responds to activation can provide clues for roles each may play. Carlson et al. demonstrated that mouse macrophages activated with LPS increase Txnrd1 expression at the levels of both mRNA and protein, although Txnrd1 enzymatic activity was not measured (35). Expression of several other selenoproteins, including Gpx enzymes, appeared to be less affected by LPS treatment, which suggests a special role for Txnrd1 in regulating redox status in activated macrophages. Human neutrophils stimulated with TNF-α increased GPX4 expression in a ROS-dependent manner, thus suggesting that this selenoprotein is important for protecting the cells against oxidative damage during activation (94).
LPS-treatment or Fcγ-receptor (FcγR)-stimulation of mouse macrophages increases expression of two ER selenoproteins, Selk and Sels (110, 243). For Sels, this could be related to its role in mitigating ER stress arising from increased protein processing that accompanies macrophage activation. Consistent with these findings, SELS mRNA was shown to increase in human PBMCs 7 days after influenza vaccine challenge (88). Sels expression is increased by LPS-treatment in mice in a manner dependent on both Se status and gender (243). For Selk, its increased expression during LPS-activation of macrophages has less to do with ER stress but be more related to its role in Ca2+ flux and cell signaling induced in activated macrophages (110, 256). Interestingly, Sep15 mRNA is highly abundant in immune cells, and its increased abundance during activation may reflect an increased requirement for folding and maturation of a restricted group of N-glycosylated proteins in the ER (140). More efficient protein folding through increased expression of SEP15 or other ER selenoproteins such as SELM may be an important mechanism by which dietary Se affects immune cell function during activation of these cells.
VI. Se and Redox Signaling in Immune Cells
A. An overview
The generation of ROS by immune cells is often associated with the killing of microbes by phagocytes. Indeed, ROS produced by macrophages and neutrophils is essential for the oxidative destruction of phagocytosed pathogens and fully effective immunity. ROS have also become recognized as important mediators of cell signaling and cell-to-cell communication for a variety of phagocytic and nonphagocytic immune cells. For example, mutations in genes encoding superoxide-generating enzymes can disrupt the oxidative burst generated by phagocytes, thus leading to chronic granulomatous disease (CGD) that is characterized by severe, life-threatening bacterial and fungal infections (106). This disease involves persistent inflammation that has largely been attributed to recurrent infections due to inadequate killing of pathogens by phagocytes. However, persistent inflammation may also occur independent of infection, and recently, it was shown that a deficiency in one of the superoxide generating enzymes, Nox2, is associated with hyperinflammation and autoimmune diseases due to the key role of this pro-oxidant enzyme in terminating immune responses (217). This example along with several others illustrate how ROS have emerged as important secondary messengers that affect signaling and functions of a variety of cell types, including immune cells. Interestingly, levels of Se intake can influence the production of ROS and their downstream effects. The next sections describe the role of redox mechanisms and how Se may affect these mechanisms. It should be noted that ROS in cells or tissues are often measured in indirect manners, such as the oxidation of fluorochromes or the evaluation of the oxidative damage of lipids, proteins, or DNA. Thus, it should be kept in mind that the term ROS is often used in reference to their effects, and often not by direct measurement of individual ROS themselves.
B. Types of ROS important for immune cell signaling
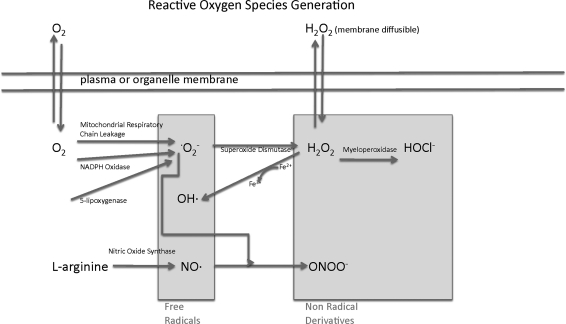
Chemically reactive molecules derived from oxygen include superoxide (· O2−), H2O2, hydroxyl radical (·OH), nitric oxide (NO·), and peroxynitrite (ONOO−; Fig. 3). These reactive molecules are divided into two major groups: free radicals (·O2−, ·OH, and nitric oxide [NO·]) and nonradical derivatives of O2 (H2O2, ONOO−) (59). Some ROS such as H2O2 are able to diffuse freely through cellular membranes, whereas others such as superoxide that are electrically non-neutral fail to do so (77). However, there is some evidence that H2O2 may not penetrate some membranes as easily as previously proposed (9, 223, 236). This is important, because it means that superoxide may or may not be restricted to the cellular compartments in which it is generated. ROS can be generated spontaneously or through enzymatic reactions. For example, superoxide can be generated through electron leakage from the electron transport chain in the mitochondria. Alternatively, superoxide can be synthesized by flavoenzymes such as xanthine oxidase (135), 5-lipoxygenase, or the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases that are described in greater detail next. In biological systems, superoxide is short lived owing to its rapid reduction to H2O2 by superoxide dismutase (SOD) (118). The superoxide anion has an estimated half-life of 1 μs, whereas H2O2 is more stable with an estimated half-life of 1 ms.
FIG. 3.
The relationship between different reactive oxygen species (ROS). The two major subsets, free radicals and nonradical derivatives, are shown with illustrations showing how members of each are related to each other. There are several sources of superoxide (·O2−), which can subsequently be converted to other ROS such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO-).
Stability of ROS is dependent on the levels and activity of enzymes responsible for their neutralization, and some of these enzymes are selenoproteins. As just mentioned, the GPX1 and 3 play important roles in reducing H2O2 to water, and Se status in immune cells can directly affect the half-life of this ROS. In addition, GPX4 educes phospholipid hydroperoxides, and this activity has recently been shown to regulate protein tyrosine phosphatase (PTP) signaling, particularly through Gpx4-mediated reduction of 12/15-lipoxygenase (45). Txnrd1 may indirectly regulate the downstream effects of H2O2 by reducing disulfide bonds generated by H2O2 in signaling molecules. Other selenoproteins such as Selp also exhibit peroxidase activity, and the antioxidant properties of Selp have been shown to affect mouse macrophage differentiation and survival during parasitic infection, but the signaling events are not clear. How individual selenoproteins affect specific signaling pathways is discussed in greater detail in later sections, but overall, the pathways and signaling molecules affected by the actions of selenoproteins is only just beginning to be understood.
C. Se levels related to the production of ROS in immune cells
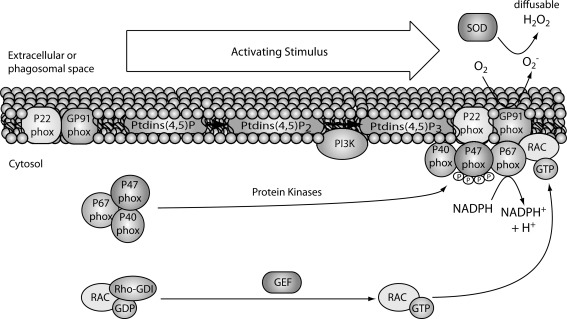
Activation of immune cells through cell surface or intracellular receptors can lead to high levels of ROS within minutes, often referred to as an oxidative burst. In general, activated phagocytes produce higher levels of ROS than nonphagocytic immune cells such as T cells. Although mitochondrial sources of ROS have been implicated in the activation of T cells and other immune cells (122, 276), the oxidative burst that is induced on activation of immune cells is predominantly of nonmitochondrial origin. The main nonmitochondrial sources of ROS are the NADPH oxidases (NOX, also called phagocytic oxidases, PHOX), which are multicomponent enzymes consisting of cytosolic and membrane-bound proteins (257). The membrane components include a stable, heterodimeric flavocytochrome (Cyt b558) comprised of two subunits, gp9phox (NOX2) and p22phox. The cytosolic components are comprised of four factors including p67phox, p47phox, p40phox, and a small G-protein (Ras-related C3 botulinum toxin substrate 1/2 [RAC1/2]). When cell surface or intracellular receptors are stimulated by their ligands, the cytosolic components just listed translocate to the plasma or phagosomal membrane, where the NADPH enzyme complex is assembled (Fig. 4). The catalytic core (b558) transfers electrons from cytosolic NADPH across the plasma membrane to oxygen located on the phagolysosomal or extracellular side to produce superoxide (207). The main source of oxidative burst in phagocytes is the NOX2-based NADPH oxidase system. NOX2-generated ROS are crucial for killing bound or ingested microbes, and NOX2-deficiency results in CGD, which is characterized by severe bacterial and fungal infections (98). In addition to NOX2, two families of NOX homologs are expressed in several tissues and cell types, including those of the immune system: the alternative NOXs (e.g., NOX4) and dual oxidases (for DUOXs) (182). They are capable of generating low amounts of superoxide that are quickly dismutated into H2O2 and are suggested to be involved in cell signaling and host defense. The small GTPase RAC is an important cytosolic regulatory component of the NOX2 complex and exists in two isoforms; RAC1 predominates in monocytes and RAC2 in neutrophils (182, 283). In resting cells, GDP-bound RAC is in complex with GDP dissociation inhibitor, and on activation, GTP is exchanged for GDP via guanine nucleotide exchange factor, and this causes RAC to interact with membrane-associated p47phox (64). This GTP-bound form of RAC positively regulates the actions of the NOX2 complex and results in generation of superoxide (99). Overall, phagocytes such as neutrophils and macrophages utilize their oxidative burst for both destruction of microbes and signaling, whereas nonphagocytic immune cells such as T cells generate an oxidative burst mainly for modulating signaling and function.
FIG. 4.
Generation of superoxide by NADPH oxidase. On activation (e.g., LPS in phagocytes or TCR in T cells), the cytosolic components including p47phox, p67phox, and p40phox, assemble at the membrane to form the enzyme complex. An electron is transferred through the catalytic core (b558) comprised of two subunits, gp91phox (NOX2) and p22 phox. In resting cells, GDP-bound RAC is in complex with its inhibitor GDI, and on activation, GTP is exchanged for GDP via guanine nucleotide exchange factor (GEF) and this causes RAC to interact with membrane-associated p47phox. This GTP-bound form of RAC positively regulates the actions of the NOX2 complex, and the result is the transfer of one electron to oxygen to generate superoxide. This superoxide can subsequently be converted by SOD to diffusable H2O2. NADPH, nicotinamide adenine dinucleotide phosphate; LPS, lipopolysaccharide; TCR, T cell receptor; PHOX, phagocytic oxidase; NOX, NADPH oxidase; RAC, Ras-related C3 botulinum toxin substrate; GDI, GDP dissociation inhibitor; SOD, superoxide dismutase.
Se levels in immune cells can affect the oxidative burst in both phagocytic and nonphagocytic cells. For example, neutrophils from Se deficient rats exhibited reduced oxidative burst when incubated for prolonged periods with stimulants such as phorbal myristate acetate (PMA) or opsonized zymosan (12). This decreased oxidative burst was due to inadequate metabolism of H2O2, which was linked to lower activity of the NADPH-dependent superoxide-generating system. There are important feedback mechanisms involving levels of H2O2 and the strength of oxidative burst, and the neutrophil results support the notion that selenoproteins regulate this mechanism. Further supporting this notion, macrophages from Selk−/− mice exhibited decreased oxidative burst when phagocytosing IgG-opsonized protein (256). Supplementing immune cells with above-adequate levels of Se (1.0 ppm Se) can also affect the oxidative burst process. In T cells, higher dietary Se produces a stronger oxidative burst in response to T cell receptor (TCR) stimulation (102). Similarly, in J774.1 mouse macrophages Se supplementation to above-adequate levels (>100 ng/ml sodium selenite in culture media) increased the oxidative burst induced by PMA (211). Higher expression of selenoproteins does not always increase oxidative burst, as demonstrated in T helper cells from Gpx1−/− mice (268). When stimulated through the TCR, Gpx1-deficient T helper cells produced higher levels of ROS compared with wild-type controls, thus suggesting that Gpx1 may be required for controlling the oxidative burst once it is generated, but not for the initial generation of ROS during the oxidative burst. Overall, it is apparent that higher levels of Se intake leading to increased expression of antioxidant selenoproteins do not diminish levels of ROS on stimulation of immune cells. This suggests that selenoproteins collectively contribute to the signal strength in T cells, but certain selenoproteins such as Gpx1 are important for regulating the half-life of ROS generated from receptor-mediated oxidative burst. The manner in which certain selenoproteins may perturb the downstream events influenced by receptor-mediated oxidative burst may involve their direct actions on the redox intermediates or on signaling molecules, and these effects are described in greater detail next.
D. Se levels related to calcium and redox signaling in immune cells
1. H2O2 as a secondary messenger in leukocyte activation
H2O2 may enter the cell from extracellular sources by diffusion through the plasma membrane. Alternatively, H2O2 may be generated within immune cells on stimulation of a variety of receptor systems in a tightly regulated manner. As just described, H2O2 is mainly generated through SOD-catalyzed dismutation of superoxide, which itself is generated through receptor-induced NOX activity. H2O2 is less reactive than ROS radicals such as superoxide and the highly reactive OH·. The actions of H2O2 are quite different from ·O2− and OH· in that H2O2 exerts its actions through the oxidation of proteins, whereas these other ROS more readily react with any molecules they encounter (especially true for OH·). H2O2 primarily targets Cys residues in various proteins, oxidizing the -SH group of Cys to sulfenic acid. Sulfenic acid on the Cys residues is reduced back to Cys by enzymatic systems that involve glutathione (GSH) or TXN. In this manner, the redox state of the Cys residue may serve as a molecular switch that can transmit different signals in reduced or oxidized states. Most Cys in proteins are not located within the proper context to be oxidized by H2O2. In particular, the deprotonated Cys state is necessary for effective oxidizing action of H2O2 to convert it to sulfenic acid. The majority of Cys residues within proteins exhibits a pKa value of 8.5 and does not exist as anions at physiological pH. However, if the Cys is located near a positively charged amino acid, then its pKa value may be lowered to value below 5.0, making it deprotonated at physiological pH and a suitable target for oxidation by H2O2. Thus, it is the context in which the Cys is located that determines whether it may act as a molecular switch for transmitting H2O2-mediated signals. An example of this signaling mechanism that is particularly important for immune cells is the PTPs, which contain a redox-regulated Cys in the proper context (HCxxGxxRS/T) (7, 184). Oxidation of this Cys by H2O2 inactivates the PTP, and reduction by GSH or TXN reverts the catalytic domain back to its active state (37, 272). In addition to H2O2, phospholipid hydroperoxides can also oxidize signaling molecules at their active-site Cys residues (45).
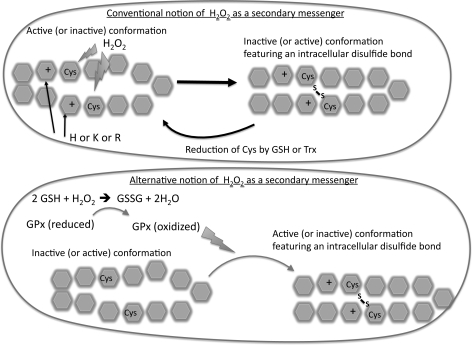
Recently, there has emerged a model of H2O2 signal transduction that differs from that just described (Fig. 5). This model involves signals that are not exerted through direct contact of H2O2 with signaling molecules, but through thiol-based peroxidase enzymes such as GPX and peroxiredoxins. During this process, these thiol peroxidases do not minimize oxidant signaling by H2O2, but actually promote the actions of H2O2 by relaying oxidants to signaling molecules (70, 253). The best example is that of Gpx3 transferring oxidative equivalents to Yap1 in yeast, whch involves formation of a Gpx3-Yap1 disulfide bridge (53). The Cys residue in Gpx3 linked to the Cys residue in Yap1 is then reduced by Txn, which restores the reduced state of Gpx3, and this results in formation of a Yap1 intramolecular disulfide bond. This model helps explain two observations in H2O2-mediated signaling not addressed by the direct oxidation model just described: (1) there appears to be some degree of specificity in the oxidant actions of H2O2, and (2) removal of GPX does not increase the oxidant signaling of H2O2 as would be suspected, but actually decreases its actions (70). In this sense, the GPX and peroxiredoxin enzymes sense increases in H2O2, detoxify this molecule while simultaneously transmitting its oxidant signal to other signaling molecules that ultimately affects transcription. This mechanism does not replace the direct actions of H2O2 on Cys, but is thought to act in conjunction with the direct actions. Much of the data for this model have been obtained using yeast systems (53, 70, 254), but there is some evidence that it occurs in mammalian cells (91).
FIG. 5.
Two alternative models for the actions of H2O2 as a secondary messenger. The conventional model (top) involves direct actions of H2O2 on adjacent Cys residues within a signaling molecule to form a disulfide bond that alters conformation of the active site and the activation state. A new model (bottom) has been proposed in which peroxidases such as GPx1 promote the oxidation of adjacent Cys residues and formation of disulfide bonds. In this sense, the actions of H2O2 are indirect and the direct affects are determined by levels and locations of GPX1 and GSH. Cys, cysteine; GSH, glutathione; GPX, glutathione peroxidase.
2. The relationship between Ca2+ flux and oxidative burst
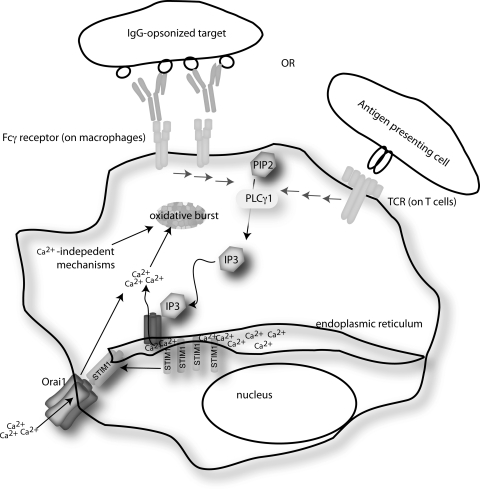
Ca2+ plays a key role as a secondary messenger of signal transduction for a wide range of cell-types. The pathway by which Ca2+ flux occurs was elegantly introduced by Putney in 1986 (200), and the model was subsequently modified to incorporate the vast amount of data generated since. Immune cells require influx of extracellular Ca2+ to initiate or propagate signals that regulate different functions including gene transcription, proliferation, chemotaxis, cytokine secretion, and oxidative destruction of phagocytosed microbes. For immune cells, Ca2+ enters from extracellular spaces but is most often initiated from release of intracellular Ca2+ stores, predominantly located in the ER (143, 201). For example, engagement of TCR, B cell receptor, Fcγ or Fcɛ receptors, or chemokine receptors will activate phospholipase Cγ (PLCγ), which cleaves phosphatidylinositol-4,5-bisphosphate (PIP2) to produce messenger molecules inositol-1,4, 5-trisphosphate (IP3) and diacylglycerol (DAG), the former of which triggers a rise in cellular Ca2+ levels (Fig. 6). The predominant pathway of Ca2+ flux involves binding of IP3 to the IP3-receptor (IP3R) located on the ER membrane, which results in Ca2+ release from ER stores. Loss of ER Ca2+ stores induces the opening of calcium release-activated Ca2+ (CRAC or Orai1) channels in the plasma membrane (20). This process is termed store-operated Ca2+ entry (SOCE) and relies on the actions of stromal interaction molecule 1 (STIM1) (148). STIM1 is located in the ER membrane and contains a luminal EF-hand domain that senses Ca2+ loss from ER stores. On efflux of Ca2+ from ER stores, STIM1 is induced to interact with Orai1 channels, causing structural changes in the Orai1 channel that allows extracellular Ca2+ to enter the cytosol.
FIG. 6.
The basic steps involved in store-operated Ca2+ release (SOCE) for either T cells or macrophages. Stores of Ca2+ for these cells are largely maintained in ER. Engagement of receptors on the surface of immune cells leads to activation of PLCγ, which converts PIP3 to DAG and IP3. IP3 rapidly binds to the IP3 receptor on the ER membrane, which causes loss of Ca2+ from the ER stores. The lower [Ca2+] in the ER lumen is sensed by EF-hand motifs in the ER luminal STIM1 molecule, and this leads to oligomerization of STIM1. Oligomerized STIM1 physically interacts with Orai1 on the plasma membrane, which activates this channel and causes the entry of high levels of Ca2+. ER, endoplasmic reticulum; PLCγ, phospholipase Cγ; IP3; inositol-1,4, 5-trisphosphate; DAG, diacylglycerol; EF, elongation factor; STIM1, stromal interaction molecule 1.
Ca2+ flux is generated within seconds of receptor stimulation in immune cells and generally precedes any measurable oxidative burst, which typically occurs within minutes. Ca2+ flux first to occur, and it is required for generation of an effective oxidative burst, as best exemplified by experiments involving neutrophils. Early studies used ethylene glycol tetraacetic acid to chelate Ca2+ during activation of neutrophils, which led to a significant decrease in superoxide generation (76, 78, 198). Within seconds of stimulation with inflammatory molecules such as formyl-Methionyl-Leucyl-Phenylalanine (fMLP), an elevation in cytoplasmic Ca2+ occurs that has been shown to directly regulate the key NOX in neutrophils, NOX2. As just described, STIM1 is the central ER membrane molecule that relays signals from the ER to the CRAC channels on the surface of immune cells, thus producing the SOCE that leads to the rise in intracellular Ca2+. Using small interfering RNA in human neutrophil-like HL-60 cells, it was shown that expression of STIM1, but not STIM2, was required for NOX2 activation during fMLP stimulation (27). However, although the Ca2+ flux is required, it is not sufficient for activation of NOX2 and O2− production (28). Thus, NOX regulation involves both Ca2+-independent and Ca2+-dependent mechanisms that act in synergy to modulate O2− production in activated phagocytes.
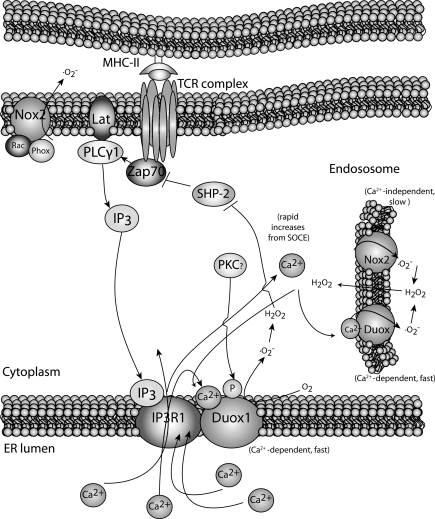
A link between Ca2+ and oxidative burst has been identified in other immune cells in addition to neutrophils. In elegant studies involving human Jurkat T cells and human CD4+ T cell blasts, Kwon et al. characterized two different oxidative bursts during TCR stimulation (137, 138). First, there appears an early (<5 min) oxidative burst generated by DUOX1 in a Ca2+-dependent manner (Fig. 7). Knocking down DUOX1 inhibited H2O2 production and specific activation events including phophorylation of Tyr319 in zeta chain-associated protein kinase 70 and extracellular signal-regulated protein kinase (ERK) activation. Interestingly, knockdown of DUOX1 decreased Ca2+ flux, including release of Ca2+ from the ER upon TCR-stimulation. This suggests that, in addition to Ca2+ regulating oxidative burst, the opposite may also be true, that is, Ca2+-dependent oxidative burst through DUOX1 is required for optimal Ca2+ flux. However, it should be noted that the knockdown of DUOX1 was carried out in Jurkat T cell lines and the role of DUOX1 in regulating Ca2+ flux in naive human or mouse T cells has not been demonstrated. Regardless, there is evidence for a role for DUOX1 in early oxidative burst during TCR stimulation, and it is important to determine whether similar results are obtained during the stimulation of truly naive human and mouse T cells.
FIG. 7.
Two different NADPH oxidase systems operate during activation of T cells. At early stages after TCR-activation, Ca2+-dependent IP3 generation leads to activation of the IP3 receptor on the ER membrane. This causes DUOX1-induced superoxide, which is dismutated into H2O2. This acts to promote T cell activation in the early stages. DUOX isoforms may also operate within the endosome. At later stages, the NOX2-based system generates superoxide in a Ca2+-independent manner. H2O2 generated from these steps may build up and negatively regulate the IP3 pathway through inhibition of SHP-2. This is believed to down-modulate T cell activation. DUOX, dual oxidase; SHP-2.
In addition to this early oxidative burst in T cells, there is a separate generation of ROS that occurs in a slower, more sustained manner (peaking at ∼15 min). This second oxidative burst appears to involve the classic NOX, NOX2, which is the main component of the enzymatic system responsible for oxidative bursts in activated phagocytes (115). This second oxidative burst may be important for inhibiting some elements of TCR signaling and, thus, acting to down-modulate the activation process. This is supported by experiments involving human T cells in which TCR-induced H2O2 dampened ERK activation in a negative feed-back manner (136). Further, there appears to be autoregulation of oxidative burst and activation in human T cells in that NOX2 expression is down-regulated 24 h after TCR-stimulation (268). Thus, the effects of a TCR-induced oxidative burst may be different on the fate of the T cells, depending on the source and the timing of ROS generation.
3. The effects of Se intake on Ca2+ flux and redox signaling in T cells
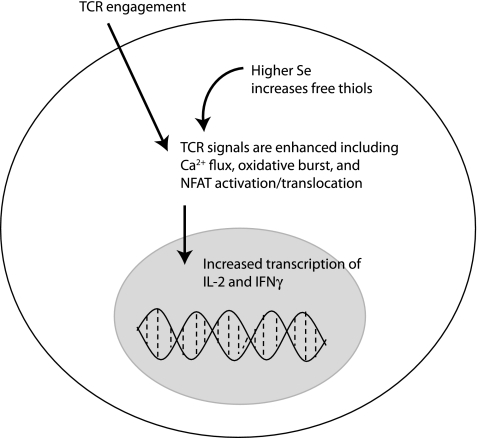
Human lymphocytes respond to Se supplementation with 100 μg Se/day as sodium selenite for 6 weeks predominantly by increasing mRNA encoding proteins involved in protein biosynthesis (192). Increased expression of the synthesis machinery may be required for increased production of selenoproteins themselves, or for protein factors that poise lymphocytes for stronger proliferative capacity. For example, dietary (2.0 ppm Se for 8 weeks) supplementation of mice or ex vivo (100 nM Se as sodium selenite) supplementation of cell cultures resulted in increased expression of the IL-2 receptor α subunit (CD25) and increased proliferative capacity of concanavalin-A-stimulated mouse lymphocytes (210). Our laboratory conducted studies on primary CD4+ T cells from mice fed diets containing moderately low (0.08 ppm), adequate (0.25 ppm), and above-adequate (1.0 ppm) Se. When these CD4+ T cells were stimulated through the TCR, higher Se intake significantly increased their proliferative capacity (102). Increases in dietary Se in mice were shown to enhance signaling strength during TCR-induced activation. In particular, increased Se intake increased Ca2+ mobilization, oxidative burst, and translocation of nuclear factor of activated T cells (NFAT; Fig. 8). Interestingly, Se intake had no effect on phosphorylated ERK levels. The ERK data are consistent with other data suggesting no influence of oxidative stress on this particular signaling event (38), but conflict with data in other T cell systems showing sensitivity of ERK activation to levels of ROS (136) This may reflect differences between truly naive T cells and cell lines. Regardless, both IL-2 and the IL-2 receptor are increased with higher Se intake (1.0–2.7 ppm Se) (102, 105), which would logically contribute to a higher proliferative capacity through autocrine and paracrine actions of this growth factor.
FIG. 8.
Activation signals in naive T cells during TCR-stimulation are enhanced by higher levels of dietary Se. Within seconds Ca2+, flux is triggered by TCR-stimulation, and this is enhanced by increasing dietary Se. In addition, later signals including oxidative burst and NFAT activation and translocation are enhanced by higher Se intake. These signals lead to induced IL-2 and IFN-γ gene expression, which are also increased with higher Se intake. The mechanisms by which Se levels affect these signaling events involves the content of higher free thiols, although specific alterations in specific disulfide bonds in signaling molecules have not yet been identified. NFAT, nuclear factor of activated T cells; IL-2, interleukin 2; IFN-γ, interferon-γ.
When the CD4+ T cells from mice fed different Se diets (0.08 ppm−1.0 ppm Se) were analyzed for oxidative stress, no differences were detected. However, levels of free thiols were increased with increasing dietary Se. Moreover, the differences in TCR-induced Ca2+ flux and proliferative capacity were eliminated when cells were treated with an exogenous source of free thiols in the form of either N-acetylcysteine (NAC) or β-mercaptoethanol (102). In a related study, T cells lacking selenoproteins exhibited increased levels of oxidative stress and decreased proliferative capacity, and addition of NAC eliminated the proliferative capacity (230). These results suggest that free thiols are a key mechanism by which dietary Se affects the activation of T cells. Consistent with these findings, studies utilizing human T cells from an individual with genetically impaired selenoprotein expression exhibited decreased proliferation when TCR-stimulated (218). The lymphocytes from this individual also had very low Txnrd activity and were unable to reduce exogenous H2O2, thus suggesting reduced antioxidant capacity.
Similar to other cell-types, a key redox mechanism by which high Se enhances the activation of T cells may involve the activation of the transcription factor, nuclear factor-kappa B (NFκB). On TCR-induced activation, NFκB translocates to the nucleus and binds to specific gene regions for inducing expression of several proactivation, proinflammatory mRNA. Binding of NFκB to target gene regions in Jurkat T cells is enhanced by reduction of a disulfide bond in the p50 subunit, which is regulated by reduced TXN (157). Levels of reduced Txn are increased with increased Txnrd1, and Txnrd1 activity is increased in T cells with increasing dietary Se (0.086–1.0 ppm) (102). This implies that higher Se and Txnrd1 activity would generate more effective NFκB binding. This also would explain why the effects of increasing Se on T cell activation are negated when the cells are flooded with free thiols, which also act to reduce the disulfide bond in NFκB (157). In certain cell-types, physiological levels of Se may be important for inhibiting the expression of proinflammatory genes and limiting the extension of the inflammatory response (62). In human HuH-7 cells cultured in media with 2% fetal calf serum and various added concentrations of sodium selenite (0, 0.05, 0.5, 1.0, and 2.0 μM) for 3 days before cell activation with TNF-α, physiological levels of Se mediated inhibition of the activation of the transcription factor NFκB, which regulates genes that encode inflammatory cytokines (153). Moreover, overexpression of GPX1 increased the half-life of the inhibitor of NFκB, IκBα, in untreated human T47D cells by twofold (132).
A recent study provided insight into a special, and somewhat surprising, role of Gpx1 in T cell activation and differentiation. During TCR-induced activation of CD4+ T cells from Gpx1−/− mice, there was a higher, more sustained oxidative burst compared with wild-type controls (268). This corresponded to increased IL-2 and IFN-γ production in the Gpx1-deficient CD4+ T cells, suggesting stronger TCR signaling and T helper (Th) 1 bias in the absence of Gpx1. This is in contrast to Se-deficient CD4+ T cells, which have lower expression of Gpx1 as well as other selenoproteins compared with Se-sufficient cells. Se-deficiency leads to lower TCR-induced oxidative burst, weaker TCR signals, and less IL-2 and IFN-γ compared with Se-sufficient CD4+ T cells (102). These effects of Se-deficiency influence the earliest of TCR-signaling events, even those such as Ca2+ flux occurring within seconds. These early events were not measured in the Gpx1-deficient T cell studies, but it may be that Gpx1 plays less of a role in the early TCR-signaling events. The differences between Gpx1-deficient T cell compared to wild-type controls for oxidative burst or cytokine production were measured 24 h after TCR-stimulation. In this sense, decreased selenoproteins collectively affect early TCR signaling events, whereas as examined so far, the absence of Gpx1 alters the ability of the cells to deal with elevated ROS long after TCR signaling has occurred.
4. Se related to calcium and redox signaling in phagocytes
Before one can appreciate the role of Se in redox signaling in phagocytes, the multiple roles that redox intermediates play in phagocyte function should be addressed. Activation of phagocytic leukocytes such as macrophages and neutrophils through a variety of receptors induces a relatively rapid increase in ROS, that is, phagocytic oxidative burst (75). This oxidative burst serves to degrade ingested or attached microbes, but also is important for mediating signals within the phagocyte. In addition, the secretion of redox mediators such as H2O2 and NO· is an important mechanism through which phagocytes communicate with neighboring cells, including other phagocytes. These redox mediators can prime neighboring phagocytes and improve functions such as phagocytic capacity (66), and Se status can affect this process. For example, J774.1 mouse macrophages cultured in media with 1% fetal bovine serum (FBS) and no added Se exhibit decreased phagocytic capacity compared with Se-adequate controls (media supplemented with 0.1 ppm Se) (211). The requirement of antioxidant nutrients such as Se and vitamin E for resistance against nematode infections highlight the importance of redox balance in phagocytes (232). For example, mice fed torula yeast-based low Se diets exhibited decreased resistance to Heligmosomoides polygyrus compared with adequate Se diets (0.2 ppm Se) on secondary infection (11). In addition to killing of parasites, other effector functions are affected by Se-deficiency including cytokine and NO· production (103). The J774.1 mouse macrophages cultured in Se deficient media just described (media with 1% FBS) secreted significantly lower levels of LPS-induced TNF-α, IL-1β, and IL-6 (211) compared with controls with added Se (0.1 ppm Se). In fact, macrophages deficient in one selenoprotein, Selk, exhibit impaired oxidative burst during FcγR-mediated phagocytosis (256). This is likely due to low Ca2+ flux that is required for an optimal oxidative burst. Phagocytes rely mostly on Nox2-based production of superoxide, and the relationship between Ca2+ flux and oxidative burst likely is different from that just described for T cells.
Overall, there are several lines of evidence suggesting that sufficient levels of Se and selenoprotein are required for optimal oxidative burst, Ca2+ flux, and effector functions in phagocytes. A separate issue from this effect on signaling is the oxidative stress before activation, which is higher under conditions of low selenoprotein expression (35). This enhanced baseline oxidative stress is not beneficial for cell signaling in the same manner as the receptor mediated oxidative burst. In this sense, Se deficiency increases baseline oxidative stress and thereby impairs phagocytic activation in the same manner. Not only is adequate Se required for optimal activation and function of these phagocytes, but also for expression of antioxidant selenoenzymes used to mitigate damage from mitochondrial and nonmitochondrial ROS. The Gpx enzymes can detoxify H2O2, whereas Txnrd1 is crucial for maintaining reduced thioredoxin and redox tone. Consistent with this notion, Txnrd1 mRNA and protein were shown to increase in macrophages on LPS-stimulation (35). Under resting conditions, macrophages lacking selenoproteins exhibited increased ROS production and without the ability to increase expression of Txnrd1, the macrophages cannot correct the redox tone from this increased ROS. Overall, it is evident that Se levels and specific selenoproteins are important for setting the redox tone in phagocytes before activation.
The differentiation of macrophages is also influenced by redox tone, and Selp has been demonstrated to play a particularly important role in this process. In particular, increased expression of Selp is induced by IL-10 during the switch of mouse macrophages from a classical (M1) to alternatively activated (M2) phenotype (23). Limiting the pathogenicity during certain diseases, such as African trypanosomiasis, requires a macrophage transition from M1 to M2 during the course of infection (85). Interestingly, the antioxidant activity, not the Se delivery role, of Selp was demonstrated to play a crucial role for limiting pathogenicity and oxidative damage to tissues and was required for survival of mice infected with trypanosomes. This raises the question of whether optimal Se intake and Selp expression is required for resolving other diseases dependent on M2. In fact, many immune responses or phases of particular immune responses require strong M1 responses followed by a resolution of inflammation that relies on the switch to M2. Whether high levels of Se intake actually skew M1 versus M2 responses one way or another should be considered. This could also be quite important for noninfectious diseases that involve transitions from M1 to M2 during the disease process, such as atherosclerosis or chronic inflammatory disorders.
The role of Ca2+ flux during phagocytosis depends on the type of cell as well as the type of phagocytic receptor involved. Perhaps the best-defined phagocytic process dependent on efficient Ca2+ flux is ingestion of IgG-opsonized particles mediated by FcγRs on the surface of macrophages (188). Macrophages play a crucial role in innate immune responses against pathogens through FcγR-mediated microbe engulfment and production of proinflammatory cytokines (49, 187). FcγRI is the high-affinity receptor that binds monomeric IgG2a in mice and IgG1 and IgG3 in humans (186). FcγRII and FcγRIII are low-affinity receptors that require a higher avidity present on multivalent immune complexes (IC) to effectively promote phagocytosis. FcγRIV is found in mice (the human ortholog is CD16A) and binds to IgG2a and IgG2b with intermediate affinity (185). FcγRI, III, and IV signal through an immunoreceptor tyrosine-based activation motif (ITAM)-containing γ chain that is associated with the small cytoplasmic domain of the receptors (266). Tyrosine phosphorylation of the ITAM results in the recruitment of Src homology (SH)2-containing molecules and adaptor proteins that propogate signals through downstream effectors. An important early effector enzyme in this signaling cascade is PLCγ, which cleaves PIP2 to produce messenger molecules IP3 and DAG, the former of which triggers a rise in cellular Ca2+ levels. Selk has been shown to be required for FcγR-dependent Ca2+ flux (256), and studies in our laboratory are currently focused on identifying other signaling molecules involved in this process.
Similar to the process just described for T cells, macrophages require rapid and efficient Ca2+ flux for activation and effector functions. However, in macrophages, it remains less clear how different receptors trigger Ca2+-dependent and Ca2+-independent signaling pathways. On efflux of Ca2+ from ER stores, STIM1 is induced to interact with CRAC channels, causing structural changes in the CRAC channel that allows extracellular Ca2+ to enter the cytosol. Stim1 knockout mice were used to demonstrate that SOCE was important for FcγR-mediated phagocytosis by macrophages (26). Both Ca2+ flux and ROS feed into NFκB activation, and Se supplementation of macrophages to above-adequate levels may be particularly disruptive for the redox balance that regulates NFκB signaling. For example, experiments involving RAW 264.7 mouse macrophages and an NFκB reporter system demonstrated that higher Se status in these cells inhibited NFκB activation induced by LPS (277). Expression of cyclooxygenase-2 (Cox-2) and inducible nitric oxide synthase (iNos), both of which depend on NFκB activation, were also decreased with Se supplementation during activation through either Toll-like receptor 3 (Tlr3) or Tlr4 using poly(i:c) or LPS, respectively. It is difficult to determine whether this reflects in vivo mechanisms by which increases in dietary Se affect macrophage activation. Experiments using the selenoorganic compound, ebselen, showed that this compound inhibited NO, Cox-2, and TNF-α in rat Kupfer cells, which are the resident macrophages of the liver (229). This suggests that small-molecular-weight selenocompounds influence redox status in these in vitro experiments, but the role of these compounds versus selenoproteins in vivo needs to be considered. Overall, high Se status in macrophages may perturb redox tone either through antioxidant selenoproteins or through small-molecular-weight selenocompounds, either of which may inhibit NFκB signaling during activation.
5. A novel link between Selk and the calpain/calpastatin system
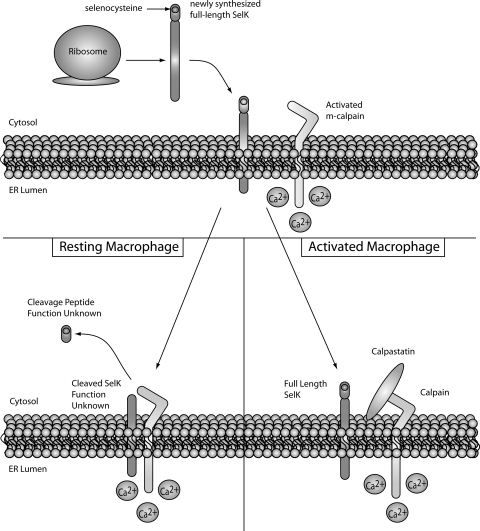
Calpains are Ca2+-activated Cys proteases that cleave specific targets to modulate cellular functions, and important functions in immune cells that may involve calpain proteolytic modulation include apoptosis, proliferation, and migration. An important role is emerging for calpains in regulating inflammation and immune responses, although specific mechanisms by which this occurs have not been clearly defined (55, 96, 248). There are two major isoforms of this enzyme, μ-calpain (or calpain 1) and m-calpain (or calpain 2), which require micromolar and millimolar Ca2+ concentrations for activity, respectively (30). These enzymes are comprised of an 80 kDa catalytic subunit and a 30 kDa regulatory subunit. Activation occurs after Ca2+-binding induces conformational changes that lead to autocleavage of the N-terminal inhibitory domain of the 80 kDa subunit (173, 246). Since the activation of calpain is an irreversible reaction, its activity should be tightly regulated by mechanisms in addition to fluctuating Ca2+ levels. A key part of this regulation is calpastatin, which is an endogenous inhibitor of calpain.
As just described, Selk is a particularly important selenoprotein for immune cell activation. In a recent study from our laboratory, Selk was identified as a novel target for m-calpain (110). Proteolysis by m-calpain produced a truncated isoform of Selk lacking the Sec residue. The cleaved isoform of Selk was found to be highly abundant in resting macrophages and, on activation with several different Tlr ligands, calpastatin expression was upregulated. Calpastatin is the endogenous inhibitor of calpains, and in activated macrophages, the increased calpastatin was shown to inhibit m-calpain cleavage and lead to increased full length, Sec-containing Selk (Fig. 9). Since calpains are activated by Ca2+ and selenoproteins such as Selk contain the Sec at their redox centers, this provides another potential linkage between Ca2+ and redox tone in immune cells.
FIG. 9.
Selk cleavage by m-calpain in macrophages. In resting macrophages, Selk synthesized on the ribosome is immediately cleaved by activated m-calpain. This results in nearly all Selk existing as inactived protein in resting macrophages as demonstrated by lower Ca2+ flux and migration in response to chemokines such as MCP-1. TLR-activation increases expression of calpastatin, which inhibits cleavage by m-calpain and results in higher levels of full-length Selk. Thus, in activated macrophages, full-length Selk is able to efficiently promote Ca2+ and migration toward chemokines. MCP-1, monocyte chemotactic protein-1; TLR, Toll-like receptor.
These data provide new insight into actions and regulation of the calpain/calpastatin system in a major cell-type involved in inflammation. In addition to Selk, other selenoproteins may also serve as targets for calpain, particularly other ER membrane selenoproteins such as Sels and Seln, as the ER membrane has been identified as an important site of calpain/calpastatin association and activity (215, 216). In addition, some selenoproteins such as Selr (methionine sulfoxide reductase B1 [MsrB1]) have been reported to exhibit truncated forms detected in different tissues (71).
VII. Se and Immune Cell Effector Functions
A. T helper cell differentiation
1. Se and T helper differentiation
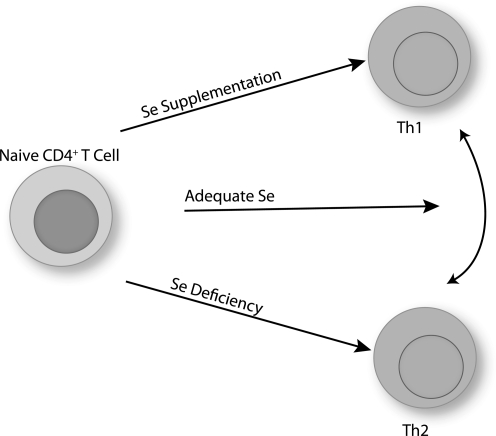
On TCR-stimulation of naive CD4+ T helper cell, these cells differentiate into effector T cells that play a central role in initiating and shaping immune responses. The number and type of CD4+ T helper cells that are generated during the first encounter with antigen-presenting cells substantially contribute to the outcome of the immune response. In particular, CD4+ T cells become polarized during activation into Th1, Th2, Th17, Treg, or other T helper subtypes (180, 213, 242). Redox tone plays an important role in this differentiation process. For example, CD4+ T cells from Nox2-deficient mice exhibit increased Th1 cytokines on activation compared with wild-type controls (115). This suggests that a higher reductive state favors Th1 differentiation. Consistent with this notion, GSH depletion in mice reduces Th1 responses, and the antigen-presenting cells are important in this effect (196). Similarly, a higher reductive state induced through increased dietary Se intake (0.086–1.0 ppm Se) had similar effects on Th1-skewing during the activation of naive CD4+ T cells (102). Higher Se intake led to increased production of IFN-γ on TCR-stimulation, whereas low dietary Se led to increased IL-4. Adequate Se intake appears to produce a more flexible differentiation state that is driven more by the environmental cues (e.g., cytokines) and antigen-presenting cell (Fig. 10). There are, however, some data that do not fit the model of higher reductive tone leading to Th1 differentiation. For example, CD4+ T cells from Gpx1 knockout mice showed a bias toward Th1, and less Th2 or Th17 differentiation (268). In contrast to results with Nox2-deficient mice or low dietary Se, this suggests that higher oxidative stress leads to increased Th1 differentiation. This may suggest that Gpx1 serves a role different from other selenoproteins in T cell differentiation, a role that is distinct from the generation of the oxidative burst and more related to scavenging H2O2 at periods beyond the initial activation stage.
FIG. 10.
Effects of Se intake on CD4+ T cell differentiation. Adequate levels of Se intake do not bias T cell differentiation and T helper (Th) 1 versus Th2 differentiation is largely determined by signals provided by the antigen-presenting cell or cytokine milleau. For example, CD4+ T cells activated in a pro-Th1 environment or a pro-Th2 environment can differentiate into either Th1 or Th2 cells. Se supplementation boosts TCR signals and skews differentiation toward a Th1 phenotype. In contrast, Se deficiency leads to low TCR signals and skews differentiation toward lowered activation states with a biase toward a Th2 phenotype.
2. Regulatory T helper cells
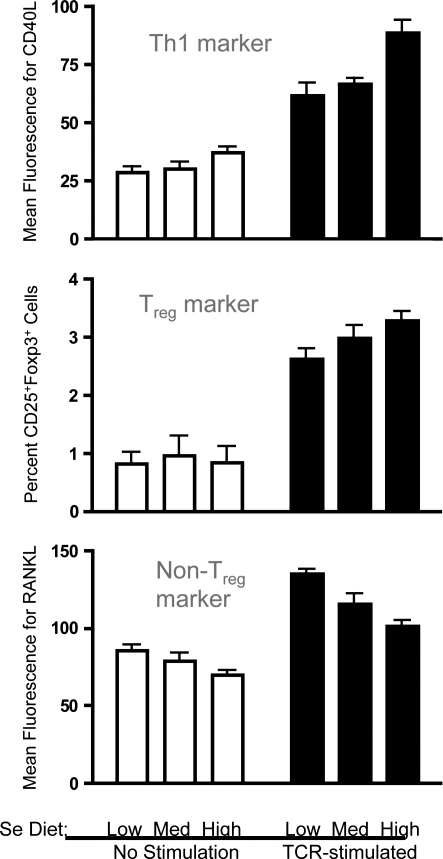
The effects of Se levels on regulatory T helper cells (Treg) cells has not been described in the literature, despite the crucial role that these T helper cells play in preventing excessive immunity and chronic inflammation. In our laboratory, we stimulated naive mouse CD4+ T helper cells through the TCR and examined surface and intracellular expression of Th cell markers to determine the effect of increasing dietary Se on differentiation. Similar to the mRNA data just described, increasing Se from adequate to above-adequate levels (0.25 to 1.0 ppm Se) suggests a skewed differentiation toward Th1 as indicated with surface expression of the Th1 marker, CD40L (Fig. 11). Interestingly, we found that differentiation into Treg cells was affected by dietary Se levels. TCR-induced differentiation of CD4+ T cells into CD25+Foxp3+ Treg cells was increased with increasing dietary Se. A surface marker for which expression has been shown to be inversely related to markers of Treg cells is receptor activator for nuclear factor-κB ligand (RANKL), also referred to as TNF-related activation-induced cytokine receptor (90, 144). Thus, levels of RANKL were evaluated on the surface of CD4+ T cells with or without TCR-stimulation, and results showed that increasing Se levels resulted in decreasing levels of RANKL for both unstimulated and stimulated CD4+ T cells. These data, although not conclusive, support the notion that increasing Se levels may promote a Treg phenotype from TCR-stimulated naïve CD4+ T cells and further investigation of how dietary Se influences immunoregulation via these important cells in vivo in needed.
FIG. 11.
Analyses of cell markers during activation of naive CD4+ T cells from mice fed different Se diets. Under conditions previously described (102), purified splenic CD4+ T cells were stimulated for 18 h through the TCR, and flow cytometry was used to measure markers for Th1 cells (CD40L), Treg cells (CD25 and FoxP3), and a marker excluded from Treg cells (RANKL). Preliminary studies in our laboratory suggest that increased Se intake leads to higher levels of Th1 and Treg markers. FoxP3, forkhead box P3; RANKL, receptor activator for nuclear factor-κB ligand.
3. Epigenetic poising in naive T helper cells
Does Se intake affect epigenetic poising of naive CD4+ T helper cells?
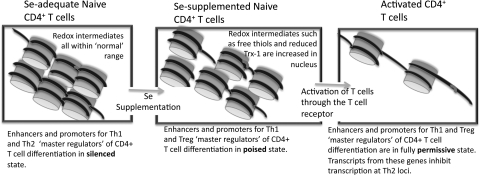
When data in Figure 11 are closely examined, there appears to be an effect of increasing dietary Se on some of the phenotypic markers for Th1 (CD40L) and Treg (RANKL) effector cells before T cell stimulation. This suggests a poising of naive CD4+ T cells by dietary Se levels that leads to a skewing of T cell differentiation prior to TCR-induced activation. Epigenetic poising is crucial for naive CD4+ T cells, because, on activation, the earliest induced transcripts are rapidly translated into proteins that provide negative- and positive-feedback mechanisms for controlling differentiation into effector cells (197). Known regulators of CD4+ T cell differentiation include T-box expressed in T cells and IL-12Rβ2 (pro-Th1), GATA binding protein 3 (pro-Th2), forkhead box P3 (FoxP3; pro-Treg), and RAR-related orphan receptor gamma (thymus) (pro-Th17). These are often referred to as “master regulators” of T cell differentiation, because they are among the earliest transcriptionally induced genes when naïve CD4+ T cells are stimulated through the TCR. Thus, influencing transcription of these genes through epigenetic modifications would, thus, provide an early signal to regulate differentiation.
Interestingly, Se supplementation from 0.086 to 0.25 to 1.0 ppm Se regulates the earliest detectable gene transcription events triggered by CD4+ T cell activation through redox intermediates (102). In addition, a recent study in rats demonstrated that increasing dietary Se using basal diets (Se-deficient) supplemented with l-Se-Met at 0.15 ppm (Se-adequate) or 4 ppm (above-adequate) for 104 days decreased global genomic DNA methylation in liver and colon mucosa, with specific genes particularly sensitive to this effect (280). In fact, Se and other dietary factors have been shown to affect epigenetic mechanisms related to cancer (13). There are findings indicating that dietary Se influence epigenetic mechanisms involved in cancer and that nutrient interactions are important in cancer susceptibility (50). Methylation in the promoter region of the tumor suppressor p53 gene is sensitive to both folate (265) and Se (51), and this may be important in the early stages of cancer development. HDAC activity in B cell lymphoma cell lines has been shown to be inhibiited by the small organic selenocompound, methylseleninic acid (125). Chronic inflammation may be an underlying risk factor in the early stages of cancer development, as well as being a key feature of inflammatory bowel disease (IBD). In fact, levels and interactions between nutrients have been shown to impact the epigentic regulation of IBD (13). Overall, Se status may influence the poising of naive CD4+ T cells through redox intermediates that epigenetically influence differentiation toward a particular phenotype upon TCR-engagement (Fig. 12).
FIG. 12.
Hypothetical effect of higher Se intake on chromatin remodeling. Evidence suggests that higher levels of dietary Se may affect epigenetic states of certain gene regions, and this may be an important factor in how Se levels influences T helper cell differentiation. This may occur by increasing levels of redox intermediates in the nucleus such as free thiols on signaling molecules or reduced Txn-1, which may influence the rate-limiting steps of enzymes involved in chromatin remodeling. This can lead a poised state of chromatin that is able to more quickly respond to TCR-stimulation and rapidly generate mRNA for master regulator proteins such as T-bet. Txn-1, thioredoxin 1; T-bet, T-box expressed in T cells.
The poising of gene regions for rapid transcription is carried out by various epigenetic enzymes, which catalyze histone methylation, acetylation, and ADP-ribosylation, as well as DNA methylation. Importantly, the rate-limiting steps of several of these epigenetic enzymes are redox dependent (48). Some of these redox-senstive enzymes have been shown to be affected by Se supplementation. For example, the enzyme responsible for catalyzing DNA methylation (DNA methylase) in Friend erythroleukemic cells is sensitive to inhibition by Se supplementation with 20 μM as sodium selenite in culture media (46). Since inhibition of DNA methylation leads to a more permissive state for transcription, this suggests that increasing Se intake may lead to increased permissiveness of certain gene regions. A key selenoenzyme in mediating these effects may be Txnrd1, which produces higher levels of reduced Txn-1 in CD4+ T cells from Se supplemented mice (102). Txnrd1 converts oxidized Txn-1 to reduced Txn-1 in the cytoplasm and nucleus, which is important because Txn-1 has been linked to regulation of H3K9 tri-methylation and -acetylation and to production of the cytokine, IL-2, which is involved in T cell proliferation and Th1 differentiation (2, 195). Thus, free thiols and Txn-1 as well as other redox intermediates may represent important mechanisms by which Se supplementation affects epigenetic events in naive CD4+ T cells.
B. B cell function and antibody production
In a double-blind study more fully described next, 22 adult subjects received 50 or 100 μg/day Se Se supplementation as sodium selenite for 15 weeks, and this was shown to increase anti-poliovirus immunity in regards to several aspects of cell-mediated immunity (29). However, antibody titers to poliovirus were not affected by levels of Se intake, thus suggesting that plasma B cell production of IgG was not affected by Se supplementation. Consistent with this notion, rodent studies described in detail next involving influenza infection also showed no significant effect of increasing dietary Se on humoral responses (18). In a very small study involving 11 men, antibody titers against diphteria vaccine, but not against influenza A or B, were increased after reinoculation in high Se-supplemented (297 μg/day) versus low Se-supplemented (13 μg/day) individuals (95). Thus, Se levels may affect B-cell-dependent antibody production in a pathogen-dependent manner, and less consistently than the effects observed on T cell immunity. Of course, antibody titers are not influenced by B cell function alone, with T helper cells playing a crucial role as well. It is difficult to uncouple B cell function from T helper cell function when using humoral responses as an experimental outcome.
Numbers of B cells in spleens of female mice responded to diets with low (0.02 ppm), adequate (0.2 ppm), or above-adequate Se-Met (2 ppm) in the diet for 50 days (255). Low Se-Met diets reduced the number of B cells in the spleen compared with adequate Se diets, whereas above-adequate Se-Met intake reduced B cell numbers. The number of circulating memory B cells is sensitive to levels of ROS such as superoxide and hydrogen peroxidase, and Se has been shown to influence levels of both of these in B cells (267). B cell activation and differentiation is influenced by oxidatively sensitive NFkB and involves leukotriene formation (21), and Se intake may impinge on these processes. Overall, there is a need for more data regarding the effects of Se and selenoproteins on redox signaling in B cells and how that affects antibody production in vivo.
C. Adherence and migration of leukocytes
1. Expression of adherence molecules
Cellular infiltration into tissues requires efficient adhesion of circulating blood monocytes and lymphocytes to endothelial cells and subsequent migration to sites of inflammation. l-selectin is a member of the selectin family that is expressed on circulating monocytes and lymphocytes and is important for tethering and rolling along the capillary wall. Somewhat surprisingly, human monocytes supplemented with Se (2 μg/ml) were found to exhibit decreased monocyte rolling and adhesion (1). This was accredited to increased matrix metalloproteinase-dependent shedding of l-selectin, and Se supplementation of mice (injected with 2 μg/ml Se as sodium selenite based on total blood volume) led to increased levels of shedded l-selectin in the sera. Exactly how this may affect cellular infiltration and inflammation in humans is unclear, but increased soluble l-selectin may be an important mechanism by which Se levels modulate migration or chemotactic capacity of various immune cells.
In addition to the circulating immune cells, Se levels can affect the endothelial cells with which they interact. For example, Se supplementation (100 nM) of human umbilical vein endothelial cells (HUVECs) inhibited expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin stimulated by high glucose (284). Se supplementation in these experiments also inhibited insulin-induced VCAM-1 and ICAM-1 expression, whereas high insulin had no inducing effect on E-selectin. The effects of Se supplementation at 100 nM involved the activation of the mitogen-activated protein kinase, p38, which was inhibited by Se. These data were similar to an earlier study showing a dose-dependent inhibition of TNF-α-induced ICAM-1, VCAM-1 and E-selectin expression on HUVECs by Se supplementation with sodium selenite (0–2 μM) (281). In this study, p38 was not examined but the effects of Se were found to be independent of NFκB translocation to the nucleus. In a smaller study involving corticoid-dependent asthmatics with lowered circulatory Se status, Se supplementation (200 μg/day for 6 months) reduced expression of adhesion molecules CD11a, CD11b and CD62L on PBMC (116). This study also examined HUVECs and, similar to results from the experiments just mentioned, Se supplementation significantly decreased expression of VCAM-1, E-selectin (after 3 months), and P-selectins and ICAM-1 (after 6 months). Thus, Se supplementation may reduce adherence through down-regulation of these surface receptors, although functional rolling/adherence assays were not included in these studies. This may reflect a mechanism by which increased Se intake reduces inflammation.
2. Migration
Data from mice in which a Cre/lox system were used to delete the tRNA necessary for selenoprotein synthesis in macrophages (ΔTrspM mice) revealed the requirement of selenoproteins for effective migration of macrophages (35). In this study, the migration of macrophages from ΔTrspM mice through matrigel compound, which mimics the extracellular matrix, was decreased compared with controls. This appeared not to be due to the intrinsic motility of the ΔTrspM macrophages, because no differences were observed between ΔTrspM macrophages and controls when the cells migrated through filter pores in the absence of the matrigel. The authors' interpretation was that eliminating selenoprotein expression was more likely to affect the ability of the macrophages to secret proteolytic enzymes to alter the extracellular matrix as they move through the gel. In support of this view, the authors found that selenoprotein deficiency led to altered expression of certain genes associated with breakdown of extracellular matrix.
In the experiments just described for ΔTrspM macrophages, serum was used as a chemotactic agent in these experiments, and no data were obtained using more common chemokines such as monocyte chemotactic protein-1 (MCP-1), which may have shown quite different results in terms of intrinsic chemotactic capacity in the absence of selenoprotein expression. In fact, data from our laboratory involving mice in which only Selk was deleted (Selk−/− mice) revealed a significant reduction in the migratory capacity of immune cells (256). Selk−/− T cells exhibited decreased migration in response to chemokines including stromal cell-derived factor-1 and regulated upon activation, normal T-cell expressed, and secreted, whereas Selk−/− neutrophils were impaired for migration in response to the murine analog of IL-8, KC. This is likely due to the role Selk plays in Ca2+ flux, which is indispensible for chemokine receptor signaling during immune cell migration (42). Selk-deficiency was not only important for response to chemokines, but also for production of certain chemokines as well. For example, in vivo production of KC and MCP-1 in response to TLR agonists or viral infection was decreased in Selk−/− mice. Thus, optimal expression of Selk is important for both aspects of immune cell migration, that is, production of chemokines to attract immune cells and chemokine receptor-driven infiltration of the responding immune cells. Given that Selk expression in various mouse tissues is sensitive to changes in dietary Se levels (0.08 –1.0 ppm Se) (256), this selenoprotein represents an important link between dietary Se and immune cell migration during inflammation and immune responses.
D. Se and eicosinoid synthesis in macrophages
Eicosinoids are fatty acid mediators derived from arachidonic acid (AA) and are made up of five different types: prostaglandins, prostacyclins, thromboxanes, leukotrienes, and lipoxins. Eicosinoids are important modulators of inflammation and immune responses, and Se levels affect the synthesis and actions of these mediators. The available data describing the effects on these mediators illustrate the notion that not all proinflammatory functions are positively correlated with increased Se intake. At extremely higher levels, Se can directly react with essential thiol groups on molecules to form RS-Se-SR adducts and inhibit cell signaling events. For example, the DNA binding capacity of NFκB in cell lysates was inhibited by addition of high levels of Se, although the levels used were toxic (>5–10 μM) (127). At more physiological levels or even superphysiological levels of intracellular Se achieved with Se supplementation (nM concentrations), there is less likely a direct inhibitory effect of inorganic Se on NFκB or other signaling molecules. However, elegant experiments involving mouse bone marrow-derived macrophages (BMDM) and RAW 264.7 mouse macrophages revealed an important, albeit complex effect of altered cellular Se status and NFκB activation. In these studies, BMDM were isolated from mice fed Se-deficient and Se-supplemented diets containing 0.01 or 0.4 ppm Se as described (174, 259). BMDM and RAW 264.7 cells were cultured in media containing 5% defined FBS with total Se of 6.0 nM (low Se) or the same media supplemented with 2 μM Se (high Se). These studies showed that, on LPS activation, lower Se status causes decreased production of the AA metabolite, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (259). Se supplementation increased 15d-PGJ2 in a Cox-1-dependent manner, which can form a 15d-PGJ2-IKKβ adduct that inhibits IκB kinase β (IKKβ) activity. This mechanism may explain earlier data showing that Se-deficient macrophages exhibit increased LPS-induced NFκB activation and subsequent Cox-2 expression, iNos expresson, and NO production compared with Se-supplemented macrophages (199, 279). As expected, lower Se status in these macrophages increased intracellular ROS, consistent with other data showing increased ROS with decreased selenoprotein expression in macrophages (35). Collectively, these data suggest an inverse relationship between Se levels and inflammatory signaling through NFκB and eicosinoids that is driven by redox tone. It remains unclear which selenoproteins regulate these processes, but likely candidates include Gpx1 and Txnrd1, which are both increased with increasing Se intake.
More recently, specific effects of Se levels on eicosinoid synthesis in macrophages were shown to influence the balance between proinflammatory and anti-inflammatory prostaglandins. The initial step of prostaglandin (PG) synthesis involves formation of PGH2 from AA by the action of COX-1 and COX-2. PGH2 is subsequently acted on by specific PG synthases, microsomal PGE synthase-1, thromboxane synthase, and PGD synthase (PGDS), to form prostaglandin E2 (PGE2), thromboxane A2, and prostaglandin D2 (PGD2), respectively (191). During the resolution phase of inflammation, AA metabolism shifts from the production of PGE2 to that of PGD2 and its downstream product, 15d-PGJ2 (235, 241). Hematopoietic PGDS (H-PGDS) is the enzyme in macrophages that catalyzes the conversion of PGH2 to PGD2, and H-PGD2 was shown to be regulated by the redox state of macrophages in a manner that depended on their Se status as well as the differential modulation of peroxisome proliferator-activated receptor γ and NFκB (79). The data suggested that Se acted to skew AA metabolism toward the production of PGD2, and in this manner promoted the resolution of inflammation. This supports the notion described further next regarding the role of low Se status in promoting cycles of inflammation and the use of Se supplementation to resolve this inflammatory process.
E. Phagocytosis
How do dietary Se levels affect phagocytosis and the signaling pathways involved? In vitro studies involving J774.1 murine macrophages stimulated with mitogens such as PMA and LPS demonstrated that Se-deficiency (RPMI with 1% FBS) resulted in decreased phagocytic activity compared with Se-adequate controls (same media supplemented with 100 nM Se as selenite) (211). However, the receptor systems involved in uptake of the beads used for this experiment were not determined. In a related study published by our laboratory, macrophages from Selk−/− mice did not exhibit differences compared with controls in the phagocytosis of similar beads (256). However, when IgG-opsonized beads were used to promote phagocytosis of beads through the FcγR, Selk-deficient macrophages exhibited decreased phagocytosis (our unpublished data). Macrophages from Selk−/− mice were impaired for FcγR-induced Ca2+ flux, but not for Ca2+ flux induced by the ionophore, thapsigargin (256). This was accompanied by reduced oxidative burst. We have subsequently shown that Selk is required for full activation of macrophages during FcγR-mediated phagocytosis and production of soluble mediators (our unpublished data). Interestingly, production and secretion of MCP-1 was not affected by Selk-deficiency. This is consistent with findings in which macrophages for the ER membrane protein crucial for Ca2+ flux, Stim1, were not different from wild-type macrophages in FcγR-induced MCP-1 secretion (26). This very likely affects the special role that chemokines like MCP-1 play in attracting monocytes and macrophages for both inflammatory functions as well and noninflammatory functions such as engulfment of apoptotic cells and wound-healing (162).
F. Inflammation linked to ER stress
One molecular mechanism linking Se status with inflammation was identified when single nucleotide polymorphisms in the human gene encoding selenoprotein S (SELS or SEPS1) were correlated to serum concentrations of proinflammatory cytokines, for example, IL-6, IL-1β, and TNF-α (47). Related to this finding, polymorphisms in the SELS gene as well as the gene encoding another ER selenoprotein, SEP15, were recently correlated to increased risk for colorectal cancer (247). SELS has been shown to be a glucose-regulated protein involved in the retrotranslocation of misfolded proteins from the ER into the cytosol for proteasomal degradation (80, 128). As just described, Sels expression is increased by LPS-treatment in mice in a manner dependent on both Se status and gender (243). In this sense, low expression of SELS and other ER selenoproteins involved in protein folding (SEP15 and SELM) may increase inflammatory cytokines through increased ER stress (Fig. 13). SELK is another ER protein linked to ER stress in the human cell line, HEPG2 (60). However, in vivo deletion of Selk by itself did not increase ER stress or affect levels of inflammation in mice (256). In addition, it remains unknown whether SELT and SELN are involved in ER stress or inflammation.
FIG. 13.
Selenoproteins in the ER regulate inflammation in part through effects on protein-folding. Evidence has been presented for roles for SelS and Selk in regulating ER stress. SelM and Sep15 have been suggested to play key roles in protein folding. Altogether, low Se intake may lead to low expression of these selenoproteins. Decreased expression of some or all of these selenoproteins may cause an increase in misfolded proteins and cause ER stress. This may lead to secretion of proinflammatory mediators by affected cells and eventually increased inflammation.
VIII. Linkages Between Se and Human Disease
A. Se supplementation to boost anti-viral immunity
1. Se levels can affect the virus itself
Viral diseases are affected by Se levels, and one disease for which Se supplementation to overcome Se-deficiency has been particularly effective as a preventive modality is Keshan disease (270), a myocarditis mainly occurring in regions of China with low soil Se. A suspected co-factor in the etiology of Keshan disease is coxsackievirus B3 (CVB3), which may become more virulent under low Se status in the infected host (17). In fact, changes in the virus itself have been more clearly established as a mechanism by which Se-deficiency promotes the development of Keshan disease than impaired immunity or chronic inflammation (119). Findings from the laboratory of Melinda Beck have shown that CVB3 and influenza A may be mutated under conditions of oxidative stress induced by low Se status (16), and virulence of other RNA viruses may also be influenced by Se intake. Thus, when considering potential immune-enhancing effects of Se supplementaiton, the effects that may be exerted on the virus itself should be considered in addition to immunity.
2. Human immunodeficiency virus 1/acquired immune deficiency syndrome
Se supplementation offers an inexpensive method for slowing disease progression for human immunodeficiency virus (HIV-1)-positive individuals. Data from animal models and human studies strongly suggest that Se status declines in the advanced stages of acquired immune deficiency syndrome (AIDS) (15, 63, 273). The implementation of highly active antiretroviral therapy (HAART) to combat HIV-1 infection has improved immune system function in infected individuals, and HAART also appears to reduce any effect of HIV-1 infection on Se status (209). The real question is whether Se supplementation in HIV-1-positive individuals can reduce morbidity and mortality, and results from intervention studies have been inconclusive (48). HIV-1 replication appears to favor intracellular oxidative stress, and whether levels of Se supplementation may be optimized to reduce oxidative stress, this may offer a means to control infection. Endogenous levels of antioxidant selenproteins such as GPX1 and 4 and TXNRD1 increase with Se supplementation, but this is diminished with HIV-1 infection (87, 109). There is evidence of a direct effect of Se supplementation on reducing HIV-1 pathogenesis. In this study, human U937 monocytes were grown in basal media containing 7 nM Se as a baseline or with Se supplementation (25–1000 nM). Data have shown that TXNRD1, a selenoprotein that is highly sensitive to Se status, targets the HIV-1 protein Tat and directly inhibits HIV-1 replication (121). HIV-1 infects immune cells including T cells, macrophages, and dendritic cells and causes immunosuppression, and increased Se status leading to higher levels of Txnrd1 may directly lower Tat-dependent viral replication in these cells. Thus, increasing Txnrd1 expression along with other selenoproteins by supplementing HIV/AIDS patients with Se may provide a potentially inexpensive therapy.
3. Influenza viruses
Studies comparing Se-deficient to Se-sufficient mice infected with influenza A virus [Influenza A/Bangkok/1/79 (H3N2)] demonstrated the importance of adequate Se intake for viral clearance and recovery (18). Viral titers were similar between Se-deficient and Se-sufficient hosts. However, Se-deficiency appeared to alter the immune cell subsets and cytokine profiles during the course of infection. The cytokines produced by immune cells responding to influenza infection were altered by Se status, but no mechanistic data were presented to explain these differences. Interestingly, Se status had no effect on antibody production, thus suggesting that Se-deficiency affects cell-mediated immunity to a greater extent than humoral immunity in this model.
Recently, Se supplementation was found to be protective against H1N1 influenza infection (278). In this study, mice were fed deficient (0 ppm), adequate (0.2 ppm), and supplemented (0.3–0.5 ppm) levels of Se in their diets and infected with H1N1 at a dose that resulted in 41% survival in mice fed adequate Se diets. Only 25% mice fed low Se diets survived, but 75% mice fed higher Se diets survived. Anti-viral cytokines, TNF-α and IFN-γ, were increased with increasing dietary Se, but no indicators of adaptive immunity or humoral immune response were evaluated. Interestingly, there was no clear correlation between dietary Se and clearance of H1N1 as measured by viral titers. This is similar to the results in experiments just described for H3N2, where viral titers were not affected and suggest that the effects of Se levels on survival were dependent on more than just improved viral clearance. More complete analyses of viral clearance using different doses of input virus or evaluation of different tissues over time may have revealed more robust anti-viral responses with increased Se intake. In addition, no information was obtained regarding RNA genomic mutations possibly induced by lower Se intake. Regardless, the correlation between increased dietary Se with increases in anti-viral cytokines and improved resistance to influenza infection suggest that higher Se intake is beneficial for fighting these infections.
4. Poliovirus
A rare study in humans demonstrated a boost in anti-poliovirus immunity in individuals supplemented with Se (29). In this study, individuals with low Se status were supplemented with either 50 or 100 μg Se daily for 15 weeks and then orally vaccinated with live, attenuated poliomyelitis vaccine and evaluated for immune responses. Se supplementation boosted T cell numbers and anti-viral cytokines like IFN-γ, and led to more rapid viral clearance. Once again, humoral responses were not affected by Se supplementation, suggesting B cell function may not be influenced to the same extent as T cells for this pathogen.
B. Critical illness stress-induced immune suppression
Studies have suggested that acquired critical illness stress-induced immune suppression (CRISIS) plays a role in the development of nosocomial infection and sepsis, particularly in critically ill children (65). Deficiencies in trace elements, Se, and zinc, and other nutrients may exacerbate CRISIS. In fact, several different inflammatory conditions in humans such as clinical sepsis have been associated with significantly decreased Se status (107, 152). Injection of LPS in rats to induce an acute phase response results in significantly decreased Se in plasma and liver (152). The mechanisms by which this occurs are becoming clearer with recent studies. For example, there is evidence from studies in mice that acute phase response to LPS causes a decrease in selenoprotein synthesis in the liver (203). LPS led to a down-regulation of factors involved in selenoprotein synthesis, including the EFsec, selenophosphate-synthetase 2 (Sps2), Sec-tRNA[Ser]Sec synthase, and Pstk. The liver is the predominant site of synthesis of SELP, which is secreted into the plasma and delivers Se to other tissues via its ten Sec residues as just described in detail. The human SELP promoter has been shown to be negatively regulated by proinflammatory cytokines in human hepatocytes in vitro (58). Thus, sepsis may trigger a pathogenic cycle in which sepsis and inflammatory cytokines decrease in SELP synthesis in the liver, which leads to lower Se levels in other tissues, which increases oxidative stress and further increases in inflammatory responses (Fig. 14). For the immune system, lower Se status may not only lower signaling capacity of lymphocytes and innate immune cells, but may also lead to stress-induced lymphopenia (34). Engulfment of apoptotic lymphocytes by macrophages could then lead to a predominant M2 phenotype and further suppress immunity.
FIG. 14.
Cyclical decrease in Se status under conditions of sepsis or other types of inflammation. Initiated by sepsis or circulating LPS, inflammatory cytokines cause down-regulation of SelP biosynthesis, which leads to decreased delivery of Se to tissues, which can further promote inflammation as described in Figure 13. In addition, inflammation can cause increased vascular permability in certain tissues, which also can contribute to possible loss of Se from circulation and exacerbate inflammation. Intervention with Se supplementation may attenuate conditions involved in this cycle by increasing overall Se in circulation and inhibiting ER stress or other oxidative stress conditions, thus leading to an overall decreased inflammatory response.
Intervention with Se supplementation has been proposed as an inexpensive means to mitigate the effects of CRISIS and other conditions involving sepsis. In this study, C57BL/6J mice were transferred at the age of 45 days to a Se-poor diet (0.1 ppm Se), then they received 100 μM Se as sodium selenite in drinking water for 3 days. Se supplementation was shown to decrease the acute inflammatory response in LPS-injected mice as measured by TNF-α, IL-6, and MCP1 (243). The effect was evident only in males, suggesting a sex-specific relationship between Se status and sepsis. An animal model of sepsis utilizing sheep demonstrated that a large-bolus injection of Se (2 mg Se as sodium selenite) had more beneficial effects than continuous administration of Se (4.0 μg/kg per h) (263), although the conclusions of this study require follow-up investigation to determine if these results are duplicated in humans. In humans, treatment of critically septic patients with Se supplementation seems to improve clinical outcome in terms of infections and organ failure (8, 205), but larger intervention studies are required before conclusive results can be obtained. Importantly, dosage and timing of Se administration (best results with >500 μg/day) must be carefully evaluated so as to not endanger these patients (97).
C. Systemic inflammatory response syndrome
In addition to the potentially important role of Se in CRISIS just described, systemic inflammatory response syndrome (SIRS) is another syndrome related to critical illness in which Se status may play a key role in clinical outcome. SIRS is characterized by a disruption of normal cytokine regulation resulting from infectious or noninfectious origin and is currently diagnosed by the presence of two or more of the following factors: fever, heart rate >90 beats/min, respiratory rate of >20 breaths/min, or abnormally low or high white blood cell count (>12,000/μl or <4000/μl). The broad definition of SIRS and the numerous underlying causes make this an especially difficult condition to characterize and treat. SIRS is characterized by an early decrease in Se status as demonstrated by low plasma Se, GPX3 activity, and SELP levels (73, 154, 214). The changes in Se status observed during SIRS suggest that this nutritional factor may serve as a potential biomarker or prognostic indicator for intensive care unit patients with SIRS. One study demonstrated that there was a correlation between initial plasma Se levels and clinical outcome, with patients with SIRS exhibiting lower Se levels (<0.07 μM) presenting with a higher rate of nosocomial pneumonia, organ system failure, and mortality (74). Despite intervention measures in SIRS cases using Se supplementation (40 μg/day), patients with low initial Se levels tended to stay low and this was particularly true in patients whose condition worsened. Another study that confirmed the association between Se levels and SIRS outcome demonstrated that GPX3 activity was decreased in patients with severe SIRS (154).
Based on the correlative data just described, there has emerged an interest in the use of Se supplementation as an intervention of SIRS. Five studies conducted between 2007 and 2011 investigated the potential beneficial effects of Se supplementation (as sodium selenite) on SIRS and sepsis patient outcomes (8, 72, 123, 169, 252). Study populations in three of these studies ranged from 35 to 150 patients with initial doses of 400 to 4000 μg Se followed by daily infusions, and results showed increases in plasma Se and GPX3 levels. The investigators noted that although mortality rates were unaffected, the disease in the Se supplemented group was less severe as assessed by sepsis-related organ failure. One study involving 238 sepsis patients used a bolus of 1000 μg Se followed by 14 days of continuous intravenous injection (1000 μg Se/day) and reported increased GPX3 and plasma Se levels along with a significant decrease in mortality rate in the Se supplemented group relative to the placebo group (8). Another study involving 35 patients with SIRS used a bolus of 2000 μg Se followed by 10 days continuous infusion with 1600 μg/day, and results showed a significant decrease in the incidence of infection and severity of the illness (154). These studies illustrate the complex roles of timing and dosage for using Se in treating SIRS while suggesting a generally negative correlation between Se levels and illness severity. None of the studies reported any negative short-term effects of pharmaceutical concentrations of Se supplementation.
There may be particular benefits with the early, transient pro-oxidant effect of sodium selenite as a therapeutic strategy to reverse the proinflammatory states that accompany severe sepsis and septic shock. A comparison of four studies of Se supplementation demonstrated that critically ill patients consistently showed decreased plasma Se concentration (258). However, supplementation with Se at <1000 μg/day showed little effect on mortality rate, whereas bolus doses of >1000 μg Se followed by continued intravenous supplementation produced mixed but potentially promising results. A bolus dose of sodium selenite in the early phase of septic shock may act as a pro-oxidant and inhibit NFκB binding to DNA through disulfide bond stabilzation, regulating gene expression and, thus, the synthesis of proinflammatory cytokines at an early stage of SIRS (157). In this sense, the pro-oxidant properties of sodium selenite may be beneficial early in the course of septic shock if they reduce inflammation either by inhibiting the activation of NFκB or by inducing a proapoptotic effect on activated circulating cells, and intervention with a bolus of Se may provide an effective treatment modality during SIRS and other critical illnesses.
D. Intestinal inflammation and food-borne illnesses
IBD is a chronic, relapsing, and remitting inflammatory condition characterized by excessive local inflammation and tissue damage that can lead to loss of the intestinal barrier function. Oxidative stress plays a major role in the pathogenesis of IBD, with reduced antioxidant capacity shown to exacerbate disease (133). In some cases, IBD has been associated with decreased Se status (84, 206), but other studies failed to confirm these findings (231). High Se supplementation (2 μg/g body weight) was shown to prevent inflammation and improve the health of affected tissue in a rat model of dextran sodium sulfate (DSS)-induced colitis (249). Selp is among the selenoproteins expressed at the highest levels in the intestine (104, 179), and this protein may play a particularly important role in the pathogenesis of colitis. In a mouse model of colitis utilizing DSS treatment, Selp mRNA was decreased in the colon of DSS-treated mice (237). In addition, the inflammatory cytokines produced during colitis reduce production of SELP in human intestinal epithelial Caco-2 in a nitric oxide-dependent manner. This may reflect a particularly important role for SELP in the M1 to M2 switch in macrophage phenotype, without which resolution of inflammation may not procede. Thus, colitis may involve a cyclical decline of bioavailable Se similar to that just described for patients with sepsis and lead to a chronic inflammatory disease state.
Listeriosis and salmonellosis are two food-borne bacterial diseases in humans. Early studies in rats and a recent study in mice have demonstrated impaired immunity in Se-deficient rodents during infection with Listeria monocytogenes, the main causative pathogen of listeriosis in humans (181, 262). The impaired responses resulting from low Se intake included decreased cytokine secretion and reduced NK cytotoxicity. In addition, antioxidant markers were higher in the Se-adequate mice compared with Se-deficient mice. Not all cell-types apparently require adequate Se levels for anti-bacterial functions. For example, killing of Salmonella typhimurium and Staphylococcus aureus by neutrophils was found to be unaffected by Se-deficiency (25). However, the full benefits of adequate Se status during S. aureus infection may not be achieved except under conditions of high bacteremia, because only injection of high doses of bacteria produced mortality differences when comparing Se-deficient and -sufficient rats. Overall, these studies highlight the importance of maintaining adequate levels of Se intake for full immunity against bacterial pathogens. Supplementation with Se above adequate levels was not included, so no conclusions can be drawn regarding any potential for further immune enhancing or protective effects from above-adequate Se diets.
Food-borne pathogens such as Escherichia coli O157:H7 and others may lead to human diseases ranging from diarrhea to hemorrhagic colitis and hemolytic uremic syndrome. Se-deficiency has been shown to exacerbate infection of mice with Citrobacter rodentium, which shares many characteristics with human enteropathogenic (EPEC13) and enterohemorrhagic E. coli (EHEC) (233). Infections with this pathogen in mice produce robust immune responses characterized by a mixed Th1/Th17 response (100, 114). Enteropathogenic E. coli (EPEC) and EHEC (e.g., E. coli O157:H7) are food-borne pathogens that are the causative agents for human diseases ranging from diarrhea to hemorrhagic colitis and hemolytic uremic syndrome. A recent study demonstrated an interesting effect of Se status on the composition of gut microbiota in mice (124). The diversity of intestinal microflora increased with increased Se intake, and there was potential competition between gut micobiota and the hosts for usage of available Se. Thus, Se intake levels may affect enteric bacteria composition and this may, in turn, influence host selenoprotein expression and immunity.
E. Allergies and asthma
1. Epidemiology
Asthma is a multi-factorial inflammatory syndrome characterized by airway hyper-responsiveness, wheezing, coughing, and shortness of breath (146, 167). The complex etiology of asthma involves genetic, allergic, environmental, infectious, emotional, and nutritional factors (151). Genetic polymorphisms in humans and studies in animals suggest that oxidative stress is a contributing factor in the development and severity of asthma (204). However, correlations between Se status and asthma have not been consistently demonstrated. For example, a number of epidemiological studies have reported that asthma incidence, prevalence, or severity is associated with reduced Se status (52, 69, 92, 120, 130, 170, 190, 202, 228, 244). This is in contrast to findings in a large, multi-regional study conducted under the Global Allergy and Asthma European Network, in which asthma prevalence/severity data from 14 centers in Europe showed no significant association between Se status and asthma levels (33). Other studies also have failed to confirm a link between Se levels or GPx activities and development of asthma. One study has even suggested that Se levels or GPx activities were positively associated with severity of bronchial responsiveness (81). A large pregnancy study demonstrated that low Se levels in umbilical cord blood were negatively associated with persistent wheeze in young children (225). Another study found that maternal plasma Se concentration, but not GPx activity, in early pregnancy and in cord blood was inversely associated with wheezing in early childhood (56). Overall, results of these studies are conflicting, and it is difficult to clearly define the relationship between Se status and asthma, particulalry with cross-sectional study designs. This may be due to the multi-factorial etiology of this disease or variations in the study populations involved in these different studies (age of allergen exposure, atopic versus nonatopic, fluctuations in Se status over the course of disease progression, etc.). Another potential reason may be that Se status also can affect the immune system and the T helper responses that drive allergic asthma (atopic asthma).
2. Mouse models of allergic asthma
Oxidative stress is a major factor contributing to the development of allergic asthma. Allergen challenge in the lung induces rapid increases in the oxidized to reduced glutathione ratio as well as ROS levels that preceed inflammatory cell infiltration (193). Dietary Se levels have been shown to alter the development of ovalbumin (OVA)-induced allergic asthma in mice (Fig. 15). Low Se status resulted in lower Th2- or Th1-type immunity compared to adequate Se status, whereas above-adequate Se status appeared to skew T helper responses away from the Th2-type responses that drive allergic asthma (102, 105). Do selenoproteins play a protective role or do they promote asthma? Data from mouse studies have suggested that expression of certain selenoproteins may be induced during asthma. For example, lung Gpx1 and liver Selp were increased in OVA-challenged mice compared with controls (105). A recent study reported that expression of Gpx2 was increased after induction of allergic airway disease, whereas mice with targeted disruption of the Gpx2 gene showed significantly enhanced airway inflammation compared with wild-type mice (57). These observations suggest that Gpx2, which is more often associated with the intestinal epithelium, may play an important role in protection from allergen-induced disease. In another study, attenuation of allergen-induced eosinophilic infiltration and airway hyper-responsiveness was observed in Gpx1-deficient mice compared with wild-type mice (268). Thus, different Gpx enzymes may have opposing effects on asthma (164). This could be due to their multiple roles in regulating both oxidative stress and immunity during the development of allergic asthma in mice. Overall, mouse dietary studies have revealed some information regarding the crosstalk between Se status, redox tone, inflammation, and T helper immune responses. Evidence is clear that T helper cell differentiation is affected by Se levels. However, further investigation is needed to better understand how Se intake may influence the development of asthma in humans and the role of specific selenoproteins in the complex pathogenicity of allergies and asthma.
FIG. 15.
Results from mouse models of allergic asthma suggest that dietary Se levels may alter disease outcome. In relationship to the affects of dietary Se on T helper cell differentiation as outlined in Figure 10, low Se status leads to an overall lower immune response to Th2-inducing allergens. Increasing Se status to adequate levels increases TCR signal strength and enables stronger Th2 responses that drive allergic asthma. Further increasing Se status with Se supplementation further increases TCR signal strength, but skews CD4+ T cell away from Th2-type that drives allergic asthma.
3. Intervention with Se supplementation for patients with asthma
Similar to the epidemiological data just described, results from intervention studies aimed at determining the effectiveness of Se supplementation for reducing the incidence or severity of asthma have also been unclear. For example, one study reported significantly decreased consumption of corticosteroids after Se supplementation with 200 μg/day for 96 weeks in corticoid-dependent asthmatics (82). However, other studies failed to confirm any benefit from Se supplementation for 99 asthmatic adults using 100 μg/day Se as high-Se yeast or for 54 allergic adults using 76 μg/day Se as high-Se garlic (61, 226). Based on these findings, Se supplementation has not generally been recommended as a therapeutic modality for reducing asthma burden. However, combining Se supplementation methods with other treatments may prove more effective. Allergen-specific immunotherapy (IT) is a promising treatment modality for allergies and asthma that involves the delivery of increasing doses of allergen with the goal of inducing long-term desensitization and relief of symptoms. Althoguh IT has proved effective for allergic conditions such as rhinitis and conjunctivitis, the efficacy of IT for treating allergic asthma has been less impressive (24). IT is currently the only disease-modifying treatment for asthma, but improvements need to be made to current IT modalities to make it more effective and safe. The overall goal of IT is to divert immune responses away from CD4+T helper Th2-type to Th1/Treg-types. Augmenting IT treatments to more effectively divert responses in this manner would improve the efficacy of this treatment. Given the effects of Se supplementation on skewed T helper responses, it may provide the ideal means to augment IT therapy.
F. Cystic fibrosis
Cystic fibrosis (CF) is a hereditary disease caused by a mutation in the gene for the protein cystic fibrosis transmembrane conductance regulator (CFTR), which is required to regulate the components of sweat, digestive juices, and mucus. Respiratory problems in CF arise from inhibited mucociliary clearance in the airways, exacerbated inflammation and persistent airway infection that leads to progressive damage of the lung tissue, due in part to oxidative stress (31). This notion has generated interest in the use of Se supplementation to mitigate the oxidative stress in the lungs of patients with CF. There have been some data demonstrating benefits of antioxidant supplementation that includes Se as an ingredient, and a demonstrated improvement in lung function in patients with CF (269). Moreover, there are epigenetic manifestations of CF that are related to Se deficiency, and antioxidant supplementation has been found to provide benefits (260, 261). However, similar to asthma, there appears to be conflicting evidence regarding the clinical effectiveness of supplementation with Se or other antioxidants in CF (227). It is interesting to note that the inflammation involved in CF may lower Se status, similar to some other types of inflammation (165). Thus, it would be worth further investigating the relationship between CF, Se status, and the cause-and-effect relationship between Se levels and the inflammatory processes that accompany CF.
G. Autoimmunity
ROS appear to have a dual role in autoimmune diseases. Rheumatoid arthritis (RA) is one example where ROS may promote inflammation and progression of disease (67). ROS have also been suggested to be involved in the pathogenesis of multiple sclerosis (86) and type-1 diabetes (40). However, there is emerging evidence that ROS also play an important role in preventing autoimmune responses (112). A mutation in the Ncf1 gene, which encodes the p47phox subunit of the NOX2 complex, leads to reduced ROS and enhanced autoimmune disorders such as RA (111, 189). This is consistent with data showing that sustained ROS generated from NOX2 during lymphocyte activation are required for turning off certain signaling components and down-modulating the activation process (136). Another mechanism by which ROS affects autoimmunity relates to the role of ROS in the controlled deletion of activated T cells that occurs on antigen restimulation, which is important for maintaining T cell equilibrium during an immune response. This process is termed activation-induced cell death (AICD), and antioxidants have been shown to inhibit AICD in T cells (89). The effects of dietary Se on AICD have not been evaluated, but increased dietary Se affects redox tone in unstimulated T cells and ROS generation in stimulated T cells (102).
There are important links between Se levels and autoimmunity, with the best example being autoimmune thyroid diseases such as Hashimoto's thyroiditis (HT). Clinical studies have demonstrated that in patients with HT, Se supplementation reduced thyroid peroxidase autoantibody titers significantly as compared with the control subjects receiving placebo (250). Since Se levels affect both the thyroid gland and the immune system, it has been difficult to determine the contribution of each to the observed effects with HT. It is worth noting that all studies using Se to successfully treat HT were conducted in or near mainland Europe with nearly all exclusively enrolling female subjects, and whether male patients similarly benefit from Se treatment remains unknown. In addition, it remains unclear how Se supplementation may affect HT in patients with higher baseline Se status. A recent study utilizing a mouse model of autoimmune thyroiditis and Se supplementation (0.3 mg/L in the drinking water) implicated Treg cells as a key factor by which Se exerts its effects (274). In particular, the autoimmune mice had fewer Treg cells and reduced Foxp3 mRNA expression in splenocytes compared with controls without disease. The percentage of Treg cells and expression of Foxp3 mRNA in autoimmune mice were increased by Se supplementation. Mice that received Se supplementation also had lower serum autoantibody titers and reduced lymphocytic infiltration in thyroids than untreated autoimmune mice.
Treg cells play an important role in preventing autoimmune responses, and Se supplementation may affect levels of Treg cells that arise from the thymus (natural Treg) or during differentiation of naive T cells (induced Treg). Treg cells have different redox properties from conventional CD4+ T helper cells and are more resistant to the proapoptotic effects of H2O2 (176). This may be related to higher expression of Txn1, which affects their effector role in preventing prolonged or excessive immune responses and, perhaps, autoimmunity (175). As just described, Txn1 enzymatically reduces a variety of molecular substrates and in the process is itself oxidized. Regeneration of reduced Txn1 in the cytosol is carried out by the selenoprotein, Txnrd1. Levels of Txnrd1 in T cells is directly correlated to Se intake and Se intake is linked to expression of Treg markers on T cells as shown in Figure 11. However, it remains unknown whether a higher intake of Se increases the suppressive function of Treg cells.
Other possible links between Se status and autoimmunity have been suggested in studies focus on RA. RA is an autoimmune disease that causes chronic inflammation of the joints that can also cause inflammation of the tissue around the joints, as well as in other organs in the body. In a small study involving 46 patients with RA and 48 age-matched controls, serum Se levels were significantly reduced in RA (194). In the RA group, there also was evidence for increased lipid peroxidation, as urinary 8-isoprostane levels were significantly elevated. Whether Se status was a causative factor in RA or an effect of the chronic inflammation caused by this disease was not clear. Interestingly, insulin and adiponectin were also significantly increased, whereas insulin sensitivity was decreased in patients with RA. Plasma glucose levels were unchanged. This is important, given the relationship between extremes in Se status and diabetes described in more detail below.
H. Se supplementation and aging immunity
The bioavailability of Se along with ∼30 other minerals and vitamins (V/M) has dramatic effects on the aging process (159). One notion of these nutrients in relation to aging is referred to as the triage theory, which proposes that when the dietary availability of a V/M is moderately inadequate, nature ensures that V/M-dependent functions that are essential from an evolutionary perspective are protected at the expense of those functions that are less essential. In other words, this guarantees that shortages do not have acute short-term negative consequences but may have long-term insidious effects that increase risk of diseases associated with aging (6). The triage theory does not imply that any particular V/M deficiency is the only cause of an age-related disease but rather that it is a contributing factor along with the sum of all contributing causal factors. The aging process leads to a progressive decline in many physiological processes, including immune responses (142). According to the triage theory, immune response would likely fall into the “less essential” category, with V/M deficiencies causing insidious problems that are less overt and accumulate over time. Consistent with this notion, there are immune deficiencies associated with V/M deficiencies that emerge mainly in postreproductive ages (158).
A major issue with immunological aging is the cumulative oxidative damage to cellular components over time, and nutritional intervention may help to prevent or limit such damage. Since Se is a potent dietary antioxidant, it should be considered in studies focused on nutritional effects on the aging immune system. This is especially true given that elderly individuals are at risk for low Se status in certain populations (10, 149). Se is incorporated into important antioxidant enzymes such as the GPX enzymes, which provide direct protection against ROS. In addition, selenoproteins such as the TXNRD enzymes, SELR (methionine sulfoxide reductase B1), and perhaps others play key roles in regulating redox tone or reversing oxidative damage inflicted on cells. This suggests that adequate or above-adequate levels of Se may be beneficial for maintaining proper immune responses in aging individuals. A small study involving 89 men and women aged 65 to 80 years evaluated several nutritional markers to determine which, if any, correlated with proliferative capacity of blood lymphocytes (264). Se was one of four nutrients found to positively correlate with proliferative capacity. Thus, the potential decline in Se status in the elderly may be a major contributing factor to decline immunity, although the data to support this are not entirely clear (224).
Aging is associated with reduced IL-2 production and decreased T cell proliferative capacity. The role of Se in maintaining optimal T cell responses should take into consideration synergism between Se and other required nutrients. For example, vitamin E has been shown to be important for improving specific age-related T cell signaling events in naive CD4+ T cells (155). Dietary Se may act in concert with vitamin E to boost aging immunity. In fact, most studies in aging individuals include several nutritional supplements in combination. The evidence is limited, but overall it suggests there are benefits of supplementation with Se and other antioxidants to prevent declining immune system function.
I. Lymphedema
Lymphedema occurs when excessive protein-rich fluid accumulates in the extravascular interstitial spaces as a consequence of impaired lymphatic drainage. This condition may arise as a potentially serious complication from treatment of patients with breast, gynecologic, or genitourinary cancers (234). There is some association of the degenerative changes that occur during lymphedema with excessive generation of oxygen radicals, which has led to the investigation of Se supplementation as a treatment modality. For example, patients who have undergone postmastectomy with lymphedema of the arm were administered sodium selenite orally (800 μg Se/day on days 1 through 4, 500 μg Se/day on days 5 through 28), and findings indicated a spontaneous reduction in lymphedema volume and normalized blood parameters in a manner consistent with diminished oxygen radical production (126). In a randomized, placebo-controlled, double-blind study with patients with postmastectomy lymphedema undergoing combined physical decongestion therapy (CPDT), sodium selenite at similar dosages increased the efficacy of CPDT and improved the mobility and heat tolerance of the affected extremity. The patients in this study received 1000 μg of Se/day orally during the first week, 300 μg Se/day during the second and third weeks, and a maintenance dose of 100 μg Se/day during 3 months of follow-up. All patients remained erysipelas-free during the 3 weeks of CPDT and the 3-month follow-up period (126). In another study, 12 patients with edema of the arm and 36 patients with edema of the head-and-neck region were treated with sodium selenite (350 μg/m2 body surface over 4 to 6 weeks) for therapy-related lymphedema (166). Ten of the 12 patients with arm edema showed improvement, and the findings with the overall study population suggested that sodium selenite had a positive effect on secondary-developing lymphedema caused by radiation therapy alone or by irradiation after surgery. Although results show a potential benefit of Se supplementation in attenuating lymphedema, meta-analysis have suggested that there are not enough data to reach a clear conclusion (54), and further research is needed.
J. Se supplementation and inflammation associated with diabetes
Some alarming and surprising data regarding Se supplementation and type-2 diabetes came to the forefront in 2009 when findings were published from the Se and vitamin E cancer prevention trial (SELECT) (145). SELECT was a phase-3 randomized, placebo-controlled trial of Se (200 μg/day from l-Se-Met), vitamin E (400 IU/day of all rac-α-tocopheryl acetate), or both for prostate prevention. SELECT was one of the largest human cancer prevention trials ever undertaken, but was discontinued well before the planned 12 year intervention period had been completed. Contributing to the early termination of this study was a slight but statistically nonsignificant increase in type-2 diabetes mellitus within the Se-supplemented group. It is important to note that the link between Se supplementation and type-2 diabetes in this study may have involved study design issues (93), and it was based on the observation that of a total of 1202 subjects, 58 diabetes cases occurred in the Se-alone group compared with 39 in the placebo group. Although these facts call into question the conclusions drawn from SELECT regarding diabetes risk from high Se intake, there are other findings that support such a correlation. For example, increased GPX1 activity has been hypothesized to interfere with insulin signaling. Mild insulin resistance associated with pregnancy was shown to be accompanied by increased GPX activity in humans (41). Further, transgenic mice overexpressing Gpx1 developed a type-2 diabetes-like phenotype characterized by insulin resistance, hyperglycemia, hyperinsulinemia, and obesity (161). In fact, perturbing the axis of selenoprotein expression toward either deficient or over-expressed levels may dysregulate glucose homeostasis and promote the development of diabetes (139).
The SELECT findings may be attributed to the fact that the serum Se levels of diabetics tend to be higher than those of diabetes-free controls not because they were taking supplemental Se, but due to disease-related changes of the serum protein levels. In a recent study, serum SELP concentrations were higher in patients with type-2 diabetes or prediabetes than those with normal glucose tolerance (275). Moreover, SELP levels correlated with serum C-reactive protein (CRP) levels and carotid intima-media thickness, which are an indicator of inflammation and atherosclerosis, respectively. The relationship between SELP and CRP was very strong (r2=0.962, p<0.001), and this association persisted after adjustment for other confounding factors. The inflammatory conditions involving adipose tissue during type-2 diabetes are quite different from those associated with sepsis, and the affects on SELP expression are different as well. As just described, sepsis actually decreases hepatically derived SELP in the serum. Misu et al. showed that hepatic SELP mRNA expression was increased in human liver samples from patients with type-2 diabetes (171). Zhang et al. found that Selp mRNA was significantly reduced in adipose tissue of ob/ob and high-fat diet-induced obese mice as well as in primary adipose cells isolated from Zucker obese rats (282). Adipocytes and hepatocytes appear to have opposite reactions to diabetic inflammation, and adipocytes may down-regulate Selp expression in response to higher insulin. However, administration of Selp increased insulin resistance in both hepatocytes and myocytes (171). Thus, there are some contrasting findings that may involve species differences and the cause-effect relationship between SELP expression in the liver and adipose tissue during type-2 diabetes requires clarification.
The link between Se and type-2 diabetes is indeed a controversial one. As just mentioned, the association found in the SELECT trial was not statistically significant. In fact, a longitudinal study showed that the risk of dysglycemia was significantly lower in men with plasma Se in the highest tertile compared with those in the lowest tertile, but no significant relationship was observed in women (3). This finding remained after controlling for potential confounders. When comparing this trial to the SELECT trial, the authors noted that the median baseline Se concentration of the the highest tertile was equal to that of the lowest tertile of the SELECT trial. This emphasizes the importance of baseline Se levels in determining how Se supplementation may affect type-2 diabetes or other health outcomes.
The effects of Se supplementation may be quite different for type-1 diabetes. In a mouse model of strepozotocin-induced diabetes, which includes elements reflecting type-1 diabetes and involves immune-driven pathology, Se appears to be more protective. For example, a combined micronutrient treatment including Se with vitamin E, vanadium, and chromium, reduced islet destruction and blood glucose parameters (39). This protective effect involved a shift in the balance between inflammatory cytokines (TNF-α) and regulatory cytokines (IL-10). Other studies have also suggested Se supplementation in rodents improved glucose homeostasis in streptozotocin-induced diabetes (14, 19, 163). In this sense, Se supplementation may increase the Treg responses similar to observed effects in autoimmune models just described.
Se levels can affect the manner in which cells respond to elevated levels of insulin, involving the inflammasome. TXN-1 plays an important role in activation of the NOD-like receptor family, pryin domain-containing 3 (NLRP3) inflammasome, which is responsible for activation of the cytokine IL-1β. Dysregulation of IL-1β is associated with several inflammatory diseases and glucose-triggered inflammasome activation and IL-1β secretion are major factors in the pathogenesis of insulin resistance and type-2 diabetes (286). ROS from receptor-linked NOX or H2O2 treatment oxidizes TXN-1, releasing thioredoxin-interacting protein (TXNIP) that then binds with and activates the NLRP3 inflammasome to activate IL-1β (285). The selenoprotein TXNRD1, by keeping TXN1 in the reduced state that binds to and inactivates TXNIP, may be crucial for regulating inflammasome activation and IL-1β secretion. Although the direct effect of Se intake on glucose metabolism and insulin production by β-cells of the pancreas and activity of glycolytic and gluconeogenic liver enzymes have been demonstrated, understanding the effects on the immune system and its contributions in the pathogenesis of diabetes requires further investigation.
IX. Can Se Supplementation Be Targeted to the Immune System?
As outlined in Table 2, a variety of Se supplementation forms and dosages have been used in different studies, and more uniformity in these aspects may allow better comparison of results in the future. Overall, Se supplementation has traditionally been carried out using oral ingestion of either sodium selenite, l-Se-Met, Se-enriched baker's yeast, or Se-enriched garlic. The form of Se that is used for supplementing human diets can be important not only for its effectiveness in enhancing Se status, but also for inducing potentially adverse side-effects (93). Most of the studies described so far in this article have involved one of these oral Se supplementation approaches and have shown that they all are effective in modulating immunity, but improvements are needed to make current Se therapy more effective and safe. As just described, the need for new Se supplementation modalities is highlighted by results of the SELECT trial and other emerging data suggesting potential adverse effects. Therefore, novel delivery systems that more selectively target the immune system could allow administration of a lower dosage of Se and decrease the associated risks.
Table 2.
Summary of Selenium Supplementation Forms and Dosages in Studies Involving Inflammation and Immunity
| Model | Forms of Se | Dosages of Se | References | |
|---|---|---|---|---|
| Cell culture studies | HUVECs | Sodium selenite added to baseline Se in media | 0.1 μM | 279 |
| 0–2 μM | 276 | |||
| 10 μg/ml | 116 | |||
| Human primary monocytes | 1–25 μg/ml | 1 | ||
| Human T cells | >5–10 μM | 177 | ||
| Human macrophages | 25 nM-1.0 μM | 121 | ||
| FELCs | 20 μM | 46 | ||
| Human HuH-7 cells | 0, 0.05, 0.5, 1.0, 2.0 μM | 152 | ||
| Mouse primary lymphocytes | 100 nM | 208 | ||
| J774.1 mouse macrophages | 100 ng/ml | 209 | ||
| RAW 264.7 mouse macrophages | 2 μM | 197, 256, 274 | ||
| Animal studies | Mouse | Sodium selenite in diets | Low, 0.08 mg/kg medium, 0.25 mg/kg high, 1.0 mg/kg | 102, 253 |
| Deficient, 0.086 mg/kg adequate, 0.15 mg/kg | 129, 177 | |||
| Low, 0.08 ppm medium, 0.25 ppm high, 2.7 ppm | 105 | |||
| 0, 0.1, 0.4, 2.25 mg/kg | 124 | |||
| 2 mg/kg | 208 | |||
| 0, 0.2, 0.3–0.5 ppm | 273 | |||
| Selenomethionine in diets | Low, 0.02 ppm adequate, 0.2 ppm above adequate, 2 ppm | 252 | ||
| Se-poor diet | 0.1 mg/kg | 241 | ||
| Drinking water, sodium selenite drinking water, sodium selenite | 100 μM 0.3 mg/L |
174, 269 | ||
| Rat | l-selenomethionine in diets | Deficient, basal diets | 275 | |
| Adequate, 0.15 ppm | ||||
| Above adequate, 4 ppm | ||||
| Sodium selenite in diets | 2 μg/g body weight | 246 | ||
| Sheep | Bolus injection, selenite | 2 mg Se | 258 | |
| Continuous infusion, selenite | 4 μg Se/kg body weight/hr | |||
| Human studies | Oral ingestion of Se | Sodium selenite in diets | 40 μg/day | 183 |
| 50–100 μg/day | 29 | |||
| 100 μg/day | 190 | |||
| 200 μg/day | 82, 116 | |||
| 1000 μg/day for 1 week | 126 | |||
| 300 μg/day for 2 weeks | ||||
| 100 μg/day for 3 months | ||||
| Low, 13 μg/day | 95 | |||
| High, 297 μg/day | ||||
| Diets, Se-enriched onions | 50 μg/day | 88 | ||
| Diets, Se-enriched yeast | 50–200 μg/day | |||
| Diets, high Se garlic | 76 μg/day | 61, 223 | ||
| Diets, high Se yeast | 100 μg/day | |||
| Diets, l-selenomethionine | 200 μg/day | 144 | ||
| Bolus intravenous administration of Se | Sodium selenite | 1000 μg followed by 1000 μg/day | 8 | |
| 2000 μg followed by 1600 μg/day | 153 | |||
Se, selenium; HUVECs, human umbilical vein endothelial cells; FELCs, Friend erythroleukemic cells.
The potential adverse effects of systemic Se supplementation emphasize the need for a more concerted effort in exploring alternative Se supplementation formulations to more selectively target the immune system. One approach may involve targeting the intestinal lymphatic regions, which have been routinely explored and used for site-specific lymphatic delivery of orally administered proteins, drugs, and vaccines (4, 83, 271). Some alternative Se-delivery approaches have been attempted and shown to ameliorate colitis in an animal model (168). Given that the gastrointestinal tract is richly supplied with lymphoid tissues, formulations targeting these tissues may provide an effective means of delivering Se to the immune system to more selectively exert its immune-deviating effects. In fact, our laboratory is currently developing novel Se formulations with this goal in mind. Overall, enhanced delivery of Se to lymphatic system is a prerequisite to fully utilizing this potent antioxidant for immune modulation.
X. Information Gaps and Future Directions
Se is conventionally regarded as a potentially effective complementary and alternative medicine modality based on the notion that it positively modulates immune function. However, not all types of immune responses are equivalently affected by increasing levels of Se. The reasons for this are unclear due to an inadequate understanding of the mechanisms by which this nutritional antioxidant affects the immune system. Thus, identifying specific cell signaling pathways and immune cell functions regulated by dietary Se levels represents the next logical, necessary step in utilizing Se supplementation to enhance or modulate immunity. Redox sensing and signaling, protein-folding, epigenetic poising, and other major points of regulation on which Se levels and individual selenoproteins may impinge should be better understood.
Once mechanistic studies have provided insights regarding the effects of Se supplementation on immune cells and networks, this information should be used to choose appropriate uses of this nutritional supplement for treating particular disorders or diseases. Published data using mouse models and limited human studies suggest that Se supplementation may provide an inexpensive means to divert immune responses away from the CD4+ Th2-type that drive allergic asthma, and promote the Th1-type that provide protection against viral infections and cancer. This provides the framework for human intervention studies for using Se supplementation to reduce allergic asthma, to boost specific vaccine responses, or to reduce progression of infectious diseases such as tuberculosis or HIV-1 in particular populations. Combining Se supplementation with other treatment modalities may also be effective, as just described in the use of IT for reducing asthma. Importantly, reducing the potential for adverse effects of long-term Se supplementation should be first addressed before clinical trials may be undertaken. This will require assessment of baseline Se status of individuals within populations to prescribe the appropriate level of Se supplementation. In this sense, Se supplementation could be administered in a more personalized manner as is the case with other therapeutic approaches. In addition, supplement formulations may be developed to provide a more targeted delivery of Se to the immune cells instead of the systemic Se supplementation approaches now in place. This will reduce the amount of Se used in supplements and decrease the development of potential side-effects such as disrupted glucose metabolism.
Abbreviations Used
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- AA
arachidonic acid
- AICD
activation-induced cell death
- AIDS
acquired immune deficiency syndrome
- BMDM
bone marrow-derived macrophages
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CGD
chronic granulomatous disease
- COX-2/Cox-2
cyclooxygenase-2
- CPDT
combined physical decongestion therapy
- CRAC
calcium release-activated Ca2+
- CRISIS
critical illness stress-induced immune suppression
- CRP
C-reactive protein
- CVB3
coxsackievirus B3
- Cys
cysteine
- Cyt b558
heterodimeric flavocytochrome
- DAG
diacylglycerol
- DIO1/Dio1
deiodinase 1
- DSS
dextran sodium sulfate
- DUOX/Duox
dual oxidase
- EFSec
selenocysteine-specific translation elongation factor
- EHEC
enterohemorrhagic Escherichia coli
- EPEC
enteropathogenic E. coli
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated protein kinase
- FBS
fetal bovine serum
- FcγR
Fcγ-receptor
- FELCs
friend erythroleukemic cells
- fMLP
formyl-Methionyl-Leucyl-Phenylalanine
- FoxP3
forkhead box P3
- GDI
GDP dissociation inhibitor
- GPX/Gpx
glutathione peroxidase
- GSH
glutathione
- HAART
highly active antiretroviral therapy
- HDAC
histone deacetylase
- HIV
human immunodeficiency virus
- H2O2
hydrogen peroxide
- H-PGDS
hematopoietic PGDS
- HT
Hashimoto's thyroiditis
- HUVECs
human umbilical vein endothelial cells
- IBD
inflammatory bowel disease
- IC
immune complexes
- ICAM-1
intercellular adhesion molecule 1
- IFN-γ
interferon-γ
- IKKβ
IκB kinase β
- IL-2
interleukin 2
- iNOS
inductible nitric oxide synthase
- IP3
inositol-1,4, 5-trisphosphate
- IP3R
IP3 receptor
- IT
immunotherapy
- ITAM
immunoreceptor tyrosine-based activation motif
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- MSRB1/Msrb1
methionine sulfoxide reductase B1
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFAT
nuclear factor of activated T cells
- NFκB
nuclear factor-kappa B
- NLRP3
NOD-like receptor family, pryin domain-containing 3
- NO·
nitric oxide
- NOX/Nox
NADPH oxidase
- ·O2-
superoxide
- ·OH
hydroxyl radical
- ONOO-
peroxynitrite
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cell
- PG
prostaglandin
- PGD2
prostaglandin D2
- PGDS
PGD synthase
- PGE2
prostaglandin E2
- PHOX
phagocytic oxidase
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PLCγ
phospholipase Cγ
- PMA
phorbal myristate acetate
- Pstk
phosphoseryl-tRNA[Ser]Sec kinase
- PTPs
protein tyrosine phosphatases
- RA
rheumatoid arthritis
- RAC1/2/Rac1/2
Ras-related C3 botulinum toxin substrate 1/2
- RANKL
receptor activator for nuclear factor-κB ligand
- ROS
reactive oxygen species
- SBP2/Sbp2
SECIS-binding protein 2
- Se
selenium
- Sec
selenocysteine
- Sec-tRNASec
selenocysteyl-tRNASec
- SECIS
selenocysteine insertion sequence
- SEL
selenoprotein (human)
- Sel
selenoprotein (mouse)
- SELECT
selenium and vitamin E cancer prevention trial
- Se-Met
selenomethionine
- SEP15/Sep15
human 15kD selenoprotein
- Ser
serine
- SHP-2
Sarc homology phosphatase-2
- SIRS
systemic inflammatory response syndrome
- SOCE
store-operated Ca2+ entry
- SOD
superoxide dismutase
- SPS2/Sps2
selenophosphate-synthetase 2
- STIM1/Stim1
stromal interaction molecule 1
- T3
liothyronine or 3,3′,5-triiodothyronine
- T4
thyroxine or 3,3′,5, 5′-tetraiodothyronine
- T-bet
T-box expressed in T cells
- TCR
T cell receptor
- Th
T helper
- TLR/Tlr
Toll-like receptor
- TNF-α
tumor necrosis factor α
- Treg
regulatory T helper cells
- TXN/Txn
thioredoxin
- TXNIP
thioredoxin-interacting protein
- TXNRD/Txnrd
thioredoxin reductase
- VCAM-1
vascular cell adhesion molecule 1
- V/M
vitamins and minerals
Acknowledgments
This research was supported by NIH grants R21AT004844 and R01AI089999. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCCAM, NIAID, or NIH. The work was also supported in part by NIH grant G12RR003061.
References
- 1.Ahrens I. Ellwanger C. Smith BK. Bassler N. Chen YC. Neudorfer I. Ludwig A. Bode C. Peter K. Selenium supplementation induces metalloproteinase-dependent l-selectin shedding from monocytes. J Leukoc Biol. 2008;83:1388–1395. doi: 10.1189/jlb.0707497. [DOI] [PubMed] [Google Scholar]
- 2.Ahsan MK. Masutani H. Yamaguchi Y. Kim YC. Nosaka K. Matsuoka M. Nishinaka Y. Maeda M. Yodoi J. Loss of interleukin-2-dependency in HTLV-I-infected T cells on gene silencing of thioredoxin-binding protein-2. Oncogene. 2006;25:2181–2191. doi: 10.1038/sj.onc.1209256. [DOI] [PubMed] [Google Scholar]
- 3.Akbaraly TN. Arnaud J. Rayman MP. Hininger-Favier I. Roussel AM. Berr C. Fontbonne A. Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing Study. Nutr Metab (Lond) 2010;7:21. doi: 10.1186/1743-7075-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldwell FE. Baird MA. Fitzpatrick CE. McLellan AD. Cross ML. Lambeth MR. Buchan GS. Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: anatomical sites of bacterial replication and immune activity. Immunol Cell Biol. 2005;83:549–553. doi: 10.1111/j.1440-1711.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 5.Allmang C. Wurth L. Krol A. The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim Biophys Acta. 2009;1790:1415–1423. doi: 10.1016/j.bbagen.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen JN. Mortensen OH. Peters GH. Drake PG. Iversen LF. Olsen OH. Jansen PG. Andersen HS. Tonks NK. Moller NP. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angstwurm MW. Engelmann L. Zimmermann T. Lehmann C. Spes CH. Abel P. Strauss R. Meier-Hellmann A. Insel R. Radke J. Schuttler J. Gartner R. Selenium in intensive care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 9.Antunes F. Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 10.Arnaud J. Akbaraly TN. Hininger I. Roussel AM. Berr C. Factors associated with longitudinal plasma selenium decline in the elderly: the EVA study. J Nutr Biochem. 2007;18:482–487. doi: 10.1016/j.jnutbio.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Au Yeung KJ. Smith A. Zhao A. Madden KB. Elfrey J. Sullivan C. Levander O. Urban JF. Shea-Donohue T. Impact of vitamin E or selenium deficiency on nematode-induced alterations in murine intestinal function. Exp Parasitol. 2005;109:201–208. doi: 10.1016/j.exppara.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Baker SS. Cohen HJ. Altered oxidative metabolism in selenium-deficient rat granulocytes. J Immunol. 1983;130:2856–2860. [PubMed] [Google Scholar]
- 13.Barnett M. Bermingham E. McNabb W. Bassett S. Armstrong K. Rounce J. Roy N. Investigating micronutrients and epigenetic mechanisms in relation to inflammatory bowel disease. Mutat Res. 2010;690:71–80. doi: 10.1016/j.mrfmmm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Battell ML. Delgatty HL. McNeill JH. Sodium selenate corrects glucose tolerance and heart function in STZ diabetic rats. Mol Cell Biochem. 1998;179:27–34. doi: 10.1023/a:1006819227506. [DOI] [PubMed] [Google Scholar]
- 15.Beck KW. Schramel P. Hedl A. Jaeger H. Kaboth W. Serum trace element levels in HIV-infected subjects. Biol Trace Elem Res. 1990;25:89–96. doi: 10.1007/BF02990269. [DOI] [PubMed] [Google Scholar]
- 16.Beck MA. Handy J. Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck MA. Kolbeck PC. Rohr LH. Shi Q. Morris VC. Levander OA. Benign human enterovirus becomes virulent in selenium-deficient mice. J Med Virol. 1994;43:166–170. doi: 10.1002/jmv.1890430213. [DOI] [PubMed] [Google Scholar]
- 18.Beck MA. Nelson HK. Shi Q. Van Dael P. Schiffrin EJ. Blum S. Barclay D. Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. Faseb J. 2001;15:1481–1483. [PubMed] [Google Scholar]
- 19.Becker DJ. Reul B. Ozcelikay AT. Buchet JP. Henquin JC. Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia. 1996;39:3–11. doi: 10.1007/BF00400407. [DOI] [PubMed] [Google Scholar]
- 20.Berridge MJ. Bootman MD. Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 21.Bonizzi G. Piette J. Schoonbroodt S. Greimers R. Havard L. Merville MP. Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosl MR. Takaku K. Oshima M. Nishimura S. Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci U S A. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosschaerts T. Guilliams M. Noel W. Herin M. Burk RF. Hill KE. Brys L. Raes G. Ghassabeh GH. De Baetselier P. Beschin A. Alternatively activated myeloid cells limit pathogenicity associated with African trypanosomiasis through the IL-10 inducible gene selenoprotein P. J Immunol. 2008;180:6168–6175. doi: 10.4049/jimmunol.180.9.6168. [DOI] [PubMed] [Google Scholar]
- 24.Bousquet J. Specific immunotherapy in asthma. Allergy. 1999;54(Suppl 56):37–38. doi: 10.1111/j.1398-9995.1999.tb04438.x. [DOI] [PubMed] [Google Scholar]
- 25.Boyne R. Mann SO. Arthur JR. Effects of Salmonella typhimurium infection on selenium deficient rats. Microbios Lett. 1984;27:83–87. [Google Scholar]
- 26.Braun A. Gessner JE. Varga-Szabo D. Syed SN. Konrad S. Stegner D. Vogtle T. Schmidt RE. Nieswandt B. STIM1 is essential for Fcgamma receptor activation and autoimmune inflammation. Blood. 2009;113:1097–1104. doi: 10.1182/blood-2008-05-158477. [DOI] [PubMed] [Google Scholar]
- 27.Brechard S. Plancon S. Melchior C. Tschirhart EJ. STIM1 but not STIM2 is an essential regulator of Ca2+ influx-mediated NADPH oxidase activity in neutrophil-like HL-60 cells. Biochem Pharmacol. 2009;78:504–513. doi: 10.1016/j.bcp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Brechard S. Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broome CS. McArdle F. Kyle JA. Andrews F. Lowe NM. Hart CA. Arthur JR. Jackson MJ. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80:154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 30.Brown N. Crawford C. Structural modifications associated with the change in Ca2+ sensitivity on activation of m-calpain. FEBS Lett. 1993;322:65–68. doi: 10.1016/0014-5793(93)81112-d. [DOI] [PubMed] [Google Scholar]
- 31.Brown RK. Wyatt H. Price JF. Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J. 1996;9:334–339. doi: 10.1183/09031936.96.09020334. [DOI] [PubMed] [Google Scholar]
- 32.Burk RF. Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta. 2009;1790:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burney P. Potts J. Makowska J. Kowalski M. Phillips J. Gnatiuc L. Shaheen S. Joos G. Van Cauwenberge P. van Zele T. Verbruggen K. van Durme Y. Derudder I. Wohrl S. Godnic-Cvar J. Salameh B. Skadhauge L. Thomsen G. Zuberbier T. Bergmann KC. Heinzerling L. Renz H. Al-Fakhri N. Kosche B. Hildenberg A. Papadopoulos NG. Xepapadaki P. Zannikos K. Gjomarkaj M. Bruno A. Pace E. Bonini S. Bresciani M. Gramiccioni C. Fokkens W. Weersink EJ. Carlsen KH. Bakkeheim E. Loureiro C. Villanueva CM. Sanjuas C. Zock JP. Lundback B. Janson C. A case-control study of the relation between plasma selenium and asthma in European populations: a GAL2EN project. Allergy. 2008;63:865–871. doi: 10.1111/j.1398-9995.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 34.Carcillo J. Holubkov R. Dean JM. Berger J. Meert KL. Anand KJ. Zimmerman J. Newth CJ. Harrison R. Willson DF. Nicholson C. Rationale and design of the pediatric critical illness stress-induced immune suppression (CRISIS) prevention trial. JPEN J Parenter Enteral Nutr. 2009;33:368–374. doi: 10.1177/0148607108327392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson BA. Yoo MH. Sano Y. Sengupta A. Kim JY. Irons R. Gladyshev VN. Hatfield DL. Park JM. Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol. 2009;10:57. doi: 10.1186/1471-2172-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson BA. Yoo MH. Shrimali RK. Irons R. Gladyshev VN. Hatfield DL. Park JM. Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc. 2010;69:300–310. doi: 10.1017/S002966511000176X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caselli A. Marzocchini R. Camici G. Manao G. Moneti G. Pieraccini G. Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 38.Cemerski S. Cantagrel A. Van Meerwijk JP. Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. 2002;277:19585–19593. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- 39.Chang Y. Zhang GZ. Piao SL. Gao S. Zheng DM. Song Y. Tsicopoulos A. Ying S. Protective effects of combined micronutrients on islet beta-cells of streptozotocin-induced diabetic mice. Int J Vitam Nutr Res. 2009;79:104–116. doi: 10.1024/0300-9831.79.2.104. [DOI] [PubMed] [Google Scholar]
- 40.Chen J. Gusdon AM. Thayer TC. Mathews CE. Role of increased ROS dissipation in prevention of T1D. Ann N Y Acad Sci. 2008;1150:157–166. doi: 10.1196/annals.1447.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X. Scholl TO. Leskiw MJ. Donaldson MR. Stein TP. Association of glutathione peroxidase activity with insulin resistance and dietary fat intake during normal pregnancy. J Clin Endocrinol Metab. 2003;88:5963–5968. doi: 10.1210/jc.2003-030544. [DOI] [PubMed] [Google Scholar]
- 42.Collins SR. Meyer T. Calcium flickers lighting the way in chemotaxis? Dev Cell. 2009;16:160–161. doi: 10.1016/j.devcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Combs GF., Jr. Watts JC. Jackson MI. Johnson LK. Zeng H. Scheett AJ. Uthus EO. Schomburg L. Hoeg A. Hoefig CS. Davis CD. Milner JA. Determinants of selenium status in healthy adults. Nutr J. 2011;10:75. doi: 10.1186/1475-2891-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad M. Jakupoglu C. Moreno SG. Lippl S. Banjac A. Schneider M. Beck H. Hatzopoulos AK. Just U. Sinowatz F. Schmahl W. Chien KR. Wurst W. Bornkamm GW. Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conrad M. Sandin A. Forster H. Seiler A. Frijhoff J. Dagnell M. Bornkamm GW. Radmark O. Hooft van Huijsduijnen R. Aspenstrom P. Bohmer F. Ostman A. 12/15-lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2010;107:15774–15779. doi: 10.1073/pnas.1007909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox R. Goorha S. A study of the mechanism of selenite-induced hypomethylated DNA and differentiation of Friend erythroleukemic cells. Carcinogenesis. 1986;7:2015–2018. doi: 10.1093/carcin/7.12.2015. [DOI] [PubMed] [Google Scholar]
- 47.Curran JE. Jowett JB. Elliott KS. Gao Y. Gluschenko K. Wang J. Abel Azim DM. Cai G. Mahaney MC. Comuzzie AG. Dyer TD. Walder KR. Zimmet P. MacCluer JW. Collier GR. Kissebah AH. Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 48.Cyr AR. Domann FE. The redox basis of epigenetic modifications: from mechanisms to functional consequences. Antioxid Redox Signal. 2011;15:551–589. doi: 10.1089/ars.2010.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 50.Davis CD. Uthus EO. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. J Nutr. 2003;133:2907–2914. doi: 10.1093/jn/133.9.2907. [DOI] [PubMed] [Google Scholar]
- 51.Davis CD. Uthus EO. Finley JW. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J Nutr. 2000;130:2903–2909. doi: 10.1093/jn/130.12.2903. [DOI] [PubMed] [Google Scholar]
- 52.de Luis DA. Izaola O. Aller R. Armentia A. Cuellar L. Antioxidant and fat intake in patients with polinic asthma. Med Clin (Barc) 2003;121:653–654. [PubMed] [Google Scholar]
- 53.Delaunay A. Pflieger D. Barrault MB. Vinh J. Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 54.Dennert G. Horneber M. Selenium for alleviating the side effects of chemotherapy, radiotherapy and surgery in cancer patients. Cochrane Database Syst Rev. 2006;3:CD005037. doi: 10.1002/14651858.CD005037.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deshpande RV. Goust JM. Chakrabarti AK. Barbosa E. Hogan EL. Banik NL. Calpain expression in lymphoid cells. Increased mRNA and protein levels after cell activation. J Biol Chem. 1995;270:2497–2505. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- 56.Devereux G. McNeill G. Newman G. Turner S. Craig L. Martindale S. Helms P. Seaton A. Early childhood wheezing symptoms in relation to plasma selenium in pregnant mothers and neonates. Clin Exp Allergy. 2007;37:1000–1008. doi: 10.1111/j.1365-2222.2007.02757.x. [DOI] [PubMed] [Google Scholar]
- 57.Dittrich AM. Meyer HA. Krokowski M. Quarcoo D. Ahrens B. Kube SM. Witzenrath M. Esworthy RS. Chu FF. Hamelmann E. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur Respir J. 2010;35:1148–1154. doi: 10.1183/09031936.00026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dreher I. Jakobs TC. Kohrle J. Cloning and characterization of the human selenoprotein P promoter. Response of selenoprotein P expression to cytokines in liver cells. J Biol Chem. 1997;272:29364–29371. doi: 10.1074/jbc.272.46.29364. [DOI] [PubMed] [Google Scholar]
- 59.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 60.Du S. Zhou J. Jia Y. Huang K. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys. 2010;502:137–143. doi: 10.1016/j.abb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Dunstan JA. Breckler L. Hale J. Lehmann H. Franklin P. Lyons G. Ching SY. Mori TA. Barden A. Prescott SL. Supplementation with vitamins C, E, beta-carotene and selenium has no effect on anti-oxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy. 2007;37:180–187. doi: 10.1111/j.1365-2222.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 62.Duntas LH. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res. 2009;41:443–447. doi: 10.1055/s-0029-1220724. [DOI] [PubMed] [Google Scholar]
- 63.Dworkin BM. Rosenthal WS. Wormser GP. Weiss L. Nunez M. Joline C. Herp A. Abnormalities of blood selenium and glutathione peroxidase activity in patients with acquired immunodeficiency syndrome and AIDS-related complex. Biol Trace Elem Res. 1988;15:167–177. doi: 10.1007/BF02990135. [DOI] [PubMed] [Google Scholar]
- 64.El-Benna J. Dang PM. Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 65.Felmet KA. Hall MW. Clark RS. Jaffe R. Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Boyanapalli R. McPhillips KA. Frasch SC. Janssen WJ. Dinauer MC. Riches DW. Henson PM. Byrne A. Bratton DL. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filippin LI. Vercelino R. Marroni NP. Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008;152:415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finley JW. Bioavailability of selenium from foods. Nutr Rev. 2006;64:146–151. doi: 10.1111/j.1753-4887.2006.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 69.Flatt A. Pearce N. Thomson CD. Sears MR. Robinson MF. Beasley R. Reduced selenium in asthmatic subjects in New Zealand. Thorax. 1990;45:95–99. doi: 10.1136/thx.45.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fomenko DE. Koc A. Agisheva N. Jacobsen M. Kaya A. Malinouski M. Rutherford JC. Siu KL. Jin DY. Winge DR. Gladyshev VN. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc Natl Acad Sci U S A. 2011;108:2729–2734. doi: 10.1073/pnas.1010721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fomenko DE. Novoselov SV. Natarajan SK. Lee BC. Koc A. Carlson BA. Lee TH. Kim HY. Hatfield DL. Gladyshev VN. MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J Biol Chem. 2009;284:5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forceville X. Laviolle B. Annane D. Vitoux D. Bleichner G. Korach JM. Cantais E. Georges H. Soubirou JL. Combes A. Bellissant E. Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care. 2007;11:R73. doi: 10.1186/cc5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forceville X. Mostert V. Pierantoni A. Vitoux D. Le Toumelin P. Plouvier E. Dehoux M. Thuillier F. Combes A. Selenoprotein P, rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur Surg Res. 2009;43:338–347. doi: 10.1159/000239763. [DOI] [PubMed] [Google Scholar]
- 74.Forceville X. Vitoux D. Gauzit R. Combes A. Lahilaire P. Chappuis P. Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med. 1998;26:1536–1544. doi: 10.1097/00003246-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 75.Forman HJ. Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 76.Foyouzi-Youssefi R. Petersson F. Lew DP. Krause KH. Nusse O. Chemoattractant-induced respiratory burst: increases in cytosolic Ca2+ concentrations are essential and synergize with a kinetically distinct second signal. Biochem J. 1997;322(Pt 3):709–718. doi: 10.1042/bj3220709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 78.Gallois A. Bueb JL. Tschirhart E. Effect of SK&F 96365 on extracellular Ca2+-dependent O2- production in neutrophil-like HL-60 cells. Eur J Pharmacol. 1998;361:293–298. doi: 10.1016/s0014-2999(98)00728-6. [DOI] [PubMed] [Google Scholar]
- 79.Gandhi UH. Kaushal N. Ravindra KC. Hegde S. Nelson SM. Narayan V. Vunta H. Paulson RF. Prabhu KS. Selenoprotein-dependent upregulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR){gamma} J Biol Chem. 2011;286:27471–27482. doi: 10.1074/jbc.M111.260547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao Y. Feng HC. Walder K. Bolton K. Sunderland T. Bishara N. Quick M. Kantham L. Collier GR. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress—SelS is a novel glucose-regulated protein. FEBS Lett. 2004;563:185–190. doi: 10.1016/S0014-5793(04)00296-0. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Larsen V. Chinn S. Arts IC. Amigo H. Rona RJ. Atopy, wheeze and bronchial responsiveness in young Chilean adults. Do dietary antioxidants matter? Allergy. 2007;62:714–715. doi: 10.1111/j.1398-9995.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 82.Gazdik F. Kadrabova J. Gazdikova K. Decreased consumption of corticosteroids after selenium supplementation in corticoid-dependent asthmatics. Bratisl Lek Listy. 2002;103:22–25. [PubMed] [Google Scholar]
- 83.Ge W. Hu PZ. Huang Y. Wang XM. Zhang XM. Sun YJ. Li ZS. Si SY. Sui YF. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following different administration routes. Oncol Rep. 2009;22:915–920. doi: 10.3892/or_00000517. [DOI] [PubMed] [Google Scholar]
- 84.Geerling BJ. Badart-Smook A. Stockbrugger RW. Brummer RJ. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514–521. doi: 10.1038/sj.ejcn.1601049. [DOI] [PubMed] [Google Scholar]
- 85.Ghassabeh GH. De Baetselier P. Brys L. Noel W. Van Ginderachter JA. Meerschaut S. Beschin A. Brombacher F. Raes G. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–583. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- 86.Gilgun-Sherki Y. Melamed E. Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 87.Gladyshev VN. Stadtman TC. Hatfield DL. Jeang KT. Levels of major selenoproteins in T cells decrease during HIV infection and low molecular mass selenium compounds increase. Proc Natl Acad Sci U S A. 1999;96:835–839. doi: 10.1073/pnas.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldson AJ. Fairweather-Tait SJ. Armah CN. Bao Y. Broadley MR. Dainty JR. Furniss C. Hart DJ. Teucher B. Hurst R. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: a randomised controlled trial. PLoS One. 2011;6:e14771. doi: 10.1371/journal.pone.0014771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green DR. Droin N. Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 90.Groux H. Sornasse T. Cottrez F. de Vries JE. Coffman RL. Roncarolo MG. Yssel H. Induction of human T helper cell type 1 differentiation results in loss of IFN-gamma receptor beta-chain expression. J Immunol. 1997;158:5627–5631. [PubMed] [Google Scholar]
- 91.Gutscher M. Sobotta MC. Wabnitz GH. Ballikaya S. Meyer AJ. Samstag Y. Dick TP. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasselmark L. Malmgren R. Unge G. Zetterstrom O. Lowered platelet glutathione peroxidase activity in patients with intrinsic asthma. Allergy. 1990;45:523–527. doi: 10.1111/j.1398-9995.1990.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 93.Hatfield DL. Gladyshev VN. The outcome of selenium and vitamin E cancer prevention trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9:18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hattori H. Imai H. Furuhama K. Sato O. Nakagawa Y. Induction of phospholipid hydroperoxide glutathione peroxidase in human polymorphonuclear neutrophils and HL60 cells stimulated with TNF-alpha. Biochem Biophys Res Commun. 2005;337:464–473. doi: 10.1016/j.bbrc.2005.09.076. [DOI] [PubMed] [Google Scholar]
- 95.Hawkes WC. Kelley DS. Taylor PC. The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res. 2001;81:189–213. doi: 10.1385/BTER:81:3:189. [DOI] [PubMed] [Google Scholar]
- 96.Hendry L. John S. Regulation of STAT signalling by proteolytic processing. Eur J Biochem. 2004;271:4613–4620. doi: 10.1111/j.1432-1033.2004.04424.x. [DOI] [PubMed] [Google Scholar]
- 97.Heyland DK. Selenium supplementation in critically ill patients: can too much of a good thing be a bad thing? Crit Care. 2007;11:153. doi: 10.1186/cc5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heyworth PG. Cross AR. Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 99.Heyworth PG. Knaus UG. Settleman J. Curnutte JT. Bokoch GM. Regulation of NADPH oxidase activity by Rac GTPase activating protein(s) Mol Biol Cell. 1993;4:1217–1223. doi: 10.1091/mbc.4.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Higgins LM. Frankel G. Douce G. Dougan G. MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hill KE. Zhou J. McMahan WJ. Motley AK. Atkins JF. Gesteland RF. Burk RF. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;278:13640–13646. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 102.Hoffmann FW. Hashimoto AC. Shafer LA. Dow S. Berry MJ. Hoffmann PR. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140:1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoffmann PR. Mechanisms by which selenium influences immune responses. Arch Immunol Ther Exp (Warsz) 2007;55:289–297. doi: 10.1007/s00005-007-0036-4. [DOI] [PubMed] [Google Scholar]
- 104.Hoffmann PR. Hoge SC. Li PA. Hoffmann FW. Hashimoto AC. Berry MJ. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007;35:3963–3973. doi: 10.1093/nar/gkm355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffmann PR. Jourdan-Le Saux C. Hoffmann FW. Chang PS. Bollt O. He Q. Tam EK. Berry MJ. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007;179:3258–3267. doi: 10.4049/jimmunol.179.5.3258. [DOI] [PubMed] [Google Scholar]
- 106.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 107.Hollenbach B. Morgenthaler NG. Struck J. Alonso C. Bergmann A. Kohrle J. Schomburg L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J Trace Elem Med Biol. 2008;22:24–32. doi: 10.1016/j.jtemb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 109.Hori K. Hatfield D. Maldarelli F. Lee BJ. Clouse KA. Selenium supplementation suppresses tumor necrosis factor alpha-induced human immunodeficiency virus type 1 replication in vitro. AIDS Res Hum Retroviruses. 1997;13:1325–1332. doi: 10.1089/aid.1997.13.1325. [DOI] [PubMed] [Google Scholar]
- 110.Huang Z. Hoffmann FW. Norton RL. Hashimoto AC. Hoffmann PR. Selenoprotein K is a novel target of m-calpain and cleavage is regulated by Toll-like receptor-induced calpastatin in macrophages. J Biol Chem. 2011;286:34830–34838. doi: 10.1074/jbc.M111.265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hultqvist M. Olofsson P. Holmberg J. Backstrom BT. Tordsson J. Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hultqvist M. Olsson LM. Gelderman KA. Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009;30:201–208. doi: 10.1016/j.it.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Ip C. Thompson HJ. Zhu Z. Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 114.Ishigame H. Kakuta S. Nagai T. Kadoki M. Nambu A. Komiyama Y. Fujikado N. Tanahashi Y. Akitsu A. Kotaki H. Sudo K. Nakae S. Sasakawa C. Iwakura Y. Differential roles of interleukin-17A and −17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 115.Jackson SH. Devadas S. Kwon J. Pinto LA. Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 116.Jahnova E. Horvathova M. Gazdik F. Weissova S. Effects of selenium supplementation on expression of adhesion molecules in corticoid-dependent asthmatics. Bratisl Lek Listy. 2002;103:12–16. [PubMed] [Google Scholar]
- 117.Jakupoglu C. Przemeck GK. Schneider M. Moreno SG. Mayr N. Hatzopoulos AK. de Angelis MH. Wurst W. Bornkamm GW. Brielmeier M. Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson F. Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 119.Jun EJ. Ye JS. Hwang IS. Kim YK. Lee H. Selenium deficiency contributes to the chronic myocarditis in coxsackievirus-infected mice. Acta Virol. 2011;55:23–29. doi: 10.4149/av_2011_01_23. [DOI] [PubMed] [Google Scholar]
- 120.Kadrabova J. Mad'aric A. Kovacikova Z. Podivinsky F. Ginter E. Gazdik F. Selenium status is decreased in patients with intrinsic asthma. Biol Trace Elem Res. 1996;52:241–248. doi: 10.1007/BF02789165. [DOI] [PubMed] [Google Scholar]
- 121.Kalantari P. Narayan V. Natarajan SK. Muralidhar K. Gandhi UH. Vunta H. Henderson AJ. Prabhu KS. Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J Biol Chem. 2008;283:33183–33190. doi: 10.1074/jbc.M807403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaminski M. Kiessling M. Suss D. Krammer PH. Gulow K. Novel role for mitochondria: protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol Cell Biol. 2007;27:3625–3639. doi: 10.1128/MCB.02295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karachevtsev AN. Medik VA. Elaboration and realization of a complex program of regional health services development. Sov Zdravookhr. 1990:57–62. [PubMed] [Google Scholar]
- 124.Kasaikina MV. Kravtsova MA. Lee BC. Seravalli J. Peterson DA. Walter J. Legge R. Benson AK. Hatfield DL. Gladyshev VN. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011;25:2492–2499. doi: 10.1096/fj.11-181990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kassam S. Goenaga-Infante H. Maharaj L. Hiley CT. Juliger S. Joel SP. Methylseleninic acid inhibits HDAC activity in diffuse large B-cell lymphoma cell lines. Cancer Chemother Pharmacol. 2011;68:815–821. doi: 10.1007/s00280-011-1649-1. [DOI] [PubMed] [Google Scholar]
- 126.Kasseroller RG. Schrauzer GN. Treatment of secondary lymphedema of the arm with physical decongestive therapy and sodium selenite: a review. Am J Ther. 2000;7:273–279. doi: 10.1097/00045391-200007040-00008. [DOI] [PubMed] [Google Scholar]
- 127.Kim IY. Stadtman TC. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc Natl Acad Sci U S A. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim KH. Gao Y. Walder K. Collier GR. Skelton J. Kissebah AH. SEPS1 protects RAW264.7 cells from pharmacological ER stress agent-induced apoptosis. Biochem Biophys Res Commun. 2007;354:127–132. doi: 10.1016/j.bbrc.2006.12.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kipp A. Banning A. van Schothorst EM. Meplan C. Schomburg L. Evelo C. Coort S. Gaj S. Keijer J. Hesketh J. Brigelius-Flohe R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res. 2009;53:1561–1572. doi: 10.1002/mnfr.200900105. [DOI] [PubMed] [Google Scholar]
- 130.Kocyigit A. Armutcu F. Gurel A. Ermis B. Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biol Trace Elem Res. 2004;97:31–41. doi: 10.1385/BTER:97:1:31. [DOI] [PubMed] [Google Scholar]
- 131.Koyama H. Omura K. Ejima A. Kasanuma Y. Watanabe C. Satoh H. Separation of selenium-containing proteins in human and mouse plasma using tandem high-performance liquid chromatography columns coupled with inductively coupled plasma-mass spectrometry. Anal Biochem. 1999;267:84–91. doi: 10.1006/abio.1998.2949. [DOI] [PubMed] [Google Scholar]
- 132.Kretz-Remy C. Arrigo AP. Selenium: a key element that controls NF-kappa B activation and I kappa B alpha half life. Biofactors. 2001;14:117–125. doi: 10.1002/biof.5520140116. [DOI] [PubMed] [Google Scholar]
- 133.Kruidenier L. Kuiper I. Lamers CB. Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 134.Kryukov GV. Castellano S. Novoselov SV. Lobanov AV. Zehtab O. Guigo R. Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 135.Kuppusamy P. Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264:9880–9884. [PubMed] [Google Scholar]
- 136.Kwon J. Devadas S. Williams MS. T cell receptor-stimulated generation of hydrogen peroxide inhibits MEK-ERK activation and lck serine phosphorylation. Free Radic Biol Med. 2003;35:406–417. doi: 10.1016/s0891-5849(03)00318-6. [DOI] [PubMed] [Google Scholar]
- 137.Kwon J. Qu CK. Maeng JS. Falahati R. Lee C. Williams MS. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 2005;24:2331–2341. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kwon J. Shatynski KE. Chen H. Morand S. de Deken X. Miot F. Leto TL. Williams MS. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci Signal. 2010;3:ra59. doi: 10.1126/scisignal.2000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Labunskyy VM. Lee BC. Handy DE. Loscalzo J. Hatfield DL. Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14:2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Labunskyy VM. Yoo MH. Hatfield DL. Gladyshev VN. Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry. 2009;48:8458–8465. doi: 10.1021/bi900717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Laclaustra M. Navas-Acien A. Stranges S. Ordovas JM. Guallar E. Serum selenium concentrations and hypertension in the US Population. Circ Cardiovasc Qual Outcomes. 2009;2:369–376. doi: 10.1161/CIRCOUTCOMES.108.831552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lesourd BM. Meaume S. Cell mediated immunity changes in ageing, relative importance of cell subpopulation switches and of nutritional factors. Immunol Lett. 1994;40:235–242. doi: 10.1016/0165-2478(94)00062-x. [DOI] [PubMed] [Google Scholar]
- 143.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 144.Lichtman AH. Chin J. Schmidt JA. Abbas AK. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci U S A. 1988;85:9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lippman SM. Klein EA. Goodman PJ. Lucia MS. Thompson IM. Ford LG. Parnes HL. Minasian LM. Gaziano JM. Hartline JA. Parsons JK. Bearden JD., 3rd Crawford ED. Goodman GE. Claudio J. Winquist E. Cook ED. Karp DD. Walther P. Lieber MM. Kristal AR. Darke AK. Arnold KB. Ganz PA. Santella RM. Albanes D. Taylor PR. Probstfield JL. Jagpal TJ. Crowley JJ. Meyskens FL., Jr. Baker LH. Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu J. Berndt C. Holmgren A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim Biophys Acta. 2009;1790:1513–1519. doi: 10.1016/j.bbagen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 148.Luik RM. Wu MM. Buchanan J. Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lymbury R. Tinggi U. Griffiths L. Rosenfeldt F. Perkins AV. Selenium status of the Australian population: effect of age, gender and cardiovascular disease. Biol Trace Elem Res. 2008;126(Suppl 1):S1–S10. doi: 10.1007/s12011-008-8208-6. [DOI] [PubMed] [Google Scholar]
- 150.MacFarquhar JK. Broussard DL. Melstrom P. Hutchinson R. Wolkin A. Martin C. Burk RF. Dunn JR. Green AL. Hammond R. Schaffner W. Jones TF. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med. 2010;170:256–261. doi: 10.1001/archinternmed.2009.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maddox L. Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- 152.Maehira F. Luyo GA. Miyagi I. Oshiro M. Yamane N. Kuba M. Nakazato Y. Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin Chim Acta. 2002;316:137–146. doi: 10.1016/s0009-8981(01)00744-6. [DOI] [PubMed] [Google Scholar]
- 153.Maehira F. Miyagi I. Eguchi Y. Selenium regulates transcription factor NF-kappaB activation during the acute phase reaction. Clin Chim Acta. 2003;334:163–171. doi: 10.1016/s0009-8981(03)00223-7. [DOI] [PubMed] [Google Scholar]
- 154.Manzanares W. Biestro A. Galusso F. Torre MH. Manay N. Pittini G. Facchin G. Hardy G. Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 2009;35:882–889. doi: 10.1007/s00134-008-1356-5. [DOI] [PubMed] [Google Scholar]
- 155.Marko MG. Ahmed T. Bunnell SC. Wu D. Chung H. Huber BT. Meydani SN. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J Immunol. 2007;178:1443–1449. doi: 10.4049/jimmunol.178.3.1443. [DOI] [PubMed] [Google Scholar]
- 156.Matsue H. Edelbaum D. Shalhevet D. Mizumoto N. Yang C. Mummert ME. Oeda J. Masayasu H. Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 157.Matthews JR. Wakasugi N. Virelizier JL. Yodoi J. Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mazari L. Lesourd BM. Nutritional influences on immune response in healthy aged persons. Mech Ageing Dev. 1998;104:25–40. doi: 10.1016/s0047-6374(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 159.McCann JC. Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011;25:1793–1814. doi: 10.1096/fj.11-180885. [DOI] [PubMed] [Google Scholar]
- 160.This reference has been deleted
- 161.McClung JP. Roneker CA. Mu W. Lisk DJ. Langlais P. Liu F. Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McDonald PP. Fadok VA. Bratton D. Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 163.McNeill JH. Delgatty HL. Battell ML. Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes. 1991;40:1675–1678. doi: 10.2337/diab.40.12.1675. [DOI] [PubMed] [Google Scholar]
- 164.Meyer HA. Dittrich AM. Hamelmann E. Different isoforms of glutathione peroxidase cause opposing effects during the development of allergic asthma in mice. Antioxid Redox Signal. 2010;14:169–170. doi: 10.1089/ars.2010.3591. [DOI] [PubMed] [Google Scholar]
- 165.Michalke B. Selenium speciation in human serum of cystic fibrosis patients compared to serum from healthy persons. J Chromatogr A. 2004;1058:203–208. [PubMed] [Google Scholar]
- 166.Micke O. Bruns F. Mucke R. Schafer U. Glatzel M. DeVries AF. Schonekaes K. Kisters K. Buntzel J. Selenium in the treatment of radiation-associated secondary lymphedema. Int J Radiat Oncol Biol Phys. 2003;56:40–49. doi: 10.1016/s0360-3016(02)04390-0. [DOI] [PubMed] [Google Scholar]
- 167.Miller AL. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern Med Rev. 2001;6:20–47. [PubMed] [Google Scholar]
- 168.Miroliaee AE. Esmaily H. Vaziri-Bami A. Baeeri M. Shahverdi AR. Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods. 2011;21:200–208. doi: 10.3109/15376516.2010.547887. [DOI] [PubMed] [Google Scholar]
- 169.Mishra V. Baines M. Perry SE. McLaughlin PJ. Carson J. Wenstone R. Shenkin A. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr. 2007;26:41–50. doi: 10.1016/j.clnu.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 170.Misso NL. Powers KA. Gillon RL. Stewart GA. Thompson PJ. Reduced platelet glutathione peroxidase activity and serum selenium concentration in atopic asthmatic patients. Clin Exp Allergy. 1996;26:838–847. [PubMed] [Google Scholar]
- 171.Misu H. Takamura T. Takayama H. Hayashi H. Matsuzawa-Nagata N. Kurita S. Ishikura K. Ando H. Takeshita Y. Ota T. Sakurai M. Yamashita T. Mizukoshi E. Honda M. Miyamoto K. Kubota T. Kubota N. Kadowaki T. Kim HJ. Lee IK. Minokoshi Y. Saito Y. Takahashi K. Yamada Y. Takakura N. Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2011;12:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 172.Mittag J. Behrends T. Hoefig CS. Vennstrom B. Schomburg L. Thyroid hormones regulate selenoprotein expression and selenium status in mice. PLoS One. 5:e12931. doi: 10.1371/journal.pone.0012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moldoveanu T. Hosfield CM. Lim D. Elce JS. Jia Z. Davies PL. A Ca(2+) switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 174.Moskovitz J. Stadtman ER. Selenium-deficient diet enhances protein oxidation and affects methionine sulfoxide reductase (MsrB) protein level in certain mouse tissues. Proc Natl Acad Sci U S A. 2003;100:7486–7490. doi: 10.1073/pnas.1332607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mougiakakos D. Johansson CC. Jitschin R. Bottcher M. Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 176.Mougiakakos D. Johansson CC. Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113:3542–3545. doi: 10.1182/blood-2008-09-181040. [DOI] [PubMed] [Google Scholar]
- 177.Muller D. Desel H. Acute selenium poisoning by paradise nuts (Lecythis ollaria) Hum Exp Toxicol. 2010;29:431–434. doi: 10.1177/0960327109360046. [DOI] [PubMed] [Google Scholar]
- 178.Muller M. Banning A. Brigelius-Flohe R. Kipp A. Nrf2 target genes are induced under marginal selenium-deficiency. Genes Nutr. 2010;5:297–307. doi: 10.1007/s12263-010-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Murawaki Y. Tsuchiya H. Kanbe T. Harada K. Yashima K. Nozaka K. Tanida O. Kohno M. Mukoyama T. Nishimuki E. Kojo H. Matsura T. Takahashi K. Osaki M. Ito H. Yodoi J. Shiota G. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259:218–230. doi: 10.1016/j.canlet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 180.Murphy KM. Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 181.Murray JM. Murray AB. The Effects of Selenium Deficiency and Repletion on Host Resistance to Infection. Cambridge: Commonwealth on Agricultural Bureaux; 1985. pp. 244–247. [Google Scholar]
- 182.Nauseef WM. Nox enzymes in immune cells. Semin Immunopathol. 2008;30:195–208. doi: 10.1007/s00281-008-0117-4. [DOI] [PubMed] [Google Scholar]
- 183.Navas-Acien A. Bleys J. Guallar E. Selenium intake and cardiovascular risk: what is new? Curr Opin Lipidol. 2008;19:43–49. doi: 10.1097/MOL.0b013e3282f2b261. [DOI] [PubMed] [Google Scholar]
- 184.Neel BG. Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 185.Nimmerjahn F. Bruhns P. Horiuchi K. Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 186.Nimmerjahn F. Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 187.Nimmerjahn F. Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 188.Nunes P. Demaurex N. The role of calcium signaling in phagocytosis. J Leukoc Biol. 88:57–68. doi: 10.1189/jlb.0110028. [DOI] [PubMed] [Google Scholar]
- 189.Olofsson P. Holmberg J. Tordsson J. Lu S. Akerstrom B. Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- 190.Omland O. Deguchi Y. Sigsgaard T. Hansen JC. Selenium serum and urine is associated to mild asthma and atopy. The SUS study. J Trace Elem Med Biol. 2002;16:123–127. doi: 10.1016/S0946-672X(02)80039-6. [DOI] [PubMed] [Google Scholar]
- 191.Osterud B. Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–1112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 192.Pagmantidis V. Meplan C. van Schothorst EM. Keijer J. Hesketh JE. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am J Clin Nutr. 2008;87:181–189. doi: 10.1093/ajcn/87.1.181. [DOI] [PubMed] [Google Scholar]
- 193.Park CS. Kim TB. Lee KY. Moon KA. Bae YJ. Jang MK. Cho YS. Moon HB. Increased oxidative stress in the airway and development of allergic inflammation in a mouse model of asthma. Ann Allergy Asthma Immunol. 2009;103:238–247. doi: 10.1016/S1081-1206(10)60188-3. [DOI] [PubMed] [Google Scholar]
- 194.Pemberton PW. Ahmad Y. Bodill H. Lokko D. Hider SL. Yates AP. Walker MG. Laing I. Bruce IN. Biomarkers of oxidant stress, insulin sensitivity and endothelial activation in rheumatoid arthritis: a cross-sectional study of their association with accelerated atherosclerosis. BMC Res Notes. 2009;2:83. doi: 10.1186/1756-0500-2-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Perrone L. Devi TS. Hosoya K. Terasaki T. Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 196.Peterson JD. Herzenberg LA. Vasquez K. Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Placek K. Coffre M. Maiella S. Bianchi E. Rogge L. Genetic and epigenetic networks controlling T helper 1 cell differentiation. Immunology. 2009;127:155–162. doi: 10.1111/j.1365-2567.2009.03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pozzan T. Lew DP. Wollheim CB. Tsien RY. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983;221:1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- 199.Prabhu KS. Zamamiri-Davis F. Stewart JB. Thompson JT. Sordillo LM. Reddy CC. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-kappaB in up-regulation. Biochem J. 2002;366:203–209. doi: 10.1042/BJ20020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 201.Putney JW., Jr. Broad LM. Braun FJ. Lievremont JP. Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 202.Qujeq D. Hidari B. Bijani K. Shirdel H. Glutathione peroxidase activity and serum selenium concentration in intrinsic asthmatic patients. Clin Chem Lab Med. 2003;41:200–202. doi: 10.1515/CCLM.2003.032. [DOI] [PubMed] [Google Scholar]
- 203.Renko K. Hofmann PJ. Stoedter M. Hollenbach B. Behrends T. Kohrle J. Schweizer U. Schomburg L. Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J. 2009;23:1758–1765. doi: 10.1096/fj.08-119370. [DOI] [PubMed] [Google Scholar]
- 204.Riedl MA. Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 205.Rinaldi S. Landucci F. De Gaudio AR. Antioxidant therapy in critically septic patients. Curr Drug Targets. 2009;10:872–880. doi: 10.2174/138945009789108774. [DOI] [PubMed] [Google Scholar]
- 206.Ringstad J. Kildebo S. Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1993;28:605–608. doi: 10.3109/00365529309096096. [DOI] [PubMed] [Google Scholar]
- 207.Rotrosen D. Yeung CL. Leto TL. Malech HL. Kwong CH. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992;256:1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- 208.Rotruck JT. Pope AL. Ganther HE. Swanson AB. Hafeman DG. Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 209.Rousseau MC. Molines C. Moreau J. Delmont J. Influence of highly active antiretroviral therapy on micronutrient profiles in HIV-infected patients. Ann Nutr Metab. 2000;44:212–216. doi: 10.1159/000046686. [DOI] [PubMed] [Google Scholar]
- 210.Roy M. Kiremidjian-Schumacher L. Wishe HI. Cohen MW. Stotzky G. Selenium supplementation enhances the expression of interleukin 2 receptor subunits and internalization of interleukin 2. Proc Soc Exp Biol Med. 1993;202:295–301. doi: 10.3181/00379727-202-43538. [DOI] [PubMed] [Google Scholar]
- 211.Safir N. Wendel A. Saile R. Chabraoui L. The effect of selenium on immune functions of J774.1 cells. Clin Chem Lab Med. 2003;41:1005–1011. doi: 10.1515/CCLM.2003.154. [DOI] [PubMed] [Google Scholar]
- 212.Saito Y. Takahashi K. Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem. 2002;269:5746–5751. doi: 10.1046/j.1432-1033.2002.03298.x. [DOI] [PubMed] [Google Scholar]
- 213.Sakaguchi S. Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 214.Sakr Y. Reinhart K. Bloos F. Marx G. Russwurm S. Bauer M. Brunkhorst F. Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br J Anaesth. 2007;98:775–784. doi: 10.1093/bja/aem091. [DOI] [PubMed] [Google Scholar]
- 215.Samanta K. Kar P. Chakraborti T. Shaikh S. Chakraborti S. Characteristic properties of endoplasmic reticulum membrane m-calpain, calpastatin and lumen m-calpain: a comparative study between membrane and lumen m-calpains. J Biochem. 2010;147:765–779. doi: 10.1093/jb/mvq009. [DOI] [PubMed] [Google Scholar]
- 216.Samanta K. Kar P. Ghosh B. Chakraborti T. Chakraborti S. Localization of m-calpain and calpastatin and studies of their association in pulmonary smooth muscle endoplasmic reticulum. Biochim Biophys Acta. 2007;1770:1297–1307. doi: 10.1016/j.bbagen.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 217.Schappi MG. Jaquet V. Belli DC. Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol. 2008;30:255–271. doi: 10.1007/s00281-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 218.Schoenmakers E. Agostini M. Mitchell C. Schoenmakers N. Papp L. Rajanayagam O. Padidela R. Ceron-Gutierrez L. Doffinger R. Prevosto C. Luan J. Montano S. Lu J. Castanet M. Clemons N. Groeneveld M. Castets P. Karbaschi M. Aitken S. Dixon A. Williams J. Campi I. Blount M. Burton H. Muntoni F. O'Donovan D. Dean A. Warren A. Brierley C. Baguley D. Guicheney P. Fitzgerald R. Coles A. Gaston H. Todd P. Holmgren A. Khanna KK. Cooke M. Semple R. Halsall D. Wareham N. Schwabe J. Grasso L. Beck-Peccoz P. Ogunko A. Dattani M. Gurnell M. Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120:4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schomburg L. Kohrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008;52:1235–1246. doi: 10.1002/mnfr.200700465. [DOI] [PubMed] [Google Scholar]
- 220.Schomburg L. Riese C. Renko K. Schweizer U. Effect of age on sexually dimorphic selenoprotein expression in mice. Biol Chem. 2007;388:1035–1041. doi: 10.1515/BC.2007.128. [DOI] [PubMed] [Google Scholar]
- 221.Schomburg L. Schweizer U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim Biophys Acta. 2009;1790:1453–1462. doi: 10.1016/j.bbagen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 222.Schweizer U. Streckfuss F. Pelt P. Carlson BA. Hatfield DL. Kohrle J. Schomburg L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem J. 2005;386:221–226. doi: 10.1042/BJ20041973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Seaver LC. Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Seiler WO. Clinical pictures of malnutrition in ill elderly subjects. Nutrition. 2001;17:496–498. doi: 10.1016/s0899-9007(01)00558-5. [DOI] [PubMed] [Google Scholar]
- 225.Shaheen SO. Newson RB. Henderson AJ. Emmett PM. Sherriff A. Cooke M. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J. 2004;24:292–297. doi: 10.1183/09031936.04.00117803. [DOI] [PubMed] [Google Scholar]
- 226.Shaheen SO. Newson RB. Rayman MP. Wong AP. Tumilty MK. Phillips JM. Potts JF. Kelly FJ. White PT. Burney PG. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 2007;62:483–490. doi: 10.1136/thx.2006.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shamseer L. Adams D. Brown N. Johnson JA. Vohra S. Antioxidant micronutrients for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2010;12:CD007020. doi: 10.1002/14651858.CD007020.pub2. [DOI] [PubMed] [Google Scholar]
- 228.Shaw R. Woodman K. Crane J. Moyes C. Kennedy J. Pearce N. Risk factors for asthma symptoms in Kawerau children. N Z Med J. 1994;107:387–391. [PubMed] [Google Scholar]
- 229.Shimohashi N. Nakamuta M. Uchimura K. Sugimoto R. Iwamoto H. Enjoji M. Nawata H. Selenoorganic compound, ebselen, inhibits nitric oxide and tumor necrosis factor-alpha production by the modulation of jun-N-terminal kinase and the NF-kappab signaling pathway in rat Kupffer cells. J Cell Biochem. 2000;78:595–606. [PubMed] [Google Scholar]
- 230.Shrimali RK. Irons RD. Carlson BA. Sano Y. Gladyshev VN. Park JM. Hatfield DL. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J Biol Chem. 2008;283:20181–20185. doi: 10.1074/jbc.M802559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sikora SK. Spady D. Prosser C. El-Matary W. Trace elements and vitamins at diagnosis in pediatric-onset inflammatory bowel disease. Clin Pediatr (Phila) 2011;50:488–492. doi: 10.1177/0009922810397041. [DOI] [PubMed] [Google Scholar]
- 232.Smith A. Madden KB. Yeung KJ. Zhao A. Elfrey J. Finkelman F. Levander O. Shea-Donohue T. Urban JF., Jr Deficiencies in selenium and/or vitamin E lower the resistance of mice to Heligmosomoides polygyrus infections. J Nutr. 2005;135:830–836. doi: 10.1093/jn/135.4.830. [DOI] [PubMed] [Google Scholar]
- 233.Smith AD. Botero S. Shea-Donohue T. Urban JF., Jr The pathogenicity of an enteric Citrobacter rodentium infection is enhanced by deficiencies in the antioxidants selenium and vitamin E. Infect Immun. 2011;79:1471–1478. doi: 10.1128/IAI.01017-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Smith BG. Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18:153–158. doi: 10.1097/MOO.0b013e32833aac21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Smith WL. DeWitt DL. Allen ML. Bimodal distribution of the prostaglandin I2 synthase antigen in smooth muscle cells. J Biol Chem. 1983;258:5922–5926. [PubMed] [Google Scholar]
- 236.Sousa-Lopes A. Antunes F. Cyrne L. Marinho HS. Decreased cellular permeability to H2O2 protects Saccharomyces cerevisiae cells in stationary phase against oxidative stress. FEBS Lett. 2004;578:152–156. doi: 10.1016/j.febslet.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 237.Speckmann B. Pinto A. Winter M. Forster I. Sies H. Steinbrenner H. Proinflammatory cytokines down-regulate intestinal selenoprotein P biosynthesis via NOS2 induction. Free Radic Biol Med. 2010;49:777–785. doi: 10.1016/j.freeradbiomed.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 238.Spiller HA. Pfiefer E. Two fatal cases of selenium toxicity. Forensic Sci Int. 2007;171:67–72. doi: 10.1016/j.forsciint.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 239.Squires JE. Berry MJ. Eukaryotic selenoprotein synthesis: mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life. 2008;60:232–235. doi: 10.1002/iub.38. [DOI] [PubMed] [Google Scholar]
- 240.Squires JE. Stoytchev I. Forry EP. Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol Cell Biol. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Stachowska E. Dolegowska B. Dziedziejko V. Rybicka M. Kaczmarczyk M. Bober J. Rac M. Machalinski B. Chlubek D. Prostaglandin E2 (PGE2) and thromboxane A2 (TXA2) synthesis is regulated by conjugated linoleic acids (CLA) in human macrophages. J Physiol Pharmacol. 2009;60:77–85. [PubMed] [Google Scholar]
- 242.Stockinger B. Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 243.Stoedter M. Renko K. Hog A. Schomburg L. Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem J. 2010;429:43–51. doi: 10.1042/BJ20091868. [DOI] [PubMed] [Google Scholar]
- 244.Stone J. Hinks LJ. Beasley R. Holgate ST. Clayton BA. Reduced selenium status of patients with asthma. Clin Sci (Lond) 1989;77:495–500. doi: 10.1042/cs0770495. [DOI] [PubMed] [Google Scholar]
- 245.Stranges S. Navas-Acien A. Rayman MP. Guallar E. Selenium status and cardiometabolic health: state of the evidence. Nutr Metab Cardiovasc Dis. 2010;20:754–760. doi: 10.1016/j.numecd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 246.Strobl S. Fernandez-Catalan C. Braun M. Huber R. Masumoto H. Nakagawa K. Irie A. Sorimachi H. Bourenkow G. Bartunik H. Suzuki K. Bode W. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc Natl Acad Sci U S A. 2000;97:588–592. doi: 10.1073/pnas.97.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sutherland A. Kim DH. Relton C. Ahn YO. Hesketh J. Polymorphisms in the selenoprotein S and 15-kDa selenoprotein genes are associated with altered susceptibility to colorectal cancer. Genes Nutr. 2010;5:215–223. doi: 10.1007/s12263-010-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Svensson L. McDowall A. Giles KM. Stanley P. Feske S. Hogg N. Calpain 2 controls turnover of LFA-1 adhesions on migrating T lymphocytes. PLoS One. 2010;5:e15090. doi: 10.1371/journal.pone.0015090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tirosh O. Levy E. Reifen R. High selenium diet protects against TNBS-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition. 2007;23:878–886. doi: 10.1016/j.nut.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 250.Toulis KA. Anastasilakis AD. Tzellos TG. Goulis DG. Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. 2010;20:1163–1173. doi: 10.1089/thy.2009.0351. [DOI] [PubMed] [Google Scholar]
- 251.Turanov AA. Kehr S. Marino SM. Yoo MH. Carlson BA. Hatfield D. Gladyshev VN. Mammalian thioredoxin reductase 1: roles in redox homeostasis and charcterization of cellular targets. Biochem J. 2010;430:285–293. doi: 10.1042/BJ20091378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Valenta J. Brodska H. Drabek T. Hendl J. Kazda A. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med. 2011;37:808–815. doi: 10.1007/s00134-011-2153-0. [DOI] [PubMed] [Google Scholar]
- 253.Veal EA. Day AM. Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 254.Veal EA. Findlay VJ. Day AM. Bozonet SM. Evans JM. Quinn J. Morgan BA. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 255.Vega L. Rodriguez-Sosa M. Garcia-Montalvo EA. Del Razo LM. Elizondo G. Non-optimal levels of dietary selenomethionine alter splenocyte response and modify oxidative stress markers in female mice. Food Chem Toxicol. 2007;45:1147–1153. doi: 10.1016/j.fct.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 256.Verma S. Hoffmann FW. Kumar M. Huang Z. Roe K. Nguyen-Wu E. Hashimoto AS. Hoffmann PR. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 186. 2011;186:2127–2137. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Vincent JL. Forceville X. Critically elucidating the role of selenium. Curr Opin Anaesthesiol. 2008;21:148–154. doi: 10.1097/ACO.0b013e3282f49afe. [DOI] [PubMed] [Google Scholar]
- 259.Vunta H. Davis F. Palempalli UD. Bhat D. Arner RJ. Thompson JT. Peterson DG. Reddy CC. Prabhu KS. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J Biol Chem. 2007;282:17964–17973. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- 260.Wallach JD. Garmaise B. Cystic fibrosis—a perinatal manifestation of selenium deficiency. In: Hemphill DD, editor. Trace Substances in Environmental Health. Columbia, MO: University of Missouri Press; 1979. pp. 469–476. [Google Scholar]
- 261.Wallach JD. Lan M. Yu WH. Gu BQ. Yu FT. Goddard RF. Common denominators in the etiology and pathology of visceral lesions of cystic fibrosis and Keshan disease. Biol Trace Elem Res. 1990;24:189–205. doi: 10.1007/BF02917207. [DOI] [PubMed] [Google Scholar]
- 262.Wang C. Wang H. Luo J. Hu Y. Wei L. Duan M. He H. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol. 2009;10:55. doi: 10.1186/1471-2172-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang Z. Forceville X. Van Antwerpen P. Piagnerelli M. Ahishakiye D. Macours P. De Backer D. Neve J. Vincent JL. A large-bolus injection, but not continuous infusion of sodium selenite improves outcome in peritonitis. Shock. 2009;32:140–146. doi: 10.1097/SHK.0b013e318193c35d. [DOI] [PubMed] [Google Scholar]
- 264.Wardwell L. Chapman-Novakofski K. Herrel S. Woods J. Nutrient intake and immune function of elderly subjects. J Am Diet Assoc. 2008;108:2005–2012. doi: 10.1016/j.jada.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wasson GR. McGlynn AP. McNulty H. O'Reilly SL. McKelvey-Martin VJ. McKerr G. Strain JJ. Scott J. Downes CS. Global DNA and p53 region-specific hypomethylation in human colonic cells is induced by folate depletion and reversed by folate supplementation. J Nutr. 2006;136:2748–2753. doi: 10.1093/jn/136.11.2748. [DOI] [PubMed] [Google Scholar]
- 266.Waterman PM. Cambier JC. The conundrum of inhibitory signaling by ITAM-containing immunoreceptors: potential molecular mechanisms. FEBS Lett. 2010;584:4878–4882. doi: 10.1016/j.febslet.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Werz O. Szellas D. Steinhilber D. Reactive oxygen species released from granulocytes stimulate 5-lipoxygenase activity in a B-lymphocytic cell line. Eur J Biochem. 2000;267:1263–1269. doi: 10.1046/j.1432-1327.2000.01000.x. [DOI] [PubMed] [Google Scholar]
- 268.Won HY. Sohn JH. Min HJ. Lee K. Woo HA. Ho YS. Park JW. Rhee SG. Hwang ES. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal. 2010;13:575–587. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- 269.Wood LG. Fitzgerald DA. Lee AK. Garg ML. Improved antioxidant and fatty acid status of patients with cystic fibrosis after antioxidant supplementation is linked to improved lung function. Am J Clin Nutr. 2003;77:150–159. doi: 10.1093/ajcn/77.1.150. [DOI] [PubMed] [Google Scholar]
- 270.Xia YM. Hill KE. Burk RF. Biochemical studies of a selenium-deficient population in China: measurement of selenium, glutathione peroxidase and other oxidant defense indices in blood. J Nutr. 1989;119:1318–1326. doi: 10.1093/jn/119.9.1318. [DOI] [PubMed] [Google Scholar]
- 271.Xie Y. Bagby TR. Cohen MS. Forrest ML. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6:785–792. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Xu D. Rovira II. Finkel T. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell. 2002;2:251–252. doi: 10.1016/s1534-5807(02)00132-6. [DOI] [PubMed] [Google Scholar]
- 273.Xu XM. Carlson BA. Grimm TA. Kutza J. Berry MJ. Arreola R. Fields KH. Shanmugam I. Jeang KT. Oroszlan S. Combs GF., Jr. Marx PA. Gladyshev VN. Clouse KA. Hatfield DL. Rhesus monkey simian immunodeficiency virus infection as a model for assessing the role of selenium in AIDS. J Acquir Immune Defic Syndr. 2002;31:453–463. doi: 10.1097/00126334-200212150-00001. [DOI] [PubMed] [Google Scholar]
- 274.Xue H. Wang W. Li Y. Shan Z. Teng X. Gao Y. Fan C. Teng W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr J. 2010;57:595–601. doi: 10.1507/endocrj.k10e-063. [DOI] [PubMed] [Google Scholar]
- 275.Yang SJ. Hwang SY. Choi HY. Yoo HJ. Seo JA. Kim SG. Kim NH. Baik SH. Choi DS. Choi KM. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011;96:E1325–E1329. doi: 10.1210/jc.2011-0620. [DOI] [PubMed] [Google Scholar]
- 276.Yi JS. Holbrook BC. Michalek RD. Laniewski NG. Grayson JM. Electron transport complex I is required for CD8+ T cell function. J Immunol. 2006;177:852–862. doi: 10.4049/jimmunol.177.2.852. [DOI] [PubMed] [Google Scholar]
- 277.Youn HS. Lim HJ. Choi YJ. Lee JY. Lee MY. Ryu JH. Selenium suppresses the activation of transcription factor NF-kappa B and IRF3 induced by TLR3 or TLR4 agonists. Int Immunopharmacol. 2008;8:495–501. doi: 10.1016/j.intimp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 278.Yu L. Sun L. Nan Y. Zhu LY. Protection from H1N1 Influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice. Biol Trace Elem Res. 2011;141:254–261. doi: 10.1007/s12011-010-8726-x. [DOI] [PubMed] [Google Scholar]
- 279.Zamamiri-Davis F. Lu Y. Thompson JT. Prabhu KS. Reddy PV. Sordillo LM. Reddy CC. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic Biol Med. 2002;32:890–897. doi: 10.1016/s0891-5849(02)00775-x. [DOI] [PubMed] [Google Scholar]
- 280.Zeng H. Yan L. Cheng WH. Uthus EO. Dietary selenomethionine increases exon-specific DNA methylation of the p53 gene in rat liver and colon mucosa. J Nutr. 2011;141:1464–1468. doi: 10.3945/jn.111.140715. [DOI] [PubMed] [Google Scholar]
- 281.Zhang F. Yu W. Hargrove JL. Greenspan P. Dean RG. Taylor EW. Hartle DK. Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium. Atherosclerosis. 2002;161:381–386. doi: 10.1016/s0021-9150(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 282.Zhang Y. Chen X. Reducing selenoprotein P expression suppresses adipocyte differentiation as a result of increased preadipocyte inflammation. Am J Physiol Endocrinol Metab. 2011;300:E77–E85. doi: 10.1152/ajpendo.00380.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhao X. Carnevale KA. Cathcart MK. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J Biol Chem. 2003;278:40788–40792. doi: 10.1074/jbc.M302208200. [DOI] [PubMed] [Google Scholar]
- 284.Zheng HT. Zhou LN. Huang CJ. Hua X. Jian R. Su BH. Fang F. Selenium inhibits high glucose- and high insulin-induced adhesion molecule expression in vascular endothelial cells. Arch Med Res. 2008;39:373–379. doi: 10.1016/j.arcmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 285.Zhou R. Tardivel A. Thorens B. Choi I. Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 286.Zhou R. Yazdi AS. Menu P. Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]